Abstract
The human papilloma virus (HPV) group comprises approximately 200 genetic types that have a special affinity for epithelial tissues and can vary from producing benign symptoms to developing into complicated pathologies, such as cancer. The HPV replicative cycle affects various cellular and molecular processes, including DNA insertions and methylation and relevant pathways related to pRb and p53, as well as ion channel expression or function. Ion channels are responsible for the flow of ions across cell membranes and play very important roles in human physiology, including the regulation of ion homeostasis, electrical excitability, and cell signaling. However, when ion channel function or expression is altered, the channels can trigger a wide range of channelopathies, including cancer. In consequence, the up- or down-regulation of ion channels in cancer makes them attractive molecular markers for the diagnosis, prognosis, and treatment of the disease. Interestingly, the activity or expression of several ion channels is dysregulated in HPV-associated cancers. Here, we review the status of ion channels and their regulation in HPV-associated cancers and discuss the potential molecular mechanisms involved. Understanding the dynamics of ion channels in these cancers should help to improve early diagnosis, prognosis, and treatment in the benefit of HPV-associated cancer patients.
1. Introduction
The human papillomavirus (HPV) group comprises members with a high affinity to the cutaneous and mucosal epithelium. Around two hundred genetic types of HPV have been described and grouped into five genera (α, β, γ, μ, and ν), according to the variability of the viral capsid protein gene L1 [1,2].
The α group includes approximately 65 types with an affinity to mucosal epithelium and is commonly sexually transmitted. Its prevalence is high in teenage women and other young adults of active reproductive age, generally around 20 years, and its prevalence decreases in older adults [3]. Some HPV α-genetic types are non-dangerous or non-oncogenic related. For example, the HPV-62, HPV-89, HPV-11, and HPV-6 types are considered to be low-risk (LR-HPV) types and are associated with genital warts and papillomatosis, representing a prevalence of 11.6% [4,5]. On the other hand, the HPV-16, HPV-18, HPV-51, HPV-52, and HPV-53 types are reported as high-risk (HR-HPV) types, with a 13% prevalence, and are associated with malignant cytology and neoplastic processes [6]. Finally, a 5.2% prevalence corresponds to the HPV-53, HPV-66, HPV-70, and HPV-68 types, which are described as probably dangerous [5]. The β group includes around 50 HPV types with cutaneous epithelium affinity and mainly produces cutaneous lesions, while the γ, μ, and ν genera are considered benign. The most important high-risk types are HPV-16 and HPV-18 [6,7,8,9], as well as other types, depending on the studied population. For example, in Asian populations, cervical cancer has been associated with HPV-52 and HPV-58 types in addition to HPV-16 [10].
Other HPV-associated cancers include vulvar, vaginal [11], and anal cancer in women [12], while in men they include penile [13], testicular, and anal cancer [14,15]. The second HPV-associated cancer group is that of oropharyngeal cancers and involves head and neck [16,17,18], tongue, and laryngeal cancers [5,19]. Finally, other less frequent HPV-related cancers are lung [20], colon [21], bladder [22], prostate, and breast cancers [23,24]. The relationship between HPV genetic types and different types of cancers is shown in Table 1.

Table 1.
HPV genetic types associated with the prevalence of different conditions.
The tumorigenesis associated with high-risk HPV infections has been mainly related to the E5, E6, and E7 viral genes, whose corresponding oncoproteins are responsible for the altered regulation of several cellular pathways. Thus, E6 and E7 genotyping is currently used in some countries, including Finland [25], Canada [26], and Mexico [7], as a complementary test together with cytology for timely cervical cancer, neoplasms, and other HPV-related cancer diagnoses [26]. The inhibition of apoptosis, the induction of cell proliferation, immune system evasion, the promotion of metastasis, and the dysregulation of the cell cycle are some of the processes orchestrated by E5, E6, and E7 proteins [27]. Interestingly, the altered expression of some ion channels has been related to viral proteins [28], making certain ion channels predictive and therapeutic molecular markers for different cancer types [29,30,31,32].
2. HPV-Replicative Cycle Association with Cellular Pathways and Ion Channels
We will first provide illustrative and general examples of how HPV infection modifies certain cellular pathways and ion channels associated with tumor progression. The high-risk HPV replicative cycle starts with the virus attachment to the host cell via L1 capsid viral protein coupling with the cellular heparin sulphate proteoglycans [33]. The HPV virus has a special affinity to active and proliferative cells, especially to basal epithelial cells, because the virus does not have DNA replicative enzymes and depends on the active cell cycle. The infection of the basal epithelia is successful due to epithelium micro-abrasion. Interestingly, it was recently found that the cervical transformation zone is very important in HPV infection [34]. Then, a secondary mechanism is activated to internalize the virus via the L2 capsid viral late protein and different cellular receptors, including epidermal growth factor receptors (EGFR), integrins, and annexin-A2. Thereafter, nuclear internalization takes place when HPV enters through nuclear pores, typically in the mitotic phase [2]. Interestingly, during the intracellular delivery of the virus, Annexin-A2 is initially a monomeric cytoplasmic protein, together with the p11 protein domain. Annexin-A2 and the p11 (S100A10) domain form a heterotetrametric complex, consisting of two Annexin-A2 units and a dimer of S100A10 [33,35]. This protein complex is translocated to the epithelial cell membrane due to tropism between the L2 HPV capsid protein and S100A10 [36]. This information suggests that a persistent HPV infection stimulates the cell membrane Annexin-A2 protein complex location and ensures HPV’s infective success (Figure 1a). Some studies propose that the heterotetramer (Annexin-A2/S100A10) modulates ion channels and other membrane receptors [37,38].
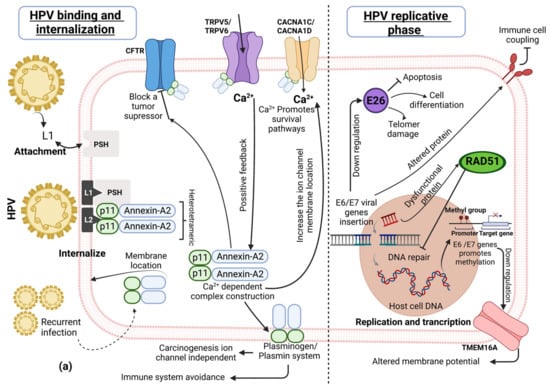
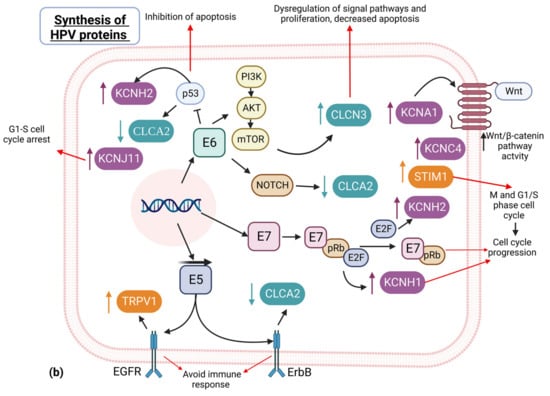
Figure 1.
HPV-replicative cycle association with cellular pathways and ion channels. (a) HPV attachment and internalization is regulated by Annexin-A2/p11, which in turn affects calcium and chloride channels. Additionally, in HPV replication and transcription, the DNA insertion and DNA methylation compromises the DNA repair process by ion channel-dependent and -independent pathways associated with cell proliferation or tumor suppression. (b) HPV oncoproteins E5, E6 and E7 alter several pathways regulating some ion channels in cervical cancer.
CFTR, TRPV5, TRPV6, CACNA1DC, and CACNA1D ion channels are modulated by Annexin-A2/S100A10. The Annexin-A2 complex binds to TRPV5/6 (transient receptor potential vanilloid) ion channels, increases their cell surface location, and promotes the entry of calcium [38]. Calcium is necessary to stimulate heterotetrameric complex construction, thus promoting positive feedback [39]. TRPV channels and other calcium channels also modulate the intracellular calcium implicated in proliferation, apoptosis, and metastasis in different cancer types [40]. Particularly, TRPV6 upregulation is involved in ovarian, prostate, pancreas, and breast adenocarcinoma [41]. Moreover, the CFTR (cystic fibrosis conductance regulator protein) channel facilitates the entry of chloride ions, is considered a tumor suppressor, and is involved in the proper epithelial differentiation. However, the Annexin-A2 complex affects chloride ion flux via CFTR [38], and could therefore lead to tumor development [42]. The Annexin-A2/S100A10 complex has itself been implicated in some cancer progression pathways that are dependent on the immune response and plasminogen production [37].
After virus attachment and internalization into the host cell nucleus, the HPV starts the replicative and transcriptional process. Initially, the virus transcribes and traduces the early E1 and E2 viral proteins [2,43]. Viral early proteins act as replication factors on E5, E6, and E7 genes to maintain and regulate the replicative cell cycle. The replication and transcription viral phases produce HPV copies using the viral episomal DNA as a template [44]. When the HPV infection is persistent, the E1 and E2 proteins also promote a level of viral DNA integration in the host chromosome, producing a particular methylation profile. E6 and E7 viral genes could be integrated into hotspot genes, such as FHIT, KLF5, LINC00392, RAD51B, CAS8, CASC21, ERBB2, TP63, MYC, CCND1, and CTTN and promote somatic alterations [45], including transcriptional modifications on oncogenes, tumor suppressor genes, cell cycle regulators, and transcriptional factors [46].
The ETS2 gene is located on chromosome 21 (encodes the E26 protein) and acts as a transcriptional factor and tumor suppressor but is affected by viral insertion [18]. HPV insertion could repress ETS2 expression, compromising telomere integrity and possibly DNA damage accumulation. Another effect is the reduction in apoptosis, resulting in optimal conditions for HPV replication [47,48]. On the other hand, the RAD51B gene encodes the RAD51 protein, but the HPV genome integration produces a dysfunctional protein, altering the genome repair and inducing the accumulation of damage in the genome [18,49].
The CD274 gene encoding the PDL1 protein is located on chromosome 9 and is affected by HPV insertion, finally producing an altered PDL1 transmembrane protein. This HPV replicative mechanism complicates the host’s immune response because altered PDL1 significantly reduces antigen recognition [45,50].
Gene hypermethylation by HPV has been observed in genes such as BARX2 (a tumor suppressor) and IRX4 (methylation sensible gene) [18]. Additionally, the transmembrane protein TMEM16A gene (which encodes the Ca2+-dependent chloride channel ANO1) is downregulated, affecting the cell membrane potential and promoting the neoplastic process, for example, in HPV+ head and neck carcinoma [28,51]. The specific methylation pattern produced by HPV may depend on the tissue and the persistent viral infection.
In the final HPV replicative cycle, E5, E6, and E7 oncoprotein expression have a key role in different cell pathways [44]. The E6 viral protein degrades the p53 protein (also known as “the guardian of the genome”) [52]. The consequence of p53 damage is the dysregulation of PI3K and AKT signal pathways, as well as cell proliferation and a decrease in apoptosis, among other effects. Interestingly, certain potassium channels are also dysregulated, potentially promoting cell cycle progression and the inhibition of apoptosis [53].
On the other hand, the tumor suppressor retinoblastoma protein (pRB) has important cell functions in the complex with the transcription factor E2F. pRB-E2F also regulates proteasomal protein degradation. The E7 protein sequesters pRB and promotes the detachment of E2F, leading to cell cycle progression [54]. Additionally, E7-pRb is more sensitive to ubiquitination and protein processing. The destabilization of pRb may be involved in the deregulation of the expression of some potassium channels, including KCNH1 [55], KCNC4 [56], and KCNA1 [57], as well as calcium channels [58] (Figure 1b).
Another interesting example of the association between HPV and ion channels is the ATP-sensitive potassium (KATP) channel, which favors the progression of the cell cycle and proliferation. E7 induces the expression of the SUR subunit of the channel, which in turn favors cell proliferation [59].
The E5 protein has a high affinity to receptors for important cytokines, such as EGF and ErbB. E5 works together with E7 to repress the gene expression of EGFR [60]. In addition, the TRPV1 calcium channel promotes the ubiquitination of EGFR, modulating the EGFR/MAPK pathway [61,62].
In summary, several cellular proteins involved in the HPV-replicative cycle affect different pathways, leading to neoplastic cell transformation and including the participation of several ion channels. These transmembrane proteins have been suggested as important components of the hallmarks of cancer because their dysfunction is associated with cell proliferation, apoptosis, metastasis, angiogenesis, and immune system avoidance, among other processes [63].
Next, we describe and discuss the expression status and relationship of ion channels with different HPV-associated cancers and their potential uses as clinical markers. HPV infection is considered an etiological factor of some types of cancers, especially cervical and head and neck cancers. In certain other cases, including anal, penile, and vulvar cancers, a potential association with HPV has been suggested; however, the possible involvement of ion channels in these cancers has not been investigated in detail [64]. Thus, here we mainly focus on HPV-associated cancers as recognized by the International Agency on Research for Cancer (IARC), such as cervical and head and neck cancers. Nevertheless, we were also interested in reviewing ion channel expression in lung cancer because its potential association with HPV infection remains controversial [65]. Unfortunately, lung cancer is a malignancy with one of the worst mortality-to-incidence ratios worldwide. Therefore, the potential use of ion channels as clinical tools for this cancer may have benefits, although its association with HPV infection remains controversial. There is no doubt that this controversy may be resolved in some decades with patients receiving the vaccine against HPV.
A few examples of the potential association of HPV and ion channels in other cancers are also mentioned to provide the reader with a view of the broader impact of this topic in cancer. The present review focuses on ion channels and HPV-associated cancers. Several extraordinary publications have reviewed the association of ion channels and cancer, and some of these are cited in this review. However, we apologize to those authors whose contributions could not be cited.
3. Ion Channels as Potential Clinical Tools in HPV-Associated Cancers
Cancer is a multifactorial disease, and ion channels are regulated by a variety of cancer-associated factors, including virus infection, hormones, carcinogens present in cigarette smoke, air pollution, etc. Therefore, it is not easy to determine whether changes in ion channel expression are solely attributed to HPV infection [66]. However, when information is available, we can distinguish whether the analyzed cells or samples from patients were identified as HPV+ and whether the channel expression was compared with the corresponding normal cells or tissues. Interestingly, several of the studies here reported also analyzed ion channel expression status with the patients’ clinical outcomes.
3.1. Potassium Ion Channels
Potassium channels are found in all excitable and non-excitable cells [67]. They are responsible for the transport of K+ ions down its electrochemical gradient and play key roles in several processes, including the establishment of resting membrane potential, the regulation of action potentials, hormone secretion, and cell signaling [68,69]. In cancer cells, K+ channels have been implicated in cell proliferation, apoptosis, and migration [68]. In the following sections, the main groups of K+ ion channels studied and involved in carcinogenesis are discussed [68].
3.1.1. Voltage-Gated Potassium Channels
Voltage-gated K+ (Kv) channels regulate cell excitability in response to changes in the cell membrane potential. Kv channels become activated with membrane depolarization and closed when the cell membrane is hyperpolarized [67]. The voltage sensing domain (VSD) movement is associated with pore opening [70]. Cancer cells undergo hyperpolarization upon exiting the G1 phase of the cell cycle, leading to the cell proliferation processes [71].
KV10.1 (KCNH1, Ether-à-go-go 1 (Eag1)) is expressed in a few normal tissues, including the brain, adrenal glands, placenta, and testis; however, in cancer tissues its expression is more ubiquitous and abundant [71]. Interestingly, the level of this channel is negatively regulated by retinoblastoma and the E2F pathway [55]. High KCNH1 expression has been demonstrated in several biological models of lung and HPV+ cervical cancers (Figure 2) [32,72]. KCNH1 has been proposed as a therapeutic target by using certain channel blockers (Table 2). For example, astemizole is a second-generation antihistamine that blocks some K+ channels, including KCNH1 and KCNH2 [73], and reduces the proliferation of HPV+ cervical cancer (CC) cells [74], while in non-small cell lung cancer (NSCLC) cell lines, this drug did not prevent the epithelial-to-mesenchymal transition (EMT) despite the fact that KCNH1 gene and protein expression were up-regulated during EMT [72]. These results suggest that the role of KCNH1 channels in EMT is not solely related to their conductive functions [72].
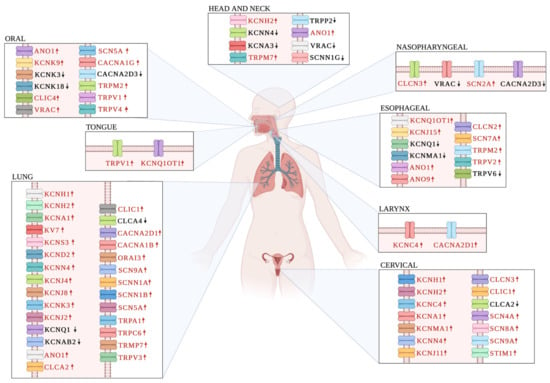
Figure 2.
Expression status of ion channels in HPV-associated cancers. The figure illustrates the tissue location of a variety of ion channels involved in carcinogenesis of HPV-associated cancers in both sexes. Ion channel overexpression is represented in red letters and up-arrow, while letters in black and down-arrow indicates downregulated ion channel expression. Together, these observations suggest a strong oncogenic or tumor suppressor role for many ion channels in HPV-associated cancers.

Table 2.
Compendium of ion channels and their potential clinical use in HPV-associated cancers.
The human Ether-à-go-go-related gene Kv11.1 (KCNH2 or hERG1) is expressed in several normal tissues, including cardiac tissue and smooth muscle, and in cancer cells it is regulated by several microRNAs relevant to tumor progression [53]. The overexpression of the channel in different head and neck cancers is associated with cell growth, invasiveness, malignant transformation, and poor prognosis [75,109,124,174]; likewise, in HPV+ cervical adenocarcinoma, the inhibition of hERG1 alters cell cycle development [125]; meanwhile, in lung cancer, the gene silencing and application of channel blockers decreases cell proliferation (Table 2) [175].
Kv3 channels participate in cancer cell proliferation, migration, and metastasis via the AKT signaling pathway and vimentin, which is involved in cell migration and EMT [56]. In CC HPV+ cell lines, the potassium voltage-gated channel subfamily C member 4 (KCNC4 or Kv3.4) and the AKT pathway have been shown to regulate vimentin expression, and the use of blood depressing substance II (BDS-II) as a channel blocker (Table 2) decreases vimentin expression and cancer cell migration [56]. Similarly, in head and neck cancer, a high expression of KCNC4 is associated with laryngeal and pharyngeal squamous cell carcinoma; in accordance, the silencing of KCNC4 by siRNA inhibits cell proliferation in the G2/M phase [121]. The transcriptional factor HIF-1α regulates cell migration and proliferation through KCNC4 channels [108].
The potassium voltage-gated channel subfamily A member 1 (KCNA1 or Kv1.1) plays an important role in the control of neuronal excitability [122]. In CC HPV+, KCNA1 overexpression in samples from patients is associated with the poor prognosis of the disease, the induction of mitochondrial dysfunction in cell lines, and the regulation of the Hhg, Wnt, and Notch signaling pathways, while KCNA1 silencing arrests cell growth, invasion, and migration, as well as improving survival time, in nude mice [57]. In non-small cell lung cancer, the application of the KCNA1 blocker dendrotoxin-K (DTX-K) reduces cell viability and tumor size in mice [126].
Kv1.3 (potassium voltage-gated channel subfamily A member 3, KCNA3) channels regulate membrane potential and Ca2+ entry in human effector memory T cells (TEM). In head and neck cancer, tumor infiltrating lymphocytes (TILs; cells that can be recruited by the tumor cells and the cytokines that they produce can help tumor cell survival) display up to a 70% reduction in functional KCNA3 channels, consequently decreasing Ca2+ entry and CD8 T cell function (Figure 2) [107].
On the other hand, a differential expression of KCNQ channels has been observed in lung cancer, with higher expressions in side-populations than in main-population cells; moreover, when side-population cells were treated with the EGFR antagonist gefitinib [176] in combination with KCNQ blockers (TEA, 4-AP) or openers (flupirtine), the gefitinib resistance decreased [76]. The deletion of potassium voltage-gated channel modifier subfamily S member 3 (KCNS3 or Kv9.3) in lung adenocarcinoma (LUAD) cell lines inhibits cell proliferation by cell cycle arrest in the G0/G1 phase (Table 2) [77]. Additionally, potassium voltage-gated channel subfamily D member 2 (KCND2 or Kv4.2) is deregulated in several tumors and its high expression in LUAD is associated with unfavorable clinical outcomes for patients [106].
Furthermore, Kv subfamily Q member 1 (KCNQ1) plays important oncogenic roles, mainly in cell cycle, apoptosis, and autophagy [127]. In esophageal squamous cell carcinoma (ESCC), miR-483e5p overexpression silences KCNQ1 promoting cell proliferation, migration, and invasion [78]. Likewise, in lung cancer, KCNQ1 overexpression has been associated with longer survival times for patients with LUAD [177]. Interestingly, the long non-coding RNA (lncRNA) KCNQ1 overlapping transcript 1 (KCNQ1OT1) in tongue cancer promotes cisplatin resistance through the action of miR-124-3p, whereas in esophageal cancer, its overexpression is associated with worse prognoses via miR-133b [128,178]. KCNQ1OT1 is overexpressed in lung adenocarcinoma and is associated with resistance to stereotactic body radiotherapy [127] and its silencing reduces the expression of the multidrug resistance gene 1 (MDR1) in lung cancer cell lines (Table 2) [129].
Kv subfamily A regulatory beta subunit 2 (KCNAB2) participates in neuroendocrine functions as well as in certain cancer processes [105]. Its low expression in LUAD is associated with accelerated tumor growth and poor prognosis, whereas its overexpression contributes to the expression of chemokines required for immune cell migration [104].
3.1.2. Calcium-Activated Potassium Channels
Calcium-activated potassium channels are involved in neurosecretion, action potential formation, and the overall regulation of neuronal excitability, among other activities [179]. The up-regulation of the calcium-activated potassium channel, subfamily M, and alpha member 1 (KCNMA1 or KCa1.1) in combination with verapamil (a drug used in the treatment of cardiac arrhythmias and the inhibition of transmembrane Ca2+ flux) [180] potentiates the cytotoxic effect of cisplatin against ESCC (Table 2) [130]. It is of interest that, in transgenic mice expressing the HPV16-E7 oncogene and treated with estradiol to induce cervical neoplasms and cancer, the KCNMA1 gene and protein expression was increased, as well as in samples from patients with cervical lesions [29].
Calcium-activated potassium channel subfamily N member 4 (KCNN4 or KCa3.1) participates in the activation of microglia, but it also influences migration, cell proliferation, activation, and cytokine release from blood cells and has been associated with apoptosis, metastasis, and drug resistance in cancer [181]. In head and neck cancer, the inhibition of programmed death receptor alpha 1 (αPD-1) increases the fluxes of KCNN4 and Kv1.3 channel activity, thus improving the immune function [131]. Likewise, pembrolizumab, which is a humanized monoclonal antibody with high specificity towards PD-1, has been used in the treatment of several malignancies, including head and neck cancer [182], and increases KCNN4 channel activity and CD8 T-cell chemotaxis (Table 2) [132].
In CC, KCNN4 activation increases the sensitivity of cells to Hoechst 33258 dye, thus improving the penetration of cytotoxic substances into cancer cells [133]. KCNN4 overexpression is associated with cell proliferation and reduced apoptosis in HPV+ CC cell lines; accordingly, clotrimazole together with KCNN4 silencing decreases channel currents and cell proliferation [79]. In lung cancer, the overexpression of KCNN4 is associated with proliferation, migration, invasion, tumorigenicity, and poor prognosis (Figure 2) via membrane potential hyperpolarization and the P13/AKT and MEK/ERK signaling pathways [103,183]. In addition, in non-small cell lung cancer, KCNN4 channel blocking improves the response to the EGFR inhibitor gefitinib [184].
3.1.3. Inwardly Rectifying Potassium Channels
Inwardly rectifying potassium (Kir) channels are responsible for the regulation of the resting membrane potential, thus modulating electrical activity, neuronal activity, insulin secretion, and K+ transport [185]. Kir channel subfamily J member 15 (KCNJ15 or Kir4.2) is part of the Kir transport channels, and its inhibition in ESCC arrests cell proliferation, migration, and invasion, whereas its high expression is associated with shorter life expectancy compared to lower expression groups [80].
Kir channel subfamily J member 2 (KCNJ2 or Kir2.1) and Kir channel subfamily J member 4 (KCNJ4 or Kir2.3) have been involved in lung cancer. KCNJ2 is responsible for maintaining the resting membrane potential and the regulation of cell excitability, although changes in its expression are associated with apoptosis, proliferation, and cell adhesion [186]. In small cell lung cancer (SCLC), KCNJ4 modulates cell growth, and its silencing sensitizes lung cancer cell lines to the cytotoxic effects of adriamycin, cisplatin, and etoposide (Table 2) [134]. KCNJ4 is up-regulated by EGFR-associated pathways [81], and KCNJ4 overexpression in LUAD is associated with poor prognosis [102].
Finally, ATP-sensitive Kir channels play a role in coupling membrane potential to cell metabolism [187]. These channels consist of a K+ channel subunit forming the channel pore (Kir6.x) and a sulfonylurea receptor (SUR) [82], and have been associated with oncogenic processes in lung and HPV+ cervical cancers. Kir6.2 (KCNJ11), SUR1, and SUR2 are overexpressed in the cell lines and biopsies of patients with CC; in addition, when treating the cell lines with glibenclamide (a drug used in the treatment of diabetes that blocks KCNJ11) [188], cell proliferation was inhibited (Table 2) [82]. In NSCLC, the overexpression of SUR1 (KCNJ8 or Kir6.1) promotes cell proliferation, and silencing KCNJ8 or glibenclamide treatment decreases cell and tumor growth, as well as increasing expression of the tumor suppressor Krüppel-like factor 4 (KLF4) [83].
3.1.4. Two-Pore Domain Potassium Channels
Two-pore domain potassium channels are involved in the regulation of physical, chemical, and mechanical stimuli and associated with several cell signaling pathways [189]. In oral squamous cell carcinoma (OSCC), the protein expression of KCNK3 (TASK1 or K2P3) and KCNK18 (TRESK or K2P18), which belong to the family of tandem pore domains in weakly inward rectifying K+ (TWIK) channels, was found to be decreased in rat tumors and cancer patient tissues compared to normal samples, unlike KCNK9 (TASK3 or K2P9), which was found to be overexpressed in the OSCC samples compared to normal tissue [84]. However, KCNK3 are overexpressed in NSCLC cell lines and their silencing reduces proliferation and enhances apoptosis [135].
3.2. Sodium Ion Channels
Sodium channels are transmembrane proteins that are highly selective for Na+ transport in favor of its electrochemical gradient. Among the main functions of these channels are the generation and propagation of action potentials and the regulation of neuronal excitability and ion transport [190]. There are two main families of these channels: voltage-gated sodium channels (VGSC, Nav) and epithelial sodium channels (ENaC) [191,192]. These two types of channels are aberrantly expressed in cancer cells and involved in proliferative processes [193].
3.2.1. Voltage-Activated Sodium Channels
Voltage-gated sodium channels play essential roles in the electrical activity of excitable cells, including the generation and propagation of action potentials in neurons and neuroendocrine cells, as well as cardiac, skeletal, and smooth muscle [194]. Nine subtypes of VGSC (Nav1.1–Nav1.9) have been characterized and are encoded by the SCN1A-SCN11A genes and are highly conserved in humans [195]. Structurally, they are formed by a complex of an α-subunit associated with one or more β-subunits, and although these channels have similar functions, some differences in their properties and isoforms determine their role in the physiology of some diseases [196,197]. The activation of sodium channels takes place after membrane depolarization, and the resulting sodium entry further depolarizes the membrane, generating action potentials [197,198].
Voltage-regulated sodium channels increase the motility and invasiveness of cancer cells, depending on the tissue in which they are expressed; therefore, understanding their regulation in cancer development can lead to innovative therapies and molecular biomarkers for several cancers [199]. The SCN5A (Nav1.5) channel has been studied in the OSCC cell line SCC-15 and tissues of patients. The up-regulation of this channel in OSCC is associated with proliferation, migration, and invasion and correlates with metastasis in lymph nodes as well as with high neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR), which are indicators of the inflammatory state of the organism [114,145].
Interestingly, the expression of Nav1.5 is induced by EGF in OSCC HSC-3 cells and channel expression is associated with cell proliferation and migration via the Wnt/β-catenin signaling pathway [146]. In addition, the inhibition of Nav1.5 channel activity with ranolazine reduces breast cancer metastasis and pulmonary colonization [147].
The voltage-regulated sodium channel SCN8A (Nav1.6) has been studied in tissue samples from HPV16+ CC patients, primary cultures, and cell lines. HeLa (HPV18+), SiHa, and Caski (both HPV16+) cells show the overexpression of SCN8A, and its induced expression increases the activity of the matrix metalloproteinase MMP-2, which leads to cancer invasion and progression [148,200] (Table 1). The inhibition of SCN8A in primary cultured CC cells using Cn2 toxin and tetrodotoxin significantly reduces the invasiveness of these cancer cells [93].
Concerning the voltage-regulated sodium channel SCN7A (Nav2.1), an in silico study in ESCC showed mutational signatures in patient tumors which were associated with tumor vascular invasion and shorter survival time, suggesting that SCN7A has a high value as a prognostic marker for patients with ESCC [92]. On the other hand, a study performed in NSCLC reported that the functional expression of SCN9A (Nav1.7) is controlled by EGF/EGFR signaling through the ERK1/2 pathway, which in turn promotes the invasion of H460 lung cancer cells.
3.2.2. Epithelial Sodium Channels
Epithelial sodium channels (ENaC) are passive transport ion channels present in the apical membrane of epithelial cells [123]. They are formed by three homologous subunits (α, β, and γ) that form the pore, allowing the movement of Na+ from the extracellular fluid to the cytoplasm in numerous re-absorptive epithelia, such as the distal part of the renal tube, lungs, the respiratory tract, the female and male reproductive tracts, placenta, and colon, among others [123,201]. Na+ reabsorption is regulated by hormones, such as aldosterone, vasopressin, and glucocorticoids; among the main functions mediated by ENaC are electrolyte homeostasis, the regulation of extracellular fluid volume, and arterial pressure [202].
The malfunction of ENaC because of deletions or loss-of-function mutations in any of its subunits leads to its over- or under-expression, which in turn is related to several human diseases, such as the Liddle syndrome, cystic fibrosis, multisystemic pseudo-hypoaldosteronism, and essential hypertension [123]. Its deregulation is also involved in tumor development and progression, for example, the ENaC alpha subunit mediates cancer cell proliferation and migration processes [149]. Thus, ENaCs are increasingly being studied as potential therapeutic targets and diagnostic and prognostic markers in cancer [203].
The SCNN1B gene encodes for the beta (β) subunit of ENaC, and bioinformatic analysis identified six candidate genes as biomarkers in LUAD, suggesting that the hypermethylation of SCNN1B significantly decreases its expression. This was associated with the short survival of patients, making the channel a prognostic molecular biomarker [203]. Further studies of the SCLC cell line H889 found that the SCNN1A gene encoding the α-subunit of ENaC directly correlates with the expression of ASCL1, which is essential in cancer cell progression and survival, for example, in a subset of lung cancer [204].
In addition, the SCNN1G gene coding for the γ subunit of ENaC has been studied in head and neck squamous cell carcinoma, where it is downregulated, and this correlates with tumor metastasis and poor prognosis in HNSCC patients, suggesting a tumor suppressor role. Moreover, the function of SCNN1G has been analyzed under overexpression conditions, where γENaC inhibits HNSCC cell migration by increasing adhesive strength and cell–cell integrity, thus highlighting its potential as a diagnostic biomarker in HNSCC. However, its use as a biomarker is limited to certain types of cancer, as other studies have reported the activity of γENaC as a tumor promoter in breast cancer [113,205] (Figure 2).
3.3. Chloride Ion Channels
Chloride channels (Cl−) are ubiquitously expressed in all cell types [206]. These ion channels are found in the plasma membrane and intracellular organelles, where they serve vital functions, including the regulation of ionic homeostasis, membrane excitability, intracellular pH, transepithelial transport, proliferation, and cell volume [207,208]. Cl− flux across the plasma membrane regulates neuronal and muscle excitability by changing the membrane potential [209]. Because of their important role in the maintenance of cellular homeostasis, mutations in genes encoding these channels can trigger the development of human pathologies, such as myotonia, epilepsy, cystic fibrosis, and cancer cell migration and infiltration [210]. The families of chloride channels that have been implicated in cancer are the voltage-dependent chloride channels (VDC), calcium-activated chloride channels (CaCl), volume-regulated chloride channels (VRAC), and chloride intracellular channels (CLIC).
3.3.1. Voltage-Dependent Chloride Channels
Voltage-dependent chloride channels (CLC) mediate Cl− flux in favor of their electrochemical gradient. Their main function is to establish the resting membrane potential and chloride concentration in intracellular compartments. There are nine subtypes of the CLC channel family, where CLCN3 (CLC-3) expression is related to cell cycle regulation and cell proliferation, migration, and apoptosis [211,212] (Figure 2). In cervical squamous cell carcinoma, the CLCN3 channel is involved in cell invasion and migration processes and includes the participation of the PI3K/AKT/mTOR signaling pathway [94].
Studies performed in nasopharyngeal carcinoma cells (CNE-2Z) indicate that the selective activation of CLCN3 chloride ion channels is closely related to apoptotic events [213]. The expression of the proapoptotic proteins caspase-3 and BAX was upregulated in zolendronic acid (ZA)-treated cells via the production of reactive oxygen species (ROS), which in turn activated the CLCN3 channel, triggering cell apoptosis [120,211] (Figure 2). Similarly, dihydroartemisinin (DHA) treatment in CNE-2Z cells increased the expression of CLCN3 chloride channels and enhanced their activity. The increase in Cl− currents induced apoptotic volume depletion (AVD) and the activation of caspase-3 [173]. Interestingly, the inhibition of this channel at specific stages of the cell cycle was able to arrest cancer cell proliferation; however, when it is persistently expressed with the help of activators, it leads to cell apoptosis. Thus, its importance as a possible therapeutic target in cancer is highlighted [214,215].
The voltage-dependent chloride channel CLCN2 (CIC-2) is involved in the maintenance of membrane potential, transepithelial transport, the regulation of cell volume, and cell proliferation and survival [216]. The lubiprostone-mediated activation and overexpression of CLCN2 in ESCC reduces cancer cell proliferation by acting as a tumor suppressor via the IFN signaling pathway [95].
3.3.2. Calcium-Activated Chloride Channels
Calcium-activated chloride channels (CaCl) are present in excitable and non-excitable cells and become activated when cytosolic Ca2+ is increased [217]. These channels play important roles in different processes, including afterdepolarization, transepithelial transport, the regulation of cellular excitability, vascular tone control, epithelial secretion, and cell proliferation in carcinogenesis processes [218,219]. The depletion of Anoctamin-9 chloride channel ANO9 (TMEM16J) by siRNA in KYSE150 and KYSE790 cells increased the number of cells under arrest in the G0/G1 phase, reducing cell proliferation and migration [119] (Figure 2).
The ANO1 channel (TMEM16A) is involved in cancer cell proliferation, invasion, survival, and apoptosis; the signaling pathways that can be regulated by ANO1 include ERK, PI3K-Akt, TNF, Ca-M, and EGFR [118,220,221]. Studies performed in head and neck squamous cell carcinoma (HNSCC) indicate that a high expression of ANO1 is related to metastatic processes in lymph nodes, poor prognosis, and the poor survival of patients with OSCC and ESCC [96,97,171,172]. It is also suggested that ANO1 directly correlates with the activation of the Erk½ pathway and increases tumor size and downregulates the expression of the proapoptotic protein Bim [98,99]. The overexpression of ANO1 in HNSCC has pointed to its development as a potential prognostic, diagnostic, and therapeutic target [170,222] (Table 2). Some ANO1 channel regulators, including fluoxetine [100] and casein kinase 2 (CK2), reduce cell migration activity and Notch signaling [117,169]. In addition, the combination of cisplatin with chelating agents, such as cuprizone, could produce a synergistic effect in the treatment of HNSCC overexpressing this channel [167,168].
ANO1 is also overexpressed in NSCLC tissues [220,223]. This overexpression and its association with clinical outcome (Figure 2) could be related to the expression and activity of EGFR [224,225]. Additionally, a study showed how the anticancer effect of verteporfin significantly reduces ANO1 protein levels and EGFR-STAT3 activation in PC9 cells [166]. Growing evidence shows that the inhibition of ANO1 expression or activity can serve as therapeutic agents in NSCLC and include matrine [165], homoharringtonine (HHT), and arctigenin [163,164] (Figure 2). Other therapeutic agents whose main effect is to decrease channel activity and the carcinogenic processes involved include benzophenanthridine, theaflavin, cepharantin, zafirlukast, and a hydrogel loaded with limonin [160,161,162,226,227].
3.3.3. Volume-Regulated Chloride Channels (VRAC)
Cell volume is finely regulated by several mechanisms [228]. After cell swelling caused by increased intracellular osmolytes, the cell restores its volume to normal conditions. This process is called regulatory volume depletion (RVD) and occurs through the loss of K+, Cl−, and organic osmolytes [229,230]. The Cl− conductance is critical for RVD and is mediated by the volume-regulated anion channel (VRAC)/organic osmolyte channel (VSOAC), also known as VSOR [231].
VRAC has been reported to regulate carcinogenic processes. When the cell is in isovolumetric conditions, the activation of VRAC causes cell shrinkage, cell contraction, or a decrease in apoptotic volume (AVD), a condition that mediates apoptosis in several cells [232]. During AVD, the Cl− conductance increases, the cell depolarizes, and K+ is lost, thereby activating Caspase-3 and favoring cell apoptosis [233]. Apoptotic resistance in cancer cells involves the functional impairment of chloride channels, where decreased VRAC activity reduces cell contraction, inhibits Caspase-3 activity, and prevents cell apoptosis [234,235].
The treatment of head and neck cancers can be affected by the development of chemoresistance to cisplatin, the first-line treatment in patients with nasopharyngeal carcinoma [236]. A study based on the bioinformatics analysis of positive HPV tumor samples identified that the high expression of VRAC correlates to the effectiveness of cisplatin treatment, and in vitro assays in resistant cells show that reduced VRAC expression reduces cisplatin uptake and favors cancer cell survival [101]. Moreover, another study confirmed that the mechanism by which cisplatin exerts its function in CNE-2Z cancer cells (a human nasopharyngeal carcinoma cell line) is via the P2Y receptor, which induces the activation of VRAC and AVD, ultimately leading to cell apoptosis [159]. While in OSCC the VRAC-specific inhibitor DCPIB significantly decreased the proliferation of HST-1 cells, in other cancer cell types, VRAC may not be relevant for cell proliferation [158,237]. At any rate, VRAC is a possible therapeutic target and prognostic marker in drug resistance for certain HPV-associated cancers [159].
3.3.4. Chloride Intracellular Channels (CLICs)
Intracellular chloride channel proteins are predominantly found in intracellular organelles [238]. These proteins function as both enzymes or channels, depending on the form in which they are found, either as soluble proteins or integral membrane proteins [239]. CLICs are a family of multifunctional proteins that can have diverse functions, including chloride transport or the regulation and modulation of gene expression or cytoskeleton structure [206,240]. Intracellular chloride channels are also involved in neurological, pulmonary, and cardiovascular functions and are related to pathologies such as cancer, as they regulate cell cycle and apoptosis [238,241,242].
The intracellular chloride channels CLIC1 and CLIC4 are promising cancer therapeutic targets because of their activities as ion channels and signal transducers in cell cycle progression and the malignant transformation of cancer cells [239]. The expression of CLIC chloride channels in the cell membrane may lead to tumor progression [243] by interfering with different signaling pathways [244] (Figure 1b).
CLIC1 (CLCNL1) can be found either as a cytosolic or transmembrane protein affecting cell cycle progression and the migration and invasion of solid tumors; however, in reality, its overexpression in several cancers has led to the suggestion that it can act as a prognostic marker and therapeutic target [240]. For example, CLIC1 shows increased expression in cell lines and tissue samples from HPV+ CC patients and has been proposed as a tumor promoter [153]. In a similar manner, CLIC1 is upregulated in LUAD and is associated with tumor metastasis and shorter survival [245]. Furthermore, the inhibition of CLIC1 in lung cancer cell lines decreases cell proliferation via the suppression of the p38 MAPK pathway, increased apoptosis, and the activation of Jun N-terminal kinases (JNK) [246,247]. This role converts this channel into a potential therapy target for this type of cancer (Figure 2).
CLIC4 (CLIC4L), in turn, fulfills functions in ionic homeostasis, transepithelial transport, and cell volume balance [248,249]. This channel is regulated by p53 and tumor necrosis factor-α (TNF-α), two relevant proteins in cancer [244]. Experiments performed in the squamous cell carcinoma of the lower lip (LLSCC) showed differential CLIC4 expression and function depending on the stages of the disease [157,250]. A proteomic study in lung cancer revealed that the down-regulation of CLIC4 is associated with carcinogenesis in some types of lung cancer. CLIC4 restoration in lung cancer cell lines attenuates cell proliferation, suggesting CLIC4 as a tumor suppressor [248,251].
3.4. Calcium Ion Channels
Calcium (Ca2+) regulates several physiological processes, including muscle contraction, transmitter release, cell differentiation and proliferation, gene transcription, and apoptosis [252]. Ca2+-permeable channels regulate Ca2+ concentrations in mitochondria, endoplasmic reticulum, lysosomes, and cytosol, with subsequent effects on cell proliferation, apoptosis, and autophagy that can trigger oncogenic processes [252]. Many Ca2+ channels are involved in carcinogenesis and will be discussed next.
3.4.1. Voltage-Gated Calcium Channels
Voltage-dependent calcium channels (Cav) play important roles in the regulation of mitosis, cell proliferation, and apoptosis [253]. Cav channel Alpha 2 delta 3 (CACNA2D3) is downregulated in nasopharyngeal carcinoma tissue samples and cell lines; when restoring its expression level, it influences the non-canonical Wnt/Ca2+ signaling pathway and induces apoptosis [156]. These reports are related to research in ESCC, where CACNA2D3 is downregulated in cancerous samples, possibly due to DNA copy number loss with promoter hypermethylation, and thus, CACNA2D3 is able to inhibit cell proliferation as well as colony and tumor formation in mice, possibly by increasing the expression of p53 and p21 [136].
Cav channel alpha 2 delta 1 (CACNA2D1) has been associated with cell proliferation [254]. CACNA2D1 protein (α2δ1) has been implicated in several types of cancer, including laryngeal squamous cell carcinoma, where its expression is associated with miR-107 expression. When miR-107 is downregulated, α2δ1 is overexpressed, favoring the progression and poor prognoses of this pathology (Table 2) [254]. In contrast, α2δ1 contributes to the drug resistance of SCLC and participates in cell proliferation, invasion, and metastasis [255]. Likewise, in non-small cell lung cancer, the inhibition of α2δ1 with the mAb1B50-1 blocker delays the tumorigenicity of tumor-initiating cells [256].
The voltage-gated calcium channel subunit alpha 1B (CACNA1B or Cav2.2) is involved in the release of neurotransmitters that are mainly related to pain signaling in the central and peripheral nervous system [257]. In NSCLC, CACNA1B gene expression is higher in tumor tissue than in normal samples, which is associated with increased intracellular Ca2+ concentration, leading to cell proliferation [110].
Cav channel subunit Alpha 1G channel (CACNA1G or Cav3.1) mediates Ca2+ entry into excitable cells and is involved in muscle contraction, hormone and neurotransmitter regulation, and cell division [258]. In human squamous cell carcinoma, its expression was significantly higher than in normal mucosa and dysplasia. This overexpression is associated with the proliferation and inhibition of apoptosis, since its silencing by siRNA decreases these processes [87]. In lung adenocarcinoma, CACNA1G expression is different in the cell lines evaluated [88]. The T-type alpha 1H subunit (CACNA1H or Cav3.2) channels play important roles in chronic pain [259]. CACNA1H is expressed in LUAD cell lines and channel blockage inhibits cancer properties [88].
3.4.2. Calcium Channels Activated by Calcium Release
Calcium release-activated calcium channels (CRACs) are important for Ca2+ regulation in neurons and the glial cells of the nervous system and have been also implicated in cytokine production and antigen response in immune cells [260]. The store-operated calcium entry (SOCE) channel Orai3 is involved in LUAD cell proliferation, since Orai3 can regulate cell cycle and cyclin expression [89].
3.4.3. Stromal Interaction Molecule 1
Stromal interaction molecules (STIM) are transmembrane proteins that respond to Ca2+ fluctuations in the endoplasmic reticulum [261]. STIM1 overexpression in HPV+ CC regulates the production of vascular endothelial growth factor A (VEGF-A), increasing the invasive capacity of cells. Likewise, its silencing arrests the cell cycle in the S and G2/M phases and increases the expression of p21 protein [58].
3.4.4. Transient Receptor Potential Cation Channels
Transient receptor potential (TRP) channels act as cellular sensors of mechanical forces, taste, thermal sensation, and other physiological processes [262]. Several TRP subfamilies, including TRPC, TRPV, TRPM, TRPA, and TRPP, are suggested to participate in cancer [263].
The transient receptor potential cation channel subfamily C member 6 (TRPC6) is activated by the second messenger diacylglycerol (DAG) [264]. In human head and neck carcinoma, TRPC6 is overexpressed in cancerous tissue samples in comparison to samples from non-cancer patients, and its inhibition significantly interferes with cell invasion, although it did not reduce cell proliferation [137]. TRPV1 (transient receptor potential cation channel subfamily V member 1 or transient receptor potential vanilloid 1) is activated by nociceptive stimuli, especially regarding capsaicin, which is present in hot peppers and is a natural agonist of TRPV1; therefore, it is involved in pain perception, thermoregulation, and osmoregulation [262]. TRPV1 is overexpressed in tongue cell carcinoma samples compared to healthy epithelium and is suggested as a tumor marker for this carcinoma [90]. However, vanilloids are cytotoxic in OSCC but are independent of TRPV1 activation; hence, capsazepine was suggested as a therapeutic candidate for this cancer (Table 2) [138]. The transient receptor potential cation channel subfamily V member 2 (or transient receptor potential vanilloid 2, TRPV2) is mainly expressed in nerve, immune, and neuroendocrine cells, and it participates in osmoregulation and autonomic and cardiovascular modulation, as well as the regulation of the cytoskeleton and cell motility [265]. Its overexpression in ESCC patient tissue samples and cell lines is associated with cell cycle progression. TRPV2 silencing leads to reduced cell proliferation, migration, and invasion, the induction of apoptosis, and an altered Wnt/β-catenin signaling pathway [139].
TRPV3 (transient receptor potential cation channel subfamily V member 3 or transient receptor potential vanilloid 3) is mainly expressed in keratinocytes and is activated by repetitive thermal stimuli [262]. The overexpression of TRPV3 in NSCLC is able to influence cell proliferation, channel blocking decreases the colony-forming capacity of cell lines, and the cell cycle is arrested in the G1/S phase (Table 2) [140]. The transient receptor potential cation channel subfamily V member 4 (or transient receptor potential vanilloid 4, TRPV4) is expressed in many tissues, is involved in the perception of various physical and chemical stimuli, and its expression has been associated with several neurodegenerative disorders [266]. In OSCC, their expression is higher in tumor samples than in the adjacent non-tumorous regions and is suggested to promote cell proliferation via AKT signaling calcium calmodulin kinase II (CAMKII) activation [91]. TRPV6 (transient receptor potential cation channel subfamily V member 6 or transient receptor potential vanilloid 6) is expressed in the placenta, epidermis, digestive tract, and salivary glands, and its dysregulation is associated with several diseases, including cancer [267]. In ESCC, the low gene and protein expression of TRPV6 is linked with the unfavorable survival of male patients but is favorable for women; thus, it may be regulated by sex steroid hormones [111].
The transient receptor potential cation channel subfamily M member 2 (or transient receptor potential melastatin 2, TRPM2) is associated with oxidative stress and has been related to cardiovascular and neurodegenerative disorders [262]. In some cancers, TRPM2 has a protective role via reducing oxidative stress, inflammation, and chromosomal instability, although in other cancers it may promote tumor progression [268]. In OSCC, TRPM2 is highly expressed in cell lines and cancerous samples from patients, and its knock-down by shRNA inhibits cell survival and migration; thus, the channel is proposed as an alternative target in the treatment of OSCC [141]. Likewise, in ESCC, the protein expression level of TRPM2 is increased compared to normal tissue [142]. TRPM7 (transient receptor potential cation channel subfamily M member 7 or transient receptor potential melastatin 7) is important in the intracellular regulation of magnesium (Mg2+), zinc (Zn2+), and Ca2+ concentration via the modulation of SOCE [269]. In head and neck carcinoma, a relevant Ca2+ current takes place via TRPM7, and its blocking or silencing reduces the growth and proliferation of cancer cells [270]. TRPM7 is overexpressed in LUAD cell lines and samples from patients with the pathology and promotes the expression of stem cells markers. In addition, the reduction in gene expression by shRNA or channel block with waixenicin A inhibits cell viability (Table 2) [143]. The transient receptor potential cation channel subfamily A member 1 (or transient receptor potential ankyrin 1, TRPA1), which is activated by a wide range of irritant substances that can be found in food or in the environment, is mainly expressed in neuronal cells and has nociceptive functions [262]. In SCLC, TRPA1 overexpression has been detected both in cell lines and patient tissue samples compared to non-malignant lung tissue, and its downregulation decreases the anchoring capacity of cancer cells [112].
TRPP2 (polycystin 2 transient receptor potential cation channel or transient receptor potential polycystic receptor 2) is involved in mechanical sensation and cell proliferation [271]. In head and neck carcinoma cell lines, TRPP2 silencing increases cell proliferation [144].
4. Conclusions
The high incidence of HPV-associated cancers causes thousands of deaths worldwide. Thus, novel diagnostic, prognostic, and therapeutic tools are urgently needed. The expression and regulation of ion channels in HPV-associated cancers here reviewed represents a plethora of opportunities and potential solutions to this problem.
Because cancer is a multifactorial disease and ion channels are regulated by a variety of cancer-associated factors, more studies are needed to determine whether changes in ion channel expression are solely or mainly associated with HPV infection. For instance, an RNA-Seq database analysis of ion channel expression from HPV+ and HPV- tissues from the same patient or cancer type should be performed. This analysis should include information concerning the exposure of the patients to other cancer-associated factors, such as hormones, cigarette smoke, etc., to define the specific influence of each risk factor on ion- channel expression. It will be very interesting in a few decades to analyze ion channel expression in “HPV-associated cancers” in those patients who have been vaccinated against HPV. As mentioned above, this will help to resolve the controversy of the association of HPV infection with lung cancer and other malignancies. The investigation of more precise molecular mechanisms associating HPV-oncoproteins with the regulation of ion channels is necessary.
A significant percentage of drugs commonly used in clinical settings target ion channels. Glibenclamide and antihistamines are examples of the extraordinary opportunity that ion channel inhibitors may provide for drug repurposing for the benefit of cancer patients. The study of the combination of ion channel inhibitors with usually prescribed anticancer drugs represents an exceptional area for cancer research. This approach may help to reduce drug-resistance and fight cancer cells via diverse molecular mechanisms and cellular pathways. The vast amount of research yet to be performed associating ion channels and HPV could help to find novel markers and targets to improve clinical outcomes in patients with these types of malignancies.
Author Contributions
A.J.C., B.A. and I.O.-B. contributed equally to this work. Conceptualization, A.J.C., B.A., I.O.-B., F.V.-M., J.d.l.G., A.R., C.B. and J.C.; investigation, A.J.C., B.A., I.O.-B., F.V.-M., J.d.l.G., P.G., R.O.-D., Y.S.-P., C.M.G.-C., A.R. and J.C.; writing—original draft preparation, A.J.C., B.A., I.O.-B., F.V.-M., A.R. and J.C.; writing—review and editing, All of the authors.; funding acquisition, J.d.l.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by Conacyt grant A1-S-9783 to J.d.l.G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bzhalava, D.; Mühr, L.S.A.; Lagheden, C.; Ekström, J.; Forslund, O.; Dillner, J.; Hultin, E. Deep Sequencing Extends the Diversity of Human Papillomaviruses in Human Skin. Sci. Rep. 2014, 4, 5807. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.V. The Human Papillomavirus Replication Cycle, and Its Links to Cancer Progression: A Comprehensive Review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef] [PubMed]
- Scott-Wittenborn, N.; Fakhry, C. Epidemiology of HPV Related Malignancies. Semin. Radiat. Oncol. 2021, 31, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Catalan Institute of Oncology; International Agency for Research on Cancer (IARC). HPV Information Centre. Available online: https://hpvcentre.net/references.php (accessed on 9 January 2023).
- Catalan Institute of Oncology (ICO); International Agency for Research on Cancer (IARC). Human Papillomavirus and Related Diseases Report World. Available online: www.hpvcentre.net (accessed on 10 January 2023).
- Mazarico, E.; Gonzalez-Bosquet, E. Prevalence of Infection by Different Genotypes of Human Papillomavirus in Women with Cervical Pathology. Gynecol. Oncol. 2012, 125, 181–185. [Google Scholar] [CrossRef]
- Torres-Ibarra, L.; Cuzick, J.; Lorincz, A.T.; Spiegelman, D.; Lazcano-Ponce, E.; Franco, E.L.; Moscicki, A.B.; Mahmud, S.M.; Wheeler, C.M.; Rivera-Paredez, B.; et al. Comparison of HPV-16 and HPV-18 Genotyping and Cytological Testing as Triage Testing within Human Papillomavirus-Based Screening in Mexico. JAMA Netw. Open 2019, 2, e1915781. [Google Scholar] [CrossRef]
- Schulte-Frohlinde, R.; Georges, D.; Clifford, G.M.; Baussano, I. Predicting Cohort-Specific Cervical Cancer Incidence from Population-Based Surveys of Human Papiloma Virus Prevalence: A Worldwide Study. Am. J. Epidemiol. 2022, 191, 402–412. [Google Scholar] [CrossRef]
- Carmen Alarcón-Romero, D.L.; Organista-Nava, J.; Gómez-Gómez, Y.; Ortiz-Ortiz, J.; Hernández-Sotelo, D.; del Moral-Hernández, O.; Mendoza-Catalán, M.A.; Antaño-Arias, R.; Leyva-Vázquez, M.A.; Sales-Linares, N.; et al. Prevalence and Distribution of Human Papillomavirus Genotypes (1997–2019) and Their Association with Cervical Cancer and Precursor Lesions in Women from Southern Mexico. Cancer Control. 2022, 29. [Google Scholar] [CrossRef]
- Li, M.; Du, X.; Lu, M.; Zhang, W.; Sun, Z.; Li, L.; Ye, M.; Fan, W.; Jiang, S.; Liu, A.; et al. Prevalence Characteristics of Single and Multiple HPV Infections in Women with Cervical Cancer and Precancerous Lesions in Beijing, China. J. Med. Virol. 2019, 91, 473–481. [Google Scholar] [CrossRef]
- Alemany, L.; Saunier, M.; Tinoco, L.; Quirós, B.; Alvarado-Cabrero, I.; Alejo, M.; Joura, E.A.; Maldonado, P.; Klaustermeier, J.; Salmerón, J.; et al. Large Contribution of Human Papillomavirus in Vaginal Neoplastic Lesions: A Worldwide Study in 597 Samples. Eur. J. Cancer 2014, 50, 2846–2854. [Google Scholar] [CrossRef]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human Papillomavirus Types from Infection to Cancer in the Anus, According to Sex and HIV Status: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef]
- Kidd, L.C.; Chaing, S.; Chipollini, J.; Giuliano, A.R.; Spiess, P.E.; Sharma, P. Relationship between Human Papillomavirus and Penile Cancer-Implications for Prevention and Treatment. Transl. Androl. Urol. 2017, 6, 791–802. [Google Scholar] [CrossRef]
- Goldstone, S.E.; Enyinna, C.S.; Davis, T.W. Detection of Oncogenic Human Papillomavirus and Other Predictors of Anal High-Grade Dysplasia in Men Who Have Sex with Men with Abnormal Cytology. Dis. Colon. Rectum. 2009, 52, 31–39. [Google Scholar] [CrossRef]
- Urbute, A.; Rasmussen, C.L.; Belmonte, F.; Obermueller, T.; Prigge, E.S.; Arbyn, M.; Verdoodt, F.; Kjaer, S.K. Prognostic Significance of HPV DNA and P16INK4a in Anal Cancer: A Systematic Review and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 703–710. [Google Scholar] [CrossRef]
- Goon, P.K.C.; Stanley, M.A.; Ebmeyer, J.; Steinsträsser, L.; Upile, T.; Jerjes, W.; Bernal-Sprekelsen, M.; Görner, M.; Sudhoff, H.H. HPV & Head and Neck Cancer: A Descriptive Update. Head Neck Oncol. 2009, 1, 36. [Google Scholar] [CrossRef]
- Tagliabue, M.; Mena, M.; Maffini, F.; Gheit, T.; Blasco, B.Q.; Holzinger, D.; Tous, S.; Scelsi, D.; Riva, D.; Grosso, E.; et al. Role of Human Papillomavirus Infection in Head and Neck Cancer in Italy: The HPV-AHEAD Study. Cancers 2020, 12, 3567. [Google Scholar] [CrossRef]
- Parfenov, M.; Pedamallu, C.S.; Gehlenborg, N.; Freeman, S.S.; Danilova, L.; Bristow, C.A.; Lee, S.; Hadjipanayis, A.G.; Ivanova, E.V.; Wilkerson, M.D.; et al. Characterization of HPV and Host Genome Interactions in Primary Head and Neck Cancers. Proc. Natl. Acad. Sci. USA 2014, 111, 15544–15549. [Google Scholar] [CrossRef]
- Hübbers, C.U.; Akgül, B. HPV and Cancer of the Oral Cavity. Virulence 2015, 6, 244–248. [Google Scholar] [CrossRef]
- Syrjänen, K.J. HPV Infections and Lung Cancer. J. Clin. Pathol. 2002, 55, 885–891. [Google Scholar] [CrossRef]
- Damin, D.C.; Ziegelmann, P.K.; Damin, A.P. Human Papillomavirus Infection and Colorectal Cancer Risk: A Meta-Analysis. Color. Dis. 2013, 15, e420–e428. [Google Scholar] [CrossRef]
- Daniela Iacobone, A.; Muresu, N.; Di Lorenzo, B.; Saderi, L.; Sechi, I.; Del Rio, A.; Piana, A.; Sotgiu, G. Prevalence of Human Papilloma Virus Infection in Bladder Cancer: A Systematic Review. Diagnostics 2022, 12, 1759. [Google Scholar] [CrossRef]
- Lawson, J.S.; Glenn, W.K.; Salyakina, D.; Clay, R.; Delprado, W.; Cheerala, B.; Tran, D.D.; Ngan, C.C.; Miyauchi, S.; Karim, M.; et al. Human Papilloma Virus Identification in Breast Cancer Patients with Previous Cervical Neoplasia. Front. Oncol. 2016, 5, 298. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.S.; Glenn, W.K.; Whitaker, N.J. Human Papilloma Viruses and Breast Cancer—Assessment of Causality. Front. Oncol. 2016, 6, 207. [Google Scholar] [CrossRef] [PubMed]
- Veijalainen, O.; Kares, S.; Kujala, P.; Tirkkonen, M.; Vuento, R.; Kholová, I.; Luukkaala, T.; Osuala, V.; Mäenpää, J. Human Papillomavirus Test with Cytology Triage in Organized Screening for Cervical Cancer. Acta Obs. Gynecol. Scand. 2016, 95, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Tota, J.E.; Bentley, J.; Blake, J.; Coutlée, F.; Duggan, M.A.; Ferenczy, A.; Franco, E.L.; Fung-Kee-Fung, M.; Gotlieb, W.; Mayrand, M.H.; et al. Approaches for Triaging Women Who Test Positive for Human Papillomavirus in Cervical Cancer Screening. Prev. Med. 2017, 98, 15–20. [Google Scholar] [CrossRef]
- Moody, C.A.; Laimins, L.A. Human Papillomavirus Oncoproteins: Pathways to Transformation. Nat. Rev. Cancer 2010, 10, 550–560. [Google Scholar] [CrossRef]
- Dixit, R.; Kemp, C.; Kulich, S.; Seethala, R.; Chiosea, S.; Ling, S.; Ha, P.K.; Duvvuri, U. TMEM16A/ANO1 Is Differentially Expressed in HPV-Negative versus HPV-Positive Head and Neck Squamous Cell Carcinoma through Promoter Methylation. Sci. Rep. 2015, 5, 16657. [Google Scholar] [CrossRef]
- Ramírez, A.; Vera, E.; Gamboa-Domínguez, A.; Lambert, P.; Gariglio, P.; Camacho, J. Calcium-Activated Potassium Channels as Potential Early Markers of Human Cervical Cancer. Oncol. Lett. 2018, 15, 7249–7254. [Google Scholar] [CrossRef]
- Ramírez, A.; Vázquez-Sánchez, A.Y.; Carrión-Robalino, N.; Camacho, J. Ion Channels and Oxidative Stress as a Potential Link for the Diagnosis or Treatment of Liver Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3928714. [Google Scholar] [CrossRef]
- Diaz, D.; Delgadillo, D.M.; Hernández-Gallegos, E.; Ramírez-Domínguez, M.E.; Hinojosa, L.M.; Ortiz, C.S.; Berumen, J.; Camacho, J.; Gomora, J.C. Functional Expression of Voltage-Gated Sodium Channels in Primary Cultures of Human Cervical Cancer. J. Cell. Physiol. 2007, 210, 469–478. [Google Scholar] [CrossRef]
- Barajas-Farias, L.; Bermpudez-Ocaña, D.; Díaz, L.; Larrea, F.; Farias, B.; Avila-Chávez, E.; Cadena, A.; Hinojosa, L.M.; Lara, G.; Villanueva, L.A.; et al. Ether á Go-Go Potassium Channels as Human Cervical Cancer Markers. Cancer Res. 2004, 64, 6996–7001. [Google Scholar] [CrossRef]
- Woodham, A.W.; da Silva, D.M.; Skeate, J.G.; Raff, A.B.; Ambroso, M.R.; Brand, H.E.; Isas, J.M.; Langen, R.; Kast, W.M. The S100A10 Subunit of the Annexin A2 Heterotetramer Facilitates L2-Mediated Human Papillomavirus Infection. PLoS ONE 2012, 7, e43519. [Google Scholar] [CrossRef]
- Erdemoglu, M.; Yilmaz, M.; Eroglu, R.; Kirat, S.; Altinordu, A.; Ozkara, A.; Can, B.; Kartal, E.; Akkus, F.; Avci, F.; et al. Current Approaches in Gynecology and Gyneco-Oncology; Yilmaz, M., Ed.; Iksad: Ankara, Turkey, 2022; pp. 223–236. [Google Scholar]
- Dziduszko, A.; Ozbun, M.A. Annexin A2 and S100A10 Regulate Human Papillomavirus Type 16 Entry and Intracellular Trafficking in Human Keratinocytes. J. Virol. 2013, 87, 7502–7515. [Google Scholar] [CrossRef]
- Taylor, J.R.; Fernandez, D.J.; Thornton, S.M.; Skeate, J.G.; Lühen, K.P.; Da Silva, D.M.; Langen, R.; Kast, W.M. Heterotetrameric Annexin A2/S100A10 (A2t) Is Essential for Oncogenic Human Papillomavirus Trafficking and Capsid Disassembly, and Protects Virions from Lysosomal Degradation. Sci. Rep. 2018, 8, 11642. [Google Scholar] [CrossRef]
- Tantyo, N.A.; Karyadi, A.S.; Rasman, S.Z.; Salim, M.R.G.; Devina, A.; Sumarpo, A. The Prognostic Value of S100A10 Expression in Cancer. Oncol. Lett. 2018, 17, 1417–1424. [Google Scholar] [CrossRef]
- Seo, J.S.; Svenningsson, P. Modulation of Ion Channels and Receptors by P11 (S100A10). Trends Pharmacol. Sci. 2020, 41, 487–497. [Google Scholar] [CrossRef]
- Bharadwaj, A.; Kempster, E.; Waisman, D.M. The Annexin A2/S100a10 Complex: The Mutualistic Symbiosis of Two Distinct Proteins. Biomolecules 2021, 11, 1849. [Google Scholar] [CrossRef]
- Lei, J.; Deng, F.; Ding, H.; Fu, M.; Xu, T.; Ji, B.; Feng, L.; Li, M.; Qiu, J.; Gao, Q. Recent Developments on the Roles of Calcium Signals and Potential Therapy Targets in Cervical Cancer. Cells 2022, 11, 3003. [Google Scholar] [CrossRef]
- Stewart, J.M. TRPV6 as a Target for Cancer Therapy. J. Cancer 2020, 11, 374–387. [Google Scholar] [CrossRef]
- Amaral, M.D.; Quaresma, M.C.; Pankonien, I. What Role Does Cftr Play in Development, Differentiation, Regeneration and Cancer? Int. J. Mol. Sci. 2020, 21, 3133. [Google Scholar] [CrossRef]
- Lechner, M.; Liu, J.; Masterson, L.; Fenton, T.R. HPV-Associated Oropharyngeal Cancer: Epidemiology, Molecular Biology and Clinical Management. Nat. Rev. Clin. Oncol. 2022, 19, 306–327. [Google Scholar] [CrossRef]
- Hoppe-Seyler, K.; Bossler, F.; Braun, J.A.; Herrmann, A.L.; Hoppe-Seyler, F. The HPV E6/E7 Oncogenes: Key Factors for Viral Carcinogenesis and Therapeutic Targets. Trends Microbiol. 2017, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qi, Y.; Cui, X.; Huo, Q.; Zhu, L.; Zhang, A.; Tan, M.; Hong, Q.; Yang, Y.; Zhang, H.; et al. Characteristic of HPV Integration in the Genome and Transcriptome of Cervical Cancer Tissues. Biomed Res. Int. 2018, 2018, 6242137. [Google Scholar] [CrossRef] [PubMed]
- Hermida-Prado, F.; Menéndez, S.T.; Albornoz-Afanasiev, P.; Granda-Diaz, R.; Álvarez-Teijeiro, S.; Villaronga, M.Á.; Allonca, E.; Alonso-Durán, L.; León, X.; Alemany, L.; et al. Distinctive Expression and Amplification of Genes at 11q13 in Relation to HPV Status with Impact on Survival in Head and Neck Cancer Patients. J. Clin. Med. 2018, 7, 501. [Google Scholar] [CrossRef] [PubMed]
- Fry, E.A.; Inoue, K. Aberrant Expression of ETS1 and ETS2 Proteins in Cancer. Cancer Rep. Rev. 2018, 2, 15761. [Google Scholar] [CrossRef]
- Wolvetang, E.J.; Wilson, T.J.; Sanij, E.; Busciglio, J.; Hatzistavrou, T.; Seth, A.; Hertzog, P.J.; Kola, I. ETS2 Overexpression in Transgenic Models and in Down Sydrome Predisposes to Apoptosis via the P53 Pathway. Hum. Mol. Genet. 2003, 12, 247–255. [Google Scholar] [CrossRef]
- Hang, D.; Zhou, W.; Jia, M.; Wang, L.; Zhou, J.; Yin, Y.; Ma, H.; Hu, Z.; Li, N.; Shen, H. Genetic Variants within MicroRNA-Binding Site of RAD51B Are Associated with Risk of Cervical Cancer in Chinese Women. Cancer Med. 2016, 5, 2596–2601. [Google Scholar] [CrossRef]
- Hong, A.M.; Ferguson, P.; Dodds, T.; Jones, D.; Li, M.; Yang, J.; Scolyer, R.A. Significant Association of PD-L1 Expression with Human Papillomavirus Positivity and Its Prognostic Impact in Oropharyngeal Cancer. Oral. Oncol. 2019, 92, 33–39. [Google Scholar] [CrossRef]
- Finegersh, A.; Kulich, S.; Guo, T.; Favorov, A.V.; Fertig, E.J.; Danilova, L.V.; Gaykalova, D.A.; Califano, J.A.; Duvvuri, U. DNA Methylation Regulates TMEM16A/ANO1 Expression through Multiple CpG Islands in Head and Neck Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 15173. [Google Scholar] [CrossRef]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Vande Pol, S.; Podjarny, A.; et al. Structure of the E6/E6AP/P53 Complex Required for HPV-Mediated Degradation of P53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- He, S.; Moutaoufik, M.T.; Islam, S.; Persad, A.; Wu, A.; Aly, K.A.; Fonge, H.; Babu, M.; Cayabyab, F.S. HERG Channel and Cancer: A Mechanistic Review of Carcinogenic Processes and Therapeutic Potential. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188355. [Google Scholar] [CrossRef]
- Tomita, T.; Huibregtse, J.M.; Matouschek, A. A Masked Initiation Region in Retinoblastoma Protein Regulates Its Proteasomal Degradation. Nat. Commun. 2020, 11, 2019. [Google Scholar] [CrossRef]
- De Guadalupe Chávez-López, M.; Zúñiga-García, V.; Castro-Magdonel, B.E.; Vera, E.; Garrido, E.; Sánchez-Ramos, J.; Ponce-Castañeda, M.V.; de Lourdes Cabrera-Muñoz, M.; Escobar, Y.; Ortiz, C.S.; et al. Eag1 Gene and Protein Expression in Human Retinoblastoma Tumors and Its Regulation by PRb in HeLa Cells. Genes 2020, 11, 119. [Google Scholar] [CrossRef]
- Sim, H.J.; Song, M.S.; Lee, S.Y. Kv3 Channels Contribute to Cancer Cell Migration via Vimentin Regulation. Biochem. Biophys. Res. Commun. 2021, 551, 140–147. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Zhang, Q.; Li, C. Silencing of KCNA1 Suppresses the Cervical Cancer Development via Mitochondria Damage. Channels 2019, 13, 321–330. [Google Scholar] [CrossRef]
- Chen, Y.F.; Chiu, W.T.; Chen, Y.T.; Lin, P.Y.; Huang, H.J.; Chou, C.Y.; Chang, H.; Tang, M.J.; Shen, M.R. Calcium Store Sensor Stromal-Interaction Molecule 1-Dependent Signaling Plays an Important Role in Cervical Cancer Growth, Migration, and Angiogenesis. Proc. Natl. Acad. Sci. USA 2011, 108, 15225–15230. [Google Scholar] [CrossRef]
- Scarth, J.A.; Wasson, C.W.; Patterson, M.R.; Evans, D.; Carden, H.; Whitehouse, A.; Mankouri, J.; Samson, A.; Morgan, E.L.; Macdonald, A. Exploitation of ATP-Sensitive Potassium Ion (KATP) Channels by HPV Promotes Cervical Cancer Cell Proliferation by Contributing to MAPK/AP-1 Signalling. Cold Spring Harb. Lab. 2022. [Google Scholar] [CrossRef]
- Westrich, J.A.; Warren, C.J.; Pyeon, D. Evasion of Host Immune Defenses by Human Papillomavirus. Virus Res. 2017, 231, 21–33. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Qiu, L. Transient Receptor Potential Vanilloid 1 Promotes EGFR Ubiquitination and Modulates EGFR/MAPK Signalling in Pancreatic Cancer Cells. Cell Biochem. Funct. 2020, 38, 401–408. [Google Scholar] [CrossRef]
- Zhai, K.; Liskova, A.; Kubatka, P.; Büsselberg, D. Calcium Entry through Trpv1: A Potential Target for the Regulation of Proliferation and Apoptosis in Cancerous and Healthy Cells. Int. J. Mol. Sci. 2020, 21, 4177. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef]
- Szymonowicz, K.A.; Chen, J. Biological and Clinical Aspects of HPV-Related Cancers. Cancer Biol. Med. 2020, 17, 864–878. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.-M.; Xu, Q.-P.; Li, X.; Xiao, R.-D.; Cai, L.; He, F. The Association between Human Papillomavirus Infection and Lung Cancer: A System Review and Meta-Analysis. Oncotarget 2017, 8, 96419–96432. Available online: www.impactjournals.com/oncotarget (accessed on 10 January 2023). [CrossRef] [PubMed]
- Clavel, J. Progress in the Epidemiological Understanding of Gene-Environment Interactions in Major Diseases: Cancer. Comptes Rendus Biol. 2007, 330, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Q.; Purhonen, P.; Hebert, H. Structure of Potassium Channels. Cell. Mol. Life Sci. 2015, 72, 3677–3693. [Google Scholar] [CrossRef]
- Huang, X.; Jan, L.Y. Targeting Potassium Channels in Cancer. J. Cell Biol. 2014, 206, 151–162. [Google Scholar] [CrossRef]
- González, C.; Baez-Nieto, D.; Valencia, I.; Oyarzún, I.; Rojas, P.; Naranjo, D.; Latorre, R. K+ Channels: Function-Structural Overview. Compr. Physiol. 2012, 2, 2087–2149. [Google Scholar] [CrossRef]
- Barros, F.; Pardo, L.A.; Domínguez, P.; Sierra, L.M.; de la Peña, P. New Structures and Gating of Voltage-Dependent Potassium (Kv) Channels and Their Relatives: A Multi-Domain and Dynamic Question. Int. J. Mol. Sci. 2019, 20, 248. [Google Scholar] [CrossRef]
- Pardo, L.A.; Contreras-Jurado, C.; Zientkowska, M.; Alves, F.; Stühmer, W. Role of Voltage-Gated Potassium Channels in Cancer. J. Membr. Biol. 2005, 205, 115–124. [Google Scholar] [CrossRef]
- Restrepo, I.; Sánchez, C.; Camacho, J. Human EAG1 Potassium Channels in the Epithelial-to-Mesenchymal Transition in Lung Cancer Cells. Anticancer. Res. 2011, 31, 1265–1270. [Google Scholar]
- García-Quiroz, J.; Camacho, J. Astemizole: And Old Anti-Histamine as a New Promising Anti-Cancer Drug. Anticancer. Agents Med. Chem. 2011, 11, 307–314. [Google Scholar] [CrossRef]
- De Guadalupe Chávez-López, M.; Hernández-Gallegos, E.; Vázquez-Sánchez, A.Y.; Gariglio, P.; Camacho, J. Antiproliferative and Proapoptotic Effects of Astemizole on Cervical Cancer Cells. Int. J. Gynecol. Cancer 2014, 24, 824–828. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Guo, Y.; Shui, L.; Li, S.; Bai, Y.; Liu, Y.; Zeng, M.; Xia, J. HERG1 Promotes Esophageal Squamous Cell Carcinoma Growth and Metastasis through TXNDC5 by Activating the PI3K/AKT Pathway. J. Exp. Clin. Cancer Res. 2019, 38. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, H.R.; Ryu, P.D.; Lee, S.Y. Regulation of Voltage-Gated Potassium Channels Attenuates Resistance of Side-Population Cells to Gefitinib in the Human Lung Cancer Cell Line NCI-H460. BMC Pharm. Toxicol 2017, 18, 14. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, J.-W.; Byun, J.K.; Kim, H.K.; Ryu, P.D.; Lee, S.Y.; Kim, D.-Y. Silencing of Voltage-Gated Potassium Channel Kv9.3 Inhibits Proliferation in Human Colon and Lung Carcinoma Cells. Oncotarget 2015, 6, 8132–8143. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Zhu, S.; Lan, X. MiR-483–5p Promotes Esophageal Cancer Progression by Targeting KCNQ1. Biochem. Biophys. Res. Commun. 2020, 531, 615–621. [Google Scholar] [CrossRef]
- Liu, L.; Zhan, P.; Nie, D.; Fan, L.; Lin, H.; Gao, L.; Mao, X. Intermediate-Conductance-Ca2-Activated K Channel IKCa1 Is Upregulated and Promotes Cell Proliferation in Cervical Cancer. Med. Sci. Monit. Basic Res. 2017, 23, 45–57. [Google Scholar] [CrossRef]
- Nakamura, S.; Kanda, M.; Koike, M.; Shimizu, D.; Umeda, S.; Hattori, N.; Hayashi, M.; Tanaka, C.; Kobayashi, D.; Yamada, S.; et al. KCNJ15 Expression and Malignant Behavior of Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2020, 27, 2559–2568. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zhang, Y.H.; Sun, H.Y.; Lau, C.P.; Li, G.R. Epidermal Growth Factor Receptor Tyrosine Kinase Regulates the Human Inward Rectifier Potassium K IR2.3 Channel, Stably Expressed in HEK 293 Cells. Br. J. Pharm. 2011, 164, 1469–1478. [Google Scholar] [CrossRef]
- Vázquez-Sánchez, A.Y.; Hinojosa, L.M.; Parraguirre-Martínez, S.; González, A.; Morales, F.; Montalvo, G.; Vera, E.; Hernández-Gallegos, E.; Camacho, J. Expression of KATP Channels in Human Cervical Cancer: Potential Tools for Diagnosis and Therapy. Oncol. Lett. 2018, 15, 6302–6308. [Google Scholar] [CrossRef]
- Xu, K.; Sun, G.; Li, M.; Chen, H.; Zhang, Z.; Qian, X.; Li, P.; Xu, L.; Huang, W.; Wang, X. Glibenclamide Targets Sulfonylurea Receptor 1 to Inhibit P70S6K Activity and Upregulate KLF4 Expression to Suppress Non-Small Cell Lung Carcinoma. Mol. Cancer 2019, 18, 2085–2096. [Google Scholar] [CrossRef]
- Zavala, W.D.; Foscolo, M.R.; Kunda, P.E.; Cavicchia, J.C.; Acosta, C.G. Changes in the Expression of the Potassium Channels TASK1, TASK3 and TRESK in a Rat Model of Oral Squamous Cell Carcinoma and Their Relation to Malignancy. Arch. Oral Biol. 2019, 100, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Walline, H.M.; Komarck, C.M.; McHugh, J.B.; Bellile, E.L.; Brenner, J.C.; Prince, M.E.; McKean, E.L.; Chepeha, D.B.; Wolf, G.T.; Worden, F.P.; et al. Genomic Integration of High-Risk HPV Alters Gene Expression in Oropharyngeal Squamous Cell. Mol. Cancer Res. 2016, 14, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Lin, N.; Zuo, W.; Luo, H.; Li, Y.; Liu, S.; Meng, L.; Fan, A.; Zhu, L.; Jacob, T.J.C.; et al. Ethanol Promotes Cell Migration via Activation of Chloride Channels in Nasopharyngeal Carcinoma Cells. Alcohol. Clin. Exp. Res. 2015, 39, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Li, R.F.; Man, Q.W.; Liu, J.Y.; Zheng, Y.Y.; Gao, X.; Liu, H.M. Overexpression of T-Type Calcium Channel Cav3.1 in Oral Squamous Cell Carcinoma: Association with Proliferation and Anti-Apoptotic Activity. J. Mol. Histol. 2021, 52, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Bhargava, A.; Rath, S.N. T-Type Calcium Channel Antagonist, TTA-A2 Exhibits Anti-Cancer Properties in 3D Spheroids of A549, a Lung Adenocarcinoma Cell Line. Life Sci. 2020, 260, 118291. [Google Scholar] [CrossRef]
- Ay, A.; Benzerdjerb, N.; Sevestre, H.; Ahidouch, A.; Ouidid, H. Orai3 Constitutes a Native Store-Operated Calcium Entry That Regulates Non Small Cell Lung Adenocarcinoma Cell Proliferation. PLoS ONE 2015, 10, e0124201. [Google Scholar] [CrossRef]
- Marincsák, R.; Tóth, B.; Czifra, G.; Márton, I.; Rédl, P.; Tar, I.; Tóth, L.; Kovács, L.; Bíró, T. Increased Expression of TRPV1 in Squamous Cell Carcinoma of the Human Tongue. Oral Dis. 2009, 15, 328–335. [Google Scholar] [CrossRef]
- Fujii, S.; Tajiri, Y.; Hasegawa, K.; Matsumoto, S.; Yoshimoto, R.U.; Wada, H.; Kishida, S.; Kido, M.A.; Yoshikawa, H.; Ozeki, S.; et al. The TRPV4-AKT Axis Promotes Oral Squamous Cell Carcinoma Cell Proliferation via CaMKII Activation. Lab. Investig. 2020, 100, 311–323. [Google Scholar] [CrossRef]
- Yuan, P.; Rao, W.; Lin, Z.; Liu, S.; Lin, X.; Wu, C.; Lin, X.; Hu, Z.; Ye, W. Genomic Analyses Reveal SCN7A Is Associated with the Prognosis of Esophageal Squamous Cell Carcinoma. Esophagus 2022, 19, 303–315. [Google Scholar] [CrossRef]
- Hernandez-Plata, E.; Ortiz, C.S.; Marquina-Castillo, B.; Medina-Martinez, I.; Alfaro, A.; Berumen, J.; Rivera, M.; Gomora, J.C. Overexpression of Nav1.6 Channels Is Associated with the Invasion Capacity of Human Cervical Cancer. Int. J. Cancer 2012, 130, 2013–2023. [Google Scholar] [CrossRef]
- Guan, Y.; Luan, Y.; Xie, Y.; Zhou, H.; Li, W.; Zhang, X.; Shen, X.; Chen, Y.; Xu, L.; Lin, Z.; et al. Chloride Channel-3 Is Required for Efficient Tumour Cell Migration and Invasion in Human Cervical Squamous Cell Carcinoma. Gynecol. Oncol. 2019, 153, 661–669. [Google Scholar] [CrossRef]
- Mitsuda, M.; Shiozaki, A.; Kudou, M.; Shimizu, H.; Arita, T.; Kosuga, T.; Konishi, H.; Komatsu, S.; Kubota, T.; Fujiwara, H.; et al. Functional Analysis and Clinical Significance of Chloride Channel 2 Expression in Esophageal Squamous Cell Carcinoma. Surg. Oncol. 2021, 28, 5384–5397. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Shang, L.; Jiang, Y.Y.; Hao, J.J.; Zhang, Y.; Zhang, T.T.; Lin, D.C.; Liu, S.G.; Wang, B.S.; Gong, T.; et al. Consistent and Differential Genetic Aberrations between Esophageal Dysplasia and Squamous Cell Carcinoma Detected by Array Comparative Genomic Hybridization. Clin. Cancer Res. 2013, 19, 5867–5878. [Google Scholar] [CrossRef]
- Ruiz, C.; Martins, J.R.; Rudin, F.; Schneider, S.; Dietsche, T.; Fischer, C.A.; Tornillo, L.; Terracciano, L.M.; Schreiber, R.; Bubendorf, L.; et al. Enhanced Expression of ANO1 in Head and Neck Squamous Cell Carcinoma Causes Cell Migration and Correlates with Poor Prognosis. PLoS ONE 2012, 7, e43265. [Google Scholar] [CrossRef]
- Godse, N.R.; Khan, N.; Yochum, Z.A.; Gomez-Casal, R.; Kemp, C.; Shiwarski, D.J.; Seethala, R.S.; Kulich, S.; Seshadri, M.; Burns, T.F.; et al. TMEM16A/ANO1 Inhibits Apoptosis via Downregulation of Bim Expression. Clin. Cancer Res. 2017, 23, 7324–7332. [Google Scholar] [CrossRef]
- Bill, A.; Gutierrez, A.; Kulkarni, S.; Kemp, C.; Bonenfant, D.; Voshol, H.; Duvvuri, U.; Gaither, L.A. ANO1/TMEM16A Interacts with EGFR and Correlates with Sensitivity to EGFR-Targeting Therapy in Head and Neck Cancer. Oncotarget 2015, 6, 9173–9188. [Google Scholar] [CrossRef]
- Ayoub, C.; Wasylyk, C.; Li, Y.; Thomas, E.; Marisa, L.; Robé, A.; Roux, M.; Abecassis, J.; De Reyniès, A.; Wasylyk, B. ANO1 Amplification and Expression in HNSCC with a High Propensity for Future Distant Metastasis and Its Functions in HNSCC Cell Lines. Br. J. Cancer 2010, 103, 715–726. [Google Scholar] [CrossRef]
- Siemer, S.; Fauth, T.; Scholz, P.; Al-zamel, Y.; Khamis, A.; Gül, D.; Freudelsperger, L.; Wollenberg, B.; Becker, S.; Stauber, R.H.; et al. Profiling Cisplatin Resistance in Head and Neck Cancer: A Critical Role of the Vrac Ion Channel for Chemoresistance. Cancers 2021, 13, 4831. [Google Scholar] [CrossRef]
- Wu, X.Y.; Yu, X.Y. Overexpression of KCNJ4 Correlates with Cancer Progression and Unfavorable Prognosis in Lung Adenocarcinoma. J. Biochem. Mol. Toxicol. 2019, 33, e22270. [Google Scholar] [CrossRef]
- Bulk, E.; Ay, A.S.; Hammadi, M.; Ouadid-Ahidouch, H.; Schelhaas, S.; Hascher, A.; Rohde, C.; Thoennissen, N.H.; Wiewrodt, R.; Schmidt, E.; et al. Epigenetic Dysregulation of KCa3.1 Channels Induces Poor Prognosis in Lung Cancer. Int. J. Cancer 2015, 137, 1306–1317. [Google Scholar] [CrossRef]
- Lyu, Y.; Wang, Q.; Liang, J.; Zhang, L.; Zhang, H. The Ion Channel Gene KCNAB2 Is Associated with Poor Prognosis and Loss of Immune Infiltration in Lung Adenocarcinoma. Cells 2022, 11, 3438. [Google Scholar] [CrossRef] [PubMed]
- Ashton, C.; Rhie, S.K.; Carmichael, J.D.; Zada, G. Role of KCNAB2 Expression in Modulating Hormone Secretion in Somatotroph Pituitary Adenoma. J. Neurosurg. 2021, 134, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, K.; Yang, J. Potassium Voltage-Gated Channel Subfamily D Member 2 Induces an Aggressive Phenotype in Lung Adenocarcinoma. Neoplasma 2021, 68, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Chimote, A.A.; Hajdu, P.; Sfyris, A.M.; Gleich, B.N.; Wise-Draper, T.; Casper, K.A.; Conforti, L. Kv1.3 Channels Mark Functionally Competent CD8+ Tumor-Infiltrating Lymphocytes in Head and Neck Cancer. Cancer Res. 2017, 77, 53–61. [Google Scholar] [CrossRef]
- Qian, C.; Dai, Y.; Xu, X.; Jiang, Y. HIF-1α Regulates Proliferation and Invasion of Oral Cancer Cells through Kv3.4 Channel. Ann. Clin. Lab. Sci. 2019, 49, 457–467. [Google Scholar]
- Lastraioli, E.; Lottini, T.; Iorio, J.; Freschi, G.; Fazi, M.; Duranti, C.; Carraresi, L.; Messerini, L.; Taddei, A.; Ringressi, M.N.; et al. HERG1 Behaves as Biomarker of Progression to Adenocarcinoma in Barrett’s Esophagus and Can Be Exploited for a Novel Endoscopic Surveillance. Oncotarget 2016, 7, 59535–59547. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, W.; Zhang, S.; Wang, X.; Tang, Z.; Gu, J.; Li, J.; Huang, J. CACNA1B (Cav2.2) Overexpression and Its Association with Clinicopathologic Characteristics and Unfavorable Prognosis in Non-Small Cell Lung Cancer. Dis. Mrk. 2017, 2017, 6136410. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xie, X.; Wen, J.; Luo, K.J.; Liu, Q.W.; Yang, H.; Hu, Y.; Fu, J.H. TRPV6 Plays a New Role in Predicting Survival of Patients with Esophageal Squamous Cell Carcinoma. Diagn. Pathol. 2016, 11, 14. [Google Scholar] [CrossRef]
- Schaefer, E.A.M.; Stohr, S.; Meister, M.; Aigner, A.; Gudermann, T.; Buech, T.R.H. Stimulation of the Chemosensory TRPA1 Cation Channel by Volatile Toxic Substances Promotes Cell Survival of Small Cell Lung Cancer Cells. Biochem. Pharm. 2013, 85, 426–438. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, X.; Lan, Y.; Liang, P.; Huang, Y.; Wang, Y.; Zhou, X.; Zhang, Z.; Liang, Y.; Xiao, X. Aberrant Inactivation of SCNN1G Promotes the Motility of Head and Neck Squamous Cell Carcinoma. Pathol. Res. Pract. 2022, 240, 154175. [Google Scholar] [CrossRef]
- Zhang, J.; Mao, W.; Dai, Y.; Qian, C.; Dong, Y.; Chen, Z.; Meng, L.; Jiang, Z.; Huang, T.; Hu, J.; et al. Voltage-Gated Sodium Channel Nav1.5 Promotes Proliferation, Migration and Invasion of Oral Squamous Cell Carcinoma. Acta Biochim. Biophys. Sin. 2019, 51, 562–570. [Google Scholar] [CrossRef]
- Campbell, T.M.; Main, M.J.; Fitzgerald, E.M. Functional Expression of the Voltage-Gated Na+- Channel Nav1.7 Is Necessary for EGF-Mediated Invasion in Human Non-Small Cell Lung Cancer Cells. J. Cell Sci. 2013, 126, 4939–4949. [Google Scholar] [CrossRef]
- Lu, A.; Shi, Y.; Liu, Y.; Lin, J.; Zhang, H.; Guo, Y.; Li, L.; Lin, Z.; Wu, J.; Ji, D.; et al. Integrative Analyses Identified Ion Channel Genes GJB2 and SCNN1B as Prognostic Biomarkers and Therapeutic Targets for Lung Adenocarcinoma. Lung Cancer 2021, 158, 29–39. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Zhao, Z.; Xiu, R.; Jia, J.; Chen, P.; Liu, Y.; Wang, Y.; Yi, J. Cepharanthine, a Novel Selective ANO1 Inhibitor with Potential for Lung Adenocarcinoma Therapy. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 119132. [Google Scholar] [CrossRef]
- Vyas, A.; Duvvuri, U.; Kiselyov, K. Copper-Dependent ATP7B up-Regulation Drives the Resistance of TMEM16A-Overexpressing Head-and-Neck Cancer Models to Platinum Toxicity. Biochem. J. 2019, 476, 3705–3719. [Google Scholar] [CrossRef]
- Vyas, A.; Gomez-Casal, R.; Cruz-Rangel, S.; Villanueva, H.; Sikora, A.G.; Rajagopalan, P.; Basu, D.; Pacheco, J.; Hammond, G.R.V.; Kiselyov, K.; et al. Lysosomal Inhibition Sensitizes TMEM16A-Expressing Cancer Cells to Chemotherapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2100670119. [Google Scholar] [CrossRef]
- Reddy, R.B.; Bhat, A.R.; James, B.L.; Govindan, S.V.; Mathew, R.; Ravindra, D.R.; Hedne, N.; Illiayaraja, J.; Kekatpure, V.; Khora, S.S.; et al. Meta-Analyses of Microarray Datasets Identifies ANO1 and FADD as Prognostic Markers of Head and Neck Cancer. PLoS ONE 2016, 11, e0147409. [Google Scholar] [CrossRef]
- Zhou, C.; Tang, X.; Xu, J.; Wang, J.; Yang, Y.; Chen, Y.; Chen, L.; Wang, L.; Zhu, L.; Yang, H. Opening of the CLC-3 Chloride Channel Induced by Dihydroartemisinin Contributed to Early Apoptotic Events in Human Poorly Differentiated Nasopharyngeal Carcinoma Cells. J. Cell. Biochem. 2018, 119, 9560–9572. [Google Scholar] [CrossRef]
- Fernández–Valle, Á.; Rodrigo, J.P.; Rodríguez–Santamarta, T.; Villaronga, M.Á.; Álvarez–Teijeiro, S.; García–Pedrero, J.M.; Suárez–Fernández, L.; Lequerica–Fernández, P.; de Vicente, J.C. HERG1 Potassium Channel Expression in Potentially Malignant Disorders of the Oral Mucosa and Prognostic Relevance in Oral Squamous Cell Carcinoma. Head Neck 2016, 38, 1672–1678. [Google Scholar] [CrossRef]
- Ariyoshi, W.; Okinaga, T.; Chaweewannakorn, W.; Akifusa, S.; Nisihara, T. Mechanisms Involved in Enhancement of Matrix Metalloproteinase-9 Expression in Macrophages by Interleukin-33. J. Cell. Physiol. 2017, 232, 3481–3495. [Google Scholar] [CrossRef]
- Pinto, M.C.; Schreiber, R.; Lerias, J.; Ousingsawat, J.; Duarte, A.; Amaral, M.; Kunzelmann, K. Regulation of TMEM16A by CK2 and Its Role in Cellular Proliferation. Cells 2020, 9, 1138. [Google Scholar] [CrossRef] [PubMed]
- Britschgi, A.; Bill, A.; Brinkhaus, H.; Rothwell, C.; Clay, I.; Duss, S.; Rebhan, M.; Raman, P.; Guy, C.T.; Wetzel, K.; et al. Calcium-Activated Chloride Channel ANO1 Promotes Breast Cancer Progression by Activating EGFR and CAMK Signaling. Proc. Natl. Acad Sci. USA 2013, 110, E1026–E1034. [Google Scholar] [CrossRef] [PubMed]
- Katsurahara, K.; Shiozaki, A.; Kosuga, T.; Kudou, M.; Shoda, K.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; Fujiwara, H.; et al. ANO9 Regulated Cell Cycle in Human Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2020, 27, 3218–3230. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, H.; Yang, X.; Liang, X.; Tan, Q.; Chen, Z.; Zhao, C.; Gu, Z.; Yu, M.; Zheng, Y.; et al. The Apoptotic Effect of Zoledronic Acid on the Nasopharyngeal Carcinoma Cells via ROS Mediated Chloride Channel Activation. Clin. Exp. Pharm. Physiol. 2018, 45, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, S.T.; Rodrigo, J.P.; Allonca, E.; García-Carracedo, D.; Álvarez-Alija, G.; Casado-Zapico, S.; Fresno, M.F.; Rodríguez, C.; Suárez, C.; García-Pedrero, J.M. Expression and Clinical Significance of the Kv3.4 Potassium Channel Subunit in the Development and Progression of Head and Neck Squamous Cell Carcinomas. J. Pathol. 2010, 221, 402–410. [Google Scholar] [CrossRef]
- D’adamo, M.C.; Liantonio, A.; Rolland, J.F.; Pessia, M.; Imbrici, P. Kv1.1 Channelopathies: Pathophysiological Mechanisms and Therapeutic Approaches. Int. J. Mol. Sci. 2020, 21, 2935. [Google Scholar] [CrossRef]
- Hanukoglu, I.; Hanukoglu, A. Epithelial Sodium Channel (ENaC) Family: Phylogeny, Structure-Function, Tissue Distribution, and Associated Inherited Diseases. Gene 2016, 579, 95–132. [Google Scholar] [CrossRef]
- Menéndez, S.T.; Rodrigo, J.P.; Álvarez-Teijeiro, S.; Villaronga, M.Á.; Allonca, E.; Vallina, A.; Astudillo, A.; Barros, F.; Suárez, C.; García-Pedrero, J.M. Role of HERG1 Potassium Channel in Both Malignant Transformation and Disease Progression in Head and Neck Carcinomas. Mod. Pathol. 2012, 25, 1069–1078. [Google Scholar] [CrossRef]
- Suzuki, T.; Takimoto, K. Selective Expression of HERG and Kv2 Channels Influences Proliferation of Uterine Cancer Cells. Int. J. Oncol. 2004, 25, 153–159. [Google Scholar] [CrossRef]
- Jeon, W.; Dong, P.; Yeong, S. Effects of Voltage-Gated K+ Channel Blockers in Gefitinib-Resistant H460 Non-Small Cell Lung Cancer Cells. Anticancer. Res. 2012, 32, 5279–5284. [Google Scholar]
- He, H.; Song, X.; Yang, Z.; Mao, Y.; Zhang, K.; Wang, Y.; Su, B.; Li, Q.; Chen, H.; Li, Y. Upregulation of KCNQ1OT1 Promotes Resistance to Stereotactic Body Radiotherapy in Lung Adenocarcinoma by Inducing ATG5/ATG12-Mediated Autophagy via MiR-372-3p. Cell Death Dis. 2020, 11, 883. [Google Scholar] [CrossRef]
- Qiao, C.; Qiao, Y.; Jin, H.; Zheng, M.; Wang, L. LncRNA KCNQ1OT1 Contributes to the Cisplatin Resistance of Tongue Cancer through the KCNQ1OT1/MiR-124-3p/TRIM14 Axis. Eur. Rev. Med. Pharm. Sci. 2020, 24, 200–212. [Google Scholar] [CrossRef]
- Ren, K.; Xu, R.; Huang, J.; Zhao, J.; Shi, W. Knockdown of Long Non-Coding RNA KCNQ1OT1 Depressed Chemoresistance to Paclitaxel in Lung Adenocarcinoma. Cancer Chemother. Pharm. 2017, 80, 243–250. [Google Scholar] [CrossRef]
- Ge, N.; Yang, G.; Zhang, Y.; Chang, N.; Kang, H.; Zhou, Q.; Fan, P. Upregulation of KCNMA1 Facilitates the Reversal Effect of Verapamil on the Chemoresistance to Cisplatin of Esophageal Squamous Cell Carcinoma Cells. Eur. Rev. Med. Pharm. Sci. 2021, 25, 1869–1880. [Google Scholar] [CrossRef]
- Gawali, V.S.; Chimote, A.A.; Newton, H.S.; Feria-Garzón, M.G.; Chirra, M.; Janssen, E.M.; Wise-Draper, T.M.; Conforti, L. Immune Checkpoint Inhibitors Regulate K+ Channel Activity in Cytotoxic T Lymphocytes of Head and Neck Cancer Patients. Front. Pharm. 2021, 12, 742862. [Google Scholar] [CrossRef]
- Newton, H.S.; Gawali, V.S.; Chimote, A.A.; Lehn, M.A.; Palackdharry, S.M.; Hinrichs, B.H.; Jandarov, R.; Hildeman, D.; Janssen, E.M.; Wise-Draper, T.M.; et al. PD1 Blockade Enhances K+ Channel Activity, Ca2+ Signaling, and Migratory Ability in Cytotoxic T Lymphocytes of Patients with Head and Neck Cancer. J. Immunother. Cancer 2020, 8, e000844. [Google Scholar] [CrossRef]
- Bukhari, M.; Deng, H.; Sipes, D.; Ruane-Foster, M.; Purdy, K.; Woodworth, C.D.; Sur, S.; Samways, D.S.K. KCa3.1-Dependent Uptake of the Cytotoxic DNA-Binding Dye Hoechst 33258 into Cancerous but Not Healthy Cervical Cells. J. Biol. Chem. 2021, 296, 100084. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.; Peng, J.; Wu, X.; Zhang, Y.; Zhu, W.; Guo, L. Upregulation of the Inwardly Rectifying Potassium Channel Kir2.1 (KCNJ2) Modulates Multidrug Resistance of Small-Cell Lung Cancer under the Regulation of MiR-7 and the Ras/MAPK Pathway. Mol. Cancer 2015, 14, 59. [Google Scholar] [CrossRef]
- Leithner, K.; Hirschmugl, B.; Li, Y.; Tang, B.; Papp, R.; Nagaraj, C.; Stacher, E.; Stiegler, P.; Lindenmann, J.; Olschewski, A.; et al. TASK-1 Regulates Apoptosis and Proliferation in a Subset of Non-Small Cell Lung Cancers. PLoS ONE 2016, 11, e0157453. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, C.L.; Nie, C.J.; Li, J.C.; Zeng, T.-T.; Zhou, J.; Chen, J.; Chen, K.; Fu, L.; Liu, H.; et al. Investigation of Tumor Suppressing Function of CACNA2D3 in Esophageal Squamous Cell Carcinoma. PLoS ONE 2013, 8, e60027. [Google Scholar] [CrossRef]
- Bernaldo De Quirós, S.; Merlo, A.; Secades, P.; Zambrano, I.; Saenz, I.; María, S.; Ugidos, N.; Jantus-Lewintre, E.; Sirera, R.; Suarez, C.; et al. Identification of TRPC6 as a Possible Candidate Target Gene within an Amplicon at 11q21-Q22.2 for Migratory Capacity in Head and Neck Squamous Cell Carcinomas. BMC Cancer 2013, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, C.B.; Kirma, N.B.; De La Chapa, J.J.; Chen, R.; Henry, M.A.; Luo, S.; Hargreaves, K.M. Vanilloids Induce Oral Cancer Apoptosis Independent of TRPV1. Oral Oncol. 2014, 50, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Kudou, M.; Shiozaki, A.; Yamazato, Y.; Katsurahara, K.; Kosuga, T.; Shoda, K.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; et al. The Expression and Role of TRPV2 in Esophageal Squamous Cell Carcinoma. Sci. Rep. 2019, 9, 16055. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Fan, K.; Li, B.; Li, H.; Qi, H.; Guo, J.; Cao, Y.; Sun, H. Overexpression of TRPV3 Correlates with Tumor Progression in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2016, 17, 437. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Xu, W.L.; Xu, Z.Q.; Qi, C.; Li, Y.; Cheng, J.; Liu, L.K.; Wu, Y.N.; Gao, J.; Ye, J.H. The Overexpressed Functional Transient Receptor Potential Channel TRPM2 in Oral Squamous Cell Carcinoma. Sci. Rep. 2016, 6, 38471. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, Y.; Huang, M.; Shen, B.; Xue, H.; Wu, K. Effect of TRPM2-Mediated Calcium Signaling on Cell Proliferation and Apoptosis in Esophageal Squamous Cell Carcinoma. Technol. Cancer Res. Treat. 2021, 20, 15330338211045213. [Google Scholar] [CrossRef]
- Liu, K.; Xu, S.H.; Chen, Z.; Zeng, Q.X.; Li, Z.J.; Chen, Z.M. TRPM7 Overexpression Enhances the Cancer Stem Cell-like and Metastatic Phenotypes of Lung Cancer through Modulation of the Hsp90α/UPA/MMP2 Signaling Pathway. BMC Cancer 2018, 18, 1167. [Google Scholar] [CrossRef]
- Li, K.; Chen, L.; Lin, Z.; Zhu, J.; Fang, Y.; Du, J.; Shen, B.; Wu, K.; Liu, Y. Role of the AMPK/ACC Signaling Pathway in TRPP2-Mediated Head and Neck Cancer Cell Proliferation. Biomed Res. Int. 2020, 2020, 4375075. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Zhu, X.; Chen, W.; Wang, H.; Jiang, Y. Preliminary Study on the Role of Voltage-Gated Sodium Channel Subtype Nav1.5 in Lymph Node Metastasis of Oral Squamous Cell Carcinoma. Chin. J. Stomatol. 2017, 52, 188–193. [Google Scholar]
- Xu, X.; Dai, Y.; Feng, L.; Zhang, H.; Hu, Y.; Xu, L.; Zhu, X.; Jiang, Y. Knockdown of Nav1.5 Inhibits Cell Proliferation, Migration and Invasion via Wnt/β-Catenin Signaling Pathway in Oral Squamous Cell Carcinoma. Acta Biochim. Biophys. Sin. 2020, 52, 527–535. [Google Scholar] [CrossRef]
- Driffort, V.; Gillet, L.; Bon, E.; Marionneau-Lambot, S.; Oullier, T.; Joulin, V.; Collin, C.; Pagès, J.C.; Jourdan, M.L.; Chevalier, S.; et al. Ranolazine Inhibits NaV1.5-Mediated Breast Cancer Cell Invasiveness and Lung Colonization. Mol. Cancer 2014, 13, 264. [Google Scholar] [CrossRef]
- Lopez-Charcas, O.; Espinosa, A.M.; Alfaro, A.; Herrera-Carrillo, Z.; Ramirez-Cordero, B.E.; Cortes-Reynosa, P.; Perez Salazar, E.; Berumen, J.; Gomora, J.C. The Invasiveness of Human Cervical Cancer Associated to the Function of NaV1.6 Channels Is Mediated by MMP-2 Activity. Sci. Rep. 2018, 8, 12955. [Google Scholar] [CrossRef]
- Ware, A.W.; Harris, J.J.; Slatter, T.L.; Cunliffe, H.E.; McDonald, F.J. The Epithelial Sodium Channel Has a Role in Breast Cancer Cell Proliferation. Breast Cancer Res. Treat. 2021, 187, 31–43. [Google Scholar] [CrossRef]
- Jia, L.; Liu, W.; Guan, L.; Lu, M.; Wang, K.W. Inhibition of Calcium-Activated Chloride Channel ANO1/TMEM16A Suppresses Tumor Growth and Invasion in Human Lung Cancer. PLoS ONE 2015, 10, e0136584. [Google Scholar] [CrossRef]
- Lee, J.R.; Lee, J.Y.; Kim, H.J.; Hahn, M.J.; Kang, J.S.; Cho, H. The Inhibition of Chloride Intracellular Channel 1 Enhances Ca2+ and Reactive Oxygen Species Signaling in A549 Human Lung Cancer Cells. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Shi, S.; Bai, X.; Ji, Q.; Wan, H.; An, H.; Kang, X.; Guo, S. Molecular Mechanism of Ion Channel Protein TMEM16A Regulated by Natural Product of Narirutin for Lung Cancer Adjuvant Treatment. Int. J. Biol. Macromol. 2022, 223, 1145–1157. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Xu, Y.; Guo, W.; Yu, H.; Zhang, L.; Wang, Y.; Chen, X. Acetylation-Stabilized Chloride Intracellular Channel 1 Exerts a Tumor-Promoting Effect on Cervical Cancer Cells by Activating NF-ΚB. Cell. Oncol. 2021, 44, 557–568. [Google Scholar] [CrossRef]
- Kakinouchi, K.; Yoshie, S.; Tsuji, S.; Murono, S.; Hazama, A. Dysfunction of Cl− Channels Promotes Epithelial to Mesenchymal Transition in Oral Squamous Cell Carcinoma via Activation of Wnt/β-Catenin Signaling Pathway. Biochem. Biophys. Res. Commun. 2021, 555, 95–101. [Google Scholar] [CrossRef]
- Kulkarni, S.; Bill, A.; Godse, N.R.; Khan, N.I.; Kass, J.I.; Steehler, K.; Kemp, C.; Davis, K.; Bertrand, C.A.; Vyas, A.R.; et al. TMEM16A/ANO1 Suppression Improves Response to Antibody-Mediated Targeted Therapy of EGFR and HER2/ERBB2. Genes Chromosom. Cancer 2017, 56, 460–471. [Google Scholar] [CrossRef]
- Wong, A.M.G.; Kong, K.L.; Chen, L.; Liu, M.; Wong, A.M.G.; Zhu, C.; Tsang, J.W.H.; Guan, X.Y. Characterization of CACNA2D3 as a Putative Tumor Suppressor Gene in the Development and Progression of Nasopharyngeal Carcinoma. Int. J. Cancer 2013, 133, 2284–2295. [Google Scholar] [CrossRef]
- Lima, F.J.; de Souza Lopes, M.L.D.; da Silva Barros, C.C.; Nonaka, C.F.W.; da Silveira, É.J.D. Modification in CLIC4 Expression Is Associated with P53, TGF-β, TNF-α and Myofibroblasts in Lip Carcinogenesis. Braz. Dent. J. 2020, 31, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Matsuda, M.; Kato, K.; Jimi, E.; Takeuchi, H.; Nakano, S.; Kajioka, S.; Matsuzaki, E.; Hirofuji, T.; Inoue, R.; et al. Volume-Regulated Chloride Channel Regulates Cell Proliferation and Is Involved in the Possible Interaction between TMEM16A and LRRC8A in Human Metastatic Oral Squamous Cell Carcinoma Cells. Eur. J. Pharm. 2021, 895, 173881. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, L.; Lin, J.; Liu, S.; Luo, H.; Mao, J.; Nie, S.; Chen, L.; Wang, L. Cisplatin Activates Volume-Sensitive Like Chloride Channels Via Purinergic Receptor Pathways in Nasopharyngeal Carcinoma Cells. J. Membr. Biol. 2015, 248, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Chang, R.; Shen, J.; Wang, Y.; Song, H.; Kang, X.; Zhao, Y.; Guo, S.; Qin, J. Self-Healing Pectin/Cellulose Hydrogel Loaded with Limonin as TMEM16A Inhibitor for Lung Adenocarcinoma Treatment. Int. J. Biol. Macromol. 2022, 219, 754–766. [Google Scholar] [CrossRef]
- Shi, S.; Ma, B.; Sun, F.; Qu, C.; Li, G.; Shi, D.; Liu, W.; Zhang, H.; Hailong, A. Zafirlukast Inhibits the Growth of Lung Adenocarcinoma via Inhibiting TMEM16A Channel Activity. J. Biol. Chem. 2022, 298, 101731. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Y.; Shi, S.; Wang, X.; Zhang, H.; Zhan, Y.; An, H. Arctigenin, a Novel TMEM16A Inhibitor for Lung Adenocarcinoma Therapy. Pharm. Res. 2020, 155, 104721. [Google Scholar] [CrossRef]
- Guo, S.; Bai, X.; Shi, S.; Deng, Y.; Kang, X.; An, H. TMEM16A, a Homoharringtonine Receptor, as a Potential Endogenic Target for Lung Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 10930. [Google Scholar] [CrossRef]
- Guo, S.; Chen, Y.; Pang, C.; Wang, X.; Shi, S.; Zhang, H.; An, H.; Zhan, Y. Matrine Is a Novel Inhibitor of the TMEM16A Chloride Channel with Antilung Adenocarcinoma Effects. J. Cell. Physiol. 2019, 234, 8698–8708. [Google Scholar] [CrossRef]
- Jeong, S.B.; Das, R.; Kim, D.H.; Lee, S.; Oh, H.I.; Jo, S.; Lee, Y.; Kim, J.; Park, S.J.; Choi, D.K.; et al. Anticancer Effect of Verteporfin on Non-Small Cell Lung Cancer via Downregulation of ANO1. Biomed. Pharmacother. 2022, 153, 113373. [Google Scholar] [CrossRef]
- Duvvuri, U.; Shiwarski, D.J.; Xiao, D.; Bertrand, C.; Huang, X.; Edinger, R.S.; Rock, J.R.; Harfe, B.D.; Henson, B.J.; Kunzelmann, K.; et al. TMEM16A Induces MAPK and Contributes Directly to Tumorigenesis and Cancer Progression. Cancer Res. 2012, 72, 3270–3281. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, J.; Wu, W.; Zhu, Q.; Tang, Y.; Zhu, C.; Dai, J.; Li, Z.; Wang, J.; Xue, L.; et al. Genome-Wide Copy Number Variation Analysis Identified ANO1 as a Novel Oncogene and Prognostic Biomarker in Esophageal Squamous Cell Cancer. Carcinogenesis 2019, 40, 1198–1208. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Zhang, Z.; Fan, Y.; Zhang, L.; Gao, K.; Luo, S.; Xiao, Q.; Sun, C. Simvastatin Inhibits Oral Squamous Cell Carcinoma by Targeting TMEM16A Ca2+-Activated Chloride Channel. J. Cancer Res. Clin. Oncol. 2021, 147, 1699–1711. [Google Scholar] [CrossRef]
- Glassmeier, G.; Hempel, K.; Wulfsen, I.; Bauer, C.K.; Schumacher, U.; Schwarz, J.R. Inhibition of HERG1 K + Channel Protein Expression Decreases Cell Proliferation of Human Small Cell Lung Cancer Cells. Pflug. Arch. 2012, 463, 365–376. [Google Scholar] [CrossRef]
- Rawluk, J.; Waller, C.F. Gefitinib. In Recent Results in Cancer Research; Springer: New York, NY, USA, 2018; Volume 211, pp. 235–246. [Google Scholar] [CrossRef]
- Chang, K.T.; Wu, H.J.; Liu, C.W.; Li, C.Y.; Lin, H.Y. A Novel Role of Arrhythmia-Related Gene KCNQ1 Revealed by Multi-Omic Analysis: Theragnostic Value and Potential Mechanisms in Lung Adenocarcinoma. Int. J. Mol. Sci. 2022, 23, 2279. [Google Scholar] [CrossRef]
- Xu, H.; Miao, J.; Liu, S.; Liu, H.; Zhang, L.; Zhang, Q. Long Non-Coding RNA KCNQ1 Overlapping Transcript 1 Promotes the Progression of Esophageal Squamous Cell Carcinoma by Adsorbing MicroRNA-133b. Clinics 2021, 76, e2175. [Google Scholar] [CrossRef]
- Vergara, C.; Latorre, R.; Marrion, N.V.; Adelmant, J.P. Calcium-Activated Potassium Channels. Curr. Opin. Neurobiol. 1998, 8, 321–329. [Google Scholar] [CrossRef]
- Simpson, W. The Calcium Channel Blocker Verapamil and Cancer Chemotherapy. Cell Calcium 1985, 6, 449–467. [Google Scholar] [CrossRef]
- Todesca, L.M.; Maskri, S.; Brömmel, K.; Thale, I.; Wünsch, B.; Koch, O.; Schwab, A. Targeting Kca3.1 Channels in Cancer. Cell. Physiol. Biochem. 2021, 22, 131–144. [Google Scholar] [CrossRef]
- De Felice, F.; Giudice, E.; Bolomini, G.; Distefano, M.G.; Scambia, G.; Fagotti, A.; Marchetti, C. Pembrolizumab for Advanced Cervical Cancer: Safety and Efficacy. Expert Rev. Anticancer. Ther. 2021, 21, 221–228. [Google Scholar] [CrossRef]
- Xu, P.; Mo, X.; Xia, R.; Jiang, L.; Zhang, C.; Xu, H.; Sun, Q.; Zhou, G.; Zhang, Y.; Wang, Y.; et al. KCNN4 Promotes the Progression of Lung Adenocarcinoma by Activating the AKT and ERK Signaling Pathways. Cancer Biomark. 2021, 31, 187–201. [Google Scholar] [CrossRef]
- Glaser, F.; Hundehege, P.; Bulk, E.; Todesca, L.M.; Schimmelpfennig, S.; Nass, E.; Budde, T.; Meuth, S.G.; Schwab, A. KCa Channel Blockers Increase Effectiveness of the EGF Receptor TK Inhibitor Erlotinib in Non-Small Cell Lung Cancer Cells (A549). Sci. Rep. 2021, 11, 18330. [Google Scholar] [CrossRef] [PubMed]
- Reimann, F.; Ashcroft, F.M. Inwardly Rectifying Potassium Channels. Curr. Opin. Cell Biol. 1999, 11, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Inanobe, A.; Furutani, K.; Murakami, S.; Findlay, I.; Kurachi, Y. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Am. Physiol. Soc. 2010, 90, 291–366. [Google Scholar] [CrossRef]
- Rodrigo, G.C.; Standen, N.B. ATP-Sensitive Potassium Channels. Curr. Pharm. Des. 2005, 11, 1915–1940. [Google Scholar] [CrossRef] [PubMed]
- Pergakis, M.; Badjatia, N.; Chaturvedi, S.; Cronin, C.A.; Kimberly, W.T.; Sheth, K.N.; Simard, J.M. BIIB093 (IV Glibenclamide): An Investigational Compound for the Prevention and Treatment of Severe Cerebral Edema. Expert Opin. Investig. Drugs 2019, 28, 1031–1040. [Google Scholar] [CrossRef]
- Kamuene, J.M.; Xu, Y.; Plant, L.D. The Pharmacology of Two-Pore Domain Potassium Channels. In Handbook of Experimental Pharmacology; Springer Science and Business Media Deutschland GmbH: Argovia, Switzerland, 2021; Volume 267, pp. 417–443. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Lewis, R.J. Sodium Channels and Pain: From Toxins to Therapies. Br. J. Pharm. 2018, 175, 2138–2157. [Google Scholar] [CrossRef]
- Chen-Izu, Y.; Shaw, R.M.; Pitt, G.S.; Yarov-Yarovoy, V.; Sack, J.T.; Abriel, H.; Aldrich, R.W.; Belardinelli, L.; Cannell, M.B.; Catterall, W.A.; et al. Na+ Channel Function, Regulation, Structure, Trafficking and Sequestration. J. Physiol. 2015, 593, 1347–1360. [Google Scholar] [CrossRef]
- Hernandez, C.M.; Richards, J.R. Physiology, Sodium Channels, StatPearls. In Treasure Island; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lopez-Charcas, O.; Pukkanasut, P.; Velu, S.E.; Brackenbury, W.J.; Hales, T.G.; Besson, P.; Gomora, J.C.; Bastien Roger, S. Pharmacological and Nutritional Targeting of Voltage-Gated Sodium Channels in the Treatment of Cancers. Science 2021, 24, 102270. [Google Scholar] [CrossRef]
- Bertil, H. Ion Channels of Excitable Membranes, 3rd ed.; Sinauer Associates, INC: Washington, WA, USA, 2001. [Google Scholar]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XLVII. Nomenclature and Structure-Function Relationships of Voltage-Gated Sodium Channels. Pharm. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef]
- Catterall, W.A. From Ionic Currents to Molecular Mechanisms. Neuron 2000, 26, 13–25. [Google Scholar] [CrossRef]
- Yu, F.H.; Catterall, W.A. Overview of the Voltage-Gated Sodium Channel Family. Genome Biol. 2003, 4, 207. [Google Scholar] [CrossRef]
- Brackenbury, W.J. Voltage-Gated Sodium Channels and Metastatic Disease. Channels 2012, 6, 352–361. [Google Scholar] [CrossRef]
- Roger, S.; Potier, M.; Vandier, C.; Besson, P.; Le Guennec, J.-Y. Voltage-Gated Sodium Channels: New Targets in Cancer Therapy? Curr. Pharm. Des. 2006, 12, 3681–3695. [Google Scholar] [CrossRef]
- Hanukoglu, I.; Boggula, V.R.; Vaknine, H.; Sharma, S.; Kleyman, T.; Hanukoglu, A. Expression of Epithelial Sodium Channel (ENaC) and CFTR in the Human Epidermis and Epidermal Appendages. Histochem. Cell Biol. 2017, 147, 733–748. [Google Scholar] [CrossRef]
- Galizia, L.; Ojea, A.; Kotsias, B.A. Regulación Por Proteasas Del Canal de Sodio Sensible al Amiloride (ENaC). Medicina 2011, 71, 179–182. [Google Scholar]
- Liu, C.; Zhu, L.L.; Xu, S.G.; Ji, H.L.; Li, X.M. ENaC/DEG in Tumor Development and Progression. J. Cancer 2016, 7, 1888–1891. [Google Scholar] [CrossRef]
- He, M.; Liu, S.; Gallolu Kankanamalage, S.; Borromeo, M.D.; Girard, L.; Gazdar, A.F.; Minna, J.D.; Johnson, J.E.; Cobb, M.H. The Epithelial Sodium Channel (AENaC) Is a Downstream Therapeutic Target of ASCL1 in Pulmonary Neuroendocrine Tumors. Transl. Oncol. 2018, 11, 292–299. [Google Scholar] [CrossRef]
- Amara, S.; Ivy, M.T.; Myles, E.L.; Tiriveedhi, V. Sodium Channel ΓENaC Mediates IL-17 Synergized High Salt Induced Inflammatory Stress in Breast Cancer Cells. Cell Immunol 2016, 302, 1–10. [Google Scholar] [CrossRef]
- Gururaja Rao, S.; Ponnalagu, D.; Patel, N.J.; Singh, H. Three Decades of Chloride Intracellular Channel Proteins: From Organelle to Organ Physiology. Curr. Protoc. Pharm. 2018, 80, 11.21.1–11.21.17. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Stein, V.; Weinreich, F.; Zdebik, A.A. Molecular Structure and Physiological Function of Chloride Channels. Physiol. Rev. 2002, 82, 503–568. [Google Scholar] [CrossRef]
- Jentsch, T.J.; Günther, W. Chloride Channels: An Emerging Molecular Picture. BioEssays 1997, 19, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Poroca, D.R.; Pelis, R.M.; Chappe, V.M. ClC Channels and Transporters: Structure, Physiological Functions, and Implications in Human Chloride Channelopathies. Front. Pharmacol. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Planells-Cases, R.; Jentsch, T.J. Chloride Channelopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 173–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, D.; Li, Y.; Chen, W.; Ruan, Z.; Deng, L.; Wang, L.; Tian, H.; Yiu, A.; Fan, C.; et al. Discovery of Bufadienolides as a Novel Class of ClC-3 Chloride Channel Activators with Antitumor Activities. J. Med. Chem. 2013, 56, 5734–5743. [Google Scholar] [CrossRef]
- Ye, D.; Luo, H.; Lai, Z.; Zou, L.; Zhu, L.; Mao, J.; Jacob, T.; Ye, W.; Wang, L.; Chen, L. ClC-3 Chloride Channel Proteins Regulate the Cell Cycle by Up-Regulating Cyclin D1-CDK4/6 through Suppressing P21/P27 Expression in Nasopharyngeal Carcinoma Cells. Sci. Rep. 2016, 6, 30276. [Google Scholar] [CrossRef]
- Wanitchakool, P.; Ousingsawat, J.; Sirianant, L.; MacAulay, N.; Schreiber, R.; Kunzelmann, K. Cl− Channels in Apoptosis. Eur. Biophys. J. 2016, 45, 599–610. [Google Scholar] [CrossRef]
- Xu, B.; Mao, J.; Wang, L.; Zhu, L.; Li, H.; Wang, W.; Jin, X.; Zhu, J.; Chen, L. ClC-3 Chloride Channels Are Essential for Cell Proliferation and Cell Cycle Progression in Nasopharyngeal Carcinoma Cells. Acta Biochim. Biophys. Sin. 2010, 42, 370–380. [Google Scholar] [CrossRef]
- Hong, S.; Bi, M.; Wang, L.; Kang, Z.; Ling, L.; Zhao, C. CLC-3 Channels in Cancer (Review). Oncol. Rep. 2015, 33, 507–514. [Google Scholar] [CrossRef]
- Dutzler, R. The ClC Family of Chloride Channels and Transporters. Curr. Opin. Struct. Biol. 2006, 16, 439–446. [Google Scholar] [CrossRef]
- Hartzell, C.; Putzier, I.; Arreola, J. Calcium-Activated Chloride Channels. Annu. Rev. Physiol. 2005, 67, 719–758. [Google Scholar] [CrossRef]
- Eggermont, J. Calcium-Activated Chloride Channels: (Un)Known, (Un)Loved? Proc. Am. Thorac. Soc. 2004, 1, 22–27. [Google Scholar] [CrossRef]
- Grigoriev, V. Calcium-Activated Chloride Channels: Structure, Properties, Role in Physiological and Pathological Processes. Biomeditsinskaya Khimiya 2021, 67, 17–33. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Hou, F.; Zhang, C.; Gao, J.; Wang, K.W. Inhibition of Ca2+ -Activated Chloride Channel ANO1 Suppresses Ovarian Cancer through Inactivating PI3K/Akt Signaling. Int. J. Cancer 2019, 144, 2215–2226. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Gao, J.; Cui, X.; Yang, S.; Liu, Z. Prognostic Significance of ANO1 Expression in Cancers. Medicine 2021, 100, e24525. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, L.; Li, N. ANO1: More Than Just Calcium-Activated Chloride Channel in Cancer. Front. Oncol. 2022, 12, 922838. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, R.; Jiang, D. TMEM16A as a Potential Biomarker in the Diagnosis and Prognosis of Lung Cancer. Iran. Med. 2019, 22, 32–38. [Google Scholar]
- He, Y.; Li, H.; Chen, Y.; Li, P.; Gao, L.; Zheng, Y.; Sun, Y.; Chen, J.; Qian, X. Expression of Anoctamin 1 Is Associated with Advanced Tumor Stage in Patients with Non-Small Cell Lung Cancer and Predicts Recurrence after Surgery. Clin. Transl. Oncol. 2017, 19, 1091–1098. [Google Scholar] [CrossRef]
- Chatterjee, S.; Browning, E.A.; Hong, N.; Debolt, K.; Sorokina, E.M.; Liu, W.; Birnbaum, M.J.; Fisher, A.B. Membrane Depolarization Is the Trigger for PI3K/Akt Activation and Leads to the Generation of ROS. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, 105–114. [Google Scholar] [CrossRef]
- Zhang, G.; Zhu, L.; Xue, Y.; Zhao, Z.; Li, H.; Niu, Z.; Wang, X.; Chen, P.; Zhang, J.; Zhang, X. Benzophenanthridine Alkaloids Suppress Lung Adenocarcinoma by Blocking TMEM16A Ca2+-Activated Cl− Channels. Pflug. Arch. 2020, 472, 1457–1467. [Google Scholar] [CrossRef]
- Shi, S.; Ma, B.; Sun, F.; Qu, C.; An, H. Theaflavin Binds to a Druggable Pocket of TMEM16A Channel and Inhibits Lung Adenocarcinoma Cell Viability. J. Biol. Chem. 2021, 297, 101016. [Google Scholar] [CrossRef]
- Lang, F.; Busch, G.L.; Ritter, M.; Vo¨lkl, H.; Vo¨lkl, V.; Waldegger, S.; Gulbins, E.; Ha¨ussinger, D.; Ha¨ussinger, H. Functional Significance of Cell Volume Regulatory Mechanisms. Physiol Rev. 1998, 78, 247–306. [Google Scholar] [CrossRef] [PubMed]
- Sardini, A.; Amey, J.S.; Weylandt, K.H.; Nobles, M.; Valverde, M.A.; Higgins, C.F. Cell Volume Regulation and Swelling-Activated Chloride Channels. Biochim. Biophys. Acta Biomembr. 2003, 1618, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.; Trouet, D.; Carton, I.; Nilius, B. Cellular Function and Control of Volume-Regulated Anion Channels. Cell Biochem. Biophys. 2001, 35, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.D.; Labron, M.W.; Boehnke, S.E.; Carnduff, L.; Kirov, S.A. VRACs CARVe a Path for Novel Mechanisms of Communication in the CNS. Sci. Signal. 2007, 17, 787–802. [Google Scholar] [CrossRef]
- Poulsen, K.A.; Andersen, E.C.; Hansen, C.F.; Klausen, T.K.; Hougaard, C.; Lambert, I.H.; Hoffmann, E.K. Deregulation of Apoptotic Volume Decrease and Ionic Movements in Multidrug-Resistant Tumor Cells: Role of Chloride Channels. Am. J. Physiol. Cell Physiol. 2010, 298, 14–25. [Google Scholar] [CrossRef]
- Lang, F.; Hoffmann, E.K. Role of Ion Transport in Control of Apoptotic Cell Death. Compr. Physiol. 2012, 2, 2037–2061. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambe, I.H. Ion Channels and Transporters in the Development of Drug Resistance in Cancer Cells. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130169. [Google Scholar] [CrossRef]
- Lee, E.L.; Shimizu, T.; Ise, T.; Numata, T.; Kohno, K.; Okada, Y. Impaired Activity of Volume-Sensitive Cl- Channel Is Involved in Cisplatin Resistance of Cancer Cells. J. Cell. Physiol. 2007, 211, 513–521. [Google Scholar] [CrossRef]
- Kanno, Y.; Chen, C.Y.; Lee, H.L.; Chiou, J.F.; Chen, Y.J. Molecular Mechanisms of Chemotherapy Resistance in Head and Neck Cancers. Front. Oncol. 2021, 11, 640392. [Google Scholar] [CrossRef]
- Liu, T.; Stauber, T. The Volume-Regulated Anion Channel Lrrc8/Vrac Is Dispensable for Cell Proliferation and Migration. Int. J. Mol. Sci. 2019, 20, 2663. [Google Scholar] [CrossRef]
- Gururaja Rao, S.; Patel, N.J.; Singh, H. Intracellular Chloride Channels: Novel Biomarkers in Diseases. Front. Physiol. 2020, 11, 96. [Google Scholar] [CrossRef]
- Peretti, M.; Angelini, M.; Savalli, N.; Florio, T.; Yuspa, S.H.; Mazzanti, M. Chloride Channels in Cancer: Focus on Chloride Intracellular Channel 1 and 4 (CLIC1 AND CLIC4) Proteins in Tumor Development and as Novel Therapeutic Targets. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2523–2531. [Google Scholar] [CrossRef]
- Edwards, J.C.; Kahl, C.R. Chloride Channels of Intracellular Membranes. FEBS Lett. 2010, 584, 2102–2111. [Google Scholar] [CrossRef]
- Suh, K.S.; Yuspa, S.H. Intracellular Chloride Channels: Critical Mediators of Cell Viability and Potential Targets for Cancer Therapy. Curr. Pharm. Des. 2005, 11, 2753. [Google Scholar] [CrossRef]
- Huang, J.J.; Lin, J.; Chen, X.; Zhu, W. Identification of Chloride Intracellular Channels as Prognostic Factors Correlated with Immune Infiltration in Hepatocellular Carcinoma Using Bioinformatics Analysis. Medicine 2021, 100, e27739. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated Copper and Oxidative Stress in Cancer Cells as a Target for Cancer Treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef]
- Fernández-Salas, E.; Suh, K.S.; Speransky, V.V.; Bowers, W.L.; Levy, J.M.; Adams, T.; Pathak, K.R.; Edwards, L.E.; Hayes, D.D.; Cheng, C.; et al. MtCLIC/CLIC4, an Organellular Chloride Channel Protein, Is Increased by DNA Damage and Participates in the Apoptotic Response to P53. Mol. Cell. Biol. 2002, 22, 3610–3620. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, W.; Huang, R.; Chen, D.; Li, Z.; Qi, X.; Sun, L.; Lin, L.; Zhang, Z. Comprehensive Analysis of Clinical Prognosis and CLIC1 Immune Invasion in Lung Adenocarcinoma. Medicine 2022, 101, e30760. [Google Scholar] [CrossRef]
- Dhanasekaran, D.N.; Reddy, E.P. JNK Signaling in Apoptosis. Oncogene 2008, 27, 6245–6251. [Google Scholar] [CrossRef]
- Yasuda, Y.; Nagano, T.; Jimbo, N.; Kiriu, T.; Suraya, R.; Hazama, D.; Yamamoto, M.; Maniwa, Y.; Nishimura, Y.; Kobayashi, K. Chloride Intracellular Channel 1 Expression Is Associated with Poor Prognosis of Lung Adenocarcinoma. Anticancer. Res. 2022, 42, 271–277. [Google Scholar] [CrossRef]
- Suh, K.S.; Crutchley, J.M.; Koochek, A.; Ryscavage, A.; Bhat, K.; Tanaka, T.; Oshima, A.; Fitzgerald, P.; Yuspa, S.H. Reciprocal Modifications of CLIC4 in Tumor Epithelium and Stroma Mark Malignant Progression of Multiple Human Cancers. Clin. Cancer Res. 2007, 13, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Yang, Y.; Madanikia, S.; Ho, Y.; Li, M.; Sanchez, V.; Cataisson, C.; Huang, J.; Yuspa, S.H. Elevating CLIC4 in Multiple Cell Types Reveals a TGF- Dependent Induction of a Dominant Negative Smad7 Splice Variant. PLoS ONE 2016, 11, e0161410. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Lu, J.; Yuan, R.; Liu, J.; Liu, Y.; Wu, K.; Wu, J.; Du, J.; Shen, B. Knockdown of CLIC4 Enhances ATP-Induced HN4 Cell Apoptosis through Mitochondrial and Endoplasmic Reticulum Pathways. Cell Biosci. 2016, 6, 5. [Google Scholar] [CrossRef]
- Okudela, K.; Katayama, A.; Woo, T.; Mitsui, H.; Suzuki, T.; Tateishi, Y.; Umeda, S.; Tajiri, M.; Masuda, M.; Nagahara, N.; et al. Proteome Analysis for Downstream Targets of Oncogenic KRAS—The Potential Participation of CLIC4 in Carcinogenesis in the Lung. PLoS ONE 2014, 9, e87193. [Google Scholar] [CrossRef] [PubMed]
- Kondratskyi, A.; Yassine, M.; Kondratska, K.; Skryma, R.; Slomianny, C.; Prevarskaya, N. Calcium-Permeable Ion Channels in Control of Autophagy and Cancer. Front. Physiol. 2013, 4, 272. [Google Scholar] [CrossRef]
- Antal, L.; Martin-Caraballo, M. T-Type Calcium Channels in Cancer. Cancers 2019, 11, 134. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Z.; Zhang, K.; Dong, Y.; Zhang, A.; Lu, C.; Liu, L. MicroRNA-107 Inhibits Proliferation and Invasion of Laryngeal Squamous Cell Carcinoma Cells by Targeting CACNA2D1 in Vitro. Anticancer. Drugs 2020, 31, 260–271. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Zhao, W.; Duan, J.; Wang, Z.; Chen, H.; Tian, Y.; Wang, D.; Zhao, J.; An, T.; et al. Mechanistic Exploration of Cancer Stem Cell Marker Voltage-Dependent Calcium Channel A2d1 Subunit-Mediated Chemotherapy Resistance in Small-Cell Lung Cancer. Clin. Cancer Res. 2018, 24, 2148–2158. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, X.; Zhao, W.; Yang, Y.; Zhang, Z. Calcium Channel A2δ1 Subunit Is a Functional Marker and Therapeutic Target for Tumor-Initiating Cells in Non-Small Cell Lung Cancer. Cell Death Dis. 2021, 12, 257. [Google Scholar] [CrossRef]
- Gao, S.; Yao, X.; Yan, N. Structure of Human Cav2.2 Channel Blocked by the Painkiller Ziconotide. Nature 2021, 596, 143–147. [Google Scholar] [CrossRef]
- Kessi, M.; Chen, B.; Peng, J.; Yan, F.; Yang, L.; Yin, F. Calcium Channelopathies and Intellectual Disability: A Systematic Review. Orphanet J. Rare Dis. 2021, 16, 219. [Google Scholar] [CrossRef]
- Cai, S.; Gomez, K.; Moutal, A.; Khanna, R. Targeting T-Type/CaV3.2 Channels for Chronic Pain. Transl. Res. 2021, 234, 20–30. [Google Scholar] [CrossRef]
- Mei, Y.; Barrett, J.E.; Hu, H. Calcium Release-Activated Calcium Channels and Pain. Cell Calcium 2018, 74, 180–185. [Google Scholar] [CrossRef]
- Novello, M.J.; Zhu, J.; Feng, Q.; Ikura, M.; Stathopulos, P.B. Structural Elements of Stromal Interaction Molecule Function. Cell Calcium 2018, 73, 88–94. [Google Scholar] [CrossRef]
- Kaneko, Y.; Szallasi, A. Transient Receptor Potential (TRP) Channels: A Clinical Perspective. Br. J. Pharm. 2013, 171, 2474–2507. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP Channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Jardin, I.; Nieto, J.; Salido, G.M.; Rosado, J.A. TRPC6 Channel and Its Implications in Breast Cancer: An Overview. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867. [Google Scholar] [CrossRef]
- Kojima, I.; Nagasawa, M. TRPV2. In Handbook of Experimental Pharmacology; Springer: New York, NY, USA, 2014; Volume 222, pp. 247–272. [Google Scholar] [CrossRef]
- Garcia-Elias, A.; Mrkonjić, S.; Jung, C.; Pardo-Pastor, C.; Vicente, R.; Valverde, M.A. The Trpv4 Channel. Handb. Exp. Pharm. 2014, 222, 293–319. [Google Scholar] [CrossRef]
- Fecher-Trost, C.; Weissgerber, P.; Wissenbach, U. TRPV6 Channels. Handb. Exp. Pharm. 2014, 222, 359–384. [Google Scholar] [CrossRef]
- Miller, B.A. TRPM2 in Cancer. Cell Calcium 2019, 80, 8–17. [Google Scholar] [CrossRef]
- Zou, Z.G.; Rios, F.J.; Montezano, A.C.; Touyz, R.M. TRPM7, Magnesium, and Signaling. Int. J. Mol. Sci. 2019, 20, 1877. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Li, M.H.; Inoue, K.; Chu, X.P.; Seeds, J.; Xiong, Z.G. Transient Receptor Potential Melastatin 7-like Current in Human Head and Neck Carcinoma Cells: Role in Cell Proliferation. Cancer Res. 2007, 67, 10929–10938. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.-F.; Sun, M.-M.; Hu, X.-Y.; Du, J.; He, W. TRPP2 Ion Channels: The Roles in Various Subcellular Locations. Biochimie 2022, 201, 116–127. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).