Abstract
Antiretroviral therapy (ART) controls HIV-1 replication in people with HIV-1 (PWH), but intestinal integrity impairment persists and fuels microbial translocation and chronic immune activation, thus heightening the cardiovascular disease (CVD) risk. Here, we sought to identify novel immunological correlates of the HIV and CVD status in ART-treated PWH (HIV+; n = 42) and uninfected participants (HIV−; n = 40) of the Canadian HIV and Aging Cohort Study (CHACS), with/without subclinical coronary atherosclerotic plaques, measured by Coronary Computed Tomography Angiography as total plaque volume (TPV, mm3). PBMCs were analyzed by flow cytometry for the expression of T-cell lineage (CD45, CD3, CD4, CD8αα, CD8αβ, TCRαβ, TCRγδ), epithelial cell (EpCAM/CD326), activation (HLA-DR), and gut-homing/residency markers (CD69, CD196/CCR6, CD199/CCR9, CD49d/Itgα4, CD103/ItgαE, Itgβ7). Alterations in the CD3+ T-cell pool, such as increased frequencies of CD8+TCRαβ+ and TCRγδ+ cells, to the detriment of CD4+TCRαβ+ subsets, were associated with the HIV status. Also, CD4+ T-cells with CD326+CD69+CCR6+ItgαE+ and CCR6+Itgβ7− phenotypes were increased in frequency in HIV+ vs. HIV− participants, together with a decreased frequency of CD8+ T-cells with an intraepithelial lymphocyte (IEL)-like CD3+CD4−TCRαβ+TCRγδ−CD8αα+CD8αβ− phenotype. Finally, multivariate logistic regression identified the frequency of ItgαE+CD8+, ItgαE−CD8+, CCR6+CD4+, and CCR6+Itgβ7−CD4+ T-cells as strong positive correlates of HIV status and atherosclerotic plaque in ART-treated PWH.
1. Introduction
HIV-1 affects an estimated 39.0 million individuals globally, with approximately 1.3 million new incidences reported in 2023 [1]. While antiretroviral therapy (ART) has significantly improved the lives of people with HIV-1 (PWH), transforming it into a manageable chronic condition, treatment is not curative [2,3,4,5]. Consequently, ART-treated PWH experience premature aging and increased risk of developing non-AIDS comorbidities, such as cardiovascular disease (CVD) [6,7,8]. Therefore, new efforts are required to find solutions for the current medical challenges faced by PWH, even in countries where ART is available.
HIV-1 infection provokes profound immunological alterations leading to inadequate mucosal barrier functions. This can result in cells displaying modified immune phenotypes related to inflammation and cell activation [9,10]. For example, the expression of HLA-DR is identified as an indicator of overt immune activation associated with HIV-1 disease progression [11,12], while the expression of CD69, an early T-cell activation marker [13], indicates the ability of cells to reside into tissues [14]. The gut-associated lymphoid tissues (GALTs) represent the primary location for CD4+ T-cell depletion during the acute phase of HIV-1 infection and also constitute an important site for HIV-1 reservoir persistence during the chronic phase [3,4,5,15,16,17]. ART initiation promptly decreases HIV-1 viral loads and results in the normalization of CD4+ T-cell counts in the bloodstream. However, even in cases where viral suppression occurs and circulating CD4+ T-cell counts are restored, PWH continue to exhibit elevated CD8+ T-cell counts, resulting in persistently imbalanced CD4/CD8 ratios, with a low CD4/CD8 ratio predicting the size of HIV reservoirs [18] and morbidity/mortality [19,20]. In addition, the replenishment of the CD4+ T-cell pools within the GALT progresses slowly and is less complete than in the peripheral blood [21]. This inadequate recovery of GALT-infiltrating CD4+ T-cells leads to a breach in the epithelial cell barrier, promoting microbial translocation and triggering immune activation [15,22]. These events, uncontrolled by ART, are documented to fuel a state of chronic inflammation and immune activation facilitating the occurrence of non-AIDS comorbidities in ART-treated PWH, including premature immunological aging and CVD [6,7,8,9,23,24,25]. Of particular relevance, our group demonstrated an increased HIV-DNA reservoir size in ART-PWH with subclinical coronary artery atherosclerotic plaques compared to those lacking atherosclerosis [26]. Furthermore, our group also documented the enriched presence of HIV reservoirs in a subset of CD4+ T-cells with a heart homing phenotype [27], thus establishing a link between the persistence of HIV reservoirs and cardiovascular disease risk.
Effective communication between intestinal epithelial cells (IECs) and immune cells is crucial for maintaining mucosal homeostasis in its capacity to fight pathogens at barrier surfaces [28,29]. Th17-polarized CD4+ T-cells are fundamental for sustaining gut immunity, significantly aiding in maintaining epithelial integrity and functionality [30,31,32,33]. Consequently, the deficit in Th17 cells profoundly alters the GALT barrier functions, leading to a compromised epithelium and chronic systemic inflammation [9,30,31,32,34]. Pioneering studies documented that HIV-1 infection is associated with changes in the expression of the gut-homing molecule integrin (Itg)α4β7 on the peripheral CD4+ T-cells [35,36,37] and that the expression of the Itgα4β7 on blood CD4+ T-cells mirrors events taking place in the gut and predicts the risk of HIV acquisition/disease progression [38]. Since the Itgα4β7+ CD4+ T-cell subset predominantly consists of CCR6+ Th17 cells, their capacity to migrate into the GALT justifies our findings that CCR6+Itgβ7+ CD4+ T-cells are selectively targeted by HIV-1 for infection [30,31,32,35]. Indeed, Itgα4β7 has been reported as a marker of CD4+ T-cells highly permissive to HIV-1 infection [35]. In addition, we demonstrated that retinoic acid promoted Itgβ7 expression in CCR6+ CD4+ T-cells, and increased HIV-1 replication and outgrowth [30,31,32]. More evidence underscores how cellular immune activation is linked to the migration and subsequent depletion of Th17 cells in the gut of PWH via CCR6/CCL20 and CCR9/CCL25-dependent mechanisms [12,15,39,40,41]. In addition to Th17 cells, other T-cell subsets important for the maintenance of gut immunity include the intraepithelial lymphocytes (IELs), a subset marked by the expression of CD103/ItgαE [42,43]. Importantly, IELs are tissue-resident T-cells that usually do not recirculate in the blood [42,44,45,46,47,48], with functional alterations in IELs being reported during HIV-1 infection [49,50].
Considering the large body of evidence suggesting the existence of a gut–heart axis in cardiovascular pathology [51,52], we hypothesized that alterations in the frequency and phenotype of circulating T-cells, as a consequence of an impaired intestinal barrier integrity, contribute to an increased risk of coronary atherosclerosis occurrence in ART-treated PWH. In an effort to identify a novel blood immunological signature indicative of HIV status and CVD risk, in this study, we had access to peripheral blood mononuclear cell (PBMC) samples from ART-treated PWH and HIV-uninfected controls included in the Canadian HIV and Aging Cohort Study (CHACS), with and without subclinical atherosclerosis, determined by Computed Coronary Tomography Angiography (CCTA) measurement of the coronary artery atherosclerotic, as we previously reported [53,54]. Polychromatic flow cytometry analysis was performed using a large panel of T-cell lineage (CD45, CD3, CD4, CD8αα, CD8αβ, TCRαβ, TCRγδ), epithelial cell (EpCAM/CD326), activation (HLA-DR), and gut-homing/residency markers (CD69, CD196/CCR6, CD199/CCR9, CD49d/Itgα4, CD103/ItgαE, Itgβ7). This allowed an in-depth characterization of circulating T-cell subsets differentially expressed in HIV+ vs. HIV− CHACS participants. Multivariate logistic regression analysis explored the relationship between the identified T-cell subsets and the HIV and CVD status, as well as total plaque volume (TPV) values, in crude models and upon adjustment for confounding factors. Together, our findings demonstrate that alterations in circulating T-cell subsets with gut-homing/residency phenotypes correlate with the HIV status and the presence of subclinical atherosclerotic plaque in ART-treated PWH.
2. Materials and Methods
2.1. Study Participants
The CHACS composition has been described in previous publications by our group [53,54,55,56,57]. In summary, the inclusion criteria for the cardiovascular imaging subset of the CHACS cohort required participants to be over 40 years old, have a 10-year CVD risk as determined by the Framingham Risk Score (FRS—which calculates risk based on factors such as age, cholesterol levels, systolic blood pressure, smoking status, and diabetes status) ranging from 5 to 20%, and have no clinical diagnosis of CVD. Participants with renal impairment or a known hypersensitivity to contrast agents were excluded from the study. Here, we used PBMCs from 82 CHACS participants, of whom n = 42 were PWH (HIV+) and n = 40 were HIV-uninfected participants (HIV−). With the exception of two untreated PWH with a history of elite controller (HIV+ 13) and long-term non progressor (HIV+ 40), all the other PWH included in the study received four major ARV classes [i.e., nucleoside reverse transcriptase inhibitor (NRTI), protease inhibitors (PI), non-nucleoside reverse transcriptase inhibitor (NNRTI), integrase strand transfer inhibitor (INSTI)] listed in the File S1 (columns AI-AH). The clinical parameters of the study participants are depicted in Table 1 and Table 2. Further stratification of both groups was conducted based on the presence or absence of subclinical atherosclerotic plaque on CCTA, determined by TPV, measured in cubic millimeters (mm3 equal to or greater than zero), as we previously reported [53,54,55,56,57].

Table 1.
Description of study CHACS participants.

Table 2.
Clinical parameters of ART-treated PWH CHACS participants with and without subclinical atherosclerosis.
2.2. Blood Collection and Phenotypic Analysis
PBMCs were isolated by Ficoll density centrifugation from peripheral blood collected by venipuncture on EDTA Vacutainer tubes. PBMCs were frozen in DMSO 10% FBS until use. Thawed PBMCs were stained with the following fluorochrome-conjugated antibodies: CD3 (AF700), CD45 (APC-H7), CD56 (PerCP-Cy5.5), CD69 (BV711), TCRαβ (BUV563), TCRγδ (BUV395), CD8αβ (RY586), CD8αα (BB515), CD33 (BV480), CD49d/Itgα4 (PE-CF594), Itgβ7 (BUV737), CD103/ItgαE (BUV805), CD196/CCR6 (BUV661), and CD199/CCR9 (APC) (BD Biosciences, Franklin Lakes, NJ, USA); CD4 (BV421), HLA-DR (BV785), CD326/EpCAM (BV650), CD19 (PerCP-Cy5.5) and CD66b (PerCP-Cy5.5; BioLegend, San Diego, CA, USA). The fixable Viability Stain 575V (BV570; BD Biosciences) was used to exclude dead cells from the analysis. Phenotypic analysis was performed by flow cytometry using a FACSymphony™ A5 Cell Analyzer, (BD Biosciences) and FlowJo software, version 10.8.1 (Tree Star, Ashland, OR, USA).
2.3. CellEngine Flow Cytometry Analysis
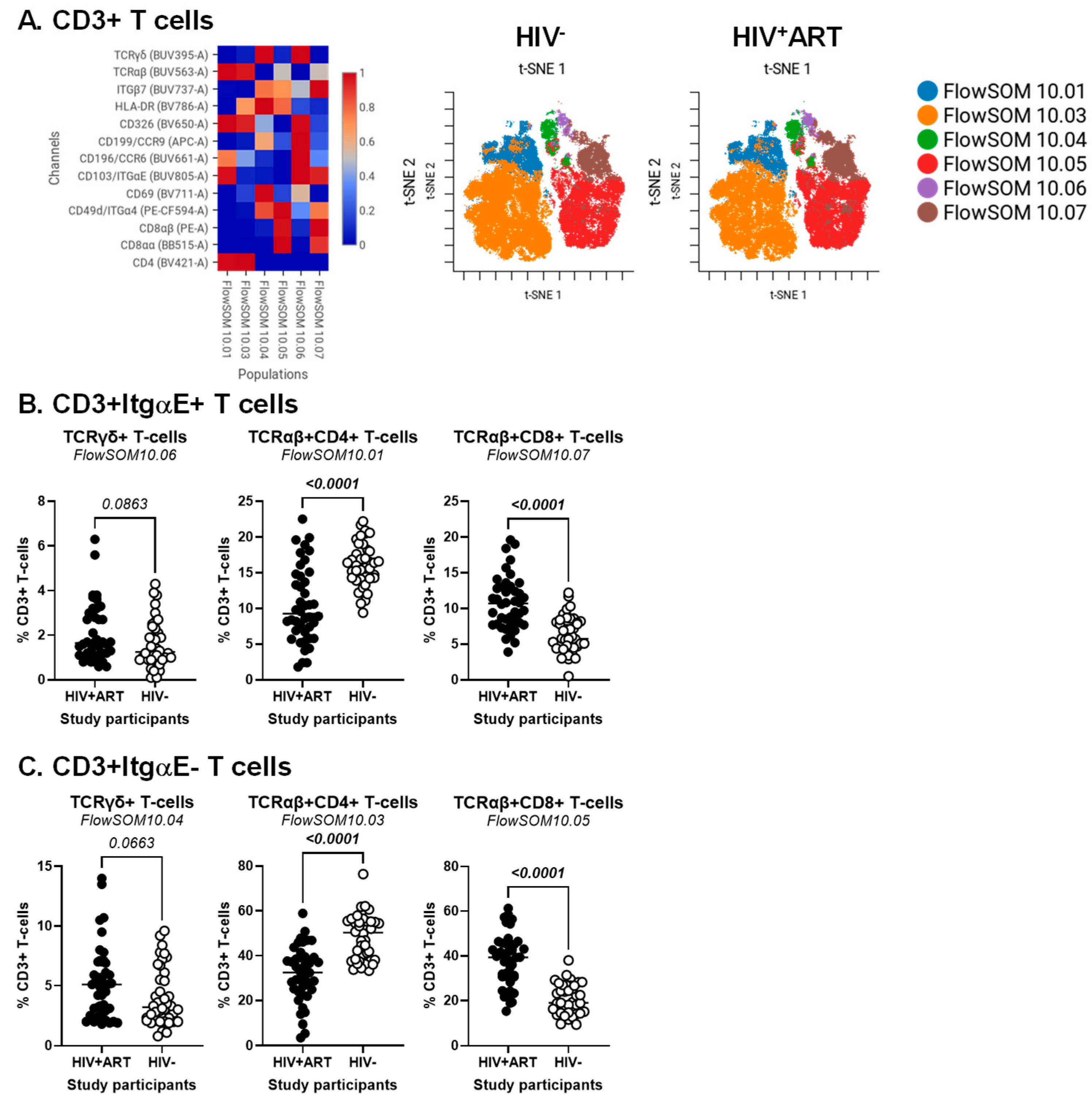
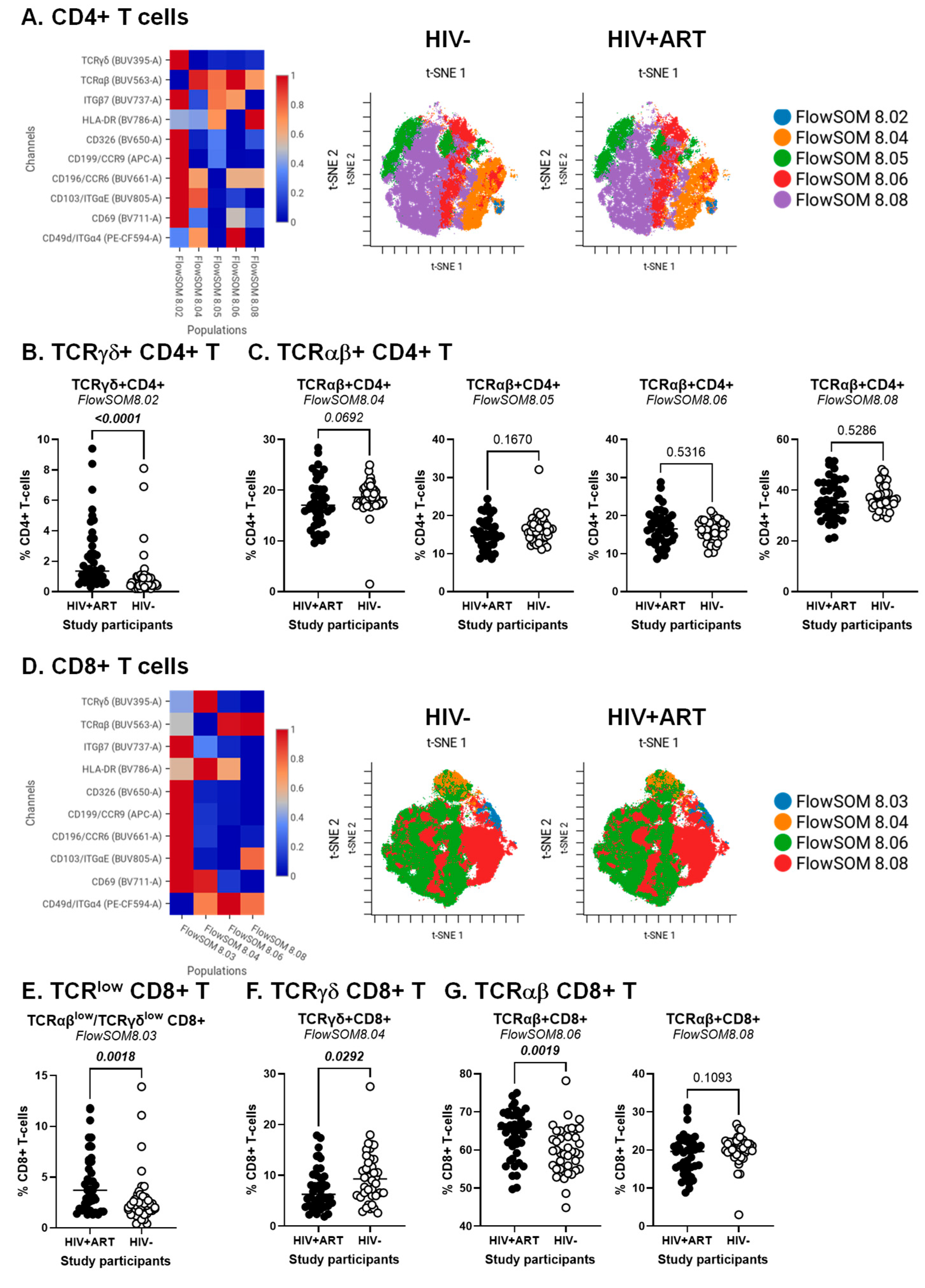
BD Diva FCS files were further exported and analyzed using the CellEngine software (CellCarta, Montreal, QC, Canada). Briefly, the t-Distributed Stochastic Neighbour Embedding (t-SNE) and Self-Organizing Map (SOM) analysis were performed on CD3+ T-cells, CD3+CD4+ T-cells or CD3+CD8+ T-cells gated manually, as illustrated in Figure S1. The following parameters were used for CD3+ T-cells: 25% subsampling, channels (TCRαβ, TCRγδ, CD4, CD8αα, CD8αβ, CD326, HLA-DR, CD69, CD196/CCR6, CD199/CCR9, CD49d/Itgα4, CD103/ItgαE, Itgβ7), t-SNE perplexity = 100, and number of nearest neighbors (k) = 400 (Figure 1). The following parameters were used for CD3+CD4+ T-cells and CD3+CD8+ T-cells: 50% subsampling, channels (TCRαβ, TCRγδ, CD326, HLA-DR, CD69, CD196/CCR6, CD199/CCR9, CD49d/Itgα4, CD103/ItgαE, Itgβ7), t-SNE perplexity = 100, and number of nearest neighbors (k) = 400 (Figure 2). The remaining parameters stayed as default settings and channels were rescaled equally (Figure 1 and Figure 2).
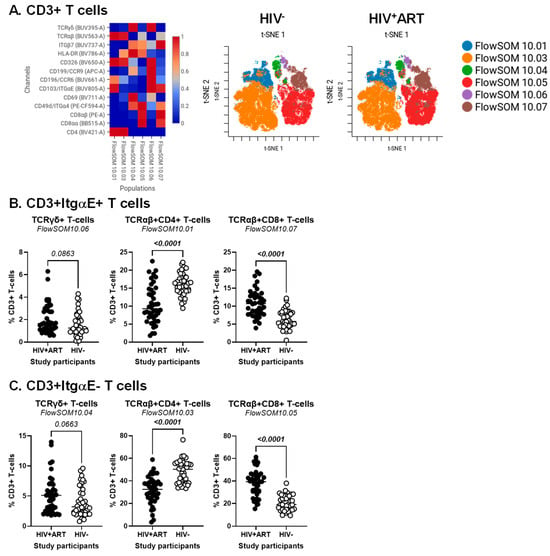
Figure 1.
Identification of CD3+ T-cell subsets differentially expressed in ART-treated PWH vs. HIV-uninfected participants. PBMCs from HIV + ART (n = 42) and HIV− (n = 40) participants were stained with a cocktail of fluorochrome-conjugated Abs for the identification of CD3+ T-cell subsets with differential expression of the T-cell lineage markers CD4, CD8αβ, CD8αα, TCRαβ, and TCRγδ, as well as the activation markers HLA-DR and CD69, the intestinal epithelial cell marker CD326, and the gut-homing chemokine receptors CD196/CCR6 and CD199/CCR9, and adhesion molecules CD49d/Itgα4, Itgβ7, CD103/ItgαE. The Fixable Viability Stain 575V was used to exclude dead cells from the analyses, as depicted in Figure S1. The flow cytometry analysis was down-sampled to 25% and analyzed using CellEngine with a perplexity = 100 and a number of nearest neighbors (k) = 400. Channels were rescaled equally on CellEngine. Shown are heatmaps (left panel) and tSNE plots (middle and right panels) generated from supervised flow cytometry analysis of CD3+ T-cells (A), as well as statistical analysis of the frequency of ItgαE+ (B) and ItgαE− (C) CD3+ T-cell subsets with a TCRγδ+CD4−CD8− (left panel), TCRαβ+CD4+ (middle panel), and TCRαβ+CD8+ T-cells (right panel). (B,C) Mann–Whitney p-values are indicated on the graphs.
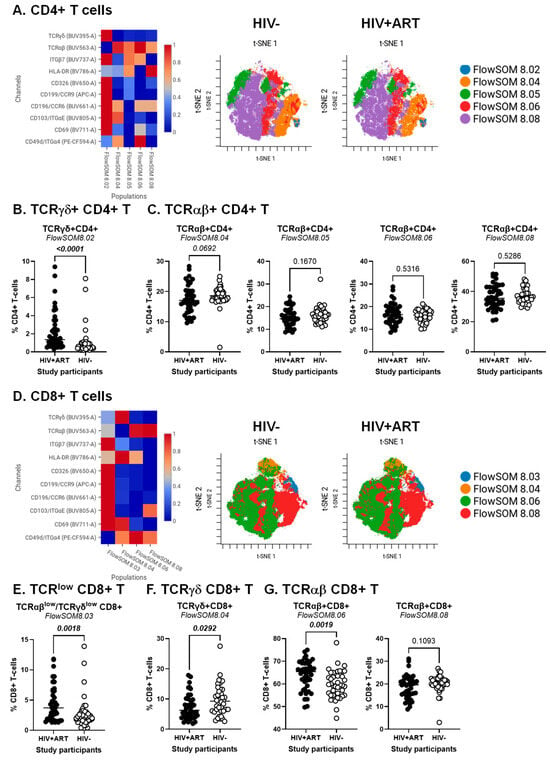
Figure 2.
Identification of CD4+ and CD8+ T-cell subsets differentially expressed in ART-treated PWH vs. HIV-uninfected participants. The flow cytometry staining of PBMCs from HIV + ART (n = 42) and HIV− (n = 40) participants was performed as detailed in the Figure 1 legend. Analysis was down-sampled to 50% and analyzed using CellEngine with a perplexity = 100 and a number of nearest neighbors (k) = 400. Channels were rescaled equally on CellEngine. (A–G) Shown are heatmaps and tSNE plots generated from supervised flow cytometry data analysis of (A–C) CD3+CD4+ T-cells and (D–G) CD3+CD8+ T cells with differential expression of TCRγδ or TCRαβ, together with CD326, CCR6, CCR9, Itgα4, Itgβ7, and/or ItgαE. Shown are statistical analyses of the frequency of CD4+ (B,C) and CD8+ T-cell subsets (E–G) with TCRγδ+ (B,F) TCRγδlowTCRαβlow (E), and TCRαβ+ phenotypes (C,G). Mann–Whitney p-values are indicated on the graphs.
2.4. Statistical Analysis
The Shapiro–Wilk test was employed to evaluate whether continuous variables followed a normal distribution. Normally distributed continuous variables were described using mean and standard deviation (SD), whereas not normally distributed variables were described using median and interquartile range (IQR). The Mann–Whitney test was used for comparing variables not normally distributed using the GraphPad Prism 10.3.0 software (Dotmatics, Boston, MA, USA). Fisher’s Exact Test was used to test if an association existed between two or three categorical variables. To identify novel immunological predictors of the HIV and CVD/TPV status and to determine the effect of potential confounding factors on the association between the circulating cell subsets and the HIV status and the presence or absence of coronary plaque within HIV+ individuals, we used a generalized linear model (GLM) regression as implemented in the R [58] package glmmTMB. For the prediction of HIV status, the modeling was performed using a logistic regression (Table S1). For the prediction of CVD status and TPV values, the modeling was performed in two parts [59]. First, a logistic regression model was used to determine the association between covariates and the presence or absence of coronary plaques (the zero-inflation model) (Table S2). Second, the association between covariates and the extent of plaque volumes among participants with the presence of coronary atherosclerosis was investigated by GLMs regression (the conditional model) (Table S3). Odds ratios (ORs) and regression coefficients with their 95% confidence interval (CI) were given for the first and second part of the modeling, respectively. The confounding effect was investigated by the change in the linear model’s estimate for each subpopulation before and after the adjustment. We compared the crude (univariate) and the multiple regression models to study the contribution of the immune subset alone and in the presence of a sets of potential confounding factors (Tables S1–S3). A change in the estimated association measure of less than 10% identified small confounding bias, while a change superior to 30% identified large confounding bias [60,61]. The p-value adjustment for the multiple testing hypothesis was performed according to the method of Benjamin and Hochberg [62], which controls the false discovery rate with adjusted p-value cutoffs of 0.05. Nominal p-values were adjusted within each T-cell subpopulation with a specific immune phenotype. Confounding modeling analysis with glmmTMB was performed using the statistical package R version 4.4.1.
3. Results
3.1. Clinical and Laboratory Characteristics of the Study Participants
The HIV+ (n = 42) and HIV− (n = 40) participants from the CHACS included in the cardiovascular imaging subgroup were categorized in TPV+ and TPV−, based on the presence or the absence of coronary atherosclerotic plaque. TPV was measured using coronary artery CCTA, as we previously reported [53,55,56,57]. Detailed demographic and clinical information on study participants are included in Table 1. Briefly, HIV+ vs. HIV− participants were similar regarding age (median 54.1 vs. 54.5 years old), male sex (95% vs. 85%), and Framingham risk score (FRS; median 9 vs. 10). HIV+ participants presented lower body mass index (BMI; median 24.1 vs. 26.35 kg/m2; p = 0.0052). Smoking habits tended to be more prevalent among HIV+ compared to HIV− participants, but differences did not reach statistical significance (p = 0.1826). For TPV values, differences did not reach statistical significance between HIV+ and HIV− when all participants where included (p = 0.617). However, among TPV+ participants, TPV values were significantly higher in HIV+ compared to HIV− groups (median 851 vs. 182 mm3; p = 0.0002). The HIV+ and HIV− groups also presented with statistically similar levels of D-dimers (p = 0.8127), fibrinogen (p = 0.5735), HDL (p = 0.1973), and triglycerides (p = 0.2154); however, HIV+ participants had lower levels of LDL than the HIV− group (median 2.55 vs. 3.1 mmol/L; p = 0.0452). The latter observation may be explained by the fact that a higher percentage of HIV+ participants were undergoing statin treatment (26% [11 of 42] compared to 12.5% [5 of 40]; p = 0.0580).
The HIV+ participants grouped based on the presence (TPV+) or the absence (TPV−) of the atherosclerotic plaque were further analyzed for multiple clinical and laboratory parameters included in Table 2. Differences between the TPV+ and TPV− groups did not reach statistical significance for age (p = 0.6573), male sex (95.8% vs. 93.7%; p > 0.9999), BMI; (p = 0.1320), FRS (p = 0.1404), statin treatment (p = 0.7275), plasma levels of D-dimer (p = 0.3029), LDL (p = 0.2972), HDL (p = 0.7596), or triglycerides (p = 0.2033), as well as the CMV co-infection status (p = 0.5227), duration of HIV infection (p = 0.2363), nadir CD4 counts (p = 0.7889), or the frequency of CD4+ T-cells (p = 0.2250). In contrast, the smoking habits tended to be higher (p = 0.0666) among TPV+ in comparison to TPV− ART-treated PWH participants, together with plasma fibrinogen levels (p = 0.0827) and the duration of ART (p = 0.0706).
3.2. Immune Profile Alterations Indicative of HIV and Subclinical CVD Status
Here, we sought to determine whether changes in the frequency and activation status of specific T-cell subsets with a gut-homing and/or gut-residency phenotype in the peripheral blood can be linked to the HIV status and the CVD risk in ART-treated PWH. To this aim, PBMCs from HIV+ and HIV− CHACS participants, with (TPV+) and without (TPV−) subclinical atherosclerosis, were analyzed for the composition of the pool of hematopoietic CD45+CD3+ T-cells (Figure S1). Among CD45+ cells, T-cells lacking the lineage markers CD19 (B cells), CD56 (NK cells), CD66b (neutrophils), and CD33 (myeloid cells), but expressing CD3 (T-cells) were further analyzed for the expression of CD4 (CD4+ T-cells) and CD8αβ (CD8+ T-cells) (Figure S1). As expected, our results showed statistically significant decreased and increased numbers of CD4+ T-cells and CD8+ T-cells, respectively, in HIV+ compared to HIV− participants, as well as decreased CD4/CD8 ratios (Figure S2A). Aiming to investigate if there was a relationship between these parameters and the presence of plaque, we found that CD4+ and CD8+ T-cell numbers, as well as CD4/CD8 ratios, were similar regardless of the presence of plaque (Figure S2B). For the HIV− group, the CD4/CD8 ratios correlated positively with age and TPV values (Figure S2C,D, right panels). These correlations were not observed in the HIV+ group (Figure S2C,D, left panels), indicative of an increased CVD risk occurring regardless of aging in the context of HIV infection.
3.3. CellEngine Identification of CD4+ and CD8+ T-Cell Subsets with Gut-Homing Phenotype and Altered Frequency and Activation Status During ART-Treated HIV Infection
Our group previously reported alterations in CD4+ T-cell phenotype and functions to be associated with the CVD status in ART-treated PWH [27,54,63]. To identify novel immunological correlates of HIV status and CVD risk, CellEngine analysis of polychromatic flow cytometry data was performed, upon staining with a set of T-cell lineage (CD45, CD3, CD4, CD8αα, CD8αβ, TCRαβ, TCRγδ), activation (HLA-DR), epithelial cell (EpCAM/CD326), and gut-homing/residency markers (CD69, CD196/CCR6, CD199/CCR9, CD49d/Itgα4, CD103/ItgαE, Itgβ7). The analysis of CD3+ T-cells revealed the presence of six major subsets, with differential expression of the T-cell lineage markers TCRαβ, TCRγδ, CD4, CD8αα, and/or CD8αβ, as well as the intraepithelial residency marker ItgαE [64], as follows: FlowSOM10.01 (TCRαβ+CD4+ItgαE+), FlowSOM10.03 (TCRαβ+CD4+ItgαE−), FlowSOM10.04 (TCRγδ+CD4−CD8−ItgαE−), FlowSOM10.06 (TCRγδ+CD4−CD8−ItgαE+), FlowSOM10.05 (TCRαβ+CD8+ItgαE−), and FlowSOM10.07 (TCRαβ+CD8+ItgαE+) (Figure 1A, left heatmap). Of note, there was preferential expression of Itgβ7 on TCRγδ+ (FlowSOM10.04 and 10.06) and TCRαβ+CD8+ T-cells (FlowSOM10.05 and 10.07), CD326 on TCRαβ+CD4+ T-cells (FlowSOM10.01 and 10.03), CCR6 on CD4+ (FlowSOM10.01) and CD8+ (FlowSOM10.06) T-cell subsets, while CD69 and CCR9 were expressed at the highest levels on TCRγδ+ (FlowSOM10.04 and 10.06) T-cells (Figure 1A, left heatmap).
The frequency of these six clustered varied between HIV+ and HIV− groups (Figure 1A, right t-SNE SOMs; Figure 1B,C). There was only a tendency for increased frequency of TCRγδ+ T-cell subsets expressing or not ItgαE (FlowSOM10.04 and 10.06) in HIV+ vs. HIV− groups (Figure 1B,C, left panels). In contrast, differences reached statistical significance for decreased TCRαβ+CD4+ (FlowSOM10.01 and 10.03) (Figure 1B,C, middle panels) and increased TCRαβ+CD8+ (FlowSOM10.05 and 10.07) T-cell subsets (Figure 1B,C, right panels) in HIV+ vs. HIV− groups.
The CellEngine analysis was further performed on CD4+ and CD8+ T-cells, manually gated as illustrated in Figure S1. The results depicted in Figure 2 reveal subtle T-cell subsets that varied in frequency and homing/activation phenotype between HIV+ and HIV− groups for TCRγδ+ and TCRαβ+CD4+ (Figure 2A–C) and TCRαβ+CD8+ T-cells (Figure 2D–F). The highest expression of the gut/homing/residency markers Itgβ7, CD326, CD69, CCR6, CCR9 and ItgαE was observed on subsets of TCRγδ+CD4+ (FlowSOM8.02; Figure 2A) and CD8+ T-cells with relatively low TCRαβ and TCRγδ expression (FlowSOM8.03; Figure 2D), with their frequency being both increased in HIV+ vs. HIV− participants (Figure 2B,E). There was only a tendency for reduced frequency of TCRαβ+CD4+ T-cells (FlowSOM8.04; Figure 2C, left panel). Other significant differences were observed for TCRγδ+CD8+ (FlowSOM8.03) and TCRαβ+CD8+ T-cells (FlowSOM8.06), with their frequency being decreased and increased, respectively, in HIV+ vs. HIV− participants (Figure 2F,G, left panel).
In addition to the expected depletion of TCRαβ+CD4+ T-cells and expansion of TCRαβ+CD8+ T-cells in the peripheral blood of PWH, these analyses reveal an altered composition of these circulating T-cell pools during ART-treated HIV-infection, with the abundance of subsets with a gut-homing/residency profile in HIV+ vs. HIV− participants. Such an accumulation may reflect the aberrant mobilization of key sentinel cells from the gut into the periphery, likely as a consequence of gut barrier impairment.
3.4. Increased Frequencies of CD4+ T-Cells with a Gut-Homing/Residency CD326+CD69+CCR6+ Phenotype in HIV+ vs. HIV− Participants
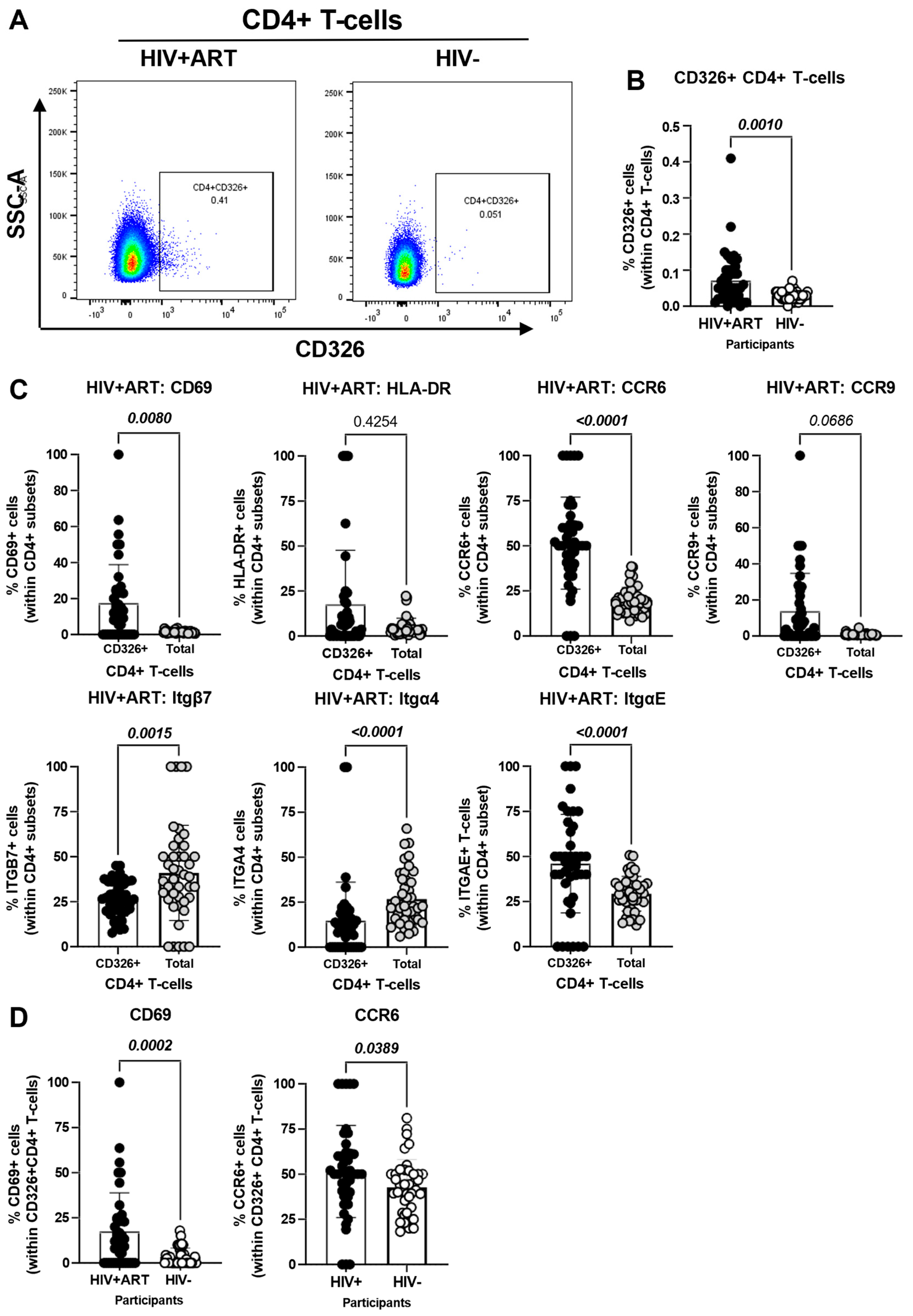
The CellEngine flow cytometry analysis (Figure 1A and Figure 2A) brought our attention to the presence of a fraction of TCRαβ+ CD4+ T-cells expressing the epithelial cell marker EpCAM/CD326 [65,66,67] and the gut-homing marker CCR6 (enriched in FlowSOM8.08, Figure 2A). This presence was further confirmed by manual gating in Figure 3A, with CD326+CD4+ T-cells being more abundant in HIV+ compared to HIV− participants (p = 0.001) (Figure 3B). Compared to total CD4+ T-cells, the CD326+CD4+ T-cell subset of HIV+ participants distinguished from total CD4+ T-cells by a statistically significant increased expression of CD69 (p = 0.008), CCR6 (p < 0.0001) and ItgαE (p < 0.0001), a tendency for increased CCR9 expression (p = 0.0686), and a decreased expression of Itgβ7 (p = 0.0015) and Itgα4 (p < 0.0001), with no significant differences for HLA-DR (Figure 3C). Of particular interest, CD326+CD4+ T-cells of HIV+ vs. HIV− participants expressed at higher frequency CD69 (p = 0.0002) and CCR6 (p = 0.0389) (Figure 3D). These results point to the enriched presence in the circulation of HIV+ vs. HIV− participants of an atypical subset of TCRαβ+CD4+ T-cells expressing the epithelial marker CD326 and overexpressing the gut homing/residency markers CD69 and CCR6.
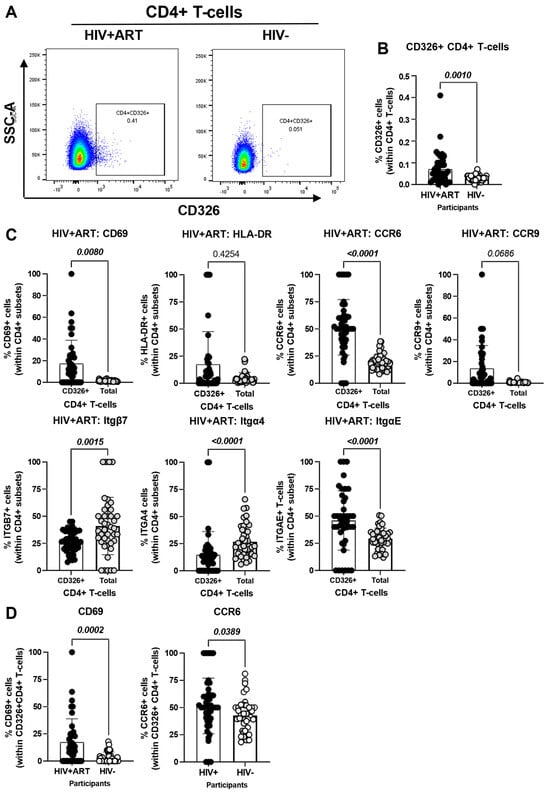
Figure 3.
Frequency and phenotype of peripheral blood CD326+ CD4+ T-cells in HIV+ vs. HIV− participants. The flow cytometry staining of PBMCs from HIV + ART (n = 42) and HIV− (n = 40) participants was performed as detailed in the Figure 1 legend. The flow cytometry analysis was performed by manual gating using the Flow Jo software, as depicted in Figure S1. Shown are the representative gating strategy for the identification of CD326+ CD4+ T-cells (A) and statistical analysis of their frequency (B) in HIV + ART vs. HIV− participants; and (C) the expression of activation stained with a cocktail of molecules (CD69 and HLA-DR), and gut-homing chemokine receptors (CCR6 and CCR9) and integrins (Itgβ7, Itgβ4 and ItgαE) on CD326+ CD4+ T-cells of HIV+ participants, as well as differences in CD69 and CCR6 expression between CD326+ CD4+ T-cells of HIV+ vs. HIV− participants (D). Mann–Whitney p-values are indicated on the graphs.
3.5. Increased Frequencies of CCR6+ItgB7− CD4+ T-Cells in the Peripheral Blood of HIV+ vs. HIV− Participants
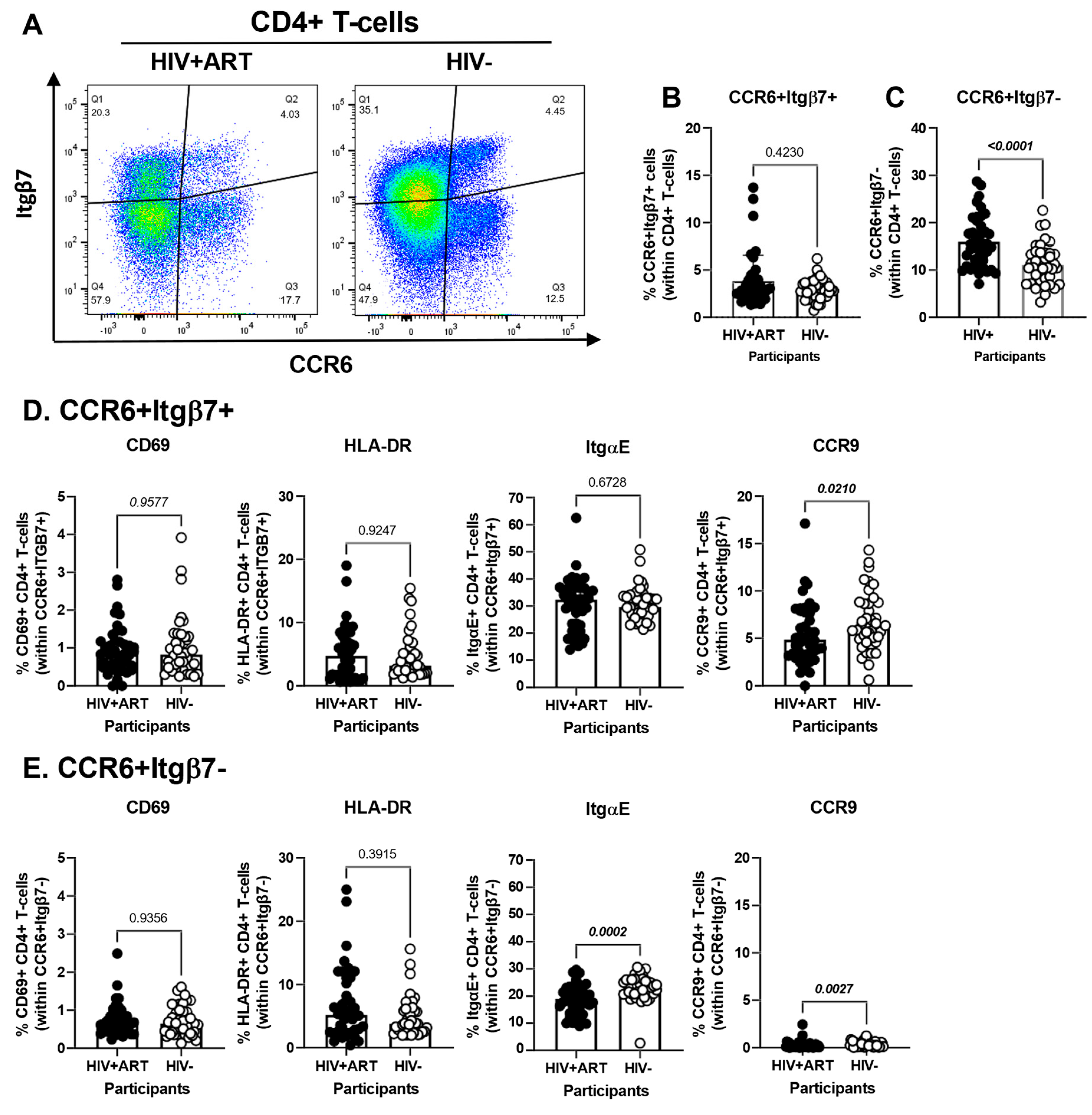
The CellEngine flow cytometry analysis pointed to the presence of four TCRαβ+CD4+ T-cells subsets with differential expression of the gut-homing markers CCR6 and Itgβ7 (Figure 2A). We further performed manual gating on FlowJo to investigate the frequency and phenotype (i.e., CD69, HLA-DR, ItgαE, CCR9) of CD4+ T-cells expressing or not CCR6 and/or Itgβ7 (Figure 4). We found no significant differences in the frequency of CCR6+Itgβ7+ CD4+ T-cells (enriched in FlowSOM8.06, Figure 2A) in HIV+ compared to HIV− participants (Figure 4A,B) and no significant differences in the expression of CD69, HLA-DR, and ItgαE on CCR6+Itgβ7+ CD4+ T-cells between HIV+ and HIV− participants, with CCR9 expression being slightly lower in HIV+ participants (Figure 4D). Contrariwise, the frequency of CCR6+Itgβ7− CD4+ T-cells (enriched in FlowSOM8.08, Figure 2A) was higher in HIV+ compared to HIV− participants (Figure 4A,C), with the expression of CD69 and HLA-DR being equivalent in both groups, and the ItgαE and CCR9 expression levels being slightly lower in HIV+ vs. HIV− participants (Figure 4D). Of note, the expression of ItgαE and CCR9 was markedly lower in CCR6+ItgB7− compared to CCR6+ItgB7+ CD4+ T-cells, indicative of an altered gut-homing potential of these cells in ART-treated PWH.
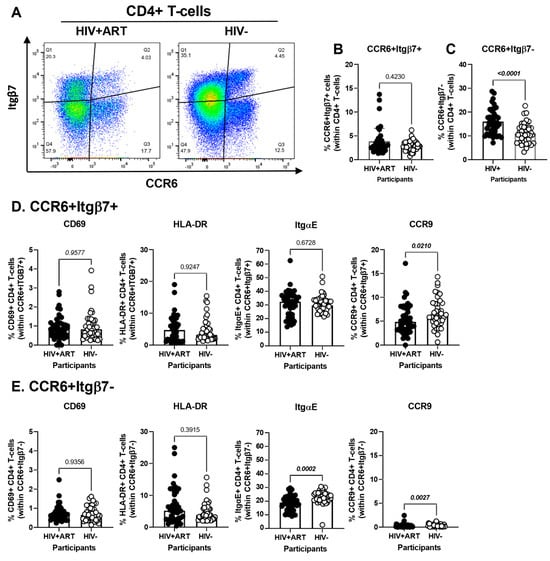
Figure 4.
Frequency and phenotype of peripheral blood CD4+ T-cells expressing CCR6 and/or Itgβ7 in ART-treated PWH and HIV-uninfected participants. (A–E) PBMCs from HIV+ART (n = 42) and HIV− (n = 40) participants were stained with a cocktail of fluorochrome-conjugated Abs, as detailed in Figure 1 legend. Cell frequencies and phenotypes were analyzed by flow cytometry manual gating (BD Fortessa and FlowJo), with CD4+ T-cells identified as in Table S1. Shown is the gating strategy for the identification of CD4+ T-cells expressing CCR6 and/or Itgβ7 (A), the frequencies of CD4+ T-cells with a CCR6+Itgβ7+ (B) and CCR6+Itgβ7− (C) phenotypes in HIV + ART vs. HIV− participants; and the characterization of the expression of CD69, HLA-DR, ItgαE, and CCR9, on circulating CCR6+Itgβ7+ (D) and CCR6+Itgβ7− (E) CD4+ T-cells in HIV + ART vs. HIV− participants. (B–E) Mann–Whitney p-values are indicated on the graphs.
3.6. Decreased Frequencies and Gut-Homing Potential of CD8+ IEL-like T-Cells in the Peripheral Blood of HIV+ vs. HIV− Participants
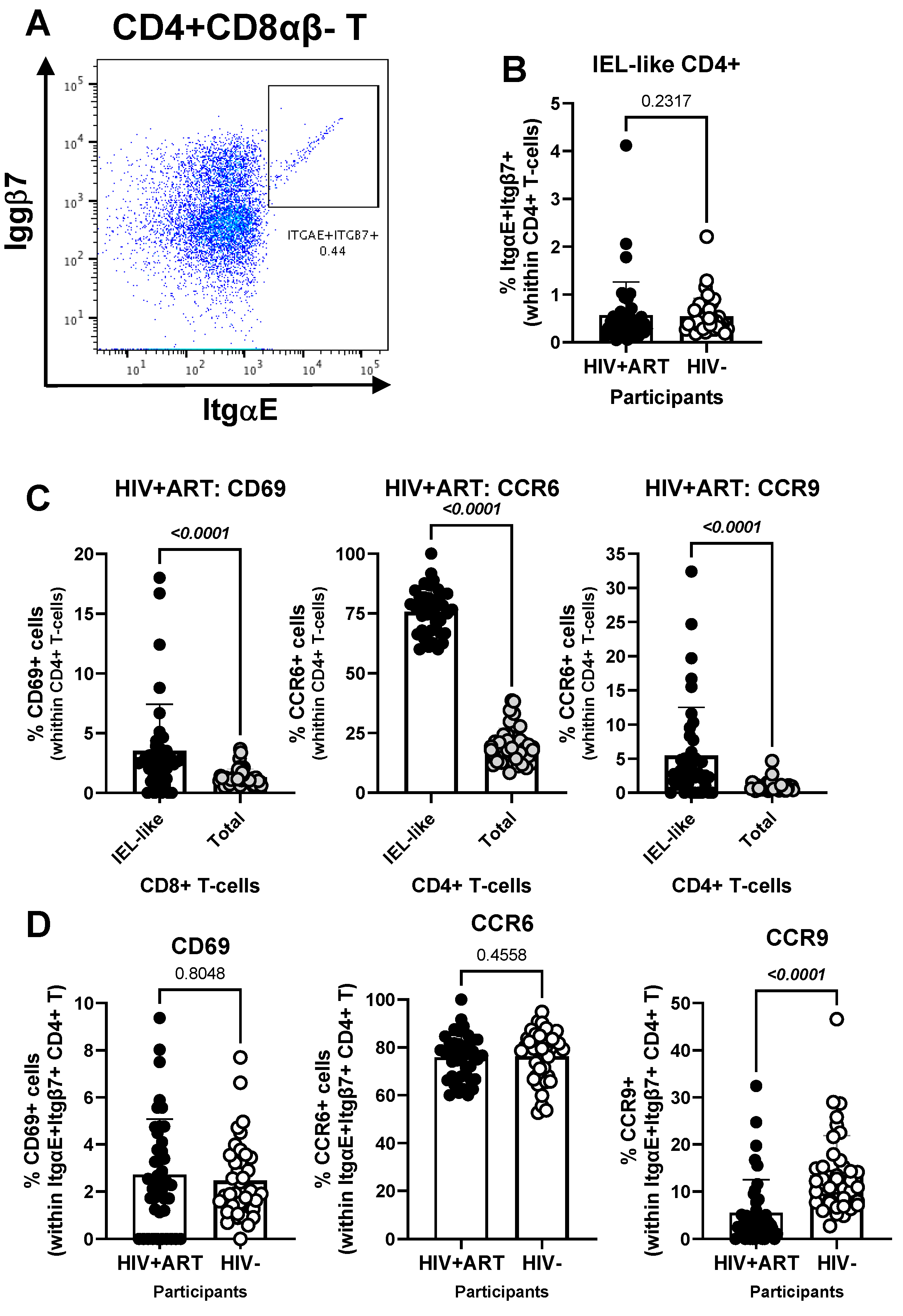
Another element of our hypothesis of observing gut immune alterations in peripheral blood was related to the presence of circulating IELs. Using well-established markers in the literature [42,44,45,46,47,48], we used manual gating on FlowJo to identify subsets of circulating CD4+ (CD3+CD4+Itgβ7+ItgαE+TCRαβ+TCRγδ−CD8αβ−) and CD8+ (CD3+CD4−Itgβ7+ItgαE+TCRαβ+TCRγδ−CD8αα+CD8αβ−) with IEL-like phenotypes (Figure 5 and Figure 6). We observed that CD4+ IEL-like T-cells (Figure 5A) did not differ in frequency between HIV+ and HIV− groups (Figure 5B), but distinguished from their total CD4+ T-cell counterparts by superior expression of CD69 (p < 0.0001), CCR6 (p < 0.0001) and CCR9 (p < 0.0001), with CCR6 being expressed on the majority of CD4+ IEL-like T-cells (Figure 5C), indicative of their imprinting for gut-homing. Of note, CD4+ IEL-like T-cells from HIV+ vs. HIV− groups expressed similar levels of CD69 and CCR6 but lower CCR9 expression (Figure 5D), indicative of a deficit in their CCR9-mediated homing.
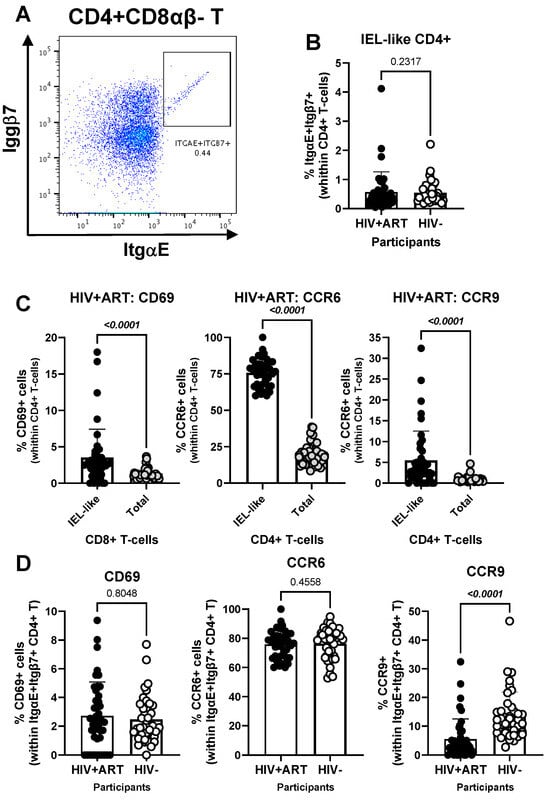
Figure 5.
Frequency and phenotype of peripheral blood IEL-like CD4+ T-cells in ART-treated PWH vs. HIV− participants. PBMCs from HIV + ART (n = 42) and HIV− (n = 40) participants were stained with a cocktail of fluorochrome-conjugated Abs, as in the Figure 1 legend. The Fixable Viability Dye 575V was used to exclude dead cells from the analyses. Cell frequencies and phenotypes were analyzed by flow cytometry (BD Fortessa and FlowJo). The flow cytometry analysis was performed by manual gating, as depicted in Figure S1. Shown is the gating strategy for the identification of CD4+ T-cells (CD45+CD3+CD4+TCRαβ+) with an IEL-like phenotype (Itgβ7+CD103/ItgαE+) (A), the analysis of their frequency in HIV+ vs. HIV− participants (B); as well as the expression of CD69, CCR6 and CCR9 on IEL-like vs. total CD4+ T-cells (C), and differences in the phenotype of IEL-like CD4+ T-cells between HIV+ART and HIV− participants (D). Mann–Whitney p-values are indicated on the graphs (B–D).
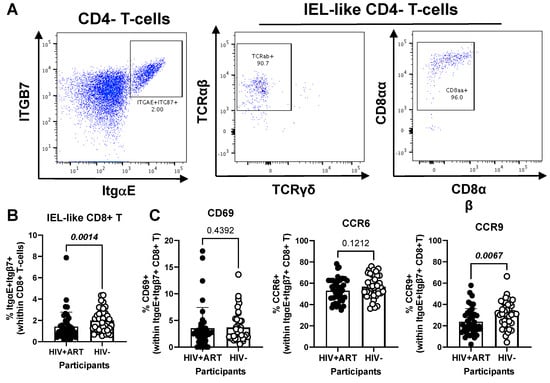
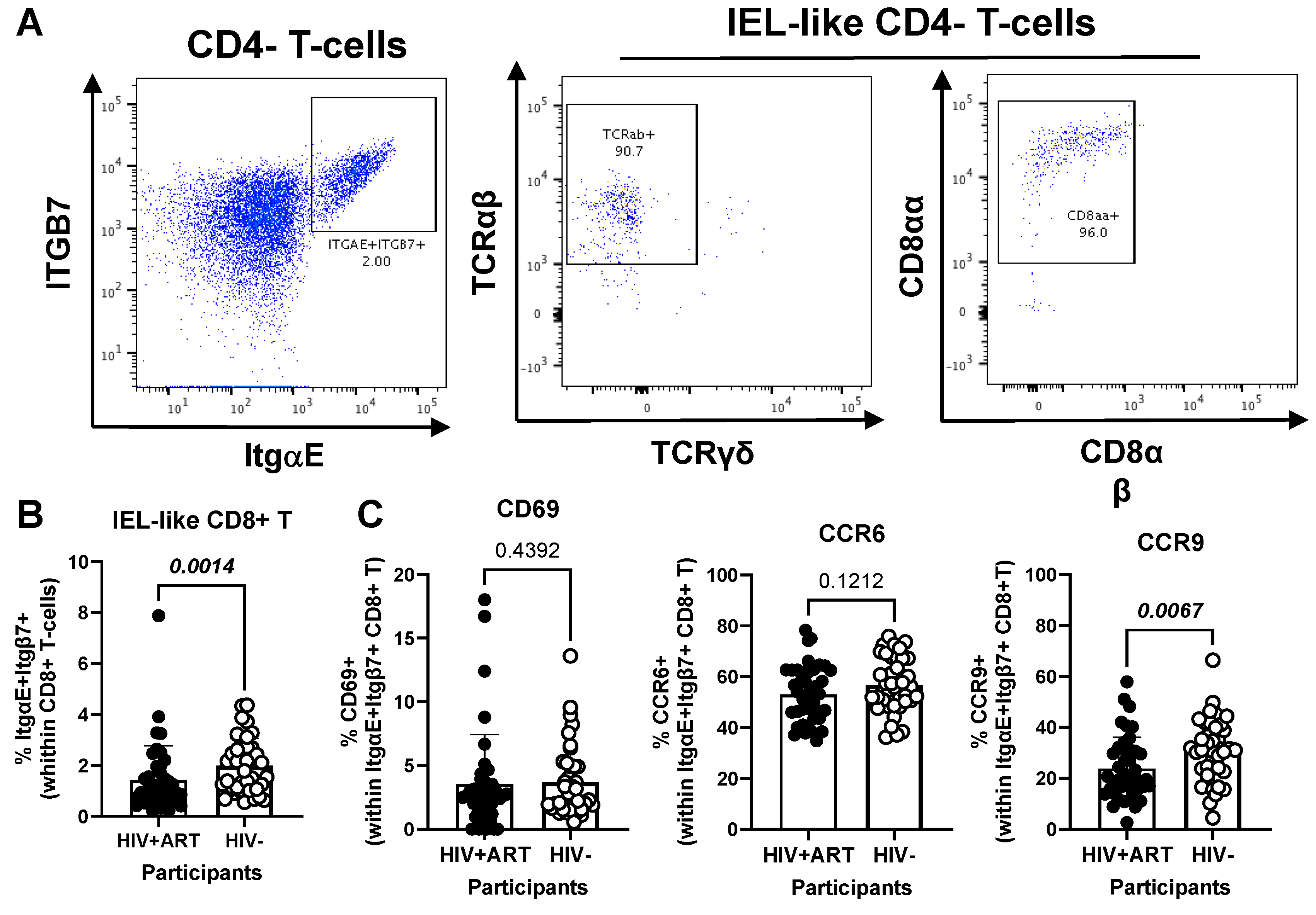
Figure 6.
Frequency and phenotype of peripheral blood CD8+ IEL-like T-cells in HIV+ vs. HIV− participants. (A–C) PBMCs from HIV + ART (n = 42) and HIV− (n = 40) participants were stained with a cocktail of fluorochrome-conjugated Abs, as detailed in the Figure 1 legend, to allow the identification of CD8+ T-cells (CD45+CD3+CD4−TCRαβ+) with an IEL-like phenotype (Itgβ7+CD103/ItgαE+CD8αα+) and the analysis of their CD69, CD196/CCR6, and CD199/CCR9 expression. Shown are representative dot plots for the identification of Itgβ7+CD103/ItgαE+ CD4− T-cells (left panel) and their co-expression of TCRαβ/TCRαα (middle panel) and CD8αα/CD8αβ (right panel) (A) and the statistical analysis of the frequency of circulating IEL-like CD8+ T-cells in HIV+ vs. HIV− participants (B); as well as the expression of CD69, CCR6 and CCR9 on IEL-like CD8+ T-cells from HIV+ vs. HIV− participants (C). Mann–Whitney p-values are indicated on the graphs (B,C).
Further, our results show a decreased frequency of CD8+ IEL-like T-cells (Figure 6A) in HIV+ compared to HIV− participants (p = 0.0014) (Figure 6B), together with similar levels of CD69 and CCR6, but decreased CCR9 expression (p = 0.0067) in cells from HIV+ vs. HIV− groups (Figure 6C). Together these results reveal the presence of circulating TCRαβ+CD4+ and TCRαβ+CD8+ T-cell subsets with IEL-like features, with CD8+ IEL-like cells being decreased in frequency and exhibiting a deficient CCR9-mediated gut-homing potential in association with the HIV status.
3.7. Multivariate Logistic Regression Analyses Identify Immune Subpopulations That Predict the HIV Status and the CVD Risk in ART-Treated PWH
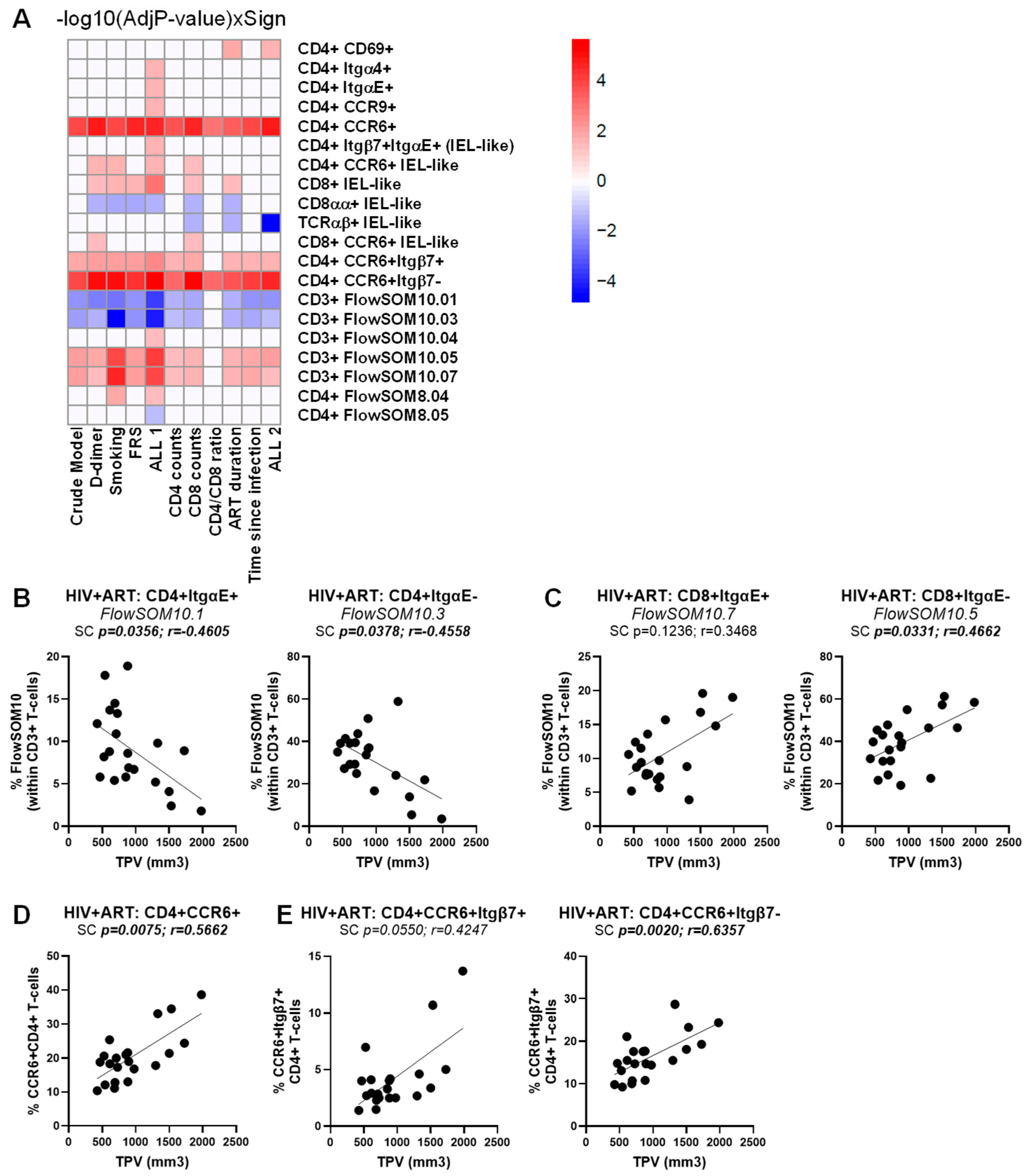
To identify novel immunological predictors the HIV status and CVD risk, we performed a multivariate logistic regression analysis considering all studied immunological subpopulations and the clinical information listed in File S1, with statistical results detailed in Tables S1–S3. To identify predictors of the HIV status, the basic logistic regression model, which only included the immune subsets (crude model), was evaluated against multivariate logistic regression models that adjusted for different potential confounding factors linked to cardiovascular health (i.e., BMI, CVD status, LDL) and HIV status (i.e., CD4 counts, CD8 counts, CD4/CD8 ratios, time on ART, time since HIV diagnosis), separately or all confounding factors together (ALL1 and ALL 2), as detailed in Table S1. Across all models, the frequency of the following TCRαβ+CD3+ T-cell subpopulations was positively associated with the HIV status CD326+CD4+, CD326+CD69+CD4+, CCR6+Itgβ7−CD4+, CD8+FlowSOM8.03 and CD8+FlowSOM10.07 T-cells, while the CD4+CCR9+ IEL-like, CD4+ItgαE+FlowSOM10.01, and CD4+ItgαE-FlowSOM10.03 were identified as negative predictors (Table S1, Figure S3). Other subpopulations significant in the crude model but that lost statistical significance with the HIV status when adjustments were made for HIV-specific cofounding factors included TCRγδ+FlowSOM10.05 and TCRαβ+CD8+FlowSOM08.06 (Figure S3).
A similar multivariate logistic regression analysis failed to identify T-cell subpopulations associated with CVD in the HIV+ group, in the crude model or upon univariate adjustment for D-dimer, smoking status, and FRS, as well as for HIV-specific confounding factors (i.e., CD4 counts, CD8 counts, CD4/CD8 ratios, time on ART, and time since HIV diagnosis) (Table S2). The only exception was for CD326+CD69+ CD4+ T cells that were identified as a weak positive predictor of the CVD status, with marginal significance reached in the crude model only (p = 0.04711) (Table S2). Nevertheless, when a similar strategy was used to identify immunological predictors of the TPV values in the ART-treated PWH group (Table S3), multiple TCRαβ+CD3+ T-cell subpopulations were identified as positive and negative correlates (Figure 7A). In particular, CD4+ItgαE+FlowSOM10.01 and CD4+ItgαE−FlowSOM10.03 were negatively correlated with the TPV values (Figure 7B), while CD8+ItgαE−FlowSOM10.05 (Figure 7C), together with CCR6+CD4+ and CCR6+Itgβ7−CD4+, and to a lower extent CCR6+Itgβ7+CD4+ T-cells (Figure 7D), were identified as positive correlates in the crude model and upon adjustment to all or the majority of CVD- and HIV-specific confounding factors (Figure 7A, Table S3).
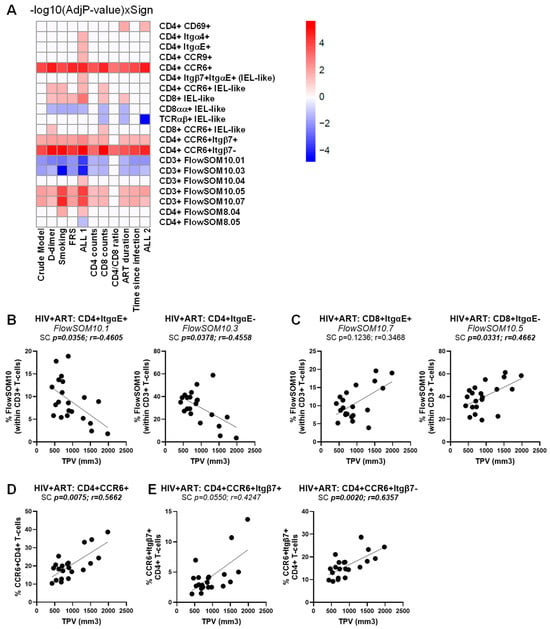
Figure 7.
Multivariate regression model identified novel immunological predictors of subclinical atherosclerosis in ART-treated PWH. The heatmap illustrates the subsets of T-cells that were identified in Table S3 as a significant predictor of TPV values in the crude model or upon adjusting for D-dimer, smoking status, FRS, and ALL 1 (D-dimer, smoking status, FRS), as well as CD4 counts, CD8 counts, CD4/CD8 ratios, time on ART, time since HIV diagnosis, and ALL 2 (CD4 counts, CD8 counts, CD4/CD8 ratios, time on ART, time since HIV diagnosis). Adjusted p-values (−log10) are indicated as a red and blue gradient for positively (Sign = 1) and negatively associated predictors (Sign = −1), respectively. (A). Shown are individual illustrations of the correlations TPV values and the frequency of CD4+ (B) and CD8+ T-cells (C) with an ItgαE+ (left panels) or ItgαE− phenotypes (right panels), as well as CD4+ T-cells with a CCR6+ phenotype (D) expressing or not Itgβ7 (E). Spearman correlation p and r values are indicated on the graphs (B–D).
Together, these results point to major changes in the frequency, activation status, and gut-homing/residency potential of specific CD4+ and CD8+ T-cell subsets circulating in the peripheral blood that robustly predict the HIV status and the presence of the subclinical atherosclerotic plaque. Such peculiar T-cell subsets may represent new peripheral blood markers for evaluating and therapeutically managing the CVD risk in ART-treated PWH.
4. Discussion
In this study, we identified alterations in the composition of the pool of circulating CD3+ T-cells expressing gut-homing/residency markers as novel predictors of HIV-associated coronary atherosclerosis among ART-treated CHACS participants. This study was inspired by the concept of liquid biopsy used in oncology [68,69,70] and the evidence that inflammatory conditions in the intestines are associated with the unusual circulation in the blood of intestinal epithelial cells and other tissue-resident immune cells [71,72,73]. Our major findings point to (i) aberrant circulation of CD326+CD4+ T-cells, TCRγδ+, and TCRαβ+ cells with IEL-like phenotypes in the peripheral blood of HIV+ vs. HIV− participants and (ii) a positive association between TPV and the abundance of CCR6+CD4+ T-cells expressing or not the gut-homing marker Itgβ7, subsets, previously identified by our group as being highly permissive to HIV infection [74]. These results are consistent with intestinal barrier alterations persisting during HIV-1 infection [15,16,30,31,32] and a documented link between HIV reservoir size in CD4+ T-cells and subclinical CVD [26].
PBMCs from n = 82 participants of the CHACS were analyzed, including 42 ART-treated PWH and 40 individuals without HIV. Both groups were further categorized based on the presence/absence of coronary atherosclerotic plaque. HIV+ individuals in the TPV+ group showed an increased burden of coronary atherosclerosis compared to HIV−TPV+ participants, consistent with previous research by our group [54,57]. The analysis of the HIV+ group revealed that TPV+ compared to TPV− participants had similar FRS and plasma D-dimer levels, two documented predictors of cardiovascular risk [75,76]. The TPV+ and TPV− HIV+ participants also showed a similar time since HIV infection, duration of ART, nadir CD4 counts, and current CD4 percentages. Interestingly, age and TPV correlated with the CD4/CD8 ratio in HIV− but not in HIV+ individuals, showing opposite trends between the two groups. These clinical similarities between TPV+ and TPV− HIV+ participants prompted us to search for new immunological correlates of the subclinical atherosclerosis in ART-treated PWH. Thus, we performed polychromatic flow cytometry analysis using a large panel of T-cell lineage (CD45, CD3, CD4, CD8αα, CD8αβ, TCRαβ, TCRγδ), epithelial cell (EpCAM/CD326), activation (HLA-DR), and gut-homing/residency markers (CD69, CD196/CCR6, CD199/CCR9, CD49d/Itgα4, CD103/ItgαE, Itgβ7). This allowed an in-depth characterization of circulating T-cell subsets differentially expressed in CHACS participants with known HIV and TPV status.
A noteworthy finding of our study was the increased abundance of a subset of TCRαβ+CD4+ T-cells expressing the epithelial cell adhesion molecule (EpCAM)/CD326 in HIV+ compared to HIV− participants. CD326 plays a crucial role in establishing a functional intestinal barrier [66,67,77], is expressed on sigmoid colon epithelial cells [78], as well as circulating tumor epithelial cells used as markers in the context of liquid biopsies [67,68,79]. CD4+ T-cells might acquire CD326 expression upon interaction with epithelial cells through trogocytosis, a process that involves the transfer of surface molecules and membrane fragments from one cell to another, thereby granting recipient cells new functionalities or the ability to eliminate donor cells [80,81]. Such a mechanism has been reported in the context of HIV-1 infection [82] and may reflect the history of cell-to-cell interactions in vivo. Nevertheless, the intrinsic expression of CD326 mRNA in T-cells requires further evaluation at transcriptional level.
Compared to total CD4+ T-cells, CD326+CD4+ T-cells expressed a CD69+ItgαE+CCR6+CCR9+ phenotype. CD69 is an early T-cell activation marker and a promotor of T-cell retention in tissues [13], with its expression being associated with T-cell activation in HIV-1 infection [83,84,85]. By comparing the phenotype of the CD326+CD4+ T-cells to total CD4+ T-cells within HIV+ participants, we observed increased expression of CD103/ItgαE, a hallmark of gut residency, considering the interaction between CD103/ItgαE on T-cells and E-cadherin on intestinal epithelial cells [42,86]. Moreover, we have also observed increased CCR6 expression in CD326+CD4+ T-cells. Previous research by our group and others indicated that CCR6-expressing T-cells are more susceptible to HIV-1 and harbor higher amounts of integrated HIV-DNA in ART-treated PHW [30,31,32]. Furthermore, CD326+CD4+ compared to total CD4+ T-cells from HIV+ individuals were characterized by heightened expression of CCR9, with the CCR9/CCL25 axis being key for gut-homing, a process altered during HIV infection [16,39,87]. Of note, CCR9 is associated with inflammatory conditions and its knockdown can reduce CVD progression [88]. This is in line with the aberrant accumulation of CCR9+ T-cells in the blood of ART-treated PWH, as a consequence of their impaired recruitment into the gut due to a deficit of mucosal CCL25 expression [39]. All together, these results support the idea that the presence of CD326+CD4+ T-cells in the circulation may reflect alterations in the intestinal barrier integrity during HIV disease progression.
Both CD4+ and CD8+ T-cells expressing CD103/ItgαE and CD69 are affected during HIV-1 infection [16,50,86,87]. Of note, CellEngine analysis also depicted high CD326 expression on TCRγδ+CD4−CD8−ItgαE+ (FlowSOM10.06) and TCRαβlowTCRγδlowCD4−CD8−ItgαE+ (FlowSOM8.03) T-cells, subsets that also showed increased abundance in HIV+ vs. HIV− participants. Of note, unconventional T-cells that co-expressed TCRαβ and TCRγδ have been described before [89,90]. Like Th17 cells, TCRγδ+ T-cells are crucial for maintaining the gut barrier integrity [47] and are significantly perturbed during HIV-1 infection, even during effective ART [91,92,93]. The aberrant presence of different CD326+ T-cell subsets in the circulation may reflect the state intestinal barrier impairment, well documented in the context of ART-treated HIV infection and associated with the CVD risk.
Another interesting finding of our study was the increased frequency of CCR6+Itgβ7− CD4+ T-cells in HIV+ vs. HIV− individuals, without significant changes on the frequency of CCR6+Itgβ7+CD4+T-cells. Of note, CCR6+Itgβ7− and CCR6+Itgβ7+ CD4+ T-cells from HIV+ vs. HIV− CHACS participants expressed lower levels of CCR9 and ItgαE, respectively, pointing to their altered gut-homing potential. While memory CD4+ T-cells expressing both integrin α4β7 and CCR6 are inclined towards a Th17 lineage and are more vulnerable to HIV, it is primarily the expression of CCR6, rather than integrin α4β7, that reflects this susceptibility [74,94,95]. The relative contribution of CCR6+Itgβ7− vs. CCR6+Itgβ7+ CD4+ T-cells to HIV reservoir persistence during ART requires further investigations. Nevertheless, our group and others have previously reported that CCR6 expression is a marker of HIV reservoirs [95,96,97]. Other research documented the presence of integrated HIV-DNA in CD4+CD103+CD69+CCR6low T-cells infiltrating the gut of PWH [49,50]. Of particular notice, we also identified a fraction of CD4+ T-cells with IEL-like (ItgαE+Itgβ7+) and gut-homing/residence phenotype (CD69+CCR6+CCR9+). Although their frequency did not differ inside the pool of CD4+ T-cells of HIV+ vs. HIV−, their role as HIV reservoirs during ART deserves further investigations.
Our study revealed a decreased frequency of CD8+ T-cells with an IEL-like phenotype (ItgαE+Itgβ7+TCRαβ+TCRγδ−CD8αα+CD8αβ−) in ART-treated PWH compared to HIV− counterparts, together with a reduced CCR9 expression, again indicative of their impaired intraepithelial homing capacity. The cell phenotype was characterized by TCRαβ and CD8αα expression, which reveal that they are “natural” IELs, distinct from “induced” IELs that primarily express CD4 or CD8αβ and derive from conventional TCRαβ+ T-cells of peripheral lymphoid tissues. Natural IELs originate from precursor cells that have undergone development in the thymus and can migrate to the intestinal epithelium without the need for a cognate antigen [42,44,48]. Initially discovered in mice, the presence of TCRαβ+CD8αα+ IELs in humans is still debatable [44], although a low frequency (<1%) is documented in humans [48]. While their TCRαβ+CD8αβ+ and TCRγδ+CD8αα+ counterparts perform distinct functions within the immune system, TCRαβ+CD8αα+ IELs are particularly noted for their potential suppressive/regulatory contributions, as well as similarities antiviral NK cells [44,48]. Due to the lack of description of these IELs in human studies, research into their role in HIV infection is limited. Studies reported a significant reduction in the population of both CD4+ and CD4+CD8αα+ IELs within the intestinal epithelium during the SIV/HIV infection [98]. Other research mentions alterations in subsets that simultaneously expressed CD8αα and αβ [99]. Notably, in this work, the subset we examined did not exhibit co-expression of these markers. Therefore, to our knowledge, this is the first study documenting the diminution of TCRαβ+CD8αα+ IELs in the peripheral blood of PWH. Since IELs are key in targeting virus-infected IECs, via perforin, granzyme B, and IFN-γ and can perform CTL functions and secrete a broad spectrum of interferons, including types I, II, and III, supporting the antiviral state of the epithelium [45,100], we speculate that their reduced frequency is associated with impaired antiviral responses during HIV infection.
Finally, multivariate logistic regression analyses linked the changes in the composition of circulating T-cell subsets expressing gut-homing/residency markers to the HIV and CVD status, as well as coronary atherosclerosis plaque values. Of particular relevance, we identified ItgαE+CD8+, ItgαE−CD8+, CCR6+CD4+, and CCR6+Itgβ7−CD4+ T-cell subsets as positive correlates, and the ItgαE+CD4+ and ItgαE−CD4+ T-cell subsets as negative correlates of TPV values in crude models or upon adjustment for HIV (i.e., CD4 counts, CD8 counts, CD4/CD8 ratios, time on ART, time since infection) and CVD (i.e., D-dimers, smoking status, FRS) confounding factors.
Our study has several limitations. The study was performed on 82 participants divided into four groups based on their HIV (42 and 40 participants per group) and CVD status (24 and 16 participants per group). Although statistically significant differences were observed for specific parameters, sample size limitations cannot be excluded, especially for stratification based on CVD status in the HIV+ group. Similarly, the study was performed in a majority of male participants and the validity of these results on female participants remains to be determined. The predictive value of the identified cellular markers need to be validated in large-scale immune monitoring studies during specific interventions designed to decrease the CVD risk in ART-treated PWH (e.g., statins) [101]. This study is descriptive and we acknowledge the difficulty of establishing a cause–effect relationship between the immune parameters measured and subclinical atherosclerosis. Future mechanistic studies, in situ investigations, as well as longitudinal investigations considering the history of antiretroviral used (e.g., protease inhibitors), are needed to link events occurring in the gut to the alterations observed in the pool of circulating T-cells and demonstrate the direct contribution the identified alterations to the occurrence of CVD in ART-treated PWH.
5. Conclusions
In conclusion, our results reveal alterations in the composition of the pool of circulating T-cell subsets with a gut-homing/residency and activated phenotype that are associated with the HIV status and the coronary atherosclerotic plaque presence and volume. The increased frequency of CD326+CD4+ T-cells and decreased numbers of natural CD8+ IELs in the peripheral blood suggest ongoing gut barrier disruption, which may predispose ART-treated PWH to increased CVD risk, as well documented [8,15,24,25]. This study also identifies a specific immune cell phenotype, CCR6+Itgβ7−CD4+ T-cells and ItgαE− CD8+ T-cells, positively associated with increased atherosclerotic plaque burden in PWH. Our results provide a compelling rationale for further investigation into targeted interventions that address not only viral suppression but also the unique immune mucosal dysregulation in ART-treated PWH, reflected in the peripheral blood, for reducing the CVD onset and mortality and improving long-term health outcomes in an aging PWH community. Future studies are required to clarify the cause–effect relationship for the immunological correlates of CVD identified in this study. For example, subsets of CCR6+ CD4+ T-cells, known to be enriched in HIV reservoirs [32,96] and depicting a heart-homing phenotype [27], may contribute to atherosclerotic plaque formation upon recruitment into vascular walls via their capacity to sustain residual HIV transcription and fuel inflammation. Alternatively, the recruitment into vascular walls of specific ItgαE− CD8+ T-cell subsets may contribute to endothelial bed damage and atherosclerotic plaque rupture, via cytotoxic/cytolytic mechanisms similar to those recently reported for CD8+ T-cell mediated gut barrier integrity disruption in PWH with [102].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells14211732/s1, Figure S1: General gating strategy for flow cytometry analysis of PBMCs of study participants; Figure S2: Frequency of CD4+ and CD8+ T-cells in HIV+ vs. HIV− participants relative to age and subclinical CVD; Figure S3: Multivariate regression model identified novel immunological predictors of HIV-1 status; File S1: Master file clinical and research data; Table S1: Multivariate regression model to identify immunological predictors of HIV status; Table S2: Multivariate regression model to identify immunological predictors of CVD status; Table S3: Multivariate regression model to identify immunological predictors of TPV values in ART-treated PWH.
Author Contributions
Conceptualization, E.M.G., J.D., M.N. and P.A.; methodology, E.M.G., J.D., A.F.-M. and P.A.; software, E.M.G., J.D. and A.F.-M.; validation, E.M.G., J.D., A.F.-M., M.E.-F. and P.A.; formal analysis, E.M.G., J.D., A.F.-M., C.C.-L., M.E.-F. and P.A.; investigation, E.M.G., J.D., R.E.C. and T.R.W.S.; resources, J.-P.R., M.E.-F., M.D. and C.T.; data curation, J.D. and A.F.-M.; writing—original draft preparation, E.M.G. and P.A.; writing—review and editing, J.D., A.F.-M. and P.A.; visualization, E.M.G., J.D., A.F.-M. and P.A.; supervision, P.A.; project administration, P.A.; funding acquisition, M.D., C.T. and P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Canadian Institutes of Health Research (CIHR; PJT-153052; PJT-178127 to P.A.), National Institutes of Health (NIH) to C.T. and P.A. (R01AG054324), as well as infrastructure funding from the Canadian Foundation for Innovation (CFI) to P.A. and C.T. Core facilities and human cohorts were supported by the Fondation du CHUM and the Fonds de recherche du Québec—Santé (FRQ-S) HIV/AIDS and Infectious Diseases Network. M.D. receives a clinician-researcher salary award from the Fonds de recherche du Québec—Santé. The CHACS cohort is supported by funds from CIHR (HAL 398643 to M.D.) and by the CHIR HIV clinical trial network (CTN-272).
Institutional Review Board Statement
The collection of peripheral blood from the participants was conducted in compliance with the Declaration of Helsinki. This research received approval from the Institutional Review Board of the Centre de recherche du Centre hospitalier de l’Université de Montréal (CRCHUM), Montréal, Québec, Canada (#CE.11.063).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author: petronela.ancuta@umontreal.ca.
Acknowledgments
The authors thank Philippe St-Onge and Gael Dulude (Flow Cytometry Core Facility, CHUM-Research Centre) for expert technical support with polychromatic flow cytometry sorting; Anita L. Ray (CellCarta, Montréal, QC, Canada) for training in CellEngine Analysis; Olfa Debbeche for managing the NLC3 Core Facility of the CHUM-Research Centre; Annie Chamberland, Stéphanie Matte, Mohamed Sylla for blood processing and managing the cell biobank; Marc Messier-Peet for providing clinical information and managing the CHACS database; and Mario Legault for his help with ethical approvals and informed consents. The authors address a special thanks to all ART-treated PWH and HIV-uninfected study participants for their crucial contribution to this work.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| ART | Antiretroviral therapy |
| BMI | Body mass index |
| CCTA | Computed Coronary Tomography Angiography |
| CHACS | Canadian HIV and aging cohort study |
| CVD | Cardiovascular disease |
| EpCAM | Epithelial cell adhesion molecule |
| FRS | Framingham risk score |
| GALT | Gut-associated lymphoid tissues |
| GLM | Generalized linear model |
| HIV-1 | Human immunodeficiency virus type 1 |
| IEC | Intestinal epithelial cells |
| IEL | Intraepithelial lymphocyte |
| IQR | Interquartile range |
| Itg | Integrin |
| PBMC | Peripheral blood mononucleated cells |
| PWH | People with HIV |
| SD | Standard deviation |
| SOM | Self-organizing map |
| TPV | Total plaque volume |
| t-SNE | t-Distributed Stochastic Neighbour Embedding |
References
- WHO. HIV Data and Statistics. 2023. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 15 September 2025).
- Barre-Sinoussi, F.; Ross, A.L.; Delfraissy, J.F. Past, present and future: 30 years of HIV research. Nat. Rev. Microbiol. 2013, 11, 877–883. [Google Scholar] [CrossRef]
- Deeks, S.G.; Archin, N.; Cannon, P.; Collins, S.; Jones, R.B.; de Jong, M.; Lambotte, O.; Lamplough, R.; Ndung’u, T.; Sugarman, J.; et al. Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat. Med. 2021, 27, 2085–2098. [Google Scholar] [CrossRef]
- Siliciano, J.D.; Siliciano, R.F. In Vivo Dynamics of the Latent Reservoir for HIV-1: New Insights and Implications for Cure. Annu. Rev. Pathol. 2022, 17, 271–294. [Google Scholar] [CrossRef]
- McMyn, N.F.; Varriale, J.; Fray, E.J.; Zitzmann, C.; MacLeod, H.; Lai, J.; Singhal, A.; Moskovljevic, M.; Garcia, M.A.; Lopez, B.M.; et al. The latent reservoir of inducible, infectious HIV-1 does not decrease despite decades of antiretroviral therapy. J. Clin. Invest. 2023, 133, e171554. [Google Scholar] [CrossRef]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef]
- Akusjarvi, S.S.; Neogi, U. Biological Aging in People Living with HIV on Successful Antiretroviral Therapy: Do They Age Faster? Curr. HIV/AIDS Rep. 2023, 20, 42–50. [Google Scholar] [CrossRef]
- Perkins, M.V.; Joseph, S.B.; Dittmer, D.P.; Mackman, N. Cardiovascular Disease and Thrombosis in HIV Infection. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 175–191. [Google Scholar] [CrossRef]
- Mudd, J.C.; Brenchley, J.M. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. J. Infect. Dis. 2016, 214 (Suppl. S2), S58–S66. [Google Scholar] [CrossRef]
- Agrati, C.; De Biasi, S.; Fidanza, L.; Gibellini, L.; Nasi, M.; Pinti, M.; Cossarizza, A. The importance of advanced cytometry in defining new immune cell types and functions relevant for the immunopathogenesis of HIV infection. AIDS 2020, 34, 2169–2185. [Google Scholar] [CrossRef]
- Paiardini, M.; Muller-Trutwin, M. HIV-associated chronic immune activation. Immunol. Rev. 2013, 254, 78–101. [Google Scholar] [CrossRef]
- Rousseau, R.K.; Szadkowski, L.; Kovacs, C.M.; Saikali, M.F.; Nadeem, R.; Malazogu, F.; Huibner, S.; Cummins, C.L.; Kaul, R.; Walmsley, S.L. Activation and gut-homing of peripheral T cells in HIV immunologic non-responders despite long term viral suppression. PLoS ONE 2021, 16, e0254149. [Google Scholar] [CrossRef]
- Cibrian, D.; Sanchez-Madrid, F. CD69: From activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef]
- Walsh, D.A.; Borges da Silva, H.; Beura, L.K.; Peng, C.; Hamilton, S.E.; Masopust, D.; Jameson, S.C. The Functional Requirement for CD69 in Establishment of Resident Memory CD8(+) T Cells Varies with Tissue Location. J. Immunol. 2019, 203, 946–955. [Google Scholar] [CrossRef]
- Moretti, S.; Schietroma, I.; Sberna, G.; Maggiorella, M.T.; Sernicola, L.; Farcomeni, S.; Giovanetti, M.; Ciccozzi, M.; Borsetti, A. HIV-1-Host Interaction in Gut-Associated Lymphoid Tissue (GALT): Effects on Local Environment and Comorbidities. Int. J. Mol. Sci. 2023, 24, 12193. [Google Scholar] [CrossRef]
- Veazey, R.S. Intestinal CD4 Depletion in HIV / SIV Infection. Curr. Immunol. Rev. 2019, 15, 76–91. [Google Scholar] [CrossRef]
- Busman-Sahay, K.; Starke, C.E.; Nekorchuk, M.D.; Estes, J.D. Eliminating HIV reservoirs for a cure: The issue is in the tissue. Curr. Opin. HIV AIDS 2021, 16, 200–208. [Google Scholar] [CrossRef]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- McBride, J.A.; Striker, R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017, 13, e1006624. [Google Scholar] [CrossRef]
- Lu, W.; Mehraj, V.; Vyboh, K.; Cao, W.; Li, T.; Routy, J.P. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J. Int. AIDS Soc. 2015, 18, 20052. [Google Scholar] [CrossRef]
- Grossman, Z.; Meier-Schellersheim, M.; Paul, W.E.; Picker, L.J. Pathogenesis of HIV infection: What the virus spares is as important as what it destroys. Nat. Med. 2006, 12, 289–295. [Google Scholar] [CrossRef]
- Thompson, C.G.; Gay, C.L.; Kashuba, A.D.M. HIV Persistence in Gut-Associated Lymphoid Tissues: Pharmacological Challenges and Opportunities. AIDS Res. Hum. Retroviruses 2017, 33, 513–523. [Google Scholar] [CrossRef]
- El-Far, M.; Tremblay, C.L. Gut microbial diversity in HIV infection post combined antiretroviral therapy: A key target for prevention of cardiovascular disease. Curr. Opin. HIV AIDS 2018, 13, 38–44. [Google Scholar]
- So-Armah, K.; Benjamin, L.A.; Bloomfield, G.S.; Feinstein, M.J.; Hsue, P.; Njuguna, B.; Freiberg, M.S. HIV and cardiovascular disease. Lancet HIV 2020, 7, e279–e293. [Google Scholar] [CrossRef]
- Lewis, C.V.; Taylor, W.R. Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H1227–H1233. [Google Scholar] [CrossRef]
- Turcotte, I.; El-Far, M.; Sadouni, M.; Chartrand-Lefebvre, C.; Filali-Mouhim, A.; Fromentin, R.; Chamberland, A.; Jenabian, M.A.; Baril, J.G.; Trottier, B.; et al. Association Between the Development of Subclinical Cardiovascular Disease and Human Immunodeficiency Virus (HIV) Reservoir Markers in People with HIV on Suppressive Antiretroviral Therapy. Clin. Infect. Dis. 2023, 76, 1318–1321. [Google Scholar] [CrossRef]
- Ramani, H.; Gosselin, A.; Bunet, R.; Jenabian, M.A.; Sylla, M.; Pagliuzza, A.; Chartrand-Lefebvre, C.; Routy, J.P.; Goulet, J.P.; Thomas, R.; et al. IL-32 Drives the Differentiation of Cardiotropic CD4+ T Cells Carrying HIV DNA in People with HIV. J. Infect. Dis. 2024, 229, 1277–1289. [Google Scholar] [CrossRef]
- Guenin-Mace, L.; Konieczny, P.; Naik, S. Immune-Epithelial Cross Talk in Regeneration and Repair. Annu. Rev. Immunol. 2023, 41, 207–228. [Google Scholar] [CrossRef]
- Mahapatro, M.; Erkert, L.; Becker, C. Cytokine-Mediated Crosstalk between Immune Cells and Epithelial Cells in the Gut. Cells 2021, 10, 111. [Google Scholar] [CrossRef]
- Wacleche, V.S.; Landay, A.; Routy, J.P.; Ancuta, P. The Th17 Lineage: From Barrier Surfaces Homeostasis to Autoimmunity, Cancer, and HIV-1 Pathogenesis. Viruses 2017, 9, 303. [Google Scholar] [CrossRef]
- Planas, D.; Routy, J.P.; Ancuta, P. New Th17-specific therapeutic strategies for HIV remission. Curr. Opin. HIV AIDS 2019, 14, 85–92. [Google Scholar] [CrossRef]
- Fert, A.; Raymond Marchand, L.; Wiche Salinas, T.R.; Ancuta, P. Targeting Th17 cells in HIV-1 remission/cure interventions. Trends Immunol. 2022, 43, 580–594. [Google Scholar] [CrossRef]
- Buckner, J.H.; Harrison, O.J. Th17 cells: From gut homeostasis to CNS pathogenesis. Trends Immunol. 2022, 43, 167–169. [Google Scholar] [CrossRef]
- Kim, C.J.; McKinnon, L.R.; Kovacs, C.; Kandel, G.; Huibner, S.; Chege, D.; Shahabi, K.; Benko, E.; Loutfy, M.; Ostrowski, M.; et al. Mucosal Th17 cell function is altered during HIV infection and is an independent predictor of systemic immune activation. J. Immunol. 2013, 191, 2164–2173. [Google Scholar] [CrossRef]
- Arthos, J.; Cicala, C.; Nawaz, F.; Byrareddy, S.N.; Villinger, F.; Santangelo, P.J.; Ansari, A.A.; Fauci, A.S. The Role of Integrin alpha4beta7 in HIV Pathogenesis and Treatment. Curr. HIV/AIDS Rep. 2018, 15, 127–135. [Google Scholar] [CrossRef]
- Pollock, J.; Kaul, R. How integral is the alpha4beta7 integrin to HIV transmission? eBioMedicine 2021, 63, 103148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, C.; Li, Y.; Liu, Z.; Wang, J.; Zhang, Y.; Yan, Z.; Zhang, Y.; Li, G.; Chen, J. Distinct chemokines selectively induce HIV-1 gp120-integrin alpha4beta7 binding via triggering conformer-specific activation of alpha4beta7. Signal Transduct. Target. Ther. 2021, 6, 265. [Google Scholar] [CrossRef]
- Sivro, A.; Schuetz, A.; Sheward, D.; Joag, V.; Yegorov, S.; Liebenberg, L.J.; Yende-Zuma, N.; Stalker, A.; Mwatelah, R.S.; Selhorst, P.; et al. Integrin alpha4beta7 expression on peripheral blood CD4(+) T cells predicts HIV acquisition and disease progression outcomes. Sci. Transl. Med. 2018, 10, eaam6354. [Google Scholar] [CrossRef] [PubMed]
- Mavigner, M.; Cazabat, M.; Dubois, M.; L’Faqihi, F.E.; Requena, M.; Pasquier, C.; Klopp, P.; Amar, J.; Alric, L.; Barange, K.; et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J. Clin. Invest. 2012, 122, 62–69. [Google Scholar] [CrossRef]
- Loiseau, C.; Requena, M.; Mavigner, M.; Cazabat, M.; Carrere, N.; Suc, B.; Barange, K.; Alric, L.; Marchou, B.; Massip, P.; et al. CCR6 regulatory T cells blunt the restoration of gut Th17 cells along the CCR6-CCL20 axis in treated HIV-1-infected individuals. Mucosal Immunol. 2016, 9, 1137–1150. [Google Scholar] [CrossRef] [PubMed]
- Nayrac, M.; Requena, M.; Loiseau, C.; Cazabat, M.; Suc, B.; Carrere, N.; Barange, K.; Alric, L.; Martin-Blondel, G.; Izopet, J.; et al. Th22 cells are efficiently recruited in the gut by CCL28 as an alternative to CCL20 but do not compensate for the loss of Th17 cells in treated HIV-1-infected individuals. Mucosal Immunol. 2021, 14, 219–228. [Google Scholar] [CrossRef]
- Sumida, H. Dynamics and clinical significance of intestinal intraepithelial lymphocytes. Immunol. Med. 2019, 42, 117–123. [Google Scholar] [CrossRef]
- Olivares-Villagomez, D.; Van Kaer, L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018, 39, 264–275. [Google Scholar] [CrossRef]
- Gui, Y.; Cheng, H.; Zhou, J.; Xu, H.; Han, J.; Zhang, D. Development and function of natural TCR(+) CD8alphaalpha(+) intraepithelial lymphocytes. Front. Immunol. 2022, 13, 1059042. [Google Scholar] [CrossRef]
- Hu, M.D.; Jia, L.; Edelblum, K.L. Policing the intestinal epithelial barrier: Innate immune functions of intraepithelial lymphocytes. Curr. Pathobiol. Rep. 2018, 6, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, A.; Mucida, D.; Bilate, A.M. Intraepithelial Lymphocytes of the Intestine. Annu. Rev. Immunol. 2024, 42, 289–316. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef]
- Van Kaer, L.; Olivares-Villagomez, D. Development, Homeostasis, and Functions of Intestinal Intraepithelial Lymphocytes. J. Immunol. 2018, 200, 2235–2244. [Google Scholar] [CrossRef]
- Yukl, S.A.; Khan, S.; Chen, T.H.; Trapecar, M.; Wu, F.; Xie, G.; Telwatte, S.; Fulop, D.; Pico, A.R.; Laird, G.M.; et al. Shared Mechanisms Govern HIV Transcriptional Suppression in Circulating CD103(+) and Gut CD4(+) T Cells. J. Virol. 2020, 95. [Google Scholar] [CrossRef]
- Kiniry, B.E.; Li, S.; Ganesh, A.; Hunt, P.W.; Somsouk, M.; Skinner, P.J.; Deeks, S.G.; Shacklett, B.L. Detection of HIV-1-specific gastrointestinal tissue resident CD8(+) T-cells in chronic infection. Mucosal Immunol. 2018, 11, 909–920. [Google Scholar] [CrossRef]
- Snelson, M.; Muralitharan, R.R.; Liu, C.F.; Marko, L.; Forslund, S.K.; Marques, F.Z.; Tang, W.H.W. Gut-Heart Axis: The Role of Gut Microbiota and Metabolites in Heart Failure. Circ. Res. 2025, 136, 1382–1406. [Google Scholar] [CrossRef] [PubMed]
- Troseid, M.; Andersen, G.O.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. eBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Giguere, K.; Chartrand-Lefebvre, C.; Baril, J.G.; Conway, B.; El-Far, M.; Falutz, J.; Harris, M.; Jenabian, M.A.; Leipsic, J.; Loutfy, M.; et al. Baseline characteristics of a prospective cohort study of aging and cardiovascular diseases among people living with HIV. HIV Med. 2023, 24, 1210–1221. [Google Scholar] [CrossRef]
- Wiche Salinas, T.R.; Zhang, Y.; Gosselin, A.; Rosario, N.F.; El-Far, M.; Filali-Mouhim, A.; Routy, J.P.; Chartrand-Lefebvre, C.; Landay, A.L.; Durand, M.; et al. Alterations in Th17 Cells and Non-Classical Monocytes as a Signature of Subclinical Coronary Artery Atherosclerosis during ART-Treated HIV-1 Infection. Cells 2024, 13, 157. [Google Scholar] [CrossRef]
- Chen, Z.; Boldeanu, I.; Nepveu, S.; Durand, M.; Chin, A.S.; Kauffmann, C.; Mansour, S.; Soulez, G.; Tremblay, C.; Chartrand-Lefebvre, C. In vivo coronary artery plaque assessment with computed tomography angiography: Is there an impact of iterative reconstruction on plaque volume and attenuation metrics? Acta Radiol. 2017, 58, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Durand, M.; Chartrand-Lefebvre, C.; Baril, J.G.; Trottier, S.; Trottier, B.; Harris, M.; Walmsley, S.; Conway, B.; Wong, A.; Routy, J.P.; et al. The Canadian HIV and aging cohort study—Determinants of increased risk of cardio-vascular diseases in HIV-infected individuals: Rationale and study protocol. BMC Infect. Dis. 2017, 17, 611. [Google Scholar] [CrossRef] [PubMed]
- Boldeanu, I.; Sadouni, M.; Mansour, S.; Baril, J.G.; Trottier, B.; Soulez, G.; Chin, A.S.; Leipsic, J.; Tremblay, C.; Durand, M.; et al. Prevalence and Characterization of Subclinical Coronary Atherosclerotic Plaque with CT among Individuals with HIV: Results from the Canadian HIV and Aging Cohort Study. Radiology 2021, 299, 571–580. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing (Version 4.4.1); R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 15 September 2025).
- Gullaksen, S.; Funck, K.L.; Laugesen, E.; Hansen, T.K.; Dey, D.; Poulsen, P.L. Volumes of coronary plaque disease in relation to body mass index, waist circumference, truncal fat mass and epicardial adipose tissue in patients with type 2 diabetes mellitus and controls. Diab Vasc. Dis. Res. 2019, 16, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Schlesselman, J.J.; Stolley, P.D. Case-Control Studies: Design, Conduct, Analysis; Oxford University Press: Oxford, UK, 1982; pp. 144–147. [Google Scholar]
- Kenward, M.G.; Roger, J.H. The use of baseline covariates in crossover studies. Biostatistics 2010, 11, 1–17. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Rothan, C.; Yero, A.; Shi, T.; Farnos, O.; Chartrand-Lefebvre, C.; El-Far, M.; Costiniuk, C.T.; Tsoukas, C.; Tremblay, C.; Durand, M.; et al. Antiretroviral therapy-treated HIV-infected adults with coronary artery disease are characterized by a distinctive regulatory T-cell signature. AIDS 2021, 35, 1003–1014. [Google Scholar] [CrossRef]
- Agace, W.W.; Higgins, J.M.; Sadasivan, B.; Brenner, M.B.; Parker, C.M. T-lymphocyte-epithelial-cell interactions: Integrin alpha(E)(CD103)beta(7), LEEP-CAM and chemokines. Curr. Opin. Cell Biol. 2000, 12, 563–568. [Google Scholar] [CrossRef]
- Huang, S.H.; Ren, Y.; Thomas, A.S.; Chan, D.; Mueller, S.; Ward, A.R.; Patel, S.; Bollard, C.M.; Cruz, C.R.; Karandish, S.; et al. Latent HIV reservoirs exhibit inherent resistance to elimination by CD8+ T cells. J. Clin. Invest. 2018, 128, 876–889. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, W.; Nie, Y.; Yang, Y.; Chen, G.; Huang, L.; Wu, H.; Lei, Y.; Chen, L.; Hu, Q.; et al. EpCAM Is Essential to Maintaining the Immune Homeostasis of Intestines via Keeping the Expression of pIgR in the Intestinal Epithelium of Mice. Front. Immunol. 2022, 13, 843378. [Google Scholar] [CrossRef] [PubMed]
- Yahyazadeh Mashhadi, S.M.; Kazemimanesh, M.; Arashkia, A.; Azadmanesh, K.; Meshkat, Z.; Golichenari, B.; Sahebkar, A. Shedding light on the EpCAM: An overview. J. Cell. Physiol. 2019, 234, 12569–12580. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabieres, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Han, D.; Li, R.; Shi, J.; Tan, P.; Zhang, R.; Li, J. Liquid biopsy for infectious diseases: A focus on microbial cell-free DNA sequencing. Theranostics 2020, 10, 5501–5513. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Khoo, B.L. Liquid biopsy technologies for hematological diseases. Med. Res. Rev. 2021, 41, 246–274. [Google Scholar] [CrossRef]
- Hegazy, A.N.; West, N.R.; Stubbington, M.J.T.; Wendt, E.; Suijker, K.I.M.; Datsi, A.; This, S.; Danne, C.; Campion, S.; Duncan, S.H.; et al. Circulating and Tissue-Resident CD4+ T Cells with Reactivity to Intestinal Microbiota Are Abundant in Healthy Individuals and Function Is Altered During Inflammation. Gastroenterology 2017, 153, 1320–1337.e16. [Google Scholar] [CrossRef] [PubMed]
- Samat, A.A.K.; van der Geest, J.; Vastert, S.J.; van Loosdregt, J.; van Wijk, F. Tissue-Resident Memory T Cells in Chronic Inflammation-Local Cells with Systemic Effects? Cells 2021, 10, 409. [Google Scholar] [CrossRef]
- Asawa, S.; Nuesch, M.; Gvozdenovic, A.; Aceto, N. Circulating tumour cells in gastrointestinal cancers: Food for thought? Br. J. Cancer 2023, 128, 1981–1990. [Google Scholar] [CrossRef]
- Monteiro, P.; Gosselin, A.; Wacleche, V.S.; El-Far, M.; Said, E.A.; Kared, H.; Grandvaux, N.; Boulassel, M.R.; Routy, J.P.; Ancuta, P. Memory CCR6+ CD4+ T cells are preferential targets for productive HIV type 1 infection regardless of their expression of integrin beta7. J. Immunol. 2011, 186, 4618–4630. [Google Scholar] [CrossRef]
- Folsom, A.R.; Gottesman, R.F.; Appiah, D.; Shahar, E.; Mosley, T.H. Plasma d-Dimer and Incident Ischemic Stroke and Coronary Heart Disease: The Atherosclerosis Risk in Communities Study. Stroke 2016, 47, 18–23. [Google Scholar] [CrossRef]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovic, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 1—Serological Markers. Heart Lung Circ. 2019, 28, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yang, Y.; Yang, F.; Liu, S.; Zhu, Z.; Lei, Z.; Guo, J. Functions of EpCAM in physiological processes and diseases (Review). Int. J. Mol. Med. 2018, 42, 1771–1785. [Google Scholar] [CrossRef]
- Planas, D.; Pagliuzza, A.; Ponte, R.; Fert, A.; Marchand, L.R.; Massanella, M.; Gosselin, A.; Mehraj, V.; Dupuy, F.P.; Isnard, S.; et al. LILAC pilot study: Effects of metformin on mTOR activation and HIV reservoir persistence during antiretroviral therapy. eBioMedicine 2021, 65, 103270. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Nakayama, M.; Hori, A.; Toyoura, S.; Yamaguchi, S.I. Shaping of T Cell Functions by Trogocytosis. Cells 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Schriek, P.; Villadangos, J.A. Trogocytosis and cross-dressing in antigen presentation. Curr. Opin. Immunol. 2023, 83, 102331. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.N.; Leventoux, N.; Campos-Mora, M.; Gimenez, S.; Corbeau, P.; Villalba, M. NK Cells Acquire CCR5 and CXCR4 by Trogocytosis in People Living with HIV-1. Vaccines 2022, 10, 688. [Google Scholar] [CrossRef]
- Couturier, J.; Suliburk, J.W.; Brown, J.M.; Luke, D.J.; Agarwal, N.; Yu, X.; Nguyen, C.; Iyer, D.; Kozinetz, C.A.; Overbeek, P.A.; et al. Human adipose tissue as a reservoir for memory CD4+ T cells and HIV. AIDS 2015, 29, 667–674. [Google Scholar] [CrossRef]
- Wanjalla, C.N.; McDonnell, W.J.; Barnett, L.; Simmons, J.D.; Furch, B.D.; Lima, M.C.; Woodward, B.O.; Fan, R.; Fei, Y.; Baker, P.G.; et al. Adipose Tissue in Persons with HIV Is Enriched for CD4(+) T Effector Memory and T Effector Memory RA(+) Cells, Which Show Higher CD69 Expression and CD57, CX3CR1, GPR56 Co-expression with Increasing Glucose Intolerance. Front. Immunol. 2019, 10, 408. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H.; Alvarez, X.; Pahar, B.; Moroney-Rasmussen, T.; Lackner, A.A.; Veazey, R.S. Distinct expression patterns of CD69 in mucosal and systemic lymphoid tissues in primary SIV infection of rhesus macaques. PLoS ONE 2011, 6, e27207. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Bergsbaken, T.; Edelblum, K.L. The multifunctional nature of CD103 (alphaEbeta7 integrin) signaling in tissue-resident lymphocytes. Am. J. Physiol. Cell Physiol. 2022, 323, C1161–C1167. [Google Scholar] [CrossRef]
- Vimonpatranon, S.; Goes, L.R.; Chan, A.; Licavoli, I.; McMurry, J.; Wertz, S.R.; Arakelyan, A.; Huang, D.; Jiang, A.; Huang, C.; et al. MAdCAM-1 costimulation in the presence of retinoic acid and TGF-beta promotes HIV infection and differentiation of CD4+ T cells into CCR5+ TRM-like cells. PLoS Pathog. 2023, 19, e1011209. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, M.; Yang, Z.; Lu, C.; Wang, Q.; Wang, H.; Deng, C.; Liu, Y.; Yang, Y. The Roles of CCR9/CCL25 in Inflammation and Inflammation-Associated Diseases. Front. Cell Dev. Biol. 2021, 9, 686548. [Google Scholar] [CrossRef]
- Mayassi, T.; Barreiro, L.B.; Rossjohn, J.; Jabri, B. A multilayered immune system through the lens of unconventional T cells. Nature 2021, 595, 501–510. [Google Scholar] [CrossRef]
- Ziegler, H.; Welker, C.; Sterk, M.; Haarer, J.; Rammensee, H.G.; Handgretinger, R.; Schilbach, K. Human Peripheral CD4(+) Vdelta1(+) gammadeltaT Cells Can Develop into alphabetaT Cells. Front. Immunol. 2014, 5, 645. [Google Scholar] [CrossRef]
- Li, H.; Pauza, C.D. HIV envelope-mediated, CCR5/alpha4beta7-dependent killing of CD4-negative gammadelta T cells which are lost during progression to AIDS. Blood 2011, 118, 5824–5831. [Google Scholar] [CrossRef]
- Pauza, C.D.; Poonia, B.; Li, H.; Cairo, C.; Chaudhry, S. gammadelta T Cells in HIV Disease: Past, Present, and Future. Front. Immunol. 2014, 5, 687. [Google Scholar]
- Walker, E.M.; Slisarenko, N.; Gerrets, G.L.; Grasperge, B.F.; Mattison, J.A.; Kissinger, P.J.; Welsh, D.A.; Veazey, R.S.; Jazwinski, S.M.; Rout, N. Dysregulation of IL-17/IL-22 Effector Functions in Blood and Gut Mucosal Gamma Delta T Cells Correlates with Increase in Circulating Leaky Gut and Inflammatory Markers During cART-Treated Chronic SIV Infection in Macaques. Front. Immunol. 2021, 12, 647398. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.S.; Korner, H. CCR6/CCL20 chemokine axis in human immunodeficiency virus immunity and pathogenesis. J. Gen. Virol. 2017, 98, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Khoury, G.; Fromentin, R.; Solomon, A.; Chomont, N.; Sinclair, E.; Milush, J.M.; Hartogensis, W.; Bacchetti, P.; Roche, M.; et al. Human Immunodeficiency Virus (HIV)-Infected CCR6+ Rectal CD4+ T Cells and HIV Persistence on Antiretroviral Therapy. J. Infect. Dis. 2020, 221, 744–755. [Google Scholar] [CrossRef]
- Gosselin, A.; Wiche Salinas, T.R.; Planas, D.; Wacleche, V.S.; Zhang, Y.; Fromentin, R.; Chomont, N.; Cohen, E.A.; Shacklett, B.; Mehraj, V.; et al. HIV persists in CCR6+ CD4+ T cells from colon and blood during antiretroviral therapy. AIDS 2017, 31, 35–48. [Google Scholar] [CrossRef]
- Khoury, G.; Anderson, J.L.; Fromentin, R.; Hartogenesis, W.; Smith, M.Z.; Bacchetti, P.; Hecht, F.M.; Chomont, N.; Cameron, P.U.; Deeks, S.G.; et al. Persistence of integrated HIV DNA in CXCR3 + CCR6 + memory CD4+ T cells in HIV-infected individuals on antiretroviral therapy. AIDS 2016, 30, 1511–1520. [Google Scholar] [CrossRef]
- Mattapallil, J.J.; Reay, E.; Dandekar, S. An early expansion of CD8alphabeta T cells, but depletion of resident CD8alphaalpha T cells, occurs in the intestinal epithelium during primary simian immunodeficiency virus infection. AIDS 2000, 14, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Konno, A.; Okada, K.; Mizuno, K.; Nishida, M.; Nagaoki, S.; Toma, T.; Uehara, T.; Ohta, K.; Kasahara, Y.; Seki, H.; et al. CD8alpha alpha memory effector T cells descend directly from clonally expanded CD8alpha +beta high TCRalpha beta T cells in vivo. Blood 2002, 100, 4090–4097. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Qiu, Y.; Yang, H. Intestinal intraepithelial lymphocytes: Maintainers of intestinal immune tolerance and regulators of intestinal immunity. J. Leukoc. Biol. 2021, 109, 339–347. [Google Scholar] [CrossRef]
- Rock, A.E.; Russell, M.L.; Triant, V.A. Balancing polypharmacy and comorbidity management: Cardiovascular health. Curr. Opin. HIV AIDS 2025, 20, 409–415. [Google Scholar] [CrossRef]
- Das Adhikari, U.; Froehle, L.M.; Pipkin, A.N.; Baharlou, H.; Linder, A.H.; Shah, P.; Hussey, A.; Zhang, Q.; Nyquist, S.; Khwaled, S.; et al. Immunometabolic defects of CD8+ T cells disrupt gut barrier integrity in people with HIV. Cell 2025, 188, 5666–5679.e19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).