Melatonin Protects Intact Rat Ovarian Transplantation via the MT1/Nrf2/ARE Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Endocrine Function
2.3. Measurement of Reactive Oxygen Species (ROS) in Ovarian Tissue
2.4. Measurement of Serum ROS
2.5. Measurement of Superoxide Dismutase (SOD), Glutathione (GSH), and Malondialdehyde (MDA) in Ovarian Tissue
2.6. Measurement of Total Antioxidant Capacity (TAC)
2.7. Measurement of Hydroxyl Radical Concentration in Ovarian Tissue Using the Fenton Assay
2.8. Nrf2 Protein Nuclear Translocation Assay in Ovarian Tissue
2.9. Western Blot Analysis
2.10. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
- Primer sequence (5′–3′)
- Nrf2 F: GATCAGGCTCAGTCACTCGATAG
- R: ACACTGTAACTCGGGAATGGAAA
- HO-1 F: CAGGGTGACAGAAGAGGCTAAGA
- R: TGGGATGAACTAGTGCTGATCTG
- SOD1 F: GAGAGGCATGTTGGAGACCTG
- R: TGTTTCTCGTGGACCACCATAG
- IL-6 F: GGC CCT TGC TTT CTC TTC G
- R: ATA ATA AAG TTT TGA TTA TGT
- TNF-α F: CCAGGTTCTCTTCAAGGGACAA
- R: GGTATGAAATGGCAAATCGGCT
- KEAP1 F: GGACTTTCGTAGCCTCCATGAA
- R: TAGCATTCCACACTGTCCAGAAA
- sMafg F: CGTTGGGATCGGTCAGTTCA
- R: CCACTTGCACTCTCGTCCAT
- GAPDH F: CTGGAGAAACCTGCCAAGTATG
- R: GGTGGAAGAATGGGAGTTGCT
2.11. Immunofluorescence Analysis
2.12. Statistical Analysis
3. Results
3.1. Melatonin Modulates Follicular Histology, Hormone Secretion, and the Estrous Cycle
3.2. Melatonin Regulates the Inflammatory Response in Intact Ovarian Transplantation
3.3. Melatonin Attenuates Oxidative Stress in Intact Ovarian Transplantation
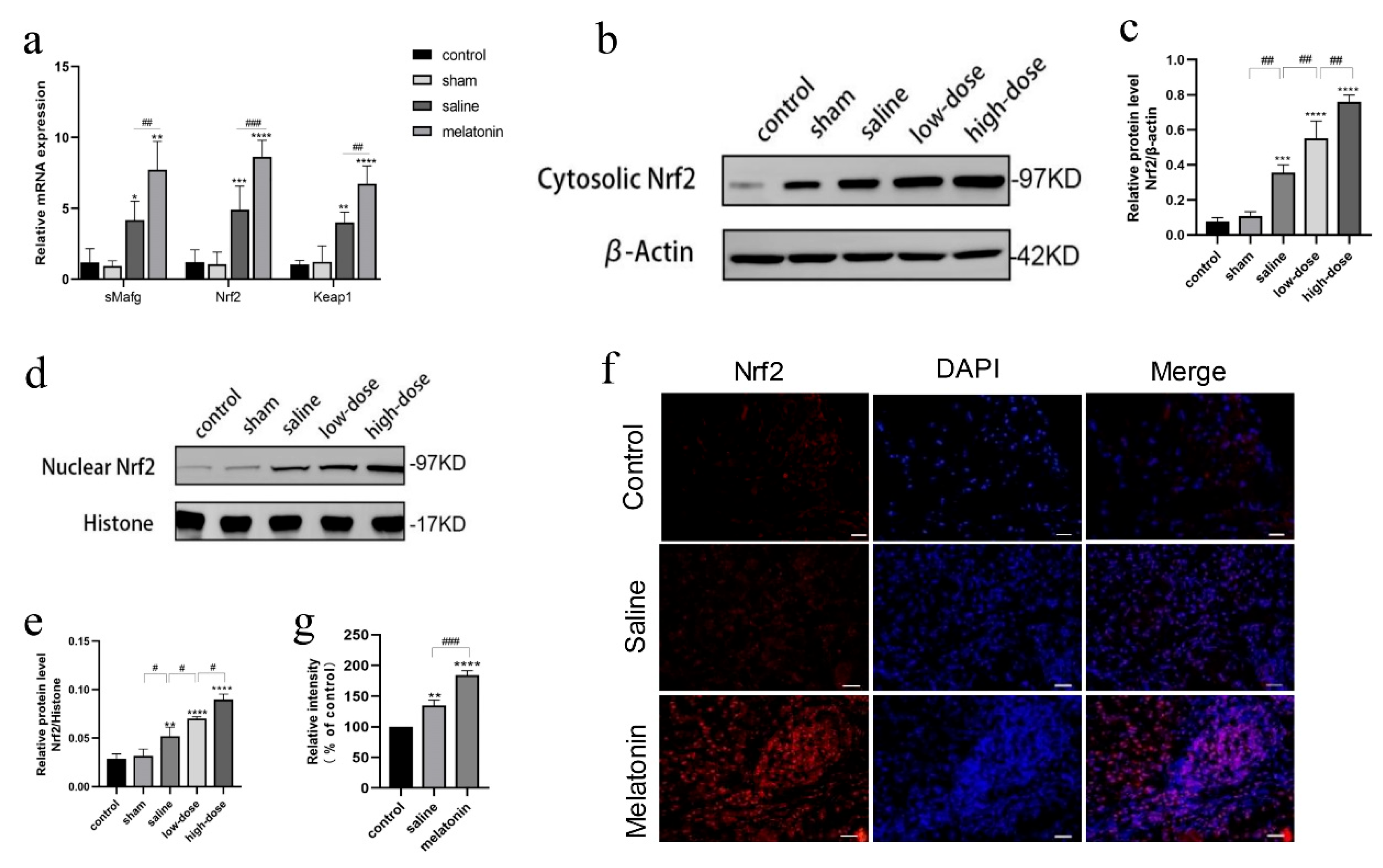
3.4. Melatonin Protects Against Oxidative and Inflammatory Damage via the Nrf2 Pathway
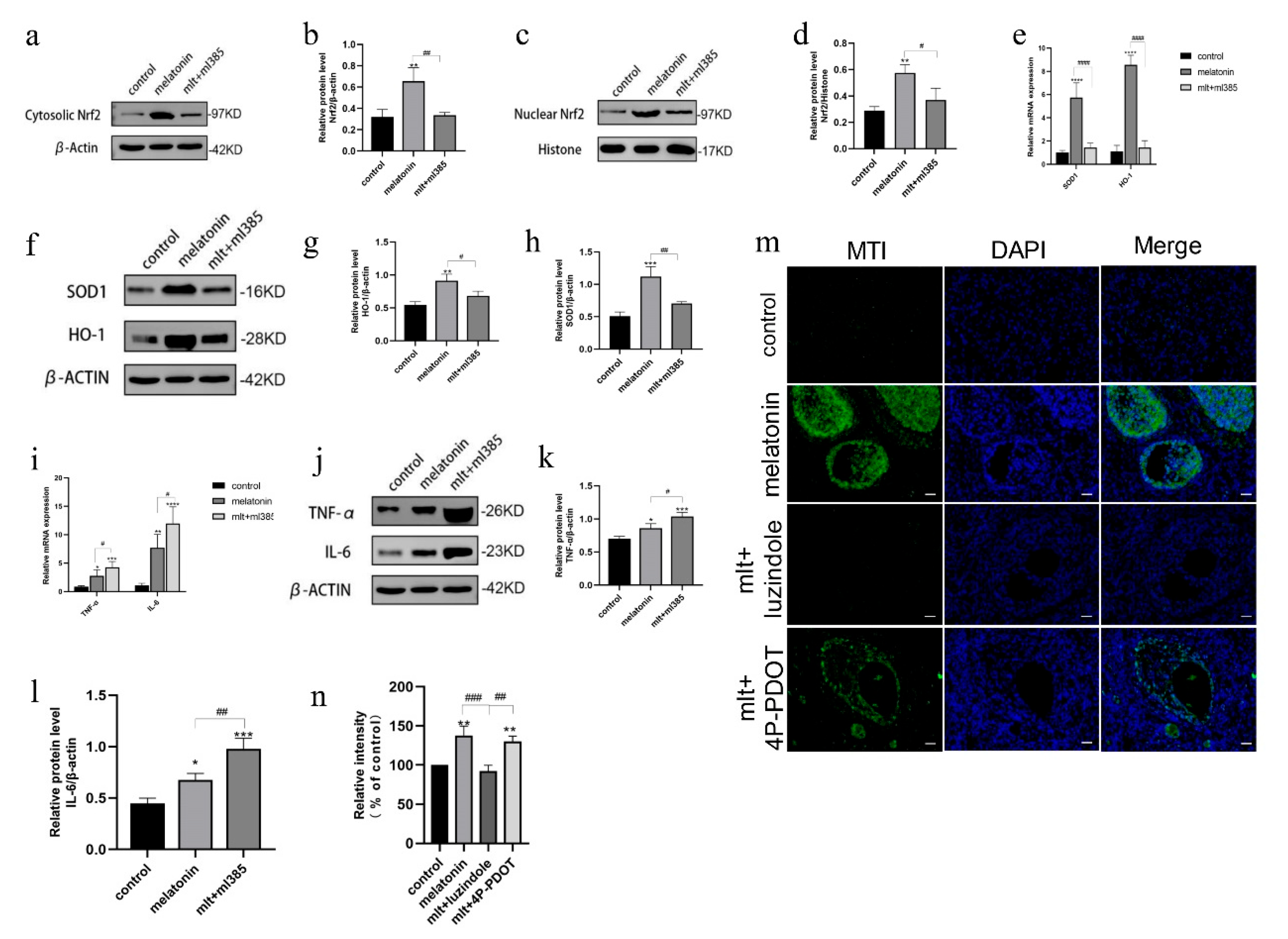
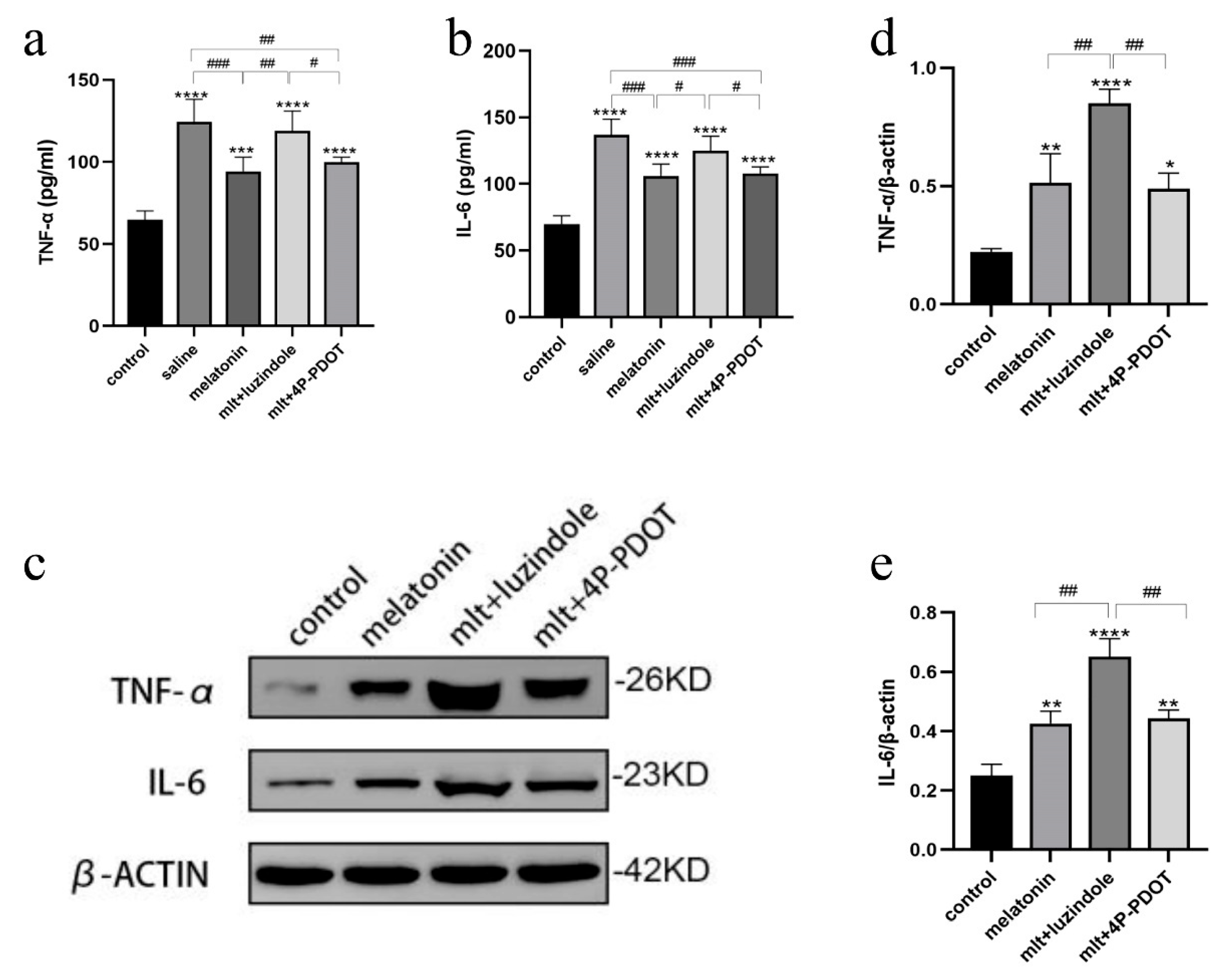
3.5. Inhibition of Melatonin Receptors Impairs Ovarian Protection Mediated by the Nrf2/ARE Pathway
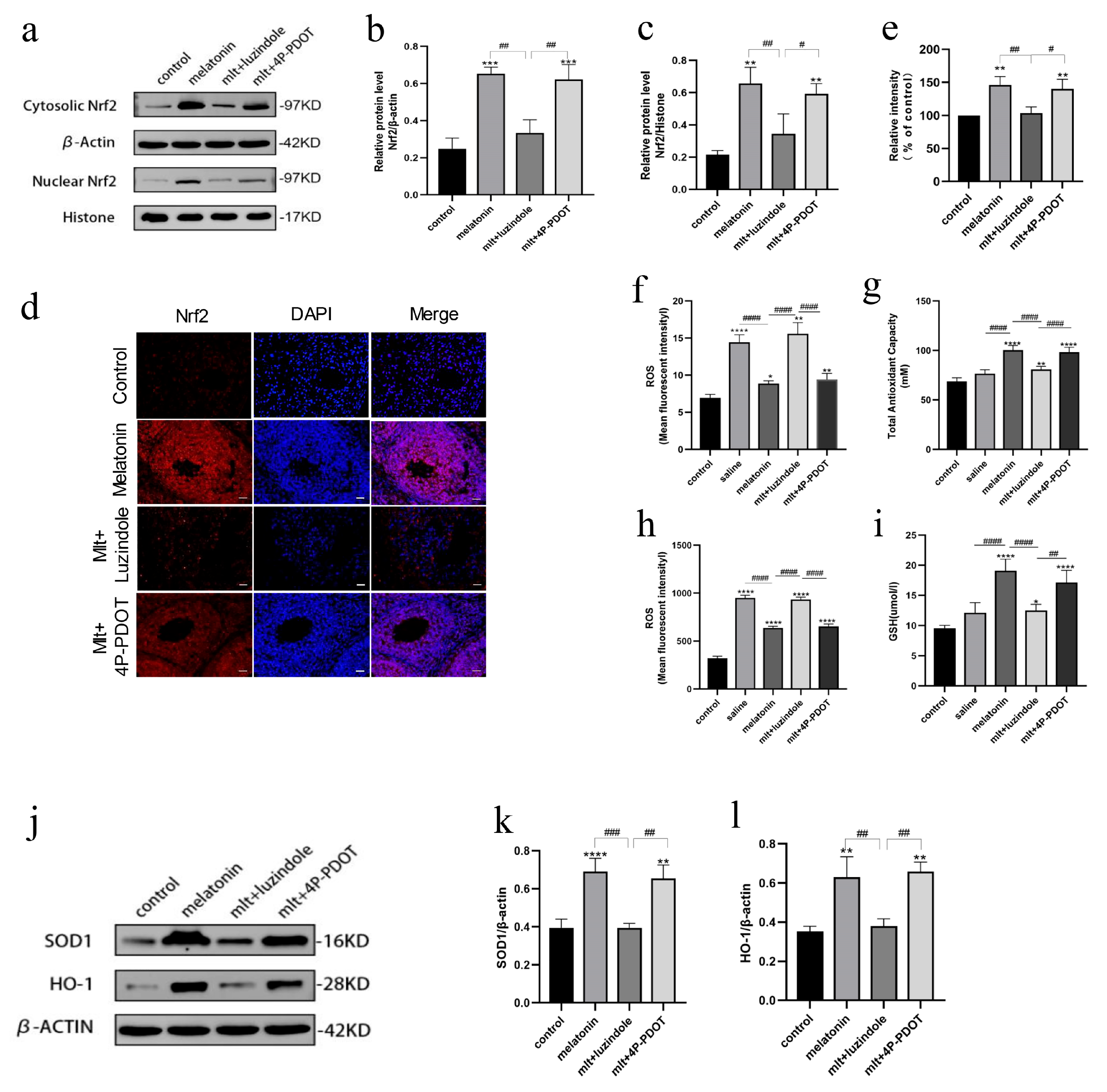
3.6. Inhibition of Melatonin Receptors Impairs the Protective Effects of Melatonin on Oxidative Stress
4. Discussion
5. Clinical Practice Perspective
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer in Women: Burden and Trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Brewster, D.H.; Wood, R.; Nowell, S.; Fischbacher, C.; Kelsey, T.W.; Wallace, W.H.B. The impact of cancer on subsequent chance of pregnancy: A population-based analysis. Hum. Reprod. 2018, 33, 1281–1290. [Google Scholar] [CrossRef]
- Rosenbrock, J.; Vásquez-Torres, A.; Mueller, H.; Behringer, K.; Zerth, M.; Celik, E.; Fan, J.; Trommer, M.; Linde, P.; Fuchs, M.; et al. Involved Site Radiotherapy Extends Time to Premature Menopause in Infra-Diaphragmatic Female Hodgkin Lymphoma Patients—An Analysis of GHSG HD14- and HD17-Patients. Front. Oncol. 2021, 11, 658358. [Google Scholar] [CrossRef] [PubMed]
- Mauri, D.; Gazouli, I.; Zarkavelis, G.; Papadaki, A.; Mavroeidis, L.; Gkoura, S.; Ntellas, P.; Amylidi, A.L.; Tsali, L.; Kampletsas, E. Chemotherapy Associated Ovarian Failure. Front. Endocrinol. 2020, 11, 572388. [Google Scholar] [CrossRef] [PubMed]
- Spears, N.; Lopes, F.; Stefansdottir, A.; Rossi, V.; De Felici, M.; Anderson, R.A.; Klinger, F.G. Ovarian damage from chemotherapy and current approaches to its protection. Hum. Reprod. Update 2019, 25, 673–693. [Google Scholar] [CrossRef]
- Cui, W.; Stern, C.; Hickey, M.; Goldblatt, F.; Anazodo, A.; Stevenson, W.S.; Phillips, K.A. Preventing ovarian failure associated with chemotherapy. Med. J. Aust. 2018, 209, 412–416. [Google Scholar] [CrossRef]
- Finkelstein, T.; Zhang, Y.; Vollenhoven, B.; Rolnik, D.L.; Horta, F. Successful pregnancy rates amongst patients undergoing ovarian tissue cryopreservation for non-malignant indications: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 292, 30–39. [Google Scholar] [CrossRef]
- Gavish, Z.; Spector, I.; Peer, G.; Schlatt, S.; Wistuba, J.; Roness, H.; Meirow, D. Follicle activation is a significant and immediate cause of follicle loss after ovarian tissue transplantation. J. Assist. Reprod. Genet. 2018, 35, 61–69. [Google Scholar] [CrossRef]
- Roness, H.; Meirow, D. FERTILITY PRESERVATION: Follicle reserve loss in ovarian tissue transplantation. Reproduction 2019, 158, F35–F44. [Google Scholar] [CrossRef]
- Han, C.; Zeng, Q.; He, L.; Luan, Z.; Liu, R.; Zhang, G.; Liu, W. Advances in the mechanisms related to follicle loss after frozen-thawed ovarian tissue transplantation. Transpl. Immunol. 2023, 81, 101935. [Google Scholar] [CrossRef]
- Olesen, H.; Pors, S.E.; Jensen, L.B.; Grønning, A.P.; Lemser, C.E.; Nguyen Heimbürger, M.T.H.; Mamsen, L.S.; Getreu, N.; Christensen, S.T.; Andersen, C.Y.; et al. N-acetylcysteine protects ovarian follicles from ischemia-reperfusion injury in xenotransplanted human ovarian tissue. Hum. Reprod. 2021, 36, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Koimtzis, G.; Stefanopoulos, L.; Brooker, V.; Geropoulos, G.; Chalklin, C.G.; Gupta, S.; Carrington-Windo, E.; Papaioannou, M.; Papavramidis, T.S. The Role of Vitamin D in Kidney Transplantation Outcomes: A Systematic Review. Life 2022, 12, 1664. [Google Scholar] [CrossRef]
- Bai, Y.-J.; Li, Y.-M.; Hu, S.-M.; Zou, Y.-G.; An, Y.-F.; Wang, L.-L.; Shi, Y.-Y. Vitamin D supplementation reduced blood inflammatory cytokines expression and improved graft function in kidney transplant recipients. Front. Immunol. 2023, 14, 1152295. [Google Scholar] [CrossRef]
- Ki, M.S.; Kim, N.E.; Woo, A.; Kim, S.Y.; Kim, Y.S.; Kim, H.E.; Lee, J.G.; Paik, H.C.; Park, M.S. Post-Transplant Vitamin D Deficiency in Lung Transplant Recipients: Impact on Outcomes and Prognosis. Transpl. Int. 2024, 37, 13313. [Google Scholar] [CrossRef] [PubMed]
- Courbebaisse, M.; Bourmaud, A.; Souberbielle, J.-C.; Sberro-Soussan, R.; Moal, V.; Le Meur, Y.; Kamar, N.; Albano, L.; Thierry, A.; Dantal, J.; et al. Nonskeletal and skeletal effects of high doses versus low doses of vitamin D3 in renal transplant recipients: Results of the VITALE (VITamin D supplementation in renAL transplant recipients) study, a randomized clinical trial. Am. J. Transplant. 2023, 23, 366–376. [Google Scholar] [CrossRef]
- Khatoon, R. Unlocking the Potential of Vitamin D: A Comprehensive Exploration of Its Role in Neurological Health and Diseases. Biology 2025, 14, 280. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Robredo, P.; González-Zamora, J.; Recalde, S.; Bilbao-Malavé, V.; Bezunartea, J.; Hernandez, M.; Garcia-Layana, A. Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants 2020, 9, 838. [Google Scholar] [CrossRef]
- Calvo, J.R.; Maldonado, M.D. Immunoregulatory properties of melatonin in the humoral immune system: A narrative review. Immunol. Lett. 2024, 269, 106901. [Google Scholar] [CrossRef]
- Andronachi, V.-C.; Simeanu, C.; Matei, M.; Radu-Rusu, R.-M.; Simeanu, D. Melatonin: An Overview on the Synthesis Processes and on Its Multiple Bioactive Roles Played in Animals and Humans. Agriculture 2025, 15, 273. [Google Scholar] [CrossRef]
- Esteban-Zubero, E.; García-Gil, F.A.; López-Pingarrón, L.; Alatorre-Jiménez, M.A.; Iñigo-Gil, P.; Tan, D.X.; García, J.J.; Reiter, R.J. Potential benefits of melatonin in organ transplantation: A review. J. Endocrinol. 2016, 229, R129–R146. [Google Scholar] [CrossRef] [PubMed]
- Zitkute, V.; Kvietkauskas, M.; Maskoliunaite, V.; Leber, B.; Ramasauskaite, D.; Strupas, K.; Stiegler, P.; Schemmer, P. Melatonin and Glycine Reduce Uterus Ischemia/Reperfusion Injury in a Rat Model of Warm Ischemia. Int. J. Mol. Sci. 2021, 22, 8373. [Google Scholar] [CrossRef] [PubMed]
- Shiroma, M.E.; Damous, L.L.; Cotrim, F.P.; Roa, C.L.; Cipolla-Neto, J.; Reiter, R.J.; Baracat, E.C.; Soares, J.M., Jr. Pretreatment with melatonin improves ovarian tissue cryopreservation for transplantation. Reprod. Biol. Endocrinol. RB&E 2021, 19, 17. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Yin, H.; Kim, S.S.; Lin Tan, S.; Gosden, R.G. Fertility after intact ovary transplantation. Nature 2002, 415, 385. [Google Scholar] [CrossRef]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef]
- Tu, W.; Wang, H.; Li, S.; Liu, Q.; Sha, H. The Anti-Inflammatory and Antioxidant Mechanisms of the Keap1/Nrf2/ARE Signaling Pathway in Chronic Diseases. Aging Dis. 2019, 10, 637–651. [Google Scholar] [CrossRef]
- Sindan, N.; Bhandari, A.; Zhao, Y.; Lu, X.; Lv, J. Expression and localization of nuclear factor erythroid 2-related factor 2 in the ovarian tissues of mice at different ages. Exp. Ther. Med. 2018, 16, 3546–3552. [Google Scholar] [CrossRef]
- Sze, S.C.W.; Zhang, L.; Zhang, S.; Lin, K.; Ng, T.B.; Ng, M.L.; Lee, K.F.; Lam, J.K.W.; Zhang, Z.; Yung, K.K.L. Aberrant Transferrin and Ferritin Upregulation Elicits Iron Accumulation and Oxidative Inflammaging Causing Ferroptosis and Undermines Estradiol Biosynthesis in Aging Rat Ovaries by Upregulating NF-Κb-Activated Inducible Nitric Oxide Synthase: First Demonstration of an Intricate Mechanism. Int. J. Mol. Sci. 2022, 23, 12689. [Google Scholar] [CrossRef]
- Lim, J.; Ortiz, L.; Nakamura, B.N.; Hoang, Y.D.; Banuelos, J.; Flores, V.N.; Chan, J.Y.; Luderer, U. Effects of deletion of the transcription factor Nrf2 and benzo [a]pyrene treatment on ovarian follicles and ovarian surface epithelial cells in mice. Reprod. Toxicol. (Elmsford N. Y.) 2015, 58, 24–32. [Google Scholar] [CrossRef]
- Opie, L.H.; Lecour, S. Melatonin has multiorgan effects. Eur. Heart J. Cardiovasc. Pharmacother. 2016, 2, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.Q.; Rohde, J.M.; Liu, L.; Nimmagadda, S.; Sudini, K.; Brimacombe, K.R.; Gajghate, S.; et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem. Biol. 2016, 11, 3214–3225. [Google Scholar] [CrossRef]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and Health: Insights of Melatonin Action, Biological Functions, and Associated Disorders. Cell. Mol. Neurobiol. 2023, 43, 2437–2458. [Google Scholar] [CrossRef]
- Al-Ghoul, W.M.; Herman, M.D.; Dubocovich, M.L. Melatonin receptor subtype expression in human cerebellum. Neuroreport 1998, 9, 4063–4068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Wang, J.; Lv, D.; Zhu, T.; Wang, F.; Tian, X.; Yao, Y.; Ji, P.; Liu, G. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal Res. 2019, 66, e12550. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Masana, M.I.; Iacob, S.; Sauri, D.M. Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1997, 355, 365–375. [Google Scholar] [CrossRef]
- Song, Z.; Han, H.; Ge, X.; Das, S.; Desert, R.; Athavale, D.; Chen, W.; Komakula, S.S.B.; Lantvit, D.; Nieto, N. Deficiency of neutrophil high-mobility group box-1 in liver transplant recipients exacerbates early allograft injury in mice. Hepatology 2023, 78, 771–786. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J. Molecular Mechanisms of Melatonin-Mediated Cell Protection and Signaling in Health and Disease. Pharmaceutics 2021, 13, 129. [Google Scholar] [CrossRef]
- Huang, C.C.; Chiou, C.H.; Liu, S.C.; Hu, S.L.; Su, C.M.; Tsai, C.H.; Tang, C.H. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: Implications for the treatment of rheumatoid arthritis. J. Pineal Res. 2019, 66, e12560. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Wang, F.; Zhang, Y.T.; Wei, S.Z.; Liu, J.Y.; Sun, H.Y.; Wang, G.H.; Liu, C.F. Microglial MT1 activation inhibits LPS-induced neuroinflammation via regulation of metabolic reprogramming. Aging Cell 2021, 20, e13375. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, C.; Zhang, K.; Lan, X.; Chen, X.; Zang, W.; Wang, Z.; Guan, F.; Zhu, C.; Yang, X.; et al. Melatonin receptor activation provides cerebral protection after traumatic brain injury by mitigating oxidative stress and inflammation via the Nrf2 signaling pathway. Free. Radic. Biol. Med. 2019, 131, 345–355. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-antioxidant response element pathway: A review of its regulation by melatonin and the proteasome. Mol. Cell. Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Zhang, R.; Ma, B.; Ni, L.; Feng, H.; Liu, C. Melatonin attenuates high glucose-induced endothelial cell pyroptosis by activating the Nrf2 pathway to inhibit NLRP3 inflammasome activation. Mol. Med. Rep. 2023, 27, 71. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tastan, B.; Tarakcioglu, E.; Tufekci, K.U.; Olcum, M.; Ersoy, N.; Bagriyanik, A.; Genc, K.; Genc, S. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front. Immunol. 2019, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, Z.; Ashrafizadeh, M. Melatonin as a potential modulator of Nrf2. Fundam. Clin. Pharmacol. 2020, 34, 11–19. [Google Scholar] [CrossRef]
- Shiroma, M.E.; Botelho, N.M.; Damous, L.L.; Baracat, E.C.; Soares, J.M., Jr. Melatonin influence in ovary transplantation: Systematic review. J. Ovarian Res. 2016, 9, 33. [Google Scholar] [CrossRef]
- Toporcerová, S.; Uhrinová, I.; Iannaccone, S.F.; Grendelová, A.; Petrášová, D.; Hudák, V.; Urdzík, P.; Toporcer, T. Effect of melatonin on frozen-thawed ovarian autograft in a rat model. Eur. J. Gynaecol. Oncol. 2019, 40, 603–608. [Google Scholar] [CrossRef]
- Monteiro, K.; Damous, L.L.; Shiroma, M.E.; Termini, L.; Cipolla-Neto, J.; Simoes, R.D.S.; da Silva, R.F.; Turri, J.A.; Baracat, E.C.; Soares-Junior, J.M. Melatonin increases superoxide dismutase 2 (SOD2) levels and improves rat ovarian graft function after transplantation. J. Ovarian Res. 2024, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Ding, Y.; Zhang, X. Melatonin and ovarian tissue transplantation: Current frontiers in research. J. Gynecol. Obstet. Hum. Reprod. 2024, 53, 102726. [Google Scholar] [CrossRef] [PubMed]
- Friedman, O.; Orvieto, R.; Fisch, B.; Felz, C.; Freud, E.; Ben-Haroush, A.; Abir, R. Possible improvements in human ovarian grafting by various host and graft treatments. Hum. Reprod. 2012, 27, 474–482. [Google Scholar] [CrossRef]
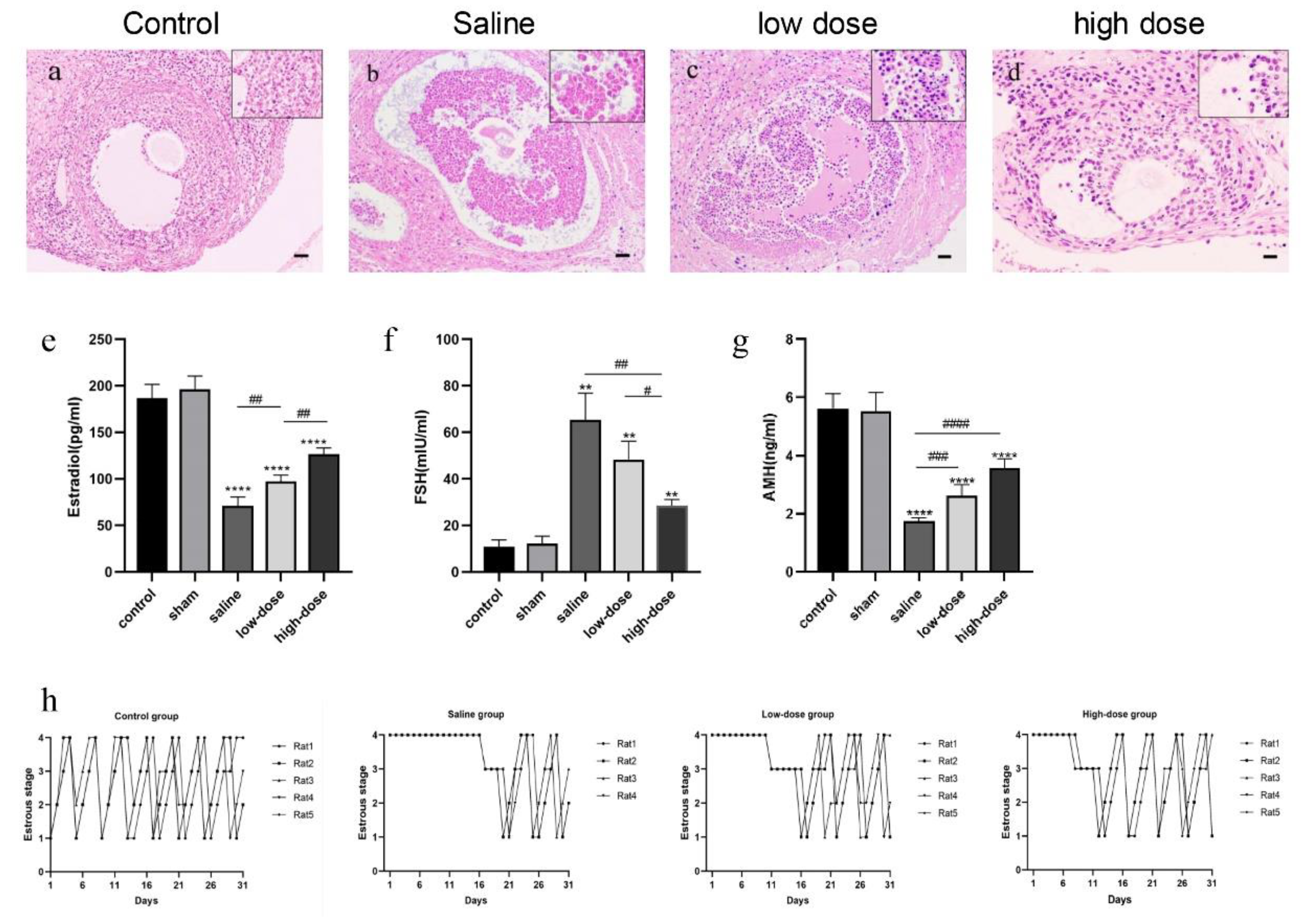


| Group | Primordial Follicles | Primary Follicles | Secondary Follicles | Antral Follicles |
|---|---|---|---|---|
| Fresh control (n = 5) | 103.6 ± 3.4 ** | 24.0 ± 2.7 ** | 13.4 ± 2.4 ** | 7.6 ± 1.1 ** |
| Saline (n = 5) | 56.4 ± 4.9 | 12.4 ± 2.1 | 5.6 ± 1.8 | 1.6 ± 1.1 |
| low dose (n = 5) | 64.4 ± 5.9 * | 17.6 ± 2.7 * | 8.4 ± 1.1 * | 2.8 ± 0.8 |
| high dose (n = 5) | 80.8 ± 2.9 ** | 19.0 ± 3.5 ** | 10 ± 1.6 ** | 5.4 ± 1.1 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Wang, S.; Wu, Y.; Zhang, X.; Ding, Y. Melatonin Protects Intact Rat Ovarian Transplantation via the MT1/Nrf2/ARE Pathway. Cells 2025, 14, 1588. https://doi.org/10.3390/cells14201588
Xie L, Wang S, Wu Y, Zhang X, Ding Y. Melatonin Protects Intact Rat Ovarian Transplantation via the MT1/Nrf2/ARE Pathway. Cells. 2025; 14(20):1588. https://doi.org/10.3390/cells14201588
Chicago/Turabian StyleXie, Lingyun, Shanshan Wang, Yuling Wu, Xuyin Zhang, and Yan Ding. 2025. "Melatonin Protects Intact Rat Ovarian Transplantation via the MT1/Nrf2/ARE Pathway" Cells 14, no. 20: 1588. https://doi.org/10.3390/cells14201588
APA StyleXie, L., Wang, S., Wu, Y., Zhang, X., & Ding, Y. (2025). Melatonin Protects Intact Rat Ovarian Transplantation via the MT1/Nrf2/ARE Pathway. Cells, 14(20), 1588. https://doi.org/10.3390/cells14201588






