Abstract
The current environmental quality standards (EQSs) for freshwater ecosystems have been established in relation to the priority substances covered by Directive 2013/39/EU. The procedure for deriving EQSs that rely on the selection of the most sensitive toxicological data, with the application of arbitrary safety factors, is probably unrealistic for the Italian freshwater ecosystem. In this work, a procedure for the evaluation of specific sensitivity of 13 taxonomic groups from bacteria to amphibians and the derivation of protective chemical reference values specifically for the Italian aquatic communities was developed. Toxicological raw data of species belonging to the same taxonomic group spending at least one phase of their life cycle in Italian freshwater ecosystems were downloaded from EnviroTox and USEPA ECOTOX databases, aggregated, and then used as input for the model called Species Sensitivity Distribution in order to estimate the predicted no effect concentrations (PNECs). The comparison of relative sensitivity factors (RFSs) made it possible to identify the amphibians as the most sensitive group toward metals, trace elements, and pesticides, whereas crustacean were identified as the most sensitive group toward towards polycyclic aromatic hydrocarbons (PAHs). PNECs were estimated to cover 62 substances, of which 37 identified by Directive 2013/39/EU, and in most of the cases, the values were higher than EQSs. The PNECs reported in this work should be considered more realistic and tailored for Italian freshwater ecosystems, having significant repercussions in the classification of water bodies and the estimation of environmental impact assessment.
1. Introduction
The Water Framework Directive (WFD) (2000/60/EC) [1] prompted the European Commission to identify priority substances among those presenting significant risk for the aquatic environment, and to establish their environmental quality standards (EQSs) in water, sediment, and/or biota. In 2001, a list of 33 Priority substances was drawn up (Decision 2455/2001) [2], and in 2008, the EQSs were established for those substances (Directive 2008/105/EC or EQS Directive, EQSD) [3]. The EQSD was then revised in 2013 by Directive 2013/39/EU [4], which modified the EQSs for seven of the existing priority substances and introduced twelve new Priority substances.
In Italy, EQSs were defined by the National Legislative Decree no. 172/2015 [5]. In particular, the water bodies in which the annual average concentrations for the substances on the priority list agree with EQSs are classified as being in “good” chemical status.
The procedure for establishing EQSs follows a complex process mainly referred to the EU Guidance document No. 27 (2011) [6], on which the Scientific Committee on Health, Environmental and Emerging Risks (SCHEER) expressed an opinion reported in a scientific advice, prepared by a collaborative framework (the common implementation strategy) for the WFD [7]. The SCHEER concluded that the overall scientific quality of the proposed changes was an improvement to the earlier 2011 version.
EQSs basically coincide with the predicted no effect concentration values (PNECs) that are mainly derived from toxicological data from single species laboratory tests, belonging to databases from international organizations, verified references, and scientific papers. PNECs are inferred by the lowest reliable values on chronic toxicity or more sensitive toxicity thresholds such as no observed effect concentrations (NOECs) or lowest observed effect concentrations (LOECs), corrected by safety factors ranging from 10 to 1000, as recommended by the Institute for Health and Consumer Protection in support of European Commission in 2003 (Table 1) [8]. Assessment factors (AF) had already been proposed by OECD in 1992 with the intention of predicting a concentration below in which an unacceptable effect will most likely not occur [9].

Table 1.
Assessment factor to derive aquatic PNECs [9].
However, guidance document No. 27 does not describe the exact procedure for deriving EQSs, nor the source of the ecotoxicological data used for each substance; in practice, most of the time EQSs coincide with the lowest toxicity threshold available, adjusted with the respective safety factors. AFs reflect the degree of uncertainty in extrapolation from laboratory toxicity test data for a limited number of species into the ‘real’ environment, leading to the conclusion that the degree of uncertainty and level of protection of the obtained PNECs remain largely unknown [10].
EQSs included in the Italian Legislative Decree no. 172/2015 [5] for the context of national aquatic ecosystems are often unrealistic and mainly subjected to the following defects:
- The original sensitivity data from which EQSs are derived frequently refer to species that do not naturally occur in Italy and are not representative of the Italian freshwater ecosystems;
- Because of the safety factors applied, EQS are often too low to be detected in field by the most common analytical techniques (i.e., Fluoranthene = 0.0063 μg L−1; Heptachlor = 2 × 10−7 μg L−1; Cypermethrin = 8 × 10−5 μg L−1);
- The safety factors applied are arbitrarily chosen (depending on data availability of toxicity data for organisms at certain trophic levels, taxonomic groups, or feeding strategies), overly precautionary, and not subjected to a process of verifying their correspondence with toxicity measured in real environments;
- EQSs are not representative of the overall sensitivity of aquatic communities, but only that of a few species (frequently the same ones and belonging mainly to fish or crustacean) chosen for their highest sensitivity.
A step forward in estimating more realistic chemical reference values has been made regarding the bioavailability properties of priority substances. SCHEER introduced some simplified variables such as pH, DOC, and Ca content to normalize and predict the bioavailable fraction of several contaminants from chemical measurements [11] using the biotic ligand model (BLM), which nevertheless implies certain assumptions [12,13]. So far, applications based on simplified BLM models are available and validated only for Ni, Cu, and Zn [8].
The purpose of this work is to identify reference chemical values for inorganic and organic contaminants that are protective for the aquatic communities and representative of Italian freshwater ecosystems in order to allow more realistic ecological risk assessments (ERA) of water bodies. With this aim, an alternative approach for deriving PNECs is proposed for a possible review of the EQSs, in function of the composition and diversity of the local biological communities.
PNECs for organic and inorganic contaminants were estimated on the basis of the Species Sensitivity Distribution method (SSD) [14] using selected sensitivity data for each contaminant related to species living in Italian aquatic ecosystems, and then aggregated in order to simulate the dose–effect relationship in entire biological community.
The SSD model is a probabilistic approach that reports the percentage of a potentially affected species as a function of pollutant concentration. The model is a well-established procedure for estimating the environmental risk of chemicals [15,16,17] and has been already used in support of the European Commission Directive 93/67/EEC on risk assessment for new notified substances [18]. However, the SSD model is based on several assumptions, which are not always met in real environments [19] such as (i) the equal importance of all species; (ii) the equal sensitivity between laboratory and field organisms; (iii) dependence on the choice of ecologically relevant endpoints to derive the SSD model; (iv) no interactions between species; and (v) requiring large number of chronic data, not always available [20].
In this study, the SSD model was applied to a large number of taxonomic groups representative of Italian freshwater ecosystems for which suitable data were available. Thus, PNECs identified are both cautionary and realistic without the need of AF applied to reduce data uncertainty. The estimated PNECs were then compared with those provided by the Italian national legislation for the protection of aquatic life (Italian Legislative Degree 172/2015).
2. Materials and Methods
2.1. Creation of Dataset
The increased need to categorize chemicals and their effects, as well as the demands on available data, have led to the creation of multiple sets of ecotoxicological metadata over the past decade, such as EnviroTox (https://envirotoxdatabase.org/ (accessed on 1 February 2023)) and USEPA ECOTOX database (https://cfpub.epa.gov/ecotox/ (accessed on 1 February 2023)).
The EnviroTox was chosen as the primary database to acquire ecotoxicological data regarding organic compounds (PAHs and pesticides) and trace metals, while ECOTOX was also consulted when the information was poor or not available. EnviroTox is part of a platform which is developed by Middle Tennessee State University (USA) on behalf of the Health and Environmental Sciences Institute [21]. It includes more than 91,000 records from acute or chronic testing referred to aquatic matrix assessment and covers over 1500 species and 4000 chemicals identified by CAS number, coming from 11 different sources (including ECHA, ECOTOX, USEPA Pesticides, OECD QSAR toolbox databases). A data quality assessment in the EnviroTox system is based on relevance, validity, and acceptability, following the stepwise information-filtering tool (SIFT) approach developed by Beasley et al. [22].
The USEPA’s ECOTOXicology Knowledgebase (ECOTOX) is a database created and maintained by the Office of Research and Development (ORD’s) in Duluth, Minnesota (USA). It includes data on chemical toxicity to aquatic life, terrestrial plants, and wildlife, derived primarily from peer-reviewed literature since 1970. ECOTOX integrates three previously developed independent databases (AQUIRE, PHYTOTOX, and TERRETOX) into a single system that to date collects ecotoxicological information of more than 12,000 chemicals, surveying more than 13,000 aquatic and terrestrial species (https://cfpub.epa.gov/ecotox/stats.cfm (accessed on 1 February 2023)). The ECOTOX database applies quality assurance procedures before entering data into its repository that consist of a review process by specifically trained personnel who verify the chemicals, species, end points, and environmental concentrations/doses used in each record.
In order to obtain a good representativeness of Italian freshwater ecosystems potentially affected by the presence of contaminants, species sensitivity data referred to pure substances for a large number of test species were selected from the two databases and organized in a specific dataset using the following procedure:
- A quality check of raw data was performed in order (i) to eliminate duplicate records referring to the same experiment but reported in two or more sources and/or with different units and (ii) to verify that data were related to pure substances rather that to molecular weight of salts or compounds;
- NOECs/LOECs values from toxicological tests with prolonged exposures and/or sublethal end points were used as a priority, and only as a subordinate EC50/LC50s from acute tests;
- For the same species and substance, when multiple values referring to tests with the same conditions (i.e., duration, data expression, measured end point) were available, the geometric mean among them was considered;
- In rare cases for which NOECs/LOECs were not available, the NOECs/LOECs were inferred as EC5 by dividing the value of EC50 by a factor of 10 (extrapolated NOEC = EC50/10), assuming that a typical sigmoid dose–response relationship can be approximately linearized on a logarithmic scale;
- A single representative NOEC value (Aggregated NOEC = NOECA) was considered for each taxonomic group (i.e., Crustacea, Mollusca, Anellida), given by the geometric mean of all available data for that group. This allowed the final PNEC to be balanced among taxonomic groups and not biased toward taxa characterized by greater availability of values (usually fishes and/or crustaceans);
- Finally, when NOECA were obtained for at least three taxonomic groups for a specific substance, the PNEC was estimated using the SSD model.
2.2. The Application of the SSD Model and PNECs Estimation
Species sensitivity distributions reflect sensitivity data for organisms to derive a hazard concentration (HCx%), for example, the HC5 where 5% of the organisms are affected (95% protected) [23].
The SSD model uses sensitivity data as input such as NOEC or effective concentration for x% (ECx) for a group of tested species, obtained through acute or chronic toxicity tests. The SSD probability distribution is extrapolated from the sample of tested species to infer a group-wide protective concentration, the hazardous concentration for p% of the group (HCp) [24].
Because the main issue of the SSD model is the agreement between the real distributional form of toxicological data and the theoretical distributions provided by log-normal and log-logistic models [17], the derivation of the PNEC for each substance was assessed following different criteria on the basis of the distribution of the data. If the NOECA values of the various taxonomic groups had a normal distribution (Shapiro–Wilk Test), the PNEC was inferred to the EC5 of the log-normal SSD model; if their distribution was not normal, the PNEC was inferred to the EC5 returned by the logistic model. A basic assumption for SSD use, in fact, is the distribution of species sensitivities in a given ecosystem following a theoretical function whereby log-transformed toxicity data are fitted to a log-logistic or log-normal distribution curve [24]. In addition, in the present study, the selected species belonging to diverse taxonomic groups could be considered representative of the entire biological community.
SSD analysis was carried out through the use of MOSAIC_SSD software (https://mosaic.univ-lyon1.fr/ssd (accessed on 1 February 2023)), developed by the Laboratory of Biometrics and Evolutionary Biology at the University of Lyon, which provides the HCp value based on scripts developed in the R statistics SW package called “fitdistrplus”. Once the toxicity data are entered, it is possible to choose both log-normal and log-logistic distribution model for SSD fitting and start the simulation. For the dataset considered, HC5 (%), which can be assumed as a PNEC, was estimated with log-normal or log-logistic functions for all chemicals for which NOECA values at least from three taxonomic groups were available (Figure 1).
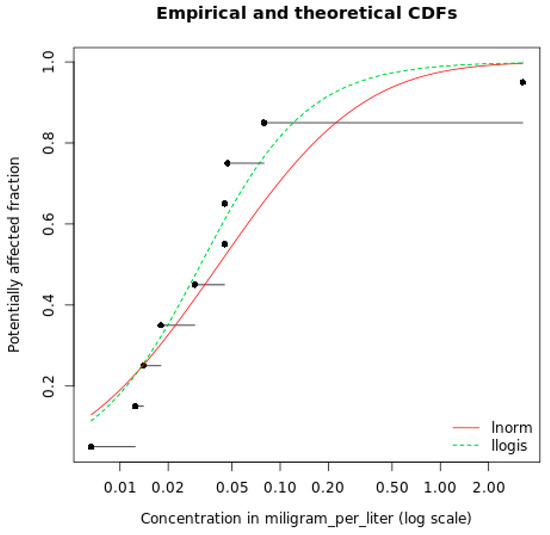
Figure 1.
Example of the interpolation performed by the SSD model with both log-norm and log-logistic modes. Each dot represents an aggregated NOEC value referring to a taxonomic group.
3. Results and Discussion
Tables S1 and S2 report the species living in Italian freshwater ecosystems and substances for which specific toxicity thresholds have been identified with the criteria previously described. In detail, Table S1 is related to metals, trace elements, and pesticides, whereas Table S2 is related to PAHs and other environmentally relevant substances. Toxicity data regarding 68 substances (18 metals and trace elements, 26 pesticides, 12 PAHs, and 12 other substances including solvents and phenols), distributed among 93 species, were processed.
The species for which toxicological data are available for the largest set of substances are those used worldwide in toxicological standardized tests, some of which occur naturally in Italian rivers and lakes. Since these species are distributed in different and widespread taxonomic groups such as green algae Chlorella vulgaris and Raphydocelis subcapitata, crustaceans Daphnia magna and Ceriodaphnia dubia, or fishes Oncorhynchus mykiss and Ciprinus carpio, a good representativeness of Italian ecosystems, covering different trophic levels from bacteria to amphibians, can be claimed for several substances.
At the same time, it is necessary to point out the heterogeneity of the available sensitivity data for different substances. Generally, the information is extensive and detailed for metals and trace elements, but relatively poor for many organic contaminants (i.e., Dibenzo(a,h)anthracene, DDE and Dibenzo(a,h)anthracene).
Table 2, Table 3 and Table 4 show NOECA values for each taxonomic group regarding metals and trace elements, pesticides and volatile solvents, PAHs and others environmentally relevant substances, respectively.

Table 2.
NOECs aggregated by geometric mean estimated for metals and trace elements for each taxon (μg L−1).

Table 3.
NOECs aggregated (NOECA) by geometric mean for pesticides and volatile solvents from each taxon (μg L−1).

Table 4.
NOECs aggregated by geometric mean for PAHs and other environmentally relevant substances from each taxon (μg L−1).
The dataset and its elaboration allowed obtaining representative NOECA values from 2 to 11 taxonomic groups out of 13 according to the substance. For example, it was possible to calculate the NOECA of Azymphos ethyl and Heptachlor epox only for crustaceans and fishes, whereas Cu, Cd, and Zn were estimated at 10 reference values, with the exception of amphibians and Euglenozoa.
Within the same taxonomic group, the reference values ranged from one to four orders of magnitude for the same substance. This confirmed the considerable difference in sensitivity among taxon, as previously reported [25,26].
Since crustaceans were the group for which it was possible to estimate NOECA for all substances, with the exception of toluene, by setting their values equal to 1, it was possible to calculate a relative sensitivity factor (RSF) that expresses the relative sensitivity of each taxonomic group through the simple ratio NOECAcrustacean/NOECAgroupX. Table 5 shows the geometric mean and median values of RSFs per category of chemicals. It can be noted that for amphibians, the RFSs were the lowest for metals and trace elements and pesticides, indicating that this group is the most sensitive for these chemical compounds, followed by cnidarians for metals and insects for pesticides. The latter case is perfectly in agreement with the insecticidal role of pesticides.

Table 5.
Geometric mean and median values of RSFs per category of chemical substances. In bold are the two most sensitive factors.
Regarding PAHs, crustaceans resulted in the most sensitive, while with respect to all the other contaminants listed in Table 4, Cyanobacteria and Pisces could be considered the most sensitive taxonomic groups.
By assuming that the ecosystem sensitivity depends on the most sensitive species, the general benchmarks should be close to the respective protective concentrations for each taxonomic group. In Table 6, the results of the normality test of the NOECA estimated in all taxonomic groups and the PNECs for the entire aquatic community are reported, which were caused by the integration of at least 3 values in the SSD model. The obtained PNECs were also compared with the EQSs of Table 1/A of Italian Legislative Decree 172/2015.

Table 6.
Normality test, selection of model applied, and PNEC estimated for substances with at least 3 NOECA in comparison with the EQSs of Table 1/A of Italian Legislative Decree 172/2015 and the PNECs estimated (EQS-AA = Environmental Quality Standard—Annual Average; SQA-MAC = Environmental Quality Standard—Maximum Admissible Concentration).
In some cases, the amount of data used to derive the PNECs seems to disagree with that suggested by Wheeler et al. [27], that advocate stability of SSDs upward of 10 to 15 data points for log-normal and log-logistic models when applied to single species experiments. Nevertheless, in the present work, the reliability of the obtained data is ensured by the aggregation of the information on two successive levels: within the same species and between species belonging to the same taxonomic group by using a geometric mean.
A similar approach was previously adopted by Gottschalk and Nowak [28]. Instead of considering one single deterministic (often averaged) toxic endpoint for each species, they probabilistically produced for all species their own sensitivity distributions, which, when taken together, comprise the generic SSD of a particular environmental compartment.
In any case, the adopted methodology, although it does not include the AF, is considered rather precautionary at different levels because of the following:
- ▪
- The procedure referred to toxicity tests, which assume that the active substance is fully bioavailable;
- ▪
- Priority was given to NOECs and/or LOECs values referring to chronic toxicity tests, with sub-lethal end points and/or prolonged exposures;
- ▪
- Where only EC50/LC50 was available, the values obtained in equal test conditions but with a longer exposure period were selected;
- ▪
- The estimated PNECs by SSD model are lower than the lowest available data of the most sensitive taxonomic group in 83.3% of the cases.
Estimated PNECs for metals and trace elements were close to NOECA for amphibians and cnidarians, just as those for pesticides to insects.
PNECs estimated in the present work cover a list of 62 substances, of which 37 were identified by 2013/39/EU Directive. By taking into account only those substances for which both PNEC and EQS are available, the comparison between the two values highlights important qualitative and quantitative differences (Table 6). Considering that the estimated PNECs have an environmental protection function, while the EQSs also have a human health significance, higher concentrations for PNECs than EQSs were observed, probably due, or at least in part, to the non-use of arbitrary AFs and to the integration of data referring to all available taxa. In particular, for PAHs, the PNECs representative of the Italian freshwater ecosystems are 1–2 orders of magnitude higher than EQSs, with an average of 240 times greater; for metals and trace elements, they are 1 order greater, with an average of 32 times. With regard to pesticides, a more heterogeneous picture emerged, with some substances having estimated PNECs much lower than EQSs (i.e., Aldrin, Cybuthrine, and Dieldrin), and others such as Dicofol, Fluoranthene, Exachlorobutadiene having three orders of magnitude higher than EQS values, with Heptachlor having up to eight orders of magnitude higher than the reference value.
4. Conclusions
In this work, a revised procedure for deriving specific protective chemical reference values tailored to the structure and composition of the biological communities in the Italian freshwater ecosystems, without the need of the application of safety factors adopted in the Directive 2013/39/EU, was proposed.
The built dataset is so broad and representative of the main taxonomic groups and trophic levels expressing the functionality of Italian aquatic ecosystems that the estimated PNECs could be considered quite realistic and more truthful than the European EQSs. However, PNECs estimated will have to be improved by reducing the heterogeneity of data availability among the taxonomic groups. For example, PNECs for crustacean are based on sensitivity data from 29 species, while those for annelids or rotifers on a single organism. Moreover, toxicological data on so-called emerging contaminants such as cosmetics and pharmaceuticals are still insufficient to estimate a representative PNEC for freshwater ecosystems, even if only approximately.
These values were estimated neither by applying arbitrary safety factors nor referring to the absolute lowest value available, but were derived from a careful balanced integration of representative data from numerous taxa. Therefore, the findings of this study pose the basis for an accurate revision of the methodologies applied for the establishment of the European EQSs.
The comparison of RFSs identified the amphibians and insects as the most sensitive groups for metals and pesticides, and crustaceans for PAHs. Those groups should therefore be taken into consideration in future works related to the determination of model species in ecotoxicology studies.
In the future, the research will be continued by deepening toxicological analyses for substances for which data are available for a few taxonomic groups (i.e., Azynphos ethyl, Benzo(a)anthracene, and Exachlorocycloesane), comparing the classification of selected examples of different water bodies according to current legislative framework with the chemical reference values developed on an ecotoxicological basis in the present work, and evaluating the effects on environmental management.
Finally, an important field of application that could be addressed is the use of these chemical reference values for the ERA of solid waste such as the application of sludge from sewage treatment plants in agriculture and the reuse of bottom ash from incineration of municipal plants for road construction.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/w15101811/s1, Table S1: List of species and substances regarding metals and trace elements and pesticides for which specific toxicity thresholds have been identified. Table S2: List of species and substances regarding PAHs and other contaminants for which specific toxicity thresholds have been identified.
Author Contributions
Conceptualization, F.O., A.T. and A.P.; Methodology, F.O., G.C., M.B. and B.C.; Formal Analysis: G.C., M.B. and B.C.; Supervision, F.O.; Validation, F.O., A.T. and A.P.; Writing—original draft, F.O.; Writing—review and editing, M.B., B.C. and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Additional data are provided in the supporting materials.
Acknowledgments
The authors thank Stefania Cioni for the revision of English language and grammar.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0060 (accessed on 1 February 2023).
- Commission Decision 2455/2001/EC of the European Parliament and of the Council of 20 November 2001 Establishing the List of Priority Substances in the Field of Water Policy and Amending Directive 2000/60/EC [cf. Annex 10 of Water Framework Directive]. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32001D2455 (accessed on 9 April 2023).
- Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Available online: http://data.europa.eu/eli/dir/2008/105/oj (accessed on 1 February 2023).
- Directive 2013/39/EU of the European Parliament and of the Council Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. Available online: http://data.europa.eu/eli/dir/2013/39/oj (accessed on 1 February 2023).
- Decreto Legislativo 13 Ottobre 2015, n. 172. Attuazione Della Direttiva 2013/39/UE, che Modifica la Direttiva 2000/60/CE per Quanto Riguarda le Sostanze Prioritarie nel Settore della Politica delle Acque. Available online: https://www.gazzettaufficiale.it/eli/id/2015/10/27/15G00186/sg (accessed on 9 April 2023). (In Italian).
- Guidance Document No. 27. In Technical Guidance for Deriving Environmental Quality Standards; Technical Report–2011–055; European Union: Maastricht, The Netherlands, 2011; ISBN 978-92-79-16228-2. [CrossRef]
- SCHEER. Scientific Advice on Technical Guidance n° 27, Technical Guidance for Deriving Environmental Quality Standards. 2017. Available online: https://data.europa.eu/doi/10.2875/018826 (accessed on 1 February 2023).
- TGD. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances, Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances, Directive 98/8/EC of the European Parliament and of the Council Concerning the Placing of Biocidal Products on the Market. Part II. Institute for Health and Consumer Protection. 2003. Available online: https://op.europa.eu/en/publication-detail/-/publication/212940b8-3e55-43f8-8448-ba258d0374bb (accessed on 9 April 2023).
- OECD. Report of the OECD Workshop on the Extrapolation of Laboratory Aquatic Toxicity Data on the Real Environment; Organisation for Economic Cooperation and Development (OECD), OECD Environment Monographs No. 59; Organisation for Economic Co-Operation and Development: Paris, France, 1992. [Google Scholar]
- Finizio, A.; Villa, S.; Vighi, M. Predicted No Effect Concentration (PNEC), Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128012383. [Google Scholar] [CrossRef]
- Di Toro, D.; Allen, H.E.; Bergman, H.L.; Meyer, J.S.; Paquin, P.R.; Santore, R.C. Biotic ligand model of the acute toxicity of metals. 1. Technical Basis. Environ. Toxicol. Chem. 2001, 20, 2383–2396. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Wood, C.M. Biotic Ligand Model, a flexible tool for developing site-specific water quality guidelines for metals. Environ. Sci. Technol. 2004, 28, 6177–6192. [Google Scholar] [CrossRef] [PubMed]
- Verschoor, A.J.; Vink, J.P.M.; Vijver, M.G. Simplification of biotic ligand models of Cu, Ni, and Zn by 1-, 2-, and 3-parameter transfer functions. Integr. Environ. Assess Manag. 2012, 8, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Posthuma, L.; Suter, G.W., II; Traas, T.P. Species Sensitivity Distributions in Cotoxicology; Lewish Publishers: New York, NY, USA, 2002; 579p. [Google Scholar] [CrossRef]
- Scott-Fordsmand, J.J.; Damgaard, C. Uncertainty analysis of single- concentration exposure data for risk assessment-introducing the species effect distribution approach. Environ. Toxicol. Chem. 2006, 25, 3078–3081. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.T.; Zheng, S.; Ni, X.; Zhao, J. Species sensitivity distribution and its application in ecotoxicology. Asian J. Ecotoxicol. 2010, 5, 491–497. [Google Scholar]
- Fox, D.R. A Bayesian approach for determining the no effect concentration and hazardous concentration in ecotoxicology. Ecotoxicol. Environ. Saf. 2010, 73, 123–131. [Google Scholar] [CrossRef]
- Commission Directive 93/67/EEC of 20 July 1993 Laying down the Principles for Assessment of Risks to Man and the Environment of Subtances Notified in Accordance with Council Directive 67/548/EEC OJ L 227 08.09.1993. p. 9. Available online: http://data.europa.eu/eli/dir/1993/67/oj (accessed on 1 February 2023).
- Forbes, V.E.; Calow, P. Population growth rate as a basis for ecological risk assessment of toxic chemicals. Phil. Trans. R. Soc. Lond. B 2002, 357, 1299–1306. [Google Scholar] [CrossRef]
- Smetanová, S.; Bláha, L.; Liess, M.; Schäfer, R.B.; Beketov, M.A. Do predictions from Species Sensitivity Distributions match with field data? Environ. Pollut. 2014, 189, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A.; Beasley, A.; Barron, M.G.; Belanger, S.E.; Bonnel, M.; Brill, J.L.; De Zwart, D.; Kienzler, A.; Krailler, J.; Otter, R.; et al. Creation of a Curated Aquatic Toxicology Database: EnviroTox. Environ. Toxicol. Chem. 2019, 38, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Beasley, A.; Belanger, S.E.; Otter, R.R. Stepwise Information-Filtering Tool (SIFT): A method for using risk assessment metadata in a nontraditional way. Environ. Toxicol. Chem. 2015, 34, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.J.; Vermeire, T.G. Risk Assessment of Chemicals: An Introduction, 2nd ed.; Springer: Dordrecht, The Netherlands, 2007; ISBN 978-1-4020-6101-1 (HB). [Google Scholar]
- Verdonck, F.A.M.; Jaworska, J.; Thas, O.; Vanrolleghem, P.A. Determining environmental standards using bootstrapping, bayesia and maximum likelihood techniques: A comparative study. Anal. Chim. Acta 2001, 446, 429–438. [Google Scholar] [CrossRef]
- Isnard, P.; Flammarion, P.; Roman, G.; Babut, M.; Bastien, P.; Binstein, S.; Essermeant, L.; Ferard, J.F.; Gallotti-Schmitt, S.; Saouter, E.; et al. Statistical analysis of regulatory ecotoxicity tests. Chemosphere 2001, 45, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.S.; Filzmoser, P.; Deutsch, R.C. A robust approach to risk assessment based on Species Sensitivity Distributions. Risk Anal. 2018, 38, 2073–2086. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, J.R.; Grist, E.P.M.; Leung, K.M.Y.; Morritt, D.; Crane, M. Species sensitivity distributions: Data and model choice. Mar. Pollut. Bull. 2002, 45, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Nowack, B. A Probabilistic Method for Species Sensitivity Distributions Taking into Account the Inheren Uncertainty and Variability of Effects to Estimate Environmental Risk. Integr. Environ. Assess. Manag. 2013, 9, 14791126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).