Abstract
To address the problems of eutrophication exacerbation in water bodies caused by low carbon-to-nitrogen ratio (C/N) wastewater and the limited nitrogen removal efficiency of conventional constructed wetlands, this study proposes the use of biochar (Corncob biochar YBC, Walnut shell biochar HBC, and Manure biochar FBC) coupled with intermittent aeration technology to enhance nitrogen removal in constructed wetlands. Through the construction of vertical flow wetland systems, hydraulic retention time (HRT = 1–3 d) and influent C/N ratios (1, 3, 5) were regulated, before being combined with material characterization (FTIR/XPS) and microbial analysis (16S rRNA) to reveal the synergistic nitrogen removal mechanisms. HBC achieved efficient NH4+-N adsorption (32.44 mg/L, Langmuir R2 = 0.990) through its high porosity (containing Si-O bonds) and acidic functional groups. Under optimal operating conditions (HRT = 3 d, C/N = 5), the CW-HBC system achieved removal efficiencies of 97.8%, 98.8%, and 79.6% for NH4+-N, TN, and COD, respectively. The addition of biochar shifted the dominant bacterial phylum toward Actinobacteriota (29.79%), with its slow-release carbon source (TOC = 18.5 mg/g) alleviating carbon limitation. Mechanistically, HBC synergistically optimized nitrogen removal pathways through “adsorption-biofilm (bacterial enrichment)-microzone oxygen regulation (pore oxygen gradient).” Based on technical validation, a dual-track institutionalization pathway of “standards-legislation” is proposed: incorporating biochar physicochemical parameters and aeration strategies into multi-level water environment technical standards; converting common mechanisms (such as Si-O adsorption) into legal requirements through legislative amendments; and innovating legislative techniques to balance precision and universality. This study provides an efficient technical solution for low C/N wastewater treatment while constructing an innovative framework for the synergy between technical specifications and legislation, supporting the improvement of watershed ecological restoration systems.
1. Introduction
With the deepening advancement of China’s ecological civilization construction, watershed ecological restoration and black-odorous water body treatment have become critical issues in environmental governance. The treatment of low carbon-to-nitrogen ratio (C/N) wastewater represents a significant challenge, as this type of wastewater exhibits poor denitrification efficiency due to insufficient carbon sources, substantially exacerbating the risk of water body eutrophication. According to research by Jin et al., Chinese lakes face severe eutrophication problems, with low C/N wastewater being one of the major contributing factors [1].
Constructed wetlands (CWs), as an ecological wastewater treatment technology, possess advantages, such as low cost and strong adaptability, and are widely applied in decentralized wastewater treatment. However, traditional constructed wetlands face significant bottlenecks when treating low C/N wastewater: substrate saturation, insufficient oxygen supply, and carbon source deficiency lead to limited denitrification efficiency [2]. Research by Wu et al. indicates that traditional constructed wetlands typically struggle to achieve ideal total nitrogen removal rates, particularly under carbon-deficient conditions [3]. Internationally, similar challenges are prevalent in tropical regions such as Brazil and Thailand, where Zhang et al.’s research demonstrates that constructed wetland performance in these areas is similarly significantly affected by C/N [4].
In recent years, biochar as a novel functional material has received widespread attention in the environmental field. Research by Mohan et al. demonstrates that biochar possesses significant adsorption properties and microbial carrier functions [5]. Wang et al. confirmed that biochar can effectively provide carbon sources and promote denitrification processes [6]. Meanwhile, research by Saeed and Sun indicates that intermittent aeration technology can significantly enhance nitrification efficiency by optimizing oxygen supply patterns [7]. Zhou et al. discovered that coupling biochar with intermittent aeration technology can significantly improve low C/N wastewater treatment efficiency, with this technological combination demonstrating breakthrough potential [8].
From the perspective of environmental resource law, although China’s current legal frameworks including the Environmental Protection Law, Water Pollution Prevention and Control Law, Wetland Protection Law, and Yangtze River Protection Law clearly establish water environment restoration objectives, they lack operational specifications for specific technical implementation. This is specifically manifested in the following reasons: legal provisions are mostly principled requirements, lacking clear guidance on technology selection and parameter control; although the Ecological Environment Standards Management Measures propose a framework for technical standard development, standardization regulations for emerging technologies such as biochar are severely insufficient; there exists an obvious disconnect between technical applications and legal regulations in practice.
Research in the field of hydraulic environmental engineering predominantly focuses on laboratory performance optimization, neglecting the integration between technical implementation and policy and legal systems [9]. For example, selection standards for different biochar materials (such as corn cob, walnut shell, and manure feedstocks) have not yet been embedded in regulatory practices; optimization of intermittent aeration modes (such as hydraulic retention time and C/N regulation) lacks supporting dynamic management regulations and legal foundations.
Therefore, this study, based on the technical foundation of “biochar-enhanced aeration for strengthening constructed wetlands in treating low C/N wastewater,” attempts to break disciplinary barriers and explore synergistic pathways between environmental resource law and hydraulic environmental engineering. The research validates the feasibility of technical solutions by comparing the adsorption efficiency, microbial community responses, and process parameter optimization of three types of biochar—Corn cob biochar (YBC), Walnut shell biochar (HBC), and Manure biochar (FBC)—while exploring effective mechanisms for transforming experimental results into environmental resource laws, regulations, and technical standards, providing scientifically sound and regulatory-compliant solutions for watershed ecological restoration.
2. Materials and Methods
2.1. Biochar Preparation
This study utilized YBC, HBC, and FBC materials provided by Henan Lize Environmental Protection Technology Co., Ltd. (Henan, China) as experimental substrates.
According to recent studies, YBC (pyrolyzed at 500 °C) exhibits a typical BET surface area of 100–200 m2/g, total carbon content > 60%, and pH 8.5–10.0 (attributed to alkali metal ash content); HBC, due to its high lignin characteristics, achieves BET surface area of 120–350 m2/g and total carbon content of 70–85% under 600 °C pyrolysis; FBC demonstrates high ash content characteristics, with a BET surface area typically <20 m2/g, strong alkalinity (pH 10.0–12.0), and significant ash content [10,11,12].
The preparation protocol for biochar carriers involved the following procedures: flaked HBC was ground into powder form (<150 μm), while YBC and FBC were inherently powdered and could be utilized directly; biochar, sodium alginate, and sodium bicarbonate were mixed at a mass ratio of 4:1:0.1, with deionized water added under stirring to form a homogeneous suspension; the mixture was dropped through a needle into 1 mol/L CaCl2 solution for 24 h solidification, forming spherical carriers with diameters of 1.0–1.5 cm; subsequently, the carriers were dried in a constant temperature oven at 60 °C for 6–12 h until constant weight was achieved, ultimately yielding biochar carriers with dense and rigid surfaces.
2.2. Constructed Wetland System Configuration
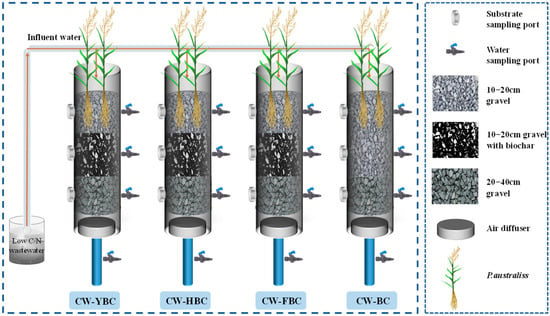
The constructed wetland simulation apparatus is illustrated in Figure 1. The experiment employed a custom-made vertical flow constructed wetland device fabricated from polymethyl methacrylate (PMMA) (diameter: 20 cm, height: 60 cm) with a three-tier filling structure: the bottom support layer consisted of 20–40 mm gravel (height: 15 cm), the middle working layer comprised 10–20 mm gravel mixed with biochar at a 1:1 ratio (height: 20 cm), and the top cover layer contained 10–20 mm gravel (height: 15 cm), with a system effective volume of 8.0 L. Reed seedlings were acclimated with 10% Hoagland’s nutrient solution for 4 weeks, after which healthy plants (height: 30 ± 2 cm) were transplanted into the cover layer with root burial depth of 10 cm. This study employed four experimental configurations: CW-YBC, CW-HBC, CW-FBC, and a biochar-free control group (CW-BC), targeting simulated low C/N wastewater under intermittent aeration mode (6:00–12:00). This study used intermittent water supply with a vertical downward flow direction.
2.3. Experimental Design
The synthetic simulated wastewater used in this study consisted of KNO3, NH4Cl, C6H12O6, CaCl2, MgSO4-7H2O, KH2PO4, CuSO4-7H2O, and FeSO4. Each experimental group was conducted with n = 3 independent replicates and tested in batch mode under controlled conditions at room temperature (25 °C) with simulated natural light. The selection of HRT (1–3 d) and C/N (1, 3, 5) in this study was based on research on denitrification inhibition under low-carbon, high-nitrogen loading conditions, with experiments conducted in 6 stages [13,14,15]. The experimental conditions and influent water quality at different stages are shown in Table 1.
2.4. Analytical Methods
COD, NH4+-N, TN, PO43−-P, NO3−-N, and NO2−-N parameters were analyzed according to Standard Methods for the Examination of Water and Wastewater; pH was measured using a pH meter (Lichen, Shanghai Xiren Scientific Instrument Co., Ltd., Shanghai, China); dissolved oxygen was determined using a portable dissolved oxygen meter (JPBJ-607A, Shanghai Leici Instrument Factory, Shanghai, China); electrical conductivity (EC) was measured using a pen-type conductivity meter (CT-20, Shanghai Lichen Instrument Technology Co., Ltd., Shanghai, China); microbial biomass was determined using membrane filtration enrichment method; microbial analysis employed 16S rRNA gene sequencing technology to analyze Alpha diversity, OTU community structure, and community composition at phylum and genus levels [16,17]. Fourier-transform infrared spectroscopy (FTIR) (MIR-FT/P, Envea, Poissy, France) was used to determine the infrared spectra of biochar prepared from three materials, analyzing the functional group types and structural characteristics of the three biochars; X-ray photoelectron spectroscopy (XPS) (AMICU, Shimadzu Corporation, Kyoto, Japan) was employed to analyze the C fitting of the three biochars, determining the functional groups constituted by C elements [18,19].
2.5. Research Methodology for Technical Standardization and Legal Coordination Mechanisms
This research focuses on standardization pathways and legal coordination mechanisms for ecological restoration technologies, primarily employing an interdisciplinary research approach that combines empirical analysis with normative analysis methods. First, based on experimental data from biochar (YBC, HBC, FBC) coupled with intermittent aeration applications in constructed wetlands for low carbon-to-nitrogen ratio wastewater treatment, the Chinese environmental technology standards analysis framework was adopted to explore pathways for optimizing technical standard systems through key technical indicators. Second, utilizing normative analysis methods from environmental and resource law research, with existing environmental and resource legal norms as the foundation, the coordination pathways between technology and legal norms were investigated. Finally, an integrated pathway of “technical standardization—legal transformation—collaborative governance” was proposed, balancing technical precision with legal normativity to improve watershed ecological restoration institutional systems.
3. Results and Discussion
3.1. Biochar Characteristics and Adsorption Performance
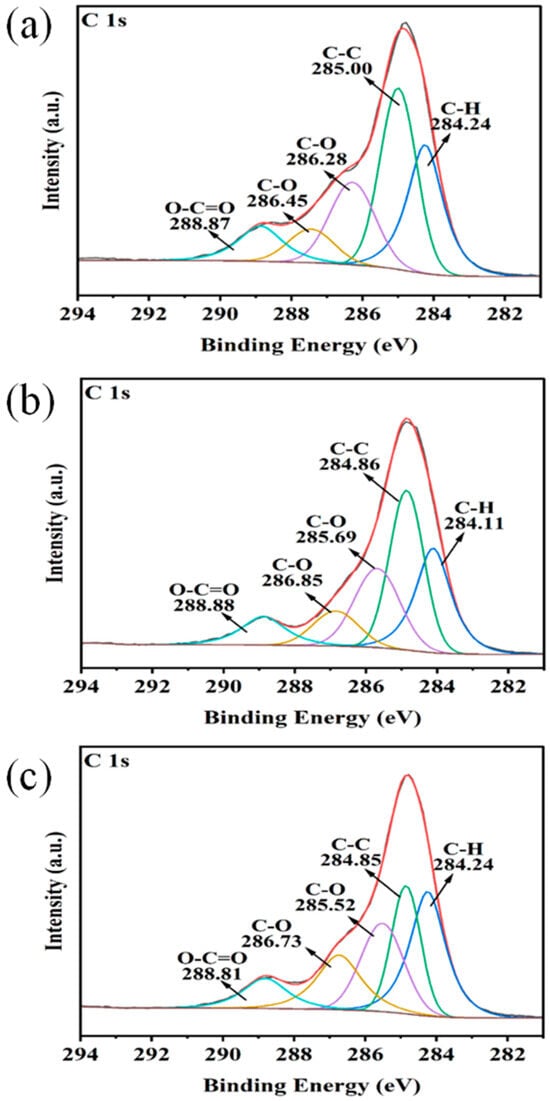
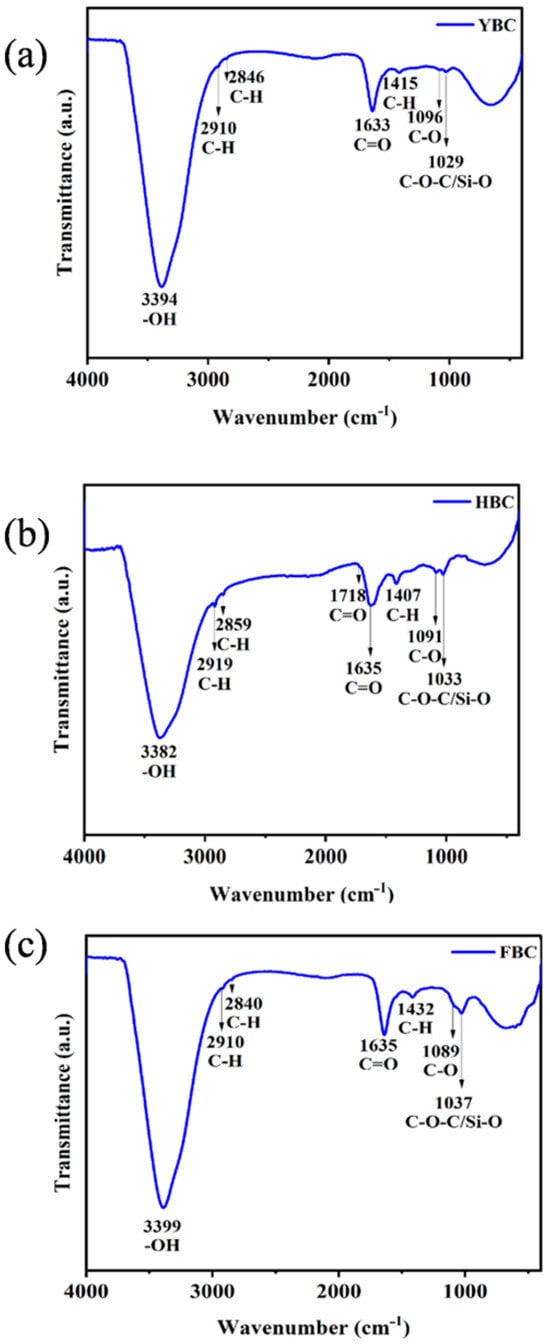
Characterization of surface functional groups on biochar through XPS and FTIR revealed C-containing functional groups and their mechanisms for NH4+ adsorption [20]. XPS analysis indicated that C-H/C-C bonds comprised the highest proportion in all three biochars (Table 2 and Figure 2), followed by C-O bonds (indicating hydroxyl/ether group enrichment), while the significant presence of C=O bonds in FBC confirmed its carbonyl functional group advantage. FTIR was employed to further elucidate the structure–function relationships of functional groups. In YBC (Figure 3a), O-H stretching vibrations at 3394 cm−1 formed hydrophilic interfaces with adsorbed water, enabling NH4+ capture through hydrogen bonding; coordination complexation was achieved synergistically through C=O at 1633 cm−1 and C-H at 1415 cm−1; Si-O vibrations at 1029 cm−1 enhanced adsorption capacity through ion exchange. In HBC (Figure 3b), -OH at 3382 cm−1 and carboxylic C=O at 1718 cm−1 collaboratively established acidic microenvironments, promoting protonation-driven ion exchange; C-H vibrations at 2919/2859 cm−1 suggested aliphatic chain participation in hydrophobic interaction regulation; conjugated carbonyls at 1635 cm−1 potentially enhanced NH4+ selectivity through π–cation interactions; C-O at 1091 cm−1 and Si-O at 1033 cm−1 not only formed rigid frameworks to stabilize adsorption sites but also enhanced adsorption capacity through electrostatic interactions, where silicon oxide compounds constituted by Si-O possessed nanoscale porous structures that significantly increased material porosity and specific surface area, enhancing NH4+ adsorption [21]. FBC (Figure 3c) exhibited synergistic formation of highly polar surfaces through O-H at 3399 cm−1 and C=O at 1635 cm−1, immobilizing NH4+ via electrostatic attraction; weakened C-H vibrations at 2910/2840 cm−1 reflected pyrolysis-induced alkane fragmentation, potentially increasing specific surface area; Si-O and C-O vibrations at 1037/1089 cm−1 constituted multi-level adsorption sites, coupling physical entrapment with chemical bonding. In summary, all three types of biochar contain Si-O, which enhances the adsorption of NH4+. The high porosity and electrostatic attraction of HBC act in a synergistic manner, thereby enhancing the adsorption capacity of NH4+.
As shown in Figure 4, the adsorption process of NH4+-N by biochar exhibited significant kinetic differences: the initial phase (0–5 min) was dominated by rapid surface adsorption, where HBC achieved higher initial adsorption quantities through ion exchange and hydrogen bonding due to its high porosity (porous structure supported by Si-O bonds) and incompletely destroyed carboxyl (-COOH) and hydroxyl (-OH) groups [22]; with time extension (5 min–24 h), mass transfer resistance of NH4+-N within pores increased, gradually reducing adsorption rates, but the uneven electron cloud distribution of conjugated olefinic carbonyls (C=O) in HBC strengthened coordination complexation capability, while silicon oxide compounds as pore fillers further expanded specific surface area, ultimately achieving an equilibrium adsorption capacity of 32.44 mg/L, significantly superior to YBC and FBC [23]. Gani et al. also confirmed that hydroxyl groups (-OH) of phenols and alcohols, olefins (C=C), and aromatic rings (C-H) are core active sites for biochar adsorption [24]; Dong et al. indicated that synergistic effects of carboxyl groups and silicon oxides significantly enhance NH4+-N immobilization efficiency, consistent with the carboxylic acid protonation (-COOH → -COO−) and Si-O− ion exchange mechanisms observed in HBC in this experiment [25].
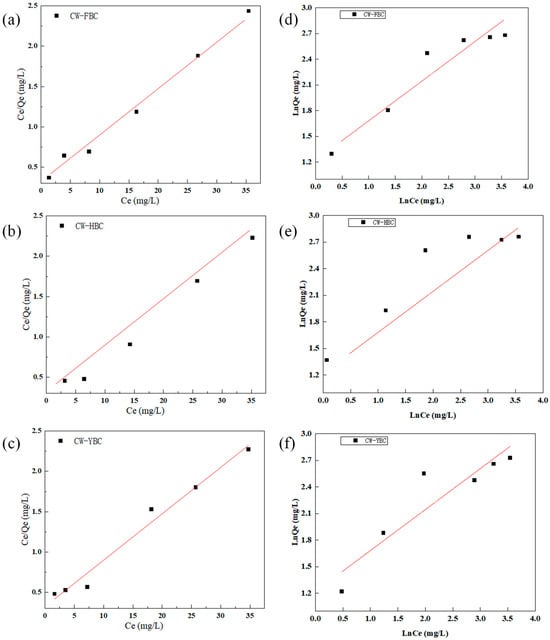
Table 3 presents adsorption isotherm fitting data calculated using Langmuir and Freundlich isothermal adsorption models. Figure 5 shows the fitted curve for the adsorption isotherm study. HBC and FBC were dominated by the Langmuir model, indicating primarily monolayer adsorption with uniform surface site distribution and no interactions between adsorbates. YBC and FBC showed better fit with the Freundlich model, reflecting surface heterogeneity (uneven distribution of pores and functional groups), with adsorption between monolayer and multilayer. HBC exhibited the highest Langmuir binding constant and lowest 1/n value, indicating strong NH4+ affinity and facile adsorption, suitable for low-concentration acidic wastewater. YBC showed the highest Langmuir maximum adsorption capacity but better Freundlich model compatibility, suitable for multimodal adsorption of high-concentration wastewater. FBC combined both Langmuir (chemical bonding) and Freundlich (physical entrapment) characteristics, demonstrating outstanding wide-concentration adaptability. C. Sun et al. discovered that the Langmuir model compatibility of walnut shell biochar was consistent with this experimental result, supporting the universality of monolayer adsorption mechanisms [26].
3.2. Removal Performance of Nitrogen and COD in Constructed Wetlands
3.2.1. Nitrogen Removal Performance in Constructed Wetlands
- 1.
- NO2−−N Removal
As an intermediate product of nitrification and denitrification, NO2−-N was rapidly reduced to N2 by denitrifying bacteria under low dissolved oxygen conditions (DO < 0.5 mg/L) in Stage I, but accumulation may occur under different conditions. In this study, the CW-HBC system exhibited the lowest NO2−-N accumulation (0.02–0.06 mg/L) due to its developed porous structure promoting anaerobic microbial proliferation and accelerating NO2−-N reduction [27]. After Stage II, increased DO inhibited denitrification while ammonia-oxidizing bacteria (AOB) activity preceded nitrite-oxidizing bacteria (NOB), leading to NO2−-N accumulation. Reduced HRT weakened biochar adsorption capacity, and decreased C/N caused insufficient electron donors for denitrification, both exacerbating NO2−-N accumulation [28].
- 2.
- NH4+−N Removal
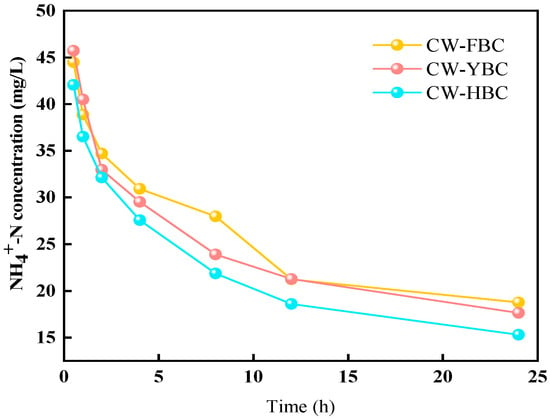
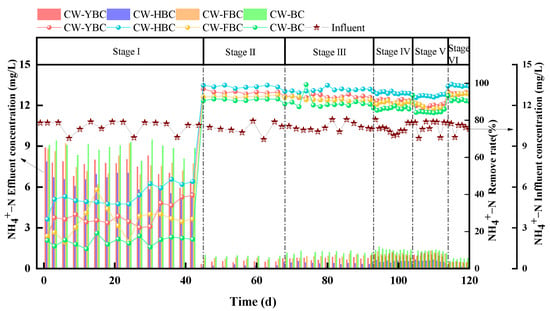
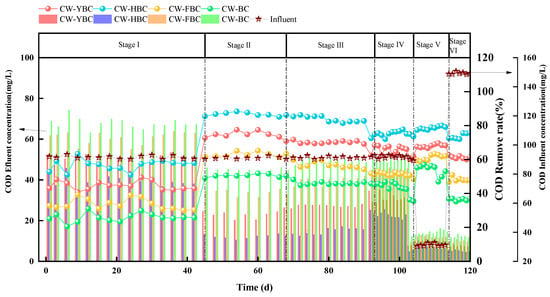
As shown in Figure 6, Stage I demonstrated significant differences in NH4+-N removal efficiency among constructed wetlands (CWs). CW-HBC exhibited superior performance with removal rates of 26.6–46.9%, significantly higher than other systems. Due to the filling effect of silicon oxide compounds in biochar, NH4+-N adsorption capacity was significantly enhanced through increased material porosity and specific surface area. In contrast, CW-BC showed the lowest removal rate due to limited adsorption capacity resulting from simple porous structure and low functional group density [29]. In Stage II, all CWs demonstrated substantially improved NH4+-N removal rates, particularly with CW-HBC achieving 97.8% NH4+-N removal (ANOVA, p < 0.01; n = 3). This result is consistent with the findings of Zhou et al., where the efficiency for NH-N exceeded 95% [30]. Aeration significantly increased DO concentration, promoting nitrifying bacteria activity and making nitrification reactions (NH4+ → NO3−) the dominant removal pathway [31].
From Stage III to Stage IV, HRT gradually decreased. During this process, all four experimental systems experienced varying degrees of decline in NH4+-N removal rates. At HRT of 1 day, CW-HBC’s NH4+-N removal rate decreased to 93.6% but remained advantageous compared to other systems. The primary reason was that reduced HRT decreased the adsorption time for NH4+-N by substrates and biochar, leading to decreased NH4+-N removal rates.
NH4+-N removal efficiency was also influenced by C/N ratios. Increased C/N led to decreased NH4+-N removal rates because heterotrophic bacteria preferentially utilized DO for aerobic respiration when organic matter was abundant, intensifying DO competition and inhibiting nitrifying bacteria activity. Xu Zhou et al. confirmed this in their study on the effects of biochar-coupled intermittent aeration treatment of low C/N wastewater on CW microbial community structure [32]. CW-FBC and CW-BC showed no significant differences in removal rates under different C/N conditions, possibly because plant oxygen release capacity was weak in CW-FBC, limiting nitrification efficiency, while biochar enhanced NH4+-N removal [33]. However, as shown in Figure 6, under different HRT and C/N conditions, effluent NH4+-N concentrations from all systems remained stably below 5 mg/L, meeting local discharge standards.
- 3.
- NO3−−N Removal
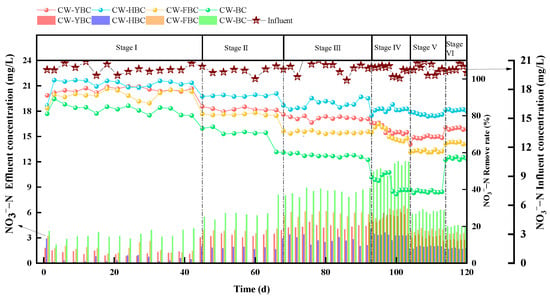
The denitrification process in CWs depends on synergistic nitrification and denitrification, where denitrification is the key step for efficient NO3−-N removal. As shown in Figure 7, in Stage I, plant root oxygen release and atmospheric reaeration within the system were far below aerobic microbial demands, resulting in prolonged anoxic environments. Under these conditions, anaerobic denitrifying bacteria proliferated extensively, with denitrification reactions dominating (Figure 7), exhibiting high NO3−-N removal rates, particularly with CW−HBC achieving 85.5–99.2% removal efficiency, while NH4+-N accumulated due to limited nitrification. In Stage II, NO3−−N removal rates for CW-YBC, CW-HBC, CW-FBC, and CW-BC decreased to 80.6%, 85.65%, 71.75%, and 60.0%, respectively. Although aerobic environments inhibited denitrifying bacteria activity, biochar partially offset the negative effects of increased DO through physical entrapment (micropore adsorption) and chemical complexation (oxygen-containing functional groups), and biochar’s special porous structure provided anoxic microenvironments for denitrifying bacteria while adsorbing and retaining NO3−, further enhancing denitrification effects to some extent [34].
NO3−−N removal efficiency was also affected by HRT. From Stage II to Stage III, all four systems experienced varying degrees of decline in NO3−−N removal rates. When HRT was further shortened to 1 day, removal rates continued to decrease to 64.5%, 81.9%, 60.3%, and 39.8%, respectively. This was primarily because shortened HRT reduced physical entrapment and chemical complexation time for NO3− by substrates, leading to NO3− ion accumulation and decreased removal efficiency [35].
Results also indicated that NO3−−N removal efficiency in CWs was significantly positively correlated with influent C/N ratios. In Stage V, NO3−−N removal rates for CW-YBC, CW-HBC, CW-FBC, and CW-BC were 64.5–73.5%, 79.6–80.1%, 60.0–60.7%, and 38.4–39.8%, respectively (Figure 7). At C/N=1, denitrifying bacteria exhibited reduced nitrate reductase activity due to insufficient electron donors, leading to NO3−−N accumulation in the system. As C/N increased, NO3−−N removal rates significantly improved across all systems. Biochar (especially HBC) provided electron donors for denitrifying bacteria through pyrolysis-derived dissolved organic matter release as slow-release carbon sources; aerobic degradation of organic matter by heterotrophic bacteria reduced system redox potential, promoting denitrifying bacteria proliferation [32,36].
- 4.
- TN Removal
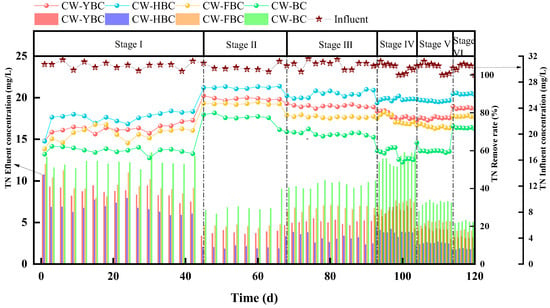
TN removal in CWs primarily relies on microbially driven nitrification–denitrification processes, supplemented by substrate adsorption and plant uptake. As shown in Figure 8, during non-aeration phases, TN removal rates were low due to DO limitation inhibiting nitrifying bacteria activity, while biochar significantly enhanced TN retention capacity through adsorption, with HBC demonstrating superior removal rates due to synergistic effects of porous structure and surface functional groups [37].
After aeration, increased DO concentrations provided necessary conditions for nitrification processes, while sufficient carbon sources ensured smooth denitrification processes, with their synergistic effects significantly improving TN removal rates across all systems. Shortened HRT led to decreased TN removal rates, primarily attributed to reduced adsorption time for pollutants by biochar while potentially limiting complete nitrification and denitrification [38,39]. Treatment results for different C/N wastewaters indicated that relying solely on biochar adsorption capacity for pollutant removal was limited; sufficient DO and carbon sources were also required. Higher C/N ratios better enabled wetland systems to create alternating aerobic–anoxic environments, forming complete nitrification–denitrification pathways and improving TN removal rates [40]. When C/N decreased, carbon source deficiency led to insufficient electron donors during denitrification, causing intermediate product NO2−−N accumulation that could not be effectively reduced to N2, ultimately resulting in decreased TN removal rates [41]. C/N regulation experiments demonstrated that biochar-enhanced CW pollutant treatment required sufficient DO and carbon sources [42].
CW-HBC outperformed other systems in this study, primarily due to the following: (1) HBC’s multi-level pore networks forming alternating aerobic–anoxic zones that, respectively, enriched nitrifying and denitrifying bacteria; (2) HBC’s rough surface providing high-density attachment sites for microorganisms, forming thick biofilms that enhanced substrate mass transfer efficiency. HBC’s slow-release carbon synergistically provided reducing power with influent organic matter. In Stage V, carbon source scarcity led to denitrification of intermediate product, NO2−−N accumulation, and decreased TN removal rates. Zhou Xu et al.’s research further verified that HBC could enhance denitrification rates and reduce effluent NO3−−N concentrations through DOM release [32]. This study demonstrated that under sufficient DO and carbon source conditions, CW-HBC achieved efficient and stable TN removal through a “adsorption-biofilm-microzone regulation” integrated mechanism, providing innovative solutions for low C/N wastewater treatment.
3.2.2. COD Removal Performance in Constructed Wetlands
Under non-aeration conditions, significant differences in COD removal rates among systems were primarily attributed to anoxic environments inhibiting heterotrophic bacteria activity, impeding organic matter degradation, while biochar significantly improved COD adsorption capacity through enhanced intermolecular interactions and electrostatic attraction from surface functional groups, achieving higher removal rates than CW-BC [43]. During aeration phases, increased DO concentrations enhanced aerobic heterotrophic bacteria metabolic activity, universally improving COD removal rates. CW-HBC performed optimally, with HBC’s porosity and high specific surface area providing ideal interfaces for microbial attachment and substrate mass transfer; FTIR and XPS analyses indicated that oxygen-containing functional groups on HBC surfaces directionally enriched degradation through hydrogen bonding and hydrophobic interactions, accelerating organic matter mineralization and achieving higher COD removal rates than other systems [44].
As HRT shortened, reduced adsorption of organic matter by biochar and gravel, along with decreased plant aerobic respiration uptake of organic matter, led to decreased COD removal rates across all four systems [45]. As shown in Figure 9, from Stage IV to Stage III, COD removal rates for CW-YBC, CW-HBC, CW-FBC, and CW-BC decreased by 12.9%, 13.2%, 13.6%, and 15%, respectively.
When C/N increased, organic loading intensified, high organic loads triggered DO competition, causing localized system anaerobic conditions where denitrification and methanogenesis dominated, decreasing COD removal rates with minimal fluctuation [46]. Compared to other systems, HBC’s efficient COD removal was attributed to “adsorption–biodegradation” synergistic mechanisms, where micropores retained macromolecular organic matter through size screening effects while surface-loaded degrading bacteria catalyzed refractory substance oxidation through extracellular enzymes. Zhou Xu et al.’s research confirmed that carboxyl and carbonyl functional groups in HBC enhanced microbial–substrate interface electron transfer, improving COD degradation rates by 30%, which had similar effects to the high COD removal observed in this study [47].
3.3. Microbial Community Response in Constructed Wetlands
This study analyzed microbial community structure in constructed wetland systems through 16S rRNA sequencing. Alpha diversity indices differed under aeration and non-aeration phases, as well as different HRT and C/N conditions (Table 4). Key findings showed that CW-FBC exhibited the highest species richness under anoxic conditions, while CW-HBC demonstrated optimal community stability in Stage I. After intermittent aeration introduction (Stage II), CW-HBC’s species richness significantly increased, possibly due to endogenous pollutant release. Community diversity peaked in Stage IV but decreased significantly in Stage V due to carbon source limitation [48].
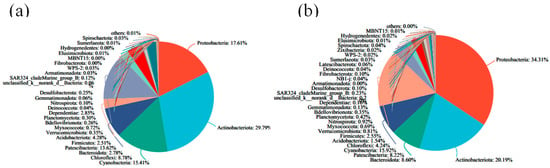
Biochar addition significantly altered dominant bacterial phyla composition. Without biochar, the top three phyla were Proteobacteria, Actinobacteriota, and Bacteroidota. After biochar addition, nitrogen-related phyla changed to Actinobacteriota (29.79%), Proteobacteria (17.61%), and Cyanobacteria (15.41%) (Figure 10). These three phyla demonstrated strong associations with nitrogen removal processes: Proteobacteria possessed electrochemically active characteristics enhancing nitrogen metabolism [49], while Actinobacteriota and Proteobacteria showed positive correlations with nitrification and denitrification under intermittent aeration conditions.
Figure 10.
(a) CW microbial phylum-level community structure diagram with biochar added and (b) without biochar added.
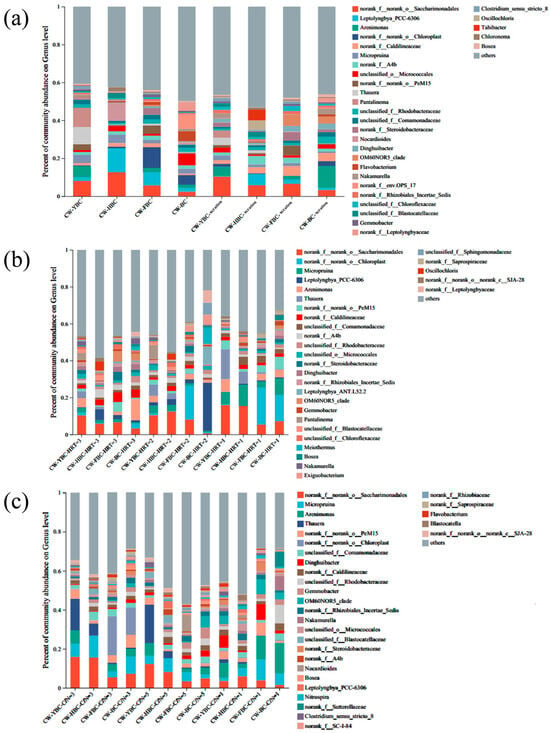
At the genus level, functional microbial groups responded differently to operational parameters (Figure 11). Denitrification-related genera included Saccharimonadales and Leptolyngbya, whose proportions varied with biochar type and aeration conditions. Competition-related genera such as Arenimonas increased under carbon-limited conditions, potentially inhibiting efficient denitrifying bacteria. Key operational effects included the following: Biochar-coupled aeration enriched efficient denitrifying groups in CW-HBC while increasing Saccharimonadales in CW-YBC through organic carbon release; shortened HRT promoted non-denitrifying genera (Chloroplast, Micropruina), reducing system efficiency; low C/N increased Arenimonas proportions (4.2–15.6%), weakening denitrification through carbon competition; high C/N decreased Saccharimonadales due to intensified DO competition among heterotrophic microorganisms [50,51].
4. Institutional and Regulatory Implications
4.1. Standards Level: Incorporating Biochar-Coupled Intermittent Aeration into Water Ecological Environment Treatment Technical Standards
Technical standards constitute a system with the highest concentration of quantitative indicators and the most frequent interactions with legal frameworks. Water environment technical standards, as technical specifications for rational categorization of environmental governance practices, can effectively enhance environmental governance efficiency and provide specific, measurable quantitative indicators as reference criteria, addressing the ambiguous status quo in water ecological restoration and environmental governance [52]. It is important to acknowledge that the present study was conducted under laboratory-scale conditions using synthetic effluent over a relatively short experimental period. Despite these limitations, experimental results demonstrate that biochar achieves efficient NH4+−N removal through surface functional group-mediated “multimodal synergistic adsorption” mechanisms (such as ion exchange, hydrogen bonding, and coordination complexation), while intermittent aeration coupled with modified biochar (such as the CW-HBC system) can stably achieve compliant NH4+−N removal. To fully leverage this technology’s role in watershed water ecological environment governance (particularly low C/N wastewater treatment) and enhance governance efficiency, it is urgent to incorporate its core elements into and improve relevant ecological environment technical standard systems. However, before this technology can be included in technical standards, it is essential to conduct pilot-scale experiments with real effluents and evaluate the long-term performance of the proposed technology. Only with more robust experimental data can the practical applicability of this technology be comprehensively assessed and its potential inclusion in technical standards be justified.
The first measure is to advance standard development and pilot applications. Given the increasingly severe eutrophication and associated black and odorous water problems, low carbon-to-nitrogen ratio (C/N) wastewater has become a common challenge in water ecological restoration, sewage treatment, and environmental engineering due to carbon source scarcity constraining denitrification efficiency. Biochar-coupled intermittent aeration technology demonstrates significant advantages through its efficient nitrogen removal and relatively economical operational costs. Given the limitations of current research based on laboratory conditions and synthetic effluent, it is necessary to prioritize pilot-scale experiments using real wastewater in the future, evaluating the long-term stability and treatment effectiveness of biochar-coupled intermittent aeration technology under actual application conditions, as well as improve technical standards based on comprehensive experimental results from scaled-up studies.
The second measure is to improve technical specifications and application guidelines. This experimental data confirms that biochar-coupled intermittent aeration technology significantly enhances the comprehensive treatment efficiency of constructed wetlands for low C/N wastewater, with key indicators demonstrating excellent performance: NH4+−N (equilibrium adsorption capacity reaching 32.44 mg/L), TN (removal rate 80.0%), and COD (removal rate 86.7%). Beyond industry self-regulatory standards, this technology should be incorporated into national and local water ecological environment technical standards through standardization procedures according to the requirements of the “Administrative Measures for Ecological Environment Standards.” Standard development must be based on ecological environment quality characteristics and baseline research, considering economic and social development needs and public environmental demands, while scientifically establishing the following protection objectives: (1) clarifying biochar physicochemical property standards (such as carbon-containing functional group composition, porosity, specific surface area, and other key parameters) to ensure adsorption performance; (2) standardizing intermittent aeration operational parameters (such as aeration intensity, cycles, duration) and developing differentiated guidelines considering various application scenarios (such as urban–rural differences and pollution loads); (3) establishing microbial community management requirements (such as key functional bacterial group structures and community stability indicators). Establishing a comprehensive technical standard system can effectively supplement environmental resource legal systems and enhance water environment governance efficiency.
The third measure is to construct a synergistic and integrated standardization system. Based on the intrinsic mechanisms (such as adsorption–biodegradation synergy and microzone oxygen regulation) and performance characteristics of biochar-coupled intermittent aeration technology for low C/N wastewater treatment, the water ecological environment technical standard system should be further improved. This system should cover the entire process including pollutant discharge limits, process monitoring methods, and treatment technology specifications. Regional differences should be fully considered, encouraging localities to develop or refine local ecological environment standards (such as technical procedures for different types of black and odorous water bodies) based on their water environment characteristics (such as rural scattered pollution sources versus urban concentrated discharge). The ultimate goal is to achieve effective integration and coordinated operation between the biochar-coupled intermittent aeration technology standard system and existing environmental management systems.
4.2. Regulatory Level: Achieving Synergy Between Treatment Technologies Such as Biochar-Coupled Intermittent Aeration and Legal Norms
China’s current environmental resource legal system has preliminarily formed, encompassing environmental regulations (such as the Water Pollution Prevention and Control Law, Air Pollution Prevention and Control Law, Soil Pollution Prevention and Control Law), resource regulations (such as the Water Law, Grassland Law, Forest Law), and ecological regulations (such as the Yangtze River Protection Law, Qinghai–Tibet Plateau Ecological Protection Law, Nature Reserve Regulations), with ongoing efforts to compile an “Ecological Environment Code.” To achieve effective integration between advanced treatment technologies such as biochar-coupled intermittent aeration and legal norms, institutionalization pathways within the environmental resource legal framework must be explored.
The first measure is to promote legal amendments and technology legalization. Relevant legal amendment procedures should be initiated promptly to transform experimentally verified key technical parameters (such as biochar porosity and specific functional group content) and common patterns (such as Si−O bonds enhancing NH4+ adsorption through ion exchange) into legally binding technical requirements or recommended specifications. Based on this technology’s process characteristics (such as “micro-ecological regulation” and “multi-pathway synergistic nitrogen removal”), core elements of technical specifications should be extracted in chapters such as “Water Pollution Prevention” and “Ecological Restoration” in the “Ecological Environment Code,” clearly establishing such treatment technologies as statutory water pollution prevention measures, providing a legal basis for optimizing low C/N wastewater treatment processes [53]. Simultaneously, legal frameworks should encourage sewage treatment facilities to adopt intermittent aeration strategies and optimized media (such as biochar) to construct suitable functional microbial habitats.
The second measure is to innovate legislative techniques and regulatory forms. Legal standardization of technology requires enhancing legislative technical capabilities and innovating regulatory forms. On the one hand, intrinsic patterns of technology application should be summarized, elevating experimentally confirmed stable correlation parameters (such as biochar surface carboxyl content, adsorption model fitting goodness (R2 values), optimal intermittent aeration cycles) to basic legal requirements or assessment benchmarks, achieving legal standardization of technical parameters. On the other hand, uncertainties in technological development must be acknowledged, adhering to “inclusive and prudent” legislative principles. When creating relevant legal norms, focus should be placed on core performance indicators and actual needs of water ecological environment governance, balancing necessary precision of technical indicators with reasonable universality of legal norms, avoiding direct incorporation of overly specific process details into legal provisions, which could lead to rigid legal application and hinder technological innovation.
The third measure is to strengthen legal implementation and integrated governance. Legal transformation of biochar-coupled intermittent aeration technology requires ensuring its scientific validity and adaptability while guaranteeing regulatory enforceability. This demands optimization of environmental legal responsibility systems and exploration of “technical compliance” mechanisms in water ecological environment governance. Specifically, by clarifying compliance with technical standard requirements as circumstances for fulfilling legal obligations or reducing liability, technology application entities are encouraged to actively follow technical specifications, achieving coordinated unity between technological innovation application and legal compliance adherence.
Environmental problems possess high degrees of professionalism, regionality, and uncertainty, making environmental resource legal norms inherently technology-dependent. Technical specifications can span the entire lifecycle of environmental governance: preventive risk warning through monitoring technology; enhanced supervision through technical means during implementation; post-incident responsibility clarification and restoration guidance through technical assessment. Efficient treatment technologies such as biochar-coupled intermittent aeration are important tools for enhancing water ecological environment governance effectiveness. Effective experiences from empowering environmental governance practices should be promptly summarized and combined with feedback on technical standard implementation effects, continuously promoting deep synergistic governance between technology and environmental legal norms.
5. Conclusions
This study confirms that HBC-modified biochar achieves optimal NH4+−N adsorption during the non-aeration phase through the synergistic effect of acidic functional groups and multi-level pores. Based on this, intermittent aeration is coupled with dynamic regulation of dissolved oxygen concentration to achieve efficient removal of TN, NH4+−N, and COD at HRT = 3 d and C/N = 5. CW-HBC, relying on its high porosity and abundant functional groups, enriches functional microbial communities centered on the Proteobacteria phylum, reshaping the community structure and strengthening electron transfer pathways to synergistically enhance denitrification performance. Concurrently, this study proposes a dual-track approach for the institutionalization of the biochar-assisted intermittent aeration technology (CW-HBC): At the standard level, it is imperative that key physical and chemical parameters, as well as operational strategies, be incorporated into a multi-tiered water ecological environment technology standard system. The development of standards at the industry, national, and local levels must be coordinated to ensure the standardization of methodologies for the treatment of low C/N wastewater. At the regulatory level, common mechanisms must be transformed into statutory technical requirements through legislative revisions. Furthermore, legislative technical innovations must be leveraged to balance technical precision and legal universality to establish legal status.
Author Contributions
Conceptualization, G.Y.; Data curation, J.H. and Q.C.; Writing—original draft, S.T.; Writing—review & editing, M.L. and S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Youth Project of the National Social Science Foundation of China grant number (24CFX101).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
Financial support from the Youth Project of the National Social Science Foundation of China (24CFX101) is highly appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jin, X.; Xu, Q.; Huang, C. Current Status and Future Tendency of Lake Eutrophication in China. Sci. China C Life Sci. 2005, 48, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Removal of Nutrients in Various Types of Constructed Wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A Review on the Sustainability of Constructed Wetlands for Wastewater Treatment: Design and Operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Jinadasa, K.B.S.N.; Gersberg, R.M.; Liu, Y.; Ng, W.J.; Tan, S.K. Application of Constructed Wetlands for Wastewater Treatment in Developing Countries—A Review of Recent Developments (2000–2013). J. Environ. Manag. 2014, 141, 116–131. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and Inorganic Contaminants Removal from Water with Biochar, a Renewable, Low Cost and Sustainable Adsorbent—A Critical Review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, L.; Zheng, J.; Tan, Y.; Li, Y.; Wei, L.; Zhang, F.; Zhu, L. Enhancing Nitrogen Removal in Low C/N Wastewater with Recycled Sludge-Derived Biochar: A Sustainable Solution. Water Res. 2024, 267, 122551. [Google Scholar] [CrossRef]
- Saeed, T.; Sun, G. A Review on Nitrogen and Organics Removal Mechanisms in Subsurface Flow Constructed Wetlands: Dependency on Environmental Parameters, Operating Conditions and Supporting Media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Zhang, H.; Wu, H. Enhanced Nitrogen Removal of Low C/N Domestic Wastewater Using a Biochar-Amended Aerated Vertical Flow Constructed Wetland. Bioresour. Technol. 2017, 241, 269–275. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, W.; Wang, W.; Yin, W.; Liu, H.; Ma, X.; Zhou, Y.; Lei, P.; Wei, D.; Zhang, L.; et al. A Review on China’s Constructed Wetlands in Recent Three Decades: Application and Practice. J. Environ. Sci. 2021, 104, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; You, Z.; Huang, Y.; Peng, J.; He, T.; Su, T.; Li, Y.; Ragauskas, A.J.; Wang, S.; Song, X.; et al. New Insights in the Formation Mechanism of Cellulose-Based Biochar. Small 2025, 21, 2410597. [Google Scholar] [CrossRef]
- Li, J.; Gu, G.; Zhang, J.; Wang, Y.; Peng, C.; Li, Y.; Yang, S.; Tao, E. MnSO4-Modified Woodchip Biochar “Dual Fixation” Mechanism: Functional Group-Electron Synergistic Stabilization of Heavy Metals and Carbon Structure. J. Environ. Chem. Eng. 2025, 13, 116706. [Google Scholar] [CrossRef]
- Abbey, A.N.A.; Frimpong, K.A.; Odoi-Yorke, F.; Ampofo, E.A.; Darko, R.O. A Review of Biochar’s Sustainability in Climate-Smart Agriculture: Recent Advances, Emerging Trends, and Future Directions. Eur. J. Agron. 2025, 169, 127690. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Zhang, Y.; Zhang, X. Effects of HRT on the Efficiency of Denitrification and Carbon Source Release in Constructed Wetland Filled with Bark. Water Sci. Technol. 2017, 75, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Kong, Q. Nitrogen Removal and Nitrous Oxide Emission in Surface Flow Constructed Wetlands for Treating Sewage Treatment Plant Effluent: Effect of C/N Ratios. Bioresour. Technol. 2017, 240, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, R.; Feng, L.; Zhou, X.; Lv, J.; Wu, H. Intensified Nitrogen Removal in Intermittently-Aerated Vertical Flow Constructed Wetlands with Agricultural Biomass: Effect of Influent C/N Ratios. Chem. Eng. J. 2018, 345, 22–30. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, X.; Shi, H.; Li, J.; Zhang, M.; Zhao, J.; Su, X. Comprehensive Assessment of 16S rRNA Gene Amplicon Sequencing for Microbiome Profiling across Multiple Habitats. Microbiol. Spectr. 2023, 11, e00563-23. [Google Scholar] [CrossRef]
- Alemu, T. Post-Treatment of Tannery Wastewater Using Pilot Scale Horizontal Subsurface Flow Constructed Wetlands (Polishing). Water Sci. Technol. 2017, 77, 988–998. [Google Scholar] [CrossRef]
- Lago, B.C.; Silva, C.A.; Melo, L.C.A.; Morais, E.G.D. Predicting Biochar Cation Exchange Capacity Using Fourier Transform Infrared Spectroscopy Combined with Partial Least Square Regression. Sci. Total Environ. 2021, 794, 148762. [Google Scholar] [CrossRef]
- Ghobeira, R.; Esbah Tabaei, P.S.; Morent, R.; De Geyter, N. Chemical Characterization of Plasma-Activated Polymeric Surfaces via XPS Analyses: A Review. Surf. Interfaces 2022, 31, 102087. [Google Scholar] [CrossRef]
- Chand, N.; Kumar, K.; Suthar, S. Enhanced Wastewater Nutrients Removal in Vertical Subsurface Flow Constructed Wetland: Effect of Biochar Addition and Tidal Flow Operation. Chemosphere 2022, 286, 131742. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, T.; Chen, Y.; Tian, G.; Sharma, V.K.; Zhu, Y.; Zong, L.; Wang, A. Mesoporous Silicate/Carbon Composites Derived from Dye-Loaded Palygorskite Clay Waste for Efficient Removal of Organic Contaminants. Sci. Total Environ. 2019, 696, 133955. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, M.; Meng, X.; Zhou, J.L.; Zhang, H.; Shen, X. The Role of Biochar on Alleviating Ammonia Toxicity in Anaerobic Digestion of Nitrogen-Rich Wastes: A Review. Bioresour. Technol. 2022, 351, 126924. [Google Scholar] [CrossRef]
- Zhou, A.; Zhu, L.; Chen, Y.; Wang, J.; Liu, Y. Fast Adsorption of Low-Concentration Ammonia Nitrogen by Persulfate-Modified Carbon Materials: Structure Influence, Performance, and Mechanism. Environ. Res. 2025, 278, 121680. [Google Scholar] [CrossRef]
- Gani, T.Z.H.; Berkson, Z.J.; Zhu, R.; Kang, J.H.; Di Iorio, J.R.; Chan, K.W.; Consoli, D.F.; Shaikh, S.K.; Copéret, C.; Román-Leshkov, Y. Promoting Active Site Renewal in Heterogeneous Olefin Metathesis Catalysts. Nature 2023, 617, 524–528. [Google Scholar] [CrossRef]
- Dong, L.; Hou, L.; Wang, Z.; Gu, P.; Chen, G.; Jiang, R. A New Function of Spent Activated Carbon in BAC Process: Removing Heavy Metals by Ion Exchange Mechanism. J. Hazard. Mater. 2018, 359, 76–84. [Google Scholar] [CrossRef]
- Sun, C.; Wang, G.; Liu, Y.; Bei, K.; Yu, G.; Zheng, W.; Liu, Y. The Adsorption Mechanism and Optimal Dosage of Walnut Shell Biochar for Chloramphenicol. Heliyon 2024, 10, e39123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, D.; Zhou, C.; Huang, X.; Chen, Y.; Wang, S.; Liu, G. Enhanced Nitrogen Removal via Partial Nitrification/Denitrification Coupled Anammox Using Three Stage Anoxic/Oxic Biofilm Process with Intermittent Aeration. Water Res. 2024, 255, 121491. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yin, J.; Zhang, Y.; Luo, A.; Tian, Y.; Liu, Y.; Peng, Y. Nitrogen Removal and Carbon Reduction of Mature Landfill Leachate under Extremely Low Dissolved Oxygen Conditions by Simultaneous Partial Nitrification Anammox and Denitrification. Bioresour. Technol. 2024, 401, 130704. [Google Scholar] [CrossRef]
- Fan, X.; Wang, S.; Zhang, Y.; Zhao, M.; Zhou, N.; Fan, S. Effect of Citric Acid Modification on the Properties of Hydrochar and Pyrochar and Their Adsorption Performance toward Methylene Blue: Crucial Roles of Minerals and Oxygen Functional Groups. Environ. Monit. Assess. 2024, 196, 664. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, L.; Zhang, H.; Wu, H. Determination of the Optimal Aeration for Nitrogen Removal in Biochar-Amended Aerated Vertical Flow Constructed Wetlands. Bioresour. Technol. 2018, 261, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Briceño, C.I.; Pozarlik, A.K.; Bramer, E.A.; Niedzwiecki, L.; Pawlak-Kruczek, H.; Brem, G. Hydrothermal Carbonization of Wet Biomass from Nitrogen and Phosphorus Approach: A Review. Renew. Energy 2021, 171, 401–415. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Z.; Li, Z.; Wu, H. Impacts of Aeration and Biochar Addition on Extracellular Polymeric Substances and Microbial Communities in Constructed Wetlands for Low C/N Wastewater Treatment: Implications for Clogging. Chem. Eng. J. 2020, 396, 125349. [Google Scholar] [CrossRef]
- Wang, J.-F.; Cai, Z.-X.; Li, Y.-H.; Sun, Y.-Y.; Wu, H.-M.; Song, X.-S.; Zhu, H. Microbiota and Genetic Potential for Reducing Nitrous Oxide Emissions by Biochar in Constructed Wetlands. Sci. Total Environ. 2023, 903, 166489. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, D.; Hou, X.; Jiang, N.; Li, Y.; Ge, S.; Mu, Y.; Shen, J. Nitrification-Denitrification Co-Metabolism in an Algal-Bacterial Aggregates System for Simultaneous Pyridine and Nitrogen Removal. J. Hazard. Mater. 2023, 460, 132390. [Google Scholar] [CrossRef]
- Cui, H.; Feng, Y.; Lu, W.; Wang, L.; Li, H.; Teng, Y.; Bai, Y.; Qu, K.; Song, Y.; Cui, Z. Effect of Hydraulic Retention Time on Denitrification Performance and Microbial Communities of Solid-Phase Denitrifying Reactors Using Polycaprolactone/Corncob Composite. Mar. Pollut. Bull. 2024, 205, 116559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, A.; Huang, P.; Min, L.; Sun, H. Sorption and Molecular Fractionation of Biochar-Derived Dissolved Organic Matter on Ferrihydrite. J. Hazard. Mater. 2020, 392, 122260. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Yang, K.; Zeng, Q.; Le, L.; Ran, H.; Liu, D. Acid Treatment for Enhancing Hg0 Removal Efficiency of Chlorine-Loaded Biochar: Mechanism and Kinetic Analysis. Environ. Sci. Pollut. Res. 2023, 31, 4897–4909. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Zhou, L.; Zhu, Z.; Wu, Z.; Zhang, K.; Wang, Y.; Ju, T.; Ji, X.; Jin, D.; et al. Metagenomics Insights into High-Rate Nitrogen Removal from Municipal Wastewater by Integrated Nitrification, Partial Denitrification and Anammox at an Extremely Short Hydraulic Retention Time. Bioresour. Technol. 2023, 387, 129606. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, Y.; Van Loosdrecht, M.C.M.; Chen, G. Development of Nitrification and Elemental Sulfur-Based Denitrification/Anammox (NS0DA) Process for Mainstream Nitrogen Removal. Water Res. 2025, 283, 123836. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, J.; Li, M.; Zhuang, L.-L. Optimization of the Pollutant Removal in Partially Unsaturated Constructed Wetland by Adding Microfiber and Solid Carbon Source Based on Oxygen and Carbon Regulation. Sci. Total Environ. 2021, 752, 141919. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Yang, Y.; Fang, Y.; Luo, S.; Cheng, H.; Wang, A. Element Sulfur-Based Autotrophic Denitrification Constructed Wetland as an Efficient Approach for Nitrogen Removal from Low C/N Wastewater. Water Res. 2022, 226, 119258. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, W.; Zhao, H.; Tan, Q. Effects of Carbon to Nitrogen Ratio on Nitrogen Removal in a Single-Stage Microaerobic System: A Model-Based Evaluation. J. Environ. Manag. 2024, 359, 121007. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-H.; Jeong, Y.-H.; Nguyen, H.-H.T.; Kwak, D.-H. Experimental Application of Micro/Nano Bubbles to Control P Release and Separate P Particles from Benthic Lake Sediment. J. Contam. Hydrol. 2025, 268, 104466. [Google Scholar] [CrossRef] [PubMed]
- Özkan, A.; Stolley, D.L.; Cressman, E.N.K.; McMillin, M.; DeMorrow, S.; Yankeelov, T.E.; Rylander, M.N. Tumor Microenvironment Alters Chemoresistance of Hepatocellular Carcinoma Through CYP3A4 Metabolic Activity. Front. Oncol. 2021, 11, 662135. [Google Scholar] [CrossRef]
- He, S.; Ding, L.; Pan, Y.; Hu, H.; Ye, L.; Ren, H. Effect of Hydraulic Retention Time on Nitrogen Removal and Functional Gene Quantity/Transcription in Biochar Packed Reactors at 5 °C: A Control-Strategy Study. Bioresour. Technol. 2018, 264, 400–405. [Google Scholar] [CrossRef]
- Chang, Y.; Meng, J.; Hu, Y.; Lee, P.-H.; Zhan, X. A Review in Fe(0)/Fe(Ⅱ) Mediated Autotrophic Denitrification for Low C/N Wastewater Treatment. Water Res. 2025, 282, 123925. [Google Scholar] [CrossRef]
- Lehmann, J. A Handful of Carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Zhou, X.; Manna, B.; Lyu, B.; Lear, G.; Kingsbury, J.M.; Singhal, N. Resource Recovery from Wastewater by Directing Microbial Metabolism toward Production of Value-Added Biochemicals. Bioresour. Technol. 2025, 419, 132061. [Google Scholar] [CrossRef]
- Xu, W.; Yang, B.; Wang, H.; Zhang, L.; Dong, J.; Liu, C. Simultaneous Removal of Antibiotics and Nitrogen by Microbial Fuel Cell-Constructed Wetlands: Microbial Response and Carbon–Nitrogen Metabolism Pathways. Sci. Total Environ. 2023, 893, 164855. [Google Scholar] [CrossRef]
- Deng, X.; Chen, G.; Zhang, C.; Gao, X.; Sun, B.; Shan, B. Manganese-Modified Biochar for Sediment Remediation: Effect, Microbial Community Response, and Mechanism. Environ. Pollut. 2024, 363, 125175. [Google Scholar] [CrossRef]
- Li, W.; Chen, X.; Yang, T.; Zhu, H.; He, Z.; Zhao, R.; Chen, Y. Sponge Iron Enriches Autotrophic/Aerobic Denitrifying Bacteria to Enhance Denitrification in Sequencing Batch Reactor. Bioresour. Technol. 2024, 407, 131097. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Technical standards as norms and their relationship with law. Chin. J. Int. Law 2022, 44, 84–100. [Google Scholar]
- Li, M. Technology is coupled with the specification structure: The Intelligent Construction of Environmental legal system in the Digital Age. Huxiang Law Rev. 2024, 4, 133–144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).