Secondhand Smoke Exposure Timing Triggers Distinct Placental Responses in Mouse Pregnancy
Abstract
1. Introduction
2. Materials and Methods
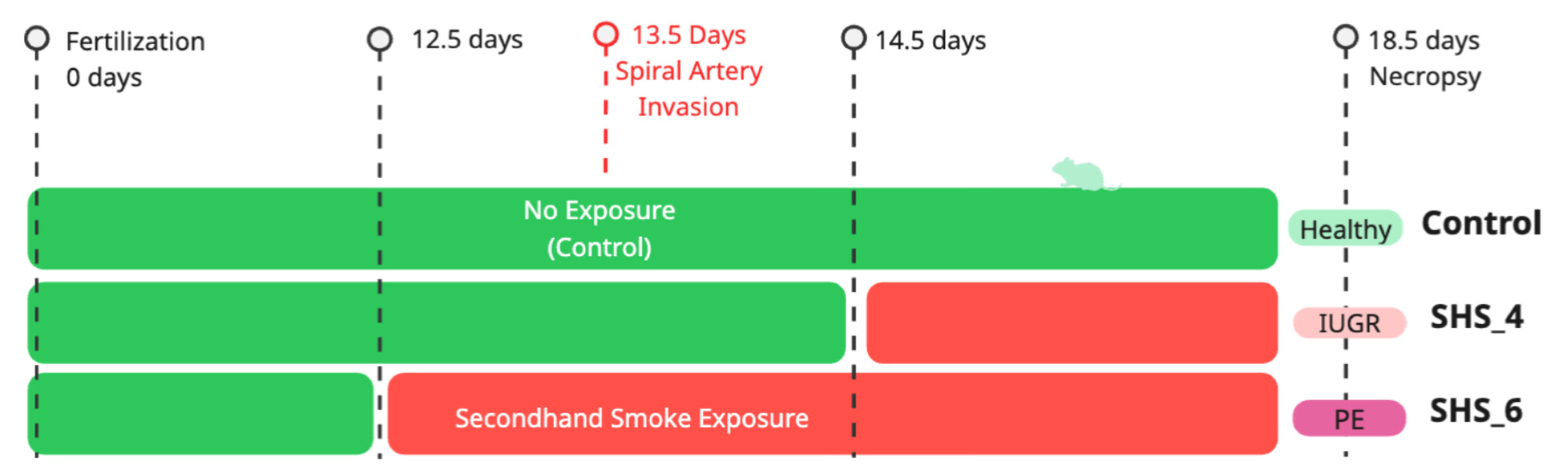
2.1. Animal Husbandry and Exposure Protocol
2.2. Evaluation of Blood Pressure
2.3. Assessment of Proteinuria
2.4. RNA Isolation, Preparation of Libraries, and Sequencing Process
2.5. Analysis of RNA-Seq Data
2.6. Statistics
3. Results
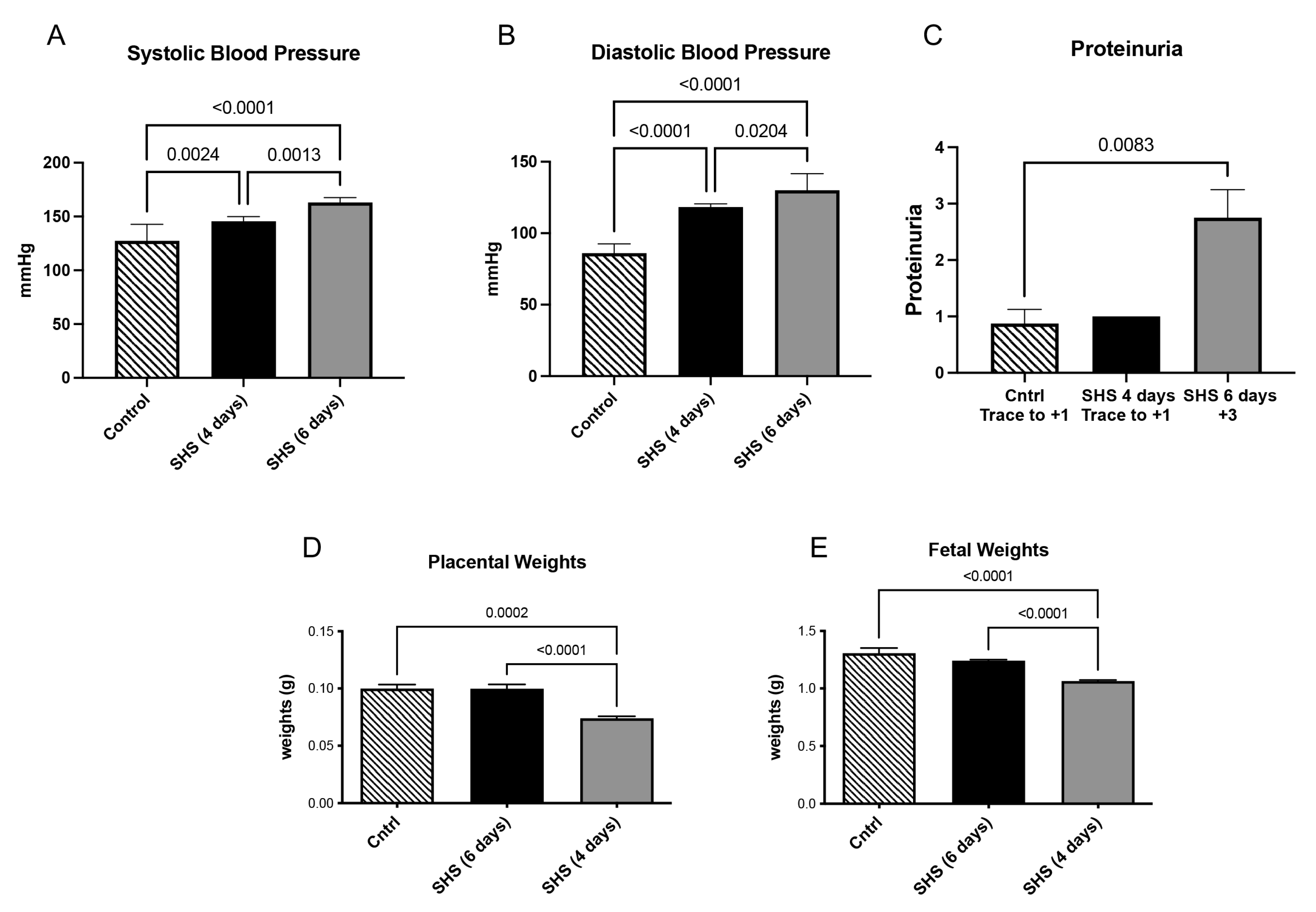
3.1. Blood Pressure, Proteinuria, and Fetal and Placental Weights
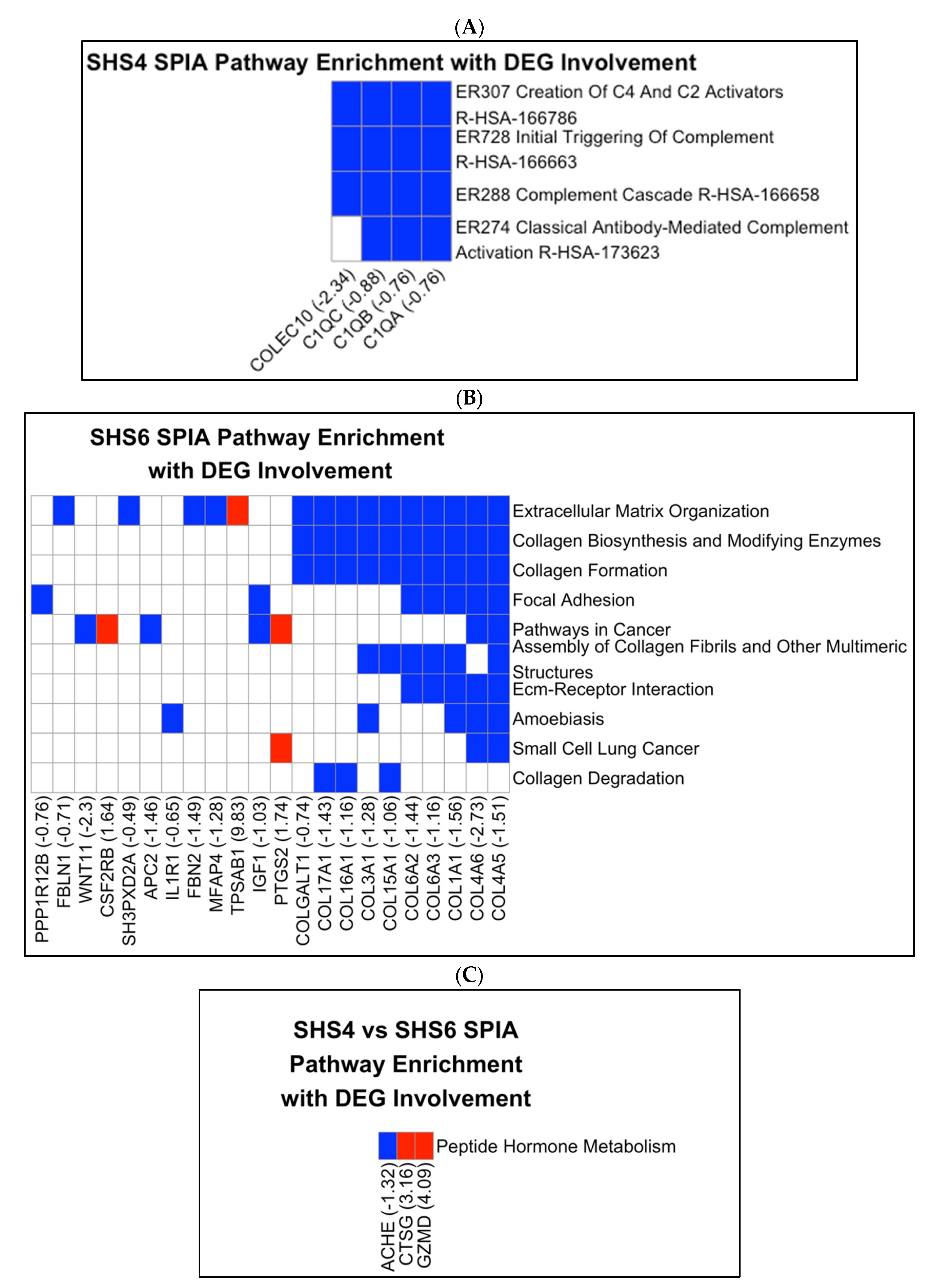
3.2. Pathway Enrichment Results
3.3. Drug Target Analysis
3.4. GSEA Pathway Analysis
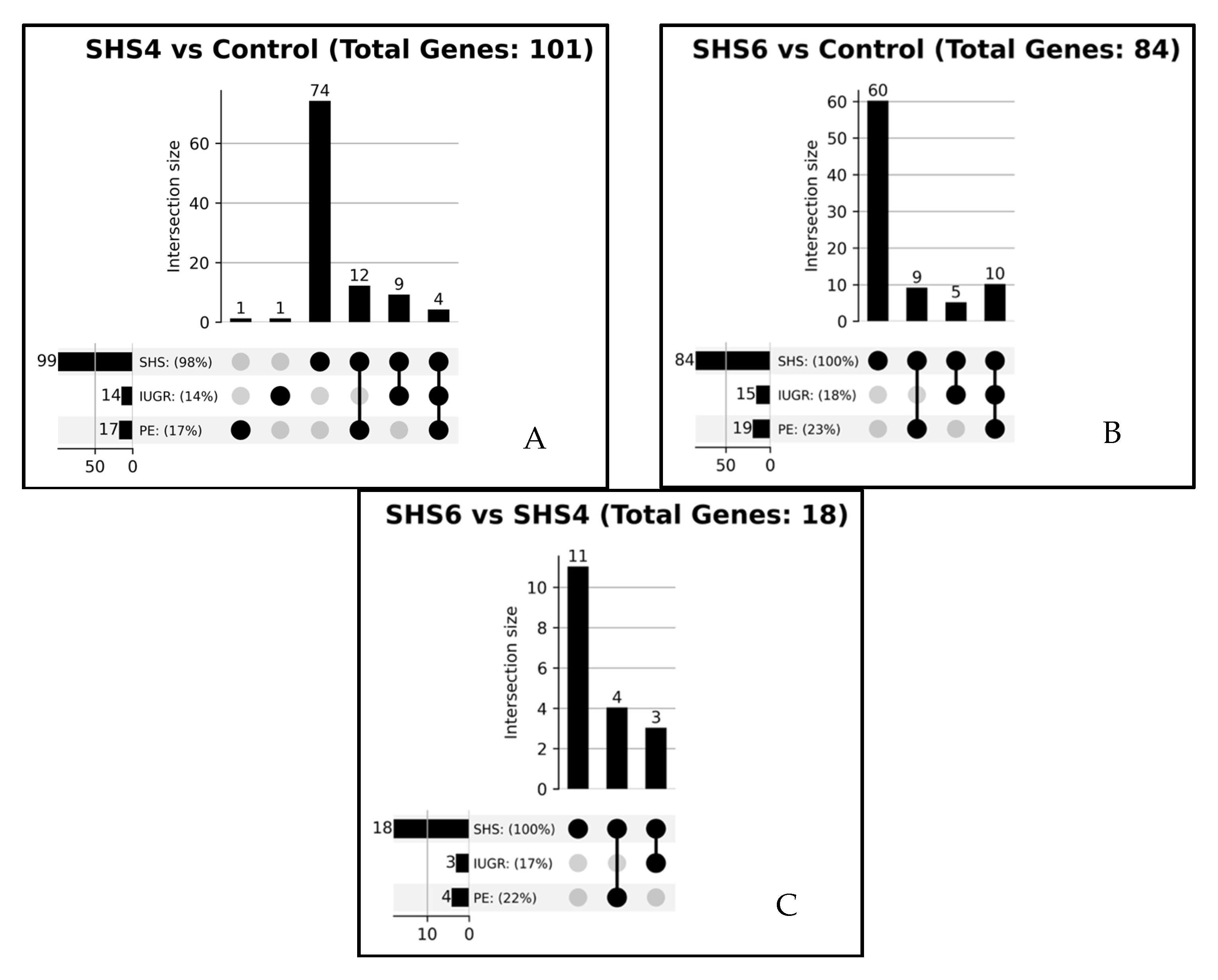
3.5. Literature Associations with DEGs
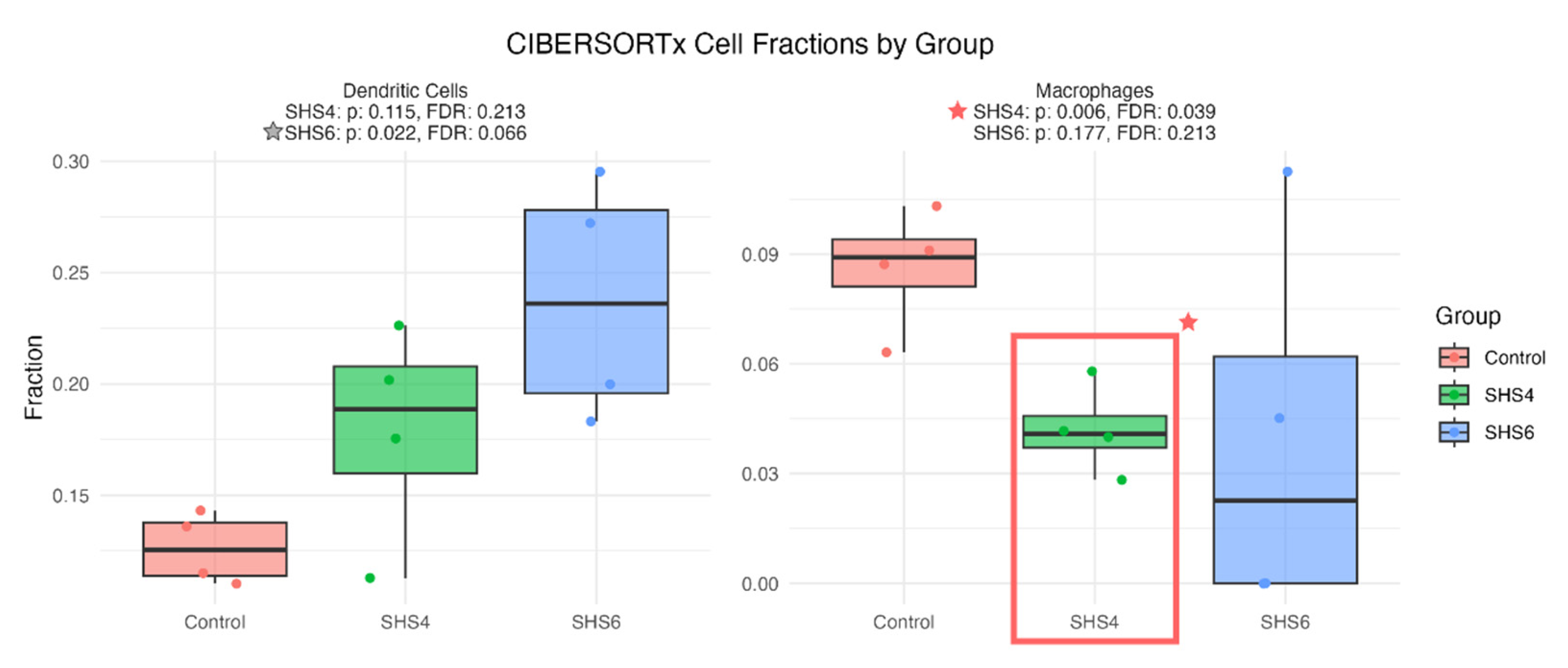
3.6. Immune Infiltration Analysis
4. Discussion
4.1. SHS Exposure and IUGR: Complement System and Immune Dysregulation
4.2. SHS Exposure and Preeclampsia: Collagen Formation and ECM Dysregulation
4.3. Timing of Exposure and Pathway-Specific Effects
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Pruss-Ustun, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Banderali, G.; Martelli, A.; Landi, M.; Moretti, F.; Betti, F.; Radaelli, G.; Lassandro, C.; Verduci, E. Short and long term health effects of parental tobacco smoking during pregnancy and lactation: A descriptive review. J. Transl. Med. 2015, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General; Publications and Reports of the Surgeon General: Atlanta, GA, USA, 2006.
- Ambroz, A.; Klema, J.; Rossnerova, A.; Milcova, A.; Pastorkova, A.; Pulkrabova, J.; Parizek, O.; Gomersall, V.; Gramblicka, T.; Zelenka, J.; et al. Associations of environmental pollution with pro-oxidant, antioxidant and inflammatory markers in pregnant mothers and newborns. Front. Toxicol. 2025, 7, 1572486. [Google Scholar] [CrossRef]
- Kirkham, M.N.; Cooper, C.; Broberg, E.; Robertson, P.; Clarke, D.; Pickett, B.E.; Bikman, B.; Reynolds, P.R.; Arroyo, J.A. Different Lengths of Gestational Exposure to Secondhand Smoke or e-Cigarette Vapor Induce the Development of Placental Disease Symptoms. Cells 2024, 13, 1009. [Google Scholar] [CrossRef]
- Chiarello, D.I.; Ustariz, J.; Marin, R.; Carrasco-Wong, I.; Farias, M.; Giordano, A.; Gallardo, F.S.; Illanes, S.E.; Gutierrez, J. Cellular mechanisms linking to outdoor and indoor air pollution damage during pregnancy. Front. Endocrinol. 2023, 14, 1084986. [Google Scholar] [CrossRef]
- Jauniaux, E.; Johns, J.; Gulbis, B.; Spasic-Boskovic, O.; Burton, G.J. Transfer of folic acid inside the first-trimester gestational sac and the effect of maternal smoking. Am. J. Obstet. Gynecol. 2007, 197, 58.e51–58.e56. [Google Scholar] [CrossRef]
- Kramer, M.S. The epidemiology of adverse pregnancy outcomes: An overview. J. Nutr. 2003, 133, 1592S–1596S. [Google Scholar] [CrossRef]
- Cnattingius, S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob. Res. 2004, 6 (Suppl. S2), S125–S140. [Google Scholar] [CrossRef]
- World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Geographic variation in the incidence of hypertension in pregnancy. Am. J. Obstet. Gynecol. 1988, 158, 80–83. [Google Scholar] [CrossRef]
- Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar] [CrossRef]
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Cresswell, J.A.; Alexander, M.; Chong, M.Y.C.; Link, H.M.; Pejchinovska, M.; Gazeley, U.; Ahmed, S.M.A.; Chou, D.; Moller, A.B.; Simpson, D.; et al. Global and regional causes of maternal deaths 2009-20: A WHO systematic analysis. Lancet Glob. Health 2025, 13, e626–e634. [Google Scholar] [CrossRef] [PubMed]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction: Relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M. Pathophysiology of ischemic placental disease. Semin. Perinatol. 2014, 38, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Bell, M.J. If we know so much about preeclampsia, why haven’t we cured the disease? J. Reprod. Immunol. 2013, 99, 1–9. [Google Scholar] [CrossRef]
- Romo, A.; Carceller, R.; Tobajas, J. Intrauterine growth retardation (IUGR): Epidemiology and etiology. Pediatr. Endocrinol. Rev. 2009, 6 (Suppl. S3), 332–336. [Google Scholar]
- Zamecznik, A.; Niewiadomska-Jarosik, K.; Wosiak, A.; Zamojska, J.; Moll, J.; Stanczyk, J. Intra-uterine growth restriction as a risk factor for hypertension in children six to 10 years old. Cardiovasc. J. Afr. 2014, 25, 73–77. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef]
- Mejia, C.; Lewis, J.; Jordan, C.; Mejia, J.; Ogden, C.; Monson, T.; Winden, D.; Watson, M.; Reynolds, P.R.; Arroyo, J.A. Decreased activation of placental mTOR family members is associated with the induction of intrauterine growth restriction by secondhand smoke in the mouse. Cell Tissue Res. 2016, 367, 387–395. [Google Scholar] [CrossRef]
- Niu, Z.; Xie, C.; Wen, X.; Tian, F.; Yuan, S.; Jia, D.; Chen, W.Q. Potential pathways by which maternal second-hand smoke exposure during pregnancy causes full-term low birth weight. Sci. Rep. 2016, 6, 24987. [Google Scholar] [CrossRef]
- Kornacki, J.; Gutaj, P.; Kalantarova, A.; Sibiak, R.; Jankowski, M.; Wender-Ozegowska, E. Endothelial Dysfunction in Pregnancy Complications. Biomedicines 2021, 9, 1756. [Google Scholar] [CrossRef] [PubMed]
- Girardi, G.; Yarilin, D.; Thurman, J.M.; Holers, V.M.; Salmon, J.E. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J. Exp. Med. 2006, 203, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Ahmed, A.; Girardi, G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension 2011, 58, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Holers, V.M.; Girardi, G.; Mo, L.; Guthridge, J.M.; Molina, H.; Pierangeli, S.S.; Espinola, R.; Xiaowei, L.E.; Mao, D.; Vialpando, C.G.; et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J. Exp. Med. 2002, 195, 211–220. [Google Scholar] [CrossRef]
- Vukoja, M.; Curlin, M.; Vukojevic, K.; Jelic-Knezovic, N.; Kolobaric, A.; Orlovic Vlaho, M.; Soljic, V. Effect of Granzyme K, FasL and Interferon-gamma Expression in Placentas with Preeclampsia. Biomedicines 2024, 12, 842. [Google Scholar] [CrossRef]
- Cheraghi, M.; Salvi, S. Environmental tobacco smoke (ETS) and respiratory health in children. Eur. J. Pediatr. 2009, 168, 897–905. [Google Scholar] [CrossRef]
- Burke, S.D.; Barrette, V.F.; Gravel, J.; Carter, A.L.; Hatta, K.; Zhang, J.; Chen, Z.; Leno-Duran, E.; Bianco, J.; Leonard, S.; et al. Uterine NK cells, spiral artery modification and the regulation of blood pressure during mouse pregnancy. Am. J. Reprod. Immunol. 2010, 63, 472–481. [Google Scholar] [CrossRef]
- Adamson, S.L.; Lu, Y.; Whiteley, K.J.; Holmyard, D.; Hemberger, M.; Pfarrer, C.; Cross, J.C. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 2002, 250, 358–373. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Lun, A.T.L.; Baldoni, P.L.; Smyth, G.K. edgeR v4: Powerful differential analysis of sequencing data with expanded functionality and improved support for small counts and larger datasets. Nucleic Acids Res. 2025, 53, gkaf018. [Google Scholar] [CrossRef]
- Ohta, T.; Nakazato, T.; Bono, H. Calculating the quality of public high-throughput sequencing data to obtain a suitable subset for reanalysis from the Sequence Read Archive. Gigascience 2017, 6, gix029. [Google Scholar] [CrossRef]
- Tarca, A.L.; Draghici, S.; Khatri, P.; Hassan, S.S.; Mittal, P.; Kim, J.S.; Kim, C.J.; Kusanovic, J.P.; Romero, R. A novel signaling pathway impact analysis. Bioinformatics 2009, 25, 75–82. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Vastrik, I.; D’Eustachio, P.; Schmidt, E.; Gopinath, G.; Croft, D.; de Bono, B.; Gillespie, M.; Jassal, B.; Lewis, S.; Matthews, L.; et al. Reactome: A knowledge base of biologic pathways and processes. Genome Biol. 2007, 8, R39. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstrale, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Spendlove, M.D.; Gibson, T.M.; McCain, S.; Stone, B.C.; Gill, T.; Pickett, B.E. Pathway2Targets: An open-source pathway-based approach to repurpose therapeutic drugs and prioritize human targets. PeerJ 2023, 11, e16088. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.M.; Jensen, S.; Pickett, B.E. A signaling pathway-driven bioinformatics pipeline for predicting therapeutics against emerging infectious diseases. F1000Research 2021, 10, 330. [Google Scholar] [CrossRef]
- Wei, C.H.; Kao, H.Y.; Lu, Z. PubTator: A web-based text mining tool for assisting biocuration. Nucleic Acids Res. 2013, 41, W518–W522. [Google Scholar] [CrossRef]
- Newman, A.M.; Steen, C.B.; Liu, C.L.; Gentles, A.J.; Chaudhuri, A.A.; Scherer, F.; Khodadoust, M.S.; Esfahani, M.S.; Luca, B.A.; Steiner, D.; et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 2019, 37, 773–782. [Google Scholar] [CrossRef]
- Flouris, A.D.; Metsios, G.S.; Carrillo, A.E.; Jamurtas, A.Z.; Gourgoulianis, K.; Kiropoulos, T.; Tzatzarakis, M.N.; Tsatsakis, A.M.; Koutedakis, Y. Acute and short-term effects of secondhand smoke on lung function and cytokine production. Am. J. Respir. Crit. Care Med. 2009, 179, 1029–1033. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Chen, D.; Chen, X.; Li, Q.; Ding, J.; Yu, F.; Zhu, X.; Zhang, N.; Chen, Y. The effect of active and passive smoking during pregnancy on birth outcomes: A cohort study in Shanghai. Tob. Induc. Dis. 2024, 22, 10–18332. [Google Scholar] [CrossRef] [PubMed]
- Kimball, R.; Wayment, M.; Merrill, D.; Wahlquist, T.; Reynolds, P.R.; Arroyo, J.A. Hypoxia reduces placental mTOR activation in a hypoxia-induced model of intrauterine growth restriction (IUGR). Physiol. Rep. 2015, 3, e12651. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.I.; Chen, Y.Y.; Wang, C.L.; Yu, J.S.; Chang, Y.S.; Yu, C.J. mTOR regulates proteasomal degradation and Dp1/E2F1-mediated transcription of KPNA2 in lung cancer cells. Oncotarget 2016, 7, 25432–25442. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Luppi, S.; Fejza, A.; Giolo, E.; Ricci, G.; Andreuzzi, E. Extracellular matrix and pregnancy: Functions and opportunities caught in the net. Reprod. Biol. Endocrinol. 2025, 23, 24. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; Wang, H.; Chen, X.; Lan, Z.; Li, P.; Cao, Y.; Liu, M.; Lv, J.; Chen, Y.; et al. Collagen I Induces Preeclampsia-Like Symptoms by Suppressing Proliferation and Invasion of Trophoblasts. Front. Endocrinol. 2021, 12, 664766. [Google Scholar] [CrossRef]
- Jauniaux, E.; Burton, G.J. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum. Dev. 2007, 83, 699–706. [Google Scholar] [CrossRef]
- Brosens, J.J.; Pijnenborg, R.; Brosens, I.A. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: A review of the literature. Am. J. Obstet. Gynecol. 2002, 187, 1416–1423. [Google Scholar] [CrossRef]
- Solt, I.; Cohen, S.M.; Admati, I.; Beharier, O.; Dominsky, O.; Yagel, S. Placenta at single-cell resolution in early and late preeclampsia: Insights and clinical implications. Am. J. Obstet. Gynecol. 2025, 232, S176–S189. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Y.; Yu, Z.; Cheng, X.; Zou, L.; Liu, X. Single cell RNA-sequencing reveals the cellular senescence of placental mesenchymal stem/stromal cell in preeclampsia. Placenta 2024, 150, 39–51. [Google Scholar] [CrossRef]
- Admati, I.; Skarbianskis, N.; Hochgerner, H.; Ophir, O.; Weiner, Z.; Yagel, S.; Solt, I.; Zeisel, A. Two distinct molecular faces of preeclampsia revealed by single-cell transcriptomics. Med 2023, 4, 687–709. [Google Scholar] [CrossRef]
| Symbol | Description | Log2FC | FDR |
|---|---|---|---|
| IL11RA3 | interleukin 11 receptor subunit alpha | 8.0 | 0.003 |
| ECE2 | endothelin converting enzyme 2 | 4.7 | 0.016 |
| CHIL3 | chitinase acidic | 2.5 | <0.001 |
| KPNA2 | karyopherin subunit alpha 2 | 2.1 | 0.014 |
| NPTX2 | neuronal pentraxin 2 | 1.9 | <0.001 |
| UPK3BL | uroplakin 3B like 1 | −4.0 | <0.001 |
| ARHGAP40 | Rho GTPase-activating protein 40 | −4.1 | <0.001 |
| SLCO1B2 | solute carrier organic anion transporter family member 1B3 | −4.6 | 0.002 |
| KRT15 | keratin 15 | −4.8 | 0.002 |
| UGT1A9 | UDP glucuronosyltransferase family one member A9 | −5.2 | 0.03 |
| Symbol | Description | Log2FC | FDR |
|---|---|---|---|
| TPSAB1 | tryptase alpha/beta 1 | 9.8 | 0.001 |
| PLCXD1 | phosphatidylinositol-specific phospholipase C X domain-containing 1 | 2.2 | 0.023 |
| RTP3 | receptor transporter protein 3 | 1.8 | 0.035 |
| CD6 | CD6 molecule | 1.8 | 0.029 |
| EDN2 | endothelin 2 | 1.8 | 0.007 |
| UPK3BL | uroplakin 3B like 1 | −2.9 | 0.008 |
| PRSS27 | serine protease 27 | −3.0 | 0.04 |
| CLEC3B | C-type lectin domain family three member B | −3.1 | 0.020 |
| MUC16 | mucin 16, cell surface-associated | −3.2 | 0.037 |
| KRT5 | keratin 5 | −3.3 | 0.007 |
| Symbol | Description | Log2FC | FDR |
|---|---|---|---|
| TPSAB1 | tryptase alpha/beta 1 | 10.0 | 0.001 |
| PPIHL | peptidylprolyl isomerase H | 8.0 | 0.036 |
| GZMB | granzyme B | 4.6 | <0.001 |
| GZMD | granzyme D | 4.1 | <0.001 |
| PRF1 | perforin 1 | 3.8 | 0.007 |
| IRX5 | Iroquois homeobox 5 | −1.6 | 0.027 |
| XRRA1 | X-ray radiation resistance associated with 1 | −1.7 | 0.036 |
| NPTX2 | neuronal pentraxin 2 | −1.7 | <0.001 |
| TBX1 | T-box transcription factor 1 | −1.8 | 0.018 |
| RGS9 | regulator of G protein signaling 9 | −2.3 | 0.001 |
| Target Symbol | Subcellular Location | Number Pathways with Target | Number Approved Drugs | Number Phase 1 | Number Phase 2 | Number Phase 3 | Number Phase 4 |
|---|---|---|---|---|---|---|---|
| IL6 | Secreted | 7 | 1 | 0 | 1 | 3 | 1 |
| TP53 | Cytoplasm | 8 | 0 | 0 | 3 | 2 | 0 |
| EGFR | Cell membrane | 9 | 5 | 0 | 0 | 0 | 5 |
| ESR1 | Nucleus | 6 | 5 | 0 | 0 | 0 | 5 |
| AKT1 | Cytoplasm | 9 | 1 | 0 | 3 | 1 | 1 |
| MTOR | Lysosome | 6 | 1 | 0 | 1 | 3 | 1 |
| PIK3CA | Cytosol | 9 | 3 | 0 | 0 | 2 | 3 |
| KIT | Cell membrane | 6 | 5 | 0 | 0 | 0 | 5 |
| PTGS2 | Microsome | 8 | 5 | 0 | 0 | 0 | 5 |
| Description | Adjusted p-Value | q-Value | Comparison |
|---|---|---|---|
| Complement and coagulation cascades | 9.14 × 10−3 | 7.54 × 10−3 | SHS-4D |
| Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell | 1.17 × 10−3 | 1.07 × 10−3 | SHS-4D |
| Complement cascade | 2.36 × 10−3 | 2.16 × 10−3 | SHS-4D |
| Collagen formation | 2.03 × 10−8 | 1.66 × 10−8 | SHS-6D |
| Collagen biosynthesis and modifying enzymes | 2.03 × 10−8 | 1.66 × 10−8 | SHS-6D |
| Assembly of collagen fibrils and other multimeric structures | 2.03 × 10−8 | 1.66 × 10−8 | SHS-6D |
| Collagen degradation | 2.03 × 10−8 | 1.66 × 10−8 | SHS-6D |
| Collagen chain trimerization | 2.03 × 10−8 | 1.66 × 10−8 | SHS-6D |
| Extracellular matrix organization | 2.03 × 10−8 | 1.66 × 10−8 | SHS-6D |
| ECM proteoglycans | 7.27 × 10−8 | 5.95 × 10−8 | SHS-6D |
| Degradation of the extracellular matrix | 3.28 × 10−7 | 2.68 × 10−7 | SHS-6D |
| Crosslinking of collagen fibrils | 1.79 × 10−6 | 1.47 × 10−6 | SHS-6D |
| Integrin cell surface interactions | 4.82 × 10−5 | 3.95 × 10−5 | SHS-6D |
| ECM-receptor interaction | 3.61 × 10−4 | 3.13 × 10−4 | SHS-6D |
| Focal adhesion | 9.29 × 10−3 | 8.05 × 10−3 | SHS-6D |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, A.; Frank, E.; Reall, M.; Hiatt, O.; Beck, L.; Reynolds, P.R.; Pickett, B.E.; Arroyo, J.A. Secondhand Smoke Exposure Timing Triggers Distinct Placental Responses in Mouse Pregnancy. Cells 2025, 14, 1735. https://doi.org/10.3390/cells14211735
Chou A, Frank E, Reall M, Hiatt O, Beck L, Reynolds PR, Pickett BE, Arroyo JA. Secondhand Smoke Exposure Timing Triggers Distinct Placental Responses in Mouse Pregnancy. Cells. 2025; 14(21):1735. https://doi.org/10.3390/cells14211735
Chicago/Turabian StyleChou, Archarlie, Ethan Frank, Matt Reall, Olivia Hiatt, Logan Beck, Paul R. Reynolds, Brett E. Pickett, and Juan A. Arroyo. 2025. "Secondhand Smoke Exposure Timing Triggers Distinct Placental Responses in Mouse Pregnancy" Cells 14, no. 21: 1735. https://doi.org/10.3390/cells14211735
APA StyleChou, A., Frank, E., Reall, M., Hiatt, O., Beck, L., Reynolds, P. R., Pickett, B. E., & Arroyo, J. A. (2025). Secondhand Smoke Exposure Timing Triggers Distinct Placental Responses in Mouse Pregnancy. Cells, 14(21), 1735. https://doi.org/10.3390/cells14211735









