Adsorption of Atrazine from Synthetic Contaminated Water Using a Packed-Bed Column with a Low-Cost Adsorbent (Moringa oleifera Lam.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorbent Treatment
2.3. Adsorbent Characterizations
2.4. Packed-Bed Column Adsorption Experiments
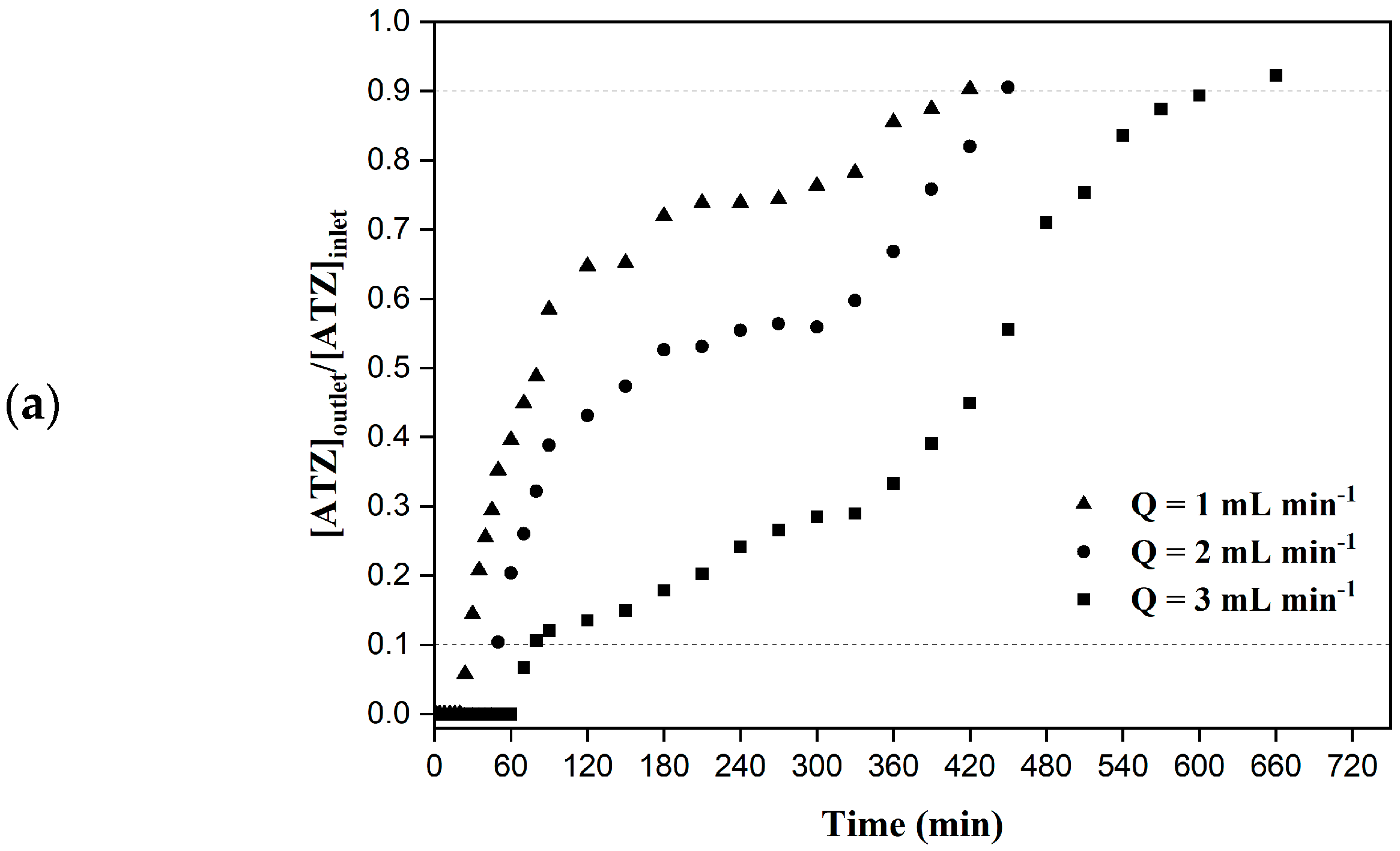
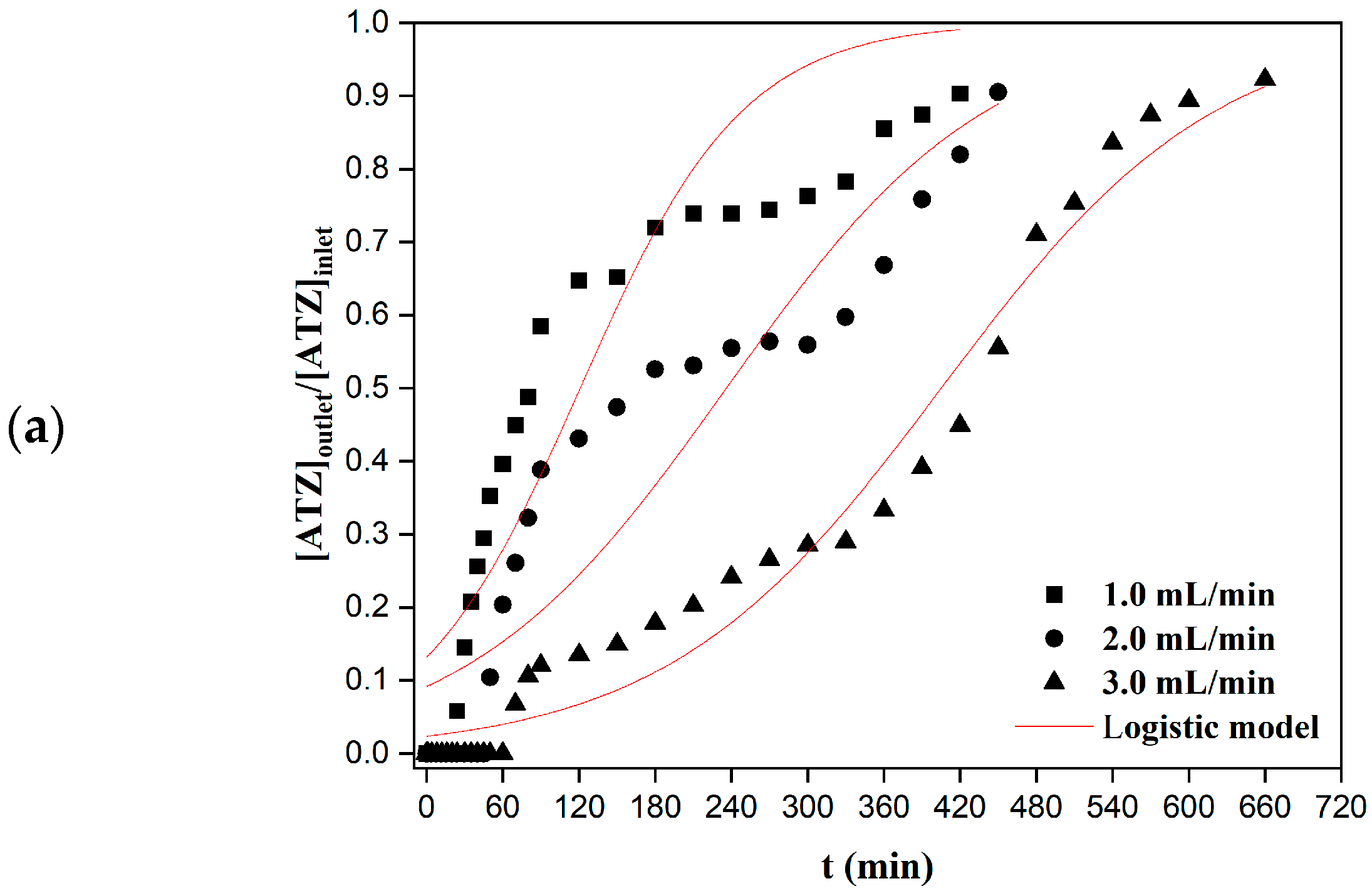
2.4.1. Effect of the Flow Rate
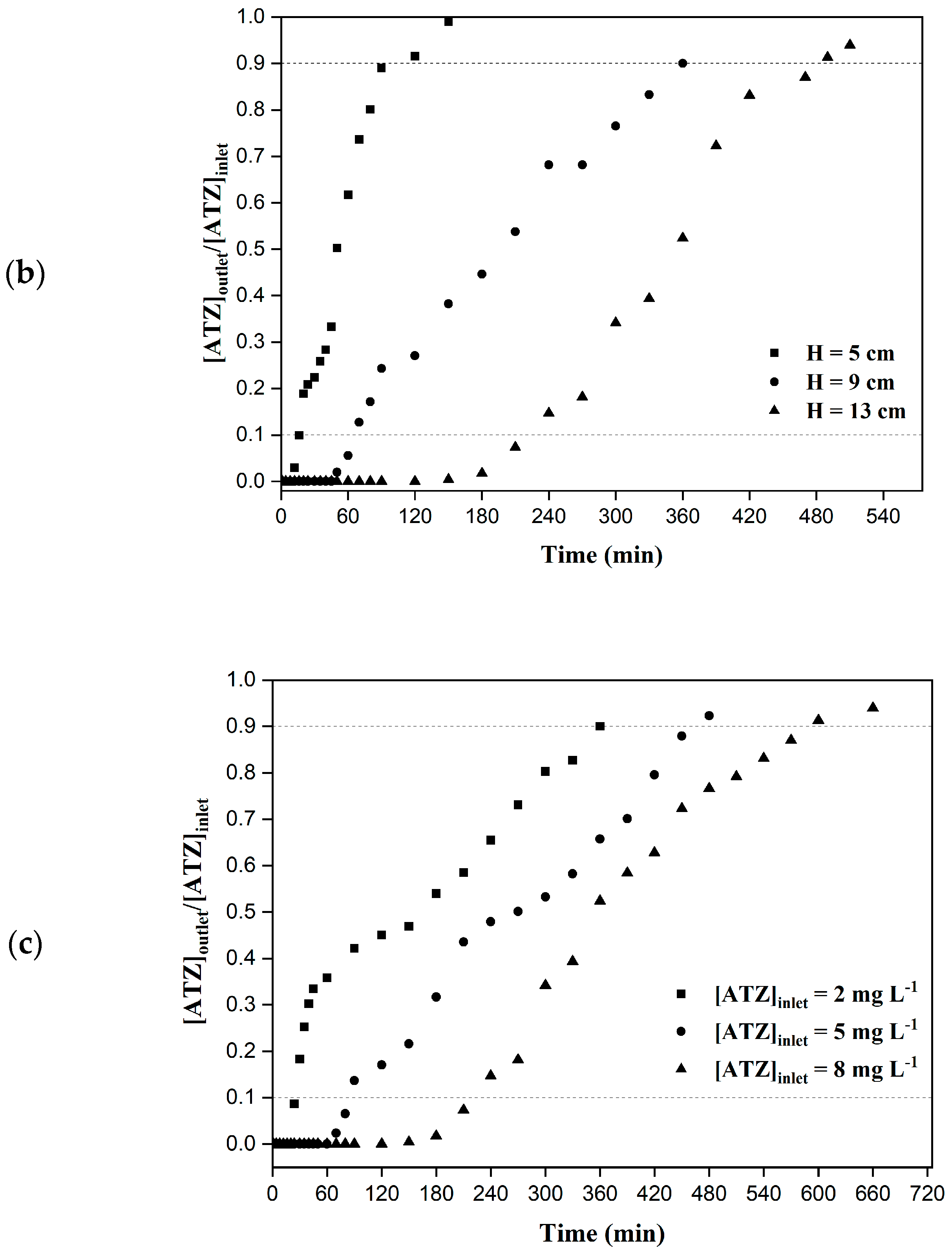
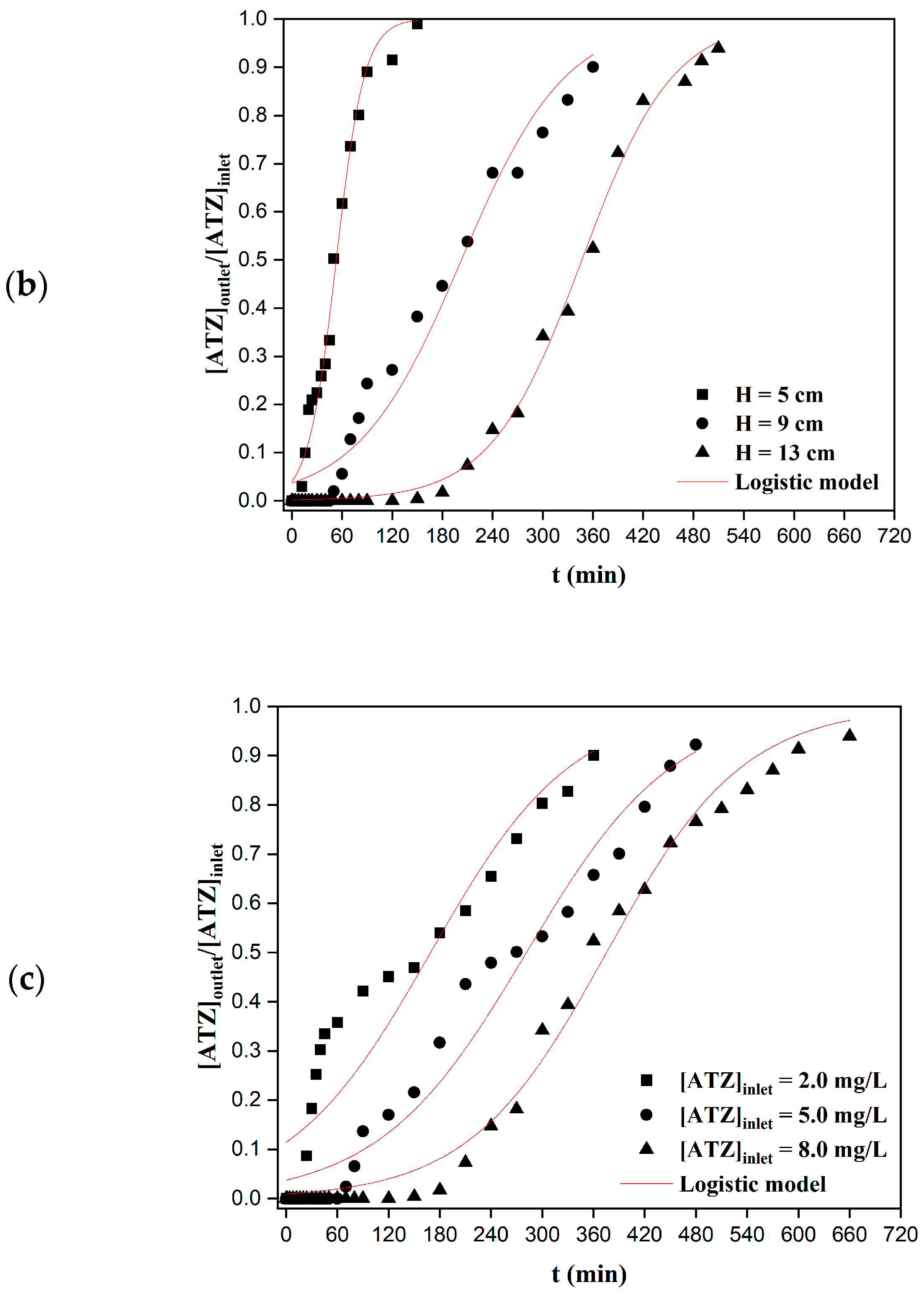
2.4.2. Effect of the Bed Heigh
2.4.3. Effect of the Inlet Concentration
2.5. Fixed-Bed Adsorption
2.6. Column Adsorption Modelling
2.7. Adsorbed Column Regeneration Experiments
3. Results and Discussion
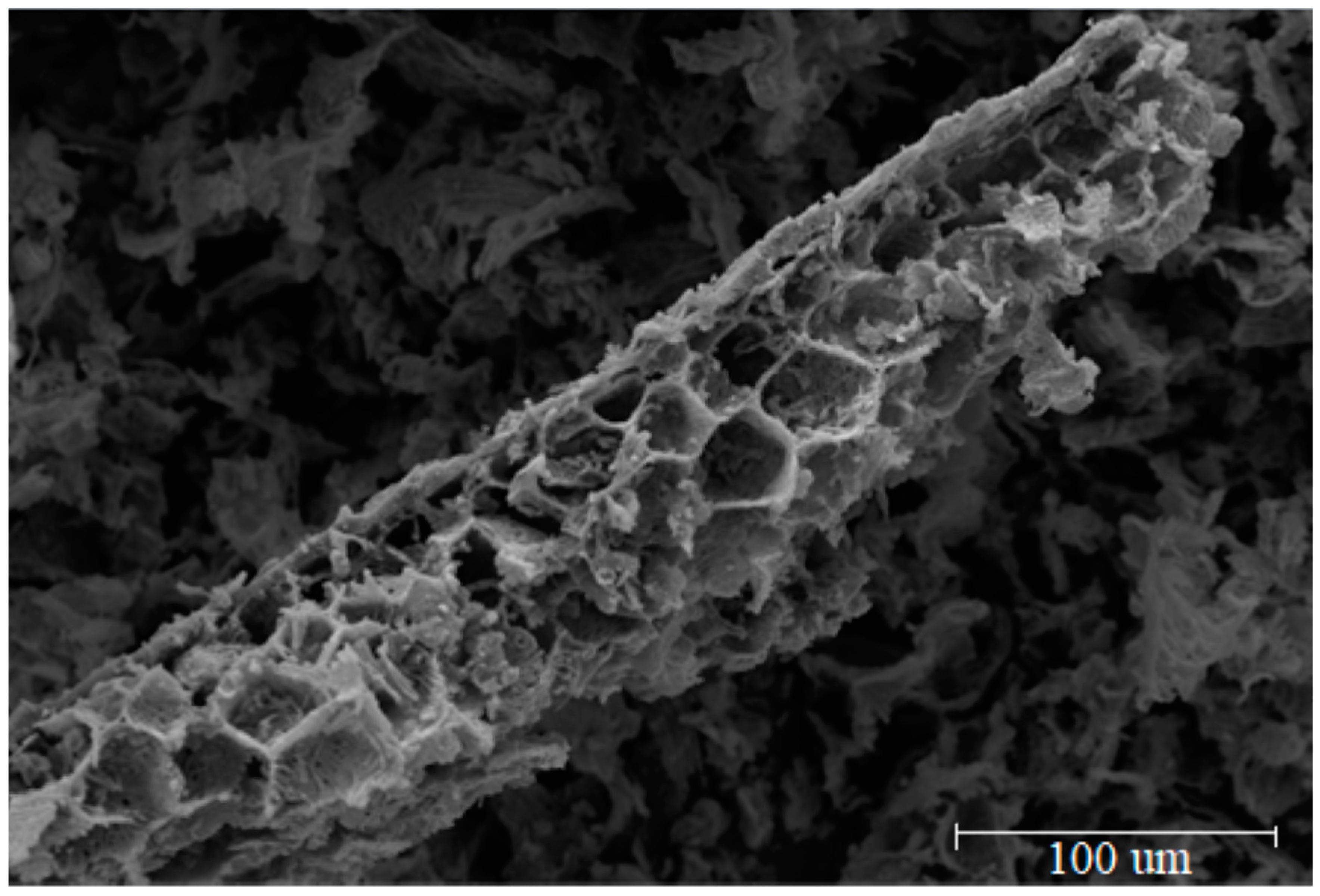
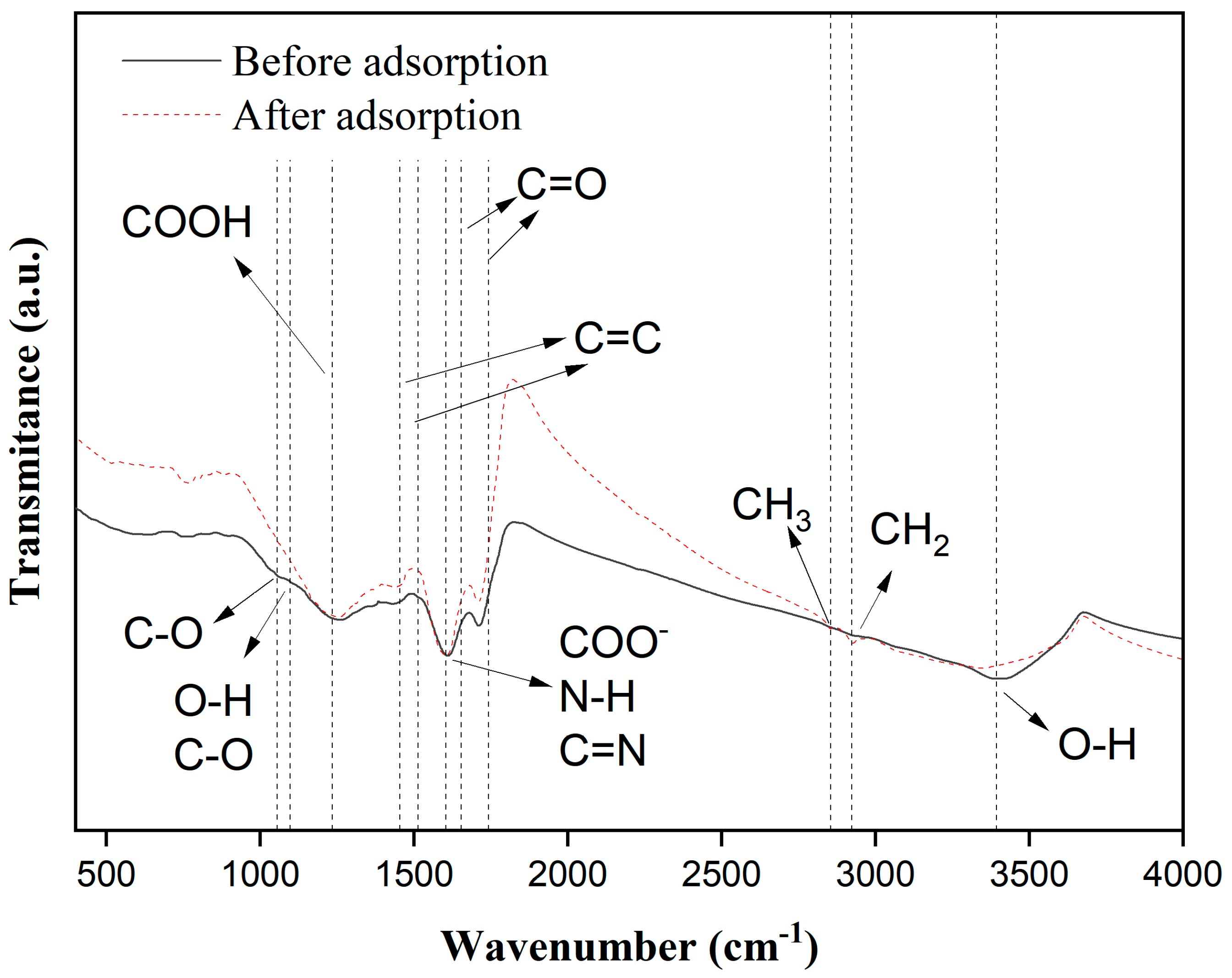
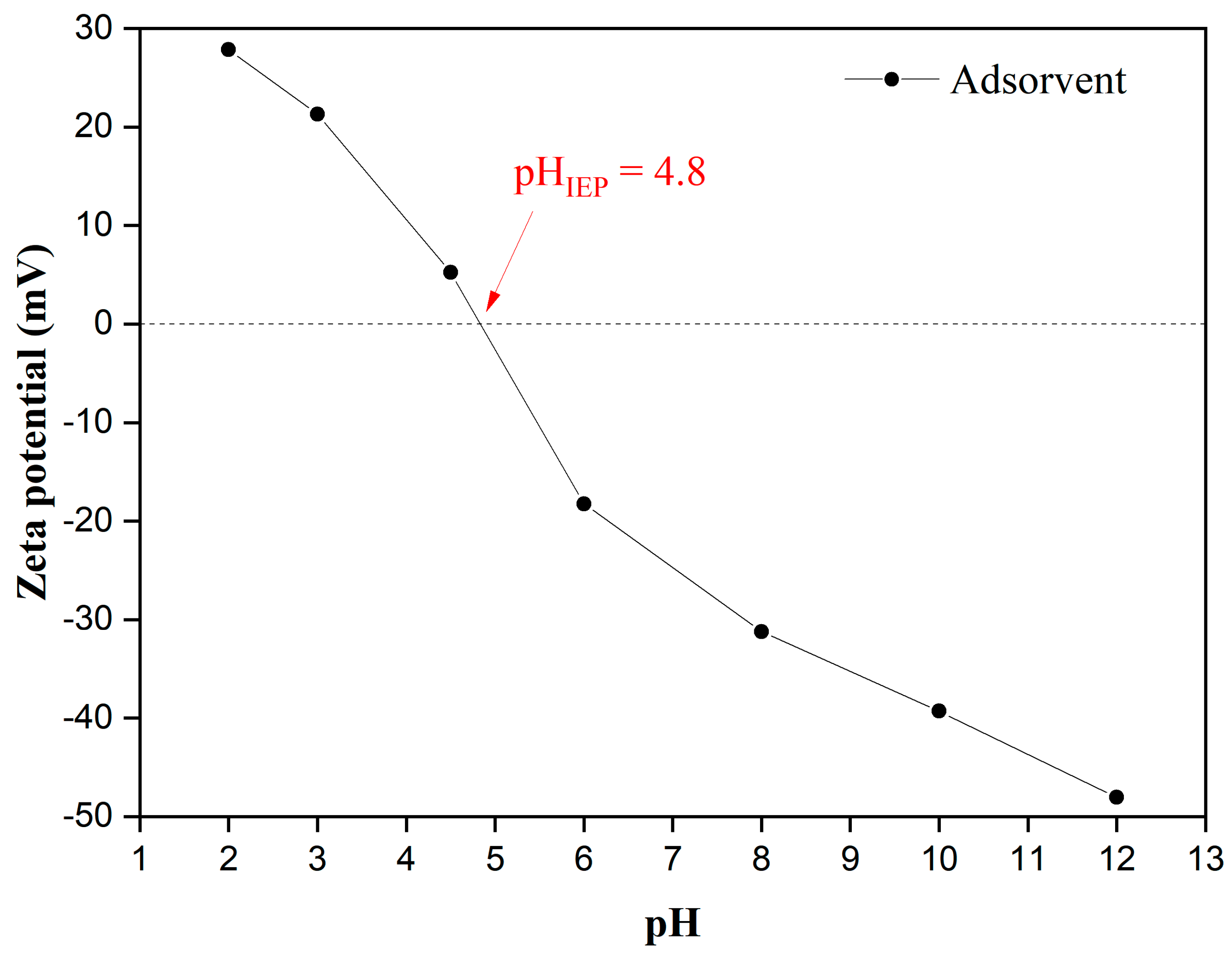
3.1. Adsorbent Characterization
3.2. Column Adsorption—Experimental Data
3.3. Column Adsorption—Model Fitting
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rostami, S.; Jafari, S.; Moeini, Z.; Jaskulak, M.; Keshtgar, L.; Badeenezhad, A.; Azhdarpoor, A.; Rostami, M.; Zorena, K.; Dehghani, M. Current Methods and Technologies for Degradation of Atrazine in Contaminated Soil and Water: A Review. Environ. Technol. Innov. 2021, 24, 102019. [Google Scholar] [CrossRef]
- MacLennan, P.A.; Delzell, E.; Sathiakumar, N.; Myers, S.L.; Cheng, H.; Grizzle, W.; Chen, V.W.; Wu, X.C. Cancer Incidence among Triazine Herbicide Manufacturing Workers. J. Occup. Environ. Med. 2002, 44, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Asim, M.; Khan, T.A. Low Cost Adsorbents for the Removal of Organic Pollutants from Wastewater. J. Environ. Manag. 2012, 113, 170–183. [Google Scholar] [CrossRef]
- Franco, P.; Navarra, W.; Sacco, O.; De Marco, I.; Mancuso, A.; Vaiano, V.; Venditto, V. Photocatalytic Degradation of Atrazine under Visible Light Using Gd-Doped ZnO Prepared by Supercritical Antisolvent Precipitation Route. Catal. Today 2022, 397–399, 240–248. [Google Scholar] [CrossRef]
- do Nascimento, C.T.; Vieira, M.G.A.; Scheufele, F.B.; Palú, F.; da Silva, E.A.; Borba, C.E. Adsorption of Atrazine from Aqueous Systems on Chemically Activated Biochar Produced from Corn Straw. J. Environ. Chem. Eng. 2022, 10, 107039. [Google Scholar] [CrossRef]
- Gil, Y.; Sinfort, C. Emission of Pesticides to the Air during Sprayer Application: A Bibliographic Review. Atmos. Environ. 2005, 39, 5183–5193. [Google Scholar] [CrossRef]
- Dehghani, M.; Gharehchahi, E.; Jafari, S.; Moeini, Z.; Derakhshan, Z.; Ferrante, M.; Conti, G.O. Health Risk Assessment of Exposure to Atrazine in the Soil of Shiraz Farmlands, Iran. Environ. Res. 2022, 204, 119090. [Google Scholar] [CrossRef]
- Shan, W.; Hu, W.; Wen, Y.; Ding, X.; Ma, X.; Yan, W.; Xia, Y. Evaluation of Atrazine Neurodevelopment Toxicity in Vitro-Application of HESC-Based Neural Differentiation Model. Reprod. Toxicol. 2021, 103, 149–158. [Google Scholar] [CrossRef]
- Luo, S.; Ren, L.; Wu, W.; Chen, Y.; Li, G.; Zhang, W.; Wei, T.; Liang, Y.Q.; Zhang, D.; Wang, X.; et al. Impacts of Earthworm Casts on Atrazine Catabolism and Bacterial Community Structure in Laterite Soil. J. Hazard. Mater. 2022, 425, 127778. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, J.H.; Guo, Q.N.; Ma, L.Y.; Yang, H. Physiochemical Assessment of Environmental Behaviors of Herbicide Atrazine in Soils Associated with Its Degradation and Bioavailability to Weeds. Chemosphere 2021, 262, 127830. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Chauhan, A.; Datta, S.; Wani, A.B.; Singh, N.; Singh, J. Toxicity, Degradation and Analysis of the Herbicide Atrazine. Environ. Chem. Lett. 2018, 16, 211–237. [Google Scholar] [CrossRef]
- Beane Freeman, L.E.; Rusiecki, J.A.; Hoppin, J.A.; Lubin, J.H.; Koutros, S.; Andreotti, G.; Zahm, S.H.; Hines, C.J.; Coble, J.B.; Barone-Adesi, F.; et al. Atrazine and Cancer Incidence among Pesticide Applicators in the Agricultural Health Study (1994-2007). Environ. Health Perspect. 2011, 119, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Edginton, A.N.; Rouleau, C. Toxicokinetics Of14C-Atrazine and Its Metabolites in Stage-66 Xenopus Laevis. Environ. Sci. Technol. 2005, 39, 8083–8089. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; Von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Biodiversidade, S. De Ministério Do Meio Ambiente—MMA. Biol. (Bratisl) 2000. [Google Scholar]
- BRASIL. Ministério Da Saúde. Secretária de Vigilância Em Saúde; 2021. [Google Scholar]
- Shirmardi, M.; Alavi, N.; Lima, E.C.; Takdastan, A.; Mahvi, A.H.; Babaei, A.A. Removal of Atrazine as an Organic Micro-Pollutant from Aqueous Solutions: A Comparative Study. Process Saf. Environ. Prot. 2016, 103, 23–35. [Google Scholar] [CrossRef]
- Binh, Q.A.; Nguyen, V.H.; Kajitvichyanukul, P. Influence of Pyrolysis Conditions of Modified Corn Cob Bio-Waste Sorbents on Adsorption Mechanism of Atrazine in Contaminated Water. Environ. Technol. Innov. 2022, 26, 102381. [Google Scholar] [CrossRef]
- Xiaozhen, F.; Bo, L.; Aijun, G. Dynamics of Solar Light Photodegradation Behavior of Atrazine on Soil Surface. J. Hazard. Mater. 2005, 117, 75–79. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Li, X.; He, H.; Yang, C. Performances and Mechanisms of Efficient Degradation of Atrazine Using Peroxymonosulfate and Ferrate as Oxidants. Chem. Eng. J. 2018, 353, 533–541. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Tan, L.S.; Shukor, S.R.A. Dimethoate and Atrazine Retention from Aqueous Solution by Nanofiltration Membranes. J. Hazard. Mater. 2008, 151, 71–77. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial Biosorbents and Biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Cusioli, L.F.; de Oliveira Bezerra, C.; Quesada, H.B.; Alves Baptista, A.T.; Nishi, L.; Vieira, M.F.; Bergamasco, R. Modified Moringa Oleifera Lam. Seed Husks as Low-Cost Biosorbent for Atrazine Removal. Environ. Technol. 2021, 42, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Moosa Hasany, S.; Bhanger, M.I.; Iqbal, S. Sorption Potential of Moringa Oleifera Pods for the Removal of Organic Pollutants from Aqueous Solutions. J. Hazard. Mater. 2007, 141, 546–556. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Regalbuto, J.R.; Robles, J.O. The Engineering of Pt/Carbon Catalyst Preparation for Application on Proton Exchange Fuel Cell Membrane. University of Illinois at Chicago, Chicago. 2004. Available online: https://amrel.bioe.uic.edu/NSFREU2004/Reports2004/Jaime%20Robles_Final%20Report.pdf (accessed on 22 February 2023).
- Cusioli, L.F.; Quesada, H.B.; de Brito Portela Castro, A.L.; Gomes, R.G.; Bergamasco, R. Development of a New Low-Cost Adsorbent Functionalized with Iron Nanoparticles for Removal of Metformin from Contaminated Water. Chemosphere 2020, 247, 125852. [Google Scholar] [CrossRef]
- Cong, L.; Chang, L.; Liu, X. Nonlinear-Wave Based Analysis and Modeling of Heat Integrated Distillation Column. Sep. Purif. Technol. 2015, 150, 119–131. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Chen, K.; Shi, Z.; Li, M.; Guo, P.; Wu, L. Dynamic Adsorption of Sulfamethoxazole from Aqueous Solution by Lignite Activated Coke. Materials 2020, 13, 1785. [Google Scholar] [CrossRef]
- Suzaki, P.Y.R.; Munaro, M.T.; Triques, C.C.; Kleinübing, S.J.; Fagundes Klen, M.R.; Bergamasco, R.; de Matos Jorge, L.M. Phenomenological Mathematical Modeling of Heavy Metal Biosorption in Fixed-Bed Columns. Chem. Eng. J. 2017, 326, 389–400. [Google Scholar] [CrossRef]
- Quesada, H.B.; Cusioli, L.F.; O Bezerra, C.; Baptista, A.T.; Nishi, L.; Gomes, R.G.; Bergamasco, R. Acetaminophen Adsorption Using a Low-cost Adsorbent Prepared from Modified Residues of Moringa Oleifera Lam. Seed Husks. J. Chem. Technol. Biotechnol. 2019, 94, 3147–3157. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Seshaiah, K.; Reddy, A.V.R.; Rao, M.M.; Wang, M.C. Biosorption of Pb2+from Aqueous Solutions by Moringa Oleifera Bark: Equilibrium and Kinetic Studies. J. Hazard. Mater. 2010, 174, 831–838. [Google Scholar] [CrossRef]
- Coldebella, P.F.; Fagundes-klen, R.; Valverde, K.C.; Cavalcanti, E.B.; Aparecida, O. Potential Effect of Chemical and Thermal Treatment on the Kinetics, Equilibrium, and Thermodynamic Studies for Atrazine Biosorption by the Moringa Oleifera Pods. Can. J. Chem. Eng. 2017, 95, 961–973. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Rodríguez, A.; Ovejero, G.; García, J. Comparative Adsorption Performance of Ibuprofen and Tetracycline from Aqueous Solution by Carbonaceous Materials. Chem. Eng. J. 2016, 283, 936–947. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Ramana, D.K.V.; Seshaiah, K.; Reddy, A.V.R. Biosorption of Ni(II) from Aqueous Phase by Moringa Oleifera Bark, a Low Cost Biosorbent. Desalination 2011, 268, 150–157. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.K.; Sandle, N.K. Effect of Different Oxidizing Agent Treatments on the Surface Properties of Activated Carbons. Carbon 1999, 37, 1323–1332. [Google Scholar] [CrossRef]
- de Oliveira Bezerra, C.; Cusioli, L.F.; Quesada, H.B.; Nishi, L.; Mantovani, D.; Vieira, M.F.; Bergamasco, R. Assessment of the Use of Moringa Oleifera Seed Husks for Removal of Pesticide Diuron from Contaminated Water. Environ. Technol. 2020, 41, 191–201. [Google Scholar] [CrossRef]
- Han, R.; Zhang, L.; Song, C.; Zhang, M.; Zhu, H.; Zhang, L.J. Characterization of Modified Wheat Straw, Kinetic and Equilibrium Study about Copper Ion and Methylene Blue Adsorption in Batch Mode. Carbohydr. Polym. 2010, 79, 1140–1149. [Google Scholar] [CrossRef]
- Ueda Yamaguchi, N.; Cusioli, L.F.; Quesada, H.B.; Camargo Ferreira, M.E.; Fagundes-Klen, M.R.; Salcedo Vieira, A.M.; Gomes, R.G.; Vieira, M.F.; Bergamasco, R. A Review of Moringa Oleifera Seeds in Water Treatment: Trends and Future Challenges. Process Saf. Environ. Prot. 2021, 147, 405–420. [Google Scholar] [CrossRef]
- Johar, N.; Ahmad, I.; Dufresne, A. Extraction, Preparation and Characterization of Cellulose Fibres and Nanocrystals from Rice Husk. Ind. Crop. Prod. 2012, 37, 93–99. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Alves, V.N.; Rezende, H.C.; Almeida, I.L.S.; De Assunção, R.M.N.; Tarley, C.R.T.; Segatelli, M.G.; Coelho, N.M.M. Characterization and Use of Moringa Oleifera Seeds as Biosorbent for Removing Metal Ions from Aqueous Effluents. Water Sci. Technol. 2010, 62, 2198–2203. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, L.A.; Rouquerol, J.; Siemieniewska, T. International Union of Pure and Applied Chemistry Physical Chemistry Division Reporting Physisorption Data for Gas/Soils Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1985, 31, 579–638. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.; Zhang, J. Determining Roles of In-Situ Measured Surface Potentials of Phase Controlled Synthesized MnO2 Nanostructures for Superficial Adsorption. Appl. Surf. Sci. 2020, 513, 145752. [Google Scholar] [CrossRef]
- Quesada, H.B.; Baptista, A.T.A.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface Water Pollution by Pharmaceuticals and an Alternative of Removal by Low-Cost Adsorbents: A Review. Chemosphere 2019, 222, 766–780. [Google Scholar] [CrossRef] [PubMed]
- da Costa, T.B.; da Silva, M.G.C.; Vieira, M.G.A. Biosorption of Lanthanum Using Sericin/Alginate/Polyvinyl Alcohol Beads as a Natural Cation Exchanger in a Continuous Fixed-Bed Column System. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127233. [Google Scholar] [CrossRef]
- Gülçek, B.; Tepe, O. Removal of Atrazine by Biogenic Manganese Oxide in Batch and Fixed-Bed Column Reactors. Geomicrobiol. J. 2022, 39, 17–27. [Google Scholar] [CrossRef]
- Bakka, A.; Mamouni, R.; Saffaj, N.; Laknifli, A.; Aziz, K.; Roudani, A. Removal of Bifenthrin Pesticide from Aqueous Solutions by Treated Patellidae Shells Using a New Fixed Bed Column Filtration Technique. Process Saf. Environ. Prot. 2020, 143, 55–65. [Google Scholar] [CrossRef]
- Chu, K.H. Improved Fixed Bed Models for Metal Biosorption. Chem. Eng. J. 2004, 97, 233–239. [Google Scholar] [CrossRef]
- Hu, Q.; Xie, Y.; Feng, C.; Zhang, Z. Prediction of Breakthrough Behaviors Using Logistic, Hyperbolic Tangent and Double Exponential Models in the Fixed-Bed Column. Sep. Purif. Technol. 2019, 212, 527–579. [Google Scholar] [CrossRef]
- Knox, J.C.; Ebner, A.D.; Levan, M.D.; Coker, R.F.; Ritter, J.A. Limitations of Breakthrough Curve Analysis in Fixed-Bed Adsorption. Ind. Eng. Chem. Res. 2016, 55, 4734–4748. [Google Scholar] [CrossRef]
- Islam, M.A.; Dada, T.K.; Parvin, M.I.; Vuppaladadiyam, A.K.; Kumar, R.; ANTUNES, E. Silver Ions and Silver Nanoparticles Removal by Coffee Derived Biochar Using a Continuous Fixed-Bed Adsorption Column. SSRN Electron. J. 2022, 48, 102935. [Google Scholar] [CrossRef]
- Aksu, Z.; Gönen, F. Biosorption of Phenol by Immobilized Activated Sludge in a Continuous Packed Bed: Prediction of Breakthrough Curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Homem, N.C.; Vieira, A.M.S.; Bergamasco, R.; Vieira, M.F. Low-Cost Biosorbent Based on Moringa Oleifera Residues for Herbicide Atrazine Removal in a Fixed-Bed Column. Can. J. Chem. Eng. 2018, 96, 1468–1478. [Google Scholar] [CrossRef]
- Lim, A.P.; Aris, A.Z. Continuous Fixed-Bed Column Study and Adsorption Modeling: Removal of Cadmium (II) and Lead (II) Ions in Aqueous Solution by Dead Calcareous Skeletons. Biochem. Eng. J. 2014, 87, 50–61. [Google Scholar] [CrossRef]
- Chu, K.H. Breakthrough Curve Analysis by Simplistic Models of Fixed Bed Adsorption: In Defense of the Century-Old Bohart-Adams Model. Chem. Eng. J. 2020, 380, 122513. [Google Scholar] [CrossRef]


| Moringa oleifera Lam. Seeds | Adsorbent | |
|---|---|---|
| BET-specific surface area (m2 g−1) | 1.822 | 2.496 |
| Average pore diameter (Å) | 36.28 | 29.46 |
| Total pore volume (cm3 g−1) | 0.0256 | 0.0437 |
| Micropore volume (cm3 g−1) | 0.0041 | 0.0274 |
| Mesopore volume (cm3 g−1) | 0.0215 | 0.0163 |
| H (cm) | Q (mL/min) | pH | [ATZ]inlet (mg/L) | tb (min) | ts (min) | qe (mg/g) | qb (mg/g) | Wt (mg) | η (%) |
|---|---|---|---|---|---|---|---|---|---|
| 13 | 1.0 | 5.0 | 2.0 | 25 | 420 | 1.51 | 1.460 | 2.92 | 50,00 |
| 13 | 2.0 | 5.0 | 2.0 | 49 | 453 | 0.98 | 0.760 | 1.68 | 45.23 |
| 13 | 3.0 | 5.0 | 2.0 | 78 | 601 | 0.73 | 0.326 | 0.84 | 38.80 |
| 5 | 1.0 | 5.0 | 2.0 | 14 | 97 | 0.26 | 0.08 | 0.26 | 30.70 |
| 9 | 1.0 | 5.0 | 2.0 | 65 | 360 | 0.54 | 0.128 | 0.37 | 34.59 |
| 13 | 1.0 | 5.0 | 2.0 | 218 | 485 | 1.62 | 1.41 | 2.96 | 47.79 |
| 13 | 1.0 | 5.0 | 2.0 | 27 | 360 | 1.38 | 1.27 | 2.63 | 48.28 |
| 13 | 1.0 | 5.0 | 5.0 | 85 | 474 | 1.05 | 0.69 | 2.04 | 33.82 |
| 13 | 1.0 | 5.0 | 8.0 | 221 | 601 | 0.87 | 0.32 | 1.55 | 20.64 |
| Q (mL min−1) | H (cm) | [ATZ]inlet (mg L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Parameter | 1 | 2 | 3 | 5 | 9 | 13 | 2 | 5 | 8 |
| Logistic | a | 1.8857 | 2.2934 | 3.7343 | 3.1690 | 3.2597 | 6.3883 | 2.0452 | 3.2493 | 4.667 |
| b | 0.0156 | 0.0097 | 0.0092 | 0.0596 | 0.0161 | 0.0184 | 0.0121 | 0.0116 | 0.0124 | |
| R2 | 0.8399 | 0.8563 | 0.9758 | 0.9843 | 0.9668 | 0.9963 | 0.886 | 0.9664 | 0.9907 | |
| RMSE | 0.0175 | 0.0132 | 0.0023 | 0.0019 | 0.0032 | 0.0004 | 0.0107 | 0.0033 | 0.0011 | |
| Bohart–Adams | kBA (L mg−1 min−1) | 0.0288 | ||||||||
| N0 (mg L−1) | 52.66 | 205.99 | 530.47 | 23.163 | 49.00 | 58.17 | 27.36 | 113.36 | 243.71 | |
| Thomas | kT (mL mg−1 min−1) | 0.0075 | 0.0047 | 0.0044 | 0.0288 | 0.0078 | 0.0089 | 0.0061 | 0.0023 | 0.0016 |
| qe (mg g−1) | 208.5 | 815.6 | 2100.5 | 91.7 | 231.5 | 292.1 | 137.4 | 569.3 | 1223.9 | |
| Yoon–Nelson | kYN (min−1) | 0.0156 | 0.0097 | 0.0092 | 0.0596 | 0.0161 | 0.0184 | 0.0121 | 0.0116 | 0.0124 |
| τ (min) | 120.8 | 236.4 | 405.9 | 53.1 | 202.4 | 347.1 | 169.0 | 280.1 | 376.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergamasco, R.; Mantovani, D.; Diório, A.; de Oliveira Bezerra, C.; Quesada, H.B.; Wernke, G.; Fagundes-Klen, M.R.; Cusioli, L.F. Adsorption of Atrazine from Synthetic Contaminated Water Using a Packed-Bed Column with a Low-Cost Adsorbent (Moringa oleifera Lam.). Water 2023, 15, 1260. https://doi.org/10.3390/w15071260
Bergamasco R, Mantovani D, Diório A, de Oliveira Bezerra C, Quesada HB, Wernke G, Fagundes-Klen MR, Cusioli LF. Adsorption of Atrazine from Synthetic Contaminated Water Using a Packed-Bed Column with a Low-Cost Adsorbent (Moringa oleifera Lam.). Water. 2023; 15(7):1260. https://doi.org/10.3390/w15071260
Chicago/Turabian StyleBergamasco, Rosângela, Daniel Mantovani, Alexandre Diório, Charleston de Oliveira Bezerra, Heloise Beatriz Quesada, Gessica Wernke, Márcia Regina Fagundes-Klen, and Luís Fernando Cusioli. 2023. "Adsorption of Atrazine from Synthetic Contaminated Water Using a Packed-Bed Column with a Low-Cost Adsorbent (Moringa oleifera Lam.)" Water 15, no. 7: 1260. https://doi.org/10.3390/w15071260
APA StyleBergamasco, R., Mantovani, D., Diório, A., de Oliveira Bezerra, C., Quesada, H. B., Wernke, G., Fagundes-Klen, M. R., & Cusioli, L. F. (2023). Adsorption of Atrazine from Synthetic Contaminated Water Using a Packed-Bed Column with a Low-Cost Adsorbent (Moringa oleifera Lam.). Water, 15(7), 1260. https://doi.org/10.3390/w15071260








