Abstract
Transient receptor potential vanilloid 1 (TRPV1) is an ion channel present on sensory neurons which is activated by heat, protons, capsaicin and a variety of endogenous lipids termed endovanilloids. As such, TRPV1 serves as a multimodal sensor of noxious stimuli which could trigger counteractive measures to avoid pain and injury. Activation of TRPV1 has been linked to chronic inflammatory pain conditions and peripheral neuropathy, as observed in diabetes. Expression of TRPV1 is also observed in non-neuronal sites such as the epithelium of bladder and lungs and in hair cells of the cochlea. At these sites, activation of TRPV1 has been implicated in the pathophysiology of diseases such as cystitis, asthma and hearing loss. Therefore, drugs which could modulate TRPV1 channel activity could be useful for the treatment of conditions ranging from chronic pain to hearing loss. This review describes the roles of TRPV1 in the normal physiology and pathophysiology of selected organs of the body and highlights how drugs targeting this channel could be important clinically.
1. Introduction
TRPV1 channels serve primarily as heat sensors, which are activated by temperatures >43 °C. These channels are present on sensory neurons, such as dorsal root ganglia and trigeminal neurons, where they are localized primarily in small diameter neurons and unmyelinated C fibers [1]. Amino acid sequence data predict a channel with six trans-membrane spanning regions and a pore located in a hydrophobic stretch between transmembrane segments 5 and 6. The pore shows a greater selectivity for Ca2+ over Na+ of 9.6:1 [1]. TRPV1 is also activated by capsaicin, an active ingredient in hot chili peppers [1,2]. Heat and capsaicin increase Ca2+ currents in cells expressing TRPV1. Protons (pH < 5.9) can directly activate TRPV1 channels and further enhance the sensitivity of these channels to capsaicin and heat [1]. Protons-induced activation could be relevant in tissue ischemia or inflammation. Thus, TRPV1 serves as an integrator of physical and chemical stimuli produced from the injury site or from external sources [1,2].
Chronic capsaicin administration desensitizes TRPV1 and renders the neurons less sensitive to noxious (painful) stimuli. This action requires the presence of extracellular Ca2+ and activation of Ca2+-calmodulin dependent protein kinase which promote channel phosphorylation [1,2]. This property of capsaicin has been employed for the treatment of pain associated with disease conditions such as diabetic peripheral neuropathy and arthritis [1,2].
2. Endogenous TRPV1 Agonists
These agonists, also referred to as endovanilloids, are expressed predominantly in the primary sensory neurons [3] and also different regions of the brain [4,5]. Endovanilloids are synthesized in the cells and released in an activity-dependent manner in adequate amounts to evoke TRPV1-mediated responses [6]. The endovanilloid signaling is terminated within a short period of time, which allows for strict control of its action.
Various endogenous lipids from the fatty acid pool have been identified as TRPV1 activators. Anandamide (N-arachidonoyl ethanolamine) (Figure 1), the endogenous ligand for the cannabinoid receptors, was reported to activate TRPV1 by binding to the same site as capsaicin [7]. However, its potency was 5–10 fold lower than that of capsaicin [8]. Endogenously produced anandamide causes TRPV1-dependent ileitis in the inflamed ileum of rats treated with Clostridium difficile toxin A [9]. AM-404 [N-(4-hydroxyphenyl)-arachidonoyl-ethanolamine] (Figure 1), an anandamide reuptake inhibitor and TRPV1 agonist, has been shown to attenuate motor disturbances by restoring GABA and dopamine transmission in an animal model for Huntington’s disease [10,11]. N-acyl ethanolamines (NAEs), like anandamide, have been studied as activators of TRPV1. One such NAE, N-oleoylethanolamine (OLEA) (Figure 1), evokes TRPV1 currents in cells previously sensitized with protein kinase C [12]. Intraperitoneal administration of OLEA induces visceral pain behavior in wild type, but not in TRPV1-null mice [13].
N-arachidonoyldopamine (NADA) (Figure 1), an endocannabinoid present in CNS, is a potent full agonist of TRPV1, with 5–10 fold higher potency than anandamide and equi-potent to capsaicin in functional assays [14]. Intradermal injection of NADA into the hind paw of mice results in thermal hyperalgesia [15]. NADA also constricts isolated bronchi and urinary bladder preparations from the guinea pig in a TRPV1-dependent manner [16]. A bioactive analogue of NADA, N-oleoyl dopamine (OLDA) (Figure 1), evokes increase in intracellular Ca2+ in TRPV1 expressing HEK293 cells. Similar to NADA, subcutaneous injection of OLDA produces thermal hyperalgesia [17].
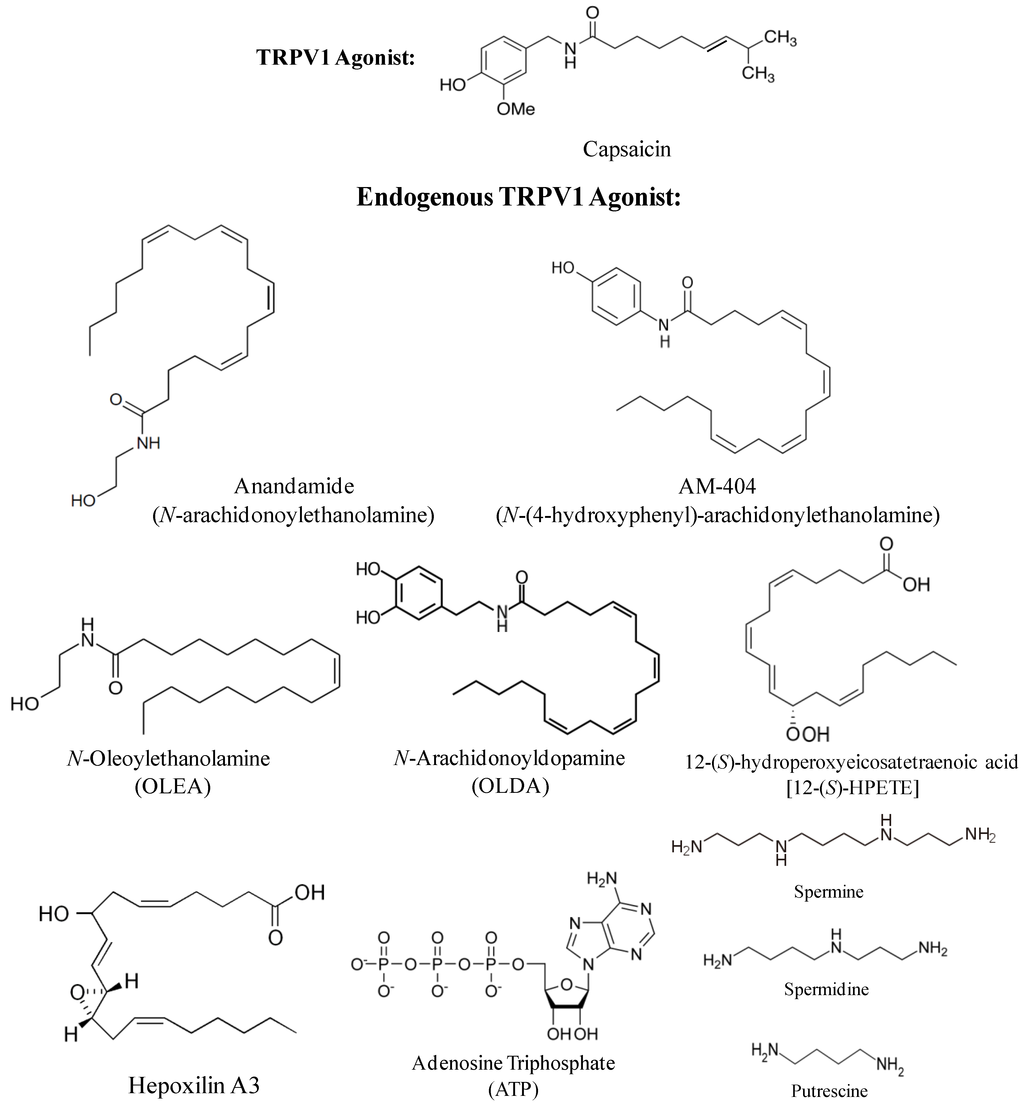
Figure 1.
Chemical structures of natural and endogenous transient receptor potential vanilloid 1 (TRPV1) receptor agonists.
Various lipooxygenase products of arachidonic acid, such as 5-(S), 8-(S), 12-(S) and 15-(S)-hydroperoxyeicosatetraenoic acids (HPETEs), are derived from the insertion of oxygen into the double bonds of the aliphatic chain of arachidonic acid. Since this reaction is catalyzed by lipoxygenases, these derivatives are called lipooxygenase products [18]. Among different HPETEs, 12-(S) HPETE (Figure 1) showed the highest potency to evoke capsazepine (a TRPV1 antagonist)-sensitive currents in DRG neurons and in HEK cells expressing TRPV1 [19]. It was reported that 12-(S) HPETE is produced in sensory neurons upon stimulation of sensory nerve endings by the inflammatory mediator bradykinin. In this study, bradykinin was shown to activate cAMP-dependent phospholipase 2 (PLA2) leading to the release of arachidonic acid, which is then metabolized by 12-lipoxygenase to produce 12-(S) HPETE. This pathway has been linked to thermal hyperalgesia induced by bradykinin [20]. In addition, 12-(S) HPETE can undergo other enzymatic reactions that lead to the formation of hepoxilins A3 (HXA3) and B3 (HXB3) (Figure 1). Intrathecal injections of these hepoxilins induce tactile allodynia via activation of TRPV1 (and TRPA1) in rats [21].
In addition to the endogenous lipids, other endogenous chemicals, including ATP, ammonia and polyamines (such as spermine, spermidine, and putrescine), as well as protons (observed during inflammation) can activate TRPV1 (for review see [22,23]) (Figure 1). ATP was identified as a TRPV1-sensitizing molecule that directly binds to TRPV1 in a region between ankyrin repeats 1–3 [24]. Moreover, by activating purinergic P2 receptors on sensory neurons, ATP stimulates phospholipase C (PLC) which produces diacylglycerol (DAG), an activator of PKC. Activators of PKC have been shown to sensitize TRPV1 channels to endogenous and exogenous agonists and reduce the temperature threshold for channel activation [25,26]. Acidic conditions normally occur during hypoxia, ischemia and inflammation [27]. Protons decrease the temperature threshold for TRPV1 activation such that even moderately acidic conditions (pH≤5.9) can activate the channel at room temperature. TRPV1 can also be activated by basic pH. Ammonia (NH3), an irritant and weak base, activates TRPV1 (and TRPA1) in sensory neurons through a mechanism that involves a cytoplasmic histidine residue [28]. Polyamines are capable of modulating inflammation and nociception by regulating the activity of TRPV1 channel. Extracellular spermine, spermidine, and putrescine, by virtue of their cationic charge, directly activate TRPV1 in a charge-dependent manner, both in heterologous expression systems and in sensory neurons. The threshold for activation by spermine is rather high (~500 μM at room temperature), but spermine can enhance capsaicin evoked currents with an effective concentration for 50% activation (EC50) value of approximately 5 μM [29].
3. Functions of TRPV1 Channel
3.1. Role of TRPV1 in Thermal Sensation
One of the remarkable features of living organisms is their ability to perceive and react to environmental conditions around them. The skin of animals has an ability to identify thermal profiles ranging from cold to extreme heat. Within this range, temperatures above 43 °C and below 15 °C evoke painful thermal sensations by activating C type nerve fibers [30,31]. Pioneering studies showed that primary afferent neurons express heat-sensitive ion channels [32,33] which were later cloned and identified as TRPV1 receptor [1,3]. The mRNA encoding TRPV1 receptor was expressed in small diameter sensory neurons within the dorsal root ganglion and trigeminal sensory ganglia, consistent with the idea that C and A-δ fibers are involved in nociception [3,34]. Immunolabeling for TRPV1 is observed in non-myelinated, low conductance nerve fibers which express neuropeptides such as substance P and neurokinin A [34,35]. TRPV1 was activated at temperatures >43 °C which are responsible for inducing pain in vivo [3,34]. TRPV1 knockout mice exhibit a reduced response to noxious stimuli induced by capsaicin, heat and protons [36,37,38]. Neurons of the dorsal root ganglion obtained from TRPV1 null mice showed greatly reduced sensitivity to heat, but a small number of neurons maintained their response when stimulated with heat temperatures above 50 °C, indicating that other receptors participate in pain perception in this temperature range [36]. The membrane potential and excitability of magnocellular neurosecretory cells of mice maintained at physiological temperature is reduced by genetic deletion or pharmacological blockade of the TRPV1 receptors. Moreover, the spontaneous electrical activity of these cells is abolished by the same experimental profile, indicating that TRPV1 plays a physiological role in the thermal sensation [39]. Human corneal epithelial cells also express functional TRPV1 receptors which are responsive to capsaicin and heat. Treatment with selective agonist 2-aminoethoxydiphenyl borate (2-APB), or exposure to temperatures above 40 °C, led to a transient increase in intracellular Ca2+ concentration, which was blocked by pretreatment with selective TRPV1 antagonists [40].
Inflammatory pain is produced following tissue damage and/or development of inflammation. It is characterized by hypersensitivity of the site of damage and nearby tissues to noxious stimuli. One of the mechanisms responsible for this aspect of inflammation is the modulation of ion channels, such as TRPV1, which become sensitized by mediators in the inflammatory micro-environment [41,42,43]. These mediators include prostaglandins, bradykinin, serotonin, ATP and adenosine [27]. Among these molecules, ATP, bradykinin, and prostaglandins sensitize TRPV1 through activating Ca2+ mobilizing receptors and PKC. These mediators reduce the threshold temperature and proton concentrations required to activate TRPV1 [26,44,45,46,47,48]. Studies using TRPV1 knockout mice and knockout of receptors for inflammatory mediators demonstrate essential roles for TRPV1 and inflammatory mediators for producing inflammatory pain [45,46,47]. The cytosolic domain of TRPV1 contains serine residues which could be phosphorylated by PKC [49,50]. Recently, it was shown in the neurons of the dorsal root ganglion and transfected cells that cyclin-dependent kinase 5 (Cdk5) controls trafficking of TRPV1 to the cell membrane through kinesin-3 family (KIF) 13B protein. Interestingly, activation of Cdk5 by injection of Freund’s adjuvant increases the trafficking of TRPV1 to the cell membrane, which likely contributes to development and maintenance of hyperalgesia [51]. The activity of TRPV1 could also be enhanced when it is associated with another receptor. In this sense, Cheng et al. [52] showed that TRPV1/TRPV3 heterodimers exhibit increased temperature sensitivity, lower threshold for activation and sensitization by heat as compared to the monomeric receptors. The microenvironment created by tissue damage and inflammation is rich in protons produced by inflammatory cells. By activating TRPV1 in these inflamed areas, protons can also contribute to pain sensations [53,54]. Thus, acidosis can enhance pain. Previous studies have shown that acidosis sensitizes visceral and cutaneous nociceptors, in large parts through activation of TRPV1 [55,56,57]. In extreme acidosis, protons can directly activate TRPV1. However, in mild acidic conditions, protons act as allosteric modulators of TRPV1 and greatly increase their sensitivity to heat, capsaicin and inflammatory agents. This phenomenon is typical of disorders characterized by increased tissue acidosis [53].
In addition to the sensitization of TRPV1 by inflammatory mediators and protons, activation of TRPV1 can enhance the release of inflammatory molecules associated with pain transmission, such as substance P, calcitonin gene-related peptide (CGRP) and bradykinin, which can contribute to peripheral sensitization of TRPV1 [58]. Moreover, TRPV1 expression is up-regulated flowing inflammation and nerve damage which can further enhance the responses mediated by these receptors [59,60].
3.2. Role of TRPV1 in Diabetes and Obesity
Type 1 diabetes has an autoimmune basis involving T cell-targeted destruction of pancreatic islet β cells. In this disease, autoreactive T cells target antigens on islet β cells or neurons, initiating a local inflammatory response and destruction of these β cells. These antigens include glutamic acid decarboxylase [61,62], myelin based proteins [63], glial fibrillary acid proteins and S100β [64]. TRPV1 is expressed on nerves innervating the islet β cells where they appear to modulate T cell function. An early study demonstrated that administration of capsaicin to neonatal mice was able to destroy these TRPV1 expressing neurons [65]. Interestingly, this treatment protected these mice from autoimmune diabetes [66]. It is believed that TRPV1 expressing neurons could induce the type 1 diabetic phenotype by modulating the proliferation and activity of T cell in the microenvironment of the islet β cells through the release of substance P. Pancreatic islet cells also include resident dendritic cells which express TRPV1 receptors [67]. Activation of these receptors by capsaicin or endovanilloids could activate dendritic cell function which includes antigen presentation to CD4+ T cells and chemotaxis. Since TRPV1 neurons play a critical role in modulating inflammation at the level of the pancreatic β cells, it could serve as a useful target for controlling inflammation and reducing diabetic symptoms. This suggests the clinical utility of TRPV1 antagonists or agonist-induced desensitization of TRPV1 to treat type 1 diabetes. The expression of TRPV1 in dendritic cells has been corroborated by some studies [67,68,69] but not others [70]. The idea that TRPV1 is an initiating factor mediating type 1 diabetes is challenged by the finding that TRPV1 isoform expressed in the non-obese diabetic (NOD) mouse possesses two mutations which render the channel less active and thereby should be less able to regulate the inflammatory process [66]. However, this discrepancy has been explained as faulty control mechanism between pancreatic islet β cells and the innervating TRPV1 expressing neurons. TRPV1 receptors are also expressed on islet β cells where they control the release of insulin [71]. Since insulin could sensitize TRPV1 receptors [72], it could positively regulate TRPV1 activity and increase the release of CGRP.
Diabetic peripheral neuropathy is a complication associated with diabetes mellitus, resulting from damage of loss of peripheral nerve terminals innervating the extremities. Affected patients normally complain of pain, tingling, and loss of feeling in the extremities. Several studies have implicated TRPV1 in the development of diabetic peripheral neuropathy in animal models of type 1 diabetes [73,74] and in humans [75]. Hong and Wiley [73] showed upregulation of TRPV1 protein and channel activity in a streptozotocin (STZ)-induced diabetic rats in large myelinated A fibers, while the normally expressing C fibers showed reduced expression. In another study, Pabbidi et al. [74] demonstrated a direct correlation between TRPV1 expression in dorsal root ganglion cells and thermal sensitivity. These investigators showed that STZ-induced diabetic mice developed an early hyperalgesic response, followed by a later phase of hypoalgesia. A similar temperature sensitivity profile was demonstrated in a double transgenic model of diabetes [74]. Interestingly, in both of these models, increased TRPV1 levels in dorsal root ganglion cells were observed in the hyperalgesic phase, while reduced levels of this protein was obtained during the hypoalgesic phase. A likely explanation for these findings is that the level of TRPV1 dictates the thermal sensitivity of diabetic animals. Similar changes in TRPV1 levels in the skin were observed in humans with diabetic neuropathy [75,76].
Type 2 diabetes is believed to be associated with inflammation, as evidenced by high levels of C-reactive proteins (CRP) in patients [77,78]. Antidiabetic drugs, such as the peroxisome proliferator activated receptor γ (PPARγ) agonist pioglitazone, are able to lower blood glucose along with CRP [79]. However, it is yet unclear whether a link exists between PPARγ agonists, CRP and TRPV1. TRPV1 has also been implicated in insulin resistance and obesity, characteristic of type 2 diabetes. It is believed that localized inflammation in the pancreas leads to increase in the activity of TRPV1 associated with aging, which contributes to increasing levels of CGRP [80]. CGRP is known to promote insulin resistance and obesity [81] by decreasing insulin release from β cells [82]. Treatment of Zucker rats with capsaicin or resiniferatoxin (RTX) (to desensitize TRPV1 expressing neurons) reduced fasting plasma insulin and improved glucose tolerance [83]. These data suggest that targeting TRPV1 for inhibition could be a novel method for treating diabetes and insulin resistance.
Various pieces of data support the contention that consumption of capsaicin or endovanilloids helps to control food intake and obesity. For example, volunteers consuming capsaicin capsules showed increased satiety and increased energy expenditure [84]. A capsaicin containing preparation was also shown to aid in weight maintenance in obese individuals [85]. The administration of the endovanilloid N-oleoylethanolamide was shown to reduce food intake in wild type mice, but not in TRPV1 knockout mice, implicating TRPV1 in the control of food intake [13]. Overall, these data support a role of TRPV1 in the control of diabetes, insulin resistance and obesity.
3.3. TRPV1 in Ototoxicity of Cisplatin and Aminoglycoside Antibiotics
Platinum containing drugs have been successfully used in the treatment of various solid tumors, including tumors of the head and neck. One such drug, cisplatin, is an important component of chemotherapeutic regimens for treating these tumors. This drug produces ototoxicity, in part, through the generation of reactive oxygen species (ROS) [86]. One target of ROS includes the organ of Corti [87], where it destroys outer hair cells [88]. These cells play an important role in hearing. Current strategies to prevent hearing loss involve the use of antioxidants [87]. However, concomitant antioxidant use could interfere with the anticancer efficacy of cisplatin and limit its efficacy in chemotherapy. As such, other treatment targets have been sought after.
One such target is TRPV1, which is expressed in the organ of Corti and spiral ganglion cells [86]. Zheng et al. [89] showed that capsaicin affected several parameters of auditory function, such as increasing the threshold of auditory nerve compound action potential (CAP) and reducing the magnitude of cochlear microphonics and electrically evoked otoacoustic emissions. Capsaicin was also shown to produce a transient increase in cochlear blood flow [89], presumably by activating TRPV1 containing neurons innervating the spiral modiolar artery and arterioles and the stria vascularis [90]. These responses were inhibited by the TRPV1 antagonist capsazepine, implicating TRPV1 in mediating these actions. However, these investigators subsequently demonstrated inhibitory effects of suprathreshold concentrations of capsaicin (300 µM) on outer hair cells potassium channels (Ik and Ik,n) [91]. A similar study by Zhou et al. [92] show increases in spiral ganglion activity following intracochlear perfusion of capsaicin.
An interesting observation is that TRPV1 and transduction channels in hair cells of the organ of Corti could gate the entry of different molecules into these cells [93]. These investigators observed rapid entry of FM1-43 styryl dye into the hair cells through stereocilia bundles on the apical surfaces of the cells [93]. Such a mechanism might allow the entry of a number of xenobiotics (such as cisplatin and aminoglycosides) into hair cells and sensory neurons. Similar findings were reported for the entry of gentamicin in Madin-Darby canine kidney (MDCK) cells [94] and in organ of Corti explant cultures [95].
In a recent study [96], we showed that TRPV1 is a target of ROS generated by cisplatin. ROS promote activation and induction of TRPV1 and the NOX3 isoform of NADPH oxidase (a major source of ROS generation in the cochlea) in the rat organ of Corti and spiral ganglion cells. Generation of ROS via NOX3 was shown to be crucial to the activation and induction of TRPV1. Subsequent studies demonstrated that TRPV1 serves as an integrator of “noxious” stimuli to activation of the inflammatory cascade in the cochlea [97]. This process involved coupling of TRPV1 to NOX3 and signal transducer and activator of transcription 1 (STAT1) [97]. Similar studies have shown induction in TRPV1 in the spiral and vestibular ganglia of mice following administration of kanamycin for 14 d [98]. Mice challenged by a single intratympanic injection of gentamicin were found to have increased intensity of TRPV1 immunoreactivity in cochlear hair cells, spiral ganglion cells, vestibular sensory cells and vestibular ganglion cells two weeks after injection [99].
It is well established that outer hair cells in the basal turn of the cochlea are more susceptible to damage than are the cells in the apical regions. Organ of Corti explants from neonatal rat cochlea were treated with gentamicin for 24 h. The greatest damage was demonstrated in the basal turn of the cochlea. Red fluorescence was observed in the basal turn of inner and outer hair cells treated with Texas Red labelled gentamicin (GTTR) [95]. Explants treated with gadolinium or ruthenium red blocked the uptake of GTTR in hair cells in a dose-dependent manner. Gadolinium blocks Ca2+-permeant, mechanosensitive cation channels and ruthenium red, a noncompetitive antagonist of TRPV1, is also a blocker of numerous cation channels [95]. Systemic injection of GTTR in neonatal rats resulted in accumulation of fluorescent label in hair cells but also in other cells in the cochlea. The authors concluded that hair cell susceptibility to damage by aminoglycosides may depend on uptake of these drugs, and that the uptake was mediated in part by TRPV1 proteins [95].
In a rat model of salicylate-induced tinnitus, induction of TRPV1 in the cochlea was observed 2 h following intraperitoneal drug administration [100]. Increases in TRPV1 levels in the cochlea of rats were also observed 24 h and 2 weeks following noise exposure [101]. These authors suggested that the increases in TRPV1 could be one mechanism underlying the development of tinnitus.
The importance of TRPV1 in mediating cisplatin ototoxicity was shown by siRNA knockdown studies. In these studies, it was shown that round window administration of TRPV1 siRNA decreased cisplatin-induced damage to outer hair cells in the organ of Corti, as evidenced from scanning electron microscopy. Round window application of TRPV1 siRNA prior to cisplatin administration significantly reduced the percentage of hair cell loss observed. Knockdown of TRPV1 also reduced cisplatin-induced hearing loss, as evidenced by preservation of auditory brainstem responses [96].
Recent studies have implicated TRPV1 in mediating inflammation in the cochlea through activation of STAT1 via a ROS-dependent pathway. Activation of TRPV1 stimulates NOX3 activity, the generation of ROS and subsequent phosphorylation of Ser727 p-STAT1 by mitogen activated protein kinase (MAPK), and also increases expression of inflammatory mediators [97,102]. Overall, these studies suggest that inhibition of TRPV1 or its downstream effector in the cochlea could provide protection against hearing loss.
3.4. TRPV1 in the Bladder
Sensory neurons innervating the urinary bladder and urethra play important roles in innate reflexes involving the storage of urine and urination. Dysfunctions of these sensory systems are probably related to disorders, such as urinary incontinence. The upper and lower urinary tracts of human and other species are richly innervated by capsaicin-sensitive neurons [103,104] which are primarily unmyelinated C nerve fibers [105]. These sensory neurons regulate urination and pain perception from the urinary bladder. On the other hand, the efferent functions include local regulation of the activity of muscle cells, excitability of nerve blood flow and leakage of plasma proteins [106]. Initial characterization of TRPV1 receptors in the urinary tract was performed by radioligand binding assays using [3H]-RTX in membranes from urinary bladder of rats [107]. Subsequently, immunohistochemistry and electron microscopy studies revealed labeling of nerve fibers innervating the bladder muscles and basal urothelial cells [108,109]. These fibers can be located inside or around the bladder mucosa in proximity to blood vessels and smooth muscle cells [108]. TRPV1 is also expressed in non-neuronal cells of the bladder, such as urothelial cells and myofibroblasts [109,110,111,112,113,114]. TRPV1 immunoreactivity was also found in a population of cells located in suburothelial space which possess characteristics of myofibroblasts and are electrically active. These cells could serve as an electrical network capable of modulating bladder sensations [115,116]. However, recently the cellular localization of TRPV1 expression in the bladder has been debated. Different studies using patch-clamp, Ca2+ imaging, RT-PCR, immunohistochemistry and Western blotting found no evidence for the functional expression of TRPV1 in bladder urothelium. Further studies are warranted to support these findings and clarify the role of TRPV1 in normal bladder physiology as well as pathologies [114,117,118].
Cultures of urothelial cells from rats and mice exhibit increased intracellular Ca2+ and nitric oxide production when challenged with selective TRPV1 agonists. No responses were observed in urothelial cells obtained from TRPV1 knockout animals [109], implicating TRPV1 in this process. Bladder distention leads to greater activity of afferent nerves which was attenuated by capsazepine and was abolished in TRPV1 knockout animals [119]. In animal models, capsaicin induces a transient increase in the frequency of bladder contractions and reduces the volume threshold needed to induce voiding [120,121,122].
The role of TRPV1 receptors has also been analyzed in various urinary tract pathologies. Patients with neurogenic detrusor overactivity exhibit increased expression of TRPV1 in urothelial cells and nerve fibers, compared to healthy subjects. Administration of RTX to these patients reduced the levels of TRPV1 in these areas [110]. Rats with detrusor muscle overactivity exhibited reduced amplitudes of bladder contraction when treated with the TRPV1 antagonist GRC-6211 [123]. Interestingly, individuals suffering from overactive bladder were effectively treated with TRPV1 agonists, capsaicin or RTX [124], which might have the capacity to destroy TRPV1 expressing neurons. Cultures of urothelial cells from patients with non-congenital bladder overactivity exhibit greater expression of TRPV1 compared with the control group and increased sensitivity to capsaicin [125].
Cystitis is an inflammation of the bladder which produces visceral pain. Cystitis induced by cyclophosphamide (a chemotherapeutic agent) in mice and rats is characterized by mechanical bladder hyperactivity which is antagonized by pretreatment with selective TRPV1 antagonist SB-366791, and is not observed in TRPV1 null mice. In contrast, the TRPV1 null mice did not show any deficits in their development of bladder inflammation [126,127]. The induced cystitis was associated with increased TRPV1 channel activity in neurons innervating the bladder and the associated dorsal root ganglia [128], along with increased TRPV1 expression in the bladder [129]. Overall, these data indicate that TRPV1 plays a principal role in the development of pain related to cystitis, suggesting the utility of TRPV1 blockers to treat this condition.
3.5. TRPV1 in the Lung
Nerve fibers expressing TRPV1 innervate different components of the respiratory tract, including the trachea, bronchi, alveoli, smooth muscles and blood vessels [130]. These receptors are also expressed in lung and cells lining the airway, but at a relatively lower level [131,132]. Interestingly, patients with emphysema (and are smokers) have a higher expression of TRPV1 receptors when compared to non-smokers [133]. Polymorphisms in the TRPV1 receptor gene are associated with a higher incidence of coughs in patients without asthma, smokers and individuals exposed to environmental irritants such as vapors, gases and dusts [134]. A recent study demonstrated a functional association between a specific polymorphism, TRPV1-I585V, with childhood asthma. Asthmatic subjects with this polymorphism exhibit a lower risk of coughing and wheezing. This result could be explained by a reduced activity of this polymorphic channel compared to the normal TRPV1. The polymorphism rendered these channels less responsive to activation by heat and capsaicin, indicating that TRPV1 plays an important role in the pathophysiology of asthma [135].
A number of studies relating to TRPV1 in the airway focus on its role on sensory nerves which stimulate the cough reflex. McLeod et al. [136] demonstrate that capsaicin can induce cough in guinea pigs by activating TRPV1. Capsaicin and citric acid were also capable of inducing cough, which was blocked in a dose-dependent manner by pretreatment with the antagonist iodo-resiniferatoxin (I-RTX) [137]. Similar effects were produced by JNJ-17203212 and capsazepine, two other TRPV1 selective antagonists [138,139]. Furthermore, in an in vivo asthma model, TRPV1 antagonist, N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), attenuated the coughing induced by allergens [136]. Increased sensitivity to TRPV1 agonists was observed in airway diseases. For example, patients with upper respiratory tract infection, asthma or chronic obstructive pulmonary disease have increased incidence of cough in response to capsaicin [140,141,142]. Children with allergic rhinitis or upper respiratory infection show worsened coughs stimulated by capsaicin, compared to unaffected children [143,144]. Patients with chronic coughs have increased expression of TRPV1 in nerve fibers [145] and airway muscle cells [146], implicating these receptors in the increased incidence of coughing. The findings in humans were reproduced in animal models of respiratory diseases. For example, exposure of these animals to allergens, tobacco smoke or viral infection causes hypersensitivity to TRPV1 agonists and morphological changes in airway nerves [147,148,149,150,151,152].
4. TRPV1 Antagonists
Two methods aimed at blocking TRPV1 in diseases have been pursued as potential treatment options. One method involves activation and desensitization of TRPV1 by agonists. Another method involves the use of antagonist drugs for TRPV1 receptor.
A surprising finding is that capsaicin has traditionally been used to reduce pain, primarily by its ability to desensitize TRPV1. Capsaicin (as 0.025%–0.075% cream preparations) is effective for treating pain produced by osteoarthritis and rheumatoid arthritis and peripheral neuropathy [153], such as diabetic peripheral neuropathy [154]. Topical capsaicin preparations have also been shown to provide relief of post-herpetic pain [155]. However, burning sensations or erythema at the site of application could decrease wide spread application of this latter treatment option [156].
Pharmaceutical companies have invested millions of dollars for drug screening and lead optimization programs that have identified selective and potent small molecule TRPV1 antagonists, many of which are undergoing clinical trials as analgesic drugs (for review see [157,158,159]). Early structure-activity relationship (SAR) studies to identify TRPV1 antagonists focused on the structure of capsaicin, a naturally occurring TRPV1 agonist. The first reported TRPV1 antagonist, capsazepine, was discovered by modifying the chemical backbone of capsaicin [160]. In capsazepine, the amide bond of capsaicin is replaced by a thiourea moiety, the amide nitrogen of which acts as tether, forcing the aromatic ring to form an orthogonal orientation with respect to the thiourea bond (see Figure 2). This tether was believed to be critical for the antagonistic activity of capsazepine. Capsazepine competes for the capsaicin-binding site on TRPV1, blocks capsaicin-induced channel activation in neonatal rat dorsal root ganglion [161] and displaces RTX from its binding site in radioligand binding studies [34]. Although capsazepine was found to be extremely useful in laboratory research, it was not considered an important candidate for clinical use. One of the reasons is its low metabolic stability and poor pharmacokinetic properties as demonstrated in rodents [23]. Another factor that impeded the clinical use of capsazepine as TRPV1 antagonist was its apparent non-selectivity [157,158,159]. In addition to inhibiting TRPV1, capsazepine also inhibited nicotinic acetylcholine receptors [162], voltage-gated Ca2+ channels [163] and TRPM8 [164]. Moreover, capsazepine illustrated species-dependent effects in various models of chronic inflammatory and neuropathic pain [165] possibly due to the species-related differences in the binding of capsazepine to TRPV1. The anti-hyperalgesic effect of capsazepine was more effective in reversing the persistent inflammatory and neuropathic pain in guinea pig than in mice or rats [165]. These shortcomings of capsazepine led to the development of potent and more selective TRPV1 antagonists. The tether, which was believed to be critical for the antagonistic effect of capsazepine, was later found to be irrelevant, as a number of compounds lacking this feature emerged as better antagonists than capsazepine, possessing excellent therapeutic potential in pain regulation and considered as promising clinical candidates [166,167].
Despite its drawbacks, the SAR of capsazepine was used as a template for developing next generation TRPV1 antagonists. A pharmacophore model for the structure of an ideal TRPV1 antagonist has been proposed based on its key binding interactions with the ion channel. This model is combined with the homology model of the TRPV1 channel which is used to filter the set of possible antagonists both by size and shape of the site and by location of appropriate interacting sites on the protein [168]. According to this model, a unifying structural feature of TRPV1 antagonists emerged that has a central hydrogen-bond acceptor/donor motif flanked by a lipophilic side chain on one side and an aromatic group that incorporates a hydrogen-bond acceptor on the other side [23,169].
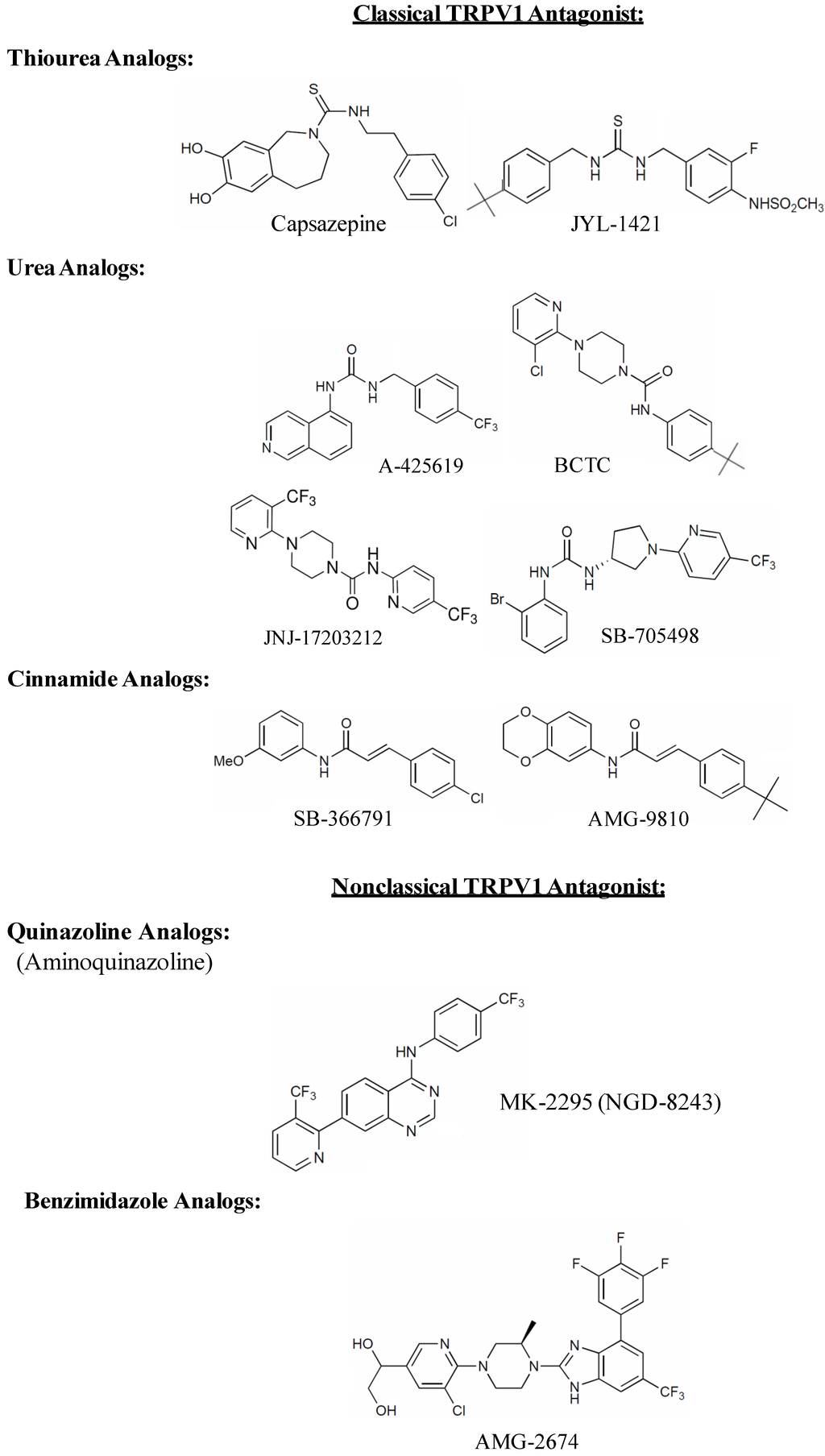
Figure 2.
Chemical structures of competitive TRPV1 receptor antagonists.
TRPV1 antagonists can be broadly classified as competitive or non-competitive antagonists. A competitive antagonist binds to the agonist binding site and locks the TRPV1 channel in closed, non-conductive state. All major competitive TRPV1 antagonists discovered so far can be divided into two major classes, classical and non-classical antagonists [23]. The classical antagonists are characterized by the presence of a carbonyl group that can act as H-bonding donor or acceptor and which is present in the form of thiourea, urea, ester or amide (see Figure 2). Many early generation TRPV1 antagonists including capsazepine had either thiourea or urea as a key moiety which was believed to be important for activity. These include JYL-1421 [170], A-425619 [171], BCTC [172], JNJ-17203212 [173] and SB-705498 [174]. Another common functionality of classical TRPV1 antagonists is cinnamide (Figure 2), the structural feature of which can be traced back to the prototypical TRPV1 antagonist capsazepine (e.g., SB-366791 [175] and AMG-9810 [176]). Non-classical TRPV1 antagonists on the other hand have a carbonyl group which is either present as a part of heterocyclic ring or is unrecognizable. These are represented as quinazoline [177] or benzimidazole analogues (AMG-2674) [178] (Figure 2), which are structurally different from capsazepine but still retain the key binding elements.
Non-competitive antagonists of TRPV1 channel are pore blockers that interact with additional binding sites (allosteric sites) thereby preventing channel opening by the agonist or blocking its aqueous pore (hence pore blockers). Non-competitive antagonists acting as open-channel blockers are therapeutically more attractive because they preferentially recognize the population of pathologically-over-activated TRPV1 channels and block them, thereby reducing the potential unwanted side effects [179]. The first ever non-competitive TRPV1 antagonist used was a trinuclear polyamine complex, ruthenium red (Figure 3). It is a nonspecific inhibitor of TRP channels that binds to the pore of the channel with high potency in a voltage dependent manner (i.e., it blocks inward currents but not outward currents) [180]. The poor channel selectivity of ruthenium red is thought to be responsible for its pro-convulsive activity in animal models that deterred its clinical development as an analgesic agent [179]. Arginine-rich hexapeptides like RRRRWW-NH2 (Figure 3) were identified to block recombinant TRPV1 channels expressed in Xenopus oocytes in a non-competitive nonselective manner with submicromolar potency [181]. However, owing to its nonselective nature, like ruthenium red, it showed severe side effects. A parallel approach identified methoctramine (Figure 3), a polymethylene tetraamine, as non-competitive capsaicin antagonist. Methoctramine, a muscarinic M2 receptor blocker, antagonized native TRPV1 receptors in DRG neurons activated by either capsaicin or protons in a voltage-dependent manner [182]. The lack of receptor selectivity has restrained its use clinically.
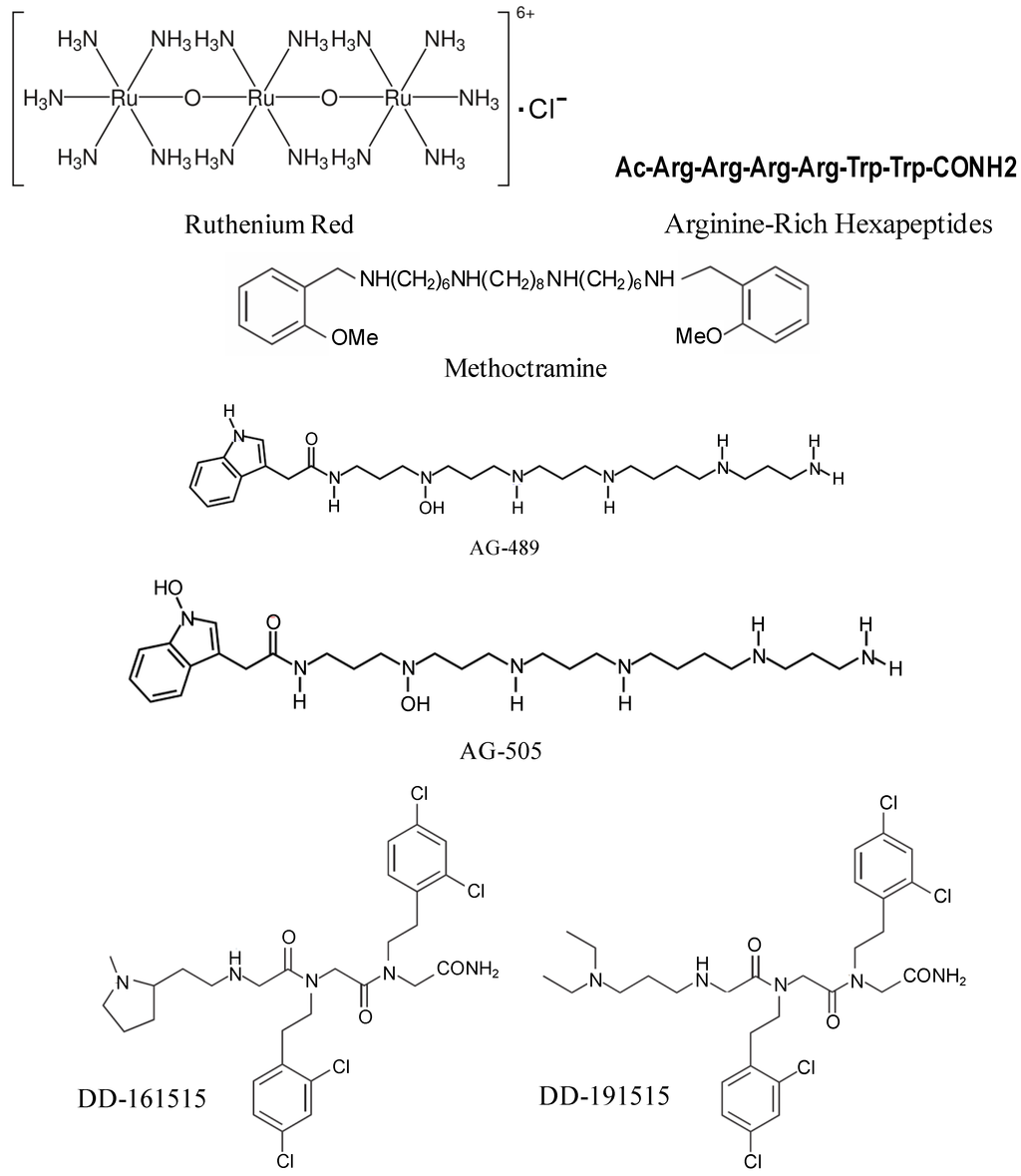
Figure 3.
Chemical structures of non-competitive TRPV1 receptor antagonists.
Two acylpolyamine toxins, AG-489 and AG-505 (Figure 3), purified from the venom of North American funnel web spider, Agelenopsis aperta, showed robust TRPV1 inhibitory activity by blocking the channel from the extracellular side of the membrane. TRPV1 blockade by AG489 was found to be strongly voltage-dependent, with relief from inhibition being observed at positive voltages. This observation is consistent with a model in which toxin inhibits the channel through a pore-blocking mechanism [23,183].
Screening of a library of trimers of N-alkylglycines (also known as peptoids) identified two compounds, DD-161515 and DD-191515 (Figure 3), which preferentially inhibit TRPV1 channel activity with micromolar potency and moderate voltage-dependency [184]. These compounds were effective in treating inflammatory pain induced by injection of capsaicin into the hind paws of mice and also reduced thermal hyperalgesia due to mustard oil-evoked tissue irritation. However, they did not treat capsaicin triggered-mechanical hypersensitivity [184] and the in vivo doses required for analgesic and anti-inflammatory effect was too high (≥25 mg/kg) to successfully use these drugs clinically.
There are two most desirable characteristics that a TRPV1 antagonist should have [159]. First, the TRPV1 antagonist should block all modes of channel activation. For example, capsazepine was ineffective in reversing inflammation-based pain behavior in rats [165,185]. In addition, capsazepine demonstrates species difference in its ability to block multiple modes of TRPV1 activation (discussed above). In contrast, other chemically distinct compounds, such as A-425619, AMG-9810 and BCTC, not only inhibited all modes of rat TRPV1 activation but also inhibited and reduced inflammation-related hyperalgesia in rats [176,186,187]. Thus, in order to achieve significant analgesic effect, the TRPV1 antagonists that inhibit all modes of TRPV1 activation are more desirable.
Another critical feature that is desirable in TRPV1 antagonists is its brain-penetration. The antagonists that penetrate the brain shows more analgesic efficacy than the ones whose actions are limited to the periphery. For example, the TRPV1 antagonist A-784168 showed good CNS penetration and hence was reported to be more potent in inhibiting pain that was presumably mediated by central sensitization than A-795614, a peripherally restricted TRPV1 antagonist [188]. However, both the compounds were equally potent when administered intrathecally, suggesting that brain penetration provides better efficacy.
In addition to the transmission of pain, TRPV1 plays an independent role in regulating body temperature. It is well known that TRPV1 agonist, capsaicin, transiently decreases body temperature in different species, including man [189]. While studying the ability of TRPV1 antagonists to inhibit capsaicin-induced hypothermia, it was observed that some of them caused hyperthermia [190,191]. These findings were surprising due to the fact that TRPV1 knock-out mice showed no difference in core body temperature than the wild-type mice [36]. Interestingly, TRPV1 antagonist, AMG-0347 and AMG-517 did not induce hyperthermia in TRPV1 knock-out mice, suggesting that the TRPV1 antagonist-mediated hyperthermia is TRPV1-dependent [192]. The hyperthermia caused by highly potent and TRPV1-selective antagonists AMG-0347 and AMG-517 were reportedly due to vasoconstriction (results in decreased heat loss through skin) and increased thermogenesis (increased metabolic heat production). Another drawback of using TRPV1 antagonists is that it elevates the threshold for detection of noxious heat [193], which could raise the possible complication of accidental burn injuries in susceptible patients. This elevation of heat threshold was reported to be pronounced for some TRPV1 antagonists than for others [194]. The question whether the hyperthermic action of TRPV1 antagonists can be separated from their analgesic action is still unanswered. Meanwhile, various strategies have been tested to alleviate the hyperthermia caused by TRPV1 antagonists while still preserving their analgesic properties. TRPV1 antagonist-induced hyperthermia is responsive to anti-pyretic agents like acetaminophen [190]. Hyperthermia caused by TRPV1 antagonists desensitizes after repeated administration of antagonists [190]. Another attractive approach is to chemically modify the pharmacophore structure of TRPV1 antagonists in order to prevent the undesirable side effect of hypothermia while inhibiting all modes of TRPV1 activation.
5. Conclusions
Since its cloning over a decade ago, research on TRPV1 has grown considerably. While a primary area of interest is the role of this channel in mediating pain, especially inflammatory and chronic pain, a number of researchers are studying the ability of agonist or antagonists of these receptors to relieve symptoms of diseases ranging from diabetes and urinary incontinence to arthritis and hearing loss. The rapid growth of research in these areas bodes well of the development of effective TRPV1 drugs to treat these and other diseases. However, the full adoption of TRPV1 antagonists into clinical practice would depend on the development of effective measures to counter drug-induced hyperthermia.
Acknowledgments
The research findings presented in this review, which was performed in the laboratory of the authors, was supported, in part, by NIH grants, R15 CA135494, R15DC011412, RO1-DC002396, and R01-CA166907, and from the Excellence in Academic Medicine Award from the SIU School of Medicine. Rafael Brito, who is a Ph.D. student, received a fellowship from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) from Brazil (Processor #2028752013-0) to work in Vickram Ramkumar’s laboratory.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef]
- Holzer, P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991, 43, 143–201. [Google Scholar]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Mezey, E.; Toth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Roberts, J.C.; Davis, J.B.; Benham, C.D. [3H]Resiniferatoxin autoradiography in the CNS of wild-type and TRPV1 null mice defines TRPV1 (VR-1) protein distribution. Brain Res. 2004, 995, 176–183. [Google Scholar] [CrossRef]
- Van der Stelt, M.; di Marzo, V. Endovanilloids. Putative endogenous ligands of transient receptor potential vanilloid 1 channels. Eur. J. Biochem. 2004, 271, 1827–1834. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sorgard, M.; di Marzo, V.; Julius, D.; Hogestatt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef]
- Ross, R.A. Anandamide and vanilloid TRPV1 receptors. Br. J. Pharmacol. 2003, 140, 790–801. [Google Scholar] [CrossRef]
- McVey, D.C.; Schmid, P.C.; Schmid, H.H.; Vigna, S.R. Endocannabinoids induce ileitis in rats via the capsaicin receptor (VR1). J. Pharmacol. Exp. Ther. 2003, 304, 713–722. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; de Miguel, R.; de Petrocellis, L.; Makriyannis, A.; di Marzo, V.; Fernandez-Ruiz, J. Compounds acting at the endocannabinoid and/or endovanilloid systems reduce hyperkinesia in a rat model of Huntington’s disease. J. Neurochem. 2003, 84, 1097–1109. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Hansen, H.H.; Berrendero, F.; de Miguel, R.; Perez-Rosado, A.; Manzanares, J.; Ramos, J.A.; Fernandez-Ruiz, J. Alleviation of motor hyperactivity and neurochemical deficits by endocannabinoid uptake inhibition in a rat model of Huntington’s disease. Synapse 2002, 44, 23–35. [Google Scholar] [CrossRef]
- Almasi, R.; Szoke, E.; Bolcskei, K.; Varga, A.; Riedl, Z.; Sandor, Z.; Szolcsanyi, J.; Petho, G. Actions of 3-methyl-N-oleoyldopamine, 4-methyl-N-oleoyldopamine and N-oleoylethanolamide on the rat TRPV1 receptor in vitro and in vivo. Life Sci. 2008, 82, 644–651. [Google Scholar] [CrossRef]
- Wang, X.; Miyares, R.L.; Ahern, G.P. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J. Physiol. 2005, 564, 541–547. [Google Scholar] [CrossRef]
- Toth, A.; Kedei, N.; Wang, Y.; Blumberg, P.M. Arachidonyl dopamine as a ligand for the vanilloid receptor VR1 of the rat. Life Sci. 2003, 73, 487–498. [Google Scholar] [CrossRef]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; de Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J.; et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef]
- Harrison, S.; de Petrocellis, L.; Trevisani, M.; Benvenuti, F.; Bifulco, M.; Geppetti, P.; di Marzo, V. Capsaicin-like effects of N-arachidonoyl-dopamine in the isolated guinea pig bronchi and urinary bladder. Eur. J. Pharmacol. 2003, 475, 107–114. [Google Scholar] [CrossRef]
- Chu, C.J.; Huang, S.M.; de Petrocellis, L.; Bisogno, T.; Ewing, S.A.; Miller, J.D.; Zipkin, R.E.; Daddario, N.; Appendino, G.; di Marzo, V.; et al. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 2003, 278, 13633–13639. [Google Scholar] [CrossRef]
- Phillis, J.W.; Horrocks, L.A.; Farooqui, A.A. Cyclooxygenases, lipoxygenases, and epoxygenasesin CNS: Their role and involvement in neurological disorders. Brain Res. Rev. 2006, 52, 201–243. [Google Scholar] [CrossRef]
- Hwang, S.W.; Cho, H.; Kwak, J.; Lee, S.Y.; Kang, C.J.; Jung, J.; Cho, S.; Min, K.H.; Suh, Y.G.; Kim, D.; Oh, U. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. USA 2000, 97, 6155–6160. [Google Scholar] [CrossRef]
- Shin, J.; Cho, H.; Hwang, S.W.; Jung, J.; Shin, C.Y.; Lee, S.Y.; Kim, S.H.; Lee, M.G.; Choi, Y.H.; Kim, J.; et al. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. USA 2002, 99, 10150–10155. [Google Scholar] [CrossRef]
- Gregus, A.M.; Doolen, S.; Dumlao, D.S.; Buczynski, M.W.; Takasusuki, T.; Fitzsimmons, B.L.; Hua, X.Y.; Taylor, B.K.; Dennis, E.A.; Yaksh, T.L. Spinal 12-lipoxygenase-derived hepoxilin A3 contributes to inflammatory hyperalgesia via activation of TRPV1 and TRPA1 receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 6721–6726. [Google Scholar]
- Morales-Lazaro, S.L.; Simon, S.A.; Rosenbaum, T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1). J. Physiol. 2013, 591, 3109–3121. [Google Scholar]
- Vriens, J.; Appendino, G.; Nilius, B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 2009, 75, 1262–1279. [Google Scholar] [CrossRef]
- Lishko, P.V.; Procko, E.; Jin, X.; Phelps, C.B.; Gaudet, R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 2007, 54, 905–918. [Google Scholar] [CrossRef]
- Premkumar, L.S.; Ahern, G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature 2000, 408, 985–990. [Google Scholar] [CrossRef]
- Tominaga, M.; Wada, M.; Masu, M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc. Natl. Acad. Sci. USA 2001, 98, 6951–6956. [Google Scholar] [CrossRef]
- Julius, D.; Basbaum, A.I. Molecular mechanisms of nociception. Nature 2001, 413, 203–210. [Google Scholar] [CrossRef]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A. TRPV1 is activated by both acidic and basic pH. J. Neurosci. 2009, 29, 153–158. [Google Scholar] [CrossRef]
- Ahern, G.P.; Wang, X.; Miyares, R.L. Polyamines are potent ligands for the capsaicin receptor TRPV1. J. Biol. Chem. 2006, 281, 8991–8995. [Google Scholar] [CrossRef]
- LaMotte, R.H.; Campbell, J.N. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J. Neurophysiol. 1978, 41, 509–528. [Google Scholar]
- Tillman, D.B.; Treede, R.D.; Meyer, R.A.; Campbell, J.N. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: Estimates of receptor depth and threshold. J. Physiol. 1995, 485Pt 3, 753–765. [Google Scholar]
- Cesare, P.; McNaughton, P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc. Natl. Acad. Sci. USA 1996, 93, 15435–15439. [Google Scholar] [CrossRef]
- Reichling, D.B.; Levine, J.D. Heat transduction in rat sensory neurons by calcium-dependent activation of a cation channel. Proc. Natl. Acad. Sci. USA 1997, 94, 7006–7011. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef]
- Walder, R.Y.; Radhakrishnan, R.; Loo, L.; Rasmussen, L.A.; Mohapatra, D.P.; Wilson, S.P.; Sluka, K.A. TRPV1 is important for mechanical and heat sensitivity in uninjured animals and development of heat hypersensitivity after muscle inflammation. Pain 2012, 153, 1664–1672. [Google Scholar] [CrossRef]
- Sudbury, J.R.; Bourque, C.W. Dynamic and permissive roles of TRPV1 and TRPV4 channels for thermosensation in mouse supraoptic magnocellular neurosecretory neurons. J. Neurosci. 2013, 33, 17160–17165. [Google Scholar] [CrossRef]
- Mergler, S.; Garreis, F.; Sahlmuller, M.; Reinach, P.S.; Paulsen, F.; Pleyer, U. Thermosensitive transient receptor potential channels in human corneal epithelial cells. J. Cell Physiol. 2011, 226, 1828–1842. [Google Scholar] [CrossRef]
- Mizumura, K.; Kumazawa, T. Modification of nociceptor responses by inflammatory mediators and second messengers implicated in their action—A study in canine testicular polymodal receptors. Prog. Brain Res. 1996, 113, 115–141. [Google Scholar] [CrossRef]
- Wood, J.N.; Perl, E.R. Pain. Curr. Opin. Genet. Dev. 1999, 9, 328–332. [Google Scholar] [CrossRef]
- Woolf, C.J.; Salter, M.W. Neuronal plasticity: Increasing the gain in pain. Science 2000, 288, 1765–1769. [Google Scholar] [CrossRef]
- Amadesi, S.; Nie, J.; Vergnolle, N.; Cottrell, G.S.; Grady, E.F.; Trevisani, M.; Manni, C.; Geppetti, P.; McRoberts, J.A.; Ennes, H.; et al. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J. Neurosci. 2004, 24, 4300–4312. [Google Scholar] [CrossRef]
- Dai, Y.; Moriyama, T.; Higashi, T.; Togashi, K.; Kobayashi, K.; Yamanaka, H.; Tominaga, M.; Noguchi, K. Proteinase-activated receptor 2-mediated potentiation of transient receptor potential vanilloid subfamily 1 activity reveals a mechanism for proteinase-induced inflammatory pain. J. Neurosci. 2004, 24, 4293–4299. [Google Scholar] [CrossRef]
- Moriyama, T.; Higashi, T.; Togashi, K.; Iida, T.; Segi, E.; Sugimoto, Y.; Tominaga, T.; Narumiya, S.; Tominaga, M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain 2005, 1, 3. [Google Scholar] [CrossRef]
- Moriyama, T.; Iida, T.; Kobayashi, K.; Higashi, T.; Fukuoka, T.; Tsumura, H.; Leon, C.; Suzuki, N.; Inoue, K.; Gachet, C.; et al. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J. Neurosci. 2003, 23, 6058–6062. [Google Scholar]
- Sugiura, T.; Tominaga, M.; Katsuya, H.; Mizumura, K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J. Neurophysiol. 2002, 88, 544–548. [Google Scholar]
- Bhave, G.; Hu, H.J.; Glauner, K.S.; Zhu, W.; Wang, H.; Brasier, D.J.; Oxford, G.S.; Gereau, R.W.T. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc. Natl. Acad. Sci. USA 2003, 100, 12480–12485. [Google Scholar]
- Numazaki, M.; Tominaga, T.; Toyooka, H.; Tominaga, M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J. Biol. Chem. 2002, 277, 13375–13378. [Google Scholar] [CrossRef]
- Xing, B.M.; Yang, Y.R.; Du, J.X.; Chen, H.J.; Qi, C.; Huang, Z.H.; Zhang, Y.; Wang, Y. Cyclin-dependent kinase 5 controls TRPV1 membrane trafficking and the heat sensitivity of nociceptors through KIF13B. J. Neurosci. 2012, 32, 14709–14721. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, F.; Liu, S.; Colton, C.K.; Wang, C.; Cui, Y.; Cao, X.; Zhu, M.X.; Sun, C.; Wang, K.; et al. Heteromeric heat-sensitive transient receptor potential channels exhibit distinct temperature and chemical response. J. Biol. Chem. 2012, 287, 7279–7288. [Google Scholar]
- Holzer, P. Acid-sensitive ion channels and receptors. Handb. Exp. Pharmacol. 2009, 194, 283–332. [Google Scholar] [CrossRef]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Leffler, A.; Monter, B.; Koltzenburg, M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 2006, 139, 699–709. [Google Scholar] [CrossRef]
- Sugiura, T.; Bielefeldt, K.; Gebhart, G.F. Mouse colon sensory neurons detect extracellular acidosis via TRPV1. Am. J. Physiol. Cell Physiol. 2007, 292, C1768–C1774. [Google Scholar]
- Yagi, J.; Wenk, H.N.; Naves, L.A.; McCleskey, E.W. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ. Res. 2006, 99, 501–509. [Google Scholar] [CrossRef]
- Planells-Cases, R.; Garcia-Sanz, N.; Morenilla-Palao, C.; Ferrer-Montiel, A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005, 451, 151–159. [Google Scholar] [CrossRef]
- Hudson, L.J.; Bevan, S.; Wotherspoon, G.; Gentry, C.; Fox, A.; Winter, J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur. J. Neurosci. 2001, 13, 2105–2114. [Google Scholar] [CrossRef]
- Ji, R.R.; Samad, T.A.; Jin, S.X.; Schmoll, R.; Woolf, C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002, 36, 57–68. [Google Scholar] [CrossRef]
- Tisch, R.; Yang, X.D.; Singer, S.M.; Liblau, R.S.; Fugger, L.; McDevitt, H.O. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 1993, 366, 72–75. [Google Scholar] [CrossRef]
- Yoshida, K.; Kikutani, H. Genetic and immunological basis of autoimmune diabetes in the NOD mouse. Rev. Immunogenet. 2000, 2, 140–146. [Google Scholar]
- Winer, S.; Astsaturov, I.; Cheung, R.; Gunaratnam, L.; Kubiak, V.; Cortez, M.A.; Moscarello, M.; O’Connor, P.W.; McKerlie, C.; Becker, D.J.; et al. Type I diabetes and multiple sclerosis patients target islet plus central nervous system autoantigens; nonimmunized nonobese diabetic mice can develop autoimmune encephalitis. J. Immunol. 2001, 166, 2831–2841. [Google Scholar] [CrossRef]
- Winer, S.; Tsui, H.; Lau, A.; Song, A.; Li, X.; Cheung, R.K.; Sampson, A.; Afifiyan, F.; Elford, A.; Jackowski, G.; et al. Autoimmune islet destruction in spontaneous type 1 diabetes is not beta-cell exclusive. Nat. Med. 2003, 9, 198–205. [Google Scholar]
- Jancso, G.; Kiraly, E.; Jancso-Gabor, A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature 1977, 270, 741–743. [Google Scholar] [CrossRef]
- Razavi, R.; Chan, Y.; Afifiyan, F.N.; Liu, X.J.; Wan, X.; Yantha, J.; Tsui, H.; Tang, L.; Tsai, S.; Santamaria, P.; et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 2006, 127, 1123–1135. [Google Scholar] [CrossRef]
- Basu, S.; Srivastava, P. Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5120–5125. [Google Scholar] [CrossRef]
- Szollosi, A.G.; Olah, A.; Toth, I.B.; Papp, F.; Czifra, G.; Panyi, G.; Biro, T. Transient receptor potential vanilloid-2 mediates the effects of transient heat shock on endocytosis of human monocyte-derived dendritic cells. FEBS Lett. 2013, 587, 1440–1445. [Google Scholar] [CrossRef]
- Toth, B.I.; Benko, S.; Szollosi, A.G.; Kovacs, L.; Rajnavolgyi, E.; Biro, T. Transient receptor potential vanilloid-1 signaling inhibits differentiation and activation of human dendritic cells. FEBS Lett. 2009, 583, 1619–1624. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Pingle, S.C.; Ahern, G.P. Dendritic cells do not transduce inflammatory stimuli via the capsaicin receptor TRPV1. FEBS Lett. 2005, 579, 5135–5139. [Google Scholar] [CrossRef]
- Akiba, Y.; Kato, S.; Katsube, K.; Nakamura, M.; Takeuchi, K.; Ishii, H.; Hibi, T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem. Biophys. Res. Commun. 2004, 321, 219–225. [Google Scholar] [CrossRef]
- Van Buren, J.J.; Bhat, S.; Rotello, R.; Pauza, M.E.; Premkumar, L.S. Sensitization and translocation of TRPV1 by insulin and IGF-I. Mol. Pain 2005, 1, 17. [Google Scholar] [CrossRef]
- Hong, S.; Wiley, J.W. Early painful diabetic neuropathy is associated with differential changes in the expression and function of vanilloid receptor 1. J. Biol. Chem. 2005, 280, 618–627. [Google Scholar]
- Pabbidi, R.M.; Yu, S.Q.; Peng, S.; Khardori, R.; Pauza, M.E.; Premkumar, L.S. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol. Pain 2008, 4, 9. [Google Scholar] [CrossRef]
- Wilder-Smith, E.P.; Ong, W.Y.; Guo, Y.; Chow, A.W. Epidermal transient receptor potential vanilloid 1 in idiopathic small nerve fibre disease, diabetic neuropathy and healthy human subjects. Histopathology 2007, 51, 674–680. [Google Scholar] [CrossRef]
- Facer, P.; Casula, M.A.; Smith, G.D.; Benham, C.D.; Chessell, I.P.; Bountra, C.; Sinisi, M.; Birch, R.; Anand, P. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007, 7, 11. [Google Scholar] [CrossRef]
- Festa, A.; D’Agostino, R., Jr.; Howard, G.; Mykkanen, L.; Tracy, R.P.; Haffner, S.M. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2000, 102, 42–47. [Google Scholar] [CrossRef]
- Wu, T.; Dorn, J.P.; Donahue, R.P.; Sempos, C.T.; Trevisan, M. Associations of serum C-reactive protein with fasting insulin, glucose, and glycosylated hemoglobin: The Third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Epidemiol. 2002, 155, 65–71. [Google Scholar] [CrossRef]
- Tajiri, Y.; Takei, R.; Mimura, K.; Umeda, F. Indicators for the efficacy of pioglitazone before and during treatment in Japanese patients with type 2 diabetes. Diabetes Technol. Ther. 2007, 9, 429–437. [Google Scholar] [CrossRef]
- Gram, D.X.; Hansen, A.J.; Wilken, M.; Elm, T.; Svendsen, O.; Carr, R.D.; Ahren, B.; Brand, C.L. Plasma calcitonin gene-related peptide is increased prior to obesity, and sensory nerve desensitization by capsaicin improves oral glucose tolerance in obese Zucker rats. Eur. J. Endocrinol. 2005, 153, 963–969. [Google Scholar] [CrossRef]
- Melnyk, A.; Himms-Hagen, J. Resistance to aging-associated obesity in capsaicin-desensitized rats one year after treatment. Obes. Res. 1995, 3, 337–344. [Google Scholar] [CrossRef]
- Pettersson, M.; Ahren, B.; Bottcher, G.; Sundler, F. Calcitonin gene-related peptide: Occurrence in pancreatic islets in the mouse and the rat and inhibition of insulin secretion in the mouse. Endocrinology 1986, 119, 865–869. [Google Scholar] [CrossRef]
- Gram, D.X.; Ahren, B.; Nagy, I.; Olsen, U.B.; Brand, C.L.; Sundler, F.; Tabanera, R.; Svendsen, O.; Carr, R.D.; Santha, P.; et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur. J. Neurosci. 2007, 25, 213–223. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Smeets, A.; Lejeune, M.P. Sensory and gastrointestinal satiety effects of capsaicin on food intake. Int. J. Obes. 2005, 29, 682–688. [Google Scholar] [CrossRef]
- Belza, A.; Frandsen, E.; Kondrup, J. Body fat loss achieved by stimulation of thermogenesis by a combination of bioactive food ingredients: A placebo-controlled, double-blind 8-week intervention in obese subjects. Int. J. Obes. 2007, 31, 121–130. [Google Scholar] [CrossRef]
- Rybak, L.P.; Ramkumar, V. Ototoxicity. Kidney Int. 2007, 72, 931–935. [Google Scholar] [CrossRef]
- Rybak, L.P.; Whitworth, C.; Somani, S. Application of antioxidants and other agents to prevent cisplatin ototoxicity. Laryngoscope 1999, 109, 1740–1744. [Google Scholar] [CrossRef]
- Kopke, R.D.; Liu, W.; Gabaizadeh, R.; Jacono, A.; Feghali, J.; Spray, D.; Garcia, P.; Steinman, H.; Malgrange, B.; Ruben, R.J.; et al. Use of organotypic cultures of Corti’s organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am. J. Otol. 1997, 18, 559–571. [Google Scholar]
- Zheng, J.; Dai, C.; Steyger, P.S.; Kim, Y.; Vass, Z.; Ren, T.; Nuttall, A.L. Vanilloid receptors in hearing: Altered cochlear sensitivity by vanilloids and expression of TRPV1 in the organ of corti. J. Neurophysiol. 2003, 90, 444–455. [Google Scholar] [CrossRef]
- Vass, Z.; Dai, C.F.; Steyger, P.S.; Jancso, G.; Trune, D.R.; Nuttall, A.L. Co-localization of the vanilloid capsaicin receptor and substance P in sensory nerve fibers innervating cochlear and vertebro-basilar arteries. Neuroscience 2004, 124, 919–927. [Google Scholar] [CrossRef]
- Wu, T.; Song, L.; Shi, X.; Jiang, Z.; Santos-Sacchi, J.; Nuttall, A.L. Effect of capsaicin on potassium conductance and electromotility of the guinea pig outer hair cell. Hear. Res. 2011, 272, 117–124. [Google Scholar] [CrossRef]
- Zhou, J.; Balaban, C.; Durrant, J.D. Effect of intracochlear perfusion of vanilloids on cochlear neural activity in the guinea pig. Hear. Res. 2006, 218, 43–49. [Google Scholar]
- Meyers, J.R.; MacDonald, R.B.; Duggan, A.; Lenzi, D.; Standaert, D.G.; Corwin, J.T.; Corey, D.P. Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels. J. Neurosci. 2003, 23, 4054–4065. [Google Scholar]
- Myrdal, S.E.; Steyger, P.S. TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hear. Res. 2005, 204, 170–182. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, C.; Kim, S.J.; Kim, H.J.; Oh, G.S.; Shen, A.; So, H.S.; Park, R. Different uptake of gentamicin through TRPV1 and TRPV4 channels determines cochlear hair cell vulnerability. Exp. Mol. Med. 2013, 45, e12. [Google Scholar] [CrossRef]
- Mukherjea, D.; Jajoo, S.; Whitworth, C.; Bunch, J.R.; Turner, J.G.; Rybak, L.P.; Ramkumar, V. Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J. Neurosci. 2008, 28, 13056–13065. [Google Scholar]
- Mukherjea, D.; Jajoo, S.; Sheehan, K.; Kaur, T.; Sheth, S.; Bunch, J.; Perro, C.; Rybak, L.P.; Ramkumar, V. NOX3 NADPH oxidase couples transient receptor potential vanilloid 1 to signal transducer and activator of transcription 1-mediated inflammation and hearing loss. Antioxid Redox Signal. 2011, 14, 999–1010. [Google Scholar] [CrossRef]
- Kitahara, T.; Li, H.S.; Balaban, C.D. Changes in transient receptor potential cation channel superfamily V (TRPV) mRNA expression in the mouse inner ear ganglia after kanamycin challenge. Hear. Res. 2005, 201, 132–144. [Google Scholar] [CrossRef]
- Ishibashi, T.; Takumida, M.; Akagi, N.; Hirakawa, K.; Anniko, M. Changes in transient receptor potential vanilloid (TRPV) 1, 2, 3 and 4 expression in mouse inner ear following gentamicin challenge. Acta Oto-Laryngol. 2009, 129, 116–126. [Google Scholar] [CrossRef]
- Kizawa, K.; Kitahara, T.; Horii, A.; Maekawa, C.; Kuramasu, T.; Kawashima, T.; Nishiike, S.; Doi, K.; Inohara, H. Behavioral assessment and identification of a molecular marker in a salicylate-induced tinnitus in rats. Neuroscience 2010, 165, 1323–1332. [Google Scholar] [CrossRef]
- Bauer, C.A.; Brozoski, T.J.; Myers, K.S. Acoustic injury and TRPV1 expression in the cochlear spiral ganglion. Int. Tinnitus J. 2007, 13, 21–28. [Google Scholar]
- Kaur, T.; Mukherjea, D.; Sheehan, K.; Jajoo, S.; Rybak, L.P.; Ramkumar, V. Short interfering RNA against STAT1 attenuates cisplatin-induced ototoxicity in the rat by suppressing inflammation. Cell Death. Dis. 2011, 2, e180. [Google Scholar] [CrossRef]
- Lecci, A.; Maggi, C.A. Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system. Regul. Pept. 2001, 101, 1–18. [Google Scholar] [CrossRef]
- Yiangou, Y.; Facer, P.; Ford, A.; Brady, C.; Wiseman, O.; Fowler, C.J.; Anand, P. Capsaicin receptor VR1 and ATP-gated ion channel P2X3 in human urinary bladder. BJU Int. 2001, 87, 774–779. [Google Scholar]
- Maggi, C.A.; Barbanti, G.; Santicioli, P.; Beneforti, P.; Misuri, D.; Meli, A.; Turini, D. Cystometric evidence that capsaicin-sensitive nerves modulate the afferent branch of micturition reflex in humans. J. Urol. 1989, 142, 150–154. [Google Scholar]
- Maggi, C.A. The dual function of capsaicin-sensitive sensory nerves in the bladder and urethra. Ciba. Found. Symp. 1990, 151, 77–90, 77–83; discussion 83–90. [Google Scholar]
- Szallasi, A.; Conte, B.; Goso, C.; Blumberg, P.M.; Manzini, S. Characterization of a peripheral vanilloid (capsaicin) receptor in the urinary bladder of the rat. Life Sci. 1993, 52, PL221–PL226. [Google Scholar]
- Avelino, A.; Cruz, C.; Nagy, I.; Cruz, F. Vanilloid receptor 1 expression in the rat urinary tract. Neuroscience 2002, 109, 787–798. [Google Scholar] [CrossRef]
- Birder, L.A.; Kanai, A.J.; de Groat, W.C.; Kiss, S.; Nealen, M.L.; Burke, N.E.; Dineley, K.E.; Watkins, S.; Reynolds, I.J.; Caterina, M.J. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc. Natl. Acad. Sci. USA 2001, 98, 13396–13401. [Google Scholar] [CrossRef]
- Apostolidis, A.; Brady, C.M.; Yiangou, Y.; Davis, J.; Fowler, C.J.; Anand, P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology 2005, 65, 400–405. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yoshiyama, M.; Zakoji, H.; Takeda, M.; Araki, I. Sex differences in the expression profile of acid-sensing ion channels in the mouse urinary bladder: A possible involvement in irritative bladder symptoms. BJU Int. 2009, 104, 1746–1751. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vannucchi, M.G.; Spinelli, M.; Bizzoco, E.; Beneforti, P.; Turini, D.; Faussone-Pellegrini, M.S. Transient receptor potential vanilloid type 1 (TRPV1) expression changes from normal urothelium to transitional cell carcinoma of human bladder. Eur. Urol. 2005, 48, 691–698. [Google Scholar] [CrossRef]
- Shabir, S.; Cross, W.; Kirkwood, L.A.; Pearson, J.F.; Appleby, P.A.; Walker, D.; Eardley, I.; Southgate, J. Functional expression of purinergic P2 receptors and transient receptor potential channels by the human urothelium. Am. J. Physiol. Renal Physiol. 2013, 305, F396–F406. [Google Scholar] [CrossRef]
- Yu, W.; Hill, W.G.; Apodaca, G.; Zeidel, M.L. Expression and distribution of transient receptor potential (TRP) channels in bladder epithelium. Am. J. Physiol. Renal Physiol. 2011, 300, F49–F59. [Google Scholar] [CrossRef]
- Ost, D.; Roskams, T.; van der Aa, F.; de Ridder, D. Topography of the vanilloid receptor in the human bladder: More than just the nerve fibers. J. Urol. 2002, 168, 293–297. [Google Scholar] [CrossRef]
- Sui, G.P.; Wu, C.; Fry, C.H. Electrical characteristics of suburothelial cells isolated from the human bladder. J. Urol. 2004, 171, 938–943. [Google Scholar] [CrossRef]
- Everaerts, W.; Vriens, J.; Owsianik, G.; Appendino, G.; Voets, T.; de Ridder, D.; Nilius, B. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am. J. Physiol. Renal Physiol. 2010, 298, F692–F701. [Google Scholar] [CrossRef]
- Yamada, T.; Ugawa, S.; Ueda, T.; Ishida, Y.; Kajita, K.; Shimada, S. Differential localizations of the transient receptor potential channels TRPV4 and TRPV1 in the mouse urinary bladder. J. Histochem. Cytochem. 2009, 57, 277–287. [Google Scholar]
- Daly, D.; Rong, W.; Chess-Williams, R.; Chapple, C.; Grundy, D. Bladder afferent sensitivity in wild-type and TRPV1 knockout mice. J. Physiol. 2007, 583, 663–674. [Google Scholar] [CrossRef]
- Cefalu, J.S.; Guillon, M.A.; Burbach, L.R.; Zhu, Q.M.; Hu, D.Q.; Ho, M.J.; Ford, A.P.; Nunn, P.A.; Cockayne, D.A. Selective pharmacological blockade of the TRPV1 receptor suppresses sensory reflexes of the rodent bladder. J. Urol. 2009, 182, 776–785. [Google Scholar] [CrossRef]
- Cheng, C.L.; Liu, J.C.; Chang, S.Y.; Ma, C.P.; de Groat, W.C. Effect of capsaicin on the micturition reflex in normal and chronic spinal cord-injured cats. Am. J. Physiol. 1999, 277, R786–R794. [Google Scholar]
- Cheng, C.L.; Ma, C.P.; de Groat, W.C. Effect of capsaicin on micturition and associated reflexes in chronic spinal rats. Brain Res. 1995, 678, 40–48. [Google Scholar] [CrossRef]
- Santos-Silva, A.; Charrua, A.; Cruz, C.D.; Gharat, L.; Avelino, A.; Cruz, F. Rat detrusor overactivity induced by chronic spinalization can be abolished by a transient receptor potential vanilloid 1 (TRPV1) antagonist. Autono. Neurosci. 2012, 166, 35–38. [Google Scholar] [CrossRef]
- Chancellor, M.B.; de Groat, W.C. Intravesical capsaicin and resiniferatoxin therapy: Spicing up the ways to treat the overactive bladder. J. Urol. 1999, 162, 3–11. [Google Scholar] [CrossRef]
- Birder, L.A.; Wolf-Johnston, A.S.; Sun, Y.; Chai, T.C. Alteration in TRPV1 and Muscarinic (M3) receptor expression and function in idiopathic overactive bladder urothelial cells. Acta Physiol. (Oxf.) 2013, 207, 123–129. [Google Scholar] [CrossRef]
- Dornelles, F.N.; Andrade, E.L.; Campos, M.M.; Calixto, J.B. Role of CXCR2 and TRPV1 in functional, inflammatory and behavioural changes in the rat model of cyclophosphamide-induced haemorrhagic cystitis. Br. J. Pharmacol. 2014, 171, 452–467. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wang, P.; Merriam, F.V.; Bjorling, D.E. Lack of TRPV1 inhibits cystitis-induced increased mechanical sensitivity in mice. Pain 2008, 139, 158–167. [Google Scholar] [CrossRef]
- Dang, K.; Bielefeldt, K.; Gebhart, G.F. Cyclophosphamide-induced cystitis reduces ASIC channel but enhances TRPV1 receptor function in rat bladder sensory neurons. J. Neurophysiol. 2013, 110, 408–417. [Google Scholar] [CrossRef]
- Homma, Y.; Nomiya, A.; Tagaya, M.; Oyama, T.; Takagaki, K.; Nishimatsu, H.; Igawa, Y. Increased mRNA expression of genes involved in pronociceptive inflammatory reactions in bladder tissue of interstitial cystitis. J. Urol. 2013, 190, 1925–1931. [Google Scholar] [CrossRef]
- Watanabe, N.; Horie, S.; Michael, G.J.; Keir, S.; Spina, D.; Page, C.P.; Priestley, J.V. Immunohistochemical co-localization of transient receptor potential vanilloid (TRPV)1 and sensory neuropeptides in the guinea-pig respiratory system. Neuroscience 2006, 141, 1533–1543. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, Y.; Kim, S.M.; Yang, Y.D.; Jung, J.; Oh, U. Quantitative analysis of TRP channel genes in mouse organs. Arch. Pharm. Res. 2012, 35, 1823–1830. [Google Scholar] [CrossRef]
- Kunert-Keil, C.; Bisping, F.; Kruger, J.; Brinkmeier, H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 2006, 7, 159. [Google Scholar] [CrossRef]
- Baxter, L.A.; Birrell, M.A.; Belvisi, M.G. The role of TRPV1 in tobacco smoke induced airway inflammatiom. Am. J. Respir. Crit. Care Med. 2012, 185, A6410. [Google Scholar]
- Smit, L.A.; Kogevinas, M.; Anto, J.M.; Bouzigon, E.; Gonzalez, J.R.; le Moual, N.; Kromhout, H.; Carsin, A.E.; Pin, I.; Jarvis, D.; et al. Transient receptor potential genes, smoking, occupational exposures and cough in adults. Respir. Res. 2012, 13, 26. [Google Scholar] [CrossRef]
- Cantero-Recasens, G.; Gonzalez, J.R.; Fandos, C.; Duran-Tauleria, E.; Smit, L.A.; Kauffmann, F.; Anto, J.M.; Valverde, M.A. Loss of function of transient receptor potential vanilloid 1 (TRPV1) genetic variant is associated with lower risk of active childhood asthma. J. Biol. Chem. 2010, 285, 27532–27535. [Google Scholar] [CrossRef]
- McLeod, R.L.; Fernandez, X.; Correll, C.C.; Phelps, T.P.; Jia, Y.; Wang, X.; Hey, J.A. TRPV1 antagonists attenuate antigen-provoked cough in ovalbumin sensitized guinea pigs. Cough 2006, 2, 10. [Google Scholar] [CrossRef]
- Trevisani, M.; Milan, A.; Gatti, R.; Zanasi, A.; Harrison, S.; Fontana, G.; Morice, A.H.; Geppetti, P. Antitussive activity of iodo-resiniferatoxin in guinea pigs. Thorax 2004, 59, 769–772. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Scott, B.P.; Nasser, N.; Ao, H.; Maher, M.P.; Dubin, A.E.; Swanson, D.M.; Shankley, N.P.; Wickenden, A.D.; Chaplan, S.R. Pharmacology and antitussive efficacy of 4-(3-trifluoromethyl-pyridin-2-yl)-piperazine-1-carboxylic acid (5-trifluoromethyl-pyridin-2-yl)-amide (JNJ17203212), a transient receptor potential vanilloid 1 antagonist in guinea pigs. J. Pharmacol. Exp. Ther. 2007, 323, 665–674. [Google Scholar] [CrossRef]
- Lalloo, U.G.; Fox, A.J.; Belvisi, M.G.; Chung, K.F.; Barnes, P.J. Capsazepine inhibits cough induced by capsaicin and citric acid but not by hypertonic saline in guinea pigs. J. Appl. Physiol. 1995, 79, 1082–1087. [Google Scholar]
- Doherty, M.J.; Mister, R.; Pearson, M.G.; Calverley, P.M. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 2000, 55, 643–649. [Google Scholar]
- Nakajima, T.; Nishimura, Y.; Nishiuma, T.; Kotani, Y.; Nakata, H.; Yokoyama, M. Cough sensitivity in pure cough variant asthma elicited using continuous capsaicin inhalation. Allergol. Int. 2006, 55, 149–155. [Google Scholar] [CrossRef]
- O’Connell, F.; Thomas, V.E.; Studham, J.M.; Pride, N.B.; Fuller, R.W. Capsaicin cough sensitivity increases during upper respiratory infection. Respir. Med. 1996, 90, 279–286. [Google Scholar] [CrossRef]
- Pecova, R.; Zucha, J.; Pec, M.; Neuschlova, M.; Hanzel, P.; Tatar, M. Cough reflex sensitivity testing in in seasonal allergic rhinitis patients and healthy volunteers. J. Physiol. Pharmacol. 2008, 59 (Suppl. 6), 557–564. [Google Scholar]
- Plevkova, J.; Varechova, S.; Brozmanova, M.; Tatar, M. Testing of cough reflex sensitivity in children suffering from allergic rhinitis and common cold. J. Physiol. Pharmacol. 2006, 57 (Suppl. 4), 289–296. [Google Scholar]
- Groneberg, D.A.; Niimi, A.; Dinh, Q.T.; Cosio, B.; Hew, M.; Fischer, A.; Chung, K.F. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am. J. Respir. Crit. Care Med. 2004, 170, 1276–1280. [Google Scholar] [CrossRef]
- Mitchell, J.E.; Campbell, A.P.; New, N.E.; Sadofsky, L.R.; Kastelik, J.A.; Mulrennan, S.A.; Compton, S.J.; Morice, A.H. Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp. Lung Res. 2005, 31, 295–306. [Google Scholar]
- Carr, M.J.; Hunter, D.D.; Jacoby, D.B.; Undem, B.J. Expression of tachykinins in nonnociceptive vagal afferent neurons during respiratory viral infection in guinea pigs. Am. J. Respir. Crit. Care Med. 2002, 165, 1071–1075. [Google Scholar] [CrossRef]
- Lewis, C.A.; Ambrose, C.; Banner, K.; Battram, C.; Butler, K.; Giddings, J.; Mok, J.; Nasra, J.; Winny, C.; Poll, C. Animal models of cough: Literature review and presentation of a novel cigarette smoke-enhanced cough model in the guinea-pig. Pulm. Pharmacol. Ther. 2007, 20, 325–333. [Google Scholar] [CrossRef]
- Lieu, T.M.; Myers, A.C.; Meeker, S.; Undem, B.J. TRPV1 induction in airway vagal low-threshold mechanosensory neurons by allergen challenge and neurotrophic factors. Am. J. Physiol. Lung. Cell Mol. Physiol. 2012, 302, L941–L948. [Google Scholar] [CrossRef]
- Myers, A.C.; Kajekar, R.; Undem, B.J. Allergic inflammation-induced neuropeptide production in rapidly adapting afferent nerves in guinea pig airways. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 282, L775–L781. [Google Scholar] [CrossRef]
- Ye, X.M.; Zhong, N.S.; Liu, C.L.; Chen, R.C. Cough reflex sensitivity is increased in guinea pigs with parainfluenza virus infection. Exp. Lung Res. 2011, 37, 186–194. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, R.L.; Wiggers, M.; Snow, D.M.; Lee, L.Y. Altered expression of TRPV1 and sensitivity to capsaicin in pulmonary myelinated afferents following chronic airway inflammation in the rat. J. Physiol. 2008, 586, 5771–5786. [Google Scholar] [CrossRef]
- Chong, M.S.; Hester, J. Diabetic painful neuropathy: Current and future treatment options. Drugs 2007, 67, 569–585. [Google Scholar] [CrossRef]
- Jensen, P.G.; Larson, J.R. Management of painful diabetic neuropathy. Drugs Aging 2001, 18, 737–749. [Google Scholar] [CrossRef]
- Zin, C.S.; Nissen, L.M.; Smith, M.T.; O’Callaghan, J.P.; Moore, B.J. An update on the pharmacological management of post-herpetic neuralgia and painful diabetic neuropathy. CNS Drugs 2008, 22, 417–442. [Google Scholar] [CrossRef]
- Galluzzi, K.E. Management strategies for herpes zoster and postherpetic neuralgia. J. Am. Osteopath. Assoc. 2007, 107, S8–S13. [Google Scholar]
- Broad, L.M.; Keding, S.J.; Blanco, M.J. Recent progress in the development of selective TRPV1 antagonists for pain. Curr. Top. Med. Chem. 2008, 8, 1431–1441. [Google Scholar] [CrossRef]
- Pal, M.; Angaru, S.; Kodimuthali, A.; Dhingra, N. Vanilloid receptor antagonists: Emerging class of novel anti-inflammatory agents for pain management. Curr. Pharm. Des. 2009, 15, 1008–1026. [Google Scholar] [CrossRef]
- Wong, G.Y.; Gavva, N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar] [CrossRef]
- Walpole, C.S.; Bevan, S.; Bovermann, G.; Boelsterli, J.J.; Breckenridge, R.; Davies, J.W.; Hughes, G.A.; James, I.; Oberer, L.; Winter, J.; et al. The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin. J. Med. Chem. 1994, 37, 1942–1954. [Google Scholar] [CrossRef]
- Bevan, S.; Hothi, S.; Hughes, G.; James, I.F.; Rang, H.P.; Shah, K.; Walpole, C.S.; Yeats, J.C. Capsazepine: A competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992, 107, 544–552. [Google Scholar] [CrossRef]
- Liu, L.; Simon, S.A. Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia. Neurosci. Lett. 1997, 228, 29–32. [Google Scholar] [CrossRef]
- Docherty, R.J.; Yeats, J.C.; Piper, A.S. Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br. J. Pharmacol. 1997, 121, 1461–1467. [Google Scholar] [CrossRef]
- Weil, A.; Moore, S.E.; Waite, N.J.; Randall, A.; Gunthorpe, M.J. Conservation of functional and pharmacological properties in the distantly related temperature sensors TRVP1 and TRPM8. Mol. Pharmacol. 2005, 68, 518–527. [Google Scholar]
- Walker, K.M.; Urban, L.; Medhurst, S.J.; Patel, S.; Panesar, M.; Fox, A.J.; McIntyre, P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2003, 304, 56–62. [Google Scholar] [CrossRef]
- Chung, J.U.; Kim, S.Y.; Lim, J.O.; Choi, H.K.; Kang, S.U.; Yoon, H.S.; Ryu, H.; Kang, D.W.; Lee, J.; Kang, B.; et al. Alpha-substituted N-(4-tert-butylbenzyl)-N'-[4-(methylsulfonylamino) benzyl]thiourea analogues as potent and stereospecific TRPV1 antagonists. Bioorg. Med. Chem. 2007, 15, 6043–6053. [Google Scholar] [CrossRef]
- Suh, Y.G.; Lee, Y.S.; Min, K.H.; Park, O.H.; Kim, J.K.; Seung, H.S.; Seo, S.Y.; Lee, B.Y.; Nam, Y.H.; Lee, K.O.; et al. Novel potent antagonists of transient receptor potential channel, vanilloid subfamily member 1: Structure-activity relationship of 1,3-diarylalkyl thioureas possessing new vanilloid equivalents. J. Med. Chem. 2005, 48, 5823–5836. [Google Scholar] [CrossRef]
- Kym, P.R.; Kort, M.E.; Hutchins, C.W. Analgesic potential of TRPV1 antagonists. Biochem. Pharmacol. 2009, 78, 211–216. [Google Scholar] [CrossRef]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S.R. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar]
- Wang, Y.; Szabo, T.; Welter, J.D.; Toth, A.; Tran, R.; Lee, J.; Kang, S.U.; Suh, Y.G.; Blumberg, P.M. High affinity antagonists of the vanilloid receptor. Mol. Pharmacol. 2002, 62, 947–956. [Google Scholar] [CrossRef]
- El Kouhen, R.; Surowy, C.S.; Bianchi, B.R.; Neelands, T.R.; McDonald, H.A.; Niforatos, W.; Gomtsyan, A.; Lee, C.H.; Honore, P.; Sullivan, J.P.; et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel and selective transient receptor potential type V1 receptor antagonist, blocks channel activation by vanilloids, heat, and acid. J. Pharmacol. Exp. Ther. 2005, 314, 400–409. [Google Scholar]
- Valenzano, K.J.; Grant, E.R.; Wu, G.; Hachicha, M.; Schmid, L.; Tafesse, L.; Sun, Q.; Rotshteyn, Y.; Francis, J.; Limberis, J.; et al. N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine -1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. In vitro characterization and pharmacokinetic properties. J. Pharmacol. Exp. Ther. 2003, 306, 377–386. [Google Scholar] [CrossRef]
- Swanson, D.M.; Dubin, A.E.; Shah, C.; Nasser, N.; Chang, L.; Dax, S.L.; Jetter, M.; Breitenbucher, J.G.; Liu, C.; Mazur, C.; et al. Identification and biological evaluation of 4-(3-trifluoromethylpyridin-2-yl)piperazine-1-carboxylic acid (5-trifluoromethylpyridin-2-yl)amide, a high affinity TRPV1 (VR1) vanilloid receptor antagonist. J. Med. Chem. 2005, 48, 1857–1872. [Google Scholar] [CrossRef]
- Rami, H.K.; Thompson, M.; Stemp, G.; Fell, S.; Jerman, J.C.; Stevens, A.J.; Smart, D.; Sargent, B.; Sanderson, D.; Randall, A.D.; et al. Discovery of SB-705498: A potent, selective and orally bioavailable TRPV1 antagonist suitable for clinical development. Bioorg. Med. Chem. Lett. 2006, 16, 3287–3291. [Google Scholar] [CrossRef]
- Gunthorpe, M.J.; Rami, H.K.; Jerman, J.C.; Smart, D.; Gill, C.H.; Soffin, E.M.; Luis Hannan, S.; Lappin, S.C.; Egerton, J.; Smith, G.D.; et al. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology 2004, 46, 133–149. [Google Scholar] [CrossRef]
- Gavva, N.R.; Tamir, R.; Qu, Y.; Klionsky, L.; Zhang, T.J.; Immke, D.; Wang, J.; Zhu, D.; Vanderah, T.W.; Porreca, F.; et al. AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo [b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J. Pharmacol. Exp. Ther. 2005, 313, 474–484. [Google Scholar]
- Zheng, X.; Hodgetts, K.J.; Brielmann, H.; Hutchison, A.; Burkamp, F.; Brian Jones, A.; Blurton, P.; Clarkson, R.; Chandrasekhar, J.; Bakthavatchalam, R.; et al. From arylureas to biarylamides to aminoquinazolines: Discovery of a novel, potent TRPV1 antagonist. Bioorg. Med. Chem. Lett. 2006, 16, 5217–5221. [Google Scholar] [CrossRef]
- Ognyanov, V.I.; Balan, C.; Bannon, A.W.; Bo, Y.; Dominguez, C.; Fotsch, C.; Gore, V.K.; Klionsky, L.; Ma, V.V.; Qian, Y.X.; et al. Design of potent, orally available antagonists of the transient receptor potential vanilloid 1. Structure-activity relationships of 2-piperazin-1-yl-1H-benzimidazoles. J. Med. Chem. 2006, 49, 3719–3742. [Google Scholar] [CrossRef]
- Messeguer, A.; Planells-Cases, R.; Ferrer-Montiel, A. Physiology and pharmacology of the vanilloid receptor. Curr. Neuropharmacol. 2006, 4, 1–15. [Google Scholar]
- Garcia-Martinez, C.; Morenilla-Palao, C.; Planells-Cases, R.; Merino, J.M.; Ferrer-Montiel, A. Identification of an aspartic residue in the P-loop of the vanilloid receptor that modulates pore properties. J. Biol. Chem. 2000, 275, 32552–32558. [Google Scholar]
- Himmel, H.M.; Kiss, T.; Borvendeg, S.J.; Gillen, C.; Illes, P. The arginine-rich hexapeptide R4W2 is a stereoselective antagonist at the vanilloid receptor 1: A Ca2+ imaging study in adult rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2002, 301, 981–986. [Google Scholar] [CrossRef]
- Mellor, I.R.; Ogilvie, J.; Pluteanu, F.; Clothier, R.H.; Parker, T.L.; Rosini, M.; Minarini, A.; Tumiatti, V.; Melchiorre, C. Methoctramine analogues inhibit responses to capsaicin and protons in rat dorsal root ganglion neurons. Eur. J. Pharmacol. 2004, 505, 37–50. [Google Scholar] [CrossRef]
- Kitaguchi, T.; Swartz, K.J. An inhibitor of TRPV1 channels isolated from funnel Web spider venom. Biochemistry 2005, 44, 15544–15549. [Google Scholar] [CrossRef]
- Garcia-Martinez, C.; Humet, M.; Planells-Cases, R.; Gomis, A.; Caprini, M.; Viana, F.; de la Pena, E.; Sanchez-Baeza, F.; Carbonell, T.; de Felipe, C.; et al. Attenuation of thermal nociception and hyperalgesia by VR1 blockers. Proc. Natl. Acad. Sci. USA 2002, 99, 2374–2379. [Google Scholar]
- McIntyre, P.; McLatchie, L.M.; Chambers, A.; Phillips, E.; Clarke, M.; Savidge, J.; Toms, C.; Peacock, M.; Shah, K.; Winter, J.; et al. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1). Br. J. Pharmacol. 2001, 132, 1084–1094. [Google Scholar] [CrossRef]
- Honore, P.; Wismer, C.T.; Mikusa, J.; Zhu, C.Z.; Zhong, C.; Gauvin, D.M.; Gomtsyan, A.; el Kouhen, R.; Lee, C.H.; Marsh, K.; et al. A-425619 [1-isoquinolin-5-yl-3-(4-trifluoromethyl-benzyl)-urea], a novel transient receptor potential type V1 receptor antagonist, relieves pathophysiological pain associated with inflammation and tissue injury in rats. J. Pharmacol. Exp. Ther. 2005, 314, 410–421. [Google Scholar] [CrossRef]
- Pomonis, J.D.; Harrison, J.E.; Mark, L.; Bristol, D.R.; Valenzano, K.J.; Walker, K. N-(4-Tertiarybutylphenyl)-4-(3-cholorphyridin-2-yl)tetrahydropyrazine-1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. in vivo characterization in rat models of inflammatory and neuropathic pain. J. Pharmacol. Exp. Ther. 2003, 306, 387–393. [Google Scholar] [CrossRef]
- Cui, M.; Honore, P.; Zhong, C.; Gauvin, D.; Mikusa, J.; Hernandez, G.; Chandran, P.; Gomtsyan, A.; Brown, B.; Bayburt, E.K.; et al. TRPV1 receptors in the CNS play a key role in broad-spectrum analgesia of TRPV1 antagonists. J. Neurosci. 2006, 26, 9385–9393. [Google Scholar] [CrossRef]
- Hori, T. Capsaicin and central control of thermoregulation. Pharmacol. Ther. 1984, 26, 389–416. [Google Scholar] [CrossRef]
- Gavva, N.R.; Bannon, A.W.; Hovland, D.N., Jr.; Lehto, S.G.; Klionsky, L.; Surapaneni, S.; Immke, D.C.; Henley, C.; Arik, L.; Bak, A.; et al. Repeated administration of vanilloid receptor TRPV1 antagonists attenuates hyperthermia elicited by TRPV1 blockade. J. Pharmacol. Exp. Ther. 2007, 323, 128–137. [Google Scholar] [CrossRef]
- Gavva, N.R.; Bannon, A.W.; Surapaneni, S.; Hovland, D.N., Jr.; Lehto, S.G.; Gore, A.; Juan, T.; Deng, H.; Han, B.; Klionsky, L.; et al. The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J. Neurosci. 2007, 27, 3366–3374. [Google Scholar] [CrossRef]
- Steiner, A.A.; Turek, V.F.; Almeida, M.C.; Burmeister, J.J.; Oliveira, D.L.; Roberts, J.L.; Bannon, A.W.; Norman, M.H.; Louis, J.C.; Treanor, J.J.; et al. Nonthermal activation of transient receptor potential vanilloid-1 channels in abdominal viscera tonically inhibits autonomic cold-defense effectors. J. Neurosci. 2007, 27, 7459–7468. [Google Scholar] [CrossRef]
- Chizh, B.A.; O’Donnell, M.B.; Napolitano, A.; Wang, J.; Brooke, A.C.; Aylott, M.C.; Bullman, J.N.; Gray, E.J.; Lai, R.Y.; Williams, P.M.; et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 2007, 132, 132–141. [Google Scholar] [CrossRef]
- Vay, L.; Gu, C.; McNaughton, P.A. The thermo-TRP ion channel family: Properties and therapeutic implications. Br. J. Pharmacol. 2012, 165, 787–801. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).