Characterization of the Meiobenthic Community Inhabiting the Zwin Coastal Lagoon (Belgium, the Netherlands) and the Role of the Sedimentary Environment
Abstract
1. Introduction
2. Materials and Methods
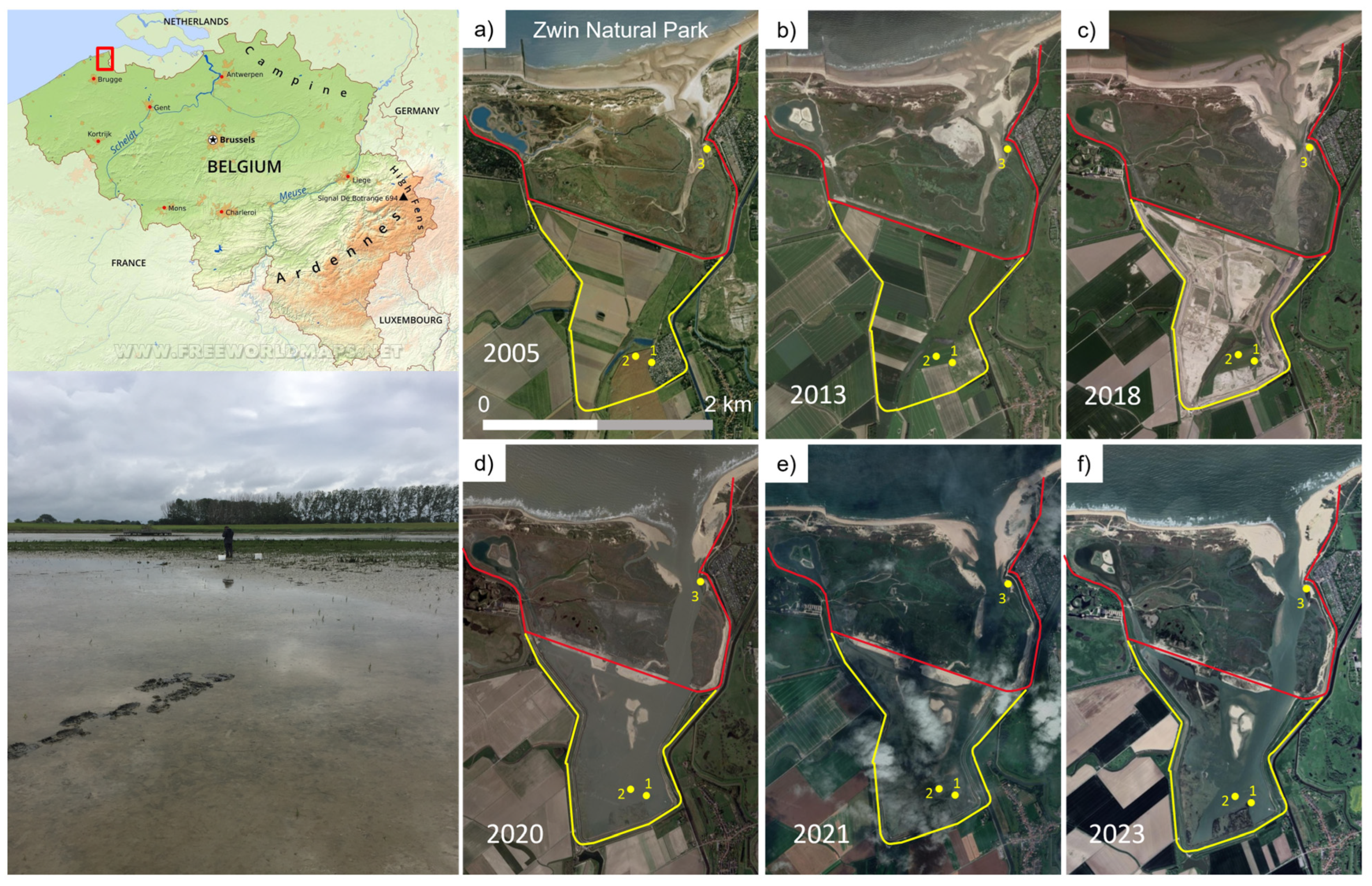
2.1. The Study Area
2.2. The Field Sampling Strategy
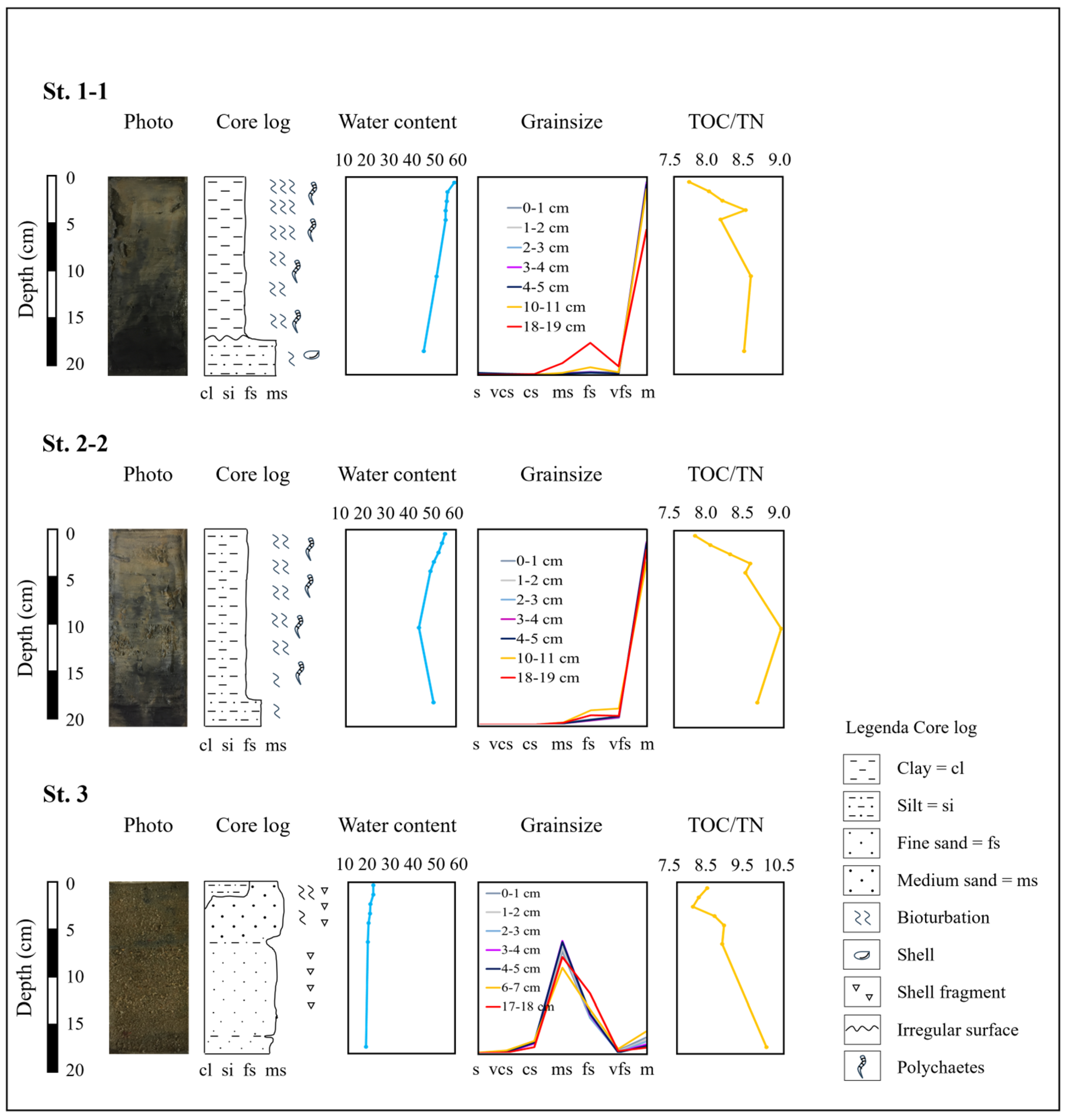
2.3. The Sedimentological Analysis
2.4. Grain-Size Analyses
2.5. TOC, TN, and Stable Isotope Analyses
2.6. Organic Matter and Chlorophyll-a Analyses
2.7. Meiofaunal Analyses
2.8. Statistical Analyses
3. Results
3.1. Environmental Variables
3.2. The Meiofaunal Community
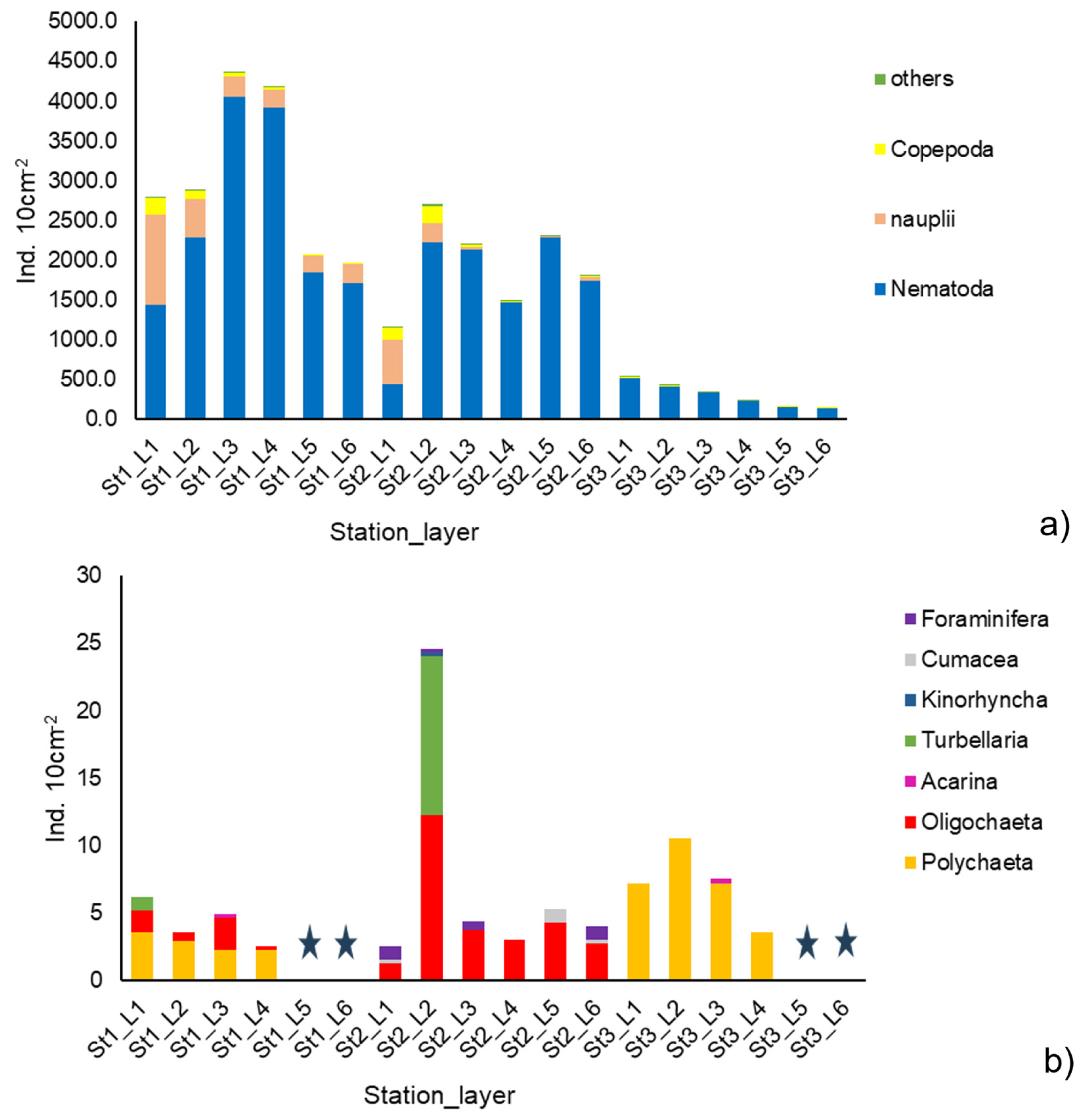
3.2.1. Meiofaunal Abundance and Diversity
3.2.2. Meiofaunal Community Composition
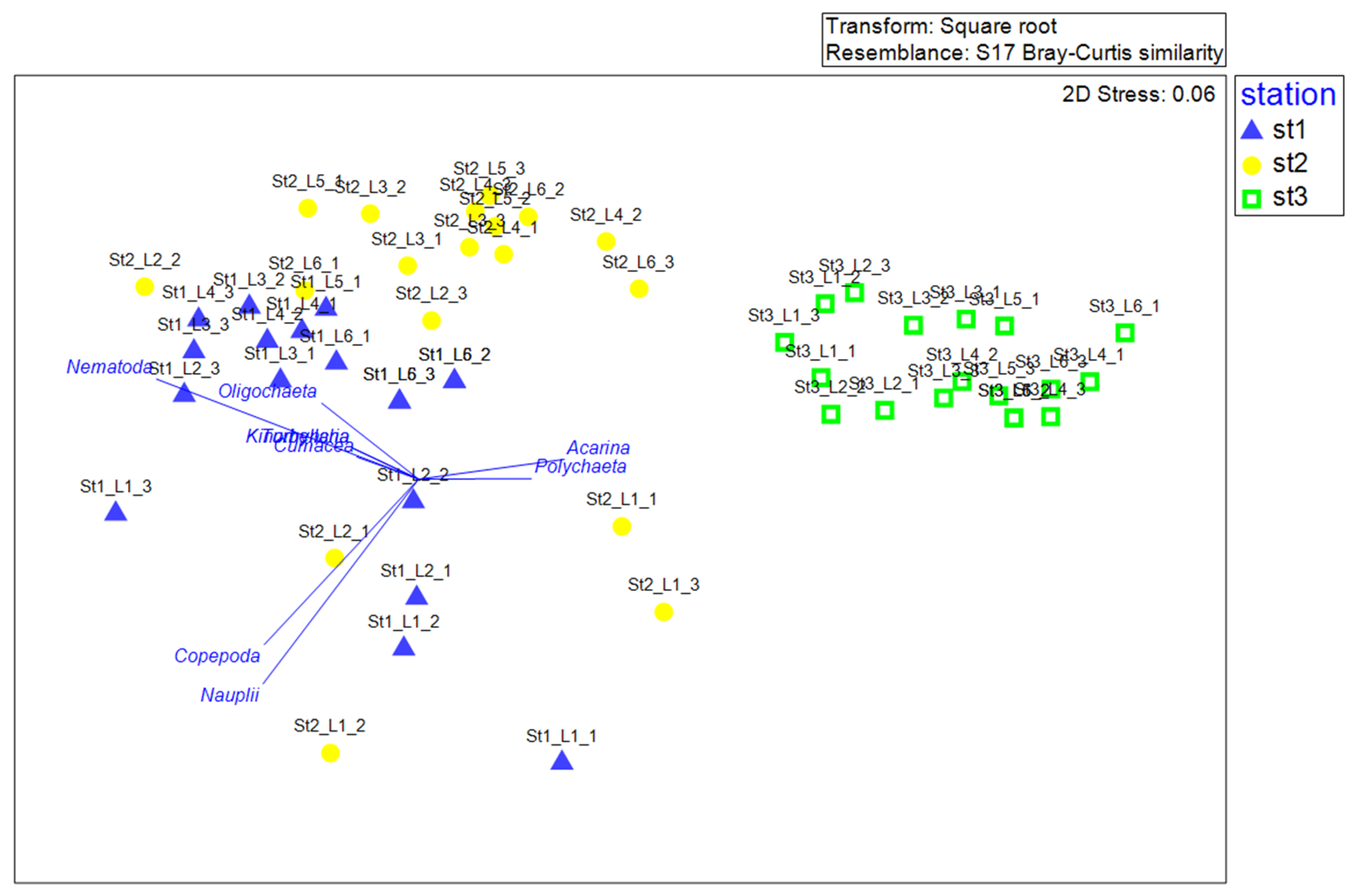
3.2.3. Linking Biological Communities to Environmental Variables
4. Discussion and Conclusions
4.1. Environmental Characterization of the Zwin Natural Park
4.2. The Meiofaunal Communities Inhabiting the Zwin Natural Park: An Anthropogenically Created Lagoon
4.3. The Influence of the Depositional Environment on the Meiobenthos
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vanden Eede, S.; Laporta, L.; Deneudt, K.; Stienen, E.; Derous, S.; Degraer, S.; Vincx, M. Marine biological valuation of the shallow Belgian coastal zone: A space-use conflict example within the context of marine spatial planning. Ocean Coast. Manag. 2014, 96, 61–72. [Google Scholar] [CrossRef][Green Version]
- Derous, S.; Agardy, T.; Hillewaert, H.; Hostens, K.; Jamieson, G.; Lieberknecht, L.; Mees, J.; Moulaert, I.; Olenin, S.; Paelinckx, D.; et al. A concept for biological valuation in the marine environment. Oceanologia 2007, 49, 99e128. Available online: https://gs.elaba.lt/object/elaba:2433053/ (accessed on 7 September 2025).
- Duck, R.W.; da Silva, J.F. Coastal lagoons and their evolution: A hydromorphological perspective. Estuar. Coast. Shelf Sci. 2012, 110, 2–14. [Google Scholar] [CrossRef]
- Beaumont, N.J.; Austen, M.C.; Atkins, J.P.; Burdon, D.; Degraer, S.; Dentinho, T.P.; Derous, S.; Holm, P.; Horton, T.; van Ierland, E.; et al. Identification, definition and quantification of goods and services provided by marine biodiversity: Implications for the ecosystem approach. Mar. Pollut. Bull. 2007, 54, 253–265. [Google Scholar] [CrossRef]
- Beaumont, N.J.; Austen, M.C.; Mangi, S.C.; Townsend, M. Economic valuation for the conservation of marine biodiversity. Mar. Pollut. Bull. 2008, 56, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Magni, P.; Semprucci, F.; Gravina, M.F. Joint analysis of macrofaunal and meiofaunal assemblages improves the assessment of lagoonal environmental heterogeneity. Estuar. Coast. Shelf Sci. 2022, 266, 107740. [Google Scholar] [CrossRef]
- Plecha, S.; Silva, P.A.; Oliveira, A.; Días, J.M. Establishing the wave climate influence on the morphodynamics of a coastal lagoon inlet. Ocean Dyn. 2012, 62, 799–814. [Google Scholar] [CrossRef]
- Alvisi, F.; Cibic, T.; Fazi, S.; Bongiorni, L.; Relitti, F.; Del Negro, P. Role of depositional dynamics and riverine input in shaping microbial benthic community structure of Po prodelta system (NW Adriatic, Italy). Estuar. Coast. Shelf Sci. 2019, 227, 106305. [Google Scholar] [CrossRef]
- Giere, O.; Schratzberger, M. New Horizons in Meiobenthos Research; Springer: Cham, Switzerland, 2023; p. 414. [Google Scholar]
- Mestdagh, S.; Bagaço, L.; Braeckman, U.; Ysebaert, T.; De Smet, B.; Moens, T.; Van Colen, C. Functional trait responses to sediment deposition reduce macrofauna-mediated ecosystem functioning in an estuarine mudflat. Biogeosciences 2018, 15, 2587–2599. [Google Scholar] [CrossRef]
- Bhuiyan, M.M.U.; Rahman, M.; Naher, S.; Shahed, Z.H.; Ali, M.M.; Towfiqul Islam, A.R.M. Oxygen declination in the coastal ocean over the twenty-first century: Driving forces, trends, and impacts. Case Stud. Chem. Environ. Eng. 2024, 9, 100621. [Google Scholar] [CrossRef]
- Alvisi, F.; Cozzi, S. Seasonal dynamics and long-term trend of hypoxia in the coastal zone of Emilia Romagna (NW Adriatic Sea, Italy). Sci. Total Environ. 2016, 541, 1448–1462. [Google Scholar] [CrossRef]
- Nasi, F.; Ferrante, L.; Alvisi, F.; Bonsdorff, E.; Auriemma, R.; Cibic, T. Macrofaunal bioturbation attributes in relation to riverine influence: What can we learn from the Po River lagoonal system (Adriatic Sea)? Estuar. Coast. Shelf Sci. 2020, 232, 106405. [Google Scholar] [CrossRef]
- Grassi, E.; Montefalcone, M.; Cesaroni, L.; Guidi, L.; Balsamo, M.; Semprucci, F. Taxonomic and functional nematode diversity in Maldivian coral degradation zones: Patterns across reef typologies and depths. PeerJ 2022, 10, e13644. [Google Scholar] [CrossRef]
- Cibic, T.; Fazi, S.; Nasi, F.; Pin, L.; Alvisi, F.; Berto, D.; Viganò, L.; Zoppini, A.; Del Negro, P. Natural and anthropogenic disturbances shape benthic phototrophic and heterotrophic microbial communities in the Po River Delta system. Estuar. Coast. Shelf Sci. 2019, 222, 168–182. [Google Scholar] [CrossRef]
- Fang, X.; Cozzoli, F.; Smolders, S.; Knights, A.; Moens, T.; Soetaert, K.; Van Colen, C. Hindcasting ecosystem functioning change in an anthropogenized estuary: Implications for an era of global change. Front. Mar. Sci. 2021, 8, 747833. [Google Scholar] [CrossRef]
- Van Colen, C.; Verbelen, D.; Devos, K.; Agten, L.; Van Tomme, J.; Vincx, M.; Degraer, S. Sediment-benthos relationships as a tool to assist in conservation practices in a coastal lagoon subjected to sediment change. Biodivers. Conserv. 2014, 23, 877–889. [Google Scholar] [CrossRef]
- Martínez, A.; Bonaglia, S.; Di Domenico, M.; Fonseca, G.; Ingels, J.; Jörger, K.M.; Laumer, C.; Leasi, F.; Zeppilli, D.; Baldrighi, E.; et al. Fundamental questions in meiofauna research highlight how small but ubiquitous animals can improve our understanding of Nature. Commun. Biol. 2025, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Baldrighi, E.; Semprucci, F.; Franzo, A.; Cvitkovic, I.; Bogner, D.; Despalatovic, M.; Berto, D.; Malgorzata Formalewicz, M.; Scarpato, A.; Frapiccini, E.; et al. Meiofaunal communities in four Adriatic ports: Baseline data for risk assessment in ballast water management. Mar. Pollut. Bull. 2019, 147, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Semprucci, F.; Jeong, R.; Kim, K.; Lee, S.; Jeon, D.; Yoo, H.; Kim, J.; Kim, J.; Yeom, J.; et al. Meiobenthic nematodes in the assessment of the relative impact of human activities on coastal marine ecosystem. Environ. Monit. Assess. 2020, 192, 81. [Google Scholar] [CrossRef] [PubMed]
- Grassi, E.; Greco, M.; Guidi, L.; Pasquariello, M.; Al-Enezi, E.; Trifuoggi, M.; Frontalini, F.; Semprucci, F. Exploring the effects of decabromodiphenyl ether on meiofaunal communities: An experimental approach. Mar. Pollut. Bull. 2025, 214, 117762. [Google Scholar] [CrossRef]
- Schratzberger, M.; Ingels, J. Meiofauna matters: The roles of meiofauna in benthic ecosystems. J. Exp. Mar. Biol. Ecol. 2018, 502, 12–25. [Google Scholar] [CrossRef]
- Grassi, E.; Catani, L.; Magni, P.; Gravina, M.F.; Semprucci, F. Taxonomic and functional diversity of nematode fauna: Two sides of the same coin in the ecological quality assessment of transitional environments. Estuar. Coast. Shelf Sci. 2023, 295, 108550. [Google Scholar] [CrossRef]
- Cocozza di Montanara, A.; Baldrighi, E.; López Correa, M.; Chianese, E.; Appolloni, L.; Simoncini, N.; Sandulli, R.; Zeppilli, D.; Semprucci, F.; Gambi, M.C.; et al. Meiobenthos and ocean acidification: Effects on meiobenthic communities inhabiting Mediterranean cold shallow CO2-vents. Estuar. Coast. Shelf Sci. 2024, 300, 108730. [Google Scholar] [CrossRef]
- Van Colen, C.; Vincx, M.; Degraer, S. Does medium-term emersion cause a mass extinction of tidal flat macrobenthos? The case of the tricolor oil pollution prevention in the Zwin nature reserve (Belgium and The Netherlands). Estuar. Coast. Shelf Sci. 2006, 68, 343–347. [Google Scholar] [CrossRef][Green Version]
- Van Colen, C.; Snoeck, F.; Struyf, K.; Vincxi, M.; Degraer, S. Macrobenthic community structure and distribution in the Zwin nature reserve (Belgium and The Netherlands). J. Mar. Biol. Assoc. UK 2009, 89, 431–438. [Google Scholar] [CrossRef]
- Rappé, K.; Fockedey, N.; Van Colen, C.; Cattrijsse, A.; Mees, J.; Vincx, M. Spatial distribution and general population characteristics of mysid shrimps in the Westerschelde estuary (SW Netherlands). Estuar. Coast. Shelf Sci. 2011, 91, 187–197. [Google Scholar] [CrossRef]
- Baeteman, C. The Coastal Plain of Belgium, Joint Product of Natural Processes and Human Activities. In Landscapes and Landforms of Belgium and Luxembourg; Demoulin, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- Cosyns, E.; Boumon, T.; De Smet, J.; Esteban, E.; Faveyts, W.; Geunens, O.; Jacobs, I.; Jacobs, M.; Jansen, J.; Lambrechts, J.; et al. Monitoring van het Natuurherstel in het Grensoverschrijdende Zwin 2011–2023; WVI, INBO, Natuurpunt Studie, Nature-ID & Universiteit Gent (Marbiol); Agentschap voor Natuur en Bos, de Vlaams Nederlandse Schelde Commissie: Zeeland, The Netherlands, 2024. [Google Scholar]
- Berner, R.A. The benthic boundary layer from the viewpoint of a geochemist. In The Benthic Boundary Layer; Springer: Boston, MA, USA, 1976; pp. 33–55. [Google Scholar]
- Tesi, T.; Belt, S.T.; Gariboldi, K.; Muschitiello, F.; Smik, L.; Finocchiaro, F.F.; Giglio, E.; Colizza, G.; Gazzurra, P.; Giordano, C.; et al. Resolving Sea ice dynamics in the north-western Ross Sea during the last 2.6 ka: From seasonal to millennial timescales. Quat. Sci. Rev. 2020, 237, 106299. [Google Scholar] [CrossRef]
- Danovaro, R. Methods for the Study of Deep-Sea Sediments, Their Functioning and Biodiversity; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- De Jonge, V.N. Fluctuations in the organic carbon to chlorophyll a ratios for estuarine benthic diatom populations. Mar. Ecol. Prog. Ser. 1980, 2, 345–353. [Google Scholar] [CrossRef]
- Fabiano, M.; Danovaro, R.; Fraschetti, S. A three-year time series of elemental and biochemical composition of organic matter in subtidal sandy sediments of the Ligurian Sea (northwestern Mediterranean). Cont. Shelf Res. 1995, 15, 1453–1469. [Google Scholar] [CrossRef]
- Pusceddu, A.; Bianchelli, S.; Sanchez Vidal, A.; Canals, M.; Durrieu De Madron, X.; Heussner, S.; Lykousis, V.; de Stigter, H.; Trincardi, F.; Danovaro, R. Organic matter in sediments of canyons and open slopes of the Portuguese, Catalan, Southern Adriatic and Cretan Sea margins. Deep Sea Res. Part I 2010, 57, 441–457. [Google Scholar] [CrossRef]
- Heip, C.; Vincx, M.; Vranken, G. The ecology of marine nematodes. Oceanogr. Mar. Biol. Annu. Rev. 1985, 23, 399–489. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/19860831851 (accessed on 8 September 2025).
- Schmidt-Rhaesa, A. Guide to the Identification of Marine Meiofauna; Verlag Dr. Friedrich Pfeil: Bayern, Germany, 2019; 607p. [Google Scholar]
- Anderson, M.; Gorley, R.; Clarke, K. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-e: Plymouth, UK, 2008. [Google Scholar]
- Anderson, M.J.; Robinson, J. Generalized discriminant analysis based on distances. Aust. N. Z. J. Stat. 2003, 45, 301–318. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer; PRIMER-e: Plymouth, UK, 2006; p. 866. [Google Scholar]
- Bonaglia, S.; Hedberg, J.; Marzocchi, U.; Iburg, S.; Glud, R.N.; Nascimento, F.J. Meiofauna improve oxygenation and accelerate sulfide removal in the seasonally hypoxic seabed. Mar. Environ. Res. 2020, 159, 104968. [Google Scholar] [CrossRef]
- Lamb, A.L.; Wilson, G.P.; Leng, M.J. A review of coastal palaeoclimate and relative sea-level reconstructions using δ13C and C/N ratios in organic material. Earth-Sci. Rev. 2006, 75, 29–57. [Google Scholar] [CrossRef]
- Yadav, V.B.; Vandana, A.P.; Vinod Kumar, A. Study of sedimentary organic carbon using δ13C, δ15N and TOC/TN as indicator in sediment core samples from Mumbai Harbor Bay. Environ. Monit. Assess. 2025, 197, 552. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Fabiano, M.; Della Croce, N. Labile organic matter and microbial biomasses in deep-sea sediments (Eastern Mediterranean Sea). Deep Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 953–965. [Google Scholar] [CrossRef]
- Hartnett, H.E.; Keil, R.G.; Hedges, J.I.; Devol, A.H. Influence of oxygen exposure time on organic carbon preservation in continental margin sediments. Nature 1998, 391, 572–575. [Google Scholar] [CrossRef]
- Dell’Anno, A.; Mei, M.L.; Pusceddu, A.; Danovaro, R. Assessing the trophic state and eutrophication of coastal marine systems: A new approach based on the biochemical composition of sediment organic matter. Mar. Pollut. Bull. 2002, 44, 611–622. [Google Scholar] [CrossRef]
- Danovaro, R.; Fabiano, M. Seasonal changes in quality and quantity of food available for benthic suspension-feeders in the Golfo Marconi (North-western Mediterranean). Estuar. Coast. Shelf Sci. 1997, 44, 723–736. [Google Scholar] [CrossRef]
- Pusceddu, A.; Gambi, C.; Manini, E.; Danovaro, R. Trophic state, ecosystem efficiency and biodiversity of transitional aquatic ecosystems: Analysis of environmental quality based on different benthic indicators. Chem. Ecol. 2007, 23, 505–515. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Kalantzi, I.; Karakassis, I. Effects of fish farming on the biological and geochemical properties of muddy and sandy sediments in the Mediterranean Sea. Mar. Environ. Res. 2010, 69, 326–336. [Google Scholar] [CrossRef]
- Manini, E.; Fiordelmondo, C.; Gambi, C.; Pusceddu, A.; Danovaro, R. Benthic microbial loop functioning in coastal lagoons: A comparative approach. Oceanol. Acta 2003, 26, 27–38. [Google Scholar] [CrossRef]
- Monnissen, J.; Thijs, S.; Artois, T.; Jouk, P.; Van de Reydt, E.; Van Dijck, T.; Monnens, M. Where meiofauna? An assessment of interstitial fauna at a Belgian beach. Diversity 2025, 17, 287. [Google Scholar] [CrossRef]
- Semprucci, F.; Sbrocca, C.; Rocchi, M.; Balsamo, M. Temporal changes of the meiofaunal assemblage as a tool for the assessment of the ecological quality status. J. Mar. Biol. Assoc. UK 2015, 95, 247–254. [Google Scholar] [CrossRef]
- Semprucci, F.; Frontalini, F.; Sbrocca, C.; Armynot du Châtelet, E.; Bout-Roumazeilles, V.; Coccioni, R.; Balsamo, M. Meiobenthos and free-living nematodes as tools for biomonitoring environments affected by riverine impact. Environ. Monit. Assess. 2015, 187, 251. [Google Scholar] [CrossRef] [PubMed]
- Moens, T.; Sroczynska, K.; Adao, H. Meiofauna in a changing world. Ecol. Indic. 2022, 138, 108769. [Google Scholar] [CrossRef]
- Gheskiere, T.; Hoste, E.; Vanaverbeke, J.; Vincx, M.; Degraer, S. Horizontal zonation patterns and feeding structure of marine nematode assemblages on a macrotidal, ultra-dissipative sandy beach (De Panne, Belgium). J. Sea Res. 2004, 55, 221–226. [Google Scholar] [CrossRef]
- Maria, T.F.; Silva Filho, M.G.; Souza, T.P.; Vanaverbeke, J.; Vanreusel, A.; Esteves, A.M. Is the vertical distribution of meiofauna similar in two contrasting microhabitats? A case study of a macrotidal sandy beach. J. Exp. Mar. Biol. Ecol. 2018, 502, 39–51. [Google Scholar] [CrossRef]
- Moens, T.; Bouillon, S.; Galluci, F. Dual stable isotope abundances unravel trophic position of estuarine nematodes. J. Mar. Biol. Assoc. UK 2005, 85, 1401–1407. [Google Scholar] [CrossRef]
- Maria, T.F.; Vanaverbeke, J.; Esteves, A.M.; De Troch, M.; Vanreusel, A. The importance of biological interactions for the vertical distribution of nematodes in a temperate ultra-dissipative Sandy beach. Estuar. Coast. Shelf Sci. 2012, 97, 114–126. [Google Scholar] [CrossRef]
- McLachlan, A.; Erasmus, T.; Furstenberg, I.P. Migrations of sandy beach meiofauna. Zool. Afr. 1977, 12, 257–277. [Google Scholar] [CrossRef]
- Maria, T.F.; De Troch, M.; Vanaverbeke, J.; Esteves, A.M.; Vanreusel, A. Use of benthic vs planktonic organic matter by Sandy-beach organisms: A food tracing experimente with 13C labelled diatoms. J. Exp. Mar. Biol. Ecol. 2011, 407, 309–314. [Google Scholar] [CrossRef]
- Thistle, D. Harpacticoid copepod emergence at a shelf site in summer and winter: Implications for hydrodynamic and mating hypotheses. Mar. Ecol. Prog. Ser. 2003, 248, 177–185. [Google Scholar] [CrossRef][Green Version]
- Pusceddu, A.; Bianchelli, S.; Gambi, C.; Danovaro, R. Assessment of benthic trophic status of marine coastal ecosystems: Significance of meiofaunal rare taxa. Estuar. Coast. Shelf Sci. 2011, 93, 420–430. [Google Scholar] [CrossRef]
- Moens, T.; Braeckman, U.; Derycke, S.; Fonseca, G.; Gallucci, F.; Gingold, R.; Guilini, K.; Ingels, J.; Leduc, D.; Vanaverbeke, J.; et al. Ecology of free-living marine nematodes. In Handbook of Zoology—Gastrotricha, Cycloneuralia and Gnathifera; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany, 2013; Volume 2, pp. 109–152. [Google Scholar]
- Semprucci, F.; Grassi, E.; Cocozza di Montanara, A.; Sandulli, R.; Baldrighi, E. Emerging Marine Nematodes as Model Organisms: Which Species for Which Question? Diversity 2025, 17, 59. [Google Scholar] [CrossRef]
- Van Damme, D.; Heip, C.; Willems, K.A. Influence of pollution on the harpacticoid copepods of two North Sea estuaries. Hydrobiologia 1984, 112, 143–160. [Google Scholar] [CrossRef]
- Grego, M.; De Troch, M.; Forte, J.; Malej, A. Main meiofauna taxa as an indicator for assessing the spatial and seasonal impact of fish farming. Mar. Pollut. Bull. 2009, 58, 1178–1186. [Google Scholar] [CrossRef]
- Baldrighi, E.; Bang, H.W.; Fast, J.; Baguley, J.G. Deep-Sea Benthic Response to the Deepwater Horizon Oil Spill: Harpacticoid Families as Sentinels of Impact Through Space and Time. Integr. Comp. Biol. 2024, 64, 867–881. [Google Scholar] [CrossRef]
- Mirto, S.; Arigò, C.; Genovese, L.; Pusceddu, A.; Gambi, C.; Danovaro, R. Nematode assemblage response to fish-farm impact in vegetated (Posidoniaoceanica) and nonvegetated habitats. Aquat. Environ. Interact. 2014, 5, 17–28. [Google Scholar] [CrossRef]
- Zeppilli, D.; Sarrazin, J.; Leduc, D.; Arbizu, P.M.; Fontaneto, D.; Fontanier, C.; Gooday, A.J.; Kristensen, R.M.; Ivanenko, V.N.; Sorensen, M.V.; et al. Is the meiofauna a good indicator for climate change and anthropogenic impacts? Mar. Biodivers. 2015, 45, 505–535. [Google Scholar] [CrossRef]
- Bianchelli, S.; Gambi, C.; Zeppilli, D.; Danovaro, R. Metazoan meiofauna in deep-sea canyons and adjacent open slopes: A large-scale comparison with focus on the rare taxa. Deep-Sea Res. Part I 2010, 57, 420–433. [Google Scholar] [CrossRef]
- Bianchelli, S.; Nizzoli, D.; Bartoli, M.; Viaroli, P.; Rastelli, E.; Pusceddu, A. Sedimentary organic matter, prokaryotes, and meiofauna across a river-lagoon-sea gradient. Diversity 2020, 12, 189. [Google Scholar] [CrossRef]
- Moreno, M.; Semprucci, F.; Vezzulli, L.; Balsamo, M.; Fabiano, M.; Albertelli, G. The use of nematodes in assessing ecological quality status in the Mediterranean coastal ecosystems. Ecol. Indic. 2011, 11, 328–336. [Google Scholar] [CrossRef]
- Dal Zotto, M.; Santulli, A.; Simonini, R.; Todaro, M.A. Organic enrichment effects on a marine meiofauna community, with focus on Kinorhyncha. Zool. Anz. 2016, 265, 127–140. [Google Scholar] [CrossRef]
- Semprucci, F.; Facca, C.; Ferrigno, F.; Balsamo, M.; Sfriso, A.; Sandulli, R. Biotic and abiotic factors affecting seasonal and spatial distribution of meiofauna and macrophytobenthos in transitional coastal waters. Estuar. Coast. Shelf Sci. 2019, 219, 328–340. [Google Scholar] [CrossRef]
- Semprucci, F.; Gravina, M.F.; Magni, P. Meiofaunal Dynamics and Heterogeneity along Salinity and Trophic Gradients in a Mediterranean Transitional System. Water 2019, 11, 1488. [Google Scholar] [CrossRef]
- Watzin, M.C. The effects of meiofauna on settling macrofauna: Meiofauna may structure macrofaunal communities. Oecologia 1983, 59, 163–166. [Google Scholar] [CrossRef]
- Grego, M.; Riedel, B.; Stachowitsch, M.; De Troch, M. Meiofauna winners and losers of coastal hypoxia: Case study harpacticoid copepods. Biogeosciences 2014, 11, 281–292. [Google Scholar] [CrossRef]
- Buzas, M.A.; Culver, S.J.; Jorissen, F.J. A statistical evaluation of the microhabitats of living (stained) infaunal benthic foraminifera. Mar. Micropaleontol. 1993, 20, 311–320. [Google Scholar] [CrossRef]
- Armynot du Châtelet, E.; Bout-Roumazeilles, V.; Coccioni, R.; Frontalini, F.; Francescangeli, F.; Margaritelli, G.; Rettori, R.; Spagnoli, F.; Semprucci, F.; Trentesaux, A.; et al. Environmental control on a land-sea transitional setting—Integrated microfaunal, sedimentological, and geochemical approaches. Environ. Earth Sci. 2016, 75, 123. [Google Scholar] [CrossRef]
- Moodley, L.; Van der Zwaan, G.J.; Herman, P.M.J.; Kempers, L.; Van Breugel, P. Differential response of benthic meiofauna to anoxia with special reference to Foraminifera (Protista: Sarcodina). Mar. Ecol. Prog. Ser. 1997, 158, 151–163. [Google Scholar] [CrossRef]
- Moodley, L.; Hess, C. Tolerance of infaunal benthic foraminifera for low and high oxygen concentrations. Biol. Bull. 1992, 183, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Austen, M.C.; Widdicombe, S. Comparison of the response of meio-and macrobenthos to disturbance and organic enrichment. J. Exp. Mar. Biol. Ecol. 2006, 330, 96–104. [Google Scholar] [CrossRef]
- De Troch, M.; Van Gansbeke, D.; Vincx, M. Resource availability and meiofauna in sediment of tropical seagrass beds: Local versus global trends. Mar. Environ. Res. 2006, 61, 59–73. [Google Scholar] [CrossRef]
- Gingold, R.; Mundo-Ocampo, M.; Holovachov, O.; Rocha-Olivares, A. The role of habitat heterogeneity in structuring the community of intertidal free-living marine nematodes. Mar. Biol. 2010, 157, 1741–1753. [Google Scholar] [CrossRef]
- Steyaert, M.; Herman, P.M.J.; Moens, T.; Widdows, J.; Vincx, M. Tidal migration of nematodes on an estuarine tidal flat (the Molenplaat, Schelde Estuary, SW Netherlands). Mar. Ecol. Prog. Ser. 2001, 224, 299–304. [Google Scholar] [CrossRef]
- Boeckner, M.J.; Sharma, J.; Proctor, H.C. Revisiting the meiofauna paradox: Dispersal and colonization of nematodes and other meiofaunal organisms in low-and high-energy environments. Hydrobiologia 2009, 624, 91–106. [Google Scholar] [CrossRef]
- Derycke, S.; Van Vynckt, R.; Vanoverbeke, J.; Vincx, M.; Moens, T. Colonization patterns of Nematoda on decomposing algae in the estuarine environment: Community assembly and genetic structure of the dominant species Pellioditis marina. Limnol. Oceanogr. 2007, 52, 992–1001. [Google Scholar] [CrossRef]
- Fonsêca-Genevois, V.D.; Somerfield, P.J.; Neves, M.H.B.; Coutinho, R.; Moens, T. Colonization and early succession on artificial hard substrata by meiofauna. Mar. Biol. 2006, 148, 1039–1050. [Google Scholar] [CrossRef][Green Version]
- Baldrighi, E.; Zeppilli, D.; Crespin, R.; Chauvaud, P.; Pradillon, F.; Sarrazin, J. Colonization of synthetic sponges at the deep-sea Lucky Strike hydrothermal vent field (Mid-Atlantic Ridge): A first insight. Mar. Biodivers. 2018, 48, 89–103. [Google Scholar] [CrossRef]
- Mevenkamp, L.; Ong, E.Z.; Van Colen, C.; Vanreusel, A.; Guilini, K. Combined, short-term exposure to reduced seawater pH and elevated temperature induces community shifts in an intertidal meiobenthic assemblage. Mar. Environ. Res. 2018, 133, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Neira, C.; Ingels, J.; Mendoza, G.; Hernandez-Lopez, E.; Levin, L.A. Distribution of meiofauna in bathyal sediments influenced by the oxygen minimum zone off Costa Rica. Front. Mar. Sci. 2018, 5, 448. [Google Scholar] [CrossRef]
- Dunne, J.A.; Williams, R.J.; Martinez, N.D. Network structure and biodiversity loss in food webs: Robustness increases with connectance. Ecol. Lett. 2002, 5, 558–567. [Google Scholar] [CrossRef]



| Station | From | To | Water Content | Porosity | BDD | Shells | Very Coarse Sand | Coarse Sand | Medium Sand | Fine Sand | Very Fine Sand | Mud | TOC | TN | δ13C | δ15N | TOC/TN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm | cm | % | g cm−3 | % d.w. | % d.w. | % d.w. | % d.w. | % d.w. | % d.w. | % d.w. | % d.w. | % d.w. | ‰ | ‰ | Molar Ratio | ||
| St.1-1 | 0 | 1 | 58.85 | 77.68 | 0.55 | 0.00 | 0.24 | 0.16 | 0.72 | 1.52 | 0.96 | 96.40 | 2.06 | 0.31 | −22.33 | 8.66 | 7.69 |
| 1 | 2 | 55.71 | 75.38 | 0.61 | 0.00 | 0.00 | 0.00 | 0.36 | 0.99 | 0.63 | 98.03 | 1.99 | 0.29 | −22.57 | 8.24 | 7.97 | |
| 2 | 3 | 55.41 | 75.16 | 0.61 | 0.00 | 0.00 | 0.00 | 0.41 | 0.90 | 0.55 | 98.14 | 1.95 | 0.28 | −22.58 | 8.27 | 8.15 | |
| 3 | 4 | 54.90 | 74.77 | 0.62 | 0.00 | 0.00 | 0.15 | 0.54 | 1.08 | 0.61 | 97.62 | 2.23 | 0.31 | −22.81 | 8.23 | 8.48 | |
| 4 | 5 | 54.97 | 74.82 | 0.62 | 0.89 | 0.44 | 0.09 | 0.35 | 0.98 | 0.53 | 96.72 | 2.11 | 0.30 | −22.57 | 8.16 | 8.13 | |
| 10 | 11 | 50.68 | 71.44 | 0.71 | 0.00 | 0.00 | 0.00 | 0.83 | 3.63 | 1.15 | 94.40 | 1.87 | 0.26 | −23.11 | 7.94 | 8.55 | |
| 18 | 19 | 44.86 | 66.45 | 0.83 | 0.00 | 0.00 | 0.21 | 5.81 | 16.12 | 4.08 | 73.77 | 1.65 | 0.23 | −23.06 | 7.54 | 8.46 | |
| St.1-2 | 0 | 2 | 54.58 | 74.52 | 0.63 | 0.00 | 0.00 | 0.00 | 0.52 | 1.14 | 0.93 | 97.41 | 1.95 | 0.29 | −22.41 | 9.33 | 7.97 |
| 2 | 4 | 52.99 | 73.29 | 0.66 | 0.00 | 0.00 | 0.00 | 0.45 | 1.01 | 0.68 | 97.86 | 1.97 | 0.29 | −22.54 | 9.17 | 8.02 | |
| 4 | 5 | 54.17 | 74.21 | 0.64 | 0.00 | 0.00 | 0.07 | 0.60 | 0.90 | 0.45 | 97.98 | 1.98 | 0.28 | −22.56 | 9.08 | 8.14 | |
| 10 | 11 | 49.50 | 70.47 | 0.73 | 0.00 | 0.00 | 0.00 | 2.11 | 8.03 | 1.25 | 88.62 | 1.84 | 0.26 | −22.74 | 8.94 | 8.15 | |
| 18 | 19 | 46.79 | 68.16 | 0.79 | 0.00 | 0.12 | 0.25 | 5.74 | 16.21 | 2.87 | 74.81 | 2.08 | 0.29 | −22.95 | 9.18 | 8.42 | |
| St.2-1 | 0 | 2 | 50.43 | 71.24 | 0.71 | 0.00 | 0.00 | 0.00 | 0.69 | 2.07 | 3.66 | 93.59 | 1.71 | 0.25 | −22.38 | 9.52 | 7.98 |
| 2 | 4 | 47.65 | 68.90 | 0.77 | 0.00 | 0.00 | 0.00 | 0.58 | 2.58 | 4.45 | 92.39 | 1.77 | 0.25 | −22.49 | 9.29 | 8.19 | |
| 4 | 5 | 46.05 | 67.51 | 0.80 | 0.00 | 0.00 | 0.00 | 0.66 | 3.22 | 4.80 | 91.32 | 1.82 | 0.26 | −22.55 | 9.40 | 8.24 | |
| 10 | 11 | 37.31 | 59.16 | 1.01 | 0.13 | 0.13 | 0.00 | 0.98 | 9.33 | 13.25 | 76.17 | 1.05 | 0.15 | −22.89 | 9.49 | 8.48 | |
| 18 | 19 | 41.73 | 63.55 | 0.90 | 0.00 | 0.14 | 0.00 | 1.43 | 5.08 | 1.72 | 91.64 | 1.89 | 0.26 | −22.38 | 8.83 | 8.38 | |
| St.2-2 | 0 | 1 | 54.75 | 74.65 | 0.63 | 0.00 | 0.00 | 0.00 | 1.00 | 2.68 | 3.98 | 92.34 | 1.73 | 0.26 | −21.83 | 8.50 | 7.78 |
| 1 | 2 | 53.32 | 73.55 | 0.65 | 0.00 | 0.00 | 0.00 | 0.77 | 2.01 | 3.48 | 93.74 | 1.83 | 0.27 | −22.26 | 8.41 | 7.99 | |
| 2 | 3 | 51.80 | 72.34 | 0.68 | 0.00 | 0.00 | 0.00 | 0.54 | 2.11 | 3.47 | 93.87 | 1.99 | 0.28 | −22.42 | 8.05 | 8.27 | |
| 3 | 4 | 49.70 | 70.64 | 0.73 | 0.00 | 0.00 | 0.00 | 0.64 | 2.06 | 3.73 | 93.57 | 1.91 | 0.26 | −22.71 | 7.82 | 8.55 | |
| 4 | 5 | 48.16 | 69.34 | 0.76 | 0.00 | 0.00 | 0.00 | 0.64 | 2.34 | 4.18 | 92.84 | 1.85 | 0.25 | −22.57 | 8.07 | 8.48 | |
| 10 | 11 | 43.00 | 64.74 | 0.87 | 0.00 | 0.00 | 0.00 | 1.06 | 7.35 | 8.27 | 83.32 | 1.60 | 0.21 | −22.95 | 7.92 | 8.98 | |
| 18 | 19 | 49.53 | 70.49 | 0.73 | 0.00 | 0.00 | 0.00 | 0.96 | 4.81 | 4.43 | 89.80 | 2.05 | 0.28 | −22.79 | 8.15 | 8.65 | |
| St.3 | 0 | 1 | 21.20 | 39.57 | 1.51 | 0.25 | 1.47 | 7.00 | 59.70 | 20.79 | 1.55 | 9.25 | 0.44 | 0.06 | −21.84 | 8.16 | 8.32 |
| 1 | 2 | 21.24 | 39.63 | 1.50 | 0.00 | 0.98 | 6.79 | 61.89 | 21.54 | 1.10 | 7.72 | 0.48 | 0.07 | −21.76 | 7.86 | 8.08 | |
| 2 | 3 | 19.93 | 37.74 | 1.55 | 0.00 | 0.47 | 6.06 | 65.40 | 20.73 | 0.69 | 6.64 | 0.32 | 0.05 | −22.01 | 7.32 | 7.92 | |
| 3 | 4 | 19.72 | 37.42 | 1.56 | 0.15 | 0.42 | 5.63 | 65.72 | 22.41 | 0.57 | 5.10 | 0.26 | 0.04 | −22.03 | 8.08 | 8.53 | |
| 4 | 5 | 18.95 | 36.27 | 1.59 | 0.00 | 0.76 | 6.24 | 65.09 | 23.18 | 0.44 | 4.29 | 0.26 | 0.04 | −22.31 | 5.01 | 8.80 | |
| 6 | 7 | 18.60 | 35.74 | 1.60 | 0.46 | 1.66 | 7.23 | 49.90 | 25.61 | 2.49 | 12.64 | 0.27 | 0.04 | −22.62 | 7.85 | 8.75 | |
| 17 | 18 | 17.71 | 34.38 | 1.64 | 0.00 | 0.42 | 3.61 | 56.35 | 35.00 | 1.35 | 3.27 | 0.16 | 0.02 | −22.80 | 5.85 | 10.01 |
| Total Abundance | |||||
|---|---|---|---|---|---|
| Source | df | SS | MS | Pseudo-F | P(MC) |
| st | 2 | 11,699 | 5849.40 | 38.64 | 0.001 |
| la(st) | 15 | 2271 | 151.40 | 1.37 | 0.211 |
| Res | 36 | 3986.7 | 110.74 | ||
| Total | 53 | 17,957 | |||
| PAIRWISE TESTS | |||||
| Groups | P(MC) | ||||
| st1, st2 | 0.049 | ||||
| st1, st3 | 0.001 | ||||
| st2, st3 | 0.001 | ||||
| N. taxa | |||||
| Source | df | SS | MS | Pseudo-F | P(MC) |
| st | 2 | 0.70 | 0.35 | 5.07 | 0.028 |
| la(st) | 15 | 1.03 | 0.07 | 2.21 | 0.023 |
| Res | 36 | 1.12 | 0.03 | ||
| Total | 53 | 2.84 | |||
| PAIRWISE TESTS | |||||
| Groups | P(MC) | Groups (St1) | P(MC) | Groups (St3) | P(MC) |
| st1, st2 | 0.381 | L1, L5 | 0.013 | L1, L5 | 0.019 |
| st1, st3 | 0.104 | L1, L6 | 0.009 | ||
| st2, st3 | 0.002 | L2, L5 | 0.014 | ||
| L2, L6 | 0.010 | ||||
| L3, L5 | 0.012 | ||||
| L3, L6 | 0.021 | ||||
| L4, L5 | 0.012 | ||||
| L4, L6 | 0.011 | ||||
| Meiofauna Community Composition | |||||
|---|---|---|---|---|---|
| Source | df | SS | MS | Pseudo-F | P(MC) |
| st | 2 | 31,458 | 15,729 | 23.76 | 0.001 |
| la(st) | 15 | 9930.60 | 662.04 | 3.93 | 0.001 |
| Res | 36 | 6071.90 | 168.67 | ||
| Total | 53 | 47,460 | |||
| PAIRWISE TESTS | |||||
| Groups | P(MC) | Groups (St1) | P(MC) | Groups (St2) | P(MC) |
| St.1, St.2 | 0.025 | L3, L5 | 0.020 | L1, L3 | 0.022 |
| St.1, St.3 | 0.001 | L3, L6 | 0.018 | L1, L4 | 0.015 |
| St.2, St.3 | 0.001 | L4, L6 | 0.023 | L1, L5 | 0.019 |
| L2, L4 | 0.046 | ||||
| Groups (St3) | P(MC) | Groups (St3) | P(MC) | ||
| L1, L3 | 0.019 | L2, L4 | 0.026 | ||
| L1, L4 | 0.009 | L2, L5 | 0.020 | ||
| L1, L5 | 0.001 | L2, L6 | 0.014 | ||
| L1, L6 | 0.002 | L3, L6 | 0.036 | ||
| Total Abundance | |||||
|---|---|---|---|---|---|
| Variable | SS (Trace) | Pseudo-F | p | Prop. | Cumul. |
| TN | 9118.5 | 93.20 | 0.001 | 0.85 | 0.85 |
| Very coarse sand | 438.03 | 5.83 | 0.005 | 0.04 | 0.89 |
| Medium sand | 327.64 | 5.74 | 0.009 | 0.03 | 0.93 |
| PRT/CHO | 209.89 | 5.35 | 0.028 | 0.02 | 0.94 |
| N. taxa | |||||
| Very fine sand | 0.59 | 8.09 | 0.021 | 0.34 | 0.34 |
| Phaeopigments | 0.44 | 9.06 | 0.013 | 0.25 | 0.59 |
| TN | 0.21 | 5.64 | 0.040 | 0.12 | 0.70 |
| Medium sand | 0.10 | 24.75 | 0.011 | 0.06 | 0.76 |
| Meiofauna community composition | |||||
| TN | 11,535.00 | 40.54 | 0.001 | 0.72 | 0.72 |
| δ15N | 1172.00 | 5.20 | 0.004 | 0.07 | 0.79 |
| TOC/TN | 1047.60 | 6.29 | 0.001 | 0.07 | 0.85 |
| Medium sand | 265.63 | 4.08 | 0.027 | 0.02 | 0.87 |
| Very coarse sand | 235.79 | 6.44 | 0.019 | 0.01 | 0.89 |
| δ13C | 119.95 | 6.01 | 0.030 | 0.01 | 0.89 |
| Site | Depth (m) | chl-a (µg/g) | Phaeo (µg/g) | CPE (µg/g) | BPC (mgC/g) | PRT/CHO | Tot. meioF. |
|---|---|---|---|---|---|---|---|
| Goro lagoon, Adr. Sea * | 1.5 | 1.7 ± 0.6 | 12.3 ± 2.2 | 14.1 ± 3.2 | 1.4 ± 0.3 | 0.9 ± 0.3 | 2713 ± 347 |
| Lesina lagoon, Adr. Sea * | 0.8 | 23.3 ± 1.7 | 20.2 ± 5.6 | 41.0 ± 4.3 | 8.1 ± 1.2 | 0.3 ± 0.03 | 1273 ± 490 |
| Marsala lagoon, Sicily * | 1.0 | 3.5 ± 0.6 | 3.1 ± 1.2 | 6.6 ± 1.8 | 6.5 ± 3.4 | 1.0 ± 0.1 | 50 ± 27 |
| Caribbean Seagrass ** | 0.5 | 1.4 ± 0.2 | 8.7 ± 1.5 | 10.1 ± 0.7 | 1.77 ± 0.42 | 0.9 ± 0.3 | 1268 ± 731 |
| Caribbean Mangrove ** | 0.5 | 4.8 ± 0.1 | 92.6 ± 5.0 | 97.4 ± 3.2 | 19.89 ± 0.89 | 0.5 ± 0.3 | 2474 ± 117 |
| Caribbean Reef ** | 3.0 | 2.7 ± 2.8 | 34.7 ± 5.9 | 37.4 ± 3.9 | 1.79 ± 0.15 | 0.3 ± 0.2 | 2871 ± 1307 |
| Red Sea Seagrass ** | 0.5 | 0.2 ± 0.1 | 16.4 ± 4.7 | 16.6 ± 2.7 | 0.54 ± 0.06 | 1.2 ± 0.1 | 1475 ± 166 |
| Red Sea Mangrove ** | 0.5 | 0.1 ± 0.0 | 13.4 ± 1.4 | 13.5 ± 0.5 | 0.18 ± 0.02 | 4.0 ± 0.3 | 343 ± 41 |
| Red Sea Reef ** | 2.0 | 0.4 ± 0.1 | 49.9 ± 8.0 | 50.3 ± 6.5 | 0.41 ±0.07 | 6.7 ± 0.4 | 488 ± 253 |
| Celebes Seagrass ** | 0.5 | 2.2 ± 1.0 | 20.4 ± 8.9 | 22.6 ± 6.4 | 7.17 ± 0.45 | 0.4 ± 0.3 | 1604 ± 267 |
| Celebes Mangrove ** | 0.5 | 0.7 ± 0.3 | 4.0 ± 1.9 | 4.7 ± 0.8 | 2.35 ± 0.19 | 1.1 ± 0.2 | 627 ± 192 |
| Celebes Reef ** | 5.0 | 1.0 ± 0.3 | 5.8 ± 1.1 | 6.8 ± 0.5 | 0.90 ± 0.08 | 0.6 ± 0.1 | 706 ± 271 |
| St.1, Zwin park # | 0.5 | 0.9 ± 0.1 | 7.6 ± 1.1 | 8.5 ± 1.1 | 8.7 ± 2.1 | 5.5 ± 1.0 | 2785 ± 2187 |
| St.2, Zwin park # | 0.5 | 3.3 ± 1.6 | 13.4 ± 7.5 | 16.7 ± 9.1 | 7.6 ± 1.1 | 4.3 ± 0.9 | 1151 ± 1093 |
| St.3, Zwin park # | 0.5 | 2.8 ± 0.6 | 8.8 ± 2.2 | 11.5 ± 2.6 | 5.2 ± 0.4 | 1.9 ± 0.5 | 544 ± 53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldrighi, E.; Alvisi, F.; Van Colen, C.; Grassi, E.; Catani, L.; Ape, F.; Vasapollo, C.; Manini, E.; Baguley, J.G.; Semprucci, F. Characterization of the Meiobenthic Community Inhabiting the Zwin Coastal Lagoon (Belgium, the Netherlands) and the Role of the Sedimentary Environment. Water 2025, 17, 2669. https://doi.org/10.3390/w17182669
Baldrighi E, Alvisi F, Van Colen C, Grassi E, Catani L, Ape F, Vasapollo C, Manini E, Baguley JG, Semprucci F. Characterization of the Meiobenthic Community Inhabiting the Zwin Coastal Lagoon (Belgium, the Netherlands) and the Role of the Sedimentary Environment. Water. 2025; 17(18):2669. https://doi.org/10.3390/w17182669
Chicago/Turabian StyleBaldrighi, Elisa, Francesca Alvisi, Carl Van Colen, Eleonora Grassi, Linda Catani, Francesca Ape, Claudio Vasapollo, Elena Manini, Jeffrey G. Baguley, and Federica Semprucci. 2025. "Characterization of the Meiobenthic Community Inhabiting the Zwin Coastal Lagoon (Belgium, the Netherlands) and the Role of the Sedimentary Environment" Water 17, no. 18: 2669. https://doi.org/10.3390/w17182669
APA StyleBaldrighi, E., Alvisi, F., Van Colen, C., Grassi, E., Catani, L., Ape, F., Vasapollo, C., Manini, E., Baguley, J. G., & Semprucci, F. (2025). Characterization of the Meiobenthic Community Inhabiting the Zwin Coastal Lagoon (Belgium, the Netherlands) and the Role of the Sedimentary Environment. Water, 17(18), 2669. https://doi.org/10.3390/w17182669








