Abstract
Arsenic poisoning constitutes a major threat to humans, causing various health problems. Almost everywhere across the world certain “hotspots” have been detected, putting in danger the local populations, due to the potential consumption of water or food contaminated with elevated concentrations of arsenic. According to the relevant studies, Asia shows the highest percentage of significantly contaminated sites, followed by North America, Europe, Africa, South America and Oceania. The presence of arsenic in ecosystems can originate from several natural or anthropogenic activities. Arsenic can be then gradually accumulated in different food sources, such as vegetables, rice and other crops, but also in seafood, etc., and in water sources (mainly in groundwater, but also to a lesser extent in surface water), potentially used as drinking-water supplies, provoking their contamination and therefore potential health problems to the consumers. This review reports the major areas worldwide that present elevated arsenic concentrations in food and water sources. Furthermore, it also discusses the sources of arsenic contamination at these sites, as well as selected treatment technologies, aiming to remove this pollutant mainly from the contaminated waters and thus the reduction and prevention of population towards arsenic exposure.
1. Introduction
Arsenic is a ubiquitous toxic metal, belonging in the metalloid group of the periodic table, found naturally in the lithosphere, hydrosphere, and atmosphere, as well as generally in the biosphere [1]. Both organic and inorganic forms of arsenic exist in nature (mostly in the form of complexes); various transportation routes in environment have been identified and rather high concentrations (mainly in water sources) have been reported in several regions around the world [2].
The World Health Organization (WHO) recommended the regulation limit (as imposed by the respective legislation) of arsenic concentration in drinking water at 10 μg/L [3]. This is also the limit of arsenic in drinking water imposed by the European Commission, the United States Environmental Protection Agency and other inter/national organizations. Contamination of water by arsenic, especially groundwater, due to arsenic’s high toxicity, is considered a major health issue in various areas worldwide. Relevant research showed that the long-term exposure to elevated concentrations of arsenic can threaten human health, causing a variety of health disorders, including skin lesions (e.g., keratosis, pigmentation) and various internal and skin cancers [4].
Nevertheless, arsenic has been used in various industries for the production of several products, such as glass, ceramics, electrical appliances, cosmetics and fireworks. In the mid-20th century, arsenic was also widely used to produce pesticides, and for the production of wood preservatives [5]. As a result of past and current uses, arsenic contamination is currently considered a problem of great concern for the scientific community, found mainly in water sources, but also in food, threatening the health of millions of people. Mondal et al. [6] revealed that arsenic exposure from food exceeds that from drinking water in the endemic region of Bihar (India), whereas additional areas are expected to be discovered in the near future, such as in Pakistan, according to a recent survey [7].
In this review, a short description of the human exposure to arsenic via water and food sources is reported and the potential pathways of its environmental cycle are presented. In addition, specific data regarding the arsenic concentration in various waters and food products from Asia, Europe, America, Australia and Africa are summarized, according to recent literature publications. The aim of this study is to summarize the world situation regarding arsenic contamination of waters and food, and human exposure to contaminated sources, which remains a threat to health. It is worth noting that the data presented relate to major published case studies and do not reflect the overall situation in each country.
2. Origin of Arsenic Contamination
High concentrations of arsenic may occur naturally (e.g., due to erosion of minerals) in several areas, which are therefore contaminated, and by anthropogenic activities (e.g., industrial production and uses). The continuous exposure of humans to arsenic via food and water consumption can lead to serious health damages, because this is a carcinogenic element with high affinity for thiols. It can also replace phosphorus in biochemical reactions owing to their similar chemical properties, as they belong in the same group of the periodic table, actually being very close, indicating the highly destructive role of this element during DNA replication and metabolic activity [8].
Soil: The predominant forms of arsenic in soils are arsenate (As5+), arsenite (As3+) and organic arsenic. Usually, in soil matrixes it can be found complexed with amorphous iron and aluminum oxides [9]. The arsenic form in the soil varies according to the different textures. For instance, the presence of clay can increase the fixation of arsenic in the respective soil, as at circum-neutral pH values arsenic is adsorbed onto the clay particles [10]. Its concentration and mobility are also dependent on pH and redox potential at the specific environmental sites [11]. Because of the toxic effects of arsenic, it has been used (mainly in the past) for the production of specific herbicides, insecticides, various toxins and decongestants. The use of intensive phosphate fertilizers in agriculture is also considered a potential source for arsenic contamination, because arsenic is a common contaminant of most phosphate minerals, which are used for the production of these fertilizers. However, the amount of arsenic in other fertilizers(e.g., nitrogen or potash) is rather low and can be considered insignificant [12]. Figure 1 shows the various routes of arsenic and arsenic-related compounds that can accumulate in an ecosystem due to anthropogenic activities, resulting in environmental deterioration.
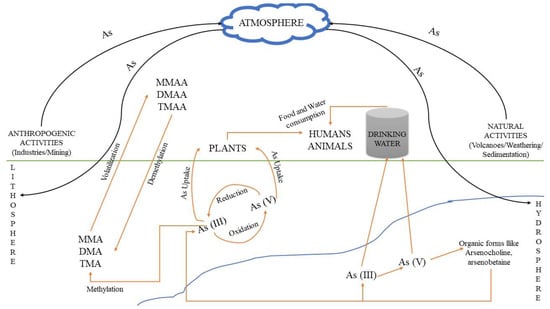
Figure 1.
Pathways through which arsenic and its relevant compounds may enter the environment and contaminate soil, atmosphere and water. Human and natural activities result in As accumulation, mainly in soil and water, where As(V) and As(III) are interconverted via oxidation and reduction bio/reactions. The respective methylated products can be produced from As(III) species, i.e., MMA, DMA and TMA, resulting in the formation of MMAA, DMAA and TMAA chemical compounds, mainly through volatilization, while the reverse process occurs through demethylation.
Atmosphere: Arsenic can be released into the atmosphere by natural or anthropogenic activities. According to a rough estimation, the global annual release of arsenic in the atmosphere is 7.8 × 107 kg/year. The natural sources are expected to release 1.2 × 107 kg arsenic per year, wherein volcanoes and microbial volatilization may supplementarily contribute an additional 8.9 × 106 and 2.1 × 107 kg/year, respectively [13]. The amount of arsenic stored in the northern hemisphere is almost five times higher than the amount of arsenic stored in the southern hemisphere, mainly due to the more intensive industrialized conditions existing in the northern hemisphere [14]. Other environmental problems, potentially causing increased arsenic emissions in the atmosphere, may include deforestation, grass burning, and the use of wood as fuel. High and significant concentrations of arsenic are also connected with the emissions of industrial wastes, especially heavy/toxic metals [15]; the concentration of arsenic in sewage sludges is considered to be an indicator of the industrialization degree for the surrounding area.
Water: Since arsenic is an element of the Earth’s crust, groundwater usually presents the most severe pollution problems among other water resources [16]. It can be found either dissolved in water or in the form of particles. Moreover, it may be transformed to dimethyl and/or trimethyl arsenic compounds by mollusks, crustaceans and fishes [17]. Arsenate is the predominant form found in seawater algae, which play an important role in the biological transport of inorganic arsenic species [18].
3. Hazards and Limits in Food and Water
Inorganic arsenic (iAs) can be found in the environment in several forms, including As(0) (metalloid arsenic), As(III) (arsenite) and As(V) (arsenate). The latter two forms are abundant in natural and drinking waters [19]. Arsenate is the dominant specie in oxic waters, whereas under mildly reducing conditions, the probability of arsenite prevalence increases [20]. As(III) is 60 times more toxic than As(V), because of its greater tendency to react with lipids, proteins and other cellular components, causing higher cellular uptake [21]. In addition, As(III) is more difficult to remove from water due to its higher mobility, as compared with As(V); therefore, it can be adsorbed less efficiently on solid surfaces [22].
Because of its toxicity, the World Health Organization (WHO) in 1993 reduced the recommended concentration limit of total As in drinking water to 10 μg/L (from the previous 50 μg/L limit) [23]. This concentration limit has been adopted by several inter/national organizations, such as the European Commission and the United States Environmental Protection Agency. However, several nations facing severe As contamination problems still currently retain the previous (higher) limit, e.g., China, India and Bangladesh. On the other hand, there are also regions where even lower limits have been applied than those proposed by WHO, such as in Australia (7 μg/L) and in the state of New Jersey, USA (5 μg/L) [24].
Equally important and hazardous is the exposure to As from food consumption. Arsenic can accumulate in plants, mainly in cereals, reducing their growth and productivity [25]. Irrigation needs of crops are often related to the amount of arsenic content in the seeds, due to the already contaminated groundwater or/and surface water application [26]. The corresponding recommended limit by Food and Agriculture Organization (FAO), in collaboration with WHO, applies currently mainly to rice grains, due to the respective higher irrigation needs, as compared with other crops, and it has been set at 0.2 mg/Kg [27]. However, it has to be noted that this concentration limit has not yet been adopted by the European Commission or the United States Environmental Protection Agency. The only country is China, which imposed (by legislation) the maximum permissible concentration of arsenic in rice at 0.15 mg/Kg, a value even stricter than the one previous recommended [28]. Regarding arsenic contamination, another food category that attracts the interest of the scientific community is fish. Through water contamination (marine or fresh), arsenic can enter aquatic organisms and accumulates. Nevertheless, it is pointed out that any relevant legislation has not been proposed or adopted by any global or local organization [29].
4. Global Status of Arsenic Contamination
It has been reported that the concentration of arsenic in groundwater of many regions exceeds the WHO recommended maximum permissible limit of 10 μg/L. In addition, more studies are published every year regarding the hazardous effects of As in humans; the aforementioned limit is already questioned, increasing the pressure to decrease the limit still further [30]. An early view of future As regulation is revealed by the even stricter limits existing in Australia and New Jersey, as aforementioned.
According to Podgorski and Berg [7], up to 220 million people worldwide are considered to be direct consumers of As-contaminated water. In their research, over 50,000 case studies were combined in order to develop a global arsenic prediction model, which revealed that the majority of those at risk, due to As consumption, are mainly the residents of Asia. The respective data are proportional since Asia is by far the most populous continent and, in addition, 50.4% of the case studies examined (selectively) are located there (Table 1). In the same context, Shaji et al. reported that more than 230 million people in India are potential consumers of arsenic-contaminated water [16].
A brief list of countries severely affected by arsenic contamination includes: Bangladesh [31], China [32], Pakistan [33], Cambodia [34], USA [35], Indonesia [36], Canada [37], Hungary [38], Mexico [39], Nepal [40], India [41] and Greece [19].
4.1. Asia
Eastern Asia: The East Asian region includes China, Hong Kong, Macau, Korea, Taiwan and Japan. China has been severely affected by arsenicosis since the 1960s [42]. A large populace is currently exposed to higher levels of arsenic in groundwater and drinking waters, which usually leads to various diseases. Sanjrani et al. stated that the health of 19 million people is at risk annually because of contaminated groundwater consumption. The study also reported that China is the top country, considering the production of arsenic, holding approximately the 50% of global market. This arsenic exposure still continues because around 10 million wells are used for drinking water, making the screening/examination process of water extremely laborious and difficult. Only 20 case studies were reported and the highest As concentration among them was 2600 μg/L [43]. Luo et al. reported a sharp increase in the arsenic levels in China because of intensive industrialization and other anthropogenic activities since the 1990s. One of the busiest regions of China is the Pearl River Delta, which is reported to contain up to 300 μg As/L in groundwater, affecting over 120 million people. This area is heavily involved in mining activities and, along with the geogenic activity, the arsenic levels in groundwater have also been raised. As a result, the rice grown in this region presents higher As content (0.13–0.43 mg/Kg), compared with China’s average value (0.05 mg/Kg), as presented in Table 2, and with the regulation limit (0.15 mg/Kg) [44,45].
Hong Kong’s population is exposed to high levels of arsenic by the contamination of food. Hong Kong has the world’s largest fish market. Shark fins are a delicacy in this region, and it is imported from all over the world. Barcia et al. revealed that the shark fins are contaminated with heavy metals, especially mercury (up to 13.6 μg/Kg) and arsenic (up to 70.6 μg/Kg) [46]. This heavy metal contamination is introduced into the aquatic ecosystem via mainly anthropogenic activities, and it reaches easily the highest trophic level of food webs, i.e., humans.
The Republic of Korea is also suffering from arsenic contamination. Crop and livestock farming are the two main sources, and these activities are continuously performed on soils contaminated by heavy metals, including arsenic (the maximum value reported is 704 mg/Kg), mainly due to poor management of mining waste (tailings). Rice is a popular food in this overall region, regularly consumed by millions of people. The growth of rice in contaminated soils can lead to arsenic bioaccumulation. The second (major) reason for arsenic contamination of water and, consequently, of food contamination in Korea is considered to be mining activity; more than 5000 mines are operational [47]. The relevant studies have also revealed arsenic accumulation in rice of certain regions of Taiwan and Japan. Abedi and Mojiri revealed that the roots of rice crops are heavily contaminated with arsenic, with concentrations up to 157,000 μg/L [25].
South Asia: The countries included in South Asian region are Bangladesh, Bhutan, India, Pakistan, Nepal, Sri Lanka, Afghanistan and Maldives. It was reported in 2021 by UNICEF that approximately 1.4 million wells in Bangladesh are contaminated with elevated concentrations of arsenic (i.e., higher than the 50 μg/L limit). In a study conducted by Adeloju et al., 12.6% of drinking-water samples tested were found to contain traces of arsenic. This statistic indicates that potentially 22 million Bangladeshi people are adversely affected by arsenicosis [48]. Agriculture is the main occupation of the local people and vegetables are commonly planted. The vegetables and crops can be heavily exposed to arsenic contamination because of the excessive use of arsenic-containing fertilizers, pesticides, and to ecologically unfriendly industrial activities [49]. According to Rahman et al., the exposure of the local population to inorganic arsenic is in the range 0.41–6.38 μg per Kg of body weight. This range is much higher when compared with a group of people not directly exposed to arsenic, i.e., 0.08–0.15 μg per Kg of body weight. The study highlighted that the arsenic intake in this area is mainly attributed to food and water consumption [50].
Bhutan is considered to be one of the countries with the highest water availability per capita, but only 1% of it is accessible for consumption, mainly from surface water sources [51]. These water resources and their consumption become the major pathway for the entrance of arsenic in the population of this country. Ayers et al. reports that approximately 100 million people are at risk of being affected by the imported contaminated water in the wider region of southern Asia [52].
India’s population is also suffering from arsenic contamination via food and water consumption. Kumar et al. conducted a relevant study in Bihar, one of the major states of India. This study showed that more than 80% of water resources are contaminated with arsenic (the highest reported value was 2 mg/L). This leads to the accumulation of arsenic in approximately 10 million people of Bihar with concentration levels greater than the maximum permitted limit recommended by WHO [53]. Das and Mondal conducted a study in India, exhibiting the role of geomorphic features in the distribution and mobilization of arsenic in the country [54]. Mukherjee et al. showed that there is a strong association of increased accumulation of arsenic in groundwater and irrigation fields, due to the movement of tectonic plates. The study reported the exposure of approximately 90 million people of India to arsenic-contaminated water [55].
Pakistan stands at 80th place out of 122 nations, which are coping with heavy arsenic contamination via water consumption. The contaminated water resources are mostly found in the plains of Punjab and Sindh, mainly due to the presence of the Chenab and Indus rivers, respectively. Groundwater samples near these rivers show concentrations of As up to 2.5 mg/L [33]. According to Rassol et al., the irrigation water used in the region of Punjab shows As concentration in the range 12–448 μg/L; the respective data resulted from 44 representative tube-well samples [56]. In addition, the presence of As in various species of fish originating from these rivers has been observed (with the concentration range 0.19–1.77 μg/g) [57]. Regarding drinking water, Sanjrani et al. collected and reviewed the conditions and levels of arsenic in the provinces of Pakistan; 3% of Punjab’s population is exposed to higher levels of arsenic, i.e., >50 μg/L, and 20% of Punjab’s population is exposed to >10 μg/L levels of arsenic. Similarly, the conditions are worse in the Sindh area compared to Punjab; 16% population is reported to be exposed to >50 μg/L and 36% of the population to >10 μg/L arsenic contamination levels [58].
The population in Nepal is also affected by arsenic in groundwater. The inhabitants of Nepal usually use the groundwater for agricultural and domestic purposes. Timalsina et al. reported that approximately 2–3 million people in Nepal are consuming arsenic-polluted water in the concentration range 10–50 μg As/L, while 0.37 million people are the consumers of arsenic-contaminated water with concentrations of more than 50 μg As/L. The study also reported the statistics of Terai district in Nepal, where almost 90% of the inhabitants depend on groundwater for their daily needs; the maximum arsenic concentration in this area was found to be 2620 μg/L [59].
Sri Lankans are also exposed to high arsenic levels via the contamination of food sources. The locals consume mainly rice, vegetables and fish products. The most popular fishes are tuna and ray species, which found to be contaminated with arsenic [60]. There are similar conditions for the case of Maldives, where the inhabitants are exposed to more than 10 μg/L arsenic via groundwater resources [61].
A large portion of Afghanistan’s population suffers from arsenicosis and the major source of contamination is considered to be thermal springs, which are the result of volcanic activity. Jawadi et al. examined 13 samples from local thermal spring waters. It was reported that the concentration of arsenic exceeds 100 μg/L in all the spring samples. People normally use the spring water on a daily basis, although it is not recommended for drinking consumption due to its elevated arsenic content [62].
Southeast Asia: The Southeast Asian region includes Vietnam, Indonesia, Philippines, Thailand, Malaysia, Cambodia and Laos. Vietnam is one of the major rice producer countries. People consume rice and rice-related products on a daily basis. These rice plants are grown in wetlands, which have caused exposure of the local population to elevated arsenic concentrations. Chu et al. conducted a study in which the arsenic levels were determined in locally produced rice. The study revealed that, along with other heavy metals, the arsenic concentration in rice was 0.14 mg/kg. Such high arsenic content was mainly attributed to the nearby mining activities, which affected the agricultural land by increasing the content of pollutants [63].
Indonesia’s inhabitants depend on groundwater for their needs. In recent years, the country is moving rapidly toward industrialization. Under this rapid economic development, pollution is increased, and groundwater is negatively affected. This further leads to various health problems and other environmental issues. The coastal cities, especially Jakarta, Mataram and Indramayu, are severely affected [64].
Regarding arsenicosis, Philippines has also witnessed several cases. Solis et al. conducted research on water samples from wells. The results revealed that 38.7% of the wells examined had arsenic concentration in excess of 10 μg/L, but not higher than 50 μg/L (average values) [65].
In Thailand, arsenic exposure occurs mainly via water consumption. Mining activities can release arsenic, contaminating groundwater [66]. In recent studies, arsenic exposure has been reported via food consumption as well. Fish is a well-known and common food among the inhabitants of Thailand; increased levels of arsenic were found in marine fish. However, according to the research conducted, arsenic was found in the form of arsenobetaine, which is considered nontoxic; nevertheless, changes from this form when it enters the human body cannot be excluded [67].
Relevant studies to assess the arsenic contamination in Malaysia have also been performed. These studies revealed that the water sources, commonly in use by the inhabitants, contain arsenic concentrations below the permissible limit in drinking water [68]. Instead, surface water, namely the Langat River, showed As concentrations sometimes higher than the limit and in the range 1–22 μg/L [69]; however, such concentrations are not considered as dangerous for human health, especially if surface water is not consumed directly as drinking water. The arsenic concentration of river water can affect the accumulation of arsenic on food produced by the use of this water, i.e., when used for irrigation. However, regarding food contamination, the health risk was proved to be low in comparison with other areas, since rice and fishes are considered to be safe for consumption, because of smaller reported concentrations. Regardless, constant monitoring and control is recommended [70,71].

Table 1.
Asian regions and concentration of arsenic in water (selected data from recent literature).
Table 1.
Asian regions and concentration of arsenic in water (selected data from recent literature).
| Country | Drinking Water (µg/L) | Groundwater (µg/L) | Surface Water (µg/L) | Highly Contaminated Region | Ref. |
|---|---|---|---|---|---|
| China | <10 | 21–2611 | 0.46–19.5 | Shanxi, Anhui | [43,44] |
| Republic of Korea | <10 | 0.02 | - | - | [72] |
| Taiwan | - | 10–1800 | - | Lanyang Plain | [16] |
| Japan | - | 0.2–7.1 | 0.2–38.3 | - | [73,74] |
| Bangladesh | >50 (~1700 samples) | <10–4730 | - | South Bangladesh | [31,75] |
| India | 0.01–9.4 | <10–390 | - | Bihar, Manipur, Jharkhand | [76] |
| Pakistan | - | <10–2580 | - | Punjab, Sindh | [33,56] |
| Nepal | - | 0–50 | - | Nawalparasi | [20] |
| Sri Lanka | - | 0–7 | - | - | [77] |
| Vietnam | 7–82 | 1–3050 | - | Red River Delta | [78] |
| Indonesia | 0–60 | 0.8–167 | - | Kamal | [64,79] |
| Thailand | - | 1–5100 | 165–985 | Nakorn Si Thammarat | [66] |
| Cambodia | - | 0.1–1300 | - | Preak Russey | [34] |
Arsenic contamination was also found in the groundwater of Cambodia. According to Ratha et al., 33–35% of examined well samples from the provinces of Kandal and Kampong Cham presented As concentrations higher than the national standard of 50 μg/L [80]. Groundwater in these areas is used for irrigation and occasionally as a drinking-water source [34]. In Laos, north of Cambodia, As contamination is related to mining activities and it affects nearby areas. Accumulation in fish tissue from the Nam Kok River is observed (3.5 mg/Kg), while the concentration of arsenic in water was determined to be below the 10 μg/L limit [81].

Table 2.
Foods affected by arsenic contamination in Asia regions (selected data from recent literature).
Table 2.
Foods affected by arsenic contamination in Asia regions (selected data from recent literature).
| Country | Product | As Concentration (mg/Kg) | Ref. |
|---|---|---|---|
| China | Rice Fish Shellfish | mean 0.05 0.11 3.6 | [44,45] |
| Hongkong | Shark fins | max. 0.07 | [46] |
| Republic of Korea | Rice | 0.03–0.77 | [47] |
| Taiwan | Rice | 0.23 | [25] |
| Japan | Rice Fish and shellfish | 0.1–0.16 0.2–8.3 | [25,82] |
| Bangladesh | Vegetables Rice Fish | <0.005–0.54 0.03–1.84 0.097–1.318 | [48] |
| India | Rice Wheat grain Potato | 0.015–0.23 <0.235 0.005–0.176 | [6] |
| Pakistan | Fish | 0.19–1.77 | [57] |
| Sri Lanka | Rice Fish Vegetables | 0.002–0.58 <0.002–66 0.001–0.025 | [60] |
| Vietnam | Rice | 0.14 | [63] |
| Indonesia | Rice Fish | 0.00–0.31 0.64–5.78 | [83,84] |
| Philippines | Fish | 0.058–0.33 | [85] |
| Thailand | Rice Fish Scrimp | 0.084–0.49 0–2.73 0.97–7.28 | [67,86] |
| Cambodia | Rice Fish Vegetables | 0.088–0.578 0.144–0.222 0.01–0.141 | [87] |
Central Asia: The major countries in the Central Asian region include Kazakhstan, Tajikistan, Turkmenistan and Uzbekistan. In Kazakhstan, arsenic gains entry into the food chain mostly via food and water consumption. Vegetable oils are widely used by the population, but the respective agroindustrial production activities can lead to the excessive entry of heavy metals, especially arsenic. Mukhametov et al. conducted a study showing that arsenic concentrations around 3 μg/kg are found in rapeseed and safflower oils. At present, these levels are safe for consumption, but through time, there may be a rise in these concentrations, which will ultimately have an adverse effect on human health and the environment [88].
In the case of Tajikistan, a relevant study in 2010 reported that none of the 1620 drinking-water samples exceeded the WHO or EU standards (i.e., 10 μg/L), regarding arsenic concentration. Data on other water sources are limited and in combination with the proved pollution problems identified in the neighboring countries, monitoring is considered to be necessary [89].
In the other countries (Turkmenistan and Uzbekistan) similar conditions prevail. The concentration of arsenic ranges below the recommended limit, but it continuously accumulates in the food web mainly via irrigation, potentially damaging human health and the environment [90].
Status in Gulf Countries: The Kingdom of Saudi Arabia is one of the rising Gulf countries at global level. However, this area is facing several pollution issues and arsenic contamination of waters is among them. Placed in an arid region, the availability of water is limited and water scarcity is considered a major problem. Groundwater is the most common water source; however, the quality is deteriorating in several cases, due to the gradual accumulation of toxic heavy metals. Arsenic exposure via water is attributed to the proximity to contaminated aquifers and rocks. The land experiences heavy weathering and sedimentation and this leads to toxic metal contamination, especially from arsenic. Water samples from the city of Madinah were tested for arsenic and it was revealed that at some sampling sites, arsenic concentration was under the permissible value, i.e., 1–2 μg/L. However, at other sites arsenic concentration was higher, especially in the southwestern region. Therefore, overall, the country is becoming exposed to arsenic via contaminated water. Recent rapid industrial development has also contributed to this pollution issue [91].
Other Gulf countries include Bahrain, Iraq, Kuwait, Oman, Qatar and United Arab Emirates. These countries are reported to have arsenic exposure mainly via food sources. Fish and shrimp are commonly consumed in these countries; the relevant studies showed that they may contain rather high arsenic content, as the average concentration found in 511 respective samples was 1.37 mg/Kg. Thus, the general population in these countries may suffer from possible arsenicosis problems [92].
4.2. Europe
The most significant European countries in terms of water pollution by arsenic are Hungary, Serbia and Romania, where 600,000 people are at risk of drinking water possibly containing elevated levels of arsenic. These countries are affected by As contamination mainly through the Pannonian Basin. For instance, in 20% of water samples from over 9000 drinking-water cases analyzed in Hungary, the concentration of arsenic measured above 10 μg/L, and most of these samples were located in the abovementioned basin [93]. Other areas at risk include Czech Republic, Croatia, Finland, Greece, Italy and Turkey. The presence of arsenic in Italian groundwater is mainly due to the dissolution of minerals through volcanic activity. Extraction and geothermal phenomena in Turkey are considered responsible for the increase in arsenic concentration in groundwater. In Greece, due to geothermal phenomena, the arsenic concentration in the corresponding springs and spa regions can vary between 30 and 4500 μg/L [19]. The oxidation of sulfide minerals during the mining process can also lead to arsenic contamination of groundwater. Furthermore, As content is increased in areas where a transition from gravel to silty clay reservoirs is observed. Arsenic is also accumulated in food sources. Fortunately, most edible products (with the exception of fresh seafood) contain very low amount of this toxic metal (<25 mg/Kg). In addition, in the northern European regions, the concentration of arsenic in soils is around 2.5 mg/Kg, while in southern Europe it is around 8 mg/Kg. However, UK imports vegetables and rice from India, Bangladesh, Italy and other countries, where the crops are mostly grown with As-polluted water [94]. A similar situation is observed in other countries, such as Germany [95]. Table 3 shows the extent of arsenic contamination in water from different regions of Europe, and Table 4 shows the respective arsenic contamination of foods.

Table 3.
European regions and concentration of arsenic in water (selected data from recent literature).

Table 4.
Foods affected by arsenic contamination in Europe (data selected from recent literature).
4.3. America
The high arsenic contamination in several American regions is also associated with volcanic eruptions and geothermal fluids, which adversely affect the environment [122]. Along with natural activities, human interventions can also lead to As contamination. In Nicaragua, over 55,000 people are drinking water with As concentration above the permissible limit, as shown in Table 5. Brazil holds 12% of planet’s fresh water, but it is partially contaminated due to the entry of As. In the region of Latin America, Argentina was the first country that reported diseases caused by As contamination because of relatively higher concentrations in water and food products (Table 6) [123].

Table 5.
American regions and concentration of arsenic in water (selected data from recent literature).

Table 6.
Foods affected by arsenic contamination in America (selected data from recent literature).
4.4. Australia
Pollution in the Australian environment is due to both biogeochemical and anthropogenic activities. The main source of pollution in the Australian continent is the mining industry. Gold mining is considered to pollute the environment with arsenic [2]. Additionally, the process of pedogenesis (i.e., the natural weathering of material), and wildland forest fires are major sources of environmental pollution in Australia. Other polluting industries may include timber treatment plants, forestry, agriculture, and ammonia production, especially in Western Australia [2]. According to previous studies in New South Wales (Australia), the process industries have released arsenic to groundwater and the environment during the production of various compounds, such as Al and Fe hydroxides and arsenopyrite [16]. The contaminated groundwater also becomes a source of arsenic accumulation in agrofood products, such as in rice, due to irrigation. In general, the concentrations of total arsenic in the Australian-grown rice were higher than in the imported rice for sale in Australia [2]. The respective concentrations of arsenic in water and food sources in the Australia regions are presented in Table 7.

Table 7.
Concentration of arsenic in water and foods in Australia and New Zealand (selected data from the recent literature).
4.5. Africa
A large part of the African population also suffers from arsenicosis. The accumulation of arsenic above the permissible limit in the food consumed has led to environmental contamination. Table 8 shows arsenic concentrations in different African regions, regarding water resources, while Table 9 presents its accumulation in edible products. According to the relevant literature, As contamination in Africa is mainly attributed to mining, such as gold, copper and uranium, and to erosion processes. For instance, topsoil around abandoned and active gold mines in western Ghana presents As concentrations in the range 1807–8401 mg/Kg, causing significant contamination in a wider area and especially in surface water and groundwater sources [158].

Table 8.
African regions and concentrations of arsenic in water sources (selected data from recent literature).

Table 9.
Foods affected by arsenic contamination in Africa regions (selected data from recent literature).
5. Main Effects of Arsenic Contamination on Human Health
Arsenic is considered a top priority contaminant due to its toxicity and as a carcinogenic chemical, whereas the intake by humans has been verified worldwide, through the consumption of contaminated water and food. Both organic and inorganic forms of arsenic can exist in the environment, but the latter ones are more poisonous and toxic. Inorganic As is found more in water sources and, consequently, in the relevant edible fish products. Regarding vegetation, the exposure to arsenic contamination (e.g., through contaminated soil) can cause the inhibition of plant growth along with the loss of or reduction in photosynthetic and reproductive activities.
The accumulation of arsenic in the food web may lead to acute and long-term effects on human health. Vomiting, abdominal pain, diarrhea, numbness and tingling, muscle pain and cramps, and death in extreme cases are reported as the main health symptoms of elevated arsenic intake [196].
The long-term effects are attributed to higher levels of inorganic arsenic in the human body. The effects can be observed mainly in skin, including pigmentation, lesions formation and patches, and acting probably as precursors to skin cancer. Bladder and lung cancers are also reported to be a result of arsenicosis [197]. Other health issues such as diabetes, pulmonary and heart diseases may also arise, due to the long-term exposure of arsenic; e.g., Taiwan has witnessed gangrene because of black-foot disease, leading to deaths, due to elevated arsenic concentrations [198]. Literature reports indicate that malnutrition also contributes to the severity of respective diseases. Infant mortality is also observed in various parts of the world, especially during pregnancy. Arsenic exposure can also lead to the reduction in cognitive development, intelligence and memory in children [199].
6. Control, Prevention and Treatment of As in Water Sources
As revealed by the data presented in this review, the intake of arsenic by humans depends mainly on arsenic concentrations in the direct or indirect use of the respective water (or food) sources. Direct exposure may occur through the consumption of contaminated drinking water and indirect exposure through the irrigation of crops with contaminated groundwater or surface water, and the subsequent consumption of agricultural products.
Regarding the prevention and control of arsenic, various measures can be proposed and applied. First, the higher or lower arsenic sources should be identified appropriately. Second, the higher arsenic-contaminated sources of groundwater should be substituted by other safer water sources, whereas the lower arsenic concentration waters can be used for various domestic purposes (probably not for dinking). Third, the higher arsenic-contaminated waters can be blended with the lower ones to achieve an average concentration, which is permissible for their respective use, according to WHO regulations. For long-term prevention and control, industrial and other wastewaters should be treated properly. The general public should be informed of the arsenicosis problem and its health effects and the population at high-risk of arsenic toxicity should be monitored regularly [3]. Fourth, the necessary arsenic treatment/removal systems should be installed. These systems, such as adsorption, precipitation, coagulation, ion-exchange, membrane filtration techniques, among others, may be centralized or applied locally.
Reducing the concentration of arsenic to below the permitted concentration limits requires the application of effective methods, since the arsenic removal mechanisms may pose certain difficulties. According to the speciation diagram of this element (Figure 2a), the dominant As(III) specie in waters is the neutral form, for which it is more difficult to apply a selective removal mechanism. A common solution proposed in the literature and employed in practice is the preliminary As(III) oxidation to the As(V) form, which is negatively charged in the water pH range usually encountered (Figure 2b) [200]. Oxidation may be achieved chemically, or biologically by using the appropriate microorganisms [201].
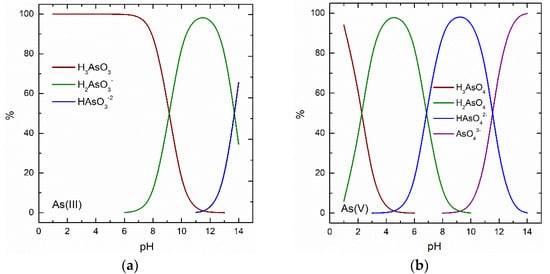
Figure 2.
Speciation diagrams of (a) As(III) and (b) As(V) at ppb concentration level in natural-like water (water composition according to National Sanitation Foundation protocols), by applying MINTEQ 3 software [206].
On the other hand, and regarding the applied treatment technology, most of the aforementioned treatment methods present specific limitations, such as higher cost, lower selectivity, insufficient removal (i.e., the As residual concentration may be higher than the 10 μg/L concentration limit), higher energy consumption, or production of large amounts of toxic sludge. Among them, the most promising seems to be the adsorption process, since by applying the appropriate sorption materials, such as conventional iron- or aluminum-based adsorbents [202], or better yet, the novel engineered inorganic nanoparticles [203], it is possible to overcome most of the previous limitations. The adsorption-based technologies are also those that have found extensive applications in the highly affected As-contaminated areas of Southeast Asia [204]. In addition, it is possible to combine the respective removal mechanism with an oxidation mechanism, producing a unique step process, and therefore, increasing the method’s effectiveness [205].
Relevant field studies in Nepal [207] and Burkina Faso [208] were conducted by applying multilayered filtration systems, where zero-valent iron (ZVI) particles were intended to fulfill the combined oxidation–adsorption removal mechanism. These systems were tested at a domestic level (i.e., for the production of 40–60 L/d), but they had difficulty removing sufficient arsenic to reduce the concentration to levels below the regulation limit. This was due to the relatively low contact time applied and the rather high initial concentration of pollutant. Additional extended field case studies at community level, including the combined application of coagulation process (Bangladesh, USA, Greece, Chile) or electrocoagulation (Mexico, India) [209], have been performed. Both of these supplementary technologies can provide a larger volume of treated water per day with residual arsenic concentration below the requested concentration limit and at a lower cost, when compared to the application of a single adsorption process.
Even though they were already adopted by several countries, they are mainly applied as batch technologies and therefore quite unsuitable for use in continuous flow systems. An oxidation method is usually mandatory as a pretreatment step. On the other hand, Argentina has invested in the reverse osmosis process for the removal of arsenic from contaminated water. For instance, 52 reverse osmosis units, 15 coagulation and 3 nanofiltration plants have been established in the Buenos Aires province and applied for drinking-water treatment [210]. However, reverse osmosis is a higher cost process; nevertheless, it was selected according to the specific characteristics of available water sources in the region. Because this is a technology of low selectivity, its application aims toward the general improvement of water quality, such as the reduction in salinity, and not particularly for the removal of arsenic.
A more ecofriendly process was tested in Australia by applying a capacitive deionization prototype unit [211]. This technology is based on the combination of electrochemistry and sorption, where As charged modes are driven and adsorbed on the porous surface of the electrodes. The electrodes were synthesized from coconut shell biochar and the unit was powered by solar panels. This unit can provide 3 L/min of treated water and residual As concentration below the regulation limit; however, owing to its low selectivity, its application in treating high salinity water is not recommended.
7. Conclusions
Arsenic concentration in food and drinking water above the maximum permissible concentration limit is a common water pollution problem in both developed and developing countries. The exposure to higher concentrations of arsenic may be life threatening. Water sources for drinking, such as surface water or groundwater, and food sources, such as fish, crops and cereals, can play a notorious role in exposing humans to arsenic. According to the literature, intense arsenic contamination is mainly attributed to mining and erosion activities, affecting mainly groundwater and surface water. By using arsenic-contaminated water for irrigation, the pollution problem can be transferred to the produced vegetation. In this case, most studies are focused on rice, as this crop requires large amounts of water for cultivation and, in addition, it is consumed in large quantities in highly populated areas. Similarly, through water, arsenic can accumulate in fish, mainly from freshwaters, and in other marine species, especially mussels. Taking into account these problems, the continuous monitoring of arsenic levels in water and food sources is mandatory in the future, allowing the application of proper treatment/removal process and the prevention of humans from arsenic intake.
Author Contributions
Conceptualization, K.K.-D. and A.I.Z.; methodology, K.K.-D.; software, K.K.-D.; validation, I.A.K., Y.R. and E.K.; formal analysis, Y.R. and E.K.; investigation, Y.R., E.K. and K.K.-D.; data curation, Y.R., E.K. and K.K.-D.; writing—original draft preparation, I.A.K., Y.R. and E.K.; writing—review and editing, I.A.K. and A.I.Z.; visualization, Y.R., E.K. and K.K.-D.; supervision, K.K.-D. and A.I.Z.; project administration, K.K.-D. and A.I.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Medunić, G.; Fiket, Ž.; Ivanić, M. Arsenic Contamination Status in Europe, Australia, and Other Parts of the World. In Arsenic in Drinking Water and Food; Srivastava, S., Ed.; Springer: Singapore, 2020; Volume 1, pp. 183–233. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Arsenic. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 10 March 2022).
- Shahid, M.; Dumat, C.; Khan Niazi, N.; Khalid, S.; Natasha, N. Global scale arsenic pollution: Increase the scientific knowledge to reduce human exposure. VertigO 2018, 31, 21331. [Google Scholar] [CrossRef]
- Ishiguro, S. Industries using arsenic and arsenic compounds. Appl. Organomet. Chem. 1992, 6, 323–331. [Google Scholar] [CrossRef]
- Mondal, D.; Rahman, M.M.; Suman, S.; Sharma, P.; Siddique, A.B.; Rahman, M.A.; Bari, A.S.M.F.; Kumar, R.; Bose, N.; Singh, S.K.; et al. Arsenic exposure from food exceeds that from drinking water in endemic area of Bihar, India. Sci. Total Environ. 2021, 754, 142082. [Google Scholar] [CrossRef] [PubMed]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Singh, A.; Giri, K. Effect of arsenate substitution on phosphate repository of cell: A computational study. R. Soc. Open Sci. 2018, 5, 181565. [Google Scholar] [CrossRef]
- Sowers, T.D.; Nelson, C.M.; Blackmon, M.D.; Jerden, M.L.; Kirby, A.M.; Diamond, G.L.; Bradham, K.D. Interconnected soil iron and arsenic speciation effects on arsenic bioaccessibility and bioavailability: A scoping review. J. Toxicol. Environ. Health Part B Crit. Rev. 2022, 25, 1–22. [Google Scholar] [CrossRef]
- Almeida, C.C.; Fontes, M.P.F.; Dias, A.C.; Pereira, T.T.C.; Ker, J.C. Adsorption and desorption of arsenic and its immobilization in soils. Sci. Agric. 2020, 78, 1–11. [Google Scholar] [CrossRef]
- Kumarathilaka, P.; Seneweera, S.; Meharg, A.; Bundschuh, J. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors—A review. Water Res. 2018, 140, 403–414. [Google Scholar] [CrossRef]
- Gao, P.; Huang, J.; Wang, Y.; Li, L.; Sun, Y.; Zhang, T.; Peng, F. Effects of nearly four decades of long-term fertilization on the availability, fraction and environmental risk of cadmium and arsenic in red soils. J. Environ. Manag. 2021, 295, 113097. [Google Scholar] [CrossRef]
- Harvey, P.J.; Handley, H.K.; Taylor, M.P. Widespread copper and lead contamination of household drinking water, New South Wales, Australia. Environ. Res. 2016, 151, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Han, C.; Hong, S.B.; Jun, S.J.; Han, Y.; Xiao, C.; Du, Z.; Hur, S.D.; Lee, J.I.; Boutron, C.F.; et al. A 300-Year High-Resolution Greenland Ice Record of Large-Scale Atmospheric Pollution by Arsenic in the Northern Hemisphere. Environ. Sci. Technol. 2019, 53, 12999–13008. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, Y.K.; Tiwari, S.; Mohan, D.; Singh, R.S. A review on health impacts, monitoring and mitigation strategies of arsenic compounds present in air. Clean. Eng. Technol. 2021, 3, 100115. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Taylor, V.; Goodale, B.; Raab, A.; Schwerdtle, T.; Reimer, K.; Conklin, S.; Karagas, M.R.; Francesconi, K.A. Human exposure to organic arsenic species from seafood. Sci. Total Environ. 2017, 580, 266–282. [Google Scholar] [CrossRef]
- Hussain, M.M.; Wang, J.; Bibi, I.; Shahid, M.; Niazi, N.K.; Iqbal, J.; Mian, I.A.; Shaheen, S.M.; Bashir, S.; Shah, N.S.; et al. Arsenic speciation and biotransformation pathways in the aquatic ecosystem: The significance of algae. J. Hazard. Mater. 2021, 403, 124027. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Mitrakas, M.; Zouboulis, A.I. Arsenic occurrence in Europe: Emphasis in Greece and description of the applied full-scale treatment plants. Desalin. Water Treat. 2015, 54, 2100–2107. [Google Scholar] [CrossRef]
- Thakur, J.K.; Thakur, R.K.; Ramanathan, A.L.; Kumar, M.; Singh, S.K. Arsenic contamination of groundwater in Nepal—An overview. Water 2011, 3, 1. [Google Scholar] [CrossRef]
- Ventura-Lima, J.; Bogo, M.R.; Monserrat, J.M. Arsenic toxicity in mammals and aquatic animals: A comparative biochemical approach. Ecotoxicol. Environ. Saf. 2011, 74, 211–218. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Zouboulis, A.I. Use of iron- and manganese-oxidizing bacteria for the combined removal of iron, manganese and arsenic from contaminated groundwater. Water Qual. Res. J. Can. 2006, 41, 117–129. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Arsenic in Drinking Water; Fact Sheet No. 210; WHO: Geneva, Switzerland, 1999. [Google Scholar]
- Appendix E. Regulation of Arsenic: A Brief Survey and Bibliography. In Arsenic; Henke, K., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 545–557. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Arsenic uptake and accumulation mechanisms in rice species. Plants 2020, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization (FAO). Codex Alimentarius Commission—Geneva 14–18 July 2014. Available online: https://www.fao.org/news/story/en/item/%20238558/icode/ (accessed on 14 March 2022).
- Biswas, J.K.; Warke, M.; Datta, R.; Sarkar, D. Is Arsenic in Rice a Major Human Health Concern? Curr. Pollut. Rep. 2020, 6, 37–42. [Google Scholar] [CrossRef]
- Mielcarek, K.; Nowakowski, P.; Puścion-Jakubik, A.; Gromkowska-Kępka, K.J.; Soroczyńska, J.; Markiewicz-Żukowska, R.; Naliwajko, S.K.; Grabia, M.; Bielecka, J.; Żmudzińska, A.; et al. Arsenic, cadmium, lead and mercury content and health risk assessment of consuming freshwater fish with elements of chemometric analysis. Food Chem. 2022, 379, 132167. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Bhattacharya, P. Arsenic in Drinking Water: Is 10 μg/L a Safe Limit? Curr. Pollut. Rep. 2019, 5, 1–3. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Mukherjee, A.; Alauddin, M.; Hassan, M.; Dutta, R.N.; Pati, S.; Mukherjee, S.C.; Roy, S.; Quamruzzman, Q.; et al. Groundwater arsenic contamination in Bangladesh-21 Years of research. J. Trace Elem. Med. Biol. 2015, 31, 237–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, B.; Guo, Z.; Han, J.; Li, H.; Jin, L.; Chen, F.; Xiong, Y. Human health risk assessment of groundwater arsenic contamination in Jinghui irrigation district, China. J. Environ. Manag. 2019, 237, 163–169. [Google Scholar] [CrossRef]
- Ali, W.; Rasool, A.; Junaid, M.; Zhang, H. A comprehensive review on current status, mechanism, and possible sources of arsenic contamination in groundwater: A global perspective with prominence of Pakistan scenario. Environ. Geochem. Health 2019, 41, 737–760. [Google Scholar] [CrossRef]
- Murphy, T.; Phan, K.; Yumvihoze, E.; Irvine, K.; Wilson, K.; Lean, D.; Ty, B.; Poulain, A.; Laird, B.; Chan, L.H.M. Groundwater irrigation and arsenic speciation in rice in Cambodia. J. Health Pollut. 2018, 8, 180911. [Google Scholar] [CrossRef]
- Mayer, J.E.; Goldman, R.H. Arsenic and skin cancer in the USA: The current evidence regarding arsenic-contaminated drinking water. Int. J. Dermatol. 2016, 55, 585–591. [Google Scholar] [CrossRef]
- Budianta, W. The use of natural zeolites from Gunungkidul, Indonesia for preventing arsenic pollution of soils and plants. IOP Conf. Ser. Earth Environ. Sci. 2021, 686, 012021. [Google Scholar] [CrossRef]
- Miller, C.B.; Parsons, M.B.; Jamieson, H.E.; Swindles, G.T.; Nasser, N.A.; Galloway, J.M. Lake-specific controls on the long-term stability of mining-related, legacy arsenic contamination and geochemical baselines in a changing northern environment, Tundra Mine, Northwest Territories, Canada. Appl. Geochem. 2019, 109, 104403. [Google Scholar] [CrossRef]
- Leonardi, G.; Vahter, M.; Clemens, F.; Goessler, W.; Gurzau, E.; Hemminki, K.; Hough, R.; Koppova, K.; Kumar, R.; Rudnai, P.; et al. Inorganic arsenic and basal cell carcinoma in areas of Hungary, Romania, and Slovakia: A case-control study. Environ. Health Perspect. 2012, 120, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Huerta, E.A.; de la Garza Varela, A.; Gómez-Bernal, J.M.; Castillo, F.; Avalos-Borja, M.; SenGupta, B.; Martínez-Villegas, N. Arsenic contamination in irrigation water, agricultural soil and maize crop from an abandoned smelter site in Matehuala, Mexico. J. Hazard. Mater. 2017, 339, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Smith, L.S.; Shrestha, S.; Maden, N. Efficacy of arsenic filtration by Kanchan Arsenic Filter in Nepal. J. Water Health 2014, 12, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Sahu, S. A decade of investigations on groundwater arsenic contamination in Middle Ganga Plain, India. Environ. Geochem. Health 2016, 38, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Rodriǵuez-Lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater arsenic contamination throughout China. Science 2013, 341, 866–868. [Google Scholar] [CrossRef]
- Sanjrani, M.A.; Zhou, B.; Zhao, H.; Bhutto, S.A.; Muneer, A.S.; Xia, S.B. Arsenic contaminated groundwater in China and its treatment options, a review. Appl. Ecol. Environ. Res. 2019, 17, 1655–1683. [Google Scholar] [CrossRef]
- Luo, C.; Routh, J.; Luo, D.; Wei, L.; Liu, Y. Arsenic in the Pearl River Delta and its related waterbody, South China: Occurrence and sources, a review. Geosci. Lett. 2021, 8, 12. [Google Scholar] [CrossRef]
- Li, G.; Sun, G.X.; Williams, P.N.; Nunes, L.; Zhu, Y.G. Inorganic arsenic in Chinese food and its cancer risk. Environ. Int. 2011, 37, 1219–1225. [Google Scholar] [CrossRef]
- Garcia Barcia, L.; Argiro, J.; Babcock, E.A.; Cai, Y.; Shea, S.K.H.; Chapman, D.D. Mercury and arsenic in processed fins from nine of the most traded shark species in the Hong Kong and China dried seafood markets: The potential health risks of shark fin soup. Mar. Pollut. Bull. 2020, 157, 111281. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.T.P.; Prinpreecha, N.; Kim, K.W. Influence of mining activities on arsenic concentration in rice in asia: A review. Minerals 2021, 11, 472. [Google Scholar] [CrossRef]
- Adeloju, S.B.; Khan, S.; Patti, A.F. Arsenic contamination of groundwater and its implications for drinking water quality and human health in under- developed countries and remote communities—A review. Appl. Sci. 2021, 11, 1926. [Google Scholar] [CrossRef]
- Haque, M.M.; Niloy, N.M.; Khirul, M.A.; Alam, M.F.; Tareq, S.M. Appraisal of probabilistic human health risks of heavy metals in vegetables from industrial, non-industrial and arsenic contaminated areas of Bangladesh. Heliyon 2021, 7, 06309. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alauddin, M.; Alauddin, S.T.; Siddique, A.B.; Islam, M.R.; Agosta, G.; Mondal, D.; Naidu, R. Bioaccessibility and speciation of arsenic in children’s diets and health risk assessment of an endemic area in Bangladesh. J. Hazard. Mater. 2021, 403, 124064. [Google Scholar] [CrossRef]
- Tariq, M.A.U.R.; Wangchuk, K.; Muttil, N. A critical review of water resources and their management in Bhutan. Hydrology 2021, 8, 31. [Google Scholar] [CrossRef]
- Ayers, J.C.; Goodbred, S.; Dietrich, M. Arsenic Contamination in South and Southeast Asia. In Environmental Science; Wohl, E., Ed.; Oxford University Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Kumar, A.; Ali, M.; Kumar, R.; Kumar, M.; Sagar, P.; Pandey, R.K.; Akhouri, V.; Kumar, V.; Anand, G.; Niraj, P.K.; et al. Arsenic exposure in Indo Gangetic plains of Bihar causing increased cancer risk. Sci. Rep. 2021, 11, 2376. [Google Scholar] [CrossRef]
- Das, A.; Mondal, S. Geomorphic controls on shallow groundwater arsenic contamination in Bengal basin, India. Environ. Sci. Pollut. Res. 2021, 28, 42177–42195. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sarkar, S.; Chakraborty, M.; Duttagupta, S.; Bhattacharya, A.; Saha, D.; Bhattacharya, P.; Mitra, A.; Gupta, S. Occurrence, predictors and hazards of elevated groundwater arsenic across India through field observations and regional-scale AI-based modeling. Sci. Total Environ. 2020, 759, 143511. [Google Scholar] [CrossRef]
- Rasool, A.; Xiao, T.; Farooqi, A.; Shafeeque, M.; Liu, Y.; Kamran, M.A.; Katsoyiannis, I.A.; Eqani, S.A.M.A.S. Quality of tube well water intended for irrigation and human consumption with special emphasis on arsenic contamination at the area of Punjab, Pakistan. Environ. Geochem. Health 2017, 39, 847–863. [Google Scholar] [CrossRef]
- Alamdar, A.; Eqani, S.A.M.A.S.; Hanif, N.; Ali, S.M.; Fasola, M.; Bokhari, H.; Katsoyiannis, I.A.; Shen, H. Human exposure to trace metals and arsenic via consumption of fish from river Chenab, Pakistan and associated health risks. Chemosphere 2017, 168, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Sanjrani, M.; Mek, T.; Sanjrani, N.; Leghari, S.; Moryani, H.; Shabnam, A. Current Situation of Aqueous Arsenic Contamination in Pakistan, Focused on Sindh and Punjab Province, Pakistan: A Review. J. Pollut. Eff. Control 2017, 5, 2. [Google Scholar] [CrossRef]
- Timalsina, H.; Mainali, B.; Angove, M.J.; Komai, T.; Paudel, S.R. Potential modification of groundwater arsenic removal filter commonly used in Nepal: A review. Groundw. Sustain. Dev. 2021, 12, 100549. [Google Scholar] [CrossRef]
- Jinadasa, B.K.K.K.; Fowler, S.W. A critical review of arsenic contamination in Sri Lankan Foods. J. Food Qual. Hazards Control 2019, 6, 134–145. [Google Scholar] [CrossRef]
- Ramanathan, A.; Johnston, S.; Mukherjee, A.; Nath, B. Safe and Sustainable Use of Arsenic-Contaminated Aquifers in the Gangetic Plain, 1st ed.; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- Jawadi, H.A.; Malistani, H.A.; Moheghy, M.A.; Sagin, J. Essential trace elements and arsenic in thermal springs, Afghanistan. Water 2021, 13, 134. [Google Scholar] [CrossRef]
- Chu, H.T.T.; Vu, T.V.; Nguyen, T.K.B.; Nguyen, H.T.H. Accumulation of arsenic and heavy metals in native and cultivated plant species in a lead recycling area in Vietnam. Minerals 2019, 9, 132. [Google Scholar] [CrossRef]
- Suryono, C.A. The Toxic Metal Arsenic Contamination of the Coastal Aquifers in the North Coast of Java, Indonesia. J. Kelaut. Trop. 2016, 18, 76–81. [Google Scholar] [CrossRef][Green Version]
- Solis, K.L.B.; Macasieb, R.Q.; Parangat, R.C.; Resurreccion, A.C.; Ocon, J.D. Spatiotemporal variation of groundwater arsenic in Pampanga, Philippines. Water 2020, 12, 2366. [Google Scholar] [CrossRef]
- Jones, H.; Visoottiviseth, P.; Bux, M.K.; Földényi, R.; Kováts, N.; Borbély, G.; Galbács, Z. Case reports: Arsenic pollution in Thailand, Bangladesh, and Hungary. Rev. Environ. Contam. Toxicol. 2008, 197, 163–187. [Google Scholar] [CrossRef]
- Pradit, S.; Noppradit, P.; Goh, B.P.; Sornplang, K.; Ong, M.C.; Towatana, P. Occurrence of microplastics and trace metals in fish and shrimp from Songkhla lake, Thailand during the COVID-19 pandemic. Appl. Ecol. Environ. Res. 2021, 19, 1085–1106. [Google Scholar] [CrossRef]
- Ab Razak, N.H.; Praveena, S.M.; Aris, A.Z.; Hashim, Z. Drinking water studies: A review on heavy metal, application of biomarker and health risk assessment (a special focus in Malaysia). J. Epidemiol. Glob. Health 2015, 5, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.F.; Lim, C.K.; Mokhtar, M.B.; Khirotdin, R.P.K. Predicting arsenic (As) exposure on human health for better management of drinking water sources. Int. J. Environ. Res. Public Health 2021, 18, 7997. [Google Scholar] [CrossRef] [PubMed]
- Alina, M.; Azrina, A.; Mohd Yunus, A.S.; Mohd Zakiuddin, S.; Mohd Izuan Effendi, H.; Muhammad Rizal, R. Heavy metals (mercury, arsenic, cadmium, plumbum) in selected marine fish and shellfish along the Straits of Malacca. Int. Food Res. J. 2012, 19, 135–140. [Google Scholar]
- Zulkafflee, N.S.; Mohd Redzuan, N.A.; Nematbakhsh, S.; Selamat, J.; Ismail, M.R.; Praveena, S.M.; Yee Lee, S.; Abdull Razis, A.F. Heavy Metal Contamination in Oryza sativa L. at the Eastern Region of Malaysia and Its Risk Assessment. Int. J. Environ. Res. Public Health 2022, 19, 739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Cha, J.; Raza, M. Groundwater development, use, and its quality in Korea: Tasks for sustainable use. Water Policy 2021, 23, 1375. [Google Scholar] [CrossRef]
- Even, E.; Masuda, H.; Shibata, T.; Nojima, A.; Sakamoto, Y.; Murasaki, Y.; Chiba, H. Geochemical distribution and fate of arsenic in water and sediments of rivers from the Hokusetsu area, Japan. J. Hydrol. Reg. Stud. 2017, 9, 34–47. [Google Scholar] [CrossRef]
- Thuyet, D.Q.; Saito, H.; Saito, T.; Moritani, S.; Kohgo, Y.; Komatsu, T. Multivariate analysis of trace elements in shallow groundwater in Fuchu in western Tokyo Metropolis, Japan. Environ. Earth Sci. 2016, 75, 559. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Khan, M.H.; Haque, M. Arsenic contamination in groundwater in bangladesh: Implications and challenges for healthcare policy. Risk Manag. Healthc. Policy 2018, 11, 251–261. [Google Scholar] [CrossRef]
- Dhillon, A.K. Arsenic Contamination of India’s Groundwater: A Review and Critical Analysis. In Arsenic Water Resources Contamination. Advances in Water Security, 1st ed.; Fares, A., Singh, S., Eds.; Springer: Cham, Switzerland, 2020; Volume 8, pp. 177–205. [Google Scholar] [CrossRef]
- Ayala Herath, H.M.S.; Kawakami, T.; Nagasawa, S.; Serikawa, Y.; Motoyama, A.; Tushara Chaminda, G.G.; Weragoda, S.K.; Yatigammana, S.K.; Amarasooriya, A.A.G.D. Arsenic, cadmium, lead, and chromium in well water, rice, and human urine in Sri Lanka in relation to chronic kidney disease of unknown etiology. J. Water Health 2018, 16, 212–222. [Google Scholar] [CrossRef]
- Le Luu, T. Remarks on the current quality of groundwater in Vietnam. Environ. Sci. Pollut. Res. 2019, 26, 1163–1169. [Google Scholar] [CrossRef]
- Irnawati, I.; Idroes, R.; Zulfiani, U.; Akmal, M.; Suhartono, E.; Idroes, G.M.; Muslem, M.; Lala, A.; Yusuf, M.; Saiful, S.; et al. Assessment of arsenic levels in water, sediment, and human hair around Ie Seu’um geothermal manifestation area, Aceh, Indonesia. Water 2021, 13, 2343. [Google Scholar] [CrossRef]
- Ratha, P.; Nandalal, K.D.W.; Pitawala, H.M.T.G.A.; Dharmagunawardhane, H.A.; Weerakoon, S.B. Arsenic Contamination in Cambodia: A Status Review. In Proceedings of the 2nd International Symposium on Conservation and Management of Tropical Lakes, Siem Reap, Cambodia, 24–26 August 2017. [Google Scholar]
- Soulivongsa, L.; Tengjaroenkul, B.; Neeratanaphan, L. Effects of contamination by heavy metals and metalloids on chromosomes, serum biochemistry and histopathology of the bonylip barb fish near sepon gold-copper mine, lao pdr. Int. J. Environ. Res. Public Health 2020, 17, 9492. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Tanibuchi, E.; Sugimoto, T.; Kawaguchi, T.; Sakakibara, N.; Yamashita, M. Determination of inorganic arsenic in seaweed and seafood by LC-ICP-MS: Method validation. J. AOAC Int. 2019, 102, 612–618. [Google Scholar] [CrossRef]
- Bentley, K.; Soebandrio, A. Dietary exposure assessment for arsenic and mercury following submarine tailings placement in Ratatotok Sub-district, North Sulawesi, Indonesia. Environ. Pollut. 2017, 227, 552–559. [Google Scholar] [CrossRef]
- Jansen, S.; Dera, R.T.S.; Margarethruth, M.S.; Nanda, S.I.; Silalahi Yosy, C.E. Analysis of arsenic in raw and cooked rice by atomic absorption spectrophotometer. IOP Conf. Ser. Earth Environ. Sci. 2018, 205, 012040. [Google Scholar] [CrossRef]
- Jalova, M.C.; Lomantong, A.D.; Calibo, L.G.; Camarin, M.A.M. Assessment of heavy metals found in commonly consumed fishes from lake Lanao, Philippines. Isr. J. Aquac. Bamidgeh 2021, 75, 1426167. [Google Scholar] [CrossRef]
- Hensawang, S.; Chanpiwat, P. Health impact assessment of arsenic and cadmium intake via rice consumption in Bangkok, Thailand. Environ. Monit. Assess. 2017, 189, 599. [Google Scholar] [CrossRef]
- Phan, K.; Sthiannopkao, S.; Heng, S.; Phan, S.; Huoy, L.; Wong, M.H.; Kim, K.W. Arsenic contamination in the food chain and its risk assessment of populations residing in the Mekong River basin of Cambodia. J. Hazard. Mater. 2013, 262, 1064–1071. [Google Scholar] [CrossRef]
- Mukhametov, A.; Yerbulekova, M.; Dautkanova, D.; Tuyakova, G.; Aitkhozhayeva, G. Heavy Metal Contents in Vegetable Oils of Kazakhstan Origin and Life Risk Assessment. Int. J. Agric. Biol. 2020, 14, 163–167. [Google Scholar]
- World Health Organization (WHO); UNICEF. Rapid Assessment of Drinking-Water Quality in the Republic of Tajikistan; World Health Organization, WHO Press: Geneva, Switzerland, 2010; Available online: https://washdata.org/report/who-unicef-radwq-tajikistan-report (accessed on 21 March 2022).
- Liu, W.; Ma, L.; Li, Y.; Abuduwaili, J.; Uulu, S.A. Heavy metals and related human health risk assessment for river waters in the Issyk−Kul basin, Kyrgyzstan, central asia. Int. J. Environ. Res. Public Health 2020, 17, 3506. [Google Scholar] [CrossRef]
- Ali, I.; Hasan, M.A.; Alharbi, O.M.L. Toxic metal ions contamination in the groundwater, Kingdom of Saudi Arabia. J. Taibah Univ. Sci. 2020, 14, 1571–1579. [Google Scholar] [CrossRef]
- Fakhri, Y.; Mohseni-Bandpei, A.; Oliveri Conti, G.; Ferrante, M.; Cristaldi, A.; Jeihooni, A.K.; Karimi Dehkordi, M.; Alinejad, A.; Rasoulzadeh, H.; Mohseni, S.M.; et al. Systematic review and health risk assessment of arsenic and lead in the fished shrimps from the Persian Gulf. Food Chem. Toxicol. 2018, 113, 278–286. [Google Scholar] [CrossRef]
- Rudnai, T.; Sándor, J.; Kádár, M.; Borsányi, M.; Béres, J.; Métneki, J.; Maráczi, G.; Rudnai, P. Arsenic in drinking water and congenital heart anomalies in Hungary. Int. J. Hyg. Environ. Health 2014, 217, 813–818. [Google Scholar] [CrossRef]
- Menon, M.; Sarkar, B.; Hufton, J.; Reynolds, C.; Reina, S.V.; Young, S. Do arsenic levels in rice pose a health risk to the UK population? Ecotoxicol. Environ. Saf. 2020, 197, 110601. [Google Scholar] [CrossRef] [PubMed]
- Hackethal, C.; Kopp, J.F.; Sarvan, I.; Schwerdtle, T.; Lindtner, O. Total arsenic and water-soluble arsenic species in foods of the first German total diet study (BfR MEAL Study). Food Chem. 2021, 346, 128913. [Google Scholar] [CrossRef] [PubMed]
- Rowland, H.A.L.; Omoregie, E.O.; Millot, R.; Jimenez, C.; Mertens, J.; Baciu, C.; Hug, S.J.; Berg, M. Geochemistry and arsenic behaviour in groundwater resources of the Pannonian Basin (Hungary and Romania). Appl. Geochem. 2011, 26, 1–17. [Google Scholar] [CrossRef]
- Jovanovic, D.; Jakovljević, B.; Rašić-Milutinović, Z.; Paunović, K.; Peković, G.; Knezević, T. Arsenic occurrence in drinking water supply systems in ten municipalities in Vojvodina Region, Serbia. Environ. Res. 2011, 111, 315–318. [Google Scholar] [CrossRef]
- Neamtiu, I.; Bloom, M.S.; Gati, G.; Goessler, W.; Surdu, S.; Pop, C.; Lupsa, I.R. Pregnant women in Timis County, Romania are exposed primarily to low-level (<10 μg/L) arsenic through residential drinking water consumption. Int. J. Hyg. Environ. Health 2015, 218, 371–379. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.; Cadar, O.; Senila, L.R.; Roman, M.; Puskas, F.; Sima, M. Assessment of Availability and Human Health Risk Posed by Arsenic Contaminated Well Waters from Timis-Bega Area, Romania. J. Anal. Methods Chem. 2017, 2017, 3037651. [Google Scholar] [CrossRef]
- McGrory, E.R.; Brown, C.; Bargary, N.; Williams, N.H.; Mannix, A.; Zhang, C.; Henry, T.; Daly, E.; Nicholas, S.; Petrunic, B.M.; et al. Arsenic contamination of drinking water in Ireland: A spatial analysis of occurrence and potential risk. Sci. Total Environ. 2017, 579, 1863–1875. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Hug, S.J.; Ammann, A.; Zikoudi, A.; Hatziliontos, C. Arsenic speciation and uranium concentrations in drinking water supply wells in Northern Greece: Correlations with redox indicative parameters and implications for groundwater treatment. Sci. Total Environ. 2007, 383, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Ujević Bošnjak, M.; Capak, K.; Jazbec, A.; Casiot, C.; Sipos, L.; Poljak, V.; Dadić, Ž. Hydrochemical characterization of arsenic contaminated alluvial aquifers in Eastern Croatia using multivariate statistical techniques and arsenic risk assessment. Sci. Total Environ. 2012, 420, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ćavar, S.; Klapec, T.; Grubešić, R.J.; Valek, M. High exposure to arsenic from drinking water at several localities in eastern Croatia. Sci. Total Environ. 2005, 339, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Carraro, A.; Fabbri, P.; Giaretta, A.; Peruzzo, L.; Tateo, F.; Tellini, F. Arsenic anomalies in shallow Venetian Plain (Northeast Italy) groundwater. Environ. Earth Sci. 2013, 70, 3067–3084. [Google Scholar] [CrossRef]
- Zuzolo, D.; Cicchella, D.; Demetriades, A.; Birke, M.; Albanese, S.; Dinelli, E.; Lima, A.; Valera, P.; De Vivo, B. Arsenic: Geochemical distribution and age-related health risk in Italy. Environ. Res. 2020, 182, 109076. [Google Scholar] [CrossRef] [PubMed]
- Şener, Ş.; Karakuş, M. Investigating water quality and arsenic contamination in drinking water resources in the Tavşanlı District (Kütahya, Western Turkey). Environ. Earth Sci. 2017, 76, 750. [Google Scholar] [CrossRef]
- Baba, A.; Uzelli, T.; Sozbilir, H. Distribution of geothermal arsenic in relation to geothermal play types: A global review and case study from the Anatolian plate (Turkey). J. Hazard. Mater. 2021, 414, 125510. [Google Scholar] [CrossRef]
- Drahota, P.; Rohovec, J.; Filippi, M.; Mihaljevič, M.; Rychlovský, P.; Červený, V.; Pertold, Z. Mineralogical and geochemical controls of arsenic speciation and mobility under different redox conditions in soil, sediment and water at the Mokrsko-West gold deposit, Czech Republic. Sci. Total Environ. 2009, 407, 3372–3384. [Google Scholar] [CrossRef]
- Monrad, M.; Ersbøll, A.K.; Sørensen, M.; Baastrup, R.; Hansen, B.; Gammelmark, A.; Tjønneland, A.; Overvad, K.; Raaschou-Nielsen, O. Low-level arsenic in drinking water and risk of incident myocardial infarction: A cohort study. Environ. Res. 2017, 154, 318–324. [Google Scholar] [CrossRef]
- Pedretti, D.; Luoma, S.; Ruskeeniemi, T.; Backman, B. A geologically-based approach to map arsenic risk in crystalline aquifers: Analysis of the Tampere region, Finland. Geosci. Front. 2019, 10, 1731–1741. [Google Scholar] [CrossRef]
- Medrano, M.J.; Boix, R.; Pastor-Barriuso, R.; Palau, M.; Damián, J.; Ramis, R.; del Barrio, J.L.; Navas-Acien, A. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ. Res. 2010, 110, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Al Rmalli, S.W.; Haris, P.I.; Harrington, C.F.; Ayub, M. A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh. Sci. Total Environ. 2005, 337, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Cubadda, F.; D’Amato, M.; Aureli, F.; Raggi, A.; Mantovani, A. Dietary exposure of the Italian population to inorganic arsenic: The 2012–2014 Total Diet Study. Food Chem. Toxicol. 2016, 98, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Golfinopoulos, S.K.; Varnavas, S.P.; Alexakis, D.E. The status of arsenic pollution in the Greek and Cyprus environment: An overview. Water 2021, 13, 224. [Google Scholar] [CrossRef]
- Kalantzi, I.; Mylona, K.; Sofoulaki, K.; Tsapakis, M.; Pergantis, S.A. Arsenic speciation in fish from Greek coastal areas. J. Environ. Sci. 2017, 56, 300–312. [Google Scholar] [CrossRef]
- Chekri, R.; Le Calvez, E.; Zinck, J.; Leblanc, J.C.; Sirot, V.; Hulin, M.; Noël, L.; Guérin, T. Trace element contents in foods from the first French total diet study on infants and toddlers. J. Food Compos. Anal. 2019, 78, 108–120. [Google Scholar] [CrossRef]
- Marín, S.; Pardo, O.; Sánchez, A.; Sanchis, Y.; Vélez, D.; Devesa, V.; Font, G.; Yusà, V. Assessment of metal levels in foodstuffs from the Region of Valencia (Spain). Toxicol. Rep. 2018, 5, 654–670. [Google Scholar] [CrossRef]
- Özden, Ö.; Erkan, N. Evaluation of Risk Characterization for Mercury, Cadmium, Lead and Arsenic Associated with Seafood Consumption in Turkey. Expo. Health 2016, 8, 43–52. [Google Scholar] [CrossRef]
- Ruttens, A.; Blanpain, A.C.; De Temmerman, L.; Waegeneers, N. Arsenic speciation in food in Belgium. Part 1: Fish, molluscs and crustaceans. J. Geochem. Explor. 2012, 121, 55–61. [Google Scholar] [CrossRef]
- Ruttens, A.; Cheyns, K.; Blanpain, A.C.; De Temmerman, L.; Waegeneers, N. Arsenic speciation in food in Belgium. Part 2: Cereals and cereal products. Food Chem. Toxicol. 2018, 118, 32–41. [Google Scholar] [CrossRef]
- Kollander, B.; Sand, S.; Almerud, P.; Ankarberg, E.H.; Concha, G.; Barregård, L.; Darnerud, P.O. Inorganic arsenic in food products on the Swedish market and a risk-based intake assessment. Sci. Total Environ. 2019, 672, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Morales-Simfors, N.; Bundschuh, J.; Herath, I.; Inguaggiato, C.; Caselli, A.T.; Tapia, J.; Choquehuayta, F.E.A.; Armienta, M.A.; Ormachea, M.; Joseph, E.; et al. Arsenic in Latin America: A critical overview on the geochemistry of arsenic originating from geothermal features and volcanic emissions for solving its environmental consequences. Sci. Total Environ. 2020, 716, 135564. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, C.F.; Hamula, C.L.A.; Huang, S.; Gabos, S.; Le, X.C. A review on arsenic concentrations in Canadian drinking water. Environ. Rev. 2010, 18, 291–307. [Google Scholar] [CrossRef]
- Saint-Jacques, N.; Brown, P.; Nauta, L.; Boxall, J.; Parker, L.; Dummer, T.J.B. Estimating the risk of bladder and kidney cancer from exposure to low-levels of arsenic in drinking water, Nova Scotia, Canada. Environ. Int. 2018, 110, 95–104. [Google Scholar] [CrossRef]
- Nigra, A.E.; Chen, Q.; Chillrud, S.N.; Wang, L.; Harvey, D.; Mailloux, B.; Factor-Litvak, P.; Navas-Acien, A. Inequalities in public water arsenic concentrations in counties and community water systems across the United States, 2006–2011. Environ. Health Perspect. 2020, 128, 127001. [Google Scholar] [CrossRef]
- Alarcón-Herrera, M.T.; Martin-Alarcon, D.A.; Gutiérrez, M.; Reynoso-Cuevas, L.; Martín-Domínguez, A.; Olmos-Márquez, M.A.; Bundschuh, J. Co-occurrence, possible origin, and health-risk assessment of arsenic and fluoride in drinking water sources in Mexico: Geographical data visualization. Sci. Total Environ. 2020, 698, 134168. [Google Scholar] [CrossRef]
- Ortiz Letechipia, J.; González-Trinidad, J.; Júnez-Ferreira, H.E.; Bautista-Capetillo, C.; Robles-Rovelo, C.O.; Contreras Rodríguez, A.R.; Dávila-Hernández, S. Aqueous Arsenic Speciation with Hydrogeochemical Modeling and Correlation with Fluorine in Groundwater in a Semiarid Region of Mexico. Water 2022, 14, 519. [Google Scholar] [CrossRef]
- Marcillo, C.E.; Prado, G.G.; Copeland, N.; Krometis, L.H. Drinking water quality and consumer perceptions at the point-of-use in san rafael las flores, guatemala. Water Pract. Technol. 2020, 15, 374–385. [Google Scholar] [CrossRef]
- Pérez Sabino, J.F.; Valladares, B.; Hernández, E.; Oliva, B.; Del Cid, M.; Jayes Reyes, P. Determinación de arsénico y mercurio en agua superficial del lago de Atitlán. Cienc. Tecnol. Y Salud 2015, 2, 127–134. [Google Scholar] [CrossRef]
- Bundschuh, J.; Armienta, M.A.; Morales-Simfors, N.; Alam, M.A.; López, D.L.; Delgado Quezada, V.; Dietrich, S.; Schneider, J.; Tapia, J.; Sracek, O.; et al. Arsenic in Latin America: New findings on source, mobilization and mobility in human environments in 20 countries based on decadal research 2010–2020. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1727–1865. [Google Scholar] [CrossRef]
- Bundschuh, J.; Litter, M.I.; Parvez, F.; Román-Ross, G.; Nicolli, H.B.; Jean, J.S.; Liu, C.W.; López, D.; Armienta, M.A.; Guilherme, L.R.G.; et al. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Sci. Total Environ. 2012, 429, 2–35. [Google Scholar] [CrossRef] [PubMed]
- Delgado Quezada, V.; Altamirano Espinoza, M.; Bundschuh, J. Arsenic in geoenvironments of Nicaragua: Exposure, health effects, mitigation and future needs. Sci. Total Environ. 2020, 716, 136527. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.V.; Pineda, A.S.; Pérez, J.V.; Zachrisson, I.R. Heavy Metals in Water, Soils and Sediments of La Villa River Basin- Panama. Int. J. Plant Soil Sci. 2021, 33, 1–12. [Google Scholar] [CrossRef]
- Alonso, D.L.; Latorre, S.; Castillo, E.; Brandão, P.F.B. Environmental occurrence of arsenic in Colombia: A review. Environ. Pollut. 2014, 186, 272–281. [Google Scholar] [CrossRef]
- George, C.M.; Sima, L.; Arias, M.H.J.; Mihalic, J.; Cabrera, L.Z.; Danz, D.; Checkley, W.; Gilman, R.H. Arsenic exposure in drinking water: An unrecognized health threat in Peru. Bull. World Health Organ. 2014, 92, 565–572. [Google Scholar] [CrossRef]
- Bolisetty, S.; Rahimi, A.; Mezzenga, R. Arsenic removal from Peruvian drinking water using milk protein nanofibril-carbon filters: A field study. Environ. Sci. Water Res. Technol. 2021, 7, 2223–2230. [Google Scholar] [CrossRef]
- Tapia, J.; Rodríguez, M.P.; Castillo, P.; Guerrero, N.; Rodríguez, C.; Valdés, A.; Townley, B.; Fuentes, G. Arsenic and Copper in Chile and the Development of Environmental Standards. In Chile Environmental History, Perspectives and Challenges; Alaniz, A.J., Ed.; Nova Science Publishers: New York, NY, USA, 2019; Volume 7, pp. 241–286. ISBN 9781536156669. [Google Scholar]
- Diaz, O.P.; Arcos, R.; Tapia, Y.; Pastene, R.; Velez, D.; Devesa, V.; Montoro, R.; Aguilera, V.; Becerra, M. Estimation of arsenic intake from drinking water and food (Raw and cooked) in a rural village of Northern Chile. urine as a biomarker of recent exposure. Int. J. Environ. Res. Public Health 2015, 12, 5614–5633. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Santos, A.C.; Fernandes, C.S.; Ng, J.C. Arsenic contamination assessment in Brazil—Past, present and future concerns: A historical and critical review. Sci. Total Environ. 2020, 730, 138217. [Google Scholar] [CrossRef]
- Richter, L.; Hernández, A.H.; Pessôa, G.S.; Arruda, M.A.Z.; Rezende-Filho, A.T.; de Almeida, R.B.; Menezes, H.A.; Valles, V.; Barbiero, L.; Fostier, A.H. Dissolved arsenic in the upper Paraguay River basin and Pantanal wetlands. Sci. Total Environ. 2019, 687, 917–928. [Google Scholar] [CrossRef]
- Mañay, N.; Pistón, M.; Cáceres, M.; Pizzorno, P.; Bühl, V. An overview of environmental arsenic issues and exposure risks in Uruguay. Sci. Total Environ. 2019, 686, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Litter, M.I.; Ingallinella, A.M.; Olmos, V.; Savio, M.; Difeo, G.; Botto, L.; Farfán Torres, E.M.; Taylor, S.; Frangie, S.; Herkovits, J.; et al. Arsenic in Argentina: Occurrence, human health, legislation and determination. Sci. Total Environ. 2019, 676, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Tanamal, C.; Blais, J.M.; Yumvihoze, E.; Chan, H.M. Health risk assessment of inorganic arsenic exposure through fish consumption in Yellowknife, Northwest Territories, Canada. Hum. Ecol. Risk Assess. 2021, 27, 1072–1093. [Google Scholar] [CrossRef]
- Guaman, S.T.Z.; Ccahua, W.C.; Rafael, N.C.; Payano, I.U.; Suazo, J.M.A.; Gioda, A.; de la Cruz, A.R.H. Estimation of arsenic contents in rice purchased on Peruvian markets and estimation of dietary intake by Peruvians through rice consumption. Sci. Agropecu. 2021, 12, 185–191. [Google Scholar] [CrossRef]
- García-Rico, L.; Valenzuela-Rodríguez, M.P.; Meza-Montenegro, M.M.; Lopez-Duarte, A.L. Arsenic in rice and rice products in Northwestern Mexico and health risk assessment. Food Addit. Contam. Part B Surveill. 2020, 13, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Nevárez, M.; Moreno, M.V.; Sosa, M.; Bundschuh, J. Arsenic in freshwater fish in the Chihuahua County water reservoirs (Mexico). J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2011, 46, 1283–1287. [Google Scholar] [CrossRef]
- López-Barrera, E.A.; Barragán-Gonzalez, R.G. Metals and metalloid in eight fish species consumed by citizens of Bogota D.C., Colombia, and potential risk to humans. J. Toxicol. Environ. Health Part A Curr. Issues 2016, 79, 232–243. [Google Scholar] [CrossRef]
- Urango-Cárdenas, I.D.; Burgos-Núñez, S.; Ospina Herrera, L.Á.; Enamorado-Montes, G.; Marrugo-Negrete, J.L. Determination of arsenic chemical species in rice grains using high-performance liquid chromatography coupled to hydride generator with atomic fluorescence detector (HPLC-HG-AFS). MethodsX 2021, 8, 101281. [Google Scholar] [CrossRef]
- Atiaga-Franco, O.L.; Otero, X.L.; Gallego-Picó, A.; Escobar-Castañeda, L.A.; Bravo-Yagüe, J.C.; Carrera-Villacrés, D. Analysis of total arsenic content in purchased rice from Ecuador. Czech J. Food Sci. 2019, 37, 425–431. [Google Scholar] [CrossRef]
- Fão, N.; Nascimento, S.; de La Cruz, A.H.; Calderon, D.; Rocha, R.; Saint’Pierre, T.; Gioda, A.; Thiesen, F.V.; Brucker, N.; Emanuelli, T.; et al. Estimation of total arsenic contamination and exposure in Brazilian rice and infant cereals. Drug Chem. Toxicol. 2021, 44, 400–408. [Google Scholar] [CrossRef]
- Oteiza, J.M.; Barril, P.A.; Quintero, C.E.; Savio, M.; Befani, R.; Cirelli, A.F.; Echegaray, N.S.; Murad, C.; Buedo, A. Arsenic in Argentinean polished rice: Situation overview and regulatory framework. Food Control 2020, 109, 106909. [Google Scholar] [CrossRef]
- Avigliano, E.; Maichak de Carvalho, B.; Invernizzi, R.; Olmedo, M.; Jasan, R.; Volpedo, A.V. Arsenic, selenium, and metals in a commercial and vulnerable fish from southwestern Atlantic estuaries: Distribution in water and tissues and public health risk assessment. Environ. Sci. Pollut. Res. 2019, 26, 7994–8006. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, E.; Molyneux, S.; Watson, S.; Miles, G.; Pearson, A. Inorganic arsenic in rice and rice products in New Zealand and Australia. Food Addit. Contam. Part B Surveill. 2019, 12, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.M.; Reichman, S.M.; Lim, R.P.; Naidu, R. Arsenic speciation in australian-grown and imported rice on sale in Australia: Implications for human health risk. J. Agric. Food Chem. 2014, 62, 6016–6024. [Google Scholar] [CrossRef]
- Rahman, M.M.; Shehzad, M.T.; Nayak, A.K.; Sharma, S.; Yeasmin, M.; Samanta, S.; Correll, R.; Naidu, R. Health risks from trace elements in muscles of some commonly available fish in Australia and India. Environ. Sci. Pollut. Res. 2020, 27, 21000–21012. [Google Scholar] [CrossRef]
- Maher, W.; Waring, J.; Krikowa, F.; Duncan, E.; Foster, S. Ecological factors affecting the accumulation and speciation of arsenic in twelve Australian coastal bivalve molluscs. Environ. Chem. 2018, 15, 46–57. [Google Scholar] [CrossRef]
- Irunde, R.; Ijumulana, J.; Ligate, F.; Maity, J.P.; Ahmad, A.; Mtamba, J.; Mtalo, F.; Bhattacharya, P. Arsenic in Africa: Potential sources, spatial variability, and the state of the art for arsenic removal using locally available materials. Groundw. Sustain. Dev. 2022, 18, 100746. [Google Scholar] [CrossRef]
- Koumolou, L.; Edorh, P.; Montcho, S.; Aklikokou, K.; Loko, F.; Boko, M.; Creppy, E.E. Health-risk market garden production linked to heavy metals in irrigation water in Benin. Comptes Rendus Biol. 2013, 336, 278–283. [Google Scholar] [CrossRef]
- Guedenon, P.; Edorh, A.P.; Kaki, C.; Yehouenou, A.P.E.; Gnandi, K.; Montcho, S.; Hounkpatin, A.; Koumolou, L.; Boko, M. Arsenic, cadmium, copper and lead accumulation in water, sediments and fish species of Oueme River in Bonou. Br. J. Pharmacol. Toxicol. 2012, 3, 13–20. [Google Scholar]
- Mladenov, N.; Wolski, P.; Hettiarachchi, G.M.; Murray-Hudson, M.; Enriquez, H.; Damaraju, S.; Galkaduwa, M.B.; McKnight, D.M.; Masamba, W. Abiotic and biotic factors influencing the mobility of arsenic in groundwater of a through-flow island in the Okavango Delta, Botswana. J. Hydrol. 2014, 518, 326–341. [Google Scholar] [CrossRef]
- Nzihou, J.F.; Bouda, M.; Hamidou, S.; Diarra, J. Arsenic in Drinking Water Toxicological Risk Assessment in the North Region of Burkina Faso. J. Water Resour. Prot. 2013, 5, 46–52. [Google Scholar] [CrossRef]
- Bretzler, A.; Lalanne, F.; Nikiema, J.; Podgorski, J.; Pfenninger, N.; Berg, M.; Schirmer, M. Groundwater arsenic contamination in Burkina Faso, West Africa: Predicting and verifying regions at risk. Sci. Total Environ. 2017, 584–585, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, O.; Amyot, M. Mercury, arsenic and selenium concentrations in water and fish from sub-Saharan semi-arid freshwater reservoirs (Burkina Faso). Sci. Total Environ. 2013, 444, 243–254. [Google Scholar] [CrossRef]
- Muimba-Kankolongo, A.; Banza Lubaba Nkulu, C.; Mwitwa, J.; Kampemba, F.M.; Mulele Nabuyanda, M.; Haufroid, V.; Smolders, E.; Nemery, B. Contamination of water and food crops by trace elements in the African Copperbelt: A collaborative cross-border study in Zambia and the Democratic Republic of Congo. Environ. Adv. 2021, 6, 100103. [Google Scholar] [CrossRef]
- Ouattara, A.A.; Yao, K.M.; Soro, M.P.; Diaco, T.; Trokourey, A. Arsenic and Trace Metals in Three West African rivers: Concentrations, Partitioning, and Distribution in Particle-Size Fractions. Arch. Environ. Contam. Toxicol. 2018, 75, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Ahoulé, D.G.; Lalanne, F.; Mendret, J.; Brosillon, S.; Maïga, A.H. Arsenic in African Waters: A Review. Water. Air. Soil Pollut. 2015, 226, 302. [Google Scholar] [CrossRef]
- Bianchini, G.; Brombin, V.; Marchina, C.; Natali, C.; Godebo, T.R.; Rasini, A.; Salani, G.M. Origin of fluoride and arsenic in the main ethiopian rift waters. Minerals 2020, 10, 453. [Google Scholar] [CrossRef]
- Merola, R.B.; Kravchenko, J.; Rango, T.; Vengosh, A. Arsenic exposure of rural populations from the Rift Valley of Ethiopia as monitored by keratin in toenails. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 121–126. [Google Scholar] [CrossRef]
- Lutz, A.; Diarra, S.; Apambire, W.B.; Thomas, J.M.; Ayamsegna, J. Drinking Water from Hand-Pumps in Mali, Niger, and Ghana, West Africa: Review of Health Effects. J. Water Resour. Prot. 2013, 5, 13–20. [Google Scholar] [CrossRef]
- Kiplangat, A.S.; Mwangi, H.; Swaleh, S.; Njue, W.M. Arsenic Contamination in Water from Selected Boreholes in Nairobi City County, Kenya. Eur. J. Adv. Chem. Res. 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Githaiga, K.B.; Njuguna, S.M.; Gituru, R.W.; Yan, X. Water quality assessment, multivariate analysis and human health risks of heavy metals in eight major lakes in Kenya. J. Environ. Manag. 2021, 297, 113410. [Google Scholar] [CrossRef] [PubMed]
- Bokar, H.; Traoré, A.Z.; Mariko, A.; Diallo, T.; Traoré, A.; Sy, A.; Soumaré, O.; Dolo, A.; Bamba, F.; Sacko, M.; et al. Geogenic influence and impact of mining activities on water soil and plants in surrounding areas of Morila Mine, Mali. J. Geochem. Explor. 2020, 209, 106429. [Google Scholar] [CrossRef]
- Lotfi, S.; Chakit, M.; Belghyti, D. Groundwater quality and pollution index for heavy metals in Sais plain, Morocco. J. Health Pollut. 2020, 10, 200603. [Google Scholar] [CrossRef]
- Bouzekri, S.; El Hachimi, M.L.; Kara, K.; El Mahi, M.; Lotfi, E.M. Metal pollution assessment of surface water from the abandoned Pb mine Zaida, high Moulouya-Morocco. Geosyst. Eng. 2020, 23, 226–233. [Google Scholar] [CrossRef]
- Orosun, M.M. Assessment of arsenic and its associated health risks due to mining activities in parts of North-central Nigeria: Probabilistic approach using Monte Carlo. J. Hazard. Mater. 2021, 412, 125262. [Google Scholar] [CrossRef] [PubMed]
- Reksten, A.M.; Victor, A.M.J.C.; Neves, E.B.N.; Christiansen, S.M.; Ahern, M.; Uzomah, A.; Lundebye, A.K.; Kolding, J.; Kjellevold, M. Nutrient and chemical contaminant levels in five marine fish species from Angola-the EAF-nansen programme. Foods 2020, 9, 629. [Google Scholar] [CrossRef] [PubMed]
- Jitaru, P.; Ingenbleek, L.; Marchond, N.; Laurent, C.; Adegboye, A.; Hossou, S.E.; Koné, A.Z.; Oyedele, A.D.; Kisito, C.S.K.J.; Dembélé, Y.K.; et al. Occurrence of 30 trace elements in foods from a multi-centre Sub-Saharan Africa Total Diet Study: Focus on Al, As, Cd, Hg, and Pb. Environ. Int. 2019, 133, 105197. [Google Scholar] [CrossRef]
- Bati, K.; Mogobe, O.; Masamba, W.R.L. Concentrations of Some Trace Elements in Vegetables Sold at Maun Market, Botswana. J. Food Res. 2016, 6, 69–77. [Google Scholar] [CrossRef]
- Bakary, T.; Flibert, G.; Bernadette, S.P.; Oumarou, Z.; François, T.; Cheikna, Z.; Maxime, D.K.; Yves, T.; Aly, S. Evaluation of heavy metals and pesticides contents in market-gardening products sold in some principal markets of Ouagadougou (Burkina Faso). J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1026–1034. [Google Scholar] [CrossRef]
- Squadrone, S.; Burioli, E.; Monaco, G.; Koya, M.K.; Prearo, M.; Gennero, S.; Dominici, A.; Abete, M.C. Human exposure to metals due to consumption of fish from an artificial lake basin close to an active mining area in Katanga (D.R. Congo). Sci. Total Environ. 2016, 568, 679–684. [Google Scholar] [CrossRef]
- Ouattara, A.A.; Yao, K.M.; Kinimo, K.C.; Trokourey, A. Assessment and bioaccumulation of arsenic and trace metals in two commercial fish species collected from three rivers of Côte d’Ivoire and health risks. Microchem. J. 2020, 154, 104604. [Google Scholar] [CrossRef]
- Kinimo, K.C.; Yao, K.M.; Marcotte, S.; Kouassi, N.L.B.; Trokourey, A. Trace metal(loid)s contamination in paddy rice (Oryza sativa L.) from wetlands near two gold mines in Côte d’Ivoire and health risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 22779–22788. [Google Scholar] [CrossRef] [PubMed]
- Sallam, K.I.; Abd-Elghany, S.M.; Mohammed, M.A. Heavy Metal Residues in Some Fishes from Manzala Lake, Egypt, and Their Health-Risk Assessment. J. Food Sci. 2019, 84, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Dsikowitzky, L.; Mengesha, M.; Dadebo, E.; De Carvalho, C.E.V.; Sindern, S. Assessment of heavy metals in water samples and tissues of edible fish species from Awassa and Koka Rift Valley Lakes, Ethiopia. Environ. Monit. Assess. 2013, 185, 3117–3131. [Google Scholar] [CrossRef]
- Gbogbo, F.; Otoo, S.D.; Asomaning, O.; Huago, R.Q. Contamination status of arsenic in fish and shellfish from three river basins in Ghana. Environ. Monit. Assess. 2017, 189, 400. [Google Scholar] [CrossRef]
- Makokha, A.O. Arsenic Levels in the Environment and Foods Around Kisumu, Kenya. Open Environ. Eng. J. 2012, 5, 119–124. [Google Scholar] [CrossRef]
- Ngure, V.; Lelo, F.; Obwanga, B. Heavy Metal Pollution from Migori Gold Mining Area, Kenya: Health Implications for Consumers of Fish and Water. J. Nat. Sci. Res. 2017, 7, 46–53. [Google Scholar]
- Bakkali, K.; Martos, N.R.; Souhail, B.; Ballesteros, E. Determination of Heavy Metal Content in Vegetables and Oils From Spain and Morocco by Inductively Coupled Plasma Mass Spectrometry. Anal. Lett. 2012, 45, 907–919. [Google Scholar] [CrossRef]
- Usese, A.; Chukwu, O.L.; Rahman, M.M.; Naidu, R.; Islam, S.; Oyewo, E.O. Concentrations of arsenic in water and fish in a tropical open lagoon, Southwest-Nigeria: Health risk assessment. Environ. Technol. Innov. 2017, 8, 164–171. [Google Scholar] [CrossRef]
- Firth, D.C.; Salie, K.; O’Neill, B.; Hoffman, L.C. Monitoring of trace metal accumulation in two South African farmed mussel species, Mytilus galloprovincialis and Choromytilus meridionalis. Mar. Pollut. Bull. 2019, 141, 529–534. [Google Scholar] [CrossRef]
- Nel, L.; Strydom, N.A.; Bouwman, H. Preliminary assessment of contaminants in the sediment and organisms of the Swartkops Estuary, South Africa. Mar. Pollut. Bull. 2015, 101, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Mataba, G.R.; Verhaert, V.; Blust, R.; Bervoets, L. Distribution of trace elements in the aquatic ecosystem of the Thigithe river and the fish Labeo victorianus in Tanzania and possible risks for human consumption. Sci. Total Environ. 2016, 547, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Meck, M.L.; Mudimbu, D.; Davies, T.C. Accumulation of potentially harmful elements in edible parts of vegetables grown on two different geological substrates in Zimbabwe. J. Geochem. Explor. 2020, 208, 106392. [Google Scholar] [CrossRef]
- Kanda, A.; Ncube, F.; Mabote, R.R.; Mudzamiri, T.; Kunaka, K.; Dhliwayo, M. Trace elements in water, sediment and commonly consumed fish from a fish farm (NE Zimbabwe) and risk assessments. SN Appl. Sci. 2020, 2, 1502. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhang, A. Assessing potential mechanisms of arsenic-induced skin lesions and cancers: Human and in vitro evidence. Environ. Pollut. 2020, 260, 113919. [Google Scholar] [CrossRef]
- Palma-Lara, I.; Martínez-Castillo, M.; Quintana-Pérez, J.C.; Arellano-Mendoza, M.G.; Tamay-Cach, F.; Valenzuela-Limón, O.L.; García-Montalvo, E.A.; Hernández-Zavala, A. Arsenic exposure: A public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 2020, 110, 104539. [Google Scholar] [CrossRef]
- Okechukwu, C.E. Exposure to a high level of arsenic in drinking water and the risk of bladder cancer in Taiwan. Cancer Res. Stat. Treat. 2021, 4, 149–151. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Tu, J.B.; Ran, R.T.; Zhang, W.X.; Tan, Q.; Tang, P.; Kuang, T.; Cheng, S.Q.; Chen, C.Z.; Jiang, X.J.; et al. Using the Metabolome to Understand the Mechanisms Linking Chronic Arsenic Exposure to Microglia Activation, and Learning and Memory Impairment. Neurotox. Res. 2021, 39, 720–739. [Google Scholar] [CrossRef]
- Weerasundara, L.; Ok, Y.S.; Bundschuh, J. Selective removal of arsenic in water: A critical review. Environ. Pollut. 2021, 268, 115668. [Google Scholar] [CrossRef]
- Das, S.; Mukherjee, S. Implementation of Biotechnological Techniques in Treatment of Groundwater Contaminated with Arsenic. Int. J. Res. Appl. Sci. Eng. Technol. 2022, 10, 993–1000. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Current trends of arsenic adsorption in continuous mode: Literature review and future perspectives. Sustainability 2021, 13, 1186. [Google Scholar] [CrossRef]
- Simeonidis, K.; Martinez-Boubeta, C.; Zamora-Pérez, P.; Rivera-Gil, P.; Kaprara, E.; Kokkinos, E.; Mitrakas, M. Implementing nanoparticles for competitive drinking water purification. Environ. Chem. Lett. 2019, 17, 705–719. [Google Scholar] [CrossRef]
- Uppal, J.S.; Zheng, Q.; Le, X.C. Arsenic in drinking water—Recent examples and updates from Southeast Asia. Curr. Opin. Environ. Sci. Health 2019, 7, 126–135. [Google Scholar] [CrossRef]
- Tresintsi, S.; Simeonidis, K.; Estradé, S.; Martinez-Boubeta, C.; Vourlias, G.; Pinakidou, F.; Katsikini, M.; Paloura, E.C.; Stavropoulos, G.; Mitrakas, M. Tetravalent manganese feroxyhyte: A novel nanoadsorbent equally selective for As(III) and As(V) removal from drinking water. Environ. Sci. Technol. 2013, 47, 9699–9705. [Google Scholar] [CrossRef]
- Kokkinos, E.; Soukakos, K.; Kostoglou, M.; Mitrakas, M. Cadmium, mercury, and nickel adsorption by tetravalent manganese feroxyhyte: Selectivity, kinetic modeling, and thermodynamic study. Environ. Sci. Pollut. Res. 2018, 25, 12263–12273. [Google Scholar] [CrossRef]
- Mueller, B.; Dangol, B.; Ngai, T.K.K.; Hug, S.J. Kanchan arsenic filters in the lowlands of Nepal: Mode of operation, arsenic removal, and future improvements. Environ. Geochem. Health 2021, 43, 375–389. [Google Scholar] [CrossRef]
- Bretzler, A.; Nikiema, J.; Lalanne, F.; Hoffmann, L.; Biswakarma, J.; Siebenaller, L.; Demange, D.; Schirmer, M.; Hug, S.J. Arsenic removal with zero-valent iron filters in Burkina Faso: Field and laboratory insights. Sci. Total Environ. 2020, 737, 139466. [Google Scholar] [CrossRef]
- Yadav, M.K.; Saidulu, D.; Gupta, A.K.; Ghosal, P.S.; Mukherjee, A. Status and management of arsenic pollution in groundwater: A comprehensive appraisal of recent global scenario, human health impacts, sustainable field-scale treatment technologies. J. Environ. Chem. Eng. 2021, 9, 105203. [Google Scholar] [CrossRef]
- Litter, M.I.; Ingallinella, A.M.; Olmos, V.; Savio, M.; Difeo, G.; Botto, L.; Torres, E.M.F.; Taylor, S.; Frangie, S.; Herkovits, J.; et al. Arsenic in Argentina: Technologies for arsenic removal from groundwater sources, investment costs and waste management practices. Sci. Total Environ. 2019, 690, 778–789. [Google Scholar] [CrossRef]
- Zhang, W.; Mossad, M.; Yazdi, J.S.; Zou, L. A statistical experimental investigation on arsenic removal using capacitive deionization. Desalin. Water Treat. 2016, 57, 3254–3260. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).