Abstract
In the present contribution, the differentiation in the molecular structure and function of the photosynthetic apparatus of the unicellular green alga Chlorella vulgaris was studied at several light intensities (0–400 μmol m−2 s−1) and various CO2 concentrations (0.04–60% CO2), in completely autotrophic conditions. Asymmetries that occur by different light intensities and CO2 concentrations induce metabolic and functional changes. Using chlorophyll fluorescence induction techniques (OJIP test), we showed that Chlorella vulgaris tolerates extremely high CO2 levels and converts them photosynthetically into valuable products, including O2 and biomass rich in carbohydrates and lipids. Interestingly, the microalga Chlorella vulgaris under extremely high CO2 concentrations induces a new metabolic state intensifying its photosynthetic activity. This leads to a new functional symmetry. The results highlight a potent CO2 bio-fixation mechanism of Chlorella vulgaris that captures up to 288 L CO2 L PCV−1 day−1 under optimal conditions, therefore, this microalga can be used for direct biological CO2-reducing strategies and other green biotechnological applications. All of the above suggest that Chlorella vulgaris is one of the most prominent competitors for a closed algae-powered bioreactor that is able to consume huge amounts of CO2. Thus, it is a sustainable and natural bioenergetic system with perspectives in dealing with major environmental issues such as global warming. In addition, Chlorella vulgaris cultures could also be used as bioregeneration systems in extraterrestrial missions for continuous atmospheric recycling of the human settlements, paving the way for astrobiological applications.
1. Introduction
Over the last couple of centuries, industrialization along with urbanization have brought an immense increase in greenhouse gases (GHGs), mostly carbon dioxide (CO2), which is considered one of the main causes of global warming [1] and other major environmental problems, such as ocean acidification [2]. Atmospheric CO2 concentration increased rapidly from 280 ppm in 1850 [3] to more than 417 ppm in 2021, with more than half of this increase happening in the last 30 years [4]. Notably, CO2 levels today are higher than at any other point in at least the past 800,000 years [5]. Human activities release about 35 gigatons of CO2 every year, as opposed to almost 10 megatons 2 centuries ago [6]. There is already clear evidence that anthropogenic emissions of GHGs, such as CO2, alter the natural carbon cycle, which leads to an accelerated warming of our planet [7]. Based on Representative Concentration Pathways (RCPs), adopted by the International Panel on Climate Change (IPCC), and other modelling scenarios, scientists predict that the amount of CO2 in the atmosphere might double or even triple at the end of the 21st century [8,9]. This will result in a planet that is hotter by more than 4 °C compared to preindustrial ages [8,9], well above the 2 °C danger mark agreed in COP21 Paris Agreement [10].
Several techniques have been examined worldwide for reducing CO2 emission levels based on chemical, physical and biological methods [11,12,13]; among these, CO2 bio-fixation via the photosynthetic procedure is considered one of the most effective approaches for CO2 capture [14,15]. Photosynthesis is the process used by phototrophic organisms to convert light into chemical energy that is then invested in carbon fixation, the conversion of inorganic carbon compounds (usually CO2) into organic carbon compounds (usually a carbohydrate such as glucose) [16]. Oxygenic photosynthesis, the mechanism that uses H2O as a reducing agent and releases O2, evolved 3.8 × 109 years ago and changed Earth’s atmosphere [17]. It slowly converted a strongly reducing, CO2 rich, atmosphere into the oxidizing one that we know today [18]. Undoubtedly, photosynthetic organisms have a fundamental regulatory role in the natural carbon cycle equilibrium [19], an attribute in which we should invest while tackling the spiraling problem of CO2 increase.
Regulations and controls on reducing our “carbon footprint” cannot help us much without serious socioeconomic impacts on existing infrastructure [1]. However, an efficient photosynthetic system, such as microalgae, could simultaneously be profitable through numerous applications, while providing a green solution for CO2 mitigation. Microalgae are the fastest growing photosynthetic organisms on earth, up to 50 times faster than their terrestrial plants [20]. They do not require cultivable land, while their use of fresh water is dramatically reduced [21]. Various microalgae tolerate a wide range of cultivation conditions [22], including extremely high CO2 concentrations [23,24]. In particualr, Chlorella vulgaris photoautotrophic cultures cultivated in bubble column photobioreactors showed a high rate of CO2 fixation up to 10% [25,26]. Comparatively, photosynthetic activity in most terrestrial plants increases when CO2 levels rise, until some saturating concentration, which is typically around 0.1%. Higher CO2 concentrations lead to adverse effects [27].
The microalga Chlorella vulgaris is an extremely promising candidate also for large-scale cultivation that has already attracted a lot of interest for numerous applications [28]. A rapid growth rate and a resistance to harsh environmental conditions are some of the features that make this microalga a prominent candidate for efficient production of high-value biomass yields [29]. Due to its high nutritional value, it is one of the few microalgae that have been used successfully as alternative aquacultures, and a source of animal and even human food [30,31,32], as it exhibits a large number of therapeutic properties widely used in pharmaceutical and cosmetic industry [29]. Furthermore, agrochemical applications of Chlorella vulgaris can benefit common crops by both biofertilization [33] and growth acceleration [34]. Another interesting feature of this particular species is that it responds to different growth parameters by modifying its rich biochemical composition [28], which is ideal for third-generation and even fourth-generation biofuels, that are not involved in the “food vs. fuel dilemma” [35]. Both bioethanol and biodiesel production can be optimized by aiming at carbohydrate and lipid accumulation, respectively [36,37].
In the present contribution, the metabolic and functional differentiation of the unicellular green alga Chlorella vulgaris was studied at various light intensities and CO2 concentrations, aiming at effective CO2 mitigation, conversion of CO2 to O2 and the production of biomass rich in carbohydrates and lipids. Such a microalgal system could be the key for environmental and astrobiological applications.
2. Materials and Methods
2.1. Organism and Cultivation Conditions
Cultures of the wild type strain of the unicellular green alga Chlorella vulgaris (211-11b, SAG Culture Collection of Algae) [38] were used for this study. According to Wong et al. (2017), Bold’s Basal Medium (pH = 6.8 ± 0.1) was found to be the best liquid medium for biomass production in Chlorella vulgaris [39,40]. The cultures were grown in elongated glass tubes (Ø 5 cm × 50 cm), and incubated at controlled temperature (25–26 °C) [41] with continuous sterile air bubbling (50–60 L∙h−1), for about a week. Light was provided with warm white LED lamps with the intensity of 40–50 μmol m−2 s−1 and a photoperiod of 16 h light:8 h dark [42]. After reaching a sufficient amount of biomass with high photosynthetic efficiency, the cultured cells were centrifuged at 1500× g for 5 min. Subcultures with an initial concentration of 1 μL packed cell volume (PCV) per mL culture were initiated with new medium. The initial cell density of 1 μL PCV mL−1 culture was the best starting point so that all the photosynthetically active cells absorb about the same amount of light, while avoiding fast nutrient depletion. All the experiments were carried out in 120 mL hermetically closed bottles (Ø 5 cm, height 9.5 cm) with rubber septa and in the absence of any organic carbon source. The final culture volume in each bottle was 50 mL autotrophic culture medium (liquid phase) and 70 mL gas phase with different CO2 concentrations (0.04–60%). Increased CO2 levels decreased the pH of the medium from 6.8 (without CO2 addition) down to 5.2 (in treatments with 60% CO2), which was then increased proportionately to the photosynthetic activity of the microalgae [43]. The bottles were kept in a temperature-controlled chamber (25–26 °C) at various light intensities (0–400 μmol m−2 s−1). Sampling took place at the same time (at the middle of the light period), using sterile gas tight needles, without opening the bottles.
2.2. Microalgae Growth Determination
The culture’s growth rate was estimated by measuring the packed cell volume (PCV) of the culture according to the method of Navakoudis et al. [44]. Briefly, a 1 mL sample of a homogenized cell suspension was centrifuged at 1500× g for 5 min using graduated capillary hematocrit tubes (TPP, Sigma-Aldrich, St. Louis, MO, USA) and the cell volume was expressed as µL PCV mL−1.
2.3. GC-TCD Measurements
Oxygen and nitrogen measurements were made utilizing gas chromatography, using a thermal conductivity detector (GC-TCD) (Shimadzu GC 2010 Plus, Kyoto, Japan). To separate O2 and N2, argon was used as the carrier gas under the pressure of 5 bars, and at an oven temperature of 120 °C. The column used was a capillary Vici Metronics MC (Poulsbo, WA, USA) with length of 30 m (diameter 0.53 mm) and film thickness of 20 μm. The temperature of TCD was set at 200 °C for the detector and 180 °C for the injector. A gas-tight syringe (250 mL) was used for sampling from the hermetically closed bottles. The quantification of all gases was carried out by injecting known quantities of O2 and N2 in the GC-TCD.
For the CO2 measurements, helium was used as the carrier gas under pressure of five bars and at oven temperature of 250 °C. The column used was the same as explained above. The temperature of TCD was set at 300 °C for the detector and 280 °C for the injector. The quantification of CO2 was carried out by injecting known quantities in the GC-TCD. Preliminary experiments with microalgal cultures in high CO2 concentrations and in closed cultivation systems confirmed that the decrease in CO2 volume (photosynthetic CO2 fixation) was about the same with the increase in O2 volume (photosynthetic O2 production) (data not shown).
2.4. Photosynthetic and Respiratory Activity Measurements
For the measurements of photosynthetic and respiratory activity of the cultivated microalgae, we used GC-TCD measurements (for details, see above). The changes in O2 levels in Chlorella vulgaris closed cultivation systems (initial cell concentration 1 μL PCV mL−1) was measured at known time intervals under light conditions and in absolute darkness (for the first 16 h of incubation in light and in continuous darkness respectively) for the determination of the photosynthetic and respiratory activity respectively. The photosynthetic activity represents the actual net microalgal photosynthetic rate in the corresponding light intensity and CO2 concentration used in each experimental treatment. Each net photosynthetic value was calculated by the difference between gross photosynthesis (total O2 production) and the O2 consumption due to respiration. The photosynthetic and respiratory activity was expressed as μL O2 μL PCV−1 h−1.
2.5. Fluorescence Induction Measurements: OJIP-Test
The Handy Plant Efficiency Analyser, PEA (Hansatech Instruments, Kings’s Lynn, Norfolk, UK) was used for the fluorescence induction measurements [45]. This protocol is based on the measurement of a fast fluorescence transient with a 10 µs resolution in a time span of 40 µs to 1 s. Fluorescence was measured at a 12-bit resolution and excited by three red light-emitting diodes providing a saturating light intensity of 3000 µmol m−2 s−1. For the fluorescence induction measurements, we put the flat bottom of the small culture bottle (Ø 5 cm) directly on the PEA sensor using a specific adaptor that ensures absolute darkness for at least 10 min (in order to open the PSII reaction centers) before the exposure to saturated light [43]. Under these conditions, and without opening the culture bottles, we measured the photosynthetic efficiency (Fv/Fm) of the culture in the ambient conditions, according to the OJIP method [45,46]. Out of all the other photosynthetic OJIP variables measured, we used the density of active photosynthetic reaction centers (RC/CS0), the functional antenna size (ABS/RC), the primary photochemistry (PSI0), the dissipated energy flux per active reaction center (DI0/RC), the performance index on absorption basis (PIabs) and the maximum photosynthetic efficiency (Fv/Fm) [47].
2.6. Lipid Extraction and Quantification
The protocol of Folch and Stanley [48] was used for the extraction of total lipids, as discussed by Sati et al. [49], with some slight modifications. Briefly, 3 mL of chloroform:methanol in a ratio 2:1 (v/v) were added in 2.5 μL PCV pellet of centrifuged microalgal cells (1500× g for 5 min) and incubated for 15–20 min with frequent vortexing. The samples were mixed with 1 mL of 0.74% NaCl solution for two phase separation and centrifuged for 5 min at 1500× g. The lower phase (chloroform with dissolved lipids) was isolated and incubated at 95 °C for complete chloroform evaporation [48,49].
For the total lipids quantification, the method of Park and Jeong [50] was used with slight modifications. Briefly, the lipids were dissolved in 200 μL of H2SO4 (99,99%), by vortexing briefly. The samples were then incubated at 95 °C for 10 min and immediately cooled at room temperature. 4 mL of phosphor-vanillin reagent (500 mL H3PO4 85%, 125 mL ddH2O and 0.75 g Vanillin) was added into the samples and incubated for an additional 10 min at room temperature, before being measured spectrophotometrically at 530 nm. The quantification of total lipids was estimated using a calibration curve with canola oil.
2.7. Carbohydrate Extraction and Quantification
For the extraction and quantification of total carbohydrates, the method of Schulze et al. [51] was used. Briefly, 1 mL of HCl 37% was added to 2.5 μL PCV of centrifuged microalgal cells (1500× g for 5 min) and incubated for 15 min. Samples were then diluted with 5 mL of ddH2O. At 400 μL of the diluted samples, we added 900 μL of thymol-sulfuric acid reagent (0.1 g thymol into 100 mL of H2SO4 99.99%), and vortexed briefly. The samples were then incubated for 30 min at 95 °C. The absorbance was measured at 505 nm. The quantification of total carbohydrates was estimated using a calibration curve with glucose [51].
2.8. Data Analysis
Each experiment was repeated at least three times, thus, each treatment included three independent samples. Standard errors of the average values are presented on all diagrams. ANOVA One Way and Tukey HSD paired tests were used for testing the significance of the values. The normality of samples was tested using the Shapiro–Wilk test and the homoscedacity using the Bartlett’s test.
3. Results
3.1. Effect of Different Light Intensities on the Microalgal Photosynthetic Mechanism
Τhe effects of various light intensities (0–400 μmol m−2 s−1) on the photosynthetic apparatus of Chlorella vulgaris under natural atmospheric conditions in closed cultivation systems were evaluated. The kinetics of the maximal photosynthetic efficiency (Fv/Fm) in Figure 1A showed that increased light intensity acts as a major stress factor for the photosynthetic mechanism under ambient CO2 (0.04%) concentration. In complete darkness (0 μmol m−2 s−1), Fv/Fm remained constantly high. After a couple of days, under extremely low light conditions (10 μmol m−2 s−1), Fv/Fm values showed a slight decrease. As light intensity increased to 50 μmol m−2 s−1, the photosynthetic efficiency drop came earlier and sharper, leading to a decrease in photosynthetic capacity. Stepping up to 100 μmol m−2 s−1 and 200 μmol m−2 s−1, Fv/Fm decreased down to 0.4 (Figure 1A). Specifically, at 400 μmol m−2 s−1 under ambient CO2, there was a total breakdown of the photosynthetic apparatus after the fourth incubation day (no reliable measurements with OJIP-test; no observed bullets in Figure 1A). These first results showed an extremely light sensitive photosynthetic mechanism under ambient carbon dioxide with limited biomass increase (Figure 1B).
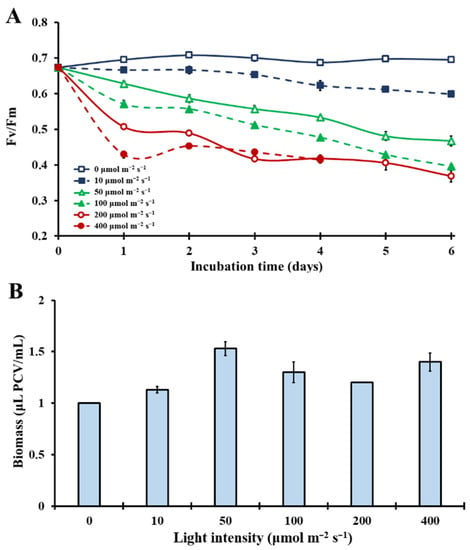
Figure 1.
(A): Kinetics of the microalgal photosynthetic efficiency, expressed in Fv/Fm, over the incubation time under different light intensities (0–400 μmol m−2 s−1) in an air atmosphere with 0.04% CO2 (p < 0.05 for each day). (B): Microalgal biomass concentration expressed in μL PCV/mL on the sixth incubation day, over different light intensities (0–400 μmol m−2 s−1). Significantly different are the biomass concentrations of the following treatments: 0 μmol m−2 s−1 versus 50, 100 and 400 μmol m−2 s−1; 10 μmol m−2 s−1 versus 50 μmol m−2 s−1 and 50 μmol m−2 s−1 versus 200 μmol m−2 s−1 (p < 0.05).
The effect of light on the molecular structure and function of the photosynthetic apparatus of the microalga Chlorella vulgaris is presented in more detail in Figure 2, which depicts a series of more specific OJIP parameters. The results reveal that stress was induced by increasing the light intensity under ambient CO2 concentration in closed cultivation systems. Concisely, the density of active photosynthetic reaction centers (RC/CS0) decreased while the antenna size per active reaction center (ABS/RC) increased, leading to decreased primary photochemistry (PSI0) and increased dissipation energy per active reaction center (DI0/RC). As a result, the performance index (PI(abs)) decreased (Figure 2). The parameters mentioned above are the most representative indicators of the sensitivity/tolerance of the photosynthetic apparatus under abiotic stress and are in agreement with the observations made in abiotic stress conditions, such as ozone elevation [52], high UVB radiation [53,54] and low temperature [55].
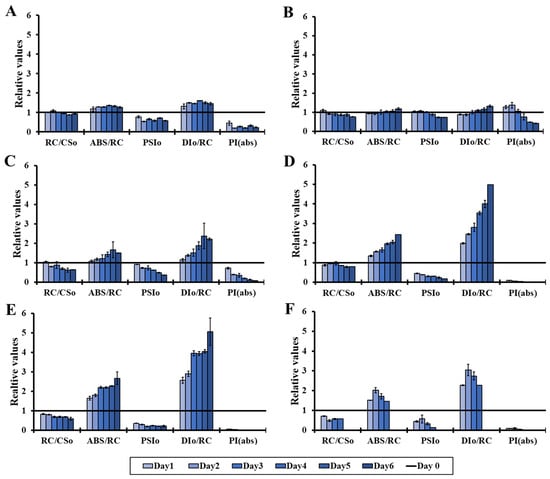
Figure 2.
Changes in RC/CS0, ABS/RC, PSI0, DI0/RC and PI(abs) (OJIP test) over the 6 days of incubation time compared to the corresponding values of day 0, under different light intensities (0–400 μmol m−2 s−1) in an air atmosphere (0.04% CO2). (A): 0 μmol m−2 s−1, (B): 10 μmol m−2 s−1, (C): 50 μmol m−2 s−1, (D): 100 μmol m−2 s−1, (E): 200 μmol m−2 s−1 and (F): 400 μmol m−2 s−1.
3.2. Effect of Various Extreme CO2 Concentrations at the Photosynthetic Mechanism under Several Light Intensities
A series of various extreme CO2 concentrations (0.04, 10, 20, 30, 40 and 60%) under different light intensities (0, 10, 50, 100, 200 and 400 μmol m−2 s−1) were combined in order to test the effects of elevated CO2 concentrations in the Chlorella vulgaris cultures (Figure 3). The results showed that under continuous darkness (0 μmol m−2 s−1), by increasing the CO2 levels, the photosynthetic efficiency (Fv/Fm) decreased proportionally. However, in the presence of light, changes induced in the Fv/Fm were completely different. Rising CO2 levels (up to 40%; 1000 times higher than the atmospheric concentration) had a protective role to the photosynthetic apparatus, expressed with higher Fv/Fm values (Figure 3), in contrast to the light-induced stress effect that appeared in treatments without any additional CO2 (Figure 3). The above-mentioned protective mechanism induced by elevated CO2 allowed microalgae to grow even at high light intensities (400 μmol m−2 s−1), inducing a new functional symmetry. That was impossible under ambient CO2 concentration. CO2 concentrations at 60% and higher were not ideal for the microalgae, since they accounted for an additional stress factor in each tested light intensity, except the 100 μmol m−2 s−1. This light intensity seems to be the only one suitable for microalgal adaptation, even at 60% CO2, since the Fv/Fm values were maintained to the levels of 40% CO2 and were higher than the corresponding values in the treatment with ambient CO2 concentration. Lower intensities (<100 μmol m−2 s−1) were not enough for efficient use of the extremely high CO2 concentration (60%), while higher intensities (>100 μmol m−2 s−1), in combination with the excess of CO2, caused further stress effects.
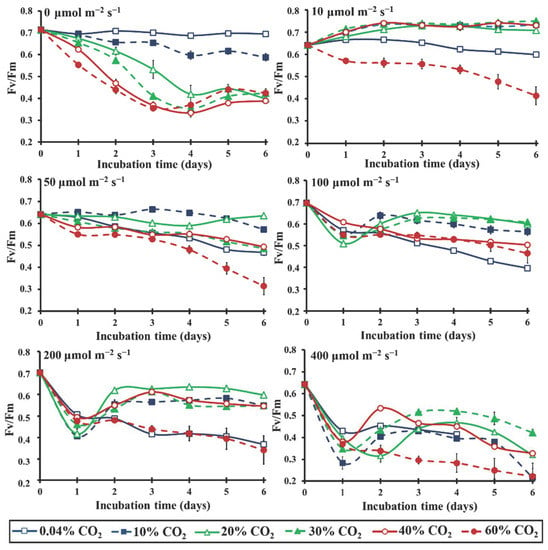
Figure 3.
Kinetics of the microalgal photosynthetic efficiency, expressed in Fv/Fm, over the incubation time under different light intensities (0–400 μmol m−2 s−1) in an air atmosphere with different CO2 concentrations (0.04–60%) (p < 0.05 for each light intensity and each day).
For the analytical parameters of the fluorescence induction measurements (OJIP-test) presented in Figure 4, three different states were chosen in order to study the combined effect of light and CO2 concentration in closed cultivation systems of Chlorella vulgaris cultures: (a) the ambient CO2 state at 0.04% CO2; (b) the concentration of 30% CO2, with the most protective effect against high light intensities (Figure 3); and (c) the extremely high concentration of 60% CO2, where the CO2 stress on the photosynthetic apparatus was detected. In the absence of light, obvious signs of stress were observed in the presence of high CO2 concentrations. In contrast, there was an improvement of the functionality of the photosynthetic apparatus under light conditions combined with high CO2 levels (30%). Briefly, the density of the reaction centers (RC/CS0) increased and the size of the antenna per active reaction center (ABS/RC) decreased in the treatments with 30% CO2 compared to the corresponding treatments with ambient (0.04%) and extremely high (60%) CO2 concentrations. These changes improved the primary photochemistry (PSI0) and decreased the non-photochemical energy dissipation per active reaction center (DI0/RC), which led to an increased photosynthetic performance (PI(abs)) (Figure 4).
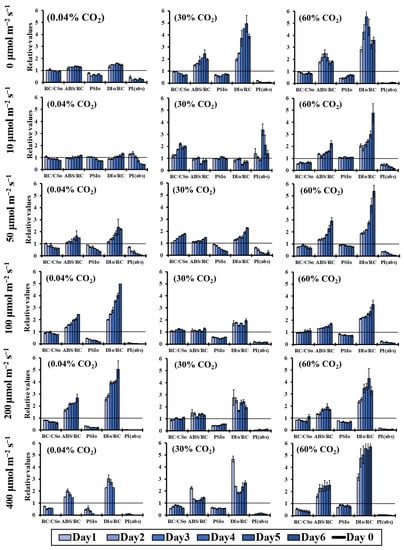
Figure 4.
Changes in RC/CS0, ABS/RC, PSI0, DI0/RC and PI(abs) (OJIP test) over the 6 days of incubation time compared to the corresponding values of day 0, under different light intensities (0–400 μmol m−2 s−1) in an air atmosphere with 0.04% CO2, 30% CO2 and 60% CO2.
Comparing and combining all the above-mentioned results, the high concentration of 30% CO2 was perfectly managed by the microalgal photosynthetic mechanism up to the light intensity of 100 μmol m−2 s−1. Higher light intensities were not so beneficial for the microalga, since the non-photochemical energy dissipation was increased, mainly in the first incubation day. After that incubation time interval, there was some adaptation signs, which could permit the microalgal survival in high light intensities (up to 400 μmol m−2 s−1), and high CO2 concentrations (up to 60%) (Figure 4). It is worth mentioning that the stress effect that appeared in the photosynthetic apparatus in 60% CO2 was quenched in the light intensity of 100 μmol m−2 s−1, compared to lower and higher light intensities, meaning that the green microalga Chlorella vulgaris could also survive in extremely high CO2 concentrations, changing its bioenergetic strategy.
3.3. Photosynthetic and Respiratory Activities under Different Light Intensities and CO2 Concentrations
The photosynthetic and respiratory activities of the microalga Chlorella vulgaris, grown in closed systems under a combination of six different light intensities and six different CO2 concentrations, were calculated (Figure 5). It was proven that the photosynthetic rate, expressed as μL O2 (μL PCV)−1 h−1, was almost six times higher at extreme CO2 levels (from 20% to 60%) compared to the ambient levels (0.04%) (Figure 5). In the case that the photosynthetic activity was expressed per chlorophyll content, the corresponding values at extreme CO2 levels also increased almost eight times with the same trend (data not shown). Furthermore, taking into consideration the overall results for each individual light intensity, it was clearly shown that the maximal photosynthetic rate was reached at light intensities of about 100–200 μmol m−2 s−1. Higher light intensities induced photoinhibition (Figure 4) and decreased the photosynthetic activity (Figure 5). It is remarkable that extreme CO2 concentrations strongly induced the photosynthetic activity (Figure 5) at low light intensities of 10–50 μmol m−2 s−1, while the measurements were definitely better at the high light intensities (100–200 μmol m−2 s−1), highlighting the extremely high photosynthetic sensitivity/efficiency of this microalga and its increased demands for CO2 consumption. Chlorella vulgaris is supposed to be a very adaptable green microalga that survives in adverse conditions, changing its bioenergetic strategy, depending on the best combination of CO2 concentration and light intensity.
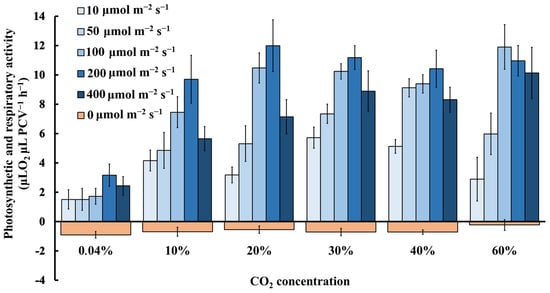
Figure 5.
Photosynthetic and respiratory activities of microalgae expressed in μL O2 μL PCV−1 h−1 cultivated in different light intensities (0–400 μmol m−2 s−1) in an air atmosphere with different CO2 concentrations (0.04–60%) (p < 0.05 for each light intensity, each CO2 concentration and each day).
Kinetics of total O2 quantities per culture during the entire incubation time are presented in Figure 6. The trend of the measurements confirmed the above-mentioned photosynthetic activity results. Even though significant amounts of O2 were produced at very low light intensities (10 μmol m−2 s−1) over the incubation period of six days, the maximum O2 production was reached at a light intensity of about 100–200 μmol m−2 s−1. Increasing CO2 concentrations improved photosynthetic O2 production, even at extreme CO2 values. Treatments with 30–40% CO2 yielded the optimal results in our closed cultivation systems (Figure 6). Notably, in higher light intensities, 20% CO2 was consumed and converted to O2 in almost 2–3 days, while by the end of the 4–6 days of incubation, concentrations as high as 40% CO2 were almost completely converted to O2.
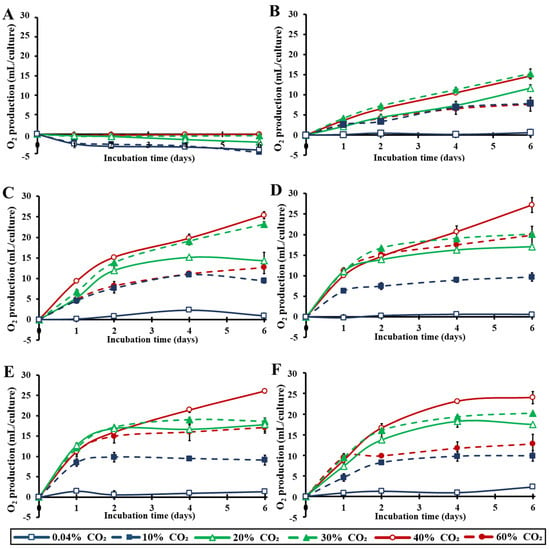
Figure 6.
Kinetics of total O2 production per microalgal culture over the incubation time under different light intensities (0–400 μmol m−2 s−1) in an air atmosphere with different CO2 concentrations (0.04–60%). (A): 0 μmol m−2 s−1, (B): 10 μmol m−2 s−1, (C): 50 μmol m−2 s−1, (D): 100 μmol m−2 s−1, (E): 200 μmol m−2 s−1 and (F): 400 μmol m−2 s−1 (p < 0.05 for each light intensity and each day).
3.4. Microalgal Biomass Production under Different Light Intensities and CO2 Concentrations
The microalgal biomass concentration under several light intensities and CO2 concentrations is presented in Figure 7. The results clearly show that the optimal biomass increase (about four times higher than the initial cell biomass) was recorded at Chlorella vulgaris cultures, which were incubated in closed cultivation systems, when exposed to light intensities higher than 50 μmol m−2 s−1 and CO2 concentrations of about 30–40% CO2. Increasing the light intensity to 100 μmol m−2 s−1, 200 μmol m−2 s−1 and 400 μmol m−2 s−1 led to a slight improvement of biomass productivity, whereas increasing the CO2 to 60% CO2 limited the microalgal biomass production (Figure 7). This extreme CO2 concentration (60%) changed the bioenergetic management of Chlorella vulgaris cultures. The microalga limited the energy yields for growth (Figure 7), in order to face the extra stress (Figure 3 and Figure 4). Oppositely, 30–40% CO2 concentrations were ideal for the closed cultivation systems used in this contribution, leading to the best growth (Figure 7) and photochemistry (Figure 3, Figure 4, Figure 5 and Figure 6), mainly to high light intensities (100–200 μmol m−2 s−1). Lower CO2 concentrations did not seem to satisfy Chlorella vulgaris demands, leading to a limited functionality of the photosynthetic apparatus and limited growth.
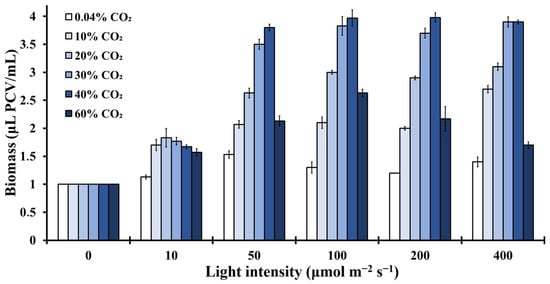
Figure 7.
Microalgal biomass concentration expressed in μL PCV/mL on the sixth incubation day, over different light intensities (0–400 μmol m−2 s−1) and CO2 concentrations (0.04–60%) (p < 0.05 for each light intensity, each CO2 concentration and each day).
To evaluate the quality of the biomass produced, we examined the levels of carbohydrates and lipids. The microalgae content in carbohydrates and lipids was measured in the three different “carbon states” mentioned above (0.04% CO2–30% CO2–60% CO2) under different light conditions (Table 1 and Table 2). In the absence of light, microalgae did not have any exploitable external energy source, thus they supplied their demand by breaking down their cellular stocks in carbohydrates and lipids, and therefore lower values were recorded. The same tendency was observed in all CO2-deprived cultures at all tested light intensities. On the contrary, optimizing the CO2 concentration at 30%, led to an accumulation of carbohydrates at approximately 30–37% of dry weight in the first incubation day (Table 1). No significant changes in the cellular lipid level between the treatments were observed, even under high light conditions (Table 2). Interestingly, pushing CO2 levels up to 60% led to an accumulation of lipids increase of up to 19% of dry weight in the first incubation day under high light intensities (Table 2). This observation could be attributed to the microalgal attempt to face the excess stress caused by the extremely high CO2 concentration of 60%. Biomass increase was restricted, due to the lipid accumulation for saving energy in a more valuable form.

Table 1.
Percentage (% dry weight) of total carbohydrate content over 0, 1, 3 and 6 days of incubation under 0.04% CO2, 30% CO2 and 60% CO2 under different light intensities (0–400 μmol m−2 s−1).

Table 2.
Percentage (% dry weight) of total lipid content over 0, 1, 3 and 6 days of incubation under 0.04% CO2, 30% CO2 and 60% CO2 under different light intensities (0–400 μmol m−2 s−1).
4. Discussion
The present study examined the metabolic and functional differentiation of the green alga Chlorella vulgaris under various light intensities and extreme CO2 concentrations. The results verified the ability of this species to modify hostile CO2 atmospheres by converting CO2 to O2, through the photosynthetic management of solar radiation, for the benefit of the organism, producing a microalgal biomass rich in carbohydrates and lipids.
In the current study, we found that high CO2 concentrations, even at extreme levels up to 40%, not only increased biomass yield of Chlorella vulgaris, but also protected the photosynthetic mechanism against photoinhibition and photodamage. Consequently, the microalga Chlorella vulgaris is a species that does not only tolerate extremely high CO2 levels, but also, extremely high CO2 levels (up to 1500 times higher concentration than the ambient atmospheric CO2 concentration) strongly increase its photosynthetic activity. The result is a pronounced CO2 conversion into O2 and valuable microalgal biomass. The potentiality of this photosynthetic system for CO2 mitigation showed clearly that today’s Earth atmospheric CO2 levels are not the optimal habitat for Chlorella vulgaris.
In this work, we report optimal growth at extreme CO2 concentrations (30–40%) with inhibitory effects appearing first at 60% CO2, where a completely different bioenergetic strategy was used by the microalga in order to face the extra stress effect caused by the extreme CO2 concentration. In a hermitically sealed cultivation system similar to ours, Papazi et al. [23] found that Chlorella minutissima grew best at around 25% CO2, verifying the CO2 tolerance of Chlorella sp. [23]. However, Chlorella minutissima was evidently stressed at 40% CO2, while Chlorella vulgaris did not show any stress effects at that level [23]. Since Chlorella vulgaris demands extremely high CO2 concentrations in order to yield the best biomass production and mitigates up to 288 L CO2 L PCV−1 day−1, it is ideal for direct biological CO2 capture from industrial fuel gases, such as in coal plants that usually generate 10–20% CO2 fumes, and other similar applications [56].
In our experiments, in light intensities above 50 μmol m−2 s−1, CO2 concentrations up to 30% and 40% were almost completely converted to O2 in 2–3 and 4–6 days, respectively. Additionally, the microalgal culture quadrupled its biomass in just 5–6 days without any additional energy input. The microalgal biomass consists of relatively high amounts of carbohydrates and lipids, ideal for biofuel production, without significant differences in the cellular concentrations (20–37% carbohydrates and 13–19% lipids), considering light intensity and CO2 concentration (Table 1 and Table 2). However, extreme CO2 concentrations led to a strong increase in carbohydrate (about 3.5 times higher amount in 3 days) and lipid (about 2.5 times higher amount in 3 days) accumulation per culture (Table 1 and Table 2).
Large scale cultivation of Chlorella vulgaris could bring valuable biomass yield, while also solving environmental problems, mainly CO2-driven climate change. The aforementioned is in agreement with recent techno-economic analyses and life-cycle assessments of microalgae-based production systems, suggesting the necessity of using the microalgae biomass in an integrated biorefinery system from which all the valuable components are extracted and utilized [57].
It is known that the annual global CO2 emissions since 2000 consist of 34.07 × 109 t CO2 from fossil fuel and industry emissions, and 5.86 × 109 t CO2 from land-use change emissions. As a result, the total CO2 emissions are 39.93 × 109 t/year or 0.11 × 109 t/day [58]. The importance of the present study lies in the fact that the microalga Chlorella vulgaris is not only resistant to extremely high CO2 concentrations, but actually requires them for intense photosynthetic activity without any stress induction. These data highlight that this particular microalga is a unique tool for solving global warming. If we consider its excellent photosynthetic activity (12 L O2 L PCV−1 h−1 or 288 L O2 L PCV−1 day−1) when exposed to 20 or 30% CO2, and the ratio of 1 L CO2 gas = 0.001812 kg CO2, a theoretical huge Chlorella vulgaris culture of 210 Km3 (210 × 109 m3) consisting of a cell density of 1 μL PCV/mL culture could mitigate the total global CO2 emissions, converting them to O2 and valuable biomass.
In recent decades, space missions have seen revolutionary progress, and sending humans to other planets, such as Mars, is now a realistic and most anticipated goal. NASA is already equipped with, and constantly updates, Environmental Control and Life Support Systems (ECLSS) for water recovery, air revitalization and oxygen generation [59]. In addition to that, bioregenerative life-support systems (BLSS) are developed with the aim of continuously recycling the above resources, while generating food via oxygenic photosynthetic microorganisms [60]. Most importantly, these systems aim for constant O2 regeneration from CO2-rich atmospheres [61], in which the results of the present contribution may seem helpful. Chlorella vulgaris is a photosynthetic microalga that demands extreme CO2 concentrations for its metabolism, having the ability to convert a CO2-rich, hostile-for-humans atmosphere to a hospitable O2-rich atmosphere. As illustrated in Figure 5, the maximal rate of O2 production happened in the treatments with CO2 concentrations of 20% and more, under a light intensity of 100–200 μmol m−2 s−1, and reached a value of about 288 L O2 L PCV−1 day−1. These results could be extrapolated and compared with a regular human’s oxygen requirements (about 590 L O2 day−1) [62], which could be completely covered from a Chlorella vulgaris culture consisting from approximately 2 L PCV microalgae. The continuous recycling of a human settlement’s atmosphere in another planet, such as Mars, in which O2 is consumed and CO2 is produced continuously, could be provided by a closed cultivation system of Chlorella vulgaris, creating an efficient and sustainable O2 regeneration system for cosmonauts and even settlers on other planets.
Author Contributions
Conceptualization, K.K.; supervision, K.K.; investigation, F.M., A.P. and K.K.; funding acquisition, K.K.; methodology, F.M., A.P. and K.K.; resources, K.K.; validation, F.M. and A.P.; visualization, F.M. and A.P.; writing—original draft, F.M.; writing—review and editing, K.K. and A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Regional Development Fund of the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call Special Actions in aquatic farming—industrial materials—open innovation in culture. Project code: Τ6ΥΒΠ-00494 (MIS 5056221).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- McNeil, B.I.; Matear, R.J. Southern Ocean acidification: A tipping point at 450-ppm atmospheric CO2. Proc. Natl. Acad. Sci. USA 2008, 105, 18860–18864. [Google Scholar] [CrossRef]
- Etheridge, D.M.; Steele, L.P.; Langenfelds, R.L.; Francey, R.J.; Barnola, J.-M.; Morgan, V.I. Natural and anthropogenic changes in atmospheric CO2 over the last 1000 years from air in Antarctic ice and firn. J. Geophys. Res. Atmos. 1996, 101, 4115–4128. [Google Scholar] [CrossRef]
- Earth System Research Laboratory National Oceanic and Atmospheric Administration. Available online: https://www.esrl.noaa.gov/gmd/ccgg/trends/ (accessed on 4 May 2021).
- Lüthi, D.; Le Floch, M.; Bereiter, B.; Blunier, T.; Barnola, J.-M.; Siegenthaler, U.; Raynaud, D.; Jouzel, J.; Fischer, H.; Kawamura, K.; et al. High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 2008, 453, 379–382. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emissions. 2020. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 1 August 2020).
- Szulejko, J.E.; Kumar, P.; Deep, A.; Kim, K.-H. Global warming projections to 2100 using simple CO2 greenhouse gas modeling and comments on CO2 climate sensitivity factor. Atmos. Pollut. Res. 2017, 8, 136–140. [Google Scholar] [CrossRef]
- Nazarenko, L.; Schmidt, G.A.; Miller, R.L.; Tausnev, N.; Kelley, M.; Ruedy, R.; Russell, G.L.; Aleinov, I.; Bauer, S.; Bleck, R.; et al. Future climate change under RCP emission scenarios with GISS ModelE2. J. Adv. Model. Earth Syst. 2015, 7, 244–267. [Google Scholar] [CrossRef]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Walsh, B.; Ciais, P.; Janssens, I.A.; Peñuelas, J.; Riahi, K.; Rydzak, F.; van Vuuren, D.P.; Obersteiner, M. Pathways for balancing CO2 emissions and sinks. Nat. Commun. 2017, 8, 14856. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.M.; Moraes, L.; Cardias, B.B.; Souza, M.; Costa, J. Chemical absorption and CO2 biofixation via the cultivation of Spirulina in semicontinuous mode with nutrient recycle. Bioresour. Technol. 2015, 192, 321–327. [Google Scholar] [CrossRef] [PubMed]
- El Mekawy, A.; Hegab, H.M.; Mohanakrishna, G.; Elbaz, A.F.; Bulut, M.; Pant, D. Technological advances in CO2 conversion electro-biorefinery: A step toward commercialization. Bioresour. Technol. 2016, 215, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.H.; Fanka, L.S.; Costa, J.A.V. Utilization of simulated flue gas containing CO2, SO2, NO and ash for Chlorella fusca cultivation. Bioresour. Technol. 2016, 214, 159–165. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Wu, N.; Lan, C.Q. CO2 bio-mitigation using microalgae. Appl. Microbiol. Biotechnol. 2008, 79, 707–718. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Mar. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Johnson, M.P. Photosynthesis. Essays Biochem. 2016, 60, 255–273. [Google Scholar] [CrossRef]
- Buick, R. When did oxygenic photosynthesis evolve? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 2731–2743. [Google Scholar] [CrossRef]
- Lyons, T.W.; Reinhard, C.T.; Planavsky, N.J. The rise of oxygen in Earth’s early ocean and atmosphere. Nature 2014, 506, 307–315. [Google Scholar] [CrossRef]
- Keller, D.P.; Lenton, A.; Littleton, E.W.; Oschlies, A.; Scott, V.; Vaughan, N.E. The Effects of Carbon Dioxide Removal on the Carbon Cycle. Curr. Clim. Chang. Rep. 2018, 4, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yeh, K.-L.; Aisyah, R.; Lee, D.-J.; Chang, J.-S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Oliveira, A.C. Microalgae as a raw material for biofuels production. J. Ind. Microbiol. Biotechnol. 2008, 36, 269–274. [Google Scholar] [CrossRef]
- Papazi, A.; Korelidou, A.; Andronis, E.; Parasyri, A.; Stamatis, N.; Kotzabasis, K. Bioenergetic reprogramming plasticity under nitrogen depletion by the unicellular green alga Scenedesmus Obliq. Planta 2018, 247, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Papazi, A.; Makridis, P.; Divanach, P.; Kotzabasis, K. Bioenergetic changes in the microalgal photosynthetic apparatus by extremely high CO2 concentrations induce an intense biomass production. Physiol. Plant. 2008, 132, 338–349. [Google Scholar] [CrossRef]
- Zerveas, S.; Kydonakis, E.; Moutidis, P.; Maragkoudakis, A.; Kotzabasis, K. Microalgae strategy in anoxic atmospheres with various CO2 concentrations—Environmental and (astro)biotechnological perspectives. Environ. Exp. Bot. 2021, 187, 104474. [Google Scholar] [CrossRef]
- Anjos, M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A.; Dragone, G. Optimization of CO2 bio-mitigation by Chlorella vulgaris. Bioresour. Technol. 2013, 139, 149–154. [Google Scholar] [CrossRef]
- Lam, M.K.; Lee, K.T. Effect of carbon source towards the growth of Chlorella vulgaris for CO2 bio-mitigation and biodiesel production. Int. J. Greenh. Gas Control 2013, 14, 169–176. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, F.; Hao, L.; Shedayi, A.A.; Guo, L.; Ma, C.; Huang, B.; Xu, M. The optimal CO2 concentrations for the growth of three perennial grass species. BMC Plant Biol. 2018, 18, 27. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. J. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef]
- Görs, M.; Schumann, R.; Hepperle, D.; Karsten, U. Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. J. Appl. Phycol. 2009, 22, 265–276. [Google Scholar] [CrossRef]
- Kholif, A.E.; Morsy, T.A.; Matloup, O.H.; Anele, U.Y.; Mohamed, A.G.; El-Sayed, A.B. Dietary Chlorella vulgaris microalgae improves feed utilization, milk production and concentrations of conjugated linoleic acids in the milk of Damascus goats. J. Agric. Sci. 2016, 155, 508–518. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Yusoff, F.; Goh, Y.M.; Banerjee, S. Applications of microalga Chlorella vulgaris in aquaculture. Rev. Aquac. 2018, 12, 328–346. [Google Scholar] [CrossRef]
- Schreiber, C.; Schiedung, H.; Harrison, L.; Briese, C.; Ackermann, B.; Kant, J.; Schrey, S.D.; Hofmann, D.; Singh, D.; Ebenhoh, O.; et al. Evaluating potential of green alga Chlorella vulgaris to accumulate phosphorus and to fertilize nutrient-poor soil substrates for crop plants. J. Appl. Phycol. 2018. [Google Scholar] [CrossRef]
- Faheed, F.A.; Abd-El Fattah, Z. Effect of Chlorella vulgaris as bio-fertilizer on growth parameters and metabolic aspects of lettuce plant. J. Agri. Soc. Sci. 2008, 4, 165–169. [Google Scholar]
- Singh, A.; Nigam, P.S.; Murphy, J.D. Renewable fuels from algae: An answer to debatable land based fuels. Bioresour. Technol. 2011, 102, 10–16. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, I.S.; Kim, H.M.; Wi, S.G.; Bae, H.-J. Bioethanol production from the nutrient stress-induced microalga Chlorella vulgaris by enzymatic hydrolysis and immobilized yeast fermentation. Bioresour. Technol. 2014, 153, 47–54. [Google Scholar] [CrossRef]
- Asadi, P.; Rad, H.A.; Qaderi, F. Lipid and biodiesel production by cultivation isolated strain Chlorella sorokiniana pa.91 and Chlorella vulgaris in dairy wastewater treatment plant effluents. J. Environ. Health Sci. Eng. 2020, 18, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Beyerinck, M.W. Culturversuche mit Zoochlorellen, Lichenengonidien und anderen niederen Algen. Bot. Ztg. 1890, 47, 725–739, 741–754, 757–768, 781–785. [Google Scholar]
- Wong, Y.K.; Ho, Y.H.; Ho, K.C.; Leung, H.M.; Yung, K.K.L. Growth Medium Screening for Chlorella vulgaris Growth and Lipid Production. J. Aquac. Mar. Biol. 2017, 6, 00143. [Google Scholar] [CrossRef]
- Nichols, H.W.; Bold, H.C. Trichosarcina polymorpha Gen. et Sp. Nov. J. Phycol. 1965, 1, 34–38. [Google Scholar] [CrossRef]
- Serra-Maia, R.; Bernard, O.; Gonçalves, A.; Bensalem, S.; Lopes, F. Influence of temperature on Chlorella vulgaris growth and mortality rates in a photobioreactor. Algal Res. 2016, 18, 352–359. [Google Scholar] [CrossRef]
- Seyfabadi, J.; Ramezanpour, Z.; Amini Khoeyi, Z. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2010, 23, 721–726. [Google Scholar] [CrossRef]
- Zerveas, S.; Mente, M.; Tsakiri, D.; Kotzabasis, K. Microalgal photosynthesis induces alkalization of aquatic environment as a result of H+ uptake independently from CO2 concentration—New perspectives for environmental applications. J. Environ. Manage. 2021, 289, 112546. [Google Scholar] [CrossRef]
- Navakoudis, E.; Vrentzou, K.; Kotzabasis, K. A polyamine- and LHCII protease activity-based mechanism regulates the plasticity and adaptation status of the photosynthetic apparatus. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 261–271. [Google Scholar] [CrossRef]
- Strasser, B.J.; Strasser, R.J. Measuring Fast Fluorescence Transients to Address Environmental Questions: The JIP-Test. In Photosynthesis: From Light to Biosphere; Mathis, P., Ed.; Kluwer Academic Press: Dordrecht, The Netherlands, 1995; pp. 977–980. [Google Scholar] [CrossRef]
- Srivastava, A.; Strasser, R.J.; Govindjee. Polyphasic rise of chlorophyll a fluorescence in herbicide-resistant D1 mutants of Chlamydomonas reinardtii. Photosynth. Res. 1995, 43, 131–141. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Sati, H.; Mitra, M.; Mishra, S.; Baredar, P. Microalgal lipid extraction strategies for biodiesel production: A review. Algal Res. 2019, 38, 101413. [Google Scholar] [CrossRef]
- Park, J.; Jeong, H. Easy and rapid quantification of lipid contents of marine dinoflagellates using the sulpho-phospho-vanillin method. Algae 2016, 31, 391–401. [Google Scholar] [CrossRef]
- Schulze, C.; Wetzel, M.; Reinhardt, J.; Schmidt, M.; Felten, L.; Mundt, S. Screening of microalgae for primary metabolites including β-glucans and the influence of nitrate starvation and irradiance on β-glucan production. J. Appl. Phycol. 2016, 28, 2719–2725. [Google Scholar] [CrossRef]
- Navakoudis, E.; Lütz, C.; Langebartels, C.; Lütz-Meindl, U.; Kotzabasis, K. Ozone impact on the photosynthetic apparatus and the protective role of polyamines. Biochim. Biophys. Acta. 2003, 1621, 160–169. [Google Scholar] [CrossRef]
- Lütz, C.; Navakoudis, E.; Seidlitz, H.K.; Kotzabasis, K. Simulated solar irradiation with enhanced UV-B adjust plastid- and thylakoid-associated polyamine changes for UV-B protection. Biochim. Biophys. Acta 2005, 1710, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Sfichi, L.; Ioanidis, E.N.; Kotzabasis, K. Thylakoid-associated polyamines adjust the UVB-sensitivity of the photosynthetic apparatus by means of LHCII changes. Photochem. Photobiol. 2004, 80, 499–506. [Google Scholar] [CrossRef]
- Sfakianaki, M.; Sfichi, L.; Kotzabasis, K. The involvement of LHCII-associated polyamines in the response of the photosynthetic apparatus to low temperature. J. Photochem. Photobiol. B 2006, 84, 181–188. [Google Scholar] [CrossRef]
- Jin, H.F.; Lim, B.R.; Lee, K. Influence of nitrate feeding on carbon dioxide fixation by microalgae. J. Environ. Sci. Health A 2006, 41, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Carrasquillo, R. ISS ECLSS Technology Evolution for Exploration. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 10–13 January 2005. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Liu, T.; Zhong, Y.; Ji, C.; Lu, Q.; Fan, L.; Li, J.; Leng, L.; Li, K.; et al. Microalgae biotechnology as an attempt for bioregenerative life support systems: Problems and prospects. J. Chem. Technol. Biotechnol. 2019, 94, 3039–3048. [Google Scholar] [CrossRef]
- Battistuzzi, M.; Cocola, L.; Salasnich, B.; Erculiani, M.S.; Alei, E.; Morosinotto, T.; Claudi, R.; Poletto, L.; La Rocca, N. A new remote sensing-based system for the monitoring and analysis of growth and gas exchange rates of photosynthetic microorganisms under simulated non-terrestrial conditions. Front. Plant Sci. 2020, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Jones, H. Design Rules for Space Life Support Systems. SAE Tech. Pap. 2003, 2356. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).