Abstract
The evolutionary conserved Notch signaling pathway functions as a mediator of direct cell–cell communication between neighboring cells during development. Notch plays a crucial role in various fundamental biological processes in a wide range of tissues. Accordingly, the aberrant signaling of this pathway underlies multiple genetic pathologies such as developmental syndromes, congenital disorders, neurodegenerative diseases, and cancer. Over the last two decades, significant data have shown that the Notch signaling pathway displays a significant function in the mature brains of vertebrates and invertebrates beyond neuronal development and specification during embryonic development. Neuronal connection, synaptic plasticity, learning, and memory appear to be regulated by this pathway. Specific mutations in human Notch family proteins have been linked to several neurodegenerative diseases including Alzheimer’s disease, CADASIL, and ischemic injury. Neurodegenerative diseases are incurable disorders of the central nervous system that cause the progressive degeneration and/or death of brain nerve cells, affecting both mental function and movement (ataxia). There is currently a lot of study being conducted to better understand the molecular mechanisms by which Notch plays an essential role in the mature brain. In this study, an in silico analysis of polymorphisms and mutations in human Notch family members that lead to neurodegenerative diseases was performed in order to investigate the correlations among Notch family proteins and neurodegenerative diseases. Particular emphasis was placed on the study of mutations in the Notch3 protein and the structure analysis of the mutant Notch3 protein that leads to the manifestation of the CADASIL syndrome in order to spot possible conserved mutations and interpret the effect of these mutations in the Notch3 protein structure. Conserved mutations of cysteine residues may be candidate pharmacological targets for the potential therapy of CADASIL syndrome.
1. Introduction
Research on Drosophila melanogaster with notched wings led to the discovery of the Notch gene in 1914 [1]. To date, it seems that the evolutionary history of the Notch family is closely related to the biological tree of life. The Notch protein and its homologs, Notch1, Notch2, Notch3, Notch4, LIN-12, and GPL-1, have been detected in the genomes of all kingdoms, demonstrating the evolutionary development of the Notch family [2]. Members of the Notch family were discovered to have a comparable structure across several kingdoms, extending from bacteria to chordates [3]. Only one Notch receptor is found in D. melanogaster. The Notch receptors LIN-12 and GLP-14 in Caenorhabditis elegans are redundant [4]. Mammals have four Notch paralogs, Notch1, Notch2, Notch3, and Notch4, displaying both redundant and distinct activities [5].
The Notch receptors (Notch1–Notch4), found in mammalian cells, are four different transmembrane proteins expressed on the cell’s surface as heterodimers not covalently bonded [6]. Notch proteins have an extracellular domain (NECD) that operates as the signal receiver and a transmembrane–intracellular domain (NICD) that operates as the signal transducer. The Notch1–Notch4 ECDs contain 36, 35, 34, and 29 epidermal growth factor-like repeats (EGF-like domain), respectively. Also, the ECD of Notch receptors has three cysteine-rich Lin12-Notch repeats (LNRs) and a heterodimerization domain (HD). The Notch ICD has an RBPJk-associated molecule domain (RAM) and nuclear localization sequences (NLSs) on both sides of the ANK domains. Also, Notch ICD consists of five to six ankyrin repeats (ANK), a transcriptional activation domain (TAD), and a C-terminal domain (PEST) rich in proline, glutamic acid, serine, and threonine. Notch family proteins function as cell surface receptors and direct regulators of gene transcription, constituting a particular signal transduction pathway that enables cells to affect the gene expression of their neighboring cells [7]. Notch signaling is activated upon cell-to-cell contact due to interactions between four transmembrane receptors encoded by Notch genes (Notch1–4) and five Notch ligands encoded by JAG1, JAG2, and DLL1, DLL3, and DLL4 [8]. Notch ECD contains EGF-like repeats that condense ligand–receptor binding [9].
The human (Homo sapiens) Notch1 gene is found at locus 9q34.3 on chromosome 9. Loss of function of the Notch1 protein is linked to abnormalities in angiogenesis, cardiogenesis, and somitogenesis, which could lead to the death of an embryo. This gene is involved in forming the first definitive adult hematopoietic stem cells (HSCs) [10]. Moreover, the development of B and T cells is regulated by Notch1 signaling. Mutations in the signaling and transcriptional regulator Notch1 result in various developmental aortic valve abnormalities, severe valve calcification, and T-cell acute lymphoblastic leukemia [10,11]. The Notch2 gene is located on chromosome 1p12. Notch2 has specific functional activity in determining cell fate and in the development of kidney, ovary, smooth muscle, T, and B cells [12]. Postnatal signaling regulates homeostasis, bone regeneration, and immune system function [13,14]. Mutations resulting in excessive Notch2 activity may lead in systemic issues typical of Alagille and Hajdu–Cheney syndromes such as heart abnormalities, chronic cholestasis, osteoporosis, polycystic kidneys, skeletal deformities, and neurological disorders [15,16]. The Notch3 gene is found between locations 13.2 and 13.1 on the short arm (p) of chromosome 19. This large type I transmembrane receptor, mostly expressed in pericytes and vascular smooth muscle cells adjacent to the local blood arteries, takes part in maintaining and renewing tissues as well as in important developmental functions [17]. Overexpression and aberrant activation of the Notch3 gene are linked to cancer, particularly breast and ovarian cancer. Mutations in Notch3 have been directly linked to the CADASIL syndrome [18]. The Notch4 gene is found at locus 6p21.32 on chromosome 6. It has been observed that both the overexpression and mutations of the Notch4 gene are related to cancer [19]. Notch4 is considered a new biomarker of cancer stem cells (CSCs) [20].
Notch genes are involved in various critical biological processes including somitogenesis, angiogenesis, vasculogenesis, cardiac development and function, neuronal development, and the specification and maintenance of neural stem cells (NSCs) [21]. All four mammalian Notch receptor paralogs and several pathway components (ligands and targets) are expressed with different cell type specificities in both the adult mouse and human brain [22]. There is evidence of Notch receptor expression in neurons (Notch1 and Notch2), neural stem cells (Notch1 and Notch2), vascular smooth muscle cells and pericytes (Notch3), endothelial cells (Notch1 and Notch4), and astrocytes (Notch1 and Notch2) [22]. Notch has been linked to maintaining NSCs in an undifferentiated state, preventing neuronal development, and even causing terminal differentiation inside the astrocyte lineage [23,24]. Notch signaling is essential for neural stem cell maintenance and neurogenesis in both the embryonic and adult brains [6]. The elderly’s brain function, cell differentiation, and neurite formation are all impacted by Notch signaling, which is crucial for the nervous system’s regular operation [5,25]. Accordingly, multiple mutations in Notch proteins have been linked to neurodegenerative conditions [21].
Quantitative data on this pathway’s structural, biochemical, and biophysical features have emerged during the last few years [26]. Various loss-of-function mutations in the embryo and adult highlight the critical role of Notch signaling. Numerous studies on neurogenesis have used Drosophila melanogaster, zebrafish, and mice as model species [6]. The conditional loss of Notch signaling in the embryo causes the precocious differentiation of NSCs and neurodevelopmental defects such as impaired survival and the aberrant migration of progenitor cells [6]. In the adult brain, NSCs are predominantly quiescent and rarely divide. However, it is likely that quiescent NSCs enter the cell cycle and transform into active NSCs before quitting the cell cycle again and reentering the quiescent state [27]. In adults, Notch signaling pathway mutations are involved in many neurodegenerative diseases and brain disorders [6].
Neurodegenerative diseases are incurable disorders of the central nervous system that present clinically and pathologically in various ways and damage particular neuronal subsets and anatomical functioning systems [28]. There are currently no therapies that target the underlying cause of neurodegenerative diseases. Therefore, it is not feasible to prevent or stop the progression of these disorders [29]. The involvement of Notch receptor genes and proteins in aging, cerebrovascular disorder, and Alzheimer’s disease is significant. Notch signaling may be a fundamental overlap between age-related vascular and Alzheimer’s pathogenesis that contributes to their comorbidity and combined impact on cognitive decline and dementia. Numerous results from genetics, cell culture model studies, and neuropathology all point to a connection between aberrant Notch signaling and the pathogenesis of Alzheimer’s disease [21]. In addition, it is generally established that the Notch3 protein plays a significant role in the development of CADASIL [30,31,32].
Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy is a hereditary dominant rare disease caused by mutations in the Notch3 protein, affecting adults beyond middle age and resulting in dementia and disability [33]. CADASIL is a fatal late-onset disease that primarily appears as a degenerative disorder of the central nervous system, and it is defined by specific clinical, neuroradiological, and pathological characteristics [30,34]. Over the last two decades, extensive efforts have been directed toward research on Notch3, identifying more than 280 mutations [35]. Some of these mutations cause a phenotype whereas others remain silent. Extensive analysis for categorizing, organizing, and mapping these mutations is required for a simple genotype–phenotype linkage [33]. Numerous pathogenic mutations in the Notch3 gene change the number of cysteine residues in the receptor’s extracellular domain, leading to protein misfolding and receptor aggregation [33]. Each EGF-like repeat contains six cysteines, which combine to create three disulfide bonds and provide the EGF repeat its three-dimensional structure [36]. However, non-Cys mutations have also been reported in recent years. These mutations do not match the disease’s typical pattern and pathology [30]. Even though most of the mutations in Notch3 are point mutations, it has been established that each one has a major impact on the three-dimensional structure of the Notch3 protein [30].
The study of the Notch family has increased, significantly, the availability of biological data on polymorphisms and mutations that are related with neurodegenerative diseases [15,21,37,38,39]. The initial purpose of this work was to gather all the cases and link them between nucleotides and protein sequences. Today, the scientific research seems to be focused on understanding the way the EGF region functions due to the significant mutagenesis it presents through a series of scientific publications [39,40,41,42,43]. The ultimate goal of this study is the holistic study of all mutations occurring in Notch3, with an emphasis on the EGF region and the CADASIL syndrome, in order to identify specific patterns of mutagenesis in the EGF-repeats that may be related to the clinical phenotype, sex, and age data of the various patients [38,39,44,45,46]. The study and analysis of all mutations can additionally open new horizons thus contributing to the identification of new pharmacological targets as well as contributing to the identification of a candidate treatment against CADASIL syndrome and, generally, neurodegenerative diseases. The outline of the integrated bioinformatic method is presented in Figure 1.
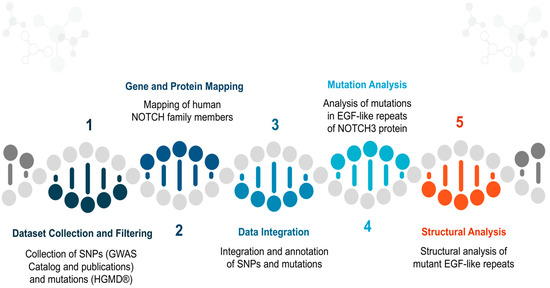
Figure 1.
Flow chart presentation of the bioinformatic method, presented in five steps.
2. Materials and Methods
2.1. Dataset Collection and Filtering
Data were collected from polymorphism databases, disease-specific mutation databases, and publications. Specifically, single-nucleotide polymorphisms (SNPs) on Notch1–Notch4 genes associated with neurodegenerative diseases were extracted from available online databases such as GWAS-Catalog, dbSNP, LitVar, and ClinVar. Likewise, a second search was carried out in the online database PubMed (https://pubmed.ncbi.nlm.nih.gov/, accessed on 18 March 2024) for publications that contained the key terms “Neurodegenerative diseases”, “Cognitive Disorders”, “Alzheimer’s disease”, “CADASIL” AND “NOTCH1”, “NOTCH2”, “NOTCH3”, and “NOTCH4” with no date restriction. The collected SNPs from all databases were extracted, filtered, and annotated using Matlab bioinformatics toolbox for data mining and semantic techniques. All SNPs causing mutations on the protein level and directly related to neurodegenerative diseases were stored the final dataset. The Human Gene Mutation Database (HGMD®) (https://www.hgmd.cf.ac.uk/ac/index.php, accessed on 18 March 2024) was searched for missense mutations on Notch1–Notch4 proteins. HGMD®® attempts to aggregate all known (published) human gene mutations responsible for human diseases. The mutations associated with neurodegenerative diseases have been collected. For each mutation, the access number, codon change, mutation position, and the phenotype it induces were recorded.
2.2. Gene and Protein Mapping
In this step, mapping of human Notch family genes and proteins was accomplished. The terms “Notch1”, “Notch2”, “Notch3”, and “Notch4” were searched on the NCBI database (https://www.ncbi.nlm.nih.gov, accessed on 18 March 2024) while the filter “Gene” was previously selected for extracting the nucleotide sequences of these genes. Furthermore, additional information like gene location, chromosome, nucleotide sequence length, access number, and alternative gene names was extracted from the NCBI database. A second search was carried out on NCBI database with the “protein” filter for extracting the amino acid sequences of human Notch family proteins. These terms were also searched on available online protein databases such as UniProt and InterPro. Information on the amino acid sequence lengths of proteins and disease involvement was obtained. Domains of each protein were also recorded. Protein domain data were also extracted from publications in the PubMed database.
2.3. Data Integration
The data collected from polymorphism databases and mutation databases were merged and annotated. SNPs and the mutations in the human Notch family associated with neurodegenerative diseases were integrated to correlate polymorphisms and mutations. The integrated data are presented in a table, providing information for SNP ID, nucleotide change, codon change, mutation position, domain in which the mutation is located, and the phenotype it causes. The finding of correlations among polymorphisms and mutations on Notch1–Notch4 was necessary to find out which ones and how many mutations are associated with a known polymorphism, which neurodegenerative disease is most often caused by Notch3 mutations, which Notch3 domain contains the majority of these mutations, and which amino acid appears to be most frequently mutated. To comment on these queries, specific diagrams have been created where the data analysis is presented.
2.4. Mutation Analysis
The majority of polymorphisms/mutations associated with neurodegeneration were located in Notch3 and specifically in the EGF region. For this reason, our work then focused on this domain (Table 7). In order to analyze the mutations identified in the EGF-like repeats of the Notch3 protein, a FASTA file with the amino acid sequences of 34 EGF-like repeats was created. This file was analyzed using the Jalview platform (https://www.jalview.org/, accessed on 18 March 2024). JalView computes and visualizes a large number of sequences with high performance. The main advantage of this method is that it allows for the identification of conserved motifs with a quick overview of alignment. In this step, a multiple alignment of 34 EGF-like amino acid sequences was performed in order to find the conserved amino acids in the EGF-like repeats. Jalview’s comments section, which displays amino acid conservation with logos and histograms, was also examined to discover novel motifs. The sequencing results were further elaborated. A histogram was created, showing the percentage of mutations at each amino acid position in the EGF-like sequences according to multiple-sequence alignment numbering. Finally, mutations in conserved amino acids in the EGF-like repeats were studied to identify conserved amino acid changes. A chart presenting the results of this analysis was also constructed.
2.5. Structural Analysis
Using the MOE (Molecular Operating Environment) platform, the mutant EGF-like repeat structure was analyzed to figure out the consequences for the Notch3 protein when including amino acid changes. MOE 2019.01 is an integrated life sciences software that supports drug design through molecular simulation, protein structure analysis, small molecule processing data, protein binding, and small molecule design. A homology modeling of the Notch3 approach was used for the structural analysis since no structure has been determined for this protein. The homology modeling of Notch3 protein structure was extracted from the AlphaFold Protein structure Database (https://alphafold.ebi.ac.uk/, accessed on 18 March 2024). Structural analysis of the EGF-like 2 repeat was performed while introducing conserved mutations associated with CADASIL syndrome.
3. Results
3.1. Dataset
As a consequence of systematic data mining, SNPs of human Notch family members correlated with neurodegenerative diseases were identified (Table 1). These polymorphisms have been derived both from the data mined from the biological database GWAS CATALOG as well as from the publications that contained the ontologies of interest based on PubMed searches. In total, 1887 relevant publications were extracted, of which 188 described polymorphisms. Only single-nucleotide polymorphisms that cause mutations at the protein level and are directly related to neurodegenerative diseases were used. In the Notch1 gene, 57 polymorphisms were collected from online polymorphism databases and publications, of which 41 missense variants were screened. Only one variant was identified in this gene related to Alzheimer’s disease (Table 4). Twenty-six polymorphisms in the Notch2 gene were retrieved. Among these, 15 missense SNPs were examined, and only one was found to be associated with autism multiplex disorder (Table 5). A total of 59 polymorphisms in the Notch3 gene were extracted from online databases and publications. Twenty-eight missense SNPs were screened, and all were found to be involved in the manifestation of neurodegenerative diseases (Table 7). Most SNPs are associated with CADASIL disease. Finally, from the Notch4 gene were extracted only two SNPs (missense variants), and neither was found to be associated with neurodegenerative disease (Table 6). Unlike SNPs in Notch1, Notch2, and Notch4, missense SNPs in the Notch3 gene seem more strongly related to neurodegenerative disorders. Additionally, through filtering the results of the HGMD database, all mutations in Notch1–Notch4 associated with neurodegenerative diseases were reported and imported. More specifically, 1, 1, 312, and 4 mutations in Notch1, Notch2, Notch3, and Notch4, respectively, were identified to be correlated with neurodegenerative diseases.

Table 1.
Dataset of collected SNPs and mutations from online databases.
3.2. Gene and Protein Mapping
Data on Notch1–Notch4 genes and proteins were retrieved from the National Center for Biotechnology Information (NCBI), UniProt, and InterPro. NCBI-Gene results for Notch1–Notch4 are shown in Table 2. The search results for Notch1–Notch4 proteins are shown in Table 3. Notch1 has the greatest amino acid sequence length of the four Notch proteins (2555 aa), followed by Notch2 (2471 aa), Notch3 (2321 aa), and Notch4 (2003 aa). Mutations in these genes are linked to the manifestation of several diseases. Mutations in the Notch1 gene are associated with diseases such as aortic valve disease, type 1 (AOVD1), Adams–Oliver syndrome type 5 (AOS5), T-cell acute lymphoblastic leukemia (T-ALL), chronic lymphocytic leukemia, and squamous cell carcinoma of the head and neck. Mutations in Notch2 are linked to Hajdu–Cheney syndrome, Alagille syndrome 2 (ALGS2), and cancer. Mutations in Notch3 have been identified as the cause of diseases such as CADASIL, infantile myofibromatosis, early-onset arteriopathy with cavitating leukodystrophy, lateral meningocele syndrome, and cancer. Finally, mutations in the Notch4 gene may be associated with schizophrenia.

Table 2.
NCBI data for Notch1–4 genes.

Table 3.
NCBI data for Notch1–Notch4 proteins.
By mining information from databases and publications, Notch1–Notch4 proteins were mapped. The Notch1–4 proteins consist of EGF, LNR, NOD, NODP, TM, RAM, NLS, ANK, TAD, and PEST domains [47]. In mammals, the TAD region is present in Notch1 and 2 but not in Notch3 and 4 [48]. The EGF domain is made up of EGF-like repeats. In human Notch1, Notch2, Notch3, and Notch4, the EGF domains consist of 36 EGF-like, 35 EGF-like, 34 EGF-like, and 29 EGF-like repeats, respectively. Each EGF-like repeat comprises 30–40 amino acids and contains six cysteine residues (C). The LNR sector consists of three micro-domains, LNR1, LNR2, and LNR3. The ANK domain in Notch1–2 proteins is made of six ankyrin repeats while in Notch3-4, proteins are made of five ankyrin repeats. Notch1–Notch4 protein domains are demonstrated with specific colors in protein sequences in Figure 2, Figure 3, Figure 4 and Figure 5.

Figure 2.
Notch1 protein domains. Colors represent protein domains.

Figure 3.
Notch2 protein domains. Colors represent protein domains.

Figure 4.
Notch3 protein domains. Amino acids marked with bold red represent the CADASIL mutations. Colors represent protein domains.
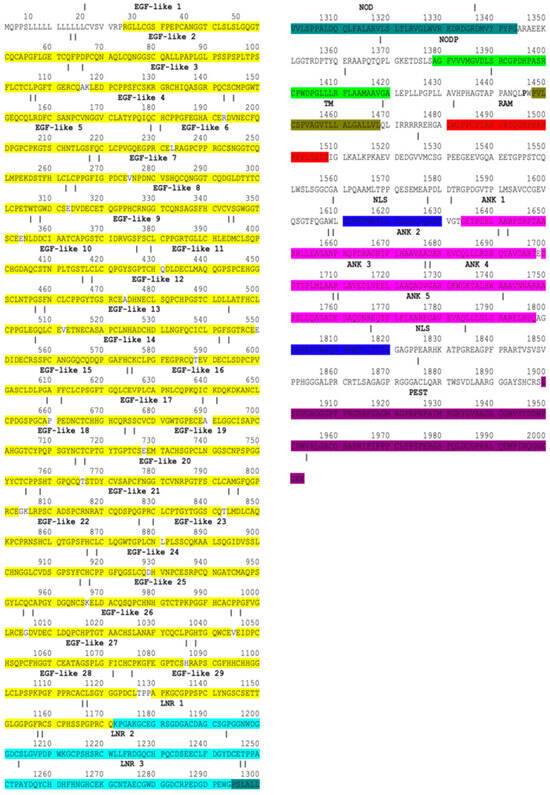
Figure 5.
Notch4 protein domains. Colors represent protein domains.
3.3. Data Integration
The integrations of polymorphism and mutation datasets of Notch1–Notch4 are shown in Table 4, Table 5, Table 6 and Table 7. Since Notch3 is associated with the manifestation of neurodegenerative diseases to a considerably more significant degree than other human Notch family members, the annotation of Notch3 data followed. In Table 6, the consolidated data from the recording of Notch3 polymorphisms and mutations are presented in order to correlate them. Information on nucleotide change, amino acid change, protein domain on which the mutation is located, and the phenotype it induces were obtained for the recorded SNPs. According to the association between polymorphisms and mutations reported, 23 polymorphisms are related to mutations not identified in the HGMD data source. A blank cell in the “Accession number” column of the table indicates the particular polymorphisms. Although there are 43 mutations associated with known SNPs, there are 292 mutations unrelated to any identified SNP (Figure 6).

Table 4.
SNPs and mutations in Notch1 associated with neurodegenerative diseases.

Table 5.
SNPs and mutations in Notch2 associated with neurodegenerative diseases.

Table 6.
SNPs and mutations in Notch3 associated with neurodegenerative diseases.

Table 7.
Mutations in Notch4 associated with neurodegenerative diseases.
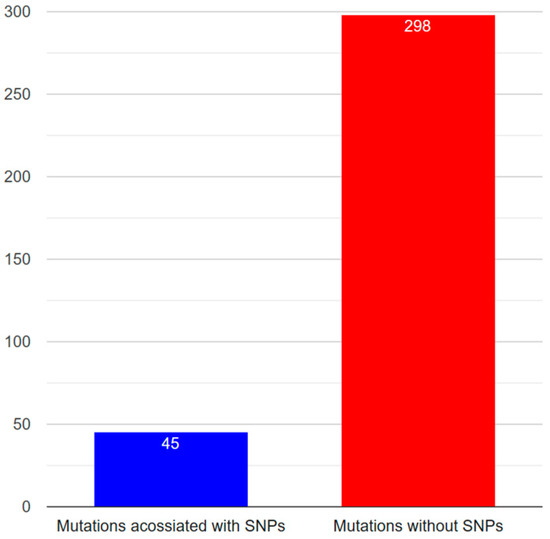
Figure 6.
Number of associated and unassociated mutations with known SNPs.
The percentage of mutations in Notch3 associated with neurodegenerative diseases is displayed graphically in a pie chart in Figure 7. According to this study, 90% of Notch3 mutations lead to CADASIL disease, 4% of Notch3 mutations lead to Alzheimer’s disease, and 4% of Notch3 mutations lead to white matter lesions. Only 2% of Notch3 mutations are associated with other neurodegenerative diseases such as the small-vessel disease of the brain, ischemic stroke, migraine, and autism. Since CADASIL is represented by 90% of mutations in Notch3, a guide map (Figure 4) was created for Notch3 mutations. Mutations in the amino acid sequence of the Notch3 protein are marked in bold red.
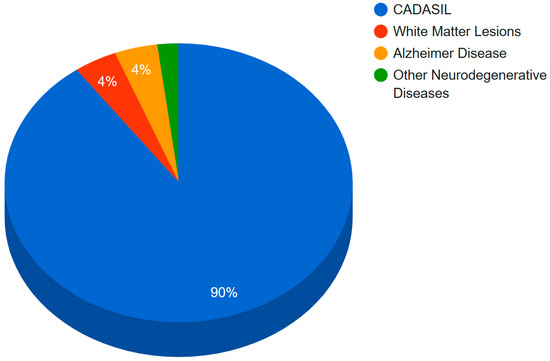
Figure 7.
Percentage of Notch3 mutations associated with a specific neurodegenerative disease.
There have been reported to be 310 mutations in Notch3 that cause CADASIL syndrome (Table 7). The majority of the mutations (305), as shown in the amino acid sequence of Figure 4, are found in the EGF domain. The distribution of mutations in the EGF-like repeats and other protein domains is illustrated through a chart (Figure 8). The highest concentration of mutations is observed in the EGF-like 3 and EGF-like 4 repeats while the lowest numbers of mutations are found in the NOD, RAM, and PEST domains. In addition, more than 60% of Notch3 protein mutations that lead to CADASIL disease occur at the cysteine residue (Figure 9).
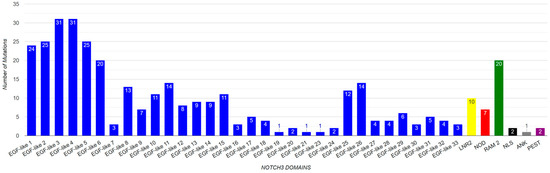
Figure 8.
Demonstration of mutation number per Notch3 domain.
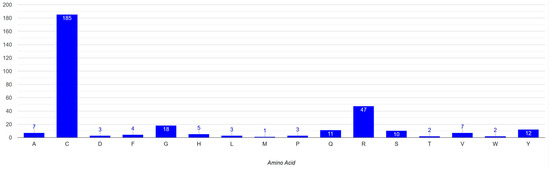
Figure 9.
Mutated amino acid residues in Notch3 protein associated with CADASIL.
3.4. Mutation Analysis
Since 90% of the mutations identified in Notch3 and related to neurodegenerative diseases were located in the EGF region, the mutation analysis was mainly focused on this specific region. The multiple-sequence alignment (MSA) of amino acid sequences of Notch3 EGF-like repeats was performed to identify highly conserved amino acids within 34 EGF-like repeats. EGF-like repeats have a significant role in Notch signaling [2]. Six cysteine residues in each EGF repeat generate disulfide bonds affecting their native three-dimensional structure. Consequently, they are a crucial component of the EGF domain, and mutations in these residues lead to a pathological phenotype, specifically in CADASIL syndrome [2,30,49]. As shown by the present multiple alignment in the visualized results of the histogram “conservation” (Figure 10), cysteine residues are conserved within all EGF-like repeats. More particularly, the “consensus” histogram (Figure 8) shows the percentage of conserved amino acids at each position. Based on the data, cysteine residues are 100% conserved at positions 27, 41, 43, and 52 while positions 6 and 21 are 97% and 94% conserved, respectively. Additionally, glycine is conserved at positions 49 (97%), 46 (91%), 25 (71%), and 24 (76%), and proline is conserved at position 20 (74%). The greatest concentration of mutations appears at the cysteine residues. Almost 60% of cysteines at position 27 and 50% at positions 6 and 52 were identified as mutated. Results for the identified mutation percentage in each amino acid position of the EGF sequences are demonstrated in the histogram “mutations” (Figure 10). Most of the mutations are identified in positions 6, 27, and 52 of EGF-like repeats.
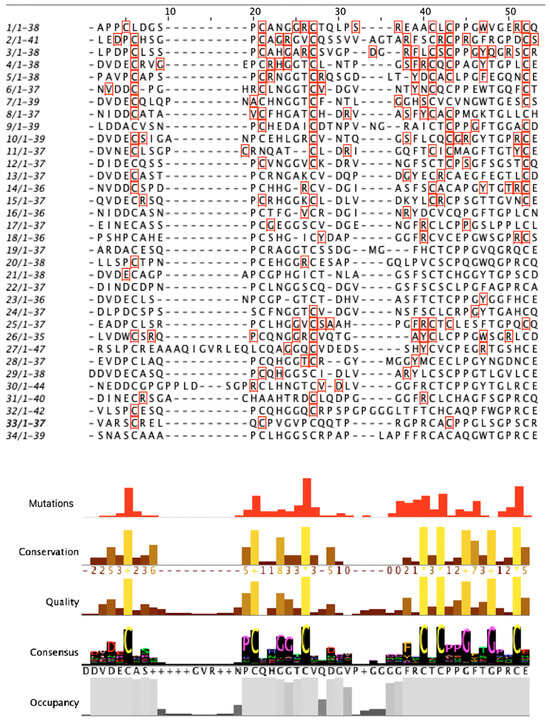
Figure 10.
Conserved amino acids based on the sequence alignment of the EGF-like repeats. Each EGF-like repeat is presented with the specific number and the sequence length. The amino acids marked with the red square are the mutated ones.
The cysteine residues with the greatest level of conservation were analyzed to determine how frequently a certain amino acid change occurs at these positions (Table 8). The conserved cysteine residues are C21, C27, C41, C43, and C52. The frequency of each mutation is represented graphically in Figure 11. Based on the genetic background, more particularly based on the triplets that code for the amino acids replacing the cysteine, the appearance of the specific mutations was expected. The changing of one nucleotide in the triplets coding for cysteine (TGT, TGC) leads to the coding of another amino acid that has two common nucleotides with cysteine. Despite the fact that all cases of cysteine mutations associated with CADASIL syndrome have been reported (Table 8), it is vital to note that no nonsense mutation (TGA) has been reported as a cause of CADASIL. Although, based on the genetic code, the specific mutations are expected, their different frequencies of occurrence lead to the conclusion that these mutations are also related to genetic drift. Most frequently, cysteines were found to be mutated into arginine and tyrosine. This analysis also revealed that cysteines C27S/R/Y/W/F (EGF 5), C43S/R/Y/W/F (EGF 2), and C52S/R/G/F/Y (EGF 4) appeared to be more sensitive to pathogenic changes.

Table 8.
Conserved amino acid changes in cysteine residues at positions (C6, C21, C27, C41, C43, and C52) of the EGF-like repeats of the Notch3 protein, accompanied with the frequency of their appearances and nucleotide changes (marked in red).
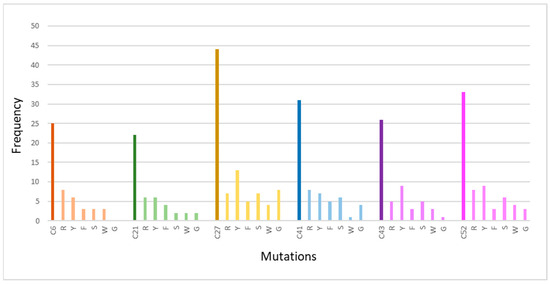
Figure 11.
Presentation of cysteine mutations at positions (C6, C21, C27, C41, C43, and C52) based on the frequency of occurrence of a specific mutation. Τhe chart’s bold-colored columns represent the total number of conserved cysteine mutations of 34 EGF-like repeats.
3.5. Structural Analysis
The structural analysis of the mutations made it possible to understand the implications of inserting specific mutations into the amino acid sequence of the Notch3 EGF domain. Each EGF-like repeat consists of a set of two anti-parallel β-sheets (Figure 12). The structural stability of the Notch3 protein is maintained via disulfide bridges established by a set of strategically positioned cysteine residues [50]. As the key element of the domain, the six cysteine residues of the EGF-like repeat are crucial for the creation of disulfide bonds determining the native 3D structure of Notch proteins (Figure 12B).
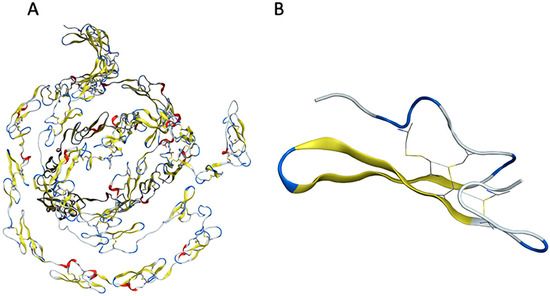
Figure 12.
Structural representation of the EGF domain and the EGF-like 2 repeat of the Notch3 protein. (A) Structure of EGF domain. (B) Structure of EGF-like 2 wild-type repeat.
Mutations in the cysteine residue at position 27 of EGF2 partly rearranged and destabilized the structure of EGF-like repeat due to the destruction of the disulfide bridge between the mutant cysteine and another cysteine residue (Figure 13). A change in the structure of the EGF 2 repeat was induced in each case of cysteine mutation.
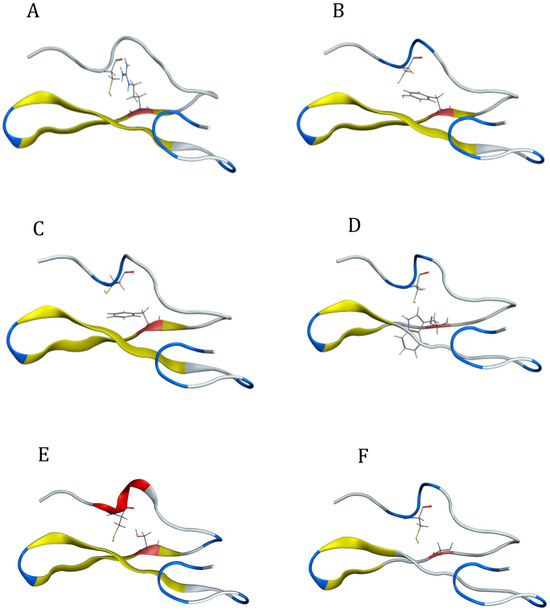
Figure 13.
Structural representation of the mutated EGF2 repeat of the Notch3 protein in the cases where the cysteine at position 27 mutated: (A) C27R, (B) C27Y, (C) C27F, (D) C27W, (E) C27S, and (F) C27G. In the EGF structure, the anti-parallel β-sheets are shown in yellow and the location of the mutated cysteine is shown in orange.
Cysteine residue has a polar, uncharged side chain. The thiol group imparts polarity to cysteine. The induced C27R mutation leads to a broken disulfide bond between the mutant cysteine in one of the β sheets and its interacting cysteine in the coil opposite the β-sheet (Figure 13A). Arginine is a positively charged amino acid with a long side chain that carries a charged guanidine group. The full positive charge of the arginine side chain interacted with the partial negative charge of the cysteine side chain in this study. Even though there was no change in the β-sheet structure, this mutation seemed to affect the coil where the cysteine is located, causing the expansion of the coil because arginine is larger than cysteine. Thus, with the opening of the peptide chain, the space that could accommodate the arginine was created, causing the disturbance of the EGF-like 2 structures.
The C27Y and C27F mutations did not significantly change the structure of EGF despite breaking the disulfide bond. The C27Y mutation results in the differentiation of the coil opposite to tyrosine since tyrosine is larger than cysteine (Figure 13B). Tyrosine is a non-polar amino acid that contains an aromatic side chain. Due to the presence of the hydroxyl group in the side chain, tyrosine is predicted to interact via hydrogen bonding with cysteine. C27F mutation leads to a similar differentiation of the EGF 2 coil since phenylalanine is also an aromatic amino acid with a larger side chain than cysteine (Figure 13C). Phenylalanine is a non-polar amino acid and does not interact with cysteine.
The C27W mutation destroys the disulfide bond and causes a partial loss of the structure of both anti-parallel β sheets (Figure 13D). The large, conjugated side chain of tryptophan got away from the EGF2 core. Due to the lack of available space, 27W was expected to be outside the β-sheet structure. On the contrary, it was found in the available space at the same level defined by the two β-sheets. As a result, the two β-sheets lost their original form as pleated surfaces and were converted into a coil structure.
The C27S mutation destroyed a disulfide bond in the EGF-like 2 structure (Figure 13E). This led to the rearrangement of the coil structure where the cysteine, which interacts with the serine, is located. Dipolar–dipolar interactions between serine and cysteine may have caused this rearrangement. Consequently, an α helix is formed in this region of the EGF2 structure.
The C27G mutation destroys the disulfide bond and causes the partial loss of the two anti-parallel β-sheet structures (Figure 13F). Glycine is a non-polar amino acid that carries a non-polar aliphatic side chain. In the absence of a side group, there is no stereochemical barrier for glycine, allowing it to adopt a variety of conformations that could result in polypeptide chain curving and enhanced flexibility. This feature of glycine may also be responsible for the partial loss of β-sheets and rearrangement into coils.
4. Discussion
Decades of research have shown the significance of the Notch signaling pathway in neural development. More recent studies have proven that Notch receptors continue to be expressed and active in numerous areas of the adult central nervous system [51,52,53].
Adult neurogenesis, memory, synaptic plasticity, acute brain trauma, and chronic neurological diseases have all been linked to Notch signaling [22]. The analysis of mutation datasets revealed that human Notch1, Notch2, and Notch4 proteins are not significantly associated with neurodegenerative diseases [21]. On the other hand, most mutations in Notch3 lead to neurodegenerative diseases, mainly CADASIL syndrome [21,33]. Consequently, the current in silico study yielded new insights that might contribute to a better understanding of the correlation between neurodegenerative disorders and the human Notch family.
Considerable focus was given to analyzing Notch3 protein mutations associated with CADASIL disease. The study of mutations in the Notch3 protein is crucial because it could contribute to a better understanding of the molecular mechanisms that cause the disease, which is easier to study due to its monogenic nature. Even though the majority of mutations are point mutations, the effect of each on the three-dimensional structure of the Notch3 protein is significant [30,54]. Clinical genetics databases, including disease-specific mutation databases and genotype-phenotype research, provide a large amount of data on bioinformatics. Nevertheless, there is a scientific gap in linking the data provided by disease mutation databases and polymorphism databases [55]. Developing a database that provides a unified mapping of nucleotide sequences, protein sequences, and their protein domains, as well as polymorphisms and mutations related to human diseases, may pose a challenge for computational biology.
To date, a series of pathogenic mutations in Notch3 affecting the number of cysteine residues in the receptor’s extracellular domain and resulting in protein misfolding and receptor aggregation have been identified [54]. Cysteine is the most active amino acid since it is involved in a wide range of biological functions [56]. Within extracellular proteins, cysteines are frequently involved in disulfide bridges in which pairs of cysteines are oxidized to create a covalent bond. Disulfide bonds’ primary function is to stabilize protein structures. Cysteine generally has no preference for substituting with any other amino acid [57]. The reported cysteine substitutions in Notch3 ECD that induce CADASIL disease are arginine, tyrosine, phenylalanine, serine, glycine, and tryptophane. Generally, the extremely varied functions that cysteines play in extracellular proteins explain the below preferences for substitution: Arg (-5), Gly (-6), Tyr (-4), Phe (-5), Trp (-5), and Ser (-5) [33,57].
In addition, it has been observed that most frequently, the mutations associated with CADASIL occur in the first two nucleotides and much less frequently in the third nucleotide of the triplet that codes for the cysteine amino acid. Mutations in the first and second nucleotide of the cysteine triplet in this study led to the replacement of cysteine with arginine and glycine, as well as with serine, phenylalanine, and tyrosine. Cysteine substitution to tryptophane was noticed when a mutation occurred in the third nucleotide of the triplet. This study suggested that the first and second nucleotides are sensitive to mutations whereas the third nucleotide appears more conserved. Based on the genetic code, the occurrence of specific mutations was expected [58]. Also, the different frequency of occurrence of each of these mutations is considered linked to the genetic draft, which slowly eliminates the variability that mutations cause, thereby achieving a steady state [59] (high frequency of cysteine mutating to tyrosine and arginine). Recording only one case of nonsense cysteine mutation in the Notch3 protein leads to two possible conclusions. There is the possibility that nonsense cysteine mutations lead to diseases, but no more cases of these mutations have been identified. It is also possible that cases of nonsense cysteine mutations have resulted in fetal death and have not been identified. Consequently, the only mutations identified are the ones that result in non-physiological protein function and therefore cause neurodegeneration. The frequent occurrence of mutations in cysteine residues that are highly conserved in the EGF-like repeats of Notch3 leads to protein misfolding and the manifestation of CADASIL syndrome [54].
Based on the results of the present work, which stem from the specialized study of the EGF-like domain of Notch3, several beneficial conclusions emerge. The accumulation of mutations appears to be different between EGF-like repeats 1 and 34, and these mutations were significantly increased in key amino acids in each EGF-like repeat such as in cysteine, glycine, and arginine (Figure 9 and Figure 10). Today, with the increasing number of experimental data from patients with CADASIL syndrome, it is possible to create a mathematical model through which we will be able to relate the order and the series of mutations in different EGF-like repeats based on a specific phenotype of the disease, as well as based on sex and age [38,50,60,61]. Some studies also have made this observation [39]. In addition, based on the literature, we know the different phenotype in characteristics displayed by each patient that can perhaps be explained by the use of this mathematical model and the use of the above characteristics [61]. On the other hand, several attempts have been made to treat the disease based on the key amino acids that most mutations show in the EGF-like domain of Notch3 [33,40,60,62]. This particular work presents all the candidate positions as a holistic atlas in a detailed analysis of the changes both at the nucleotide and protein level for a contribution in this effort to fight CADASIL syndrome and neurodegenerative diseases in general [21,53].
5. Conclusions
To summarize, the present in silico study focused on analyzing mutations in Notch1–Notch4 proteins correlated with neurodegenerative diseases. The Notch pathway is crucial for the nervous system’s development and pathogenesis due to its strong association with stem/progenitor cell progression and extensive pleiotropy. Neurodegenerative diseases are conditions characterized by the progressive and slow degeneration of neurons resulting in aberrant cell function and cell death. So far, no therapies that cure or prevent the progression of neurodegenerative diseases by targeting their underlying causes have been developed. Current therapies for these disorders are limited to symptom treatment. The integration of molecular methods, such as nanomedicine, genomics, proteomics, bioinformatics, and the measurement of environmental toxic body burdens, holds great promise for accelerating the process of identifying specific risk factors and mechanisms of pathogenesis in order to develop effective therapies for these diseases. Due to their role in cell fate determination and cell communication, as well as the proteolytic process they undergo in the signaling pathway, Notch proteins could be used as promising therapeutic targets for neurodegenerative diseases.
The ultimate aim of the in silico study was to uncover potential CADASIL disease-causing conserved mutations and analyze the consequences of these mutations in the protein structure. Based on the results obtained from the present work, the correlation of Notch3 polymorphisms—mutations with neurodegenerative diseases, especially in CADASIL syndrome—are clearly evident. In particular, the results show the accumulation of most of them in the EGF region of the protein. This specific protein region appears to be very crucial in the biomolecule’s functionality, with changes in the EGF region appearing to lead to neurological pathologies. Through our analysis, we studied the contribution of specific sequence alterations, their frequency of occurrence at candidate sites in each EGF-like repeat, and their frequency of occurrence at specific key amino acids that appear to be conserved in each EGF-like repeat. In this direction, detailed molecular dynamics simulations showed that these conserved mutations trigger local rearrangements in the structure of the mutant EGF-like repeat of the Notch3 protein. The identified conserved mutations of cysteine residues could be used as supplementary pharmacological targets for the development of effective therapeutic schemes against CADASIL.
Since CADASIL syndrome is a monogenic disease, the opportunity to better interpret the mode of function of Notch proteins and their association with neurodegenerative diseases through mutations occurring in Notch3 was utilized. Therefore, we propose the creation of a mathematical model through which we will be able to study, in detail, the importance and contribution of mutations in EGF-like repeats based on both their concentration, frequency of occurrence, and mutation pattern in each specific numbered EGF-like repeat as well as their detection in specific key positions described in this work. As it is known, EGF-like domains are prevalent in numerous protein families, suggesting that the employment of this specific mathematical model could potentially implicate both other proteins in neurodegenerative diseases as well as various other disorders. Furthermore, future objectives should encompass the comprehensive examination of the mutations delineated in this study, particularly those occurring within the intracellular domain, from both evolutionary and structural perspectives.
Author Contributions
Conceptualization, L.P. (Louis Papageorgiou), L.P. (Lefteria Papa), E.P., A.M., K.D., D.C., A.B., E.B.-R., G.P.C., S.K., E.E. and D.V.; methodology, L.P. (Louis Papageorgiou), L.P. (Lefteria Papa), K.D., A.B., C.I., E.B.-R., G.P.C., S.K., E.E. and D.V.; software, L.P. (Louis Papageorgiou), C.I. and D.V.; validation, L.P. (Louis Papageorgiou), L.P. (Lefteria Papa), E.E. and D.V.; formal analysis, L.P. (Louis Papageorgiou), L.P. (Lefteria Papa), E.P. and D.V.; investigation, L.P. (Louis Papageorgiou), S.K., E.E. and D.V.; resources, L.P. (Louis Papageorgiou), L.P. (Lefteria Papa), E.P., A.M., K.D., E.E. and D.V.; data curation, L.P. (Louis Papageorgiou), L.P. (Lefteria Papa), A.B., E.E. and D.V.; writing—original draft preparation, L.P. (Louis Papageorgiou), L.P. (Lefteria Papa), K.D., E.E. and D.V.; writing—review and editing, L.P. (Louis Papageorgiou), D.C., S.K., E.E. and D.V.; visualization, L.P. (Louis Papageorgiou) and L.P. (Lefteria Papa); supervision, L.P. (Louis Papageorgiou), E.E. and D.V.; project administration, D.V. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mohr, O.L. Character Changes Caused by Mutation of an Entire Region of a Chromosome in Drosophila. Genetics 1919, 4, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Vlachakis, D.; Papageorgiou, L.; Papadaki, A.; Georga, M.; Kossida, S.; Eliopoulos, E. An updated evolutionary study of the Notch family reveals a new ancient origin and novel invariable motifs as potential pharmacological targets. PeerJ 2020, 8, e10334. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, S.; Muskavitch, M.A.; Yedvobnick, B. Molecular cloning of Notch, a locus affecting neurogenesis in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1983, 80, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Yochem, J.; Weston, K.; Greenwald, I. The Caenorhabditis elegans lin-12 gene encodes a transmembrane protein with overall similarity to Drosophila Notch. Nature 1988, 335, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Engler, A.; Taylor, V. Notch: An interactive player in neurogenesis and disease. Cell Tissue Res. 2018, 371, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Milner, L.A.; Bigas, A. Notch as a mediator of cell fate determination in hematopoiesis: Evidence and speculation. Blood 1999, 93, 2431–2448. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, A.; Wilson, A.; MacDonald, H.R.; Radtke, F. Paradigms of notch signaling in mammals. Int. J. Hematol. 2005, 82, 277–284. [Google Scholar] [CrossRef]
- Shen, W.; Huang, J.; Wang, Y. Biological Significance of NOTCH Signaling Strength. Front. Cell Dev. Biol. 2021, 9, 652273. [Google Scholar] [CrossRef]
- Sanchez-Martin, M.; Ferrando, A. The NOTCH1-MYC highway toward T-cell acute lymphoblastic leukemia. Blood 2017, 129, 1124–1133. [Google Scholar] [CrossRef]
- Garg, V.; Muth, A.N.; Ransom, J.F.; Schluterman, M.K.; Barnes, R.; King, I.N.; Grossfeld, P.D.; Srivastava, D. Mutations in NOTCH1 cause aortic valve disease. Nature 2005, 437, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Xiu, M.X.; Liu, Y.M. The role of oncogenic Notch2 signaling in cancer: A novel therapeutic target. Am. J. Cancer Res. 2019, 9, 837–854. [Google Scholar] [PubMed]
- Arruga, F.; Vaisitti, T.; Deaglio, S. The NOTCH Pathway and Its Mutations in Mature B Cell Malignancies. Front. Oncol. 2018, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Maekawa, Y.; Kitamura, A.; Nishida, J.; Koyanagi, A.; Yagita, H.; Kojima, H.; Chiba, S.; Shimada, M.; Yasutomo, K. Notch2 signaling is required for potent antitumor immunity in vivo. J. Immunol. 2010, 184, 4673–4678. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Zanotti, S. Hajdu-Cheney Syndrome, a Disease Associated with NOTCH2 Mutations. Curr. Osteoporos. Rep. 2016, 14, 126–131. [Google Scholar] [CrossRef]
- Turnpenny, P.D.; Ellard, S. Alagille syndrome: Pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 2012, 20, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Manini, A.; Pantoni, L. CADASIL from Bench to Bedside: Disease Models and Novel Therapeutic Approaches. Mol. Neurobiol. 2021, 58, 2558–2573. [Google Scholar] [CrossRef]
- Hosseini-Alghaderi, S.; Baron, M. Notch3 in Development, Health and Disease. Biomolecules 2020, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Snijders, A.M.; Schmidt, B.L.; Fridlyand, J.; Dekker, N.; Pinkel, D.; Jordan, R.C.; Albertson, D.G. Rare amplicons implicate frequent deregulation of cell fate specification pathways in oral squamous cell carcinoma. Oncogene 2005, 24, 4232–4242. [Google Scholar] [CrossRef]
- Xiu, M.; Zeng, X.; Shan, R.; Wen, W.; Li, J.; Wan, R. Targeting Notch4 in Cancer: Molecular Mechanisms and Therapeutic Perspectives. Cancer Manag. Res. 2021, 13, 7033–7045. [Google Scholar] [CrossRef]
- Kapoor, A.; Nation, D.A. Role of Notch signaling in neurovascular aging and Alzheimer’s disease. Semin. Cell Dev. Biol. 2021, 116, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Guerra, J.; Valadao, A.L.; Vlachakis, D.; Polak, K.; Vila, I.K.; Taffoni, C.; Prabakaran, T.; Marriott, A.S.; Kaczmarek, R.; Houel, A.; et al. Lysyl-tRNA synthetase produces diadenosine tetraphosphate to curb STING-dependent inflammation. Sci. Adv. 2020, 6, eaax3333. [Google Scholar] [CrossRef] [PubMed]
- Basak, O.; Giachino, C.; Fiorini, E.; Macdonald, H.R.; Taylor, V. Neurogenic subventricular zone stem/progenitor cells are Notch1-dependent in their active but not quiescent state. J. Neurosci. 2012, 32, 5654–5666. [Google Scholar] [CrossRef]
- Nyfeler, Y.; Kirch, R.D.; Mantei, N.; Leone, D.P.; Radtke, F.; Suter, U.; Taylor, V. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 2005, 24, 3504–3515. [Google Scholar] [CrossRef]
- Lv, Y.; Pang, X.; Cao, Z.; Song, C.; Liu, B.; Wu, W.; Pang, Q. Evolution and Function of the Notch Signaling Pathway: An Invertebrate Perspective. Int. J. Mol. Sci. 2024, 25, 3322. [Google Scholar] [CrossRef]
- Sprinzak, D.; Blacklow, S.C. Biophysics of Notch Signaling. Annu. Rev. Biophys. 2021, 50, 157–189. [Google Scholar] [CrossRef]
- Urban, N.; van den Berg, D.L.; Forget, A.; Andersen, J.; Demmers, J.A.; Hunt, C.; Ayrault, O.; Guillemot, F. Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science 2016, 353, 292–295. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Jagaran, K.; Singh, M. Nanomedicine for Neurodegenerative Disorders: Focus on Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2021, 22, 9082. [Google Scholar] [CrossRef] [PubMed]
- Vlachakis, D.; Tsaniras, S.C.; Ioannidou, K.; Papageorgiou, L.; Baumann, M.; Kossida, S. A series of Notch3 mutations in CADASIL; insights from 3D molecular modelling and evolutionary analyses. J. Mol. Biochem. 2014, 3, 134. [Google Scholar]
- Ables, J.L.; Breunig, J.J.; Eisch, A.J.; Rakic, P. Not(ch) just development: Notch signalling in the adult brain. Nat. Rev. Neurosci. 2011, 12, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M. Cadasil. Handb. Clin. Neurol. 2018, 148, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, I.; Nakao-Azuma, Y.; Yoshida, H.; Yamaguchi, M.; Mizuno, T. Progress to Clarify How NOTCH3 Mutations Lead to CADASIL, a Hereditary Cerebral Small Vessel Disease. Biomolecules 2024, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Louvi, A.; Arboleda-Velasquez, J.F.; Artavanis-Tsakonas, S. CADASIL: A critical look at a Notch disease. Dev. Neurosci. 2006, 28, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N.; Kittner, S.J. Top-NOTCH3 Variants in the Population at Large. Stroke 2020, 51, 3482–3484. [Google Scholar] [CrossRef] [PubMed]
- Haltom, A.R.; Jafar-Nejad, H. The multiple roles of epidermal growth factor repeat O-glycans in animal development. Glycobiology 2015, 25, 1027–1042. [Google Scholar] [CrossRef]
- Zeng, Q.; Pan, H.; Zhao, Y.; Wang, Y.; Xu, Q.; Tan, J.; Yan, X.; Li, J.; Tang, B.; Guo, J. Association between NOTCH3 gene and Parkinson’s disease based on whole-exome sequencing. Front. Aging Neurosci. 2022, 14, 995330. [Google Scholar] [CrossRef] [PubMed]
- Szymanowicz, O.; Korczowska-Lacka, I.; Slowikowski, B.; Wiszniewska, M.; Piotrowska, A.; Goutor, U.; Jagodzinski, P.P.; Kozubski, W.; Dorszewska, J. Headache and NOTCH3 Gene Variants in Patients with CADASIL. Neurol. Int. 2023, 15, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Rutten, J.W.; Van Eijsden, B.J.; Duering, M.; Jouvent, E.; Opherk, C.; Pantoni, L.; Federico, A.; Dichgans, M.; Markus, H.S.; Chabriat, H.; et al. The effect of NOTCH3 pathogenic variant position on CADASIL disease severity: NOTCH3 EGFr 1-6 pathogenic variant are associated with a more severe phenotype and lower survival compared with EGFr 7-34 pathogenic variant. Genet. Med. 2019, 21, 676–682. [Google Scholar] [CrossRef]
- Matsushima, T.; Conedera, S.; Tanaka, R.; Li, Y.; Yoshino, H.; Funayama, M.; Ikeda, A.; Hosaka, Y.; Okuzumi, A.; Shimada, Y.; et al. Genotype-phenotype correlations of cysteine replacement in CADASIL. Neurobiol. Aging 2017, 50, 169.e7–169.e14. [Google Scholar] [CrossRef]
- Young, K.Z.; Lee, S.J.; Zhang, X.; Cartee, N.M.P.; Torres, M.; Keep, S.G.; Gabbireddy, S.R.; Fontana, J.L.; Qi, L.; Wang, M.M. NOTCH3 is non-enzymatically fragmented in inherited cerebral small-vessel disease. J. Biol. Chem. 2020, 295, 1960–1972. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, M.; Ludwig, H.; Muller-Hocker, J.; Messerschmidt, A.; Gasser, T. Small in-frame deletions and missense mutations in CADASIL: 3D models predict misfolding of Notch3 EGF-like repeat domains. Eur. J. Hum. Genet. 2000, 8, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ren, Z.; Shi, Y.; Zhang, J. A Novel Mutation Outside of the EGFr Encoding Exons of NOTCH3 Gene in a Chinese with CADASIL. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020, 29, 105410. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Mizuta, I.; Watanabe-Hosomi, A.; Mukai, M.; Koizumi, T. Clinical and Genetic Aspects of CADASIL. Front. Aging Neurosci. 2020, 12, 91. [Google Scholar] [CrossRef]
- Sari, U.S.; Kisabay, A.; Batum, M.; Tarhan, S.; Dogan, N.; Coskunoglu, A.; Cam, S.; Yilmaz, H.; Selcuki, D. CADASIL with Atypical Clinical Symptoms, Magnetic Resonance Imaging, and Novel Mutations: Two Case Reports and a Review of the Literature. J. Mol. Neurosci. 2019, 68, 529–538. [Google Scholar] [CrossRef]
- Servito, M.; Gill, I.; Durbin, J.; Ghasemlou, N.; Popov, A.F.; Stephen, C.D.; El-Diasty, M. Management of Coronary Artery Disease in CADASIL Patients: Review of Current Literature. Medicina 2023, 59, 586. [Google Scholar] [CrossRef]
- Mitchell, A.; Chang, H.Y.; Daugherty, L.; Fraser, M.; Hunter, S.; Lopez, R.; McAnulla, C.; McMenamin, C.; Nuka, G.; Pesseat, S.; et al. The InterPro protein families database: The classification resource after 15 years. Nucleic Acids Res. 2015, 43, D213–D221. [Google Scholar] [CrossRef] [PubMed]
- Chillakuri, C.R.; Sheppard, D.; Lea, S.M.; Handford, P.A. Notch receptor-ligand binding and activation: Insights from molecular studies. Semin. Cell Dev. Biol. 2012, 23, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, E.; Bacopoulou, F.; Brouzas, D.; Megalooikonomou, V.; D’Elia, D.; Bongcam-Rudloff, E.; Vlachakis, D. NOTCH3 and CADASIL syndrome: A genetic and structural overview. EMBnet J. 2019, 24, e921. [Google Scholar] [CrossRef]
- Muino, E.; Gallego-Fabrega, C.; Cullell, N.; Carrera, C.; Torres, N.; Krupinski, J.; Roquer, J.; Montaner, J.; Fernandez-Cadenas, I. Systematic Review of Cysteine-Sparing NOTCH3 Missense Mutations in Patients with Clinical Suspicion of CADASIL. Int. J. Mol. Sci. 2017, 18, 1964. [Google Scholar] [CrossRef]
- Salazar, J.L.; Yang, S.A.; Yamamoto, S. Post-Developmental Roles of Notch Signaling in the Nervous System. Biomolecules 2020, 10, 985. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.M.; Artavanis-Tsakonas, S.; Louvi, A. The Notch pathway in CNS homeostasis and neurodegeneration. Wiley Interdiscip. Rev. Dev. Biol. 2020, 9, e358. [Google Scholar] [CrossRef] [PubMed]
- Lampada, A.; Taylor, V. Notch signaling as a master regulator of adult neurogenesis. Front. Neurosci. 2023, 17, 1179011. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Zhang, X.; Wu, E.; Sukpraphrute, R.; Sukpraphrute, C.; Ye, A.; Wang, M.M. Structural changes in NOTCH3 induced by CADASIL mutations: Role of cysteine and non-cysteine alterations. J. Biol. Chem. 2023, 299, 104838. [Google Scholar] [CrossRef] [PubMed]
- Webb, E.A.; Smith, T.D.; Cotton, R.G. Difficulties in finding DNA mutations and associated phenotypic data in web resources using simple, uncomplicated search terms, and a suggested solution. Hum. Genom. 2011, 5, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Nallapareddy, V.; Bogam, S.; Devarakonda, H.; Paliwal, S.; Bandyopadhyay, D. DeepCys: Structure-based multiple cysteine function prediction method trained on deep neural network: Case study on domains of unknown functions belonging to COX2 domains. Proteins 2021, 89, 745–761. [Google Scholar] [CrossRef] [PubMed]
- Barnes, M.R. Bioinformatics for Geneticists a Bioinformatics Primer for the Analysis of Genetic Data, 2nd ed.; Wiley: Chichester, UK, 2007. [Google Scholar] [CrossRef]
- Saier, M.H.J. Understanding the Genetic Code. J. Bacteriol. 2019, 201. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.; Maloy, S.R.; Hughes, K.T. Brenner’s encyclopedia of genetics. In Encyclopedia of Genetics, 2nd ed.; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Locatelli, M.; Padovani, A.; Pezzini, A. Pathophysiological Mechanisms and Potential Therapeutic Targets in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL). Front. Pharmacol. 2020, 11, 321. [Google Scholar] [CrossRef] [PubMed]
- Dupe, C.; Guey, S.; Biard, L.; Dieng, S.; Lebenberg, J.; Grosset, L.; Alili, N.; Herve, D.; Tournier-Lasserve, E.; Jouvent, E.; et al. Phenotypic variability in 446 CADASIL patients: Impact of NOTCH3 gene mutation location in addition to the effects of age, sex and vascular risk factors. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow. Metab. 2023, 43, 153–166. [Google Scholar] [CrossRef]
- Oliveira, D.V.; Coupland, K.G.; Shao, W.; Jin, S.; Del Gaudio, F.; Wang, S.; Fox, R.; Rutten, J.W.; Sandin, J.; Zetterberg, H.; et al. Active immunotherapy reduces NOTCH3 deposition in brain capillaries in a CADASIL mouse model. EMBO Mol. Med. 2023, 15, e16556. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).