Abstract
Blaine specific surface area (SSA) is an essential physical property of iron concentrate and is availably improved by a high-pressure grinding roller (HPGR) to enhance the pelletability and the qualities of oxidized pellets. In this study, the four concentrates with various SSA were obtained by HPGR treatment to produce the oxidized pellets. The results show that the high degree of lattice deformation corresponds to high SSA which is beneficial for improving the quality of green, preheated, and fired pellets. The compressive strength increases from 600 to 1870 N·pellet−1 for preheated pellets and increases from 2530 to 4270 N·pellet−1 for fired pellets when SSA increased from 849 cm2/g to 1601 cm2/g, under the optimal condition of preheating at 1050 °C for 12 min and firing at 1250 °C for 15 min. The oxidation of magnetite is accelerated. Low porosity and microphotographs with integrated hematite joined crystallization support the strength enhancement by high SSA.
1. Introduction
Pellets and sinter are the main burdens of blast furnace ironmaking. Compared with the sintering process, the pollutant emission and energy consumption of the pelletizing process are significantly minor [1]. Recently, augmenting the proportion of pellets in the blast furnace burden has attracted much attention in the Chinese steel industry due to precise environmental policies [2,3]. For improving the transport and the blast furnace performance, the mechanical strength and metallurgical performance of the pellets are required to be improved by oxidizing and roasting [4,5].
Recent years have witnessed a growing academic interest in the effect of the chemical properties of iron concentrate on the strength of pellets [6,7,8,9]. Iron concentrates account for the major composition in pellets. The optimization of concentrates matching according to chemical compositions is a key determinant of producing superior pellets. However, although different iron concentrates might have similar chemical compositions, their disintegrated characteristics were vitally discrepant [10]. Therefore, the physical property impacts equally on the strength of pellets. It features mainly the specific surface area (labeled SSA), granularity, and pelletability. Ball grinding, wet grinding, and grinding with a high-pressure grinding roller (HPGR), are prevalently applied to ameliorate the physical properties of iron concentrates.
HPGR is beneficial for improving the pelletability of ore pellet feed material. It provides extrusion force and shear stress for iron concentrate particles to form cakes, which boosts the lattice deformation in the fresh surfaces that are generated from particles to improve the SSA [11]. In the studies [12,13], the mechanism of the increase of SSA with pellet feed by HPGR was revealed that the efficient transfer of mechanical energy to chemical energy leads to particle deformation. The evidence from several experimental studies [14,15,16] has established that the pelletizing performance of iron concentrates could be positively improved by HPGR with lower energy consumption.
The impact of SSA on the strength of green pellets has recently been studied. Zhang et al. investigated the pellets that were produced by an iron ore with low silicon and the strength of which was examined by comparing the SSA fitness and particles fitness [17]. The conclusions were established that both the smaller particle size and high SSA have a positive effect on pellets strength. Abazarpoor et al. found that the strength and the roughness of particles were improved, which corresponds to the higher SSA of the iron concentrate by HPGR than by a ball mill [18]. Pal et al. demonstrated that both too low SSA and too high SSA hamper the improvement of pellets strength [19]. All the mentioned studies confirmed the positive effects of SSA on green pellets strength. However, the studies have suffered from a lack of the effects of preheating and firing on the quality of oxidized pellets.
This study aims to examine the influence of SSA on the roasting system at different temperatures at different times for producing oxidized pellets with high strength. The Blaine SSA of the tested iron concentrates that were treated with HPGR ranged from 849 cm2/g to 1601 cm2/g. The fineness of the concentrates was evaluated by particle size distribution, BET SSA, and lattice deformation. With the strength of pellets tested, the influence of time and temperature was studied to optimize the preheating and firing. The effect of SSA on oxidation kinetics was investigated. The porosity and morphology of the roasted pellets were examined under the optimal roasting conditions.
2. Materials and Methods
2.1. Raw Materials
The iron concentrate and bentonite were obtained from LiuSteel China, and the chemical compositions that were analyzed by an X-ray Fluorescence Spectrometer (Thermo Fisher Scientific, Shanghai, China) are listed in Table 1. The mass fraction of Fe3O4 is estimated to be approximately 46.11% based on the 14.31% FeO in Table 1. Fe3O4, which could be oxidized to Fe2O3, is prevalently regarded as Fe2O3·FeO for Fe2+ and Fe3+ in it. The iron concentrate with high Fe3O4 content is suitable for preparing oxidized pellets by roasting at high temperatures. As shown in Table 2, the bentonite was used as a binder which presents the montmorillonite content of 51.56%, the swelling volume of 9.6 mL/g, and water absorption of 302.63%.

Table 1.
Chemical composition of the iron concentrate and Bentonite (mass fraction %).

Table 2.
Physical properties of Bentonite.
Concentrate A is the raw iron ore concentrate with the Blaine SSA of 849 cm2/g. Concentrates B, C, and D were obtained by treating Concentrate A for adequate grinding times in HPRG treatment to possess high SSA. The particle morphologies of the four concentrates under SEM (scanning electron microscopy) (Japanese Electronics Corporation, Beijing, China) are displayed in Figure 1. It can be seen that the particles of the initial Concentrate A seem smooth and large. For the high SSA concentrates, the surface roughness of particles is enlarged and the tiny particles that are generated by HPGR adhere to other large ones with microcracks.
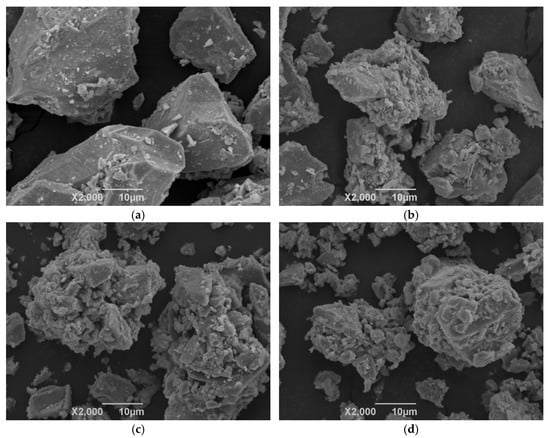
Figure 1.
Morphology of concentrates with varying specific surface area 2000×. (a) Concentrate A, SSA 849 cm2/g; (b) Concentrate B, SSA 1282 cm2/g; (c) Concentrate C, SSA 1445 cm2/g; (d) Concentrate D, SSA 1601 cm2/g.
2.2. Experimental Methods
Concentrates B, C, and D with the required SSA were prepared by treating Concentrate A with the HPGR (2PG400 × 250, manufactured by Jinpeng Mining Machinery Co., LTD, Yantai, China). The parameters of HPGR were set with the grinding roller of 400 mm in diameter and 250 mm in length, the roller pressure of 1.5 MPa, the roller speed of 120 r/min, and the moisture of 8% in the concentrate. The particle size distribution of concentrates was measured by sieving. SSA was measured both by the Blaine permeability apparatus (SBT-127) according to the standard of ASTMC 204-2007 and by the nitrogen gas adsorption apparatus (ASAP 2460) according to the BET method. The X-Ray diffraction (XRD) patterns of the concentrates were tested by the X-ray diffractometer (D8ADVANCE) to analyze the lattice deformation.
The pellets that were pelletized by Concentrate A, B, C, and D are labeled as Pellet A, B, C, and D, respectively. The conventional pelletizing flowsheet that is shown in Figure 2 was utilized in the present study. It involves blending the concentrate with 2.1% bentonite, pelletizing for 15 min in a disc pelletizer (0.8 m in diameter, 0.2 m rim depth, 32 rpm rotational speed, 45° inclination angle), sieving green pellets to have a size of 10-12 mm, and drying in an oven at 105 °C for 4 h. The pellets were introduced to the furnace (CX-1400G) with two heating zones where the preheating and firing temperature were raised to a predetermined temperature. The quality of the roasted pellets was evaluated by testing the compressive strength according to the standard of ISO 4700. Drop number test for green pellets was carried out at 0.5 m height.
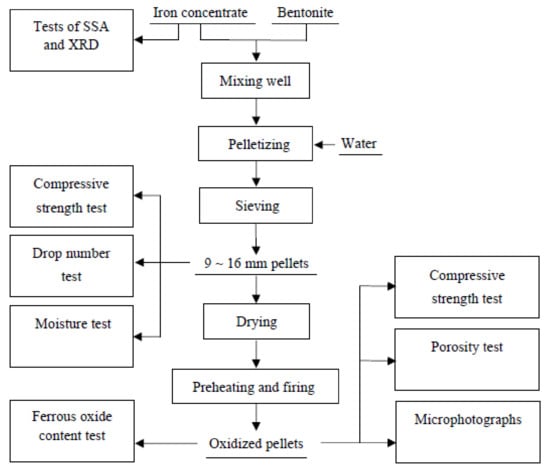
Figure 2.
Pelletizing flowsheet.
The porosity of the preheated and fired pellets was measured by conforming to the Chinese Standard (GB/T 24586-2009). The microphotographs of the concentrates and pellets were obtained with SEM (JSM-6490LV). The ferrous oxide content of the pellets was determined by chemical titration according to the standard (GB/T 6730.8-2016). The magnetite oxidizability of the roasted pellets was obtained by the formula as follows:
where α, Cp, and C0 represent the magnetite oxidizability (%), the ferrous oxide content of the roasted pellets, and the ferrous oxide content of the dry green pellets, respectively.
α = 1 − Cp/C0
3. Results and Discussion
3.1. Fineness of Concentrates
The size fraction and SSA of the iron concentrate by HPGR are presented in Table 3. Concentrate A possesses the SSA of 849 cm2/g and the particles passing 0.074 mm are over 98%. Concentrates B, C, and D possessing a high Blaine SSA of 1282–1601 cm2/g were obtained by treating Concentrate A for adequate grinding times. By sieving, their particles passing 0.037 mm gradually increase with the increase of Blaine SSA. Although the particles of all the four concentrates passing 0.045 mm are over 90%, their fraction also increases slowly with the enhancement of Blaine SSA. The BET SSA by gas adsorption, however, has a larger order of magnitude than the Blaine SSA, but it varies with the same trend. The high BET SSA indicates the improvement of surface activation, which is beneficial for the oxidation in the roasting of pellets.

Table 3.
Size fraction and Blaine specific surface area of mixed iron concentrates.
The Blaine SSA measured by air permeability and by comparing with a standard cement sample shows relative fineness of powder samples. It should be controlled in an appropriate range for iron-concentrate pelletization. The SSA of the feed material by HPGR treatment is with an increase of 300–600 cm2/g but it can be affected by the edge material. [20] A recommended range is 1700–2250 cm2/g [21] for iron concentrate pelletization. However, the appropriate range is not absolute. For instance, the ultra-fine iron concentrate after beneficiation is prevalent with poor pelletization but possesses the SSA above 2600 cm2/g. [19] Therefore, the XRD patterns of the four concentrates were further refined to study the correlation between SSA and lattice deformation.
The fineness of concentrates is considerably determined by the lattice structure and the surface activation depends mainly on the lattice deformation. This activated surface forms the force among particles in pelletization. The XRD patterns of the concentrates are shown in Figure 3. The raw concentrate mainly consists of hematite and magnetite. The intensity reduces and the full width of the peaks at half maximum (FWHM) widens when the concentrates with high SSA were diffracted. The result implies that the HPGR treatment augmented the lattice deformation of the concentrates. The lattice deformation can be described by the strain which follows the Scherrer equation:
where K, λ, F, S, εi, and θ represent the crystal shape factor, wavelength of the X-ray source, FWHM, mean crystallite size, crystallite strain of one concentrate, and diffraction angle, respectively. The crystallite strain and mean size were obtained by refining XRD patterns, as shown in Figure 4. The crystallite strain in Concentrate B, C, and D increases by 8.43, 16.87 and 61.45%, respectively, relative to Concentrate A. The crystallite size also reduces as the SSA increases. These microscopic results are consistent with the fineness results, which suggests that the higher degree of lattice deformation and smaller crystallite size support the increase of SSA. The increased active surface among particles is expected to enhance the recrystallization in the pellet to produce higher-strength oxidation pellets.
Fcosθ = Kλ/S + 4εisinθ
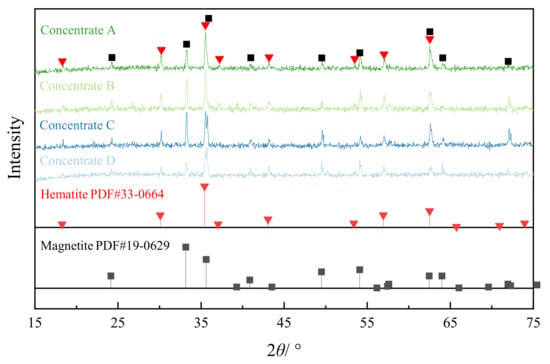
Figure 3.
XRD patterns of the concentrates with different SSA by HPGR treatment.
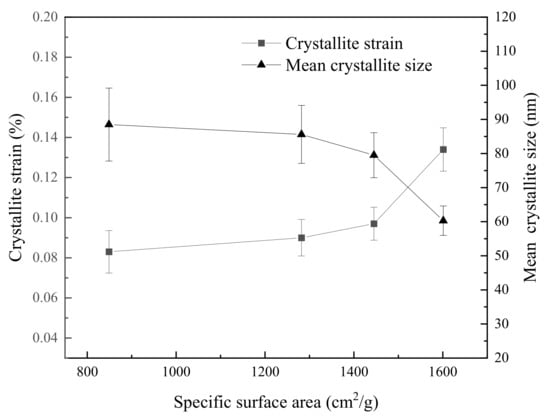
Figure 4.
Crystallite strain and the size of concentrates with different SSA.
3.2. Effect of Specific Surface Area on Green Pellet Quality
The drop number and compressive strength of green pellets with various SSA of 849–1601 cm2/g are shown in Figure 5. Both the two indexes are improved by increasing SSA. Green Pellets B, C, and D seem to have a positive linear relationship in terms of strength versus SSA. The result can be traced to the particle size (Figure 1). The considerable fraction of particles below 0.037 mm in Table 3 shows evidence of the positive correlation between SSA and fine particle size. In pelletizing, the tiny particles that are generated by HPGR treatment filled in the gap that was caused by large particles, which results in a compaction pellet. In addition, the roughness on fresh surfaces contributes to the enhancement of the adhesion force between particles, the capillary force for water absorption, and surface free energy [12]. Therefore, the quality of green pellets can be prevalently improved by augmenting the fraction of fineness in particles.
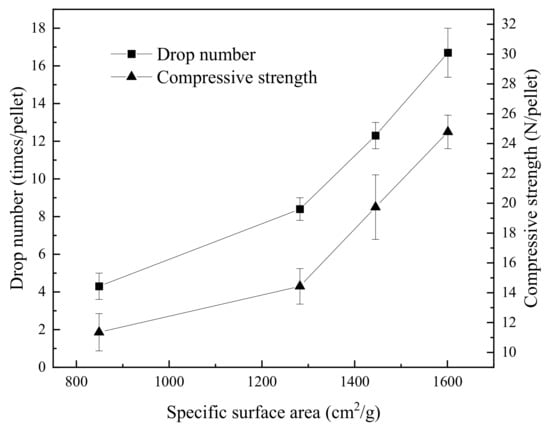
Figure 5.
Various drop numbers and the compressive strength with specific surface area for green pellets.
3.3. Effect of Specific Surface Area on the Properties of Preheated Pellets
3.3.1. Effect of Preheating Temperature
Figure 6 illustrates the effect of preheating temperature on the compressive strength of the pellets with various SSA. All the pellets were preheated at different temperatures in the range of 1000 to 1075 °C for 15 min. The compressive strength of pellets increases gradually with an increase in preheating temperature and SSA. The compressive strength of preheated pellets increased dramatically when the SSA increased from 849 to 1445 cm2/g, and then increased slightly when the SSA increased from 1445 to 1601 cm2/g. The preheating temperature of 1050 °C is appropriate because the effect of temperature on strength decreases when the preheating temperature raises from 1050 to 1075 °C. High SSA accelerates the oxidation reaction of fine particles in pellets but the improvement may be restricted at a higher temperature.
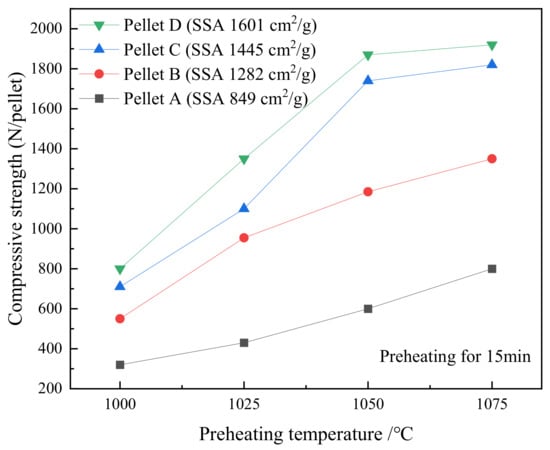
Figure 6.
The effect of temperature on the compressive strength of various SSA pellets that were preheated for 15 min.
The strength of the roasted pellets is determined by the recrystallization of hematite (Fe2O3). The oxidation of magnetite to hematite can be facilitated not only by increasing the preheated temperature and time but also by improving the lattice deformation. The transformation of smooth and large particles to rough and tiny particles can improve the oxidation rate due to more reactive sites. The high degree of lattice deformation corresponds to high SSA [12]. The fresh hematite with high crystalline activity is thus formed. Thus, the high-SSA concentrates that were pretreated by HPGR can produce high-strength oxidized pellets due to the improvement of magnetite oxidation.
3.3.2. Effect of Preheating Time
Figure 7 displays the effect of preheating time on the compressive strength of the pellets that were preheated at 1050 °C. The compressive strength of the preheated pellets improved when the preheating time was prolonged or the SSA increased. Pellets B, C, and D show faster growth of compressive strength than Pellet A, the strength of which seems to be linearly correlated with preheating time. According to the requirement of the compressive strength that is higher than 500 N·pellet−1 for the grate-kiln process, the optimal preheating time of 12 min was selected to undertake the production demand and save energy.
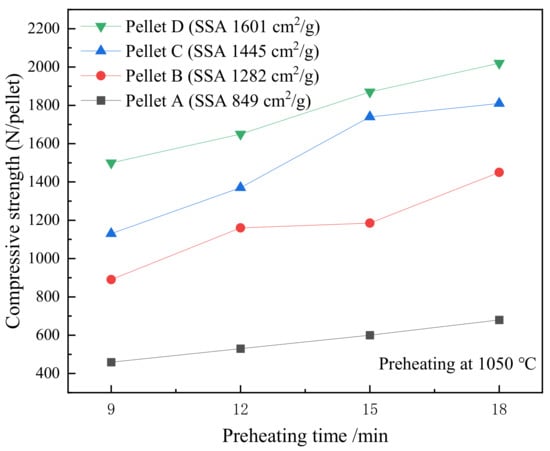
Figure 7.
The effect of time on the compressive strength of various SSA pellets that were preheated at 1050 °C.
3.4. Effect of Specific Surface Area on the Properties of the Fired Pellets
3.4.1. Effect of Firing Temperature
Figure 8 presents the effect of firing temperature on the fired pellets strength. The strength of the fired pellets increases with the increased temperature. The higher SSA, the higher the compressive strength. The compressive strength of Pellet C and D reached approximately 4000 to 4500 N·pellet−1 when the pellets were fired at the temperature in the range of 1250 to 1275 °C because of the higher SSA. The optimal firing temperature is considered to be 1250 °C due to the slow increase of strength above 1250 °C.
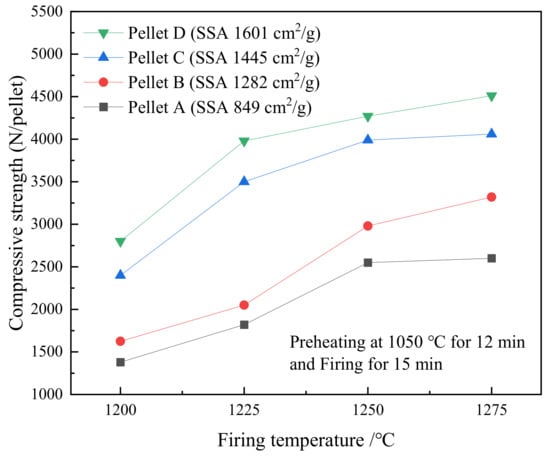
Figure 8.
The effect of temperature on the compressive strength of various SSA pellets that were fired for 15 min after preheating at 1050 °C for 12 min.
A portion of the heat in firing at high temperature has the potential to be substituted by the specific energy [22] in HPGR at room temperature when high-SSA concentrates produce high-strength pellets. In terms of producing the oxidized pellets with high quality, the energy consumption may be lowered by reducing the roasting temperature. The estimated energy saving can be obtained by comparing the power consumption of HPGR and the heat consumption of firing. From Figure 8, to achieve the strength above 2000 N·pellet−1, firing Pellet C is at 1200 °C whereas firing Pellet A is at 1250 °C, which indicates a heat decline of 4%. For a pellet production in a shaft furnace, per ton the pellets approximately consumed 7 kWh (25 MJ) in HPGR and 800 MJ in roasting. The reduction of heat consumption is 32 MJ calculated by the 4% heat decline from 1250 °C to 1200 °C. Thus, 7 MJ can be saved per ton of pellets, which indicates 2.1 TJ may be saved per year under the pellets production of 300 thousand tons.
3.4.2. Effect of Firing Time
The effect of firing time on the compressive strength of various SSA pellets that were fired at 1250 °C is shown in Figure 9. The compressive strength of pellets increased when the firing time was prolonged from 9 to 18 min. The pellets with high SSA presented a higher compressive strength. Pellet D reaches the highest strength of 4650 N·pellet−1 when the pellets are fired at 1250 °C for 18 min. As usual, the compressive strength of 2500 N·pellet−1 could meet the requirement for a blast furnace. Overall, the optimal firing time is 15 min since all the pellets possess a strength above 2250 N·pellet−1.
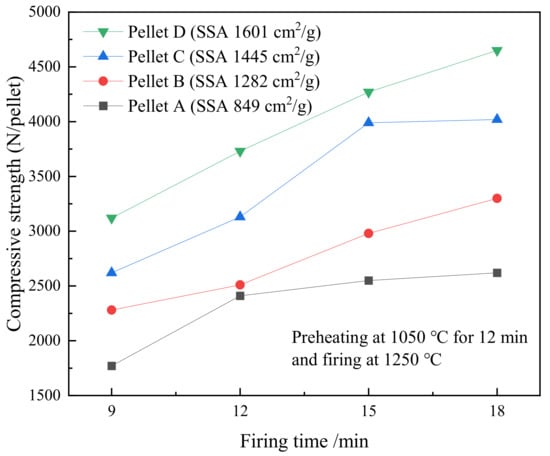
Figure 9.
The effect of time on the compressive strength of various SSA pellets that were fired at 1250 °C after preheating at 1050 °C for 12 min.
3.5. Effect of Specific Surface Area on the Oxidation Kinetics of Magnetite
Complete magnetite oxidation is beneficial for the hematite recrystallization building compact structures of fired pellets. To reveal the strength enhancement mechanism of roasted pellets, the effect of SSA on the magnetite oxidizability (α) was investigated. The variation of α versus preheating time at 1050 °C is shown in Figure 10. The α gradually increases with time and the high α is accompanied with low SSA in 12 min. The α of all the four pellets rapidly reaches more than 60% at 3 min and then slowly increases. The result is consistent with the research on the oxidation of magnetite pellets [23,24]. A low SSA implies larger concentrate particle size, which leads to larger pellet porosity. More large holes in pellets facilitate the diffusion of oxygen to the surface of interior magnetite, which accelerates the α in a shorter period. However, this rapid oxidation may be inhibited in the later stages of oxidation since oxygen is difficult to diffuse into the non-porous large particles such as Figure 1a. The slower oxidation rate in the later stages is thus mainly controlled by internal diffusion at high temperatures. To investigate the effect of SSA on the oxidation rate, the α after 3 min was discussed.
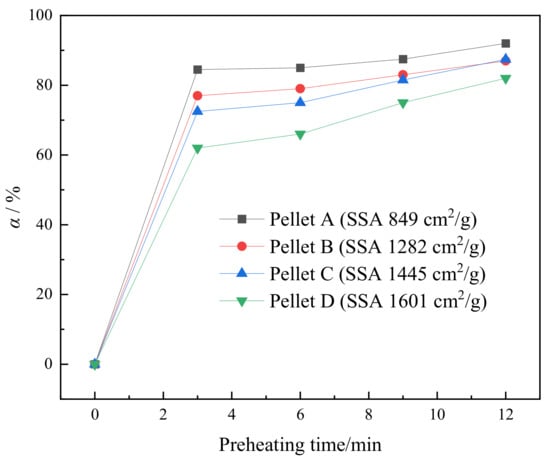
Figure 10.
The variation of α versus the preheating time at 1050 °C.
Magnetite oxidation is a first-order reaction conforming to the following rate equation [25].
where α0, k, and t represent the oxidizability at 3 min, rate constant, and preheating time, respectively. The rate constant is obtained by fitting the slope to the graph of ln(α/α0) versus t. As shown in Figure 11, the rate constants increase with the increase of SSA. Despite the low oxidation rate, the oxidation with high SSA is accelerated after preheating for 3 min. The results suggest that high SSA is more favorable for pellets to achieve a high oxidizability.
ln(α/α0) = kt
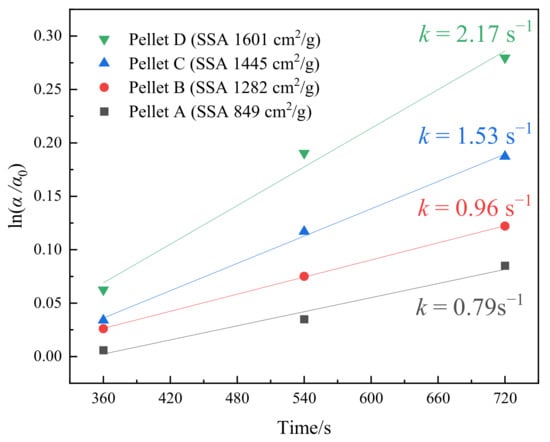
Figure 11.
The value of ln(α/α0) against t at 1050 °C.
More transformation of magnetite to hematite definitely contributes to the recrystallization of hematite. Higher SSA indicates more lattice deformation and fissures, and oxygen transports through particle cracks to the reaction interface on the nascent surface of magnetite. The oxygen diffusion and the oxidation are thus facilitated. Meanwhile, the easily forming hematite microcrystals are recrystallized by the Ostwald ripening effect which leads to the formation and recrystallization of large grain bridge crystals from minor grains [19]. To support the strength enhancement mechanism, hematite recrystallization inside the pellet needs to be observed.
3.6. Porosity and Microphotographs of Roasted Pellets
The above-mentioned discussion provides the relatively optimal conditions for producing high-quality oxidized pellets with the different SSA concentrates: preheating at 1050 °C for 12 min and firing at 1250 °C for 15 min. The compressive strength of the preheated and fired pellets is shown in Figure 12. The strong growth of all four pellets during firing is approximately 2000 N·pellet−1. It suggests that high SSA concentrates probably contribute to pelletizing high-strength oxidized pellets. The roasting temperature and time may also be lowered due to the enhancement of compressive strength by the increase of SSA.
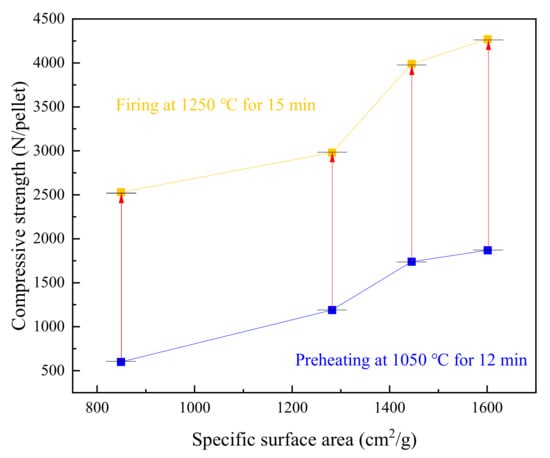
Figure 12.
The compressive strength of the preheated and fired pellets under optimal preheating and firing.
Low porosity, which represents compaction, responds to the high strength of the roasted pellets. Figure 13 displays the porosity of the pellets that were preheated at 1050 °C for 12 min and the pellets that were fired at 1250 °C for 15 min. The porosity of the roasted pellets reduces as SSA grows. The firing process augments the compaction in the pellets with high SSA due to the decline of porosity. Due to the HPGR treatment, the porosity of the green pellets that are pelletized by disintegrated particles has definitely decreased. The extrusion among adjacent particles indicates a low dispersion degree of particles [11]. The crystallization connection of Fe2O3 particles is thus improved. Additionally, the low porosity with high SSA implies a reduction in the hole size instead of the number of holes, since the oxidation is accelerated by oxygen transfer in the microcracks. The hematite crystals that are generated from complete oxidation can be further recrystallized in firing by the Ostwald ripening effect.
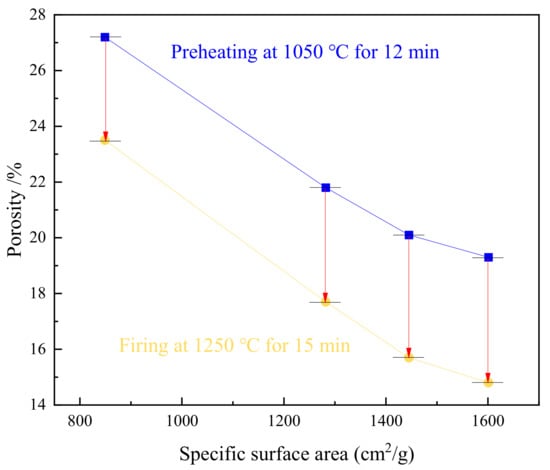
Figure 13.
The compressive strength of the preheated and fired pellets under optimal preheating and firing.
The compacted structure inside the pellets can be seen from the microphotographs in Figure 14. Pellet A both preheated and fired contains large holes and features the intertwined blend of Fe2O3 and peridotite particles, which indicates the Fe2O3 particles have not evolved completely for pelletizing by low SSA concentrates. As SSA increases, the oxidized pellets exhibit a reduction in the number of black holes, the shrinkage in the size of the holes, and the decline of porosity. As can be seen from Figure 14d, in the fired Pellet D with the highest SSA, no significant boundary of Fe2O3 might be found, and the peridotite and holes seem extremely small.

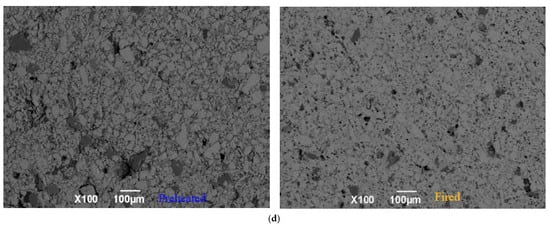
Figure 14.
Microphotographs of the preheated and fired pellets under optimal preheating and firing 100×. Fe2O3—off-white, granular, or interconnected crystals; peridotite—dark grey, granular; holes—black, irregular. (a) Concentrate A, SSA 849 cm2/g; (b) Concentrate B, SSA 1282 cm2/g; (c) Concentrate C, SSA 1445 cm2/g; (d) Concentrate D, SSA 1601 cm2/g.
4. Conclusions
With the improvement of the SSA of the iron concentrate, the oxidized pellets achieve high strength. The high SSA characterizes fine particle distribution and a high degree of lattice deformation, which not only compacts the particles in pellets but also improves the recrystallization and oxidation in roasting. The high-strength oxidized pellets are with reduced porosity and tight hematite crystallization. The increase in SSA by HPGR treatment allows the pellets to be roasted at a lower temperature and time to reduce energy consumption.
Author Contributions
Conceptualization, T.-J.C.; Formal analysis, B.-C.L.; Investigation Z.-Y.R. and Z.-H.W.; Methodology, M.-B.D. and T.-J.C.; Project administration, T.-J.C.; Resources, Z.-Y.R. and Z.-H.W.; Validation, M.-B.D.; Visualization, B.-S.G.; Writing—original draft, M.-B.D. and B.-S.G.; Writing—review and editing, M.-B.D. and T.-J.C.; Software, B.-C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Anhui Provincial Natural Science Foundation, grant number 2008085ME175, and the Graduate Natural Science Research Project of Colleges and Universities of Anhui Province in 2021, grant number YJS20210328.
Acknowledgments
This work was supported by the Anhui Provincial Natural Science Foundation (grant number 2008085ME175), and the Graduate Natural Science Research Project of Colleges and Universities of Anhui Province in 2021 (grant number YJS20210328).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, H.; Peng, S.H.; Zhang, K.; Qin, Y.L.; Meng, F.; He, W.C.; Liu, W.Q.; Chen, M.; Yan, L.X. Effect of finely ground limestone and dolomite on compression strength and reduction swelling of vanadium-titanium pellets. Materials 2021, 14, 4433. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Ryberg, M.; Yang, Y.; Feng, K.S.; Kara, S.; Hauschild, M.; Chen, W.Q. Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts. Nat. Commun. 2021, 12, 2066. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Dai, M.B.; Chun, T.J.; Long, H.M.; Wei, J. Influence of the gangue compositions on the reduction swelling index of hematite briquettes. Metall. Mater. Trans. B 2021, 52, 2139–2150. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Schenk, J.; Zhang, J.L.; Liu, Z.J.; Wang, J.; Niu, L.L.; Cheng, Q. Novel sintering indexes to evaluate and correlate the crystal characteristics and compressive strength in magnetite pellets. Powder Technol. 2020, 36, 517–526. [Google Scholar] [CrossRef]
- Copeland, C.R.; Claremboux, V.; Kawatra, S.K. A comparison of pellet quality from straight–grate and grate–kiln furnaces. Miner. Processing Extr. Metall. Rev. 2018, 40, 218–223. [Google Scholar] [CrossRef]
- Meyer, M.M.; Lagoeiro, L.E.; Graça, L.M.; Silva, C.J. Phase and microstructural characterization of iron ore pellet and their relation with cold crushing strength test. Miner. Processing Extr. Metall. Rev. 2016, 37, 295–304. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Q. Experimental Research on the Roasting of Brazilian Iron Concentrate in a Tube Furnace. In The Minerals, Metals & Materials Series, Proceedings of the Materials Engineering—From Ideas to Practice: An EPD Symposium in Honor of Jiann–Yang Hwang; Luby, M., Ed.; Springer Nature: Berlin, Germany, 2021. [Google Scholar]
- Lu, J.G.; Lan, C.C.; Liu, Q.; Zhang, S.H.; Sun, J.N. Effects of SiO2 on the preparation and metallurgical properties of acid oxidized pellets. Int. J. Miner. Metall. Mater. 2021, 28, 629. [Google Scholar] [CrossRef]
- Chun, T.J.; Mu, G.T.; Meng, Q.M.; Long, H.M.; Wang, P.; Bi, C.G. Preparation of MgO added iron ore pellets and effects on a pilot scale blast furnace operation. J. Min. Metall. Sect. B Metall. 2019, 55, 165–175. [Google Scholar] [CrossRef] [Green Version]
- Campos, T.M.; Bueno, G.; Rodriguez, V.A.; Böttcher, A.C.; Kwade, A.; Mayerhofer, F.; Tavares, L.M. Relationships between particle breakage characteristics and comminution response of fine iron ore concentrates. Miner. Eng. 2021, 164, 106818. [Google Scholar] [CrossRef]
- Fan, J.J.; Qiu, G.Z.; Jiang, T.; Guo, Y.F.; Hao, H.Z.; Yang, Y.B. Mechanism of high-pressure roll grinding on compression strength of oxidized hematite pellets. J. Cent. South Univ. 2012, 19, 2611–2619. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Pan, J.; Qiu, G.Z.; Clout, J.; Wang, C.A.; Guo, Y.F.; Hu, C.F. Mechano–chemical activation of magnetite concentrate for improving its pelletability by high pressure roll grinding. ISIJ Int. 2004, 44, 310–315. [Google Scholar] [CrossRef]
- Aminalroaya, A.; Pourghahramani, P. Investigation of particles breakage and weakening behaviors in multi–component feed grinding by high pressure grinding rolls (HPGR). Miner. Processing Extr. Metall. Rev. 2022, 43, 217–232. [Google Scholar] [CrossRef]
- Mo, Y.P.; Fan, J.J.; Wang, Y. Improving of Balling performance by High Pressure Roll Grinding. Key Eng. Mater. 2011, 481, 533–538. [Google Scholar]
- Zhu, D.Q.; Yang, C.C.; Pan, J.; Zhong, Y. Improving the Pelletization of Chromite Concentrate by hpgr and Its Mechanism. In 6th International Symposium on High–Temperature Metallurgical Processing; The Minerals, Metals & Materials Society: Warrendale, PA, USA, 2016. [Google Scholar]
- Abazarpoor, A.; Halali, M.; Hejazi, R.; Saghaeian, M. HPGR effect on the particle size and shape of iron ore pellet feed using response surface methodology. Miner. Processing Extr. Metall. 2018, 127, 40–48. [Google Scholar] [CrossRef]
- Zhang, Y.; Qing, G.L.; Tian, Y.Q.; Zhao, Z.X.; Liu, G.Y.; Wang, K.; Liu, W.W.; Li, M.; Sun, D.W.; Zhao, L.Y.; et al. Pelletizing of Iron Ore with High Iron Grade and Low Silicon Content. In 12th International Symposium on High–Temperature Metallurgical Processing; The Minerals, Metals & Materials Society: Warrendale, PA, USA, 2016. [Google Scholar]
- Abazarpoor, A.; Halali, M.; Hejazi, R.; Saghaeian, M.; Zadeh, V.S. Investigation of iron ore particle size and shape on green pellet quality. Can. Metall. Q. 2020, 59, 242–250. [Google Scholar] [CrossRef]
- Pal, J.; Ghorai, S.; Venugopalan, T. Effect of high Blaine iron ore fines in hematite ore pelletization for blast furnace. Miner. Processing Extr. Metall. 2020, 129, 299–307. [Google Scholar] [CrossRef]
- Van Der Meer, F.P. Pellet feed grinding by HPGR. Miner. Eng. 2015, 73, 21–30. [Google Scholar] [CrossRef]
- Pal, J.; Ghorai, S.; Nandi, B.; Chakraborty, T.; Das, G.; Venugopalan, T. Effect of pyroxenite and olivine minerals as source of MgO in hematite pellet on improvement of metallurgical properties. J. Cent. South Univ. 2015, 22, 3302–3310. [Google Scholar] [CrossRef]
- Campos, T.M.; Bueno, G.; Barrios, G.K.P.; Tavares, L.M. Pressing iron ore concentrate in a pilot–scale HPGR. Part 1: Experimental results. Miner. Eng. 2019, 140, 105875. [Google Scholar] [CrossRef]
- Tang, W.D.; Xue, X.X.; Yang, S.T.; Jiang, T. Mineralogical characteristics and isothermal oxidation kinetics of Hongge chromium containing vanadium and titanium magnetite pellets. Chin. J. Eng. 2018, 40, 548–556. [Google Scholar]
- Zhu, D.Q.; Luo, Y.H.; Pan, J.; Zhou, W.T. Study on High Temperature Oxidation Kinetics of Magnetite. Metal Mine 2011, 418, 89–93. [Google Scholar]
- Qiu, G.Z.; Zhu, D.Q.; Pan, J.; Wang, C.A.; Guo, Y.F.; Jiang, T.; Hu, C.F.; Clout, J.; Shu, F.H. Improving the oxidizing kinetics of pelletization of magnetite concentrate by high press roll grinding. ISIJ Int. 2004, 44, 69–73. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).