Abstract
Bone loss is a common problem that ranges from small defects to large defects after trauma, surgery, or congenital malformations. The oral cavity is a rich source of mesenchymal stromal cells (MSCs). Researchers have documented their isolation and studied their osteogenic potential. Therefore, the objective of this review was to analyze and compare the potential of MSCs from the oral cavity for use in bone regeneration. Methods: A scoping review was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines. The databases reviewed were PubMed, SCOPUS, Scientific Electronic Library Online (SciELO), and Web of Science. Studies using stem cells from the oral cavity to promote bone regeneration were included. Results: A total of 726 studies were found, of which 27 were selected. The MSCs used to repair bone defects were (I) dental pulp stem cells of permanent teeth, (II) stem cells derived from inflamed dental pulp, (III) stem cells from exfoliated deciduous teeth, (IV) periodontal ligament stem cells, (V) cultured autogenous periosteal cells, (VI) buccal fat pad-derived cells, and (VII) autologous bone-derived mesenchymal stem cells. Stem cells associate with scaffolds to facilitate insertion into the bone defect and to enhance bone regeneration. The biological risk and morbidity of the MSC-grafted site were minimal. Successful bone formation after MSC grafting has been shown for small defects with stem cells from the periodontal ligament and dental pulp as well as larger defects with stem cells from the periosteum, bone, and buccal fat pad. Conclusions: Stem cells of maxillofacial origin are a promising alternative to treat small and large craniofacial bone defects; however, an additional scaffold complement is required for stem cell delivery.
1. Introduction
Bone regeneration currently represents an important challenge in the field of regenerative medicine and craniofacial regeneration. Often in critically sized bone defects, the human body is unable to heal the bone on its own, which leads to nonunion and scar tissue formation [1]. Bone loss is caused by many diseases, trauma, and surgical procedures that lead to functionality problems, and its social impact is growing [2,3]. Autogenous bone grafting remains the gold standard for reconstructing bone defects; however, it is limited by the volume of bone that can be harvested, harvest site morbidity, local hematoma, and remodeling problems of the implanted bone [4,5]. Therefore, the limited supply of autogenous bone grafts and the risk of infection associated with allograft materials have prompted the search for an alternative approach to repair bone defects [1,4].
Bone regeneration is a complex process that requires the migration and proliferation of specific cells to the healing area in order to provide the biological substrate for new tissue growth [3,4]. For this approach, three essential components are typically required: (i) progenitor cells, to form tissues together with available host cells; (ii) stimulatory factors, to direct cellular processes; and (iii) a biomaterial template, to provide cells with a 3D cue to form tissue after implantation in vivo [4,6]. Stem cells are a promising alternative as they are a component of progenitor cells for bone formation that can be supplied exogenously [7]. Mesenchymal stromal cells (MSCs) are multipotent cells present in most adult connective tissues [8,9]. MSCs have the ability to promote better regeneration of soft tissues [10] and mineralized tissues [2]. They have been widely studied due to their ability to differentiate into multiple cell types [8]. Bone marrow (BM) is considered the main source of mesenchymal stem and progenitor cells (MSPCs) for experimental and clinical applications. However, due to the limited number of BM-MSPCs available for autogenous use, the implementation of alternative sources of MSPCs is particularly important [11,12].
Although there are several “loci” or “niches” within the adult human body made up of significant numbers of stem cells, these niches are often not easily accessible and have high residual anatomical site morbidity [13]. A number of studies have emerged which identified the presence of neural crest-derived stem cells (NCSCs) within different adult craniofacial tissues [14]. NCSCs may exist as a dormant multipotent stem cell population in the adult, as their pluripotent state becomes gradually more restricted after migration [14]. Due to their embryonic neural crest origin [11,15] and easy accessibility [16], intraoral tissues are a promising and rich source of stem cells for tissue engineering approaches with potential clinical applications [14], such as in regenerative dentistry [17]. In the oral cavity, stem cells can be isolated from various locations; among those that stand out are the dental pulp of deciduous and permanent teeth, dental follicle, apical papilla, periosteum, and periodontal ligament [1,7,18]. Dental stem cells are able to differentiate into osteoblasts, chondroblasts, and adipocytes [17,19]. Extensive research has been carried out to determine their differentiation mechanisms and efficacy in bone tissue regenerative medicine [1,18,20]. To date, different approaches have been used to induce bone repair in the injured area using stem cells from the oral cavity. However, despite the efforts made to describe the regenerative capacity of stem cells from the oral cavity, no exhaustive review has been found in the literature that details and compares the different sources of stem cells from the oral cavity and the bone regenerative results of each. This review aims to analyze and compare the potential of stem cells from different intraoral tissues for use in bone regeneration, focusing on the bone regenerative result achieved with stem cells from the oral cavity.
2. Materials and Methods
2.1. Systematic Literature Search
A scoping review was performed on stem cells from the oral cavity used for bone regeneration. Our scoping review was performed according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) guidelines [21].
An electronic search was carried out in four digital databases (PubMed, SCOPUS, Scientific Electronic Library Online (SciELO), and Web of Science). The search terms selected were: “stem cell *”, “progenitor cell *”, “autogenous periosteal cells”, “Mesenchymal Stem Cells”, “Mesenchymal Stromal Cells”, “Stem Cells” [Mesh], “Mesenchymal Stem Cells” [Mesh], “Multipotent Stem Cells” [Mesh], “Neural Crest Stem Cells”, “Bone Regeneration”, “Regenerative treatment”, “Regeneration, Guided Tissue”, “Bone”, “Formation *”, “Repair *”, “Densit *”””, “Tissue Regeneration”, “Guided Tissue Regeneration” [Mesh], “Bone and Bones” [Mesh], “Bone Density” [Mesh], “tooth,” “teeth”, “pulp”, “periodontal ligament”, “periosteum”, “Buccal Fat”, “apical papilla”, “deciduous tooth”, “dental follicle”, “oral cavity”, “dental papilla, “dental sac”, “Tooth” [Mesh], “Natal Teeth” [Mesh], “Tooth, Deciduous” [Mesh], “Dental Pulp” [Mesh], “Periodontal Ligament” [Mesh], “Periosteum” [Mesh], “Dental Papilla” [Mesh], and “Dental Sac” [Mesh]. The keywords were combined with Boolean terms OR and AND. The search was performed between May and December 2022. The bibliographies of potentially eligible clinical trials, case reports, case studies, and systematic reviews were also screened for any additional studies which were possibly fit for inclusion. The following search equation was used in PubMed:
((((((((“stem cell *” [Title/Abstract]) OR (“Neural Crest Stem Cells”)) OR (“progenitor cell *” [Title/Abstract])) OR (“autogenous periosteal cells” [Title/Abstract])) OR (“Mesenchymal Stem Cells” [Title/Abstract])) OR (“mesenchymal stromal cells” [Title/Abstract])) OR (((“Stem Cells” [Mesh]) OR “Mesenchymal Stem Cells” [Mesh]) OR “Multipotent Stem Cells” [Mesh])) AND (((((((((“Bone Regeneration” [Title/Abstract]) OR (“Regenerative treatment” [Title/Abstract])) OR ((“Regeneration, Guided Tissue” [Title/Abstract]) AND (bone [Title/Abstract]))) OR ((Bone [Title/Abstract]) AND (“formation *” [Title/Abstract] OR “repair *” [Title/Abstract] OR “densit *” [Title/Abstract] OR “Regeneration *” [Title/Abstract]))) OR ((“Guided Tissue Regeneration” [Mesh]) AND (BONE))) OR ((“Tissue Regeneration”) AND (“Bone and Bones” [Mesh]))) OR ((“Regeneration” [Mesh]) AND (bone))) OR ((regeneration) AND (“Bone and Bones” [Mesh]))) OR ((“Bone Regeneration” [Mesh]) OR “Bone Density” [Mesh]))) AND (((((((((((((tooth [Title/Abstract]) OR (teeth)) OR (pulp)) OR (“periodontal ligament” [Title/Abstract])) OR (periosteum [Title/Abstract])) OR (“Buccal Fat” [Title/Abstract])) OR (“apical papilla” [Title/Abstract])) OR (“deciduous tooth” [Title/Abstract])) OR (“dental follicle” [Title/Abstract])) OR (“oral cavity”)) OR (“dental papilla” [Title/Abstract])) OR (“dental sac”)) OR ((((((((Tooth [Mesh]) OR “Natal Teeth” [Mesh]) OR “Tooth, Deciduous” [Mesh]) OR “Dental Pulp” [Mesh]) OR “Periodontal Ligament” [Mesh]) OR “Periosteum” [Mesh]) OR “Dental Papilla” [Mesh]) OR “Dental Sac” [Mesh])).
The same search equation was adapted for the other search engines. A summary of the factors considered in this review is presented in Table 1.

Table 1.
The details of the scoping review.
2.2. Eligible Criteria
Observational (case reports and case series) and experimental (randomized and controlled clinical trials) studies were included where the general objective was to study stem cells from the oral cavity used for bone regeneration. The potentially eligible articles were screened based on the inclusion criteria: studies in English, Spanish, and Portuguese, full text with no publication date limit, and studies in which stem cells from the oral cavity were used for the treatment of bone defects. Animal and in vitro studies, studies using stem cells from a site other than the oral cavity, and articles not evaluating bone regeneration were excluded.
2.3. Article Selection and Data Extraction
Two independent reviewers analyzed articles obtained in the systematic search process by reviewing the titles and abstracts. The articles that met the eligibility criteria were then analyzed in their full text to confirm their relevance. In cases of disagreement between the two reviewers, a third reviewer was invited to help resolve the differences of opinion. From the full-text articles that made up the final selection, relevant aspects of bone regeneration and stem cells from the oral cavity were compiled. The following information was collected and shown in Table 2: author, year of publication, study design, number of participants, sex and age of the subjects, source of origin of the stem cells, stem cells, bone defect treated, material/fact associated with stem cells, study groups, and the main result in bone regeneration. For Table 3, information was collected on the methodology and results of the studies, including the experimental procedure or isolation of stem cells, the post-surgical evaluation of the defect treated with stem cells, and the complications after treatment of the bone defect. The tables used in data extraction were designed by the authors of this review to obtain data relevant to the subject studied.

Table 2.
Studies using stem cells to repair bone defects.

Table 3.
Cell culture methodology and bone regeneration analysis.
3. Results
3.1. Study Selection
The search and selection process for suitable articles is summarized in Figure 1. The total number of articles found in the databases was 708: 18 were identified from the manual search, and 254 articles were duplicates. After the initial reading by title, 213 were discarded, of which 84 were animal studies, 72 articles studied stem cells that did not come from the oral cavity, 47 were systematic reviews, and 10 were not related to the subject under study. After examination of the abstract, a further 157 studies were discarded, of which 89 studied stem cells that did not come from the oral cavity, 38 were studies that did not analyze bone regeneration, and 30 were not related to the subject of the review. After reading the full-text articles (102 articles), 75 were excluded, of which 37 studied stem cells that did not come from the oral cavity, 25 did not study bone regeneration, 13 were not related to the subject of study, and three were reviews of literature. A total of 27 articles corresponding to observational and experimental studies were finally included in this review.
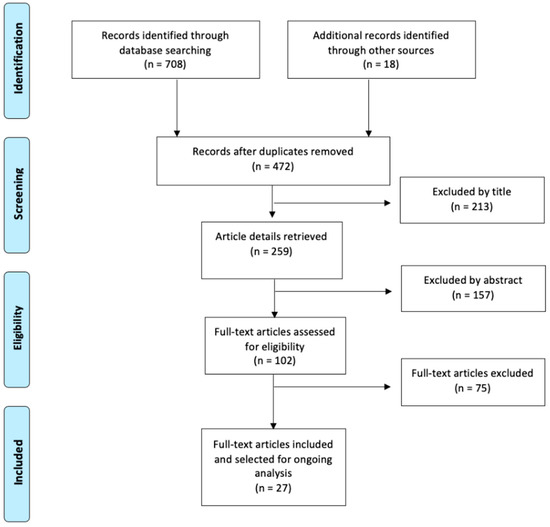
Figure 1.
Flow chart for study selection.
3.2. Characteristics of the Selected Studies
This article analyzes the bone regenerative potential of stem cells from the oral cavity since they are a promising alternative to stem cell niches that are difficult to access. Data were extracted from human studies using Table 2 and Table 3, which detail the relevant information for studies examining the use of stem cells originating from the oral cavity for bone repair.
There have been extensive studies of bone regeneration using stem cells from the oral cavity in humans in the last two decades, beginning in 2003 [36]. The articles are mainly descriptive and observational: case reports [13,23,25,28,29,30,31,32,42,43], case series [24,27,41], or pilot studies [3,36,37,38,40]. However, in recent years, randomized clinical trials have been reported [2,18,22,26,33,34,35,39,44] which use stem cells from the oral cavity, obtaining promising results in bone repair.
Stem cells play an important role in bone repair. To this end, different niches in the oral cavity have been described as a source of stem cells; among them, the periosteum, from which periosteal cells are obtained [3,36,37]; deciduous teeth [29] and permanent teeth (mainly third molars and teeth with orthodontic extraction indication), to obtain periodontal ligament stem cells (PDLP) [30,31,32,33,34,35] and dental pulp stem cells [2,13,18,22,23,24,25,26,27,28]; buccal fat pads, for buccal fat pad-derived stem cells (BFPSCs) [38,39,40]; and cancellous bone, from which autologous bone-derived stem cells (H-MSVs) are obtained [41,42,43,44].
Tissue engineering allows the synthetic scaffold to be combined with stem cells to form hybrid constructs [45]. The analyzed studies have used different scaffold alternatives for seeding cells and to form a biocomplex to replace lost bone tissue. Collagen sponge is the most widely used biomaterial as a carrier for cell micrografts in bone regeneration [2,3,13,18,22,23,24,25,26,27,29,31,39]; however, there have been other promising alternatives with significant results, such as gelatin sponge [32], platelet-rich plasma [37], polymeric [36] and mineral-based biomaterials [28,30], an autogenous bone from the oral cavity [37,39] and the iliac crest [37,39], xenografts [33,35,41,42,43], and allografts [38]. A single study used isolated stem cells applied via a drip [40].
Stem cells obtained from the oral cavity have been used only to repair bone in oral and maxillofacial defects. The main condition studied has been the intraosseous periodontal defect [23,24,26,27,28,29,30,31,32,33,34,35], using stem cells from the dental pulp [23,24,26,27,28,29] and the periodontal ligament [31,32,33,34,35]. Other conditions in which bone repairs have been carried out with stem cells include the increase in the edentulous atrophic alveolus [3,36,37,38], elevation of the maxillary sinus [13,37,42] post-extraction alveolus or alveolar ridge [2,18,22,25,32], bone defect secondary to enucleation of cysts [40,41,44], and cleft lip and palate [39,43].
Cell culture was described by all the studies analyzed, in which four main modalities were described: processing by the Rigenera * system [3,13,18,25,27,31,38], cell culture in α-MEM [2,30,33,36,38,39] or DMEM [28,44], cell culture in osteogenic medium [40], and without cell culture, that is, where the stem cell-bearing tissue was immediately mixed with the scaffold [32,34].
The post-surgical evaluation of bone regeneration was evaluated through histological, radiographic, and clinical analysis. Information on clinical complications of the stem cell-grafted site was available in all studies. The complications evaluated were mainly signs of infection, such as pain [2,25,26,33,35,40], edema [2,3,25,31,40], inflammation [2,3,22,24,25,27,30,33,35,38,44], and functionality [2,3,22,25]. In addition, some studies evaluated healing of the area [2,3,33,36,37,39], paresthesia [40], foreign body reaction [38], and morbidity [2,18].
4. Discussion
4.1. Stem Cells from the Oral Cavity in Bone Regeneration
MSCs can be isolated from various cellular niches, and some of the most accessible ones are located in the oro-maxillo-facial (OMF) area. In the oral cavity, stem cells of dental origin, such as dental pulp stem cells and periodontal ligament stem cells, can be found, which are exclusive to this area and which exhibit features of NCSCs [14]. Additionally, stem cells that are not exclusive to the oral cavity can also be found in other structures of the body, such as cultured autogenous periosteal cells, fat-derived cells, and autologous bone-derived mesenchymal stem cells. Figure 2 illustrates the origin of oral cavity stem cells used to repair bone defects.
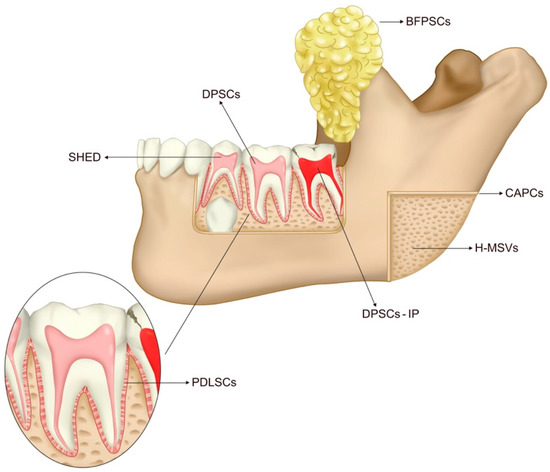
Figure 2.
Oral cavity and its sources of stem cells for bone defect repair. Dental Pulp stem cells of permanent teeth (DPSCs) are derived from the healthy dental pulp of permanent teeth. Stem Cells Derived from Inflammatory Dental Pulp (DPSCs-IP) derived from inflammatory dental pulp. Stem cells from exfoliated deciduous teeth (SHED) are derived from the healthy dental pulp of deciduous teeth. Periodontal Ligament Stem Cells (PDLSCs) are derived from the periodontal ligament. Cultured Autogenous Periosteal Cells (CAPCs) derived from the periosteum. Buccal Fat Pad Derived Cells (BFPSCs) derived from the buccal fat pad. Autologous Bone-derived mesenchymal stem cells (H-MSVs) derived from bone.
The use of oral cavity stem cell therapy for bone regeneration has been extensively studied through in vivo experiments. Animal studies have shown the efficacy of MSCs derived from the oral cavity, such as from dental pulp [46], periodontal ligament [47], and periosteal cells [48], in bone regeneration. In the last two decades, their effectiveness in humans has been demonstrated. Table 4 summarizes the types of stem cells from the oral cavity and the associated scaffolds used to insert them into the bone defect that have been employed in human studies for the regeneration of bone tissue.

Table 4.
Comparative table of oral stem cells and associated scaffolds for bone regeneration.
4.2. Dental-Origin Stem Cells
4.2.1. Dental Pulp Stem Cells
Dental pulp stem cells (DPSC) were derived from the dental pulp of permanent and deciduous teeth. The differentiation capacity of dental pulp tissue has been extensively studied since they were first identified by Gronthos et al. in 2000 [49]. The first report on DPSCs revealed that their properties are comparable to those of stem cells from the bone marrow (BMSC) in vitro and in vivo [49,50]. DPSCs have been used to regenerate structures within the oral cavity and elsewhere. They can be helpful both for the regeneration of soft tissue components and for the regeneration of mineralized structures [51]. Human pulp stem cells include dental pulp stem cells isolated from dental pulp tissues of extracted permanent teeth, stem cells derived from inflamed dental pulp, and stem cells from human exfoliated deciduous teeth.
Dental Pulp Stem Cells of Permanent Teeth
Dental pulp stem cells (DPSCs) cells of permanent teeth are used in the repair and regeneration of bone, periodontal intrabony defects, and dental defects [19]. It is an easily accessible source of MSCs derived from the dental pulp of caries-free third molars [2,13,18,22,23,24,25,26] (teeth in need of extraction due to impaction or poor positioning [27,28]) or teeth supernumeraries [28]. The dental pulp is easily collected using sterile Gracey curettes after root–crown separation to open the pulp chamber and expose pulp tissue [13,23].
Papaccio et al. [52] have conducted several studies on dental pulp stem cells (DPSCs) and have found that these are mainly multipotent cells that can be safely cryopreserved. DPSCs widely proliferate, with a doubling time of 24 h [53], and can have a long lifespan, up to 2 years after cryopreservation [52]. Dental pulp stem cells have been shown to express the MSC markers STRO-1, CD90, CD29, CD44, CD166, CD105, CD106, CD146, CD13, and are also negative for CD14 and CD34 [45].
The classical approach for bone regeneration requires a synthetic or natural scaffold for the implantation of stem cells in the bone defect [54]. All the studies analyzed used DPSCs seeded onto a collagen-based sponge scaffold [2,13,18,22,23,24,25,26,27]. This allows the formation of a biocomplex constituted by the collagen sponge as a carrier of cell micrografts that has no radiopacity at all [31]. Various studies have amply demonstrated that if DPSCs have seeded on a collagen I scaffold, the resulting biocomplex will allow the formation of well-differentiated bone of critical sizes [3,13,22,25,26].
Different bone repairs of the oral cavity with DPSCs have been reported, such as infra bony periodontal defects [23,24,26,27], post-extraction sockets [2,18,22,25], and maxillary sinus lifts [13]. For deep periodontal intrabony defects [23,24,27], the application of DPSCs significantly improved the clinical parameters of periodontal regeneration after one year of treatment compared with a defect treated without DPSCs [26]. On the other hand, DPSCs have been used for larger bone defects such as impacted lower third molar (ITM) post-extraction sockets. Barbier et al. [18] found no significant differences in the clinical, radiological, and surgical characteristics of the ITM between the groups treated with and without cells. However, it was shown that DPSCs allowed the formation of well-differentiated bone in post-extraction sockets with the formation of the Haversian system containing a critical amount of bone tissue [25]. Studies by d’Aquino et al. [2] and Giuliani et al. [22] evaluated alveolar repair secondary to third molar impaction. Using clinical and radiographic analysis, d’Aquino et al. [19] determined that at three months, there was greater clinical insertion compared with the group treated without cells. Giuliani et al. [22] evaluated the same subjects after 3 years, at which time greater bone hardness and less exposure of molar roots were observed compared to cell-free sites. Therefore, the dental pulp can be considered an interesting and potentially important source of autologous stem cells for therapeutic use in craniofacial bone regeneration.
Stem Cells Derived from Inflamed Dental Pulp
The discovery of DPSCs has provided new perspectives for bone tissue repair. However, a limitation for clinical application is the availability of DPSCs since these come from healthy tissue. Recently, some studies found that a certain proportion of ectomesenchymal stem cells were contained within inflamed tissues of the dental pulp, and that these had the potential for tissue regeneration [55,56,57]. Inflammation is a complex process that varies widely from one individual to another. Depending on the intensity of the inflammation, some stimuli can activate some stem cell properties, thus inducing their proliferation and differentiation. Hypoxia has been shown to increase DPSC proliferation [55,56,57,58,59] and the angiogenic potential of dental pulp cells [60]. Pereira et al., 2012 [61] compared cells of normal and inflamed human dental pulp and found that the morphology, proliferation rate, and differentiation potential of inflamed DPSCs were similar to those observed for normal DPSCs, thus demonstrating that the inflammatory process did not affect the stem cell properties that were assessed. However, Li et al. [28] later determined that the proliferative and osteogenic differentiation capacity of DPSC-IPs was slightly decreased, while the adipogenic and chondrogenic differentiation capacity did not show any significant differences compared with normal DPSCs (DPSC-NP). Therefore, to a certain extent, DPSC-IPs preserved the properties of DPSCs, including the expression of certain surface markers of mesenchymal stromal cells. DPSC-IPs showed highly positive expression levels of CD44 and CD90, while the levels of CD34 and CD45 were negative, in line with characteristics of mesenchymal stromal cells [28]. Previous studies have shown that although they lose some of the properties of stem cells, DPSC-IPs retain the potential for tissue regeneration [28,55,56]. These results suggested that although osteogenic capacity was impaired to some extent, DPSC-IPs could still be successfully cultured and amplified for the replacement of DPSC-NPs in clinical practice. Li et al. [28] provided evidence that DPSC-IP/β-TCP compounds may have a certain repair effect on periodontal hard tissue defects caused by periodontitis and may be a new source of oral tissue regeneration for potential future clinical applications.
Stem Cells from Exfoliated Deciduous Teeth
Stem cells from exfoliated deciduous teeth (SHEDS) are DPSCs derived from human exfoliated deciduous teeth. In 2003, Miura et al. [7] performed the first isolation of a population of MSCs from the pulp tissue of the crown of exfoliated deciduous teeth. It was identified that SHEDs are a population of highly proliferative postnatal stem cells [7,62] capable of differentiating into a variety of cell types with neurogenic [63,64], adipogenic, odontogenic, and osteogenic potential [7,64,65,66,67,68]. The degree of bone regeneration with SHEDS relative to the bone defect is almost equivalent to that with BMSCs [69]. Kunimatsu et al. [67] determined by in vitro experimentation that SHEDS exhibit greater proliferative activity, odontogenic and osteogenic differentiation potential, and osteoinductive capacity compared with DPSCs from permanent teeth.
Kim et al. [70] and Vakhurushey et al. [71] found that in vitro osteogenic differentiation of SHEDS enhances hard tissue formation when transplanted subcutaneously [70,71]. Similarly, SHEDS produce mineralized structures in vivo. SHEDS effectively repaired orofacial defects of critical size in animal models such as mice [66] rats, [65,72] minipig [68], and dogs [62] without any immune reaction [62,65]. Behnia [62] and Ma et al. [73] showed in their in vitro and in vivo experiments that cryopreservation of SHEDS for more than two years did not affect their multipotent properties and that SHEDS could be successfully used as a therapeutic approach. Thus, from a practical perspective, stem cells from deciduous teeth were an easily accessible, widely proliferating source of autologous stem cells capable of engrafting and regenerating bone to repair bone defects of critical size, indicating that SHEDs constitute a promising model for possible therapeutic applications [7,66,72,74]. Due to the few bone lesions in children, SHEDs have not been used in infants; however, a study has described their osteoregenerative capacity as an allograft [29]. Despite the significant osteoinductive results observed with SHEDS in vitro and animal models, further experimental studies are required to demonstrate their regenerative capacity in humans, as only one case report has been published.
4.2.2. Periodontal Ligament Stem Cells
The potential of periodontal ligament (PDL) stem cells was first described by Seo et al. [75]. These were isolated from extracted human third molars and transplanted into immunocompromised mice and rats to assess their regenerative potential [75]. Since then, numerous in vitro and in vivo studies have been performed to further evaluate the regenerative capacity of PDL stem cells [76,77,78]. This has been followed by experimental applications to study its clinical efficacy in humans. The human PDL contains a group of stem cells (PDLSCs) that express the surface markers of MSCs, present self-renewal capacity, and have multipotent capacity. PDLSCs are the most studied source and are considered the most suitable for periodontal intrabony defects [31]. These cells are easily accessible from the adherend of the extracted tooth roots [34] and are capable of secreting the mineralized structure [14]. The third molars have mainly been used to obtain periodontal tissue [30,32,33,34,35].
Ex vivo cultured periodontal ligament stem cells (PDLSCs) isolated from soft tissues adherent to extracted teeth have shown the ability to regenerate periodontal intrabony defects in animal models [79], a finding that has also been replicated in human studies [30,31,32,33,34,35]. Pilot studies, randomized controlled trials (RCTs) [33], and case reports [31] have demonstrated the potential of PDLSCs to be a powerful tool for periodontal intrabony therapy.
Intraosseous pockets, which result from bacterial infection and lead to bone resorption, are bone defects in the periodontal complex. Although adequate therapy can resolve the infection, it is not always possible to restore the injured tissue [31]. However, PDL tissue-derived cells have been shown to have the ability to regenerate alveolar bone tissue [30,31,32,33,34,35]. This regenerative capacity is attributed to a small number of progenitor cells within the PDL that retain their potential for proliferation and differentiation. To promote this regenerative potential, these stem cells must be combined with scaffolds made from various biomaterials, such as xenogenic bone substitute (XBS) [33,35], gelatin sponge [32,34], CALCITITE 4060-2 bone graft material [30], or collagen sponge scaffold [31].
Although all patients in the studies analyzed showed clinical benefits after PDLSC transplantation, no statistically significant differences in clinical parameters were detected between the cell group and the control group [33,34,35]. However, the radiographic analysis revealed a significant difference in bone defect density [34] and mineralization rate [31] in the cell-treated groups. These improvements in defect area and density are promising results of PDLSC application for the treatment of periodontal intrabony defects [33,35].
4.3. Non-Dental Origin Stem Cells from the Orofacial Region
4.3.1. Cultured Autogenous Periosteal Cells
In 1742, Duhamel was the first investigator to study the osteogenic potential of the periosteum. A century later, Ollier discovered that the transplanted periosteum could induce new bone formation. Based on the studies mentioned above and the advances in cell culture, H.B. Fell, in 1932, was the first to report the culture of the periosteum and its cells. Fell used an in vitro experiment to determine the ability of this tissue to form mineralized tissue. In the 1990s, the research group of A.L. Caplan pioneered the in vivo investigation of the osteogenic potential of periosteal cells in the field of bone engineering [80].
The periosteum is a highly vascular connective tissue that covers bone surfaces. It is composed of an external fibrous layer containing elastic fibers and micro vessels and an inner cambium layer where periosteum-derived progenitor cells (PDPCs), major players in bone development and fracture healing, reside [81]. Periosteal cell micrografts have been shown to maintain high cell viability and high positivity for stem cell markers such as CD73, CD90, and CD105 [82]. Three studies have looked at periosteal-derived autologous cells for bone regeneration [3,36,37]. Cultured autogenous periosteal cells (CAPCs) have been used for alveolar ridge augmentation [3,37], edentulous atrophic posterior maxillary alveolus [36], and maxillary sinus lift repair [37] in combination with biocompatible materials in specific collagen membranes soaked in cell suspensions to build a biocomplex that can be grafted directly onto the site [3].
A study by d’Aquino et al. [3] revealed significantly lower overall resorption of the alveolar ridges after extraction of a multi-rooted tooth in the group treated with periosteal cells and collagen compared with that treated solely with collagen, achieving 36% less horizontal and vertical resorption of than the group treated with collagen. Furthermore, it has been shown histologically [3] and radiographically [37] that the ossification process was much faster in the group treated with these cells at 45 days compared with the control group without cells. On the other hand, Nagata et al. [37] mixed CAPCs with particulate autogenous bone and platelet-rich plasma and achieved satisfactory results, even in cases of advanced atrophy, revealing prominent recruitment of osteoblasts and osteoclasts accompanied by angiogenesis around the regenerated bone. Therefore, the use of stem cells derived from the periosteum offers bone formation and remodeling with successful results, allowing the reduction of autogenous bone content if used as a complement to the cells [37]. This makes the procedure less invasive, and it is even possible to completely dispense with the use of autogenous bone and use collagen matrices instead [3,37]. In addition, the periosteum is freely accessible through the superficial layer of the oral cavity throughout its lifespan, and this is another important advantage of the use of the periosteum [37].
4.3.2. Buccal Fat Pad-Derived Cells
Adipose stem cells (ASCs) were first discovered in 2001 by Zuk et al. [83] and are now widely used in tissue engineering. Their advantage over other sources is that they are generally obtained from disposable liposuction tissues, and some studies have found their properties comparable to those of bone marrow-derived stem cells (BMMSCs) [84]. The buccal fat pad (BFP) is an ideal tool in the hands of an oral and maxillofacial surgeon for tissue engineering for bone tissue repair [40]. Promising results have been obtained in bone defects produced by the enucleation of cysts and tumors [40], cleft lip and palate [39], and atrophic alveolar ridges [38]. BFP was harvested from healthy subjects through a buccal incision distal to the maxillary second molar [38]. To isolate BFPSCs, 3 to 10 mL is excised under aseptic conditions [38,39]. BFPSCs have the capacity for osteogenic differentiation in vitro and have shown good adhesion to scaffolds [38]. In humans, BFPSCs have been applied in different ways to bone defects. Meshram et al. [40] collected the BFPSCs and applied them via a drip to fill the bone defect left by the enucleation procedure in a dry surgical field [40]. Khojasteh et al. [38] and Khojasteh et al. [39] used an allograft and a collagen membrane with autograft, respectively, to fill the bone defect.
Three studies demonstrated the feasibility of reconstructing bone defects with BFPSCs [38,39,40]. Meshram et al. [40] observed an increase in bone density between the preoperative and postoperative stages, going from thick irregular trabecular bone in the first month to dense compact bone at six months [40]. On the other hand, two studies by Khojasteh et al. [38,39], achieved a significantly higher percentage of newly formed bone in the BFPSC-treated group compared with the control. Therefore, the application of MSCs derived from buccal fat pads together with different scaffolds is promising for bone repair [38]. However, age is an important factor to consider in the effectiveness of this treatment. The total number of cells in the oldest patient was lower and took the longest time to culture compared with samples from younger patients [40,44]. This suggests that with increasing age, the proliferative capacity of stem cells deteriorates.
4.3.3. Autologous Bone-Derived Mesenchymal Stem Cells
MSCs can be isolated by minimally invasive means from craniofacial bone, including alveolar bone [12,83,84]. Alveolar bone stem cells have osteogenic potential [12] and immunomodulatory properties comparable to those of bone marrow-derived stem cells commonly used in bone regeneration (BMMSCs) [12,85].
Tissue engineering that combines a scaffold with mesenchymal stromal cells derived from cancellous bone has shown excellent results for the repair of bone defects in animal models [85,86,87]. For this reason, Redondo et al. [44] and Pradel et al. [41] presented clinical trials using autologous bone-derived mesenchymal stem cells (H-MSVs). Redondo et al. 2017 [44] obtained H-MSVs from the intraoral bone using a 2 mm trephine cultured on a serum cross-linked scaffold (BioMax) for the treatment of maxillary cysts. Biomax favors cell nesting and growth and is very well tolerated by the host [88]. Two to four disks were used for each cystic bone defect [41,44]. A significant increase in computed tomography (CT) density inside the cyst after treatment could be observed. By contrast, the density of the control area did not present changes.
Pradel et al. has investigated different bone defects using autologous bone-derived mesenchymal stem cells [41,42,43]. They transplanted stem cells from jaws into an enucleation of cysts [41]. By radiographic analysis, the group treated with stem cells showed considerably greater ossification in cystic cavities grafted with autogenous osteoblasts in collagen-based scaffolds [41]. In 2008, Pradel et al. investigated sinus lift using stem cells obtained from the maxilla seeded in the demineralized bone matrix (DBBM) and solvent-dehydrated mineralized bovine bone (SDBB), achieving better results in SDBB [42]. Subsequently, in 2012, Pradel and Lauer [43], using the same cell culture, achieved greater ossification of the bone defect in the test group compared with the control using spongeous iliac bone. Therefore, cell therapy with H-MSVs associated with a scaffold could be considered as an alternative for bone defects.
4.4. Mesenchymal Staminal Cell Biomarkers
Stem cells obtained from the oral cavity are characterized by the negative expression of hemopoietic antigens such as CD14, CD19, CD24, CD34, CD45, and HLA-DR, and positive expression of mesenchymal stromal cell markers such as CD10, CD13, CD29, CD44, CD73, CD90, and CD105 (Table 5) [89,90,91,92,93]. The biomarkers expressed in stromal cells can vary depending on their origin and state of differentiation. Even within the same source of stem cells, the expression of biomarkers may present variations, as observed in the studies analyzed. The osteogenic biomarkers of oral cavity stem cells indicate their ability to differentiate into bone cells and, therefore, their potential for bone regeneration (Table 5).

Table 5.
Oral cavity mesenchymal stromal cell biomarkers.
MSCs are capable of differentiating into various cell types, including bone cells, and have the potential to regenerate damaged tissues and bone structures. The mechanisms of action of stem cells for osteogenesis mainly involve cell differentiation, as they have the ability to differentiate into osteoblasts and produce bone matrix. This process is favored by growth factors such as TGF-β (Transforming Growth Factor-β), BMPs (Bone Morphogenetic Proteins), and PDGF (Platelet-Derived Growth Factor), which are found in the local environment of the lesion and the expressed biomarkers [94].
The reviewed studies that used CAPCs and H-MSVs for bone regeneration did not assess bone markers. However, the presence of various osteogenic biomarkers in stem cells of bone tissue and periosteum of other bone structures of the body has been widely described in the literature [95], which would support the osteogenic capacity of CAPCs and H-MSV obtained from the oral cavity. Despite differences in the expression of certain markers, MSCs from the oral cavity have similar therapeutic potential in bone regeneration. Therefore, the choice of the origin source would seem to depend to a great extent on the availability and ease of obtaining the stem cells. Therefore, oral cavity-derived mesenchymal stromal cells are believed to be a very important and valuable resource for the eventual development of cells for clinical/therapeutic applications in dentistry and medicine due to their easy access and low risk of complications.
4.5. Cell Processing
Osteogenic pre-differentiation has been reported to increase the bone repair potency of MSCs [86]. For this reason, several studies seeded stem cells in osteogenic media before implantation [38,40,44]. However, similar results have been obtained by incubating third to fourth-passage stem cells without an osteogenic medium. Cell culture by the enzymatic method has been used for 40 years in the laboratory to isolate cells and is considered the best available method; however, they are not compatible with clinical practice due to the extensive manipulation of the tissue and its long process of preparation [18]. It has been described that their isolation, differentiation, expansion, and proliferation can be avoided, facilitating clinical management [18]. The Rigenera Protocol allows the production of adult mesenchymal stromal cells from a minimum amount of tissue, without the need for cell culture, using long-term enzymatic experimental methods. The Rigenera® device is a technology that performs dental tissue disaggregation and the necessary filtering to obtain an autologous product for immediate application in clinical practice that is capable of promoting bone regeneration [3,13,18,22,25,26,27,31]
Biobanking
Biobanks are not-for-profit services that collect, process, store, and distribute biological samples and data. Thanks to their versatility and easy accessibility of the tissue of origin, dental stem cells are a promising resource for both research and clinical applications [96]. The great potential of stem cells for applications in the field of regenerative medicine has been demonstrated, leading to the development of numerous biobanks specialized in their collection [96]. The first tooth bank, named “Three Brackets”, was established at Hiroshima University in 2005. This was followed by the opening of other institutional centers or private companies for storing autologous dental stem cells [97].
Dental stem cell banking has focused on cells contained in the pulp of human deciduous and permanent teeth, especially wisdom teeth. Cryopreservation has proven to be an effective method for the biobanking of tooth and dental pulp [97]. The harvested dental stem cells can be stored as biological insurance for the individual or blood relatives until a relevant disease requires their usage [96].
4.6. Complications
Clinical studies have shown a wide clinical potential of MSC application. However, there have been numerous reports of adverse events and side effects associated with MSC therapy [98]. It has been proposed that these reflect aspects of cell processing and culture as they can drastically influence the cell population profile and change protein expression [98]. Furthermore, rare but prominent issues with hemocompatibility have become apparent [99].
However, the clinical studies reviewed here highlight the use of MSCs as being safe and feasible, with only minor side effects. Similar results were obtained in previous reviews [100]. It was shown that the donor sites presented no adverse alterations, with a very similar postoperative period between the groups. On the other hand, for the grafted site, no study reported serious adverse effects or morbidity after stem cell grafting [2,3,13,22,24,25,26,27,28,30,31,33,34,35,36,38,44], apart from the common side effects of regenerative surgeries such as mild-moderate pain and swelling during the first week [2,3,24,27,35,38], and mild hypersensitivity during the following weeks [35]. Postoperative clinical observations revealed healing without functional alterations [2,21].
One study described the development of partial dehiscence in one of the patients [39], and d’Aquino et al. 2009 [2] and Meshram et al. 2018 [40] described complications at the end of the first week. However, these complications gradually abated over time. Only Nagata et al. [37] described a case of progressive alveolar resorption after the sinus lift procedure. Therefore, the biological risk and morbidity of a site grafted with stem cells are minimal. These favorable results may be explained by cell differentiation prior to implantation. Therefore, the use of highly differentiated cells could be essential to avoid adverse effects [90].
Despite the positive results observed in the analyzed studies, MSC therapy remains a risky therapy. Drawbacks of approaches that include the culture of stem cells have prompted investigations into regeneration based on endogenous MSC recruitment with in situ tissue engineering. Stem cell migration is required for morphogenesis and organogenesis during development and for tissue maintenance and injury repair in adults. Successful endogenous MSC recruitment is the first step toward successful tissue regeneration. The identification of stem cell niches in the oral cavity with promising results in bone regeneration lays the foundation for the application of in situ tissue engineering [91].
4.7. Bone Repair Evaluation
The healing sequences of the grafted tissues were evaluated by clinical, radiographic, and histological analysis.
4.7.1. Radiographic
A radiographic analysis is the most commonly used test to evaluate success in the bone repair of the grafted site. 2D images such as panoramic [2,36,40,44] and standardized periapical [2,3,22,23,24,26,27,28,30,31,32,33,34,35] radiographs have been used as well as 3D radiographs such as computed tomography (CT) [13,18,31,37,38,39,40].
All periodontal intrabony defects were evaluated by standardized periapical radiographs using parallel techniques and individual custom bite blocks. These images allow the rate of increase in bone height after grafting to be analyzed in two dimensions. Panoramic radiography is used to evaluate large defects such as cystic enucleation [40,44] or alveolar ridges due to the impaction of third molars [2,36]. In the panoramic radiograph, bone regeneration is evaluated by analyzing the change in radiopacity. On the other hand, CT is used to analyze the sections obtained to determine the preoperative defect and postoperative defect through the variation in bone fill volume in large bone defects such as cleft lip and palate [39], cystic enucleation [13,40], and maxillary sinus lift [37].
4.7.2. Clinical Analysis
After surgery, soft tissue healing and normal healing sequences of the grafted tissues were evaluated. For periodontal intrabony defects, various clinical parameters that assess the bone gain were analyzed, such as the tooth mobility before and after grafting [23,24,26,27,28,30,32,34,35]. On the other hand, signs of morbidity, pain, edema, bleeding, inflammation, functionality, and healing of the grafted site were evaluated at different times after bone graft surgery [25].
4.7.3. Histological Analysis
For histological analysis, 2–3 mm trephine biopsy samples were collected from the surgical site [3,25,37,38,39]. Five studies obtained biopsies from the grafted site to later receive an implant [3,25,37,38,39], and two studies obtained the sample for histological evidence without replacement of the bone tissue obtained in the biopsy [22,40]. Histological analysis was performed on different regenerated bone defects such as defects secondary to cystic enucleation [40], alveolar ridges due to third molar impaction [22], atrophic ridges [38], cleft lip, and cleft palate [39]. Histologic results indicated active new bone formation at stem cell-treated sites [25,39,40].
4.8. Considerations and Limitations in the Use of Stem Cells
MSCs are cells with the capacity for self-renewal and multilineage differentiation [95]. Oral cavity stem cells have been studied as a possible source of stem cells for bone regeneration. However, there are limitations that must be taken into consideration. Dental stem cells are found in limited amounts, which could make their use in regenerating large defects difficult. Furthermore, dental tissues are specialized tissues that do not undergo continuous remodeling like bone tissue. Therefore, stem cells derived from dental tissues may be restricted in their differentiation potency compared to BMMSCs. Previous studies have demonstrated higher mineral deposition, proliferation rate, and levels of expression of osteogenic marker genes with bone marrow-derived mesenchymal stem cells (BMSCs) compared with oral cavity-derived stem cells, such as DPSCs, BFPSCs, and PDLSCs, in in vitro studies [95,100]. However, it is important to consider that in vivo results have shown that the bone regeneration capacity of oral cavity stem cells is similar to that of BMSCs. Therefore, the pain and morbidity accompanying MSCs obtained from bone marrow are justification for the use of oral cavity stem cells based on successful results reported in the literature. It is important to continue advancing in the study and analysis of the osteogenic capacity of oral cavity stem cells for the development of new therapies to expand therapeutic options.
5. Conclusions
Bone regeneration is a complex process that requires the migration of specific cells to form tissues along with available host cells. Stem cells of maxillofacial origin have been proven to be capable of differentiating into different cell types, including bone cells, and therefore have the potential to regenerate damaged tissues and bone structures. However, an additional scaffold complement is required to facilitate the insertion of stem cells into the defect and improve bone regeneration. Seven cell types used for different bone defects have been described: (I) dental pulp stem cells of permanent teeth, (II) stem cells derived from inflamed dental pulp, (III) stem cells from exfoliated deciduous teeth, (IV) periodontal ligament stem cells, (V) cultured autogenous periosteal cells, (VI) buccal fat pad-derived cells, and (VII) autologous bone-derived mesenchymal stem cells. MSCs show differences in the expression of certain markers; however, MSCs from the oral cavity presented similar therapeutic potential in bone regeneration. Therefore, the choice of the source of origin seems to depend to a great extent on the availability and ease of obtaining the required cells.
Author Contributions
Conceptualization, R.F. and J.A.-A.; methodology, J.A.-A. and R.F.; validation, R.F. and J.A.-A.; formal analysis, J.A.-A., R.F., R.P. and M.R.; investigation, R.F. and J.A.-A.; resources, R.F.; data curation, R.F. and J.A.-A.; writing—original draft preparation, J.A.-A.; writing—review and editing, J.A.-A., R.F., R.P. and M.R.; visualization, J.A.-A.; supervision, R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- Walmsley, G.G.; Ransom, R.C.; Zielins, E.R.; Leavitt, T.; Flacco, J.S.; Hu, M.S.; Lee, A.S.; Longaker, M.T.; Wan, D.C. Stem Cells in Bone Regeneration. Stem Cell Rev. Rep. 2016, 12, 524–529. [Google Scholar] [CrossRef] [PubMed]
- d’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur. Cell Mater. 2009, 12, 75–83. [Google Scholar] [CrossRef] [PubMed]
- d’Aquino, R.; Trovato, L.; Graziano, A.; Ceccarelli, G.; de Angelis, G.C.; Marangini, A.; Nisio, A.; Galli, M.; Pasi, M.; Finotti, M.; et al. Periosteum-derived micro-grafts for tissue regeneration of human maxillary bone. J. Transl. Sci. 2016, 2, 125–129. [Google Scholar] [CrossRef]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Pradel, W.; Tausche, E.; Gollogly, J.; Lauer, G. Spontaneous tooth eruption after alveolar cleft osteoplasty using tissue-engineered bone: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 440–444. [Google Scholar] [CrossRef]
- Dhote, R.; Charde, P.; Bhongade, M.; Rao, J. Stem Cells Cultured on Beta Tricalcium Phosphate (β-TCP) in Combination with Recombinant Human Platelet-Derived Growth Factor-BB (rh-PDGF-BB) for the Treatment of Human Infrabony Defects. J. Stem Cells 2015, 10, 243–254. [Google Scholar]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 13, 5807–5812. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baghaban Eslaminejad, M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Ding, D.C.; Shyu, W.C.; Lin, S.Z. Mesenchymal stem cells. Cell Transpl. 2011, 20, 5–14. [Google Scholar] [CrossRef]
- Kulakov, A.; Kogan, E.; Brailovskaya, T.; Vedyaeva, A.; Zharkov, N.; Krasilnikova, O.; Krasheninnikov, M.; Baranovskii, D.; Rasulov, T.; Klabukov, I. Mesenchymal Stromal Cells Enhance Vascularization and Epithelialization within 7 Days after Gingival Augmentation with Collagen Matrices in Rabbits. Dent. J. 2021, 9, 101. [Google Scholar] [CrossRef]
- Lohberger, B.; Kaltenegger, H.; Stuendl, N.; Payer, M.; Rinner, B.; Leithner, A. Effect of cyclic mechanical stimulation on the expression of osteogenesis genes in human intraoral mesenchymal stromal and progenitor cells. BioMed Res. Int. 2014, 2014, 189516. [Google Scholar] [CrossRef]
- Matsubara, T.; Suardita, K.; Ishii, M.; Sugiyama, M.; Igarashi, A.; Oda, R.; Nishimura, M.; Saito, M.; Nakagawa, K.; Yamanaka, K.; et al. Alveolar bone marrow as a cell source for regenerative medicine: Differences between alveolar and iliac bone marrow stromal cells. J. Bone Miner. Res. 2005, 20, 399–409. [Google Scholar] [CrossRef]
- Brunelli, G.; Motroni, A.; Graziano, A.; D’Aquino, R.; Zollino, I.; Carinci, F. Sinus lift tissue engineering using autologous pulp micro-grafts: A case report of bone density evaluation. J. Indian Soc. Periodontol. 2013, 17, 644–647. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Kaltschmidt, C.; Widera, D. Adult craniofacial stem cells: Sources and relation to the neural crest. Stem Cell Rev. Rep. 2012, 8, 658–671. [Google Scholar] [CrossRef]
- Roato, I.; Chinigò, G.; Genova, T.; Munaron, L.; Mussano, F. Oral Cavity as a Source of Mesenchymal Stem Cells Useful for Regenerative Medicine in Dentistry. Biomedicines 2021, 9, 1085. [Google Scholar] [CrossRef]
- Capparè, P.; Tetè, G.; Sberna, M.T.; Panina-Bordignon, P. The emerging role of stem cells in regenerative dentistry. Curr. Gene Ther. 2020, 20, 259–268. [Google Scholar] [CrossRef]
- Mozaffari, M.S.; Emami, G.; Khodadadi, H.; Baban, B. Stem cells and tooth regeneration: Prospects for personalized dentistry. EPMA J. 2019, 10, 31–42. [Google Scholar] [CrossRef]
- Barbier, L.; Ramos, E.; Mendiola, J.; Rodriguez, O.; Santamaria, G.; Santamaria, J.; Arteagoitia, I. Autologous dental pulp mesenchymal stem cells for inferior third molar post-extraction socket healing: A split-mouth randomised clinical trial. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e469–e477. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sybil, D.; Jain, V.; Mohanty, S.; Husain, S.A. Oral stem cells in intraoral bone formation. J. Oral Biosci. 2020, 62, 36–43. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Manescu, A.; Langer, M.; Rustichelli, F.; Desiderio, V.; Paino, F.; De Rosa, A.; Laino, L.; d’Aquino, R.; Tirino, V.; et al. Three years after transplants in human mandibles, histological and in-line holotomography revealed that stem cells regenerated a compact rather than a spongy bone: Biological and clinical implications. Stem Cells Transl. Med. 2013, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Ferrarotti, F.; Cricenti, L.; Mariani, G.M.; Romano, F. Autologous dental pulp stem cells in periodontal regeneration: A case report. Int. J. Periodontics Restor. Dent. 2014, 34 (Suppl. S3), s27–s33. [Google Scholar] [CrossRef]
- Aimetti, M.; Ferrarotti, F.; Mariani, G.M.; Cricenti, L.; Romano, F. Use of Dental Pulp Stem Cells/Collagen Sponge Biocomplex in the Treatment of Non-Contained Intrabony Defects: A Case Series. Clin. Adv. Periodontics 2015, 5, 104–109. [Google Scholar] [CrossRef]
- Monti, M.; Graziano, A.; Rizzo, S.; Perotti, C.; Del Fante, C.; d’Aquino, R.; Redi, C.A.; Rodriguez Y Baena, R. In Vitro and In Vivo Differentiation of Progenitor Stem Cells Obtained after Mechanical Digestion of Human Dental Pulp. J. Cell. Physiol. 2017, 232, 548–555. [Google Scholar] [CrossRef]
- Ferrarotti, F.; Romano, F.; Gamba, M.N.; Quirico, A.; Giraudi, M.; Audagna, M.; Aimetti, M. Human intrabony defect regeneration with micrografts containing dental pulp stem cells: A randomized controlled clinical trial. J. Clin. Periodontol. 2018, 45, 841–850. [Google Scholar] [CrossRef]
- Aimetti, M.; Ferrarotti, F.; Gamba, M.N.; Giraudi, M.; Romano, F. Regenerative Treatment of Periodontal Intrabony Defects Using Autologous Dental Pulp Stem Cells: A 1-Year Follow-Up Case Series. Int. J. Periodontics Restor. Dent. 2018, 38, 51–58. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Nan, X.; Wei, H.; Shi, J.; Li, A.; Gou, J. Repair of human periodontal bone defects by autologous grafting stem cells derived from inflammatory dental pulp tissues. Stem Cell Res. Ther. 2016, 7, 141. [Google Scholar] [CrossRef]
- Hernández-Monjaraz, B.; Santiago-Osorio, E.; Ledesma-Martínez, E.; Alcauter-Zavala, A.; Mendoza-Núñez, V.M. Retrieval of a periodontally compromised tooth by allogeneic grafting of mesenchymal stem cells from dental pulp: A case report. J. Int. Med. Res. 2018, 46, 2983–2993. [Google Scholar] [CrossRef]
- Feng, F.; Akiyama, K.; Liu, Y.; Yamaza, T.; Wang, T.M.; Chen, J.H.; Wang, B.B.; Huang, G.T.; Wang, S.; Shi, S. Utility of PDL progenitors for in vivo tissue regeneration: A report of 3 cases. Oral Dis. 2010, 16, 20–28. [Google Scholar] [CrossRef]
- Graziano, A.; Carinci, F.; Scolaro, S.; D’Aquino, R. Periodontal tissue generation using autologous dental ligament micro-grafts: Case report with 6 months follow-up. Ann. Maxillofac. Surg. 2013, 1, 20. [Google Scholar] [CrossRef]
- Vandana, K.L.; Praveen, N.C.; Chauhan, G.; Mittal, D. Autologous direct stem cell application in periodontal regeneration technique in the treatment of periodontal intrabony defects: An 1-year follow-up study. Int. J. Oral Health Sci. 2016, 6, 83–87. [Google Scholar] [CrossRef]
- Chen, F.-M.; Gao, L.-N.; Tian, B.-M.; Zhang, X.-Y.; Zhang, Y.-J.; Dong, G.-Y.; Lu, H.; Chu, Q.; Xu, J.; Yu, Y.; et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Shalini, H.S.; Vandana, K.L. Direct application of autologous periodontal ligament stem cell niche in treatment of periodontal osseous defects: A randomized controlled trial. J. Indian Soc. Periodontol. 2018, 22, 503–512. [Google Scholar] [CrossRef]
- Sánchez, N.; Fierravanti, L.; Núñez, J.; Vignoletti, F.; González-Zamora, M.; Santamaría, S.; Suárez-Sancho, S.; Fernández-Santos, M.E.; Figuero, E.; Herrera, D.; et al. Periodontal regeneration using a xenogeneic bone substitute seeded with autologous periodontal ligament-derived mesenchymal stem cells: A 12-month quasi-randomized controlled pilot clinical trial. J. Clin. Periodontol. 2020, 47, 1391–1402. [Google Scholar] [CrossRef]
- Schmelzeisen, R.; Schimming, R.; Sittinger, M. Making bone: Implant insertion into tissue-engineered bone for maxillary sinus floor augmentation-a preliminary report. J. Craniomaxillofac. Surg. 2003, 31, 34–39. [Google Scholar] [CrossRef]
- Nagata, M.; Hoshina, H.; Li, M.; Arasawa, M.; Uematsu, K.; Ogawa, S.; Yamada, K.; Kawase, T.; Suzuki, K.; Ogose, A.; et al. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: Coordinated activation of bone formation and resorption. Bone 2012, 50, 1123–1129. [Google Scholar] [CrossRef]
- Khojasteh, A.; Sadeghi, N. Application of buccal fat pad-derived stem cells in combination with autogenous iliac bone graft in the treatment of maxillomandibular atrophy: A preliminary human study. Int. J. Oral Maxillofac. Surg. 2016, 45, 864–871. [Google Scholar] [CrossRef]
- Khojasteh, A.; Kheiri, L.; Behnia, H.; Tehranchi, A.; Nazeman, P.; Nadjmi, N.; Soleimani, M. Lateral Ramus Cortical Bone Plate in Alveolar Cleft Osteoplasty with Concomitant Use of Buccal Fat Pad Derived Cells and Autogenous Bone: Phase I Clinical Trial. BioMed Res. Int. 2017, 2017, 6560234. [Google Scholar] [CrossRef]
- Meshram, M.; Anchlia, S.; Shah, H.; Vyas, S.; Dhuvad, J.; Sagarka, L. Buccal Fat Pad-Derived Stem Cells for Repair of Maxillofacial Bony Defects. J. Maxillofac. Oral Surg. 2019, 18, 112–123. [Google Scholar] [CrossRef]
- Pradel, W.; Eckelt, U.; Lauer, G. Bone regeneration after enucleation of mandibular cysts: Comparing autogenous grafts from tissue-engineered bone and iliac bone. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Pradel, W.; Mai, R.; Manolo Hagedorn, G.; Lauer, G.; Allegrini, S. The biomaterial influences the ossification after sinus floor elevation using tissue-engineered bone grafts. Biomed. Tech. 2008, 53, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Pradel, W.; Lauer, G. Tissue-engineered bone grafts for osteoplasty in patients with cleft alveolus. Ann. Anat. 2012, 194, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Redondo, L.M.; García, V.; Peral, B.; Verrier, A.; Becerra, J.; Sánchez, A.; García-Sancho, J. Repair of maxillary cystic bone defects with mesenchymal stem cells seeded on a cross-linked serum scaffold. J. Craniomaxillofac. Surg. 2018, 46, 222–229. [Google Scholar] [CrossRef]
- Arvidson, K.; Abdallah, B.M.; Applegate, L.A.; Baldini, N.; Cenni, E.; Gomez-Barrena, E.; Granchi, D.; Kassem, M.; Konttinen, Y.T.; Mustafa, K.; et al. Bone regeneration and stem cells. J. Cell. Mol. Med. 2011, 15, 718–746. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, S.; Ito, K.; Sugito, T.; Yoshimi, R.; Nagasaka, T.; Ueda, M. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng. Part A 2010, 16, 1891–1900. [Google Scholar] [CrossRef]
- Tsumanuma, Y.; Iwata, T.; Washio, K.; Yoshida, T.; Yamada, A.; Takagi, R.; Ohno, T.; Lin, K.; Yamato, M.; Ishikawa, I.; et al. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials 2011, 32, 5819–5825. [Google Scholar] [CrossRef]
- Kawase, T.; Okuda, K.; Kogami, H.; Nakayama, H.; Nagata, M.; Nakata, K.; Yoshie, H. Characterization of human cultured periosteal sheets expressing bone-forming potential: In vitro and in vivo animal studies. J. Tissue Eng. Regen. Med. 2009, 3, 218–229. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Tsutsui, T.W. Dental Pulp Stem Cells: Advances to Applications. Stem Cells Cloning Adv. Appl. 2020, 13, 33–42. [Google Scholar] [CrossRef]
- Morsczeck, C.; Reichert, T.E. Dental stem cells in tooth regeneration and repair in the future. Expert. Opin. Biol. Ther. 2018, 18, 187–196. [Google Scholar] [CrossRef]
- Papaccio, G.; Graziano, A.; d’Aquino, R.; Graziano, M.F.; Pirozzi, G.; Menditti, D.; De Rosa, A.; Carinci, F.; Laino, G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: A cell source for tissue repair. J. Cell. Physiol. 2006, 208, 319–325. [Google Scholar] [CrossRef]
- Suchánek, J.; Visek, B.; Soukup, T.; El-Din Mohamed, S.K.; Ivancaková, R.; Mokrỳ, J.; Aboul-Ezz, E.H.; Omran, A. Stem cells from human exfoliated deciduous teeth–isolation, long term cultivation and phenotypical analysis. Acta Med. Hradec Kral. 2010, 53, 93–99. [Google Scholar] [CrossRef]
- Petite, H.; Viateau, V.; Bensaïd, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef]
- Alongi, D.J.; Yamaza, T.; Song, Y.; Fouad, A.F.; Romberg, E.E.; Shi, S.; Tuan, R.S.; Huang, G.T. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen. Med. 2010, 5, 617–631. [Google Scholar] [CrossRef]
- Werle, S.B.; Lindemann, D.; Steffens, D.; Demarco, F.F.; de Araujo, F.B.; Pranke, P.; Casagrande, L. Carious deciduous teeth are a potential source for dental pulp stem cells. Clin. Oral Investig. 2016, 20, 75–81. [Google Scholar] [CrossRef]
- Malekfar, A.; Valli, K.S.; Kanafi, M.M.; Bhonde, R.R. Isolation and Characterization of Human Dental Pulp Stem Cells from Cryopreserved Pulp Tissues Obtained from Teeth with Irreversible Pulpitis. J. Endod. 2016, 42, 76–81. [Google Scholar] [CrossRef]
- Sakdee, J.B.; White, R.R.; Pagonis, T.C.; Hauschka, P.V. Hypoxia-amplified proliferation of human dental pulp cells. J. Endod. 2009, 35, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Takeda-Kawaguchi, T.; Tezuka, Y.; Kunisada, T.; Shibata, T.; Tezuka, K. Hypoxia enhances colony formation and proliferation but inhibits differentiation of human dental pulp cells. Arch. Oral Biol. 2010, 55, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Aranha, A.M.; Zhang, Z.; Neiva, K.G.; Costa, C.A.; Hebling, J.; Nör, J.E. Hypoxia enhances the angiogenic potential of human dental pulp cells. J. Endod. 2010, 36, 1633–1637. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.O.; Rubini, M.R.; Silva, J.R.; Oliveira, D.M.; Silva, I.C.; Poças-Fonseca, M.J.; Azevedo, R.B. Comparison of stem cell properties of cells isolated from normal and inflamed dental pulps. Int. Endod. J. 2012, 45, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Behnia, A. Transplantation of stem cells from human exfoliated deciduous teeth for bone regeneration in the dog mandibular defect. World J. Stem Cells 2014, 6, 505. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lozano, A.P.; Arevalo-Niño, K.; Gutierrez-Puente, Y.; Montiel-Hernandez, J.L.; Urrutia-Baca, V.H.; Del Angel-Mosqueda, C.; De la Garza-Ramos, M.A. SSEA-4 positive dental pulp stem cells from deciduous teeth and their induction to neural precursor cells. Head Face Med. 2022, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, Y.; Guo, W.; Yang, B.; Tian, W. Stem cells from human exfoliated deciduous teeth as an alternative cell source in bio-root regeneration. Theranostics 2019, 9, 2694–2711. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça Costa, A.; Bueno, D.F.; Martins, M.T.; Kerkis, I.; Kerkis, A.; Fanganiello, R.D.; Cerruti, H.; Alonso, N.; Passos-Bueno, M.R. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J. Craniofac. Surg. 2008, 19, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Sonoyama, W.; Yamaza, T.; Coppe, C.; Kikuiri, T.; Akiyama, K.; Lee, J.S.; Shi, S. SHED repair critical- size calvarial defects in mice. Oral Dis. 2008, 14, 428–434. [Google Scholar] [CrossRef]
- Kunimatsu, R.; Nakajima, K.; Awada, T.; Tsuka, Y.; Abe, T.; Ando, K.; Hiraki, T.; Kimura, A.; Tanimoto, K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 501, 193–198. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Y.; Zhang, C.M.; Zhang, H.Y.; Li, W.H.; Shi, S.; Le, A.D.; Wang, S.L. Stem cells from deciduous tooth repair mandibular defect in swine. J. Dent. Res. 2009, 88, 249–254. [Google Scholar] [CrossRef]
- Nakajima, K.; Kunimatsu, R.; Ando, K.; Ando, T.; Hayashi, Y.; Kihara, T.; Hiraki, T.; Tsuka, Y.; Abe, T.; Kaku, M.; et al. Comparison of the bone regeneration ability between stem cells from human exfoliated deciduous teeth, human dental pulp stem cells and human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Song, J.S.; Jeon, M.; Shin, D.M.; Kim, S.O.; Lee, J.H. Ectopic hard tissue formation by odonto/ osteogenically in vitro differentiated human deciduous teeth pulp stem cells. Calcif. Tissue Int. 2015, 97, 80–89. [Google Scholar] [CrossRef]
- Vakhrushev, I.V.; Antonov, E.N.; Popova, A.V.; Konstantinova, E.V.; Karalkin, P.A.; Kholodenko, I.V.; Lupatov, A.Y.; Popov, V.K.; Bagratashvili, V.N.; Yarygin, K.N. Design of tissue engineering implants for bone tissue regeneration of the basis of new generation polylactoglycolide scaffolds and multipotent mesenchymal stem cells from human exfoliated deciduous teeth (SHED cells). Bull. Exp. Biol. Med. 2012, 153, 143–147. [Google Scholar] [CrossRef]
- Jahanbin, A.; Rashed, R.; Alamdari, D.H.; Koohestanian, N.; Ezzati, A.; Kazemian, M.; Saghafi, S.; Raisolsadat, M.A. Success of maxillary alveolar defect repair in rats using osteoblast-differentiated human deciduous dental pulp stem cells. J. Oral Maxillofac. Surg. 2016, 74, 829.e1–829.e9. [Google Scholar] [CrossRef]
- Ma, L.; Makino, Y.; Yamaza, H.; Akiyama, K.; Hoshino, Y.; Song, G.; Kukita, T.; Nonaka, K.; Shi, S.; Yamaza, T. Cryopreserved dental pulp tissues of exfoliated deciduous teeth is a feasible stem cell resource for regenerative medicine. PLoS ONE 2012, 7, e51777. [Google Scholar] [CrossRef]
- Rikitake, K.; Kunimatsu, R.; Yoshimi, Y.; Nakajima, K.; Hiraki, T.; Aisyah Rizky Putranti, N.; Tsuka, Y.; Abe, T.; Ando, K.; Hayashi, Y.; et al. Effect of CD146+ SHED on bone regeneration in a mouse calvaria defect model. Oral Dis. 2021, 29, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- He, H.; Yu, J.; Cao, J.; E, L.; Wang, D.; Zhang, H.; Liu, H. Biocompatibility and Osteogenic Capacity of Periodontal Ligament Stem Cells on nHAC/PLA and HA/TCP Scaffolds. J. Biomater. Sci. Polym. Ed. 2011, 22, 179–194. [Google Scholar] [CrossRef]
- Kämmerer, P.W.; Scholz, M.; Baudisch, M.; Liese, J.; Wegner, K.; Frerich, B.; Lang, H. Guided bone regeneration using collagen scaffolds, growth factors, and periodontal ligament stem cells for treatment of peri-implant bone defects in vivo. Stem Cells Int. 2017, 2017, 3548435. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, B.; Cui, Z.; Cui, S.; Zhang, T.; Wang, X.; Liu, D.; Yang, R.; Jiang, N.; Zhou, Y.; et al. Bone regeneration in minipigs by intrafibrillarly-mineralized collagen loaded with autologous periodontal ligament stem cells. Sci. Rep. 2017, 7, 10519. [Google Scholar] [CrossRef]
- Yan, X.Z.; Yang, F.; Jansen, J.A.; de Vries, R.B.; van den Beucken, J.J. Cell-Based Approaches in Periodontal Regeneration: A Systematic Review and Meta-Analysis of Periodontal Defect Models in Animal Experimental Work. Tissue Eng. Part B Rev. 2015, 21, 411–426. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Sittinger, M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003, 9 (Suppl. S1), 45–64. [Google Scholar] [CrossRef]
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Trovato, L.; Monti, M.; Del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G. A New Medical Device Rigeneracons Allows to Obtain Viable Micro-Grafts From Mechanical Disaggregation of Human Tissues. J. Cell. Physiol. 2015, 230, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef]
- Cao, C.; Tarlé, S.; Kaigler, D. Characterization of the immunomodulatory properties of alveolar bone-derived mesenchymal stem cells. Stem Cell Res. Ther. 2020, 11, 102. [Google Scholar] [CrossRef]
- Mason, S.; Tarle, S.A.; Osibin, W.; Kinfu, Y.; Kaigler, D. Standardization and safety of alveolar bone-derived stem cell isolation. J. Dent. Res. 2014, 93, 55–61. [Google Scholar] [CrossRef]
- Wang, X.; Xing, H.; Zhang, G.; Wu, X.; Zou, X.; Feng, L.; Wang, D.; Li, M.; Zhao, J.; Du, J.; et al. Restoration of a critical mandibular bone defect using human alveolar bone-derived stem cells and porous nano-HA/collagen/PLA scaffold. Stem Cells Int. 2016, 2016, 8741641. [Google Scholar] [CrossRef]
- Gallego, L.; Junquera, L.; García, E.; García, V.; Alvarez-Viejo, M.; Costilla, S.; Fresno, M.F.; Meana, A. Repair of rat mandibular bone defects by alveolar osteoblasts in a novel plasma-derived albumin scaffold. Tissue Eng. Part A 2010, 16, 1179–1187. [Google Scholar] [CrossRef]
- Huang, G.T.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Bar, J.K.; Lis-Nawara, A.; Grelewski, P.G. Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential. Int. J. Mol. Sci. 2021, 22, 12018. [Google Scholar] [CrossRef]
- Kadkhoda, Z.; Rafiei, S.C.; Azizi, B.; Khoshzaban, A. Assessment of Surface Markers Derived from Human Periodontal Ligament Stem Cells: An In Vitro Study. J. Dent. (Tehran Iran) 2016, 13, 325–332. [Google Scholar]
- Yasui, T.; Mabuchi, Y.; Morikawa, S.; Onizawa, K.; Akazawa, C.; Nakagawa, T.; Okano, H.; Matsuzaki, Y. Isolation of dental pulp stem cells with high osteogenic potential. Inflamm. Regen. 2017, 37, 8. [Google Scholar] [CrossRef]
- Tomasello, L.; Mauceri, R.; Coppola, A.; Pitrone, M.; Pizzo, G.; Campisi, G.; Pizzolanti, G.; Giordano, C. Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: A potential application for bone formation. Stem Cell Res. Ther. 2017, 8, 179. [Google Scholar] [CrossRef]
- Fernandez-Tresguerres, I.; Alobera Garcia, M.A.; Canto Pingarrón, M.; Blanco Jerez, L. Bases fisiológicas de la regeneración ósea II: El proceso de remodelado. Med. Oral Patol. Oral Cirugía Bucal 2006, 11, 151–157. [Google Scholar]
- Gimble, J.M.; Guilak, F.; Nuttall, M.E.; Sathishkumar, S.; Vidal, M.; Bunnell, B.A. In vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus. Med. Hemother. 2008, 35, 228–238. [Google Scholar] [CrossRef]
- Collart-Dutilleul, P.Y.; Chaubron, F.; De Vos, J.; Cuisinier, F.J. Allogenic banking of dental pulp stem cells for innovative therapeutics. World J. Stem Cells 2015, 7, 1010–1021. [Google Scholar] [CrossRef]
- Sivolella, S.; Scanu, A.; Xie, Z.; Vianello, S.; Stellini, E. Biobanking in dentistry: A review. Jpn. Dent. Sci. Rev. 2022, 58, 31–40. [Google Scholar] [CrossRef]
- Baranovskii, D.S.; Klabukov, I.D.; Arguchinskaya, N.V.; Yakimova, A.O.; Kisel, A.A.; Yatsenko, E.M.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D. Adverse events, side effects and complications in mesenchymal stromal cell-based therapies. Stem Cell Investig. 2022, 9, 7. [Google Scholar] [CrossRef]
- Moll, G.; Ankrum, J.A.; Olson, S.D.; Nolta, J.A. Improved MSC minimal criteria to maximize patient safety: A call to embrace tissue factor and hemocompatibility assessment of MSC products. Stem Cells Transl. Med. 2022, 11, 2–13. [Google Scholar] [CrossRef]
- Abdel Meguid, E.; Ke, Y.; Ji, J.; El-Hashash, A.H.K. Stem cells applications in bone and tooth repair and regeneration: New insights, tools, and hopes. J. Cell. Physiol. 2018, 233, 1825–1835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).