Abstract
Recent advances in molecular technologies enable sensitive and quantitative assessment of circulating tumor DNA, offering a noninvasive disease monitoring tool for patients with malignant disorders. Here, we demonstrated on four follicular lymphoma cases that circulating tumor DNA based EZH2 mutation analysis performed by a highly sensitive droplet digital PCR method may be a valuable treatment monitoring approach in EZH2 mutant follicular lymphoma. EZH2 variant allele frequencies changed in parallel with the volume of metabolically active tumor sites observed on 18F-fluorodeoxyglucose positron emission tomography combined with computer tomography (PET-CT) scans. Variant allele frequencies of EZH2 mutations decreased or were eliminated rapidly upon successful treatment, with treatment failure being associated with elevated EZH2 variant allele frequencies. We also demonstrated spatial heterogeneity in a patient with two different EZH2 mutations in distinct anatomical sites, with both mutations simultaneously detected in the liquid biopsy specimen. In summary, circulating tumor DNA based EZH2 mutation analysis offers a rapid, real-time, radiation-free monitoring tool for sensitive detection of EZH2 mutations deriving from different anatomical sites in follicular lymphoma patients receiving immunochemotherapy.
1. Introduction
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma (NHL) among adults in developed countries [,]. Although the disease has an indolent biology with a median overall survival of 15 years, it is characterized by a remitting, relapsing clinical course with the occurrence of high-grade transformation to a more aggressive lymphoma in around 3% of patients per year [,,,], generating a need to develop sensitive and broadly accessible tools for disease monitoring. As relapsed and transformed FL is characterized by decreased sensitivity to immunochemotherapeutic regimens, development of new treatment modalities represents an unmet clinical need.
Tumor biopsy represents the cornerstone of the diagnosis of FL, but it is not ideal for disease monitoring due to the invasiveness of the procedure []. The process of 18F-fluorodeoxyglucose positron emission tomography combined with computer tomography (PET-CT) is the most widely used method for patient follow-up in lymphoid malignancies []; however, it is hampered by its limited sensitivity and specificity [] and with interpretation of the results being highly dependent on the evaluating radiologist []. Liquid biopsy is a novel and emerging radiation-free technique to noninvasively monitor patients with malignant disease and to evaluate their response to treatment [,]. Investigating circulating tumor DNA (ctDNA) in patient derived plasma using highly sensitive technologies may overcome the limitations of the other patient evaluating tools mentioned above, as it is less invasive, radiation-free, quick, sensitive and capable of conveying genetic information from a spatially heterogeneous or disseminated tumor [].
In recent years, several studies focused on ctDNA based treatment monitoring in NHLs, mainly in diffuse large B cell lymphoma (DLBCL). The first two pioneering studies demonstrated that ctDNA based disease monitoring predicts relapse at a median of 3 months earlier compared to imaging based methods [,]. Recently, it was found that molecular responses detected with ctDNA analysis after one or two cycles of therapy predict two-year progression free survival (PFS) in DLBCL, defined as early and major molecular responses []. However, only limited data is available on the utility of ctDNA based treatment monitoring in FL. The retrospective analysis of pretreatment plasma samples from FL patients enrolled in the PRIMA trial (NCT00140582) demonstrated inferior PFS in patients with higher ctDNA levels []. In another study, an elevated amount of pretreatment ctDNA levels correlated with increased baseline total metabolic tumor volume and shorter PFS as determined by PET-CT [].
Recently, activating mutations of the epigenetic modifier gene, an enhancer of zeste homolog 2 (EZH2) in well-defined hotspots of exon 16 and exon 18 (Y646X, A682G and A692V) were described in around 25% percent of FL patients [,,]. EZH2 represents an attractive therapeutic target in FL, indeed, the United States Food and Drug Administration (FDA) has just granted accelerated approval for the selective EZH2 inhibitor tazemetostat in relapsed/refractory FL [,]. In addition to its potential for disease monitoring, noninvasive detection of EZH2 mutations in the plasma of FL patients could help identify patients who will most likely benefit from EZH2 targeted therapies in the future.
Here, we present four individual cases of FL, where ctDNA based patient monitoring was performed using a sensitive ddPCR assay for the detection of activating EZH2 mutations. The obtained mutation profiles were correlated with PET-CT based imaging data where available. Our proof of principle study demonstrates the power of detection and time-resolved monitoring of EZH2 mutations in ctDNA of FL patients receiving immunochemotherapy.
2. Materials and Methods
We analyzed plasma ctDNA samples of four FL patients with known EZH2 activating mutations determined from formalin fixed paraffin embedded tissue specimens. All patients were treated at the 3rd Department of Internal Medicine, Semmelweis University, Budapest, Hungary. Diagnoses in all cases were based on lymph node histology and immunohistochemistry. For staging and monitoring of the treatment response, PET-CT scans were performed. Routinely, rituximab-based immunochemotherapy was commenced as first line treatment to all patients as described in Table S1. At first relapse, autologous bone marrow transplant was planned for consolidation, however none of the relapsed patients reached a second remission.
At the time of the first liquid biopsy specimen collection, all patients displayed active disease (FL or transformed FL (tFL)). The number of follow-up liquid biopsies ranged between 1 and 6 per patient. All patients received rituximab in combination with chemotherapy as outlined in Figure 1, Figure 2, Figure 3 and Figure 4. Two patients achieved complete metabolic remission (CMR) with the other two patients succumbing to their disease due to high grade transformation.
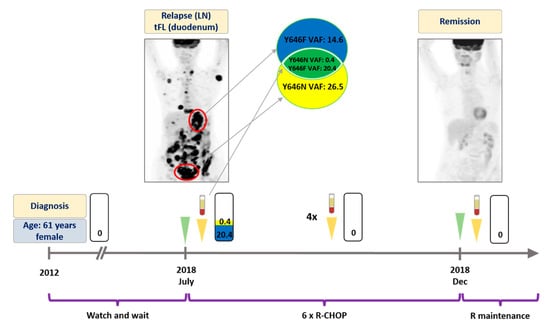
Figure 1.
Detailed illustration of treatment monitoring in Patient #1. Spatial heterogeneity was captured in pretreatment plasma and rapid elimination of EZH2 Y646N and Y646F mutations was observed upon successful R-CHOP treatment. Since then the patient has been receiving rituximab maintenance therapy with good general health conditions. Numbers in the rectangles represent the VAF of the mutations, yellow color and blue colors indicate Y646N and Y646F variants, respectively. Green arrows indicate the time when the PET scans were performed, yellow arrows indicate the time when liquid biopsy specimens were collected. LN: lymph node. tFL: transformed follicular lymphoma. VAF: variant allele frequency. R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone. R: rituximab.
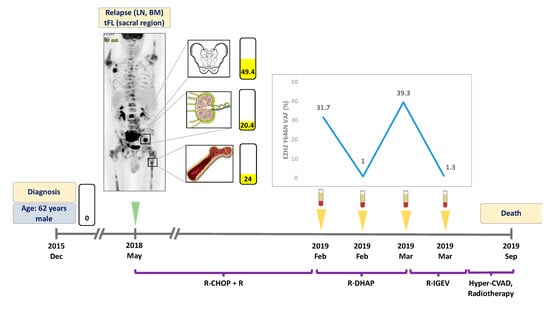
Figure 2.
Detailed illustration of treatment monitoring in Patient #2. EZH2 Y646N mutation was found in three sites at relapse. ctDNA EZH2 Y646N allele frequencies fluctuated in parallel with treatment efficacy and refractoriness. Failure to eliminate EZH2 mutations from the plasma indicated poor prognosis. Numbers in the rectangles represent the VAF of the mutation, yellow color indicates the Y646N variant. Green arrow indicates the time when the PET scans were performed, yellow arrows indicate the time when liquid biopsy specimens were collected. LN: lymph node, BM: bone marrow, tFL: transformed follicular lymphoma. VAF: variant allele frequency. R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone. R-DHAP: rituximab, dexamethasone, high-dose cytarabine, cisplatin. R-IGEV: rituximab, ifosfamide, gemcitabine, vinorelbine. Hyper-CVAD: Course A: cyclophosphamide, vincristine, doxorubicin, dexamethasone. Course B: methotrexate, cytarabine.
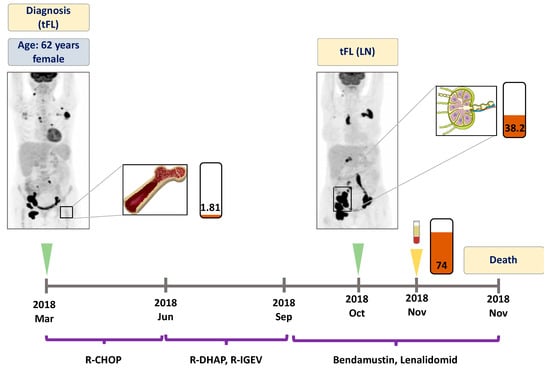
Figure 3.
Detailed illustration of treatment monitoring in Patient #3. Liquid biopsy obtained one week prior to the death of the patient revealed high allele frequency of EZH2 A682G mutation. Numbers in the rectangles represent the VAF of the mutation, brown color indicates the A682G variant. Green arrows indicate the time when the PET scans were performed, the yellow arrow indicates the time when liquid biopsy specimens were collected. LN: lymph node, BM: bone marrow, tFL: transformed follicular lymphoma. R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone. R-DHAP: rituximab, dexamethasone, high-dose cytarabine, cisplatin. R-IGEV: rituximab, ifosfamide, gemcitabine, vinorelbine.
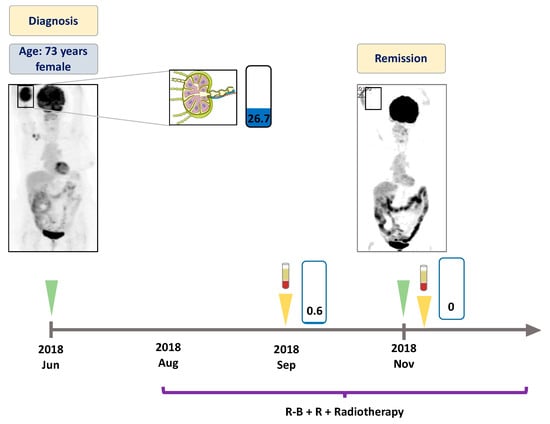
Figure 4.
Detailed illustration of treatment monitoring in Patient #4. Low variant allele frequency of EZH2 Y646F mutation was found in the plasma after one week of treatment initiation. End of treatment ctDNA at three months was found to be EZH2 wild type, with the corresponding PET-CT revealing complete metabolic response of the disease. Since then the patient has received rituximab maintenance therapy with good general health conditions. Numbers in the rectangles represent the VAF of the mutation, blue color indicates the Y646F variant. Green arrows indicate the time when the PET scans were performed, yellow arrows indicate the time when liquid biopsy specimens were collected. R-B + R: rituximab, bendamustin plus maintenance rituximab.
Peripheral blood specimens were collected using PAXgene Blood ccfDNA tubes (Qiagen, Germany). Plasma was isolated within six hours in two steps (whole blood was centrifuged at 1.600 g for 20 min at 4 °C, subsequently the plasma fraction was centrifuged at 16.000 g for 10 minutes at 4 °C) following previously published protocols [,]. The plasma samples were stored at −70 °C until further processing. ctDNA was isolated using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Germany) following the manufacturer’s instructions. Isolated cell-free DNA samples were stored at −20 °C. Quantity and quality of ctDNA were measured using the Qubit 4 Fluorometer (Thermo Fisher Scientific, USA) and 4200 TapeStation System (Agilent Technologies, USA), respectively.
Mutation specific assays for EZH2 Y646N, Y646F and EZH2 A682G (assay IDs: dHsaMDV2516844, dHsaMDV2516772, dHsaMDS555888133, respectively; Bio-Rad Laboratories, USA) were used to evaluate the EZH2 mutation status with a QX200 ddPCR System (Bio-Rad Laboratories, USA). All reactions were performed using 25 ng of ctDNA. Results were analyzed using the QuantaSoft software (version 1.7; Bio-Rad). All ddPCR reactions were performed with the detection of adequate events (>10,000 droplets per sample) and DNA copies (>10 copies/ul).
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Hungarian Medical Research Council (45371-2/2016/EKU).
3. Results
3.1. Patient #1
A 61 year-old female patient presented with cervical lymphadenopathy in 2012. The histology revealed grade II FL at that time negative for EZH2 mutation (Figure 1). Based on the limited stage IIIA disease, a watch and wait strategy was chosen. In July 2018, the patient developed B symptoms, with the PET-CT scan revealing metabolically active disseminated disease. Inguinal lymph node and duodenal biopsy showed grade II FL and DLBCL, suggesting high grade transformation of the original lymphoma. The ddPCR analysis of these specimens identified an EZH2 Y646N mutation in the inguinal (variant allele frequency, VAF: 26.5%) and an EZH2 Y646F mutation (VAF: 14.6%) in the duodenal biopsy. The analysis of a ctDNA specimen obtained prior to treatment revealed the presence of both Y646N (VAF: 0.4%) and Y646F (VAF: 20.4%) mutations in the bloodstream (Figure 1). The patient received immunochemotherapy (ICT) with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine and prednisone) as first line treatment. Four weeks later, the second liquid biopsy specimen was found to be an EZH2 wild type, with the subsequent five liquid biopsies collected every 4 weeks also confirming elimination of the EZH2 mutations (Figure 1). The corresponding control PET-CT also demonstrated CMR (Figure 1). To date, the patient is receiving rituximab maintenance therapy with good general health conditions.
3.2. Patient #2
A 62 year-old male patient was diagnosed with grade I FL, initially not requiring treatment, in December 2015; the diagnostic tissue sample proved to be EZH2 wild type. In May 2018, disease progression was detected as the PET-CT scan revealed metabolically active areas in the left inguinal and sacral regions (Figure 2). Inguinal lymph node and bone marrow biopsy showed grade II FL and lymphoid infiltration of the bone marrow, respectively, with an EZH2 Y646N mutation detected in both locations with VAFs of 24% and 20.4%. The patient received R-CHOP with rituximab maintenance therapy as first line treatment. In January 2019, the patient relapsed again, and R-DHAP (rituximab plus dexamethasone, high-dose cytarabine and cisplatin) treatment was administered as second line therapy. The liquid biopsy specimen obtained at the time of relapse was positive for the Y646N mutation with a VAF of 31.7%, with the ctDNA sample demonstrating reduction of this mutation (VAF: 1.0%) in response to therapy (Figure 2). In March 2019, R-IGEV (rituximab plus ifosfamide, gemcitabine, and vinorelbine) was started due to clinical refractoriness to the previous treatment line. At this time point, the plasma ctDNA showed an elevated Y646N mutation level (VAF: 39.3%) which was followed by a rapid reduction of the mutant clone (VAF: 1.3%) one week after the initiation of third line treatment. Later, the patient proved to be refractory to all salvage therapies and underwent a palliative surgical intervention due to the growing sacral mass, which caused urinary difficulties. Histological and molecular analyses of the surgically resected area revealed tFL and re-emergence of the previously detected EZH2 Y646N mutation (VAF: 49.4%). The patient deceased in September 2019.
3.3. Patient #3
A 62 year-old female patient who presented with generalized lymphadenopathy was diagnosed with an aggressive lymphoproliferative disease, which was proved to be an already transformed follicular lymphoma. Namely, core biopsy of the inguinal lymph node displayed tFL, meanwhile the bone marrow biopsy revealed FL infiltration. In March 2018, the PET-CT scan showed disseminated metabolically active areas. EZH2 A682G mutation was detected in the bone marrow with a VAF of 1.8% (Figure 3). The patient showed no response to first (R-CHOP) and subsequent therapeutic lines (R-DHAP, R-IGEV). Inguinal lymph node biopsy collected in October 2018 reinforced the previous tFL diagnosis and proved to be positive for the original EZH2 A682G mutation (VAF: 38.2%). At this time point, the plasma ctDNA showed an elevated A682G mutation level (VAF: 74.0%). The patient succumbed to the disease in November 2018.
3.4. Patient #4
In June 2018, a 73 year-old female patient presented with cubital mass and B-symptoms. A core biopsy of the mass resulted in diagnosis of follicular lymphoma with a 20% proliferation rate. The initial PET-CT described a stage II disease with increased metabolic activity in the right cubital area and in the right axillary lymph node region (Figure 4). At this time, an EZH2 Y646F mutation was detected in the pericubital soft tissue biopsy specimen. Rituximab plus bendamustin (R-B) treatment was commenced as first line therapy. The subsequently analysed ctDNA sample demonstrated reduction of the EZH2 variant down to a VAF of 0.56%. The control PET-CT performed in November 2018 revealed CMR of the disease, with the corresponding ctDNA also found to be the EZH2 wild type. The treatment was completed with local irradiation. Currently, the patient is still in remission with no need of further therapy.
4. Discussion
Liquid biopsy-based mutation analysis is becoming a valuable tool for cancer detection and sensitive disease monitoring. In various solid cancers, including lung and colorectal cancer, tumor derived plasma DNA analysis is already incorporated into the routine follow-up and screening guidelines [,]. Scrutiny of the clinical applicability of this approach is gaining momentum in hematological malignancies as well, mainly in B-cell disorders. Here, we present four cases of EZH2 mutant FLs, where ctDNA based treatment monitoring was performed using a highly sensitive ddPCR method to detect EZH2 mutations in patients treated with ICT. In three out of four cases, the ctDNA based mutation detection was accompanied by parallel PET-CT scan evaluation.
In the field of B-cell lymphomas, the majority of liquid biopsy studies published so far focused on DLBCL. Rossi et al. found liquid biopsy based mutation detection to be eligible for treatment monitoring in DLBCL: in their study, response to therapy was characterized by elimination of the mutations in the peripheral blood, meanwhile treatment refractory disease was associated with the persistence of the previously identified mutations or emergence of novel mutational events []. In other studies, two log reduction in ctDNA levels after two cycles of therapy in DLBCL and in Hodgkin lymphoma indicated excellent prognosis [,]. In our study, Patient #1 demonstrated a rapid ctDNA clearance upon one cycle of first line treatment which was associated with excellent treatment outcome. Patient #2 had a rapid decrease in EZH2 Y646N mutant VAF levels after initiation of second- and third-line treatment. The rapid reduction and the subsequent increase of mutant EZH2 levels can be explained by the cytoreductive effect of chemotherapy and the subsequent emergence of therapy refractory clones. Importantly, this patient has never achieved total clearance of mutant EZH2 DNA fragments in the plasma which can be linked to inferior outcome. A similar phenomenon was presented in a case report of a patient with colorectal carcinoma where a poor prognosis was observed after incomplete elimination of KRAS mutant ctDNA fragments []. The occurrence of treatment refractory disease after failure to achieve undetectable EZH2 mutation levels illuminates the possibility of detecting minimal residual disease (MRD) with the investigation of EZH2 mutations in the ctDNA of EZH2 mutant FL patients.
There are only a few studies investigating liquid biopsy based therapy monitoring in parallel with imaging in B-cell lymphomas. Camus et al. found that two thirds of patients with Hodgkin lymphoma presented with negative PET-CT scan results when MRD was detected with liquid biopsy []. In FL, Delfau-Larue et al. showed that pretreatment ctDNA levels correlated with the total metabolic tumor volumes measured with PET-CT []. In our study, we present three FL cases where ctDNA based treatment monitoring was performed in parallel with PET-CT imaging. In two cases (Patients #1 and #4) elimination of EZH2 mutations upon treatment was followed by CMR, documented by the PET-CT scans. In these cases, treatment monitoring with liquid biopsy represented a radiation-free, ‘real-time’ therapy monitoring approach compared to PET-CT imaging. In Patient #3, refractoriness to different treatment regimens was associated with persisting metabolically active tumor areas seen on the PET-CT scans and synchronously high VAF of EZH2 mutated ctDNA was detected. This is in line with previous observations reporting that ctDNA levels correlate with tumor metabolism and aggressiveness in B-cell lymphomas [,,].
As demonstrated by Rossi et al., a single ctDNA specimen is capable of revealing the intra- and intertumoral genetic heterogeneity observed in B cell lymphomas []. Recently, we have also reported the applicability of ctDNA based mutation monitoring in unveiling the spatial clonal heterogeneity in the context of therapy resistance in chronic lymphocytic leukemia []. In the present study, two different EZH2 mutations were found in the inguinal FL lymph node and in the duodenal tFL sample of Patient #1; meanwhile, both mutations were detected simultaneously in the liquid biopsy specimen. Indeed, in patients with multiple tumorous areas, a larger portion of the plasma DNA originates from the more aggressive disease site [,,]. Accordingly, in Patient #1, the EZH2 mutant DNA from the tFL localization was represented by a higher VAF in the liquid biopsy specimen, compared to the EZH2 mutation originally identified in the lymph node affected by grade II FL (20.4% vs. 0.4%, respectively). Scherer et al. also demonstrated that aggressive lymphomas such as DLBCL and tFL are accompanied by significantly higher ctDNA release compared to FL [].
Previous studies showed that detection and monitoring of the rearranged immunoglobulin heavy chain gene (IGH) is not ideal in FL due to high rates of somatic hypermutation []. The utility of the IgH-Bcl2 translocation in disease monitoring is also controversial, due to the variability of the breakpoint region and presence of this translocation in a proportion of healthy individuals [,,,]. Previously, several lymphoma-specific hotspot mutations, including EZH2, were shown to be detectable from plasma derived ctDNA of FL and DLBCL patients [,]. Here, we investigated the feasibility of EZH2 mutation monitoring in the plasma of FL patients in the context of ICT treatment. In Patients #1 and #4, clearance of EZH2 mutations was observed upon successful therapy. In Patient #2, EZH2 mutation allele frequencies changed in parallel with treatment efficacy, while high allele frequency of EZH2 mutation was detected in the plasma prior to the death of Patient #3. Considering the frequency of EZH2 mutations in FL, this approach can be applied in approximately 25% of FL cases with a sensitivity of as low as 0.01% depending on the amount of cfDNA. Our findings will most likely gain special importance in the context of EZH2 targeted therapies currently being evaluated in clinical trials and may serve as a basis for a regular disease monitoring approach in the near future. The selective EZH2 inhibitor tazemetostat demonstrated objective response rates in 77% of FL patients with EZH2 mutations in an ongoing phase II clinical trial [], with EZH2 mutations detected in both tumor tissue and plasma representing the only predictive biomarker of therapy response [].
5. Conclusions
In our study presenting four cases of EZH2 mutant FL treated with ICT, liquid biopsy based EZH2 mutation analysis offered a rapid, real-time, radiation-free monitoring tool with distinct mutations derived from different anatomical sites detected simultaneously in the ctDNA specimens.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/7/785/s1. Table S1: Detailed clinical characteristics of Patient #1–#4.
Author Contributions
Á.N. contributed to the study conception and design, sample collection, sample processing, ddPCR measurements, collection and assembly of data, data analysis and interpretation, preparation of figures and manuscript writing. B.B., N.N. and L.K. (Laura Kiss) and L.K. (Lili Kotmayer) contributed to sample collection, sample processing, ddPCR measurements. A.B., S.I. and A.M. (András Masszi) contributed to sample collection and assembly of clinical data. G.M., A.M. (András Matolcsy), D.A. and T.M. contributed to the study conception and design. C.B. contributed to the study conception and design, general supervision of the research group, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Hungarian National Research, Development and Innovation Office (NKFIH) (KH17-126718, NVKP_16-1-2016-0004, FK20_134253 and K_16 #119950 grants), as well as by the Momentum grant (LP-95021) and János Bolyai Research Scholarship (BO/00320/18/5) of the Hungarian Academy of Sciences. The study was also supported by the ÚNKP-19-4-SE-77, ÚNKP-19-2-I-SE-47 and ÚNKP-20-5-SE-22 grants of the New National Excellence Program of the Ministry for Innovation and Technology, as well as by the Complementary Research Excellence Program and Kerpel Talent Award of Semmelweis University (EFOP-3.6.3-VEKOP-16-2017-00009), and the Higher Education Institutional Excellence Programme of the Ministry of Human Capacities in Hungary within the framework of the Molecular Biology thematic programme of the Semmelweis University and the ELIXIR Hungary.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Anderson, J.R.; Armitage, J.O.; Weisenburger, D.D. Epidemiology of the non-Hodgkin’s lymphomas: Distributions of the major subtypes differ by geographic locations. Non-Hodgkin’s Lymphoma Classification Project. Ann. Oncol. 1998, 9, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Roulland, S.; Gloghini, A.; Younes, A.; von Keudell, G.; Lopez-Guillermo, A.; Fitzgibbon, J. Follicular lymphoma. Nat. Rev. Dis. Prim. 2019, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Relander, T.; Johnson, N.A.; Farinha, P.; Connors, J.M.; Sehn, L.H.; Gascoyne, R.D. Prognostic factors in follicular lymphoma. J. Clin. Oncol. 2010, 28, 2902–2913. [Google Scholar] [CrossRef]
- Bastion, Y.; Sebban, C.; Berger, F.; Felman, P.; Salles, G.; Dumontet, C.; Bryon, P.A.; Coiffier, B. Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J. Clin. Oncol. 1997, 15, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, C.; Trneny, M.; Xerri, L.; Wickham, N.; Feugier, P.; Leppa, S.; Brice, P.; Soubeyran, P.; Gomes Da Silva, M.; Mounier, C.; et al. Risk Factors and Outcomes for Patients With Follicular Lymphoma Who Had Histologic Transformation After Response to First-Line Immunochemotherapy in the PRIMA Trial. J. Clin. Oncol. 2016, 34, 2575–2582. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Ghielmini, M.; Rule, S.; Salles, G.; Vitolo, U.; Ladetto, M.; Committee, E.G. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v83–v90. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765. [Google Scholar] [CrossRef]
- El-Galaly, T.C.; Villa, D.; Gormsen, L.C.; Baech, J.; Lo, A.; Cheah, C.Y. FDG-PET/CT in the management of lymphomas: Current status and future directions. J. Intern. Med. 2018, 284, 358–376. [Google Scholar] [CrossRef]
- Han, H.S.; Escalon, M.P.; Hsiao, B.; Serafini, A.; Lossos, I.S. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann. Oncol. 2009, 20, 309–318. [Google Scholar] [CrossRef]
- Ansell, S.M.; Armitage, J.O. Positron emission tomographic scans in lymphoma: Convention and controversy. Mayo Clin. Proc. 2012, 87, 571–580. [Google Scholar] [CrossRef]
- Forshew, T.; Murtaza, M.; Parkinson, C.; Gale, D.; Tsui, D.W.; Kaper, F.; Dawson, S.J.; Piskorz, A.M.; Jimenez-Linan, M.; Bentley, D.; et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci. Transl. Med. 2012, 4, 136ra168. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Fettke, H.; Kwan, E.M.; Azad, A.A. Cell-free DNA in cancer: Current insights. Cell. Oncol. (Dordrecht) 2019, 42, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, D.M.; Green, M.R.; Bratman, S.V.; Scherer, F.; Liu, C.L.; Kunder, C.A.; Takahashi, K.; Glover, C.; Keane, C.; Kihira, S.; et al. Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing. Blood 2015, 125, 3679–3687. [Google Scholar] [CrossRef]
- Roschewski, M.; Dunleavy, K.; Pittaluga, S.; Moorhead, M.; Pepin, F.; Kong, K.; Shovlin, M.; Jaffe, E.S.; Staudt, L.M.; Lai, C.; et al. Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: A correlative biomarker study. Lancet Oncol. 2015, 16, 541–549. [Google Scholar] [CrossRef]
- Kurtz, D.M.; Scherer, F.; Jin, M.C.; Soo, J.; Craig, A.F.M.; Esfahani, M.S.; Chabon, J.J.; Stehr, H.; Liu, C.L.; Tibshirani, R.; et al. Circulating Tumor DNA Measurements As Early Outcome Predictors in Diffuse Large B-Cell Lymphoma. J. Clin. Oncol. 2018, 36, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, C.; Huet, S.; Carlton, V.E.; Fabiani, B.; Delmer, A.; Jardin, F.; Delfau-Larue, M.H.; Hacini, M.; Ribrag, V.; Guidez, S.; et al. The prognostic value of clonal heterogeneity and quantitative assessment of plasma circulating clonal IG-VDJ sequences at diagnosis in patients with follicular lymphoma. Oncotarget 2017, 8, 8765–8774. [Google Scholar] [CrossRef] [PubMed]
- Delfau-Larue, M.H.; van der Gucht, A.; Dupuis, J.; Jais, J.P.; Nel, I.; Beldi-Ferchiou, A.; Hamdane, S.; Benmaad, I.; Laboure, G.; Verret, B.; et al. Total metabolic tumor volume, circulating tumor cells, cell-free DNA: Distinct prognostic value in follicular lymphoma. Blood Adv. 2018, 2, 807–816. [Google Scholar] [CrossRef]
- Bodor, C.; Grossmann, V.; Popov, N.; Okosun, J.; O’Riain, C.; Tan, K.; Marzec, J.; Araf, S.; Wang, J.; Lee, A.M.; et al. EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood 2013, 122, 3165–3168. [Google Scholar] [CrossRef]
- Morin, R.D.; Johnson, N.A.; Severson, T.M.; Mungall, A.J.; An, J.; Goya, R.; Paul, J.E.; Boyle, M.; Woolcock, B.W.; Kuchenbauer, F.; et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat. Genet. 2010, 42, 181–185. [Google Scholar] [CrossRef]
- Morin, R.D.; Mendez-Lago, M.; Mungall, A.J.; Goya, R.; Mungall, K.L.; Corbett, R.D.; Johnson, N.A.; Severson, T.M.; Chiu, R.; Field, M.; et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 2011, 476, 298–303. [Google Scholar] [CrossRef]
- United States Food and Drug Administration. FDA granted accelerated approval to tazemetostat for follicular lymphoma. Available online: https://www.fda.gov/drugs/fda-granted-accelerated-approval-tazemetostatfollicular- lymphoma (accessed on 18 June 2020).
- Italiano, A.; Soria, J.C.; Toulmonde, M.; Michot, J.M.; Lucchesi, C.; Varga, A.; Coindre, J.M.; Blakemore, S.J.; Clawson, A.; Suttle, B.; et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: A first-in-human, open-label, phase 1 study. Lancet Oncol. 2018, 19, 649–659. [Google Scholar] [CrossRef]
- Chiu, R.W.; Poon, L.L.; Lau, T.K.; Leung, T.N.; Wong, E.M.; Lo, Y.M. Effects of blood-processing protocols on fetal and total DNA quantification in maternal plasma. Clin. Chem. 2001, 47, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Franczak, C.; Filhine-Tresarrieu, P.; Gilson, P.; Merlin, J.L.; Au, L.; Harle, A. Technical considerations for circulating tumor DNA detection in oncology. Expert Rev. Mol. Diagn. 2019, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Malapelle, U.; Sirera, R.; Jantus-Lewintre, E.; Reclusa, P.; Calabuig-Farinas, S.; Blasco, A.; Pisapia, P.; Rolfo, C.; Camps, C. Profile of the Roche cobas(R) EGFR mutation test v2 for non-small cell lung cancer. Expert Rev. Mol. Diagn. 2017, 17, 209–215. [Google Scholar] [CrossRef]
- Warren, J.D.; Xiong, W.; Bunker, A.M.; Vaughn, C.P.; Furtado, L.V.; Roberts, W.L.; Fang, J.C.; Samowitz, W.S.; Heichman, K.A. Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Diop, F.; Spaccarotella, E.; Monti, S.; Zanni, M.; Rasi, S.; Deambrogi, C.; Spina, V.; Bruscaggin, A.; Favini, C.; et al. Diffuse large B-cell lymphoma genotyping on the liquid biopsy. Blood 2017, 129, 1947–1957. [Google Scholar] [CrossRef]
- Spina, V.; Bruscaggin, A.; Cuccaro, A.; Martini, M.; Di Trani, M.; Forestieri, G.; Manzoni, M.; Condoluci, A.; Arribas, A.; Terzi-Di-Bergamo, L.; et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lymphoma. Blood 2018, 131, 2413–2425. [Google Scholar] [CrossRef] [PubMed]
- Knebel, F.H.; Bettoni, F.; da Fonseca, L.G.; Camargo, A.A.; Sabbaga, J.; Jardim, D.L. Circulating Tumor DNA Detection in the Management of Anti-EGFR Therapy for Advanced Colorectal Cancer. Front. Oncol. 2019, 9, 170. [Google Scholar] [CrossRef]
- Camus, V.; Stamatoullas, A.; Mareschal, S.; Viailly, P.J.; Sarafan-Vasseur, N.; Bohers, E.; Dubois, S.; Picquenot, J.M.; Ruminy, P.; Maingonnat, C.; et al. Detection and prognostic value of recurrent exportin 1 mutations in tumor and cell-free circulating DNA of patients with classical Hodgkin lymphoma. Haematologica 2016, 101, 1094–1101. [Google Scholar] [CrossRef]
- Scherer, F.; Kurtz, D.M.; Newman, A.M.; Stehr, H.; Craig, A.F.; Esfahani, M.S.; Lovejoy, A.F.; Chabon, J.J.; Klass, D.M.; Liu, C.L.; et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci. Transl. Med. 2016, 8, 364ra155. [Google Scholar] [CrossRef] [PubMed]
- Kiss, R.; Alpar, D.; Gango, A.; Nagy, N.; Eyupoglu, E.; Aczel, D.; Matolcsy, A.; Csomor, J.; Matrai, Z.; Bodor, C. Spatial clonal evolution leading to ibrutinib resistance and disease progression in chronic lymphocytic leukemia. Haematologica 2019, 104, e38–e41. [Google Scholar] [CrossRef] [PubMed]
- Liebs, S.; Nonnenmacher, A.; Klauschen, F.; Keilholz, U.; Vecchione, L. Liquid biopsy assessment of synchronous malignancies: A case report and review of the literature. ESMO Open 2019, 4, e000528. [Google Scholar] [CrossRef]
- Beije, N.; Helmijr, J.C.; Weerts, M.J.A.; Beaufort, C.M.; Wiggin, M.; Marziali, A.; Verhoef, C.; Sleijfer, S.; Jansen, M.; Martens, J.W.M. Somatic mutation detection using various targeted detection assays in paired samples of circulating tumor DNA, primary tumor and metastases from patients undergoing resection of colorectal liver metastases. Mol. Oncol. 2016, 10, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Lakis, S.; Heukamp, L.C.; Griesinger, F. Detection of Discrepant Driver Mutations in a Patient with Two Synchronous Primary Non-Small Cell Lung Cancers (NSCLCs) with Liquid Biopsy. J. Thorac. Oncol. 2017, 12, e186–e188. [Google Scholar] [CrossRef]
- Scherer, F.; Kurtz, D.M.; Diehn, M.; Alizadeh, A.A. High-throughput sequencing for noninvasive disease detection in hematologic malignancies. Blood 2017, 130, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Gribben, J.G.; Neuberg, D.; Freedman, A.S.; Gimmi, C.D.; Pesek, K.W.; Barber, M.; Saporito, L.; Woo, S.D.; Coral, F.; Spector, N.; et al. Detection by polymerase chain reaction of residual cells with the bcl-2 translocation is associated with increased risk of relapse after autologous bone marrow transplantation for B-cell lymphoma. Blood 1993, 81, 3449–3457. [Google Scholar] [CrossRef]
- Mandigers, C.M.; Meijerink, J.P.; Mensink, E.J.; Tonnissen, E.L.; Hebeda, K.M.; Bogman, M.J.; Raemaekers, J.M. Lack of correlation between numbers of circulating t(14;18)-positive cells and response to first-line treatment in follicular lymphoma. Blood 2001, 98, 940–944. [Google Scholar] [CrossRef]
- Rambaldi, A.; Carlotti, E.; Oldani, E.; Della Starza, I.; Baccarani, M.; Cortelazzo, S.; Lauria, F.; Arcaini, L.; Morra, E.; Pulsoni, A.; et al. Quantitative PCR of bone marrow BCL2/IgH+ cells at diagnosis predicts treatment response and long-term outcome in follicular non-Hodgkin lymphoma. Blood 2005, 105, 3428–3433. [Google Scholar] [CrossRef]
- Van Oers, M.H.; Tonnissen, E.; Van Glabbeke, M.; Giurgea, L.; Jansen, J.H.; Klasa, R.; Marcus, R.E.; Wolf, M.; Kimby, E.; Vranovsky, A.; et al. BCL-2/IgH polymerase chain reaction status at the end of induction treatment is not predictive for progression-free survival in relapsed/resistant follicular lymphoma: Results of a prospective randomized EORTC 20981 phase III intergroup study. J. Clin. Oncol. 2010, 28, 2246–2252. [Google Scholar] [CrossRef]
- Alcaide, M.; Yu, S.; Bushell, K.; Fornika, D.; Nielsen, J.S.; Nelson, B.H.; Mann, K.K.; Assouline, S.; Johnson, N.A.; Morin, R.D. Multiplex Droplet Digital PCR Quantification of Recurrent Somatic Mutations in Diffuse Large B-Cell and Follicular Lymphoma. Clin. Chem. 2016, 62, 1238–1247. [Google Scholar] [CrossRef]
- Camus, V.; Sarafan-Vasseur, N.; Bohers, E.; Dubois, S.; Mareschal, S.; Bertrand, P.; Viailly, P.J.; Ruminy, P.; Maingonnat, C.; Lemasle, E.; et al. Digital PCR for quantification of recurrent and potentially actionable somatic mutations in circulating free DNA from patients with diffuse large B-cell lymphoma. Leuk. Lymphoma 2016, 57, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Morschhauser, F.; Tilly, H.; Chaidos, A.; Phillips, T.J.; Ribrag, V.; Campbell, P.; Gandhi Laurent, D.; Jurczak, W.; McKay, P.; Opat, S.; et al. Phase 2 Multicenter Study of Tazemetostat, an EZH2 Inhibitor, in Patients with Relapsed or Refractory Follicular Lymphoma. Blood 2019, 134, 123. [Google Scholar] [CrossRef]
- McDonald, A.; Thomas, E.; Daigle, S.; Morschhauser, F.; Salles, G.; Ribrag, V.; McKay, P.; Tilly, H.; Schmitt, A.; Batlevi, C.L.; et al. Updated Report on Identification of Molecular Predictors of Tazemetostat Response in an Ongoing NHL Phase 2 Study. Blood 2018, 132, 4097. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).