Abstract
This experiment was conducted to evaluate the effects of dietary Chinese yam polysaccharides (CYP) on myogenic differentiation 1 (MYOD1), myogenin (MYOG), and myostatin (MSTN) mRNA expression of breast and thigh muscle tissues in broilers. A total of 360 (1-day-old, gender-balanced) crossbred broilers chicks with similar body weight (BW) were randomly distributed into four groups, with three replicates in each group and each replicate included 30 broilers. The feeding trial lasted for 48 days. Experimental broilers were fed 0.00 mg/kg basal diet (control group), 250 mg/kg, 500 mg/kg, and 1000 mg/kg CYP, respectively. The results showed that CYP250 and CYP500 groups had higher thigh muscle percentage (TMP) compared to the control group (p < 0.05). Meanwhile, the expression of MYOD1, MYOG mRNA in breast muscle tissues of CYP500 and CYP1000 groups was higher (p < 0.05), and the expression of MSTN mRNA in thigh muscle of CYP250, CYP500, and CYP1000 groups was lower than that of the control group (p < 0.05). In addition, there was no significant difference in the expression of MYOD1 mRNA in the thigh muscle tissue of each group (p > 0.05). Bivariate correlation analysis showed that the expression levels of MYOD1, MYOG, and MSTN mRNA in the thigh muscle tissue of broiler chickens in the CYP500 group were positively correlated with TMP. However, the expression of MYOG mRNA in thigh muscle tissue of the CYP1000 group was negatively correlated with TMP. In general, this study indicated that appropriate dietary CYP supplementation influenced the growth and development of thigh muscle tissue in broilers by altering TMP and muscle tissue development-related genes expression. Therefore, CYP could be used as a potential feed additive to promote the development of muscle tissues in broilers.
1. Introduction
As the biological resources of natural plants, Chinese herbs are a source of green feed additive with no residue, wide source, and low cost, which contains polysaccharides, organic acids, flavonoids, and other bioactive substances [1]. Chinese herbal polysaccharides (CHPs) are secondary metabolites extracted from Chinese herbal medicines such as the Chinese yam (CY) and Lycium barbarum. They have a variety of biological functions, such as regulating intestinal microflora, antioxidant and anti-inflammatory responses, anti-tumor, and so on [2,3,4], which can improve the growth performance and immunity of animals [5], therefore, in recent years, polysaccharides of Chinese herbal medicine have become the research focus of many scholars. CY belongs to the dry rhizome of Dioscorea opposita Thunb [6], which is widely distributed in the tropical and subtropical regions of China [7]. The 2010 edition of The Pharmacopoeia of the People’s Republic of China clearly records that yam, as one of the traditional Chinese herbs, has edible and medicinal value in China for more than 1500 years [8]. It contains polysaccharides, proteins, starches, amino acids, etc., biologically active ingredients [9,10], among which CYP extracted from CY are mainly included in glucose (Glu) and galactose (Gal) [11]. Animal experiments research found that CYP has many functions, such as promoting animal growth, enhancing antioxidation, and immune function. The average daily food intake and average daily body weight gain of the rats fed the containing CYP diet were greater than those of the control group [12]. CYP supplements showed scavenging activity on the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical, hydroxyl radical and superoxide anion radical in increasing concentration [13]. Adding CYP to the diet increases the proliferation activity of lymphocytes and cytokine levels in weaned rats [12]. Our previous study also confirmed that CYP has a positive effect on improving immune function of crossbred broilers [14].
Skeletal muscle is one of the important tissues for weight gain in broilers, including breast and thigh muscles [15], with its development regulated by MYOD1, MYOG, MSTN, and other regulatory factors [16,17]. MYOD1 is a skeletal muscle-specific transcription factor, which can act on activation, proliferation, and differentiation of satellite cells, and plays an important biological regulatory role in fast- and slow-muscle formation [18,19]. MYOG is a key regulator of skeletal muscle differentiation and plays an important role in terminal myoblast differentiation [20,21]. MSTN, also known as growth differentiation factor 8 (GDF-8), negatively regulates skeletal muscle growth by blocking myoblast proliferation and differentiation to control the number of muscle fibers [22,23]. However, there is no report about the effect of adding CYP on the differential expression of MYOD1, MYOG, and MSTN gene mRNA in broiler breast muscle and thigh muscle tissue. In order to explore the relationship between CYP and the growth and development of muscle tissues in broilers, the purpose of this experiment is to study the effect of adding CYP in the diet on broiler breast muscle percentage (BMP), TMP and gene expression related to muscle development, and the relationship between gene expression and BMP or TMP. These results will help us understand the regulatory function of CYP in the growth and development of muscle tissue and the expression of related genes in broilers.
2. Materials and Methods
2.1. Experimental Design, Diets and Broilers
All procedures involving broilers feeding were reviewed and approved by the Animal Protection and Utilization Committee of Henan Institute of Science and Technology (No. 2021HIST018, Xinxiang, China). A total of 360 (1-day-old, gender-balanced) crossbred broilers chicks with similar initial BW (39.54 ± 0.51 g) were randomly allotted to 4 groups (Control, CYP250, CYP500, and CYP1000, respectively) with 3 replicates in each group and each replicate included 30 broilers (sex balanced). The control group was fed a basal diet without CYP, and the CYP250, CYP500, and CYP1000 groups received the same basal diets included 250, 500, and 1000 mg/kg CYP, respectively. Feeding trials were divided into early (1–28 days) and late (29–48 days). The basal diet meeting the National Research Council (NRC, 1994) requirements is shown in Table 1. All broilers had free access to food and water during the experiment. Crossbred broilers chicks are raised in appropriate closed cages to control the air temperature. The lamp is turned on continuously for 24 h, and the temperature is kept at 32 ℃ for the first 3 days, then it is gradually cooled until 26 ℃ from days 4 to 21. The CYP in this study were provided by Shanxi Hanna Biotechnology Co., Ltd. (Xian, Shanxi, China) carbohydrate level more than 90%, polysaccharide content exceeds 30.00% (of which monosaccharides include 99.48% Glu and 0.52% Gal).

Table 1.
Ingredient and nutrient levels of the basal diet in each feeding phase for broilers.
2.2. Slaughter and Samples Collection
On day 48, after fasting for 12 h, 6 crossbred broilers were randomly selected from each group (2 broilers per replicate, gender balance). After the neck was bled and slaughtered, breast and thigh muscle were harvested and weighed respectively, and used for carcass performance determination. The percentage of breast and thigh muscle tissues are calculated as a percentage of the eviscerated weight. Meanwhile, breast and thigh muscle tissue were collected from the same site, preserved in liquid nitrogen, and then stored at −80 °C in a freezer until further analysis.
2.3. Messenger RNA Expression Analysis
Total RNA was detached from breast and thigh muscle tissues by employing TRizol reagent (Takara Biomedical Technology Co., Ltd., Beijing, China). After extraction, total RNA utilizing a spectrophotometer (Biodrop µLite, Cambridge, United Kingdom) measured the concentration of RNA within the A260:A280 ratio range. The RNA was reverse transcribed using test kits by Thermo Fisher Scientific to make cDNA. Each sample was measured in triplicate. Relative mRNA expression of β-actin, MYOD1, MYOG, and MSTN was determined by the 2−ΔΔCt method.
2.4. Primer Design
Based on GenBank (accessed on 18 June 2021 https://www.ncbi.nlm.nih.gov/genbank/) known sequences were designed with Primer 5.0 software β-actin, MYOD1, MYOG, and MSTN mRNA primers [24]. All primer sequences were provided by Sangon Biotech Co. (Shanghai, China) and all primer information is shown in Table 2.

Table 2.
Primer sequences for RT-qPCR in this experiment.
2.5. Statistical Analysis
All data were presented as mean ± SEM (standard error of the means). Muscles percentage and gene expression data were analyzed using a one-way ANOVA test. Prism 8 (GraphPad Prism, La Jolla, CA, USA) was used to generate graphs. SPSS 26.0 for Windows (IBM Corp., Chicago, IL, USA) was used to perform the Pearson’s correlation coefficient two tail test to evaluate the correlation between muscle development-related genes expression and BMP or TMP. p < 0.05 was considered statistically significant.
3. Results
3.1. BMP and TMP
Effects of dietary CYP on the BMP and TMP in broilers are shown in Figure 1. No differences in BMP were found among all groups (p > 0.05). However, compared with the control group, the TMP of broilers in the CYP250 and CYP500 groups was higher (p < 0.05).
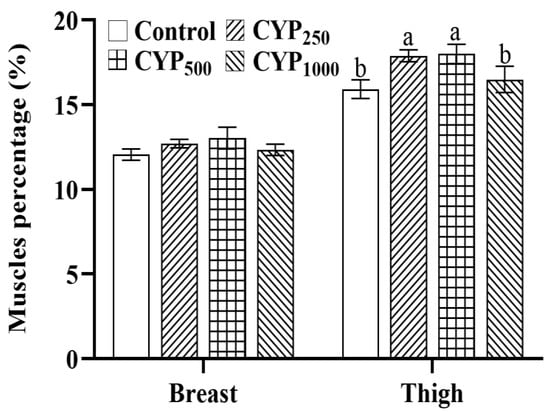
Figure 1.
Effects of dietary CYP supplementation on BMP and TMP in broilers. Different lowercase superscripts indicate significant differences (p < 0.05).
3.2. Muscle Development-Related Genes Expression
As shown in Figure 2, in breast muscle tissues, CYP500 and CYP1000 groups had higher MYOD1 mRNA expression compared to the control group (p < 0.05). Meanwhile, compared to the control group, the CYP500 and CYP1000 groups had higher MYOG expression, CYP250 and CYP500 groups had higher MSTN expression (p < 0.05).
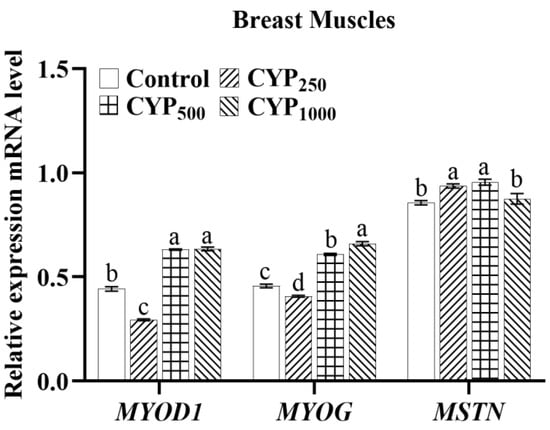
Figure 2.
The MYOD1, MYOG, and MSTN mRNA expressions of breast muscle in broilers. Different lowercase superscripts indicate significant differences (p < 0.05). MYOD1, myogenic differentiation 1; MYOG, myogenin; and MSTN, myostatin.
As shown in Figure 3, there was no difference in the expression of MYOD1 mRNA in the thigh muscle tissues of each group (p > 0.05), while MYOG and MSTN mRNA expression in CYP250, CYP500, and CYP1000 groups was significantly lower than that in the control group (p < 0.05). In addition, MYOG and MSTN mRNA expression of the CYP500 group was higher than that in the CYP250 and CYP1000 groups (p < 0.05).
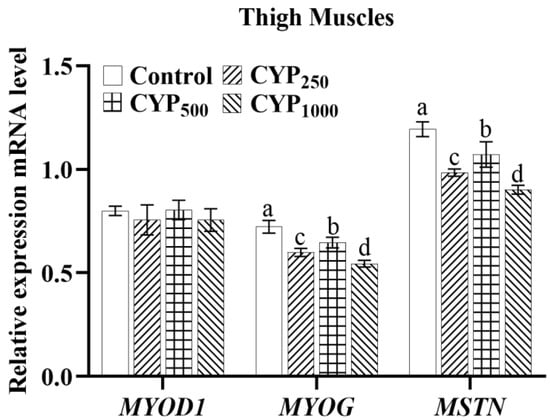
Figure 3.
The MYOD1, MYOG, and MSTN mRNA expressions of thigh muscle in broilers. Different lowercase superscripts indicate significant differences (p < 0.05). MYOD1, myogenic differentiation 1; MYOG, myogenin; and MSTN, myostatin.
3.3. Correlations between Genes Expression and BMP or TMP
As shown in Table 3, in breast muscle tissue, the MYOD1, MYOG, and MSTN mRNA expression showed no significant correlation with BMP, TMP in each group (p > 0.05).

Table 3.
The correlation between MYOD1, MYOG, and MSTN mRNA expression of breast muscle and BMP or TMP.
As shown in Table 4, the MYOD1, MYOG, and MSTN mRNA expression was significantly positively correlated with TMP in the CYP500 group (MYOD1/TMP, content: r = 0.819, p = 0.046; MYOG/TMP, content: r = 0.912, p = 0.011; and MSTN/TMP, content: r = 0.851, p = 0.032, respectively), while the MYOG mRNA expression was negatively correlated with TMP in the CYP1000 group (MYOG/TMP, content: r = −0.973, p = 0.001).

Table 4.
The correlation between MYOD1, MYOG, and MSTN mRNA expression of thigh muscle and BMP or TMP.
4. Discussion
Previous study showed that BMP and TMP are important traits of carcass yield, which are closely related to the increase of broiler growth and carcass yield [25,26]. Many studies have shown that different kinds of CHPs can increase the TMP of poultry. Sun et al. [27] observed that 1000 mg/kg supplemented with Astragalus membranaceus polysaccharides (AMP) significantly enhanced the TMP of chickens. Zhang et al. [28] found that 1% supplemented with AMP can significantly increase the TMP in broilers, thus confirming that AMP could enhance the carcass yield. Sun et al. [29] also found that L. barbarum polysaccharides (LBP) additives could improve TMP and enhance the muscle tissue development of early chickens. The present study found that dietary appropriate CYP supplementation could significantly increase TMP of broilers. Our study was similar to previous reports showing that CHPs can increase TMP and promote muscle tissues development in broiler chickens.
Numerous evidence demonstrated that MYOD1, MYOG, and MSTN play key roles in the muscle growth and development. MYOD1 and MYOG are members of the myogenic regulatory factors (MRFs) family, and their expression levels are closely related to the growth rate of muscle tissues [30,31]. As an effective growth and differentiation factor, MSTN has been shown to be involved in inhibiting muscle precursor cell proliferation, which determines muscle tissue growth and development [32]. In addition, MSTN is also involved in the formation of skeletal muscle in broilers [33]. In the current study, CYP500 and CYP1000 significantly enhanced the mRNA expression of the MYOD1 and MYOG in breast muscle tissues. In addition, a diet which is CYP supplemented significantly downregulated the mRNA expression of the MSTN in thigh muscle tissues of broilers. These results indicate that dietary appropriate CYP supplementation has a positive effect on the expression of MYOD1 and MYOG genes related to muscle development in broilers, and can also inhibit the expression of the negative regulator MSTN. All of these results suggest that the CYP promotes broiler muscle development by regulating the expression of muscle-related genes, which may be related to intestinal absorption and utilization of nutrients or digestive enzyme activities. However, how the mechanisms CYP regulate gene expression need to be further studied.
During muscle development, MYOD1 is a primary myogenic regulator, which is expressed before differentiation of myoblasts [34], and MYOG is a secondary myogenic regulator, which is expressed during and after differentiation of myoblasts [35], both of which play a positive regulating role. While MSTN is an important negative regulator of muscle tissue growth and negatively regulates muscle growth in animals [36,37]. According to bivariate correlation analysis, MYOD1, MYOG, and MSTN mRNA expression in breast muscle tissues showed no significant correlation with BMP and TMP of the broilers. Moreover, the MYOD1, MYOG, and MSTN mRNA expression in thigh muscle tissues of the CYP500 supplemented group was significantly positively correlated with TMP of broilers. Meanwhile, the MYOG mRNA expression in thigh muscle tissues of the CYP1000 supplementation group was negatively correlated with TMP of broilers. The results demonstrated that diets supplemented with CYP500 and CYP1000 might affect the growth and development of thigh muscle tissues in broilers. Overall, the MYOD1, MYOG, and MSTN mRNA expression was correlated with TMP of broilers in thigh muscle tissues. Additionally, dietary appropriate CYP supplementation may affect muscle tissue development of broilers by regulating the expression of MYOD1, MYOG, and MSTN mRNA. However, the exact molecular mechanism needs further study.
5. Conclusions
In this study, the mRNA expression of MYOD1, MYOG, and MSTN in thigh muscle tissue was significantly positively correlated with TMP in the CYP500 supplemented group. MYOG mRNA expression in thigh muscle tissues of the dietary CYP1000 supplementation group was significantly negatively correlated with TMP. At the same time, adding appropriate CYP to the diet can increase the expression of MYOD1 and MYOG and reduce the expression of MSTN mRNA, thereby improving the breast and thigh muscles yield in broilers. Therefore, CYP could be used as a potential feed additive to promote the development of muscle tissues in broilers.
Author Contributions
Investigation, writing—original manuscript, and writing—review and editing: J.D.; data curation and formal analysis: Y.J. and Y.C.; conceptualization, methodology, and supervision: J.Z. and M.S.; project administration and funding acquisition; Z.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Program for Innovative Research Team (in Science and Technology) at the University of Henan Province (22IRTSTHN026) and the Provincial Key Technology Research and Development Program of Henan (212102110180 and 212102110169).
Institutional Review Board Statement
Animal Protection and Utilization Committee of Henan Institute of Science and Technology, No. 2021HIST018.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
Thanks to all the authors for their contributions to the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Misaki, R.; Fujiyama, K.; Kajiura, H. Structure and biological functions of plant glycans and polysaccharides. Ref. Module. Chem. Mol. Sci. Chem. Eng. 2021, 5, 93–109. [Google Scholar]
- Zhang, J.; Cai, K.; Mishra, R.; Jha, R. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affects cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult. Sci. 2020, 99, 4776–4785. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, Z.; Pan, Z.; An, L.; Balasubramanian, B.; Liu, W. New insights into the role of dietary marine-derived polysaccharides on productive performance, egg quality, antioxidant capacity, and jejunal morphology in late-phase laying hens. Poult. Sci. 2020, 99, 2100–2107. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Qu, H.; Jia, J.; Kuang, C.; Wen, Y.; Yan, H.; Gui, Z. Characterization, antioxidant and antitumor activities of polysaccharides from purple sweet potato. Carbohydr. Polym. 2015, 132, 31–40. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Chang, B.; Gong, L.; Dai, J.; Yi, G. Effects of Chinese herbal polysaccharides on the immunity and growth performance of young broilers. Poult. Sci. 2003, 82, 364. [Google Scholar] [CrossRef]
- Li, Z.; Xiao, W.; Xie, J.; Chen, Y.; Yu, Q.; Zhang, W.; Shen, M. Isolation, Characterization and Antioxidant Activity of Yam Polysaccharides. Foods 2022, 11, 800. [Google Scholar] [CrossRef]
- Cheng, Z.; Hu, M.; Tao, J.; Yang, H.; Yan, P.; An, G.; Wang, H. The protective effects of Chinese yam polysaccharide against obesity-induced insulin resistance. J. Funct. Foods 2019, 55, 238–247. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Fan, Y.; Man, S.; Liu, Z.; Gao, W.; Wang, T. Antioxidant and Antitumor Activities of the Extracts from Chinese Yam (Dioscorea opposite Thunb.) Flesh and Peel and the Effective Compounds. J. Food Sci. 2016, 81, H1553–H1564. [Google Scholar] [CrossRef]
- Judith, B.; Denise, L.; Stephan, H.; Zakaria, F.; Felix, E.; Petit, B. Isolation, Physicochemical Characterization and Application of Yam (Dioscorea spp.) Starch as Thickening and Gelling Agent. Starch-Stärke 2005, 57, 107–117. [Google Scholar]
- Huang, R.; Xie, J.; Yu, Y.; Shen, M. Recent progress in the research of yam mucilage polysaccharides: Isolation, structure and bioactivities. Int. J. Biol. Macromol. 2020, 155, 1262–1269. [Google Scholar] [CrossRef]
- Yang, W.; Ying, W.; Li, X.; Ping, Y. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr. Polym. 2014, 117, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, Y.; Yin, Y.; Wu, G.; Zhou, H.; Tan, Z.; Yang, F.; Bo, M.; Huang, R.; Li, T.; et al. Chinese Yam polysaccharide enhances growth performance and cellular immune response in weanling rats. J. Sci. Food Agric. 2009, 89, 2039–2044. [Google Scholar] [CrossRef]
- Ju, Y.; Xue, Y.; Huang, J.; Zhai, Q.; Wang, X. Antioxidant Chinese yam polysaccharides and its pro-proliferative effect on endometrial epithelial cells. Int. J. Biol. Macromol. 2014, 66, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhang, J.; Chang, Y.; Wang, S.; Shi, M.; Miao, G. Effects of Chinese yam polysaccharides on the immune function and serum biochemical indexes of broilers. Front. Vet. Sci. 2022, 9, 1013888. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xu, W.; Hu, Y.; Zhu, W.; Song, C.; Chen, W.; Li, H. Early Development and Developmental Expression of Insulin-like Growth Factor 1 Receptor (IGF-IR) mRNA in Gaoyou Duck and Jinding Duck (Anas platyrhynchos domestica) Skeletal Muscle. J. Agric. Biotechnol. 2013, 21, 192–198. [Google Scholar]
- Wang, Q.; Mcpherron, A. Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cells activation. J. Physiol. 2012, 590, 2151–2165. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yao, H.; Zhang, Z.; Zhang, B.; Meng, F.; Xu, S.; Wang, X. Possible Correlation between Selenoprotein W and Myogenic Regulatory Factors in Chicken Embryonic Myoblasts. Biol. Trace. Elem. Res. 2012, 150, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, M.; Jaenisch, R. The MYOD family of transcription factors and skeletal Myogenesis. Bioessays 1995, 17, 203–209. [Google Scholar] [CrossRef]
- Maves, L.; Waskiewicz, A.; Paul, B.; Cao, Y.; Tyler, A.; Cecilia, B.; Stephen, S. Pbx homeodomain proteins direct Myod activity to promote fast-muscle differentiation. Development 2007, 134, 3371–3382. [Google Scholar] [CrossRef]
- Du, C.; Jin, Y.; Qi, J.; Ji, Z.; Li, S.; An, G.; Jia, H.; Ni, J. Effects of myogenin on expression of late muscle genes through MyoD-dependent chromatin remodeling ability of myogenin. Mol. Cells 2012, 34, 133–142. [Google Scholar] [CrossRef]
- Jawasreh, K.; Athamneh, S.; Al-Zghoul, M.; Amareen, A.; Alsukhni, I.; Aad, P. Evaluation of growth performance and muscle marker genes expression in four different broiler strains in Jordan. Ital. J. Anim. Sci. 2019, 18, 766–776. [Google Scholar] [CrossRef]
- Carnac, G.; Vernus, B.; Bonnieu, A. Myostatin in the pathophysiology of skeletal muscle. Curr. Genom. 2007, 8, 415–422. [Google Scholar]
- Li, F.; Shan, A.; Hu, J.; Zheng, Y.; Xu, L.; Chen, Z. Changes to daily feed intake during the laying period alters embryonic MSTN and MYOG gene expression in genetically fat and lean lines of chickens. Br. Poult. Sci. 2013, 54, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, S. Primer Premier 5. Biotech Softw. Internet Rep. Comput. Softw. J. Sci. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Musa, H.; Chen, G.; Cheng, J.; Li, B.; Mekki, D. Study on carcass characteristics of chicken breeds raised under the intensive condition. Int. J. Poult. Sci. 2006, 5, 530–533. [Google Scholar]
- Jin, S.; Yang, L.; Zang, H.; Xu, Y.; Chen, X.; Chen, X.; Liu, P.; Geng, Z. Influence of free-range days on growth performance, carcass traits, meat quality, lymphoid organ indices, and blood biochemistry of Wannan Yellow chickens. Poult. Sci. 2019, 98, 6602–6610. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xiao, H.; Zhou, M.; Lou, Y.; He, Z.; Pan, G.; Zhang, Q.; Li, W.; Hui, X. Effect of Astragalus Polysaccharide in Diets on Slaughter Performance and Meat Quality of Broilers. Acta. Ecologiae. Anim. Domastici. 2015, 36, 26–29. [Google Scholar]
- Zhang, J.; Shen, H.; Zhang, X.; Dai, L.; Zhang, Q.; Wang, J. Fly maggot powder combined with astragalus polysaccharide on the growth performance of yellow feather broilers, Effects of slaughter performance and immune organ index. Feed. Res. 2020, 43, 31–35. [Google Scholar]
- Sun, T.; Gao, Y.; Zhou, H.; Sun, Z.; Lou, Y. Effects of Lycium barbarum polysaccharide on performance and muscle amino acid composition of 21-day-old broilers. Heilongjiang Anim. Sci. Vet. Med. 2019, 20, 118–122. [Google Scholar]
- Beilharz, M.; Lareu, R.; Garrett, K.; Grounds, M.; Fletcher, S. Quantitation of muscle precursor cell activity in skeletal muscle by Northern analysis of MyoD and myogenin expression: Application to dystrophic (mdx) mouse muscle. Mol. Cell Neurosci. 1992, 3, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, M.; Xu, S.; Sun, J.; Liu, G. Expression of Myostatin (Mstn) and Myogenin (Myog) Genes in Zi And Rhine Goose and Their Correlation with Carcass Traits. Braz. J. Poult. Sci. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- Ríos, R.; Carneiro, I.; Arce, V.; Devesa, J. Myostatin is an inhibitor of myogenic differentiation. Am. J. Physiol. Cell. Physiol. 2002, 282, C993–C999. [Google Scholar] [CrossRef] [PubMed]
- Castelhano-Barbosa, E.; Gabriel, J.; Alvares, L.; Monteiro-Vitorello, C.; Coutinho, L. Temporal and spatial expression of the myostatin gene during chicken embryo development. Growth Dev. Aging 2005, 69, 3–12. [Google Scholar] [PubMed]
- Gizak, A.; Wrobel, E.; Moraczewski, J.; Dzugaj, A. Changes in subcellular localization of fructose 1,6-bisphosphatase during differentiation of isolated muscle satellite cells. FEBS Lett. 2006, 580, 4042–4046. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, L.; Rudnicki, M. The molecular regulation of myogenesis. Clin. Genet. 2000, 57, 16. [Google Scholar] [CrossRef] [PubMed]
- Manceau, M.; Gros, J.; Savage, K.; Thomé, V.; McPherron, A.; Paterson, B.; Marcelle, C. Myostatin promotes the terminal differentiation of embryonic muscle progenitors. Genes Dev. 2008, 22, 668–681. [Google Scholar] [CrossRef]
- Liu, H.H.; Mao, H.G.; Dong, X.Y.; Cao, H.Y.; Yin, Z.Z. Expression of MSTN gene and its correlation with pectoralis muscle fiber traits in the domestic pigeons (Columba livia)-ScienceDirect. Poult. Sci. 2019, 98, 5265–5271. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).