Abstract
The transplacental transfer of maternal immunoglobulin G from the mother to the foetus is central for providing immunity in early life, resulting in full-term newborns having IgG repertoires and levels similar to those of their mothers. The neonatal Fc receptor is recognised as the primary transporter of IgGs across the placental epithelium. Understanding the mechanisms of transplacental antibody transfer and factors that affect them is essential in optimising maternal vaccination strategies, ultimately protecting infants from various environmental pathogens. This review first outlines the biological mechanisms governing transplacental IgG transfer, followed by a discussion of how this process may be disrupted by physiological and pathological conditions during pregnancy, including preterm birth, hypergammaglobulinemia, maternal pathogenic IgG, maternal infections, hyperglycaemia, and exposure to biological therapies. We also summarise currently available models used to study transplacental IgG transfer, highlighting existing knowledge gaps and future directions for research in this field.
1. Introduction
At birth, newborns face exposure to numerous pathogens while possessing an immature immune system, which makes them especially susceptible to infections. Accordingly, maternal immunoglobulin G (IgG) begins to cross the placenta as early as 8–10 weeks of gestation, with the highest rate occurring in the third trimester (28–40 weeks of gestation), offering immune protection during the early stages of life [1,2,3]. Maternal plasma cells secrete antigen-specific antibodies essential for immune defence, among which IgA and IgG are predominantly responsible for protecting the newborn [4,5,6]. Only IgG however crosses the placenta to reach the foetal circulation, with different subclasses of IgG crossing the placenta with differential efficiency [4,7]. In the intervillous space, floating chorionic villi act as a barrier separating maternal and foetal circulations (Figure 1). They generally consist of a bi-layered epithelial structure with an outer multinuclear syncytiotrophoblast (SCT) layer and an inner core of mononuclear cytotrophoblasts, stroma containing fibroblasts and Hofbauer cells, and endothelial cells of the foetal capillaries [8,9]. There is general consensus that two receptors for the Fc region of IgGs, namely neonatal Fc receptor (FcRn) and Fcγ receptor III-A (FcγRIIIa), are co-localised on SCTs and considered to mediate the transplacental transfer of IgGs [10,11]. FcRn is considered as the primary transporter of IgGs across the placental barrier [12,13,14] while there is still considerable debate on the contribution of FcγRs in this process, with data from different laboratories showing evidence for [11,15] and against [12,16,17] direct FcγR involvement. Based on current knowledges, it is hypothesized that FcγRs fine-tune the kinetics of TPAT through several possible mechanisms: acting as a parallel transcytosis pathways independent from FcRn or co-receptors of FcRn [11,18,19], involving in multi-step mechanisms with FcRn [20], or immune complex clearance [21].
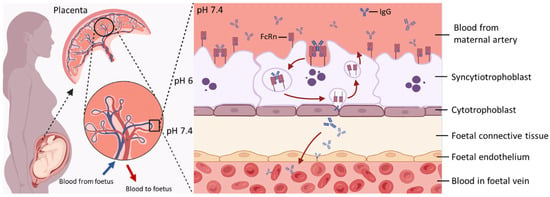
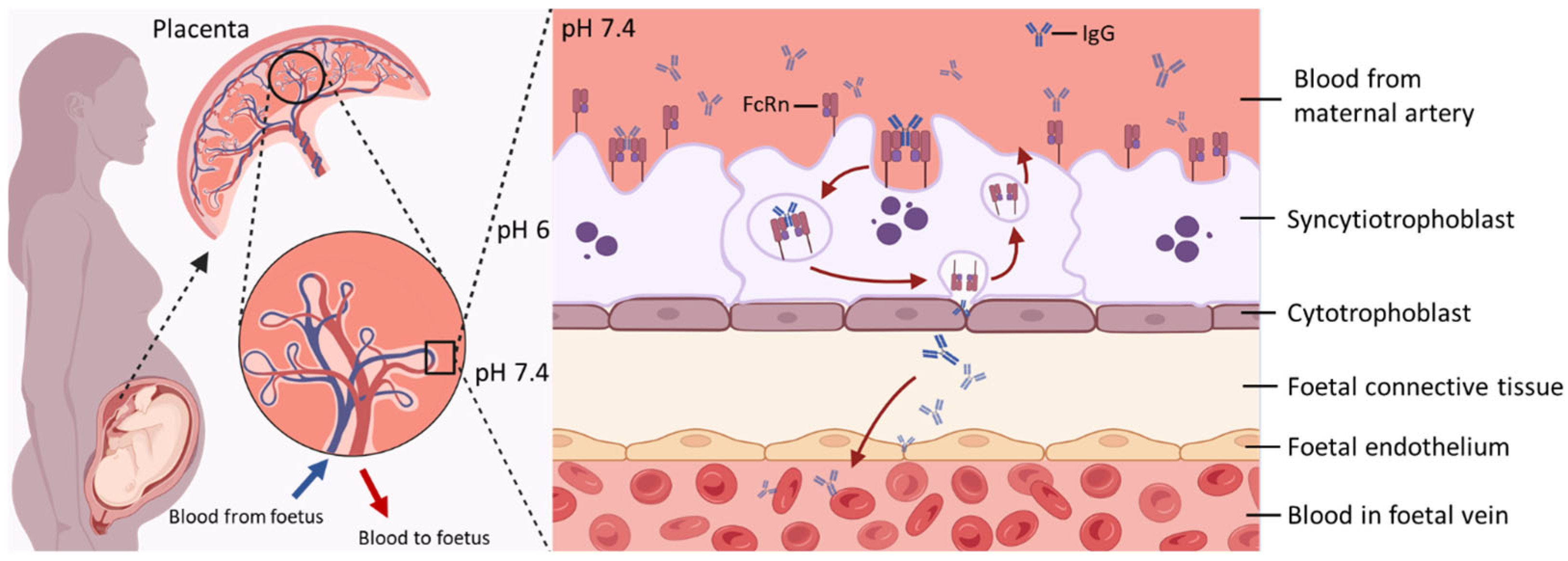
Figure 1.
Key Features of Maternal–Foetal IgG Transfer via FcRn-Mediated Transcytosis. At the maternal–foetal interface, maternal IgG binds to FcRn expressed on the apical surface of SCT. The IgG–FcRn complex is internalised into endosomes, where acidic pH stabilises their interaction. This complex is then trafficked across the cell to the basal membrane. Upon exposure to physiological pH, IgG is released into the foetal stroma and subsequently enters the foetal circulation. After release, FcRn is recycled back to the apical membrane for reuse, enabling continuous transcytosis of maternal IgG during gestation. Created in https://www.BioRender.com.
The transfer of IgGs through the SCT layer is achieved by fluid-phase pinocytosis, which comprises the pinocytic uptake of IgG by SCTs, fusion of vesicles containing IgGs with endosomes under a mildly acidic pH, and the release into stroma at a neutral pH [22]. Although the precise mechanisms that allow IgGs to cross both the villous stroma and foetal endothelium, and finally enter foetal circulation in vivo, are poorly elucidated, evidence suggests that alternative Fc receptors expressed on Hofbauer cells and the foetal endothelium might be involved in this process [18,23,24,25]. By the time the pregnancy reaches term, foetal IgG levels have surpassed maternal plasma IgG levels [3]. After birth, maternally derived IgG persists in the infant’s circulation and provides passive immune protection, typically lasting from approximately 6 weeks to 6 months, depending on factors such as the maternal immune status, antigen specificity, and IgG subclass or glycosylation profile [4,26,27,28].
Given the critical role of transplacental antibody transfer (TPAT) in providing immune protection to the foetus during pregnancy and to the newborn in the immediate period after birth, strategies to enhance immunity via the maternal–neonatal dyad have become a valuable public health practice [29]. The emergence of SARS-CoV-2 in early 2020 brought renewed attention to the safety and effectiveness of vaccination in pregnancy, reinvigorating clinical and scientific interest in maternal immunisation. Understanding the maternal factors that facilitate—or impair—optimal TPAT is therefore essential for maximising the success of this strategy [30,31]. A better understanding of the mechanisms of TPAT and the factors that affect them is crucial to optimize strategies of both maternal and neonatal vaccination. In this review, we first highlight the biological mechanism of TPAT and then discuss how this process may be altered in the context of various physiological and pathological challenges during pregnancy, including preterm birth, hypergammaglobulinemia, maternal pathogenic IgG, maternal infections, hyperglycaemia, and the use of immunotherapies. A comprehensive understanding of these factors can inform the development of maternal vaccination strategies, guide therapeutic interventions during pregnancy, and ultimately improve neonatal health outcomes. Currently available models of placental function to study the transplacental transfer of IgGs are also summarised in this review to highlight our knowledge gaps in this process.
2. Mechanisms of FcRn-Mediated Transport of IgG in the Placenta
Using FcγR/FcRn humanised mice to model maternal–foetal transfer, Borghi et al. found that FcRn, rather than FcγRIIIa, contributes to transplacental transfer of IgG [12]. Ex vivo human placental transfer models also highlighted the dominant role of FcRn in this process [14]. The engagement of IgG with FcγRs has been related to IgG-mediated inflammatory activities, while whether and how FcγRs expressed in placental cells contribute to the transplacental transfer of maternal IgG remain largely elusive [32,33]. In this review, we therefore focus on the latest research studying FcRn-mediated IgG transcytosis in physiological placentae.
The structural characteristics of IgG determine its interaction with FcRn in terms of binding manner, avidity, and distinct pH-dependant release. IgGs form a heterotetramer (comprised of two heavy and two light chain proteins covalently bonded by disulphide bridges), physiologically interacting with FcRn at a 1:2 ratio when imaged by electron microscopy [34]. The IgG population is further divided into four subclasses (IgG1–4) which exhibit different efficiencies in transplacental transfer (IgG1 > IgG3 > IgG2 ≈ IgG4) [4]. Although these four IgG subclasses are highly conserved, they differ in their constant region particularly in their hinges and upper CH2 domains [35]. Distinct structures among IgG subclasses might contribute to their different efficiencies in transplacental transfer because the FcRn engagement of IgG Fc region occurs at the CH3 and CH2 domain interface, which is involved with several residues (Ile253, Thr254, His310, His433, and His435) in the Fc region mediating various hydrogen-bonded and salt bridge interactions with FcRn [36,37]. This provides the structural basis for a pH-dependant switch as the FcRn-IgG interaction requires positively charged imidazole side chains on histidine, which occur at pH 5–6, while the binding is lost at pH 7.4 (Figure 1) as the side chains become neutrally charged [36,38,39]. Notably, FcRn plays a crucial role in prolonging the half-life of IgG by protecting it from lysosomal degradation, which accounts for the substantially longer serum persistence of IgG compared with other antibody isotypes [10,40]. Following pinocytosis by SCT, maternal IgG is internalised into endosomal compartments that subsequently acidify. The acidic environment promotes high-affinity binding of IgG to FcRn within these endosomes, thereby preventing its degradation and extending its half-life [10].
Through the development of therapeutic antibodies, the effects of post-translational modification on stability, half-life, and function of IgG have become increasingly clear [41]. Two commonly studied IgG modifications, glycosylation and methionine oxidation, have been shown to affect IgG activity [42,43]. IgG has a single N-linked glycan attached to Asn297 on each of the heavy chains of its Fc tail, largely occupying the cavity between two heavy chains [32,41]. An overall increase in both galactosylation and sialylation but decreased abundance of bisecting N-acetylglucosamine are observed in the Fc-glycan profile of maternal IgG with advancing gestation [16]. The N-linked glycans have been reported to alter both orientation and flexibility of CH2 domains, which eventually affects the Fc binding to the FcγRs backbone [44,45]. The removal of IgG glycans increases the flexibility of the FcRn binding site in the Fc region but leads to a small decrease in FcRn binding [46] while this effect is more pronounced in FcγRIIIa binding, where removal of the glycans almost completely disrupts receptor binding [47]. An analysis of ten pairs of foetal and maternal IgG samples concluded that the transplacental transfer of IgG is not Fc glycosylation-selective based on the fact that the profiles of Fc glycosylation of all the analysed IgG subclasses are largely comparable [16]. A more recent study by Borghi et al. also found no consistent difference in di-galactosylation of maternal and cord IgG1 (the most abundant IgG subclass) between two clinical cohorts from Uganda and Nicaragua [12]. Another recent study argued that IgG Fc glycosylation may contribute to the preference observed during TPAT, by fine-tuning receptor engagement within specific IgG subclasses. In particular, maternal IgGs with di-galactosylated Fc-glycans and ability to activate foetal NK cells are selectively transferred across the placenta to the neonate because of their enhanced bindings to both FcRn and FcγRIIIa [11], suggesting that antigen-specific Fc-glycan profiles ensure a preferential transfer. Further studies are required to reach a consensus on the role of IgG Fc region modification in modulating the transplacental IgG transfer. Additionally, about 10–20% of the Fab have N-glycosylation sites in the binding region [48,49]. IgG with Fab glycans has been called asymmetric antibody, and amounts increase during pregnancy [48,50]. A recent study in the context of the cell membrane has however suggested a suppressive role of IgG Fab glycans in FcRn-mediated transport [51]. The Fc fragment of IgG1 molecules also contains two conserved methionine residues at positions 252 and 428 that are subject to oxidation. These residues, which are located in the CH2 and CH3 domains, respectively, sit at the interface of the CH2/CH3 domains in the folded protein where FcRn binds [52]. Elevated levels of methionine oxidation on IgG1 were shown to increase its equilibrium dissociation constant for both FcRn and protein A [42], indicating reduced binding affinity. Using transgenic mice with human FcRn, Wang et al. further found that methionine oxidation of humanised IgG1 leads to a reduction in its half-life in the circulation, with the magnitude correlating well with the extent of methionine oxidation and changes in FcRn binding affinities [53]. Moreover, monoclonal antibodies possessing distinct Fab domains do exhibit different FcRn affinities even with an identical Fc region, which eventually affects their half-life [54,55]. These observations highlight that Fc modifications are not the sole determinants of IgG–FcRn interactions; rather, these interactions reflect whole-molecule effects that extend beyond the Fc region alone.
The expression of FcRn itself can also be modulated. Factors such as cytokines or infectious stimuli can either up- or down-regulate the expression of FcRn, and with binding sites for a wide range of transcription factors being found in the gene encoding for it, FCGRT [56,57,58,59,60]. There is some evidence that TPAT is limited by the potential saturation of placental Fc receptors at higher maternal IgG levels [29]. However, the association of placental FcRn expression and IgG transfer efficiency is still under debate because of controversial outcomes from different studies on both physiological and pathological placentae [4,15,61,62]; the controversy is potentially due to differential FcRn expression at different placental sites of collection and different manners of statistical analysis. Therefore, multicentre studies meeting both technical and interpretative integrity are required. More evidence is available indicating that the transmission efficiency of IgG subclasses varies due to differences in placental weight, birth weight, gestational age, and maternal antigen exposure [4,63].
3. Maternal Factors Affecting Antibody Transplacental Transfer
Given the critical role of TPAT in providing immune protection to both the foetus and the newborn, understanding the maternal factors that enhance or impair this process is equally essential [6]. Various maternal and placental conditions may be linked to influence the efficiency of TPAT, including hypertensive disorders of pregnancy (e.g., pre-eclampsia), placental insufficiency and intrauterine growth restriction, maternal obesity, smoking, inflammatory placental pathologies such as chorioamnionitis or villitis, and maternal malnutrition [64,65,66,67]. While these factors are clinically relevant, the available evidence remains limited and often indirect. Accordingly, this narrative review focuses on preterm birth, hypergammaglobulinemia, maternal pathogenic IgG, maternal infections, hyperglycaemia, as well as clinical interventions like immunotherapies (Figure 2) [68,69,70,71]. These factors were prioritised on the basis of more clinical evidence linking them to altered TPAT, most commonly assessed using cord-to-maternal antibody titre ratios (C/M ratios) [72,73,74]. A comprehensive understanding of these factors can inform the development of maternal vaccination strategies, guide therapeutic interventions during pregnancy, and ultimately improve neonatal health outcomes.
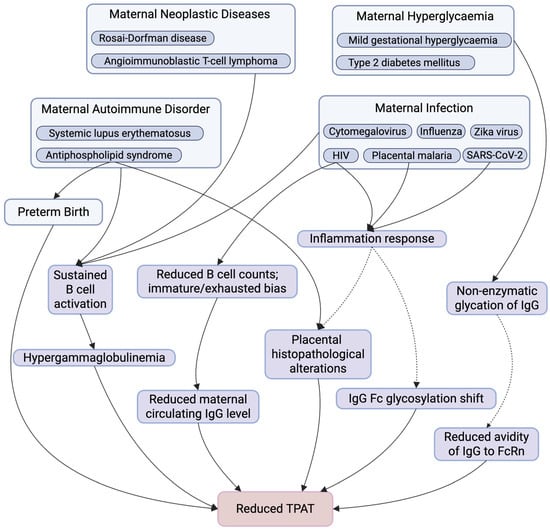
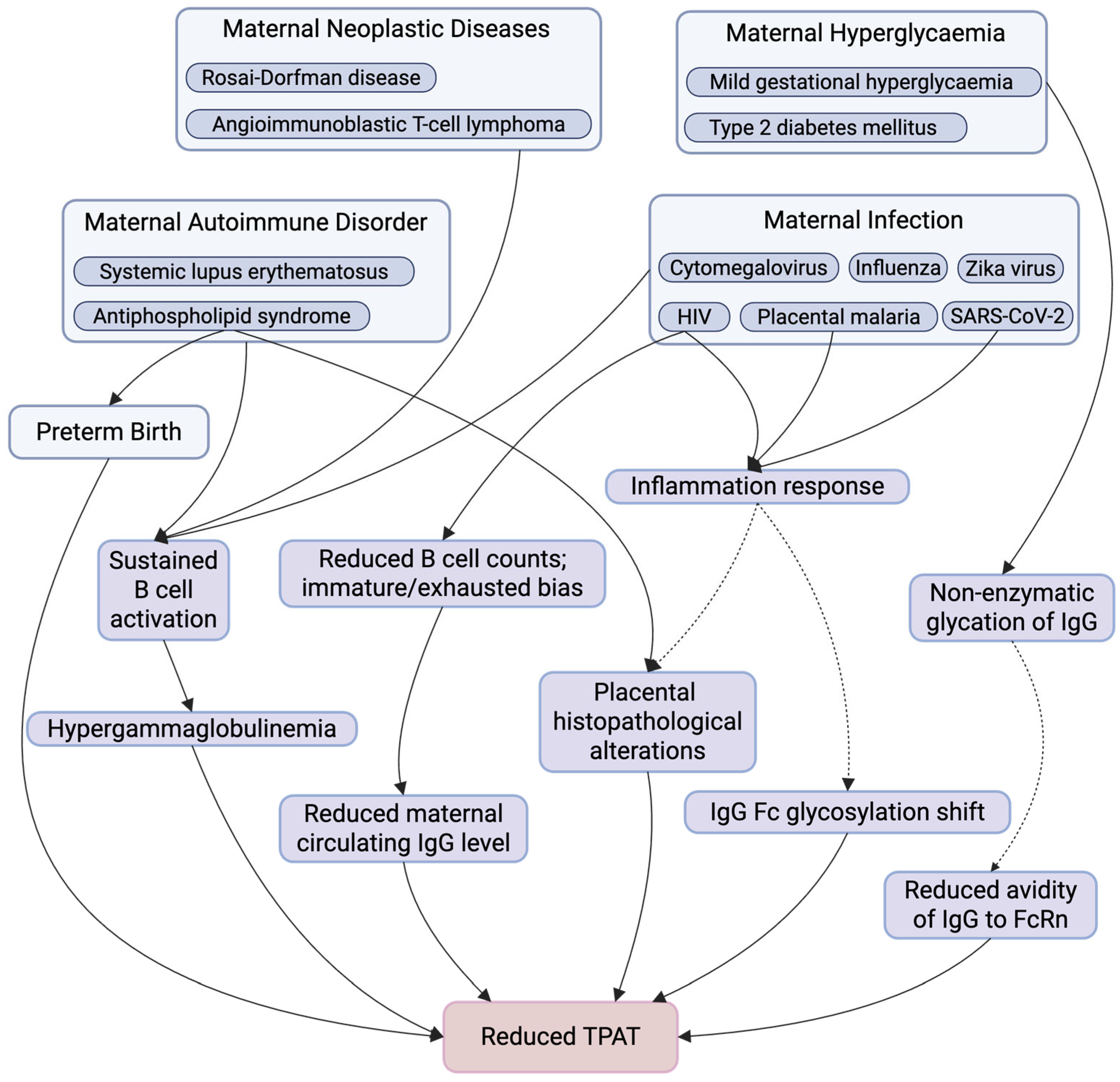
Figure 2.
Summary of Maternal Factors Affecting TPAT. This figure summarises key maternal physiological and pathological conditions and downstream immunological and placental alterations that have been implicated in TPAT. Maternal conditions include autoimmune disorders, neoplastic diseases, infections, hyperglycaemia, and preterm birth, which may influence TPAT through multiple, potentially overlapping pathways. These include sustained B cell activation, altered B cell composition (with reduced total B cell counts and an immature or exhausted bias), hypergammaglobulinemia or reduced maternal circulating IgG levels, inflammatory responses, non-enzymatic IgG glycation, shifts in IgG Fc glycosylation, placental structure and function alterations, and reduce IgG–FcRn avidity, collectively contributing to impaired TPAT. Solid arrows indicate pathways with supportive evidence, whereas dashed arrows denote proposed or associative relationships that may not be direct, are context-dependent, or require further mechanistic investigation. Created in https://www.BioRender.com.
3.1. Kinetics of TPAT During Pregnancy
TPAT begins in the first trimester and increases exponentially as gestation progresses, a process that correlates positively with the placental FcRn expression throughout pregnancy. As early as 6 weeks’ gestation, small amounts of maternal IgG can reach the embryo and have been detected in the extraembryonic coelomic fluid and villous stroma [75]. Studies involving cordocentesis showed that by approximately 17 to 22 weeks of gestation, around 10% of maternal IgG concentrations are detectable in foetal circulation. The cord blood IgG concentration progressively increases from 17 weeks of gestation through late gestation and delivery, reaching around 50% of maternal levels by 28 to 32 weeks and eventually surpassing maternal levels between 37 and 40 weeks [3,64]. By full term, foetal IgG levels vary but are typically around 25% higher than those of the mother [76,77,78]. Since higher FcRn expression has been observed in placental tissue from 36 weeks of gestation onward, the transplacental transfer of IgG mostly occurs during the third trimester. Consequently, infants born prematurely, particularly those delivered before 32 weeks, have markedly reduced IgG concentrations relative to those born at term [61,76,79,80].
The kinetics of TPAT are therefore critical for optimising the timing of maternal vaccination. To ensure sufficient IgG transferred to the foetus, particularly in cases where preterm delivery is likely, vaccination during early pregnancy may be necessary. However, later administration may be preferable in some cases, as it aligns more closely with the peak period of IgG transferred across the placenta [81]. The optimal timing of vaccination during pregnancy varies depending on the specific vaccine. While current guidelines recommend maternal pertussis vaccination between 16 and 32 weeks of gestation [82], emerging evidence suggests that this window could be broadened, as optimal neonatal antibody levels and expected infant seropositivity can be achieved between weeks 13 and 33 [80]. Tetanus, diphtheria, and acellular pertussis vaccination between 30 and 33 weeks of gestation was associated with significantly higher geometric mean concentrations of anti-pertussis toxin IgG in infants at birth compared to vaccination at 20–24 weeks (27.3 IU/mL vs. 14.7 IU/mL), suggesting an improved TPAT with later gestational immunisation [4,83]. However, to achieve optimal cord blood antibody titres in both term and preterm infants requires scheduling pertussis vaccination at least 7.5 weeks prior to delivery. These findings support the administration of tetanus, diphtheria, and acellular pertussis vaccination during the second and third trimester, with earlier vaccination within this window recommended to provide comparable levels of protection for both term and preterm infants [84]. Similar principles apply to the respiratory syncytial virus (RSV) prefusion F protein maternal vaccine. Phase 3 data show that vaccination in the late second or third trimester reduces infant RSV-associated lower respiratory tract disease [85]. Accordingly, UK guidance recommends administration from 28 weeks’ gestation onwards (ideally at 28 weeks or soon after) to ensure adequate time for maternal antibody generation and efficient TPAT, including for infants born preterm [86,87]. Additionally, SARS-CoV-2 vaccination during the first trimester has been associated with higher C/M ratios compared to vaccination in later trimesters, suggesting that early immunisation can enhance passive immunity in neonates [88].
3.2. Maternal IgG Competition—Hypergammaglobulinemia
Normally, foetal IgG concentrations are positively correlated with maternal levels [7,64]. However, under pathological conditions where maternal IgG concentrations exceed 15 g/L, a condition defined as hypergammaglobulinemia, the limited capacity of FcRn becomes saturated. As a result, IgG molecules must compete for FcRn binding, and those that remain unbound are subject to cellular lysosomal degradation [64,89,90,91]. This saturation impairs the efficiency of transplacental IgG transfer and is associated with a negative correlation between maternal IgG levels and C/M ratios, observed for both total and antigen specific antibodies [4,92,93]. Hypergammaglobulinemia is characterised by the overproduction of multiple or a single immunoglobulin class, often driven by aberrant B cell activation, resulting in elevated levels of non-specific and/or functionally impaired antibodies, which can saturate the FcRn-mediated transport system, reducing the efficiency of transplacental transfer antigen-specific IgG [62,64,79,94,95,96]. Consequently, the quality of maternally derived IgG transferred to the foetus may be compromised, reducing the effectiveness of passive immune protection in the foetus and newborn.
Hypergammaglobulinemia is observed across a wide spectrum of pathological conditions. Transient hypergammaglobulinemia, often triggered by acute immune stimulation such as Epstein–Barr virus infection or Plasmodium falciparum in placental malaria (PM), can cause a temporary spike in maternal IgG levels [97,98,99]. While potentially detrimental to the shaping of the neonatal antibody repertoire, transient hypergammaglobulinemia may be avoidable or manageable through appropriate infection control and vaccination timing [100]. In contrast, chronic hypergammaglobulinemia is commonly associated with persistent immune dysregulation, including chronic infections such as cytomegalovirus and human immunodeficiency virus (HIV), as well as autoimmune conditions (e.g., systemic lupus erythematosus) and neoplastic diseases (e.g., Rosai–Dorfman disease, angioimmunoblastic T-cell lymphoma) [94,101,102,103,104]. These chronic states are characterised by sustained B cell activation and ongoing immunoglobulin overproduction, leading to long-term saturation of the FcRn pathway. Unlike transient forms, chronic hypergammaglobulinemia is difficult to modify during pregnancy, limiting the scope for intervention. Hypergammaglobulinemia may serve as a common underlying mechanism contributing to the impaired maternal–foetal IgG transfer observed in the context of various maternal infections or immune dysregulation.
3.3. Pathogenic Maternal IgG—Antiphospholipid Syndrome
Beyond antibody quantity, the qualitative properties of maternal IgG are equally critical in shaping placental function and pregnancy outcome, as illustrated by pathological settings in which IgG acts not as a passive mediator of neonatal protection but as an active driver of placental pathology. Antiphospholipid syndrome (APS) represents a paradigmatic IgG-mediated autoimmune disorder associated with obstetric morbidity and placental dysfunction, including recurrent miscarriage, late foetal loss, early-onset pre-eclampsia, foetal growth restriction, preterm birth, and placental insufficiency [105,106]. As such, APS may provide a clinically relevant model for examining how pathogenic maternal IgG may disrupt placental biology and TPAT. APS is characterised by circulating antiphospholipid antibodies, most notably IgG directed against β2-glycoprotein I (β2GPI), which exert direct effects on endothelial cells, trophoblasts and the coagulation system [107]. Beyond the classical laboratory criteria, the emerging concept of a “mosaic” of antiphospholipid antibodies, including non-criteria antibodies, has expanded the spectrum of pathogenic IgG species implicated in so-called seronegative APS, particularly in obstetric presentations [108]. Mechanistically, recent studies demonstrate that anti-β2GPI IgG can signal through LRP8/ApoER2 within lipid raft microdomains in endothelial cells, leading to impaired nitric oxide production and endothelial dysfunction, processes highly relevant to placental vascular homeostasis [109]. Although APS has not been directly linked to quantitative defects in TPAT, the profound alterations in placental inflammation, vascular integrity and Fc-dependent IgG signalling suggest that the placental microenvironment in APS may be suboptimal for efficient IgG transport.
3.4. Maternal Infection
With the increasing frequency of epidemics [110], pregnant individuals are at heightened risk of exposure to emerging infectious pathogens. Consequently, elucidating the impact of maternal infection on TPAT is essential for informing pandemic preparedness strategies and for optimising the protective effects of maternal vaccination and treatment on neonatal health outcomes. Different infections can act on distinct regulatory levels of the antibody transfer process, including maternal antibody quantity and quality, placental structure and functional integrity, and FcRn-mediated transport pathways [111,112,113,114,115,116,117]. Nevertheless, overlapping mechanistic themes can be identified across diverse infectious contexts, such as immune activation-driven inflammation, infection-associated placental pathology, and qualitative remodelling of the maternal IgG repertoire, including alterations in Fc glycosylation [112,113,114,115,116,117]. The specific combination of these factors, together with their timing during gestation, collectively determines the degree of impact on TPAT [118,119]. The following contents delineate how these mechanisms are differentially engaged in HIV infection, PM and SARS-CoV-2 infection during pregnancy. Table 1 summarises common infectious diseases that have been reported to affect maternal–foetal IgG transfer efficiency, along with their associated immunological alterations.

Table 1.
Impact of maternal infections on TPAT efficiency.
3.4.1. HIV
With the widespread and effective use of antiretroviral therapy (ART), vertical transmission of HIV has significantly declined worldwide [125]. Nevertheless, in early infancy, neonates who are HIV-exposed but uninfected remain at a heightened risk for vaccine-preventable diseases relative to those unexposed to HIV [126,127]. This increased vulnerability is likely multifactorial, but one contributing factor is the impairment of TPAT [66,120,128]; at birth, HIV-exposed but uninfected neonates exhibit reduced C/M ratios for Haemophilus influenzae type b (23%), pneumococcus (15%), Bordetella pertussis (40%), and tetanus toxoid (27%) compared with HIV-unexposed counterparts [66].
Maternal HIV infection impairs transplacental IgG transfer by altering maternal B cell populations [120,121]. These immunological alterations are influenced by multiple factors and should be interpreted in relation to ART treatment status and the gestational timing of maternal infection. Patients with untreated HIV infection exhibit reduced total B cell counts in peripheral blood compared to uninfected individuals [111], which may impair antibody production. Additionally, chronic HIV infection is linked to a higher frequency of immature and exhausted B cells, which are less effective at generating antibodies [129]. As a result, HIV-infected pregnant women tend to have lower total antibody titre baseline, resulting in decreased levels of IgG specific to vaccines and pathogens being transferred via TPAT, thereby leaving infants with antibody levels below the protective threshold [121]. Fortunately, ART treatment has been shown to increase B cell counts and promote the normalisation of B cell subpopulations [111]. The extent to which these improvements can mitigate the HIV-induced reduction in maternal antibody production, however, remains dependent on the timing and efficacy of the intervention [102].
HIV infection is associated with persistent systemic inflammation, which drives antigen-specific remodelling of IgG Fc glycosylation. This glycosylation shift, characterised by reduced galactosylation, sialylation and fucosylation, is associated with enhanced FcγR binding and antiviral effector activity [122,130]. However, these modifications appear suboptimal for efficient TPAT. Consistent with this, Taylor et al. reported that HIV-infected pregnant women exhibited altered maternal IgG glycosylation profiles compared with uninfected counterparts, including higher proportions of agalactosylated and lower levels of digalactosylated and sialylated glycoforms. These glycosylation patterns were associated with increased binding to FcγRIIa and FcγRIIb but reduced transplacental transfer of vaccine-specific antibodies [112,113], indicating a trade-off between antiviral control and passive immunity conferred to the foetus.
3.4.2. Malaria
PM, primarily caused by Plasmodium falciparum infection during pregnancy, is characterised by the sequestration of parasitised erythrocytes within the placental intervillous space [131]. These infected erythrocytes adhere to placental receptors, triggering inflammatory response. This immune activation is marked by the infiltration of maternal mononuclear cells and elevated levels of pro-inflammatory cytokines, which can lead to structural abnormalities in the placenta, including disruption of SCT integrity, thickening trophoblast basal lamina and increased syncytial knot formation [114,115,116,117]. This pathological environment has been linked to a significantly reduced efficiency of maternal IgG transfer. For instance, Okoko et al. reported a marked decrease in transplacental IgG transfer for herpes simplex virus 1 (69%), respiratory syncytial virus (58%), and varicella-zoster virus (55%), in malaria-infected placenta [62]. Similarly, multivariate analysis from western Kenya found that PM was significantly associated with reduced cord blood levels of Epstein–Barr virus antibodies, specifically anti-VCA-p18 and anti-EBNA1 [132].
3.4.3. SARS-CoV-2
The angiotensin-converting enzyme 2 receptor, which facilitates SARS-CoV-2 cellular entry, has been identified in placental tissue, prompting initial concerns regarding potential viral replication within the placenta and consequent vertical transmission to the foetus [133,134,135]. Despite these concerns, current evidence indicates that transplacental transmission of SARS-CoV-2 is rare (0.63%) [119]. This limited vertical transmission is further supported by the absence of SARS-CoV-2-specific IgM antibodies in neonates, despite the presence of maternally transferred IgG [136]. In pregnancies affected by SARS-CoV-2 infection during the third trimester, placental pathological features such as maternal vascular malperfusion, including increased syncytial knots and focal perivillous fibrin deposition, are frequently observed [137]. These changes may reflect compromised placental function, potentially impairing the efficiency of transplacental IgG transfer [138].
Evaluation of TPAT following maternal SARS-CoV-2 infection has yielded mixed findings. Partey et al. demonstrated that levels of IgG specific to the SARS-CoV-2 nucleoprotein (N-protein) and spike receptor-binding domain in cord blood strongly correlate with maternal antibody concentrations (N-protein: Rs = 0.7155, p < 0.001; receptor-binding domain: Rs = 0.8693, p < 0.001), indicating effective TPAT that confer passive immunity to the neonate [139]. Furthermore, a reported mean C/M ratio of 1.27 (95% CI: 0.69–2.89) suggested no significant difference in TPAT efficiency based on maternal disease severity [119]. However, a comparative analysis suggests that hospitalised patients, who typically exhibit more severe symptoms, develop higher antibody titres than mildly symptomatic or asymptomatic individuals [140], which may potentially imply an enhanced efficiency of TPAT in severe maternal cases [119]. This hypothesis is supported by findings demonstrating significantly higher C/M ratios in mothers who experienced severe to critical illness compared to those with mild or no symptoms [141].
Furthermore, a positive correlation has been established between TPAT efficiency and the time interval between maternal infection and delivery. Maternal SARS-CoV-2 infection in late pregnancy (third trimester) is associated with measurably reduced placental transfer of SARS-CoV-2-specific antibodies compared with other vaccine- or pathogen-specific antibodies, potentially linked to acute inflammation-induced alterations in Fc glycosylation [118]. Maternal infections occurring during the early trimesters (first and second) were associated with more effective antibody being transferred to the foetus [119]. Importantly, the efficiency of TPAT observed in vaccinated pregnant individuals is comparable to that seen in those with natural infection [142]. These findings collectively support the administration of SARS-CoV-2 vaccines during early pregnancy as a strategy to enhance neonatal protection via passive immunity. This recommendation is further reinforced by evidence from Atyeo et al., in which median C/M ratios for SARS-CoV-2-specific IgG were approximately 1.5, 1.3, and 1.0 after first-, second-, and third-trimester vaccination, respectively, consistent with greater TPAT efficiency with earlier immunisation [88]. The insights gained from maternal SARS-CoV-2 immunisation also offer valuable guidance for optimising vaccination strategies against other emerging infectious diseases during pregnancy.
3.5. Maternal Hyperglycaemia
Hyperglycaemia during pregnancy, including conditions such as gestational diabetes mellitus and type 2 diabetes mellitus (DM-2), is increasingly prevalent worldwide and may significantly affect the efficiency of TPAT [143]. Maternal hyperglycaemia alters both maternal systemic immune regulation and placental histology, thereby influencing IgG being transferred to the foetus through various mechanisms.
Maternal and cord blood IgG levels vary depending on the type and severity of glycaemic disturbance. In women with mild gestational hyperglycaemia (MGH), maternal IgG levels are comparable to those in normoglycaemic pregnancies, while levels are significantly reduced in individuals with DM-2. Despite this, cord blood IgG concentrations remain similar across all groups [144]. This discrepancy suggests that compensatory or alternative mechanisms may preserve foetal IgG acquisition even in more severe diabetic conditions, though the underlying mechanisms remain to be fully elucidated. Structural changes in the placenta may contribute to altered transplacental IgG transfer in hyperglycaemic pregnancies. In cases of MGH, increased placental villous capillarisation and a higher number of SCT have been reported, which may facilitate TPAT and the movement of other macromolecules across the placenta [145,146]. While total IgG C/M ratios do not significantly differ between normoglycaemic and hyperglycaemic pregnancies, subclass-specific analyses reveal more nuanced differences. In the DM-2 group, transfer ratios of IgG1, IgG3, and IgG4 are reduced, suggesting impaired subclass-specific transfer. In contrast, in the MGH group, the ratio of IgG3 was increased compared to normoglycaemic controls, indicating that mild hyperglycaemia may differentially modulate IgG subclass trafficking [144].
Moreover, in diabetes mellitus, elevated circulating glucose levels can lead to non-enzymatic glycation of IgG molecules, potentially altering their structure and function [147,148]. These glycation-induced modifications have been hypothesised to reduce the binding avidity of IgG to FcRn, thereby impairing its transplacental transfer [65]. However, evidence remains inconclusive. For instance, Goetze et al. experimentally generated highly glycated IgG1 and IgG2 and assessed their binding to various Fc receptors, including FcγRIIIa and FcRn. Their findings indicated that glycation does not significantly affect receptor-binding affinity [149], challenging the assumption that glycation disrupts FcRn-mediated transport. Additionally, a study reported significant associations between maternal glucose homeostasis markers (e.g., HOMA2-IR, HOMA2-%B) and specific IgG glycosylation traits. Yet, no significant differences in overall IgG N-glycosylation patterns are detected between women with and without gestational diabetes mellitus [150]. This discrepancy may reflect the complex physiological adaptations during pregnancy that obscure subtle glycosylation-related effects, underscoring the need for more refined longitudinal analyses.
3.6. Transplacental Transfer of Bioengineering IgG—Pregnant Women Require Immunotherapies
The number of effective monoclonal antibody (mAb) biologics used to treat immune-mediated inflammatory diseases and cancer is increasing [151,152]. Like the endogenous IgG, those mAb biologics applied in immunotherapies that are necessary to manage maternal health can interact with FcRn, facilitating their transplacental transfer to foetal circulation during pregnancy [153]. In most cases, mAb biologics with modifications of their binding site with FcRs have a prolonged half-life and higher avidity with FcRn [154,155,156], but the impact on transplacental transfer of conventional antigen-specific antibodies has not been well elucidated, and their safety in relation to foetal development has become a significant concern. A study evaluating the amount of transplacental transfer observed between IgG1 mAb products treating inflammatory bowel disease on the day of birth confirmed the TPAT of IgG1 subclass mAbs (e.g., infliximab, adalimumab, and ustekinumab) targeting tumour necrosis factor alpha (TNFα), IL-12 and IL-23 [157]. Infants exposed to rituximab (an anti-CD20 mAb) in utero have been reported to exhibit the down-regulation of both B cell numbers and circulating IgG levels, as well as potential infection complications [158,159,160]. Accordingly, modifications of some mAb biologics have been used to avoid TPAT. For instance, Certolizumab, an anti-TNFα mAb, lacks an Fc portion, making it unable to bind to FcRn, and is considered safe to use throughout pregnancy; another anti-TNFα mAb, Etanercept, which possesses a modified Fc portion, also exhibits a low capacity to cross the placenta, and is not associated with an increased risk of foetal malformations, preterm delivery or pregnancy [161,162,163]. There has also been an enormous expansion in the development of engineering strategies involving FcRn to modulate the dynamic behaviour of antibodies, antigens and albumin, showing considerable potential for use in the clinic [164]. Novel strategies have potential in striking the balance between maternal health management and foetal harm minimalisation.
Furthermore, concerns about if early exposure to mAb biologics in utero affects the timing of neonatal vaccination and the effectiveness and safety of vaccination administered to neonates have also been raised [165]. Neonatal B cell depletion has been reported in pregnancies exposed to rituximab; however, none of these neonates experienced infectious complications or adverse reactions to vaccination, and B cell counts normalized in all cases within six months [166]. In contrast, early exposure to belimumab, a BAFF neutralising mAb, in utero is reported to be significantly associated with neonatal infections [167]. The safety of vaccinations during the first year of life in infants exposed to biologics in utero has also been evaluated in a systematic study [168], highlighting that the incidences of adverse event are different among vaccination (e.g., Bacillus Calmette–Guérin vaccine, rotavirus vaccine, and measles–mumps–rubella vaccine). Therefore, therapeutic strategies should be individualised accordingly to ensure the safety of both mother and infant. Although TNF inhibitors (TNFis) are commonly used biologics for immune-mediated inflammatory diseases and considered to be essentially safe for the foetus during pregnancy [169], the continued therapy of TNFis during late pregnancy is reserved only for patients with active disease, considering their possible effects on neonatal infection risk and vaccination schedules [170]. A recent study suggested that treatment with TNFis in pregnant patients with rheumatic disease induces lower maternal anti-pertussis IgG antibody levels in newborns in spite of no major differences in IgG antibody levels upon maternal tetanus-diphtheria-and-acellular-pertussis vaccination in pregnant women with or without immune-modulating treatment. As for neonates born to mothers who were treated with rituximab or belimumab during pregnancy, close monitoring of B cell counts and serum biologic levels after birth are recommended to facilitate early detection and management of potential infections [170]. The European Alliance of Associations for Rheumatology recommends that infants exposed to biologic agents before 22 weeks of gestation can follow the standard vaccination schedule, including live vaccine, while those exposed to biologics during the second and third trimester are advised to avoid the administration of live vaccines within the first six months of life [171].
4. Future Perspectives on Models of Placental Function Available to Study IgG Transplacental Transfer
4.1. Murine Model
Despite that marked differences between human and mouse placentae [172,173], in terms of unveiling the mechanism of FcRn-mediated transport of IgG, the direct masking or mutating of human FcRn in vivo is challenging, making murine models a valuable and appealing alternative. Transgenic murine models such as mice lacking endogenous FcRn but expressing a human FcRn transgene (Table 2) are currently proving to be a productive model for the assessment of FcRn-mediated transport of IgG [12], safety of vaccination for pregnant women [174], or potential therapeutics using bioengineering IgG molecules [175] because mouse FcRn can be bound to by IgGs from many different species with high affinity [176].

Table 2.
Murine and human models used for the investigation of transplacental transfer of IgG.
4.2. Placenta-Originated Model
In the meantime, diverse in vitro and ex vivo models of placental function relevant to humans or derived from human placental tissue (Table 2), such as primary cells or cell lines of placental origin, organoid, placental explant, or placental perfusion, have also been intensively developed to advance the research on transplacental transfer of IgG and other substances over the years, of which the critical evaluation has been previously reviewed [185,186]. Although human placental perfusion models more closely simulate the functional units of term placental tissue than cell monolayers or placental explants, it is also necessary to consider how morphological changes and increasing FcRn expression during placental development [3,61] influence the transplacental transfer of IgG.
4.3. Computational Modelling
The development of novel techniques, for example, a quantitative mechanistic model to unveil the determinants of transplacental transfer of IgG [187] or using the ‘placenta-on-a-chip’ model combining microfluidics and diverse human placental cells in 3D structures to study placental translocation and inflammatory responses [188,189], is providing more insights into understanding the transplacental transfer of IgG. Wessel and Dolatshahi employed a quantitative mechanistic model to reveal several key factors that contribute to IgG inter-subclass competition and potentially inter- and intra-patient IgG transfer heterogeneity [187]. Filippo et al. developed a low-dimensional machine learning model compiling a database of 248 compounds with experimental information about their placental transfer, which is considered to be useful in predicting placental drug transfer during pregnancy [190].
4.4. Strengths and Limitations
Compared to the murine model and in vitro culture of cell monolayer culture, the primary advantage of the perfusion model is its preservation of tissue functional integrity and cellular barrier function. Notably, significant differences in IgG processing have been observed between in vitro cell culture systems and placental perfusion models, and gestation-dependent transplacental IgG transfer in humans has been demonstrated beginning in the first trimester [17,191]. A recent review has offered important insights into the role of dual ex vivo perfusion models in investigating IgG transfer, while critically evaluating the various placental perfusion methodologies [192]. Nevertheless, despite their advantages, these models are limited by their inability to recapitulate the longitudinal placental development and the progressive accumulation of maternal antibodies throughout gestation. Mathematical modelling, as an emerging methodological approach, is therefore leveraged to elucidate nonlinear dynamics across diverse physiological systems and to inform the rational design of therapies and vaccines. Such models allow the concurrent analysis of multiple interdependent components, highlight the critical role of temporal dynamics, and integrate regulatory structures such as feedback loops [187]. Furthermore, mathematical modelling can better identify kinetic constraints and provide a quantitative framework in the context of TPAT for evaluating those relative factors governing this special transplacental transfer.
5. Conclusions
Pregnancy and early life represent periods of pronounced immunological complexity. Maternal adaptations maintain foetal tolerance while preserving host defence, and newborns’ immunity depends largely on the acquisition of maternal IgG [4,7,193]. TPAT therefore constitutes a critical conduit of immunity across the maternal–neonatal dyad, offering protection both in utero and in the immediate postnatal period, and serves as the biological basis for maternal vaccination strategies [1,2,3]. Consequently, optimising maternal immunisation to enhance TPAT has become an increasingly important public health priority [29]. The efficiency of TPAT varies and can be affected by multiple clinical and physiological factors, including preterm birth, hypergammaglobulinemia, maternal pathogenic IgG, maternal infection, hyperglycaemia, and immunomodulatory therapies [68,69,70,71]. These observations indicate that key determinants of IgG transfer remain insufficiently defined, particularly with respect to how maternal immune status, placental transport pathways, and antibody structural features collectively influence transfer across the SCT. Recent technological advances offer potential avenues to address these gaps. Fc-engineered IgG molecules generated through rational design exhibit extended half-life and enhanced therapeutic efficacy [194]. Such bioengineering approaches may be leveraged to improve passive immunity in newborns, especially as understanding increases regarding how molecular and cellular regulators govern the selective transfer of antigen-specific antibodies. Current understanding of FcRn–IgG immunobiology indicates that blocking FcRn represents a promising and effective approach to lowering circulating pathogenic IgG autoantibodies and mitigating IgG-driven diseases. Multiple FcRn inhibitors that specifically disrupt IgG recycling are now advancing swiftly toward clinical application in both neurology and haematology [195], exhibiting potential for future use in a much wider variety of antibody-mediated autoimmune diseases rather than optimising maternal and neonatal vaccination only. In parallel, although the inadequacy of existing experimental models was once considered to hinder the investigation in dynamics of IgG transfer in pregnant women and the rational design of maternal vaccines, computational modelling or in silico mechanistic models in combination with experimental data has now emerged as a powerful strategy to dissect the process regulating TPAT [196]. Continued progress in these areas is likely to support the development of maternal vaccines and antibody-based interventions that more consistently achieve protective immunity in both pregnant women and their infants.
Author Contributions
Conceptualization, Q.L. and Z.H., with input from N.M.S.; investigation and literature synthesis, Q.L. and Z.H.; writing—original draft preparation, Q.L. and Z.H.; writing—review and editing, Z.H., Z.S., O.G., J.A.H. and N.M.S.; critical appraisal, Z.S. and O.G.; supervision, N.M.S. and J.A.H.; resources and scholarly support, N.M.S. and J.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study.
Acknowledgments
Acknowledgements are made to the Department of Metabolism, Digestion and Reproduction, and National Heart and Lung Institute, Imperial College London, for their administrative support. Infrastructure support was provided by the NIHR Imperial Biomedical Research Centre and the NIHR Imperial Clinical Research Facility. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. Figures were created with Biorender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| IgG | Immunoglobulin G |
| FcRn | Neonatal Fc receptor |
| TPAT | Transplacental antibody transfer |
| SCT | Syncytiotrophoblast |
| FcγRIIIa | Fcγ receptor III-A |
| C/M ratios | Cord-to-maternal antibody titre ratios |
| RSV | Respiratory syncytial virus |
| PM | Placental malaria |
| APS | Antiphospholipid syndrome |
| β2GPI | β2-glycoprotein I |
| HIV | Human immunodeficiency virus |
| ART | Antiretroviral therapy |
| N-protein | Nucleoprotein |
| DM-2 | Type 2 diabetes mellitus |
| MGH | Mild gestational hyperglycaemia |
| mAb | Monoclonal antibody |
| TNFα | Tumour necrosis factor alpha |
| TNFi | Tumour necrosis factor alpha inhibitor |
References
- Glezen, W.P.; Alpers, M. Maternal immunization. Clin. Infect. Dis. 1999, 28, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Barrau, M.; Roblin, X.; Andromaque, L.; Rozieres, A.; Faure, M.; Paul, S.; Nancey, S. What Should We Know about Drug Levels and Therapeutic Drug Monitoring during Pregnancy and Breastfeeding in Inflammatory Bowel Disease under Biologic Therapy? J. Clin. Med. 2023, 12, 7495. [Google Scholar] [CrossRef]
- Malek, A.; Sager, R.; Kuhn, P.; Nicolaides, K.H.; Schneider, H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am. J. Reprod. Immunol. 1996, 36, 248–255. [Google Scholar] [CrossRef]
- Clements, T.; Rice, T.F.; Vamvakas, G.; Barnett, S.; Barnes, M.; Donaldson, B.; Jones, C.E.; Kampmann, B.; Holder, B. Update on Transplacental Transfer of IgG Subclasses: Impact of Maternal and Fetal Factors. Front. Immunol. 2020, 11, 1920. [Google Scholar] [CrossRef]
- Donald, K.; Petersen, C.; Turvey, S.E.; Finlay, B.B.; Azad, M.B. Secretory IgA: Linking microbes, maternal health, and infant health through human milk. Cell Host Microbe 2022, 30, 650–659. [Google Scholar] [CrossRef]
- Albrecht, M.; Arck, P.C. Vertically Transferred Immunity in Neonates: Mothers, Mechanisms and Mediators. Front. Immunol. 2020, 11, 555. [Google Scholar] [CrossRef]
- Ciobanu, A.M.; Dumitru, A.E.; Gica, N.; Botezatu, R.; Peltecu, G.; Panaitescu, A.M. Benefits and Risks of IgG Transplacental Transfer. Diagnostics 2020, 10, 583. [Google Scholar] [CrossRef]
- Kingdom, J.; Huppertz, B.; Seaward, G.; Kaufmann, P. Development of the placental villous tree and its consequences for fetal growth. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 92, 35–43. [Google Scholar] [CrossRef]
- DeSesso, J.M.; Williams, A.L.; Ahuja, A.; Bowman, C.J.; Hurtt, M.E. The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy. Crit. Rev. Toxicol. 2012, 42, 185–210. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal Fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef]
- Jennewein, M.F.; Goldfarb, I.; Dolatshahi, S.; Cosgrove, C.; Noelette, F.J.; Krykbaeva, M.; Das, J.; Sarkar, A.; Gorman, M.J.; Fischinger, S.; et al. Fc Glycan-Mediated Regulation of Placental Antibody Transfer. Cell 2019, 178, 202–215.e14. [Google Scholar] [CrossRef]
- Borghi, S.; Bournazos, S.; Thulin, N.K.; Li, C.; Gajewski, A.; Sherwood, R.W.; Zhang, S.; Harris, E.; Jagannathan, P.; Wang, L.X.; et al. FcRn, but not FcγRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 12943–12951. [Google Scholar] [CrossRef]
- Vaccaro, C.; Bawdon, R.; Wanjie, S.; Ober, R.J.; Ward, E.S. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc. Natl. Acad. Sci. USA 2006, 103, 18709–18714. [Google Scholar] [CrossRef]
- Firan, M.; Bawdon, R.; Radu, C.; Ober, R.J.; Eaken, D.; Antohe, F.; Ghetie, V.; Ward, E.S. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int. Immunol. 2001, 13, 993–1002. [Google Scholar] [CrossRef]
- Martinez, D.R.; Fong, Y.; Li, S.H.; Yang, F.; Jennewein, M.F.; Weiner, J.A.; Harrell, E.A.; Mangold, J.F.; Goswami, R.; Seage, G.R., 3rd; et al. Fc Characteristics Mediate Selective Placental Transfer of IgG in HIV-Infected Women. Cell 2019, 178, 190–201.e111. [Google Scholar] [CrossRef]
- Einarsdottir, H.K.; Selman, M.H.; Kapur, R.; Scherjon, S.; Koeleman, C.A.; Deelder, A.M.; van der Schoot, C.E.; Vidarsson, G.; Wuhrer, M. Comparison of the Fc glycosylation of fetal and maternal immunoglobulin G. Glycoconj. J. 2013, 30, 147–157. [Google Scholar] [CrossRef]
- Mathiesen, L.; Nielsen, L.K.; Andersen, J.T.; Grevys, A.; Sandlie, I.; Michaelsen, T.E.; Hedegaard, M.; Knudsen, L.E.; Dziegiel, M.H. Maternofetal transplacental transport of recombinant IgG antibodies lacking effector functions. Blood 2013, 122, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Takizawa, T.; Iwaki, J.; Mishima, T.; Ui-Tei, K.; Takeshita, T.; Matsubara, S.; Takizawa, T. Fc gamma receptor IIb participates in maternal IgG trafficking of human placental endothelial cells. Int. J. Mol. Med. 2015, 35, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, J.J.; Pyzik, M.; Rath, T.; Kozicky, L.K.; Sand, K.M.K.; Gandhi, A.K.; Grevys, A.; Foss, S.; Menzies, S.C.; Glickman, J.N.; et al. FcRn is a CD32a coreceptor that determines susceptibility to IgG immune complex-driven autoimmunity. J. Exp. Med. 2020, 217, e20200359. [Google Scholar] [CrossRef] [PubMed]
- Bergtold, A.; Desai, D.D.; Gavhane, A.; Clynes, R. Cell surface recycling of internalized antigen permits dendritic cell priming of B cells. Immunity 2005, 23, 503–514. [Google Scholar] [CrossRef]
- Ganesan, L.P.; Kim, J.; Wu, Y.; Mohanty, S.; Phillips, G.S.; Birmingham, D.J.; Robinson, J.M.; Anderson, C.L. FcγRIIb on liver sinusoidal endothelium clears small immune complexes. J. Immunol. 2012, 189, 4981–4988. [Google Scholar] [CrossRef]
- Pyzik, M.; Kozicky, L.K.; Gandhi, A.K.; Blumberg, R.S. The therapeutic age of the neonatal Fc receptor. Nat. Rev. Immunol. 2023, 23, 415–432. [Google Scholar] [CrossRef]
- Mishima, T.; Kurasawa, G.; Ishikawa, G.; Mori, M.; Kawahigashi, Y.; Ishikawa, T.; Luo, S.S.; Takizawa, T.; Goto, T.; Matsubara, S.; et al. Endothelial expression of Fc gamma receptor IIb in the full-term human placenta. Placenta 2007, 28, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Anderson, C.L.; Robinson, J.M. A novel Fc gamma R-defined, IgG-containing organelle in placental endothelium. J. Immunol. 2005, 175, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, M.F.; Abu-Raya, B.; Jiang, Y.; Alter, G.; Marchant, A. Transfer of maternal immunity and programming of the newborn immune system. Semin. Immunopathol. 2017, 39, 605–613. [Google Scholar] [CrossRef]
- Braibant, M.; Barin, F. The role of neutralizing antibodies in prevention of HIV-1 infection: What can we learn from the mother-to-child transmission context? Retrovirology 2013, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Van Savage, J.; Decker, M.D.; Edwards, K.M.; Sell, S.H.; Karzon, D.T. Natural history of pertussis antibody in the infant and effect on vaccine response. J. Infect. Dis. 1990, 161, 487–492. [Google Scholar] [CrossRef]
- Fouda, G.G.; Martinez, D.R.; Swamy, G.K.; Permar, S.R. The Impact of IgG transplacental transfer on early life immunity. Immunohorizons 2018, 2, 14–25. [Google Scholar] [CrossRef]
- Medoro, A.K.; Puopolo, K.M. Transplacental Antibodies: Role of Maternal Vaccines and Immunity. Clin. Perinatol. 2025, 52, 101–113. [Google Scholar] [CrossRef]
- Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 2022, 22, 277–282. [Google Scholar] [CrossRef]
- Langel, S.N.; Blasi, M.; Permar, S.R. Maternal immune protection against infectious diseases. Cell Host Microbe 2022, 30, 660–674. [Google Scholar] [CrossRef]
- Dekkers, G.; Treffers, L.; Plomp, R.; Bentlage, A.E.H.; de Boer, M.; Koeleman, C.A.M.; Lissenberg-Thunnissen, S.N.; Visser, R.; Brouwer, M.; Mok, J.Y.; et al. Decoding the Human Immunoglobulin G-Glycan Repertoire Reveals a Spectrum of Fc-Receptor- and Complement-Mediated-Effector Activities. Front. Immunol. 2017, 8, 877. [Google Scholar] [CrossRef]
- Martinez, D.R.; Fouda, G.G.; Peng, X.; Ackerman, M.E.; Permar, S.R. Noncanonical placental Fc receptors: What is their role in modulating transplacental transfer of maternal IgG? PLoS Pathog. 2018, 14, e1007161. [Google Scholar] [CrossRef]
- Sun, Y.; Estevez, A.; Schlothauer, T.; Wecksler, A.T. Antigen physiochemical properties allosterically effect the IgG Fc-region and Fc neonatal receptor affinity. mAbs 2020, 12, 1802135. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Oganesyan, V.; Damschroder, M.M.; Cook, K.E.; Li, Q.; Gao, C.; Wu, H.; Dall’Acqua, W.F. Structural insights into neonatal Fc receptor-based recycling mechanisms. J. Biol. Chem. 2014, 289, 7812–7824. [Google Scholar] [CrossRef]
- Martin, W.L.; West, A.P., Jr.; Gan, L.; Bjorkman, P.J. Crystal structure at 2.8 A of an FcRn/heterodimeric Fc complex: Mechanism of pH-dependent binding. Mol. Cell 2001, 7, 867–877. [Google Scholar] [CrossRef]
- Raghavan, M.; Bonagura, V.R.; Morrison, S.L.; Bjorkman, P.J. Analysis of the pH dependence of the neonatal Fc receptor/immunoglobulin G interaction using antibody and receptor variants. Biochemistry 1995, 34, 14649–14657. [Google Scholar] [CrossRef]
- Kim, J.K.; Firan, M.; Radu, C.G.; Kim, C.H.; Ghetie, V.; Ward, E.S. Mapping the site on human IgG for binding of the MHC class I-related receptor, FcRn. Eur. J. Immunol. 1999, 29, 2819–2825. [Google Scholar] [CrossRef]
- Suzuki, T.; Ishii-Watabe, A.; Tada, M.; Kobayashi, T.; Kanayasu-Toyoda, T.; Kawanishi, T.; Yamaguchi, T. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: A comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol. 2010, 184, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Vidarsson, G.; Cragg, M.S. Effect of posttranslational modifications and subclass on IgG activity: From immunity to immunotherapy. Nat. Immunol. 2023, 24, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Bertolotti-Ciarlet, A.; Wang, W.; Lownes, R.; Pristatsky, P.; Fang, Y.; McKelvey, T.; Li, Y.; Li, Y.; Drummond, J.; Prueksaritanont, T.; et al. Impact of methionine oxidation on the binding of human IgG1 to Fc Rn and Fc gamma receptors. Mol. Immunol. 2009, 46, 1878–1882. [Google Scholar] [CrossRef]
- Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 2009, 8, 226–234. [Google Scholar] [CrossRef]
- Lu, J.; Chu, J.; Zou, Z.; Hamacher, N.B.; Rixon, M.W.; Sun, P.D. Structure of FcγRI in complex with Fc reveals the importance of glycan recognition for high-affinity IgG binding. Proc. Natl. Acad. Sci. USA 2015, 112, 833–838. [Google Scholar] [CrossRef]
- Subedi, G.P.; Barb, A.W. The Structural Role of Antibody N-Glycosylation in Receptor Interactions. Structure 2015, 23, 1573–1583. [Google Scholar] [CrossRef]
- Jensen, P.F.; Larraillet, V.; Schlothauer, T.; Kettenberger, H.; Hilger, M.; Rand, K.D. Investigating the interaction between the neonatal Fc receptor and monoclonal antibody variants by hydrogen/deuterium exchange mass spectrometry. Mol. Cell. Proteom. 2015, 14, 148–161. [Google Scholar] [CrossRef]
- Houde, D.; Peng, Y.; Berkowitz, S.A.; Engen, J.R. Post-translational modifications differentially affect IgG1 conformation and receptor binding. Mol. Cell. Proteom. 2010, 9, 1716–1728. [Google Scholar] [CrossRef]
- Anumula, K.R. Quantitative glycan profiling of normal human plasma derived immunoglobulin and its fragments Fab and Fc. J. Immunol. Methods 2012, 382, 167–176. [Google Scholar] [CrossRef]
- Holland, M.; Yagi, H.; Takahashi, N.; Kato, K.; Savage, C.O.; Goodall, D.M.; Jefferis, R. Differential glycosylation of polyclonal IgG, IgG-Fc and IgG-Fab isolated from the sera of patients with ANCA-associated systemic vasculitis. Biochim. Biophys. Acta 2006, 1760, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Malan Borel, I.; Gentile, T.; Angelucci, J.; Pividori, J.; Guala, M.C.; Binaghi, R.A.; Margni, R.A. IgG asymmetric molecules with antipaternal activity isolated from sera and placenta of pregnant human. J. Reprod. Immunol. 1991, 20, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Volkov, M.; Brinkhaus, M.; van Schie, K.A.; Bondt, A.; Kissel, T.; van der Kooi, E.J.; Bentlage, A.E.H.; Koeleman, C.A.M.; de Taeye, S.W.; Derksen, N.I.; et al. IgG Fab Glycans Hinder FcRn-Mediated Placental Transport. J. Immunol. 2023, 210, 158–167. [Google Scholar] [CrossRef]
- Jefferis, R.; Lefranc, M.P. Human immunoglobulin allotypes: Possible implications for immunogenicity. mAbs 2009, 1, 332–338. [Google Scholar] [CrossRef]
- Wang, W.; Vlasak, J.; Li, Y.; Pristatsky, P.; Fang, Y.; Pittman, T.; Roman, J.; Wang, Y.; Prueksaritanont, T.; Ionescu, R. Impact of methionine oxidation in human IgG1 Fc on serum half-life of monoclonal antibodies. Mol. Immunol. 2011, 48, 860–866. [Google Scholar] [CrossRef]
- Wang, W.; Lu, P.; Fang, Y.; Hamuro, L.; Pittman, T.; Carr, B.; Hochman, J.; Prueksaritanont, T. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab. Dispos. 2011, 39, 1469–1477. [Google Scholar] [CrossRef]
- Schlothauer, T.; Rueger, P.; Stracke, J.O.; Hertenberger, H.; Fingas, F.; Kling, L.; Emrich, T.; Drabner, G.; Seeber, S.; Auer, J.; et al. Analytical FcRn affinity chromatography for functional characterization of monoclonal antibodies. mAbs 2013, 5, 576–586. [Google Scholar] [CrossRef]
- Liu, X.; Ye, L.; Christianson, G.J.; Yang, J.Q.; Roopenian, D.C.; Zhu, X. NF-kappaB signaling regulates functional expression of the MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J. Immunol. 2007, 179, 2999–3011. [Google Scholar] [CrossRef]
- Qian, S.; Li, C.; Liu, X.; Jia, X.; Xiao, Y.; Li, Z. Activation of the JNK/MAPK Signaling Pathway by TGF-β1 Enhances Neonatal Fc Receptor Expression and IgG Transcytosis. Microorganisms 2021, 9, 879. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, F.; Qian, S.; Bi, D.; He, Q.; Jin, H.; Luo, R.; Li, S.; Meng, X.; Li, Z. TGEV infection up-regulates FcRn expression via activation of NF-κB signaling. Sci. Rep. 2016, 6, 32154. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; Saron, W.A.A.; Lim, T.; Jahan, N.; St John, A.L. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci. Adv. 2019, 5, eaav3208. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, L.; Bai, Y.; Mojidi, H.; Simister, N.E.; Zhu, X. Activation of the JAK/STAT-1 signaling pathway by IFN-gamma can down-regulate functional expression of the MHC class I-related neonatal Fc receptor for IgG. J. Immunol. 2008, 181, 449–463. [Google Scholar] [CrossRef]
- Lozano, N.A.; Lozano, A.; Marini, V.; Saranz, R.J.; Blumberg, R.S.; Baker, K.; Agresta, M.F.; Ponzio, M.F. Expression of FcRn receptor in placental tissue and its relationship with IgG levels in term and preterm newborns. Am. J. Reprod. Immunol. 2018, 80, e12972. [Google Scholar] [CrossRef]
- Okoko, B.J.; Wesumperuma, L.H.; Ota, M.O.; Pinder, M.; Banya, W.; Gomez, S.F.; McAdam, K.P.; Hart, A.C. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J. Infect. Dis. 2001, 184, 627–632. [Google Scholar] [CrossRef]
- Schlaudecker, E.P.; Ambroggio, L.; McNeal, M.M.; Finkelman, F.D.; Way, S.S. Declining responsiveness to influenza vaccination with progression of human pregnancy. Vaccine 2018, 36, 4734–4741. [Google Scholar] [CrossRef]
- Palmeira, P.; Quinello, C.; Silveira-Lessa, A.L.; Zago, C.A.; Carneiro-Sampaio, M. IgG placental transfer in healthy and pathological pregnancies. Clin. Dev. Immunol. 2012, 2012, 985646. [Google Scholar] [CrossRef]
- Wilcox, C.R.; Holder, B.; Jones, C.E. Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front. Immunol. 2017, 8, 1294. [Google Scholar] [CrossRef]
- Jones, C.E.; Naidoo, S.; De Beer, C.; Esser, M.; Kampmann, B.; Hesseling, A.C. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA 2011, 305, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef]
- van den Berg, J.P.; Westerbeek, E.A.; Smits, G.P.; van der Klis, F.R.; Berbers, G.A.; van Elburg, R.M. Lower transplacental antibody transport for measles, mumps, rubella and varicella zoster in very preterm infants. PLoS ONE 2014, 9, e94714. [Google Scholar] [CrossRef]
- Atwell, J.E.; Thumar, B.; Formica, M.A.; Robinson, L.J.; Walsh, E.E.; King, C.L.; Karron, R.A. Hypergammaglobulinemia and Impaired Transplacental Transfer of Respiratory Syncytial Virus Antibody in Papua New Guinea. Pediatr. Infect. Dis. J. 2019, 38, e199–e202. [Google Scholar] [CrossRef] [PubMed]
- Otero, S.; Miller, E.S.; Sunderraj, A.; Shanes, E.D.; Sakowicz, A.; Goldstein, J.A.; Mithal, L.B. Maternal Antibody Response and Transplacental Transfer Following Severe Acute Respiratory Syndrome Coronavirus 2 Infection or Vaccination in Pregnancy. Clin. Infect. Dis. 2023, 76, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Taylor, P.N.; Gillespie, K.M.; Oram, R.A.; Dayan, C.M. Maternal type 1 diabetes and relative protection against offspring transmission. Lancet Diabetes Endocrinol. 2023, 11, 755–767. [Google Scholar] [CrossRef]
- Jasset, O.J.; Lopez Zapana, P.A.; Bahadir, Z.; Shook, L.; Dennis, M.; Gilbert, E.; Liu, Z.A.; Yinger, R.V.; Bald, C.; Bradford, C.G.; et al. Enhanced placental antibody transfer efficiency with longer interval between maternal respiratory syncytial virus vaccination and birth. Am. J. Obstet. Gynecol. 2024, 232, 554.e1–554.e15. [Google Scholar] [CrossRef]
- Jasset, M.O.J.; Andrea Lopez Zapana, P.; Bahadir, Z.; Shook, L.; Dennis, M.M.; Gilbert, M.E.; Liu, M.Z.A.; Yinger, M.R.V.; Bald, M.C.; Bradford, M.C.G.; et al. Longer interval between maternal RSV vaccination and birth increases placental transfer efficiency. medRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Kachikis, A.B.; Rumfelt, K.; Pike, M.; Sosa, M.; Stolarczuk, J.E.; Cho, H.; Eckert, L.O.; Martin, E.T.; Englund, J.A. Transfer of Respiratory Syncytial Virus Prefusion F Protein Antibody in Low Birthweight Infants. Open Forum Infect. Dis. 2024, 11, ofae314. [Google Scholar] [CrossRef]
- Jauniaux, E.; Jurkovic, D.; Gulbis, B.; Liesnard, C.; Lees, C.; Campbell, S. Materno-fetal immunoglobulin transfer and passive immunity during the first trimester of human pregnancy. Hum. Reprod. 1995, 10, 3297–3300. [Google Scholar] [CrossRef]
- Silveira Lessa, A.L.; Krebs, V.L.; Brasil, T.B.; Pontes, G.N.; Carneiro-Sampaio, M.; Palmeira, P. Preterm and term neonates transplacentally acquire IgG antibodies specific to LPS from Klebsiella pneumoniae, Escherichia coli and Pseudomonas aeruginosa. FEMS Immunol. Med. Microbiol. 2011, 62, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Simister, N.E. Placental transport of immunoglobulin G. Vaccine 2003, 21, 3365–3369. [Google Scholar] [CrossRef] [PubMed]
- Kohler, P.F.; Farr, R.S. Elevation of cord over maternal IgG immunoglobulin: Evidence for an active placental IgG transport. Nature 1966, 210, 1070–1071. [Google Scholar] [CrossRef]
- van den Berg, J.P.; Westerbeek, E.A.; Berbers, G.A.; van Gageldonk, P.G.; van der Klis, F.R.; van Elburg, R.M. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr. Infect. Dis. J. 2010, 29, 801–805. [Google Scholar] [CrossRef]
- Eberhardt, C.S.; Blanchard-Rohner, G.; Lemaître, B.; Boukrid, M.; Combescure, C.; Othenin-Girard, V.; Chilin, A.; Petre, J.; de Tejada, B.M.; Siegrist, C.A. Maternal Immunization Earlier in Pregnancy Maximizes Antibody Transfer and Expected Infant Seropositivity Against Pertussis. Clin. Infect. Dis. 2016, 62, 829–836. [Google Scholar] [CrossRef]
- Calvert, A.; Jones, C.E. Placental transfer of antibody and its relationship to vaccination in pregnancy. Curr. Opin. Infect. Dis. 2017, 30, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Calvert, A.; Amirthalingam, G.; Andrews, N.; Basude, S.; Coleman, M.; Cuthbertson, H.; England, A.; Greening, V.; Hallis, B.; Johnstone, E.; et al. Optimising the timing of whooping cough immunisation in mums (OpTIMUM) through investigating pertussis vaccination in pregnancy: An open-label, equivalence, randomised controlled trial. Lancet Microbe 2023, 4, e300–e308. [Google Scholar] [CrossRef]
- Immink, M.M.; Bekker, M.N.; de Melker, H.E.; den Hartog, G.; Rots, N.Y.; van Gageldonk, P.G.M.; Groenendaal, F.; Sanders, E.A.M.; van der Maas, N.A.T. Maternal Pertussis Immunization and Immunoglobulin G Levels in Early- to Late-Term and Preterm Infants. JAMA Netw. Open 2024, 7, e2424608. [Google Scholar] [CrossRef]
- Gomme, J.; Wanlapakorn, N.; Ha, H.T.T.; Leuridan, E.; Herzog, S.A.; Maertens, K. The Impact of Timing of Pertussis Vaccination During Pregnancy on Infant Antibody Levels at Birth: A Multi-Country Analysis. Front. Immunol. 2022, 13, 913922. [Google Scholar] [CrossRef]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Pérez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, L.; Tang, S.; Shi, Y. Efficacy and safety of respiratory syncytial virus vaccination during pregnancy to prevent lower respiratory tract illness in newborns and infants: A systematic review and meta-analysis of randomized controlled trials. Front. Pediatr. 2023, 11, 1260740. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.C.; Marlow, R.; Cunningham, S.; Drysdale, S.B.; Groves, H.E.; Hunt, S.; Iskander, D.; Liu, X.; Lyttle, M.D.; Mpamhanga, C.D.; et al. Bivalent prefusion F vaccination in pregnancy and respiratory syncytial virus hospitalisation in infants in the UK: Results of a multicentre, test-negative, case-control study. Lancet Child Adolesc. Health 2025, 9, 655–662. [Google Scholar] [CrossRef]
- Atyeo, C.G.; Shook, L.L.; Brigida, S.; De Guzman, R.M.; Demidkin, S.; Muir, C.; Akinwunmi, B.; Baez, A.M.; Sheehan, M.L.; McSweeney, E.; et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat. Commun. 2022, 13, 3571. [Google Scholar] [CrossRef]
- Baroncelli, S.; Galluzzo, C.M.; Orlando, S.; Mphwere, R.; Kavalo, T.; Luhanga, R.; Amici, R.; Floridia, M.; Andreotti, M.; Ciccacci, F.; et al. Immunoglobulin G passive transfer from mothers to infants: Total IgG, IgG subclasses and specific antipneumococcal IgG in 6-week Malawian infants exposed or unexposed to HIV. BMC Infect. Dis. 2022, 22, 342. [Google Scholar] [CrossRef]
- Gao, C.; Chen, Q.; Hao, X.; Wang, Q. Immunomodulation of Antibody Glycosylation through the Placental Transfer. Int. J. Mol. Sci. 2023, 24, 16772. [Google Scholar] [CrossRef]
- Qi, T.; Cao, Y. In Translation: FcRn across the Therapeutic Spectrum. Int. J. Mol. Sci. 2021, 22, 3048. [Google Scholar] [CrossRef]
- Hartter, H.K.; Oyedele, O.I.; Dietz, K.; Kreis, S.; Hoffman, J.P.; Muller, C.P. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr. Infect. Dis. J. 2000, 19, 635–641. [Google Scholar] [CrossRef]
- Stach, S.C.; Brizot, M.L.; Liao, A.W.; Palmeira, P.; Francisco, R.P.; Carneiro-Sampaio, M.M.; Zugaib, M. Placental transfer of IgG antibodies specific to Klebsiella and Pseudomonas LPS and to group B Streptococcus in twin pregnancies. Scand. J. Immunol. 2015, 81, 135–141. [Google Scholar] [CrossRef]
- Kardava, L.; Moir, S. B-cell abnormalities in HIV-1 infection: Roles for IgG3 and T-bet. Curr. Opin. HIV AIDS 2019, 14, 240–245. [Google Scholar] [CrossRef]
- Stögerer, T.; Silva-Barrios, S.; Carmona-Pérez, L.; Swaminathan, S.; Mai, L.T.; Leroux, L.P.; Jaramillo, M.; Descoteaux, A.; Stäger, S. Leishmania donovani Exploits Tunneling Nanotubes for Dissemination and Propagation of B Cell Activation. Microbiol. Spectr. 2023, 11, e0509622. [Google Scholar] [CrossRef] [PubMed]
- Greczmiel, U.; Kräutler, N.J.; Borsa, M.; Pedrioli, A.; Bartsch, I.; Richter, K.; Agnellini, P.; Bedenikovic, G.; Oxenius, A. LCMV-specific CD4 T cell dependent polyclonal B-cell activation upon persistent viral infection is short lived and extrafollicular. Eur. J. Immunol. 2020, 50, 396–403. [Google Scholar] [CrossRef]
- Gregorek, H.; Jankowska, I.; Dzierzanowska-Fangrat, K.; Teisseyre, J.; Sawicka, A.; Kasztelewicz, B.; Pawłowska, J. Long-term monitoring of Epstein-Barr virus DNA load and humoral parameter abnormalities in pediatric liver transplant recipients before development of malignancy. Pediatr. Transplant. 2010, 14, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhang, Q.; Xu, S.; Yin, T.; Li, F. Successful treatment of refractory retroperitoneal Epstein-Barr virus-positive diffuse large B-cell lymphoma with secondary hemophagocytic syndrome by sequential combination regimen of PD-1 blockade and chimeric antigen receptor T cells: A case report. Anticancer Drugs 2022, 33, e769–e775. [Google Scholar] [CrossRef]
- Atwell, J.E.; Thumar, B.; Robinson, L.J.; Tobby, R.; Yambo, P.; Ome-Kaius, M.; Siba, P.M.; Unger, H.W.; Rogerson, S.J.; King, C.L.; et al. Impact of Placental Malaria and Hypergammaglobulinemia on Transplacental Transfer of Respiratory Syncytial Virus Antibody in Papua New Guinea. J. Infect. Dis. 2016, 213, 423–431. [Google Scholar] [CrossRef]
- Pou, C.; Nkulikiyimfura, D.; Henckel, E.; Olin, A.; Lakshmikanth, T.; Mikes, J.; Wang, J.; Chen, Y.; Bernhardsson, A.K.; Gustafsson, A.; et al. The repertoire of maternal anti-viral antibodies in human newborns. Nat. Med. 2019, 25, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Henrie, R.; Cherniawsky, H.; Marcon, K.; Zhao, E.J.; Marinkovic, A.; Pourshahnazari, P.; Parkin, S.; Chen, L.Y.C. Inflammatory diseases in hematology: A review. Am. J. Physiol. Cell Physiol. 2022, 323, C1121–C1136. [Google Scholar] [CrossRef]
- Coler, C.; King-Nakaoka, E.; Every, E.; Chima, S.; Vong, A.; Del Rosario, B.; VanAbel, R.; Adams Waldorf, K.M. Impact of Infections During Pregnancy on Transplacental Antibody Transfer. Vaccines 2024, 12, 1199. [Google Scholar] [CrossRef]
- De Milito, A.; Nilsson, A.; Titanji, K.; Thorstensson, R.; Reizenstein, E.; Narita, M.; Grutzmeier, S.; Sönnerborg, A.; Chiodi, F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 2004, 103, 2180–2186. [Google Scholar] [CrossRef]
- Mok, M.Y. The immunological basis of B-cell therapy in systemic lupus erythematosus. Int. J. Rheum. Dis. 2010, 13, 3–11. [Google Scholar] [CrossRef]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Anunciación-Llunell, A.; Marques-Soares, J.; Pardos-Gea, J.; Miró-Mur, F. Pathogenesis, Diagnosis and Management of Obstetric Antiphospholipid Syndrome: A Comprehensive Review. J. Clin. Med. 2022, 11, 675. [Google Scholar] [CrossRef]
- Dzirba, D.; Glinko, M.; Skoczyńska, M.; Gruszecka, K.; Trzeszcz, M.; Benedyczak, A.; Szmyrka, M. Placental Pathology in Obstetric Antiphospholipid Syndrome Beyond Thrombosis: A Case Report and Literature Review. J. Clin. Med. 2025, 14, 5172. [Google Scholar] [CrossRef]
- Abrahams, V.M. Mechanisms of antiphospholipid antibody-associated pregnancy complications. Thromb. Res. 2009, 124, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Truglia, S.; Riitano, G.; Mancuso, S.; Recalchi, S.; Rapino, L.; Garufi, C.; Manganelli, V.; Garofalo, T.; Misasi, R.; Alessandri, C.; et al. Antibody profiles in the mosaic of ‘seronegative’ APS syndrome. Clin. Exp. Immunol. 2024, 218, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Riitano, G.; Capozzi, A.; Recalchi, S.; Augusto, M.; Conti, F.; Misasi, R.; Garofalo, T.; Sorice, M.; Manganelli, V. Role of Lipid Rafts on LRP8 Signaling Triggered by Anti-β2-GPI Antibodies in Endothelial Cells. Biomedicines 2023, 11, 3135. [Google Scholar] [CrossRef]
- Marani, M.; Katul, G.G.; Pan, W.K.; Parolari, A.J. Intensity and frequency of extreme novel epidemics. Proc. Natl. Acad. Sci. USA 2021, 118, e2105482118, Correction in Proc. Natl. Acad. Sci. USA 2023, 120, e2302169120. https://doi.org/10.1073/pnas.2302169120. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Malaspina, A.; Ho, J.; Wang, W.; Dipoto, A.C.; O’Shea, M.A.; Roby, G.; Mican, J.M.; Kottilil, S.; Chun, T.W.; et al. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J. Infect. Dis. 2008, 197, 572–579. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Dugast, A.S.; McAndrew, E.G.; Tsoukas, S.; Licht, A.F.; Irvine, D.J.; Alter, G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J. Virol. 2013, 87, 5468–5476. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Sharma, S.; Remmel, C.A.L.; Holder, B.; Jones, C.E.; Marchant, A.; Ackerman, M.E. HIV-Associated Alterations of the Biophysical Features of Maternal Antibodies Correlate With Their Reduced Transfer Across the Placenta. J. Infect. Dis. 2022, 226, 1441–1450. [Google Scholar] [CrossRef]
- Crocker, I.P.; Tanner, O.M.; Myers, J.E.; Bulmer, J.N.; Walraven, G.; Baker, P.N. Syncytiotrophoblast degradation and the pathophysiology of the malaria-infected placenta. Placenta 2004, 25, 273–282. [Google Scholar] [CrossRef]
- Elphinstone, R.E.; Weckman, A.M.; McDonald, C.R.; Tran, V.; Zhong, K.; Madanitsa, M.; Kalilani-Phiri, L.; Khairallah, C.; Taylor, S.M.; Meshnick, S.R.; et al. Early malaria infection, dysregulation of angiogenesis, metabolism and inflammation across pregnancy, and risk of preterm birth in Malawi: A cohort study. PLoS Med. 2019, 16, e1002914. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.L.; Nyengaard, J.R.; Larsen, L.G.; Nielsen, K.; Bygbjerg, I.C.; Msemo, O.A.; Lusingu, J.P.A.; Minja, D.T.R.; Theander, T.G.; Schmiegelow, C. Malaria in Early Pregnancy and the Development of the Placental Vasculature. J. Infect. Dis. 2019, 220, 1425–1434. [Google Scholar] [CrossRef]
- Chaikitgosiyakul, S.; Rijken, M.J.; Muehlenbachs, A.; Lee, S.J.; Chaisri, U.; Viriyavejakul, P.; Turner, G.D.; Pongponratn, E.; Nosten, F.; McGready, R. A morphometric and histological study of placental malaria shows significant changes to villous architecture in both Plasmodium falciparum and Plasmodium vivax infection. Malar. J. 2014, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; Pullen, K.M.; Bordt, E.A.; Fischinger, S.; Burke, J.; Michell, A.; Slein, M.D.; Loos, C.; Shook, L.L.; Boatin, A.A.; et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell 2021, 184, 628–642.e610. [Google Scholar] [CrossRef]
- Lucot-Royer, L.; Nallet, C.; Vouga, M.; Puyraveau, M.; Mauny, F.; Marty-Quinternet, S.; Bertholdt, C.; Bory, J.P.; Devalland, C.; Canaguier, M.; et al. Analysis of the transplacental transmission of SARS CoV-2 virus and antibody transfer according to the gestational age at maternal infection. Sci. Rep. 2024, 14, 3458. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Smolen, K.K.; Willems, F.; Kollmann, T.R.; Marchant, A. Transfer of Maternal Antimicrobial Immunity to HIV-Exposed Uninfected Newborns. Front. Immunol. 2016, 7, 338. [Google Scholar] [CrossRef]
- Atwell, J.E.; Lutz, C.S.; Sparrow, E.G.; Feikin, D.R. Biological factors that may impair transplacental transfer of RSV antibodies: Implications for maternal immunization policy and research priorities for low- and middle-income countries. Vaccine 2022, 40, 4361–4370. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Crispin, M.; Yu, X.; Baruah, K.; Boesch, A.W.; Harvey, D.J.; Dugast, A.S.; Heizen, E.L.; Ercan, A.; Choi, I.; et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Investig. 2013, 123, 2183–2192. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Marchant, A.; Way, S.S. Vaccination strategies to enhance immunity in neonates. Science 2020, 368, 612–615. [Google Scholar] [CrossRef]
- Miner, J.J.; Cao, B.; Govero, J.; Smith, A.M.; Fernandez, E.; Cabrera, O.H.; Garber, C.; Noll, M.; Klein, R.S.; Noguchi, K.K.; et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell 2016, 165, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.J.; Kumar, A.; Giorgi, E.E.; Eudailey, J.; LaBranche, C.C.; Martinez, D.R.; Fouda, G.G.; Moreau, Y.; Thomas, A.; Montefiori, D.; et al. Vertical HIV-1 Transmission in the Setting of Maternal Broad and Potent Antibody Responses. J. Virol. 2022, 96, e0023122. [Google Scholar] [CrossRef]
- Dauby, N.; Gagneux-Brunon, A.; Martin, C.; Mussi-Pinhata, M.M.; Goetghebuer, T. Maternal immunization in women living with HIV. Aids 2024, 38, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Dangor, Z.; Nunes, M.C.; Kwatra, G.; Lala, S.G.; Madhi, S.A. Vaccination of HIV-infected pregnant women: Implications for protection of their young infants. Trop. Dis. Travel. Med. Vaccines 2017, 3, 1. [Google Scholar] [CrossRef]
- Taton, M.; Willems, F.; Widomski, C.; Georges, D.; Martin, C.; Jiang, Y.; Renard, K.; Konopnicki, D.; Cogan, A.; Necsoi, C.; et al. HIV-related immune activation attenuates polyfunctional IgG and memory B-cell responses to Tdap immunization during pregnancy. eBioMedicine 2024, 104, 105179. [Google Scholar] [CrossRef] [PubMed]
- Moir, S.; Fauci, A.S. B-cell exhaustion in HIV infection: The role of immune activation. Curr. Opin. HIV AIDS 2014, 9, 472–477. [Google Scholar] [CrossRef]
- Golay, J.; Andrea, A.E.; Cattaneo, I. Role of Fc Core Fucosylation in the Effector Function of IgG1 Antibodies. Front. Immunol. 2022, 13, 929895. [Google Scholar] [CrossRef]
- Zakama, A.K.; Ozarslan, N.; Gaw, S.L. Placental Malaria. Curr. Trop. Med. Rep. 2020, 7, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ogolla, S.; Daud, I.I.; Asito, A.S.; Sumba, O.P.; Ouma, C.; Vulule, J.; Middeldorp, J.M.; Dent, A.E.; Mehta, S.; Rochford, R. Reduced Transplacental Transfer of a Subset of Epstein-Barr Virus-Specific Antibodies to Neonates of Mothers Infected with Plasmodium falciparum Malaria during Pregnancy. Clin. Vaccine Immunol. 2015, 22, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Seyed Hosseini, E.; Riahi Kashani, N.; Nikzad, H.; Azadbakht, J.; Hassani Bafrani, H.; Haddad Kashani, H. The novel coronavirus Disease-2019 (COVID-19): Mechanism of action, detection and recent therapeutic strategies. Virology 2020, 551, 1–9. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef]
- Mourad, M.; Jacob, T.; Sadovsky, E.; Bejerano, S.; Simone, G.S.; Bagalkot, T.R.; Zucker, J.; Yin, M.T.; Chang, J.Y.; Liu, L.; et al. Placental response to maternal SARS-CoV-2 infection. Sci. Rep. 2021, 11, 14390. [Google Scholar] [CrossRef]
- Gee, S.; Chandiramani, M.; Seow, J.; Pollock, E.; Modestini, C.; Das, A.; Tree, T.; Doores, K.J.; Tribe, R.M.; Gibbons, D.L. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat. Immunol. 2021, 22, 1490–1502. [Google Scholar] [CrossRef]
- Gao, L.; Ren, J.; Xu, L.; Ke, X.; Xiong, L.; Tian, X.; Fan, C.; Yan, H.; Yuan, J. Placental pathology of the third trimester pregnant women from COVID-19. Diagn. Pathol. 2021, 16, 8. [Google Scholar] [CrossRef]
- Chucri, T.M.; Monteiro, J.M.; Lima, A.R.; Salvadori, M.L.; Kfoury, J.R., Jr.; Miglino, M.A. A review of immune transfer by the placenta. J. Reprod. Immunol. 2010, 87, 14–20. [Google Scholar] [CrossRef]
- Partey, F.D.; Obiri, D.; Bonney, E.Y.; Pobee, A.N.A.; Damptey, I.K.; Ennuson, K.; Akwetea-Foli, J.; Nuokpem, F.Y.; Courtin, D.; Kusi, K.A.; et al. Efficient transplacental transfer of SARS-CoV-2 antibodies between naturally exposed mothers and infants in Accra, Ghana. Sci. Rep. 2024, 14, 10772. [Google Scholar] [CrossRef] [PubMed]
- Trinité, B.; Tarrés-Freixas, F.; Rodon, J.; Pradenas, E.; Urrea, V.; Marfil, S.; Rodríguez de la Concepción, M.L.; Ávila-Nieto, C.; Aguilar-Gurrieri, C.; Barajas, A.; et al. SARS-CoV-2 infection elicits a rapid neutralizing antibody response that correlates with disease severity. Sci. Rep. 2021, 11, 2608. [Google Scholar] [CrossRef]
- Song, D.; Prahl, M.; Gaw, S.L.; Narasimhan, S.R.; Rai, D.S.; Huang, A.; Flores, C.V.; Lin, C.Y.; Jigmeddagva, U.; Wu, A.; et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open 2021, 11, e053036. [Google Scholar] [CrossRef] [PubMed]
- Beharier, O.; Plitman Mayo, R.; Raz, T.; Nahum Sacks, K.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131, e150319, Correction in J. Clin. Investig. 2021, 131, e154834. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, H.D.; Fuglsang, J.; Kampmann, U.; Knorr, S.; Ovesen, P. Hyperglycemia in Pregnancy and Women’s Health in the 21st Century. Int. J. Environ. Res. Public Health 2022, 19, 16827. [Google Scholar] [CrossRef]
- de Souza, E.G.; Hara, C.C.; Fagundes, D.L.; de Queiroz, A.A.; Morceli, G.; Calderon, I.M.; França, E.L.; Honorio-França, A.C. Maternal-Foetal Diabetes Modifies Neonatal Fc Receptor Expression on Human Leucocytes. Scand. J. Immunol. 2016, 84, 237–244. [Google Scholar] [CrossRef]
- Calderon, I.M.; Damasceno, D.C.; Amorin, R.L.; Costa, R.A.; Brasil, M.A.; Rudge, M.V. Morphometric study of placental villi and vessels in women with mild hyperglycemia or gestational or overt diabetes. Diabetes Res. Clin. Pract. 2007, 78, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Wong, I.; Moller, A.; Giachini, F.R.; Lima, V.V.; Toledo, F.; Stojanova, J.; Sobrevia, L.; San Martín, S. Placental structure in gestational diabetes mellitus. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165535. [Google Scholar] [CrossRef]
- Hammes, H.P.; Kiefel, V.; Laube, H.; Federlin, K. Impaired agglutination of IgM resulting from non-enzymatic glycation in diabetes mellitus. Diabetes Res. Clin. Pract. 1990, 9, 37–42. [Google Scholar] [CrossRef]
- Perween, S.; Abidi, M.; Faiz Faizy, A.; Moinuddin. Biophysical changes in methylglyoxal modified fibrinogen and its role in the immunopathology of type 2 diabetes mellitus. Int. J. Biol. Macromol. 2022, 202, 199–214. [Google Scholar] [CrossRef]
- Goetze, A.M.; Liu, Y.D.; Arroll, T.; Chu, L.; Flynn, G.C. Rates and impact of human antibody glycation in vivo. Glycobiology 2012, 22, 221–234. [Google Scholar] [CrossRef]
- Štambuk, T.; Kifer, D.; Smirčić-Duvnjak, L.; Vučić Lovrenčić, M.; Gornik, O. Associations between plasma protein, IgG and IgA N-glycosylation and metabolic health markers in pregnancy and gestational diabetes. PLoS ONE 2023, 18, e0284838. [Google Scholar] [CrossRef]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Shepard, H.M.; Phillips, G.L.; Thanos, C.D.; Feldmann, M. Developments in therapy with monoclonal antibodies and related proteins. Clin. Med. 2017, 17, 220–232. [Google Scholar] [CrossRef]
- Pham-Huy, A.; Top, K.A.; Constantinescu, C.; Seow, C.H.; El-Chaâr, D. The use and impact of monoclonal antibody biologics during pregnancy. Can. Med Assoc. J. 2021, 193, E1129–E1136. [Google Scholar] [CrossRef]
- Reusch, J.; Andersen, J.T.; Rant, U.; Schlothauer, T. Insight into the avidity-affinity relationship of the bivalent, pH-dependent interaction between IgG and FcRn. mAbs 2024, 16, 2361585. [Google Scholar] [CrossRef]
- Suzuki, T.; Hashii, N.; Tada, M.; Ishii-Watabe, A. The influence of antibody engineering on Fc conformation and Fc receptor binding properties: Analysis of FcRn-binding engineered antibodies and an Fc fusion protein. mAbs 2021, 13, 1923366. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.; Jo, M.; Kyung, M.; Lee, W.; Ko, W.H.; Na, J.H.; Chun, Y.S.; Ko, B.J.; Jung, S.T. Engineering FcRn binding kinetics dramatically extends antibody serum half-life and enhances therapeutic potential. J. Biol. Eng. 2025, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, U.; Long, M.D.; Kane, S.V.; Roy, A.; Dubinsky, M.C.; Sands, B.E.; Cohen, R.D.; Chambers, C.D.; Sandborn, W.J. Pregnancy and Neonatal Outcomes After Fetal Exposure to Biologics and Thiopurines Among Women With Inflammatory Bowel Disease. Gastroenterology 2021, 160, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Hay, S.; Burchett, S.; Odejide, O.; Cataltepe, S. Septic Episodes in a Premature Infant After In Utero Exposure to Rituximab. Pediatrics 2017, 140, e20162819. [Google Scholar] [CrossRef]
- Chakravarty, E.F.; Murray, E.R.; Kelman, A.; Farmer, P. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011, 117, 1499–1506. [Google Scholar] [CrossRef]
- Mandal, P.K.; Dolai, T.K.; Bagchi, B.; Ghosh, M.K.; Bose, S.; Bhattacharyya, M. B cell suppression in newborn following treatment of pregnant diffuse large B-cell lymphoma patient with rituximab containing regimen. Indian J. Pediatr. 2014, 81, 1092–1094. [Google Scholar] [CrossRef]
- Chaparro, M.; Verreth, A.; Lobaton, T.; Gravito-Soares, E.; Julsgaard, M.; Savarino, E.; Magro, F.; Biron, A.I.; Lopez-Serrano, P.; Casanova, M.J.; et al. Long-Term Safety of In Utero Exposure to Anti-TNFα Drugs for the Treatment of Inflammatory Bowel Disease: Results from the Multicenter European TEDDY Study. Am. J. Gastroenterol. 2018, 113, 396–403. [Google Scholar] [CrossRef]
- Komaki, F.; Komaki, Y.; Micic, D.; Ido, A.; Sakuraba, A. Outcome of pregnancy and neonatal complications with anti-tumor necrosis factor-α use in females with immune mediated diseases; a systematic review and meta-analysis. J. Autoimmun. 2017, 76, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Bröms, G.; Kieler, H.; Ekbom, A.; Gissler, M.; Hellgren, K.; Lahesmaa-Korpinen, A.M.; Pedersen, L.; Schmitt-Egenolf, M.; Sørensen, H.T.; Granath, F. Anti-TNF treatment during pregnancy and birth outcomes: A population-based study from Denmark, Finland, and Sweden. Pharmacoepidemiol. Drug Saf. 2020, 29, 316–327. [Google Scholar] [CrossRef]
- Ward, E.S.; Ober, R.J. Targeting FcRn to Generate Antibody-Based Therapeutics. Trends Pharmacol. Sci. 2018, 39, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; Chaparro, M. Vaccines in Children Exposed to Biological Agents In Utero and/or During Breastfeeding: Are They Effective and Safe? J. Crohns Colitis 2023, 17, 995–1009. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Damotte, V.; Gelfand, J.M.; Bevan, C.; Cree, B.A.C.; Do, L.; Green, A.J.; Hauser, S.L.; Bove, R. Rituximab before and during pregnancy: A systematic review, and a case series in MS and NMOSD. Neurol. Neuroimmunol. Neuroinflamm. 2018, 5, e453. [Google Scholar] [CrossRef]
- Dernoncourt, A.; Liabeuf, S.; Bennis, Y.; Masmoudi, K.; Bodeau, S.; Laville, S.; Hurtel-Lemaire, A.S.; Gras-Champel, V.; Batteux, B. Fetal and Neonatal Adverse Drug Reactions Associated with Biologics Taken During Pregnancy by Women with Autoimmune Diseases: Insights from an Analysis of the World Health Organization Pharmacovigilance Database (VigiBase®). BioDrugs 2023, 37, 73–87. [Google Scholar] [CrossRef]
- Goulden, B.; Chua, N.; Parker, E.; Giles, I. P202 Administering live vaccines to infants exposed to biologic and targeted synthetic DMARDs in-utero for maternal treatment of rheumatic disease: A systematic review of the literature. Rheumatology 2021, 60, keab247.197. [Google Scholar] [CrossRef]
- Ghalandari, N.; Dolhain, R.; Hazes, J.M.W.; van Puijenbroek, E.P.; Kapur, M.; Crijns, H. Intrauterine Exposure to Biologics in Inflammatory Autoimmune Diseases: A Systematic Review. Drugs 2020, 80, 1699–1722. [Google Scholar] [CrossRef]
- Han, L.; Hou, Z.; Li, J.; Wang, Y.; Li, H. Biologic therapy for immune-mediated inflammatory diseases during pregnancy and lactation: Efficacy, safety, and challenges. Precis. Medicat. 2025, 2, 100029. [Google Scholar] [CrossRef]
- Götestam Skorpen, C.; Hoeltzenbein, M.; Tincani, A.; Fischer-Betz, R.; Elefant, E.; Chambers, C.; da Silva, J.; Nelson-Piercy, C.; Cetin, I.; Costedoat-Chalumeau, N.; et al. The EULAR points to consider for use of antirheumatic drugs before pregnancy, and during pregnancy and lactation. Ann. Rheum. Dis. 2016, 75, 795–810. [Google Scholar] [CrossRef]
- Lowe, D.E.; Robbins, J.R.; Bakardjiev, A.I. Animal and Human Tissue Models of Vertical Listeria monocytogenes Transmission and Implications for Other Pregnancy-Associated Infections. Infect. Immun. 2018, 86, e00801-17. [Google Scholar] [CrossRef]
- Hemberger, M.; Hanna, C.W.; Dean, W. Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 2020, 21, 27–43. [Google Scholar] [CrossRef]
- Warang, P.; Singh, G.; Moshir, M.; Binazon, O.; Laghlali, G.; Chang, L.A.; Wouters, H.; Vanhoenacker, P.; Notebaert, M.; Elhemdaoui, N.; et al. Impact of FcRn antagonism on vaccine-induced protective immune responses against viral challenge in COVID-19 and influenza mouse vaccination models. Hum. Vaccin. Immunother. 2025, 21, 2470542. [Google Scholar] [CrossRef]
- Proetzel, G.; Roopenian, D.C. Humanized FcRn mouse models for evaluating pharmacokinetics of human IgG antibodies. Methods 2014, 65, 148–153. [Google Scholar] [CrossRef]
- Roopenian, D.C.; Christianson, G.J.; Sproule, T.J.; Brown, A.C.; Akilesh, S.; Jung, N.; Petkova, S.; Avanessian, L.; Choi, E.Y.; Shaffer, D.J.; et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J. Immunol. 2003, 170, 3528–3533. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mohanty, S.; Ganesan, L.P.; Hua, K.; Jarjoura, D.; Hayton, W.L.; Robinson, J.M.; Anderson, C.L. FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J. Immunol. 2009, 182, 2583–2589. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, I.; Rothe, A.; Grill, M.; Fuchs, R. Apical to basolateral transcytosis and apical recycling of immunoglobulin G in trophoblast-derived BeWo cells: Effects of low temperature, nocodazole, and cytochalasin D. Exp. Cell Res. 2001, 269, 322–331. [Google Scholar] [CrossRef]
- Ellinger, I.; Schwab, M.; Stefanescu, A.; Hunziker, W.; Fuchs, R. IgG transport across trophoblast-derived BeWo cells: A model system to study IgG transport in the placenta. Eur. J. Immunol. 1999, 29, 733–744. [Google Scholar] [CrossRef]
- Mathiesen, L.; Mørck, T.A.; Zuri, G.; Andersen, M.H.; Pehrson, C.; Frederiksen, M.; Mose, T.; Rytting, E.; Poulsen, M.S.; Nielsen, J.K.; et al. Modelling of human transplacental transport as performed in Copenhagen, Denmark. Basic. Clin. Pharmacol. Toxicol. 2014, 115, 93–100. [Google Scholar] [CrossRef]
- Hori, T.; Okae, H.; Shibata, S.; Kobayashi, N.; Kobayashi, E.H.; Oike, A.; Sekiya, A.; Arima, T.; Kaji, H. Trophoblast stem cell-based organoid models of the human placental barrier. Nat. Commun. 2024, 15, 962. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Armstrong-Fisher, S.; Kopotsha, T.; Smith, B.; Baker, T.; Kevorkian, L.; Nesbitt, A. Certolizumab pegol does not bind the neonatal Fc receptor (FcRn): Consequences for FcRn-mediated in vitro transcytosis and ex vivo human placental transfer. J. Reprod. Immunol. 2016, 116, 7–12. [Google Scholar] [CrossRef]
- Viall, C.A.; Chen, Q.; Liu, B.; Hickey, A.; Snowise, S.; Salmon, J.E.; Stone, P.R.; Chamley, L.W. Antiphospholipid antibodies internalised by human syncytiotrophoblast cause aberrant cell death and the release of necrotic trophoblast debris. J. Autoimmun. 2013, 47, 45–57. [Google Scholar] [CrossRef]
- Roy, S.; Nanovskaya, T.; Patrikeeva, S.; Cochran, E.; Parge, V.; Guess, J.; Schaeck, J.; Choudhury, A.; Ahmed, M.; Ling, L.E. M281, an anti-FcRn antibody, inhibits IgG transfer in a human ex vivo placental perfusion model. Am. J. Obstet. Gynecol. 2019, 220, 498.e491–498.e499. [Google Scholar] [CrossRef]
- Dusza, H.M.; van Boxel, J.; van Duursen, M.B.M.; Forsberg, M.M.; Legler, J.; Vähäkangas, K.H. Experimental human placental models for studying uptake, transport and toxicity of micro- and nanoplastics. Sci. Total Environ. 2023, 860, 160403. [Google Scholar] [CrossRef]
- Myllynen, P.; Vähäkangas, K. Placental transfer and metabolism: An overview of the experimental models utilizing human placental tissue. Toxicol. In Vitro 2013, 27, 507–512. [Google Scholar] [CrossRef]
- Wessel, R.E.; Dolatshahi, S. Quantitative mechanistic model reveals key determinants of placental IgG transfer and informs prenatal immunization strategies. PLoS Comput. Biol. 2023, 19, e1011109. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, F.; Wang, H.; Wang, L.; Yuan, J.; Qin, J. Placental Barrier-on-a-Chip: Modeling Placental Inflammatory Responses to Bacterial Infection. ACS Biomater. Sci. Eng. 2018, 4, 3356–3363. [Google Scholar] [CrossRef]
- Lee, J.S.; Romero, R.; Han, Y.M.; Kim, H.C.; Kim, C.J.; Hong, J.S.; Huh, D. Placenta-on-a-chip: A novel platform to study the biology of the human placenta. J. Matern. Fetal Neonatal Med. 2016, 29, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, J.I.; Bollini, M.; Cavasotto, C.N. A Machine Learning Model to Predict Drug Transfer Across the Human Placenta Barrier. Front. Chem. 2021, 9, 714678. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, N.M.; Armstrong-Fisher, S.S.; Andersen, J.T.; van der Schoot, C.E.; Porter, C.; Page, K.R.; Falconer, D.; de Haas, M.; Williamson, L.M.; Clark, M.R.; et al. Human IgG lacking effector functions demonstrate lower FcRn-binding and reduced transplacental transport. Mol. Immunol. 2018, 95, 1–9. [Google Scholar] [CrossRef]
- Sand, K.M.K.; Gruber, M.M.; Sandlie, I.; Mathiesen, L.; Andersen, J.T.; Wadsack, C. Contribution of the ex vivo placental perfusion model in understanding transplacental immunoglobulin G transfer. Placenta 2022, 127, 77–87. [Google Scholar] [CrossRef]
- Wang, J.; Han, T.; Zhu, X. Role of maternal-fetal immune tolerance in the establishment and maintenance of pregnancy. Chin. Med. J. 2024, 137, 1399–1406. [Google Scholar] [CrossRef]
- Zalevsky, J.; Chamberlain, A.K.; Horton, H.M.; Karki, S.; Leung, I.W.; Sproule, T.J.; Lazar, G.A.; Roopenian, D.C.; Desjarlais, J.R. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010, 28, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Peter, H.H.; Ochs, H.D.; Cunningham-Rundles, C.; Vinh, D.C.; Kiessling, P.; Greve, B.; Jolles, S. Targeting FcRn for immunomodulation: Benefits, risks, and practical considerations. J. Allergy Clin. Immunol. 2020, 146, 479–491.e475. [Google Scholar] [CrossRef] [PubMed]
- Wessel, R.E.; Dolatshahi, S. Regulators of placental antibody transfer through a modeling lens. Nat. Immunol. 2024, 25, 2024–2036. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.