Recent Advances in Selected Asymmetric Reactions Promoted by Chiral Catalysts: Cyclopropanations, Friedel–Crafts, Mannich, Michael and Other Zinc-Mediated Processes—An Update
Abstract
:1. Introduction
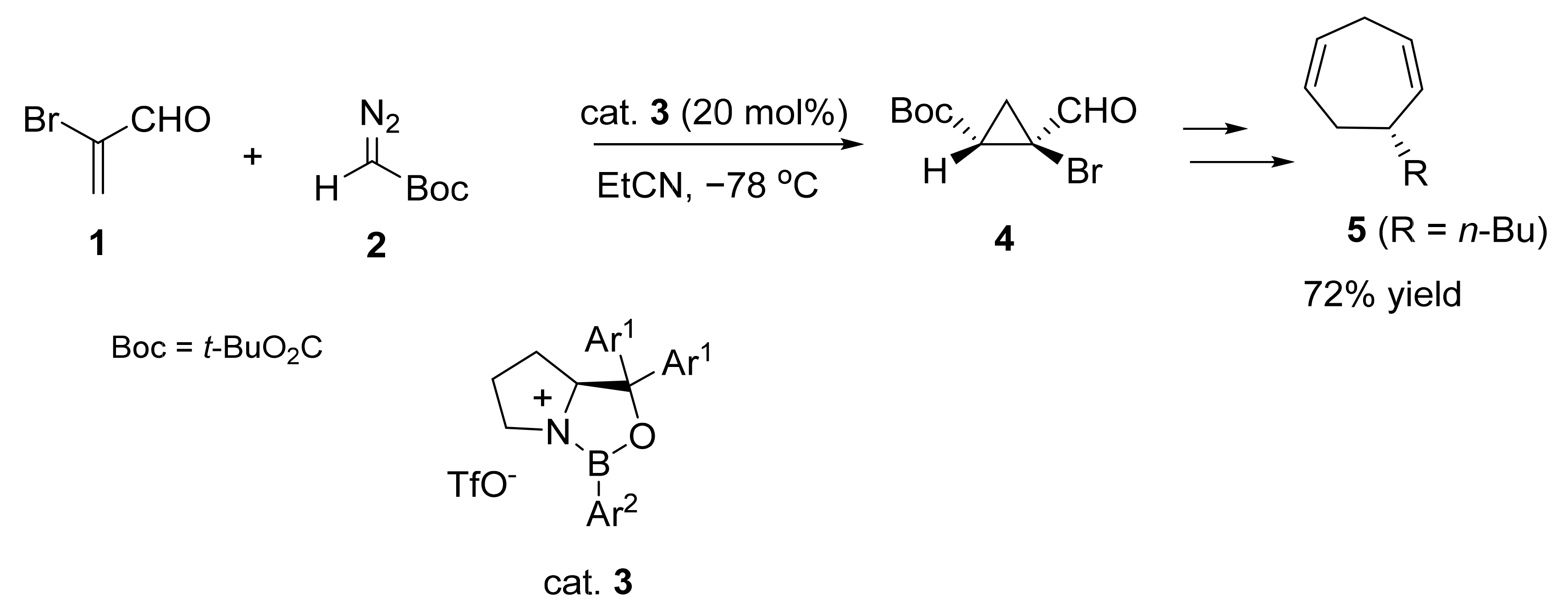
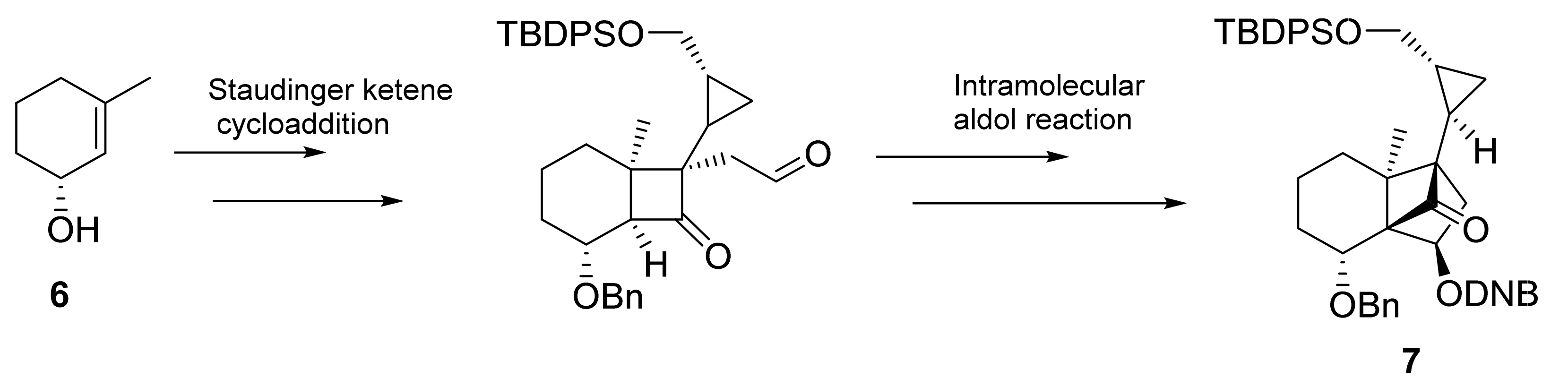
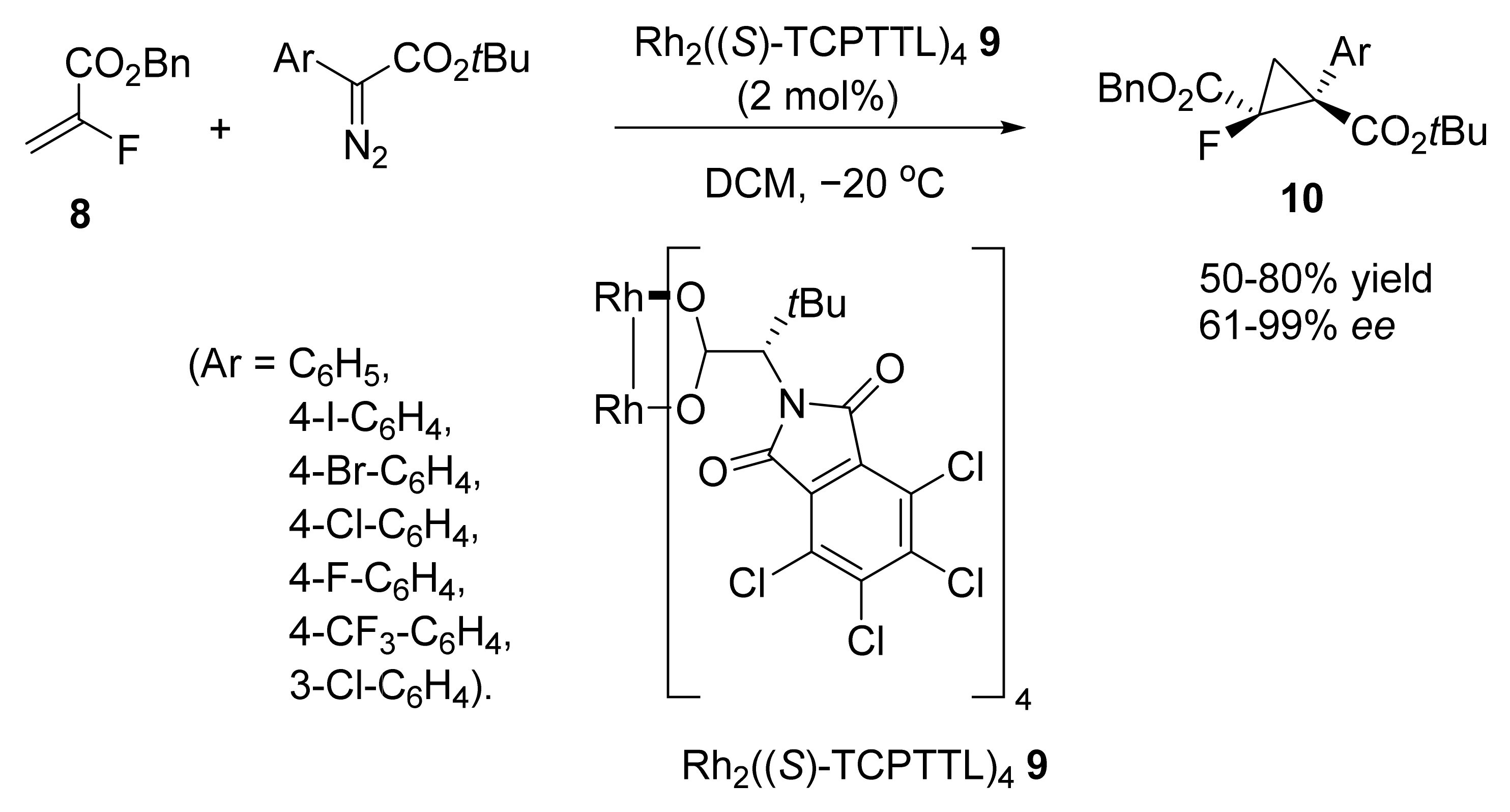
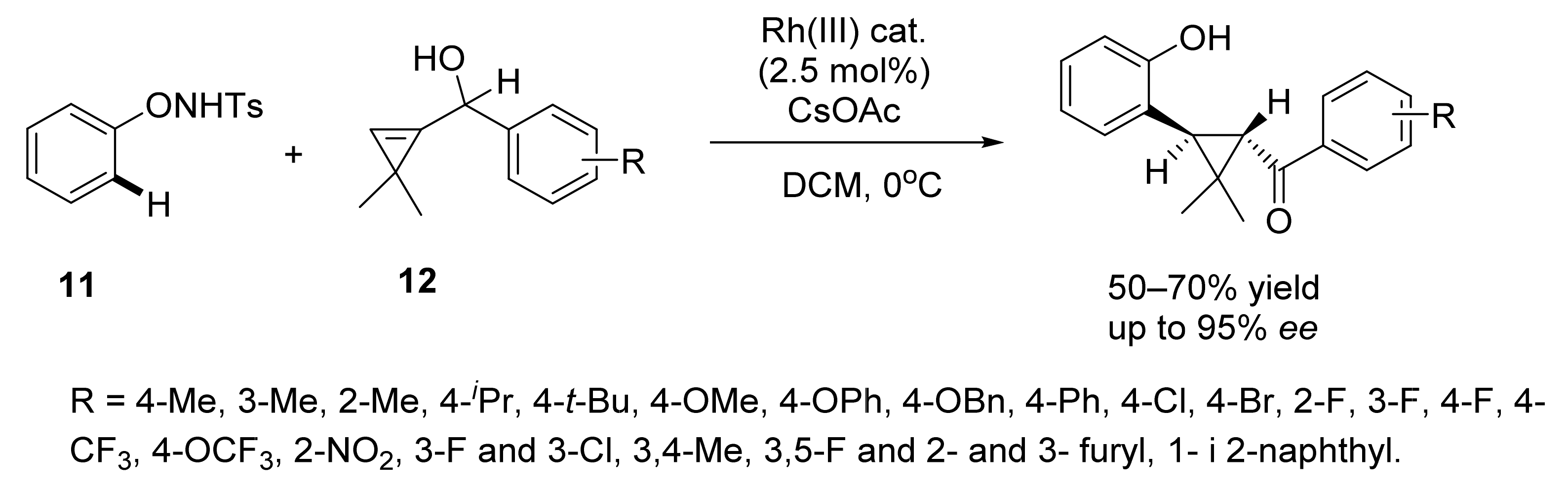
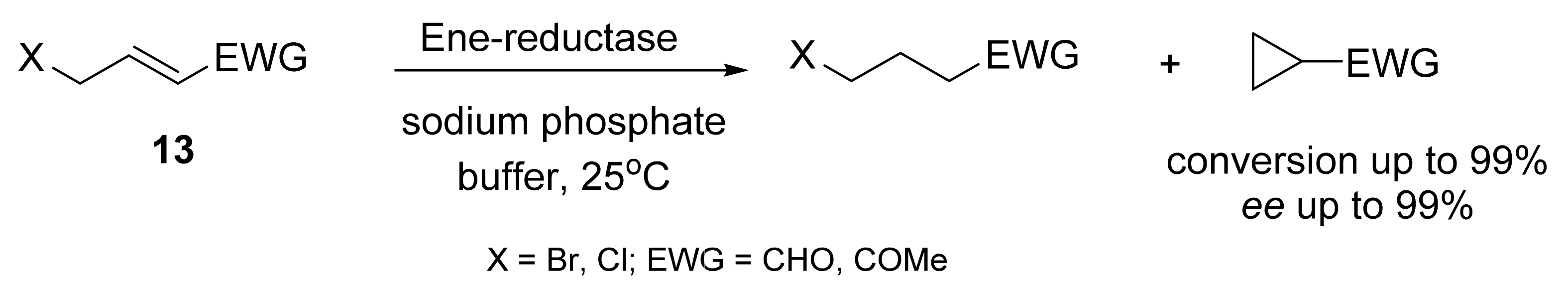
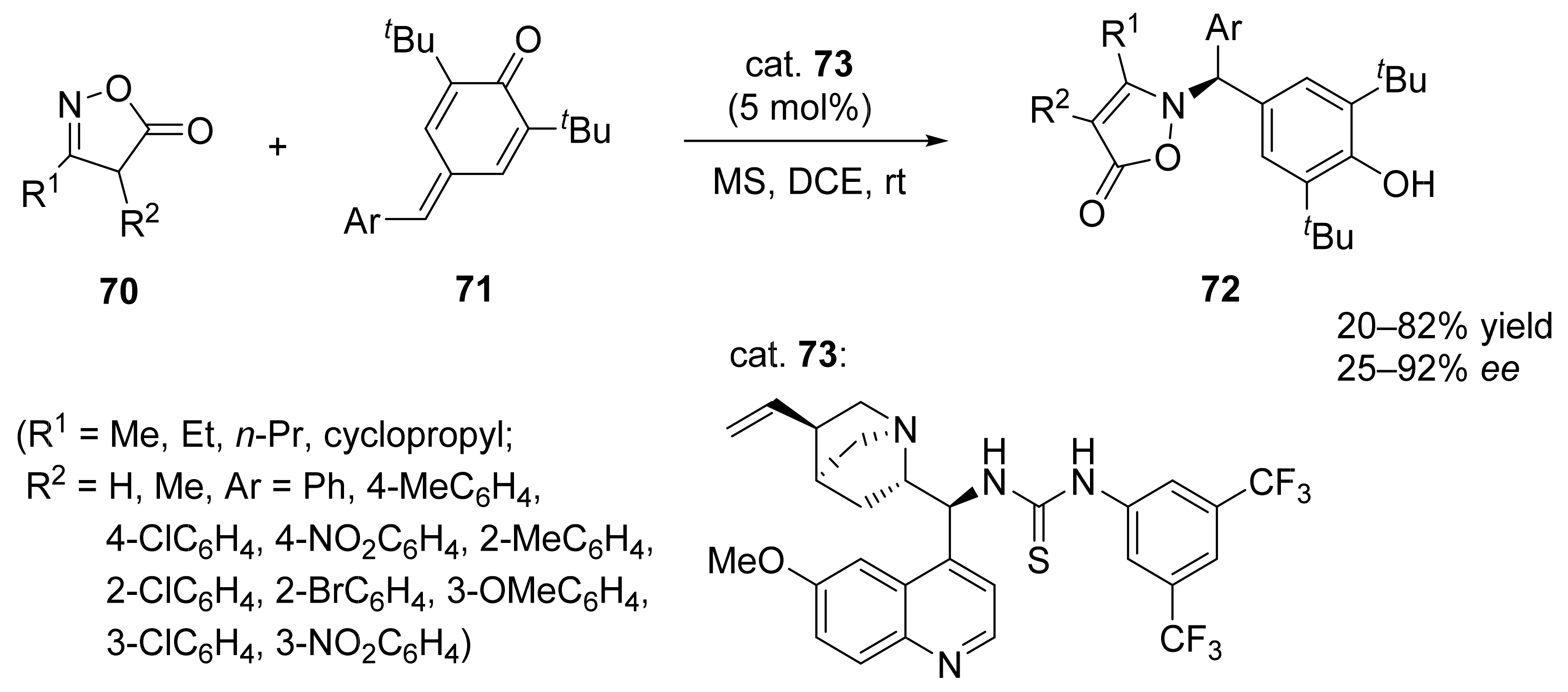
2. Asymmetric Cyclopropanation Reactions
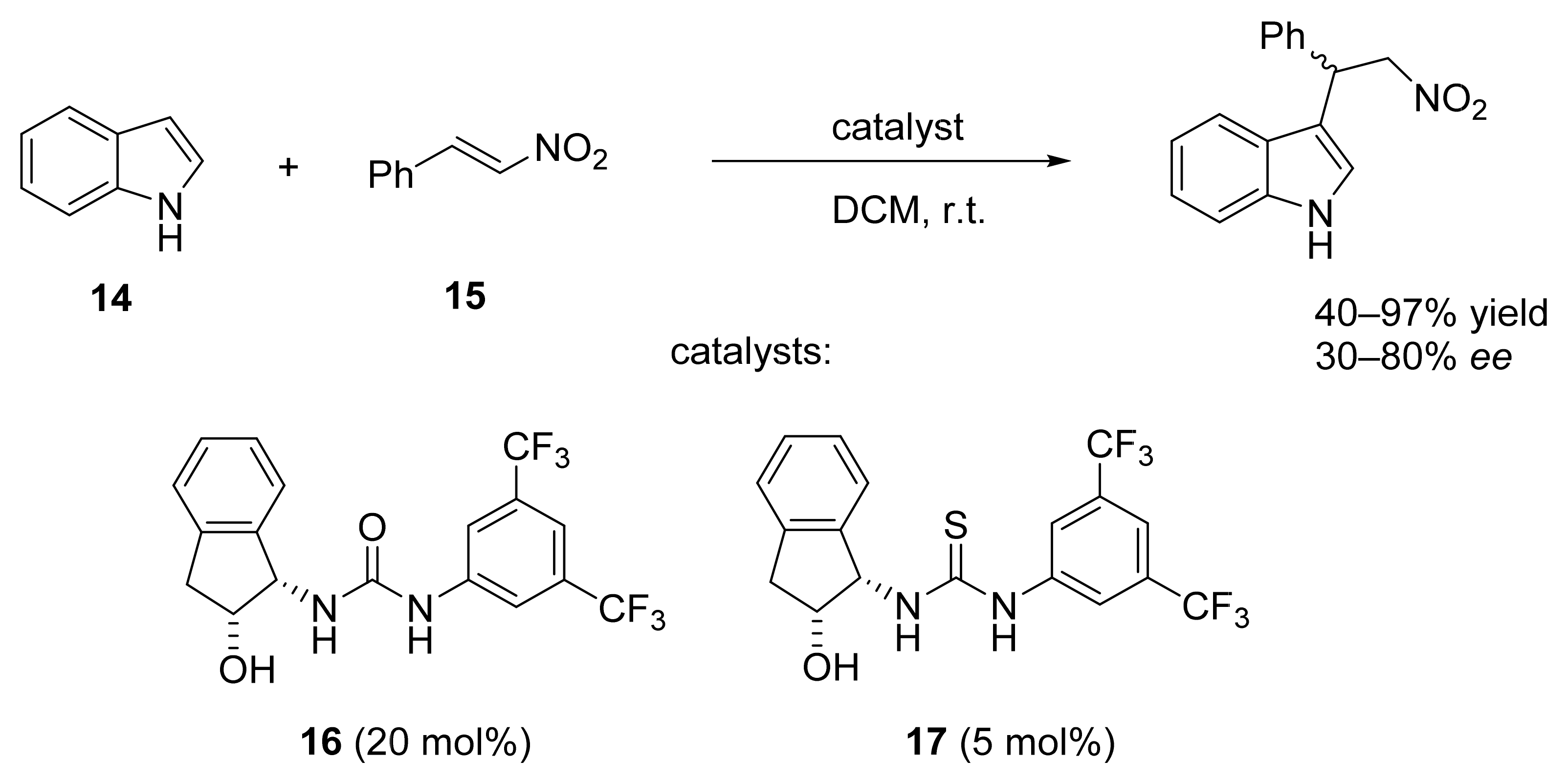
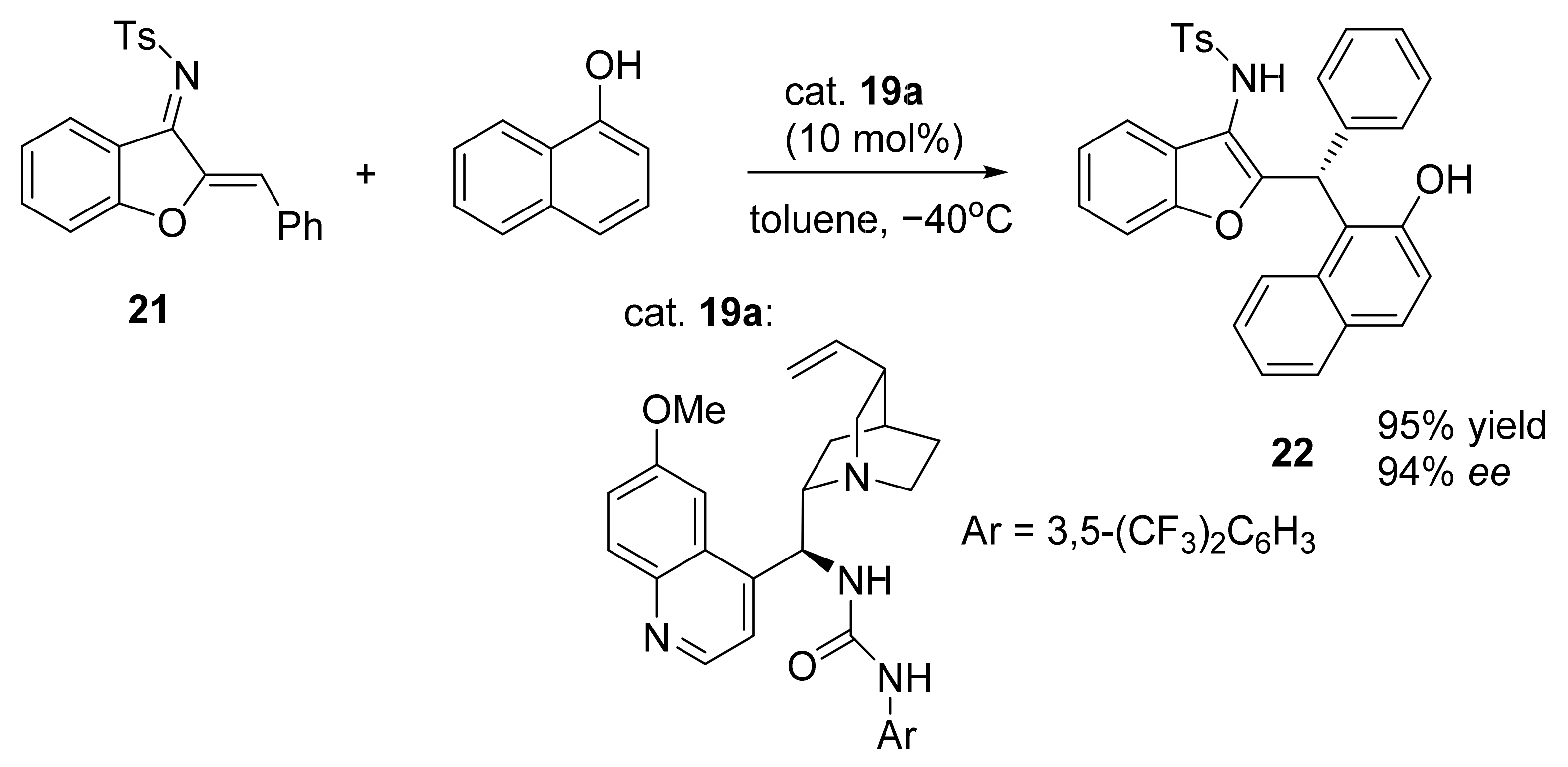
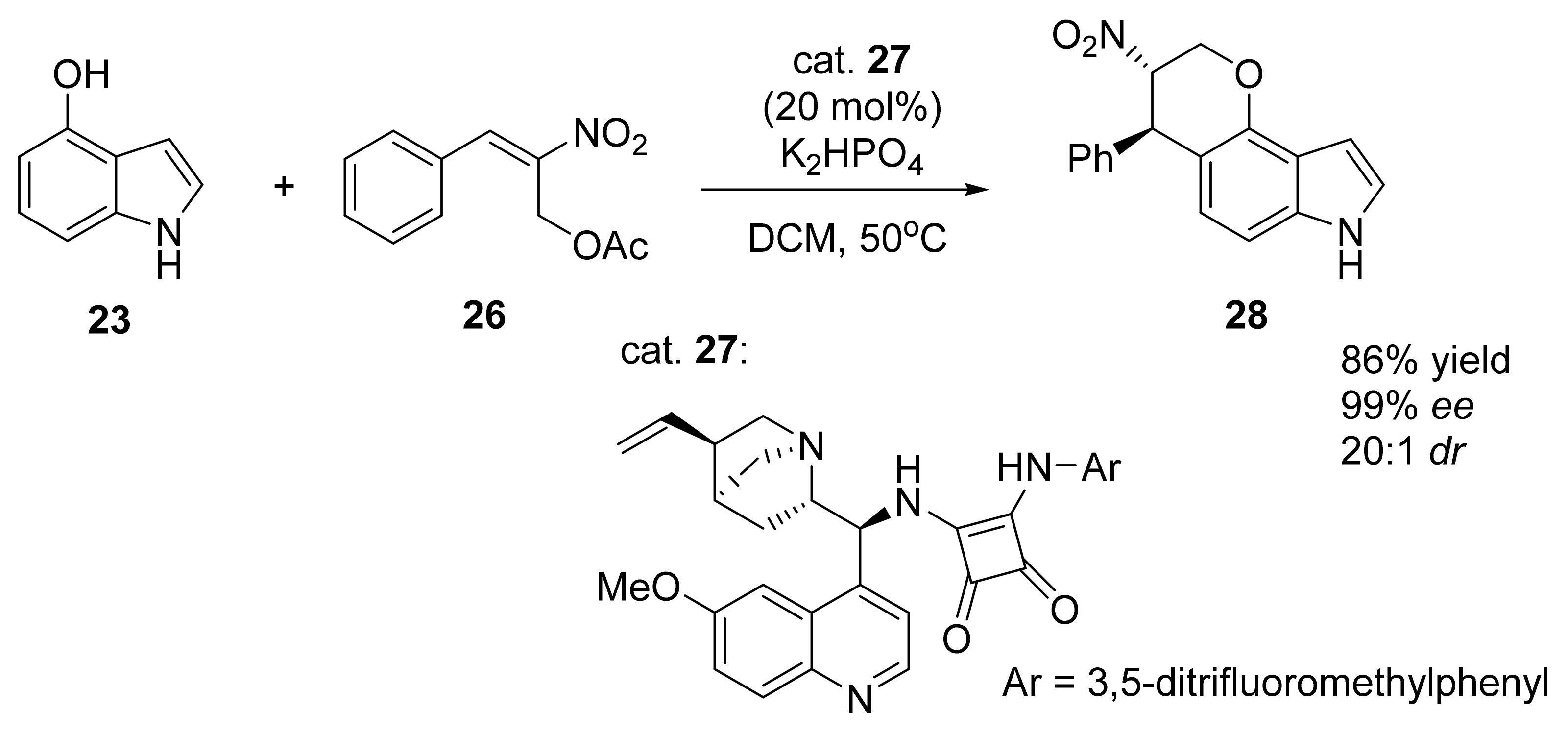
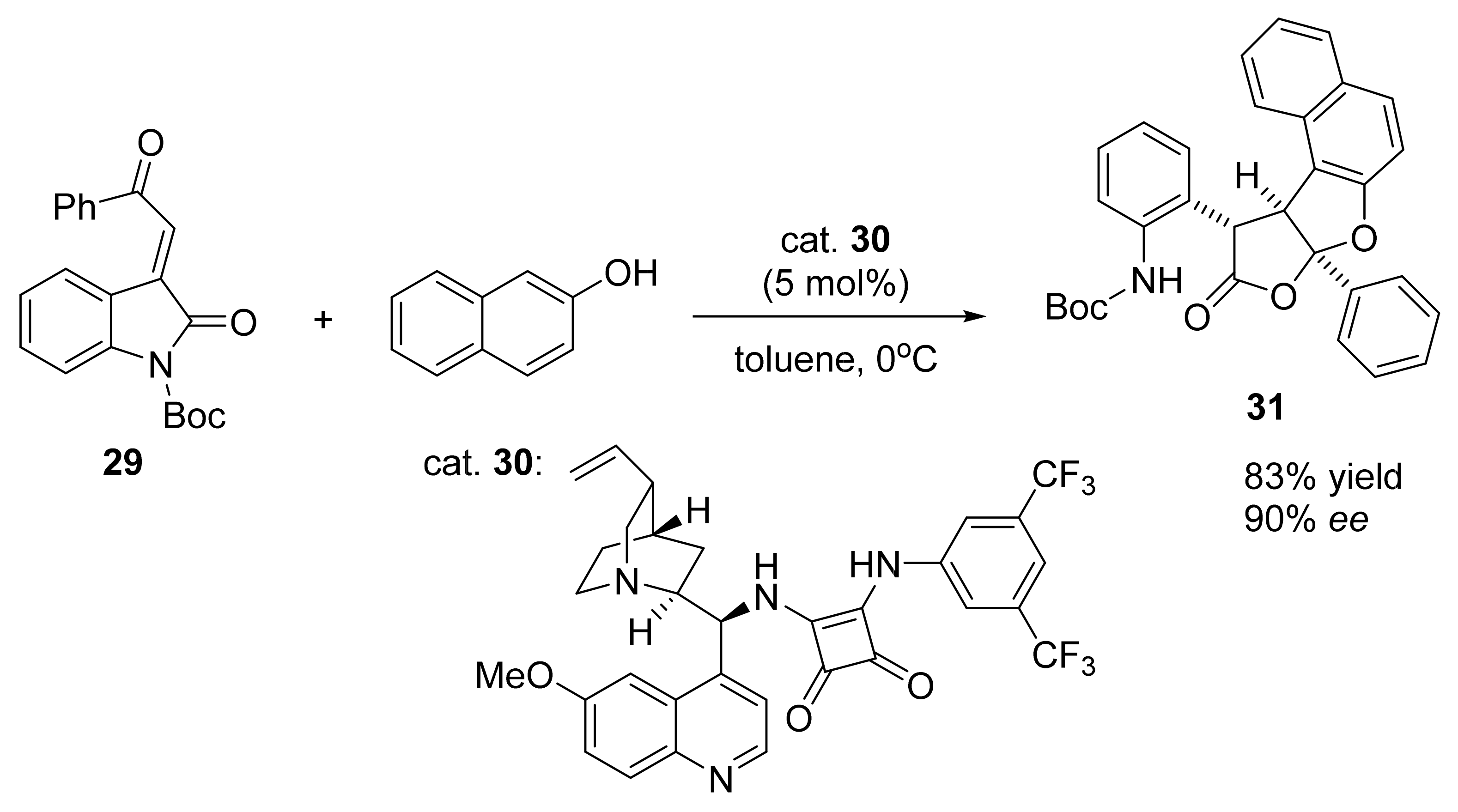
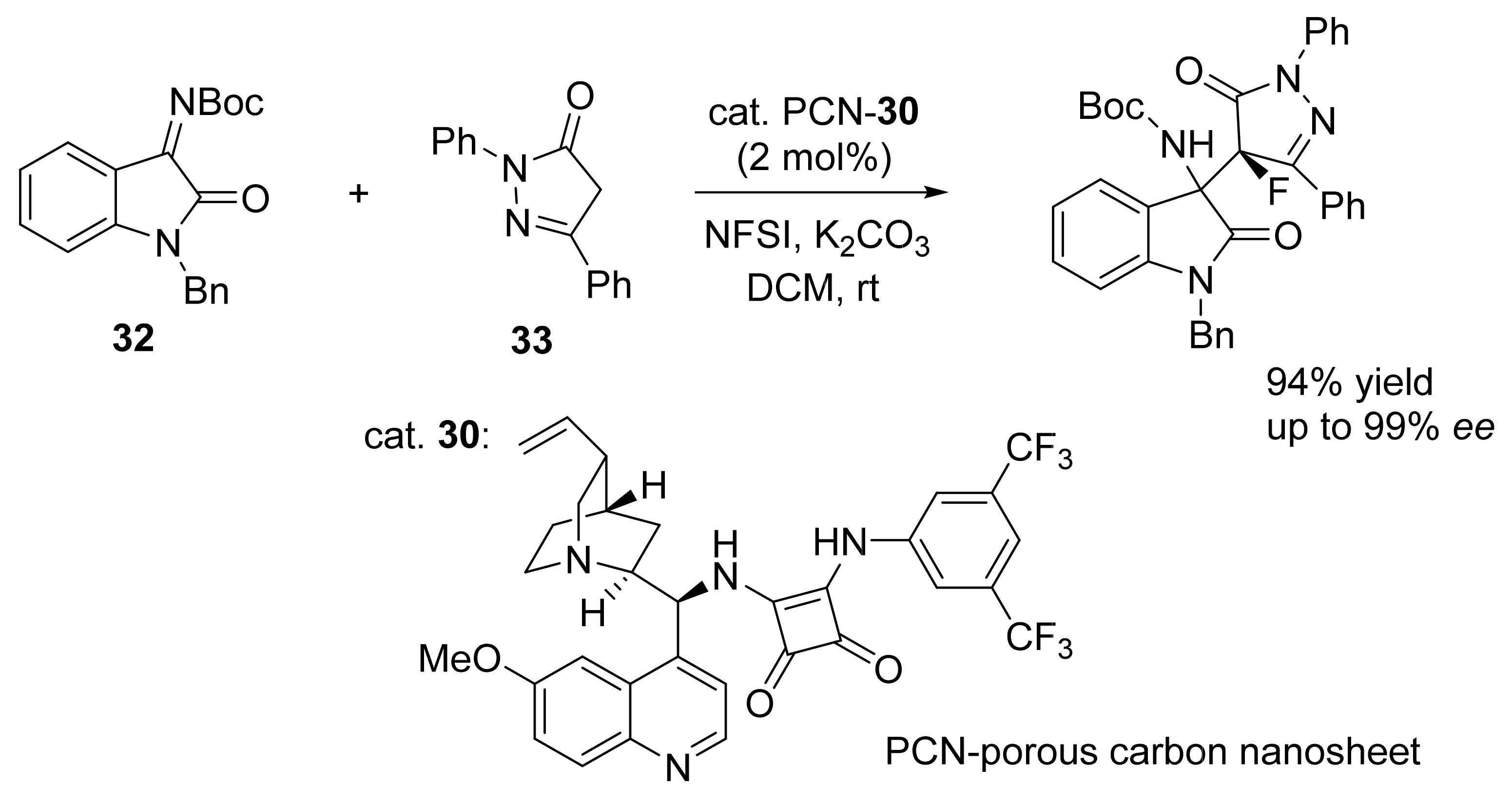
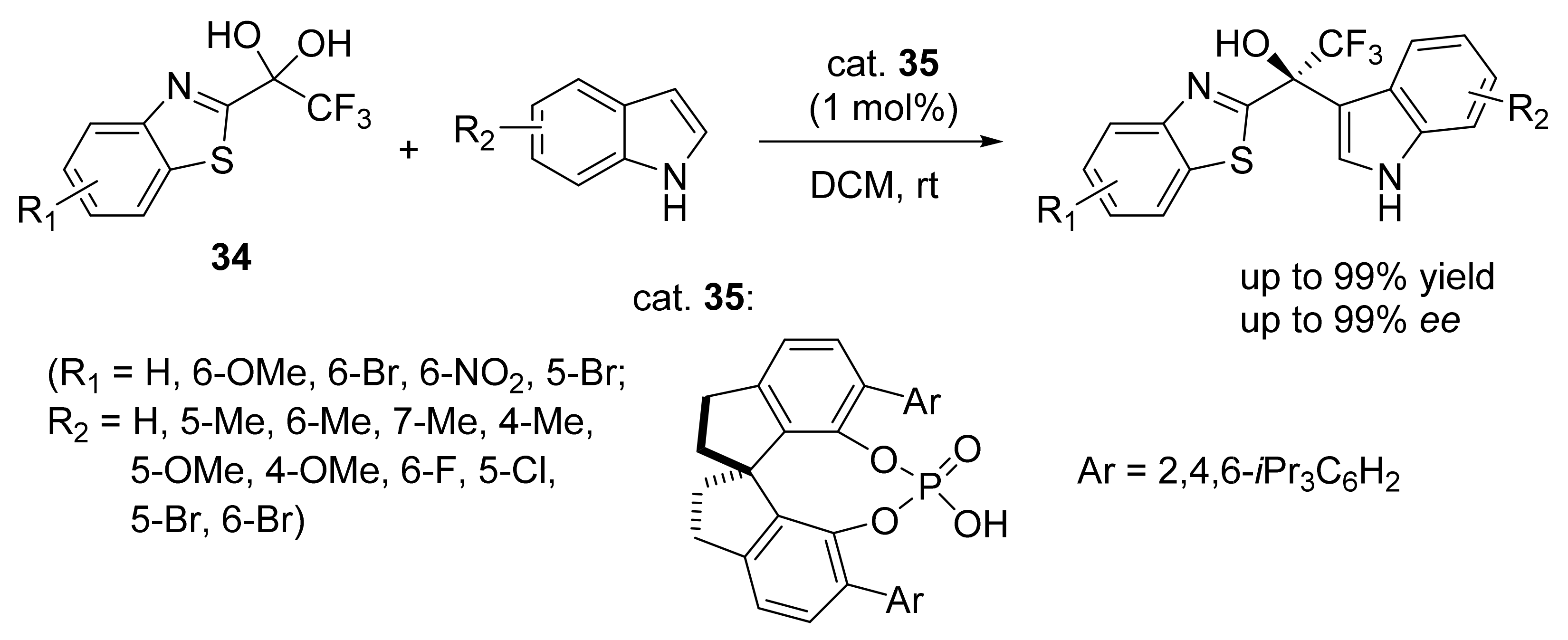
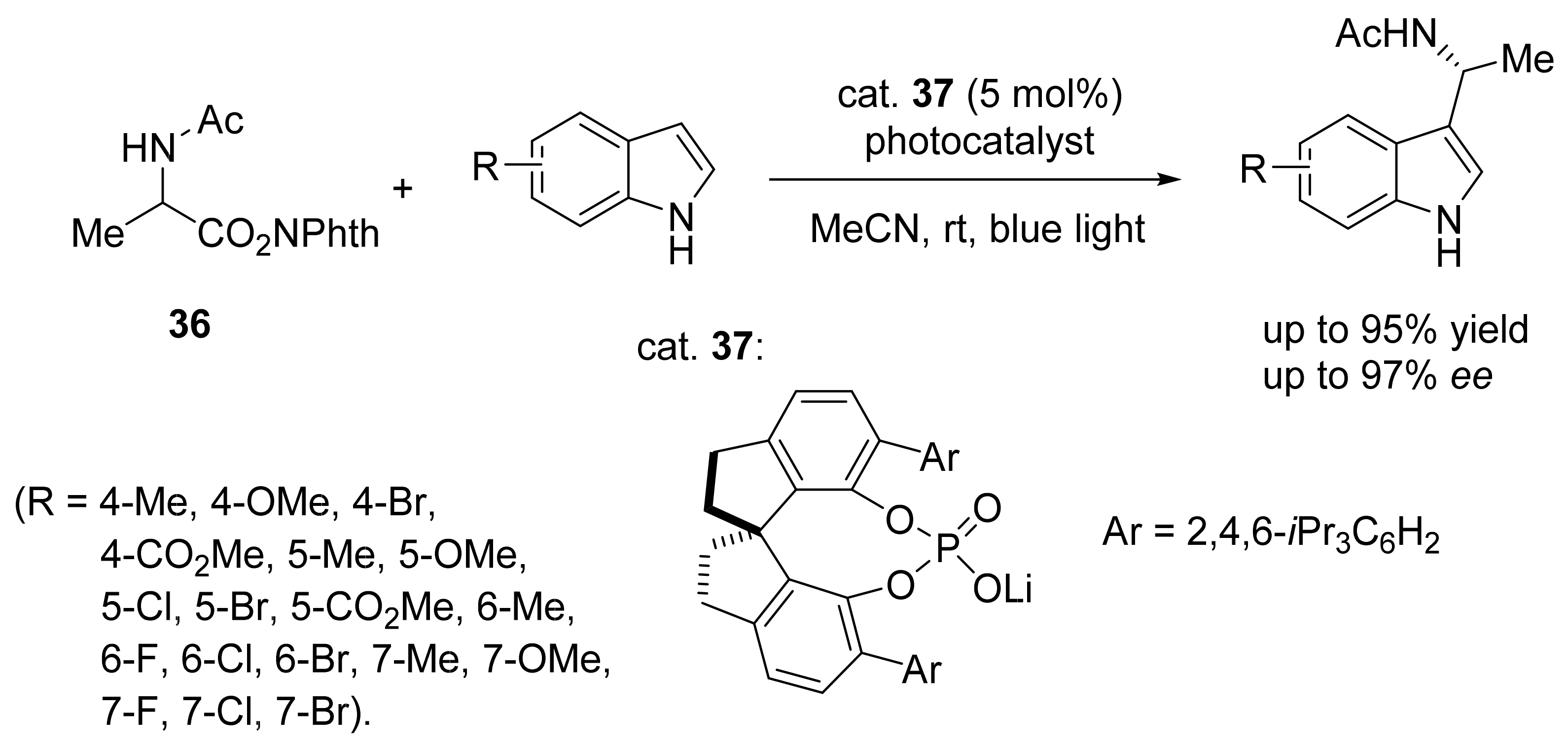
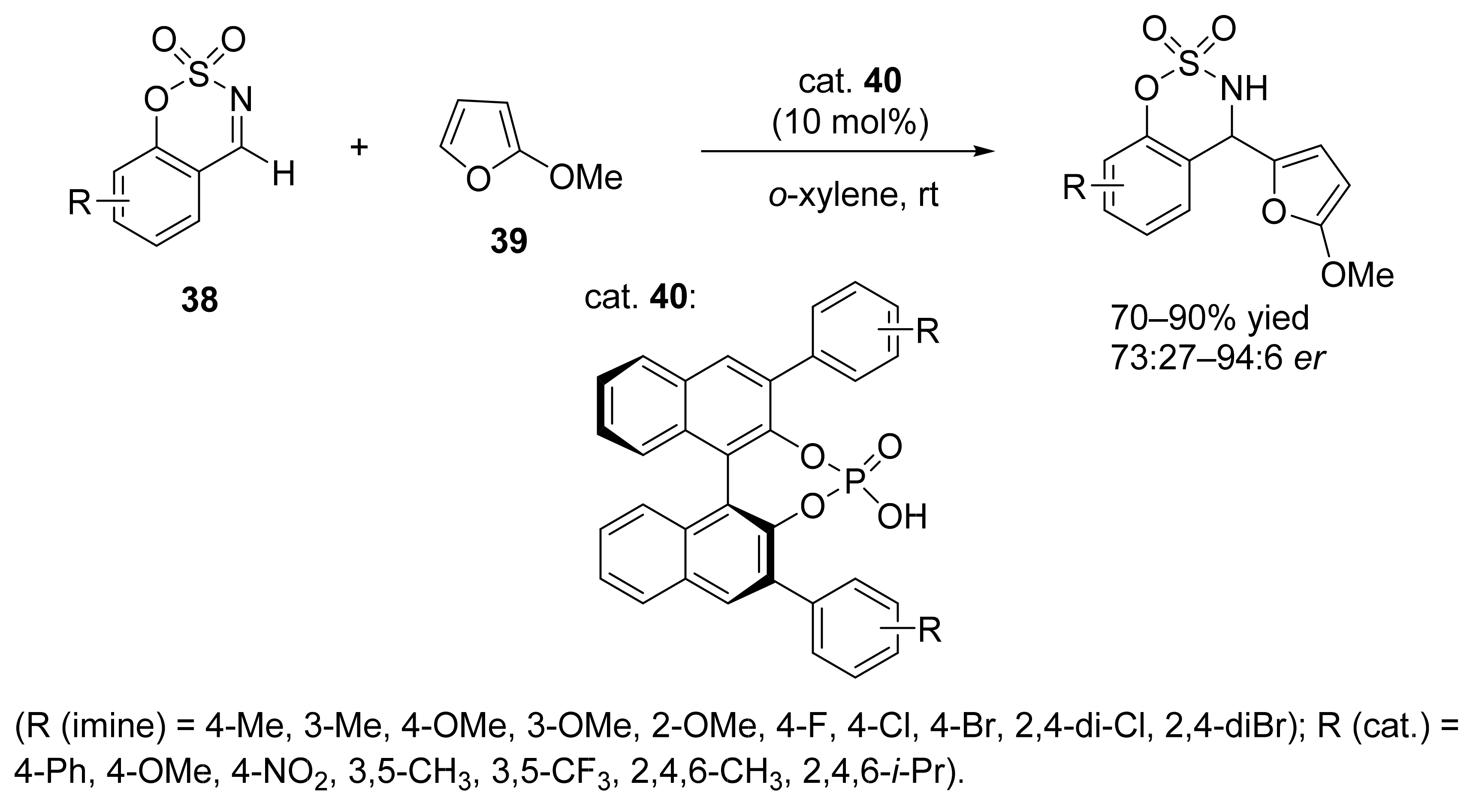
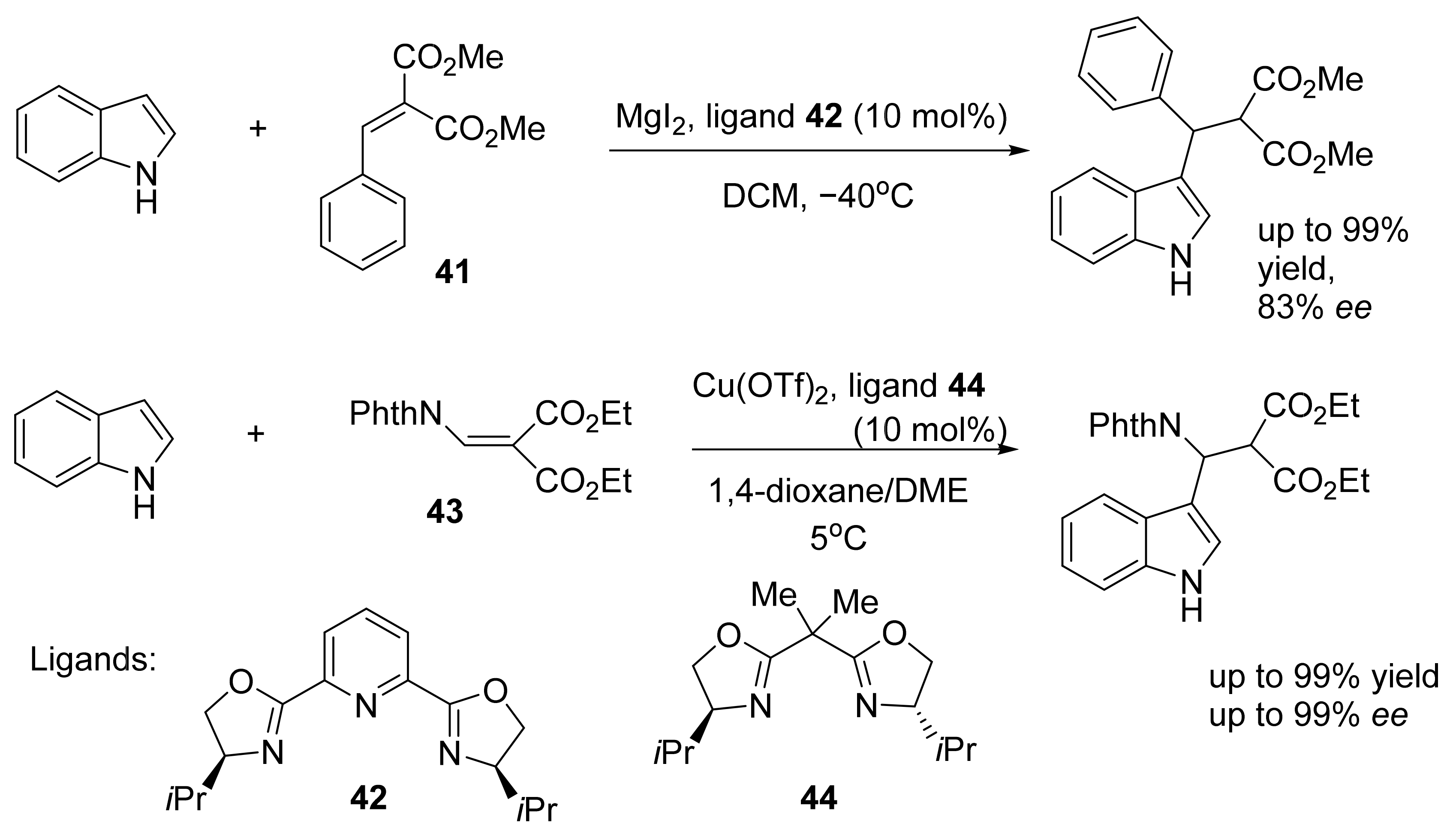
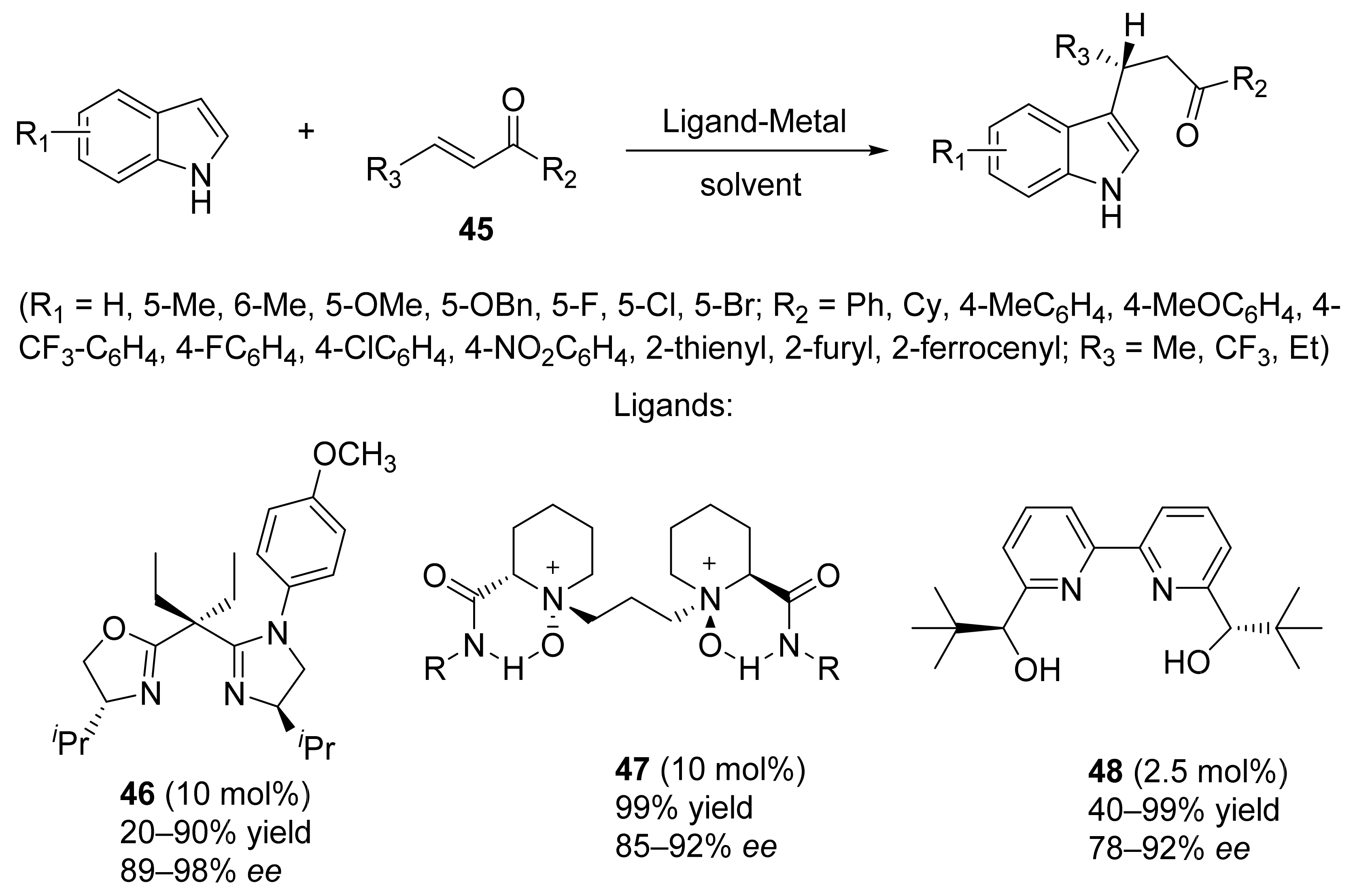
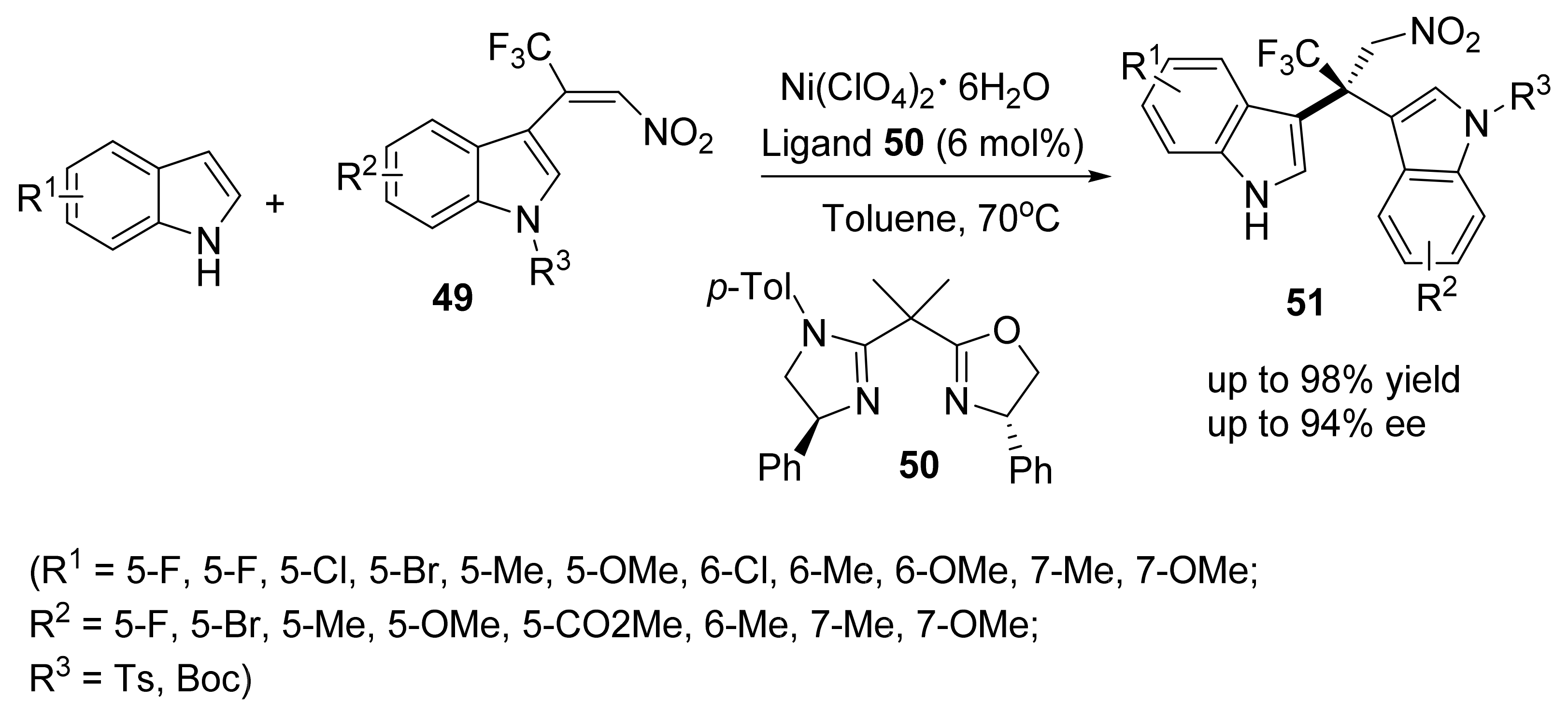
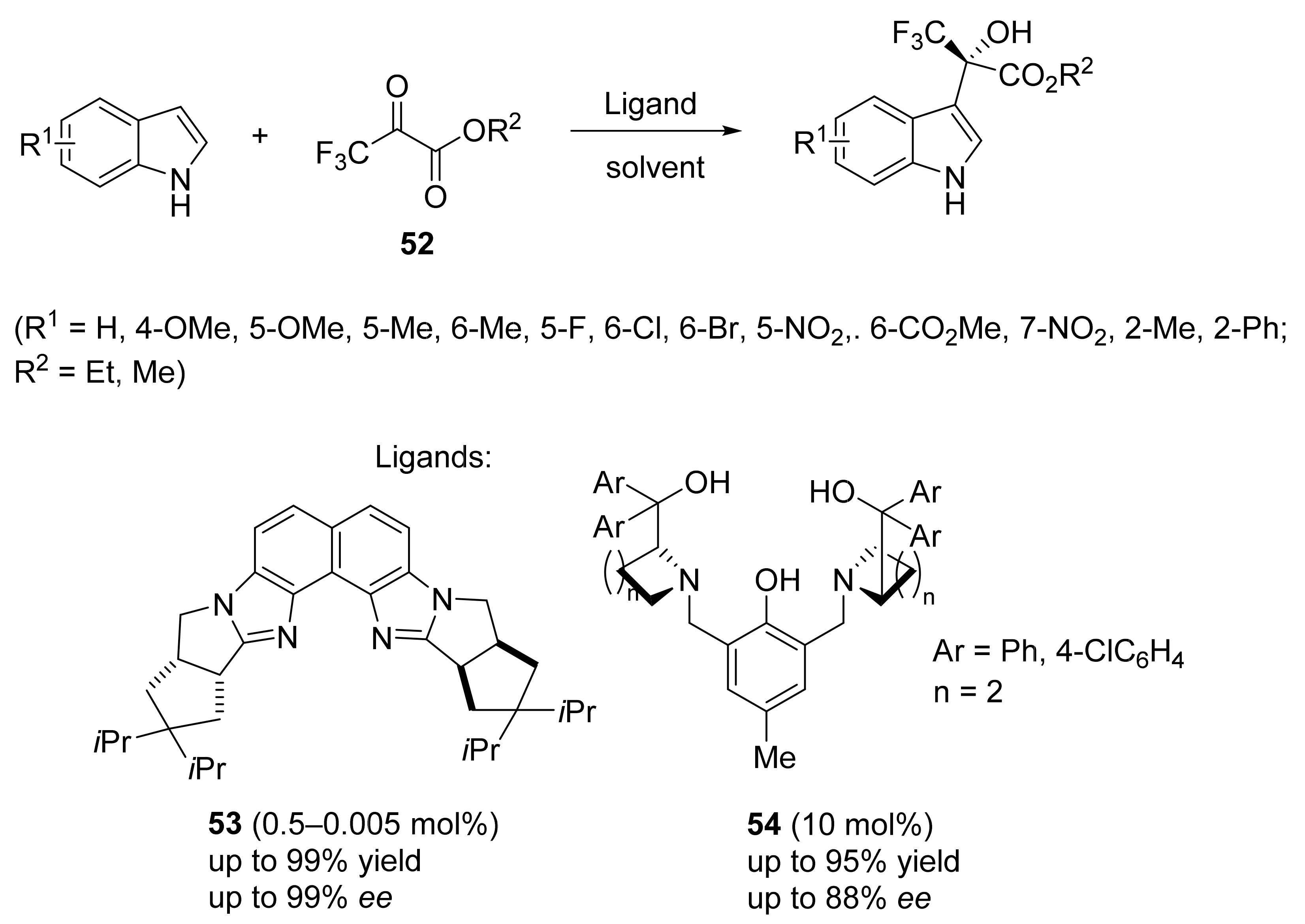
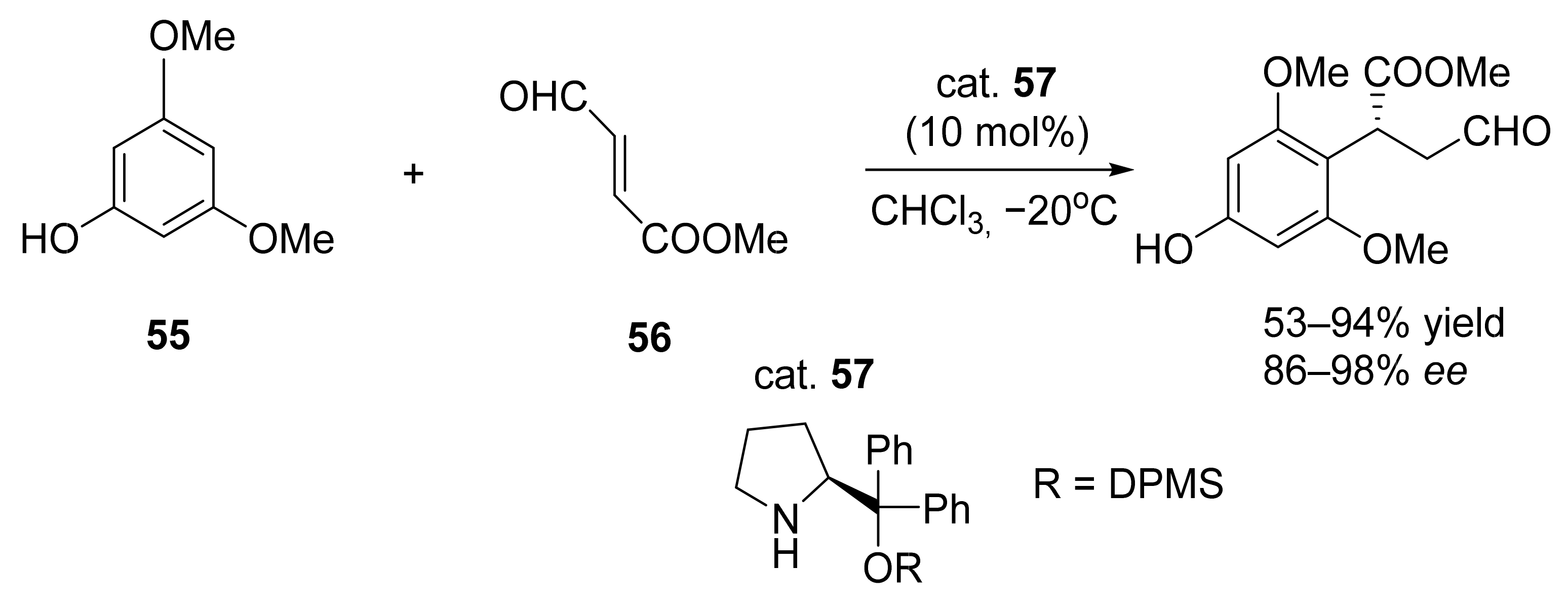
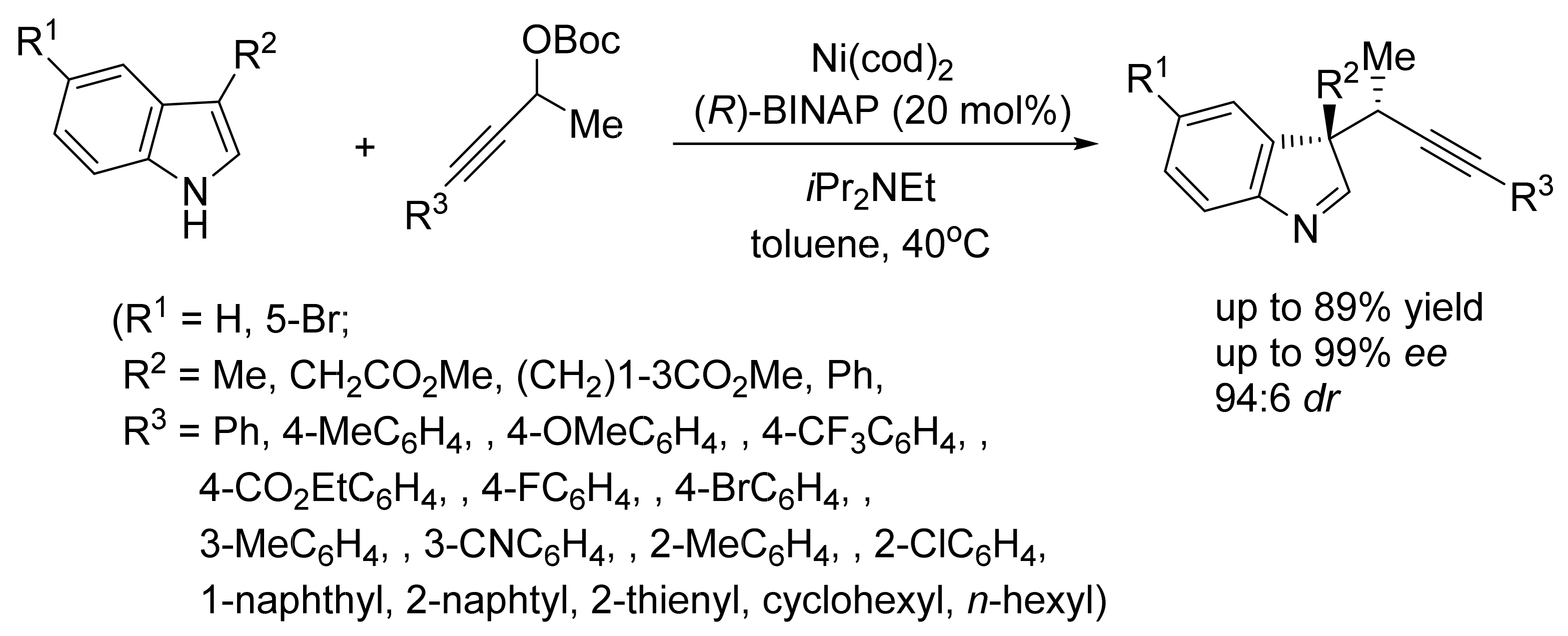
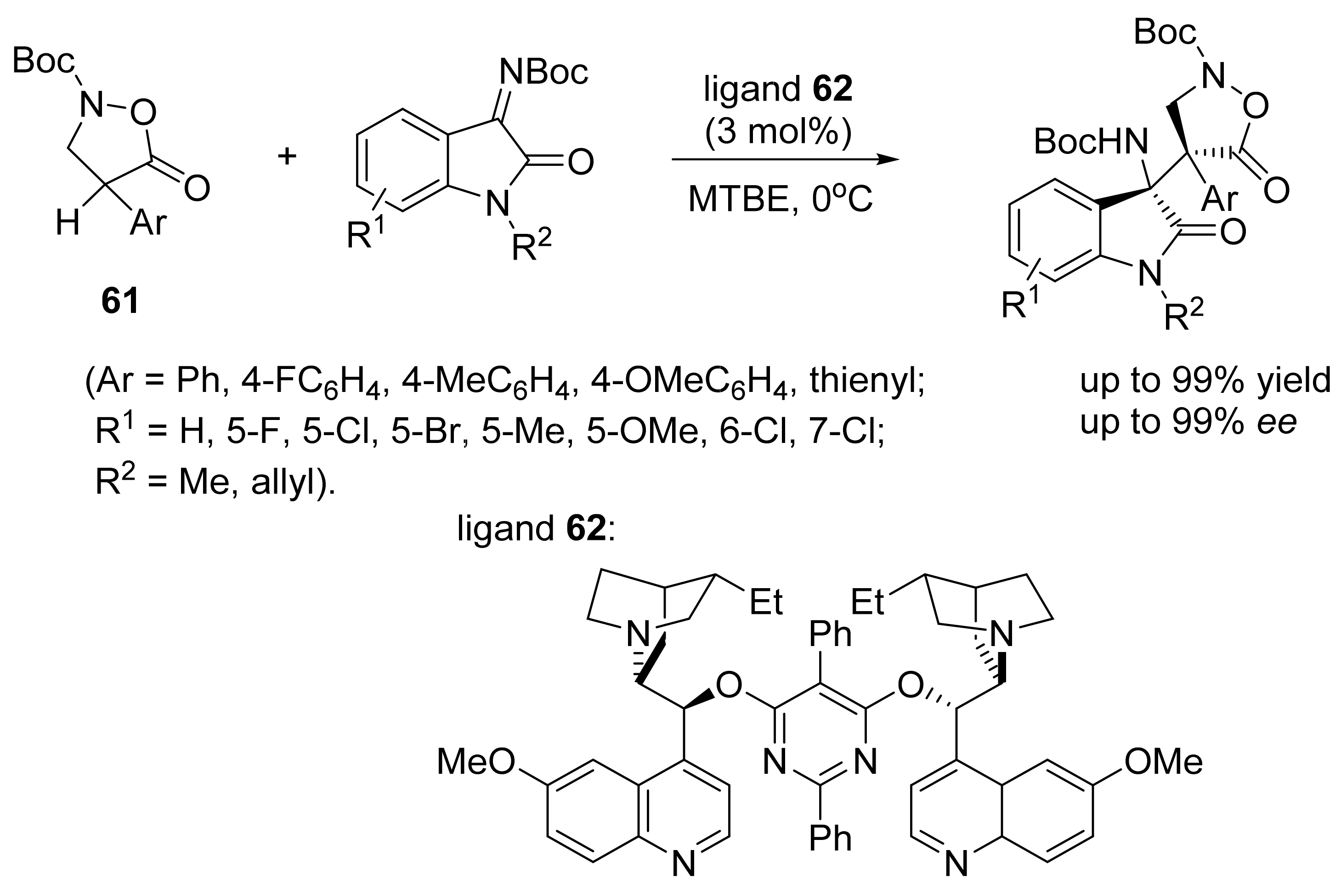
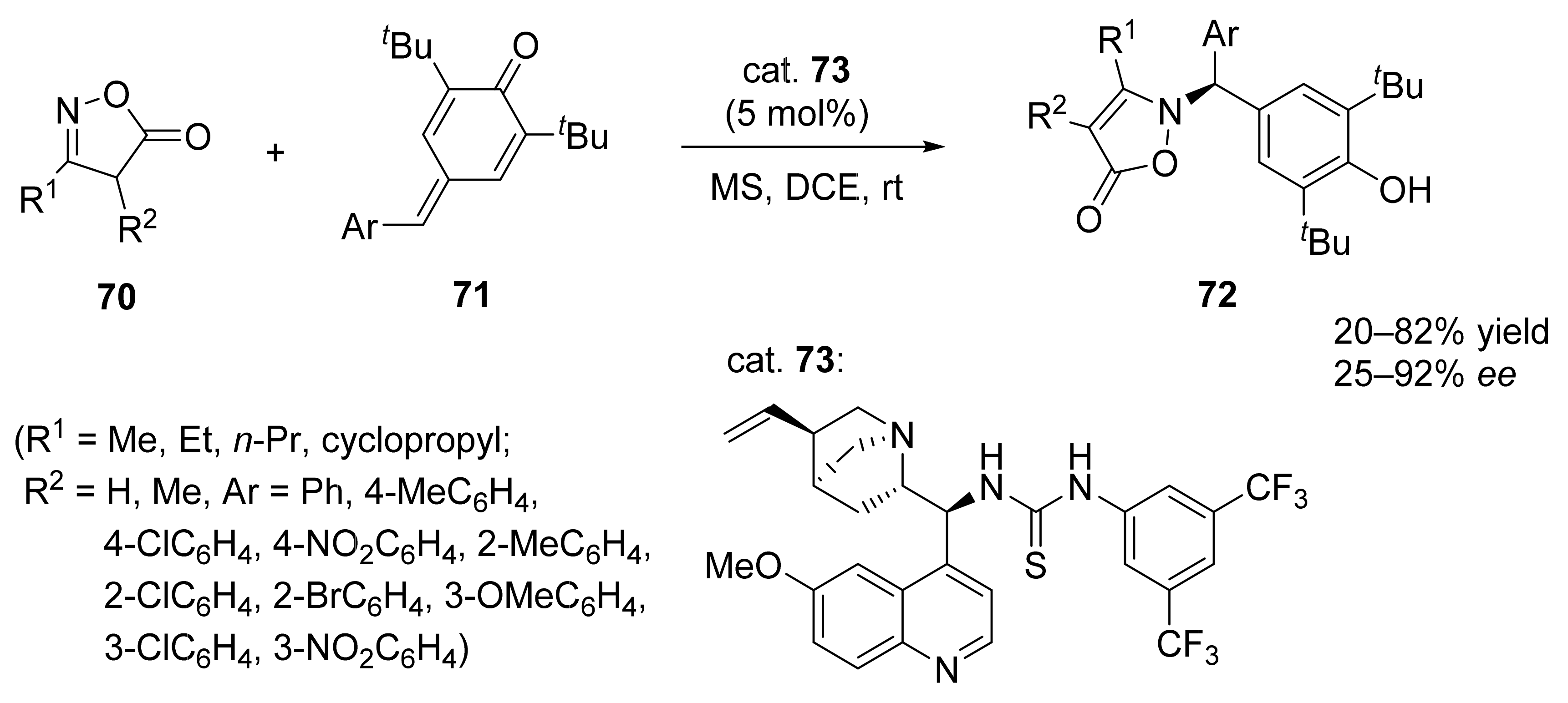
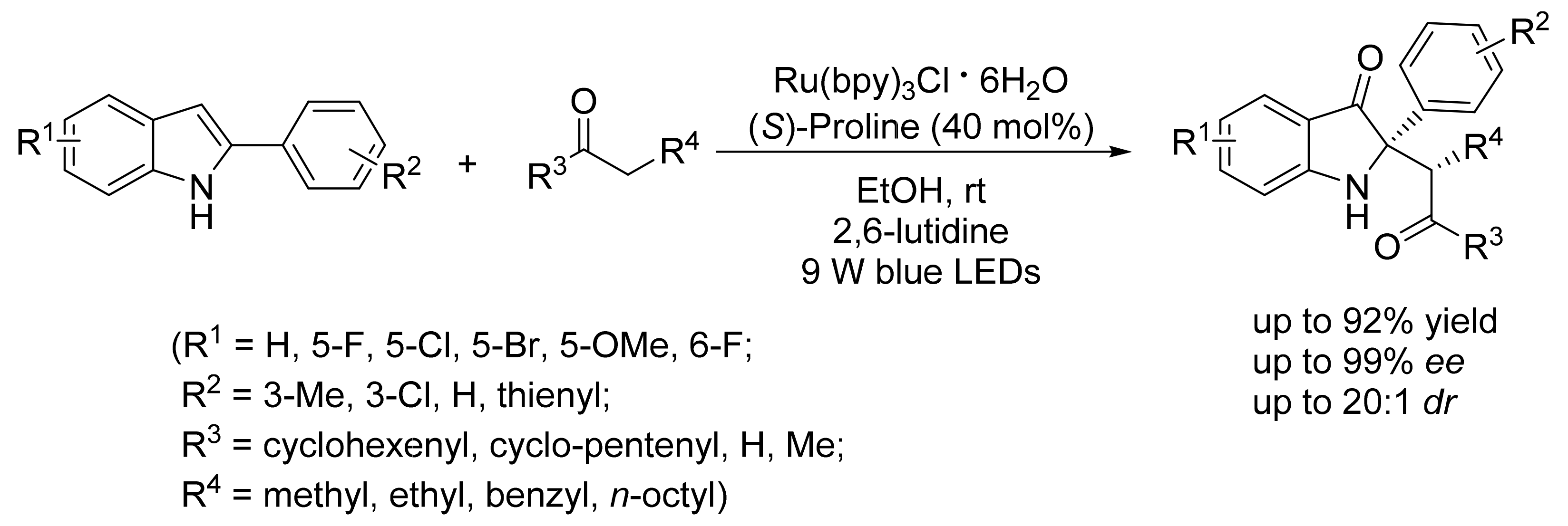
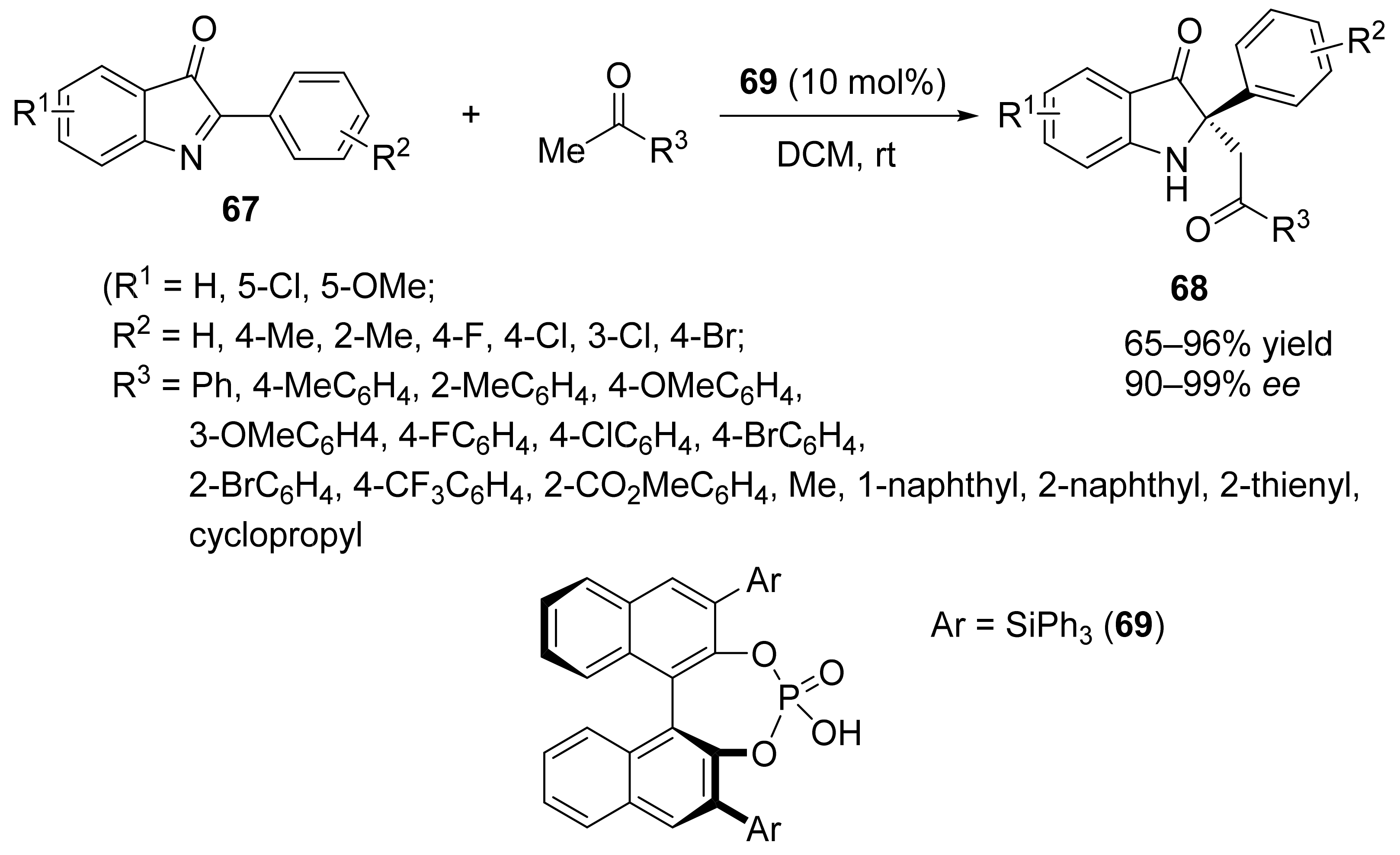
3. Asymmetric Friedel–Crafts Reactions
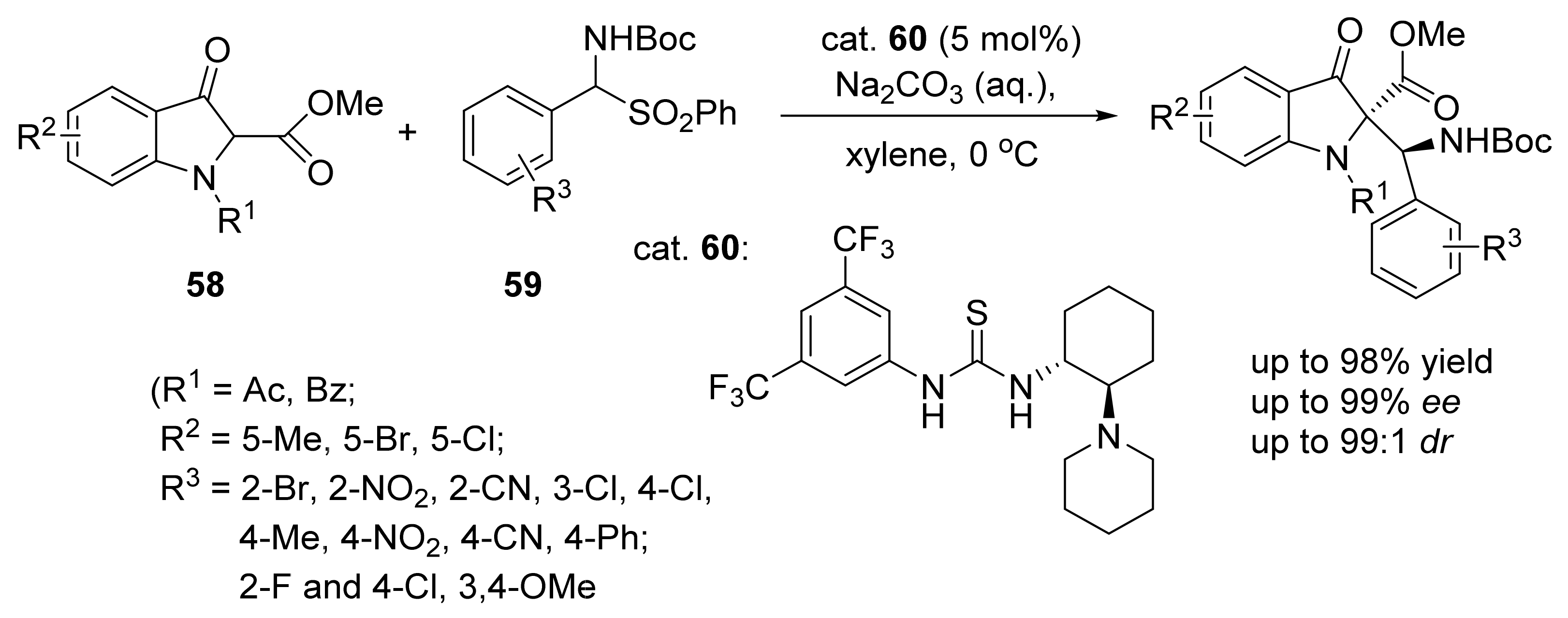
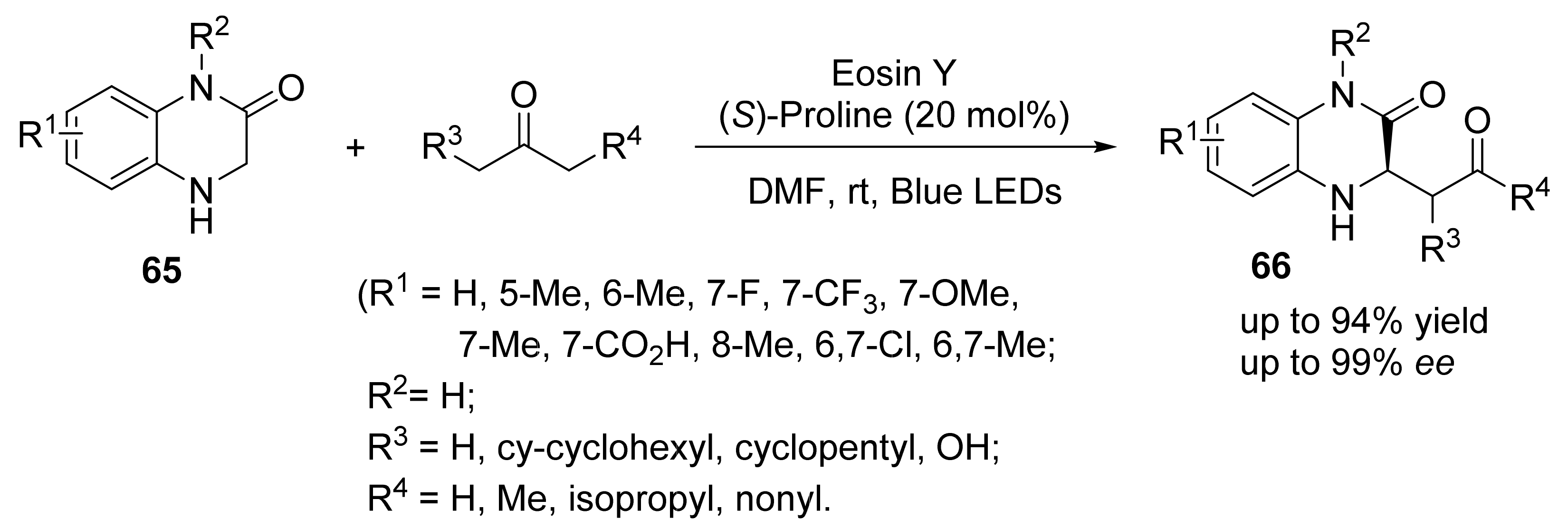
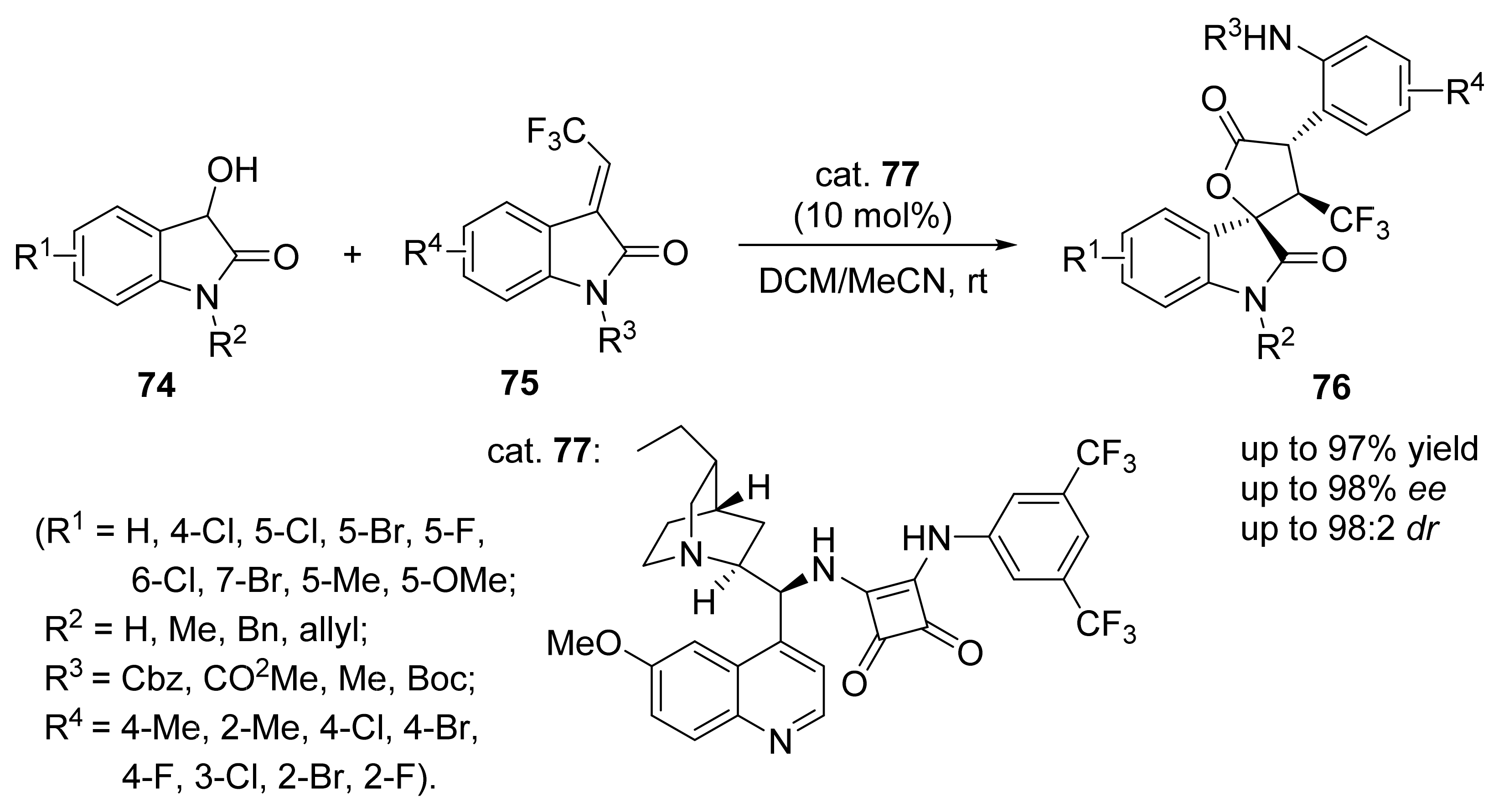
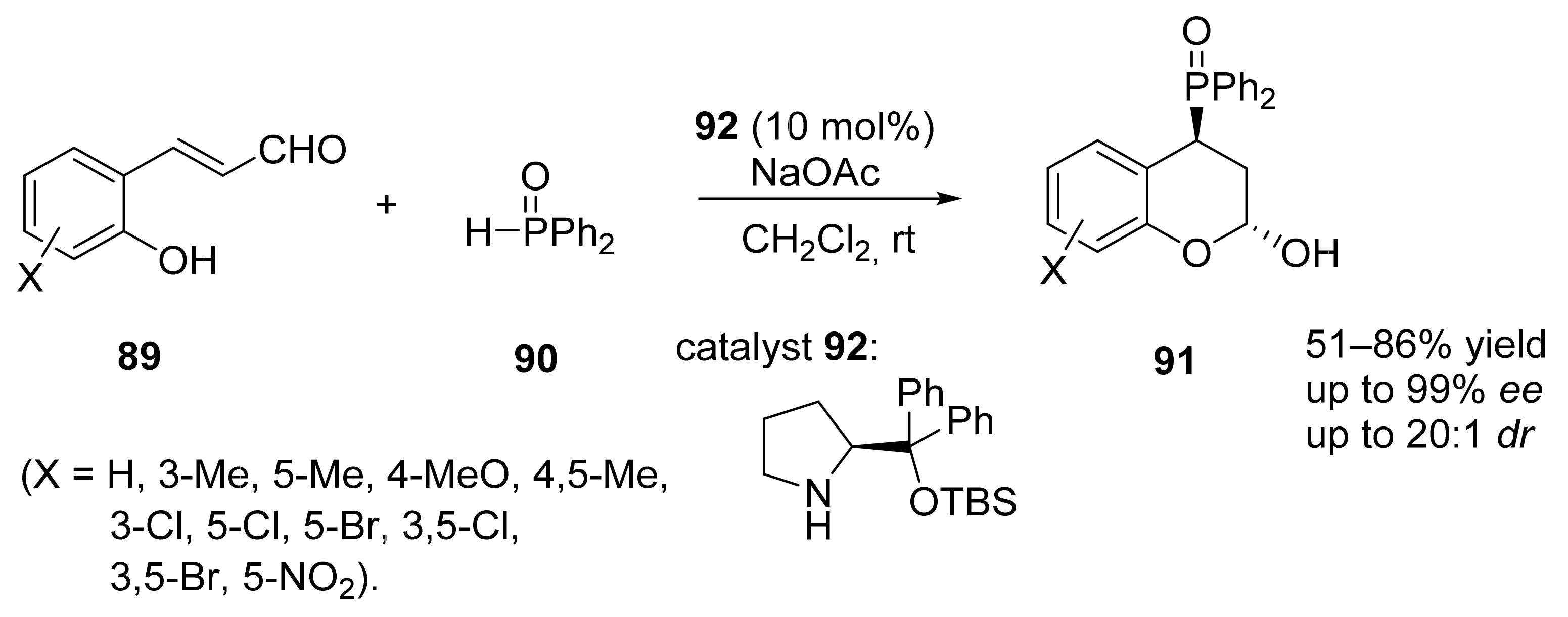
4. Asymmetric Mannich Reactions
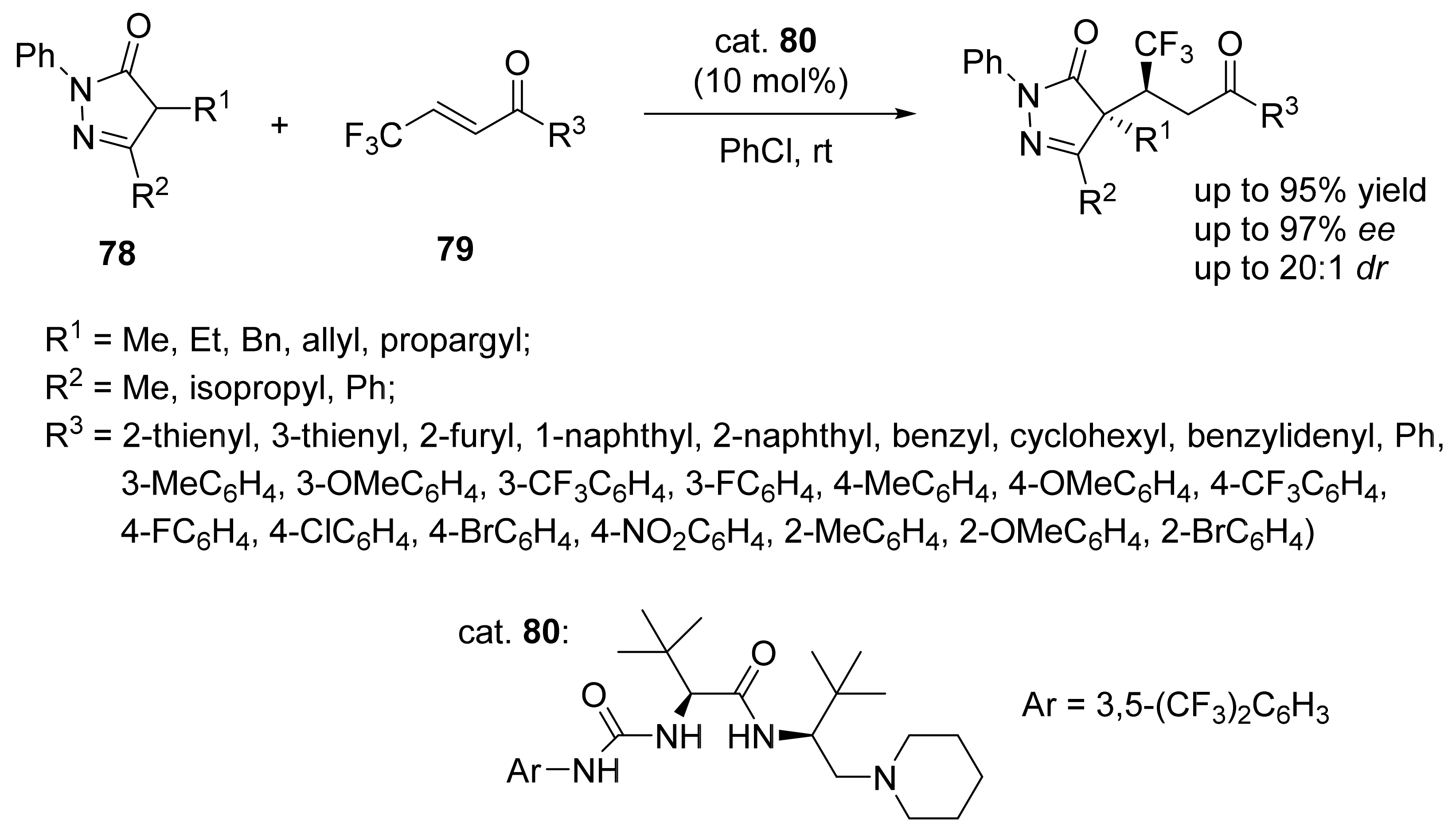
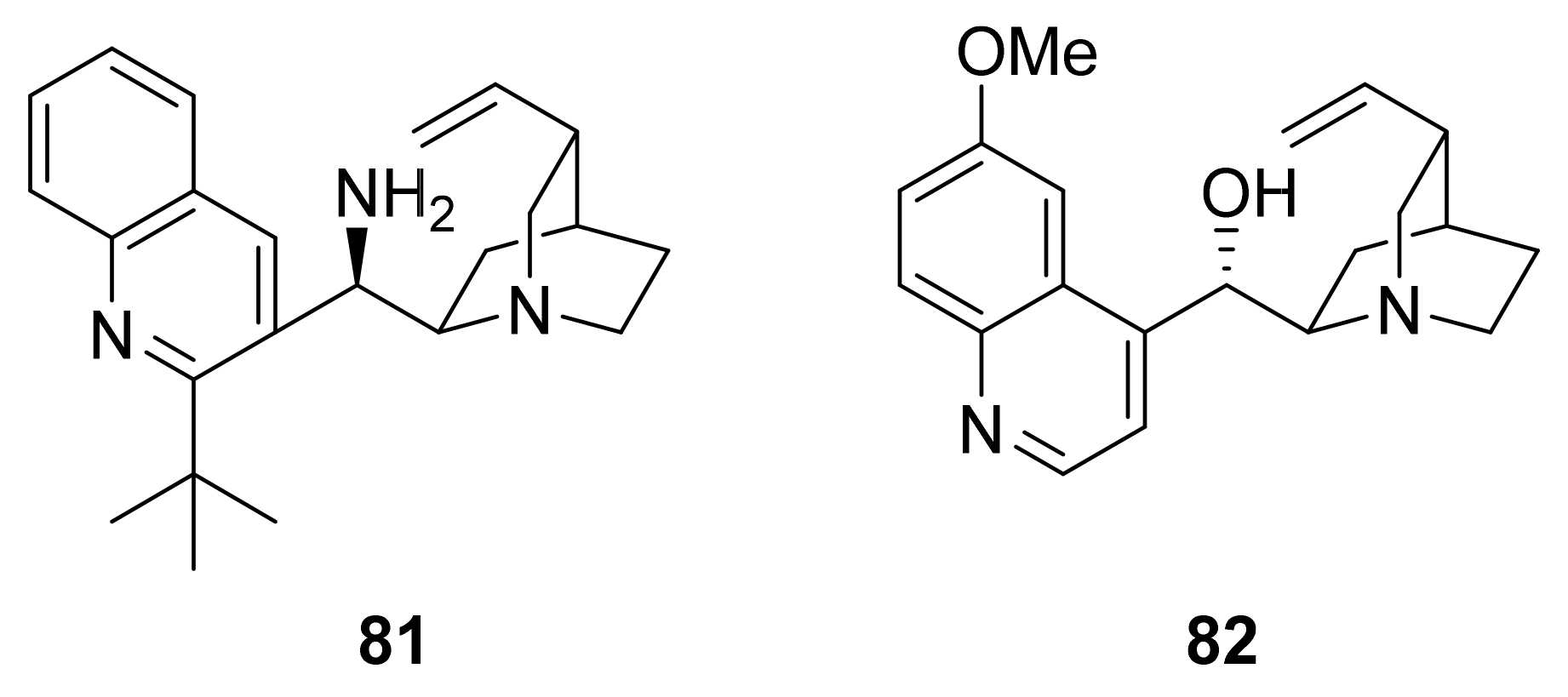
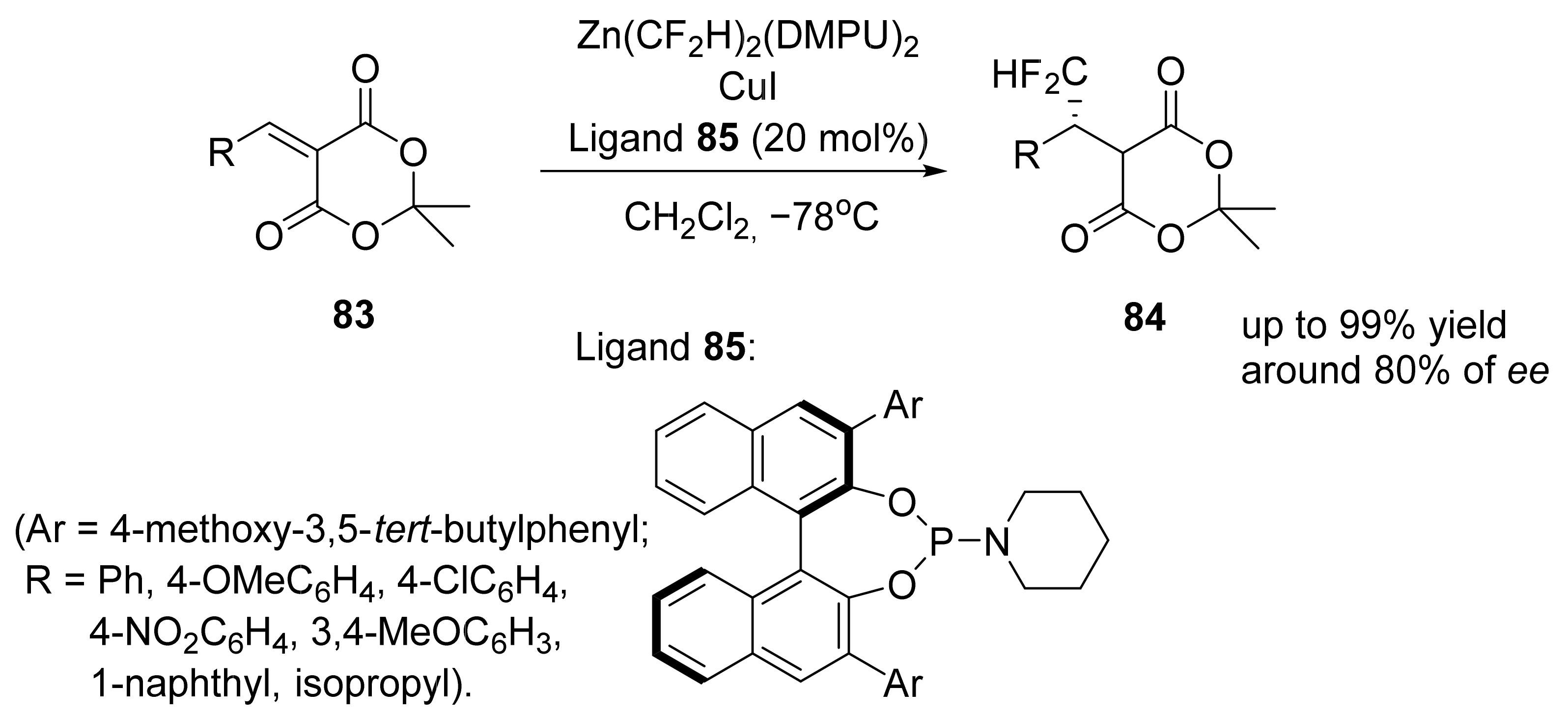
5. Asymmetric Michael Reactions
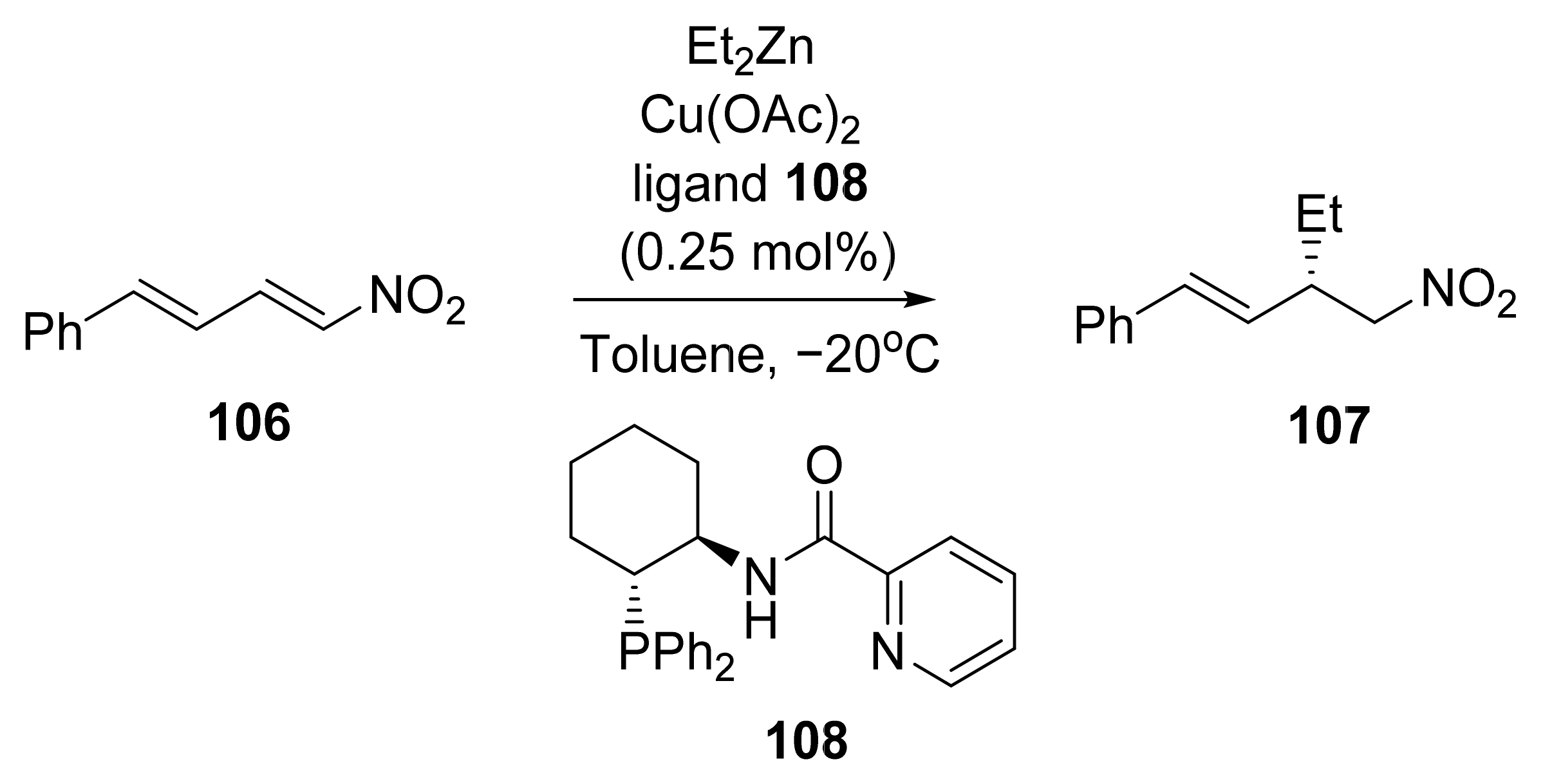
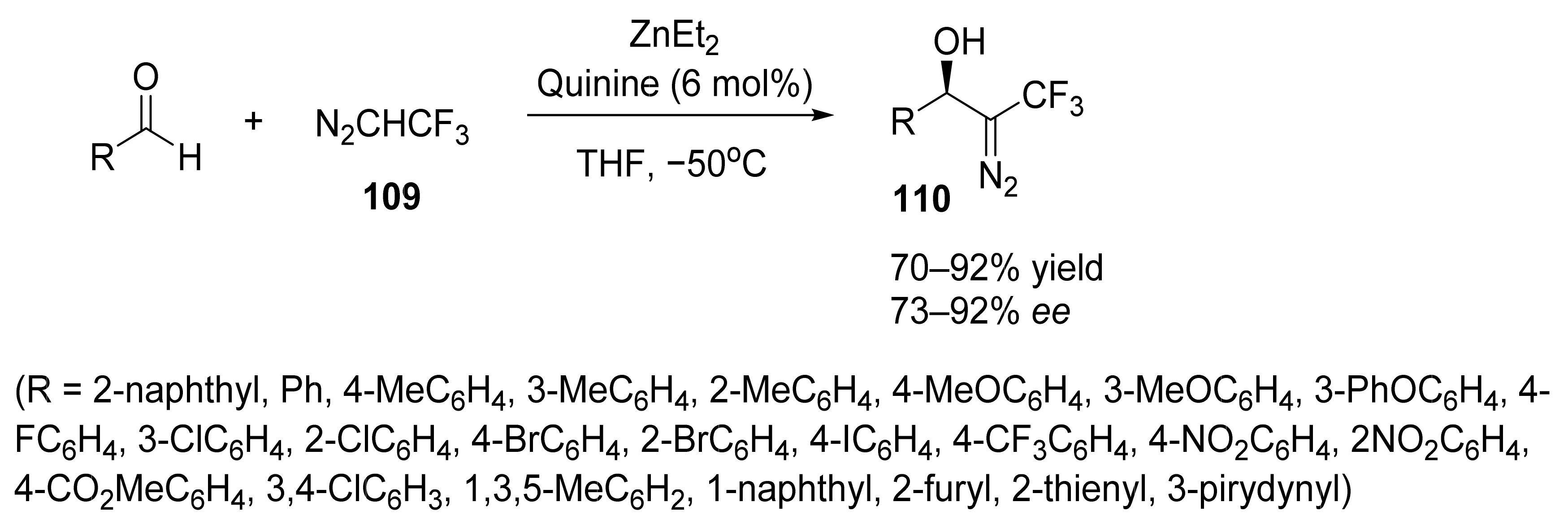
6. Asymmetric Reactions in the Presence of Zinc Ions
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eliel, E.L.; Wilen, S.H. Stereochemistry of Organic Compounds; John Wiley & Sons, Ltd.: New York, NY, USA, 1994; pp. 1–1296. [Google Scholar]
- Ariëns, E.J. Stereochemistry: A source of problems in medicinal chemistry. Med. Res. Rev. 1986, 6, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Stephens, T.D.; Bunde, C.J.W.; Fillmore, B.J. Mechanism of action in thalidomide teratogenesis. Biochem. Pharmacol. 2000, 59, 1489–1499. [Google Scholar] [CrossRef]
- Botting, J. The history of thalidomide. Drug News Perspect. 2002, 15, 604–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachwalski, M.; Vermue, N.; Rutjes, F.P.J.T. Recent advances in enzymatic and chemical deracemisation of racemic compounds. Chem. Soc. Rev. 2013, 42, 926. [Google Scholar] [CrossRef] [Green Version]
- Rachwalski, M.; Kaczmarczyk, S.; Leśniak, S.; Kiełbasiński, P. Highly Efficient Asymmetric Simmons–Smith cyclopropanation promoted by chiral heteroorganic aziridinyl ligands. ChemCatChem 2014, 6, 873–875. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Asymmetric friedel-crafts alkylation of indoles catalyzed by chiral aziridine-phosphines. Catalysts 2020, 10, 971. [Google Scholar] [CrossRef]
- Rachwalski, M.; Leenders, T.; Kaczmarczyk, S.; Kiełbasiński, P.; Leśniak, S.; Rutjes, F.P.J.T. Efficient catalysts for asymmetric Mannich reactions. Org. Biomol. Chem. 2013, 11, 4207–4213. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Adamczyk, J.; Pieczonka, A.M.; Rachwalski, M. Enantioselective mannich reaction promoted by chiral phosphinoyl-aziridines. Catalysts 2019, 9, 837. [Google Scholar] [CrossRef] [Green Version]
- Wujkowska, Z.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Phosphinoyl-aziridines as a new class of chiral catalysts for enantioselective Michael addition. Tetrahedron 2019, 75, 230–235. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Pieczonka, A.M. Optically pure aziridinyl ligands as useful catalysts in the stereocontrolled synthesis. Curr. Org. Chem. 2014, 18, 3045–3065. [Google Scholar] [CrossRef]
- Časar, Z. Synthetic approaches to contemporary drugs that contain the cyclopropyl moiety. Synthesis 2020, 52, 1315–1345. [Google Scholar] [CrossRef]
- Wu, W.; Lin, Z.; Jiang, H. Recent advances in the synthesis of cyclopropanes. Org. Biomol. Chem. 2018, 16, 7315–7329. [Google Scholar] [CrossRef]
- Novakov, I.A.; Babushkin, A.S.; Yablokov, A.S.; Nawrozkij, M.B.; Vostrikova, O.V.; Shejkin, D.S.; Mkrtchyan, A.S.; Balakin, K.V. Synthesis and structure—Activity relationships of cyclopropane-containing analogs of pharmacologically active compounds. Russ. Chem. Bull. Int. Ed. 2018, 67, 395–418. [Google Scholar] [CrossRef]
- Jüttner, F.; Wurster, K. Evidence of ectocarpene and dictyopterenes A and C’ in the water of a freshwater lake. Limnol. Oceanogr. 1984, 29, 1322–1324. [Google Scholar] [CrossRef]
- Kim, T.; Kim, J.Y.; Park, K.Y.; Ryu, D.H. Asymmetric synthesis of (-)-Dictyopterene C’ and its derivatives via catalytic enantioselective cyclopropanation. Bull. Korean Chem. Soc. 2021, 42, 675–678. [Google Scholar] [CrossRef]
- Sun, M.; Li, W.-D.Z.; Qiu, F.G. Asymmetric synthesis of the DEFG rings of solanoeclepin A. Org. Lett. 2019, 21, 644–647. [Google Scholar] [CrossRef]
- Pons, A.; Tognetti, V.; Joubert, L.; Poisson, T.; Pannecoucke, X.; Charette, A.B.; Jubault, P. Catalytic enantioselective cyclopropanation of α-fluoroacrylates: An experimental and theoretical study. ACS Catal. 2019, 9, 2594–2598. [Google Scholar] [CrossRef]
- Zheng, G.; Zhou, Z.; Zhu, G.; Zhai, S.; Xu, H.; Duan, X.; Yi, W.; Li, X. Rhodium(III)-catalyzed enantio- and diastereoselective C-H Cyclopropylation of N-Phenoxylsulfonamides: Combined experimental and computational studies. Angew. Chem. Int. Ed. 2020, 59, 2890–2896. [Google Scholar] [CrossRef]
- Heckenbichler, K.; Schweiger, A.; Brandner, L.A.; Binter, A.; Toplak, M.; Macheroux, P.; Gruber, K.; Breinbauer, R. Asymmetric reductive carbocyclization using engineered ene reductases. Angew. Chem. Int. Ed. 2018, 57, 7240–7244. [Google Scholar] [CrossRef]
- Poulsen, T.B.; Jörgensen, K.A. Catalytic asymmetric friedel-crafts alkylation reactions—Copper showed the way. Chem. Rev. 2008, 108, 2903–2915. [Google Scholar] [CrossRef]
- Maltsev, O.V.; Beletskaya, I.P.; Zlotin, S.G. Organocatalytic Michael and Friedel-Crafts reactions in enantioselective synthesis of biologically active compounds. Russ. Chem. Rev. 2011, 80, 1067–1113. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V.; Heydari, M.; Masoumi, B. Organocatalyzed asymmetric Friedel-Crafts reaction: An update. Chem. Rec. 2019, 19, 2236–2340. [Google Scholar] [CrossRef]
- Concepción Gimeno, M.; Herrera, R.P. Hydrogen bonding and internal or external Lewis or Brønsted acid assisted (Thio)urea catalysts. Eur. J. Org. Chem. 2020, 1057–1068. [Google Scholar] [CrossRef]
- Sonsona, I.G.; Marqués-López, E.; Häring, M.; Díaz Díaz, D.; Herrera, R.P. Urea activation by an external Brønsted acid: Breaking self-association and tuning catalytic performance. Catalysts 2018, 8, 305. [Google Scholar] [CrossRef] [Green Version]
- Izaga, A.; Herrera, R.P.; Concepción Gimeno, M. Gold(I)-Mediated Thiourea Organocatalyst Activation: A synergic effect for asymmetric catalysis. ChemCatChem 2017, 9, 1313–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.-L.; Liao, Y.-T.; Chang, C.-H. Asymmetric organocatalytic conjugate addition of electron-rich phenols and 1,3-dicarbonyls to arylsulfonyl indoles in an oil-water biphasic system. Eur. J. Org. Chem. 2019, 5815–5823. [Google Scholar] [CrossRef]
- Wang, C.-J.; Yang, Q.-Q.; Wang, M.-X.; Shang, Y.-H.; Tong, X.-Y.; Deng, Y.-H.; Shao, Z. Catalytic asymmetric 1,4-type Friedel-Crafts (hetero)arylations of 1-azadienes: The highly enantioselective syntheses of chiral hetero-triarylmethanes. Org. Chem. Front. 2020, 7, 609–616. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Y.; Li, F.; Luan, Y.; Li, P.; Li, W. Organocatalytic enantioselective regiodivergent C-H bond functionalization of 1-Naphthols with 1-Azadienes. Adv. Synth. Catal. 2020, 362, 1286–1291. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Wie, Z.; Cao, J.; Liang, D.; Lin, Y.; Duan, H. Asymmetric synthesis of spirooxindole-pyranoindole products via Friedel-Crafts alkylation/cyclization of the indole carbocyclic ring. New J. Chem. 2020, 44, 9788–9792. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Yang, X.-C.; Lu, H.; Gu, Y.-C.; Xu, P.-F. Organocatalytic, enantioselective friedel-crafts reaction of indoles in the carbocyclic ring and electron-rich phenols. Org. Lett. 2018, 20, 2190–2194. [Google Scholar] [CrossRef]
- Xu, K.; Chen, W.; Chen, X.; Wang, B.; Huang, J.; Tian, X. Organocatalytic asymmetric Friedel-Crafts alkylation/hemiketalization/lactonization cascade reactions: Highly enantioselective synthesis of furo[2,3-b]benzofuranones. Org. Chem. Front. 2020, 7, 1679–1684. [Google Scholar] [CrossRef]
- Zhao, L.; Bao, X.; Hu, Q.; Wang, B.; Lu, A.-H. Porous carbon nanosheet-supported chiral squaramide for highly enantioselective Friedel-crafts reaction. ChemCatChem 2018, 10, 1248–1252. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, W.; Wang, J.; Wang, Q.-A.; Yang, W. Brønsted acid-catalyzed asymmetric Friedel-crafts alkylation of indoles with benzothiazole-bearing trifluoromethyl ketone hydrates. J. Org. Chem. 2020, 85, 4398–4407. [Google Scholar] [CrossRef]
- Shen, M.-L.; Shen, Y.; Wang, P.-S. Merging visible-light photoredox and chiral phosphate catalysis for asymmetric friedel-crafts reaction with in situ generation of N-acyl imines. Org. Lett. 2019, 21, 2993–2997. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.-G. Asymmetric phosphoric acid-catalyzed aza-friedel crafts reaction of furan with cyclic N-sulfimines. Bull. Korean Chem. Soc. 2019, 40, 606–609. [Google Scholar] [CrossRef]
- Ma, J.; Kass, S.R. Electrostatically enhanced phosphoric acids and their applications in asymmetric Friedel-crafts alkylations. J. Org. Chem. 2019, 84, 11125–11134. [Google Scholar] [CrossRef]
- Liu, W.; Rajkumar, S.; Wu, W.; Huang, Z.; Yang, X. Asymmetric synthesis of β-indolyl cyclopentanones and cyclopentylamides with an all-carbon quaternary stereocenter via chiral phosphoric acid catalyzed friedel-crafts alkylation reactions. Org. Lett. 2019, 21, 3563–3567. [Google Scholar] [CrossRef]
- Ma, J.; Kass, S.R. Asymmetric arylation of 2,2,2-trifluoroacetophenones catalyzed by chiral electrostatically-enhanced phosphoric acids. Org. Lett. 2018, 20, 2689–2692. [Google Scholar] [CrossRef]
- Maestro, A.; Martinez de Marigorta, E.; Palacios, F.; Vicario, J. Enantioselective α-aminophosphonate functionalization of indole ring through an organocatalyzed friedel-crafts reaction. J. Org. Chem. 2019, 84, 1094–1102. [Google Scholar] [CrossRef]
- Gong, W.; Chen, X.; Jiang, H.; Chu, D.; Cui, Y.; Liu, Y. Highly stable Zr(IV)-based metal-organic frameworks with chiral phosphoric acids for catalytic asymmetric tandem reactions. J. Am. Chem. Soc. 2019, 141, 7498–7508. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, J.; Jung, J.; Kim, S.-G. Chiral Brønsted acid-catalyzed Friedel-Crafts reaction of 3-indolylsulfamidates with indoles: Synthesis of enantioenriched bisindolylmethane sulfamates. Tetrahedron Lett. 2019, 60, 1625–1630. [Google Scholar] [CrossRef]
- Anokhin, M.V.; Feofanov, M.N.; Averin, A.D.; Beletskaya, I.P. The asymmetric Friedel-Crafts reaction of indoles with arylidenemalonates catalyzed by MgI2/PyBox complexes. ChemistrySelect 2018, 3, 1388–1391. [Google Scholar] [CrossRef]
- Tarasenko, E.A.; Shestakov, I.V.; Rybakov, V.B.; Beletskaya, I.P. Enantioselective Copper(II)/box-catalyzed synthesis of chiral β3-tryptophan derivatives. ChemCatChem 2019, 11, 3913–3918. [Google Scholar] [CrossRef] [Green Version]
- Shahidul Islam, M.; Barakat, A.; Al-Majid, A.M.; Ali, M.; Yousuf, S.; Iqbal Choudhary, M.; Khalid, R.; Ul-Haq, Z. Catalytic asymmetric synthesis of indole derivatives as novel α-glucosidase inhibitors in vitro. Bioorg. Chem. 2018, 79, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yang, N.; Huang, X.; Hu, C.; Su, Z. Mechanism and origins of stereoinduction in an asymmetric Friedel-Crafts alkylation reaction of chalcone catalyzed by chiral N,N′-dioxide-Sc(III) complex. J. Org. Chem. 2018, 83, 4628–4640. [Google Scholar] [CrossRef]
- Kitanosono, T.; Hisada, T.; Yamashita, Y.; Kobayashi, S. Hydrogen-bonding-assisted cationic aqua Palladium(II) complex enables highly efficient asymmetric reactions in water. Angew. Chem. Int. Ed. 2021, 60, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-J.; Gong, J.-F.; Song, M.-P. Synthesis of chiral Bis(3-indolyl)methanes bearing a trifluoromethylated all-carbon quaternary stereocenter via nickel-catalyzed asymmetric Friedel-crafts alkylation reaction. J. Org. Chem. 2020, 85, 9525–9537. [Google Scholar] [CrossRef] [PubMed]
- Phuc Le, T.; Higashita, K.; Tanaka, S.; Yoshimura, M.; Kitamura, M. Revisiting the Cuii-catalyzed asymmetric Friedel-Crafts reaction of indole with trifluoropyruvate. Org. Lett. 2018, 20, 7149–7153. [Google Scholar] [CrossRef]
- Hua, Y.-Z.; Chen, J.-W.; Yang, H.; Wang, M.-C. Asymmetric Friedel-Crafts alkylation of indoles with trifluoromethyl pyruvate catalyzed by a dinuclear zinc catalyst. J. Org. Chem. 2018, 83, 1160–1166. [Google Scholar] [CrossRef]
- Yang, X.-C.; Liu, M.-M.; Mathey, F.; Yang, H.; Hua, Y.-Z. Access to chiral 2,5-pyrrolidinyl dispirooxindoles via dinuclear zinc-catalyzed asymmetric cascade reactions. J. Org. Chem. 2019, 84, 7762–7775. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Guo, X.; Kong, D.-Z.; Lu, H.-J.; Liu, L.-T.; Hua, Y.-Z.; Wang, M.-C. Catalytic asymmetric synthesis of tetrahydrofuran spirooxindoles via a dinuclear zinc catalyst. J. Org. Chem. 2020, 85, 4195–4206. [Google Scholar] [CrossRef]
- Hua, Y.-Z.; Han, X.-W.; Huang, L.-H.; Wang, M.-C. Asymmetric Friedel-Crafts alkylation of pyrrole with chalcones catalyzed by a dinuclear zinc catalyst. Chin. J. Org. Chem. 2018, 38, 237–245. [Google Scholar] [CrossRef]
- Wang, Z.; Zu, L. Organocatalytic enantioselective direct alkylation of phloroglucinol derivatives: Asymmetric total synthesis of (+)-aflatoxin B2. Chem. Commun. 2019, 55, 5171–5174. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Zhou, B.; Tsuji, H.; Kawatsura, M. Nickel-catalyzed asymmetric friedel-crafts propargylation of 3-substituted indoles with propargylic carbonates bearing an internal alkyne group. Org. Lett. 2020, 22, 2049–2053. [Google Scholar] [CrossRef]
- Anwar, M.; Yang, S.; Xu, W.; Liu, J.-G.; Perveen, S.; Kong, X.-W.; Tazeen Zehra, S.; Fang, X.-Q. Carbene-catalyzed asymmetric Friedel-Crafts alkylation-annulation sequence and rapid synthesis of indole-fused polycyclic alkaloids. Commun. Chem. 2019, 2, 85. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Sun, X.; Wang, H.-S.; Li, C.; Qiao, R.-Z. Guanosine-based sel-assembly as an enantioselective catalyst scaffold. J. Org. Chem. 2020, 85, 2010–2018. [Google Scholar] [CrossRef]
- Bai, J.K.; Chen, D.; Li, C.; Wang, H.S.; Qiao, R.Z. PNA as hybrid catalyst scaffold catalyzed asymmetric friedel-crafts alkylation. Catal. Lett. 2020, 150, 2082–2090. [Google Scholar] [CrossRef]
- Mansot, J.; Aubert, S.; Duchemin, N.; Vasseur, J.-J.; Arseniyadis, S.; Smietana, M. A rational quest for selectivity through precise ligand-positioning in tandem DNA-catalysed Friedel-Crafts alkylation/asymmetric protonation. Chem. Sci. 2019, 10, 2875–2881. [Google Scholar] [CrossRef] [Green Version]
- Dey, S.; Jäschke, A. Covalently functionalized DNA duplexes and quadruplexes as hybrid catalysts in an enantioselective friedel-crafts reaction. Molecules 2020, 25, 3121. [Google Scholar] [CrossRef]
- Ravi Kumar, G.; Ramesh, B.; Yarlagadda, S.; Sridhar, B.; Subba Reddy, B.V. Organocatalytic enantioselective Mannich reaction: Direct access to chiral β-amino esters. ACS Omega 2019, 4, 2168–2177. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.-S.; Noda, H.; Shibasaki, M. Exploiting β-amino acid enolates in direct catalytic diastereo-and enantioselective C-C bond-forming reactions. Chem. Eur. J. 2018, 24, 15796–15800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-J.; Zhong, F.; Xie, Y.-C.; Yin, L. Catalytic asymmetric Mannich-type reaction enabled by efficient dienolization of α,β-unsaturated pyrazoleamides. Chin. J. Chem. 2021, 39, 55–61. [Google Scholar] [CrossRef]
- Rostoll-Berenguer, J.; Blay, G.; Carmen-Muñoz, M.; Pedro, J.R.; Vila, C. A Combination of visible-light organophotoredox catalysis and asymmetric organocatalysis for the enantioselective Mannich reaction of dihydroquinoxalinones with ketones. Org. Lett. 2019, 21, 6011–6015. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-L.; Ding, X.; Huang, L.-Q.; He, Y.-H.; Guan, Z. Merging visible light photocatalysis and l-/d-proline catalysis: Direct asymmetric oxidative dearomatization of 2-arylindoles to access C2-quaternary indolin-3-ones. Org. Lett. 2020, 22, 1076–1080. [Google Scholar] [CrossRef]
- Li, J.-S.; Liu, Y.-J.; Li, S.; Ma, J.-A. Chiral phosphoric acid-catalyzed direct asymmetric mannich reaction of cyclic C-acylimines with simple ketones: Facile access to C2-quaternary indolin-3-ones. Chem. Commun. 2018, 54, 9151–9154. [Google Scholar] [CrossRef]
- Hui, C.; Pu, F.; Xu, J. Metal-catalyzed asymmetric michael addition in natural product synthesis. Chem. Eur. J. 2017, 23, 4023–4036. [Google Scholar] [CrossRef]
- Malkar, R.S.; Jadhav, A.L.; Yadav, G.D. Innovative catalysis in Michael addition reactions for C-X bond formation. Mol. Catal. 2020, 485, 110814. [Google Scholar] [CrossRef]
- Gandhi, S.; Sivadas, V.; Baire, B. Thiourea-tertiary amine promoted cascade catalysis: A tool for complexity generation. Eur. J. Org. Chem. 2021, 220–234. [Google Scholar] [CrossRef]
- Rohit, K.R.; Ujwaldev, S.M.; Krishnan, K.K.; Anilkumar, G. Recent developments and perspectives in the zinc-catalysed Michael addition. Asian, J. Org. Chem. 2018, 7, 85–102. [Google Scholar] [CrossRef]
- Macchia, A.; Eitzinger, A.; Brière, J.-F. Asymmetric synthesis of isoxazol-5-ones and isoxazolidin-5-ones. Synthesis 2021, 53, 107–122. [Google Scholar] [CrossRef]
- Carceller-Ferrer, L.; Blay, G.; Pedro, J.R.; Vila, C. Recent advances in catalytic enantioselective synthesis of pyrazolones with a tetrasubstituted stereogenic center at the 4-position. Synthesis 2020, 53, 215–237. [Google Scholar] [CrossRef]
- Torán, R.; Vila, C.; Sanz-Marco, A.; Carmen-Muñoz, M.; Pedro, J.R.; Blay, G. Organocatalytic enantioselective 1,6-aza-Michael addition of isoxazolin-5-ones to p-quinone methides. Eur. J. Org. Chem. 2020, 627–630. [Google Scholar] [CrossRef]
- Yang, Z.-T.; Zhao, J.; Yang, W.-L.; Deng, W.-P. Enantioselective construction of CF3-Containing Spirooxindole γ-Lactones via organocatalytic asymmetric michael/lactonization. Org. Lett. 2019, 21, 1015–1020. [Google Scholar] [CrossRef]
- Xu, X.; He, Y.; Zhou, J.; Li, X.; Zhu, B.; Chang, J. Organocatalytic asymmetric michael addition of pyrazol-5-ones to β-trifluoromethyl-α,β-unsaturated ketones: Stereocontrolled construction of vicinal quaternary and tertiary stereocenters. J. Org. Chem. 2020, 85, 574–584. [Google Scholar] [CrossRef]
- Xiao, W.; Zhou, Z.; Yang, Q.-Q.; Du, W.; Chen, Y.-C. Organocatalytic asymmetric four-component [5+1+1+1] cycloadditions via a quintuple cascade process. Adv. Synth. Catal. 2018, 360, 3526–3533. [Google Scholar] [CrossRef]
- Macchia, A.; Dafnae Cuomo, V.; Di Mola, A.; Pierri, G.; Tedesco, C.; Palombi, L.; Massa, A. On the necessity of one-pot tautomer trapping in asymmetric michael reactions of arylideneisoxazol-5-ones. Eur. J. Org. Chem. 2020, 2264–2270. [Google Scholar] [CrossRef]
- Pair, E.; Cadart, T.; Levacher, V.; Brière, J.-F. Meldrum’s acid: A useful platform in asymmetric organocatalysis. ChemCatChem 2016, 8, 1882–1890. [Google Scholar] [CrossRef]
- Endo, Y.; Ishii, K.; Mikami, K. Chiral copper-catalyzed enantioselective Michael difluoromethylation of arylidene meldrum’s acids with (difluoromethyl)zinc reagents. Tetrahedron 2019, 75, 4099–4103. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Hou, C.-J.; Chu, T.-T.; Hu, X.-P. Copper-catalyzed asymmetric tandem double Michael reactions of diethylzinc to α,β-unsaturated ketones followed by trapping with nitroolefins. Tetrahedron 2019, 75, 3943–3950. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Liu, W.; Zheng, Y.; He, Z. Organocatalytic asymmetric cascade cyclization reaction of o-hydroxycinnamaldehydes with diphenylphosphine oxide. Chin. Chem. Lett. 2018, 29, 1625–1628. [Google Scholar] [CrossRef]
- Putatunda, S.; Alegre-Requena, J.V.; Meazza, M.; Franc, M.; Rohalová, D.; Vemuri, P.; Císařová, I.; Herrera, R.P.; Rios, R.; Veselý, J. Proline bulky substituents consecutively act as steric hindrances and directing groups in a Michael/Conia-ene cascade reaction under synergistic catalysis. Chem. Sci. 2019, 10, 4107–4115. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y.; Takikawa, A.; Koshino, S.; Ishida, K. Asymmetric Synthesis of Biaryl Atropoisomers Using an Organocatalyst-Mediated Domino Reaction as the Key Step. Chem. Eur. J. 2019, 25, 10319–10322. [Google Scholar] [CrossRef]
- Wang, M.-C. Enantioselective analysis: Logic of chiral ligand design for asymmetric addition of diethylzinc to benzaldehyde. Chin. J. Org. Chem. 2018, 38, 162–170. [Google Scholar] [CrossRef]
- Mátravölgyi, B.; Deák, S.; Erdélyi, Z.; Hergert, T.; Ábrányi-Balogh, P.; Faigl, F. Effect of regioisomerism on the efficiency of 1-phenylpyrrole-type atropisomeric amino alcohol ligands in enantioselective organometallic reactions. Synlett 2018, 29, 2171–2175. [Google Scholar] [CrossRef] [Green Version]
- Prause, F.; Wagner, S.; Breuning, M. Enantioselective addition of diethylzinc to aldehydes catalyzed by 5-cis-substituted proline derivatives. Tetrahedron 2019, 75, 94–101. [Google Scholar] [CrossRef]
- Gao, E.; Li, Q.; Duan, L.; Li, L.; Li, Y.-M. Isosterically designed chiral catalysts: Rationale, optimization and their application in enantioselective nucleophilic addition to aldehydes. Tetrahedron 2020, 76, 131648. [Google Scholar] [CrossRef]
- Raji, M.; Minh Le, T.; Fülöp, F.; Szakonyi, Z. Synthesis and investigation of pinane-based chiral tridentate ligands in the asymmetric addition of diethylzinc to aldehydes. Catalysts 2020, 10, 474. [Google Scholar] [CrossRef]
- Sweetman, B.A.; Guiry, P.J. Axially chiral tridentate isoquinoline derived ligands for diethylzinc addition to aldehydes. Tetrahedron 2018, 74, 5567–5581. [Google Scholar] [CrossRef]
- Aydin, A.E. Enantioselective addition of diethylzinc to aromatic aldehydes using chiral oxazoline-based ligands. Russ. J. Org. Chem. 2020, 56, 1304–1312. [Google Scholar] [CrossRef]
- Aydin, A.E. Enantioselective addition of diethylzinc to aromatic aldehydes using novel thiophene-based chiral ligands. Russ. J. Org. Chem. 2020, 56, 901–909. [Google Scholar] [CrossRef]
- Sappino, C.; Mari, A.; Mantineo, A.; Moliterno, M.; Palagri, M.; Tatangelo, C.; Suber, L.; Bovicelli, P.; Ricelli, A.; Righi, G. New chiral amino alcohol ligands for catalytic enantioselective addition of diethylzinc to aldehydes. Org. Biomol. Chem. 2018, 16, 1860–1870. [Google Scholar] [CrossRef] [PubMed]
- Mohebbi, M.; Salehi, P.; Bararjanian, M.; Ebrahimi, S.N. Noscapine-derived β-amino alcohols as new organocatalysts for enantioselective addition of diethylzinc to aldehydes. J. Iran. Chem. Soc. 2018, 15, 47–53. [Google Scholar] [CrossRef]
- Mohebbi, M.; Bararjanian, M.; Ebrahimi, S.N.; Smieško, M.; Salehi, P. Noscapine derivatives as new chiral catalysts in asymmetric synthesis: Highly enantioselective addition of diethylzinc to aldehydes. Synthesis 2018, 50, 1841–1848. [Google Scholar] [CrossRef] [Green Version]
- Dikova, K.; Kostova, K.; Simova, S.; Linden, A.; Chimov, A.; Dimitrov, V. Synthesis and crystal structures of chiral ferrocene and ruthenocene substituted aminomethylnaphthols obtained through Betti-condensation. Polyhedron 2019, 165, 177–187. [Google Scholar] [CrossRef]
- Wang, Q.; Li, S.; Hou, C.-J.; Chu, T.-T.; Hu, X.-P. Chiral P,N-ligands for the highly enantioselective addition of diethylzinc to aromatic aldehydes. Appl. Organometal. Chem. 2019, 33, e5108. [Google Scholar] [CrossRef]
- Rexiti, R.; Zhang, Z.-G.; Lu, J.; Sha, F.; Wu, X.-Y. Regioselective and enantioselective Cu(II)-catalyzed 1,4-conjugate addition of diethylzinc reagent to nitrodienes. J. Org. Chem. 2019, 84, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.-Y.; Yang, L.; Nie, J.; Zhang, F.-G.; Ma, J.-A. Construction of chiral β-trifluoromethyl alcohols enabled by catalytic enantioselective aldol-type reaction of CF3CHN2. Org. Lett. 2019, 21, 4280–4283. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachwalski, M.; Buchcic-Szychowska, A.; Leśniak, S. Recent Advances in Selected Asymmetric Reactions Promoted by Chiral Catalysts: Cyclopropanations, Friedel–Crafts, Mannich, Michael and Other Zinc-Mediated Processes—An Update. Symmetry 2021, 13, 1762. https://doi.org/10.3390/sym13101762
Rachwalski M, Buchcic-Szychowska A, Leśniak S. Recent Advances in Selected Asymmetric Reactions Promoted by Chiral Catalysts: Cyclopropanations, Friedel–Crafts, Mannich, Michael and Other Zinc-Mediated Processes—An Update. Symmetry. 2021; 13(10):1762. https://doi.org/10.3390/sym13101762
Chicago/Turabian StyleRachwalski, Michał, Aleksandra Buchcic-Szychowska, and Stanisław Leśniak. 2021. "Recent Advances in Selected Asymmetric Reactions Promoted by Chiral Catalysts: Cyclopropanations, Friedel–Crafts, Mannich, Michael and Other Zinc-Mediated Processes—An Update" Symmetry 13, no. 10: 1762. https://doi.org/10.3390/sym13101762
APA StyleRachwalski, M., Buchcic-Szychowska, A., & Leśniak, S. (2021). Recent Advances in Selected Asymmetric Reactions Promoted by Chiral Catalysts: Cyclopropanations, Friedel–Crafts, Mannich, Michael and Other Zinc-Mediated Processes—An Update. Symmetry, 13(10), 1762. https://doi.org/10.3390/sym13101762






