Abstract
The cycling of the trace gas dimethyl sulfide (DMS) and its precursor dimethylsulfoniopropionate (DMSP) may be affected by future ocean acidification and warming. DMSP and DMS concentrations were monitored over 20-days in four mesocosm experiments in which the temperature and pH of coastal water were manipulated to projected values for the year 2100 and 2150. This had no effect on DMSP in the two-initial nutrient-depleted experiments; however, in the two nutrient-amended experiments, warmer temperature combined with lower pH had a more significant effect on DMSP & DMS concentrations than lower pH alone. Overall, this indicates that future warming may have greater influence on DMS production than ocean acidification. The observed reduction in DMSP at warmer temperatures was associated with changes in phytoplankton community and in particular with small flagellate biomass. A small decrease in DMS concentration was measured in the treatments relative to other studies, from −2% in the nutrient-amended low pH treatment to −16% in the year 2150 pH and temperature conditions. Temporal variation was also observed with DMS concentration increasing earlier in the higher temperature treatment. Nutrient availability and community composition should be considered in models of future DMS.
1. Introduction
The ocean is the major source of the natural sulfur compound dimethyl sulfide (DMS) to the atmosphere [1]. The latest model from Lana et al. [2] estimated the annual flux at 28.1 Tg of sulfur which represents 50% of the global biogenic sulfur emission to the atmosphere. This trace gas has a potentially important role in Earth’s climate, as hypothesized by Charlson et al. [3] under the CLAW hypothesis (derived from its authors’ names Charlson, Lovelock, Andreae and Warren), via its influence on the radiative budget. Once in the atmosphere, DMS is oxidized to non-sea-salt sulphate (NSS-SO42−) which contributes to the formation and growth of cloud condensation nuclei (CCN). The CLAW hypothesis states that the resulting increase in albedo and irradiance back-scatter may decrease light availability with negative feedback on marine phytoplankton and a corresponding reduction in the production of DMS. However, DMS is not the only contributor to the formation of CCN, and it is now recognized that the processes in the marine boundary layer are more complicated than initially hypothesized by CLAW [4].
DMS is produced by the enzymatic cleavage of the precursor dimethylsulfoniopropionate (DMSP) by DMSP-lyase. DMSP is mainly produced by phytoplankton, and is considered to have osmotic, cryoprotectant and antioxidant roles [5,6,7,8]. Intracellular DMSP content is dependent upon phytoplanktonic taxonomy, with the volumetric content in the surface ocean reflecting relative species abundance [9,10,11]. The main producers of DMSP are Dinophyceae, Prymnesiophyceae (primarily coccolithophores) and certain Chromophyceae, with other groups such as cryptomonads, cyanobacteria and diatoms being relatively minor producers [9,10,11]. As DMSP concentration depends on phytoplanktonic type it is also controlled by the environmental conditions influencing the physiology of the phytoplankton [12]. Bacteria are also involved in the DMSP cycle via production of DMS or methanethiol (MeSH) via demethylation [13]. Bacterial demethylation is one of the main DMSP sinks [13,14], and the bacterial capacity to convert DMSP to DMS may vary with nutrient availability [15]. The influence of environmental conditions on community composition means that changes in temperature, pH, light, and nutrient availability may indirectly impact DMSP production, and so DMS emissions to the atmosphere [10].
The oceans play a critical role in global climate regulation by absorbing CO2 emitted naturally by respiration, weathering, and degassing. Since the industrial era, anthropogenic CO2 emissions to the atmosphere have increased, leading to a decrease of oceanic pH, a phenomenon known as ocean acidification (OA), and an increase in oceanic surface temperatures [16]. Laboratory and mesocosm studies indicate that warming and ocean acidification will alter marine biogeochemistry and ecology [17,18,19,20]. In particular, studies to date suggest an indirect effect on DMSP and DMS via influences on phytoplanktonic community composition [19,21,22,23,24] and processes such as bacterial degradation [17,18,25,26,27,28]. Most mesocosm studies have observed a decrease in DMS concentration under low pH [18,22,23,25,27,28,29] and under low pH combined with warming [19,23]. Only a few have reported an increase in DMS concentration under low pH [17,30,31] and when combined with warming [31]. On the other hand, microcosm shipboard experiments carried out over shorter time-scales with smaller volumes, have shown varying results with no significant effect on DMS concentration under low pH in the sub-Arctic, Arctic and Antarctic [32], and a decrease of DMS in the Changjiang estuary [20]. In addition to the micro and mesocosm experiments, variation in DMS production and emission to the atmosphere under different climate change scenarios has been modelled, with a global decrease predicted and greater changes in the Southern Hemisphere (Schwinger et al., 2017; Six et al., 2013).
Earth System Models indicate that by the end of the century New Zealand open waters are projected to experience a pH decrease of 0.33 unit under the RCP8.5 scenario [33] and will warm by +3.5 °C by 2100 [33]. To gain insight into how OA and warming will affect New Zealand coastal endemic species, particularly those of relevance to coastal economies, the CARIM project (Coastal Acidification: Rates, Impacts and Management, [34]) was carried out. During CARIM four mesocosm experiments using coastal water took place over four years with the initial phytoplankton community differing in composition and bloom status. The mesocosms compared the effect of OA, and OA + warming, as projected for the year 2100 to present-day conditions. In addition, one of the experiments compared two future projected scenarios for the years 2100 and 2150. The study presented here uses this experimental framework to assess whether a future change in DMSP and DMS concentrations can be expected in New Zealand waters and assesses the factors responsible for this change. To our knowledge, this is the first study of the sensitivity of DMSP and DMS production to OA and warming in coastal waters in the Southern Hemisphere.
2. Experiments
2.1. Mesocosm Experiments
The mesocosm experiments (ME) took place in Wellington, New Zealand, at different times of the year (see Table 1). The installation consisted of nine 3.9 m3 bags containing ambient coastal water from Evans Bay, Wellington immersed in a 128 m3 pool supplied with a continuous input of coastal water to maintain consistent ambient temperature. The bags had transparent Perspex lids enclosing a small headspace, with each lid covered by mesh to attenuate light penetration. The temperature and pH of the bags were controlled by a dedicated Labview application using in-line heaters and/or a CO2 dosing system, with an integrated mixing system to ensure vertical uniformity of temperature and pH in the bags. The pH in the treatments was controlled by dosing with pure CO2 administrated through permeable tubing, with pH and temperature held constant at a target value. The target values were set by adjusting the observed ambient values through each experiment, using the projected future temperature or pH anomaly. The experiments lasted between 18 and 22 days (Table 1).

Table 1.
CARIM Mesocosm parameters with the target values for 2100 low pH treatment in blue, 2100 pH/T in dark yellow and dark red for 2150 pH/T treatment.
MEs 1 and 4 consisted of 2 different treatments compared to an ambient control: low pH alone (referred as “2100 low pH”) and low pH & warmer temperature in combination (“2100 pH/T”). In ME2, only a low pH treatment was compared to ambient controls, with no warming treatment. ME3 simulated the future low pH and warming scenarios predicted for the years 2100 (“2100 pH/T”) and 2150 (“2150 pH/T”) and compared these to ambient controls. The nutrient regimes varied with each experiment, with details of initial conditions and treatments summarized in Table 1. In ME1 & ME2, the nutrients were drawn down, particularly nitrate, but accumulated in ME3 & ME4 due to regular addition at 1–2-day intervals. ME1 was the only experiment carried out in autumn, but the initial conditions were similar to ME3 which started in spring, with similar initial nitrate concentrations of 0.37 ± 0.04 µmol L−1 and 0.39 ± 0.04 µmol L−1 respectively. The initial temperature, phosphate and silicate concentrations were higher in ME1 compared to ME3. As for ME2 & ME4 which both started in spring, the initial temperatures were similar, but nutrients’ concentrations slightly higher in ME2 than ME4, at 0.49 ± 0.01 µmol L−1 and 0.19 ± 0.05 µmol L−1 respectively for nitrate. The effects of different seasonal conditions will not be discussed as there were no major or consistent differences between the initial conditions to draw a conclusion. All treatments and controls were carried out in triplicate except in ME3, where only duplicates were available from day 6 onwards. Sampling took place every other day between 09:00 and 10:00, with water from each bag sampled by gravity into twice rinsed 20 L containers. Subsamples were taken from the containers for analysis of parameters.
2.2. Ancillary Measures
Water was sampled every day for total chlorophyll-a (Chl-a) (and every second day during ME4), with 250 mL of water filtered onto a GF/F filter and then snap frozen in liquid nitrogen and stored at −80 °C. Chl-a was extracted in 90% acetone before being measured with a Turner Design 10-AU fluorometer.
The Chl-a temporal variation was used to divide the ME experiments into 3 phases for ease of interpretation. Phase 1 reflected the adjustment period of the phytoplankton to the bag environment, Phase 2 the plateau/growth phase in Chl-a which varied between experiments, and Phase 3 the response phase of the phytoplankton community to experimental conditions.
Optical microscopy was used to determine the phytoplanktonic community composition and cell numbers for species > ~5 µm. Every 2 to 4 days, 1 L of water was sampled from the bags and preserved in Lugols solution. Twenty-four hours before examination on a Leica DMI3000B inverted microscope, a 150 mL subsample was left to settle in Utermohl chambers, as described in Safi et al. [35] and references therein. The genus or species of all the abundant organisms were identified when possible, before counting. The cell volume for each species was calculated using formulae representing the geometrical solids that approximated cell shape following Sun et al. [36] and Olenina et al. [37]. Phytoplankton carbon (mg C L−1) was calculated using the conversion equations of Montagnes et al.; Eppley et al. and Menden-Deuer et al. [38,39,40]. Phytoplankton carbon is presented for diatoms and small flagellates (80% < ~5 µm) which included the groups Prymnesiophyceae, Cryptophyceae, Chrysophyceae and monads.
2.3. DMSP and DMS Analysis
For DMS analysis, amber glass bottles were overflowed by gravity and sealed with no headspace, before analysis within 0.5 to 4 h. In the laboratory, the water was slowly injected through a 25 mm GF/F filter by syringe before being sparged in a silanized glass chamber. The DMS was then trapped on a 60/80 Tenax® TA (Supelco, Bellefonte, PA, USA) trap at −20 °C which was subsequently heated to 110 °C with injection into an Agilent Technology 6850 Gas Chromatography (DB-megabore sulfur SCD column, 70 m length, 0.530 megabore diameter and film thickness 4.30 µm) coupled with an Agilent 355 sulfur detector (SCD). Aside from these details the method is the same as described in Walker et al. [41,42]. For total DMSP measurement (will be referred as DMSP from now on), 20 mL vials were filled with sample water before adding 2 NaOH pellets and then gas-tight sealed. DMSP was analyzed one day after sampling using the same semi-automated purge and trap system followed by a GC-SCD as described above. Calibration was carried out using DMS and methyl ethyl sulfide Dynacal® permeation tubes (VICI Metronics Inc, Poulsbo, WA, USA) [42]. A wet standard calibration curve was made daily from a stock solution of DMSP diluted in milli-Q, with calibration concentrations ranging from 4 nM to 181 nM. These were decanted into 20 mL gas tight glass vials, hydrolyzed with 2 pellets of NaOH and then injected into the sparging unit and processed as samples. The detection limit was 0.13 (±0.02) pgS s−1. DMS was measured only during ME3 & ME4.
2.4. Culture and Incubation of Dominant Diatom
At the end of ME3, water was subsampled from the bags and the dominant diatom species, Cylindrotheca closterium, was isolated. To investigate the effect of future temperature and pH change on this diatom, it was incubated at “Now” and “Future” conditions expected at 2100. Cells in “Now”, ambient, conditions were incubated at 15 °C and pH 8.1 and “Future” cells were incubated at 18 °C and pH 7.8. All cells were grown in f/20, a 10 dilution of Guillards f/2 medium (Sigma G0154) added to sterile coastal seawater. Five µmol L−1 of silicate was also added. The pH of the “Future” medium was amended by bubbling 10% CO2 through the medium prior to the addition of any cells and checked using a spectrophotometric method [43]. Incubations were performed in 250 mL acid-cleaned, sterile polycarbonate bottles (Nalgene) with a 12 h light:dark cycle at 60 uE m−2 s−1 for both scenarios. The diatoms were incubated for 19 days in a semi-batch style so that cells remained in exponential growth, and cell density was kept low so that the medium pH was not altered by phytoplankton growth. Each culture was grown in triplicates. Samples for total DMSP measurements were taken in triplicate from each incubation bottle after 20 days along with samples for cell counts and Chl-a. The differences between the means of “Now” and “Future” conditions were compared using a t-test approach.
2.5. Statistical Analysis
The mean values of DMSP and DMS for the control and each treatment were determined for measurements across each of the 3 different phases (as defined by Chl-a). The differences between the means of the treatments’ and the control during each of the different phases were compared using Wilcoxon rank sum approaches. The Holm-Bonferroni correction was used to reduce the number of Type I errors (false positives).
GAMM (Generalised Additive Mixed Model) [44] models were used to investigate if there were any significant temporal variation of treatments relative to the control over the duration of the experiment. Initially, three GAMM models were considered similar to models (G), (S) and (GS) as described by Pedersen et al. [45]. Model (G) is a “Null” model of a “global” smooth function for all observations across the days of analysis without considering the control or treatments as a factor. Model (S) comprised of control/treatment specific smooth functions across the days of analysis and represented different processes effecting change in each treatment. Model (GS) comprised a single “global” smooth function with the addition of a smooth function factored by the control and two treatments, and represented a singular underlying process being affected by the treatment conditions. For each model an AR1 correlation structure was assumed to account for repeated measures and the replicate experiments for each ME were treated as random effects. The Akaike Information Criterion (AIC) was utilised to determine which of the 3 models provided the fit best with the measured data.
3. Results
3.1. Ancillary Measures
3.1.1. Chl-a
The mesocosms started at different times of the year with varying initial phytoplankton compositions and Chl-a concentrations. ME1 took place in austral autumn with a lower initial Chl-a, whereas the other mesocosms began in spring with intermediate initial Chl-a concentrations for ME2 & ME3 and high Chl-a concentrations in ME4 which started during a bloom. The average values over the full experiment and in Phase 3 of Chl-a concentrations are summarised in Table 2. Chl-a showed a different temporal trend in each of the four experiments, with an overall decline in ME1 & ME2, and an increase in ME3 & ME4 in which nutrients were added on a daily basis. The observed differences between ME1 & ME2 in Chl-a biomass were within 10% of each other and non-significant (p > 0.1), whereas significant differences relative to the Control were apparent in the Phase 3 means in the 2100 pH/T treatment of ME4 (+28%; p-value = 0.01) and the 2150 pH/T treatment of ME3 (+70%; p-value < 0.01). There was no significant difference in Chl-a biomass in the 2100 low pH treatment in any experiment, with observed differences within 10% for ME1 and ME2 and 1% for ME4.

Table 2.
Average Chl-a concentration in µg L−1 in the four mesocosms experiments over the full experiment duration and in Phase 3 with the associated standard deviation.
3.1.2. Phytoplanktonic Composition
Diatoms were the dominant phytoplankton group in all four experiments, based upon total cell Carbon content (Table 3), followed by the small flagellates (Table 3 and Figure 1). In ME1 & ME2, the observed differences in the diatom biomass between the treatments and Control were within 26% and 7% (from day 6 onwards) of each other, for ME1 & ME2 respectively, and non-significant. In ME3 diatom biomass increased during the experiment, with significantly higher biomass in the 2100 pH/T and 2150 pH/T treatments relative to the control during Phase 3 (+415% and +365% for 2100 pH/T and 2150 pH/T scenarios respectively; p-values < 0.01; see Table 3 for averages). During ME4, the high initial biomass of diatoms decreased during Phase 1 and 2 before increasing again. Both ME3 & ME4 saw an increase in the pennate diatom species, Cylindrotheca closterium in the Control treatment of Phase 3, which appeared to be related to nutrient availability. Small flagellate biomass was higher than diatoms at the start of ME1 & ME2 and higher at the end of ME3 & ME4 (Figure 1). During ME1 & ME2 the observed differences between controls and treatments were within 18% and 15% of each other, and non-significant; the small flagellates’ biomass increased in Phase 1 & 2 of ME1 and then decreased in Phase 3. However, in ME3 & ME4, small flagellate biomass increased throughout the experiment and was higher in Phase 3 of the Control, relative to the lower biomass in the 2150 pH/T scenario of ME3 (−33%; p-value = 0.06; see Table 3 for averages) and combined treatment of ME4 (−24%; p-value = 0.08; see Table 3 for averages).

Table 3.
Average per treatment during Phase 3 of Chl-a concentration in µg L−1, diatoms and small flagellates carbon content in µg L-1 and DMSP concentration in nmol L-1 in the different mesocosms with the associated standard deviation.
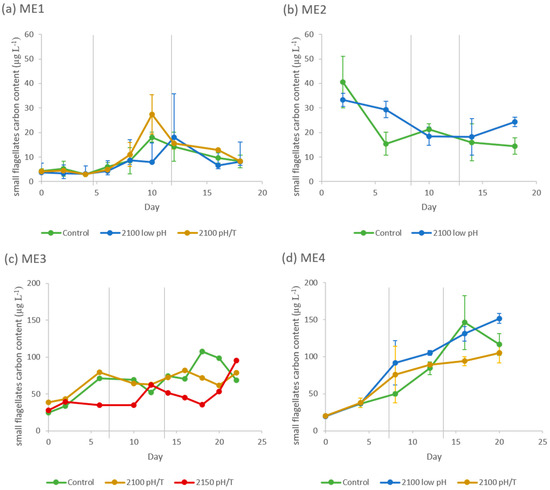
Figure 1.
Small flagellates carbon content (µg L−1) during (a) ME1, (b) ME2, (c) ME3 & (d) ME4. The average of the 3 treatment replicates are plotted against time with standard deviation as error bars. There is no error bar for ME3 as there were only 2 replicates per treatment from day 6. Note the different vertical scales.
3.2. DMSP
DMSP concentrations were generally higher in ME3 & ME4 compared to ME1 & ME2. In general, there was an increase of DMSP across all the experiments with a plateau or decline in Phase 3 (Figure 2). In the treatments of ME3 & ME4, DMSP concentrations were significantly lower compared to the Controls during Phase 3 (−19% and −26% for 2100 pH/T and 2150 pH/T for ME3 and −21% and −37% in 2100 low pH and 2100 pH/T for ME4 respectively with p-values < 0.09). For ME1 & ME2, the (G) model was preferred suggesting that there is no deviation in the data and so no effect of the treatment (Table 4). For ME3 & ME4, the (S) and (GS) models were preferred, suggesting that there is a treatment effect (Table 4). Although the response differed between ME1 & ME2, with DMSP increasing sharply in Phase 2 in ME1 and Phase 1 in ME2, the observed differences in DMSP between the treatments and Controls were within 9% and 8% of each other and non-significant in either ME1 & ME2 (Figure 2a,b). Conversely, ME3 & ME4 exhibited similar increases over Phase 1 and Phase 2 with a treatment effect from day 10 onwards. The DMSP maxima in the Control occurred during Phase 3 in the Controls whereas DMSP plateaued in the treatments (Figure 2c,d). Significant differences were observed in Phase 3 between Control and treatments (−16% and −24% of 2100 pH/T and 2150 pH/T scenarios of ME3 respectively; p-values < 0.03; and −32% and −16% of 2100 pH/T and 2100 low pH of ME4 respectively; p-values < 0.09).
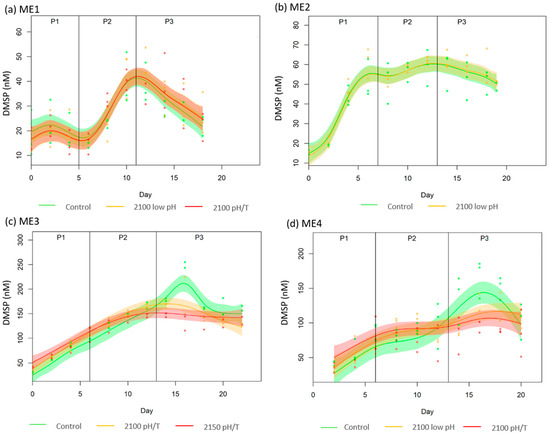
Figure 2.
GAMMs plot of DMSP concentrations (in nmol L−1) during (a) ME1, (b) ME2, (c) ME3, and (d) ME4, in each treatment against time. The bold line represents the average for each treatment or control of the data points and the shaded area represents 2 standard errors. Note differences in vertical scales.

Table 4.
DMSP concentration AICs from models (G), (S) and (GS) for the 4 mesocosms. The model providing the best fit is the one with the lowest AIC (in bold). The (G) model fits a common trajectory regardless of treatment, while the (S) and (GS) models incorporate treatment effects.
3.3. DMS
The trends in DMS concentration (Figure 3a,b) differed to those of DMSP concentration during ME3 & ME4. DMS increased during Phase 1 & 2 in all mesocosms and peaked in the pH/T treatments (18 and 24.6 nM). Concentrations peaked in Phase 3 in the Control in both experiments and also the 2100 low pH treatment in ME4 (17.63 and 16.94 nM, respectively) before declining. In ME4, the observed differences between 2100 low pH and Control were within 2% of each other, and non-significant. In both experiments, there was temporal differences in the DMS response between treatments and Control with the DMS maximum occurring 4 to 6 days earlier in the 2100 pH/T treatments. The (GS) model was preferred for both mesocosms suggesting that the deviation in the data can be modelled by considering the treatments a single function (a global process) with individual treatment effects (Table 5). Despite the difference between Control and treatments in ME3 the deviations were within the uncertainties of the replicate mesocosms and so not significant (p-values > 0.1 in Phase 3), whereas there was a difference of −58% between Control and 2100 pH/T in ME4 (p-value < 0.1 in Phase 3). The integrated DMS concentration over the entire experiment were 9% and 16% lower in the 2100 pH/T and 2150 pH/T treatments, respectively, in ME3, and 2% and 12% lower in 2100 low pH and 2100 pH/T, respectively, in ME4, relative to the ambient Control.
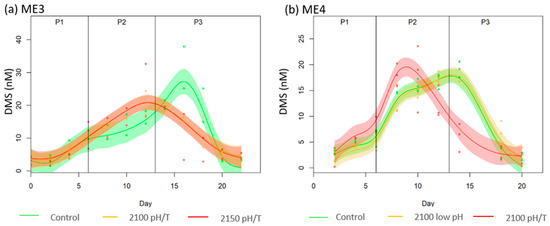
Figure 3.
GAMMs plot of DMS concentrations (in nmol L−1) during (a) ME3, and (b) ME4 against time. The bold line represents the average of the treatment’s duplicates, the points are the actual measurements and the clearer area around the average represents the deviation. Note the differences in vertical scales.

Table 5.
DMS concentration AICs from models (G), (S) and (GS) for ME3 & ME4. The model providing the best fit is the one with the lowest AIC (in bold). The (G) model fits a common trajectory regardless of treatment, while the (S) and (GS) models incorporate treatment effects.
3.4. DMSP Production by Cylindrotheca Cultures
The significant growth response of Cylindrotheca closterium during ME3 prompted further study of this species’ response to future conditions in uni-algal incubations, with DMSP cell concentration measured to determine whether there was a treatment effect on the production of DMSP. For the “Now” conditions, the cellular DMSP content was 8.59 ± 0.87 nmol cell−1 (mean ± standard deviation) compared to 7.64 ± 0.27 nmol cell−1 (mean ± standard deviation) under “Future” conditions (Figure 4). These are higher concentrations than the 2.28 nmol cell−1 reported for this species by Keller et al. [46], although the total DMSP (DMS + DMSP particulate + DMSP dissolved) is reported in the current study. Consequently, there was no significant treatment effect on the production of DMSP when comparing “Now” and “Future” conditions (t = 1.8085; df = 2.3889; p-value = 0.19).
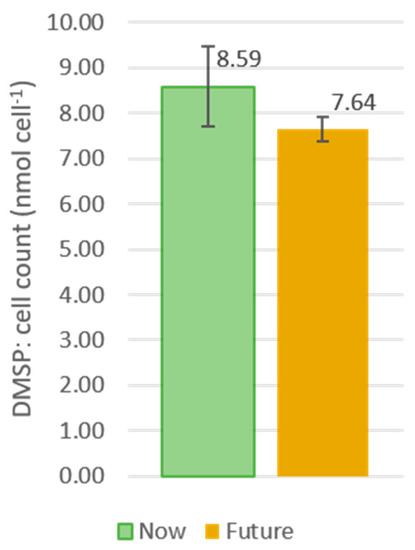
Figure 4.
Averages of “Now” and “Future” DMSP content (in nmol cell−1) with associated standard deviations as error bars.
4. Discussion
It is established that DMSP and DMS concentrations vary with phytoplankton biomass, bloom status and community composition [9,10]. The four mesocosm experiments using coastal water from the same location at different times of the year and in different years show variation in DMSP & DMS response to future conditions. Nevertheless, there were consistent responses between experiments, with the combined treatment of lower pH and warmer temperature generally having a greater influence on DMSP and DMS production than lower pH alone.
The results suggest that nutrients influence the response to future conditions, with the response in ME1 & ME2, which were carried out in nutrient-limited or deplete conditions, differing from ME3 & ME4 in which nutrients were supplemented every one to two days (see Table 1). This variation in nutrient availability may have influenced phytoplankton community composition and consequently DMSP and DMS production. The general increase in diatoms observed in the treatments, is consistent with the positive response of diatoms to increasing pCO2 reported in other studies [19,21,22,23,24], many of which have added nutrients. Although diatoms contain lower intracellular DMSP than other phytoplankton groups [9], their increase in biomass during Phase 3 in the treatments of both ME3 & ME4 should be reflected in an increase in DMSP. However, DMSP concentrations generally plateaued or declined in the treatments in Phase 3 but increased in the Control indicating that the diatom response was not the primary factor determining DMSP [23]. The C. closterium incubations confirmed that the DMSP intracellular concentration is not affected by future conditions, consistent with Li et al. [47] who reported no significant difference in DMSP particulate (DMSPp) and DMSP dissolved (DMSPd) of C. closterium under elevated CO2.
The DMSP trends may instead reflect change in other components of the phytoplankton community. The small flagellates’ carbon content, which included Prymnesiophyceae, Cryptophyceae, Chrysophyceae and monads, show similar trends to DMSP concentration in ME3 & ME4, with higher Phase 3 values in the Control relative to the low pH/T treatments (see Figure 1). Small flagellates have higher cellular DMSP than diatoms; for example, Chrysophyceae DMSP to Chl-a ratio is reported to be 47.95 mmol g−1 and between 15.21 and 43.97 mmol g−1 for Prymnesiophyceae, both of which were significant contributors to the small flagellates in the mesocosm experiments. Conversely, the larger diatom Cylindrotheca closterium has only 1.12 mmol g−1 [46]. Small flagellates were more abundant in the Controls of ME3 & ME4, and their concentrations were positively correlated with DMSP concentrations but the strength of the relationship differs between the experiments (Figure 5). This correlation between DMSP and small flagellates suggests the small flagellates were the primary contributors to DMSP concentration and consequently, infers that they were responsible for the elevated DMSP concentration in the Controls. The lower small flagellate biomass in the pH/T treatments may reflect increased competition with the diatoms [19], or alternatively increased microzooplankton grazing at higher temperatures [48] and a corresponding decline in DMSP. However, there were no significant differences in microzooplankton grazing between the control and treatments in ME3 (Gutierrez-Rodrigues, A. personal communication).
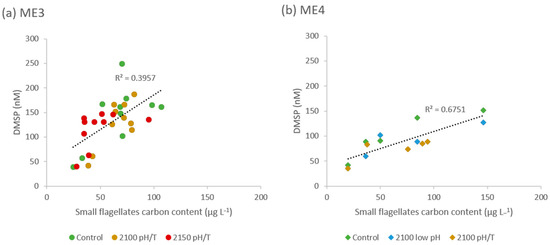
Figure 5.
Relationship between DMSP concentration (in nmol L−1) and small flagellates’ carbon content (in µg L−1) in the different treatments of (a) ME3 and (b) ME4.
Whereas phytoplanktonic community is a primary driver of DMSP concentration, DMS may be more influenced by processes [10,27]. The ME4 results indicate, contrary to other studies (see Figure 6), that low pH had no effect, with no significant difference in the timing or magnitude of the DMS maximum between the low pH treatment and Control (Figure 3). It is possible that the DMSP-lyase activity, which is responsible for the conversion of DMSP-to-DMS is not impacted by a lower pH contrary to the observations of Park et al. [19]. The optimum pH of DMSP-lyase differs with phytoplankton species [19,28], and only a few have a pH optimum higher than 8 [5,49,50]. In the pH/T treatments in both ME3 & ME4 the DMS maximum occurred earlier than in the Controls, indicating an effect of temperature. It is well established that bacterial activity or growth rate increase at higher temperature [51,52,53], although there was no evidence of a treatment effect on bacterial production in ME3 (Deans, F. personal communication). A decrease in bacterial yield of DMS under higher CO2 and warmer temperature can lead to reduced DMS concentrations [23], and the difference in DMS dynamics may reflect a shift in bacterial DMSP metabolism [17], potentially in response to a shift in the phytoplankton community. An analogous temporal variation in DMS dynamics was observed during the SERIES iron addition experiment [54], in which elevated DMS concentrations occurred earlier in the iron-induced bloom relative to external waters, and was followed by rapid removal of DMS due to elevated bacterial carbon and sulfur requirements [54,55]. The implications of an earlier DMS maximum under warmer temperatures is unclear but may result in more rapid DMS cycling. Variation in the timing and magnitude of the DMS air-sea gradient may also affect emissions to the atmosphere and subsequently influence aerosol and cloud formation.
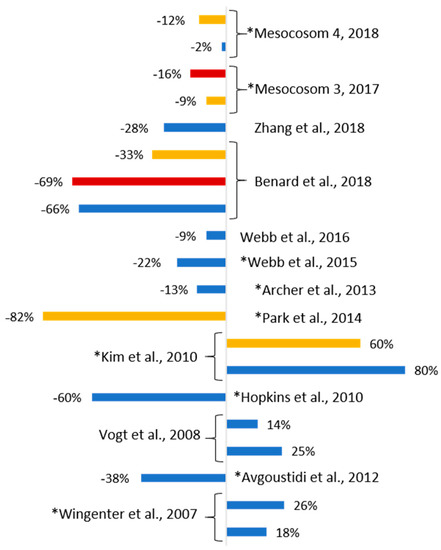
Figure 6.
Comparison of DMS change in mesocosm studies. The percentage of DMS variation future to present is plotted. Blue bars represent “low pH” treatment (lowest pH of the experiments); yellow bars “pH/T” of end the 2100 scenario; red bar “2150 scenario”; * indicates nutrients addition. The results from the lowest pH and highest temperature are plotted for each study.
Figure 6 presents a comparison of the results of this study with other published mesocosm studies that have examined the effect of ocean acidification and warming on DMS production (see Figure 6’s legend for attribution). These were carried out in different coastal waters at Northern hemisphere sites (Norwegian fjords, Korea, Baltic sea) with different initial conditions at different times of the year. In addition, they cover a broad pH range (from 8.3 to 7.2) and some include nutrient addition. For each reported study the difference in total DMS concentration, integrated over the full duration of each experiment, between the control and the most extreme future scenario is presented. The decrease in DMS production in New Zealand coastal waters is relatively low compared to other coastal regions, although this comparison is influenced by differences in biomass and community composition between studies. The seasonal Evans Bay pH range is between 7.85 to 8.10 [56] and its minimum pH is not significantly different from the experiments’ targeted pH. However, although coastal phytoplankton experience this pH range over the year [57], the experiments were primarily carried out during spring when Evans Bay’s pH is usually >8.0, and so the phytoplankton community at this time would not experience the low pH tested in the experiments. In contrast to the current study, most studies report a significant decrease in DMS concentration with OA, with only Vogt et al. and Wingenter et al. [17,30] showing an increase in DMS production under low pH, which they attribute to increased bacterial and viral activity. In the combined low pH & elevated temperature treatment of Kim et al. [31] significant production of DMS relative to the control was attributed to micro-zooplankton grazing on high DMSP phytoplankton species which were more abundant in their treatments. Consequently, although the observed response in DMS is not consistent with this study, the underlying driver of phytoplankton community structure is a primary factor. In other experiments where warmer temperatures were combined with lower pH the DMS yield is lower, consistent with the results presented here [19,23]. A strong correlation was observed between DMS production and grazing by Park et al. [19] but did not result in a DMS increase. The results from Park et al. and Kim et al. [19,31] highlight how the biological communities (phytoplanktonic and micro-grazers) and processes influence DMSP and DMS concentrations.
As shown in our experiments, temperature combined with low pH has a greater impact than low pH alone on DMSP & DMS concentrations, and the phytoplankton communities from which they derive [58], in New Zealand coastal waters. Single stressor studies, of pH or temperature, allow the individual effects of these drivers to be determined but future projections and models need to incorporate their combined and interactive effects. It is recommended that further experimental mesocosm studies are carried out with these two parameters in combination, so that the results can be applied in models to provide more accurate projections of future DMS. Warmer temperatures are projected to increase stratification leading to reduced macronutrient availability for phytoplanktonic growth [59], as reported for tropical and sub-tropical regions [60]. Models predicting a decrease in DMS in several oceanic regions are based upon a decrease in nutrient availability [61,62]. However, smaller phytoplankton are favoured under lower nutrient availability [63], as in the Southern Ocean where small flagellates dominate when nutrients decrease (see references in [64]). As demonstrated in this study, DMSP production is related to small flagellate biomass and so their response to future change in nutrient availability may influence DMS. Nutrients then play a critical role in shaping not just the community composition but also sulfur cycling. Consequently, further studies examining the influence of nutrient availability, combined with improved representation of phytoplankton groups in models, should enable more accurate projection of future DMSP & DMS.
5. Summary and Conclusions
Mesocosms experiments in New Zealand coastal water, identified that:
- OA is not a critical determinant of future DMSP & DMS whereas warmer temperatures have a significant impact;
- Under future temperature and pH, with nutrient availability maintained, shifts in phytoplankton community structure that include a decrease in small flagellate biomass result in decreased DMSP concentrations;
- although DMS concentration decreased under OA and warmer temperature this decrease was not as significant as reported by other studies;
- future changes in the temporal evolution of DMSP & DMS may have implications for sulfur and carbon cycling, and DMS emissions.
Author Contributions
A.D.S.-M. and A.M. analyzed the DMSP and DMS samples. N.B., M.G. and C.S.L. developed the mesocosms experimental design. E.A. maintained the C. closterium cultures. K.S. carried out the optical microscopy and with M.G. calculated the carbon content of the different phytoplankton species. P.W.D. and K.M. applied the statistical analysis. C.S.L. initiated and coordinated the experiments. A.D.S.-M. and C.S.L. carried out the data interpretation and A.D.S.-M. prepared the manuscript with the contributions from E.A., M.G., K.S., P.W.D., K.M. and N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the New Zealand Ministry of Business, Industry and Employment, with support from NZ Fisheries and NIWA SSIF.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to short-term contractual obligations.
Acknowledgments
We would like to thank Stacy Deppeler, Lisa Northcote, Rory Graham, Phil Fisher for their help and assistance with the mesocosm experiments. The experiments were part of the CARIM (Coastal Acidification: Rate, Impact and Management) project, funded by the New Zealand Ministry of Business, Industry and Employment, with support from NZ Fisheries and NIWA SSIF. I would like to thank too the University of Otago for the Departmental Award that is funding my PhD and Christina McGraw, my co-supervisor, for her feedbacks on the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Lovelock, J.E.; Maggs, R.J.; Rasmussen, R.A. Atmospheric Dimethyl Sulfide and the Natural Sulfur Cycle. Nature 1972, 237, 452–453. [Google Scholar] [CrossRef]
- Lana, A.; Simó, R.; Vallina, S.M.; Dachs, J. Re-examination of global emerging patterns of ocean DMS concentration. Biogeochemistry 2011, 110, 173–182. [Google Scholar] [CrossRef]
- Charlson, R.J.; Lovelock, J.E.; Andreae, M.O.; Warren, S.G. Oceanic phytoplankton, atmospheric sulphur, cloud albedo and climate. Nature 1987, 326, 655–661. [Google Scholar] [CrossRef]
- Quinn, P.K.; Bates, T.S. The case against climate regulation via oceanic phytoplankton sulphur emissions. Nature 2011, 480, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Stefels, J.; Dijkhuizen, L. Characteristics of DMSP-lyase in Phaeocystis sp. (prymnesiophyceae). Mar. Ecol. Prog. Ser. 1996, 131, 307–313. [Google Scholar] [CrossRef]
- Kirst, G.O.; Thiel, C.; Wolff, H.; Nothnagel, J.; Wanzek, M.; Ulmke, R. Dimethylsulfoniopropionate (DMSP) in icealgae and its possible biological role. Mar. Chem. 1991, 35, 381–388. [Google Scholar] [CrossRef]
- Nishiguchi, M.K.; Somero, G.N. Temperature and concentration dependence of compatibility of the organic osmolyte B-dimethylsulfoniopropionate. Cryobiology 1992, 29, 118–124. [Google Scholar]
- Sunda, W.G.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Lett. Nat. 2002, 418, 317–320. [Google Scholar]
- Keller, M.D.; Bellows, W.K.; Guillard, R.L. Dimethyl Sulfide Production in Marine Phytoplankton. Biog. Sulfur Environ. 1989, 393, 167–182. [Google Scholar]
- Stefels, J.; Steinke, M.; Turner, S.; Malin, G.; Belviso, S. Environmental constraints on the production and removal of the climatically active gas dimethylsulphide (DMS) and implications for ecosystem modelling. Biogeochemistry 2007, 83, 245–275. [Google Scholar] [CrossRef]
- Yoch, D.C. Dimethylsulfoniopropionate: Its sources, role in the marine food web, and biological degradation to dimethylsulfide. Appl. Environ. Microbiol. 2002, 68, 5804–5815. [Google Scholar] [PubMed]
- Stefels, J. Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J. Sea Res. 2000, 43, 183–197. [Google Scholar] [CrossRef]
- Kiene, R.P.; Linn, L.J. The fate of dissolved dimethylsulfiniopropionate (DMSP) in seawater: Tracer studies using 35S-DMSP. Geochim. Cosmochim. Acta 2000, 64, 2797–2810. [Google Scholar] [CrossRef]
- Kiene, R.P.; Linn, L.J.; González, J.; Moran, M.A.; Bruton, J.A. Dimethylsulfoniopropionate and methanethiol are important precursors of methionine and protein-sulfur in marine bacterioplankton. Appl. Environ. Microbiol. 1999, 65, 4549–4558. [Google Scholar] [CrossRef] [PubMed]
- Simó, R.; Dachs, J. Global ocean emission of dimethylsulfide predicted from biogeophysical data. Glob. Biogeochem. Cycles 2002, 16, 26-1–26-10. [Google Scholar] [CrossRef]
- Poloczanska, E.; Mintenbeck, K.; Portner, H.O.; Roberts, D.; Levin, L.A. The IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. In Proceedings of the 2018 Ocean Sciences Meeting, AGU, Portland, OR, USA, 11–16 February 2018. [Google Scholar]
- Vogt, M.; Steinke, M.; Turner, S.; Paulino, A.; Meyerhöfer, M.; Riebesell, U.; LeQuéré, C.; Liss, P. Dynamics of dimethylsulphoniopropionate and dimethylsulphide under different CO2 concentrations during a mesocosm experiment. Biogeosciences 2008, 5, 407–419. [Google Scholar] [CrossRef]
- Hopkins, F.E.; Turner, S.M.; Nightingale, P.D.; Steinke, M.; Bakker, D.; Liss, P.S. Ocean acidification and marine trace gas emissions. Proc. Natl. Acad. Sci. USA 2010, 107, 760–765. [Google Scholar]
- Park, K.T.; Lee, K.; Shin, K.; Yang, E.J.; Hyun, B.; Kim, J.M.; Noh, J.H.; Kim, M.; Kong, B.; Choi, D.H.; et al. Direct linkage between dimethyl sulfide production and microzooplankton grazing, resulting from prey composition change under high partial pressure of carbon dioxide conditions. Env. Sci. Technol. 2014, 48, 4750–4756. [Google Scholar] [CrossRef]
- Jian, S.; Zhang, J.; Zhang, H.-H.; Yang, G.-P. Effects of ocean acidification and short-term light/temperature stress on biogenic dimethylated sulfur compounds cycling in the Changjiang River Estuary. Environ. Chem. 2019, 16, 197–211. [Google Scholar]
- Hussherr, R.; Levasseur, M.; Lizotte, M.; Tremblay, J.-E.; Mol, J.; Thomas, H.; Gosselin, M.; Starr, M.; Miller, L.A.; Jarniková, T.; et al. Impact of ocean acidification on Arctic phytoplankton blooms and dimethyl sulfide concentration under simulated ice-free and under-ice conditions. Biogeosciences 2017, 14, 2407–2427. [Google Scholar] [CrossRef]
- Zhang, S.-H.; Yu, J.; Ding, Q.-Y.; Yang, G.-P.; Gao, K.-S.; Zhang, H.-H.; Pan, D.-W. Effect of elevated pCO2 on trace gas production during an ocean acidification mesocosm experiment. Biogeosciences 2018, 15, 6649–6658. [Google Scholar] [CrossRef]
- Bénard, R.; Levasseur, M.; Scarratt, M.; Michaud, S.; Starr, M.; Mucci, A.; Ferreyra, G.; Gosselin, M.; Tremblay, J.-E.; Lizotte, M.; et al. Contrasting effects of acidification and warming on dimethylsulfide concentrations during a temperate estuarine fall bloom mesocosm experiment. Biogeosciences 2019, 16, 1167–1185. [Google Scholar] [CrossRef]
- Mélançon, J.; Levasseur, M.; Lizotte, M.; Scarratt, M.; Tremblay, J.-E.; Tortell, P.; Yang, G.-P.; Shi, G.-Y.; Gao, H.; Semeniuk, D.; et al. Impact of ocean acidification on phytoplankton assemblage, growth, and DMS production following Fe-dust additions in the NE Pacific high-nutrient, low-chlorophyll waters. Biogeosciences 2016, 13, 1677–1692. [Google Scholar]
- Avgoustidi, V.; Nightingale, P.D.; Joint, I.; Steinke, M.; Turner, S.M.; Hopkins, F.E.; Liss, P.S. Decreased marine dimethyl sulfide production under elevated CO2 levels in mesocosm and in vitro studies. Environ. Chem. 2012, 9, 399–404. [Google Scholar] [CrossRef]
- Archer, S.D.; Suffrian, K.; Posman, K.M.; Bach, L.T.; Matrai, P.A.; Countway, P.D.; Ludwig, A.; Riebesell, U. Processes That Contribute to Decreased Dimethyl Sulfide Production in Response to Ocean Acidification in Subtropical Waters. Front. Mar. Sci. 2018, 5, 245. [Google Scholar] [CrossRef]
- Archer, S.D.; Kimmance, S.A.; Stephens, J.A.; Hopkins, F.E.; Bellerby, R.G.J.; Schulz, K.G.; Piontek, J.; Engel, A. Contrasting responses of DMS and DMSP to ocean acidification in Arctic waters. Biogeosciences 2013, 10, 1893–1908. [Google Scholar] [CrossRef]
- Webb, A.L.; Malin, G.; Hopkins, F.E.; Ho, K.L.; Riesebell, U.; Schulz, K.; Liss, P.S. Ocean acidification has different effects on the production of dimethylsulphide and dimethylsulphoniopropionate measured in cultures of Emiliania huxleyi RCC1229 and mesocosm study: A comparison of laboratory monocultures and community interactions. Environ. Chem. 2015, 13, 314–329. [Google Scholar] [CrossRef]
- Webb, A.L.; Leedham-Elvidge, E.; Hughes, C.; Hopkins, F.E.; Malin, G.; Bach, L.T.; Schulz, K.; Crawfurd, K.; Brussaard, C.P.D.; Stuhr, A.; et al. Effect of ocean acidification and elevated fCO2 on trace gas production by a Baltic Sea summer phytoplankton community. Biogeosciences 2016, 13, 4595–4613. [Google Scholar] [CrossRef]
- Wingenter, O.W.; Haase, K.B.; Zeigler, M.; Blake, D.R.; Rowland, F.S.; Sive, B.C.; Paulino, A.; Thyrhaug, R.; Larsen, A.; Schulz, K.; et al. Unexpected consequences of increasing CO2and ocean acidity on marine production of DMS and CH2ClI: Potential climate impacts. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Kim, J.M.; Lee, K.; Yang, E.J.; Shin, K.; Noh, J.H.; Park, K.T.; Hyun, B.; Jeong, H.J.; Kim, J.H.; Kim, K.Y.; et al. Enhanced production of oceanic dimethylsulfide resulting from co2-induced grazing activity in a high CO2 world. Environ. Sci. Technol. 2010, 44, 8140–8143. [Google Scholar] [CrossRef]
- Hopkins, F.E.; Nightingale, P.D.; Stephens, J.A.; Moore, C.M.; Richier, S.; Cripps, G.L.; Archer, S.D. Dimethylsulfide (DMS) production in polar oceans may be resilient to ocean acidification. Biogeosciences 2018. [Google Scholar] [CrossRef]
- Law, C.S.; Rickard, G.J.; Mikaloff-fletcher, S.E.; Pinkerton, M.H.; Behrens, E.; Chiswell, S.M.; Currie, K. Climate change projections for the surface ocean around New Zealand. N. Z. J. Mar. Freshw. Res. 2018, 52, 309–335. [Google Scholar] [CrossRef]
- Available online: http://www.carim.nz/ (accessed on 28 November 2020).
- Safi, K.A.; Brian Griffiths, F.; Hall, J.A. Microzooplankton composition, biomass and grazing rates along the WOCE SR3 line between Tasmania and Antarctica. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 1025–1041. [Google Scholar] [CrossRef]
- Sun, J.; Liu, D. Geometric models for calculating cell biovolume and surface area for phytoplankton. J. Plankton Res. 2003, 25, 1331–1346. [Google Scholar] [CrossRef]
- Olenina, I.; Hajdu, S.; Edler, L.; Andersson, A.; Wasmund, N.; Busch, S.; Gobel, J.; Gromisz, S.; Huseby, S.; Huttunen, M.; et al. Biovolumes and size-classes of phytoplankton in the Baltic Sea. In Proceedings of the HELCOM Baltic Sea Environmental Proceedings, Helsinki, Finland, January 2006; p. 144. Available online: https://epic.awi.de/id/eprint/30141/1/bsep106.pdf (accessed on 28 January 2021).
- Montagnes, D.J.S.; Franklin, D.J. Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: Reconsidering some paradigms. Limnol. Oceanogr. 2001, 46, 2008–2018. [Google Scholar] [CrossRef]
- Eppley, R.W.; Reid, F.M.H.; Strickland, J.D.H. The Ecology of the Plankton Off La Jolla, California, in the Period April Through September, 1967. 1970: UC San Diego: Library–Scripps Digital Collection. Bull. Scripps Inst. Oceanogr. 1967, 17, 33–42. [Google Scholar]
- Menden-Deuer, S.; Lessard, E.J. Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol. Oceanogr. 2000, 45, 569–579. [Google Scholar] [CrossRef]
- Walker, C.F.; Harvey, M.J.; Bury, S.J.; Chang, F.H. Biological and physical controls on dissolved dimethylsulfide over the north-eastern continental shelf of New Zealand. J. Sea Res. 2000, 43, 253–264. [Google Scholar] [CrossRef]
- Walker, C.F.; Harvey, M.J.; Smith, M.J.; Bell, T.G.; Saltzman, E.S.; Marriner, A.S.; McGregor, J.A.; Law, C.S. Assessing the potential for dimethylsulfide enrichment at the sea surface and its influence on air-sea flux. Ocean Sci. 2016, 12, 1033–1048. [Google Scholar] [CrossRef]
- Dickson, A.G.; Goyet, C. Handbook of Methods for the Analysis of the Various Parameters of the Carbon Dioxide System in Sea Water; Version 2; Oak Ridge National Lab: Oak Ridge, TN, USA, 1994. [Google Scholar]
- Hastie, T.; Tibshirani, R. Generalized Additive Models. Stat. Sci. 1986, 1, 297–318. [Google Scholar] [CrossRef]
- Pedersen, E.J.; Miller, D.L.; Simpson, G.L.; Ross, N. Hierarchical generalized additive models in ecology: An introduction with mgcv. PeerJ 2019, 7, e6876. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D.; Korjeff-Bellows, W. Physiological Aspects of the Production of Dimeyhtlsulfoniopropionate (DMSP) by Marine Phytoplankton, in Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds; Springer: Berlin, Germany, 1996; pp. 131–142. [Google Scholar]
- Li, P.-F.; Yang, G.-P.; Zhang, J.; Levasseur, M.; Liu, C.-Y.; Sun, J.; Yang, W. Impacts of elevated pCO2 on trace gas emissions in two microalgae: Phaeocystis globosa and Nitzschia closterium. Environ. Chem. 2017, 14, 425–441. [Google Scholar] [CrossRef]
- Chen, B.; Landry, M.R.; Huang, B.; Liu, H. Does warming enhance the effect of microzooplankton grazing on marine phytoplankton in the ocean? Limnol. Oceanogr. 2012, 57, 519–526. [Google Scholar] [CrossRef]
- De Souza, M.P.; Yoch, D.C. Purification and characterization of dimethylsulfoniopropionate lyase from an Alcaligenes-like dimethyl sulfide-producing marine isolate. Appl. Environ. Microbiol. 1995, 61, 21–26. [Google Scholar] [CrossRef]
- Li, C.-Y.; Wei, T.-D.; Zhang, S.-H.; Chen, X.-L.; Gao, X.; Wang, P.; Xie, B.-B.; Su, H.-N.; Qin, G.-L.; Zhang, X.-Y.; et al. Molecular insight into bacterial cleavage of oceanic dimethylsulfoniopropionate into dimethyl sulfide. Proc. Natl. Acad. Sci. USA 2014, 111, 1026–1031. [Google Scholar] [CrossRef]
- Vaqué, D.; Guagayol, O.; Peters, F.; Felipe, J.; Malits, A.; Pedrós-Alió, C. Differential response of grazing and bacterial heterotrophic production to experimental warming in Antarctic waters. Aquat. Microb. Ecol. 2009, 54, 101–112. [Google Scholar] [CrossRef]
- Vázquez-Domínguez, E.; Vaqué, D.; Gasol, J.M. Temperature effects on the heterotrophic bacteria, heterotrophic nanoflagellates, and microbial top predators of the NW Mediterranean. Aquat. Microb. Ecol. 2012, 67, 107–121. [Google Scholar]
- Tsai, A.Y.; Gong, G.C.; Shiau, W. Impact of short-term warming on seasonal variations in bacterial growth, grazing, and viral lysis in coastal waters of Taiwan. Aquat. Microb. Ecol. 2016, 76, 195–205. [Google Scholar] [CrossRef][Green Version]
- Levasseur, M.; Scarratt, M.G.; Michaud, S.; Merzouk, A.; Wong, C.S.; Ary Chuk, M.; Richardson, W.; Rivkin, R.B.; Hale, M.; Wong, E.; et al. DMSP and DMS dynamics during a mesoscale iron fertilization experiment in the Northeast Pacific—Part I: Temporal and vertical distributions. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 2353–2369. [Google Scholar] [CrossRef]
- Merzouk, A.; Levasseur, A.; Scarratt, M.G.; Michaud, S.; Rivkin, R.B.; Hale, M.S.; Kiene, R.P.; Price, N.M.; Li, W.K.W. DMSP and DMS dynamics during a mesoscale iron fertilization experiment in the Northeast Pacific–Part II: Biological cycling. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 2370–2383. [Google Scholar] [CrossRef]
- Vance, J.; Currie, K.I.; Law, C.S.; Murdoch, J.; Zeldis, J. NZOA-ON: The New Zealand Ocean Acidification Observing Network. Mar. Freshw. Res. 2020, 71, 281–299. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS ONE 2011, 6, e28983. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.A.; Rudisill, J.R.; Neeley, A.R.; Maucher, J.M.; Hutchins, D.A.; Feng, Y.; Hare, C.E.; Leblanc, K.; Rose, J.M.; Wihelm, S.W.; et al. Effects of increased pCO2 and temperature on the North Atlantic spring bloom. III. Dimethylsulfoniopropionate. Mar. Ecol. Prog. Ser. 2009, 388, 41–49. [Google Scholar] [CrossRef]
- Moore, J.K.; Lindsay, K.; Doney, S.C.; Long, M.C.; Misumi, K. Marine ecosystem dynamics and biogeochemical cycling in the community earth system model [CESM1(BGC)]: Comparison of the 1990s with the 2090s under the RCP4.5 and RCP8.5 scenarios. J. Clim. 2013, 26, 9291–9312. [Google Scholar] [CrossRef]
- Boyce, D.G.; Lewis, M.R.; Worm, B. Global phytoplankton decline over the past century. Nature 2010, 466, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Schwinger, J.; Tjiputra, J.; Goris, N.; Six, K.D.; Kirkevåg, A.; Selang, O.; Heinze, C.; Ilyina, T. Amplification of global warming through pH dependence of DMS production simulated with a fully coupled Earth system model. Biogeosciences 2017, 14, 3633–3648. [Google Scholar] [CrossRef]
- Six, K.D.; Kloster, S.; Ilyina, T.; Archer, S.D.; Zhang, K.; Maier-Reimer, E. Global warming amplified by reduced sulphur fluxes as a result of ocean acidification. Nat. Clim. Chang. 2013, 3, 975–978. [Google Scholar] [CrossRef]
- Wang, S.; Maltrud, M.; Elliott, S.; Cameron-Smith, P.; Jonko, A. Influence of dimethyl sulfide on the carbon cycle and biological production. Biogeochemistry 2018, 138, 49–68. [Google Scholar]
- Deppeler, S.; Petrou, K.; Schulz, K.G.; Westwoog, K.; Pearce, I.; McKinlay, J.; Davidson, A. Ocean acidification of a coastal Antarctic marine microbial community reveals a critical threshold for CO2; tolerance in phytoplankton productivity. Biogeosciences 2018, 15, 209–231. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).