Abstract
Invasive species are a major threat to global biodiversity. Therefore, it is crucial to monitor their presence and expansion within invaded areas and carry out studies to improve our knowledge of their biology and ecology. One of the most effective and spectacular invaders among freshwater snails is the acute bladder snail (Physella acuta) (Draparnaud, 1805). This study aims to update the available data on P. acuta in Morocco and determine the main environmental factors that favor its distribution and expansion in this country. Field surveys were conducted in northern Morocco between 2014 and 2023, with a focus on protected areas such as Ramsar sites, and especially great geographical barriers such as the Middle Atlas Mountains and the Sebou and Moulouya River basins. The gastropods were collected using Surber samplers (20 × 25 cm surface area, 400 µm mesh), together with measurements of the physicochemical parameters of the water and other abiotic factors. The bladder snail is probably the most widespread freshwater snail in Morocco, where the species appears to be highly adaptable and can thrive in different habitats, including degraded ones, showing great plasticity in terms of the physicochemical parameters of the water. The main factor limiting the geographical distribution and abundance of P. acuta in the study area was water velocity and conductivity. However, further studies are required to address the future range of expansion of P. acuta in relation to climate change. Although one of the consequences of climate change is reduced water flow speed, which may promote its range of expansion in Morocco, salinization of streams may also reduce its ability to colonize new environments.
1. Introduction
The introduction of exotic species is considered one of the main factors endangering the biodiversity of aquatic ecosystems [1,2,3]. In recent years, the introduction of exotic species has also been seen as a new form of global change [4], especially in aquatic ecosystems; the significant growth of international trade and concurrent increases in transport capacities have accelerated the rate of introduction of alien species throughout the world [5]. Invasive species have important worldwide consequences on native biodiversity [6], but also on public health [7] and local economy [8,9].
Among freshwater invaders, non-native freshwater gastropods can have an important effect on the biodiversity of colonized areas. Indeed, many snail species have proved to be very successful invaders in recent decades, even able to cross oceans and continents [10,11,12,13]. In addition, freshwater snails can be carriers and transmitters of several parasites and pathogens [14,15,16,17] that spread through being carried by their hosts. A good example of a successful biological invasion among the Gastropoda class is the acute bladder snail (Physella acuta) (Draparnaud, 1805)—an aquatic pulmonate snail with a left-handed light and glossy yellowish corneous shell and pointed apex, and no operculum. The body is greyish. The upper mantle under the shell is covered with spots; the animal has digitations (finger-like processes) along the edge of the mantle against the columella and there is no pseudobranch (false gill). The shell has six sinistral fast increasing whorls, slightly convex with a clear suture, which is in many specimens whitish, as the columellar border, and shell size varies between 8 and 15 mm high and from 5 to 7 mm wide. It may be confused in North Africa with the species Physa fontinalis (Linnaeus, 1758), which has a generally smaller shell with an obtuse apex, whereas P. acuta has an acute apex [18].
Although the type locality of Physella acuta is in the Garonne River basin (France) [19] (it was once thought to be native to the Mediterranean), the origin of the acute bladder snail is from the Nearctic (native to the northeastern United States and adjacent Canada) [20]. It was believed that the original description was made when the species was already introduced to Europe [13], as for another North American invader Gammarus trigrinus described in England. The invasive species may have spread from North America to France across Europe and is now considered cosmopolitan, invading all continents except Antarctica and colonizing different habitats representing a wide range of abiotic factors [21,22,23]. The invasive species is often referred to in the literature as Physa acuta (Draparnaud, 1805) or sometimes Haitia acuta (Draparnaud, 1805). In this study, we used Physella acuta following Vinarski [13].
The first record of P. acuta in Morocco probably dates back to 1972. This species, like most of the non-native species known in Morocco, was certainly established either from aquarium plants or with fish species introduced into Moroccan freshwaters after the Second World War [24]. Since then, the species has been recorded all over the country and is now considered common in its continental waters [25,26,27,28,29]. However, its ecological niche and geographical range of expansion have been little monitored following the first report in Morocco. In this study, we first aim to update the available data on P. acuta in Morocco and to determine the main environmental factors that favor its distribution in this country, while trying to confirm its ecological plasticity. The second goal of our study is to estimate the potential impact of climate change on its future range of expansion in Morocco and adjacent countries.
2. Materials and Methods
2.1. Field Surveys
Field surveys were conducted between 2014 and 2023 in 140 sampling sites covering various environmental conditions and types of water bodies (estuaries and streams, irrigation canals, lagoons, ponds, and brackish and salty marshes) observed in Morocco, with a particular focus on protected areas such as Ramsar sites and sites of ecological and biological interest (known as SEBI or SIBE). Quantitative samples of the different microhabitats present on the site were taken against the current between 10am and noon. The choice of microhabitats was based on their biogenic capacity (favorable for aquatic life), their representativeness within the site and the different classes of current velocity at the sampled site. We collected relative estimates of macroinvertebrate taxa, focusing primarily on P. acuta in different microhabitats surveyed at each locality. Macroinvertebrates were collected using sweep nets, dip nets and Surber samplers (surface area 20 × 25 cm; 8 samples equating to 0.40 m2). Abundance data were converted to density per m2 (see the Suplementary file for the complete list of localities and P. acuta abundance).
2.2. Environmental Data
Water pH, electrical conductivity (µs·cm−1) and dissolved oxygen concentrations were measured directly on the field with a portable apparatus (WTW, MPP350) and the water temperature was measured (±0.1 °C) using both a digital and mercury thermometer. The flow velocity (in m.s−1) was measured as the time taken by a floating body (a cork stopper) to cover at least one meter. Elevation was measured using a GPS device (Garmin eTrex 10, Schaffhausen, Switzerland). In addition, we analyzed the physicochemical parameters of the waters harboring P. acuta. For each station, two replicates of each water sample were collected in 500 mL polyethylene bottles and preserved with 2 mL of concentrated hydrochloric acid. Samples were carried out in a cooler according to the ISO 5667-6, ISO 5667-2 and ISO 5667-3 [30,31,32] standards. Sulfates (SO42– in in mg·L−1), the biological oxygen demand after 5 days (BOD5 in mg·L−1), and orthophosphate (PO43− in mg·L−1) and nitrate (N-NO3− in mg·L−1) concentrations were measured in the laboratory according to AFNOR standards [33] and Rodier et al. [34].
2.3. Statistical Analysis
Out of the 140 sampled sites, 74 were chosen to ensure homogeneous data, and 44 of these 74 sites harbored P. acuta. Other sites harboring P. acuta were not included in the following analyses because some environmental parameters were missing. To compare physicochemical parameters between sites, we used ANOVAs followed by Tukey’s test if conditions of normality and/or homoscedasticity were satisfied; otherwise, we used the Kruskal–Wallis test followed by Wilcoxon tests. The analyses were carried out using R software version 4.1.3, and the ggeffects, MASS and ggplot2 packages.
To investigate the relationship between environmental factors and the presence of P. acuta, we firstly conducted a principal component analysis (PCA) with the ade4.0, factoextra and ggeffects packages. We applied a logistic regression to determine the environmental factors explaining the distribution of P. acuta in Morocco using MASS, ggplot2 and agricolae packages. Logistic regression is a modeling tool that primarily attempts to forecast and clarify the outcomes of a binary categorical parameter Y using a set of continuous, discrete or binary variables X. Abundance data were transformed into presence/absence data before conducting a logistic regression [35]. We implemented a step-by-step top-down selection approach to enhance the explanatory model. We evaluated the quality of the final model based on the Akaike information criterion (AIC), whereby lower values indicate better model performance. We considered the model valid and accepted when it satisfied the logistic regression assumptions, including sufficient cases and no overdispersion.
2.4. Gathering Distribution Data
To complete and update the distribution map of P. acuta in Morocco, the geographical coordinates were compiled from the Global Biodiversity Information Facility GBIF [36] and an extensive literature search of published articles [24,25,26,27,28,29,37].
3. Results
Physella acuta is widely distributed throughout Morocco, with the southernmost record from the Laayoune-Sakia El Hamra region (Figure 1).
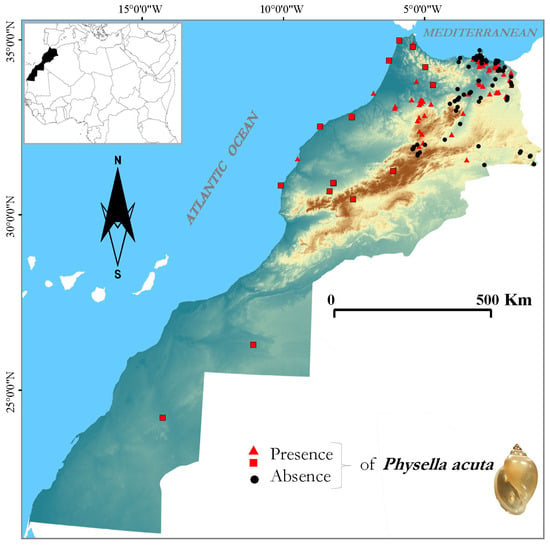
Figure 1.
Updated map of P. acuta distribution in the continental waters of Morocco. Red triangles: own records; red squares: bibliographic records from GBIF and published articles [24,25,26,27,28,29,36,37].
The species was detected at 96 sites (out of 140 sampled sites) along the northern part of Morocco, occupying a wide range of habitats: high-altitude lakes (e.g., Sidi Ali, Zerrouka and Tifounassine), the potamal section of big rivers (e.g., Moulouya and Sebou) or anthropogenic habitats (e.g., the artificial canal of Saidia). Most of the surveyed inland Ramsar sites were infested by the invasive gastropod. In the sites surveyed, P. acuta was often found in association with shallow, stagnant or slowly flowing waters. Future surveys could reveal other populations of this species and extend its known range in Morocco. The complete list of records for P. acuta in Morocco is provided in the Supplementary Material.
The bladder snail showed great plasticity in terms of the physicochemical parameters of the water, as illustrated by the abundance of P. acuta according to the physicochemical parameters of the water and abiotic factors in each new locality (Table S1 in the Supplementary File). Regarding electrical conductivity in particular, P. acuta was recorded in low mineralized waters with a minimum of 213 μs·cm−1 at Ain Sfa (perennial spring in the Oriental region of Morocco) and in the brackish waters of the coastal system, with a maximum of 30,340 μs.cm−1 registered at Sidi Boughaba Lake (a Ramsar site on the Atlantic coast of Morocco). The species appears to tolerate significant spatiotemporal fluctuations in dissolved oxygen levels; it has been observed in both highly oxygenated waters, with a concentration of 9.6 mg.L−1 at Dardoura River, an affluent of the Marchica lagoon (Ramsar site on the Mediterranean coast of Morocco), and in weakly oxygenated waters, with a minimum concentration of 1.5 mg·L−1 registered at Ouzej R (upstream of another affluent of the Marchica lagoon). The species demonstrates great plasticity, as evidenced by its ability to tolerate a wide range of organic pollution indicators. Specifically, it can tolerate nitrate concentrations ranging from 0.03 to 53.62 mg·L−1, sulfate concentrations ranging from 34 to 403 mg·L−1, phosphate concentrations ranging from 0.005 to 4.31 mg·L−1 and BOD5 concentrations ranging from 0.49 to 32.0 mg·L−1. During the study period, P. acuta was found in waters with a neutral pH in general, ranging between 6.5 and 8.5, and in water temperatures ranging from 16.9 to 28 °C.
The mesological parameters of the sites were initially analyzed using standardized principal components. The first two axes F1 and F2 (Figure 2) accounted for 51.6% of the total inertia and held the bulk of the information. Axis F1 (38.9% of total inertia) showed a conductivity gradient that increased from left to right. Axis F2 (12.7% of total inertia) showed water velocity rising from bottom to top and being negatively correlated with depth. The species obviously grew absent when conductivity or water velocity increased.
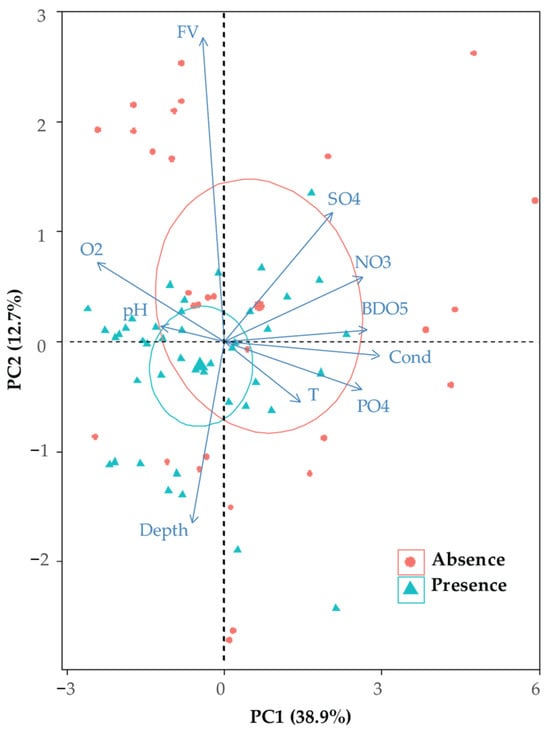
Figure 2.
Biplot of the first plane of the principal component analysis of physicochemical parameters (T: temperature, Cond: electrical conductivity, FV: flow velocity, O2: dissolved oxygen, NO3: nitrate, SO4: sulfates, PO4: orthophosphate, BOD5: biological oxygen demand and Dep: depth). Circles indicate 95% confidence intervals for the sites colonized by P. acuta (blue) and the non-colonized sites (red).
The analysis of variance (see Figure 3) showed that of the ten mesological variables considered in this study, four had a direct influence on the presence or absence of P. acuta and one had an influence on its abundance.
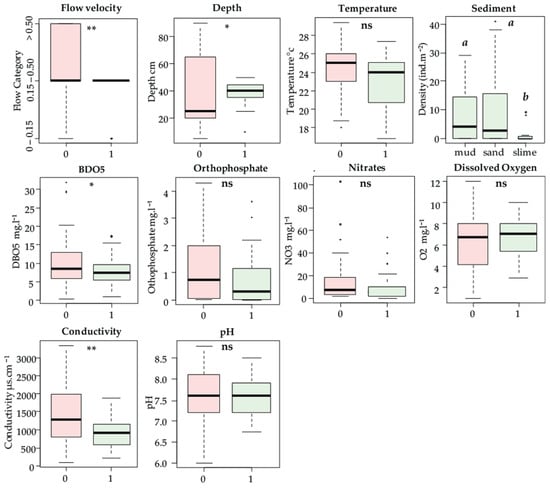
Figure 3.
Boxplots of environmental variables classified according to P. acuta presence (1)/absence (0) or abundance. The letters above indicate whether there is a significant difference between sites in pairwise post hoc comparisons (ns: not significant. p-values: * ≤ 0.05; ** ≤ 0.01).
Based on the results of the variance analysis (Figure 3), we inferred that P. acuta occurrence was affected by flow velocity, electrical conductivity, depth and BOD5, while sediment impacted its abundance.
The AIC of the best logistic model was 71.554. Based on this model, we concluded that there was a significant correlation between P. acuta presence and three environmental factors (conductivity, flow velocity and substrate type) with 95% confidence (Table 1).

Table 1.
Final model fit to the study data, n = 74.
The explanatory variables of the ultimate model accounted for 38.39% of the variation in the response variable, as noted by McFadden’s pseudo-R2. The predictions were flawed in 13 instances out of a total of 74, equating to a 17.5% misclassification rate.
The logistic model used in this study was able to forecast the likelihood of P. acuta presence (Figure 4). It suggested that the species was more likely to be present when water velocity and electrical conductivity were low. Nevertheless, the chance of the species being present decreased significantly in the presence of slime substrate (25%), whereas it significantly increased when the substrate was muddy (76%) or sandy (63%).
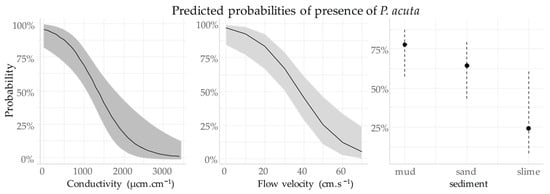
Figure 4.
Likelihood of P. acuta presence based on the model used in this study with 95% confidence bands.
Figure 4 shows a significant decrease in the probability of the presence of P. acuta according to conductivity and flow velocity. P. acuta has a 25% chance of inhabiting an environment with a conductivity of 2000 µs·cm−1 or a flow velocity above 50 cm·s−1.
However, we found a strong interaction between environmental factors; for instance, the probability of the species’ presence at a conductivity of 2000 µs·cm−1 exceeds 80% when the flow velocity is low (Figure 5). The substrate has the same impact on the probability of P. acuta presence in relation to the flow speed. The probability is close to 99% for a mud substrate (see Figure 5), compared with 78% (see Figure 4 and Table 2) for a slime substrate, as long as the flow velocity does not exceed 0.15 m·s−1.
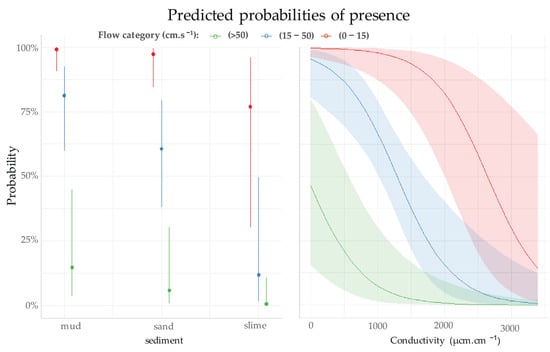
Figure 5.
Probability of presence (±95% CI) of P. acuta according to the electrical conductivity and type of sediment for each category of flow velocity.

Table 2.
Likelihood of P. acuta presence based on the model used in this study with 95% confidence bands.
4. Discussion
Since its introduction into Morocco in 1972, probably via the Aquarium/ornamental plant trade or by migratory birds, the North American P. acuta has become the most widespread invasive mollusk in Morocco [24], where the species appears to be highly euryceous and can thrive in various habitats, including degraded ones. The habitats of this species vary widely, from large permanent streams at high and low altitudes to reservoirs, natural and managed springs, and small temporary tributaries, as long as they are connected to a permanent source or underground water. It can also be found in human-made structures, such as irrigation canals and cement tanks used for water retention. Its southernmost record is presently in the Laayoune-Sakia El Hamra region, where it seems to adapt easily and to have low requirements in terms of habitat quality. In Morocco, the species has been found in freshwaters with high levels of organic matter and low dissolved oxygen levels. Specimens have been found in the waters of the Selouan and Za Rivers—some of the most polluted rivers in eastern Morocco [38]. This result is congruent with the literature on P. acuta in which it is considered a globally invasive species living in all kinds of freshwater environments, including altered habitats [18,39,40]. Once established, P. acuta can adapt to a wide range of habitat conditions including highly polluted freshwaters [40]. It is highly tolerant to elevated temperature and often abundant in reservoirs affected by thermal waters from heat and power plants [41,42]. Its high capacity to tolerate great variability in abiotic factors and to successfully withstand extreme physical and chemical parameter values might be key to its biological success [43,44,45]. Physella acuta can survive in unstable, heated freshwater environments and colonize environmentally toxic habitats inaccessible to other snail species thanks to its high resistance to pollution [46,47]. It can stand anaerobic conditions for prolonged periods because snails are pulmonates and can use atmospheric oxygen [48]. One of the most important traits of P. acuta is its huge evolutionary potential which allows it to adapt to new environmental conditions [49]. P. acuta has remarkable reproductive plasticity and a greater foraging ability than native species. This feature might contribute to its invasion success and allow it to displace native gastropods and become the dominant species over very short periods of time [50,51,52].
Among the environmental parameters explaining the current distribution of P. acuta in Morocco, our models highlighted that flow velocity and conductivity were the two main limiting factors (alongside other factors) explaining its distribution. Its low tolerance to flow velocity explains why it can invade flowing water ecosystems only in summer, after water levels and flow rates have decreased and when the current velocity slows down. This phenomenon is particularly noticeable in the Moulouya, Za and Melloulou Rivers [28,29,37]. This finding is congruent with those of previous studies on the sensitivity of P. acuta to flow velocity [53,54]. It is indeed known to inhabit standing water ecosystems such as wetlands, ponds, lakes and downstream stretches of rivers [18]. Its current distribution may be strongly enlarged by the impact of climate change in Morocco because climate change is impacting the water discharge of streams and rivers worldwide [55].
This is particularly true in North African countries which are particularly impacted by anthropogenic activities, and Morocco is a good example. The country currently faces a crisis due to climate change and the overexploitation of its resources in freshwaters. The country extends latitudinally through five different bioclimatic zones [56], which results in a declining rainfall gradient from north to south and a longitudinal gradient influenced by the Atlas Mountains. Precipitation trends in Morocco are highly variable. However, projections from the USIAD indicate a significant decrease in average annual rainfall across the country from 10–20% to as much as 30% in the Saharan region [57]. The construction of hydraulic facilities has increased considerably since the 1990s to compensate for this scarce rainfall, and several surface water mobilization structures have been built on watercourses in catchment basins, mainly in the northern part of the country [58]. The National Water Strategy of Morocco has already planned the construction of about 60 large dams by 2030 in addition the 150 existing ones and also 1000 small dams for the development and transfer of raw water resources from the north to the south and for the safeguarding of hydraulic infrastructures. Moreover, there are already 13 water transfer systems between watersheds that may promote the dispersion of P. acuta between hydrosystems [59]. As a consequence, the distribution of P. acuta in Morocco may strongly increase over the next few decades.
The range of expansion of P. acuta may be restricted by the rising salinity of freshwaters due to climate change. Our study shows that P. acuta is sensitive to electrical conductivity above 400 µs.cm−1. This confirms the results of previous experimental studies that highlighted the sensitivity of pulmonate gastropoda to salinity, especially of P. acuta [60]. In freshwaters, the concentration of dissolved ions can increase through evaporation, especially in semi-arid and arid regions and regions with seasonally hot dry climates [61]. This trend is expected to increase due to climate change and the reduction in available surface water volumes [57]. Several processes can contribute to salinization in arid and semi-arid areas. For instance, irrigation leaves salt residues behind after evaporation, and groundwater levels rise following vegetation removal. All of this brings salt ions from weathering geological sources toward the soil surface [62]. This can be expected in Morocco where the water management strategy will increase groundwater pumping and promote evaporation in dams and irrigation channels. The resulting potential rising conductivity may restrict the range of expansion of P. acuta if conductivity reaches the threshold of 400 µs.cm−1 highlighted in our study.
Molluscan invasions can lead to fauna homogenization, extirpation of vulnerable endemic species and alterations in the biotic composition of the invaded ecosystems, as in the case of P. acuta. This invasive species can cause several negative impacts on the invaded freshwater ecosystems [13,63,64]. In addition to P. acuta, five other alien and notorious invasive snails can be found in the freshwaters of Morocco, i.e., the seminole rams-horn Helisoma duryi (Wetherby, 1879), the New Zealand mudsnail Potamopyrgus antipodarum (J.E. Gray, 1843), the North American freshwater limpet Ferrissia californica (Tryon 1863), the Malayan livebearing snail Melanoides tuberculata (O.F. Müller, 1774) and the Fountain bladder snail Physa fontinalis (Linnaeus, 1758). Worse still, many invasive species have been recorded recently to be present in the freshwater ecosystems of Morocco, including fish, annelids, mollusks and arthropods, leading to the formation of new communities in the area through diverse unknown interactions with unpredictable damage [24,65,66,67,68].
In 2023, we have seen huge gaps between the number of known alien species and the number of studies devoted to examining their impacts in the freshwater ecosystems in Morocco and how management strategies against biological invasion are still lacking [24]. Even worse, except for the ruddy-headedduck Oxyura jamaicensis (Gmelin, 1789), there is no eradication program to eliminate or stop the spread of exotic species in Morocco. Management plans for the control or eradication of invasive alien species, such as P. acuta and other notorious snails, must be implemented and are urgently needed.
5. Conclusions
The bladder snail is one of the most widespread freshwater exotic species in Morocco, and the fact that its spread has gone undetected is linked to the lack of studies on alien species. The results of this study once again demonstrate the adaptability of P. acuta and identify current velocity (among others) as the primary factor hindering its spread in North Africa. As a result of ongoing anthropogenic changes in natural habitats and climate patterns (irregular and scarce rainfall), the lotic ecosystems of Morocco are expected to experience a decrease in their flow rate and velocity. This could lead to significant expansion of the dispersal range of invasive species such as P. acuta. However, climate change may also lead to an increase in conductivity that may limit its range of expansion. Further studies on the impact of salinization in Morocco are required to better predict the range of expansion of P. acuta.
Although the relationship between P. acuta populations and environmental factors is well documented in the invaded areas of the North Palearctic regions, our understanding of how environmental factors influence P. acuta populations in the freshwaters of North Africa is limited. This study represents an important step toward a better understanding of the invasion process of P. acuta and its population dynamics in response to environmental factors and climate change in Morocco and North Africa as a whole.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16060803/s1, Table S1: Values of abundance of Physella acuta and physico-chemical parameters recorded at each site.
Author Contributions
Conceptualization, methodology, software, formal analysis, investigation, resources, data curation and writing—original draft preparation, Y.M., A.F.T. and P.G.; writing—review and editing, visualization and supervision, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. Site locations are available in Taybi et al. 2023 [24] and GBIF (https://www.gbif.org, (assessed on 10 October 2023)).
Acknowledgments
We thank the anonymous reviewers for valuable corrections and comments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef]
- Rahel, F.J.; Olden, J.D. Assessing the effects of climate change on aquatic invasive species. Conserv. Biol. 2008, 22, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Leape, J.; Marton-Lefevre, J.; Mittermeier, R.A.; Rose, M.; Robinson, J.G.; Stuart, S.N.; Waldman, B.; Genovesi, P. Invasives: A major conservation threat. Science 2011, 333, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Cohen, J. The invasiveness of an introduced species does not predict its impact. Biol. Invasions 2007, 9, 309–315. [Google Scholar] [CrossRef]
- Macdonald, J.; Tonkin, Z. A Review of the Impact of Eastern Gambusia on Native Fishes of the Murray-Darling Basin; Authority Publication No. 38/09; Murray-Darling Basin Authority: Canberra, Australia, 2008; p. 52. [Google Scholar]
- Strauss, S.Y.; Lau, J.A.; Carroll, S.P. Evolutionary responses of natives to introduced species: What do introductions tell us about natural communities? Ecol. Lett. 2006, 9, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, R.N.; Darriet, F.; Chabrerie, O.; Lenoir, J.; Courchamp, F.; Claeys, C.; Robert, V.; Jourdain, F.; Ulmer, R.; Diagne, C.; et al. Invasive hematophagous arthropods and associated diseases in a changing world. Parasit. Vectors 2023, 16, 291. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.A.; Haubrock, P.J.; Cuthbert, R.N.; Bang, A.; Soto, I.; Balzani, P.; Tarkan, A.S.; Macêdo, R.L.; Carneiro, L.; Bodey, T.W.; et al. Recent advances in availability and synthesis of the economic costs of biological invasions. BioScience 2023, 73, 560–574. [Google Scholar] [CrossRef]
- Pointier, J.P.; David, P.; Jarne, P. Biological invasions: The case of planorbid snails. J. Helminthol. 2005, 79, 249–256. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Burlakova, L.E.; Karatayev, V.A. Introduction, distribution, spread, and impacts of exotic freshwater gastropods in Texas. Hydrobiologia 2009, 619, 181–194. [Google Scholar] [CrossRef]
- Gittenberger, E. Long-distance dispersal of molluscs: ‘Their distribution at first perplexed me much’. J. Biogeogr. 2011, 39, 10–11. [Google Scholar] [CrossRef]
- Vinarski, M. The history of an invasion: Phases of the explosive spread of the physid snail Physella acuta through Europe, Transcaucasia and Central Asia. Biol. Invasions 2017, 19, 1299–1314. [Google Scholar] [CrossRef]
- Villavicencio, A.; Gorochov, V.; Carvalho, M. Lymnaea truncatula Müller, 1774 (Pulmonata: Lymnaeidae) infected with Fasciola hepatica (Linnaeus, 1758) (Trematoda: Digenea), in Moscow districts, Russian Federation. Rev. Patol. Trop. 2006, 35, 59–64. [Google Scholar] [CrossRef][Green Version]
- Zumaquero-Ríos, J.L.; Sarracent-Perez, J.; Rojas-Garcia, R.; Rojas-Rivero, L.; Martinez-Tovilla, Y.; Valero, M.A.; Mas-Coma, S. Fascioliasis and intestinal parasitoses affecting schoolchildren in Atlixco, Puebla State, Mexico: Epidemiology and treatment with nitazoxanide. PLoS Negl. Trop. Dis. 2013, 7, e2553. [Google Scholar] [CrossRef]
- Rondelaud, D.; Vignoles, P.; Dreyfuss, G.; Pointier, J.P.; Vázquez, A.A. Control of Fasciolosis-Transmitting Lymnaeids in the Field. In The Lymnaeidae; Vinarski, M.V., Vázquez, A.A., Eds.; Zoological, Monographs; Springer: Cham, Switzerland, 2023; Volume 7. [Google Scholar] [CrossRef]
- Vázquez, A.A.; Alba, A.; Alda, P.; Vittecoq, M.; Chapuis, E.; Faugère, D.; Pointier, J.-P. Lymnaeid Snails and the Transmission of Fasciolosis: Understanding the Differential Risks from Local to Global Scale. In The Lymnaeidae; Vinarski, M.V., Vázquez, A.A., Eds.; Zoological, Monographs; Springer: Cham, Switzerland, 2023; Volume 7. [Google Scholar] [CrossRef]
- Glöer, P. The freshwater gastropods of the West-Palaearctis. In Volume I Fresh- and Brackish Waters Except Spring and Subterranean Snails; Identification Key, Anatomy, Ecology, Distribution; Biodiversity Research Lab: Hetlingen, Germany, 2019; 399p. [Google Scholar]
- Paraense, W.L. Sinonímia entre Physa acuta e Physa cubensis: Morfologia e genética. In Tópicos em Malacologia: Ecos do XIX Encontro Brasileiro de Malacologia; Fernandez Santos, M.A.S.B., Pimenta, A., Thiengo, S.C., Eds.; Sociedade Brasileira de Malacologia: Rio de Janeiro, Brazil, 2011; pp. 32–35. [Google Scholar]
- Dillon Junior, R.T.; Wethington, A.R.; Rhett, J.; Smith, T. Populations of the European freshwater pulmonated Physa acuta are not reproductively isolated from American Physa heterostropha or Physa integra. Invertebr. Biol. 2002, 121, 226–234. [Google Scholar] [CrossRef]
- Ebbs, E.T.; Loker, E.S.; Brant, S.V. Phylogeography and genetics of the globally invasive snail Physa acuta Draparnaud 1805, and its potential to serve as an intermediate host to larval digenetic trematodes. BMC Evol. Biol. 2018, 18, 103. [Google Scholar] [CrossRef]
- Butkus, R.; Višinskienė, G.; Arbačiauskas, K. First record of the acute bladder snail Physella acuta (Draparnaud, 1805) in the wild waters of Lithuania. Bioinvasions Rec. 2019, 8, 281–286. [Google Scholar] [CrossRef]
- Cieplok, A.; Spyra, A. The roles of spatial and environmental variables in the appearance of a globally invasive Physa acuta in water bodies created due to human activity. Sci. Total Environ. 2020, 744, 140928. [Google Scholar] [CrossRef] [PubMed]
- Taybi, A.F.; Mabrouki, Y.; Piscart, C. Distribution of Freshwater Alien Animal Species in Morocco: Current Knowledge and Management Issues. Diversity 2023, 15, 169. [Google Scholar] [CrossRef]
- Ramdani, M.; Dakki, M.; Kharboua, M.; El Agbani, M.A.; Metge, G. Les Gastéropodes dulcicoles du Maroc: Inventaire commenté. Bull. Inst. Sci. Rabat. 1987, 11, 135–140. [Google Scholar]
- Ghamizi, M. Les Mollusques des Eaux Continentales du Maroc. Systématique, bio-Ecologie et Malacologie Appliquée. Ph.D. Thesis, University of Cadi Ayyad, Marrakesh, Morocco, 1998; pp. 1–560. [Google Scholar]
- Touabay, M.; Aouad, N.; Mathieu, J. Etude hydrobiologique d’un cours d’eau du Moyen-Atlas: L’oued Tizguit (Maroc). Ann. Limnol. 2002, 38, 65–80. [Google Scholar] [CrossRef][Green Version]
- Taybi, A.F.; Mabrouki, Y.; Ghamizi, M.; Berrahou, A. The freshwater malacological composition of Moulouya’s watershed and Oriental Morocco. J. Mater. Environ. Sci. 2017, 8, 1401–1416. [Google Scholar]
- Mabrouki, Y.; Taybi, A.F.; El Alami, M.; Berrahou, A. Biotypology of stream macroinvertebrates from North African and semi arid catchment: Oued Za (Morocco). Knowl. Manag. Aquat. Ecosyst. 2019, 420, 17. [Google Scholar] [CrossRef]
- ISO 5667-6; Water Quality Sampling. Part 6: Guidelines for Sampling of Rivers and Streams. ISO (International Organization for Standardization): Geneve, Switzerland, 1990; p. 26.
- ISO 5667-2; Water Quality Sampling. Part 2: Guidelines for Sampling of Rivers and Streams. ISO (International Organization for Standardization): Geneve, Switzerland, 1991; p. 9.
- ISO 5667-3; Water Quality Sampling. Part 3: Guidance on the Preservation and Handling of Samples. ISO (International Organization for Standardization): Geneve, Switzerland, 1994; p. 31.
- AFNOR (Association française de Normalisation). Qualité de l’eau. Recueil des Normes Françaises Environnement, Tomes 1, 2, 3 and 4; AFNOR Editions: Saint-Denis, France, 1997; p. 2500. [Google Scholar]
- Rodier, J.; Bazin, C.; Broutin, J.P.; Chambon, P.; Champsaur, H.; Rodi, L. L’analyse de L’eau, 8th ed.; Edition Dunod: Paris, France, 1996; p. 1383. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2012; p. 1006. [Google Scholar]
- GBIF. Global Biodiversity Information Facility. Physella acuta (Draparnaud, 1805) in GBIF Secretariat (2023). GBIF BackboneTaxonomy. 2023. Available online: https://www.gbif.org (accessed on 10 October 2023).
- Taybi, A.F.; Mabrouki, Y.; Berrahou, A.; Dakki, A.; Millán, A. Longitudinal distribution of macroinvertebrate in a very wet NorthAfrican basin, Oued Melloulou (Morocco). Ann. Limnol. 2020, 56, 17. [Google Scholar] [CrossRef]
- Taybi, A.F.; Mabrouki, Y.; Berrahou, A.; Legssyer, B. Spatio-temporal typology of the physico-chemical parameters of the Moulouya and its main tributaries. Afr. J. Aquat. Sci. 2020, 45, 431–441. [Google Scholar] [CrossRef]
- Ibáñez, M.; Alonso, M.R. Physella (Costatella) acuta (Draparnaud, 1805) in the Canary Islands (Pulmonata Basommatophora: Planorboidea: Physidae). Vieraea 2003, 31, 133–144. [Google Scholar]
- Vázquez-Capote, R.; Diéguez-Fernández, L.; Fimia-Duarte, R.; Iannacone, J. Environmental influence on the abundance of two populations of Physella acuta (Pulmonata: Physidae) from Camagüey (Cuba). Neotrop. Helminthol. 2015, 9, 243–252. [Google Scholar] [CrossRef]
- Protasov, A.A.; Silayeva, A.A.S. Raspredeleniye i Obili’e Molluskov v Vodoemakh, Podvergennykh Vozde’stviyu Podogretykh Vod Elektrostantsciy. Vestn. Zhitomirskogo Pedagog. Univ. 2002, 10, 16–17. [Google Scholar]
- Sharapova, T.A. Osobennosti Raspredeleniya i Ecologii MolluskovVselenscev v VodoemeOkhladitele Tumenskoy TESc v Zapadnoy Sibiri. Vestn. Zool. 2008, 42, 185–187. [Google Scholar]
- Semenchenko, V.; Laenko, T.; Razlutskij, V. A new record of the North American gastropod Physella acuta (Draparnaud, 1805) from the Neman River Basin, Belarus. Aquat. Invasions 2008, 3, 359–360. [Google Scholar] [CrossRef]
- Vasileva, S.Y. Shell size of the freshwater snail Physella acuta (Draparnaud, 1805) collected from water vegetation: A case study from South-East Bulgaria. Ecol. Balk. 2011, 3, 61–64. [Google Scholar]
- Banha, F.; Marques, M.M.; Anastácio, P.M. Dispersal of two freshwater invasive macroinvertebrates, Procambarus clarkii and Physella acuta, byoff-roadvehicles. Aquat. Conserv. Mar. Freshw. 2014, 24, 582–591. [Google Scholar] [CrossRef]
- Tomkins, A.R.; Scott, R.R. Effects of treated sevage effluent on the macroinvertebrates of a fine sediment substrate stream. Mauri Ora 1986, 13, 1–12. [Google Scholar]
- Strzelec, M. The effect of elevated water temperature on the occurrence of freshwater snails in the Rybnik dam reservoir (upper Silesia, Poland). Folia Malacol. 1999, 7, 93–98. [Google Scholar] [CrossRef]
- Harman, W.N. Snails (Mollusca: Gastropoda). In Pollution Ecology of Freshwater Invertebrates; Hart, C.W., Jr., Fuller, S.L.H., Eds.; Academic Press: New York, NY, USA, 1974; pp. 275–312. [Google Scholar]
- Hänfling, B.; Kollmann, J. An evolutionary perspective of biological invasions. Tree 2002, 17, 545–546. [Google Scholar] [CrossRef]
- Núñez, V. Fecundity and survival advantages of an exotic gastropod compared to a native species. Am. Malacol. Bull. 2011, 29, 95–103. [Google Scholar] [CrossRef]
- Saha, C.; Chakraborty, J.; Pramanik, S.; Parveen, S.; Aditya, G. Observations on the abundance and fecundity of the invasive snail Physa acuta in West Bengal, India: Implications for management. Ecol. Environ. Conserv. 2016, 23, 333–338. [Google Scholar]
- Paul, P.; Aditya, G. Invasion of the freshwater snail Physella acuta (Draparnaud, 1805) in selected ponds of North Dinajpur, India. J. Environ. Biol. 2021, 42, 577–581. [Google Scholar] [CrossRef]
- Hoffman, A.L.; Olden, J.D.; Monroe, J.B.; Poff, L.; Wellnitz, T.; Wiens, J.A. Current velocity and habitat patchiness shape stream herbivore movement. Oikos 2006, 115, 358–368. [Google Scholar] [CrossRef]
- Schössow, M.; Arndt, H.; Becker, G. Response of gastropod grazers to food conditions, current velocity, and substratum roughness. Limnologica 2016, 58, 49–58. [Google Scholar] [CrossRef]
- van Vliet, M.T.H.; Franssen, W.H.P.; Yearsley, J.R.; Ludwig, F.; Haddeland, I.; Lettenmaier, D.P.; Kabat, P. Global river discharge and water temperature under climate change. Glob. Environ. Chang. 2013, 23, 450–464. [Google Scholar] [CrossRef]
- Mokhtari, N.; Mrabet, R.; Lebailly, P.; Bock, L. Spatialisation des bioclimats, de l’aridité et des étages de végétation du Maroc. Rev. Mar. Sci. Agron. Vét. 2014, 2, 50–66. [Google Scholar]
- USAID. Climate Change Risk Profile—Morocco. 2016. Available online: https://www.climatelinks.org/sites/default/files/asset/document/2016_USAID_Climate%20Risk%20Profile%20-%20Morocco.pdf (accessed on 19 November 2023).
- Alaoui, M. Water sector in Morocco: Situation and perspectives. J. Water Resour. Ocean Sci. 2013, 2, 108–114. [Google Scholar]
- Houzir, M.; Mokass, M.; Schalatek, L. Climate Governance and the Role of Climate Finance in Morocco. 2016. Available online: https://us.boell.org/sites/default/files/morocco_study_climate_governance_final_english_nov.2.pdf (accessed on 15 December 2023).
- Kefford, B.J.; Hickey, G.L.; Ben-David, E.; Dunlop, J.E.; Palmer, C.G.; Allan, K.; Choy, S.; Piscart, C. Global Scale Variation in the Salinity Sensitivity of Riverine Macroinvertebrates: Eastern Australia, France, Israel and South Africa. PLoS ONE 2012, 7, e35224. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.L.; Kiehl, J.T.; Ramanathan, V.; Dickinson, R.E.; Hack, J.J. Description of NCAR Community Climatemodel (CCM1); Report Number: NCAR Technical Note NCAR/TN-285+STR; National Center for Atmospheric Research: Boulder, CO, USA, 1987; 109p. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Kefford, B.J.; Piscart, C.; Prat, N.; Schäfer, R.; Schulz, C.J. Salinisation of rivers: An urgent ecological issue. Environ. Pollut. 2013, 173, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Cowie, R. Invertebrate invasions on Pacific Islands and the replacement of unique native faunas: A synthesis of the land and freshwater snails. Biol. Invasions 2001, 3, 119–136. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55, 152–174. [Google Scholar] [CrossRef]
- Mabrouki, Y.; Taybi, A.F.; Skalli, A.; Sánchez-Vialas, A. Amphibians of the Oriental Region and the Moulouya River Basin of Morocco: Distribution and conservation notes. Basic Appl. Herpetol. 2019, 33, 19–32. [Google Scholar] [CrossRef]
- Mabrouki, Y.; Ben Ahmed, R.; Taybi, A.F.; Rueda, J. Annotated checklist of the leech (Annelida: Hirudinida) species of the Moulouya river basin, Morocco with several new distribution records and an historical overview. Afr. Zool. 2019, 54, 199–214. [Google Scholar] [CrossRef]
- Taybi, A.F.; Mabrouki, Y.; Haaren, T.V. Distribution of the alien Tubificid worm Branchiura sowerbyi (Beddard, 1892) in Morocco. Arx. Misc. Zool. 2023, 21, 253–260. [Google Scholar] [CrossRef]
- Mabrouki, Y.; Taybi, A.F.; Glöer, P. First record of Fountain bladder snail Physa fontinalis (Linnaeus, 1758) in Morocco. Folia Malacol. 2024, 32, 71–75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).