Abstract
To increase the low utilization rate of spodumene ore during lithium extraction, spodumene ore was subjected to carbothermic reduction to enrich lithium and prepare a manganese-silicon alloy. The experimental results showed that during thermal reduction, lithium was volatilized and collected in the condensation zone. The Li2O content in the lithium condensate was 41.72%, which was 10.85 times higher than that of the raw material. The effects of varying reduction temperatures and times on the lithium volatilization rate and direct yield of Mn5Si3 alloy were investigated. The best process conditions were 1873 K for 6 h. Under these conditions, the lithium volatilization rate was 97.65%, and the direct yield of Mn5Si3 was 86.47%.
1. Introduction
With the rapid development of science and technology, lithium is an important material for nuclear energy, energy storage, aerospace, and other fields [1,2,3,4]. Lithium extraction from spodumene ore is an important process in the production of lithium products [5,6,7]. As an aluminosilicate ore [8,9,10], the theoretical composition of spodumene is 8.03% Li2O [11,12], 27.4% Al2O3, and 64.6% SiO2 [13]. Lithium extraction from spodumene ore is still mainly performed by the sulfuric acid method [14,15,16,17]. When lithium is extracted by the sulfuric acid method, the spodumene ore is transformed and roasted at 900–1050 °C, and the dense α-spodumene (α-LiAlSi2O6) is transformed into loose β-spodumene (β-LiAlSi2O6). β-LiAlSi2O6 is mixed with excess concentrated sulfuric acid and subjected to secondary calcination at 170–250 °C. The calcined product is concentrated, immersed in water, and filtered to obtain purified Li2SO4 concentrate. Then, Na2CO3 is added to the concentrate to undergo ion exchange with Li2SO4 to obtain insoluble Li2CO3 precipitate [18,19,20,21]. The sulfuric acid method only considers the extraction of Li and converts Si2O and Al2O3 in spodumene ore into hazardous waste residues [22,23]. Taking spodumene concentrate with 6% Li2O content as an example, for every ton of lithium carbonate (Li2CO3) that is produced, 8–10 tons of hazardous waste slag and 6–9 tons of high-salt wastewater are produced [24,25,26]. This causes environmental pollution and wastes resources.
Owing to the problems associated with the acid–sulfur method, in this paper, lithium was enriched by adding coke and manganese dioxide (MnO2) to spodumene by thermal reduction. SiO2 and Al2O3 were recovered in spodumene ore. The reported method represents a new technology for lithium extraction from spodumene for the comprehensive recovery and utilization of its valuable components.
2. Materials and Methods
2.1. Materials
The spodumene ore used in the experiment was obtained from Africa. X-ray diffraction was used to analyze the crystal phases of spodumene; the results are shown in Figure 1. The ore was composed of spodumene (LiAlSi2O6) and quartz (SiO2). Inductively coupled plasma emission spectroscopy (ICP-OES) was used for quantitative element analysis of the ore; the results are shown in Table 1. The coke was produced by Ningxia Huiheng (China), and its composition is shown in Table 2.
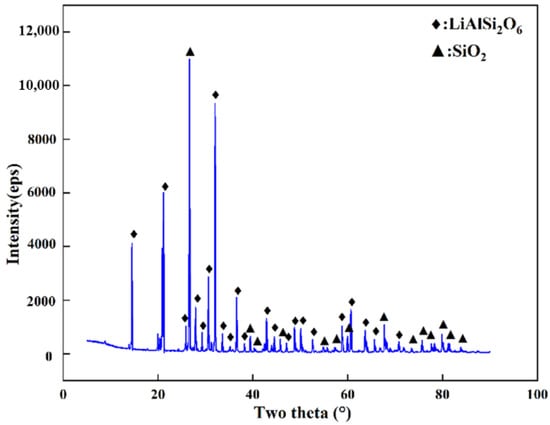
Figure 1.
XRD pattern of spodumene ore.

Table 1.
Spodumene ore composition.

Table 2.
Coke composition.
2.2. Methods
First, the spodumene ore and coke were crushed and screened through 200 mesh. The spodumene ore, coke, and manganese dioxide were mixed evenly (), pressed into blocks under a pressure of 18 MPa, and placed into the graphite crucible of a vacuum furnace. The vacuum pump was turned on, and after reaching the vacuum state, argon gas was passed until the pressure reached 3 × 104–3.5 × 104 Pa (the atmospheric pressure in the experimental area was about 8 × 104 Pa). Heating was started according to the preset experimental conditions at a rate of approximately 10 K/min. At the end of the reaction (the pressure in the furnace was about 7 × 104–7.5 × 104 Pa), the residue in the graphite crucible and the condensate in the stainless-steel crucible were removed for characterization. The reaction process flow was shown in Figure 2.
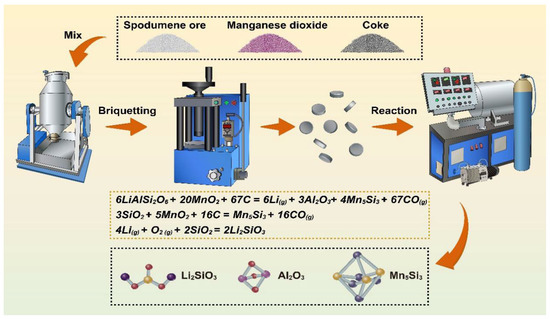
Figure 2.
Schematic diagram of the reaction process. The raw materials were first mixed by a blender; then, the mixture was pressed into a round cake shape, entering the vacuum furnace for the preset experimental conditions.
2.3. Characterization Methods
2.3.1. Lithium Volatilization Rate
In the formula, is the volatilization rate of lithium, is the mass of lithium in the reduced residue, and is the mass of lithium in the raw material.
2.3.2. Lithium Enrichment Rate
In the formula, is the enrichment ratio of lithium, is the content of Li in the condensate, and is the content of Li in the raw material.
2.3.3. Alloy Direct Yield
In the formula, is the direct yield of manganese-silicon alloy, is the mass of manganese-silicon alloy in the reduced residue, and is the mass of manganese-silicon alloy in the raw material.
2.3.4. Testing Equipment
The phases in spodumene, condensate, and reduction products were detected by X-ray diffractometry (XRD, X’Pert Pro MPD, Nalytical, Heracles, Almelo, The Netherlands). Inductively coupled plasma emission spectroscopy (ICP-OES, Optima 8300, PerkinElmer, Waltham, MA, USA) was used to quantitatively analyze the main chemical components in the raw materials. The morphology of the reduced products was observed and characterized by scanning electron microscopy (TM-3030 Plus, Hitachi, Tokyo, Japan) and energy-dispersive spectrometry (INCA, Oxford, UK). The lithium content of the condensate and reduction products was measured by an atomic absorption spectrophotometer (AAS; iCE 3500, Thermo, Waltham, MA, USA).
3. Results and Discussion
3.1. Thermodynamic Calculations
HSC6.0 thermodynamic software was used to calculate the possible reactions; the results are shown in Figure 3. SiO2 in the ore participated during the reaction and preferentially formed LiAlSi2O6, indicating that Mn5Si3 was preferentially formed over lithium vapor and alumina (Al2O3). When MnO2 was added to the reaction, the reaction temperature dropped significantly to 651 K and 667 K lower than that of SiO2 + C and LiAlSi2O6 + C, respectively, indicating that adding MnO2 reduced the reaction temperature and facilitated the reaction. Therefore, this process was thermodynamically feasible.
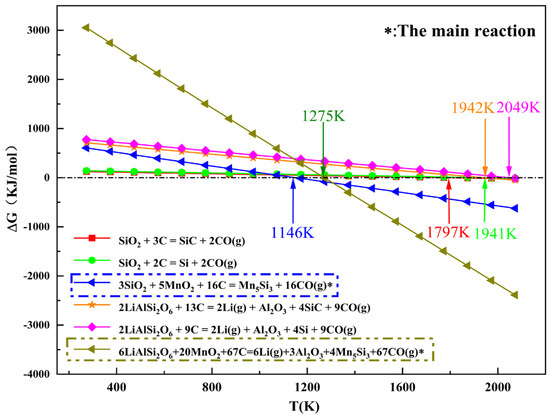
Figure 3.
Thermodynamic calculations of possible reactions.
3.2. Condensate Analysis
Due to the limited feed amount and the low lithium content in spodumene ore, it was difficult to collect the lithium condensate in a single step, so the lithium-rich condensate was collected through multiple experiments. The phase analysis of the condensate was carried out by XRD; the results are shown in Figure 4. Li mainly existed in the condensate in the form of Li2SiO3. By referring to the literature [27], we found that Li2O and SiO2 formed eutectic compounds in the range of 1028–1201 °C. Therefore, it is believed that the Li2SiO3 generated in the condensation zone was formed by Li in the condensation zone and O2 in air to generate Li2O, which then reacted with SiO2 to form eutectic compounds. The reaction equation is shown in Reaction 4. The Li content in the condensate was 19.47%, as detected by atomic absorption spectrophotometry, and the Li2O content was 41.72%, which was 10.85 times more enriched compared with spodumene.
4Li (g) + 2O2 (g) + SiO2 = 2Li2SiO3,
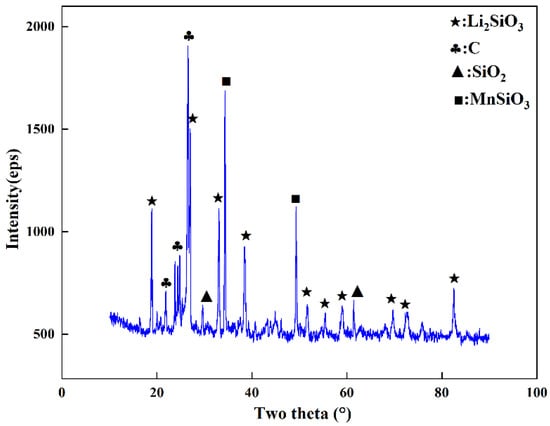
Figure 4.
XRD pattern of the condensate.
3.3. The Effect of Temperature
Based on the theoretical calculations and experimental exploration, the reduction time was set to 6 h to ensure the completion of the reaction. After the reaction, due to differences in density, the graphite crucible underwent delamination. Figure 5 and Figure 6 show the XRD patterns of the lower- and upper-layer materials from 1673 K to 1973 K with a holding time of 6 h. Figure 5 shows that LiAlSi2O6 did not begin to react at 1673 K or 1723 K, indicating that the reaction temperature was too low. The Mn5Si2 diffraction peak appeared because SiO2 in the ore preferentially reacted with C and MnO2, which was consistent with the thermodynamic calculations showing that SiO2 should preferentially produce LiAlSi2O6. However, as shown in Figure 6, SiC and Mn2C5 were present in the upper-layer material, and SiC and Mn2C5 were intermediates during the formation of Mn5Si3. This indicates that the reaction between SiO2 and MnO2 was incomplete. When the temperature reached 1773 K and 1823 K, the diffraction peaks shown in Figure 6 all changed to those of Mn5Si3, indicating that the lower-layer material was composed of alloys at these temperatures. The diffraction peaks of Al2O3 and LiAlSi2O6 shown in Figure 6 indicate that LiAlSi2O6 began to react but did so incompletely. When the temperature was 1873 K, the diffraction peak of LiAlSi2O6 shown in Figure 6 disappeared, and the intensity of the diffraction peaks of Al2O3 and SiC was enhanced. At this time, LiAlSi2O6 completely reacted, but the diffraction peak of Mn5Si3 remained, indicating that a small amount of Mn5Si3 was mixed into the upper-layer material. As the reaction temperature continued to increase, the Mn5Si3 diffraction peak remained. When the temperature was 1923 K, the intensity of the SiC diffraction peak shown in Figure 6 was enhanced, indicating that the SiC content increased. The high content of SiC affected the viscosity of the upper-layer material, which inhibited the upward diffusion of lithium vapor, resulting in a decrease in the volatilization rate of lithium. Therefore, the formation of SiC should be controlled during the reaction.
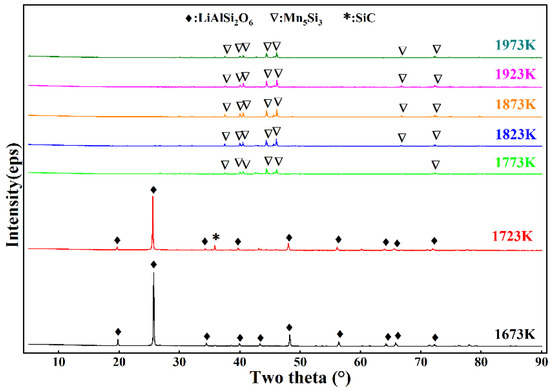
Figure 5.
XRD patterns of the underlying material at varying reduction temperatures.
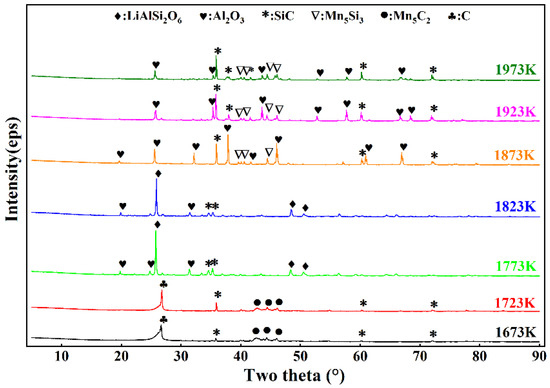
Figure 6.
XRD patterns of the upper layer at varying reduction temperatures.
The direct yield of Mn5Si3 was obtained by weighing the alloy in the lower-layer material, as shown in Figure 7. The direct yield of Mn5Si3 was low at temperatures below 1873 K because the spodumene ore had not completely reacted to form an alloy. When the temperature was 1873 K, the direct yield of Mn5Si3 was 86.47%, which was the maximum yield. Continuing to increase the reaction temperature resulted in a slight decrease in the direct yield of Mn5Si3. The reason for the low direct yield of Mn5Si3 was that the formation of SiC caused the loss of Si, and a small amount of Mn5Si3 entered the upper layer. After grinding and mixing the reduced residues in a graphite crucible at varying reduction temperatures, the Li content was detected by atomic absorption spectrophotometry. The influence of varying reduction temperatures on the volatilization rate of Li during the experimental process was investigated; the results are shown in Figure 8. At temperatures below 1723 K, spodumene ore did not participate in the reaction, so the volatilization rate of lithium was 0%. When the reduction temperature was 1773 K, the volatilization rate of Li was 32.75%, indicating that LiAlSi2O6 had begun to be reduced at this temperature, although the reduction degree was low. Upon increasing the temperature, the volatilization rate of Li gradually increased. When the temperature was 1873 K, the volatilization rate of Li was 97.65%. Upon continuing to increase the temperature, the Li volatilization rate remained identical to that at 1873 K, indicating that spodumene completely reacted at 1873 K. This was consistent with the analysis of the results presented in Figure 5 and Figure 6.
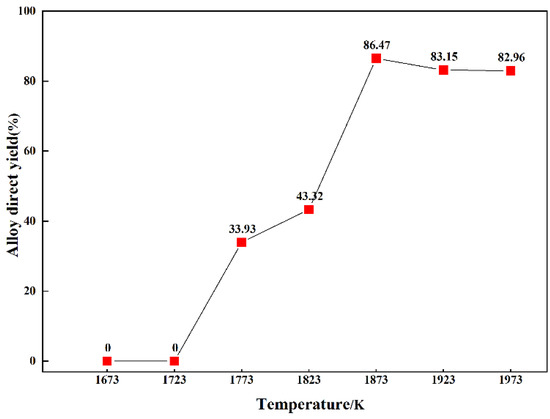
Figure 7.
Direct yields of alloys at varying reduction temperatures.
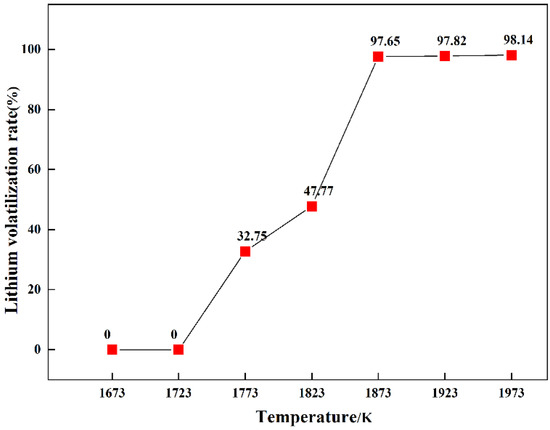
Figure 8.
Reduction rates of lithium at varying reaction temperatures.
3.4. The Effect of Time
In the previous section, the optimal reduction temperature was determined to be 1873 K by exploring the influence of temperature. In this section, we designed a gradient pair experiment with a reduction time of 1–10 h at the optimal reduction temperature. The material ratio and detection and treatment method used in the experiment were the same as those described in the previous section. Figure 9 shows that before 4 h, in addition to the Mn5Si3 diffraction peaks, there were Mn5C2 and SiC diffraction peaks in the lower-layer material. These peaks indicate that the reaction was incomplete. Upon increasing the reduction time to 6 h, the diffraction peaks of the lower-layer material were due to Mn5Si3 and Mn5C2. The SiC diffraction peaks disappeared, which showed that 6 h was enough to completely separate the upper and lower layers. Figure 10 shows that when the reduction time was 4 h, the diffraction peak of LiAlSi2O6 disappeared. Thus, LiAlSi2O6 was completely reduced, but MnO2 was not completely reduced, indicating that MnO2 required a longer reduction time than LiAlSi2O6.
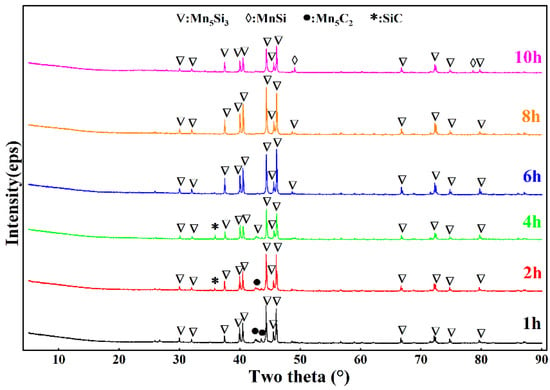
Figure 9.
XRD patterns of the middle and lower alloys in the reduction residue with varying holding times.
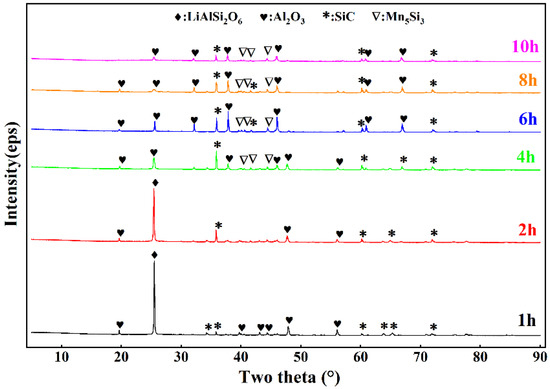
Figure 10.
XRD patterns of the upper and middle slag in the reduction residue with varying holding times.
The direct yield of Mn5Si3 with varying reduction times is shown in Figure 11. The direct yield of Mn5Si3 increased significantly when the reduction time was increased 2 h to 4 h, from 56.33% to 81.55%, indicating that LiAlSi2O6 was involved in the reaction from 2 h to 4 h. When the reduction time was 6 h, the direct yield of the alloy was the highest. When the reduction time was increased to 8 h, the direct yield of Mn5Si3 decreased slightly, but the change was insignificant.
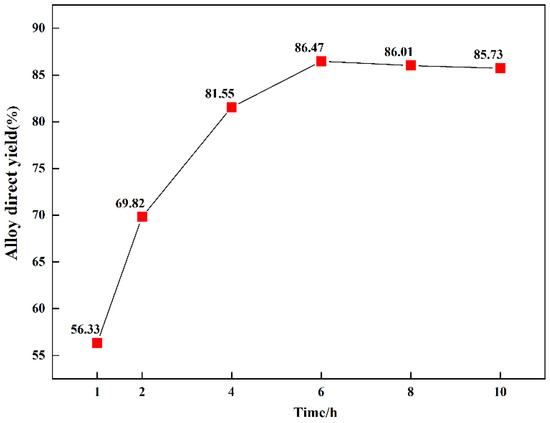
Figure 11.
Direct yield of the manganese-silicon alloy with varying holding times.
The volatilization rate of lithium with varying reduction times is shown in Figure 12. There was a large increase in 2–4 h, which further verified the conclusion that LiAlSi2O6 was considerably reduced with reaction times of 2–4 h. Upon extending the time, the volatilization rate of lithium increased gradually. When the reduction time was 6 h, the volatilization rate of lithium reached 97.65%, and the reduction of lithium was complete.
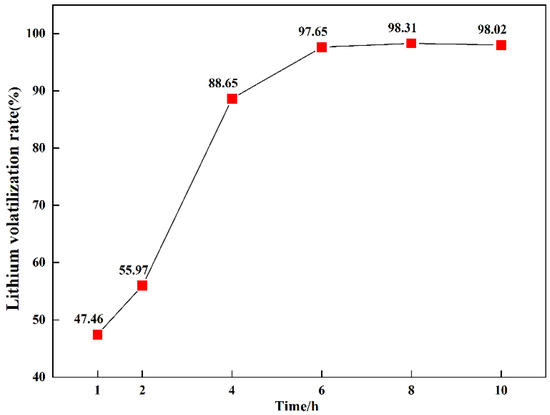
Figure 12.
Reduction rates of lithium with varying reduction times.
Based on single-factor experimental analysis, it can be concluded that the optimal parameters for studying lithium enrichment by the carbothermal reduction of spodumene to prepare manganese-silicon alloy were 1873 K and 6 h.
3.5. SEM-EDS Analysis
The upper- and lower-layer materials under the abovementioned optimal conditions were analyzed by SEM-EDS; the results are shown in Figure 13 and Figure 14. Figure 13a shows that the alloy was bright white with a smooth surface and no cracks or other defects. Figure 13b,c shows that the coincidence degree of Mn and Si was high.
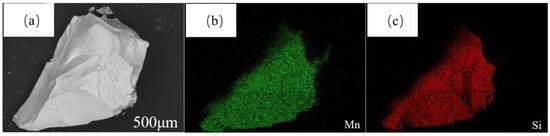
Figure 13.
SEM-EDS image of the upper layer at 1873 K and 6 h: (a) SEM image; (b) Mn element distribution; (c) Si element distribution.
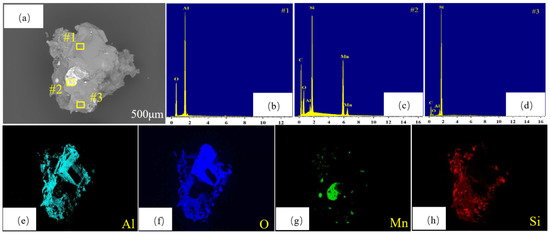
Figure 14.
SEM-EDS image of the upper layer at 1873 K and 6 h: (a) SEM image; (b) #1 point scan; (c) #2 point scan; (d) #3 point scan; (e) Al element distribution; (f) O element distribution; (g) Mn element distribution; (h) Si element distribution.
Figure 14a shows the topography of the upper-layer material, which was mainly a gray material, mixed with a small amount of bright white material. Three points were selected for point scanning; the results are shown in Figure 14b–d. The compound at point 1 was Al2O3, and that at point 2 was Mn5Si3. The inclusions were Al2O3 and SiC. The compound at point 3 was SiC, and a small amount of Al2O3 was included, which was consistent with the previous XRD analysis results. Figure 14e–h shows the element distribution of the upper-layer material. The surface scan of the upper material showed the presence of highly overlapping Al and O elements, indicating that the upper-layer material mainly comprised Al2O3, with a small amount of Mn5Si3 mixed in. This indicates that even under the optimal conditions, a small amount of Mn5Si3 still entered the upper-layer material because when LiAlSi2O6 formed Mn5Si3, Al2O3 was also decomposed, which caused a small amount of Mn5Si3 to be carried into the upper layer by Al2O3. The mechanistic process of the reaction was shown in Figure 15.
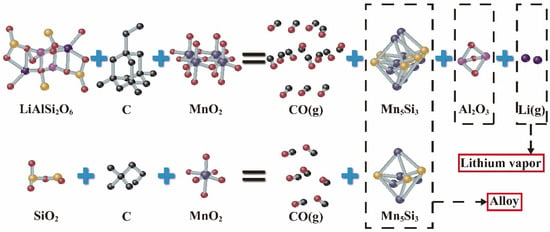
Figure 15.
Reaction mechanism diagram.
4. Conclusions
- Thermodynamic calculations were carried out using HSC6.0 thermodynamic software. We found that SiO2 in the ore preferentially participated in the reaction compared with LiAlSi2O6. Compared with the reaction of SiO2 + C and LiAlSi2O6 + C, the decrease was 651 K and 667 K, respectively, indicating that the addition of MnO2 reduced the reaction temperature and increased the feasibility of the reaction.
- Due to the limited feed amount and the low lithium content in spodumene ore, it was difficult to collect the lithium condensate in a single step, so the lithium-rich condensate was collected through multiple experiments. The collected condensate contained 19.47% Li, which was 10.85 times more enriched compared with spodumene.
- Reduction temperature and time were two important factors affecting the reaction. With increased temperature and time, spodumene ore was gradually reduced; when the reduction temperature was 1873 K and the reduction time was 6 h, the spodumene ore reaction was complete, the volatilization rate of lithium was 97.65%, and the direct yield of Mn5Si3 was 86.47%.
Author Contributions
In this joint work, each author contributed according to their expertise and capability. Conceptualization, M.Y. and T.Q.; methodology, M.Y., K.Y., R.J., X.C., W.Z. and T.Q.; theoretical basis, M.Y. and K.Y.; formal analysis, M.Y.; investigation, X.C., R.J. and W.Z.; resources, T.Q.; data curation, M.Y. and T.Q.; writing—original draft preparation, M.Y.; writing—review and editing, M.Y.; visualization, K.Y.; supervision, T.Q.; project administration, T.Q.; funding acquisition, T.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Yunnan Provincial Academician Free Exploration Project (2022HA006).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available because related studies are ongoing.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y. The flotation behavior and adsorption mechanism of a new cationic collector on the separation of spodumene from feldspar and quartz. Sep. Purif. Technol. 2021, 264, 118445. [Google Scholar] [CrossRef]
- Lajoie-Leroux, F.; Dessemond, C.; Soucy, G.; Laroche, N.; Magnan, J.F. Impact of the impurities on lithium extraction from β-spodumene in the sulfuric acid process. Miner. Eng. 2018, 129, 1–8. [Google Scholar] [CrossRef]
- Fosu, A.Y.; Kanari, N.; Vaughan, J.; Chagnes, A. Literature Review and Thermodynamic Modelling of Roasting Processes for Lithium Extraction from Spodumene. Metals 2020, 10, 1312. [Google Scholar] [CrossRef]
- Xie, R.; Zhu, Y.; Liu, J.; Li, Y.; Wang, X.; Shumin, Z. Research Status of Spodumene Flotation: A Review. Miner. Process. Extr. Metall. Rev. 2020, 42, 321–334. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Acid roasting of spodumene: Microwave vs. conventional heating. Miner. Eng. 2019, 138, 161–167. [Google Scholar] [CrossRef]
- Rosales, G.D.; Resentera, A.C.J.; Gonzalez, J.A.; Wuilloud, R.G.; Rodriguez, M.H. Efficient extraction of lithium from β-spodumene by direct roasting with NaF and leaching. Chem. Eng. Res. Des. 2019, 150, 320–326. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, G.; Wang, H.; Yu, H.; Zhao, X. Investigation of Enhanced Leaching of Lithium from α-Spodumene Using Hydrofluoric and Sulfuric Acid. Minerals 2017, 7, 205. [Google Scholar] [CrossRef]
- Fosu, A.Y.; Kanari, N.; Bartier, D.; Vaughan, J.; Chagnes, A. Novel extraction route of lithium from alpha-spodumene by dry chlorination. RSC Adv. 2022, 12, 21468–21481. [Google Scholar] [CrossRef]
- Abdullah, A.A.; Oskierski, H.C.; Altarawneh, M.; Senanayake, G.; Lumpkin, G.; Dlugogorski, B.Z. Phase transformation mechanism of spodumene during its calcination. Miner. Eng. 2019, 140, 105883. [Google Scholar] [CrossRef]
- Fosu, A.Y.; Kanari, N.; Bartier, D.; Hodge, H.; Vaughan, J.; Chagnes, A. Physico-Chemical Characteristics of Spodumene Concentrate and Its Thermal Transformations. Materials 2021, 14, 7423. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, C.; Yu, J. Conversion from α-spodumene to intermediate product Li2SiO3 by hydrothermal alkaline treatment in the lithium extraction process. Miner. Eng. 2022, 183, 107599. [Google Scholar] [CrossRef]
- Guo, H.; Lv, M.; Kuang, G.; Wang, H. Enhanced lithium extraction from α-spodumene with fluorine-based chemical method: A stepwise heat treatment for fluorine removal. Miner. Eng. 2021, 174, 107246. [Google Scholar] [CrossRef]
- Rezaee, M.; Han, S.; Sagzhanov, D.; Vaziri Hassas, B.; Slawecki, T.M.; Agrawal, D.; Akbari, H.; Mensah-Biney, R. Microwave-assisted calcination of spodumene for efficient, low-cost and environmentally friendly extraction of lithium. Powder Technol. 2022, 397, 116992. [Google Scholar] [CrossRef]
- Barbosa, L.I.; Valente, G.; Orosco, R.P.; González, J.A. Lithium extraction from β-spodumene through chlorination with chlorine gas. Miner. Eng. 2014, 56, 29–34. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, Q.; Chen, B.; Shi, X.; Liao, T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process. Hydrometallurgy 2011, 109, 43–46. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, T.; He, L.; Zhao, Z.; Liu, X. A promising approach for directly extracting lithium from α-spodumene by alkaline digestion and precipitation as phosphate. Hydrometallurgy 2019, 189, 105141. [Google Scholar] [CrossRef]
- Dessemond, C.; Lajoie-Leroux, F.; Soucy, G.; Laroche, N.; Magnan, J.-F. Spodumene: The Lithium Market, Resources and Processes. Minerals 2019, 9, 334. [Google Scholar] [CrossRef]
- Kuang, G.; Liu, Y.; Li, H.; Xing, S.; Li, F.; Guo, H. Extraction of lithium from β-spodumene using sodium sulfate solution. Hydrometallurgy 2018, 177, 49–56. [Google Scholar] [CrossRef]
- Guo, H.; Yu, H.-Z.; Zhou, A.-A.; Lü, M.-H.; Wang, Q.; Kuang, G.; Wang, H.-D. Kinetics of leaching lithium from α-spodumene in enhanced acid treatment using HF/H2SO4 as medium. Trans. Nonferrous Met. Soc. China 2019, 29, 407–415. [Google Scholar] [CrossRef]
- Grasso, M.L.; González, J.A.; Gennari, F.C. Lithium extraction from β-LiAlSi2O6 using Na2CO3 through thermal reaction. Miner. Eng. 2022, 176, 107349. [Google Scholar] [CrossRef]
- Rosales, G.D.; Resentera, A.C.J.; Wuilloud, R.G.; Rodriguez, M.H.; Esquivel, M.R. Optimization of combined mechanical activation-leaching parameters of low-grade α-spodumene/NaF mixture using response surface methodology. Miner. Eng. 2022, 184, 107633. [Google Scholar] [CrossRef]
- Salakjani, N.K.; Singh, P.; Nikoloski, A.N. Production of Lithium—A Literature Review. Part 2. Extraction from Spodumene. Miner. Process. Extr. Metall. Rev. 2019, 42, 268–283. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, X.; Shan, Y.; Li, Z.; Zeng, Y.; Asselin, E. Solubility and Modeling of Li2SO4·H2O in Aqueous H2SO4–MgSO4 Solutions for Lithium Extraction from Spodumene. J. Chem. Eng. Data 2022, 67, 919–931. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Liu, M.; Li, Y.; Tang, Y.; Zhuang, L.; Tian, B. Comprehensive utilization of waste residue from lithium extraction process of spodumene. Miner. Eng. 2021, 170, 06986. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Han, G.; Gu, D.; Lin, G.; Cui, Q.; Wang, H. Recovery of lithium from a synthetic solution using spodumene leach residue. Hydrometallurgy 2018, 177, 109–115. [Google Scholar] [CrossRef]
- Kracek, F.C. The Binary System Li2O–SiO2. J. Phys. Chem. 1930, 34, 2641–2650. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).