Autophagy and Apoptosis in Rabies Virus Replication
Abstract
1. Introduction
2. Overview of Autophagy
2.1. General Mechanisms of Autophagy
2.2. Complex Interactions between Autophagy and Viruses
2.3. Induction of Autophagy by RABV
2.4. Regulation of Autophagy-Related Pathways by RABV
2.4.1. RABV Causes Autophagy by Inhibiting mTOR with AMPK
2.4.2. RABV N/P-Induced Autophagy by Downregulating CASP2
2.4.3. RABV P-Induced Autophagy by Binding to BECN1
2.4.4. IFITM3 Inhibition of Autophagy by Inhibiting mTORC1 and ULK1
2.4.5. Effect of Autophagy on RABV Proliferation
3. Overview of Apoptosis
3.1. Mechanisms of Apoptosis
3.2. Complex Interactions between Apoptosis and Viruses
3.3. RABV and Cell Apoptosis
3.3.1. RABV G-Induced Apoptosis
3.3.2. RABV M-Induced Intrinsic Apoptosis
3.3.3. RABV P-Induced Apoptosis
4. Interplay between Autophagy and Apoptosis in RABV
4.1. Inhibition of Autophagic Flux and Promotion of Apoptosis by M Protein
4.2. Enhanced Autophagy Flux and Inhibition of Apoptosis by Bif-1
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brunker, K.; Mollentze, N. Rabies Virus. Trends Microbiol. 2018, 26, 886–887. [Google Scholar] [CrossRef]
- Guo, Y.; Duan, M.; Wang, X.; Gao, J.; Guan, Z.; Zhang, M. Early events in rabies virus infection-Attachment, entry, and intracellular trafficking. Virus Res. 2019, 263, 217–225. [Google Scholar]
- Feige, L.; Zaeck, L.M.; Sehl-Ewert, J.; Finke, S.; Bourhy, H. Innate Immune Signaling and Role of Glial Cells in Herpes Simplex Virus- and Rabies Virus-Induced Encephalitis. Viruses 2021, 13, 2364. [Google Scholar] [CrossRef]
- Davis, B.M.; Rall, G.F.; Schnell, M.J. Everything You Always Wanted to Know About Rabies Virus (But Were Afraid to Ask). Annu. Rev. Virol. 2015, 2, 451–471. [Google Scholar] [CrossRef]
- Onorati, A.V.; Dyczynski, M.; Ojha, R.; Amaravadi, R.K. Targeting autophagy in cancer. Cancer 2018, 124, 3307–3318. [Google Scholar] [CrossRef]
- Besson, B.; Kim, S.; Kim, T.; Ko, Y.; Lee, S.; Larrous, F.; Song, J.; Shum, D.; Grailhe, R.; Bourhy, H.; et al. Kinome-Wide RNA Interference Screening Identifies Mitogen-Activated Protein Kinases and Phosphatidylinositol Metabolism as Key Factors for Rabies Virus Infection. mSphere 2019, 4, e00047-19. [Google Scholar] [CrossRef]
- Yin, J.; Wang, X.; Mao, R.; Zhang, Z.; Gao, X.; Luo, Y.; Sun, Y.; Yin, X. Research Advances on the Interactions between Rabies Virus Structural Proteins and Host Target Cells: Accrued Knowledge from the Application of Reverse Genetics Systems. Viruses 2021, 13, 2288. [Google Scholar] [CrossRef]
- Albertini, A.A.; Ruigrok, R.W.; Blondel, D. Rabies virus transcription and replication. Adv. Virus Res. 2011, 79, 1–22. [Google Scholar]
- Scrima, N.; Le Bars, R.; Nevers, Q.; Glon, D.; Chevreux, G.; Civas, A.; Blondel, D.; Lagaudrière-Gesbert, C.; Gaudin, Y. Rabies virus P protein binds to TBK1 and interferes with the formation of innate immunity-related liquid condensates. Cell Rep. 2023, 42, 111949. [Google Scholar] [PubMed]
- Ammar, E.; Tsai, C.W.; Whitfield, A.E.; Redinbaugh, M.G.; Hogenhout, S.A. Cellular and molecular aspects of rhabdovirus interactions with insect and plant hosts. Annu. Rev. Entomol. 2009, 54, 447–468. [Google Scholar] [PubMed]
- Ng, W.M.; Fedosyuk, S.; English, S.; Augusto, G.; Berg, A.; Thorley, L.; Haselon, A.S.; Segireddy, R.R.; Bowden, T.A.; Douglas, A.D. Structure of trimeric pre-fusion rabies virus glycoprotein in complex with two protective antibodies. Cell Host Microbe. 2022, 30, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lin, S.; Ye, F.; Yang, J.; Qi, J.; Chen, Z.; Lin, X.; Wang, J.; Yue, D.; Cheng, Y.; et al. Structural Analysis of Rabies Virus Glycoprotein Reveals pH-Dependent Conformational Changes and Interactions with a Neutralizing Antibody. Cell Host Microbe. 2020, 27, 441–453. [Google Scholar] [PubMed]
- Lafon, M. Modulation of the immune response in the nervous system by rabies virus. Curr. Top. Microbiol. Immunol. 2005, 289, 239–258. [Google Scholar] [PubMed]
- Fu, Z.F.; Li, X.; Dhingra, V. Pathogenic rabies virus alters host protein expression in the central nervous system: Implications for neuronal dysfunction. Dev. Biol. 2008, 131, 83–91. [Google Scholar]
- Chung, C.; Seo, W.; Silwal, P.; Jo, E.K. Crosstalks between inflammasome and autophagy in cancer. J. Hematol. Oncol. 2020, 13, 100. [Google Scholar] [CrossRef]
- Krause, G.J.; Cuervo, A.M. Assessment of mammalian endosomal microautophagy. Methods Cell Biol. 2021, 164, 167–185. [Google Scholar]
- Mesquita, A.; Glenn, J.; Jenny, A. Differential activation of eMI by distinct forms of cellular stress. Autophagy 2021, 17, 1828–1840. [Google Scholar] [CrossRef]
- Wang, L.; Klionsky, D.J.; Shen, H.M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 2023, 24, 186–203. [Google Scholar]
- Mahapatra, K.K.; Mishra, S.R.; Behera, B.P.; Patil, S.; Gewirtz, D.A.; Bhutia, S.K. The lysosome as an imperative regulator of autophagy and cell death. Cell. Mol. Life Sci. 2021, 78, 7435–7449. [Google Scholar]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Kudchodkar, S.B.; Levine, B. Viruses and autophagy. Rev. Med. Virol. 2009, 19, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, K.N.; Smit, J.M.; Reggiori, F. Strategies employed by viruses to manipulate autophagy. Prog. Mol. Biol. Transl. Sci. 2020, 172, 203–237. [Google Scholar]
- Ke, P.Y. Autophagy and antiviral defense. IUBMB Life 2022, 74, 317–338. [Google Scholar] [CrossRef] [PubMed]
- Pradel, B.; Robert-Hebmann, V.; Espert, L. Regulation of Innate Immune Responses by Autophagy: A Goldmine for Viruses. Front. Immunol. 2020, 11, 578038. [Google Scholar] [PubMed]
- Li, L.; Jin, H.; Wang, H.; Cao, Z.; Feng, N.; Wang, J.; Zhao, Y.; Zheng, X.; Hou, P.; Li, N.; et al. Autophagy is highly targeted among host comparative proteomes during infection with different virulent RABV strains. Oncotarget 2017, 8, 21336–21350. [Google Scholar] [CrossRef] [PubMed]
- Pirooz, S.; He, S.; O’Connell, D.; Khalilzadeh, P.; Yang, Y.; Liang, C. Viruses customize autophagy protein for efficient viral entry. Autophagy 2014, 10, 1355–1356. [Google Scholar]
- Peng, J.; Zhu, S.; Hu, L.; Ye, P.; Wang, Y.; Tian, Q.; Mei, M.; Chen, H.; Guo, X. Wild-type rabies virus induces autophagy in human and mouse neuroblastoma cell lines. Autophagy 2016, 12, 1704–1720. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Wang, Y.; Kanneganti, T.D. From pyroptosis, apoptosis and necroptosis to PANoptosis: A mechanistic compendium of programmed cell death pathways. Comput. Struct. Biotechnol. J. 2021, 19, 4641–4657. [Google Scholar]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar]
- Miller, D.R.; Thorburn, A. Autophagy and organelle homeostasis in cancer. Dev. Cell 2021, 56, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San, P.J.; Cadwell, K.; Cecconi, F.; Choi, A.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef]
- Kocaturk, N.M.; Akkoc, Y.; Kig, C.; Bayraktar, O.; Gozuacik, D.; Kutlu, O. Autophagy as a molecular target for cancer treatment. Eur. J. Pharm. Sci. 2019, 134, 116–137. [Google Scholar] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar]
- Vargas, J.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar]
- Mizushima, N.; Levine, B. Autophagy in Human Diseases. N. Engl. J. Med. 2020, 383, 1564–1576. [Google Scholar]
- Pao, K.C.; Rape, M. Tug of War in the Xenophagy World. Trends Cell Biol. 2019, 29, 767–769. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C. Xenophagy in innate immunity: A battle between host and pathogen. Dev. Comp. Immunol. 2020, 109, 103693. [Google Scholar] [CrossRef]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An overview of autophagy: Mechanism, regulation and research progress.Bulletin du cancer. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Zachari, M.; Ganley, I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar]
- Wirth, M.; Joachim, J.; Tooze, S.A. Autophagosome formation--the role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin. Cancer Biol. 2013, 23, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Lamark, T.; Johansen, T. Mechanisms of Selective Autophagy. Annu. Rev. Cell Dev. Biol. 2021, 37, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Szwed, A.; Kim, E.; Jacinto, E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol. Rev. 2021, 101, 1371–1426. [Google Scholar] [CrossRef]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol. Cell. Biol. 2012, 32, 2–11. [Google Scholar] [CrossRef]
- Scrivo, A.; Bourdenx, M.; Pampliega, O.; Cuervo, A.M. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 2018, 17, 802–815. [Google Scholar] [CrossRef]
- Choi, Y.; Bowman, J.W.; Jung, J.U. Autophagy during viral infection—A double-edged sword. Nat. Rev. Microbiol. 2018, 16, 341–354. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Keulers, T.G.; Vooijs, M.A.; Rouschop, K.M. LC3/GABARAP family proteins: Autophagy-(un)related functions. FASEB J. 2016, 30, 3961–3978. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Pan, L. ATG16L1 is equipped with two distinct WIPI2-binding sites to drive autophagy. Autophagy 2023, 14, 1–3. [Google Scholar]
- McCullough, J.; Frost, A.; Sundquist, W.I. Structures, Functions, and Dynamics of ESCRT-III/Vps4 Membrane Remodeling and Fission Complexes. Annu. Rev. Cell Dev. Biol. 2018, 34, 85–109. [Google Scholar] [PubMed]
- Feng, Q.; Luo, Y.; Zhang, X.N.; Yang, X.F.; Hong, X.Y.; Sun, D.S.; Li, X.C.; Hu, Y.; Li, X.G.; Zhang, J.F.; et al. MAPT/Tau accumulation represses autophagy flux by disrupting IST1-regulated ESCRT-III complex formation: A vicious cycle in Alzheimer neurodegeneration. Autophagy 2020, 16, 641–658. [Google Scholar] [CrossRef]
- Kuo, C.J.; Hansen, M.; Troemel, E. Autophagy and innate immunity: Insights from invertebrate model organisms. Autophagy 2018, 14, 233–242. [Google Scholar] [CrossRef]
- Hiramel, A.I.; Best, S.M. Role of autophagy in Zika virus infection and pathogenesis. Virus Res. 2018, 254, 34–40. [Google Scholar] [CrossRef]
- Jordan, T.X.; Randall, G. Manipulation or capitulation: Virus interactions with autophagy. Microbes Infect. 2012, 14, 126–139. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Gu, J.; Deng, T.; Yuan, Z.; Hu, B.; Xu, Y.; Yan, Y.; Zan, J.; Liao, M.; et al. BECN1-dependent CASP2 incomplete autophagy induction by binding to rabies virus phosphoprotein. Autophagy 2017, 13, 739–753. [Google Scholar]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.; Sher, A.A.; Coombs, K.M.; et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, W.; Dong, X.; Liu, M.; Chen, H.; Tang, B. The battle for autophagy between host and influenza A virus. Virulence 2022, 13, 46–59. [Google Scholar]
- Zhang, H.; Huang, J.; Song, Y.; Liu, X.; Qian, M.; Huang, P.; Li, Y.; Zhao, L.; Wang, H. Regulation of innate immune responses by rabies virus. Anim. Models Exp. Med. 2022, 5, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Li, J.; Chen, L.; Luo, J.; Kuang, Y.; Lv, Z.; Fan, R.; Zhang, B.; Luo, Y.; et al. Rabies Virus-Induced Autophagy Is Dependent on Viral Load in BV2 Cells. Front. Microbiol. 2021, 12, 595678. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H. Regulation of Autophagy by mTOR Signaling Pathway. Adv. Exp. Med. Biol. 2019, 1206, 67–83. [Google Scholar]
- Al-Bari, M.; Xu, P. Molecular regulation of autophagy machinery by mTOR-dependent and -independent pathways. Ann. N. Y. Acad. Sci. 2020, 1467, 3–20. [Google Scholar]
- Liu, J.; Liao, M.; Yan, Y.; Yang, H.; Wang, H.; Zhou, J. Rabies virus phosphoprotein P5 binding to BECN1 regulates self-replication by BECN1-mediated autophagy signaling pathway. Cell Commun. Signal. 2020, 18, 153. [Google Scholar] [CrossRef]
- Hu, F.; Song, D.; Yan, Y.; Huang, C.; Shen, C.; Lan, J.; Chen, Y.; Liu, A.; Wu, Q.; Sun, L.; et al. IL-6 regulates autophagy and chemotherapy resistance by promoting BECN1 phosphorylation. Nat. Commun. 2021, 12, 3651. [Google Scholar] [CrossRef]
- Tran, S.; Fairlie, W.D.; Lee, E.F. BECLIN1: Protein Structure, Function and Regulation. Cells 2021, 10, 1522. [Google Scholar] [CrossRef]
- Tu, Z.; Gong, W.; Zhang, Y.; Feng, Y.; Liu, Y.; Tu, C. Inhibition of Rabies Virus by 1,2,3,4,6-Penta-O-galloyl-β-d-Glucose Involves mTOR-Dependent Autophagy. Viruses 2018, 10, 201. [Google Scholar] [CrossRef]
- Xie, Y.; Chi, Y.L.; Liu, S.Q.; Zhu, W.Y. BCX4430 inhibits the replication of rabies virus by suppressing mTOR-dependent autophagy invitro. Virology 2023, 585, 21–31. [Google Scholar]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Sharma, L.K.; Vanegas, D.; Callaway, D.A.; Bai, Y.; Lechleiter, J.D.; Herman, B. A nonapoptotic role for CASP2/caspase 2: Modulation of autophagy. Autophagy 2014, 10, 10541070. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer. 2020, 19, 12. [Google Scholar] [PubMed]
- Prerna, K.; Dubey, V.K. Beclin1-mediated interplay between autophagy and apoptosis: New understanding. Int. J. Biol. Macromol. 2022, 204, 258273. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Fang, A.; Wang, Z.; Tian, B.; Zhang, Y.; Sui, B.; Luo, Z.; Li, Y.; Zhou, M.; Chen, H.; et al. Trim25 restricts rabies virus replication by destabilizing phosphoprotein. Cell Insight. 2022, 1, 100057. [Google Scholar] [CrossRef]
- Ma, J.; Tu, Z.; Du, S.; Zhang, X.; Wang, J.; Guo, J.; Feng, Y.; He, H.; Wang, H.; Li, C.; et al. IFITM3 restricts RABV infection through inhibiting viral entry and mTORC1 dependent autophagy. Vet. Microbiol. 2023, 284, 109823. [Google Scholar]
- Hur, J.Y.; Frost, G.R.; Wu, X.; Crump, C.; Pan, S.J.; Wong, E.; Barros, M.; Li, T.; Nie, P.; Zhai, Y.; et al. The innate immunity protein IFITM3 modulates γ-secretase in Alzheimer’s disease. Nature 2020, 586, 735740. [Google Scholar] [CrossRef]
- Cui, Z.; Napolitano, G.; de Araujo, M.; Esposito, A.; Monfregola, J.; Huber, L.A.; Ballabio, A.; Hurley, J.H. Structure of the lysosomal mTORC1-TFEB-Rag-Ragulator megacomplex. Nature 2023, 614, 572–579. [Google Scholar] [CrossRef]
- Chen, T.; Tu, S.; Ding, L.; Jin, M.; Chen, H.; Zhou, H. The role of autophagy in viral infections.Journal of biomedical science. J. Biomed. Sci. 2023, 30, 5. [Google Scholar]
- Yan, J.M.; Zhang, W.K.; Yan, L.N.; Jiao, Y.J.; Zhou, C.M.; Yu, X.J. Bunyavirus SFTSV exploits autophagic flux for viral assembly and egress. Autophagy 2022, 18, 1599–1612. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar]
- Bock, F.J.; Tait, S. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef]
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 2022, 479, 357–384. [Google Scholar] [CrossRef]
- Cullen, S.P.; Martin, S.J. Fas and TRAIL ‘death receptors’ as initiators of inflammation: Implications for cancer. Sem. Cell Dev. Biol. 2015, 39, 26–34. [Google Scholar] [CrossRef]
- Siegmund, D.; Lang, I.; Wajant, H. Cell death-independent activities of the death receptors CD95, TRAILR1, and TRAILR2. FEBS J. 2017, 284, 1131–1159. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhou, X.; Wang, J.; Ao, X. FADD as a key molecular player in cancer progression. Mol. Med. 2022, 28, 132. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, H.; Jin, M.; Zhu, H.; Shan, B.; Geng, J.; Dziedzic, S.A.; Amin, P.; Mifflin, L.; Naito, M.G.; et al. Modulating TRADD to restore cellular homeostasis and inhibit apoptosis. Nature 2020, 587, 133–138. [Google Scholar] [CrossRef]
- Seyrek, K.; Ivanisenko, N.V.; Richter, M.; Hillert, L.K.; König, C.; Lavrik, I.N. Controlling Cell Death through Post-translational Modifications of DED Proteins. Trends Cell Biol. 2020, 30, 354–369. [Google Scholar] [CrossRef]
- Fritsch, M.; Günther, S.D.; Schwarzer, R.; Albert, M.C.; Schorn, F.; Werthenbach, J.P.; Schiffmann, L.M.; Stair, N.; Stocks, H.; Seeger, J.M.; et al. Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 2019, 575, 683–687. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [PubMed]
- Smyth, P.; Sessler, T.; Scott, C.J.; Longley, D.B. FLIP(L): The pseudo-caspase. FEBS J. 2020, 287, 4246–4260. [Google Scholar] [CrossRef] [PubMed]
- Abate, M.; Festa, A.; Falco, M.; Lombardi, A.; Luce, A.; Grimaldi, A.; Zappavigna, S.; Sperlongano, P.; Irace, C.; Caraglia, M.; et al. Mitochondria as playmakers of apoptosis, autophagy and senescence. Semin. Cell Dev. Biol. 2020, 98, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar]
- Voss, A.K.; Strasser, A. The essentials of developmental apoptosis. F1000Research 2020, 9, F1000. [Google Scholar] [CrossRef] [PubMed]
- Candé, C.; Cohen, I.; Daugas, E.; Ravagnan, L.; Larochette, N.; Zamzami, N.; Kroemer, G. Apoptosis-inducing factor (AIF): A novel caspase-independent death effector released from mitochondria. Biochimie 2002, 84, 215–222. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Bishr, M.K.; Almutairi, F.M.; Ali, A.G. Inhibitors of apoptosis: Clinical implications in cancer. Apoptosis 2017, 22, 1487–1509. [Google Scholar]
- Dumétier, B.; Zadoroznyj, A.; Dubrez, L. IAP-Mediated Protein Ubiquitination in Regulating Cell Signaling. Cells 2020, 9, 1118. [Google Scholar]
- Luo, X.; Budihardjo, I.; Zou, H.; Slaughter, C.; Wang, X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998, 94, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Roulston, A.; Marcellus, R.C.; Branton, P.E. Viruses and apoptosis. Annu. Rev. Microbiol. 1999, 53, 577–628. [Google Scholar] [CrossRef] [PubMed]
- Quarleri, J.; Cevallos, C.; Delpino, M.V. Apoptosis in infectious diseases as a mechanism of immune evasion and survival. Adv. Protein Chem. Struct. Biol. 2021, 125, 1–24. [Google Scholar] [PubMed]
- Chu, Z.; Wang, C.; Tang, Q.; Shi, X.; Gao, X.; Ma, J.; Lu, K.; Han, Q.; Jia, Y.; Wang, X.; et al. Newcastle Disease Virus V Protein Inhibits Cell Apoptosis and Promotes Viral Replication by Targeting CacyBP/SIP. Front. Cell Infect. Microbiol. 2018, 8, 304. [Google Scholar] [CrossRef]
- Wang, C.; Chu, Z.; Liu, W.; Pang, Y.; Gao, X.; Tang, Q.; Ma, J.; Lu, K.; Adam, F.; Dang, R.; et al. Newcastle disease virus V protein inhibits apoptosis in DF-1 cells by downregulating TXNL1. Vet. Res. 2018, 49, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, L.; Mao, L.; Li, W.; Sun, M.; Liu, C.; Xue, T.; Zhang, W.; Liu, M.; Li, B. Caprine parainfluenza virus type 3 N protein promotes viral replication via inducing apoptosis. Vet. Microbiol. 2021, 259, 109129. [Google Scholar] [CrossRef]
- Ampomah, P.B.; Lim, L. Influenza A virus-induced apoptosis and virus propagation. Apoptosis 2020, 25, 1–11. [Google Scholar]
- Ubol, S.; Kasisith, J.; Pitidhammabhorn, D.; Tepsumethanol, V. Screening of pro-apoptotic genes upregulated in an experimental street rabies virus-infected neonatal mouse brain. Microbiol. Immunol. 2005, 49, 423–431. [Google Scholar]
- Li, C.; Wang, Y.; Liu, H.; Zhang, X.; Baolige, D.; Zhao, S.; Hu, W.; Yang, Y. Change in the Single Amino Acid Site 83 in Rabies Virus Glycoprotein Enhances the BBB Permeability and Reduces Viral Pathogenicity. Front. Cell Dev. Biol. 2020, 8, 632957. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Yan, G.; Luo, Y.; Zhao, J.; Yang, X.; Mei, M.; Wu, X.; Guo, X. iTRAQ protein profile analysis of neuroblastoma (NA) cells infected with the rabies viruses rHep-Flury and Hep-dG. Front. Microbiol. 2015, 6, 691. [Google Scholar] [CrossRef]
- Pei, J.; Huang, F.; Wu, Q.; Luo, Z.; Zhang, Y.; Ruan, J.; Li, Y.; Zhou, M.; Fu, Z.; Zhao, L. Codon optimization of G protein enhances rabies virus-induced humoral immunity. J. Gen. Virol. 2019, 100, 1222–1233. [Google Scholar] [CrossRef]
- Préhaud, C.; Lay, S.; Dietzschold, B.; Lafon, M. Glycoprotein of nonpathogenic rabies viruses is a key determinant of human cell apoptosis. J. Viorol. 2003, 77, 10537–10547. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Yao, Q.; Gao, Z.; Cheng, Y.; Zhou, M.; Tang, Y.; Sun, L.; Dai, J.; Cao, G.; et al. AAV-expressed G protein induces robust humoral and cellular immune response and provides durable protection from rabies virus challenges in mice. Vet. Microbiol. 2020, 242, 108578. [Google Scholar] [CrossRef]
- Tian, B.; Zhou, M.; Yang, Y.; Yu, L.; Luo, Z.; Tian, D.; Wang, K.; Cui, M.; Chen, H.; Fu, Z.F.; et al. Lab-Attenuated Rabies Virus Causes Abortive Infection and Induces Cytokine Expression in Astrocytes by Activating Mitochondrial Antiviral-Signaling Protein Signaling Pathway. Front. Immunol. 2017, 8, 2011. [Google Scholar] [CrossRef]
- Fasciani, I.; Carli, M.; Petragnano, F.; Colaianni, F.; Aloisi, G.; Maggio, R.; Scarselli, M.; Rossi, M. GPCRs in Intracellular Compartments: New Targets for Drug Discovery. Biomolecules 2022, 12, 1343. [Google Scholar] [CrossRef]
- Marucci, G.; Dal Ben, D.; Lambertucci, C.; Martí, N.A.; Spinaci, A.; Volpini, R.; Buccioni, M. GPR17 receptor modulators and their therapeutic implications: Review of recent patents. Expert Opin. Ther. Pat. 2019, 29, 85–95. [Google Scholar]
- Liu, W.; Yang, Y.; Zeng, Z.; Tian, Y.; Wu, Q.; Zhou, M.; Fu, Z.F.; Zhao, L. G protein-coupled receptor 17 restricts rabies virus replication via BAK-mediated apoptosis. Vet. Microbiol. 2022, 265, 109326. [Google Scholar] [CrossRef]
- Sarmento, L.; Tseggai, T.; Dhingra, V.; Fu, Z.F. Rabies virus-induced apoptosis involves caspase-dependent and caspase-independent pathways. Virus Res. 2006, 121, 144–151. [Google Scholar] [CrossRef]
- Huang, Q.; Li, F.; Liu, X.; Li, W.; Shi, W.; Liu, F.F.; O’Sullivan, B.; He, Z.; Peng, Y.; Tan, A.C.; et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011, 17, 860–866. [Google Scholar] [CrossRef]
- Harrington, J.S.; Ryter, S.W.; Plataki, M.; Price, D.R.; Choi, A. Mitochondria in health, disease, and aging. Physiol. Rev. 2023, 103, 2349–2422. [Google Scholar]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar]
- Tian, Q.; Wang, Y.; Zhang, Q.; Luo, J.; Jiang, H.; Zhang, B.; Mei, M.; Wu, F.; Wu, Y.; Peng, J.; et al. Phosphoprotein Gene Contributes to the Enhanced Apoptosis Induced by Wild-Type Rabies Virus GD-SH-01 In Vitro. Front. Microbiol. 2017, 8, 1697. [Google Scholar] [CrossRef]
- Zan, J.; Liu, J.; Zhou, J.W.; Wang, H.L.; Mo, K.K.; Yan, Y.; Xu, Y.B.; Liao, M.; Su, S.; Hu, R.L.; et al. Rabies virus matrix protein induces apoptosis by targeting mitochondria. Exp. Cell Res. 2016, 347, 83–94. [Google Scholar]
- Luo, J.; Zhang, Y.; Zhang, Q.; Wu, Y.; Zhang, B.; Mo, M.; Tian, Q.; Zhao, J.; Mei, M.; Guo, X. The Deoptimization of Rabies Virus Matrix Protein Impacts Viral Transcription and Replication. Viruses 2019, 12, 4. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, L.; Qin, J.; Lu, Y.; Shen, H.M.; Chen, H.B. Targeting mitophagy to promote apoptosis is a potential therapeutic strategy for cancer. Autophagy 2023, 19, 1031–1033. [Google Scholar]
- Kalkavan, H.; Chen, M.J.; Crawford, J.C.; Quarato, G.; Fitzgerald, P.; Tait, S.; Goding, C.R.; Green, D.R. Sublethal cytochrome c release generates drug-tolerant persister cells. Cell 2022, 185, 3356–3374. [Google Scholar] [CrossRef]
- Hadian, K.; Stockwell, B.R. The therapeutic potential of targeting regulated non-apoptotic cell death. Nat. Rev. Drug Discov. 2023, 22, 723–742. [Google Scholar]
- Mei, M.; Long, T.; Zhang, Q.; Zhao, J.; Tian, Q.; Peng, J.; Luo, J.; Wang, Y.; Lin, Y.; Guo, X. Phenotypic Consequences In vivo and In vitro of Rearranging the P Gene of RABV HEP-Flury. Front. Microbiol. 2017, 8, 120. [Google Scholar] [CrossRef]
- Sorice, M. Crosstalk of Autophagy and Apoptosis. Cells 2022, 11, 1479. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, J.E. P2 × 7 Receptor Inhibits Astroglial Autophagy via Regulating FAK- and PHLPP1/2-Mediated AKT-S473 Phosphorylation Following Kainic Acid-Induced Seizures. Int. J. Mol. Sci. 2020, 21, 6476. [Google Scholar] [CrossRef]
- Takahashi, Y.; Meyerkord, C.L.; Wang, H.G. Bif-1/endophilin B1: A candidate for crescent driving force in autophagy. Cell Death Differ. 2009, 16, 947–955. [Google Scholar] [CrossRef]
- Takahashi, Y.; Coppola, D.; Matsushita, N.; Cualing, H.D.; Sun, M.; Sato, Y.; Liang, C.; Jung, J.U.; Cheng, J.Q.; Mulé, J.J.; et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat. Cell Biol. 2007, 9, 1142–1151. [Google Scholar]
- Hou, P.; Guo, Y.; Jin, H.; Sun, J.; Bai, Y.; Li, W.; Li, L.; Cao, Z.; Wu, F.; Zhang, H.; et al. Bif-1c Attenuates Viral Proliferation by Regulating Autophagic Flux Blockade Induced by the Rabies Virus CVS-11 Strain in N2a Cells. Microbiol. Spectr. 2023, 11, e307922. [Google Scholar] [CrossRef]
- Wang, D.B.; Uo, T.; Kinoshita, C.; Sopher, B.L.; Lee, R.J.; Murphy, S.P.; Kinoshita, Y.; Garden, G.A.; Wang, H.G.; Morrison, R.S. Bax interacting factor-1 promotes survival and mitochondrial elongation in neurons. J. Neurosci. 2014, 34, 2674–2683. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1. Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
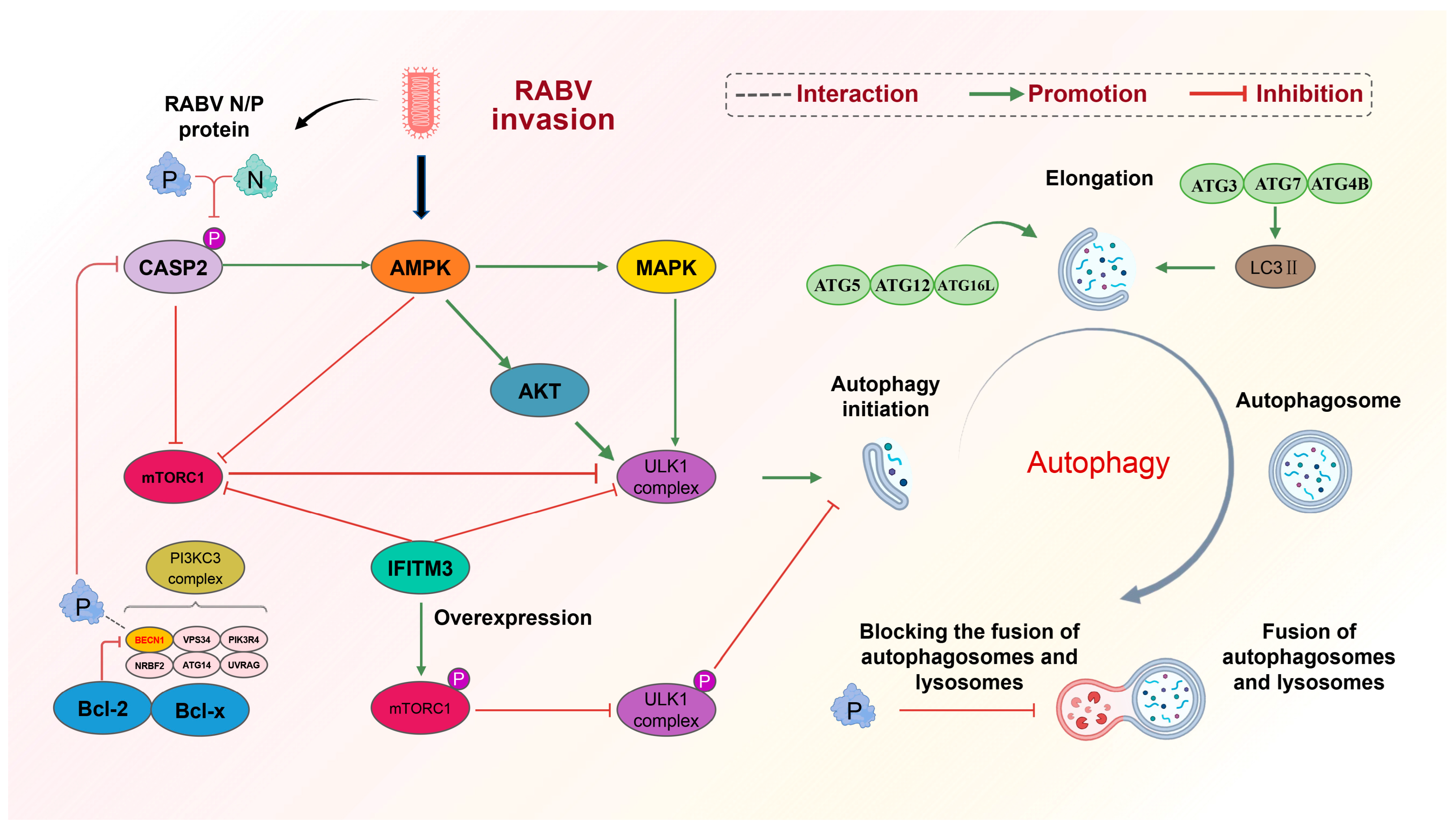
 : promotion
: promotion  : inhabition. Mechanism of RABV-induced mitochondrial apoptosis. RABV is able to cause mitochondrial apoptosis in late replication and is regulated by the BCL-2 family of genes. The three proteins of RABV, M, G, and P are closely related to apoptosis. The M protein inhibits the anti-apoptotic gene Bcl2, causing mitochondrial apoptosis and the release of cytochrome C and AIF factors. Caspase-dependent pathway (➀): released cytochrome C activates caspase-9, which further activates the downstream caspase-3, causing apoptosis. Non-caspase-dependent pathway (➁): released AIF can directly cause apoptosis.
: inhabition. Mechanism of RABV-induced mitochondrial apoptosis. RABV is able to cause mitochondrial apoptosis in late replication and is regulated by the BCL-2 family of genes. The three proteins of RABV, M, G, and P are closely related to apoptosis. The M protein inhibits the anti-apoptotic gene Bcl2, causing mitochondrial apoptosis and the release of cytochrome C and AIF factors. Caspase-dependent pathway (➀): released cytochrome C activates caspase-9, which further activates the downstream caspase-3, causing apoptosis. Non-caspase-dependent pathway (➁): released AIF can directly cause apoptosis.
 : promotion
: promotion  : inhabition. Mechanism of RABV-induced mitochondrial apoptosis. RABV is able to cause mitochondrial apoptosis in late replication and is regulated by the BCL-2 family of genes. The three proteins of RABV, M, G, and P are closely related to apoptosis. The M protein inhibits the anti-apoptotic gene Bcl2, causing mitochondrial apoptosis and the release of cytochrome C and AIF factors. Caspase-dependent pathway (➀): released cytochrome C activates caspase-9, which further activates the downstream caspase-3, causing apoptosis. Non-caspase-dependent pathway (➁): released AIF can directly cause apoptosis.
: inhabition. Mechanism of RABV-induced mitochondrial apoptosis. RABV is able to cause mitochondrial apoptosis in late replication and is regulated by the BCL-2 family of genes. The three proteins of RABV, M, G, and P are closely related to apoptosis. The M protein inhibits the anti-apoptotic gene Bcl2, causing mitochondrial apoptosis and the release of cytochrome C and AIF factors. Caspase-dependent pathway (➀): released cytochrome C activates caspase-9, which further activates the downstream caspase-3, causing apoptosis. Non-caspase-dependent pathway (➁): released AIF can directly cause apoptosis.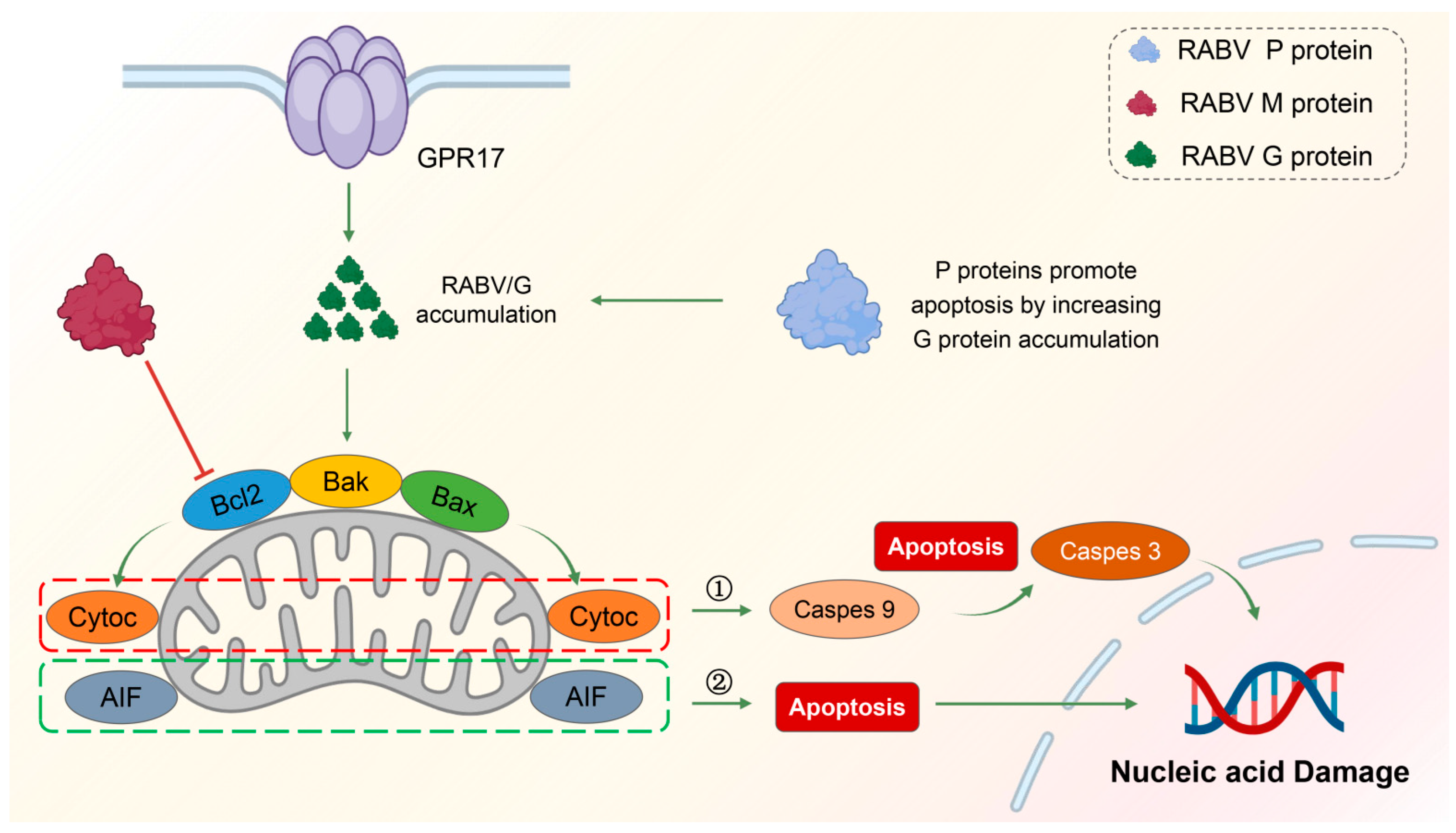
| Proteins | G | M | P | N | Effect on RABV Replication | References |
|---|---|---|---|---|---|---|
| Autophagy | Induce autophagy | 1. Decrease CASP2 2. Activate mTOR 3. Interact with BECN1 4. Inhibit the fusion of autophagolysosomes | 1. Decrease CASP2 2. Activate mTOR | Promotion | [27,58,61,63,64,65,66,67,68,69] | |
| Apoptosis | 1. Expression level proportional to the level of apoptosis 2. Target PTPN4 via C-terminal PDZ domain 3. Upregulate pro-apoptotic gene BAK | 1. Target mitochondria 2. Upregulate AIF and increase cytochrome c release 3. Co-localize with TOMM20 | 1. Involved in intrinsic apoptosis 2. Downregulate of anti-apoptotic gene Bcl-2 3. Assist G protein in inducing apoptosis | Inhibition | [27,108,109,110,112,115,118,119,122,126,127,128] | |
| Crosstalk between autophagy and apoptosis | Inhibition of autophagic flux by caspase-3 induces apoptosis | Involved in autophagy and apoptosis | [27,114,117,128,131,132,133,134,135,136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Xu, B.; Luo, Y.; Luo, J.; Huang, S.; Guo, X. Autophagy and Apoptosis in Rabies Virus Replication. Cells 2024, 13, 183. https://doi.org/10.3390/cells13020183
Li S, Xu B, Luo Y, Luo J, Huang S, Guo X. Autophagy and Apoptosis in Rabies Virus Replication. Cells. 2024; 13(2):183. https://doi.org/10.3390/cells13020183
Chicago/Turabian StyleLi, Saisai, Bowen Xu, Yongwen Luo, Jun Luo, Shile Huang, and Xiaofeng Guo. 2024. "Autophagy and Apoptosis in Rabies Virus Replication" Cells 13, no. 2: 183. https://doi.org/10.3390/cells13020183
APA StyleLi, S., Xu, B., Luo, Y., Luo, J., Huang, S., & Guo, X. (2024). Autophagy and Apoptosis in Rabies Virus Replication. Cells, 13(2), 183. https://doi.org/10.3390/cells13020183







