From COPD to Smoke-Related Arteriopathy: The Mechanical and Immune–Inflammatory Landscape Underlying Lung Cancer Distant Spreading—A Narrative Review
Abstract
1. Introduction
2. Methods
3. The Lung Region
3.1. The Complex Landscape Linking COPD to Malignant Transformation and Cardiovascular Disease
3.1.1. Inflammation and Tissue Remodeling
3.1.2. Gene Reprogramming
3.1.3. COPD and Cardiovascular Events
3.2. Lung Cancer in Smokers
3.2.1. Smoke-Induced Tumorigenesis
3.2.2. Molecular Basis of Smoke-Associated Lung Carcinogenesis
4. Vascular Compartment
4.1. Smoke-Induced Vascular Pathology
4.1.1. Vascular Physiopathology
4.1.2. Blood Flow Rheology
4.1.3. Smoke-Associated Vascular Disease
4.2. Circulating Tumor Cell Dynamics in Vasculopathy
4.3. Apolipoproteins
5. The Immune–Inflammatory Axis in Vasculopathy and Lung Cancer Dissemination
Immune Checkpoint Inhibitors, Bronchodilation, and Anti-Platelet Therapy
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| nAChRs | Nicotinic Acetylcholine Receptor |
| EMT | Epithelial to Mesenchymal Transition |
| AMPK-α2 | AMP-activated Protein Kinase α2 |
| SCC | Squamous Cell Carcinoma |
| HBP17 | Heparin-Binding Protein/Azurocidin |
| TOB1 | Transducer of ERBB2, 1 |
| DUSP6 | Dual-Specificity Phosphatase 6 |
| BRD2 | part of the Bromodomain and Extra-Terminal motif (BET) protein family |
| LOH | Loss Of Heterozygosity |
| FHIT | Fragile Histidine Triad |
| CSC | Cancer Stem Cell |
| uPA | urokinase Plasminogen Activator |
| OPN | Osteopontin |
| NET | Neutrophil Extracellular Trap |
| CTC | Circulating Tumor Cell |
| vSMCs | vascular Smooth Muscle Cells |
| EC | Endothelial Cell |
| HDL | High-Density Lipoprotein |
| SP-C | Surfactant Protein C |
| CFD | Computational Fluid Dynamics |
| PAD | Peripheral Vascular Disease |
| MRI | Magnetic Resonance Imaging |
| WSS | Wall Shear Stress |
References
- Cancer Facts & Figures 2023; American Cancer Society, Inc.: Atlanta, GA, USA, 2022; Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2023/2023-cancer-facts-and-figures.pdf (accessed on 9 January 2024).
- Kızılırmak, D.; Yılmaz, Z.; Havlucu, Y.; Çelik, P. Impact of the COVID-19 Pandemic on Diagnosis of Lung Cancer. SN Compr. Clin. Med. 2023, 5, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantini, L.; Mentrasti, G.; Russo, G.L.; Signorelli, D.; Pasello, G.; Rijavec, E.; Russano, M.; Antonuzzo, L.; Rocco, D.; Giusti, R.; et al. Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of lung cancer patients in Italy (COVID-DELAY study): Fewer cases and higher stages from a real-world scenario. ESMO Open 2022, 7, 100406, Erratum in ESMO Open 2022, 7, 100471. https://doi.org/10.1016/j.esmoop.2022.100471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49, Erratum in CA Cancer J. Clin. 2024, 74, 203. https://doi.org/10.3322/caac.21830. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non–small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Skoulidis, F.; Heymach, J.V. Co-occurring genomic alterations in non-small-cell lung cancer biology and therapy. Nat. Rev. Cancer 2019, 19, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Šutić, M.; Vukić, A.; Baranašić, J.; Försti, A.; Džubur, F.; Samaržija, M.; Jakopović, M.; Brčić, L.; Knežević, J. Diagnostic, Predictive, and Prognostic Biomarkers in Non-Small Cell Lung Cancer (NSCLC) Management. J. Pers. Med. 2021, 11, 1102. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Brennan, P.; Hainaut, P.; Boffetta, P. Genetics of lung-cancer susceptibility. Lancet Oncol. 2011, 12, 399–408. [Google Scholar] [CrossRef]
- e Alencar, V.T.L.; Formiga, M.N.; de Lima, V.C.C. Inherited lung cancer: A review. Ecancermedicalscience 2020, 14, 1008. [Google Scholar] [CrossRef]
- Kanwal, M.; Ding, X.J.; Cao, Y. Familial risk for lung cancer. Oncol. Lett. 2017, 13, 535–542. [Google Scholar] [CrossRef]
- Subramanian, J.; Govindan, R. Molecular genetics of lung cancer in people who have never smoked. Lancet Oncol. 2008, 9, 676–682. [Google Scholar] [CrossRef]
- Stella, G.M.; Luisetti, M.; Pozzi, E.; Comoglio, P.M. Oncogenes in non-small-cell lung cancer: Emerging connections and novel therapeutic dynamics. Lancet Respir. Med. 2013, 1, 251–261. [Google Scholar] [CrossRef]
- Benusiglio, P.R.; Fallet, V.; Sanchis-Borja, M.; Coulet, F.; Cadranel, J. Lung cancer is also a hereditary disease. Eur. Respir. Rev. 2021, 30, 210045. [Google Scholar] [CrossRef] [PubMed]
- Smolle, E.; Pichler, M. Non-Smoking-Associated Lung Cancer: A distinct Entity in Terms of Tumor Biology, Patient Characteristics and Impact of Hereditary Cancer Predisposition. Cancers 2019, 11, 204. [Google Scholar] [CrossRef]
- Vancheri, C.; Failla, M.; Crimi, N.; Raghu, G. Idiopathic pulmonary fibrosis: A disease with similarities and links to cancer biology. Eur. Respir. J. 2010, 35, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Stella, G.M.; D’Agnano, V.; Piloni, D.; Saracino, L.; Lettieri, S.; Mariani, F.; Lancia, A.; Bortolotto, C.; Rinaldi, P.; Falanga, F.; et al. The oncogenic landscape of the idiopathic pulmonary fibrosis: A narrative review. Transl. Lung Cancer Res. 2022, 11, 472–496. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, S.; Oggionni, T.; Lancia, A.; Bortolotto, C.; Stella, G.M. Immune Stroma in Lung Cancer and Idiopathic Pulmonary Fibrosis: A Common Biologic Landscape? Int. J. Mol. Sci. 2021, 22, 2882. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.; Chino, V.; Allocca, V.; D’Agnano, V.; Bortolotto, C.; Bianco, A.; Corsico, A.G.; Stella, G.M. Idiopathic pulmonary fibrosis and lung cancer: Targeting the complexity of the pharmacological interconnection. Expert. Rev. Respir. Med. 2022, 16, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- CDC. Available online: https://www.cdc.gov/lung-cancer/risk-factors/index.html#:~:text=People%20who%20smoke%20cigarettes%20are,the%20risk%20of%20lung%20cancer (accessed on 15 July 2025).
- Crispo, A.; Brennan, P.; Jöckel, K.H.; Schaffrath-Rosario, A.; Wichmann, H.E.; Nyberg, F.; Simonato, L.; Merletti, F.; Forastiere, F.; Boffetta, P.; et al. The cumulative risk of lung cancer among current, ex- and never-smokers in European men. Br. J. Cancer 2004, 91, 1280–1286. [Google Scholar] [CrossRef]
- Asomaning, K.; Miller, D.P.; Liu, G.; Wain, J.C.; Lynch, T.J.; Su, L.; Christiani, D.C. Second hand smoke, age of exposure and lung cancer risk. Lung Cancer 2008, 61, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.D.; Schiller, J.H.; Boffetta, P.; Cai, J.; Connolly, C.; Kerpel-Fronius, A.; Kitts, A.B.; Lam, D.C.L.; Mohan, A.; Myers, R.; et al. Air Pollution and Lung Cancer: A Review by International Association for the Study of Lung Cancer Early Detection and Screening Committee. J. Thorac. Oncol. 2023, 18, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Huang, W.; Chen, G. The International Agency for Research on Cancer (IARC) evaluation of the carcinogenicity of outdoor air pollution: Focus on China. Chin. J. Cancer 2014, 33, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.; Brauer, M.; Dummer, T.; Atkar-Khattra, S.; Yee, J.; Melosky, B.; Ho, C.; McGuire, A.L.; Sun, S.; Grant, K.; et al. High-Ambient Air Pollution Exposure Among Never Smokers Versus Ever Smokers with Lung Cancer. J. Thorac. Oncol. 2021, 16, 1850–1858. [Google Scholar] [CrossRef]
- Brunekreef, B.; Beelen, R.; Hoek, G.; Schouten, L.; Bausch-Goldbohm, S.; Fischer, P.; Armstrong, B.; Hughes, E.; Jerrett, M.; van den Brandt, P. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: The NLCS-AIR study. Res. Rep. Health Eff. Inst. 2009, 139, 5–71; discussion 73–89. [Google Scholar] [PubMed]
- Shahadin, M.S.; Ab Mutalib, N.S.; Latif, M.T.; Greene, C.M.; Hassan, T. Challenges and future direction of molecular research in air pollution-related lung cancers. Lung Cancer 2018, 118, 69–75. [Google Scholar] [CrossRef]
- Celià-Terrassa, T.; Kang, Y. Metastatic niche functions and therapeutic opportunities. Nat. Cell Biol. 2018, 20, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Rodrigues, P.; Wesolowski, L.; Vanharanta, S. Genomic control of metastasis. Br. J. Cancer 2021, 124, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kiri, S.; Ryba, T. Cancer, metastasis, and the epigenome. Mol. Cancer 2024, 23, 154. [Google Scholar] [CrossRef]
- Stella, G.M.; Senetta, R.; Cassenti, A.; Ronco, M.; Cassoni, P. Cancers of unknown primary origin: Current perspectives and future therapeutic strategies. J. Transl. Med. 2012, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016, 138, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Timens, W. The pathology of chronic obstructive pulmonary disease. Annu. Rev. Pathol. 2009, 4, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Brusselle, G.G.; Joos, G.F.; Bracke, K.R. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011, 378, 1015–1026. [Google Scholar] [CrossRef]
- Chong, L.; Guo, P.; Zhu, Y. Mediator Complex: A Pivotal Regulator of ABA Signaling Pathway and Abiotic Stress Response in Plants. Int. J. Mol. Sci. 2020, 21, 7755. [Google Scholar] [CrossRef] [PubMed]
- Yang, I.A.; Relan, V.; Wright, C.M.; Davidson, M.R.; Sriram, K.B.; Savarimuthu Francis, S.M.; Clarke, B.E.; Duhig, E.E.; Bowman, R.V.; Fong, K.M. Common pathogenic mechanisms and epigenetic changes in lung cancer and COPD. Expert Opin. Ther. Targets 2011, 15, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Caramori, G.; Ruggeri, P.; Mumby, S.; Ieni, A.; Lo Bello, F.; Chimankar, V.; Donovan, C.; Andò, F.; Nucera, F.; Coppolino, I.; et al. Molecular links between COPD and lung cancer: New targets for drug discovery? Expert Opin. Ther. Targets 2019, 23, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Nurwidya, F.; Damayanti, T.; Yunus, F. The Role of Innate and Adaptive Immune Cells in the Immunopathogenesis of Chronic Obstructive Pulmonary Disease. Tuberc. Respir. Dis. 2016, 79, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.K.; Scott, A.; Tatler, A.L.; Belchamber, K.B.R.; Cox, M.J. Macrophages and the microbiome in chronic obstructive pulmonary disease. Eur. Respir. Rev. 2024, 33, 240053. [Google Scholar] [CrossRef] [PubMed]
- Bender, E.C.; Tareq, H.S.; Suggs, L.J. Inflammation: A matter of immune cell life and death. Npj Biomed. Innov. 2025, 2, 7. [Google Scholar] [CrossRef]
- Sohal, S.S.; Mahmood, M.Q.; Walters, E.H. Clinical significance of epithelial mesenchymal transition (EMT) in chronic obstructive pulmonary disease (COPD): Potential target for prevention of airway fibrosis and lung cancer. Clin. Trans. Med. 2014, 3, e33. [Google Scholar] [CrossRef]
- Sohal, S.; Ward, C.; Danial, W.; Wood-Baker, R.; Walters, E. Recent advances in understanding inflammation and remodeling in the airways in chronic obstructive pulmonary disease. Exp. Rev. Respir. Med. 2013, 7, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Wang, L.F.; Yin, Y.Q. How Do Innate Immune Cells Contribute to Airway Remodeling in COPD Progression? Int. J. Chron. Obstruct. Pulmon. Dis. 2020, 15, 107–116. [Google Scholar] [CrossRef]
- Brake, S.J.; Lu, W.; Chia, C.; Haug, G.; Larby, J.; Hardikar, A.; Singhera, G.K.; Hackett, T.L.; Eapen, M.S.; Sohal, S.S. Transforming growth factor-β1 and SMAD signalling pathway in the small airways of smokers and patients with COPD: Potential role in driving fibrotic type-2 epithelial mesenchymal transition. Front. Immunol. 2023, 14, 1216506. [Google Scholar] [CrossRef]
- Lu, W.; Eapen, M.S.; Hardikar, A.; Chia, C.; Robertson, I.; Singhera, G.K.; Hackett, T.L.; Sohal, S.S. Epithelial-mesenchymal transition changes in nonsmall cell lung cancer patients with early COPD. ERJ Open Res. 2023, 9, 00581-2023. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428, Erratum in J. Clin. Investig. 2010, 120, 1786. https://doi.org/10.1172/JCI39104C1. [Google Scholar] [CrossRef]
- Marconi, G.D.; Fonticoli, L.; Rajan, T.S.; Pierdomenico, S.D.; Trubiani, O.; Pizzicannella, J.; Diomede, F. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells 2021, 10, 1587. [Google Scholar] [CrossRef]
- Boccaccio, C.; Comoglio, P.M. Invasive growth: A MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer 2006, 6, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Perelli, L.; Zhang, L.; Mangiameli, S.; Giannese, F.; Mahadevan, K.K.; Peng, F.; Citron, F.; Khan, H.; Le, C.; Gurreri, E.; et al. Evolutionary fingerprints of epithelial-to-mesenchymal transition. Nature 2025, 640, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Carstens, J.L.; Lovisa, S. Epithelial-to-mesenchymal transition drives cancer genomic instability. J. Exp. Clin. Cancer Res. 2025, 44, 135. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.Q.; Sohal, S.S.; Shukla, S.D. Epithelial mesenchymal transition in smokers: Large versus small airways and relation to airflow obstruction. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 1515–1524. [Google Scholar] [CrossRef]
- Sohal, S.S. Chronic Obstructive Pulmonary Disease (COPD) and Lung Cancer: Epithelial Mesenchymal Transition (EMT), the Missing Link? EBioMedicine 2015, 2, 1578–1579. [Google Scholar] [CrossRef][Green Version]
- Soltani, A.; Mahmood, M.Q.; Reid, D.W.; Walters, E.H. Cancer-protective effects of inhaled corticosteroids in COPD are likely related to modification of epithelial activation. Eur. Respir. J. 2019, 54, 1901088. [Google Scholar] [CrossRef]
- Mottais, A.; Riberi, L.; Falco, A.; Soccal, S.; Gohy, S.; De Rose, V. Epithelial-Mesenchymal Transition Mechanisms in Chronic Airway Diseases: A Common Process to Target? Int. J. Mol. Sci. 2023, 24, 12412. [Google Scholar] [CrossRef]
- Su, X.; Wu, W.; Zhu, Z.; Lin, X.; Zeng, Y. The effects of epithelial-mesenchymal transitions in COPD induced by cigarette smoke: An update. Respir. Res. 2022, 23, 225. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Guo, Z. Stem cell therapies for chronic obstructive pulmonary disease: Mesenchymal stem cells as a promising treatment option. Stem Cell Res. Ther. 2024, 15, 312. [Google Scholar] [CrossRef]
- Ritzmann, F.; Brand, M.; Bals, R.; Wegmann, M.; Beisswenger, C. Role of Epigenetics in Chronic Lung Disease. Cells 2025, 14, 251. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Castaldi, P.J.; Wan, E.S.; Siedlinski, M.; Hersh, C.P.; Demeo, D.L.; Himes, B.E.; Sylvia, J.S.; Klanderman, B.J.; Ziniti, J.P.; et al. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum. Mol. Genet. 2012, 21, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Basma, H.; Gunji, Y.; Iwasawa, S.; Nelson, A.; Farid, M.; Ikari, J.; Liu, X.; Wang, X.; Michalski, J.; Smith, L.; et al. Reprogramming of COPD lung fibroblasts through formation of induced pluripotent stem cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L552–L565. [Google Scholar] [CrossRef]
- Botelho, F.M.; Bauer, C.M.; Finch, D.; Nikota, J.K.; Zavitz, C.C.; Kelly, A.; Lambert, K.N.; Piper, S.; Foster, M.L.; Goldring, J.J.; et al. IL-1α/IL-1R1 expression in chronic obstructive pulmonary disease and mechanistic relevance to smoke-induced neutrophilia in mice. PLoS ONE 2011, 6, e28457. [Google Scholar] [CrossRef]
- Zhou, X.; Baron, R.M.; Hardin, M.; Cho, M.H.; Zielinski, J.; Hawrylkiewicz, I.; Sliwinski, P.; Hersh, C.P.; Mancini, J.D.; Lu, K.; et al. Identification of a chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum. Mol. Genet. 2012, 21, 1325–1335. [Google Scholar] [CrossRef]
- Ghosh, B.; Loube, J.; Thapa, S.; Ryan, H.; Capodanno, E.; Chen, D.; Swaby, C.; Chen, S.; Mahmud, S.; Girgis, M.; et al. Loss of E-cadherin is causal to pathologic changes in chronic lung disease. Commun. Biol. 2022, 5, 1149. [Google Scholar] [CrossRef]
- Yeung-Luk, B.H.; Wally, A.; Swaby, C.; Jauregui, S.; Lee, E.; Zhang, R.; Chen, D.; Luk, S.H.; Upadya, N.; Tieng, E.; et al. Epigenetic Reprogramming Drives Epithelial Disruption in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2024, 70, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, U.; Llamazares Prada, M.; Pohl, S.T.; Richter, M.; Tamas, R.; Schuler, M.; Keller, C.; Mijosek, V.; Muley, T.; Schneider, M.A.; et al. High-resolution transcriptomic and epigenetic profiling identifies novel regulators of COPD. EMBO J. 2023, 42, e111272. [Google Scholar] [CrossRef] [PubMed]
- van Gestel, A.J.; Clarenbach, C.F.; Stöwhas, A.C.; Rossi, V.A.; Sievi, N.A.; Camen, G.; Kohler, M. The speed of blood pressure fluctuations in patients with chronic obstructive pulmonary disease. Heart Lung Circ. 2014, 23, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Wu, L.; Man, S.F. The relationship between reduced lung function and cardiovascular mortality: A population-based study and a systematic review of the literature. Chest 2005, 127, 1952–1959. [Google Scholar] [CrossRef]
- Calverley, P.M.; Anderson, J.A.; Celli, B.; Ferguson, G.T.; Jenkins, C.; Jones, P.W.; Yates, J.C.; Vestbo, J.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N. Engl. J. Med. 2007, 356, 775–789. [Google Scholar] [CrossRef]
- Barnes, P.J.; Celli, B.R. Systemic manifestations and comorbidities of COPD. Eur. Respir. J. 2009, 33, 1165–1185. [Google Scholar] [CrossRef]
- Barr, R.G.; Mesia-Vela, S.; Austin, J.H.; Basner, R.C.; Keller, B.M.; Reeves, A.P.; Shimbo, D.; Stevenson, L. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: The Emphysema and Cancer Action Project (EMCAP) Study. Am. J. Respir. Crit. Care Med. 2007, 176, 1200–1207. [Google Scholar] [CrossRef]
- Imaizumi, Y.; Eguchi, K.; Kario, K. Lung Disease and Hypertension. Pulse 2014, 2, 103–112. [Google Scholar] [CrossRef]
- Fujikura, K.; Albini, A.; Barr, R.G.; Parikh, M.; Kern, J.; Hoffman, E.; Hiura, G.T.; Bluemke, D.A.; Carr, J.; Lima, J.A.C.; et al. Aortic enlargement in chronic obstructive pulmonary disease (COPD) and emphysema: The Multi-Ethnic Study of Atherosclerosis (MESA) COPD study. Int. J. Cardiol. 2021, 331, 214–220. [Google Scholar] [CrossRef]
- Ando, K.; Kaneko, N.; Doi, T.; Aoshima, M.; Takahashi, K. Prevalence and risk factors of aortic aneurysm in patients with chronic obstructive pulmonary disease. J. Thorac. Dis. 2014, 6, 1388–1395. [Google Scholar] [CrossRef]
- Bellamkonda, K.S.; Suckow, B.D.; Columbo, J.A.; Upchurch, G.R., Jr.; Jacobs, B.; Ochoa Chaar, C.I.; Scully, R.E.; Goodney, P.P.; Scali, S.T.; Stone, D.H. The Implications of Oxygen-Dependent Chronic Obstructive Pulmonary Disease on Sac Growth and Mortality Following Endovascular Aneurysm Repair. Ann. Vasc. Surg. 2025, 115, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic aromatic hydrocarbons: From metabolism to lung cancer. Toxicol. Sci. 2015, 145, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.; Enewold, L.; Pellizzari, E.; Beach, J.B.; Bowman, E.D.; Krishnan, S.S.; Shields, P.G. Smoking increases carcinogenic polycyclic aromatic hydrocarbons in human lung tissue. Cancer Res. 2001, 61, 6367–6371. [Google Scholar]
- Martey, C.A.; Baglole, C.J.; Gasiewicz, T.A.; Sime, P.J.; Phipps, R.P. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L391–L399. [Google Scholar] [CrossRef]
- Shehata, S.A.; Toraih, E.A.; Ismail, E.A.; Hagras, A.M.; Elmorsy, E.; Fawzy, M.S. Vaping, Environmental Toxicants Exposure, and Lung Cancer Risk. Cancers 2023, 15, 4525. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, C.; Hsu, M.L.; Wisnivesky, J.; Dowlati, A.; Boffetta, P.; Kong, C.Y. E-Cigarette Use and Lung Cancer Screening Uptake. JAMA Netw. Open. 2024, 7, e2419648. [Google Scholar] [CrossRef]
- Allbright, K.; Villandre, J.; Crotty Alexander, L.E.; Zhang, M.; Benam, K.H.; Evankovich, J.; Königshoff, M.; Chandra, D. The paradox of the safer cigarette: Understanding the pulmonary effects of electronic cigarettes. Eur. Respir. J. 2024, 63, 2301494. [Google Scholar] [CrossRef]
- Available online: https://www.cdc.gov/tobacco/e-cigarettes/youth.html (accessed on 15 July 2025).
- Klebe, S.; Leigh, J.; Henderson, D.W.; Nurminen, M. Asbestos, Smoking and Lung Cancer: An Update. Int. J. Environ. Res. Public Health 2019, 17, 258. [Google Scholar] [CrossRef]
- van Zandwijk, N.; Frank, A.L.; Reid, G.; Dimitri Røe, O.; Amos, C.I. Asbestos-Related lung Cancer: An underappreciated oncological issue. Lung Cancer 2024, 194, 107861. [Google Scholar] [CrossRef] [PubMed]
- Saracino, L.; Zorzetto, M.; Inghilleri, S.; Pozzi, E.; Stella, G.M. Non-neuronal cholinergic system in airways and lung cancer susceptibility. Transl. Lung Cancer Res. 2013, 2, 284–294. [Google Scholar] [CrossRef]
- Dasgupta, P.; Rizwani, W.; Pillai, S.; Kinkade, R.; Kovacs, M.; Rastogi, S.; Banerjee, S.; Carless, M.; Kim, E.; Coppola, D.; et al. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int. J. Cancer 2009, 124, 36–45. [Google Scholar] [CrossRef]
- Di Cello, F.; Flowers, V.L.; Li, H.; Vecchio-Pagán, B.; Gordon, B.; Harbom, K.; Shin, J.; Beaty, R.; Wang, W.; Brayton, C.; et al. Cigarette smoke induces epithelial to mesenchymal transition and increases the metastatic ability of breast cancer cells. Mol. Cancer 2013, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Prashanth, N.; Meghana, P.; Sandeep Kumar Jain, R.; Pooja SRajaput Satyanarayan, N.D.; Raja Naika, H.; Kumaraswamy, H.M. Nicotine promotes epithelial to mesenchymal transition and gemcitabine resistance via hENT1/RRM1 signalling in pancreatic cancer and chemosensitizing effects of Embelin-a naturally occurring benzoquinone. Sci. Total Environ. 2024, 914, 169727. [Google Scholar] [CrossRef]
- Chen, P.C.; Lee, W.Y.; Ling, H.H.; Cheng, C.H.; Chen, K.C.; Lin, C.W. Activation of fibroblasts by nicotine promotes the epithelial-mesenchymal transition and motility of breast cancer cells. J. Cell Physiol. 2018, 233, 4972–4980. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Masiello, M.G.; Proietti, S.; Coluccia, P.; Fabrizi, G.; Catizone, A.; Ricci, G.; de Toma, G.; Bizzarri, M.; Cucina, A. Nicotine increases colon cancer cell migration and invasion through epithelial to mesenchymal transition (EMT): COX-2 involvement. J. Cell Physiol. 2018, 233, 4935–4948. [Google Scholar] [CrossRef]
- Raveendran, M.; Senthil, D.; Utama, B.; Shen, Y.; Dudley, D.; Wang, J.; Zhang, Y.; Wang, X.L. Cigarette suppresses the expression of P4Hα and vascular collagen production. Biochem. Biophys. Res. Commun. 2004, 323, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Lemaître, V.; Dabo, A.J.; D’Armiento, J. Cigarette smoke components induce matrix metalloproteinase-1 in aortic endothelial cells through inhibition of mTOR signaling. Toxicol. Sci. 2011, 123, 542–549. [Google Scholar] [CrossRef]
- Arif, B.; Garcia-Fernandez, F.; Ennis, T.L.; Jin, J.; Davis, E.C.; Thompson, R.W.; Curci, J.A. Novel mechanism of aortic aneurysm development in mice associated with smoking and leukocytes. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2901–2909. [Google Scholar] [CrossRef]
- Liao, S.; Curci, J.A.; Kelley, B.J.; Sicard, G.A.; Thompson, R.W. Accelerated replicative senescence of medial smooth muscle cells derived from abdominal aortic aneurysms compared to the adjacent inferior mesenteric artery. J. Surg. Res. 2000, 92, 85–95. [Google Scholar] [CrossRef]
- Polytarchou, C.; Iliopoulos, D.; Hatziapostolou, M.; Kottakis, F.; Maroulakou, I.; Struhl, K.; Tsichlis, P.N. Akt2 regulates all Akt isoforms and promotes resistance to hypoxia through induction of miR-21 upon oxygen deprivation. Cancer Res. 2011, 71, 4720–4731. [Google Scholar] [CrossRef]
- Husgafvel-Pursiainen, K. Genotoxicity of environmental tobacco smoke: A review. Mutat Res. 2004, 567, 427–445. [Google Scholar] [CrossRef]
- Stannard, L.; Doak, S.H.; Doherty, A.; Jenkins, G.J. Is Nickel Chloride really a Non-Genotoxic Carcinogen? Basic Clin. Pharmacol. Toxicol. 2017, 121 (Suppl. S3), 10–15. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Chu, G.; Zhou, G.; Jiang, J.; Huang, F.; Xu, J.; Zheng, S.; Chen, Z.; Jiang, W.; Lu, Y.; et al. Assessing cytogenotoxicity of cigarette smoke condensates using three in vitro assays. Mutat. Res. 2009, 677, 21–26. [Google Scholar] [CrossRef]
- Messner, B.; Frotschnig, S.; Steinacher-Nigisch, A.; Winter, B.; Eichmair, E.; Gebetsberger, J.; Schwaiger, S.; Ploner, C.; Laufer, G.; Bernhard, D. Apoptosis and necrosis: Two different outcomes of cigarette smoke condensate-induced endothelial cell death. Cell Death Dis. 2012, 3, e424. [Google Scholar] [CrossRef] [PubMed]
- Khulan, B.; Ye, K.; Shi, M.K.; Waldman, S.; Marsh, A.; Siddiqui, T.; Okorozo, A.; Desai, A.; Patel, D.; Dobkin, J.; et al. Normal bronchial field basal cells show persistent methylome-wide impact of tobacco smoking, including in known cancer genes. Epigenetics 2025, 20, 2466382. [Google Scholar] [CrossRef]
- Herzog, C.; Jones, A.; Evans, I.; Raut, J.R.; Zikan, M.; Cibula, D.; Wong, A.; Brenner, H.; Richmond, R.C.; Widschwendter, M. Cigarette Smoking and E-cigarette Use Induce Shared DNA Methylation Changes Linked to Carcinogenesis. Cancer Res. 2024, 84, 1898–1914. [Google Scholar] [CrossRef] [PubMed]
- Essogmo, F.E.; Zhilenkova, A.V.; Tchawe, Y.S.N.; Owoicho, A.M.; Rusanov, A.S.; Boroda, A.; Pirogova, Y.N.; Sangadzhieva, Z.D.; Sanikovich, V.D.; Bagmet, N.N.; et al. Cytokine Profile in Lung Cancer Patients: Anti-Tumor and Oncogenic Cytokines. Cancers 2023, 15, 5383. [Google Scholar] [CrossRef]
- Cook, J.H.; Melloni, G.E.M.; Gulhan, D.C.; Park, P.J.; Haigis, K.M. The origins and genetic interactions of KRAS mutations are allele- and tissue-specific. Nat. Commun. 2021, 12, 1808. [Google Scholar] [CrossRef]
- O’Sullivan, É.; Keogh, A.; Henderson, B.; Finn, S.P.; Gray, S.G.; Gately, K. Treatment Strategies for KRAS-Mutated Non-Small-Cell Lung Cancer. Cancers 2023, 15, 1635. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRASp.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- Jänne, P.A.; Riely, G.J.; Gadgeel, S.M.; Heist, R.S.; Ou, S.I.; Pacheco, J.M.; Johnson, M.L.; Sabari, J.K.; Leventakos, K.; Yau, E.; et al. Adagrasib in Non-Small-Cell Lung Cancer Harboring a KRASG12C Mutation. N. Engl. J. Med. 2022, 387, 120–131. [Google Scholar] [CrossRef]
- Sidaway, P. Sotorasib effective in KRAS-mutant NSCLC. Nat. Rev. Clin. Oncol. 2021, 18, 470. [Google Scholar] [CrossRef]
- Longo, D.L.; Rosen, N. Targeting Oncogenic RAS Protein. N. Engl. J. Med. 2022, 387, 184–186. [Google Scholar] [CrossRef]
- Le Calvez, F.; Mukeria, A.; Hunt, J.D.; Kelm, O.; Hung, R.J.; Tanière, P.; Brennan, P.; Boffetta, P.; Zaridze, D.G.; Hainaut, P. TP53 and KRAS mutation load and types in lung cancers in relation to tobacco smoke: Distinct patterns in never, former, and current smokers. Cancer Res. 2005, 65, 5076–5083. [Google Scholar] [CrossRef]
- Joehanes, R.; Just, A.C.; Marioni, R.E.; Pilling, L.C.; Reynolds, L.M.; Mandaviya, P.R.; Guan, W.; Xu, T.; Elks, C.E.; Aslibekyan, S.; et al. Epigenetic Signatures of Cigarette Smoking. Circ. Cardiovasc. Genet. 2016, 9, 436–447. [Google Scholar] [CrossRef]
- Raso, M.G.; Wistuba, I.I. Molecular pathogenesis of early-stage non-small cell lung cancer and a proposal for tissue banking to facilitate identification of new biomarkers. J. Thorac. Oncol. 2007, 2 (Suppl. S3), S128–S135. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Suzuki, Y.; Fujishita, T.; Kitahara, H.; Shimamatsu, S.; Kohno, M.; Morodomi, Y.; Kawano, D.; Maehara, Y. The prognostic impact of the amount of tobacco smoking in non-small cell lung cancer--differences between adenocarcinoma and squamous cell carcinoma. Lung Cancer 2014, 85, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Maeda, R.; Yoshida, J.; Ishii, G.; Hishida, T.; Nishimura, M.; Nagai, K. The prognostic impact of cigarette smoking on patients with non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 735–742. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bhandare, R.R.; Boddu, S.H.S.; Shaik, A.B.; Saktivel, L.P.; Gupta, G.; Negi, P.; Barakat, M.; Singh, S.K.; Dua, K.; et al. Molecular mechanisms underlying the regulation of tumour suppressor genes in lung cancer. Biomed. Pharmacother. 2024, 173, 116275. [Google Scholar] [CrossRef]
- Tao, S.; Pu, Y.; Yang, E.J.; Ren, G.; Shi, C.; Chen, L.J.; Chen, L.; Shim, J.S. Inhibition of GSK3β is synthetic lethal with FHIT loss in lung cancer by blocking homologous recombination repair. Exp. Mol. Med. 2025, 57, 167–183. [Google Scholar] [CrossRef]
- Spira, A.; Beane, J.; Shah, V.; Liu, G.; Schembri, F.; Yang, X.; Palma, J.; Brody, J.S. Effects of cigarette smoke on the human airway epithelial cell transcriptome. Proc. Natl. Acad. Sci. USA 2004, 101, 10143–10148. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.; Beane, J.E.; Shah, V.; Steiling, K.; Liu, G.; Schembri, F.; Gilman, S.; Dumas, Y.M.; Calner, P.; Sebastiani, P.; et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat. Med. 2007, 13, 361–366. [Google Scholar] [CrossRef]
- Castaldi, P.; Sauler, M. Cigarette Smoking and the Airway Epithelium: Characterizing Changes in Gene Expression over Time. Am. J. Respir. Crit. Care Med. 2023, 208, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.C.; Li, Q.Y.; Tian, L.; Liu, H.L.; Liu, X.H.; Shi, Y.; He, C.; Ding, S.S.; Yan, J.; Li, K.; et al. Identification of apoptosis-associated protein factors distinctly expressed in cigarette smoke condensate-exposed airway bronchial epithelial cells. J. Biochem. Mol. Toxicol. 2020, 34, e22444. [Google Scholar] [CrossRef]
- Alamgeer, M.; Peacock, C.D.; Matsui, W.; Ganju, V.; Watkins, D.N. Cancer stem cells in lung cancer: Evidence and controversies. Respirology 2013, 18, 757–764. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: Advances in knowledge and implications for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 170. [Google Scholar] [CrossRef]
- Jordan, C.T.; Guzman, M.L.; Noble, M. Cancer stem cells. N. Engl. J. Med. 2006, 355, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Bomken, S.; Fiser, K.; Heidenreich, O.; Vormoor, J. Understanding the cancer stem cell. Br. J. Cancer 2010, 103, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; Sprick, M.R.; Kemper, K.; Stassi, G.; Medema, J.P. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008, 15, 947–958. [Google Scholar] [CrossRef]
- Alam, H.; Sehgal, L.; Kundu, S.T.; Dalal, S.N.; Vaidya, M.M. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol. Biol. Cell 2011, 22, 4068–4078. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yamaguchi, M.; Hirai, S.; Sumi, T.; Tada, M.; Saito, A.; Chiba, H.; Kojima, T.; Watanabe, A.; Takahashi, H.; et al. Characterization of distal airway stem-like cells expressing N-terminally truncated p63 and thyroid transcription factor-1 in the human lung. Exp. Cell Res. 2018, 372, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, P.S.; Bourdin, A. Club cells, CC10 and self-control at the epithelial surface. Eur. Respir. J. 2014, 44, 831–832. [Google Scholar] [CrossRef] [PubMed]
- Kathiriya, J.J.; Brumwell, A.N.; Jackson, J.R.; Tang, X.; Chapman, H.A. Distinct Airway Epithelial Stem Cells Hide among Club Cells but Mobilize to Promote Alveolar Regeneration. Cell Stem Cell 2020, 26, 346–358.e4. [Google Scholar] [CrossRef]
- Salwig, I.; Spitznagel, B.; Vazquez-Armendariz, A.I.; Khalooghi, K.; Guenther, S.; Herold, S.; Szibor, M.; Braun, T. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019, 38, e102099. [Google Scholar] [CrossRef]
- Campo-Trapero, J.; Cano-Sánchez, J.; Palacios-Sánchez, B.; Sánchez-Gutierrez, J.J.; González-Moles, M.A.; Bascones-Martínez, A. Update on molecular pathology in oral cancer and precancer. Anticancer Res. 2008, 28, 1197–1205. [Google Scholar] [PubMed]
- Vairaktaris, E.; Spyridonidou, S.; Papakosta, V.; Vylliotis, A.; Lazaris, A.; Perrea, D.; Yapijakis, C.; Patsouris, E. The hamster model of sequential oral oncogenesis. Oral. Oncol. 2008, 44, 315–324. [Google Scholar] [CrossRef]
- Willenbrink, T.J.; Ruiz, E.S.; Cornejo, C.M.; Schmults, C.D.; Arron, S.T.; Jambusaria-Pahlajani, A. Field cancerization: Definition, epidemiology, risk factors, and outcomes. J. Am. Acad. Dermatol. 2020, 83, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, D.P.; Southwick, H.W.; Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 1953, 6, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Simple, M.; Suresh, A.; Das, D.; Kuriakose, M.A. Cancer stem cells and field cancerization of oral squamous cell carcinoma. Oral Oncol. 2015, 51, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Aramini, B.; Masciale, V.; Grisendi, G.; Bertolini, F.; Maur, M.; Guaitoli, G.; Chrystel, I.; Morandi, U.; Stella, F.; Dominici, M.; et al. Dissecting Tumor Growth: The Role of Cancer Stem Cells in Drug Resistance and Recurrence. Cancers 2022, 14, 976. [Google Scholar] [CrossRef]
- Kitamura, H.; Okudela, K.; Yazawa, T.; Sato, H.; Shimoyamada, H. Cancer stem cell: Implications in cancer biology and therapy with special reference to lung cancer. Lung Cancer 2009, 66, 275–281. [Google Scholar] [CrossRef]
- Tura-Ceide, O.; Lobo, B.; Paul, T.; Puig-Pey, R.; Coll-Bonfill, N.; García-Lucio, J.; Smolders, V.; Blanco, I.; Barberà, J.A.; Peinado, V.I. Cigarette smoke challenges bone marrow mesenchymal stem cell capacities in guinea pig. Respir. Res. 2017, 18, 50. [Google Scholar] [CrossRef]
- Zhu, F.; Guo, G.H.; Chen, W.; Wang, N.Y. Effects of bone marrow-derived mesenchymal stem cells engraftment on vascular endothelial cell growth factor in lung tissue and plasma at early stage of smoke inhalation injury. World J. Emerg. Med. 2010, 1, 224–228. [Google Scholar] [PubMed]
- Chen, J.T.; Lin, T.S.; Chow, K.C.; Huang, H.H.; Chiou, S.H.; Chiang, S.F.; Chen, H.C.; Chuang, T.L.; Lin, T.Y.; Chen, C.Y. Cigarette smoking induces overexpression of hepatocyte growth factor in type II pneumocytes and lung cancer cells. Am. J. Respir. Cell Mol. Biol. 2006, 34, 264–273. [Google Scholar] [CrossRef]
- Yoneyama, R.; Aoshiba, K.; Furukawa, K.; Saito, M.; Kataba, H.; Nakamura, H.; Ikeda, N. Nicotine enhances hepatocyte growth factor-mediated lung cancer cell migration by activating the α7 nicotine acetylcholine receptor and phosphoinositide kinase-3-dependent pathway. Oncol. Lett. 2016, 11, 673–677. [Google Scholar] [CrossRef][Green Version]
- Du, B.; Leung, H.; Khan, K.M.; Miller, C.G.; Subbaramaiah, K.; Falcone, D.J.; Dannenberg, A.J. Tobacco smoke induces urokinase-type plasminogen activator and cell invasiveness: Evidence for an epidermal growth factor receptor dependent mechanism. Cancer Res. 2007, 67, 8966–8972. [Google Scholar] [CrossRef][Green Version]
- Jiang, Y.J.; Chao, C.C.; Chang, A.C.; Chen, P.C.; Cheng, F.J.; Liu, J.F.; Liu, P.I.; Huang, C.L.; Guo, J.H.; Huang, W.C.; et al. Cigarette smoke-promoted increases in osteopontin expression attract mesenchymal stem cell recruitment and facilitate lung cancer metastasis. J. Adv. Res. 2022, 41, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Park, J.H.; Park, D.I.; Sohn, C.I.; Lee, J.M.; Kim, T.I. Impact of Smoking on Human Natural Killer Cell Activity: A Large Cohort Study. J. Cancer Prev. 2020, 25, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Morini, V.; Coluccia, P.; Proietti, S.; D’Anselmi, F.; Pasqualato, A.; Masiello, M.G.; Palombo, A.; De Toma, G.; Bizzarri, M.; et al. Nicotine increases survival in human colon cancer cells treated with chemotherapeutic drugs. Toxicol Vitr. 2013, 27, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Sun, W.; Cao, J.; Ma, Z. COX-2 in lung cancer: Mechanisms, development, and targeted therapies. Chronic Dis. Transl. Med. 2024, 10, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.S.; Aggarwal, B.B. Transcription factor NF-kappaB: A sensor for smoke and stress signals. Ann. N. Y. Acad. Sci. 2005, 1056, 218–233. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.H.; Thuong, L.H.H.; Jiang, Y.J.; Huang, C.L.; Huang, Y.W.; Cheng, F.J.; Liu, P.I.; Liu, C.L.; Huang, W.C.; Tang, C.H. Cigarette smoke promotes IL-6-dependent lung cancer migration and osteolytic bone metastasis. Int. J. Biol. Sci. 2024, 20, 3257–3268. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Sun, R.; Zhu, Y.; Li, Z.; She, X.; Jian, X.; Yu, F.; Deng, X.; Sai, B.; Wang, L.; et al. Lung microbiome alterations in NSCLC patients. Sci. Rep. 2021, 11, 11736. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yin, Z.; Yang, L.; Fan, J.; Xu, J.; Jin, Y.; Yu, J.; Zhang, D.; Yang, G. Smoking Induced Extracellular Vesicles Release and Their Distinct Properties in Non-Small Cell Lung Cancer. J. Cancer 2019, 10, 3435–3443. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, N.; Hu, X.; Wang, H. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol. Cancer 2021, 20, 117. [Google Scholar] [CrossRef]
- Liang, Z.; Fang, S.; Zhang, Y.; Zhang, X.; Xu, Y.; Qian, H.; Geng, H. Cigarette Smoke-Induced Gastric Cancer Cell Exosomes Affected the Fate of Surrounding Normal Cells via the Circ0000670/Wnt/β-Catenin Axis. Toxics 2023, 11, 465. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung cancer in never smokers—A different disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.C.; Liam, C.K.; Andarini, S.; Park, S.; Tan, D.S.W.; Singh, N.; Jang, S.H.; Vardhanabhuti, V.; Ramos, A.B.; Nakayama, T.; et al. Lung Cancer Screening in Asia: An Expert Consensus Report. J. Thorac. Oncol. 2023, 18, 1303–1322. [Google Scholar] [CrossRef]
- Luo, G.; Zhang, Y.; Rumgay, H.; Morgan, E.; Langselius, O.; Vignat, J.; Colombet, M.; Bray, F. Estimated worldwide variation and trends in incidence of lung cancer by histological subtype in 2022 and over time: A population-based study. Lancet Respir. Med. 2025, 13, 348–363. [Google Scholar] [CrossRef]
- Tang, F.H.; Wong, H.Y.T.; Tsang, P.S.W.; Yau, M.; Tam, S.Y.; Law, L.; Yau, K.; Wong, J.; Farah, F.H.M.; Wong, J. Recent advancements in lung cancer research: A narrative review. Transl. Lung Cancer Res. 2025, 14, 975–990. [Google Scholar] [CrossRef]
- Rzucidlo, E.M.; Powell, R.J. Arterial aneurysms in smokers and patients with chronic obstructive pulmonary disease: A common pathophysiologic link. Vasc. Med. 2009, 14, 195–204. [Google Scholar]
- Hamilton, G.; Rath, B. Mesenchymal-Epithelial Transition and Circulating Tumor Cells in Small Cell Lung Cancer. Adv. Exp. Med. Biol. 2017, 994, 229–245. [Google Scholar] [CrossRef]
- Pei, D.; Shu, X.; Gassama-Diagne, A.; Thiery, J.P. Mesenchymal-epithelial transition in development and reprogramming. Nat. Cell Biol. 2019, 21, 44–53. [Google Scholar] [CrossRef]
- Gostomczyk, K.; Marsool, M.D.M.; Tayyab, H.; Pandey, A.; Borowczak, J.; Macome, F.; Chacon, J.; Dave, T.; Maniewski, M.; Szylberg, Ł. Targeting circulating tumor cells to prevent metastases. Hum. Cell 2024, 37, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Cambria, E.; Coughlin, M.F.; Floryan, M.A.; Offeddu, G.S.; Shelton, S.E.; Kamm, R.D. Linking cell mechanical memory and cancer metastasis. Nat. Rev. Cancer 2024, 24, 216–228. [Google Scholar] [CrossRef]
- Malinverno, C.; Corallino, S.; Giavazzi, F.; Bergert, M.; Li, Q.; Leoni, M.; Disanza, A.; Frittoli, E.; Oldani, A.; Martini, E.; et al. Endocytic reawakening of motility in jammed epithelia. Nat. Mater. 2017, 16, 587–596. [Google Scholar] [CrossRef]
- Yang, C.; Tibbitt, M.W.; Basta, L.; Anseth, K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014, 13, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, W.; Guo, M. The Cell as Matter: Connecting Molecular Biology to Cellular Functions. Matter 2021, 4, 1863–1891. [Google Scholar] [CrossRef] [PubMed]
- D’Aniello, C.; Cermola, F.; Palamidessi, A.; Wanderlingh, L.G.; Gagliardi, M.; Migliaccio, A.; Varrone, F.; Casalino, L.; Matarazzo, M.R.; De Cesare, D.; et al. Collagen Prolyl Hydroxylation-Dependent Metabolic Perturbation Governs Epigenetic Remodeling and Mesenchymal Transition in Pluripotent and Cancer Cells. Cancer Res. 2019, 79, 3235–3250. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Ganss, R. Modulation of the Vascular-Immune Environment in Metastatic Cancer. Cancers 2021, 13, 810. [Google Scholar] [CrossRef]
- Thaxton, C.; Kano, M.; Mendes-Pinto, D.; Navarro, T.P.; Nishibe, T.; Dardik, A. Implications of preoperative arterial stiffness for patients treated with endovascular repair of abdominal aortic aneurysms. JVS Vasc. Sci. 2024, 5, 100209. [Google Scholar] [CrossRef]
- Westerhof, N.; Lankhaar, J.W.; Westerhof, B.E. The arterial Windkessel. Med. Biol. Eng. Comput. 2009, 47, 131–141. [Google Scholar] [CrossRef]
- Schwartz, J.A.; Keagy, B.A.; Johnson, G., Jr. Determination of whole blood apparent viscosity: Experience with a new hemorheologic technique. J. Surg. Res. 1988, 45, 238–247. [Google Scholar] [CrossRef]
- Baieth, H.E. Physical parameters of blood as a non—Newtonian fluid. Int. J. Biomed. Sci. 2008, 4, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, Y.W.; Shin, S.Y.; Lee, S.L.; Kim, C.H.; Chung, K.S.; Lee, J.S. A Physics-Integrated Deep Learning Approach for Patient-Specific Non-Newtonian Blood Viscosity Assessment using PPG. Comput. Methods Programs Biomed. 2025, 265, 108740. [Google Scholar] [CrossRef]
- Willigendael, E.M.; Teijink, J.A.; Bartelink, M.L.; Kuiken, B.W.; Boiten, J.; Moll, F.L.; Büller, H.R.; Prins, M.H. Influence of smoking on incidence and prevalence of peripheral arterial disease. J. Vasc. Surg. 2004, 40, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Mackay, D.F.; Pell, J.P. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart. 2014, 100, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, T.; Geng, K.; Yuan, G.; Chen, Y.; Xu, Y. Smoking and the Pathophysiology of Peripheral Artery Disease. Front. Cardiovasc. Med. 2021, 8, 704106. [Google Scholar] [CrossRef]
- Leone, A.; Landini, L. Vascular pathology from smoking: Look at the microcirculation! Curr. Vasc. Pharmacol. 2013, 11, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.K.; Erwin, A.P.; Yue, X. Nicotine and vascular dysfunction. Acta Physiol. 2021, 231, e13631. [Google Scholar] [CrossRef]
- Lietz, M.; Berges, A.; Lebrun, S.; Meurrens, K.; Steffen, Y.; Stolle, K.; Schueller, J.; Boue, S.; Vuillaume, G.; Vanscheeuwijck, P.; et al. Cigarette-smoke-induced atherogenic lipid profiles in plasma and vascular tissue of apolipoprotein E-deficient mice are attenuated by smoking cessation. Atherosclerosis 2013, 229, 86–93. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Matsuno, S.; Kagota, S.; Haginaka, J.; Kunitomo, M. Oxidants in cigarette smoke extract modify low-density lipoprotein in the plasma and facilitate atherogenesis in the aorta of Watanabe heritable hyperlipidemic rabbits. Atherosclerosis 2001, 156, 109–117. [Google Scholar] [CrossRef]
- Centner, A.M.; Bhide, P.G.; Salazar, G. Nicotine in Senescence and Atherosclerosis. Cells 2020, 9, 1035. [Google Scholar] [CrossRef] [PubMed]
- Siasos, G.; Tsigkou, V.; Kokkou, E.; Oikonomou, E.; Vavuranakis, M.; Vlachopoulos, C.; Verveniotis, A.; Limperi, M.; Genimata, V.; Papavassiliou, A.G.; et al. Smoking and atherosclerosis: Mechanisms of disease and new therapeutic approaches. Curr. Med. Chem. 2014, 21, 3936–3948. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Aceto, N. Bring it on: Blood-based analysis of circulating tumor cells in lung cancer. EMBO Mol. Med. 2020, 12, e12802. [Google Scholar]
- Xin, Y.; Li, K.; Yang, M.; Tan, Y. Fluid Shear Stress Induces EMT of Circulating Tumor Cells via JNK Signaling in Favor of Their Survival during Hematogenous Dissemination. Int. J. Mol. Sci. 2020, 21, 8115. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, K.; Xin, Y.; Tang, K.; Yang, M.; Wang, G.; Tan, Y. Fluid shear stress regulates the survival of circulating tumor cells via nuclear expansion. J. Cell Sci. 2022, 135, jcs259586, Erratum in J. Cell Sci. 2023, 136, jcs261030. https://doi.org/10.1242/jcs.261030. [Google Scholar] [CrossRef] [PubMed]
- Krog, B.L.; Henry, M.D. Biomechanical regulation of CTC metastasis in lung cancer. Biophys. J. 2018, 115, 186–195. [Google Scholar]
- Kurma, K.; Alix-Panabières, C. Platelet–CTC interactions: Mechanisms and implications in metastasis. Cancer Cell 2023, 41, 439–452. [Google Scholar]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Mitchell, M.J.; King, M.R. Fluid shear stress sensitizes cancer cells to receptor-mediated apoptosis via trimeric death receptors. New J. Phys. 2013, 15, 015008. [Google Scholar] [CrossRef]
- Chien, S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1209–H1224. [Google Scholar] [CrossRef] [PubMed]
- Au, S.H.; Storey, B.D.; Moore, J.C.; Tang, Q.; Chen, Y.-L.; Javaid, S.; Sarioglu, A.F.; Sullivan, R.; Madden, M.W.; O’Keefe, R.; et al. Clusters of circulating tumor cells traverse capillary-sized vessels. Proc. Natl. Acad. Sci. USA 2016, 113, 4947–4952. [Google Scholar] [CrossRef]
- Labelle, M.; Begum, S.; Hynes, R.O. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011, 20, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Roh-Johnson, M.; Bravo-Cordero, J.J.; Patsialou, A.; Sharma, V.P.; Guo, P.; Liu, H.; Hodgson, L.; Condeelis, J. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Cell 2014, 158, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Secomb, T.W. Microvascular blood viscosity in vivo and the endothelial surface layer. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2657–H2664. [Google Scholar] [CrossRef]
- Chiu, J.J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Chute, E.P.; Littooy, F.N.; Bandyk, D.; Krupski, W.C.; Barone, G.W.; Acher, C.W.; Ballard, D.J. Prevalence and Associations of Abdominal Aortic Aneurysm Detected through Screening. Ann. Intern. Med. 1997, 126, 441–449. [Google Scholar] [CrossRef]
- Bluestein, D.; Chandran, K.B.; Manning, K.B. Towards non-thrombogenic performance of blood recirculating devices. Ann. Biomed. Eng. 2010, 38, 1236–1256. [Google Scholar] [CrossRef] [PubMed]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Vorp, D.A.; Lee, P.C.; Wang, D.H.; Makaroun, M.S.; Nemoto, E.M.; Ogawa, S.; Webster, M.W. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J. Vasc. Surg. 2001, 34, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Wagner, D.D. NETosis: A new factor in tumor progression and cancer-associated thrombosis. Semin. Thromb. Hemost. 2014, 40, 277–283. [Google Scholar] [CrossRef]
- Park, J.; Wysocki, R.W.; Amoozgar, Z.; Maiorino, L.; Fein, M.R.; Jorns, J.; Schott, A.F.; Kinugasa-Katayama, Y.; Lee, Y.; Won, N.H.; et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 2016, 8, 361ra138. [Google Scholar] [CrossRef]
- Les, A.S.; Shadden, S.C.; Figueroa, C.A.; Park, J.M.; Tedesco, M.M.; Herfkens, R.J.; Dalman, R.L.; Taylor, C.A. Quantification of hemodynamics in abdominal aortic aneurysms during rest and exercise using magnetic resonance imaging and computational fluid dynamics. Ann. Biomed. Eng. 2010, 38, 1288–1313. [Google Scholar] [CrossRef] [PubMed]
- Fillinger, M.F.; Marra, S.P.; Raghavan, M.L.; Kennedy, F.E. Prediction of rupture risk in abdominal aortic aneurysm during observation: Wall stress versus diameter. J. Vasc. Surg. 2003, 37, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Liquid biopsy: From discovery to clinical application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef] [PubMed]
- Stroka, K.M.; Jiang, H.; Chen, S.H.; Tong, Z.; Wirtz, D.; Sun, S.X.; Konstantopoulos, K. Water permeation drives tumor cell migration in confined microenvironments. Cell 2014, 157, 611–623. [Google Scholar] [CrossRef]
- Polacheck, W.J.; Charest, J.L.; Kamm, R.D. Interstitial flow influences direction of tumor cell migration through competing mechanisms. Proc. Natl. Acad. Sci. USA 2011, 108, 11115–11120. [Google Scholar] [CrossRef]
- Ma, H.; Wells, A.; Wei, L.; Li, Y.; Yu, F. Emerging microfluidic platforms for cancer metastasis research. Trends Biotechnol. 2020, 38, 556–572. [Google Scholar]
- Huang, L.; Liu, Y.; Yu, L.; Cheng, A.; Cao, J.; Wang, R.; Liu, Y.; Song, S.; Zhao, W.; Liu, Q.; et al. Association of metal elements deposition with symptomatic carotid artery stenosis and their spatial distribution in atherosclerosis plaques. Metallomics 2025, 17, mfaf019. [Google Scholar] [CrossRef]
- Angeli, J.K.; Cruz Pereira, C.A.; de Oliveira Faria, T.; Stefanon, I.; Padilha, A.S.; Vassallo, D.V. Cadmium exposure induces vascular injury due to endothelial oxidative stress: The role of local angiotensin II and COX-2. Free Radic. Biol. Med. 2013, 65, 838–848. [Google Scholar] [CrossRef]
- Bozzani, A.; Arici, V.; Cutti, S.; Marzo, L.D.; Sterpetti, A.V. Increased rupture of Abdominal Aortic Aneurysm in patients with COPD correlates with high atmospheric levels of PM2.5 and PM10. Int. J. Cardiol. Cardiovasc. Risk Prev. 2024, 21, 200266. [Google Scholar] [CrossRef]
- Bozzani, A.; Cutti, S.; Marzo, L.D.; Gabriele, R.; Sterpetti, A.V. Spatio-temporal correlation between admissions for ruptured abdominal aortic aneurysms and levels of atmospheric pollution in Italy. Curr. Probl. Cardiol. 2024, 49, 102249. [Google Scholar] [CrossRef] [PubMed]
- Zha, H.; Wang, R.; Feng, X.; An, C.; Qian, J. Spatial characteristics of the PM2.5/PM10 ratio and its indicative significance regarding air pollution in Hebei Province, China. Environ. Monit. Assess. 2021, 193, 486. [Google Scholar] [CrossRef]
- Urbanek, T.; Juśko, M.; Niewiem, A.; Kuczmik, W.; Ziaja, D.; Ziaja, K. The influence of atmospheric pressure on aortic aneurysm rupture--is the diameter of the aneurysm important? Kardiol. Pol. 2015, 73, 1327–1333. [Google Scholar] [CrossRef]
- Delk, S.C.; Chattopadhyay, A.; Escola-Gil, J.C.; Fogelman, A.M.; Reddy, S.T. Apolipoprotein mimetics in cancer. Semin Cancer Biol. 2021, 73, 158–168. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, Y.; Weng, H.; Wang, B. Apolipoprotein E enhances lung cancer metastasis by interacting with endothelial LRP1 receptors. Cancer Lett. 2018, 431, 157–167. [Google Scholar] [CrossRef]
- Kotlyarov, S. High-Density Lipoproteins: A Role in Inflammation in COPD. Int. J. Mol. Sci. 2022, 23, 8128. [Google Scholar] [CrossRef]
- Tibuakuu, M.; Kamimura, D.; Kianoush, S.; DeFilippis, A.P.; Al Rifai, M.; Reynolds, L.M.; White, W.B.; Butler, K.R.; Mosley, T.H.; Turner, S.T.; et al. The association between cigarette smoking and inflammation: The Genetic Epidemiology Network of Arteriopathy (GENOA) study. PLoS ONE 2017, 12, e0184914. [Google Scholar] [CrossRef]
- Elisia, I.; Lam, V.; Cho, B.; Hay, M.; Li, M.Y.; Yeung, M.; Bu, L.; Jia, W.; Norton, N.; Lam, S.; et al. The effect of smoking on chronic inflammation, immune function and blood cell composition. Sci. Rep. 2020, 10, 19480. [Google Scholar] [CrossRef] [PubMed]
- Leduc, C.; Antoni, D.; Charloux, A.; Falcoz, P.E.; Quoix, E. Comorbidities in the management of patients with lung cancer. Eur. Respir. J. 2017, 49, 1601721. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.W.; Sobus, S.; Gritz, E.R. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. 2014, 15, e568–e580. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M. Tumor-altered dendritic cell function: Implications for anti-tumor immunity. Front. Immunol. 2013, 4, 192. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, S.; Zhu, Z.; Gatt, A.; Liu, J. E-selectin in vascular pathophysiology. Front. Immunol. 2024, 15, 1401399. [Google Scholar] [CrossRef]
- Turhan, H.; Saydam, G.S.; Erbay, A.R.; Ayaz, S.; Yasar, A.S.; Aksoy, Y.; Basar, N.; Yetkin, E. Increased plasma soluble adhesion molecules; ICAM-1, VCAM-1, and E-selectin levels in patients with slow coronary flow. Int. J. Cardiol. 2006, 108, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Park-Windhol, C.; D’Amore, P.A. Disorders of Vascular Permeability. Annu. Rev. Pathol. 2016, 11, 251–281. [Google Scholar] [CrossRef] [PubMed]
- Geindreau, M.; Bruchard, M.; Vegran, F. Role of Cytokines and Chemokines in Angiogenesis in a Tumor Context. Cancers 2022, 14, 2446. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Subramanian, J. Long-Term Survival by Number of Immune Checkpoint Inhibitors in PD-L1-Negative Metastatic NSCLC: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e2457357. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Braun, A.; Anders, H.J.; Gudermann, T.; Mammadova-Bach, E. Platelet-Cancer Interplay: Molecular Mechanisms and New Therapeutic Avenues. Front. Oncol. 2021, 11, 665534. [Google Scholar] [CrossRef]
- Lycan, T.W., Jr.; Norton, D.L.; Ohar, J.A. COPD and Immune Checkpoint Inhibitors for Cancer: A Literature Review. Int. J. Chron. Obstruct Pulmon Dis. 2024, 19, 2689–2703. [Google Scholar] [CrossRef] [PubMed]
| Pro-Metastatic Smoke-Related Mechanisms | Refs. |
|---|---|
| ↑ invasive g rowth program mediated by scatter factors | [105,106] |
| ↑ uPA through activation of EGFR/ERK1/MAPK-mediated signaling | [107] |
| ↑ OPN expression via the JAK2/STAT3 pathway; attraction of mesenchymal stromal cells | [108] |
| ↓ NK cell function; altered innate immunity | [109] |
| Nicotine-mediated selective advantage and prevention of drug-mediated apoptosis | [110] |
| Chronic inflammation (↑ NF-κB, COX-2, 5-LOX) | [111,112] |
| Smoke-related IL-6-associated bone metastasization | [113] |
| Altered microbioma | [114] |
| Deregulation of EV and exosomes and the subsequent release of pro-metastatic RNAs | [115,116,117] |
| l | Factor | Mechanism | Potential Actionable Target |
|---|---|---|---|
| Immune–Inflammatory | IL-1β, TNF-α, IL-6 | Pro-inflammatory cytokines promoting chronic inflammation, tumor promotion, angiogenesis | Anti-cytokine therapies (e.g., IL-1β inhibitors, anti-TNF agents) |
| NF-κB, STAT3, HIF-1α signaling | Sustained pro-survival and inflammatory signaling pathways | NF-κB/STAT3 inhibitors, HIF-1α modulators | |
| T-reg depletion, CD8+ T-cell predominance | Immune imbalance, reduced immunosurveillance | T-reg restoration, immune checkpoint modulation | |
| Macrophage polarization (M1 dominance, tumor-associated macrophages) | Pro-tumor inflammation, matrix remodeling | CSF-1R inhibitors, macrophage reprogramming | |
| Oxidative stress | ROS, mitochondrial dysfunction | DNA damage, impaired apoptosis | Antioxidants, mitochondrial protective agents |
| Vascular dysfunction | Endothelial adhesion molecules (VCAM-1, ICAM-1, E-selectin) | Promotes leukocyte adhesion, CTC arrest | Anti-adhesion therapies (e.g., selectin blockers) |
| Reduced NO bioavailability | Endothelial dysfunction, impaired vasodilation | NO donors, endothelial stabilizers | |
| Pathologic angiogenesis (VEGF, Angiopoietin-2) | Abnormal, leaky vessels facilitating metastasis | Anti-VEGF therapies, angiopoietin pathway inhibitors | |
| Biomechanical | Low shear stress, turbulent flow, flow stagnation | Facilitates CTC adhesion, extravasation | Vascular normalization, flow modulation |
| Elevated IFP | Drives outward migration of tumor cells | Anti-VEGF, normalization of tumor IFP | |
| Extracellular matrix | MMP-2, MMP-9 | ECM degradation enabling invasion | MMP inhibitors |
| Epigenetic/Genetic | DNA methylation, histone modifications | Silencing of tumor suppressor genes | Epigenetic drugs (e.g., DNMT inhibitors, HDAC inhibitors) |
| Apolipoproteins/Lipids | Oxidized LDL, ApoB/ApoE dysregulation | Endothelial activation, macrophage recruitment | Lipid-lowering agents, ApoE modulators |
| Aneurysmal niche | MMP overexpression, inflammatory cell infiltration | Vessel wall degradation, permissive microenvironment | MMP inhibitors, anti-inflammatory therapies |
| Hemodynamic abnormalities in aneurysm (low WSS, recirculation zones) | Increased CTC residence time and adhesion | Flow-altering endovascular interventions | |
| Pharmacologic interactions | Corticosteroids, ICSs | Immune suppression, impaired antigen presentation | Tapering strategies, ICS alternatives |
| Aspirin, P2Y12 inhibitors | Reduce platelet cloaking of CTCs, inhibit thrombosis | Consider as adjunct to anti-metastatic therapy | |
| Triple inhaled therapy (ICS/LABA/LAMA) | Reduces inflammation, improves oxygenation | May indirectly mitigate hypoxia-driven tumor progression | |
| PD-L1 expression | Immune evasion by inhibiting T cell-mediated cytotoxicity | Enhances tumor immune escape and metastatic spread | Anti-PD-1/PD-L1 therapy (e.g., pembrolizumab) |
| Tumor–stromal crosstalk | EMT promotion (TGF-β, matrix degradation) | Increases invasiveness, resistance to apoptosis | TGF-β inhibitors, EMT blockers |
| Smoke | Cells | Mechanism | Molecules/Mediators | Effects |
|---|---|---|---|---|
| Lung | Neutrophils | Activation–Degranulation | ROS, MMP, NET | ↑ Invasive capacity |
| Macrophages | M2 polarization | VEGF, TGF-β, IL-10 | Tissue remodeling, immunosuppression, tumor angiogenesis | |
| T lymphocytes | ↓ Cytotoxic CD8+ T ↑ Tregs | ↓ Immune surveillance ↑ Invasive capacity | ||
| Vessels | Endothelial cells | Activation ↑ Permeability | ICAM-1, VCAM-1, E-selectin | ↑Adhesion, transmigration of CTCs |
| Vascular Niche | Endothelial cells, pericytes, smooth muscle cells, inflammatory cells, stem cells | Systemic inflammation, vascular disease | Cytokine: IL-6, IL-1β, TNF-α Chemokines: CXCL12, CCL2 Angiogenic factors: VEGF-A, Ang2 | ↑ Paracellular passage of tumor cells to distant tissues |
| Key Points | Knowledge Gap |
|---|---|
| LUNG CANCER → Immune checkpoint inhibitors Inflamed or remodeled vasculature in COPD or systemic vasculopathy may be a critical determinant of response to immunotherapy. | |
|
|
| COPD → Triple bronchodilation Beyond airway-specific effects, it can have systemic implications relevant to tumor–immune dynamics. | |
|
|
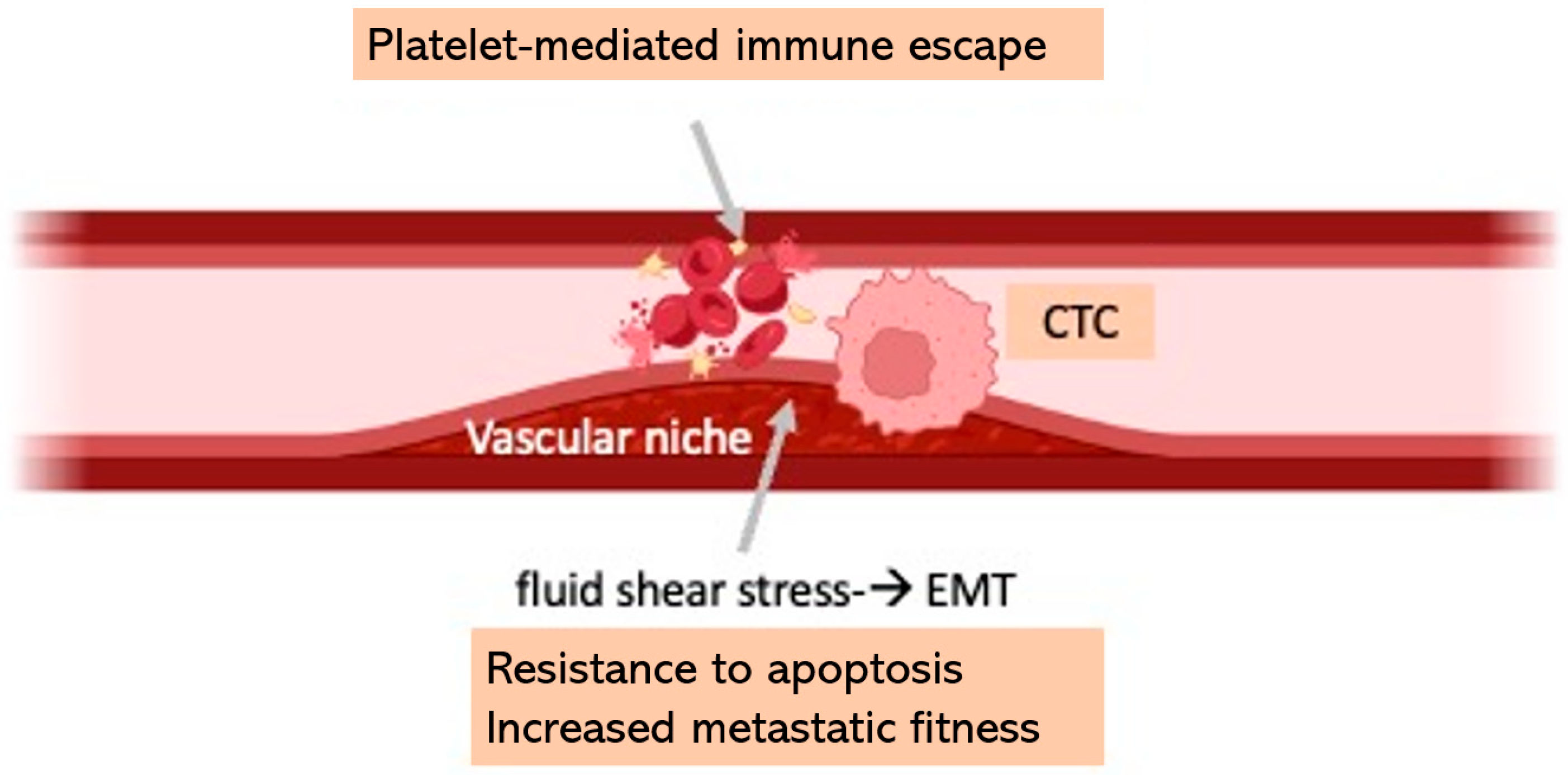
| VASCULOPATHY → Antiplatelet therapy Platelets can shield CTCs from immune recognition and promote their extravasation. | |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stella, G.M.; Bertuccio, F.R.; Novy, C.; Bortolotto, C.; Salzillo, I.; Perrotta, F.; D’Agnano, V.; Conio, V.; Arici, V.; Cerveri, P.; et al. From COPD to Smoke-Related Arteriopathy: The Mechanical and Immune–Inflammatory Landscape Underlying Lung Cancer Distant Spreading—A Narrative Review. Cells 2025, 14, 1225. https://doi.org/10.3390/cells14161225
Stella GM, Bertuccio FR, Novy C, Bortolotto C, Salzillo I, Perrotta F, D’Agnano V, Conio V, Arici V, Cerveri P, et al. From COPD to Smoke-Related Arteriopathy: The Mechanical and Immune–Inflammatory Landscape Underlying Lung Cancer Distant Spreading—A Narrative Review. Cells. 2025; 14(16):1225. https://doi.org/10.3390/cells14161225
Chicago/Turabian StyleStella, Giulia M., Francesco Rocco Bertuccio, Cristina Novy, Chandra Bortolotto, Ilaria Salzillo, Fabio Perrotta, Vito D’Agnano, Valentina Conio, Vittorio Arici, Pietro Cerveri, and et al. 2025. "From COPD to Smoke-Related Arteriopathy: The Mechanical and Immune–Inflammatory Landscape Underlying Lung Cancer Distant Spreading—A Narrative Review" Cells 14, no. 16: 1225. https://doi.org/10.3390/cells14161225
APA StyleStella, G. M., Bertuccio, F. R., Novy, C., Bortolotto, C., Salzillo, I., Perrotta, F., D’Agnano, V., Conio, V., Arici, V., Cerveri, P., Bianco, A., Corsico, A. G., & Bozzani, A. (2025). From COPD to Smoke-Related Arteriopathy: The Mechanical and Immune–Inflammatory Landscape Underlying Lung Cancer Distant Spreading—A Narrative Review. Cells, 14(16), 1225. https://doi.org/10.3390/cells14161225







