Abstract
Brain-derived neurotrophic factor (BDNF) has previously been associated with the pathogenesis of both emotionally unstable personality disorder (EUPD) and suicidal behavior. No study has yet investigated BDNF-associated epigenetic alterations in a group of severely impaired EUPD and suicidal patients. The discovery cohort consisted of 97 women with emotionally unstable personality disorder (EUPD) with at least two serious suicide attempts (SAs) and 32 healthy female controls. The genome-wide methylation pattern was measured by the Illumina EPIC BeadChip and analyzed by robust linear regression models to investigate mean BDNF methylation levels in a targeted analysis conditioned upon severity of suicide attempt. The validation cohort encompassed 60 female suicide attempters, stratified into low- (n = 45) and high-risk groups (n = 15) based on degree of intent-to-die and lethality of SA method, and occurrence of death-by-suicide at follow-up. Mean BDNF methylation levels exhibited increased methylation in relation to EUPD (p = 0.0159, percentage mean group difference ~3.8%). Similarly, this locus was confirmed as higher-methylated in an independent cohort of females with severe suicidal behavior (p = 0.0300). Results were independent of age and BMI. This is the first study to reveal emerging evidence of epigenetic dysregulation of BDNF with dependence on features known to confer increased risk of suicide deaths (lethality of suicide-attempt method and presence of EUPD diagnosis with history of recent SAs). Further studies investigating epigenetic and genetic effects of BDNF on severe suicidal behavior and EUPD are needed to further elucidate the role of epigenetic regulatory mechanisms and neurotrophic factors in relation to suicide and EUPD, and hold potential to result in novel treatment methods.
1. Introduction
Emotionally unstable personality disorder (EUPD) is a psychiatric disorder characterized by an enduring pattern of affective instability, unstable relationships, efforts to avoid real or perceived abandonment, identity disturbance, and impulse-driven self-destructive behavior [1]. EUPD is a severely debilitating condition entailing a significant burden on those affected and their loved ones [2] and co-occur with other psychiatric disorders at a higher rate than any other personality disorder [3]. EUPD confers a high mortality rate with reported several-fold increases in both suicides and all-cause mortality in comparison to the general population [4]. EUPD has also been demonstrated to be associated with a wide array of non-psychiatric medical conditions that by themselves confer a high rate of morbidity and mortality [5]. Although EUPD patients consume healthcare at a high rate, they still have higher rates of health problems compared to the general population, possibly due to low compliance [6]. In addition to the burden of physical co-morbidity, EUPD is associated with a behavioral phenotype of poor health habits, i.e., smoking, physical inactivity, and rates of substance abuse of up to 50% [7]. EUPD is known to often co-occur with PTSD and around 30% of EUPD patients have PTSD [8].
EUPD is today thought to develop in an interplay between genetic predisposition and exposure to neglect and abuse in early life [9]. Several neurobiological mechanisms have been suggested as possible pathophysiological drivers of EUPD [10] and other personality disorders. A study measuring inflammatory factors in the plasma of patients with antisocial personality disorder with or without substance abuse disorder found an increase in pro-inflammatory factors and a heavy decrease in neurotrophic factors (including brain-derived-neurotrophic factor) regardless of the history of substance abuse, suggesting a possible systemic inflammatory mechanism and a lack of neuroprotective proteins underpinning the disorder [11]. The proposed relevance of dysregulation of the hypothalamic–pituitary–adrenal axis (HPA-axis) in EUPD has been extensively studied and emerging evidence implicates both epigenetic [12] and physiological [13] alterations in the condition. In a study of 49 patients with EUPD, the plasma levels of a wide array of enzymes involved in oxidative pathways were measured as well as several enzymes involved in anti-oxidative pathways. The study found a significant increase in inflammatory factors and a significant decrease in anti-inflammatory factors when compared to 33 healthy control subjects [14].
Early-life adversity (ELA)—an established risk factor of EUPD [9]—has been previously associated with increased circulating plasma levels of C-reactive protein in adults, a central component to systemic inflammation [15]. Furthermore, inflammatory marker interleukin 6 (IL-6), another protein central in inflammation, has been associated with high neuroticism, impulsivity [16], and low conscientiousness [17]—core traits of EUPD [18]. Attempts have been made to unify research pointing to childhood adversity with neurobiological models involving increased systemic inflammation and alteration in the HPA-axis [19,20]. Epigenetic changes of the genome with a resulting alteration in expression of both neuroprotective and inflammatory proteins have been suggested to play a role in EUPD, with previous research identifying methylation of BDNF-coupled promotor sites as particularly significant [21].
BDNF is a protein central in neurogenesis and the growth of neurons in the central nervous system. It supports the survival of neurons and is central also in their growth, differentiation, and plasticity throughout life. A decrease in BDNF expression has been linked to several psychiatric disorders including schizophrenia and depression. An absence of BDNF, as shown in knockout mouse models, leads to a failure of development and death shortly after birth. Decreased BDNF has been shown in layers IV and V of the dorsolateral prefrontal cortex—an area involved in working memory—in patients with schizophrenia spectrum disorder (SSD). Albeit inconclusive, previous studies have implicated that such effects may contribute to explaining working memory impairments seen in SSD. Furthermore, an inverse relationship between cortisol levels and BDNF has been found both in animal and human post-mortem studies of SSD [22]. Emerging evidence further implicates BDNF in the pathophysiology of depression. For example, rodents subjected to persistent stress were shown to exhibit decreased BDNF expression levels and hippocampal atrophy—an alteration that has also been evinced in major depressive disorder in humans. The relevance of BDNF in depression is further supported by studies demonstrating that antidepressant drugs are associated with a reversal of hippocampal atrophy and contemporary increases in BDNF expression levels [23]. A rat study exposed postnatal animals to stressed caretakers that displayed abusive behaviors. The maltreated rats displayed an increase in methylation in the BDNF gene encoding the protein. On the other hand, no differences in circulating BDNF were evinced in a systematic review of studies comparing whole-blood-derived gene expression levels of psychiatric patients with a history of suicidal attempts with non-suicidal controls [24]. The studies included did, however, not discriminate between severe and less severe suicide attempts, and the authors emphasized the need for rigorous case–control studies of suicidality and BDNF [25].
Aims
The main goal of the present study was to investigate whether epigenetic dysregulation in BDNF was associated with EUPD and the severity of suicidal behavior (putatively with higher risk of later-completed suicide [26]) in females. To increase probabilities of deriving biologically meaningful results, we only considered methylation sites classified as differentially methylated regions (DMRs) and that were annotated to be located within 2000 bp of the transcriptional start site (TSS2000; a region where DNA methylation has been previously shown to exert larger influences on gene expression compared to other regions [27]). Using these stricter methylation site inclusion criteria, 16 BDNF-coupled sites were identified. To further increase robustness, the mean methylation value was calculated across methylation M-values of these 16 BDNF-coupled sites. As a first step, associations between candidate methylation sites and EUPD were investigated in a group of 97 females with emotionally unstable personality disorder (EUPD, previously borderline personality disorder) with severe suicidal behavior (a history of at least two recent suicide attempts) and 32 healthy volunteers. As a second step, candidate methylation markers were investigated in an independent group of 60 female suicide attempters, stratified into in silico generated high- and low-risk subgroups according to (a) a violent method of suicide attempt (i.e., hanging, shooting, or drowning) [28], (b) exhibiting a high intent-to-die as measured by the Freeman scale [29], or (c) having later died by suicide. These analyses pertained exclusively to female subjects.
2. Methods
2.1. Characterization of Discovery Group
The studied patient group consisted of 106 Caucasian females living in the Stockholm County, originally recruited as part of a randomized controlled trial (RCT) comparing two psychotherapy modalities (dialectical behavioral therapy and psychodynamic therapy) to treatment as usual (TAU) in women with EUPD with a history of severe suicidal behavior. Patients were referred to the study from all Stockholm County Council psychiatric clinics (a catchment area of 1.8 million inhabitants). Study inclusion criteria included a prior history of two or more potentially lethal suicide attempts (as defined by the patient’s belief that the attempt could have been lethal), with at least one attempt during the six months preceding referral. Subjects were excluded if presenting with a current life-threatening eating disorder, current psychotic disorder, or major depressive illness with melancholic features, evidence of dementia, or other irreversible organic brain syndrome or an active diagnosis of substance dependence [30]. The Structured Clinical Interview for DSM-IV Axis I and II interview (SCID-I and II) [31] schedules and the Comprehensive Psychopathological Rating Scale interview [32] were administered at baseline and EUPD (at the time, referred to as BPD) diagnosis, and psychiatric comorbidities were established after consensus diagnostic conference amongst experienced clinicians. Subjects were required to be in the 18–50-year-old age group. A total of 162 women with BPD were invited to take part in the SKIP project between 1999 and 2004. Of these individuals, 14 declined to join the study, 41 were excluded due to not fulfilling inclusion criteria or to fulfilling exclusion criteria, and one completed suicide before joining the study. Thus, out of 162 women, 106 (65%) Caucasian females took part in the SKIP study. The baseline measurements of this trial included—amongst others—a blood sample, which was analyzed to extract epigenetic data for the current project. Through the unique personal identification number in Sweden, patients were linked to the Cause of Death Register maintained by the Swedish National Board of Health and Welfare. Eight suicides were ascertained from the death certificates. Follow-up times ended in 2011.
2.1.1. Ethics and Patient Consent
Details on the EUPD cohort have been previously published [30,33,34]. The original and the complementary study protocols were approved by the Committee for Ethical Research at Karolinska Institutet (Dnrs: 95–83; 2021-06929-01) and participants provided written informed consent.
2.1.2. Blood Sample Collection, Methylation Profiling, and Data Processing (EUPD Group)
Standard procedures were utilized in blood sampling. Extraction from non-fasting subjects occurred in the morning. Retrieval of DNA from 97 EUPD participants and 32 control subjects were conducted by the phenol-chloroform method and samples were subjected to bisulfite conversion according to the EZ DNA Methylation GoldTM kit (ZymoResearch, Irvine, CA, USA). Hybridization of the resulting DNA to the Illumina Infinium Methylation EPIC BeadChip was executed. Thereafter, methylation values for 850 K probes for each sample were obtained through array imaging by an Illumina iScan system (Illumina, San Diego, CA, USA). Quality control and normalization of raw methylation IDAT data were conducted with the mefill package for R statistics (https://github.com/perishky/meffil/ accessed on 1 June 2022), utilizing control probes to isolate biological variations from technical variations. All samples passed QC, resulting in extraction of methylation β-values in a total of 129 subjects (97 EUPD and 32 control subjects).
2.1.3. Annotation and Selection of DNA Methylation Probes
First, we identified 17 individual CpG-sites annotated to the gene BDNF that were classified as differentially methylated regions (DMRs) in the Illumina EPIC ManiFest annotation file. The DMR-regions were annotated to the following island shores: ‘Island’ (n = 11), N_Shore (n = 1), and S_Shore (n = 5). To increase the chance of biologically relevant results, we only considered CpG-sites annotated to be located within 2000 bp of the transcriptional start site—a region whereby the relationship between DNA methylation and gene expression have been previously shown to be more closely related compared to other regions [27]. Methylation loci situated more than 2000 base pairs away from the TSS were, thus, excluded from the subsequent analysis. One out of 17 BDNF-associated loci was situated more than 2000 base pairs from the TSS and was excluded, resulting in 16 individual CpG-sites included in the subsequent analysis. To increase the robustness of the derived values, individual CpG-sites were first transformed from betas into M-values, which have been shown to be statistically more robust [35]. Transformed values were subsequently aggregated by both mean and median values across these 16 BDNF-associated sites. The individual CpG-site values were plotted in boxplots together with derived mean and median values. From visual inspection of these boxplots for the EUPD and Suicide study groups, it was determined that the mean and median value were adequate representations of these individual values (Supplementary Figures S1 and S2). The mean value was implemented for subsequent analyses in both datasets. Following these procedures, each subject was represented by one mean value derived across these 16 BDNF-coupled CpG-sites.
2.1.4. DNA Methylation Association Study
Associations across M-transformed mean BDNF-methylation M-values and clinical groups (EUPD vs. Control) were investigated by robust linear regression models using the R-package ‘robustbase’ [36], specifying the recommended setting (KS2014). Chain-of-regression estimates included the standard MM-regression estimator (guaranteeing an acceptable compromise between high breakdown (i.e., 50%) and very high efficiency (i.e., 95%) [37]). As methylation levels have been previously shown to be heavily influenced by age and BMI, the analysis was adjusted for age and BMI. Models exhibiting p-values for the primary explanatory variable <0.05 were considered significant. As a post hoc analysis, individual M-transformed values for CpG-sites were individually investigated by robust linear regression models, stating the same specifications as in the main analysis. Thereafter, we analyzed whether the investigated CpG-sites exhibited an abundance of differentially methylated CpG-sites using binomial tests (as previously performed by Boström et al. [38]). p-value thresholds were set to the highest observed below a p-value of 0.05 to stratify probes according to significant and non-significant methylation changes. Subsequently, binomial tests were performed in R using the function ‘binom.test’, contrasting the number of nominally significant CpG-sites to the total number (n = 16) of investigated probes annotated as BDNF exhibiting methylation alterations in the same direction. Binomial test p-values were not adjusted for multiple testing, as they pertained to one gene only. A non-adjusted binomial test p-value < 0.05 was considered significant and indicative of an abundance of BDNF-associated probes differentially methylated in EUPD or the severe suicide attempt group.
2.2. Characterization of the Validation Dataset
2.2.1. Ethics and Patient Consent
Details on the cohort of death by suicides and stratification of subjects have been previously published [26]. In brief, the study protocols were approved by the Regional Ethical Board in Stockholm, Sweden (Dnrs: 00-194, 2015/1454.32) and the participants gave their informed consent to the study, which was conducted in accordance with guidelines and regulations.
2.2.2. Validation Cohort of Suicide Attempters
The study included adult patients that were clinically assessed in 2000–2005 at a Suicide Prevention Clinic (Karolinska university hospital) after conducting documented acts of self-destruction with variable intentions to die. Subjects with psychotic disorders, dementia, mental disability, or (intravenous) substance abuse were excluded. To improve the clinical generalizability of the study, non-suicidal self-injury and/or non-intravenous substance abuse were not included in the participant exclusion criteria. After exclusion of subjects declining to participate (n = 50), not meeting inclusion criteria (n = 61), or unable to attend clinical follow-up visits (n = 47), 100 patients were included in the study (33 men and 67 women). A total of 26 subjects were medication-free at the time of assessment. The medications reported in more than five individuals were antidepressants (Sertraline (n = 20), Citalopram (n = 12), Mirtazapine (n = 12), Venlafaxine (n = 9), and Fluoxetine (n = 7)). No patients received lithium treatment [26]. DNA samples were extracted from 88 of these subjects. The information about suicide and the cause thereof was available from matching unique identification numbers with the national Cause of Death register. Later death by suicide was established in four cases in the years 2004, 2006, 2007, and 2014, three of which were from hanging and one of which was from substance intoxication. Subjects that used a violent method of attempt but not death by suicide (i.e., hanging, shooting, or drowning) [28], exhibiting a Freeman scale of >6 [29], or having later died by suicide were classified as high-risk.
2.2.3. Descriptive Statistics (Validation Group)
The classification of violent and non-violent suicide attempts into low- or high-risk groups has been previously described in detail [26]. In brief, dichotomization was performed with relevance concerning putative biological differences, and subjects fulfilling any of the following criteria were classified as high-risk: violent suicide attempt method, or a high score in the Freeman Scale, or later death by suicide. In accordance with the literature, non-violent attempts included substance intoxication or a single wrist-cut, whereas violent attempts pertained to all other available suicide methods, i.e., attempted drowning, shooting, gassing, several deep cuts, or hanging [28].
The Freeman scale captures key elements of suicide intent in assessing two primary objectives in relation to suicide attempts: interruption and reversibility probability. The inter-rater reliability of interruption and reversibility probability amounted to 0.8 and 0.97, respectively [29]. The second part of the scale measures likelihoods of interruption of attempted suicide by others whereby a high score exerts this as unlikely, conferring a higher risk of suicide completion. The reversibility scale assesses the probability of reversing the suicide attempt by, for example, assessing the amount and class of substance consumed or the extent of inflicted self-injury. Similarly, a lower reversibility probability is associated with a higher risk of death and confers a higher score on the Freeman scale. The subscales are rated 1–5 and combined for a total score of 2–10 [29]. Severe suicide attempts conferring a higher risk of death by suicide are inferred from scores >6. The discriminating validity of the scale was considered very good in a previous study consisting of a large sample of attempted-suicide subjects and suicide victims [29].
The Shapiro–Wilk’s test was implemented to assess skewness and kurtosis of the distribution of included clinical outcome variables, inferring a normal distribution (ND) for body-mass index (BMI) and age of participants. Independent samples t-tests were used to assess group differences for ND continuous variables, Mann–Whitney U-tests for non-ND continuous variables, and Fisher’s Exact test in the case of categorical variables.
2.2.4. Blood Sample Collection, Methylation Profiling, and Data Processing (Validation Group)
Peripheral blood samples were collected according to standardized procedures. Study participants were required to be fasting at the time of blood sampling, which occurred in the morning. DNA was retrieved using the phenol-chloroform method, after which bisulfite conversion was performed using the EZ DNA Methylation GoldTM kit (ZymoResearch, Irvine, CA, USA). DNA specimens were thereafter hybridized to the Illumina Infinium Methylation EPIC beadchip and the array was imaged using the Illumina Iscan system (Illumina, San Diego, CA, USA), resulting in the quantification of methylation values at approximately 850,000 unique methylation identifiers across all samples. Preprocessing of methylation data included background correction, adjustment for methylation site measurement techniques (i.e., type I and type II probes), adjusting for any potential batch effects, and the removal of putatively unreliable probes. DNA methylation data were corrected for in silico generated surrogate measures of white blood cell type heterogeneity. Evaluation of sample outliers based on methylation data was performed by principal component analysis (PCA). Methylation preprocessing steps were performed using R software, version 3.3.0, and the following bioconductor packages—minfi [39], watermelon [40], sva [41], champ [42], and FactomineR [43]. A detailed description of the preprocessing steps has been previously published [26].
2.2.5. DNA Methylation Association Study (Validation)
To investigate associations between mean BDNF-associated promoter methylation levels and severe clinical psychiatric phenotypes, we studied mean values across 16 BDNF-associated CpG-sites. In this analysis, as clinical variables were largely comparable between the in silico stratified groups, mean BDNF DNA-methylation M-values were compared between groups in using the one-sided Wilcoxon signed rank sum exact test, specifying the alternative hypothesis that methylation values are higher in severe suicide attempters (the same loci were greater-methylated in female EUPD patients). Post hoc univariate analyses were performed to exclude confounders from age and BMI on DNA methylation levels, correlating mean BDNF methylation levels with these descriptive variables in using Pearson’s moment correlation coefficients. p-values < 0.05 were considered significant.
3. Results
3.1. Promotor-Associated BDNF Is Higher-Methylated in Whole Blood of EUPD Patients (n = 129)
The 97 EUPD participants were all females with a mean age and BMI of 29.4 years (SD = 7.6) and 24.5 kg/m2 (SD = 4.7), respectively. Approximately a third had attained a university-level education and 10.3% had biological children. Active or previous tobacco usage was recorded in 57.7% of the EUPD-group. All subjects fulfilled criteria for one or more Axis I psychiatric diagnoses. Anxiety disorders were most commonly prevalent (60.8%) and a substantial proportion had major depressive disorder (MDD, 42.3%), of which a subset presented with severe MDD (13.4%). Naturally, all participants met criteria for EUPD, 32% of which fulfilled criteria for 7 or more items. Subjects had a mean global assessment of functioning (GAF) score of 49.2, corresponding to serious symptoms or serious impairment in social, occupational, or school functioning. History of alcohol and substance abuse was highly prevalent (33.0% and 26.8%, respectively). Benzodiazepines constituted the most frequent psychotropic medication (36.1%), closely followed by selective-serotonin reuptake inhibitors (SSRI; 33.0%), non-SSRI antidepressants (20.6%), neuroleptics (12.4%), and mood stabilizers (4.1%). All EUPD subjects had a history of recent suicide attempts (constituting inclusion criteria whereby the mean age at first suicide attempt was 20 years (SD = 7.5). Eight women (8.25%) died by suicide after the study was concluded, in the years 2002-2012. These deaths occurred by intoxication (N = 5), hanging (N = 2), and railway suicide (N = 1). One additional subject died of unknown cause, which was not categorized as a suicide-related death (Table 1).

Table 1.
Characteristics of subjects (EUPD grp).
In the association analysis between whole-blood-derived BDNF-DNA methylation and EUPD, we studied mean values across 16 BDNF-associated promoter CpG-sites. Mean and median-derived values did not significantly differ, as measured by unpaired independent samples t-tests (p = 0.661), and the mean value was determined upon visual inspection as representative of the underlying data (Supplemental Figure S1). In the subsequent analysis—implementing robust linear regression models adjusted for age and BMI—mean BDNF DNA-methylation M-values were increased with dependence on EUPD (compared to controls) (p = 0.0159). Across all samples, non-M-transformed mean BDNF methylation beta-values were 0.056 (SD = 0.0059) with a mean between-group difference of ~3.8% (Table 2 and Figure 1).

Table 2.
Robust Linear Regression Contrasting Mean BDNF Methylation Levels to Disease Status (EUPD or Control).

Figure 1.
Violin Boxplots Contrasting Mean BDNF Methylation Beta-values by Group (EUPD or Control) (n = 129) [FEMALES]. On the association analysis between whole-blood-derived BDNF-DNA methylation and EUPD, we studied mean values across 16 BDNF-associated promoter CpG-sites. In this analysis—implementing robust linear regression models adjusted for age and BMI—mean BDNF DNA-methylation was increased with dependence on EUPD (compared to controls) (p = 0.0159). Across all samples, non-M-transformed mean BDNF methylation beta-values were 0.056 (SD = 0.00595) with a mean between-group difference of ~3.8%.
As a post hoc sensitivity analysis, we re-performed the above analysis for the 16 individual CpG-sites separately and evaluated whether there was an abundance of individual BDNF-associated probes differentially methylated in EUPD. Three probes exhibited higher methylation with dependence on EUPD (i.e., cg11718030, p-value = 0.0002; cg15462887, p-value 0.0434; cg24377657, p-value = 0.0057). The number of nominally significant probes (n = 3) were contrasted to the total number of probes (n = 16) by the highest nominally significant p-value observed (i.e., p = 0.0434) in an exact binomial test. This analysis yielded a p-value of 0.0299, indicative of an abundance of individual BDNF-associated probes differentially methylated in EUPD (Table 3).

Table 3.
Robust Linear Regression Contrasting Individual BDNF Methylation M-values to Disease Status (EUPD or Control).
3.2. Cohort Description (Validation Group)
All participants were females (males were excluded). A total of 15 patients (25%) were classified into the high-risk/severe attempt category and 45 (75%) into the low-risk group. Clinical outcome variables were largely comparable between the two groups (p > 0.05), i.e., BMI, psychiatric diagnoses, exposure to violence in childhood or adulthood [26], alcohol dependence, or substance abuse (Table 4).

Table 4.
Characteristics of subjects (Suicide grp).
3.3. Promotor-Associated BDNF-Methylation Levels Are Higher-Methylated with Dependence on Severity of Suicide Attempt (n = 60)
In the association analysis between whole-blood-derived BDNF-DNA methylation and severity of suicide attempt, we studied mean values across 16 BDNF promoter-associated CpG-sites. Mean and median-derived values did not significantly differ, as measured by unpaired independent samples t-tests (p = 0.939), and the mean value was determined upon visual inspection as representative of the underlying data (Supplemental Figure S2). DNA methylation levels were higher-methylated with dependence on the severity of suicidal behavior (compared to lower-risk suicide attempters) (W = 623, p = 0.0231) (n = 60) (Figure 2).
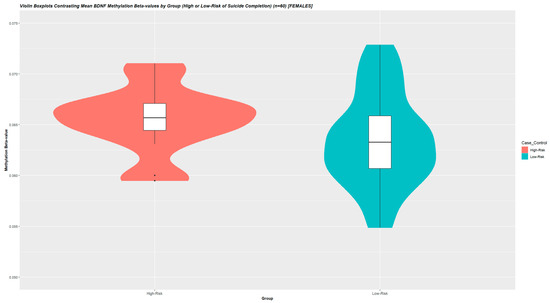
Figure 2.
Violin Boxplots Contrasting Mean BDNF Methylation Beta-values by Group (High or Low-Risk of Suicide Completion) (n = 60) [FEMALES]. To investigate associations between mean BDNF-associated promoter methylation and severe clinical psychiatric phenotypes, we studied mean methylation M-values across 16 BDNF-associated promoter CpG-sites. Beta-values were used for illustration. In this analysis, as clinical variables were largely comparable between the in silico stratified groups, mean BDNF DNA-methylation was compared between groups using the one-sided Wilcoxon signed rank sum exact test. Post hoc univariate analyses were performed to exclude confounders from age and BMI on DNA methylation levels, correlating mean BDNF methylation levels with these descriptive variables in using Pearson’s moment correlation coefficients. p-values < 0.05 were considered significant. DNA methylation levels were higher-methylated with dependence on severity of suicidal behavior (compared to lower-risk suicide attempters) (W = 227, p = 0.0299) (n = 60). Across all samples, non-M-transformed mean BDNF methylation beta-values were 0.06885 (SD = 0.0306) with a mean between-group difference of ~2.0%.
Post hoc analyses by Pearson’s product moment correlation coefficient yielded that derived DNA methylation levels were unassociated with age and BMI (p > 0.1). Across all samples, non-M-transformed mean BDNF methylation beta-values were 0.0689 (SD = 0.0305) with a mean between-group difference of ~2.0% (Figure 2). As a post hoc sensitivity analysis, we re-performed the above analysis for the 16 individual CpG-sites separately and evaluated whether there was an abundance of individual BDNF-associated probes differentially methylated in severe suicidal behavior. Two probes exhibited higher methylation with dependence on EUPD (i.e., cg15462887, p-value = 0.005; cg23497217, p-value = 0.002). The number of nominally significant probes (n = 2) were contrasted to the total number of probes (n = 16) by the highest nominally significant p-value observed (i.e., p = 0.005) in an exact binomial test. This analysis yielded a p-value of 0.00286, indicative of an abundance of individual BDNF-associated probes differentially methylated in severe suicidal behavior (Table 5).

Table 5.
Wilcoxon Rank Sum Exact Test Contrasting Individual BDNF Methylation M-values to Severity of Suicidal Behavior.
4. Discussion
This study reports on a targeted DNA methylation analysis of BDNF-coupled CpG-sites in a cohort of 97 severely impaired EUPD patients with a history of suicide attempts and 32 non-psychiatric controls. Validation of these findings was investigated in an independent cohort of 60 female suicide attempters, stratified into high and low risk based on the severity of suicide attempts. In these analyses, mean BDNF methylation levels were higher-methylated in relation to EUPD and severity of suicidal behavior—independent of age and BMI. This is the first study to reveal emerging evidence of BDNF-coupled increased methylation with dependence on EUPD in women with severe suicidal behavior. These results are strengthened by the validation—of similar magnitude and direction—in a wholly independent cohort of women with dependence on the severity of suicidal behavior. These findings could also be interpreted as more robust compared to studies investigating individual CpG-sites, by accounting for the mean value across several putatively inter-linked BDNF-coupled methylation loci. Thus, epigenetic alterations in BDNF are associated with both EUPD and severe suicidal behavior in female subjects. Causal inferences are prevented by the cross-sectional study design, which did not allow for the determination of whether the observed alterations contribute to the pathophysiology of the condition or arise as an effect of the condition. Nevertheless, extensive previous research on the topic of BDNF and psychiatric disorders adds to the relevance of these findings. According to classical models of epigenetic regulatory mechanisms, increased DNA methylation inhibits gene transcription, implicating, although not directly studied in this population, that decreased BDNF-levels may be associated with EUPD and severity of suicidal behavior. Targeted studies measuring both DNA methylation and gene expression levels in the brain of concerned subjects are needed to further elucidate the role of BDNF in EUPD and suicidality.
Strengths of the study include two phenotypically well-defined, representative patient cohorts of suicide attempters with thorough diagnostics of the psychiatric disorders and a careful assessment of the severity of suicidal behavior [26]. In addition, possible confounders such as psychotropic medication usage, childhood adversity, and comorbidity patterns were considered. It is clear that suicide attempts are highly heterogeneous and prospective studies demonstrate that high intent to die and choice of suicide method evince a higher risk of death by suicide [26,28,44]. Consequently, research suggests that suicide victims are more similar to high-intent or violent attempters as compared to non-violent attempters [28]. It can, thus, be argued that a lack of deep phenotyping and/or stratification of suicide attempters may prevent assured conclusions from previous studies exploring this field [45]. Moreover, the study pertained only to promotor-associated DNA methylation markers measured in whole blood deemed more influential on gene expression compared to other DNA-methylation regions, and the differential methylation status of the candidate site was consistently higher-methylated across the two investigated cohorts with dependence on phenotypes sharing similar features (i.e., severe suicidality).
Our study is burdened by several limitations. First, given the relatively small sample size, the main analysis would be underpowered to comprehensively detect subtle changes in DNA methylation. Yet, lower-powered studies are appropriate in detecting group-differences with a larger effect size, lending further support for the relevance of the presented findings. Second, as derived methylation changes were small (i.e., mean absolute difference in methylation beta-value of 0.06–3.6%), the biological relevance of such subtle changes could be questioned. Recent studies, however, implicate that methylation changes in the 1–5% range are associated with extensive transcriptional and translational consequences—a magnitude of alteration deemed particularly relevant in the pathophysiology of complex multifactorial psychiatric conditions [46]. Third, whole-blood-derived BDNF methylation levels were higher-methylated with dependence on EUPD and severity of suicide attempt. While this cohort of severely impaired EUPD patients with a history recent of suicide attempts could be argued to exhibit similar features to the cohort assessing the severity of suicidal behavior, replication of our findings in relation to EUPD or severe suicidal behavior would be of value to discern whether the observed alterations are related to EUPD or suicidality (or both). Fourth, the appropriateness of the assumption of blood–brain transferability of epigenetic alterations has come under increasing scrutiny [47]. Comparing individual Illumina-array-derived methylation samples extracted from whole blood and several brain regions in 122 individuals, Hannon et al. showed that, for a majority of epigenetic sites, blood-based alterations provide limited causative information as to disease-inducing epigenetic changes occurring in brain tissue [47]. Thus, the uncritical extrapolation of our findings to putative alterations in brain tissue is cautioned against. The aim of the present study was to explore any associations between epigenetic markers of BDNF in whole blood and severe clinical psychiatric phenotypes. Further studies are needed to elucidate any downstream effects of the observed results on gene expression in brain tissue. Fifth, this study pertained exclusively to female subjects. Thus, no assured conclusions can be drawn in relation to BDNF blood methylation levels in males with EUPD or severe suicidal behavior. Sixth, given the limited scope of the study objectives—to investigate blood-derived DNA methylation markers in relation to EUPD and severity of suicide attempt—genotyping of BDNF was not performed. Future studies investigating associations between BDNF polymorphisms and promotor methylation levels are needed to fully elucidate whether putative underlying pathophysiological mechanisms are conferred by epigenetic, genetic, or combined effects. Seventh, associations between methylation levels and gene expression levels were not investigated. This was a conscious choice of the authors as the putative prevalence of driving BDNF gene mutations would need to be accounted for the adequate interpretation of such analyses [48]. Moreover, openly accessible data were not available for the examination of DNA methylation and gene expression in representative cohorts of severely impaired EUPD patients or suicide attempters. Thus, the adjunctive value of investigating methylation-expression correlations in whole blood of control subjects was considered of marginal relevance and was, thus, not explored. Lastly, the DNA methylation analysis did not account for potential confounders from non-antidepressant medication use. Antidepressants were the only medication class reported in more than five subjects and there were no significant between-group differences in any disorders precipitating the prescription of such medications (i.e., MDD or anxiety disorders). In addition, the use of SSRI medication was independent of BDNF methylation levels in the EUPD group. Thus, while it cannot be completely excluded, substantial bias from medication use to the present findings would be highly unlikely. Lastly, we restrict pre-specified inclusion criteria to only include CpG-sites annotated as differentially methylated regions (DMRs) and located within 2000 base pairs of the transcriptional start site. These criteria precluded the investigation of other genes that have previously been associated with neuroinflammation. For example, CRP, IL-1, IL-2, and IL-6 were not annotated to any DMR probe within the TSS2000 and were, thus, not investigated.
In conclusion, whole-blood-derived BDNF promotor methylation levels are higher-methylated with dependence on EUPD and severity of suicide attempts in females. Ample research previously implicated BDNF gene variations in relation to suicide deaths and EUPD, arguably providing strong support to the relevance of the presented findings. Future studies performed in brain tissue, males, or investigating associations between BDNF polymorphisms and such methylation levels are needed to fully elucidate whether the observed associations contribute to major underlying pathophysiological mechanisms in EUPD and/or severe suicidal behavior. Results of such studies hold potential to aid efforts aimed at preventing suicide and managing EUPD by granting complementary insights into the underlying pathophysiological processes and could aid in advancing novel treatments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells12030350/s1, Figure S1: Boxplots DNA methylation M-values for BDNF-coupled CpG-sites, their Median and Mean in the Discovery Group; Figure S2: Boxplots DNA methylation M-values for BDNF-coupled CpG-sites, their Median and Mean in the Validation Group.
Author Contributions
Conceptualization, A.E.D.B. and J.J.; methodology, A.E.D.B. and E.J.; software, A.E.D.B. and E.J.; validation, A.E.D.B., E.J. and J.J.; formal analysis, A.E.D.B. and E.J.; investigation, A.E.D.B. and E.J.; resources, J.J. and M.Å.; data curation, A.W, Å.N., J.J. and M.Å; writing—original draft preparation, A.E.D.B. and E.J.; writing—review and editing, A.E.D.B., E.J., A.W., Å.N., M.Å. and J.J.; visualization, A.E.D.B. and J.J.; supervision, A.E.D.B. and J.J.; project administration, A.E.D.B. and J.J.; funding acquisition, J.J. and M.Å. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this study was provided by the Swedish Research Council (Project number K2009-61P-21304-04-4), Stockholm County Council (ALF, FoUI-954107) and from Region Västerbotten (ALF, RV-967864). The APC was funded by Umeå University.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Committee for Ethical Research at Karolinska Institutet (Dnrs: 95–83; 2021-06929-01) [Discovery Group] and the Regional Ethical Board in Stockholm, Sweden (Dnrs: 00-194, 2015/1454.32) [Validation Group].
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying the findings presented in this study are available upon reasonable request.
Conflicts of Interest
J.J. previously participated in the Advisory Board of Janssen concerning esketamine for the treatment of patients with major depressive disorder with current suicidal ideation. The authors have no other competing interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- (UK) NCC for MH. Borderline Personality Disorder. Am. J. Psychiatry 2009, 166, 505–508. [Google Scholar]
- Zanarini, M.C.; Frances Frankenburg, E.R.; Hennen, J.; Bradford Reich, D.; Silk, K.R. Article Axis I Comorbidity in Patients with Borderline Personality Disorder: 6-Year Follow-Up and Prediction of Time to Remission. Am. J. Psychiatry 2004, 161, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Temes, C.M.; Frankenburg, F.R.; Fitzmaurice, G.M.; Zanarini, M.C. Deaths by Suicide and Other Causes Among Patients with Borderline Personality Disorder and Personality-Disordered Comparison Subjects Over 24 Years of Prospective Follow-Up. J. Clin. Psychiatry 2019, 80, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Keuroghlian, A.S.; Frankenburg, F.R.; Zanarini, M.C. The Relationship of Chronic Medical Illnesses, Poor Health-Related Lifestyle Choices, and Health Care Utilization to Recovery Status in Borderline Patients over a Decade of Prospective Follow-up. J. Psychiatr. Res. 2013, 47, 1499–1506. [Google Scholar] [CrossRef]
- Douzenis, A.; Tsopelas, C.; Tzeferakos, G. Medical comorbidity of cluster B personality disorders. Curr. Opin. Psychiatry 2012, 25, 398–404. [Google Scholar] [CrossRef]
- Grant, B.F.; Chou, S.P.; Goldstein, R.B.; Huang, B.; Stinson, F.S.; Saha, T.D.; Smith, S.M.; Dawson, D.A.; Pulay, A.J.; Pickering, R.P.; et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: Results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 2008, 69, 533–545. [Google Scholar] [CrossRef]
- Pagura, J.; Stein, M.B.; Bolton, J.M.; Cox, B.J.; Grant, B.; Sareen, J. Comorbidity of borderline personality disorder and posttraumatic stress disorder in the U.S. population. J. Psychiatr. Res. 2010, 44, 1190–1198. [Google Scholar] [CrossRef]
- Cattane, N.; Rossi, R.; Lanfredi, M.; Cattaneo, A. Borderline personality disorder and childhood trauma: Exploring the affected biological systems and mechanisms. BMC Psychiatry 2017, 17, 221. [Google Scholar] [CrossRef]
- Ruocco, A.C.; Carcone, D. A neurobiological model of borderline personality disorder: Systematic and integrative review. Harv. Rev. Psychiatry 2016, 24, 311–329. [Google Scholar] [CrossRef]
- Wang, T.Y.; Lee, S.Y.; Hu, M.C.; Chen, S.L.; Chang, Y.H.; Chu, C.H.; Lin, S.H.; Li, C.L.; Wang, L.J.; Chen, P.S.; et al. More inflammation but less brain-derived neurotrophic factor in antisocial personality disorder. Psychoneuroendocrinology 2017, 85, 42–48. [Google Scholar] [CrossRef]
- Wilson, N.; Robb, E.; Gajwani, R.; Minnis, H. Nature and nurture? A review of the literature on childhood maltreatment and genetic factors in the pathogenesis of borderline personality disorder. J. Psychiatr. Res. 2021, 137, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Drews, E.; Fertuck, E.A.; Koenig, J.; Kaess, M.; Arntz, A. Hypothalamic-pituitary-adrenal axis functioning in borderline personality disorder: A meta-analysis. Neurosci. Biobehav. Rev. 2019, 96, 316–334. [Google Scholar] [CrossRef] [PubMed]
- MacDowell, K.S.; Marsá, M.D.; Buenache, E.; Villatoro, J.M.L.; Moreno, B.; Leza, J.C.; Carrasco, J.L. Inflammatory and antioxidant pathway dysfunction in borderline personality disorder. Psychiatry Res. 2020, 284, 112782. [Google Scholar] [CrossRef] [PubMed]
- Danese, A.; Moffitt, T.E.; Pariante, C.M.; Ambler, A.; Poulton, R.; Caspi, A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch. Gen. Psychiatry 2008, 65, 409–416. [Google Scholar] [CrossRef]
- Isung, J.; Aeinehband, S.; Mobarrez, F.; Nordström, P.; Runeson, B.; Asberg, M.; Piehl, F.; Jokinen, J. High interleukin-6 and impulsivity: Determining the role of endophenotypes in attempted suicide. Transl. Psychiatry 2014, 4, e470. [Google Scholar] [CrossRef]
- Sutin, A.R.; Terracciano, A.; Deiana, B.; Naitza, S.; Ferrucci, L.; Uda, M.; Schlessinger, D.; Costa, P.T. High Neuroticism and low Conscientiousness are associated with interleukin-6. Psychol. Med. 2010, 40, 1485–1493. [Google Scholar] [CrossRef]
- Hopwood, C.J.; Zanarini, M.C. Five-Factor Trait Instability in Borderline Relative to Other Personality Disorders. Personal. Disord. 2010, 1, 58. [Google Scholar] [CrossRef]
- Moriceau, S.; Sullivan, R.M. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006, 9, 1004. [Google Scholar] [CrossRef]
- Santiago, A.; Aoki, C.; Sullivan, R.M. From attachment to independence: Stress hormone control of ecologically relevant emergence of infants’ responses to threat. Curr. Opin. Behav. Sci. 2017, 14, 78–85. [Google Scholar] [CrossRef]
- Thomas, M.; Knoblich, N.; Wallisch, A.; Glowacz, K.; Becker-Sadzio, J.; Gundel, F.; Brückmann, C.; Nieratschker, V. Increased BDNF methylation in saliva, but not blood, of patients with borderline personality disorder. Clin. Epigenet. 2018, 10, 1–12. [Google Scholar] [CrossRef]
- Issa, G.; Wilson, C.; Terry, A.V.; Pillai, A. An inverse relationship between cortisol and BDNF levels in schizophrenia: Data from human postmortem and animal studies. Neurobiol. Dis. 2010, 39, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Drzyzga, Ł.R.; Marcinowska, A.; Obuchowicz, E. Antiapoptotic and neurotrophic effects of antidepressants: A review of clinical and experimental studies. Brain Res. Bull. 2009, 79, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L.; Lubin, F.D.; Funk, A.J.; Sweatt, J.D. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol. Psychiatry 2009, 65, 760. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.B.; Perera, S.; Banfield, L.; Anglin, R.; Minuzzi, L.; Samaan, Z. Association between BDNF levels and suicidal behaviour: A systematic review and meta-analysis. Syst. Rev. 2015, 4, 1–12. [Google Scholar] [CrossRef]
- Jokinen, J.; Boström, A.E.; Dadfar, A.; Ciuculete, D.M.; Chatzittofis, A.; Åsberg, M.; Schiöth, H.B. Epigenetic Changes in the CRH Gene are Related to Severity of Suicide Attempt and a General Psychiatric Risk Score in Adolescents. EBioMedicine 2017, 27, 123–133. [Google Scholar] [CrossRef]
- Wagner, J.R.; Busche, S.; Ge, B.; Kwan, T.; Pastinen, T.; Blanchette, M. The relationship between DNA methylation, genetic and expression inter-individual variation in untransformed human fibroblasts. Genome Biol. 2014, 15, R37. [Google Scholar] [CrossRef]
- Stenbacka, M.; Jokinen, J. Violent and non-violent methods of attempted and completed suicide in Swedish young men: The role of early risk factors. BMC Psychiatry 2015, 15, 196. [Google Scholar] [CrossRef]
- Freeman, D.J.; Wilson, K.; Thigpen, J.; McGee, R.K. Assessing intention to die in self-injury behavior. In Psychological Assessment of Suicidal Risk BT-Psychological Assessment of Suicidal Risk; Neuringer, C., Ed.; Centre For Suicide Prevention: Calgary, AB, Canada, 1974. [Google Scholar]
- Zaboli, G.; Gizatullin, R.; Nilsonne, Å.; Wilczek, A.; Jönsson, E.G.; Ahnemark, E.; Åsberg, M.; Leopardi, R. Tryptophan Hydroxylase-1 Gene Variants Associate with a Group of Suicidal Borderline Women. Neuropsychopharmacology 2006, 31, 1982–1990. [Google Scholar] [CrossRef]
- Wakefield, J.C. Diagnosing DSM-IV--Part I: DSM-IV and the concept of disorder. Behav. Res. Ther. 1997, 35, 633–649. [Google Scholar] [CrossRef]
- Asberg, M.; Montgomery, S.A.; Perris, C.; Schalling, D.; Sedvall, G. A comprehensive psychopathological rating scale. Acta Psychiatr. Scand. Suppl. 1978, 271, 5–27. [Google Scholar] [CrossRef]
- Sinai, C.; Hirvikoski, T.; Nordström, A.-L.; Nordström, P.; Sa Nilsonne, A.; Wilczek, A.; Åsberg, M.A.; Jokinen, J. Hypothalamic Pituitary Thyroid Axis and Exposure to Interpersonal Violence in Childhood among Women with Borderline Personality Disorder. Eur. J. Psychotraumatol. 2014, 5, 23911. [Google Scholar] [CrossRef] [PubMed]
- Sinai, C.; Hirvikoski, T.; Wiklander, M.; Nordström, A.L.; Nordström, P.; Nilsonne, Å.; Wilczek, A.; Åsberg, M.; Jokinen, J. Exposure to interpersonal violence and risk of post-traumatic stress disorder among women with borderline personality disorder. Psychiatry Res. 2018, 262, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Zhang, X.; Huang, C.C.; Jafari, N.; Kibbe, W.A.; Hou, L.; Lin, S.M. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinform. 2010, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Todorov, V.; Filzmoser, P. An Object-Oriented Framework for Robust Multivariate Analysis. J. Stat. Softw. 2010, 32, 1–47. [Google Scholar] [CrossRef]
- Yohai, V.J. High Breakdown-Point and High Efficiency Robust Estimates for Regression. Ann. Statist. 1987, 15, 642–656. [Google Scholar] [CrossRef]
- Boström, A.E.; Chatzittofis, A.; Ciuculete, D.-M.; Flanagan, J.N.; Krattinger, R.; Bandstein, M.; Mwinyi, J.; Kullak-Ublick, G.A.; Öberg, K.G.; Arver, S.; et al. Hypermethylation-associated downregulation of microRNA-4456 in hypersexual disorder with putative influence on oxytocin signalling: A DNA methylation analysis of miRNA genes. Epigenetics 2020, 15, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Aryee, M.J.; Jaffe, A.E.; Corrada-Bravo, H.; Ladd-Acosta, C.; Feinberg, A.P.; Hansen, K.D.; Irizarry, R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014, 30, 1363–1369. [Google Scholar] [CrossRef]
- Schalkwyk, L.C.; Pidsley, R.; Wong, C.C.Y. wateRmelon: Illumina 450 methylation array normalization and metrics. R package version 1.2.2. 2013. Available online: https://rdrr.io/bioc/wateRmelon/ (accessed on 1 June 2022).
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The SVA package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef]
- Morris, T.J.; Butcher, L.M.; Feber, A.; Teschendorff, A.E.; Chakravarthy, A.R.; Wojdacz, T.K.; Beck, S. ChAMP: 450k Chip Analysis Methylation Pipeline. Bioinformatics 2014, 30, 428–430. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Mazet, F. Package ‘FactoMineR’. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar]
- Jollant, F.; Bellivier, F.; Leboyer, M.; Astruc, B.; Torres, S.; Verdier, R.; Castelnau, D.; Malafosse, A.; Courtet, P. Impaired decision making in suicide attempters. Am. J. Psychiatry 2005, 162, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Jokinen, J.; Andersson, P.; Chatzittofis, A.; Savard, J.; Rask-Andersen, M.; Åsberg, M.; Boström, A.D.E. Accelerated epigenetic aging in suicide attempters uninfluenced by high intent-to-die and choice of lethal methods. Transl Psychiatry 2022, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Leenen, F.A.D.; Muller, C.P.; Turner, J.D. DNA methylation: Conducting the orchestra from exposure to phenotype? Clin. Epigenetics 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Hannon, E.; Lunnon, K.; Schalkwyk, L.; Mill, J. Interindividual methylomic variation across blood, cortex, and cerebellum: Implications for epigenetic studies of neurological and neuropsychiatric phenotypes. Epigenetics 2015, 10, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Ciuculete, D.M.; Boström, A.E.; Voisin, S.; Philipps, H.; Titova, O.E.; Bandstein, M.; Nikontovic, L.; Williams, M.J.; Mwinyi, J.; Schiöth, H.B. A methylome-wide mQTL analysis reveals associations of methylation sites with GAD1 and HDAC3 SNPs and a general psychiatric risk score. Transl. Psychiatry 2017, 7, e1002. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).