Assessing the Interaction Between Geologically Sourced Hydrocarbons and Thermal–Mineral Groundwater: An Overview of Methodologies
Abstract
1. Introduction
2. Hydrocarbons in Geological Environments
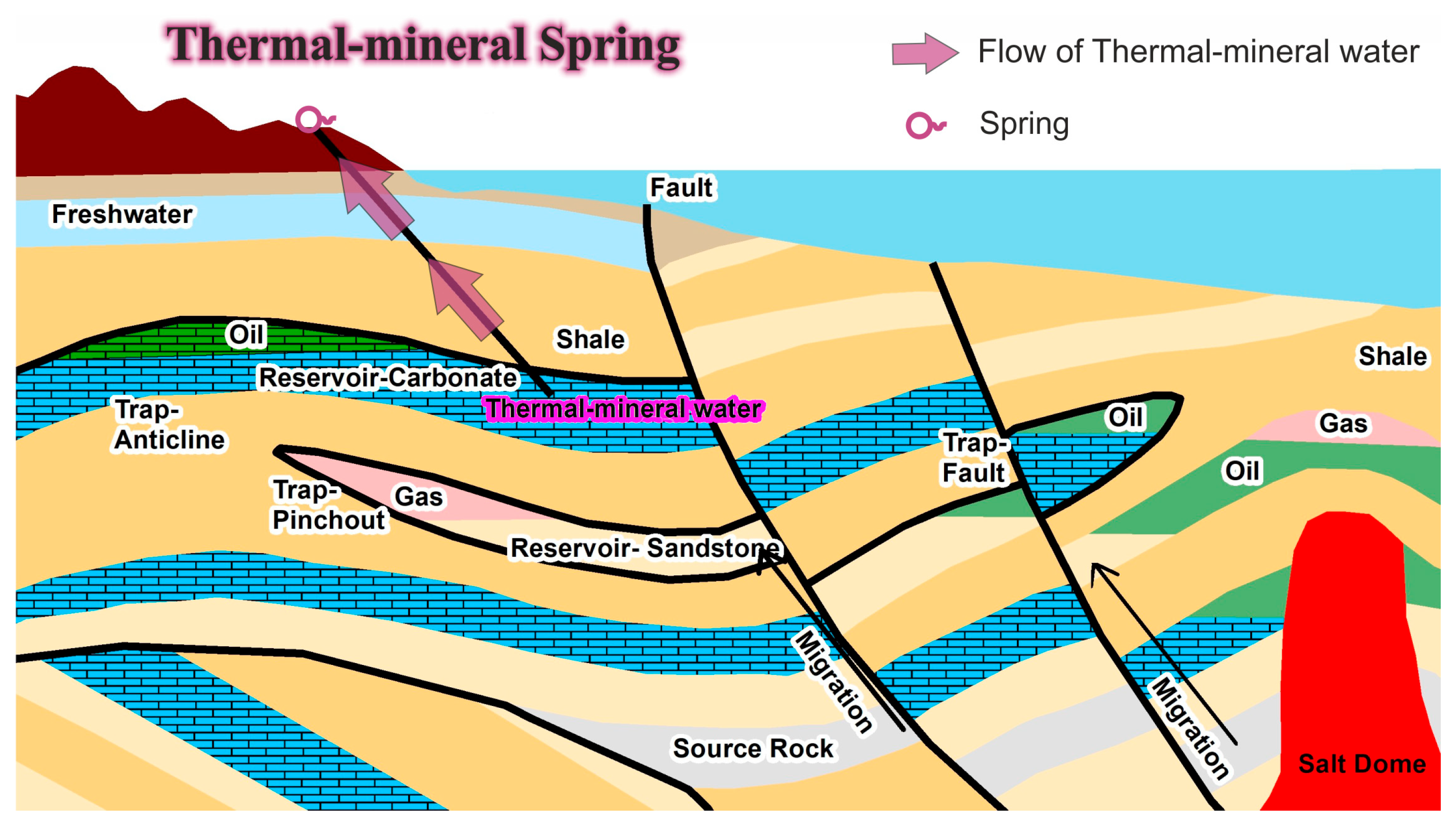
3. Groundwater Interaction with the Hydrocarbon Reservoir
4. Methods of Identifying Hydrocarbons in Groundwater
| A/A | Reference | Year | Location | Methodology | Aquifer Type |
|---|---|---|---|---|---|
| 1 | Warner et al. [101] | 2012 | Pennsylvania | Geochemical data of produced water (PW) samples/processes that control the quality of PW generated from hydrocarbon bearing formations by analyzing relationships between their major ion concentrations. | Shallow |
| 2 | Peterman Z. et al. [102] | 2012 | Northeastern Montana, USA | Analyzing water quality parameters before and after petroleum hydrocarbon pollution. Studying hydrogeochemical mechanisms including desulfurization, denigration, and ion exchange processes. | Deep (brine) |
| 3 | Li H. et al. [103] | 2017 | Colorado, USA | Isotope mixing model based on δ2H and δ18O. Cl mixing model for PW contribution assessment. Validation of Na, Cl, Br, Sr, Ba, Li, and B tracers. | Confined and shallow |
| 4 | Wang Q. et al. [104] | 2020 | NW China | Using hydrochemical indicators (Ca2+, Mg2+, Na+, K+, HCO3−, NO3−, Cl−, F−, and SO42−) and pH with the help of GIS and origin platforms, statistical analyses, and graphical methods. | Confined and shallow |
| 5 | Apango F. et al. [105] | 2021 | Mexico | Inorganic geochemistry (major cations and anions). Stable isotopes of select inorganic constituents (Sr, B, Li, and C). Select hydrocarbon molecular and isotopic tracers. Tritium and noble gas elemental and isotopic composition. | Deep/confined |
| 6 | McMahon P. et al. [106] | 2021 | USA | Hydrochemistry and water type (HCO3-Ca), δ13C (−16.1 to −11.7‰) indicating the equilibrium fractionation between CO2-DIC. Based on the analysis above and published; other shale gas production projects, TDS; major elements and stable isotopes of δ13CDIC can be chosen as inorganic geochemical monitoring indicators. | Confined and shallow |
| 7 | Rosecrans Z.C. et al. [107] | 2021 | California, USA | Chemical and isotopic tracers were analyzed in groundwater samples to assess the presence of thermogenic gas or water from hydrocarbon-bearing formations mixing with surrounding groundwater. Investigating the occurrence of light hydrocarbon gases in groundwater. | Confined and shallow |
| 8 | Stephens M. et al. [37] | 2021 | California, USA | Produced water TDS samples were utilized for model construction. Borehole geophysics data were incorporated into the TDS model. The study analyzed stratigraphy and structure impact on TDS. | Confined and shallow |
| 9 | Yang Z. et al. [83] | 2022 | Shandong Province, N China | Analysis of geochemical composition of formation water. Gini coefficient used for weighted calculation of parameters. Decision tree model applied for correlation analysis. | Deep/formation water |
| 10 | Gillespie J. et al. [35] | 2022 | California, USA | Geophysical log analysis maps groundwater salinity distribution. Data includes formation picks and water quality information. Log-calculated TDS used in TDS versus depth plots. | Deep aquifer |
| 11 | McMahon P. et al. [108] | 2023 | California, USA | Sampling of groundwater wells, springs, or seeps. Isotopic and gas compositional analysis. Standard methods for analyzing water and gas components. Collection of replicate and single sample wells. | Confined and shallow |
| 12 | Warden J. et al. [109] | 2024 | California, USA | The collected samples were analyzed for a broad suite of constituents, including major and minor ions, nutrients, trace elements, stable isotopes of water, groundwater-age tracers, noble gases, hydrocarbon gases, and volatile organic compounds (VOCs). | Deep aquifer |
| 13 | McMahon P. et al. [110] | 2024 | California, USA | Groundwater was sampled laterally and vertically within the aquifer for multiple geochemical tracers. This comprehensive sampling approach helped reveal the complex interactions affecting groundwater quality. | Confined and shallow |
| 14 | Zhang H. et al. [111] | 2024 | Northwest China | Absorbance measurements, DOC concentrations from oil field samples ranged widely from 68 mg/L to nearly 3000 mg/L, BTEX concentration. PCA analysis. | Confined and shallow |
| 15 | Rusi S. et al. [112] | 2018 | Apennine, Italy | Conducted over 6 years (2011–2017) with multiple groundwater sampling campaigns in the Gran Sasso aquifer system. Samples were analyzed for dissolved hydrocarbons, geochemical tracers, and isotopic analyses (δ13C of methane and DIC). | Carbonate/karst |
| 16 | Schloemer J. et al. [113] | 2016 | L. Saxony, Germany | Focus was on dissolved methane (CH4), ethane (C2H6), and propane (C3H8) concentrations. Accompanied by measurements of δ13C–CH4 and δ2H–CH4 for source differentiation. | Shallow |
5. Characteristics of Thermal–Mineral Water
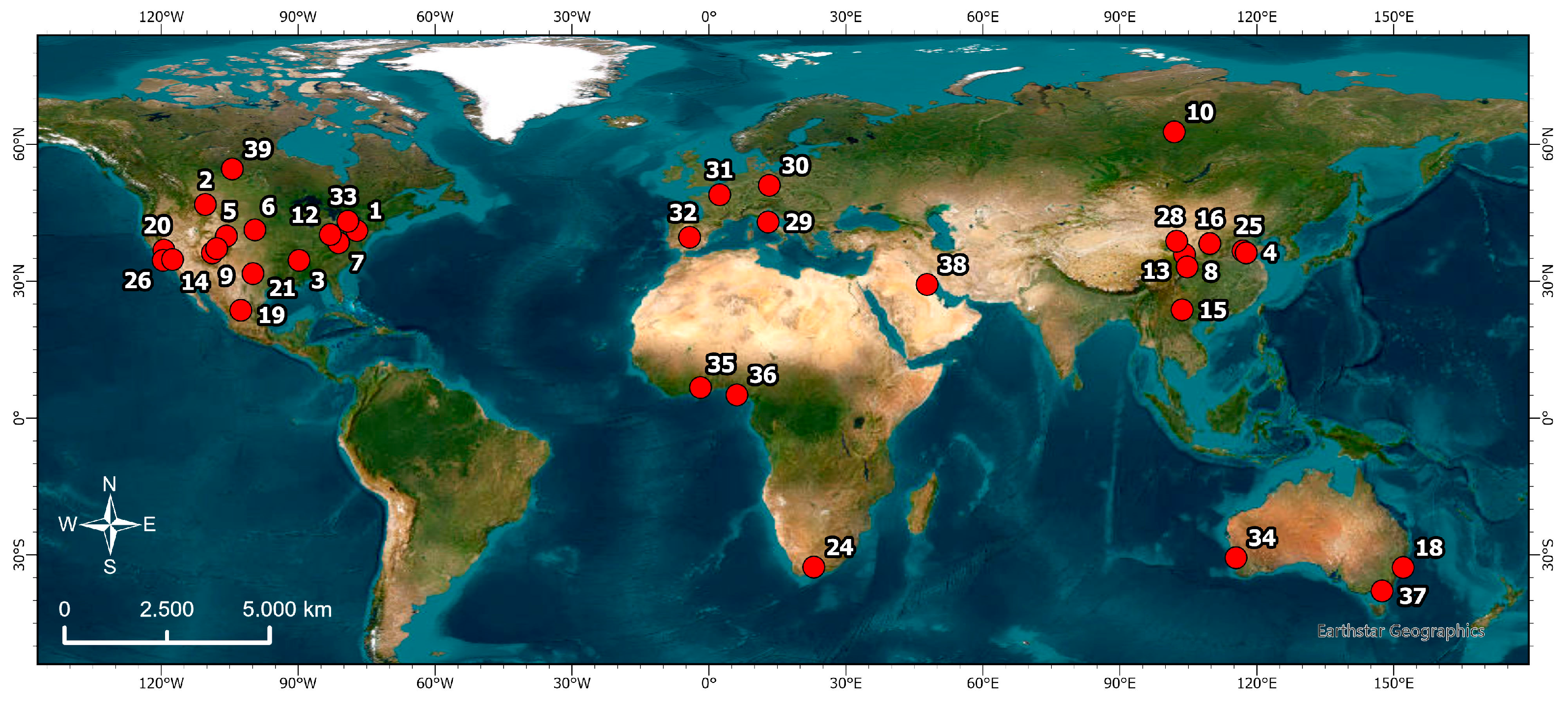
6. Examples of Thermal–Mineral Groundwaters and Hydrocarbons
| A/A | Reference | Year | Location | Methodology | Type |
|---|---|---|---|---|---|
| 1 | Kárpáti Z. et al. [126] | 1999 | Hungary—Pannonia basin | Gas chromatography–mass spectrometry analysis of thermal water. | Thermal waters |
| 2 | Gioia M.L.D. et al. [127] | 2006 | Italy (Calabria) | Gas chromatography–mass spectrometry analysis of thermal water. | Thermal sulfurous waters |
| 3 | Gonzαlez-Barreiro C. et al. [128] | 2009 | Spain (Galicia) | Hydrochemical analysis, gas chromatographic analysis, and different extraction techniques (LLE, SPME, and SPE). | Thermal–mineral medicinal waters |
| 4 | Varga Csaba [129] | 2012 | Hungary | Gas chromatographic analysis and mass spectrometry. | Healing spa water |
| 5 | Kompanichenko et al. [122] | 2016 | Russia—Mutnovskii | Hydrochemistry and organic compounds. | Thermal waters |
| 6 | Kompanichenko et al. [130] | 2022 | Russia—Kuriles and Kamchatka Peninsula | Investigation of extractable organic compounds in hydrothermal vent fluids. Thermodynamic modeling of water–rock systems. Comparative analysis of organic matter in thermal waters. | Thermal waters |
7. Future Challenges and Current Trends
8. Conclusions
- Formation water may help identify the process of hydrocarbon mixing with thermal–mineral waters, revealing a fingerprint of surface hydrocarbons.
- Analysis of groundwater quality and contamination is vital for its extraction, utilization, and safeguarding, particularly in regions with petroleum and petrochemical industries.
- Monitoring the salinity of groundwater can provide valuable insights into the potential presence of oil in a specific region.
- The interaction between water and hydrocarbons can affect the migration and accumulation of hydrocarbons in tight formations.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazakis, N.; Karakatsanis, D.; Ntona, M.M.; Polydoropoulos, K.; Zavridou, E.; Voudouri, K.A.; Busico, G.; Kalaitzidou, K.; Patsialis, T.; Perdikaki, M.; et al. Groundwater Depletion. Are Environmentally Friendly Energy Recharge Dams a Solution? Water 2024, 16, 1541. [Google Scholar] [CrossRef]
- Jasechko, S.; Perrone, D.; Befus, K.M.; Bayani Cardenas, M.; Ferguson, G.; Gleeson, T.; Luijendijk, E.; McDonnell, J.J.; Taylor, R.G.; Wada, Y.; et al. Global Aquifers Dominated by Fossil Groundwaters but Wells Vulnerable to Modern Contamination. Nat. Geosci. 2017, 10, 425–429. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Hanor, J.S. Deep Fluids in the Continents: I. Sedimentary Basins. In Treatise on Geochemistry; Holland, H., Turekian, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–48. ISBN 978-0-08-043751-4. [Google Scholar]
- Waring, G.A. Thermal Springs of the United States and Other Countries of the World; A Summary. In Geological Survey Professional Paper 492; U.S. Government Publishing Office: Washington, DC, USA, 1965. [Google Scholar]
- Mahala, S.C. Geology, Chemistry and Genesis of Thermal Springs of Odisha, India. In SpringerBriefs in Earth Sciences; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-319-90001-8. [Google Scholar]
- Kifouche, R.; Bouaıcha, F.; Bouteraa, O. Impact of Thermal Water on Environment Case Study of Mila and Guelma Region, Algeria. Bull. Min. Res. Exp. 2023, 171, 143–157. [Google Scholar] [CrossRef]
- Haile, T.; Abiye, T.A. The Interference of a Deep Thermal System with a Shallow Aquifer: The Case of Sodere and Gergedi Thermal Springs, Main Ethiopian Rift, Ethiopia. Hydrogeol. J. 2012, 20, 561–574. [Google Scholar] [CrossRef]
- Cuthbert, M.O.; Gleeson, T.; Ferguson, G.; Bierkens, M.; Taylor, R. Defining Renewable Groundwater Use and Its Relevance to Sustainable Groundwater Management. Water Resour. Res. 2023, 59, e2022WR032831. [Google Scholar] [CrossRef]
- Foster, S.; Loucks, D.P. Non-Renewable Groundwater Resources; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2006; Volume 10, p. 103. [Google Scholar]
- Conti, A.; Sacchi, E.; Chiarle, M.; Martinelli, G.; Zuppi, G.M. Geochemistry of the Formation Waters in the Po Plain (Northern Italy): An Overview. Appl. Geochem. 2000, 15, 51–65. [Google Scholar] [CrossRef]
- Coustau, H. Formation Waters and Hydrodynamics. J. Geochem. Explor. 1977, 7, 213–241. [Google Scholar] [CrossRef]
- Abba, M.K.; Al-Othaibi, A.; Abbas, A.J.; Nasr, G.G.; Mukhtar, A. Experimental Investigation on the Impact of Connate Water Salinity on Dispersion Coefficient in Consolidated Rocks Cores during Enhanced Gas Recovery by CO2 Injection. J. Nat. Gas. Sci. Eng. 2018, 60, 190–201. [Google Scholar] [CrossRef]
- Munk, L.A.; Boutt, D.F.; Moran, B.J.; McKnight, S.V.; Jenckes, J. Hydrogeologic and Geochemical Distinctions in Freshwater-Brine Systems of an Andean Salar. Geochem. Geophys. Geosyst. 2021, 22, e2020GC009345. [Google Scholar] [CrossRef]
- Sahai, N.; Adebayo, S.; Schoonen, M.A. Freshwater and Evaporite Brine Compositions on Hadean Earth: Priming the Origins of Life. Astrobiology 2022, 22, 641–671. [Google Scholar] [CrossRef]
- Lalasari, L.H.; Widowati, M.K.; Natasha, N.C.; Sulistiyono, E.; Prasetyo, A.B. The Synthesis of Calcium Salt from Brine Water by Partial Evaporation and Chemical Precipitation. In IOP Conference Series: Materials Science and Engineering, Proceedings of the International Conference on Advanced Materials for Better Future 20163, Surakarta, Indonesia, 4 October 2016; IOP Publishing Ltd.: Bristol, UK, 2017; Volume 176, p. 012040. [Google Scholar] [CrossRef]
- Brady, P.; Lopez, C.; Sassani, D. Granite Hydrolysis to Form Deep Brines. Energies 2019, 12, 2180. [Google Scholar] [CrossRef]
- Rosenthal, E.; Flexer, A.; Möller, P. The Paleoenvironment and the Evolution of Brines in the Jordan-Dead Sea Transform and in Adjoining Areas. Int. J. Earth Sci. (Geol. Rundsch.) 2006, 95, 725–740. [Google Scholar] [CrossRef]
- Panagopoulos, A. Study and Evaluation of the Characteristics of Saline Wastewater (Brine) Produced by Desalination and Industrial Plants. Environ. Sci. Pollut. Res. 2022, 29, 23736–23749. [Google Scholar] [CrossRef]
- Lee, H.; Ostadhassan, M.; Sun, Z.; Pu, H.; Liu, B.; Varma, R.S.; Jang, H.W.; Shokouhimher, M. Diffusivity and Hydrophobic Hydration of Hydrocarbons in Supercritical CO2 and Aqueous Brine. RSC Adv. 2020, 10, 37938–37946. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Leonenko, Y. Hydrogen-Brine Interfacial Tension at Subsurface Conditions: Implication for Hydrogen Geo-Storage; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2023; Volume 2023, pp. 1–5. [Google Scholar]
- Emami Baghdadi, M.H.; Darvish, H.; Rezaei, H.; Savadinezhad, M. Applying LSSVM Algorithm as a Novel and Accurate Method for Estimation of Interfacial Tension of Brine and Hydrocarbons. Pet. Sci. Technol. 2018, 36, 1170–1174. [Google Scholar] [CrossRef]
- Sośnicka, M.; Lüders, V.; Duschl, F.; Kraemer, D.; Laurent, O.; Niedermann, S.; Banks, D.A.; Wilke, F.; Wohlgemuth-Ueberwasser, C.; Wiedenbeck, M. Metal Budget and Origin of Aqueous Brines Depositing Deep-Seated Zn-Pb Mineralization Linked to Hydrocarbon Reservoirs, North German Basin. Min. Depos. 2023, 58, 1143–1170. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Kaduna, R.; Kaduna, N.; Tanko, A.I. Cogitation on Hydrocarbon Contaminants in Shallow Groundwater around Kaduna Refining and Petrochemical Company, Nigeria. Ambient. Sci. 2019, 6, 42–49. [Google Scholar] [CrossRef]
- Makri, P.; Stathopoulou, E.; Hermides, D.; Kontakiotis, G.; Zarkogiannis, S.D.; Skilodimou, H.D.; Bathrellos, G.D.; Antonarakou, A.; Scoullos, M. The Environmental Impact of a Complex Hydrogeological System on Hydrocarbon-Pollutants’ Natural Attenuation: The Case of the Coastal Aquifers in Eleusis, West Attica, Greece. J. Mar. Sci. Eng. 2020, 8, 1018. [Google Scholar] [CrossRef]
- Tan, Y.; Al-Huqail, A.A.; Chen, Q.; Majdi, H.S.; Algethami, J.S.; Ali, H.E. Analysis of Groundwater Pollution in a Petroleum Refinery Energy Contributed in Rock Mechanics through ANFIS-AHP. Int. J. Energy Res. 2022, 46, 20928–20938. [Google Scholar] [CrossRef]
- Xiao, X.; Zheng, Q.; Shen, R.; Huang, K.; Xu, H.; Tu, B.; Zhao, Y. Patterns of Groundwater Bacterial Communities along the Petroleum Hydrocarbon Gradient. J. Environ. Chem. Eng. 2022, 10, 108773. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Z.; Guo, X.; Lu, H. Source Apportionment of Petroleum Hydrocarbon Contamination in Karst Fissure Groundwater. DEStech Trans. Environ. Energy Earth Sci. 2017. [Google Scholar] [CrossRef][Green Version]
- Adeoti, L.; Anukwu, G.; Ademoye, S.; Adegbite, J.; Adigun, E.; Adeogun, O. Assessment of Hydrocarbon Pollution in Groundwater Using Electrical Resistivity Method. Curr. Appl. Sci. Technol. 2023, 23. [Google Scholar] [CrossRef]
- Samara, H.; Ke, L.; Ostrowski, T.V.; Ganzer, L.; Jaeger, P. Unconventional Oil Recovery from Al Sultani Tight Rock Formations Using Supercritical CO2. J. Supercrit. Fluids 2019, 152, 104562. [Google Scholar] [CrossRef]
- de Hemptinne, J.-C.; Peumery, R.; Ruffier-Meray, V.; Moracchini, G.; Naiglin, J.; Carpentier, B.; Oudin, J.L.; Connan, J. Compositional Changes Resulting from the Water-Washing of a Petroleum Fluid. J. Pet. Sci. Eng. 2001, 29, 39–51. [Google Scholar] [CrossRef]
- Gupta, P.K. Advances in Hydrocarbon Bioremediation Products: Natural Solutions. In Advances in Remediation Techniques for Polluted Soils and Groundwater; Gupta, P.K., Yadav, B., Himanshu, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 16; pp. 309–318. ISBN 978-0-12-823830-1. [Google Scholar]
- Gomes, K.J.M.; Oliva, P.A.C.; Da Rocha, H.O.; De Alcantara Mendes, R.; Da Costa, A.C.G.; Dos Santos Miranda, C.; De Oliveira Almeida, N. Evaluation of the Contamination of the Subsurface and Groundwater by Monoaromatic Hydrocarbons in an Eastern Amazonian Town in Northern Brazil. Environ. Earth Sci. 2023, 82, 23. [Google Scholar] [CrossRef]
- McMahon, P.B.; Kulongoski, J.T.; Vengosh, A.; Cozzarelli, I.M.; Landon, M.K.; Kharaka, Y.K.; Gillespie, J.M.; Davis, T.A. Regional Patterns in the Geochemistry of Oil-Field Water, Southern San Joaquin Valley, California, USA. Appl. Geochem. 2018, 98, 127–140. [Google Scholar] [CrossRef]
- Gillespie, J.M.; Davis, T.A.; Stephens, M.J.; Ball, L.B.; Landon, M.K. Groundwater Salinity and the Effects of Produced Water Disposal in the Lost Hills—Belridge Oil Fields, Kern County, California. Environ. Geosci. 2019, 26, 73–96. [Google Scholar] [CrossRef]
- Gillespie, J.M.; Stephens, M.J.; Chang, W.; Warden, J.G. Mapping Aquifer Salinity Gradients and Effects of Oil Field Produced Water Disposal Using Geophysical Logs: Elk Hills, Buena Vista and Coles Levee Oil Fields, San Joaquin Valley, California. PLoS ONE 2022, 17, e0263477. [Google Scholar] [CrossRef]
- Stephens, M.J.; Shimabukuro, D.H.; Gillespie, J.M.; Chang, W. Groundwater Salinity Mapping Using Geophysical Log Analysis within the Fruitvale and Rosedale Ranch Oil Fields, Kern County, California, USA. Hydrogeol. J. 2019, 27, 731–746. [Google Scholar] [CrossRef]
- Stephens, M.J.; Shimabukuro, D.H.; Chang, W.; Gillespie, J.M.; Levinson, Z. Stratigraphic and Structural Controls on Groundwater Salinity Variations in the Poso Creek Oil Field, Kern County, California, USA. Hydrogeol. J. 2021, 29, 2803–2820. [Google Scholar] [CrossRef]
- Anders, R.; Landon, M.K.; McMahon, P.B.; Kulongoski, J.T.; Hunt, A.G.; Davis, T.A. Occurrence of Water and Thermogenic Gas from Oil-Bearing Formations in Groundwater near the Orcutt Oil Field, California, USA. J. Hydrol. Reg. Stud. 2022, 41, 101065. [Google Scholar] [CrossRef]
- Vinson, D.S.; Tagma, T.; Bouchaou, L.; Dwyer, G.S.; Warner, N.R.; Vengosh, A. Occurrence and Mobilization of Radium in Fresh to Saline Coastal Groundwater Inferred from Geochemical and Isotopic Tracers (Sr, S, O, H, Ra, Rn). Appl. Geochem. 2013, 38, 161–175. [Google Scholar] [CrossRef]
- Van Sice, K.; Cravotta, C.A.; McDevitt, B.; Tasker, T.L.; Landis, J.D.; Puhr, J.; Warner, N.R. Radium Attenuation and Mobilization in Stream Sediments Following Oil and Gas Wastewater Disposal in Western Pennsylvania. Appl. Geochem. 2018, 98, 393–403. [Google Scholar] [CrossRef]
- Boehm, P.D. 15-Polycyclic Aromatic Hydrocarbons (PAHs). In Environmental Forensics; Morrison, R.D., Murphy, B.L., Eds.; Academic Press: Cambridge, MA, USA, 1964; pp. 313–337. ISBN 978-0-12-507751-4. [Google Scholar]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A Review on Polycyclic Aromatic Hydrocarbons: Source, Environmental Impact, Effect on Human Health and Remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef]
- Volkman, J.K.; Holdsworth, D.G.; Neill, G.P.; Bavor, H.J. Identification of Natural, Anthropogenic and Petroleum Hydrocarbons in Aquatic Sediments. Sci. Total Environ. 1992, 112, 203–219. [Google Scholar] [CrossRef]
- Liu, S.; Qi, S.; Luo, Z.; Mapoma, H.W.T.; Chen, Z.; Cheng, S. The Origin of High Hydrocarbon Groundwater in Shallow Aquifer: Experimental Evidences from Water-Rock Interaction. Environ. Sci. Pollut. Res. 2019, 26, 32574–32588. [Google Scholar] [CrossRef]
- Cui, J.; Jia, D.; Wang, H.; Yin, H.; Wang, Y.; Ma, D.; Wu, W.; Jing, Z.; Fan, X.; Shen, L.; et al. A Method to Simulate the Migration and Accumulation of Hydrocarbon with Analogue Modeling. Geofluids 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Jiang, H.; Ma, Z.; Fang, X. Constraining Hydrocarbon Migration and Accumulation by Two-Dimensional Numerical Simulation: Ordovician Carbonate Reservoirs of the Daniudi Area, Ordos Basin. Energy Explor. Exploit. 2023, 41, 309–325. [Google Scholar] [CrossRef]
- Sun, Q.; Cartwright, J.; Foschi, M.; Lu, X.; Xie, X. The Interplay of Stratal and Vertical Migration Pathways in Shallow Hydrocarbon Plumbing Systems. Basin Res. 2021, 33, 2157–2178. [Google Scholar] [CrossRef]
- Humez, P.; Lions, J.; Négrel, P.; Lagneau, V. CO2 Intrusion in Freshwater Aquifers: Review of Geochemical Tracers and Monitoring Tools, Classical Uses and Innovative Approaches. Appl. Geochem. 2014, 46, 95–108. [Google Scholar] [CrossRef]
- Raimi, M.O.; Ezekwe, C.I.; Bowale, A.; Samson, T.K. Hydrogeochemical and Multivariate Statistical Techniques to Trace the Sources of Ground Water Contaminants and Affecting Factors of Groundwater Pollution in an Oil and Gas Producing Wetland in Rivers State, Nigeria. Open J. Yangtze Oil Gas 2022, 7, 166–202. [Google Scholar]
- Jekayinfa, S.M. Effects of Groundwater Flow on the Distribution of Bitumen Contaminants in a Shallow Coastal Plain Sand Aquifer of the Nigerian Dahomey Basin. J. Afr. Earth Sci. 2023, 203, 104946. [Google Scholar] [CrossRef]
- Escobar, J.F.; Naranjo-Fernández, D.; Lopera, S.; Quiroz, O.M.; Ocampo, A.; Zarate, G.; Escobar, J.F.; Naranjo-Fernández, D.; Lopera, S.; Quiroz, O.M.; et al. Aquifer Management in Hydrocarbon Exploitation Operations. In Groundwater-New Advances and Challenges; Tarhouni, J., Ed.; IntechOpen: London, UK, 2023; ISBN 978-1-80355-484-6. [Google Scholar]
- Kreuzer, R.L.; Darrah, T.H.; Grove, B.S.; Moore, M.T.; Warner, N.R.; Eymold, W.K.; Whyte, C.J.; Mitra, G.; Jackson, R.B.; Vengosh, A.; et al. Structural and Hydrogeological Controls on Hydrocarbon and Brine Migration into Drinking Water Aquifers in Southern New York. Groundwater 2018, 56, 225–244. [Google Scholar] [CrossRef]
- Valsala, R.; Govindarajan, S.K. Modeling Investigations on Sorption of Petroleum Hydrocarbons to Clay Minerals in a Saturated Porous Aquifer. In Proceedings of the Fourth International Conference in Ocean Engineering (ICOE2018), Chennai, India, 18 February 2018; Murali, K., Sriram, V., Samad, A., Saha, N., Eds.; Springer: Singapore, 2019; pp. 997–1007. [Google Scholar]
- Tilley, B.S.; Ueckert, M.; Baumann, T. Porosity Dynamics through Carbonate-Reaction Kinetics in High-Temperature Aquifer Storage Applications. Math. Geosci. 2021, 53, 1535–1565. [Google Scholar] [CrossRef]
- Parra, J.O.; Hackert, C.; Bennett, M.; Collier, H.A. Permeability and Porosity Images Based on NMR, Sonic, and Seismic Reflectivity: Application to a Carbonate Aquifer. Lead. Edge 2003, 22, 1102–1108. [Google Scholar] [CrossRef]
- Underschultz, J.R.; Otto, C.J.; Bartlett, R. Formation Fluids in Faulted Aquifers: Examples from the Foothills of Western Canada and the North West Shelf of Australia. In Evaluating Fault and Cap Rock Seals (AAPG Hedberg Series); Boult, P., Kaldi, J., Eds.; American Association of Petroleum Geologists: Tulsa, OK, USA, 2005; Volume 2, pp. 247–260. [Google Scholar] [CrossRef]
- Dracos, T.H. Immiscible transport of hydrocarbons infiltrating in unconfined aquifers. In Oil in Freshwater: Chemistry, Biology, Countermeasure Technology; Elsevier Inc.: Pergamon, Turkey, 1987; pp. 161–175. ISBN 978-0-08-031862-2. [Google Scholar]
- Selley, R.C.; Sonnenberg, S.A. Nonconventional Petroleum Resources. In Elements of Petroleum Geology, 4th ed.; Selley, R.C., Sonnenberg, S.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 473–565. ISBN 978-0-12-822316-1. [Google Scholar]
- Kundu, S.N. Unconventional Hydrocarbons Resources. In Geoscience for Petroleum Engineers; Kundu, S.N., Ed.; Springer Nature: Singapore, 2023; pp. 173–192. ISBN 978-981-19-7640-7. [Google Scholar]
- Kalu, E.O.; Okeke, O.C.; Amadi, C.C.; Akudike, J.C.; Dozie, O.T.; Sunday, E.U.; Okonkwo, S.I. A Review on the Geologic Occurrence, Development and Associated Environmental Problems of Unconventional Hydrocarbon Energy Resources. Int. J. Innov. Sci. Res. Technol. (IJISRT) 2020, 5, 1411–1423. [Google Scholar] [CrossRef]
- Arciniega-Esparza, S.; Hernández-Espriú, A.; Young, M.H. Implications of Unconventional Oil and Gas Development on Groundwater Resources. Curr. Opin. Environ. Sci. Health 2022, 27, 100346. [Google Scholar] [CrossRef]
- Stolz, J.; Bain, D.; Griffin, M. (Eds.) Environmental Impacts from the Development of Unconventional Oil and Gas Reserves; Cambridge University Press: Cambridge, UK, 2022; ISBN 978-1-108-48919-5. [Google Scholar]
- Akhmiyeva, R.B. Environmental Consequences of Pollution of the Natural Environment with Oil Products. AIP Conf. Proc. 2022, 2657, 020010. [Google Scholar] [CrossRef]
- Yu, X.; Michael, H.A. Impacts of the Scale of Representation of Heterogeneity on Simulated Salinity and Saltwater Circulation in Coastal Aquifers. Water Resour. Res. 2022, 58, e2020WR029523. [Google Scholar] [CrossRef]
- Gasda, S.E.; Nilsen, H.M.; Dahle, H.K. Impact of Structural Heterogeneity on Upscaled Models for Large-Scale CO2 Migration and Trapping in Saline Aquifers. Adv. Water Resour. 2013, 62, 520–532. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Y.; Zhang, P.; Wang, M.; Qiu, Y.; Zhu, X.; Zhang, X. Comparison of Evaporite-Related Source Rocks and Implications for Petroleum Exploration: A Case Study of the Dongying Depression, Bohai Bay Basin, Eastern China. Energies 2023, 16, 5000. [Google Scholar] [CrossRef]
- Hudson, S.M.; Hanson, A.D. Thermal Maturation and Hydrocarbon Migration within La Popa Basin, Northeastern Mexico, with Implications for Other Salt Structures. AAPG Bull. 2010, 94, 273–291. [Google Scholar] [CrossRef]
- Zelilidis, A.; Bourli, N.; Andriopoulos, K.; Georgoulas, E.; Peridis, S.; Asimakopoulos, D.; Maravelis, A.G. Unraveling the Origin of the Messinian? Evaporites in Zakynthos Island, Ionian Sea: Implications for the Sealing Capacity in the Mediterranean Sea. J. Mar. Sci. Eng. 2023, 11, 271. [Google Scholar] [CrossRef]
- Wen, Z.; He, D.F.; Tong, X.G. Global Evaporites Development Characteristics and Their Effects on Formation of Giant Oil Fields. Xinjiang Pet. Geol. 2012, 33, 373–378. [Google Scholar]
- Sarg, J.F. The Sequence Stratigraphy, Sedimentology, and Economic Importance of Evaporite–Carbonate Transitions: A Review. Sediment. Geol. 2001, 140, 9–34. [Google Scholar] [CrossRef]
- Sinha, R.; Kumar, A. Characterization of a Deep Saline Aquifer Using Oil Exploration Data in an Arid Region of Rajasthan, India. In Ground Water Development-Issues and Sustainable Solutions; Ray, S.P.S., Ed.; Springer: Singapore, 2018; pp. 69–83. ISBN 978-981-13-1771-2. [Google Scholar]
- Morsy, E.A.; Othman, A. Assessing the Impact of Groundwater Mixing and Sea Water Intrusion on Oil Production in Coastal Oil Fields Using Resistivity Sounding Methods. Arab. J. Geosci. 2020, 13, 434. [Google Scholar] [CrossRef]
- Aliewi, A.; Al-Enezi, H.; Al-Maheimid, I.; Al-Kandari, J.; Al-Haddad, A.; Al-Qallaf, H.; Rashid, T.; Sadeqi, D. Sustainability of Brackish Groundwater Utilization from the Eocene Aquifer for Oil Exploration Operations in Central Kuwait. Environ. Dev. Sustain. 2020, 22, 4639–4653. [Google Scholar] [CrossRef]
- Marić, N.; Štrbački, J.; Polk, J.; Slavković Beškoski, L.; Avdalović, J.; Lješević, M.; Joksimović, K.; Žerađanin, A.; Beškoski, V.P. Spatial-Temporal Assessment of Hydrocarbon Biodegradation Mechanisms at a Contaminated Groundwater Site in Serbia. Chem. Ecol. 2022, 38, 95–107. [Google Scholar] [CrossRef]
- Rao, V.; Knight, R. Chapter 5-Methane in Groundwater. In Sustainable Shale Oil and Gas; Rao, V., Knight, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 61–74. ISBN 978-0-12-810389-0. [Google Scholar]
- Wang, J.; Zhao, Y.; Sun, J.; Zhang, Y.; Liu, C. The Distribution and Sources of Polycyclic Aromatic Hydrocarbons in Shallow Groundwater from an Alluvial-Diluvial Fan of the Hutuo River in North China. Front. Earth Sci. 2019, 13, 33–42. [Google Scholar] [CrossRef]
- Khelfi, A. Sources of Groundwater Pollution. Available online: https://www.igi-global.com/gateway/chapter/www.igi-global.com/gateway/chapter/208486 (accessed on 22 May 2024).
- Liu, S.; Qi, S.; Luo, Z.; Liu, F.; Ding, Y.; Huang, H.; Chen, Z.; Cheng, S. The Origin of High Hydrocarbon Groundwater in Shallow Triassic Aquifer in Northwest Guizhou, China. Environ. Geochem. Health 2018, 40, 415–433. [Google Scholar] [CrossRef]
- Lan, J.; Sun, Y.; Yuan, D. Transport of Polycyclic Aromatic Hydrocarbons in a Highly Vulnerable Karst Underground River System of Southwest China. Environ. Sci. Pollut. Res. 2018, 25, 34519–34530. [Google Scholar] [CrossRef]
- Poros, Z.; Mindszenty, A.; Molnár, F.; Pironon, J.; Győri, O.; Ronchi, P.; Szekeres, Z. Imprints of Hydrocarbon-Bearing Basinal Fluids on a Karst System: Mineralogical and Fluid Inclusion Studies from the Buda Hills, Hungary. Int. J. Earth Sci. (Geol. Rundsch.) 2012, 101, 429–452. [Google Scholar] [CrossRef]
- Rice, A.K.; Lackey, G.; Proctor, J.; Singha, K. Groundwater-quality Hazards of Methane Leakage from Hydrocarbon Wells: A Review of Observational and Numerical Studies and Four Testable Hypotheses. WIREs Water 2018, 5, e1283. [Google Scholar] [CrossRef]
- Sun, Z.; Yang, Z.; He, X.; Wan, C.; He, G.; Ren, Q.; Zhao, S.; Cheng, W.; Chen, S. Geochemical Characteristics of Formation Water in Carbonate Reservoirs and Its Indication to Hydrocarbon Accumulation. Geofluids 2022, 2022, 6095178. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Wang, S.; Li, X.; Cheng, W.; Chen, S. Indication of Formation Water Geochemistry for Hydrocarbon Preservation: New Applications of Machine Learning in Tight Sandstone Gas Reservoirs. ACS Omega 2022, 7, 36263–36276. [Google Scholar] [CrossRef]
- Bondu, R.; Kloppmann, W.; Naumenko-Dèzes, M.O.; Humez, P.; Mayer, B. Potential Impacts of Shale Gas Development on Inorganic Groundwater Chemistry: Implications for Environmental Baseline Assessment in Shallow Aquifers. Environ. Sci. Technol. 2021, 55, 9657–9671. [Google Scholar] [CrossRef]
- Harkness, J.S.; Darrah, T.H.; Warner, N.R.; Whyte, C.J.; Moore, M.T.; Millot, R.; Kloppmann, W.; Jackson, R.B.; Vengosh, A. The Geochemistry of Naturally Occurring Methane and Saline Groundwater in an Area of Unconventional Shale Gas Development. Geochim. Cosmochim. Acta 2017, 208, 302–334. [Google Scholar] [CrossRef]
- Zhou, Z.; Ballentine, C.J. 4He Dating of Groundwater Associated with Hydrocarbon Reservoirs. Chem. Geol. 2006, 226, 309–327. [Google Scholar] [CrossRef]
- Fine, P.; Graber, E.R.; Yaron, B. Soil Interactions with Petroleum Hydrocarbons: Abiotic Processes. Soil Technol. 1997, 10, 133–153. [Google Scholar] [CrossRef]
- Labar, S.; Hani, A.; Djabri, L. Biochemical Approach to Assess Groundwater Pollution by Petroleum Hydrocarbons (Case Skikda Algeria). J. Water Resour. Prot. 2012, 4, 493–496. [Google Scholar] [CrossRef][Green Version]
- Grattoni, C.A.; Al-Hinai, S.; Guise, P.; Fisher, Q. The Role of Interstitial Water in Hydrocarbon Flow for Tight Rocks. In Proceedings of the International Symposium of the Society of Core Analysts, Calgary, AB, Canada, 10–12 September 2007. [Google Scholar]
- Jiang, L.; Zhao, W.; Huang, J.; Fan, Y.; Hao, J. Effects of Interactions in Natural Gas/Water/Rock System on Hydrocarbon Migration and Accumulation. Sci. Rep. 2021, 11, 22070. [Google Scholar] [CrossRef] [PubMed]
- Kumar Gupta, P. Hydrocarbon Pollution Assessment and Analysis Using GC–MS. In Advances in Remediation Techniques for Polluted Soils and Groundwater; Gupta, P.K., Yadav, B., Himanshu, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Chapter 19; pp. 361–377. ISBN 978-0-12-823830-1. [Google Scholar]
- Carvalho, F.I.M.; Dantas Filho, H.A.; Dantas, K.D.G.F. Simultaneous Determination of 16 Polycyclic Aromatic Hydrocarbons in Groundwater by GC-FID after Solid-Phase Extraction. SN Appl. Sci. 2019, 1, 804. [Google Scholar] [CrossRef]
- Faustorilla, M.V.; Chen, Z.; Dharmarajan, R.; Naidu, R. Determination of Total Petroleum Hydrocarbons in Australian Groundwater Through the Improvised Gas Chromatography—Flame Ionization Detection Technique. J. Chromatogr. Sci. 2017, 55, 775–783. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Yang, X.; Song, X.; Du, X.; Lu, Y. Molecular Fingerprinting of the Biodegradation of Petroleum Organic Pollutants in Groundwater and under Site-Specific Environmental Impacts. Water 2024, 16, 1773. [Google Scholar] [CrossRef]
- Meredith, K.T.; Baker, A.; Andersen, M.S.; O’Carroll, D.M.; Rutlidge, H.; McDonough, L.K.; Oudone, P.; Bryan, E.; Zainuddin, N.S. Isotopic and Chromatographic Fingerprinting of the Sources of Dissolved Organic Carbon in a Shallow Coastal Aquifer. Hydrol. Earth Syst. Sci. 2020, 24, 2167–2178. [Google Scholar] [CrossRef]
- O’Reilly, K.T.; Mohler, R.E.; Zemo, D.A.; Ahn, S.; Magaw, R.I.; Espino Devine, C. Oxygen-Containing Compounds Identified in Groundwater from Fuel Release Sites Using GCxGC-TOF-MS. Groundw. Monit. Remediat. 2019, 39, 32–40. [Google Scholar] [CrossRef]
- Zhu, T.; Rao, Z.; Guo, F.; Zhan, N.; Wang, Y.; Arandiyan, H.; Li, X. Simultaneous Determination of 32 Polycyclic Aromatic Hydrocarbon Derivatives and Parent PAHs Using Gas Chromatography—Mass Spectrometry: Application in Groundwater Screening. Bull. Environ. Contam. Toxicol. 2018, 101, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Wigger, J.W.; Beckmann, D.D.; Torkelson, B.E.; Narang, A.X. Petroleum Hydrocarbon Fingerprinting Quantitative Interpretation: Development and Case Study for Use in Environmental Forensic Investigations. Environ. Geosci. 1999, 6, 155–156. [Google Scholar] [CrossRef]
- Douglas, G.S.; Emsbo-Mattingly, S.D.; Stout, S.A.; Uhler, A.D.; McCarthy, K.J. Chapter 8-Hydrocarbon Fingerprinting Methods. In Introduction to Environmental Forensics, 3rd ed.; Murphy, B.L., Morrison, R.D., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 201–309. ISBN 978-0-12-404696-2. [Google Scholar]
- Wade, M.J.; Stainken, D. The History of Hydrocarbon Analyses: Whence Has Forensic Geochemical Hydrocarbon Fingerprinting Come. J. Environ. Prot. 2016, 07, 303. [Google Scholar] [CrossRef]
- Warner, N.R.; Jackson, R.B.; Darrah, T.H.; Osborn, S.G.; Down, A.; Zhao, K.; White, A.; Vengosh, A. Geochemical Evidence for Possible Natural Migration of Marcellus Formation Brine to Shallow Aquifers in Pennsylvania. Proc. Natl. Acad. Sci. USA 2012, 109, 11961–11966. [Google Scholar] [CrossRef]
- Peterman, Z.E.; Thamke, J.; Futa, K.; Preston, T. Strontium Isotope Systematics of Mixing Groundwater and Oil-Field Brine at Goose Lake in Northeastern Montana, USA. Appl. Geochem. 2012, 27, 2403–2408. [Google Scholar] [CrossRef]
- Li, H.; Son, J.-H.; Hanif, A.; Gu, J.; Dhanasekar, A.; Carlson, K. Colorado Water Watch: Real-Time Groundwater Monitoring for Possible Contamination from Oil and Gas Activities. J. Water Resour. Prot. (JWARP) 2017, 9, 1660–1687. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, S.; Wang, H.; Yang, J.; Huang, H.; Dong, X.; Yu, B. Hydrogeochemical Processes and Groundwater Quality Assessment for Different Aquifers in the Caojiatan Coal Mine of Ordos Basin, Northwestern China. Environ. Earth Sci. 2020, 79, 199. [Google Scholar] [CrossRef]
- Apango, F.; Snedden, J.W.; Daigle, H.; Yurchenko, I.A. Analysis of Hydrocarbon Under-Filled and Water-Filled Miocene Deepwater Reservoirs, Eastern Mexico Offshore. Mar. Pet. Geol. 2021, 131, 105158. [Google Scholar] [CrossRef]
- McMahon, P.B.; Galloway, J.M.; Hunt, A.G.; Belitz, K.; Jurgens, B.C.; Johnson, T.D. Geochemistry and Age of Groundwater in the Williston Basin, USA: Assessing Potential Effects of Shale-Oil Production on Groundwater Quality. Appl. Geochem. 2021, 125, 104833. [Google Scholar] [CrossRef]
- Rosecrans, C.Z.; Landon, M.K.; McMahon, P.B.; Gillespie, J.M.; Kulongoski, J.T.; Stephens, M.J.; Hunt, A.G.; Shimabukuro, D.H.; Davis, T.A. Groundwater Quality of Aquifers Overlying the Oxnard Oil Field, Ventura County, California. Sci. Total Environ. 2021, 771, 144822. [Google Scholar] [CrossRef]
- McMahon, P.B.; Landon, M.K.; Stephens, M.J.; Taylor, K.A.; Gillespie, J.M.; Davis, T.A.; Shimabukuro, D.H. Fluid Migration Pathways to Groundwater in Mature Oil Fields: Exploring the Roles of Water Injection/Production and Oil-Well Integrity in California, USA. Sci. Total Environ. 2023, 900, 166400. [Google Scholar] [CrossRef]
- Warden, J.G.; Landon, M.K.; Stephens, M.J.; Davis, T.A.; Gillespie, J.M.; McMahon, P.B.; Kulongoski, J.T.; Hunt, A.G.; Shimabukuro, D.H.; Gannon, R.S.; et al. Assessing Potential Effects of Oil and Gas Development Activities on Groundwater Quality near and Overlying the Elk Hills and North Coles Levee Oil Fields, San Joaquin Valley, California. PLoS Water 2024, 3, e0000258. [Google Scholar] [CrossRef]
- McMahon, P.B.; Landon, M.K.; Stephens, M.J.; Taylor, K.A.; Wright, M.T.; Hansen, A.M.; Kraus, T.E.C.; Cozzarelli, I.M.; Shimabukuro, D.H.; Sowers, T.A.; et al. Land-Use Interactions, Oil-Field Infrastructure, and Natural Processes Control Hydrocarbon and Arsenic Concentrations in Groundwater, Poso Creek Oil Field, California, USA. Appl. Geochem. 2024, 168, 106025. [Google Scholar] [CrossRef]
- Zhang, H.; Han, X.; Wang, G.; Zhou, L.; Huang, D.; Chen, X.; Zhang, F. Hydrogeochemical and Isotopic Evidences of the Underlying Produced Water Intrusion into Shallow Groundwater in an Oil Production Area, Northwest China. Sci. Total Environ. 2024, 916, 170242. [Google Scholar] [CrossRef]
- Rusi, S.; Di Curzio, D.; Palmucci, W.; Petaccia, R. Detection of the Natural Origin Hydrocarbon Contamination in Carbonate Aquifers (Central Apennine, Italy). Environ. Sci. Pollut. Res. 2018, 25, 15577–15596. [Google Scholar] [CrossRef]
- Schloemer, S.; Elbracht, J.; Blumenberg, M.; Illing, C.J. Distribution and Origin of Dissolved Methane, Ethane and Propane in Shallow Groundwater of Lower Saxony, Germany. Appl. Geochem. 2016, 67, 118–132. [Google Scholar] [CrossRef]
- Guimbaud, C.; Colombano, S.; Noel, C.; Verardo, E.; Grossel, A.; Jourdain, L.; Jégou, F.; Hu, Z.; Jacob, J.; Ignatiadis, I.; et al. Quantification of Biodegradation Rate of Hydrocarbons in a Contaminated Aquifer by CO2 δ13C Monitoring at Ground Surface. J. Contam. Hydrol. 2023, 256, 104168. [Google Scholar] [CrossRef]
- Ma, Z.M.; Luo, Y.Y.; Fang, Y.Z.; Hou, Y.S. Hydrogeochemical Mechanism of the Petroleum Hydrocarbon Pollution in Karst Fissure Groundwater System. Appl. Mech. Mater. 2013, 295, 159–163. [Google Scholar] [CrossRef]
- Heine, F.; Zosseder, K.; Einsiedl, F. Hydrochemical Zoning and Chemical Evolution of the Deep Upper Jurassic Thermal Groundwater Reservoir Using Water Chemical and Environmental Isotope Data. Water 2021, 13, 1162. [Google Scholar] [CrossRef]
- Dvorski, S.E.-M.; Gonsior, M.; Hertkorn, N.; Uhl, J.; Müller, H.; Griebler, C.; Schmitt-Kopplin, P. Geochemistry of Dissolved Organic Matter in a Spatially Highly Resolved Groundwater Petroleum Hydrocarbon Plume Cross-Section. Environ. Sci. Technol. 2016, 50, 5536–5546. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, H.; Li, M.; Zhang, C.; Su, S.; Xiao, H. A Review of Groundwater Iodine Mobilization, and Application of Isotopes in High Iodine Groundwater. Environ. Geochem. Health 2024, 46, 388. [Google Scholar] [CrossRef]
- Vengosh, A.; Jackson, R.B.; Warner, N.; Darrah, T.H.; Kondash, A. A Critical Review of the Risks to Water Resources from Unconventional Shale Gas Development and Hydraulic Fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef]
- Visser, P.W.; Kooi, H.; Stuyfzand, P.J. The Thermal Impact of Aquifer Thermal Energy Storage (ATES) Systems: A Case Study in the Netherlands, Combining Monitoring and Modeling. Hydrogeol. J. 2015, 23, 507–532. [Google Scholar] [CrossRef]
- Jesußek, A.; Grandel, S.; Dahmke, A. Impacts of Subsurface Heat Storage on Aquifer Hydrogeochemistry. Environ. Earth Sci. 2013, 69, 1999–2012. [Google Scholar] [CrossRef]
- Kompanichenko, V.N.; Poturay, V.A.; Karpov, G.A. Organic Compounds in Thermal Water: The Mutnovskii Area and the Uzon Caldera. J. Volcanol. Seismol. 2016, 10, 305–319. [Google Scholar] [CrossRef]
- Rasulov, S.M.; Orakova, S.M.; Isaev, I.A. Thermal Properties and Phase Diagrams of Water–Hydrocarbon Systems. High Temp. 2016, 54, 210–214. [Google Scholar] [CrossRef]
- Novikov, D.A.; Van, P.T.K.; Van Tuyen, D.; Thu, D.T.; Dultsev, F.F.; Chernykh, A.V.; Hoan, T.V. Interaction of Thermal Waters with Carbonate and Aluminosilicate Minerals: A Case Study of Bang Mineral Hot Spring, Central Vietnam. E3S Web Conf. 2019, 98, 01039. [Google Scholar] [CrossRef]
- Esterhuyse, S.; Redelinghuys, N.; Charvet, P.; Fearnside, P.; Daga, V.; Braga, R.; Okello, W.; Vitule, J.; Verheyen, E.; Van Steenberge, M. Effects of Hydrocarbon Extraction on Freshwaters. In Encyclopedia of Inland Waters, 2nd ed.; Mehner, T., Tockner, K., Eds.; Elsevier: Oxford, UK, 2022; pp. 189–209. ISBN 978-0-12-822041-2. [Google Scholar]
- Kárpáti, Z.; Sajgó, C.; Vető, I.; Klopp, G.; Horváth, I. Organic Matter in Thermal Waters of the Pannonian Basin—a Preliminary Report on Aromatic Compounds. Org. Geochem. 1999, 30, 701–712. [Google Scholar] [CrossRef]
- Gioia, M.L.D.; Leggio, A.; Pera, A.L.; Liguori, A.; Perri, F. Occurrence of Organic Compounds in the Thermal Sulfurous Waters of Calabria, Italy. Chroma 2006, 63, 585–590. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Cancho-Grande, B.; Araujo-Nespereira, P.; Cid-Fernández, J.A.; Simal-Gándara, J. Occurrence of Soluble Organic Compounds in Thermal Waters by Ion Trap Mass Detection. Chemosphere 2009, 75, 34–47. [Google Scholar] [CrossRef]
- Varga, C. Volatile Organics in Thermal Spa Waters: Active Ingredients or Enviromental Toxicants? Thermae Spa Med. 2012, 1, 1–8. [Google Scholar]
- Kompanichenko, V.N.; Poturay, V.A. Organic Compounds of Medium Volatility in the Thermal Fields of Urup Island, Kuriles, and the Kamchatka Peninsula: A Comparative Analysis. Geochem. Int. 2022, 60, 256–265. [Google Scholar] [CrossRef]
- Lambrakis, N.; Zagana, E.; Katsanou, K. Geochemical Patterns and Origin of Alkaline Thermal Waters in Central Greece (Platystomo and Smokovo Areas). Environ. Earth Sci. 2013, 69, 2475–2486. [Google Scholar] [CrossRef]
- Stavropoulou, V.; Pyrgaki, A.; Zagana, E.; Pouliaris, C.; Kazakis, N. The Contributions of Tectonics, Hydrochemistry and Stable Isotopes to the Water Resource Management of a Thermal—Mineral Aquifer: The Case Study of Kyllini, Northwest Peloponnese. Geosciences 2024, 14, 205. [Google Scholar] [CrossRef]
- Larikova, O.I.; Subbota, M.I.; Kleymenov, V.F. Hydrogeological Conditions and the Possibility of Oil and Gas Bearing Deposits in the Kyzyl Kums. Foreign Technol. Div. 1978, 2349, 8. [Google Scholar]
- Rouhi, H.; Kalantari, N. Hydrogeochemistry and Groundwater Mixing Close to an Oil Field: An Example from Asmari Karstic Aquifer, Khuzestan, Iran. Water Supply 2017, 18, 357–370. [Google Scholar] [CrossRef]
- Li, H.; Son, J.-H.; Carlson, K.H. Concurrence of Aqueous and Gas Phase Contamination of Groundwater in the Wattenberg Oil and Gas Field of Northern Colorado. Water Res. 2016, 88, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, J.P.T.; Melo, C.L.; Iglesias, R.S.; Reginato, P.R. CO2-Shallow Groundwater Interaction and Related Hydrogeochemical Mechanisms: A Review on Reduced-Scale CO2 Release Field Experiments. Greenh. Gases Sci. Technol. 2023, 13, 829–859. [Google Scholar] [CrossRef]
- Teramoto, E.H.; Navarro, J.; Kiang, C.H. Avaliação geoquímica das águas envasadas de aquíferos cristalinos no sul e sudeste do Brasil. Rev. Inst. Geol. 2019, 40, 53–67. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, H.; He, C.; Li, Z. Differential Thermal Evolution between Oil and Source Rocks in the Carboniferous Shale Reservoir of the Qaidam Basin, NW China. Energies 2021, 14, 7088. [Google Scholar] [CrossRef]
- Chen, J.; Yang, X.; Zhang, X.; Pang, X.; Luo, J.; Pang, B.; Jiang, F.; Yang, H.; Li, J.; Shi, K. Hydrocarbon Expulsion Mechanism of Lower Cambrian Argillaceous Source Rocks in the Tarim Basin, China. Geol. J. 2024, 59, 1998–2021. [Google Scholar] [CrossRef]
- Han, Y.; Han, Y.; Wang, L.; Volmer, D.A.; Qi, Y. Impact of Hydrothermal Fluids on Hydrocarbon Generation and Solid Bitumen Formation in the Kongdian Formation, Huanghua Depression, China. J. Am. Soc. Mass Spectrom. 2024, 35, 3274–3285. [Google Scholar] [CrossRef]
- Li, C.; Zeng, J.; Wang, M.; Long, H.; Liu, S. Influence of Magmatic Intrusion on Abnormal Hydrocarbon Generation and Expulsion of Source Rock: A Case Study of the Dongying Sag, Bohai Bay Basin. Acta Geol. Sin.-Engl. Ed. 2024, 98, 1322–1337. [Google Scholar] [CrossRef]
- Lu, J.; Li, C.; Wang, M.; Zhang, C. Review of Deep Fluids in Sedimentary Basins and Their Influence on Resources, with a Focus on Oil and Geothermal Exploitation. Front. Earth Sci. 2023, 10, 896629. [Google Scholar] [CrossRef]
- Rashid, T.; Sabarathinam, C.; Saravana Kumar, U.; Al-Jumaa, M.; Al Salman, B.; Naseeb, H. Terrigenic Helium in Brackish Groundwaters of Kuwait, Probable Influences from Hydrocarbon Resources. Groundw. Sustain. Dev. 2023, 23, 101048. [Google Scholar] [CrossRef]
- Eymold, W.K.; Swana, K.; Moore, M.T.; Whyte, C.J.; Harkness, J.S.; Talma, S.; Murray, R.; Moortgat, J.B.; Miller, J.; Vengosh, A.; et al. Hydrocarbon-Rich Groundwater above Shale-Gas Formations: A Karoo Basin Case Study. Groundwater 2018, 56, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Pindado Jiménez, O.; Pérez Pastor, R.M.; Escolano Segovia, O.; del Reino Querencia, S. Exploring Petroleum Hydrocarbons in Groundwater by Double Solid Phase Extraction Coupled to Gas Chromatography–Flame Ionization Detector. Talanta 2015, 131, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Lesage, S.; Xu, H.; Novakowski, K.S. Distinguishing Natural Hydrocarbons from Anthropogenic Contamination in Ground Water. Groundwater 1997, 35, 149–160. [Google Scholar] [CrossRef]
- Prommer, H.; Davis, G.B.; Barry, D.A. Geochemical Changes during Biodegradation of Petroleum Hydrocarbons: Æeld Investigations and Biogeochemical Modelling. Org. Geochem. 1999, 30, 423–435. [Google Scholar] [CrossRef]
- Fei-Baffoe, B.; Badu, E.; Miezah, K.; Sackey, L.N.A.; Sulemana, A.; Amuah, E.E.Y. Contamination of Groundwater by Petroleum Hydrocarbons: Impact of Fuel Stations in Residential Areas. Heliyon 2024, 10, e25924. [Google Scholar] [CrossRef]
- Ite, A.; Harry, T.; Obadimu, C.; Asuaiko, E.R.; Inim, I.J. Petroleum Hydrocarbons Contamination of Surface Water and Groundwater in the Niger Delta Region of Nigeria. JEPHH 2018, 6, 51–61. [Google Scholar] [CrossRef]
- Currell, M.; Banfield, D.; Cartwright, I.; Cendón, D.I. Geochemical Indicators of the Origins and Evolution of Methane in Groundwater: Gippsland Basin, Australia. Environ. Sci. Pollut. Res. 2017, 24, 13168–13183. [Google Scholar] [CrossRef]
- Jellicoe, K.; McIntosh, J.C.; Ferguson, G. Changes in Deep Groundwater Flow Patterns Related to Oil and Gas Activities. Groundwater 2022, 60, 47–63. [Google Scholar] [CrossRef]
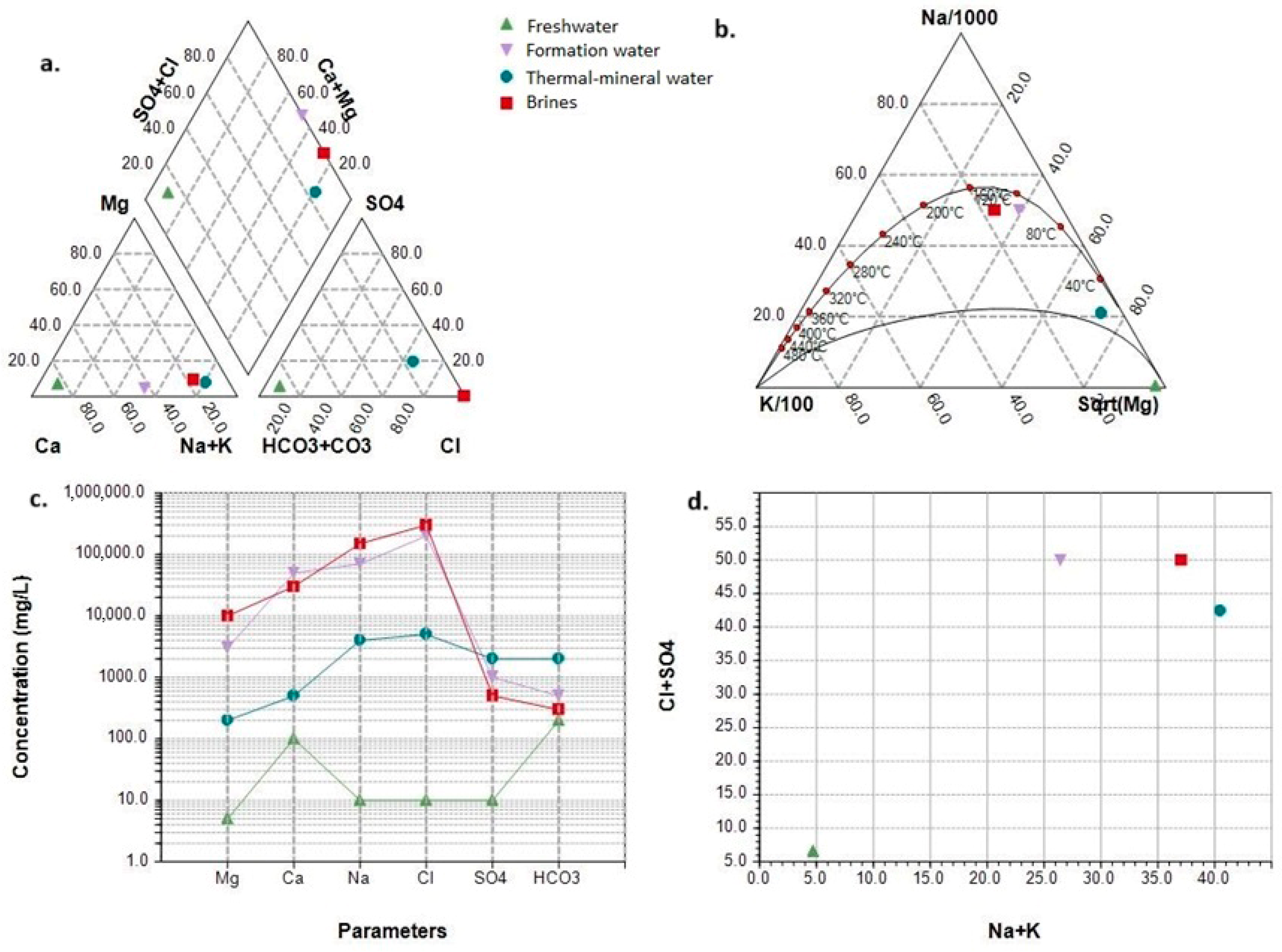
| Water Type/ Elements (mg/L) | Fresh Water | Thermal Water | Formation Water | Brine Water |
|---|---|---|---|---|
| Chloride (Cl−) | 1–10 | 50–5000 | 10,000–200,000 | 50,000–300,000 |
| Sodium (Na+) | 1–10 | 50–4000 | 5000–70,000 | 30,000–150,000 |
| Calcium (Ca2+) | 10–100 | 10–500 | 1000–50,000 | 5000–30,000 |
| Magnesium (Mg2+) | 1–5 | 10–200 | 50–3000 | 1000–10,000 |
| Sulfate (SO42−) | 1–10 | 50–2000 | 100–1000 | 100–500 |
| Bicarbonate (HCO3−) | 30–200 | 100–2000 | 100–500 | 10–300 |
| Potassium (K+) | 0.5–5 | 5–50 | 10–1500 | 500–5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavropoulou, V.; Zagana, E.; Pouliaris, C.; Kazakis, N. Assessing the Interaction Between Geologically Sourced Hydrocarbons and Thermal–Mineral Groundwater: An Overview of Methodologies. Water 2025, 17, 1940. https://doi.org/10.3390/w17131940
Stavropoulou V, Zagana E, Pouliaris C, Kazakis N. Assessing the Interaction Between Geologically Sourced Hydrocarbons and Thermal–Mineral Groundwater: An Overview of Methodologies. Water. 2025; 17(13):1940. https://doi.org/10.3390/w17131940
Chicago/Turabian StyleStavropoulou, Vasiliki, Eleni Zagana, Christos Pouliaris, and Nerantzis Kazakis. 2025. "Assessing the Interaction Between Geologically Sourced Hydrocarbons and Thermal–Mineral Groundwater: An Overview of Methodologies" Water 17, no. 13: 1940. https://doi.org/10.3390/w17131940
APA StyleStavropoulou, V., Zagana, E., Pouliaris, C., & Kazakis, N. (2025). Assessing the Interaction Between Geologically Sourced Hydrocarbons and Thermal–Mineral Groundwater: An Overview of Methodologies. Water, 17(13), 1940. https://doi.org/10.3390/w17131940








