Abstract
Neurodevelopmental disorders (NDDs) are considered synaptopathies, as they are due to anomalies in neuronal connectivity during development. DLG2 is a gene involved insynaptic function; the phenotypic effect of itsalterations in NDDs has been underestimated since few cases have been thoroughly described.We report on eight patients with 11q14.1 imbalances involving DLG2, underlining its potential effects on clinical presentation and its contribution to NDD comorbidity by accurate neuropsychiatric data collection. DLG2 is a very large gene in 11q14.1, extending over 2.172 Mb, with alternative splicing that gives rise to numerous isoforms differentially expressed in brain tissues. A thorough bioinformatic analysis of the altered transcripts was conducted for each patient. The different expression profiles of the isoforms of this gene and their influence on the excitatory–inhibitory balance in crucial brain structures could contribute to the phenotypic variability related to DLG2 alterations. Further studies on patients would be helpful to enrich clinical and neurodevelopmental findings and elucidate the molecular mechanisms subtended to NDDs.
1. Introduction
Neurodevelopmental disorders (NDDs) such as intellectual disability (ID), autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), epilepsy, and atypical cognitive or behavior features, are considered synaptopathies (i.e., provoked by synaptic function abnormalities) [1,2,3,4]. The precise patterns of neuronal assembly and connectivity during development are fundamental for neuromodulation and have been increasingly studied in NDDs [5]. Mutations and copy number variations (CNVs), inherited or de novo, in genes encoding for cell adhesion molecules (e.g., for neurexin–neuroligin, such as NRXN1–3 or NLGN1–4) and scaffolding proteins (e.g., discs large MAGUK scaffold proteins or Shank 1–3) or for modulating synaptic gene transcription, protein synthesis and degradation, and synaptic elimination (e.g., FMR1, TSC1–2, MECP2, and UBE3A) have been related to NDDs, since they interfere with synapse structure and function [2]. Both ‘gain-of-function’ and ‘loss-of-function’ mechanisms should be considered at the basis of synaptopathies, due to provoking imbalanced synaptic functioning [4].
Among genes involved in synapse formation, discs large MAGUK scaffold protein 2 (DLG2) has recently received attention. DLG2 (*603583; ENSG00000150672) is a very large gene in 11q14.1, extending over 2.172 Mb, with numerous alternative transcripts (https://www.ensembl.org on 5 April 2022). Its products are scaffolding postsynaptic density protein-93 (PSD-93) or channel-associated protein of synapses-110 (Chapsyn-110) [6], which acts as a postsynaptic multimeric scaffold protein belonging to the membrane-associated guanylate kinase (MAGUK) family. It binds to both cytoskeleton proteins and signaling complexes, influencing the development, plasticity, and stability of synapses [7]. It is involved in signaling and in the molecular organization of multiprotein complexes in postsynaptic density [6], and it is distributed in glutamatergic excitatory brain synapses [7]. In particular, it is recruited in NMDA receptors, regulating their surface expression and interacting with their cytoplasmic tails (www.genecards.org, accessed on 5 April 2022), and inwardly rectifying potassium channel (Kir) clusters [8]. All these data have arisen from electron microscopy studies, in vitro analyses, and experiments in mice models.
In humans, copy number variations (CNVs) involving DLG2 were first associated with schizophrenia [9,10] and, subsequently, with NDDs, including ASD, ID, and bipolar disorder [11,12,13,14]. Epilepsy has been rarely reported [15,16]. In most cases, the clinical descriptions are very scanty, as patients are part of large cohorts for case-control analyses in which recurrent CNVs in association with specific neuropsychiatric disorders have been investigated.
Patients with NDDs and DLG2 alterations are reported in Decipher (https://www.deciphergenomics.org/, accessed on 5 April 2022), but the phenotypic features are limited also in these cases.
Here, we report on a cohort of eight patients with neuropsychiatric features and CNVs in 11q14.1 that include DLG2 in order to provide a more extensive clinical characterization.
2. Materials and Methods
2.1. Patients
The 8 patients were recruited during a 10-year period at the IRCCS Stella Maris Foundation and at AOU Pisana. They presented neurodevelopmental and neuropsychiatric disorders that deserved follow-up after diagnosis. Patients with CNVs in the 11q14.1 region encompassing DLG2, either isolated or associated with other CNVs, were selected for this study.
All the patients underwent a detailed neuropsychiatric and psychological standardized observation. Brain MRI was acquired for 7/8 patients and EEG polygraphy was recorded in 3/8.
2.2. Molecular Analysis
Array comparative genomic hybridization (array-CGH) was performed in all 8 patients. The genomic DNA of the patients was isolated from peripheral blood by standard methods; DNA from healthy subjects (one male and one female) was used as the control (Agilent Technologies, Santa Clara, CA, USA). A total of 200 ng of genomic DNA, both from each patient (test sample) and the control (reference sample), was differentially labelled with Cy5-dCTP or with Cy3-dCTP using random primer labelling according to the manufacturer’s protocol (Agilent). The labelling reactions were applied to the 60K oligoarrays (Agilent, Santa Clara, CA, USA) and incubated for 24 h at 65 °C in an oven. The slides were washed and scanned using an Agilent scanner, and the identification of individual spots on the scanned arrays and quality slide evaluation were performed with Agilent-dedicated software (Feature Extraction, Agilent, Santa Clara, CA, USA). The 60K slides had a 41 Kb overall median probe spacing (33 KB in RefSeq genes).
The copy number variants (CNVs) were identified with Cytogenomics 3.0.6.6. (Agilent, Santa Clara, CA, USA) using an ADM-2 (aberration detection method-2) algorithm. This algorithm identifies aberrant intervals in a sample that has consistently high or low log ratios based on statistical score. ADM-2 uses an iterative procedure to find all genomic intervals with a score above a user-specified statistical threshold value. The threshold was set to a minimum of 6, with the minimum number of probes required in a region of 3 and a minimum absolute log ratio of 0.25. The score represented the deviation of the weighted average of the normalized log ratios from its expected value of zero and incorporated quality information about each probe measurement.
CNVs classified as pathogenic, likely pathogenic, or variant of unknown significance (VUS) were reported according to the guidelines of the Italian Society of Human Genetics (https://www.sigu.net, accessed on 5 April 2022), the American College of Medical Genetics guidelines [17], and the European Guidelines for constitutional cytogenetic analysis [18].The CNV classification was performed using databases such as the Database of Genomic Variants (DGV) (http://projects.tcag.ca/variation, accessed on 5 April 2022), the Database of Chromosome Imbalance and Phenotype in Humans using Ensembl Resources (Decipher) (https://www.deciphergenomics.org/, accessed on 5 April 2022), and the University of California Santa Cruz (UCSC) Genome Browser (https://genome.ucsc.edu/, accessed on 5 April 2022).
Pubmed (http://www.ncbi.nlm.nih.gov/pubmed, accessed on 5 April 2022), Online Mendelian Inheritance in Man (OMIM) (http://www.omim.org, accessed on 5 April 2022), and GeneCards (https://www.genecards.org, accessed on 5 April 2022) were also consulted for evaluating genotype–phenotype association.
The pathogenic CNVs detected in the 8 patients were reported according to the Genome Reference Consortium Human Build 38 (GRCh38/hg38). The segregation analyses in the parental DNA were performed by qPCR or by array-CGH.
The transcripts were researched using Ensembl (https://www.ensembl.org, accessed on 5 April 2022); the data on the expression profiles were researched using GTEx (https://www.gtexportal.org/home, accessed on 5 April 2022), and UCSC (https://genome.ucsc.edu/, accessed on 5 April 2022).
3. Results
3.1. Description of the Patient Cohort
The age of the patients ranged from 4 years old to 16 years old. Seven patients were male, and one was female. The neuropsychiatric and phenotypic features of the eight patients are summarized in Table 1 and Table S1 (supplementary material).

Table 1.
Neuropsychiatric features of the patients.
3.2. CNVs Detected in the Patient Cohort
The 11q14.1 CNVs detected by array-CGHranged from 68 Kb to 1.27 Mb; they included one intragenic duplication of DLG2 (Pt.8), six intragenic deletions (Pt.2–7), two patients (Pt.6 and 7) sharing an identical genomic imbalance, and one larger deletion (Pt.1) harboring additional genes at the 5’ of DLG2; two cases (Pt.3 and 6) presented additional rare CNVs (Table 2).

Table 2.
Genetic data about the CNVs detected in the 8 patients.
The pattern of inheritance was assessed in six patients: in three cases, the CNVs were inherited from the mother and, in three, from the father; the segregation analysis was not available for two patients (Table 2).
The biological roles of genes included in these CNVs are summarized in the supplementary material (Table S2).
3.3. DLG2 Transcripts and Expression
DLG2 extends over 2.172 Mb and is encoded on the reverse strand. According to Ensembl, DLG2 (ENSG00000150672) gives rise to 32 transcripts. All the protein-coding transcripts that overlapped with the CNVs of our patients were selected, and for each of them, the exons involved in these imbalances were exactly localized (Figure 1; Table 2; Table S3 of supplementary material).
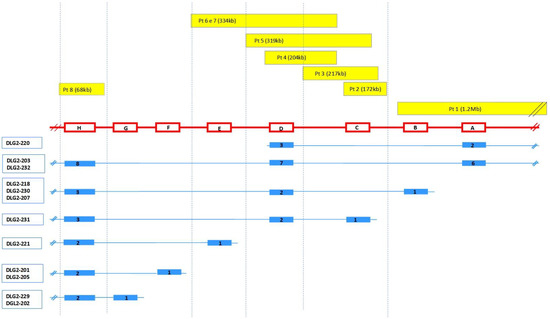
Figure 1.
Genetic overview of DLG2 CNVs and the relative transcripts involved. Top: the yellow bars indicate the imbalances detected for each patient and their extent. Middle: coding regions are indicated by red boxes (right to left) and introns by red lines; A: ENSE00001469378; B: ENSE00002179742, ENSE00002149728, ENSE00002188311; C: ENSE00003840932; D: ENSE00003487678, ENSE00002174027; E: ENSE00002152856; F: ENSE00001532570; G: ENSE00003587836, ENSE00001469384; H: ENSE00003471737. For details, see Table S3. Bottom: DLG2 isoforms (blue) with the number of exons relative to each transcript are reported. Relative lengths are not to scale. Pt = patient.
These data highlight that all the CNVs detected in our patients altered the nucleotide sequence of one or more transcripts; also, the duplication of patient 8 gave rise to a transcript that harbored two copies of the same exon, modifying the amino acid sequence of the protein.
The main features of the 13 transcripts selected are summarized in Table S4 (supplementary material); DLG2-321 and DLG2-221 are the same transcripts described by Reggiani et al., 2017 [12].
The expression of the transcripts across tissues in GTEx, UCSC, and in [12] was also evaluated. These transcripts showed very different expression profiles: some of them were scarcely expressed (e.g., DLG2-218, DLG2-202, DLG2-205, and DLG2-230), others were ubiquitous (e.g., DLG2-203 and DLG2-215), and several (i.e., DLG2-207, DLG2-201, DLG2-220, DLG2-229, and DLG2-221) showed a high and prevalent expression in the central nervous system (CNS). Some expression profiles are exemplified in Figure 2, while the rest are visualized in Figure S1 of the supplementary material.
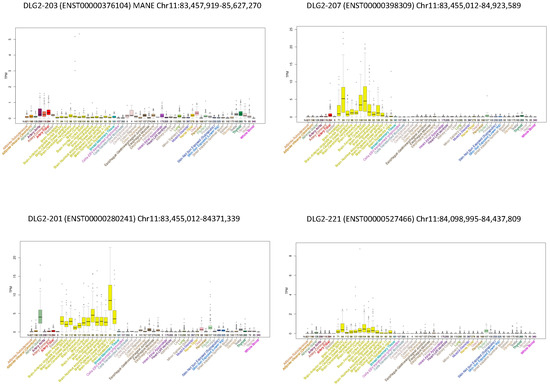
Figure 2.
Expression of some DLG2 transcripts across tissues from the GTEx Project. Examples of expression profiles: DLG2-203 (MANE) was ubiquitous; DLG2-207, DLG2-201, and DLG2-221 were prevalently expressed in CNS, with different patterns in brain areas.
4. Discussion
This paper reported on eight patients with CNVs involving DLG2 and highlighted the association between this gene and NDDs.
In this cohort, ID appeared as a leitmotif since it was diagnosed in the totality of our patients. In addition to ID, the most common NDDs were ADHD (five out of eight patients), verbal-motor dyspraxia (two patients, as well as one reported with motor clumsiness), and receptive-expressive language disorder (two patients). One patient presented focal epilepsy, and the other three showed EEG anomalies. In addition, one patient obtained a diagnosis of ASD, and autistic traits were described in two other cases (Table 1).
DLG2 is a very large gene in 11q14.1, extending over 2.172 Mb, with more than 40 exons that give rise to 32 transcripts. Among these patients, seven had intragenic alterations of DLG2, whereas one had a larger deletion harboring additional genes.
In humans, the role of this gene in NDDs has been underestimated, probably due to the fact that DLG2 shows incomplete penetrance; alterations of this gene have been reported also in healthy people (DGV database), and some patients with NDDs inherited the CNVs from an asymptomatic or paucisymptomatic parent [12], such as six cases in our cohort.
Furthermore, DLG2-203, the MANE transcript (matched annotation from NCBI and EMBL-EBI), seems not to be the most representative transcript for the synaptopathies since it was ubiquitous and was not particularly expressed in the CNS (Figure 2). Additionally, several patients with NDDs reported both in Decipher and in the literature had only intronic deletions in DLG2-203. We added two new cases whose imbalances did not affect the coding sequence of this transcript.
In view of this, all the protein-coding transcripts that overlapped with the CNVs in this cohort were evaluated. Among these transcripts, we focused on those with altered nucleotide sequences with one or more exons harbored in the imbalanced regions (Figure 1; Table 2).
Since the expression of alternative transcripts is highly tissue-specific [19], we analyzed the expression profiles of the altered transcripts with bioinformatic tools, and interestingly, all the patients had at least one transcript that was highly expressed in the CNS (Figure 2 and Figure S1).
Three patients had an altered dosage of additional genes in addition toDLG2 that influenced their phenotype. Among the genes harbored in the deletion of patient 1, PICALM deserves attention due to its role in cognition and synaptic plasticity [20]. The two duplications of patient 3in 1q42.2 and 5q14.1 contained several genes. Among them, SLC35F3 and JMY are brain-enriched genes [21]. The former is expressed in excitatory–glutamatergic and inhibitory–γ-aminobutyric acidergic neurons, while the latter is crucial during CNS development, especially in myelination [22,23]. Nothing is known about the phenotypic effects of their duplications. Correlation between the Xp22.31 duplication (patient 6) and NDDs is debated [24] (Table S2).
An almost constant feature of NDDs is their comorbidity, which can originate complex neuropsychiatric pictures characterized by the concomitance of different disorders [25]. Comorbidity was also evident in this cohort, carrying alterations of DLG2 and strengthening the idea that the same molecular mechanism could be at the basis of NDDs.
A strict association between ASD and ADHD was evidenced [26], and DLG2 may concur with both disorders [13,14,27]. Although in our cohort only one patient presented with ASD, ADHD is very frequent, further underlining the overlapping genetic basis between these NDDs.
To the best of our knowledge, epilepsy has been only rarely related to DLG2 deletion [15,16]; four patients of this cohort showed epilepsy or EEG anomalies. DLG2 belongs to the gene motif “BGNADP”, which is a network associated with ID and epilepsy, including also BTD, GALNT10, NMUR2, AUTS2, and PTPRD. Furthermore, DLG2 has been related to a pathway including GRIN2B, and they share an influence on NMDA receptor function [28,29].
The direct interaction between the DLG2 and Kirchannels [8,30] determining the functional maturation of spiny projection neurons during a critical period of striatal circuit development [6] may concur with the strong association between epilepsy and ASD [31,32]. It is worth noting that, among patients with epileptiform anomalies, one met the criteria for a diagnosis of ASD, and the other two presented autistic traits.
Psychiatric disorders such as mood disorder, anxiety disorder, obsessive-compulsive disorder (OCD), oppositional-defiant disorder (ODD), and binge eating were present in some of our patients. Mood disorder has been recognized as a neurodevelopmental psychiatric disorder [33]. Shared genetic pathways among synaptopathies including psychiatric disorders have been related to DLG2 haploinsufficiency both in mice models and in human studies [11,34]. In this study, we focused on a pediatric population in which NDDs were frequent, and we cannot exclude that, following a prospective trajectory until adult age [35], there could be a higher risk of developing psychiatric disorders such as mood disorder, which is especially continuous with ADHD [36].
The interference with the excitatory synaptic transmission in the striatum may explain DLG2 contribution to such disorders [6,37,38]. The striatum itself is involved in decision making and in executive function with common circuitries in ASD and OCD [39], further corroborating the continuity between NDDs and psychiatric disorders. In addition, reward responsiveness alterations in critical brain regions, including the striatum, have been identified in several psychiatric disorders, such as ADHD, ODD, and binge eating [40,41].
Verbal and motor dyspraxia, clumsiness, and slowness in executive function appeared quite frequently in this population and were signaled also in Decipher patients. These disorders may recognize similar neural substrates due to connectivity anomalies [42] even if, in mice models, the association between Dlg2 deletion and motor coordination has not been confirmed [34].
The presented data confirmed the association between DLG2 alterations and multiple NDDs; however, the molecular mechanisms underlying the pleiotropic effect of this gene are not fully understood. Different DLG2 isoforms are deemed to have some functional differences: in addition to not sharing the same exact sequence, they do not have the same pattern of expression in different regions of the CNS. However, according to these data and those available from public databases, it is not yet possible to determine the functional peculiarities of each DLG2 isoform.
With regards to NDDs, functional characterization of the transcripts is even more challenging due to the comorbidity of these conditions. It can be speculated that one of the mechanisms underlying the comorbidity of NDDs is a genetic imbalance of DLG2, such as a CNV, that causes the concomitant alteration of several transcripts with different functional roles. Interestingly, magnetic resonance imaging revealed only aspecificanomalies in some of our patients; however, they were not related to the prevalent transcript expression profiles, confirming that NDDs should be considered synaptopathies [1,2,3,4].
Moving from neuropsychiatric to physical features, prenatal and postnatal growth alterations, overweightand obesity, facial dysmorphisms, heart anomalies, and endocrinologic anomalies were other characteristics detected in our cohort of patients (Table S1) and in patients reported in Decipher. Again, the role of DLG2 in these clinical features remains to be clarified.
5. Conclusions
DLG2 haploinsufficiency is related to NDDs, with a wide clinical spectrum, ranging from ID, ASD, and ADHD to psychiatric disorders such as mood disorders, anxiety disorders, OCD, ODD, and even eating disorders. The different expression profiles of the isoforms and their significant influence on the excitatory–inhibitory balance in crucial brain structures may explain the phenotypic variability. A thorough analysis of the altered transcripts is mandatory to understand their role in the phenotypic outcome. Further studies on patients carrying DLG2 alterations will be helpful to enrich clinical description and to elucidate the molecular mechanisms subtended to NDDs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13050859/s1, Table S1: Anamnesis, exams and physical features of the patients; Table S2: Genes harbored in the additional CNVs detected in patient 1, 3 and 6. Table S3: Coding sequences involved in the CNVs of DLG2 gene. Table S4: DLG2 protein coding transcripts involved in patients’ imbalances. Figure S1: Expression of some DLG2 transcripts across tissues from GTEx Pro.
Author Contributions
Conceptualization, R.M. and R.B.; methodology, R.M., V.B. and A.V.; software, R.M., V.B., F.C. and A.V.; validation, V.B., G.C., A.V. and R.B.; formal analysis, R.M., V.B., C.B., F.C., G.C. and A.V.; investigation, R.M., V.B., P.C., F.B. and R.B.; resources, S.B., G.C. and R.B.; data curation, R.M., V.B., P.C., C.B., A.V. and R.B.; writing—original draft preparation, R.M. and V.B.; writing—review and editing R.M., V.B., A.V., G.C. and R.B.; visualization, P.C., C.B., A.V. and R.B.; supervision, S.B., F.B., A.V. and R.B.; project administration, G.C. and R.B.; funding acquisition, R.M., G.C. and R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work belongs to the project “Study on phenotype–genotype correlations of genetic rare diseases expressing in developmental age with neuropsychiatric symptoms” funded by the “Sarzana Family Donation”. Furthermore, it was partially supported by the Italian Ministry of Health (Grant-RC2021) and taxpayer contributions (‘5 × 1000’) to the IRCCS Stella Maris Foundation for the year 2021 (RB, RM).
Institutional Review Board Statement
The Pediatric Ethical Committee approved this study (CC-2017-2365068-AUTOSNP), number registry CEP 19/2018.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.deciphergenomics.org/ (accessed on 5 April 2022); https://www.genome.ucsc.edu/ (accessed on 5 April 2022); https://www.genecards.org/ (accessed on 5 April 2022); https://omim.org/ (accessed on 5 April 2022); https://www.ensembl.org (accessed on 5 April 2022); https://www.gtexportal.org/home (accessed on 5 April 2022); http://projects.tcag.ca/variation (accessed on 5 April 2022); http://www.ncbi.nlm.nih.gov/pubmed (accessed on 5 April 2022); and https://www.sigu.net (accessed on 5 April 2022).
Acknowledgments
The authors are grateful to Dario Bernardi, who kindly revised the English of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Won, H.; Mah, W.; Kim, E. Autism spectrum disorder causes, mechanisms, and treatments: Focus on neuronal synapses. Front. Mol. Neurosci. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiqi, G.; Nan, P.; Xiaolu, D.; Lifen, Y.; Fang, H.; Liwen, W.; Chen, C.; Yin, F.; Peng, J. Synaptopathology Involved in Autism Spectrum Disorder. Front. Cell. Neurosci. 2018, 12, 470. [Google Scholar]
- Bacchelli, E.; Loi, E.; Cameli, C.; Moi, L.; Vega Benedetti, A.F.; Blois, S.; Fadda, A.; Bonora, E.; Mattu, S.; Fadda, R.; et al. Analysis of a Sardinian Multiplex Family with Autism Spectrum Disorder Points to Post-Synaptic Density Gene Variants and Identifies CAPG as a Functionally Relevant Candidate Gene. J. Clin. Med. 2019, 8, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoghbi, H.Y.; Bear, M.F. Synaptic Dysfunction in Neurodevelopmental Disorders Associated with Autism and Intellectual Disabilities. Cold Spring Harb. Perspect. Biol. 2012, 4, a009886. [Google Scholar] [CrossRef] [Green Version]
- Batool, S.; Raza, H.; Zaidi, J.; Riaz, S.; Hasan, S.; Syed, N.I. Synapse formation: From cellular and molecular mechanisms to neurodevelopmental and neurodegenerative disorders. J. Neurophysiol. 2019, 121, 1381–1397. [Google Scholar] [CrossRef]
- Yoo, T.; Kim, S.G.; Yang, S.H.; Kim, H.; Kim, E.; Kim, S.Y. A DLG2 deficiency in mice leads to reduced sociability and increased repetitive behavior accompanied by aberrant synaptic transmission in the dorsal striatum. Mol. Autism 2020, 11, 19. [Google Scholar] [CrossRef]
- Zhu, J.; Shang, Y.; Zhang, M. Mechanistic basis of MAGUK-organized complexes in synaptic development and signalling. Nat. Rev. Neurosci. 2016, 17, 209–223. [Google Scholar] [CrossRef]
- Kim, E.; Cho, K.O.; Rothschild, A.; Sheng, M. Heteromultimerization and NMDA receptor-clustering activity of chapsyn-110, a member of the PSD-95 family of proteins. Neuron 1996, 17, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Kirov, G.; Pocklington, A.J.; Holmans, P.; Ivanov, D.; Ikeda, M.; Ruderfer, D.; Moran, J.; Chambert, K.; Toncheva, D.; Georgieva, L.; et al. De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol. Psychiatry 2012, 17, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Nithianantharajah, J.; Komiyama, N.H.; McKechanie, A.; Johnstone, M.; Blackwood, D.H.; St Clair, D.; Emes, R.D.; van de Lagemaat, L.N.; Saksida, L.M.; Bussey, T.J.; et al. Synaptic scaffold evolution generated components of vertebrate cognitive complexity. Nat. Neurosci. 2013, 16, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Noor, A.; Lionel, A.C.; Cohen-Woods, S.; Moghimi, N.; Rucker, J.; Fennell, A.; Thiruvahindrapuram, B.; Kaufman, L.; Degagne, B.; Wei, J.; et al. Copy number variant study of bipolar disorder in Canadian and UK populations implicates synaptic genes. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2014, 165, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, C.; Coppens, S.; Sekhara, T.; Dimov, I.; Pichon, B.; Lufin, N.; Addor, M.-C.; Belligni, E.F.; Digilio, M.C.; Faletra, F.; et al. Novel promoters and coding first exons in DLG2 linked to developmental disorders and intellectual disability. Genome Med. 2017, 9, 67. [Google Scholar] [CrossRef]
- Egger, G.; Roetzer, K.M.; Noor, A.; Lionel, A.C.; Mahmood, H.; Schwarzbraun, T.; Boright, O.; Mikhailov, A.; Marshall, C.R.; Windpassinger, C.; et al. Identification of risk genes for autism spectrum disorder through copy number variation analysis in Austrian families. Neurogenetics 2014, 15, 117–127. [Google Scholar] [CrossRef]
- Ruzzo, E.K.; Perez-Cano, L.; Jung, J.Y.; Wang, L.K.; Kashef-Haghighi, D.; Hartl, C.; Singh, C.; Xu, J.; Hoekstra, J.N.; Leventhal, O.; et al. Inherited and de novo genetic risk for autism impacts shared networks. Cell 2019, 178, 850–866.e26. [Google Scholar] [CrossRef] [Green Version]
- Vulto-van Silfhout, A.T.; Hehir-Kwa, J.Y.; van Bon, B.W.; Schuurs-Hoeijmakers, J.H.; Meader, S.; Hellebrekers, C.J.; Thoonon, I.J.M.; de Brouwer, A.P.M.; Brunner, H.G.; Webber, C.; et al. Clinical significance of de novo and inherited copy-number variation. Hum. Mutat. 2013, 34, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.Y.; Wang, X.F.; Li, M.W.; Li, J.M.; Xi, Z.Q.; Luan, G.M.; Zhang, J.-G.; Wang, Y.-P.; Sun, J.-J.; Li, Y.-L. Upregulated expression of postsynaptic density-93 and N-methyl-D-aspartate receptors subunits 2B mRNA in temporal lobe tissue of epilepsy. Biochem. Biophys. Res. Commun. 2007, 358, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Kearney, H.M.; Thorland, E.C.; Brown, K.K.; Quintero-Rivera, F.; South, S.T.; Working Group of the American College of Medical Genetics Laboratory Quality Assurance Committee. American College of Medical Genetics standards and guidelines for interpretation and reporting of postnatal constitutional copy number variants. Genet. Med. 2011, 13, 680–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.; de Leeuw, N.; Mann, K.; Schuring-Blom, H.; Morgan, S.; Giardino, D.; Rack, K.; Hastings, R. European guidelines for constitutional cytogenomic analysis. Eur. J. Hum. Genet. 2019, 27, 1–16. [Google Scholar] [CrossRef]
- Hu, Z.; Scott, H.S.; Qin, G.; Zheng, G.; Chu, X.; Xie, L.; Adelson, D.L.; Oftedal, B.E.; Venugopal, P.; Babic, M.; et al. Revealing missing human protein isoforms based on ab initio prediction. RNA-seq and proteomics. Sci. Rep. 2015, 5, 10940. [Google Scholar] [CrossRef] [Green Version]
- Bushlin, I.; Petralia, R.S.; Wu, F.; Harel, A.; Mughal, M.R.; Mattson, M.P.; Yao, P.J. Clathrin assembly protein AP180 and CALM differentially control axogenesis and dendrite outgrowth in embryonic hippocampal neurons. J. Neurosci. 2008, 28, 10257–10271. [Google Scholar] [CrossRef] [Green Version]
- Alinejad-Rokny, H.; Heng, J.I.T.; Forrest, A.R.R. Brain-Enriched Coding and Long Non-coding RNA Genes Are Overrepresented in Recurrent Neurodevelopmental Disorder CNVs. Cell Rep. 2020, 33, 108307. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.M.; Coombes, B.J.; Nguyen, T.T.L.; Liu, D.; McElroy, S.L.; Singh, B.; Nassan, M.; Colby, C.L.; Larrabee, B.R.; Weinshilboum, R.M.; et al. Mood-Stabilizing Antiepileptic Treatment Response in Bipolar Disorder: A Genome-Wide Association Study. Clin. Pharmacol. Ther. 2020, 108, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.M.; Domingues, H.S.; Cordelières, F.P.; Sampaio, P.; Seixas, A.I.; Relvas, J.B. Jmy regulates oligodendrocyte differentiation via modulation of actin cytoskeleton dynamics. Glia 2018, 66, 1826–1844. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Shen, Y.; Köhler, U.; Sharkey, F.H.; Menon, D.; Coulleaux, L.; Malan, V.; Rio, M.; McMullan, D.J.; Cox, H.; et al. Interstitial microduplication of Xp22.31: Causative of intellectual disability or benign copy number variant? Eur. J. Med. Genet. 2010, 53, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.H.; Oerbeck, B.; Skirbekk, B.; Petrovski, B.É.; Kristensen, H. Neurodevelopmental disorders: Prevalence and comorbidity in children referred to mental health services. Nord. J. Psychiatry 2018, 72, 285–291. [Google Scholar] [CrossRef]
- Ronald, A.; Simonoff, E.; Kuntsi, J.; Asherson, P.; Plomin, R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J. Child Psychol. Psychiatry 2008, 49, 535–542. [Google Scholar] [CrossRef]
- Alemany, S.; Ribasés, M.; Vilor-Tejedor, N.; Bustamante, M.; Sánchez-Mora, C.; Bosch, R.; Richarte, V.; Cormand, B.; Casas, M.; Ramos-Quiroga, J.A.; et al. New suggestive genetic loci and biological pathways for attention function in adult attention-deficit/hyperactivity disorder. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015, 168, 459–470. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Y.; Zhang, L.; Kong, W.; Xie, H.; Wang, J.; Wu, Y.; Wu, X.; Liu, X.; Zhang, Y.; et al. Large De Novo Microdeletion in Epilepsy with Intellectual and Developmental Disabilities, with a Systems Biology Analysis. Adv. Neurobiol. 2018, 21, 247–266. [Google Scholar]
- Hu, C.; Chen, W.; Myers, S.J.; Yuan, H.; Traynelis, S.F. Human GRIN2B variants in neurodevelopmental disorders. J. Pharmacol. Sci. 2016, 132, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Leonoudakis, D.; Conti, L.R.; Anderson, S.; Radeke, C.M.; McGuire, L.M.; Adams, M.E.; Froehner, S.C.; Yates, J.R., 3rd; Vandenberg, C.A. Protein trafficking and anchoring complexes revealed by proteomic analysis of inward rectifier potassium channel (Kir2.x)-associated proteins. J. Biol. Chem. 2004, 279, 22331–22346. [Google Scholar] [CrossRef] [Green Version]
- Sicca, F.; Ambrosini, E.; Marchese, M.; Sforna, L.; Servettini, I.; Valvo, G.; Brignone, M.S.; Lanciotti, A.; Moro, F.; Grottesi, A.; et al. Gain-of-function defects of astrocytic Kir4.1 channels in children with autism spectrum disorders and epilepsy. Sci. Rep. 2016, 6, 34325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spurling Jeste, S.; Tuchman, R. Autism Spectrum Disorder and Epilepsy: Two Sides of the Same Coin? J. Child Neurol. 2015, 30, 1963–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arlington, V. Diagnostic and Statistical Manual of Mental Disorders, 5th ed; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Pass, R.; Haan, N.; Humby, T.; Wilkinson, L.S.; Hall, J.; Thomas, K.L. Selective behavioural impairments in mice heterozygous for the cross disorder psychiatric risk gene DLG2. Genes Brain Behav. 2022, 21, e12799. [Google Scholar] [CrossRef] [PubMed]
- Klassen, L.J.; Katzman, M.A.; Chokka, P. Adult ADHD and its comorbidities, with a focus on bipolar disorder. J. Affect. Disord. 2010, 124, 1–8. [Google Scholar] [CrossRef]
- Arnold, L.E.; Van Meter, A.R.; Fristad, M.A.; Youngstrom, E.A.; Birmaher, B.B.; Findling, R.L.; Horwitz, S.; Black, S.R. Development of bipolar disorder and other comorbidity among youth with attention-deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 2020, 61, 175–181. [Google Scholar] [CrossRef]
- Han, K.M.; De Berardis, D.; Fornaro, M.; Kim, Y.K. Differentiating between bipolar and unipolar depression in functional and structural MRI studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 91, 20–27. [Google Scholar] [CrossRef]
- He, Z.; Sheng, W.; Lu, F.; Long, Z.; Han, S.; Pang, Y.; Chen, Y.; Luo, W.; Yu, Y.; Nan, X.; et al. Altered resting-state cerebral blood flow and functional connectivity of striatum in bipolar disorder and major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 177–185. [Google Scholar] [CrossRef]
- Carlisi, C.O.; Norman, L.; Murphy, C.M.; Christakou, A.; Chantiluke, K.; Giampietro, V.; Simmons, A.; Brammer, M.; Murphy, D.G.; MRC AIMS consortium; et al. Shared and Disorder-Specific Neurocomputational Mechanisms of Decision-Making in Autism Spectrum Disorder and Obsessive-Compulsive Disorder. Cereb. Cortex 2017, 27, 5804–5816. [Google Scholar] [CrossRef]
- Noordermeer, S.D.; Luman, M.; Oosterlaan, J. A Systematic Review and Meta-analysis of Neuroimaging in Oppositional Defiant Disorder (ODD) and Conduct Disorder (CD) Taking Attention-Deficit Hyperactivity Disorder (ADHD) Into Account. Neuropsychol. Rev. 2016, 26, 44–72. [Google Scholar] [CrossRef] [Green Version]
- Val-Laillet, D.; Aarts, E.; Weber, B.; Ferrari, M.; Quaresima, V.; Stoeckel, L.E.; Alonso-Alonso, M.; Audette, M.; Malbert, C.H.; Stice, E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015, 8, 1–31. [Google Scholar] [CrossRef]
- Fiori, S.; Guzzetta, A.; Mitra, J.; Pannek, K.; Pasquariello, R.; Cipriani, P.; Tosetti, M.; Cioni, G.; Rose, S.E.; Chilosi, A. Neuroanatomical correlates of childhood apraxia of speech: A connectomic approach. Neuroimage Clin. 2016, 12, 894–901. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).