Abstract
Viruses have played an important role in human evolution and have evolved diverse strategies to co-exist with their hosts. As obligate intracellular pathogens, viruses exploit and manipulate different host cell processes, including cellular trafficking, metabolism and immunity-related functions, for their own survival. In this article, we review evidence for how autophagy, a highly conserved cellular degradative pathway, serves either as an antiviral defense mechanism or, alternatively, as a pro-viral process during virus infection. Furthermore, we highlight recent reports concerning the role of selective autophagy in virus infection and how viruses manipulate autophagy to evade lysosomal capture and degradation.
1. Introduction
To co-exist with their hosts viruses have evolved diverse mechanisms to exploit cellular processes and to evade from host defenses. One such central cell pathway is an ancient degradative process, designated autophagy. In contrast to proteasomal degradation that specifically targets ubiquitinated proteins, autophagy is mediated by the lysosome, which serves as an end point degradative organelle. Autophagy is thus a catabolic process that maintains cellular homeostasis by degradative removal of damaged or excess cellular organelles and protein aggregates from the cytoplasm, thereby enabling cell survival. Both cell culture and in vivo studies revealed the fundamental roles of autophagy in numerous diseases, including cancer or neurodegeneration, in aging, but also in innate and adaptive immunity to pathogen infection.
In general, autophagy can be broadly classified as macroautophagy, microautophagy and chaperone-mediated autophagy (CMA). All three pathways share the same mode of degradation via the lysosome, but are mechanistically distinct from each other [1]. Microautophagy, mainly characterized in yeast [2], involves a direct engulfment of the cytoplasm at the lysosomal membrane, facilitated by protrusion of arm-like structures, and CMA specifically directs translocation of unfolded proteins with an inherent consensus motif (KFERQ) across the limiting membrane of the lysosome for degradation [1]. Furthermore, a crucial component for CMA is the lysosomal membrane receptor, lysosome-associated membrane protein (LAMP) type 2A [1]. In contrast to CMA, macroautophagy (called autophagy henceforth) involves sequestration of intact organelles (e.g., mitochondria) and portions of the cytosol via a membrane referred to as the phagophore or isolation membrane [3,4]. This phagophore expands to form double-membrane vesicles, termed autophagosomes [3,4]. Subsequently, autophagosomes mature by fusing with endosomes and/or lysosomes to form autolysosomes, where degradation of the internal contents occurs by resident lysosomal hydrolases [5]. The membrane source for the biogenesis of the phagophore is debatable; however, several recent studies have shown that the endoplasmic reticulum (ER), mitochondria and plasma membrane (PM) serve as source for the phagophore [6,7,8].
Although the autophagy concept was developed more than 50 years ago [1], insights into the molecular mechanisms came to light only in the late 1990s from yeast genetic screens that identified mutants of autophagy-related genes (Atgs). These yeast-specific Atgs provided great insight into the molecular aspects of autophagy in higher eukaryotes. Moreover, the first identified mammalian Atgs, Atg5 and Atg12, were shown to be highly homologous to those in yeast [1]. Furthermore, the morphology of autophagosomes in yeast is similar to those in mammals [9]. The majority of proteins involved in the autophagy machinery are required for formation and elongation of the phagophore [1] (Figure 1). Moreover, the entire pathway of autophagy, from the formation of autophagosomes to their fusion with endosomes and lysosomes, is highly dynamic.
Autophagy can operate either as a non-selective or a highly selective process: first, a starvation induced, non-selective pathway; second, a target-specific and selective autophagic pathway [10]. Non-selective or starvation-induced autophagy is thought to play an important role in supplying energy to the cell by the bulk degradation of proteins and organelles, whereas selective autophagy involves the recruitment of specific adaptor proteins such as p62, NBR1 (neighbor of BRCA1 gene 1), BNIP3 (BCL2 and adenovirus E1B 19 kDa-interacting protein 3) and NIX (NIP3-like protein X) that recognize ubiquitinated protein complexes and organelles, such as mitochondria (mitophagy), peroxisomes (pexophagy) and ribosomes (ribophagy), to target them to autophagosomes for degradation [10,11,12]. More recently, studies have shown that selective autophagy plays a crucial role in the elimination of pathogenic bacteria and viruses, termed xenophagy, thus extending the functions of autophagy to innate and adaptive immunity against pathogens [11]. Moreover, studies on selective autophagy have underlined the relevance of post-translational modifications, such as phosphorylation and ubiquitination, in linking autophagy adaptor protein function to autophagy substrate recognition [13,14]. However, the role of adaptor proteins in non-selective autophagy still remains to be fully determined.
In this review we will discuss the current understanding of how virus infection regulates the crosstalk between intracellular signaling and autophagy. Furthermore, we will highlight recent reports on how autophagy exerts its antiviral function and the countermeasures adopted by viruses to exploit and subvert autophagy.
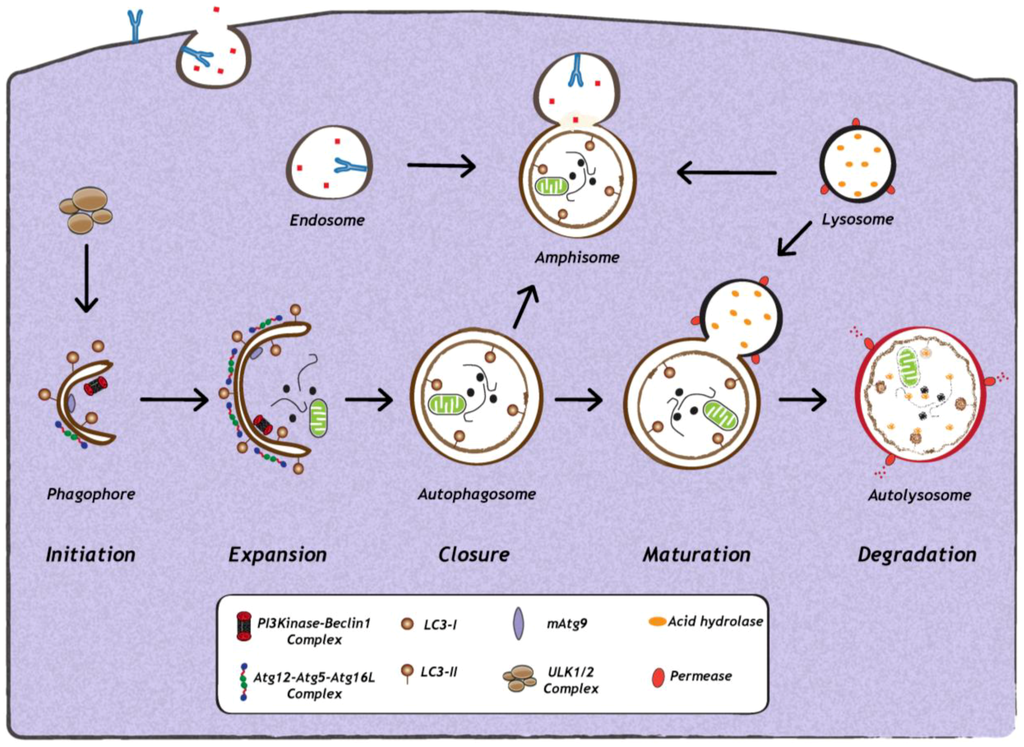
Figure 1.
Schematic representation of autophagy. The first step in the initiation of mammalian autophagy is the formation of the phagophore, followed by subsequent steps, which include elongation and expansion of the phagophore, closure and completion of a double-membrane autophagosome (which engulfs a portion of the cytoplasm), autophagosome maturation via docking and fusion with endosomal compartments (to form a hybrid vesicular product known as an amphisome) and/or with a lysosome (to form the autolysosome). The inner autophagosomal membrane and cargo is degraded by acid hydrolases inside the autolysosome, and recycling of the resulting macromolecules is mediated via permeases. The molecular machinery regulating autophagy is also depicted. ULK1 and ULK2 complexes that are crucial for induction of autophagy, PI3Kinase-Beclin1 complexes, are required for autophagosome formation, the delivery of membranes to the forming autophagosome is mediated by mammalian Atg9 (mAtg9) and two conjugation systems: LC3-II and Atg12–Atg5–Atg16L complex, which are required during elongation and expansion of the phagophore.
2. Impact of Viral Infection on Autophagy
Autophagy induction involves the coordinated action of several key factors (Figure 1). Among them are members of the Atg8 ubiquitin-like (Ubl) protein family, which include MAP1LC3A (microtubule-associated protein 1 light chain 3A), MAP1LC3B, MAP1LC3C, GABARAP (у-aminobutyric acid type A [GABA] receptor-associated protein), GABARAPL1 (GABARAP—like 1), GABARAPL2 and GABARAPL3, all of which are important for phagophore formation and closure in mammals [15]. In principle, any of the above mentioned Atg8 family proteins can serve as a marker of autophagosomes in mammalian cells, however, conventionally only MAP1LC3B (referred to as LC3 henceforth) is used as marker of autophagosomes in mammalian cells [16,17]. During autophagosome formation and elongation, the cytosolic LC3, termed LC3-I, is proteolytically cleaved and coupled to phosphatidyl-ethanolamine (PE) to form LC3-II, which is inserted into the autophagosomal membrane [1,9]. The two main characteristics of autophagy detection is, first, the increase in intracellular levels of LC3-II and, second, the re-localization of evenly distributed cytosolic LC3-I into LC3-II positive punctuate structures, representing autophagosomes [1,9]. Indeed, infections with a wide range of DNA or RNA viruses increase abundance of autophagosomes or autophagic vesicles in infected cells [18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Furthermore, several electron microscopy-based studies revealed the presence of double-membrane structures that are characteristic of autophagosomes. However, it is important to note that increased amounts of autophagosomes in infected cells can either be due to their enhanced formation or to their accumulation due to a block in their maturation or degradation. Therefore, to understand the reason for increased autophagosome numbers in virus-infected cells, it is important to study the impact of viral infection on autophagic activity or autophagic flux, which is defined as the measurement of the balance between the rate of autophagosome formation and degradation. Assays to measure autophagic activity in mammalian cells have been described in detail in other reviews and, therefore, will only be mentioned here [16,17].
2.1. Induction and Inhibition of Autophagy by Viruses
Various studies reported the activation of autophagy upon virus infection as inferred from the increased number of autophagic vesicles, the monitoring of LC3-I to LC3-II conversion and the elevated number of LC3-positive punctae in infected cells [21,22,23,29,30,32,33,35]. However, considering the dynamic nature of autophagy, it is important to determine the rate of autophagic flux to gain mechanistic insight into how viral infection affects autophagic activity in cells. In fact, recent reports demonstrated that Sindbis virus and hepatitis C virus (HCV) infection increase autophagic flux [36,37]. Infection of mouse embryonic fibroblasts (MEFs) expressing GFP-LC3 with Sindbis virus resulted in an increase in the percentage of cells containing GFP-LC3 positive dots concomitant with an increase in LC3-II abundance. In addition, infected cells contained reduced levels of p62 (an accepted marker of autophagic flux), with no significant changes of its mRNA amounts, indicating a complete autophagic response during virus infection [37]. In case of HCV infection, a study by Ke and Chen [36] demonstrated that despite unaltered levels of p62 in infected cells, LC3-II protein levels were increased upon treatment with lysosomal protease inhibitors (E64 and pepstatin A), or chloroquine (a vacuolar ATPase inhibitor) or bafilomycin A1 (an inhibitor of lysosomal acidification), [36] arguing that HCV does not block autophagy. In line with this conclusion, the authors showed that treatment of infected cells with chloroquine and bafilomycin A1 resulted in increased LC3-II levels in HCV infected cells as compared to uninfected cells, clearly indicating enhanced autophagic flux. In addition, by employing a tandem-LC3 reporter (mRFP-GFP-LC3), a tool to analyze both autophagosome and autolysosome formation, the authors found that HCV induced autophagosomes that matured into autolysosomes, thus revealing a complete autophagic response in HCV-infected cells. In contrast, an earlier study by Sir and co-workers proposed that autophagy is incomplete, and cells transfected with HCV RNA formed autophagosomes that, due to inefficient fusion with lysosomes, did not convert into autolysosomes [23]. The discrepancy between these two studies might be due to differences in the experimental approaches: HCV infection versus electroporation of HCV RNA.
As opposed to Sindbis virus and HCV, a number of viruses have evolved strategies to interfere with autophagosome formation or maturation (Figure 2). For instance, Kaposi’s Sarcoma herpesvirus (KSHV), murine γ-herpesvirus 68 (MHV68) and Herpes Simplex virus 1 (HSV-1) target Beclin-1, thereby inhibiting autophagosome formation. These viruses encode proteins that competitively bind to Beclin-1 and thus inhibit its interaction with Vps34. KSHV encodes a viral Bcl-2 homologue, which binds Beclin-1 with much higher affinity than cellular Bcl-2, thereby preventing incorporation of Beclin-1 into Vsp34 complexes [38]. M11, the viral Bcl-2 homologue encoded by MHV68, also interacts with Beclin-1 and inhibits autophagosome formation [39]. In the case of HSV-1, the virally encoded protein ICP34.5 interacts with Beclin-1 via a 20 amino acid residues-long region (aa 68-87) to inhibit autophagosome biogenesis [40]. Interestingly, deletion of ICP34.5 leads to accumulation of HSV-1 particles in autophagosome-like structures, which can be visualized by electron microscopy [41]. Other viruse,s such as human immunodeficiency virus-1 (HIV-1), influenza A virus (FluAv), coxsackievirus B3 (CVB3) and poliovirus inhibit autophagy by blocking autophagosome maturation or degradation. In the case of HIV-1 and FluAv, the viral proteins Nef and M2, respectively, block autophagosome maturation by a still poorly defined mechanism that depends on their interaction with Beclin-1 [27,28]. Studies with CVB3 and poliovirus show that autophagy is inhibited at the stage of autolysosomal degradation [18]. Moreover, in the case of coxsackievirus B3 (CVB3) [25,42], it was demonstrated that protein levels of LC3-II and p62 increase over time, suggesting that viral infection leads to an accumulation of autophagosomes due to a block in autolysosomal degradation [25,42]. However, it remains to be determined how these viruses specifically target autolysosomal degradation.
2.2. Cellular Signaling Events Influencing Autophagy During Viral Infection
The induction of autophagy is triggered by a variety of stress stimuli, including nutrient deprivation, ER stress, danger-associated molecular patterns (DAMPs), hypoxia, redox stress and mitochondrial damage. These stimuli involve a diverse range of cellular signals that have overlapping functions in autophagy and the control of other cellular stress responses. Viruses can trigger many of these stimuli during different stages of their replication cycle. For instance, several reports have shown that upon binding to the surface of target cells, some viruses can stimulate autophagy. One example is CD46 (cluster of differentiation 46), a cell surface receptor which is recognized by several viral pathogens, including human herpesvirus 6, adenovirus types B and D, and measles virus [43] (Figure 2). In case of the latter, CD46 engagement was shown to be sufficient to induce autophagy; viral binding of CD46 leads to its interaction with GOPC (Golgi-associated PDZ and coiled-coil motif-containing protein) (Figure 2), which then associates with the Vps34-Beclin-1 complex, leading to activation of autophagy [43]. While it is not known whether other viruses entering cells via CD46 also induce autophagy, in the case of HIV-1 binding to CD4+, T-cells trigger autophagy, and this is mediated by the C-terminal domain of the fusogenic gp41 subunit of the viral envelope protein [44,45]. Recent studies with the vesicular stomatitis virus (VSV) demonstrated that virus binding to Drosophila S2 cells was required to induce autophagy [31], which occurred via down regulation of mTOR activity and inactivation of Akt signaling [31]. Finally, a very recent study demonstrated that VSV-induced autophagy depends on Toll-7 in S2 cells and in adult flies [46]. The authors show that VSV interacted with Toll-7 at the plasma membrane to induce antiviral autophagy independent of the canonical Toll signaling pathway [46] (Figure 2).
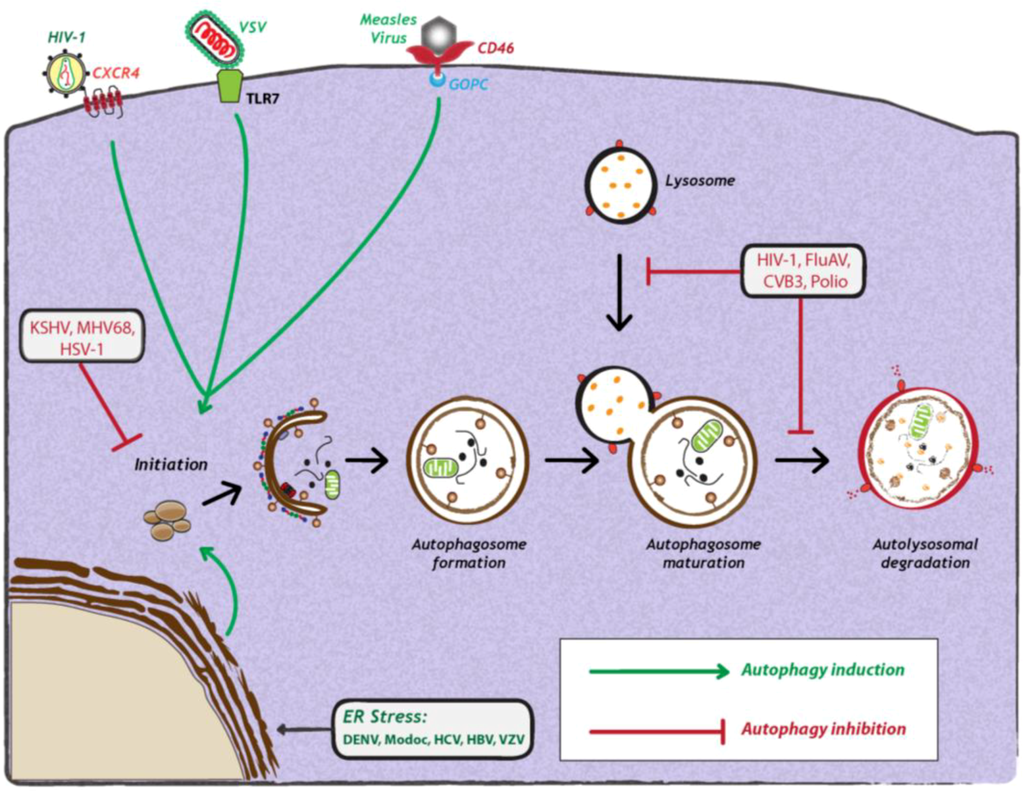
Figure 2.
Viral manipulation of autophagy. Viruses can either induce (green arrows) or inhibit the autophagy pathway (red studded lines). Viral induction of autophagy can be achieved during viral entry via interaction with cell surface receptors, via interaction with stress sensors or during viral replication. Viruses also inhibit autophagy at early or late stages of the pathway, during initiation or maturation, respectively. For further details, see the legend for Figure 1. Abbreviations: HIV-1 (human immunodeficiency virus-1); CXCR4 (C-X-C chemokine receptor type 4); VSV (vesicular stomatitis virus); TLR7 (Toll-like receptor 7); CD46 (cluster of differentiation 46); GOPC (Golgi-associated PDZ and coiled-coil motif-containing protein); KSHV (Kaposi’s sarcoma herpesvirus); MHV68 (murine γ-herpesvirus 68); HSV-1 (herpes simplex virus-1); FluAV (influenza A virus); CVB3 (coxsackievirus B3); DENV (dengue virus); HCV (Hepatitis C virus); HBV (Hepatitis B virus); VZV (varicella-zoster virus) ER (endoplasmic reticulum).
Apart from attachment of the virus particle to the host cell, viral replication itself frequently elicits stress responses, such as ER stress or production of reactive oxygen species (ROS) that induce autophagy [47]. The endoplasmic reticulum (ER) is the major site for protein synthesis. An accumulation of misfolded or unfolded proteins in this compartment results in ER stress and activates an unfolded protein response (UPR) mediated by three ER membrane-associated proteins: PERK (PKR-like eIF2α kinase), ATF6 (activating transcription factor-6) and IRE1 (inositol requiring enzyme 1). All three proteins are normally bound to and inactivated by the chaperone Bip/GRP78 at the side of the ER lumen. When ER stress is triggered, Bip interacts with unfolded luminal proteins, thus leading to the release of PERK, IRE1 and ATF6. PERK mediates the phosphorylation of eukaryotic translation initiation factor 2 α (eIF2α), resulting in rapid reduction of mRNA translation, thereby reducing the load of new proteins in the ER [48]. In addition, PERK phosphorylation of eIF2α allows the translation of activating transcription factor 4 (ATF4) to induce transcription of genes involved in amino acid synthesis and apoptosis [48]. IRE1 is autophosphorylated to function as an endoribonuclease, responsible for the unconventional splicing of the X box-binding protein 1 (XBP1) mRNA. The resulting translation product XBP1 regulates transcription of genes encoding for ER chaperones, biogenesis of phospholipids and components of the ER-associated protein degradation (ERAD) machinery. Finally, activated ATF6 translocates from the ER to the Golgi to undergo cleavage by regulated intramembrane proteolysis (RIP) by site 1 and site 2 proteases. The cytoplasmic domain of ATF6 functions as a transcription factor to transactivate genes encoding ER chaperones, ERAD components and protein foldases [48].
Among these, PERK and ATF6 function as inducers of autophagy, while IRE1 acts as a negative regulator [47]. It was recently shown that HCV infection can induce ER stress and activate all three sensors of UPR [47]. siRNA-mediated silencing of any of the three UPR signaling proteins not only resulted in a significant reduction of LC3-II abundance, but also inhibited HCV replication, arguing that all three UPR signaling pathways are required for HCV-induced autophagy [36] (Figure 2). The exact mechanism by which HCV mediates induction of autophagy via ER stress and UPR remains to be investigated. In contrast, a very recent study by Mohl et al provided evidence that HCV-induced autophagy is independent of UPR [49]. Subgenomic replicons expressing nonstructural (NS3-5B) proteins, as well as a mutant virus lacking the envelope glycoproteins, potently induced autophagy in the absence of detectable UPR [49]. Thus, taking both studies together [36,49], it is unclear how autophagy is induced in HCV infection. Recent studies with other related flaviviruses, such as Dengue virus (DENV) and Modoc virus, showed that virus infection induced autophagy [50]. In fact, expression of the replicase protein NS4A from either virus was sufficient for autophagy induction via UPR [50] (Figure 2). However, the impact of these viral proteins on autophagic flux remains to be determined. Lastly, DNA viruses can also induce autophagy via ER-stress and UPR-related pathways. In the case of varicella-zoster virus (VZV) infection, it was demonstrated that virus-induced ER stress and UPR response precede autophagosome formation [51]. The authors show that ER stress upon VZV infection is due to abundant VZV glycoprotein biosynthesis, which in turn triggers to UPR and thus, autophagy to maintain cellular homeostasis.
3. Relationship Between Autophagy and Viruses
Depending on the virus and the host cell, autophagy-mediated responses can have different effects on the outcome of viral infections. As an integral part of the immune system, autophagy is involved in the sensing of viruses and mounting of an antiviral defense. It is therefore of little surprise that viruses have evolved mechanisms to subvert the autophagic response.
4. Future Directions
Organisms have developed highly complex defense networks against invading pathogens, and in turn, pathogens either disable or manipulate host defenses to hijack their host and eventually to co-exist with it. As described in this review, autophagy fits into this theme, because on one hand, it can promote viral replication; on the other hand, it contributes to innate and adaptive immunity and thus, limits pathogen replication. Obviously as a result of co-evolution, viruses developed multiple strategies to evade autophagy or—more extremely—to reverse the antiviral effects of autophagy into proviral ones. In spite of the tremendous progress made in the last couple of years, many important aspects remain to be addressed. For instance, autophagy selectively degrades viruses, but can viruses induce autophagy to selectively degrade antiviral platforms like mitochondria or peroxisomes? Such events are plausible considering the mitochondrial or peroxisomal localization of essential innate signaling molecules, such as MAVS (mitochondrial antiviral-signaling protein) or TRIF [92,93]. Another important question relates to selective autophagy. This process requires cargo-binding adaptor proteins, which all have a molecular signature, termed the ‘LIR’ motif. It enables interaction with LC3 in order to feed bound cargo to the autophagosome. Since viruses can also induce selective autophagy, it will be important to investigate if viral proteins can directly bind LC3 and whether this interaction is LIR dependent. Studies of these and many other aspects will certainly foster our understanding to the pathogen-host interaction, but might also open new avenues for the development of novel prophylactic or therapeutic antiviral strategies.
Conflict of Interest
The authors declare no conflict of interest.
References
- Yang, Z.; Klionsky, D.J. Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar]
- Mijaljica, D.; Prescott, M.; Devenish, R.J. Microautophagy in mammalian cells: Revisiting a 40-year-old conundrum. Autophagy 2011, 7, 673–682. [Google Scholar] [CrossRef]
- Dunn, W.A., Jr. Studies on the mechanisms of autophagy: Formation of the autophagic vacuole. J. Cell Biol. 1990, 110, 1923–1933. [Google Scholar] [CrossRef]
- Fengsrud, M.; Erichsen, E.S.; Berg, T.O.; Raiborg, C.; Seglen, P.O. Ultrastructural characterization of the delimiting membranes of isolated autophagosomes and amphisomes by freeze-fracture electron microscopy. Eur. J. Cell Biol. 2000, 79, 871–882. [Google Scholar] [CrossRef]
- Eskelinen, E.L. Maturation of autophagic vacuoles in Mammalian cells. Autophagy 2005, 1, 1–10. [Google Scholar] [CrossRef]
- Axe, E.L.; Walker, S.A.; Manifava, M.; Chandra, P.; Roderick, H.L.; Habermann, A.; Griffiths, G.; Ktistakis, N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008, 182, 685–701. [Google Scholar] [CrossRef]
- Hailey, D.W.; Rambold, A.S.; Satpute-Krishnan, P.; Mitra, K.; Sougrat, R.; Kim, P.K.; Lippincott-Schwartz, J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 2010, 141, 656–667. [Google Scholar] [CrossRef]
- Ravikumar, B.; Moreau, K.; Jahreiss, L.; Puri, C.; Rubinsztein, D.C. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nature Cell Biol. 2010, 12, 747–757. [Google Scholar] [CrossRef]
- Inoue, Y.; Klionsky, D.J. Regulation of macroautophagy in Saccharomyces cerevisiae. Semin. Cell Dev. Biol. 2010, 21, 664–670. [Google Scholar] [CrossRef]
- Kirkin, V.; McEwan, D.G.; Novak, I.; Dikic, I. A role for ubiquitin in selective autophagy. Mol. Cell 2009, 34, 259–269. [Google Scholar] [CrossRef]
- Behrends, C.; Fulda, S. Receptor proteins in selective autophagy. Int. J. Cell Biol. 2012, 2012, 673290. [Google Scholar]
- Novak, I.; Dikic, I. Autophagy receptors in developmental clearance of mitochondria. Autophagy 2011, 7, 301–303. [Google Scholar] [CrossRef]
- Deribe, Y.L.; Pawson, T.; Dikic, I. Post-translational modifications in signal integration. Nat. Struct. Mol. Biol. 2010, 17, 666–672. [Google Scholar]
- McEwan, D.G.; Dikic, I. The Three Musketeers of Autophagy: phosphorylation, ubiquitylation and acetylation. Trends Cell Biol. 2011, 21, 195–201. [Google Scholar] [CrossRef]
- Behrends, C.; Sowa, M.E.; Gygi, S.P.; Harper, J.W. Network organization of the human autophagy system. Nature 2010, 466, 68–76. [Google Scholar]
- Galluzzi, L.; Aaronson, S.A.; Abrams, J.; Alnemri, E.S.; Andrews, D.W.; Baehrecke, E.H.; Bazan, N.G.; Blagosklonny, M.V.; Blomgren, K.; Borner, C.; Bredesen, D.E.; Brenner, C.; Castedo, M.; Cidlowski, J.A.; Ciechanover, A.; Cohen, G.M.; De Laurenzi, V.; De Maria, R.; Deshmukh, M.; Dynlacht, B.D.; El-Deiry, W.S.; Flavell, R.A.; Fulda, S.; Garrido, C.; Golstein, P.; Gougeon, M.L.; Green, D.R.; Gronemeyer, H.; Hajnoczky, G.; Hardwick, J.M.; Hengartner, M.O.; Ichijo, H.; Jaattela, M.; Kepp, O.; Kimchi, A.; Klionsky, D.J.; Knight, R.A.; Kornbluth, S.; Kumar, S.; Levine, B.; Lipton, S.A.; Lugli, E.; Madeo, F.; Malomi, W.; Marine, J.C.; Martin, S.J.; Medema, J.P.; Mehlen, P.; Melino, G.; Moll, U.M.; Morselli, E.; Nagata, S.; Nicholson, D.W.; Nicotera, P.; Nunez, G.; Oren, M.; Penninger, J.; Pervaiz, S.; Peter, M.E.; Piacentini, M.; Prehn, J.H.; Puthalakath, H.; Rabinovich, G.A.; Rizzuto, R.; Rodrigues, C.M.; Rubinsztein, D.C.; Rudel, T.; Scorrano, L.; Simon, H.U.; Steller, H.; Tschopp, J.; Tsujimoto, Y.; Vandenabeele, P.; Vitale, I.; Vousden, K.H.; Youle, R.J.; Yuan, J.; Zhivotovsky, B.; Kroemer, G. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009, 16, 1093–1107. [Google Scholar]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar]
- Jackson, W.T.; Giddings, T.H., Jr.; Taylor, M.P.; Mulinyawe, S.; Rabinovitch, M.; Kopito, R.R.; Kirkegaard, K. Subversion of cellular autophagosomal machinery by RNA viruses. Plos Biol. 2005, 3, e156. [Google Scholar] [CrossRef]
- Berkova, Z.; Crawford, S.E.; Trugnan, G.; Yoshimori, T.; Morris, A.P.; Estes, M.K. Rotavirus NSP4 induces a novel vesicular compartment regulated by calcium and associated with viroplasms. JVI 2006, 80, 6061–6071. [Google Scholar] [CrossRef]
- Nakashima, A.; Tanaka, N.; Tamai, K.; Kyuuma, M.; Ishikawa, Y.; Sato, H.; Yoshimori, T.; Saito, S.; Sugamura, K. Survival of parvovirus B19-infected cells by cellular autophagy. Virology 2006, 349, 254–263. [Google Scholar] [CrossRef]
- Taylor, M.P.; Kirkegaard, K. Modification of cellular autophagy protein LC3 by poliovirus. JVI 2007, 81, 12543–12553. [Google Scholar] [CrossRef]
- Ait-Goughoulte, M.; Kanda, T.; Meyer, K.; Ryerse, J.S.; Ray, R.B.; Ray, R. Hepatitis C virus genotype 1a growth and induction of autophagy. JVI 2008, 82, 2241–2249. [Google Scholar] [CrossRef]
- Sir, D.; Chen, W.L.; Choi, J.; Wakita, T.; Yen, T.S.; Ou, J.H. Induction of incomplete autophagic response by hepatitis C virus via the unfolded protein response. Hepatology 2008, 48, 1054–1061. [Google Scholar] [CrossRef]
- Taylor, M.P.; Kirkegaard, K. Potential subversion of autophagosomal pathway by picornaviruses. Autophagy 2008, 4, 286–289. [Google Scholar]
- Wong, J.; Zhang, J.; Si, X.; Gao, G.; Mao, I.; McManus, B.M.; Luo, H. Autophagosome supports coxsackievirus B3 replication in host cells. JVI 2008, 82, 9143–9153. [Google Scholar] [CrossRef]
- Dreux, M.; Gastaminza, P.; Wieland, S.F.; Chisari, F.V. The autophagy machinery is required to initiate hepatitis C virus replication. PNAS 2009, 106, 14046–14051. [Google Scholar]
- Gannage, M.; Dormann, D.; Albrecht, R.; Dengjel, J.; Torossi, T.; Ramer, P.C.; Lee, M.; Strowig, T.; Arrey, F.; Conenello, G.; Pypaert, M.; Andersen, J.; Garcia-Sastre, A.; Munz, C. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe 2009, 6, 367–380. [Google Scholar] [CrossRef]
- Kyei, G.B.; Dinkins, C.; Davis, A.S.; Roberts, E.; Singh, S.B.; Dong, C.; Wu, L.; Kominami, E.; Ueno, T.; Yamamoto, A.; Federico, M.; Panganiban, A.; Vergne, I.; Deretic, V. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J. Cell Biol. 2009, 186, 255–268. [Google Scholar] [CrossRef]
- Panyasrivanit, M.; Khakpoor, A.; Wikan, N.; Smith, D.R. Linking dengue virus entry and translation/replication through amphisomes. Autophagy 2009, 5, 434–435. [Google Scholar] [CrossRef]
- Panyasrivanit, M.; Khakpoor, A.; Wikan, N.; Smith, D.R. Co-localization of constituents of the dengue virus translation and replication machinery with amphisomes. J. Gen. Virol. 2009, 90, 448–456. [Google Scholar] [CrossRef]
- Shelly, S.; Lukinova, N.; Bambina, S.; Berman, A.; Cherry, S. Autophagy is an essential component of Drosophila immunity against vesicular stomatitis virus. Immunity 2009, 30, 588–598. [Google Scholar] [CrossRef]
- Takahashi, M.N.; Jackson, W.; Laird, D.T.; Culp, T.D.; Grose, C.; Haynes, J.I., 2nd; Benetti, L. Varicella-zoster virus infection induces autophagy in both cultured cells and human skin vesicles. JVI 2009, 83, 5466–5476. [Google Scholar] [CrossRef]
- Tang, H.; Da, L.; Mao, Y.; Li, Y.; Li, D.; Xu, Z.; Li, F.; Wang, Y.; Tiollais, P.; Li, T.; Zhao, M. Hepatitis B virus X protein sensitizes cells to starvation-induced autophagy via up-regulation of beclin 1 expression. Hepatology 2009, 49, 60–71. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, X.; Liu, D.; Fan, Z.; Hu, X.; Yan, J.; Wang, M.; Gao, G.F. Autophagy is involved in influenza A virus replication. Autophagy 2009, 5, 321–328. [Google Scholar] [CrossRef]
- Khakpoor, A.; Panyasrivanit, M.; Wikan, N.; Smith, D.R. A role for autophagolysosomes in dengue virus 3 production in HepG2 cells. J. Gen. Virol. 2009, 90, 1093–1103. [Google Scholar] [CrossRef]
- Ke, P.Y.; Chen, S.S. Activation of the unfolded protein response and autophagy after hepatitis C virus infection suppresses innate antiviral immunity in vitro. J. Clin. Invest. 2011, 121, 37–56. [Google Scholar] [CrossRef]
- Orvedahl, A.; MacPherson, S.; Sumpter, R., Jr.; Talloczy, Z.; Zou, Z.; Levine, B. Autophagy protects against Sindbis virus infection of the central nervous system. Cell Host Microbe 2010, 7, 115–127. [Google Scholar] [CrossRef]
- Liang, C.; E, X.; Jung, J.U. Downregulation of autophagy by herpesvirus Bcl-2 homologs. Autophagy 2008, 4, 268–272. [Google Scholar]
- Ku, B.; Woo, J.S.; Liang, C.; Lee, K.H.; Hong, H.S.; E, X.; Kim, K.S.; Jung, J.U.; Oh, B.H. Structural and biochemical bases for the inhibition of autophagy and apoptosis by viral BCL-2 of murine gamma-herpesvirus 68. PLoS Pathog. 2008, 4, e25. [Google Scholar] [CrossRef]
- Orvedahl, A.; Alexander, D.; Talloczy, Z.; Sun, Q.; Wei, Y.; Zhang, W.; Burns, D.; Leib, D.A.; Levine, B. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe 2007, 1, 23–35. [Google Scholar] [CrossRef]
- Levine, B.; Deretic, V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat. Rev. Immunol. 2007, 7, 767–777. [Google Scholar] [CrossRef]
- Kemball, C.C.; Alirezaei, M.; Flynn, C.T.; Wood, M.R.; Harkins, S.; Kiosses, W.B.; Whitton, J.L. Coxsackievirus infection induces autophagy-like vesicles and megaphagosomes in pancreatic acinar cells in vivo. JVI 2010, 84, 12110–12124. [Google Scholar] [CrossRef]
- Joubert, P.E.; Meiffren, G.; Gregoire, I.P.; Pontini, G.; Richetta, C.; Flacher, M.; Azocar, O.; Vidalain, P.O.; Vidal, M.; Lotteau, V.; Codogno, P.; Rabourdin-Combe, C.; Faure, M. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe 2009, 6, 354–366. [Google Scholar] [CrossRef]
- Denizot, M.; Varbanov, M.; Espert, L.; Robert-Hebmann, V.; Sagnier, S.; Garcia, E.; Curriu, M.; Mamoun, R.; Blanco, J.; Biard-Piechaczyk, M. HIV-1 gp41 fusogenic function triggers autophagy in uninfected cells. Autophagy 2008, 4, 998–1008. [Google Scholar]
- Espert, L.; Denizot, M.; Grimaldi, M.; Robert-Hebmann, V.; Gay, B.; Varbanov, M.; Codogno, P.; Biard-Piechaczyk, M. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J. Clin. Invest. 2006, 116, 2161–2172. [Google Scholar] [CrossRef]
- Nakamoto, M.; Moy, R.H.; Xu, J.; Bambina, S.; Yasunaga, A.; Shelly, S.S.; Gold, B.; Cherry, S. Virus recognition by Toll-7 activates antiviral autophagy in Drosophila. Immunity 2012, 36, 658–667. [Google Scholar] [CrossRef]
- Kroemer, G.; Marino, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef]
- Martinon, F. The endoplasmic reticulum: A sensor of cellular stress that modulates immune responses. Microbes Infect. 2012, 14, 1293–1300. [Google Scholar] [CrossRef]
- Mohl, B.P.; Tedbury, P.R.; Griffin, S.; Harris, M. Hepatitis C virus-induced autophagy is independent of the unfolded protein response. JVI 2012, 86, 10724–10732. [Google Scholar] [CrossRef]
- McLean, J.E.; Wudzinska, A.; Datan, E.; Quaglino, D.; Zakeri, Z. Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication. J. Biol. Chem. 2011, 286, 22147–22159. [Google Scholar]
- Carpenter, J.E.; Jackson, W.; Benetti, L.; Grose, C. Autophagosome formation during varicella-zoster virus infection following endoplasmic reticulum stress and the unfolded protein response. JVI 2011, 85, 9414–9424. [Google Scholar] [CrossRef]
- Rathinam, V.A.; Fitzgerald, K.A. Cytosolic surveillance and antiviral immunity. Curr. Opin. Virol. 2011, 1, 455–462. [Google Scholar] [CrossRef]
- Ramos, H.J.; Gale, M., Jr. RIG-I like receptors and their signaling crosstalk in the regulation of antiviral immunity. Curr. Opin. Virol. 2011, 1, 167–176. [Google Scholar] [CrossRef]
- Arpaia, N.; Barton, G.M. Toll-like receptors: key players in antiviral immunity. Curr. Opin. Virol. 2011, 1, 447–454. [Google Scholar] [CrossRef]
- Lee, H.K.; Lund, J.M.; Ramanathan, B.; Mizushima, N.; Iwasaki, A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 2007, 315, 1398–1401. [Google Scholar]
- Gorbea, C.; Makar, K.A.; Pauschinger, M.; Pratt, G.; Bersola, J.L.; Varela, J.; David, R.M.; Banks, L.; Huang, C.H.; Li, H.; Schultheiss, H.P.; Towbin, J.A.; Vallejo, J.G.; Bowles, N.E. A role for Toll-like receptor 3 variants in host susceptibility to enteroviral myocarditis and dilated cardiomyopathy. J. Biol. Chem. 2010, 285, 23208–23223. [Google Scholar]
- Into, T.; Inomata, M.; Takayama, E.; Takigawa, T. Autophagy in regulation of Toll-like receptor signaling. Cell Signal. 2012, 24, 1150–1162. [Google Scholar] [CrossRef]
- Delgado, M.A.; Deretic, V. Toll-like receptors in control of immunological autophagy. Cell Death Differ. 2009, 16, 976–983. [Google Scholar] [CrossRef]
- Shi, C.S.; Kehrl, J.H. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J. Biol. Chem. 2008, 283, 33175–33182. [Google Scholar]
- Deretic, V. Autophagy: an emerging immunological paradigm. J. Immunol. 2012, 189, 15–20. [Google Scholar] [CrossRef]
- Wild, P.; Farhan, H.; McEwan, D.G.; Wagner, S.; Rogov, V.V.; Brady, N.R.; Richter, B.; Korac, J.; Waidmann, O.; Choudhary, C.; Dotsch, V.; Bumann, D.; Dikic, I. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 2011, 333, 228–233. [Google Scholar]
- Orvedahl, A.; Sumpter, R., Jr.; Xiao, G.; Ng, A.; Zou, Z.; Tang, Y.; Narimatsu, M.; Gilpin, C.; Sun, Q.; Roth, M.; Forst, C.V.; Wrana, J.L.; Zhang, Y.E.; Luby-Phelps, K.; Xavier, R.J.; Xie, Y.; Levine, B. Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature 2011, 480, 113–117. [Google Scholar]
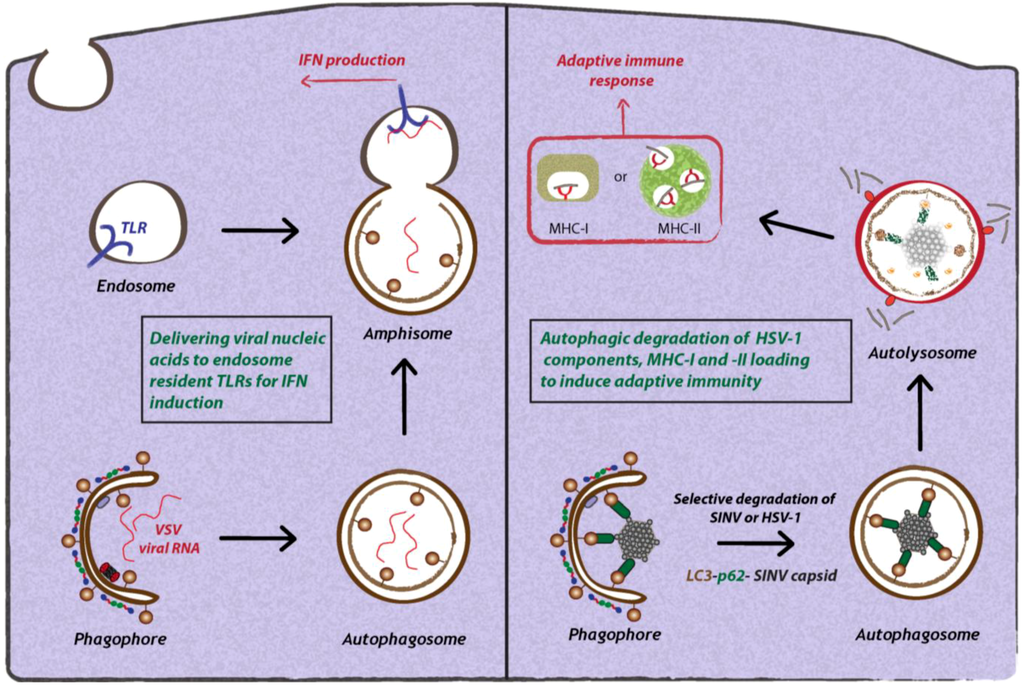
- Munz, C. Antigen processing by macroautophagy for MHC presentation. FIMMU 2011, 2, 42. [Google Scholar]
- Martinez-Borra, J.; Lopez-Larrea, C. The emergence of the major histocompatilibility complex. Adv. Exp. Med. Biol. 2012, 738, 277–289. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar]
- Gannage, M.; Munz, C. MHC presentation via autophagy and how viruses escape from it. Semin. Immunopathol. 2010, 32, 373–381. [Google Scholar] [CrossRef]
- English, L.; Chemali, M.; Duron, J.; Rondeau, C.; Laplante, A.; Gingras, D.; Alexander, D.; Leib, D.; Norbury, C.; Lippe, R.; Desjardins, M. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat. Immunol. 2009, 10, 480–487. [Google Scholar] [CrossRef]
- Leung, C.S.; Taylor, G.S. Nuclear shelter: The influence of subcellular location on the processing of antigens by macroautophagy. Autophagy 2010, 6. [Google Scholar] [Green Version]
- Paludan, C.; Schmid, D.; Landthaler, M.; Vockerodt, M.; Kube, D.; Tuschl, T.; Munz, C. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 2005, 307, 593–596. [Google Scholar]
- Schmid, D.; Pypaert, M.; Munz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 2007, 26, 79–92. [Google Scholar] [CrossRef]
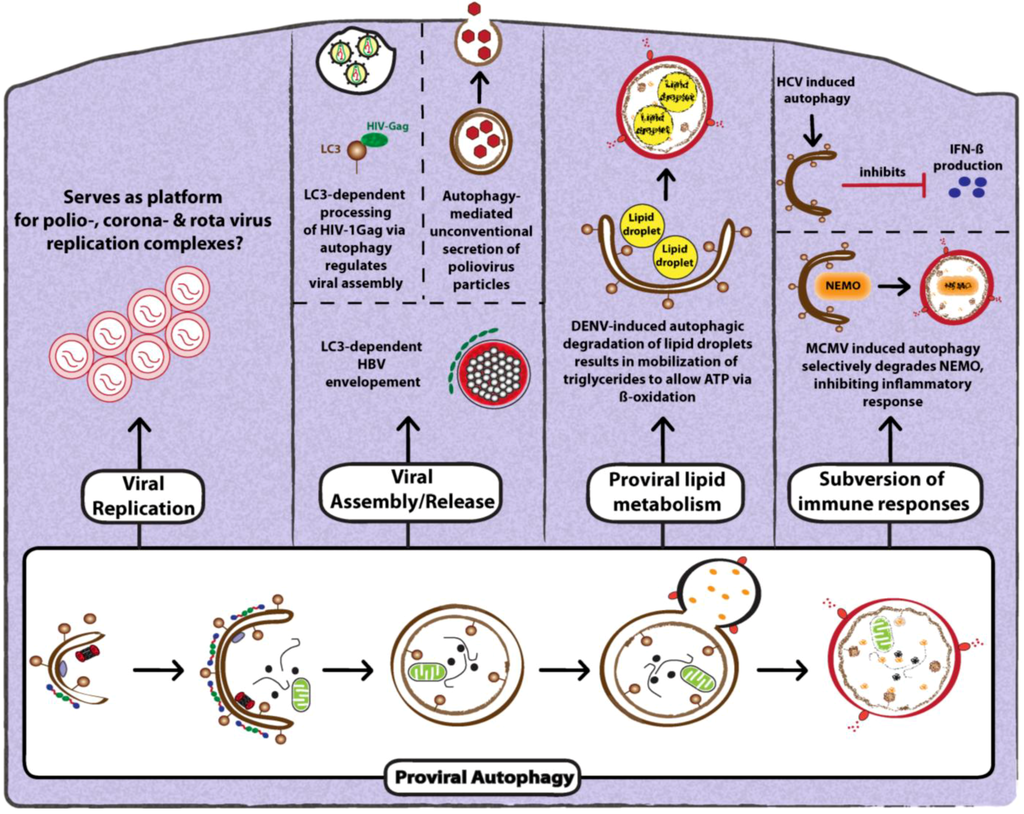
- Miller, S.; Krijnse-Locker, J. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 2008, 6, 363–374. [Google Scholar] [CrossRef]
- Schlegel, A.; Giddings, T.H., Jr.; Ladinsky, M.S.; Kirkegaard, K. Cellular origin and ultrastructure of membranes induced during poliovirus infection. JVI 1996, 70, 6576–6588. [Google Scholar]
- Huang, S.C.; Chang, C.L.; Wang, P.S.; Tsai, Y.; Liu, H.S. Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication. J. Med. Virol. 2009, 81, 1241–1252. [Google Scholar]
- O'Donnell, V.; Pacheco, J.M.; LaRocco, M.; Burrage, T.; Jackson, W.; Rodriguez, L.L.; Borca, M.V.; Baxt, B. Foot-and-mouth disease virus utilizes an autophagic pathway during viral replication. Virology 2011, 410, 142–150. [Google Scholar] [CrossRef]
- Belov, G.A.; Altan-Bonnet, N.; Kovtunovych, G.; Jackson, C.L.; Lippincott-Schwartz, J.; Ehrenfeld, E. Hijacking components of the cellular secretory pathway for replication of poliovirus RNA. JVI 2007, 81, 558–567. [Google Scholar] [CrossRef]
- Belov, G.A.; Feng, Q.; Nikovics, K.; Jackson, C.L.; Ehrenfeld, E. A critical role of a cellular membrane traffic protein in poliovirus RNA replication. PLoS Pathog. 2008, 4, e1000216. [Google Scholar]
- Hsu, N.Y.; Ilnytska, O.; Belov, G.; Santiana, M.; Chen, Y.H.; Takvorian, P.M.; Pau, C.; van der Schaar, H.; Kaushik-Basu, N.; Balla, T.; Cameron, C.E.; Ehrenfeld, E.; van Kuppeveld, F.J.; Altan-Bonnet, N. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell 2010, 141, 799–811. [Google Scholar] [CrossRef]
- Taylor, M.P.; Burgon, T.B.; Kirkegaard, K.; Jackson, W.T. Role of microtubules in extracellular release of poliovirus. JVI 2009, 83, 6599–6609. [Google Scholar] [CrossRef]
- Sir, D.; Kuo, C.F.; Tian, Y.; Liu, H.M.; Huang, E.J.; Jung, J.U.; Machida, K.; Ou, J.H. Replication of hepatitis C virus RNA on autophagosomal membranes. J. Biol. Chem. 2012, 287, 18036–18043. [Google Scholar]
- Tanida, I.; Fukasawa, M.; Ueno, T.; Kominami, E.; Wakita, T.; Hanada, K. Knockdown of autophagy-related gene decreases the production of infectious hepatitis C virus particles. Autophagy 2009, 5, 937–945. [Google Scholar] [CrossRef]
- Taguwa, S.; Kambara, H.; Fujita, N.; Noda, T.; Yoshimori, T.; Koike, K.; Moriishi, K.; Matsuura, Y. Dysfunction of autophagy participates in vacuole formation and cell death in cells replicating hepatitis C virus. JVI 2011, 85, 13185–13194. [Google Scholar] [CrossRef]
- Shrivastava, S.; Raychoudhuri, A.; Steele, R.; Ray, R.; Ray, R.B. Knockdown of autophagy enhances the innate immune response in hepatitis C virus-infected hepatocytes. Hepatology 2011, 53, 406–414. [Google Scholar] [CrossRef]
- Prentice, E.; Jerome, W.G.; Yoshimori, T.; Mizushima, N.; Denison, M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 2004, 279, 10136–10141. [Google Scholar]
- Zhao, Z.; Thackray, L.B.; Miller, B.C.; Lynn, T.M.; Becker, M.M.; Ward, E.; Mizushima, N.N.; Denison, M.R.; Virgin, H.W.T. Coronavirus replication does not require the autophagy gene ATG5. Autophagy 2007, 3, 581–585. [Google Scholar]
- Knoops, K.; Kikkert, M.; Worm, S.H.; Zevenhoven-Dobbe, J.C.; van der Meer, Y.; Koster, A.J.; Mommaas, A.M.; Snijder, E.J. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008, 6, e226. [Google Scholar] [CrossRef]
- Reggiori, F.; Monastyrska, I.; Verheije, M.H.; Cali, T.; Ulasli, M.; Bianchi, S.; Bernasconi, R.; de Haan, C.A.; Molinari, M. Coronaviruses Hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe 2010, 7, 500–508. [Google Scholar] [CrossRef]
- Heaton, N.S.; Randall, G. Dengue virus and autophagy. Viruses 2011, 3, 1332–1341. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, Z.; Liu, K.; Wang, Y.; Liu, J.; Ding, H.; Yuan, Z. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. JVI 2011, 85, 6319–6333. [Google Scholar] [CrossRef]
- Deretic, V.; Jiang, S.; Dupont, N. Autophagy intersections with conventional and unconventional secretion in tissue development, remodeling and inflammation. Trends Cell Biol. 2012. [Green Version]
- Fliss, P.M.; Jowers, T.P.; Brinkmann, M.M.; Holstermann, B.; Mack, C.; Dickinson, P.; Hohenberg, H.; Ghazal, P.; Brune, W. Viral mediated redirection of NEMO/IKKgamma to autophagosomes curtails the inflammatory cascade. PLoS Pathog. 2012, 8, e1002517. [Google Scholar]
- Gregoire, I.P.; Richetta, C.; Meyniel-Schicklin, L.; Borel, S.; Pradezynski, F.; Diaz, O.; Deloire, A.; Azocar, O.; Baguet, J.; Le Breton, M.; Mangeot, P.E.; Navratil, V.; Joubert, P.E.; Flacher, M.; Vidalain, P.O.; Andre, P.; Lotteau, V.; Biard-Piechaczyk, M.; Rabourdin-Combe, C.; Faure, M. IRGM is a common target of RNA viruses that subvert the autophagy network. PLoS Pathog. 2011, 7, e1002422. [Google Scholar]
- Dixit, E.; Boulant, S.; Zhang, Y.; Lee, A.S.; Odendall, C.; Shum, B.; Hacohen, N.; Chen, Z.J.; Whelan, S.P.; Fransen, M.; Nibert, M.L.; Superti-Furga, G.; Kagan, J.C. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 2010, 141, 668–681. [Google Scholar]
- Zemirli, N.; Arnoult, D. Mitochondrial anti-viral immunity. Int. J. Biochem. Cell Biol. 2012, 44, 1473–1476. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).