Abstract
Chronic exposure to indoor biomass smoke from the combustion of solid organic fuels is a major cause of disease burden worldwide. Almost 3 billion people use solid fuels such as wood, charcoal, and crop residues for indoor cooking and heating, accounting for approximately 50% of all households and 90% of rural households globally. Biomass smoke contains many hazardous pollutants, resulting in household air pollution (HAP) exposure that often exceeds international standards. Long-term biomass-smoke exposure is associated with Chronic Obstructive Pulmonary Disease (COPD) in adults, a leading cause of morbidity and mortality worldwide, chronic bronchitis, and other lung conditions. Biomass smoke-associated COPD differs from the best-known cigarette smoke-induced COPD in several aspects, such as a slower decline in lung function, greater airway involvement, and less emphysema, which suggests a different phenotype and pathophysiology. Despite the high burden of biomass-associated COPD, the molecular, genetic, and epigenetic mechanisms underlying its pathogenesis are poorly understood. This review describes the pathogenic mechanisms potentially involved in lung damage, the development of COPD associated with wood-derived smoke exposure, and the influence of genetic and epigenetic factors on the development of this disease.
1. Introduction
Nearly 3 billion people worldwide use solid organic materials as the primary fuel for indoor cooking and heating, mainly in developing countries, resulting in biomass smoke and household air pollution (HAP) [1,2]. Biomass smoke is generated from the incomplete combustion of organic material such as wood, crop residues, twigs, dried animal dung, and charcoal in rudimentary stoves or open fires. This biomass smoke contains high concentrations of hazardous inhalable particulate matter [3] and chemical volatiles, such as carbon monoxide, sulfurs, and polycyclic aromatic hydrocarbons, which are harmful to human health [4,5,6]. According to the World Health Organization (WHO), over 3.8 million people die prematurely every year from diseases attributable to HAP from using organic material as fuel for cooking. These deaths are due to childhood and adult pneumonia (27%), ischemic heart disease (27%), chronic obstructive pulmonary disease (COPD) (20%), strokes (18%), and lung cancer (8%) [1].
In 2010, the American Thoracic Society (ATS) issued an official statement including indoor biomass smoke as a likely new risk factor for COPD [7]. Further accumulative evidence confirmed that chronic exposure to indoor biomass smoke was an independent risk factor for COPD, with woodsmoke being the most important among the indoor biomass smoke from fuels [8].
COPD is a complex and heterogeneous disease characterized by an atypical inflammatory response to chronic exposure to inhaled toxic particles, causing damage to lung tissue and in susceptible individuals [9]. The disease shows high heterogeneity in terms of pulmonary pathology, immune response, clinical features, and phenotype. Affected lungs exhibit destruction of the parenchyma, emphysema, mucus hypersecretion, airway thickening, scarring, focal fibrosis, and loss of the small airways [9,10].
Cigarette smoking is the main risk factor for COPD. Most knowledge about the clinical symptoms, phenotype, physiology, pathology, and treatment has been obtained from studies on smoking-related COPD patients. However, 25–45% of COPD patients are nonsmokers [11,12], with biomass-smoke exposure being an important risk factor (OR: 2.44) [13], and pooled estimates by fuel type showed that woodsmoke (OR: 4.29) is the most important risk factor for COPD [14]. Several studies have shown that, compared to smoking-related COPD, biomass-related COPD exhibits a predominant airway involvement, with higher bronchial hyperreactivity and bronchodilator response. It predominantly affects small airways with less emphysema and causes a slower rate of decline in lung function [15,16,17]. These findings suggest that biomass smoke-related lung damage and COPD may involve different mechanisms of lung pathophysiology, which have been poorly studied. Although biomass smoke contains high concentrations of particulate matter and chemical compounds that are as hazardous as cigarette smoke, the exposure to pollutants may be milder in women exposed to biomass smoke while cooking due to predominant nose inhalation and normal flow rates compared with tobacco smoking, for which the inhalation pattern involves higher flow rates through the mouth [18]. Importantly, in addition to exposure to environmental risk factors, the development of COPD depends on genetic susceptibility, epigenetic genetic regulation, and other intrinsic factors that have been better studied in smoking-related COPD but little investigated in biomass-related COPD.
A better understanding of the molecular mechanisms underlying biomass-induced COPD and the genetic and epigenetic factors influencing COPD outcomes will provide insights into pathophysiology and disease progression that may guide the development of phenotype-targeted treatment.
In this article, we describe the currently available evidence for the mechanisms of lung damage due to biomass exposure and those potentially involved in biomass-related COPD related to household wood burning for cooking and heating, which is the leading risk factor among the use of different fuel types that produce indoor biomass smoke. We highlight the evidence for the influence of genetic and epigenetic factors on developing this disease phenotype.
2. Components of Woodsmoke Pollutants
Wood burning generates inefficient and incomplete combustion with higher emissions of harmful chemicals and fine particulate matter compared to burning more efficient fuels, such as liquefied petroleum gas (LPG) [3,19].
Woodsmoke components include a complex mixture of hundreds of chemical volatiles with toxic, carcinogenic, and irritant properties and respirable particulate matter (PM) that can penetrate the airways and the lungs, many shared by tobacco smoke. Each component can be present in the gas phase, particle phase, or both phases, depending on its volatility [20]. Although the exact composition of the volatile chemicals and PM varies according to the specific wood type and combustion conditions, its major harmful components include carbon monoxide (CO), benzene, aldehydes, polycyclic aromatic hydrocarbons (PAHs), nitrogen oxides (such as NO2), sulfur oxides (such as SO2), free radicals, and fine particulate matter with a diameter less than 10 μm (PM10) [20,21,22]. A résumé of relevant health-hazardous volatile chemicals and particulate matter present in woodsmoke is listed in Table 1. Because the effect of biomass and cigarette smoke exposure are compared in many of the published cohort studies [21,23,24,25,26], Supplementary Table S1 compares reported pollutant chemicals and PM components of wood and cigarette smoke [27,28].

Table 1.
Health-hazardous chemicals and particulate matter present in wood smoke.
2.1. Particulate Matter (PM)
PM is classified according to the mass median aerodynamic diameter into coarse particles with a diameter of 2.5–10 μm (PM10), fine particles with a diameter < 2.5 μm (PM2.5), and ultrafine particles (UFP) with a diameter < 0.1 μm (PM0.1). The primary source of PM2.5 and PM0.1 are emissions from the combustion of vehicle fuels (gasoline, diesel), wood, and coal. Meanwhile, PM10 particles are derived predominantly from abraded soil, road dust, construction debris, and oil combustion products with bioaerosols. Importantly, PM varies in size, shape, and chemical composition depending on the source, which includes inorganic ions (e.g., chloride, nitrates, ammonium, and sulfates), heavy metals (e.g., cadmium, copper, and zinc), black carbon, and organic compounds (e.g., phenol, and PAHs) [29,30]. Therefore, the interpretation of the biological effects and health impact of PM depends on the diverse chemical and physical characteristics, sources, and the potential dynamic changes of PM suspended in the air [29]. Nevertheless, the general focus of the impact of ambient PM on health outcomes has been on particles with aerodynamic diameters less than or equal to 2.5 or 10 μm (PM2.5 and PM10) [29]. Both PM2.5 and PM10 are associated with increased mortality from all causes, and specific causes include cardiovascular disease, respiratory disease (asthma and COPD), and lung cancer [25,31,32]. Exposure to PM from woodsmoke is toxic to humans, animals, and in vitro models, due to several mechanisms, such as airway inflammation, exacerbations of respiratory symptoms, lung inflammation, cell toxicity, and mucin expression [33,34,35]. Woodsmoke particulate matter’s physicochemical and toxicological properties depend on wood species and combustion conditions [36,37].
Burning biomass fuels in cookstoves or open fires in poorly ventilated spaces produces very high concentrations of PM10 and PM2.5 in the range of 300–3000 μg/m3, which can reach 30,000 μg/m3 during periods of cooking [3,5,38,39]. These levels exceed the WHO’s recommendations in terms of the annual mean air quality guideline (AQG) levels of 15 and 5 μg/m3 for PM10 and PM2.5, respectively [29], with interim target levels of 70 and 35 μg/m3 for PM10 and PM2.5, respectively. However, studies indicate that most woodsmoke particles are smaller than 1 μm (UFP or PM0.1), with a peak in diameter distribution between 0.15 and 0.4 μm [40,41], which is in the inhalable size range and effect on lung inflammation and cell toxicity [25,36,37,42,43]. Therefore, woodsmoke is a dangerous combination of highly concentrated chemical components with toxic and carcinogenic properties and fine inhalable particles that can penetrate the lungs.
2.2. Woodsmoke Pattern of Particulate Matter Deposition in the Lungs
Using high-fidelity computational fluid-particle dynamic (CFPD) simulations of airflow and particle deposition analysis [18], a study showed that the concentrations of cigarette smoke inhaled are three orders of magnitude higher than those of biomass smoke in the lung. In addition, cigarette smoke has higher PM intrathoracic deposition in the lungs and higher inhalation flow rates than biomass smoke. Biomass smoke consisted of a bimodal log-normal distribution with PM50–0.05, while cigarette smoke consisted mostly of PM1–0.1. Higher fractions of cigarette smoke were in the last few airway generations compared to biomass smoke due to the higher flow rates. Therefore, biomass smoke nasally inhaled at low flow rates at an average tidal volume likely prevents smoke penetration beyond the small airways such that biomass smoke results in an airway-dominant COPD phenotype with less emphysema [44,45].
3. Biomass-Smoke Exposure Burden and Lung Disease Outcomes
The household use of solid fuels for cooking and heating, primarily wood, is the most widespread source of indoor air pollution worldwide. About 2.8 billion people—36% of the world’s population—use solid fuels as their primary energy source [46]. Populations in less developed countries and rural areas are most exposed to indoor air pollution due to solid fuel use. On a global scale, the proportion of people mainly using polluting fuels for cooking is 61% in rural areas and less than 20% in urban areas. Most exposed people live in sub-Saharan Africa, with about 84% of exposed individuals, and Central Asia and South Asia, with approximately 30%. In contrast, the proportion of people using solid fuel in developed countries, such as those in North America and Europe, is about 6% [46].
Several meta-analyses support the association of household air pollution secondary to biomass smoke with lung disease outcomes, including COPD [6,8,13,14]. Two health outcomes have strong evidence of association with indoor biomass-smoke exposure: acute lower respiratory infections (ALRIs) in children under five, and COPD in men and women 30 or over, whereas lung cancer has been associated mostly with burning coal for those aged 30 or over [6]. The overall risk of COPD in women exposed to indoor air pollution from the combustion of biomass such as wood was estimated at 3.2 (95% CI: 2.3–4.8), and that for men was 1.8 (95% CI: 1.0–3.2) [6]. Two major meta-analyses published in 2010 reported that exposure to the products of biomass combustion for domestic purposes is an independent risk factor for COPD, with an odds ratio (OR) of 2.44 (95% CI: 1.9–3.33) for developing COPD [13] in men (OR: 4.30; 95% CI: 1.85–10.01) and women (OR: 2.73; 95% CI: 2.28–3.28), and in the Asian (OR: 2.31; 95% CI: 1.41–3.78) and non-Asian populations (OR: 2.56; 95% CI: 1.71–3.83) [13]. According to another pooled estimate, woodsmoke exposure could be the most important risk factor for COPD (OR: 4.29; 95% CI: 1.35–13.70) [14]. A more recent meta-analysis from population-based studies [8] confirmed that indoor biomass exposure was among the five main risk factors for COPD globally, although the magnitude of the OR (1.4; 95% CI: 1.2–1.7) was considerably lower than that in previous studies. Two large prospective cross-sectional observational studies reported that biomass smoke [47] and particulate matter with a diameter less than 2.5 μm [48] were major risk factors for COPD.
Exposure to biomass smoke is strongly associated with poverty, also a risk factor for several health issues, including COPD. Epidemiological studies often lack quantitative exposure measurements, and observational studies cannot separate poverty from exposure to biomass smoke. Interventional studies in which households or communities are randomized into a strategy to reduce indoor pollution may provide solid information but are unacceptable to maintain for a long time in a community without improving the interventions. In addition, developing lung diseases such as COPD takes decades [49]. Most published studies rely on a questionnaire to assess exposure to household air pollution, asking about the type of fuel used, possibly ventilation, but lacking estimates of the dose inhaled or toxins reaching target organs and tissue. Studies of tobacco smoking-related COPD often use an estimate of lifetime cigarettes smoked called pack-years, the product of the average number of cigarettes smoked per day divided by 20 cigarettes per pack and years of smoking to include individuals with at least 10 pack-years. Similarly, the total duration of exposure to biomass smoke or household air pollution from solid fuels can be estimated from hour-years of exposure, the product of the years of exposure, and the number of hours per day using biomass as fuel during cooking. Ten years of exposure to HAP or >60 hour-years may be relevant for COPD, although the development of the disease depends not only on exposure but also on susceptibility; hence sharp cutoffs are arbitrary and used mainly for classification purposes. Contrasting to pack-years, hour-years of biomass-smoke exposure contain no measured biomass burned, just time, and assume a virtually uniform concentration of pollutants. Pack-years and hour-years include years of exposure and are used as simple cumulative exposure indicators. It is important to remember that more years of exposure means older age, which is associated with an increasing prevalence of COPD and other chronic diseases.
3.1. Developed Countries
Most studies on household air pollution secondary to biomass smoke and COPD are reported in low- and middle-income developing countries. However, according to surveillance and census data, wood is also used as fuel for household heating in developed countries such as Canada, Australia, and the US [4,46,50]. Nevertheless, different from developing countries, the use of wood as fuel is mainly seasonal during winter and with milder exposure levels due to improved stove efficiency and adequate venting in developed countries [50].
Most studies in developed countries refer to outdoor environmental pollution indirectly attributed to seasonal wood burning and the effects on respiratory symptoms, lung function, and asthma exacerbations in several parts of the US, Canada, Denmark, Australia, and New Zealand [4]. One study analyzed the indoor exposure of PM10 from woodstoves and respiratory health in Navajo children in Arizona [51]. Additionally, only one case–control study in Spain found that self-reported combined wood and charcoal smoke exposure was associated with a 4.5-fold greater OR of having COPD [52]. The lack of more studies indicates the underestimation of the relevance of indoor biomass exposure and COPD and lung diseases in developed countries.
3.2. Outdoor Air Pollution Due to Wood Burning in Wildfires
Smoke from wood burning during wildfires is a significant source of short-term exposure to high air pollution worldwide. Climate change has intensified the severity and frequency of wildfire occurrences [53,54], resulting in more people being exposed yearly. Several systematic reviews have analyzed the literature on the health effects of exposure to smoke from wildfires [4,55,56,57,58]. Most studies concluded an association between exposure, all-cause mortality, and exacerbation of respiratory symptoms in individuals with preexisting illnesses such as asthma, COPD, bronchitis, and pneumonia [55,57]. Wildfire smoke exposure association with hospital admissions for COPD among the general public was also reported in previous studies [4,59,60,61]. Adetona et al. [56] concluded that the current evidence of adverse impacts on respiratory health among the general public is strong, but regarding firefighters is weak. Wildfire smoke exposure in the general public is associated with hospital admission for COPD and asthma exacerbation, acute bronchitis, bronchiolitis, and pneumonia. Although environmental PM concentration is associated with cardiovascular morbidity and mortality [62], the authors concluded that current evidence of an association of PM exposure from wildfire smoke with cardiovascular effects is weak. In one study, only the risk of asthma-related hospitalization increased during smoke exposure periods [63].
In contrast to long-term exposure to household air pollution (HAP) secondary to woodsmoke, the current evidence shows no association between acute exposure to smoke pollutants from wildfires and new cases of COPD or the development of biomass-induced COPD.
3.3. Outdoor Air Pollution Due to Other Types of Environmental Contaminants
High levels of urban air pollution increase the risk of respiratory mortality, and several studies have reported that high levels of PM of outdoor air pollution derived from traffic (PM2.5 and PM10) are associated with COPD exacerbations and prevalence. The type and concentration of the volatile chemical, biological material, and PM components of ambient air pollutants vary greatly depending on the primary source of those pollutants; therefore, more studies are needed. COPD from urban air pollution exposure likely shares some common pathogenic paths with that due to cigarette smoking and to exposure to biomass smoke; however, there is no conclusive evidence yet.
Larger roads and higher PM2.5 concentrations near where subjects lived were associated with greater risk for the development of COPD [64]. Long-term exposure to low-level traffic-related air pollution, especially NO2 and black carbon, is associated with the development of COPD [65]. COPD incidence was associated with the 35-year mean NO2 level in a cohort of participants living in residential areas near traffic, with stronger associations in subjects with diabetes and asthma [66], and living close to busy roads was also associated with COPD and lower lung function (FEV1 and FVC) in adult women. [67]
Current in vitro studies show that ambient air-derived particulate matter-induced human alveolar macrophages and airway epithelial cells produce proinflammatory cytokines and oxidative response [68,69,70]. This evidence pointed to inflammatory–oxidative mechanisms of lung damage, which involve Toll-like receptors (TLRs) 2 and 4 [71,72,73]. In addition, ambient PM2.5 triggers increased oxidized phospholipids in the lungs and a systemic cellular inflammatory response through TLR4/NADPH oxidase-dependent mechanisms in a mouse model [74].
4. The Effect of Chronic Exposure to Woodsmoke on Lung Function and Respiratory Symptoms
Various cross-sectional studies have reported that chronic exposure to household biomass smoke is associated with an increased prevalence of respiratory symptoms, reduced lung function, and the development of COPD. Ventilatory function (forced expiratory volume in 1 s, FEV1; forced vital capacity, FVC; and FEV1/FVC ratio) was significantly reduced in women cooking with biomass fuels compared to those that use LPG [75,76,77], and in male and female participants across all age-groups [78]. Chronic exposure to biomass smoke is associated with increased respiratory symptoms such as cough, wheezing, mucus overproduction, dyspnea, and chronic bronchitis [75,76,77,79]. A recent large study showed that indoor cooking with solid fuel is associated with reduced peak expiratory flow (PEF) in middle-aged and older adults from a large cohort in China over a 4-year follow-up [80]. A case–control study of asthma nested within a US rural cohort showed that frequent exposure to residential wood burning was associated with increased fractional exhaled nitric oxide (FeNO) among all individuals and with lower pulmonary function among individuals with asthma [81].
5. Biomass-Related COPD
Natural History and Phenotype of Biomass-Related COPD
In contrast to smoking-related COPD, biomass-related COPD’s natural history and phenotype are poorly characterized. Ramírez-Venegas et al. [82] reported a 15-year follow-up of 112 COPD patients chronically exposed to wood-derived biomass smoke during cooking (87% female, 243 ± 122 hour-years of exposure), and 302 smoking COPD patients, 26% female, with cumulative smoking of 57 ± 32 pack-years. They observed a slower decline in lung function, absence of rapid decliners, and less variability in the lung function decline in biomass-related COPD compared with smoking-related COPD. Salvi et al. [17] confirmed the slower rate of decline in lung function among nonsmoking biomass-related COPD patients compared to smoking-related COPD patients in their two-year longitudinal study.
Several cross-sectional studies have shown that biomass-related COPD is associated with less emphysema (according to CT scans or a substantially reduced DLCO) [16,44,83,84]; a higher rate of bronchial hyperresponsiveness and response to bronchodilators [17]; more fibrosis in the lung parenchyma and in the walls of the bronchi, bronchioles, and blood vessels; and stronger anthracosis compared to smoking-related COPD [45]. Biomass-smoke exposure was typically >100 hour-years or a lifetime exposure of 10 years or more. Mahesh et al. reported a minimum threshold biomass-smoke exposure of 60 hour-years to confer a significant risk of developing chronic bronchitis in women [85]. In summary, biomass-related COPD is predominantly a small-airway disease phenotype exhibiting less emphysema, preserved lung diffusion capacity, more scarring, greater anthracosis, and a slower decline in lung function. It can be considered another phenotype of COPD.
6. Mechanisms of Lung Damage Due to Biomass-Smoke Exposure
Exposure to wood biomass smoke from cooking or heating typically is lifelong, starting before birth. Evidence from clinical studies on exposed disease-free individuals and in vivo and in vitro experimental models have shown that biomass smoke induces airway inflammation, systemic inflammation, increased oxidative stress, and cellular DNA damage. The following sections focus on studies investigating the effect of biomass smoke from wood combustion, the most common source of indoor biomass smoke and the leading risk factor for COPD, including studies in human patients and volunteers (Section 6.1), exposed animal models dealing with mechanisms of lung pathogenesis, and in vitro models testing potential components of specific mechanisms of pathogenesis (Section 6.2).
6.1. Effect of Woodsmoke Exposure on Healthy Adult Individuals
6.1.1. Chronic Exposure to Woodsmoke in Healthy Adult Individuals
Overall, studies have consistently shown that chronic exposure to woodsmoke induces airway inflammation, systemic inflammation, oxidative stress, and genotoxicity, and affects populations of immune cells such as B lymphocytes in healthy individuals.
Falfán-Valencia et al. [86] examined 27 cytokines in the sera of 30 never-smokers with chronic biomass exposure (CS−/BS+), 40 smokers with no exposure to biomass (CS+/BS-), and 30 never-smokers/unexposed to biomass controls (CS-/BS-). Compared to the unexposed controls, CS-/BS+ showed higher levels of IL-1ra, IL-6, IL-8, eotaxin, IP-10, RANTES, and VEGF, which may indicate an inflammatory profile favoring an eosinophil-derived inflammatory response, whereas CS+/BS- had higher levels of IL-2, IL-9, MCP-1, MIP-1β, and VEGF. The cytokines altered in CS-/BS+ have been associated with asthma, COPD, lung fibrosis, and lung cancer. In this study, the participants self-reported their biomass exposure times by answering a standardized questionnaire.
Several cohorts of women from rural areas of West Bengal who had cooked daily with wood, cow dung cake, and agricultural waste for at least five years were compared to women who had cooked with LPG. [87,88,89,90,91,92]. Biomass users were exposed to higher indoor concentrations of PM10 and PM2.5 than LPG users, and presented increased DNA damage, increased generation of reactive oxygen species (ROS), and diminished levels of superoxide dismutase (SOD) in the buccal epithelial cells [87] and sputum cells [88]. In addition, biomass users showed an increase in the number of neutrophils, eosinophils, lymphocytes, and alveolar macrophages in the sputum [89,90,91]. In a large cohort of 635 biomass-exposed women and 452 age-matched control women, biomass users showed increased serum levels of IL-6, IL-8, TNF-α, CRP, and ROS generation, while SOD was depleted in leukocytes [92], indicating systemic inflammatory responses and increased oxidative stress due to biomass-smoke exposure. The increased systemic levels of IL-6 and IL-8 were consistent with what Falfán-Valencia et al. reported [86].
In 200 healthy nonsmoking women in rural Bangladesh exposed to household biomass smoke (assessed by measuring PM2.5, black carbon (BC), and carbon monoxide (CO) with personal monitors), Raqib et al. [93] found a reduction in CD19+ B lymphocytes and memory B cells (CD19+CD27+), with increased plasma IgE levels and reduced production of CCL5 and TNF-α by monocyte-derived macrophages. A decreased number of CD19+ B lymphocytes in biomass-exposed women was previously reported by Dutta et al. [94] in 2012.
Table 2 summarizes the effects of chronic exposure to woodsmoke on healthy adults.

Table 2.
Summary of the studies analyzing the effects of chronic exposure to biomass smoke (BS) on healthy adults.
6.1.2. Controlled Short-Term Exposure to Woodsmoke in Healthy Adult Individuals
Short-term exposure to woodsmoke for periods varying between 2 and 4 h in length, with or without intervening no-exposure intervals, and with PM concentrations of 200–500 μg/m3, has been studied in small groups of 10–40 individuals at rest, and some of the studies added exercise intervals aiming to investigate airways and systemic inflammation, oxidative stress, DNA damage, and changes in cardiovascular physiology [34,95,96,97,98,99,100,101,102,103,104,105,106,107]. Overall, the findings have been inconsistent, with some finding airway inflammation [34,95,96,97], but not others [98,99]. Blood neutrophils, serum inflammatory cytokines, and adhesion receptors have been reported as undergoing no change after short exposure [99,100,101], while others have shown changes indicative of systemic inflammation, such as an increased number of neutrophils in the blood [34] and an increased concentration of serum Clara cell protein (CC16) [96,102]. Evidence of oxidative stress has also been inconsistent, while evidence suggests no genotoxic effect of short-term exposure to woodsmoke [101,103,104]. On the other hand, some studies suggested that short-term exposure to woodsmoke may affect cardiovascular health by causing an increase in systolic pressure, central arterial stiffness, a simultaneous reduction in heart rate variability, and an increased concentration of serum amyloid A [105,106,107].
Inconsistencies may be due to the heterogeneity in the selected endpoints, the variety of combustion conditions, the time and dose of exposure, the inclusion of exercise, and varying intervals between exposures.
6.2. In Vitro and In Vivo Studies on the Effect of Woodsmoke Exposure
In vitro studies have consistently shown that wood biomass smoke induces an inflammatory response, oxidative stress, cytotoxicity, genotoxicity, mitochondrial dysfunction, mucin expression, decreased epithelial barrier function, lung parenchymal damage, and endoplasmic reticulum stress.
6.2.1. Biological Effects of Woodsmoke on the Airway Epithelium
Airway epithelial cells are directly exposed to pollutant components following the inhalation of biomass smoke, triggering the production of reactive oxygen species and inflammatory mediators followed by disruption of the epithelial barrier and an innate immune response. Due to the heterogeneity and diverse biological effects, particle components from other sources, such as traffic or ambient-derived particles, are not included.
Human airway epithelial cells, 16HBE, produce higher levels of proinflammatory IL1-β, TNF-α, IL-8, and IL-6 mRNA after in vitro exposure to PM2.5 (40 mg/L) from wood [108]. In addition, PM2.5-exposed 16HBE cells showed mitochondrial dysfunction and altered mitochondrial metabolism by a decreased mitochondrial membrane potential, increased expression of the cleavage protein phospho-DRP1, increased mitochondrial ROS (mtROS), decreased ATP levels, decreased oxygen consumption and glycolysis and a change in mitochondrial morphology. In vivo experiments with mice exposed to woodsmoke PM2.5 for six months showed an increased number of swollen mitochondria with depletion of cristae in lung tissue, confirming the mitochondrial dysfunction.
Ex vivo-cultured human lung parenchymal tissue (mostly from ex-smokers without reported disease) responded to woodsmoke PM extracts (collected near stoves during cooking, 276.1 mg/m3, PM2.5) by producing IL1-β, IL-6, IL-8, TNF-α, CCL2, CCL3, and CCL13, confirming the inflammatory effects of woodsmoke on tissue level [109]
Woodsmoke infusion (from ignited birchwood toothpicks) reduced the epithelial barrier function and E-cadherin expression, induced a loss of the cobblestone patterning, and caused a reduction in migration without affecting the viability of adenocarcinoma-derived A549 epithelial cells [110] through a p44/42 MAPK-dependent pathway. Therefore, lung epithelial cell lines or primary lung epithelial cells from noncancerous donors should also be tested.
Woodsmoke at a concentration 25-fold lower than that of CS increased the expression of mucin 5AC oligomeric mucus/gel-forming (MUC5AC) and SAM tip domain containing the ETS transcription factor (SPDEF) mRNA in mice and human airway epithelial cells (AECs) [111], especially in AECs homozygous for the p53Arg.
The results support the idea that woodsmoke increased airway mucin production more than CS and that p53 may be involved in the induction mechanism.
Water-soluble filtrates (2 mg/mL) of wood tar on mice and on human BEAS-2B virus-immortalized epithelial cells derived from normal lung tissue [112] showed increased gene expression of IL-1β, TNF-α, and IL-8; increased cell death; increased production of superoxide anions; lower rates of oxygen consumption; and a reduction in the extracellular acidification rate. In addition, mice exposed to aerosols of water-soluble fractions (2 or 10 mg/mL for 15 min) displayed increased expression of IL-1β, TNF-α, and IL-6 in lung tissue; increased levels of neutrophils, macrophages, and monocytes in the BALF; and increases in oxidative stress markers such as heme oxygenase-1 (HO-1), metallothionein-2 (MT-2), and cytochrome P450 2E (CYP2E). In addition, water-soluble wood tar fractions (containing phenolic compounds) increased total reactive oxygen species and H2O2 production, decreased the mitochondrial membrane potential (MMP), and induced oxidative damage and cell death. Whereas the wood tar organic-soluble fraction (containing more polycyclic aromatic hydrocarbons (PAHs) and oxygenated PAHs) increased superoxide anion production and decreased MMP on BEAS-2B and A549 cells [113]. Exposure to both fractions caused cell-cycle alterations in the G2/M phase induced by the upregulation of p21 and p16 and cell death.
Pine-woodsmoke particles induce cytotoxicity [114,115,116] and endoplasmic reticulum stress (ERS) [116] in human bronchial epithelial cells (HBECs), primary human lobar bronchial epithelial cells (lobar HBECs), BEAS-2B, and cancer-derived A549 cells.
Early studies showed that woodsmoke PM induced DNA damage and oxidative stress [117,118] on A549 cells, with increased levels of IL-8 [117] or without affecting IL-8 and IL-6 expression levels [118].
Table 3 and Figure 1 summarize the biological responses of human lung epithelium to woodsmoke exposure.

Table 3.
Summary of the in vitro and in vivo biological responses of the human airway epithelium to woodsmoke exposure.
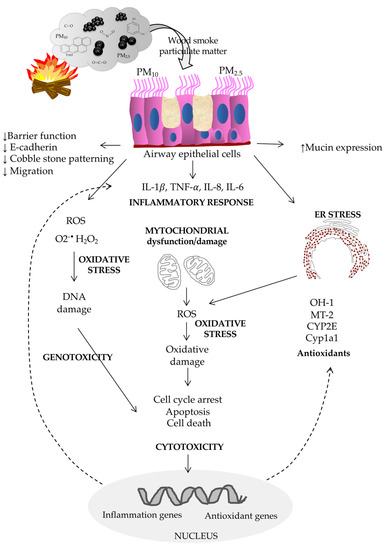
Figure 1.
Scheme of the biological responses of human airway epithelium to woodsmoke exposure.
6.2.2. Biological Effects of Woodsmoke on Alveolar Macrophages (AMs)
The function of alveolar macrophages is to detect, phagocytose, and eliminate all foreign material from the airway space, which includes PM2.5 and PM10 from inhaled air pollution [119,120,121,122]. AMs can phagocytose unopsonized environmental particles through scavenger receptors, mainly the class A scavenger receptor (SR-A) and the macrophage receptor with collagenous structure (MARCO) [123,124]. AMs also phagocytose environmental particles containing bacteria or microbial material, such as endotoxins, through Toll-like receptors (TLRs), mainly TLR4 and TLR2 [73]. After ambient PM exposure, AMs are activated and, together with airway epithelial cells, initiate an inflammatory response that involves the production of reactive oxygen species and inflammatory mediators. However, alveolar macrophages produce higher levels of inflammatory mediators such as IL-1, IL-6, and TNF-α than airway epithelial cells when exposed to the same dose of PM, suggesting that alveolar macrophages are the predominant inductors of the inflammatory response [125]. Atmospheric particles induce proinflammatory cytokines (such as IL-1β, IL-6, IL-8, TNF-α, and GM-CSF), chemokines macrophage (such as inflammatory protein 2 MIP-2, and regulated upon activation, normal T-cell expressed and secreted, RANTES), and interferons [119,126]. These cytokines promote the infiltration of neutrophils, dendritic cells, and T cells while inducing the recruitment and maturation of monocytes from the bone marrow [68,127]. However, the cytokines produced by PM-exposed AM depend on the PM’s size and chemical composition, source, time of exposure, and perhaps the macrophage phenotype [126,128]. Upon activation, macrophages acquire polarized M1 or M2 phenotypes that induce Th1- or Th2-mediated immune responses, respectively. Exposure to environmental PM2.5 induces M1 polarization in bronchoalveolar lavage fluid (BALF) from PM-exposed mice [129]. However, in a model of COPD murine model, environmental PM2.5 promotes M2 polarization in AMs [130].
All these studies have addressed the effect of PM derived from diverse sources of air pollution (e.g., ambient particulate matter, urban particulate matter, diesel exhaust particles) on alveolar macrophages [69,70,121,122,125,126,131].
Similar to AMs exposed to atmospheric particulate matter [121], AMs and monocyte-derived macrophages (MDMs) from volunteers exposed to household air pollution can phagocytize woodsmoke particles dose-dependently [132]. In addition, woodsmoke particles induce a dose-dependent production of IL-6 and IL-8 in both cells, while reducing the phagocytosis capacity and oxidative burst activity in AMs [132].
MDMs from BS-COPD and CS-COPD patients exhibit defective phagocytosis [133] with higher bacterial loads of S. pneumoniae, H. influenzae, and P. aeruginosa in induced sputum and with a decline in spirometric lung function indices compared to healthy subjects.
Infiltrated AMs in the BALF of rats chronically exposed to woodsmoke-derived PM1, PM2.5, and PM10, [134] produced increased levels of IL-1β, TNFα, and LIX after one month of exposure, with only increased levels of VEGF after six months of exposure. After exposure, AMs also showed a decreased expression of the M2 marker CD206 after four days, a recovery in CD206 expression after one month, and an increase in expression levels after six months of exposure. The increased expression of CD206 was accompanied by increased TGF-β1 and p-Smad3 expression, leading the authors to suggest that chronic biomass PM affects alveolar macrophage polarization and activation. Bazzan et al. [135] found a dual polarization of human alveolar macrophages that progressively increases with smoking and COPD severity in CS-COPD patients. In contrast, Shaykhiev et al. [136] reported that AM of healthy smokers exhibited M2 polarization.
6.2.3. Biological Effects of Woodsmoke on Fibroblasts and Endothelial Cells
Due to its intrinsic toxicity, woodsmoke affects other cell lineages in the airways, such as fibroblasts and endothelial cells. Its effects include cell activation, the expression of inflammatory cytokines, the deposition of extracellular matrix components, and genotoxicity.
Woodsmoke PM (0.3–10 mm in size) enhanced proinflammatory IL-8 production, increased pERK1/2 levels, and led to higher deposition of the extracellular matrix components perlecan and fibronectin in human lung fibroblasts (from donors with thoracic malignancies or undergoing lung transplantation) [137].
Woodsmoke particles (PM size 113 nm, high PAH content) induced the adhesion of monocytic THP-1 cells onto human umbilical endothelial cells (HUVECs) in co-cultures, which correlated with increased expression of VCAM-1 on exposed HUVECs and increased expression of the TNF and IL8 mRNAs in THP-1 cells. [138] In addition, woodsmoke increased DNA strand breaks and oxidized guanines, with low cytotoxicity toward HUVECs. This study showed that woodsmoke induces oxidative damage to endothelial cell DNA and the adhesion of monocytes, with the activation of both cell types.
6.2.4. Potential Role of Transient Receptor Potential (TRP) in the Cytotoxicity and Endoplasmic Reticulum Stress (ERS) Response Induced by Woodsmoke Particles in Human Lung Epithelial Cells
Woodsmoke particles generated from pine wood are highly cytotoxic to human bronchial epithelial cells in vitro, induce ERS and activate transient receptor potential ankyrin-1 (TRPA1) and transient receptor potential vanilloid-3 (TRPV3) ion channel receptors differentially in human bronchial epithelial cells [114,116,139,140]; however, evidence on their role in cytotoxicity and ERS has been conflicting.
Pine- and mesquite-woodsmoke particles activate TRPV3 in several human normal bronchial epithelial cells and A549 cells, with more cytotoxicity in BEAS-2B cells overexpressing TRPV3 (TRPV3-OE BEAS-2B), and partial protection by addition of antagonists of TRPV3 [114]. Using antagonists for TRPA1, TRPAV1, and TRPV4 does not affect woodsmoke particle-induced cytotoxicity.
Further, pine-woodsmoke particles caused cytotoxicity, activation of TRPA1 and TRPV3, and endoplasmic reticulum stress (ERS) responses in lobal human lobar bronchial epithelial cells (HBECs) and BEAS-2B [116]. TRPA1 transcripts remained low after treatment with woodsmoke particles, while TRPV3 transcripts increased, indicating distinct transient expression. Contrary to the previous findings, the inhibition of TRPV3 increased woodsmoke particle-induced cytotoxicity in lobal HBECs, while the inhibition of TRP1 decreased it. In addition, TRPV3 antagonists increased ERS in lobal HBECs, while TRP1 antagonists decreased it. Furthermore, the overexpression of TRPV3 in TRPV3-OE BEAS-2B cells conferred resistance to ERS by woodsmoke particles, whereas TRPV3 knockdown in BEAS-2B sensitized the cells to the induction of ERS [116]. These results indicate that TRPA1 promotes cytotoxicity and ERS response induced by woodsmoke particles, and TRPV3 protects against these phenomena, which are inconsistent with previous findings.
6.2.5. TRPA1 and the Epidermal Growth Factor Receptor (EGFR) Signaling Pathway Are Involved in MUC5AC Expression and Cytotoxicity Induced by Woodsmoke Particles in Human Bronchial Epithelial Cells
Woodsmoke particles stimulated MUC5AC overexpression by human bronchial epithelial cells [111,115,141]. However, mechanistically, these studies found that different components of the EGFR pathway were involved in woodsmoke-particle-induced MUC5AC.
Memon et al. [115] found that pine-woodsmoke particles (PM2.5) and coniferaldehyde, a highly concentrated component and TRPA1 agonist, induced cytotoxicity and the expression and secretion of MUC5AC by primary human bronchial epithelial cells (HBECs). The synthetic TRPA1 antagonist A967079 attenuated the overexpression of MUC5AC after treatment with pine-woodsmoke particles, indicating that TRPA1 is involved but not essential.
Pine-woodsmoke particles and coniferaldehyde increased the expression of the EGFR signaling components epigen (EPGN), heparin-binding epidermal growth factor (HB-EGF), and phosphor-EGFR, and induced the accumulation and perinuclear/nuclear localization of β-catenin. Furthermore, antagonists for EGFR/ErbB1, p38 MAPK, and GSK3β inhibited but did not abolish the MUC5AC overexpression induced by woodsmoke particles. These results suggest that the EGFR-dependent activation of p38 MAPK, GSK3β, and β-catenin contributes to pine-woodsmoke-particle-induced MUC5AC overproduction in HBECs, as well as the activation of TRP1. The authors suggested that MUC5AC production results from the selective and coordinated activation of TRPA1 and EGFR. A potential coordinated effect between TRP1 and EGFR activation could be investigated by inhibiting one participant (e.g., by using an effective antagonist, siRNA, or knockdown) while testing the participation of the other in the induction of MUC5AC overexpression by woodsmoke particles.
Woodsmoke PM2.5 also induced MUC5AC expression in human primary epithelial cells (carcinoma mucoepidermoid-derived NCI-H292 cell line) and in the airways of rats exposed to woodsmoke for three months. Expression of MUC5AC was inhibited using an EGFR-selective tyrosine kinase inhibitor, an EGFR-neutralizing antibody, the ERK kinase inhibitor PD98059, and specific neutralizing antibodies for AREG [141]. In contrast to the study by Memon et al. [115], woodsmoke particles increased the AREG mRNA and protein levels in epithelial cells. This discrepancy may be related to the use of carcinoma-derived cells instead of normal epithelial cells in this study. This study showed that woodsmoke PM2.5 induced MUC5AC via the AREG–EGFR–ERK pathway in the cancer-derived NCI-H292 cell line.
Overall, both studies showed that the activation of the EGFR signaling pathway contributes to woodsmoke-particle-induced MUC5AC expression and cytotoxicity in human bronchial epithelial cells, in addition to the contribution of TRPA1, which may or may not be coordinated with it.
6.2.6. Potential Role of Toll-Like Receptors (TLRs) in Inflammation Induced by Woodsmoke Particles
Mice exposed to woodsmoke-derived particles for 24 h (acute exposure, by nose aspiration during anesthesia) showed increased proinflammatory cytokine production, neutrophilic inflammation, and hyperresponsiveness. Meanwhile, in treated mice, subchronic exposure (three doses per week and examination eight weeks after exposure) induced eosinophilic inflammation and alveolar destruction. Only subchronic wood-particle exposure resulted in significant increases in airspace enlargement in the lungs of the treated mice. Furthermore, subchronic exposure to woodsmoke particles increased eosinophil cytokines (IL-5 and eotaxin). In contrast, exposure to cow-dung particles increased neutrophil and macrophage chemokines (G-CSF, MIP-1a, MIP-1b, IP-10, and IFNγ), indicating a differential effect on mice according to the fuel source [130]. Using only the acute exposure model, TLR2-, TLR3-, TLR4-, TLR5-, and IL-1R–deficient mice were exposed to wood- or dung-smoke particles, and the measurement of neutrophils in the BALF indicated that IL-1R, TLR4, and TLR2 are necessary to elicit that inflammatory response in mice [142].
It would be interesting to know the role of TLRs in the inflammatory responses induced by chronic exposure to woodsmoke and obtain models exposing awake animals in chambers controlling concentrations of particulate matter and other toxics. Chronic exposure to woodsmoke associated with developing COPD-like lesions in animal models [134].
7. Mechanisms of Pathogenesis Involved in Biomass-Related COPD
In addition to smoking, chronic exposure to woodsmoke is the most well-known environmental burden contributing to COPD development. However, COPD is a complex and multifactorial disease that develops after the inhalation of toxics, most often tobacco smoke or biomass smoke, and has genetic and epigenetic factors of susceptibility (Figure 2). Studies on nonsmoking COPD patients with chronic exposure to woodsmoke have shown changes in the inflammatory response, increased DNA damage, oxidative stress, and genetic and epigenetic susceptibility factors.
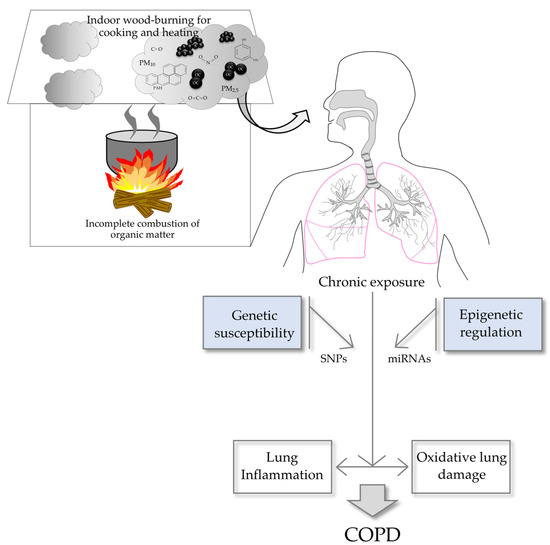
Figure 2.
Biomass-related COPD is a complex and multifactorial disease that develops after the combination of chronic exposure to the hazardous components of woodsmoke and genetic and epigenetic factors of susceptibility. Organic carbon (OC), soot (s).
7.1. Inflammation
In contrast to those for COPD related to cigarette smoke, few studies have consistently assessed the inflammatory profile and potential inflammatory mechanism of pathogenesis in COPD related to wood-biomass-smoke exposure.
Shyam et al. [143] examined the levels of the proinflammatory cytokines IL-1β and TNF-α and the potential correlation with the severity of airflow limitation in 40 patients with COPD associated with cigarette smoke (CS-COPD) and 40 biomass-smoke exposure (BS-COPD) compared to 80 healthy controls. COPD patients presented higher levels of IL-1β and TNF-α compared to controls. Among the COPD cases, there was a negative linear relationship between TNF-α and IL-1β levels with the FEV1 and 6 min walking distance. According to linear regression, TNF-α levels were associated with CS-COPD, while IL-1β levels were associated with both CS- and BS-COPD. In addition, BS-COPD patients had higher levels of IL1-β, but lower levels of TNF-α than the CS-COPD, suggesting differences in the inflammatory responses between them.
In a comparison of 29 BS-COPD female subjects, 24 BS-exposed women without COPD (BS-CTRL), 23 CS-COPD male patients, and 22 male smokers without COPD (CS-CTRL), the chemokines CCL15, CCL27, and CXCL13 were significantly lower in the BS-COPD (vs BS-CTRL) whereas the chemokines CCL1, CCL7, CCL15, CCL17, CCL19, CXCL2, IFNγ, and MIF were significantly different between the CS-COPD and CS-CONTROL [144].
Sputum eosinophilia (sputum eosinophils ≥ 3%) in 28 BS-COPD cases, was more common than in 85 CS-COPD while both BS-COPD and CS-COPD presented neutrophils in sputum [145].
In a comparison of 31 BS-COPD, 49 CS-COPD, 46 COPD patients with both exposures, and 52 healthy controls [146], COPD with combined CS + BS exposure showed lower oxygenation spirometric function and DLCO. BS-COPD and combined exposure-COPD patients showed higher levels of IgE, suggesting a Th2-type response, whereas smokers with COPD showed higher levels of CRP and fibrinogen than BS-COPD patients [146].
BS-COPD and CS-COPD have higher inflammatory responses compared to healthy controls, but the inflammatory profile showed some differences between the subgroups of COPD patients [147]. The BS-COPD group expressed significantly lower levels of IL-6, IL-8, and IL-5 than CS-COPD.
Serum levels of secretoglobin family 1A member 1 (SCGB1A1) also known as club cell protein 16 or CC16, were lower in both the BS-COPD and CS-COPD compared to the CS controls and healthy controls. In addition, the SCGB1A1 levels were positively correlated with FEV1, FVC, and exercise capacity [148].
Plasma levels of matrix metalloproteinase-(MMP)-1, MMP-7, MMP-9, MMP-9/TIMP-1, and CRP were similar in women with BS-COPD and CS-COPD but higher than those in unexposed controls [149].
Table 4 summarizes the studies on inflammatory markers in biomass-related COPD. Although the results depict inflammatory responses, those are difficult to compare because different studies primarily examined different components (Table 4). Further, the results were inconsistent between studies when similar endpoints were tested. For example, sputum eosinophilia and higher IgE levels in BS-COPD compared with CS-COPD have been reported in some studies, but not in others (Table 4). The same inconsistency is observed with high levels of IL-1β and TNF-α in both BS-COPD and CS-COPD, and elevated IL-6 and IL-8 in BS-COPD and CS-COPD (Table 4). Further studies on the inflammatory response in BS-COPD are needed, as inflammatory features may be distinct from this COPD phenotype.

Table 4.
Summary of studies on inflammatory markers in biomass smoke-related COPD (BS-COPD).
7.2. Oxidative Stress and DNA Damage
Patients with BS-COPD (n = 30) and CS-COPD (n = 30, ex-smokers) had higher plasma levels of malondialdehyde (MDA) and increased activity of superoxide dismutase (SOD) compared to unexposed healthy controls (n = 30), with no significant differences in glutathione peroxidase (GPx), glutathione reductase (GR), and glutathione-S-transferase (GST), indicating a similar pattern of oxidative stress [150]. In addition, MDA levels and SOD activity were inversely correlated with FEV1 in both groups of COPD patients.
Higher levels of DNA strand breaks in peripheral blood mononuclear cells and higher levels of plasma MDA and protein carbonyl (PC) have been found in both BS-COPD patients and CS-COPD patients compared to controls. DNA damage levels were higher in CS-COPD compared to BS-COPD [151].
7.3. MicroRNAs
7.3.1. MicroRNAs in COPD
The development of COPD involves complex interactions between environmental insults and genetic/epigenetic determinants. Among the epigenetic regulatory mechanisms, microRNAs (miRNAs) have been extensively studied as master gene regulators in health and disease. miRNAs are small noncoding RNA molecules (22 nucleotides long) that regulate the expression of genes involved in multiple biological processes at the posttranscriptional level by blocking the translation of target messenger RNAs (mRNAs).
Several studies examining changes in the microRNA profiles of bronchial biopsies, lung tissue, airway cells, sputum, and peripheral blood in COPD patients have linked these changes to disease status, diagnosis, survival, pathogenesis, and response to corticosteroid treatment [152,153,154,155,156,157]. Furthermore, miRNA and mRNA expression studies on COPD patients have revealed miRNA–mRNA network interactions associated with potential pathogenesis mechanisms [153,158]. Therefore, increasing evidence suggests that miRNAs may play a crucial role in the pathogenesis and development of COPD. Notably, these studies included ex-smoking or smoking COPD patients, with only one study including nonsmoking COPD patients with no occupational exposure to dust, soot, or coal dust [158].
7.3.2. MicroRNAs in Biomass Smoke-Related COPD
In contrast to cigarette smoke-related COPD, few studies have aimed to specifically elucidate the role of miRNAs in biomass-related COPD (Table 5).

Table 5.
Studies of miRNAs in biomass smoke-related COPD (BS-COPD) compared to cigarette smoke-related COPD (CS-COPD).
The expression of miR-22 in the sera of 25 BS-COPD female patients was decreased compared to those in 25 with CS-COPD [159]. Accordingly, the BS-COPD group had significantly elevated levels of histone deacetylase 4 (HDAC4), a predicted target of miR-22, compared with the CS-COPD. Levels of HDAC4 were positively correlated with carbon monoxide diffusing capacity as a percentage of predicted (DLCOsb%) in both groups of COPD patients. However, HDAC4 targeting by miR-22 or disease-free controls were not studied.
In comparing 96 miRNAs in three serum samples from each of the three study groups (BS-COPD, CS-COPD, and healthy controls) [160], six differentially expressed miRNAs were subsequently tested by qPCR using 25 serum samples from each study group. Out of four validated miRNAs, the authors selected miR-34a-5p, downregulated in BS-COPD compared to CS-COPD, for further analysis, and chose Notch1 (a predictive target of miR-34a-5p) for quantification in the sera of the same cohort by ELISA. They found increased levels of Notch1 in BS-COPD compared with CS-COPD.
Based on those results, the authors inferred that the downregulation of miR-34a-5p induces upregulation of Notch1, which may play a role in the differentiation of the airway epithelium associated with bronchitis in BS-COPD.
However, the downregulation of miR-34a-5p among COPD patients with different risk factors does not imply an association with the disease status. Furthermore, the downregulation of miR-34a-5p cannot be associated with the risk factor either, as differential expression was not detected between disease-free individuals with different exposure types. Notch1 targeting by miR-34a-5p was not assessed but was based on computational target prediction and (possibly) other studies, such as that of Long et al. [161]. Rather, miR-34 may be useful for discriminating between COPD patients with different risk factors, but also for supporting the notion that biomass-related COPD may have a different phenotype and should be further investigated. On the other hand, miR-374a-5p was downregulated in BS-COPD compared to unexposed controls, while miR-191-5p was upregulated in BS-COPD patients compared to controls exposed to biomass. Further examination of these miRNAs may shed light on whether circulating miRNAs are involved in the diagnosis, phenotype, or pathology of BS-COPD patients. However, these findings were not discussed in this study.
Díaz-Peña et al. [162] analyzed 2069 miRNAs in the sera of 15 BS-COPD and 15 CS-COPD patients using high-throughput sequencing without quantitative validation. Disease-free controls were not included. They reported 45 miRNAs differentially expressed in BS-COPD compared to CS-COPD. Then they performed a gene ontology (GO) analysis to find related biological mechanisms. Finding a different miRNA expression profile in BS-COPD compared to CS-COPD is hypothesis-generating, but causality would be supported further by comparison with disease-free controls, quantitative verification of sequencing data, and larger and independent validation cohorts.
Together, these three studies provide preliminary evidence that BS-COPD patients might have a different miRNA expression profile from CS-COPD patients and possibly a different phenotype.
Nevertheless, further studies are needed to identify and validate miRNAs potentially associated with disease status. Once miRNAs are discovered and validated, mechanistic studies will assess the biological role in the pathogenesis of biomass-related COPD.
7.4. Genetic Susceptibility
7.4.1. COPD Genetic Studies
There is strong evidence for the association of multiple genes with lung function and susceptibility to COPD. Early family-based studies showed COPD clusters in families in response to smoking, with a threefold increased risk of airway obstruction in smoking first-degree relatives of patients with severe COPD, but not nonsmokers [163,164]. Twin [165] and unrelated population-based studies [166] indicated significant heritability for COPD in smokers.
Severe alpha-1 antitrypsin (A1AT) deficiency, a rare genetic variant, is the best-described genetic risk factor for COPD and accounts for approximately 1% of COPD cases, especially in individuals of European origin [167].
Multiple genomic regions associated with COPD susceptibility have been identified by genome-wide association studies (GWASs), including single-nucleotide polymorphism (SNP) genotyping [168]. Multicenter longitudinal studies, such as Genetic Epidemiology of COPD (COPDGene), Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS), Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE), and subsequent joint meta-analyses have enabled the validation of more than 20 loci associated with COPD status [168,169,170,171,172,173]. Among the loci that have been replicated by different studies are HHIP (4q31), FAM13A (4q11), and CHRNA3/CHRNA5/IREB2 (15q25) [168]. Notably, these studies included COPD patients with a history of smoking, but not COPD patients who were nonsmokers.
Wain et al. [174] published the first GWAS that included COPD cases from heavy and never-smokers from subjects of European descent from the UK Biobank Lung Exome Variant Evaluation (UK BiLEVE) study cohort. They reported six novel genome-wide significant signals of association with extremes of FEV1 in both that showed associations with COPD in both ever-smokers and nonsmokers. To date, four other combined GWAS meta-analyses have been published that included the British BiLEVE cohort [175,176,177,178], but no information was provided on whether exposure to biomass smoke or another pollutant occurred in the cohort of nonsmokers.
7.4.2. Genetic Studies on Biomass Smoke-Related COPD
A few recent case–control studies performed by the same research group have analyzed associations of selected SNPs with COPD status in never-smoking women with long-term biomass exposure due to wood burning for indoor cooking in rural Mexico (Table 6). Women exposed to biomass smoke in rural areas of Mexico and several other Latin-American countries often have strong Amerindian ancestry.

Table 6.
Genetic studies of biomass smoke-related COPD (BS-COPD) compared with biomass-exposed healthy controls (BS-controls) in predominantly female populations.
The rs2070908 in HSP90B1 (in the heat shock protein 90 gene complex) was associated with a decreased risk (p < 0.01, OR = 0.6) of suffering from COPD among the subjects chronically exposed to biomass, and the rs13296 GG genotype with a lower risk (p = 0.01, OR = 0.22) of severe COPD in smokers [179].
The rs2275913 and rs8193036, two selected interleukin 17A (IL-17A) SNPs, were associated with an increased risk of CS-COPD (p < 0.01; OR = 2.91) and BS-COPD (p = 4.52 × 10−17, OR = 1.11; p = 3.15 × 10−17, OR = 1.11) [180]. IL-17A has previously been associated with chronic inflammation and the progression of COPD [181].
The rs13118928 GG genotype (p = 0.021; OR = 0.51; 95% CI: 0.27–0.97) and the rs13118928–rs1828591 (GG) haplotype (p = 0.04; OR = 0.65; 95% CI: 0.43–0.98) from the Hedgehog-interacting protein (HHIP) gene were associated with a decreased risk of COPD in women exposed to biomass smoke [182].
The HHIP loci are among the replicated loci associated with COPD in several studies [168]. rs1828591 was previously associated with FEV1/FVC in a general population cohort [183] and a Chinese COPD smoking cohort [184], while rs13118928 and rs1828591 were associated with FEV1 in a familial early-onset COPD cohort [172]. Although the rs13141641 SNP of HHIP was associated with COPD status [169,176], no significant association between rs13118928 and rs1828591 and COPD risk had previously been reported.
The alpha-1 antitrypsin (A1AT) protein polymorphisms PiS (rs17580) were associated with CS-COPD (p < 0.001, OR = 2.16) and BS-COPD (p < 0.0001, OR = 11.46). Severe deficiency of alpha-1 antitrypsin is uncommon in women exposed to biomass in Mexico, often with a strong Amerindian ancestry. Another study found a marginal association between the CT haplotype in the SERPINA1 gene rs1303-rs709932 and a reduced risk of COPD related to smoking (p = 0.048, OR = 0.81). Still, no significance was found for COPD related to biomass smoke [185].
The rs1800629 GA genotype for tumor necrosis factor TNF was associated with susceptibility to CS-COPD, but no significance was found for BS-COPD [186]. TNF polymorphisms have been associated with the clinical phenotype and progression of smoking-related COPD, such as rs361525 [187] and rs1800629 [188,189], but no significant association between TNF SNPs and COPD in nonsmoking patients had been previously reported. Also, elevated TNF-α levels have been linked to the inflammatory response in COPD in several studies [190].
The previous studies provide data on a possible association between selected SNPs and genetic susceptibility to COPD in a population of nonsmokers, primarily women chronically exposed to wood biomass smoke (Table 6). The authors tested few SNPs, and the size of the analyzed cohorts was limited.
7.5. Summary of the Pathobiology of Biomass Smoke-Induced COPD Compared to Cigarette Smoke-Induced COPD
In addition to the similarities in the hazardous particulate matter and chemical compounds between biomass and cigarette smoke, both insults require long exposure to induce COPD development. However, differences in inhalation patterns (nasal in biomass smoke and deep oral in tobacco smoke) may contribute to a less severe degree of lung tissue damage from biomass smoke than from cigarette smoke. Both biomass and cigarette smoke induce damage and injury at the cellular, tissue, organ, and systemic levels; however, not all exposed individuals develop the disease as it is clinically defined. Therefore, there are intrinsic factors contributing to the development of COPD.
In searching for differences and similarities in the pathobiology of these different phenotypes, it is relevant to compare their known characteristics. Table 7 summarizes the pathobiology of biomass smoke-related COPD compared to cigarette smoke-related COPD [192,193,194].

Table 7.
Pathobiology of biomass smoke-related COPD compared to cigarette smoke-related COPD.
The information described in this review points to classic inflammatory–oxidative mechanisms of lung damage and the potential role of genetic susceptibility and miRNAs associated with chronic biomass-smoke exposure. Therefore, we extrapolated the model of inflammatory–oxidative damage from the best-known cigarette smoke-related COPD for comparison. However, there are new insights regarding the pathophysiology and molecular and cellular mechanisms of the pathogenesis of COPD in smokers. Besides the airway inflammation model of COPD pathogenesis, emerging literature indicates that airway remodeling and epithelial–mesenchymal transition (EMT) are driving mechanisms of airway fibrosis disease in COPD in smokers [195,196,197]. Moreover, studies have revealed the role of chronic colonization or recurrent infections by a specific group of bacteria in luminal inflammation and oxidative stress on the epithelium in smokers and smoking COPD patients [198,199,200]. Therefore, further research on mechanisms of lung damage and the development of COPD secondary to biomass-smoke exposure may include these potential driving mechanisms of pathology.
8. Potential Phenotype-Driving Therapeutics
Targeted therapies based on phenotypes are common, aiming to enhance benefits and reduce adverse consequences of treatments. COPD research is particularly challenging due to COPD’s heterogeneous nature and basically syndromic diagnosis. Most studies on COPD derive from tobacco smokers, and few identify the clinical, histopathological, pulmonary function, and medical imaging characteristics of biomass-related COPD patients, a potentially new phenotype [10,15,17].
In biomass-related COPD, the inflammatory response and other pathogenesis mechanisms have not been fully characterized, and no specific clinical trials have yet been reported for biomass-related COPD.
Scattered reports of bronchial hyperresponsiveness, eosinophilia, or increased IgE suggest that inhaled corticosteroids (ICSs) might be a reasonable strategy to test [10].
Eliminating biomass-smoke exposure, or reducing indoor pollution considerably, is important for primary and secondary prevention [201], but it is difficult to achieve as household air pollution is mostly associated with poverty, and improved biomass stoves in most places lack long-term acceptance.
Recently, Siddharthan et al. [202] published a randomized, double-blind, placebo-controlled study evaluating the clinical efficacy and cost-effectiveness of low-dose theophylline for the management of biomass-associated COPD (moderate to severe) in central Uganda. The reasoning was that the recommended inhaler-based therapy for COPD is not available or affordable in low- and middle-income countries; one option is to use low-dose theophylline—an oral, once-daily therapy. Therefore, the study was designed based on availability and affordability, not necessarily on a phenotype-driven strategy.
9. Discussion and Conclusions
Despite the high burden of biomass-related COPD, few studies have evaluated the molecular, genetic, and epigenetic mechanisms underlying the pathogenesis. Significant efforts have been made to identify this phenotype’s clinical, histopathological, and pulmonary imaging characteristics, but a phenotype-derived drug treatment has not been proposed to date. To date, the only recommended intervention is the elimination of biomass exposure, and prescribed treatments are extrapolations of known studies on tobacco smokers with COPD.
Importantly, biomass smoke contains similarly high concentrations of hazardous particulate matter and chemical compounds compared to cigarette smoke; however, variations in the pattern of inhalation and differences in the concentration and deposition of harmful components in the airways and lungs may contribute to the different characteristics of biomass-related COPD compared to smoking-related COPD. As for smoking-related COPD, exposure to toxic environmental risk factors alone is insufficient for developing biomass-related COPD. Instead, an inflammatory response that damages the airways and lungs and a complex combination of intrinsic factors in each individual is required. The relevance of elucidating such mechanisms may derive in more specific biomass-related COPD treatments.
In conclusion, more research is needed to elucidate the mechanisms of biomass-induced pathogenesis and susceptibility to COPD and other lung conditions. Furthermore, more data on exposure–response relationships from well-designed longitudinal studies in children, adolescents, and adults are needed to capture the adverse effects associated with exposure to biomass before birth and at an early age and the natural history of COPD development. In studies of the inflammatory response in biomass-related COPD, the assessment of homogeneous and disease-relevant biological endpoints by comparing them to those already discovered for smoking-related COPD may be of help. Genome-wide association studies on biomass-related COPD and association with Amerindian and other ethnic ancestries are needed to discover a potential gene association with lung function and susceptibility to COPD. Similar to those for smoking-related COPD, studies examining changes in microRNA profiles should be analyzed to elucidate possible associations with disease status, diagnosis, survival, and pathogenesis.
We look forward to future advances in biomass-related COPD research and clinical applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cells12010067/s1. Table S1: Comparison between the pollutant components present in wood smoke and cigarette smoke.
Author Contributions
R.P.-P. visualized the original idea, wrote sections, and reviewed and edited the manuscript. I.M.-E. edited tables and figures. B.O.-Q. wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing does not apply to this article.
Conflicts of Interest
The authors declare no conflict of interest associated with the manuscript.
References
- World Health Organization. Household air pollution and health. Available online: https://www.who.int/news-room/fact-sheets/detail/household-air-pollution-and-health (accessed on 20 July 2022).
- Bonjour, S.; Adair-Rohani, H.; Wolf, J.; Bruce, N.G.; Mehta, S.; Pruss-Ustun, A.; Lahiff, M.; Rehfuess, E.A.; Mishra, V.; Smith, K.R. Solid fuel use for household cooking: Country and regional estimates for 1980-2010. Environ. Health Perspect. 2013, 121, 784–790. [Google Scholar] [CrossRef]
- Shupler, M. Household and personal air pollution exposure measurements from 120 communities in eight countries: Results from the PURE-AIR study. Lancet Planet Health 2020, 4, e451–e462. [Google Scholar] [CrossRef]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef]
- Rehfuess, E.; World Health Organization. Fuel for life: Household energy and health. Available online: https://apps.who.int/iris/handle/10665/43421 (accessed on 30 June 2022).
- Smith, K.R.; Mehta, S.; Maeusezahl-Feuz, M. Indoor smoke from household solid fuels. In Comparative quantification of health risks: Global and regional burden of disease due to selected major risk factors; Ezzati, M., Rodgers, A.D., Lopez, A.D., Murray, C.J.L., Eds.; World Health Organization: Geneva, Switzerland, 2004; Volume 1, pp. 1435–1493. [Google Scholar]
- Eisner, M.D.; Anthonisen, N.; Coultas, D.; Kuenzli, N.; Perez-Padilla, R.; Postma, D.; Romieu, I.; Silverman, E.K.; Balmes, J.R.; Committee on Nonsmoking Copd, E.; et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 693–718. [Google Scholar] [CrossRef] [PubMed]
- Adeloye, D.; Song, P.; Zhu, Y.; Campbell, H.; Sheikh, A.; Rudan, I.; Unit, N.R.G.R.H. Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: A systematic review and modelling analysis. Lancet Respir. Med. 2022, 10, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease Global Initiative for Chronic Obstructive Lung Disease. Available online: http://www.goldcopd.org/ (accessed on 22 August 2022).
- Turner, A.M.; Tamasi, L.; Schleich, F.; Hoxha, M.; Horvath, I.; Louis, R.; Barnes, N. Clinically relevant subgroups in COPD and asthma. Eur. Respir. Rev. 2015, 24, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.S.; Barnes, P.J. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef]
- Terzikhan, N.; Verhamme, K.M.; Hofman, A.; Stricker, B.H.; Brusselle, G.G.; Lahousse, L. Prevalence and incidence of COPD in smokers and non-smokers: The Rotterdam Study. Eur. J. Epidemiol. 2016, 31, 785–792. [Google Scholar] [CrossRef]
- Hu, G.; Zhou, Y.; Tian, J.; Yao, W.; Li, J.; Li, B.; Ran, P. Risk of COPD from exposure to biomass smoke: A metaanalysis. Chest 2010, 138, 20–31. [Google Scholar] [CrossRef]
- Kurmi, O.P.; Semple, S.; Simkhada, P.; Smith, W.C.; Ayres, J.G. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: A systematic review and meta-analysis. Thorax 2010, 65, 221–228. [Google Scholar] [CrossRef]
- Ramirez-Venegas, A.; Montiel-Lopez, F.; Falfan-Valencia, R.; Perez-Rubio, G.; Sansores, R.H. The "Slow Horse Racing Effect" on Lung Function in Adult Life in Chronic Obstructive Pulmonary Disease Associated to Biomass Exposure. Front. Med. Lausanne 2021, 8, 700836. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, Y.; Jiang, C.; Zhao, Z.; He, F.; Ran, P. Small airway disease: A different phenotype of early stage COPD associated with biomass smoke exposure. Respirology 2018, 23, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Salvi, S.S.; Brashier, B.B.; Londhe, J.; Pyasi, K.; Vincent, V.; Kajale, S.S.; Tambe, S.; Mandani, K.; Nair, A.; Mak, S.M.; et al. Phenotypic comparison between smoking and non-smoking chronic obstructive pulmonary disease. Respir Res 2020, 21, 50. [Google Scholar] [CrossRef]
- Nicolaou, L.; Checkley, W. Differences between cigarette smoking and biomass smoke exposure: An in silico comparative assessment of particulate deposition in the lungs. Environ. Res. 2021, 197, 111116. [Google Scholar] [CrossRef]
- Smith, K.R.; Zhang, J.; Uma, R.; Kishore, V.V.N.; Joshi, V.; Khalil, M.A.K. Greenhouse implications of household fuels: An analysis for India. Ann. Rev. Energy Environ. 2000, 25, 741–763. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Household use of solid fuels and high-temperature frying. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 95, 1–430. [Google Scholar]
- Larson, T.V.; Koenig, J.Q. Wood smoke: Emissions and noncancer respiratory effects. Annu. Rev. Public Health 1994, 15, 133–156. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R. Measurement of emissions from air pollution sources. 3. C1-C29 organic compounds from fireplace combustion of wood. Environ. Sci. Technol. 2001, 35, 1716–1728. [Google Scholar] [CrossRef]
- Jacobson, T.A.; Kler, J.S.; Hernke, M.T.; Braun, R.K.; Meyer, K.C.; Funk, W.E. Direct human health risks of increased atmospheric carbon dioxide. Nat. Sustain. 2019, 2, 691–701. [Google Scholar] [CrossRef]
- Zelikoff, J.T.; Chen, L.C.; Cohen, M.D.; Schlesinger, R.B. The toxicology of inhaled woodsmoke. J. Toxicol. Environ. Health. B. Crit. Rev. 2002, 5, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hoek, G. Long-term exposure to PM and all-cause and cause-specific mortality: A systematic review and meta-analysis. Environ. Int. 2020, 143, 105974. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Orellano, P.; Lin, H.L.; Jiang, M.; Guan, W.J. Short-term exposure to ozone, nitrogen dioxide, and sulphur dioxide and emergency department visits and hospital admissions due to asthma: A systematic review and meta-analysis. Environ. Int. 2021, 150, 106435. [Google Scholar] [CrossRef]
- Jinot, J.; Bayard, S.P. Chemicals identified from Table 3-1 for Mainstream Cigarette Smoke. In EPA Report: Respiratory Health Effects of Passive Smoking: Lung Cancers and Other Disorders; US Environmental Protection Agency: Washington, DC, USA, 1992. [Google Scholar]
- Soleimani, F.; Dobaradaran, S.; De-la-Torre, G.E.; Schmidt, T.C.; Saeedi, R. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: A comprehensive systematic review. Sci. Total Environ. 2022, 813, 152667. [Google Scholar] [CrossRef]
- World Health Organization. WHO global air quality guidelines: Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Available online: https://apps.who.int/iris/handle/10665/345329 (accessed on 4 July 2022).
- Cheung, K.D., N.; Kam, W.; Shafer, M.M.; Ning, Z.; Schauer, J.J.; Sioutas, C. Spatial and temporal variation of chemical composition and mass closure of ambient coarse particulate matter (PM10–2.5) in the Los Angeles area. Atmos. Environ. 2011, 45, 2651–2662. [Google Scholar] [CrossRef]
- Orellano, P.; Reynoso, J.; Quaranta, N.; Bardach, A.; Ciapponi, A. Short-term exposure to particulate matter (PM10 and PM2.5), nitrogen dioxide (NO2), and ozone (O3) and all-cause and cause-specific mortality: Systematic review and meta-analysis. Environ. Int. 2020, 142, 105876. [Google Scholar] [CrossRef]
- Bert, B.; Maciej, S.; Jie, C.; Zorana, J.A.; Richard, A.; Mariska, B.; Tom, B.; Marie-Christine, B.; Jorgen, B.; Iain, C.; et al. Mortality and Morbidity Effects of Long-Term Exposure to Low-Level PM2.5, BC, NO2, and O3: An Analysis of European Cohorts in the ELAPSE Project. Res. Rep. Health Eff. Inst. 2021, 2021, 208. [Google Scholar]
- Dilger, M.; Orasche, J.; Zimmermann, R.; Paur, H.R.; Diabate, S.; Weiss, C. Toxicity of wood smoke particles in human A549 lung epithelial cells: The role of PAHs, soot and zinc. Arch. Toxicol. 2016, 90, 3029–3044. [Google Scholar] [CrossRef]
- Ghio, A.J.; Soukup, J.M.; Case, M.; Dailey, L.A.; Richards, J.; Berntsen, J.; Devlin, R.B.; Stone, S.; Rappold, A. Exposure to wood smoke particles produces inflammation in healthy volunteers. Occup. Environ. Med. 2012, 69, 170–175. [Google Scholar] [CrossRef]
- Kim, Y.H.; Warren, S.H.; Krantz, Q.T.; King, C.; Jaskot, R.; Preston, W.T.; George, B.J.; Hays, M.D.; Landis, M.S.; Higuchi, M.; et al. Mutagenicity and Lung Toxicity of Smoldering vs. Flaming Emissions from Various Biomass Fuels: Implications for Health Effects from Wildland Fires. Environ. Health Perspect. 2018, 126, 017011. [Google Scholar] [CrossRef]
- Singh, D.; Tassew, D.D.; Nelson, J.; Chalbot, M.G.; Kavouras, I.G.; Tesfaigzi, Y.; Demokritou, P. Physicochemical and toxicological properties of wood smoke particulate matter as a function of wood species and combustion condition. J. Hazard. Mater. 2023, 441, 129874. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Tassew, D.D.; Nelson, J.; Chalbot, M.G.; Kavouras, I.G.; Demokritou, P.; Tesfaigzi, Y. Development of an Integrated Platform to Assess the Physicochemical and Toxicological Properties of Wood Combustion Particulate Matter. Chem. Res. Toxicol. 2022, 35, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; Sankar, S.; Parikh, J.; Padmavathi, R.; Srividya, K.; Venugopal, V.; Prasad, S.; Pandey, V.L. Daily average exposures to respirable particulate matter from combustion of biomass fuels in rural households of southern India. Environ. Health Perspect. 2002, 110, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, L.; Fandino-Del-Rio, M.; Koehler, K.; Checkley, W. Size distribution and lung-deposited doses of particulate matter from household exposure to biomass smoke. Indoor Air 2021, 31, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Kleeman, M.J.; Schauer, J.J.; Cass, G.R. Size and composition distribution of fine particulate matter emitted from wood burning, meat charbroiling, and cigarettes. Environ. Sci. Technol. 1999, 33, 3516–3523. [Google Scholar] [CrossRef]
- Hays, M.D.; Geron, C.D.; Linna, K.J.; Smith, N.D.; Schauer, J.J. Speciation of gas-phase and fine particle emissions from burning of foliar fuels. Environ. Sci. Technol. 2002, 36, 2281–2295. [Google Scholar] [CrossRef]
- Maji, K.J.; Dikshit, A.K.; Arora, M.; Deshpande, A. Estimating premature mortality attributable to PM2.5 exposure and benefit of air pollution control policies in China for 2020. Sci. Total Environ. 2018, 612, 683–693. [Google Scholar] [CrossRef]
- Ihantola, T.; Di Bucchianico, S.; Happo, M.; Ihalainen, M.; Uski, O.; Bauer, S.; Kuuspalo, K.; Sippula, O.; Tissari, J.; Oeder, S.; et al. Influence of wood species on toxicity of log-wood stove combustion aerosols: A parallel animal and air-liquid interface cell exposure study on spruce and pine smoke. Part Fibre Toxicol. 2020, 17, 27. [Google Scholar] [CrossRef]
- Camp, P.G.; Ramirez-Venegas, A.; Sansores, R.H.; Alva, L.F.; McDougall, J.E.; Sin, D.D.; Pare, P.D.; Muller, N.L.; Silva, C.I.; Rojas, C.E.; et al. COPD phenotypes in biomass smoke- versus tobacco smoke-exposed Mexican women. Eur. Respir. J. 2014, 43, 725–734. [Google Scholar] [CrossRef]
- Rivera, R.M.; Cosio, M.G.; Ghezzo, H.; Salazar, M.; Perez-Padilla, R. Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int. J. Tuberc. Lung Dis. 2008, 12, 972–977. [Google Scholar]
- Stoner, O.; Lewis, J.; Martinez, I.L.; Gumy, S.; Economou, T.; Adair-Rohani, H. Household cooking fuel estimates at global and country level for 1990 to 2030. Nat. Commun. 2021, 12, 5793. [Google Scholar] [CrossRef]
- van Gemert, F.; Kirenga, B.; Chavannes, N.; Kamya, M.; Luzige, S.; Musinguzi, P.; Turyagaruka, J.; Jones, R.; Tsiligianni, I.; Williams, S.; et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): A prospective cross-sectional observational study. Lancet Glob. Health 2015, 3, e44–e51. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Yang, L.; Xu, Y.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; Wen, F.; et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): A national cross-sectional study. Lancet 2018, 391, 1706–1717. [Google Scholar] [CrossRef]
- Mortimer, K.; Montes de Oca, M.; Salvi, S.; Balakrishnan, K.; Hadfield, R.M.; Ramirez-Venegas, A.; Halpin, D.M.G.; Ozoh Obianuju, B.; Han MeiLan, K.; Perez Padilla, R.; et al. Household air pollution and COPD: Cause and effect or confounding by other aspects of poverty? Int. J. Tuberc. Lung Dis. 2022, 26, 206–216. [Google Scholar] [CrossRef]
- Rogalsky, D.K.; Mendola, P.; Metts, T.A.; Martin, W.J., 2nd. Estimating the number of low-income americans exposed to household air pollution from burning solid fuels. Environ. Health Perspect. 2014, 122, 806–810. [Google Scholar] [CrossRef]
- Robin, L.F.; Less, P.S.; Winget, M.; Steinhoff, M.; Moulton, L.H.; Santosham, M.; Correa, A. Wood-burning stoves and lower respiratory illnesses in Navajo children. Pediatr. Infect. Dis. J. 1996, 15, 859–865. [Google Scholar] [CrossRef]
- Orozco-Levi, M.; Garcia-Aymerich, J.; Villar, J.; Ramirez-Sarmiento, A.; Anto, J.M.; Gea, J. Wood smoke exposure and risk of chronic obstructive pulmonary disease. Eur. Respir. J. 2006, 27, 542–546. [Google Scholar] [CrossRef]
- Westerling, A.L.; Hidalgo, H.G.; Cayan, D.R.; Swetnam, T.W. Warming and earlier spring increase western U.S. forest wildfire activity. Science 2006, 313, 940–943. [Google Scholar] [CrossRef]
- Bedia, J.; Herrera, S.; Camia, A.; Moreno, J.M.; Gutiérrez, J.M.; García, S.H. Forest fire danger projections in the Mediterranean using ENSEMBLES regional climate change scenarios. Clim. Change 2014, 122, 185–199. [Google Scholar] [CrossRef]
- Reid, C.E.; Brauer, M.; Johnston, F.H.; Jerrett, M.; Balmes, J.R.; Elliott, C.T. Critical Review of Health Impacts of Wildfire Smoke Exposure. Environ. Health Perspect. 2016, 124, 1334–1343. [Google Scholar] [CrossRef]
- Adetona, O.; Reinhardt, T.E.; Domitrovich, J.; Broyles, G.; Adetona, A.M.; Kleinman, M.T.; Ottmar, R.D.; Naeher, L.P. Review of the health effects of wildland fire smoke on wildland firefighters and the public. Inhal. Toxicol. 2016, 28, 95–139. [Google Scholar] [CrossRef] [PubMed]
- Cascio, W.E. Wildland fire smoke and human health. Sci. Total Environ. 2018, 624, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.E.; Maestas, M.M. Wildfire smoke exposure under climate change: Impact on respiratory health of affected communities. Curr. Opin. Pulm. Med. 2019, 25, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.J.; Brummel, S.; Wu, J.; Stern, H.; Ostro, B.; Lipsett, M.; Winer, A.; Street, D.H.; Zhang, L.; Tjoa, T.; et al. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup. Environ. Med. 2009, 66, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Morgan, G.; Sheppeard, V.; Khalaj, B.; Ayyar, A.; Lincoln, D.; Jalaludin, B.; Beard, J.; Corbett, S.; Lumley, T. Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology 2010, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.L.; Hanigan, I.C.; Morgan, G.G.; Henderson, S.B.; Johnston, F.H. Air pollution from bushfires and their association with hospital admissions in Sydney, Newcastle and Wollongong, Australia 1994-2007. Aust. N. Z. J. Public Health 2013, 37, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef]
- DeFlorio-Barker, S.; Crooks, J.; Reyes, J.; Rappold, A.G. Cardiopulmonary Effects of Fine Particulate Matter Exposure among Older Adults, during Wildfire and Non-Wildfire Periods, in the United States 2008-2010. Environ. Health Perspect. 2019, 127, 37006. [Google Scholar] [CrossRef]
- Hsu, H.T.; Wu, C.D.; Chung, M.C.; Shen, T.C.; Lai, T.J.; Chen, C.Y.; Wang, R.Y.; Chung, C.J. The effects of traffic-related air pollutants on chronic obstructive pulmonary disease in the community-based general population. Respir. Res. 2021, 22, 217. [Google Scholar] [CrossRef]
- Liu, S.; Jorgensen, J.T.; Ljungman, P.; Pershagen, G.; Bellander, T.; Leander, K.; Magnusson, P.K.E.; Rizzuto, D.; Hvidtfeldt, U.A.; Raaschou-Nielsen, O.; et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: The ELAPSE project. Environ. Int. 2021, 146, 106267. [Google Scholar] [CrossRef]
- Andersen, Z.J.; Hvidberg, M.; Jensen, S.S.; Ketzel, M.; Loft, S.; Sorensen, M.; Tjonneland, A.; Overvad, K.; Raaschou-Nielsen, O. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: A cohort study. Am. J. Respir. Crit. Care Med. 2011, 183, 455–461. [Google Scholar] [CrossRef]
- Schikowski, T.; Sugiri, D.; Ranft, U.; Gehring, U.; Heinrich, J.; Wichmann, H.E.; Kramer, U. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir. Res. 2005, 6, 152. [Google Scholar] [CrossRef]
- van Eeden, S.F.; Tan, W.C.; Suwa, T.; Mukae, H.; Terashima, T.; Fujii, T.; Qui, D.; Vincent, R.; Hogg, J.C. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am. J. Respir. Crit. Care Med. 2001, 164, 826–830. [Google Scholar] [CrossRef]
- Sawyer, K.; Mundandhara, S.; Ghio, A.J.; Madden, M.C. The effects of ambient particulate matter on human alveolar macrophage oxidative and inflammatory responses. J. Toxicol. Environ. Health A 2010, 73, 41–57. [Google Scholar] [CrossRef]
- Becker, S.; Mundandhara, S.; Devlin, R.B.; Madden, M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicol. Appl. Pharmacol. 2005, 207, 269–275. [Google Scholar] [CrossRef]
- Shoenfelt, J.; Mitkus, R.J.; Zeisler, R.; Spatz, R.O.; Powell, J.; Fenton, M.J.; Squibb, K.A.; Medvedev, A.E. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J. Leukoc. Biol. 2009, 86, 303–312. [Google Scholar] [CrossRef]
- Becker, S.; Dailey, L.; Soukup, J.M.; Silbajoris, R.; Devlin, R.B. TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol. Appl. Pharmacol. 2005, 203, 45–52. [Google Scholar] [CrossRef]
- Becker, S.; Fenton, M.J.; Soukup, J.M. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am. J. Respir. Cell Mol. Biol. 2002, 27, 611–618. [Google Scholar] [CrossRef]
- Kampfrath, T.; Maiseyeu, A.; Ying, Z.; Shah, Z.; Deiuliis, J.A.; Xu, X.; Kherada, N.; Brook, R.D.; Reddy, K.M.; Padture, N.P.; et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 2011, 108, 716–726. [Google Scholar] [CrossRef]
- da Silva, L.F.; Saldiva, S.R.; Saldiva, P.H.; Dolhnikoff, M.; Bandeira Cientifica, P. Impaired lung function in individuals chronically exposed to biomass combustion. Environ. Res. 2012, 112, 111–117. [Google Scholar] [CrossRef]
- Regalado, J.; Perez-Padilla, R.; Sansores, R.; Paramo Ramirez, J.I.; Brauer, M.; Pare, P.; Vedal, S. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am. J. Respir. Crit. Care Med. 2006, 174, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Roychoudhury, S.; Siddique, S.; Banerjee, M.; Bhattacharya, P.; Lahiri, T.; Ray, M.R. Respiratory symptoms, lung function decrement and chronic obstructive pulmonary disease in pre-menopausal Indian women exposed to biomass smoke. Inhal. Toxicol. 2014, 26, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Kurmi, O.P.; Devereux, G.S.; Smith, W.C.; Semple, S.; Steiner, M.F.; Simkhada, P.; Lam, K.B.; Ayres, J.G. Reduced lung function due to biomass smoke exposure in young adults in rural Nepal. Eur. Respir. J. 2013, 41, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Moran-Mendoza, O.; Perez-Padilla, J.R.; Salazar-Flores, M.; Vazquez-Alfaro, F. Wood smoke-associated lung disease: A clinical, functional, radiological and pathological description. Int. J. Tuberc. Lung Dis. 2008, 12, 1092–1098. [Google Scholar] [PubMed]
- Xia, Y.; Zhang, H.; Cao, L.; Zhao, Y. Household solid fuel use and peak expiratory flow in middle-aged and older adults in China: A large cohort study (2011–2015). Environ. Res. 2021, 193, 110566. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; Wyss, A.B.; Hoang, T.T.; Lee, M.; Richards, M.; Parks, C.G.; Beane-Freeman, L.E.; Hankinson, J.L.; Umbach, D.M.; London, S.J. Residential Wood Burning and Pulmonary Function in the Agricultural Lung Health Study. Environ. Health Perspect. 2022, 130, 87008. [Google Scholar] [CrossRef]
- Ramirez-Venegas, A.; Sansores, R.H.; Quintana-Carrillo, R.H.; Velazquez-Uncal, M.; Hernandez-Zenteno, R.J.; Sanchez-Romero, C.; Velazquez-Montero, A.; Flores-Trujillo, F. FEV1 decline in patients with chronic obstructive pulmonary disease associated with biomass exposure. Am. J. Respir. Crit. Care Med. 2014, 190, 996–1002. [Google Scholar] [CrossRef]
- Tan, W.C.; Sin, D.D.; Bourbeau, J.; Hernandez, P.; Chapman, K.R.; Cowie, R.; FitzGerald, J.M.; Marciniuk, D.D.; Maltais, F.; Buist, A.S.; et al. Characteristics of COPD in never-smokers and ever-smokers in the general population: Results from the CanCOLD study. Thorax 2015, 70, 822–829. [Google Scholar] [CrossRef]
- Golpe, R.; Sanjuan Lopez, P.; Cano Jimenez, E.; Castro Anon, O.; Perez de Llano, L.A. Distribution of clinical phenotypes in patients with chronic obstructive pulmonary disease caused by biomass and tobacco smoke. Arch. Bronconeumol. 2014, 50, 318–324. [Google Scholar] [CrossRef]
- Mahesh, P.A.; Jayaraj, B.S.; Prabhakar, A.K.; Chaya, S.K.; Vijaysimha, R. Identification of a threshold for biomass exposure index for chronic bronchitis in rural women of Mysore district, Karnataka, India. Indian J. Med. Res. 2013, 137, 87–94. [Google Scholar]
- Falfan-Valencia, R.; Ramirez-Venegas, A.; Perez Lara-Albisua, J.L.; Ramirez-Rodriguez, S.L.; Marquez-Garcia, J.E.; Buendia-Roldan, I.; Gayosso-Gomez, L.V.; Perez-Padilla, R.; Ortiz-Quintero, B. Smoke exposure from chronic biomass burning induces distinct accumulative systemic inflammatory cytokine alterations compared to tobacco smoking in healthy women. Cytokine 2020, 131, 155089. [Google Scholar] [CrossRef]
- Mondal, N.K.; Bhattacharya, P.; Ray, M.R. Assessment of DNA damage by comet assay and fast halo assay in buccal epithelial cells of Indian women chronically exposed to biomass smoke. Int. J. Hyg. Environ. Health 2011, 214, 311–318. [Google Scholar] [CrossRef]
- Mukherjee, B.; Dutta, A.; Roychoudhury, S.; Ray, M.R. Chronic inhalation of biomass smoke is associated with DNA damage in airway cells: Involvement of particulate pollutants and benzene. J. Appl. Toxicol. 2013, 33, 281–289. [Google Scholar] [CrossRef]
- Banerjee, A.; Mondal, N.K.; Das, D.; Ray, M.R. Neutrophilic inflammatory response and oxidative stress in premenopausal women chronically exposed to indoor air pollution from biomass burning. Inflammation 2012, 35, 671–683. [Google Scholar] [CrossRef]
- Mondal, N.K.; Saha, H.; Mukherjee, B.; Tyagi, N.; Ray, M.R. Inflammation, oxidative stress, and higher expression levels of Nrf2 and NQO1 proteins in the airways of women chronically exposed to biomass fuel smoke. Mol. Cell. Biochem. 2018, 447, 63–76. [Google Scholar] [CrossRef]
- Dutta, A.; Roychoudhury, S.; Chowdhury, S.; Ray, M.R. Changes in sputum cytology, airway inflammation and oxidative stress due to chronic inhalation of biomass smoke during cooking in premenopausal rural Indian women. Int. J. Hyg. Environ. Health 2013, 216, 301–308. [Google Scholar] [CrossRef]
- Dutta, A.; Ray, M.R.; Banerjee, A. Systemic inflammatory changes and increased oxidative stress in rural Indian women cooking with biomass fuels. Toxicol. Appl. Pharmacol. 2012, 261, 255–262. [Google Scholar] [CrossRef]
- Raqib, R.; Akhtar, E.; Sultana, T.; Ahmed, S.; Chowdhury, M.A.H.; Shahriar, M.H.; Kader, S.B.; Eunus, M.; Haq, M.A.; Sarwar, G.; et al. Association of household air pollution with cellular and humoral immune responses among women in rural Bangladesh. Environ. Pollut. 2022, 299, 118892. [Google Scholar] [CrossRef]
- Dutta, A.; Bhattacharya, P.; Lahiri, T.; Ray, M.R. Immune cells and cardiovascular health in premenopausal women of rural India chronically exposed to biomass smoke during daily household cooking. Sci. Total Environ. 2012, 438, 293–298. [Google Scholar] [CrossRef]
- Burbank, A.J.; Vadlamudi, A.; Mills, K.H.; Alt, E.M.; Wells, H.; Zhou, H.; Alexis, N.; Hernandez, M.L.; Peden, D.B. The glutathione-S-transferase mu-1 null genotype increases wood smoke-induced airway inflammation. J. Allergy Clin. Immunol. 2019, 143, 2299–2302. [Google Scholar] [CrossRef]
- Barregard, L.; Sallsten, G.; Andersson, L.; Almstrand, A.C.; Gustafson, P.; Andersson, M.; Olin, A.C. Experimental exposure to wood smoke: Effects on airway inflammation and oxidative stress. Occup. Environ. Med. 2008, 65, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Rebuli, M.E.; Speen, A.M.; Martin, E.M.; Addo, K.A.; Pawlak, E.A.; Glista-Baker, E.; Robinette, C.; Zhou, H.; Noah, T.L.; Jaspers, I. Wood Smoke Exposure Alters Human Inflammatory Responses to Viral Infection in a Sex-Specific Manner. A Randomized, Placebo-controlled Study. Am. J. Respir. Crit. Care Med. 2019, 199, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Muala, A.; Rankin, G.; Sehlstedt, M.; Unosson, J.; Bosson, J.A.; Behndig, A.; Pourazar, J.; Nystrom, R.; Pettersson, E.; Bergvall, C.; et al. Acute exposure to wood smoke from incomplete combustion—Indications of cytotoxicity. Part Fibre Toxicol. 2015, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Sehlstedt, M.; Dove, R.; Boman, C.; Pagels, J.; Swietlicki, E.; Londahl, J.; Westerholm, R.; Bosson, J.; Barath, S.; Behndig, A.F.; et al. Antioxidant airway responses following experimental exposure to wood smoke in man. Part Fibre Toxicol. 2010, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Stockfelt, L.; Sallsten, G.; Almerud, P.; Basu, S.; Barregard, L. Short-term chamber exposure to low doses of two kinds of wood smoke does not induce systemic inflammation, coagulation or oxidative stress in healthy humans. Inhal. Toxicol. 2013, 25, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Forchhammer, L.; Moller, P.; Riddervold, I.S.; Bonlokke, J.; Massling, A.; Sigsgaard, T.; Loft, S. Controlled human wood smoke exposure: Oxidative stress, inflammation and microvascular function. Part Fibre Toxicol. 2012, 9, 7. [Google Scholar] [CrossRef]
- Stockfelt, L.; Sallsten, G.; Olin, A.C.; Almerud, P.; Samuelsson, L.; Johannesson, S.; Molnar, P.; Strandberg, B.; Almstrand, A.C.; Bergemalm-Rynell, K.; et al. Effects on airways of short-term exposure to two kinds of wood smoke in a chamber study of healthy humans. Inhal. Toxicol. 2012, 24, 47–59. [Google Scholar] [CrossRef]
- Peters, B.; Ballmann, C.; Quindry, T.; Zehner, E.G.; McCroskey, J.; Ferguson, M.; Ward, T.; Dumke, C.; Quindry, J.C. Experimental Woodsmoke Exposure During Exercise and Blood Oxidative Stress. J. Occup. Environ. Med. 2018, 60, 1073–1081. [Google Scholar] [CrossRef]
- Danielsen, P.H.; Brauner, E.V.; Barregard, L.; Sallsten, G.; Wallin, M.; Olinski, R.; Rozalski, R.; Moller, P.; Loft, S. Oxidatively damaged DNA and its repair after experimental exposure to wood smoke in healthy humans. Mutat. Res. 2008, 642, 37–42. [Google Scholar] [CrossRef]
- Fedak, K.M.; Good, N.; Walker, E.S.; Balmes, J.; Brook, R.D.; Clark, M.L.; Cole-Hunter, T.; Devlin, R.; L'Orange, C.; Luckasen, G.; et al. Acute Effects on Blood Pressure Following Controlled Exposure to Cookstove Air Pollution in the STOVES Study. J. Am. Heart Assoc. 2019, 8, e012246. [Google Scholar] [CrossRef]
- Unosson, J.; Blomberg, A.; Sandstrom, T.; Muala, A.; Boman, C.; Nystrom, R.; Westerholm, R.; Mills, N.L.; Newby, D.E.; Langrish, J.P.; et al. Exposure to wood smoke increases arterial stiffness and decreases heart rate variability in humans. Part Fibre Toxicol. 2013, 10, 20. [Google Scholar] [CrossRef]
- Barregard, L.; Sallsten, G.; Gustafson, P.; Andersson, L.; Johansson, L.; Basu, S.; Stigendal, L. Experimental exposure to wood-smoke particles in healthy humans: Effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal. Toxicol. 2006, 18, 845–853. [Google Scholar] [CrossRef]
- Gao, M.; Liang, C.; Hong, W.; Yu, X.; Zhou, Y.; Sun, R.; Li, H.; Huang, H.; Gan, X.; Yuan, Z.; et al. Biomass-related PM2.5 induces mitochondrial fragmentation and dysfunction in human airway epithelial cells. Environ. Pollut. 2022, 292, 118464. [Google Scholar] [CrossRef]
- Kc, B.; Mahapatra, P.S.; Thakker, D.; Henry, A.P.; Billington, C.K.; Sayers, I.; Puppala, S.P.; Hall, I.P. Proinflammatory Effects in Ex Vivo Human Lung Tissue of Respirable Smoke Extracts from Indoor Cooking in Nepal. Ann. Am. Thorac. Soc. 2020, 17, 688–698. [Google Scholar] [CrossRef]
- Zeglinski, M.R.; Turner, C.T.; Zeng, R.; Schwartz, C.; Santacruz, S.; Pawluk, M.A.; Zhao, H.; Chan, A.W.H.; Carlsten, C.; Granville, D.J. Soluble Wood Smoke Extract Promotes Barrier Dysfunction in Alveolar Epithelial Cells through a MAPK Signaling Pathway. Sci. Rep. 2019, 9, 10027. [Google Scholar] [CrossRef]
- Tassew, D.; Fort, S.; Mebratu, Y.; McDonald, J.; Chu, H.W.; Petersen, H.; Tesfaigzi, Y. Effects of Wood Smoke Constituents on Mucin Gene Expression in Mice and Human Airway Epithelial Cells and on Nasal Epithelia of Subjects with a Susceptibility Gene Variant in Tp53. Environ. Health Perspect. 2022, 130, 17010. [Google Scholar] [CrossRef]
- Pardo, M.; Li, C.; He, Q.; Levin-Zaidman, S.; Tsoory, M.; Yu, Q.; Wang, X.; Rudich, Y. Mechanisms of lung toxicity induced by biomass burning aerosols. Part Fibre Toxicol. 2020, 17, 4. [Google Scholar] [CrossRef]
- Pardo, M.; Li, C.; Fang, Z.; Levin-Zaidman, S.; Dezorella, N.; Czech, H.; Martens, P.; Kafer, U.; Groger, T.; Ruger, C.P.; et al. Toxicity of Water- and Organic-Soluble Wood Tar Fractions from Biomass Burning in Lung Epithelial Cells. Chem. Res. Toxicol. 2021, 34, 1588–1603. [Google Scholar] [CrossRef]
- Deering-Rice, C.E.; Nguyen, N.; Lu, Z.; Cox, J.E.; Shapiro, D.; Romero, E.G.; Mitchell, V.K.; Burrell, K.L.; Veranth, J.M.; Reilly, C.A. Activation of TRPV3 by Wood Smoke Particles and Roles in Pneumotoxicity. Chem. Res. Toxicol. 2018, 31, 291–301. [Google Scholar] [CrossRef]
- Memon, T.A.; Nguyen, N.D.; Burrell, K.L.; Scott, A.F.; Almestica-Roberts, M.; Rapp, E.; Deering-Rice, C.E.; Reilly, C.A. Wood Smoke Particles Stimulate MUC5AC Overproduction by Human Bronchial Epithelial Cells Through TRPA1 and EGFR Signaling. Toxicol. Sci. 2020, 174, 278–290. [Google Scholar] [CrossRef]
- Nguyen, N.D.; Memon, T.A.; Burrell, K.L.; Almestica-Roberts, M.; Rapp, E.; Sun, L.; Scott, A.F.; Rower, J.E.; Deering-Rice, C.E.; Reilly, C.A. Transient Receptor Potential Ankyrin-1 and Vanilloid-3 Differentially Regulate Endoplasmic Reticulum Stress and Cytotoxicity in Human Lung Epithelial Cells After Pneumotoxic Wood Smoke Particle Exposure. Mol. Pharmacol. 2020, 98, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Budello, S.; Marabini, L.; Galbiati, V.; Piazzalunga, A.; Barbieri, P.; Cozzutto, S.; Marinovich, M.; Pitea, D.; Galli, C.L. Comparison of wood smoke PM2.5 obtained from the combustion of FIR and beech pellets on inflammation and DNA damage in A549 and THP-1 human cell lines. Arch. Toxicol. 2013, 87, 2187–2199. [Google Scholar] [CrossRef] [PubMed]
- Danielsen, P.H.; Moller, P.; Jensen, K.A.; Sharma, A.K.; Wallin, H.; Bossi, R.; Autrup, H.; Molhave, L.; Ravanat, J.L.; Briede, J.J.; et al. Oxidative stress, DNA damage, and inflammation induced by ambient air and wood smoke particulate matter in human A549 and THP-1 cell lines. Chem. Res. Toxicol. 2011, 24, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Monn, C.; Becker, S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10-2.5) in outdoor and indoor air. Toxicol. Appl. Pharmacol. 1999, 155, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Palecanda, A.; Paulauskis, J.; Al-Mutairi, E.; Imrich, A.; Qin, G.; Suzuki, H.; Kodama, T.; Tryggvason, K.; Koziel, H.; Kobzik, L. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J. Exp. Med. 1999, 189, 1497–1506. [Google Scholar] [CrossRef]
- Mukae, H.; Hogg, J.C.; English, D.; Vincent, R.; van Eeden, S.F. Phagocytosis of particulate air pollutants by human alveolar macrophages stimulates the bone marrow. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L924–L931. [Google Scholar] [CrossRef]
- Hiraiwa, K.; van Eeden, S.F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediat. Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef]
- Arredouani, M.S.; Yang, Z.; Imrich, A.; Ning, Y.; Qin, G.; Kobzik, L. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am. J. Respir. Cell Mol. Biol. 2006, 35, 474–478. [Google Scholar] [CrossRef]
- Arredouani, M.; Yang, Z.; Ning, Y.; Qin, G.; Soininen, R.; Tryggvason, K.; Kobzik, L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J. Exp. Med. 2004, 200, 267–272. [Google Scholar] [CrossRef]
- van Eeden, S.F.; Hogg, J.C. Systemic inflammatory response induced by particulate matter air pollution: The importance of bone-marrow stimulation. J. Toxicol. Environ. Health A 2002, 65, 1597–1613. [Google Scholar] [CrossRef]
- Miyata, R.; van Eeden, S.F. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol. Appl. Pharmacol. 2011, 257, 209–226. [Google Scholar] [CrossRef]
- Ishii, H.; Hayashi, S.; Hogg, J.C.; Fujii, T.; Goto, Y.; Sakamoto, N.; Mukae, H.; Vincent, R.; van Eeden, S.F. Alveolar macrophage-epithelial cell interaction following exposure to atmospheric particles induces the release of mediators involved in monocyte mobilization and recruitment. Respir. Res. 2005, 6, 87. [Google Scholar] [CrossRef]
- Schins, R.P.; Lightbody, J.H.; Borm, P.J.; Shi, T.; Donaldson, K.; Stone, V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol. Appl. Pharmacol. 2004, 195, 1–11. [Google Scholar] [CrossRef]
- Park, E.J.; Roh, J.; Kim, Y.; Park, K.; Kim, D.S.; Yu, S.D. PM 2.5 collected in a residential area induced Th1-type inflammatory responses with oxidative stress in mice. Environ. Res. 2011, 111, 348–355. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, Y.; Wang, Q.; Chen, H.; Zhou, X. Fine particulate matter exposure promotes M2 macrophage polarization through inhibiting histone deacetylase 2 in the pathogenesis of chronic obstructive pulmonary disease. Ann. Transl. Med. 2020, 8, 1303. [Google Scholar] [CrossRef]
- Sakamoto, N.; Hayashi, S.; Mukae, H.; Vincent, R.; Hogg, J.C.; van Eeden, S.F. Effect of atorvastatin on PM10-induced cytokine production by human alveolar macrophages and bronchial epithelial cells. Int. J. Toxicol. 2009, 28, 17–23. [Google Scholar] [CrossRef]
- Rylance, J.; Fullerton, D.G.; Scriven, J.; Aljurayyan, A.N.; Mzinza, D.; Barrett, S.; Wright, A.K.; Wootton, D.G.; Glennie, S.J.; Baple, K.; et al. Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 52, 584–593. [Google Scholar] [CrossRef]
- Ghosh, B.; Gaike, A.H.; Pyasi, K.; Brashier, B.; Das, V.V.; Londhe, J.D.; Juvekar, S.; Shouche, Y.S.; Donnelly, L.E.; Salvi, S.S.; et al. Bacterial load and defective monocyte-derived macrophage bacterial phagocytosis in biomass smoke-related COPD. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Hong, W.; Li, B.; Zhou, Y.; Ran, P. Chronic exposure to biomass ambient particulate matter triggers alveolar macrophage polarization and activation in the rat lung. J. Cell Mol. Med. 2022, 26, 1156–1168. [Google Scholar] [CrossRef]
- Bazzan, E.; Turato, G.; Tinè, M.; Radu, C.M.; Balestro, E.; Rigobello, C.; Biondini, D.; Schiavon, M.; Lunardi, F.; Baraldo, S.; et al. Dual polarization of human alveolar macrophages progressively increases with smoking and COPD severity. Respir. Res. 2017, 18, 40. [Google Scholar]
- Shaykhiev, R.; Krause, A.; Salit, J.; Strulovici-Barel, Y.; Harvey, B.G.; O'Connor, T.P.; Crystal, R.G. Smoking-dependent reprogramming of alveolar macrophage polarization: Implication for pathogenesis of chronic obstructive pulmonary disease. J. Immunol. 2009, 183, 2867–2883. [Google Scholar] [CrossRef] [PubMed]
- Krimmer, D.; Ichimaru, Y.; Burgess, J.; Black, J.; Oliver, B. Exposure to biomass smoke extract enhances fibronectin release from fibroblasts. PLoS ONE 2013, 8, e83938. [Google Scholar] [CrossRef]
- Forchhammer, L.; Loft, S.; Roursgaard, M.; Cao, Y.; Riddervold, I.S.; Sigsgaard, T.; Moller, P. Expression of adhesion molecules, monocyte interactions and oxidative stress in human endothelial cells exposed to wood smoke and diesel exhaust particulate matter. Toxicol. Lett. 2012, 209, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, D.; Deering-Rice, C.E.; Romero, E.G.; Hughen, R.W.; Light, A.R.; Veranth, J.M.; Reilly, C.A. Activation of transient receptor potential ankyrin-1 (TRPA1) in lung cells by wood smoke particulate material. Chem. Res. Toxicol. 2013, 26, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Burrell, K.L.; Nguyen, N.D.; Deering-Rice, C.E.; Memon, T.A.; Almestica-Roberts, M.; Rapp, E.; Serna, S.N.; Lamb, J.G.; Reilly, C.A. Dynamic Expression of Transient Receptor Potential Vanilloid-3 and Integrated Signaling with Growth Factor Pathways during Lung Epithelial Wound Repair following Wood Smoke Particle and Other Forms of Lung Cell Injury. Mol. Pharmacol. 2021, 100, 295–307. [Google Scholar] [CrossRef]
- Huang, L.; Pu, J.; He, F.; Liao, B.; Hao, B.; Hong, W.; Ye, X.; Chen, J.; Zhao, J.; Liu, S.; et al. Positive feedback of the amphiregulin-EGFR-ERK pathway mediates PM2.5 from wood smoke-induced MUC5AC expression in epithelial cells. Sci. Rep. 2017, 7, 11084. [Google Scholar] [CrossRef]
- Sussan, T.E.; Ingole, V.; Kim, J.H.; McCormick, S.; Negherbon, J.; Fallica, J.; Akulian, J.; Yarmus, L.; Feller-Kopman, D.; Wills-Karp, M.; et al. Source of biomass cooking fuel determines pulmonary response to household air pollution. Am. J. Respir. Cell Mol. Biol. 2014, 50, 538–548. [Google Scholar] [CrossRef]
- Shyam Prasad Shetty, B.; Chaya, S.K.; Kumar, V.S.; Mahendra, M.; Jayaraj, B.S.; Lokesh, K.S.; Ganguly, K.; Mahesh, P.A. Inflammatory Biomarkers Interleukin 1 Beta (IL-1beta) and Tumour Necrosis Factor Alpha (TNF-alpha) Are Differentially Elevated in Tobacco Smoke Associated COPD and Biomass Smoke Associated COPD. Toxics 2021, 9, 72. [Google Scholar] [CrossRef]
- Vishweswaraiah, S.; Thimraj, T.A.; George, L.; Krishnarao, C.S.; Lokesh, K.S.; Siddaiah, J.B.; Larsson, K.; Upadhyay, S.; Palmberg, L.; Anand, M.P.; et al. Putative Systemic Biomarkers of Biomass Smoke-Induced Chronic Obstructive Pulmonary Disease among Women in a Rural South Indian Population. Dis. Markers 2018, 2018, 4949175. [Google Scholar] [CrossRef]
- Fernandes, L.; Rane, S.; Mandrekar, S.; Mesquita, A.M. Eosinophilic Airway Inflammation in Patients with Stable Biomass Smoke- versus Tobacco Smoke-Associated Chronic Obstructive Pulmonary Disease. J Health Pollut 2019, 9, 191209. [Google Scholar] [CrossRef]
- Olloquequi, J.; Jaime, S.; Parra, V.; Cornejo-Cordova, E.; Valdivia, G.; Agusti, A.; Silva, O.R. Comparative analysis of COPD associated with tobacco smoking, biomass smoke exposure or both. Respir. Res. 2018, 19, 13. [Google Scholar] [CrossRef]
- Golpe, R.; Martin-Robles, I.; Sanjuan-Lopez, P.; Perez-de-Llano, L.; Gonzalez-Juanatey, C.; Lopez-Campos, J.L.; Arellano-Orden, E. Differences in systemic inflammation between cigarette and biomass smoke-induced COPD. Int. J. Chron. Obstr. Pulmon. Dis. 2017, 12, 2639–2646. [Google Scholar] [CrossRef]
- Veerapaneni, V.V.; Upadhyay, S.; Thimraj, T.A.; Siddaiah, J.B.; Krishnarao, C.S.; Lokesh, K.S.; Thimmulappa, R.; Palmberg, L.; Ganguly, K.; Anand, M.P. Circulating Secretoglobin Family 1A Member 1 (SCGB1A1) Levels as a Marker of Biomass Smoke Induced Chronic Obstructive Pulmonary Disease. Toxics 2021, 9, 208. [Google Scholar] [CrossRef]
- Montano, M.; Sansores, R.H.; Becerril, C.; Cisneros, J.; Gonzalez-Avila, G.; Sommer, B.; Ochoa, L.; Herrera, I.; Ramirez-Venegas, A.; Ramos, C. FEV1 inversely correlates with metalloproteinases 1, 7, 9 and CRP in COPD by biomass smoke exposure. Respir. Res. 2014, 15, 74. [Google Scholar] [CrossRef]
- Montano, M.; Cisneros, J.; Ramirez-Venegas, A.; Pedraza-Chaverri, J.; Mercado, D.; Ramos, C.; Sansores, R.H. Malondialdehyde and superoxide dismutase correlate with FEV(1) in patients with COPD associated with wood smoke exposure and tobacco smoking. Inhal. Toxicol. 2010, 22, 868–874. [Google Scholar] [CrossRef]
- Ceylan, E.; Kocyigit, A.; Gencer, M.; Aksoy, N.; Selek, S. Increased DNA damage in patients with chronic obstructive pulmonary disease who had once smoked or been exposed to biomass. Respir. Med. 2006, 100, 1270–1276. [Google Scholar] [CrossRef]
- Faiz, A.; Steiling, K.; Roffel, M.P.; Postma, D.S.; Spira, A.; Lenburg, M.E.; Borggrewe, M.; Eijgenraam, T.R.; Jonker, M.R.; Koppelman, G.H.; et al. Effect of long-term corticosteroid treatment on microRNA and gene-expression profiles in COPD. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; Raman, I.; Yan, M.; Chen, Q.; Li, Q.Z. Peripheral Blood Mononuclear Cell Gene Expression in Chronic Obstructive Pulmonary Disease: miRNA and mRNA Regulation. J. Inflamm. Res. 2022, 15, 2167–2180. [Google Scholar] [CrossRef]
- Conickx, G.; Mestdagh, P.; Avila Cobos, F.; Verhamme, F.M.; Maes, T.; Vanaudenaerde, B.M.; Seys, L.J.; Lahousse, L.; Kim, R.Y.; Hsu, A.C.; et al. MicroRNA Profiling Reveals a Role for MicroRNA-218-5p in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2017, 195, 43–56. [Google Scholar] [CrossRef]
- Tang, K.; Zhao, J.; Xie, J.; Wang, J. Decreased miR-29b expression is associated with airway inflammation in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L621–L629. [Google Scholar] [CrossRef]
- Van Pottelberge, G.R.; Mestdagh, P.; Bracke, K.R.; Thas, O.; van Durme, Y.M.; Joos, G.F.; Vandesompele, J.; Brusselle, G.G. MicroRNA expression in induced sputum of smokers and patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 183, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Ludwig, N.; Fehlmann, T.; Kahraman, M.; Backes, C.; Kern, F.; Vogelmeier, C.F.; Diener, C.; Fischer, U.; Biertz, F.; et al. Low miR-150-5p and miR-320b Expression Predicts Reduced Survival of COPD Patients. Cells 2019, 8, 1162. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Mao, Z.D.; Shi, Y.J.; Liu, Z.G.; Cao, Q.; Zhang, Q. Comprehensive Analysis of miRNA-mRNA-lncRNA Networks in Non-Smoking and Smoking Patients with Chronic Obstructive Pulmonary Disease. Cell. Physiol. Biochem. 2018, 50, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Torres, Y.; Ruiz, V.; Montano, M.; Perez-Padilla, R.; Falfan-Valencia, R.; Perez-Ramos, J.; Perez-Bautista, O.; Ramos, C. Participation of the miR-22-HDAC4-DLCO Axis in Patients with COPD by Tobacco and Biomass. Biomolecules 2019, 9, 837. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Torres, Y.; Ruiz-Lopez, V.; Perez-Bautista, O.; Buendia-Roldan, I.; Ramirez-Venegas, A.; Perez-Ramos, J.; Falfan-Valencia, R.; Ramos, C.; Montano, M. miR-34a in serum is involved in mild-to-moderate COPD in women exposed to biomass smoke. BMC Pulm. Med. 2019, 19, 227. [Google Scholar] [CrossRef]
- Long, Y.J.; Liu, X.P.; Chen, S.S.; Zong, D.D.; Chen, Y.; Chen, P. miR-34a is involved in CSE-induced apoptosis of human pulmonary microvascular endothelial cells by targeting Notch-1 receptor protein. Respir. Res. 2018, 19, 21. [Google Scholar] [CrossRef]
- Diaz-Pena, R.; Silva, R.S.; Hosgood, H.D., 3rd; Agusti, A.; Olloquequi, J. Specific miRNA Profile in Chronic Obstructive Pulmonary Disease Related to Biomass Smoke Exposure. Arch. Bronconeumol. 2022, 58, 177–179. [Google Scholar] [CrossRef]
- Silverman, E.K.; Chapman, H.A.; Drazen, J.M.; Weiss, S.T.; Rosner, B.; Campbell, E.J.; O'Donnell, W.J.; Reilly, J.J.; Ginns, L.; Mentzer, S.; et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am. J. Respir. Crit. Care Med. 1998, 157, 1770–1778. [Google Scholar] [CrossRef]
- McCloskey, S.C.; Patel, B.D.; Hinchliffe, S.J.; Reid, E.D.; Wareham, N.J.; Lomas, D.A. Siblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstruction. Am. J. Respir. Crit. Care Med. 2001, 164, 1419–1424. [Google Scholar] [CrossRef]
- Ingebrigtsen, T.; Thomsen, S.F.; Vestbo, J.; van der Sluis, S.; Kyvik, K.O.; Silverman, E.K.; Svartengren, M.; Backer, V. Genetic influences on Chronic Obstructive Pulmonary Disease - a twin study. Respir. Med. 2010, 104, 1890–1895. [Google Scholar] [CrossRef]
- Zhou, J.J.; Cho, M.H.; Castaldi, P.J.; Hersh, C.P.; Silverman, E.K.; Laird, N.M. Heritability of chronic obstructive pulmonary disease and related phenotypes in smokers. Am. J. Respir. Crit. Care Med. 2013, 188, 941–947. [Google Scholar] [CrossRef]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha1-Antitrypsin Deficiency. N. Engl. J. Med. 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- Ragland, M.F.; Benway, C.J.; Lutz, S.M.; Bowler, R.P.; Hecker, J.; Hokanson, J.E.; Crapo, J.D.; Castaldi, P.J.; DeMeo, D.L.; Hersh, C.P.; et al. Genetic Advances in Chronic Obstructive Pulmonary Disease. Insights from COPDGene. Am. J. Respir. Crit. Care Med. 2019, 200, 677–690. [Google Scholar] [CrossRef]
- Cho, M.H.; McDonald, M.L.; Zhou, X.; Mattheisen, M.; Castaldi, P.J.; Hersh, C.P.; Demeo, D.L.; Sylvia, J.S.; Ziniti, J.; Laird, N.M.; et al. Risk loci for chronic obstructive pulmonary disease: A genome-wide association study and meta-analysis. Lancet Respir. Med. 2014, 2, 214–225. [Google Scholar] [CrossRef]
- Oelsner, E.C.; Ortega, V.E.; Smith, B.M.; Nguyen, J.N.; Manichaikul, A.W.; Hoffman, E.A.; Guo, X.; Taylor, K.D.; Woodruff, P.G.; Couper, D.J.; et al. A Genetic Risk Score Associated with Chronic Obstructive Pulmonary Disease Susceptibility and Lung Structure on Computed Tomography. Am. J. Respir. Crit. Care Med. 2019, 200, 721–731. [Google Scholar] [CrossRef]
- Cho, M.H.; Boutaoui, N.; Klanderman, B.J.; Sylvia, J.S.; Ziniti, J.P.; Hersh, C.P.; DeMeo, D.L.; Hunninghake, G.M.; Litonjua, A.A.; Sparrow, D.; et al. Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat. Genet. 2010, 42, 200–202. [Google Scholar] [CrossRef]
- Pillai, S.G.; Ge, D.; Zhu, G.; Kong, X.; Shianna, K.V.; Need, A.C.; Feng, S.; Hersh, C.P.; Bakke, P.; Gulsvik, A.; et al. A genome-wide association study in chronic obstructive pulmonary disease (COPD): Identification of two major susceptibility loci. PLoS Genet. 2009, 5, e1000421. [Google Scholar] [CrossRef]
- Cho, M.H.; Castaldi, P.J.; Wan, E.S.; Siedlinski, M.; Hersh, C.P.; Demeo, D.L.; Himes, B.E.; Sylvia, J.S.; Klanderman, B.J.; Ziniti, J.P.; et al. A genome-wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum. Mol. Genet. 2012, 21, 947–957. [Google Scholar] [CrossRef]
- Wain, L.V.; Shrine, N.; Miller, S.; Jackson, V.E.; Ntalla, I.; Soler Artigas, M.; Billington, C.K.; Kheirallah, A.K.; Allen, R.; Cook, J.P.; et al. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): A genetic association study in UK Biobank. Lancet Respir. Med. 2015, 3, 769–781. [Google Scholar] [CrossRef]
- Wain, L.V.; Shrine, N.; Artigas, M.S.; Erzurumluoglu, A.M.; Noyvert, B.; Bossini-Castillo, L.; Obeidat, M.; Henry, A.P.; Portelli, M.A.; Hall, R.J.; et al. Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat. Genet. 2017, 49, 416–425. [Google Scholar] [CrossRef]
- Hobbs, B.D.; de Jong, K.; Lamontagne, M.; Bosse, Y.; Shrine, N.; Artigas, M.S.; Wain, L.V.; Hall, I.P.; Jackson, V.E.; Wyss, A.B.; et al. Genetic loci associated with chronic obstructive pulmonary disease overlap with loci for lung function and pulmonary fibrosis. Nat. Genet. 2017, 49, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Sakornsakolpat, P.; Prokopenko, D.; Lamontagne, M.; Reeve, N.F.; Guyatt, A.L.; Jackson, V.E.; Shrine, N.; Qiao, D.; Bartz, T.M.; Kim, D.K.; et al. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat. Genet. 2019, 51, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qiao, D.; Yang, C.; Kasela, S.; Kim, W.; Ma, Y.; Shrine, N.; Batini, C.; Sofer, T.; Taliun, S.A.G.; et al. Whole genome sequence analysis of pulmonary function and COPD in 19,996 multi-ethnic participants. Nat. Commun. 2020, 11, 5182. [Google Scholar] [CrossRef] [PubMed]
- Ambrocio-Ortiz, E.; Perez-Rubio, G.; Ramirez-Venegas, A.; Hernandez-Zenteno, R.J.; Paredes-Lopez, A.; Sansores, R.H.; Ramirez-Diaz, M.E.; Cruz-Vicente, F.; Martinez-Gomez, M.L.; Falfan-Valencia, R. Protective Role of Genetic Variants in HSP90 Genes-Complex in COPD Secondary to Biomass-Burning Smoke Exposure and Non-Severe COPD Forms in Tobacco Smoking Subjects. Curr. Issues. Mol. Biol. 2021, 43, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Gallegos, M.A.; Perez-Rubio, G.; Ambrocio-Ortiz, E.; Partida-Zavala, N.; Hernandez-Zenteno, R.; Flores-Trujillo, F.; Garcia-Gomez, L.; Hernandez-Perez, A.; Ramirez-Venegas, A.; Falfan-Valencia, R. Genetic variants in IL17A and serum levels of IL-17A are associated with COPD related to tobacco smoking and biomass burning. Sci. Rep. 2020, 10, 784. [Google Scholar] [CrossRef]
- Liu, M.; Wu, K.; Lin, J.; Xie, Q.; Liu, Y.; Huang, Y.; Zeng, J.; Yang, Z.; Wang, Y.; Dong, S.; et al. Emerging Biological Functions of IL-17A: A New Target in Chronic Obstructive Pulmonary Disease? Front. Pharm. 2021, 12, 695957. [Google Scholar] [CrossRef]
- Ortega-Martinez, A.; Perez-Rubio, G.; Ramirez-Venegas, A.; Ramirez-Diaz, M.E.; Cruz-Vicente, F.; Martinez-Gomez, M.L.; Ramos-Martinez, E.; Abarca-Rojano, E.; Falfan-Valencia, R. Participation of HHIP Gene Variants in COPD Susceptibility, Lung Function, and Serum and Sputum Protein Levels in Women Exposed to Biomass-Burning Smoke. Diagnostics 2020, 10, 734. [Google Scholar] [CrossRef]
- Wilk, J.B.; Chen, T.H.; Gottlieb, D.J.; Walter, R.E.; Nagle, M.W.; Brandler, B.J.; Myers, R.H.; Borecki, I.B.; Silverman, E.K.; Weiss, S.T.; et al. A genome-wide association study of pulmonary function measures in the Framingham Heart Study. PLoS Genet. 2009, 5, e1000429. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zheng, Z.; Chen, X.; Zeng, X.; Zhang, Y.; Li, D.; Shu, J.; Yang, K.; Lai, N.; et al. Genetic Variants in the Hedgehog Interacting Protein Gene Are Associated with the FEV1/FVC Ratio in Southern Han Chinese Subjects with Chronic Obstructive Pulmonary Disease. Biomed. Res. Int. 2017, 2017, 2756726. [Google Scholar] [CrossRef]
- Ponce-Gallegos, M.A.; Perez-Rubio, G.; Garcia-Carmona, A.; Garcia-Gomez, J.; Hernandez-Zenteno, R.; Ramirez-Venegas, A.; Falfan-Valencia, R. Haplotype in SERPINA1 (AAT) Is Associated with Reduced Risk for COPD in a Mexican Mestizo Population. Int. J. Mol. Sci. 2019, 21, 195. [Google Scholar] [CrossRef]
- Resendiz-Hernandez, J.M.; Ambrocio-Ortiz, E.; Perez-Rubio, G.; Lopez-Flores, L.A.; Abarca-Rojano, E.; Pavon-Romero, G.F.; Flores-Trujillo, F.; de Jesus Hernandez-Zenteno, R.; Camarena, A.; Perez-Rodriguez, M.; et al. TNF promoter polymorphisms are associated with genetic susceptibility in COPD secondary to tobacco smoking and biomass burning. Int. J. Chron. Obs. Pulmon. Dis. 2018, 13, 627–637. [Google Scholar] [CrossRef]
- Sapey, E.; Wood, A.M.; Ahmad, A.; Stockley, R.A. Tumor necrosis factor-{alpha}, rs361525 polymorphism is associated with increased local production and downstream inflammation in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 192–199. [Google Scholar] [CrossRef]
- Sakao, S.; Tatsumi, K.; Igari, H.; Shino, Y.; Shirasawa, H.; Kuriyama, T. Association of tumor necrosis factor alpha gene promoter polymorphism with the presence of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 163, 420–422. [Google Scholar] [CrossRef]
- Gingo, M.R.; Silveira, L.J.; Miller, Y.E.; Friedlander, A.L.; Cosgrove, G.P.; Chan, E.D.; Maier, L.A.; Bowler, R.P. Tumour necrosis factor gene polymorphisms are associated with COPD. Eur. Respir. J. 2008, 31, 1005–1012. [Google Scholar] [CrossRef]
- Malaviya, R.; Laskin, J.D.; Laskin, D.L. Anti-TNFalpha therapy in inflammatory lung diseases. Pharmacol. Ther. 2017, 180, 90–98. [Google Scholar] [CrossRef]
- Perez-Rubio, G.; Ambrocio-Ortiz, E.; Lopez-Flores, L.A.; Juarez-Martin, A.I.; Jimenez-Valverde, L.O.; Zoreque-Cabrera, S.; Galicia-Negrete, G.; Ramirez-Diaz, M.E.; Cruz-Vicente, F.; Castillejos-Lopez, M.J.; et al. Heterozygous Genotype rs17580 AT (PiS) in SERPINA1 is Associated with COPD Secondary to Biomass-Burning and Tobacco Smoking: A Case-Control and Populational Study. Int. J. Chron. Obs. Pulmon. Dis. 2020, 15, 1181–1190. [Google Scholar] [CrossRef]
- Yoshida, T.; Tuder, R.M. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol. Rev. 2007, 87, 1047–1082. [Google Scholar] [CrossRef]
- Agusti, A.; Hogg, J.C. Update on the Pathogenesis of Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2019, 381, 1248–1256. [Google Scholar] [CrossRef]
- Kang, M.J.; Shadel, G.S. A Mitochondrial Perspective of Chronic Obstructive Pulmonary Disease Pathogenesis. Tuberc. Respir. Dis. 2016, 79, 207–213. [Google Scholar] [CrossRef]
- Gohy, S.T.; Hupin, C.; Fregimilicka, C.; Detry, B.R.; Bouzin, C.; Gaide Chevronay, H.; Lecocq, M.; Weynand, B.; Ladjemi, M.Z.; Pierreux, C.E.; et al. Imprinting of the COPD airway epithelium for dedifferentiation and mesenchymal transition. Eur. Respir. J. 2015, 45, 1258–1272. [Google Scholar] [CrossRef]
- Shaykhiev, R.; Crystal, R.G. Basal cell origins of smoking-induced airway epithelial disorders. Cell Cycle 2014, 13, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.H.; Shukla, S.D.; Mahmood, M.Q.; Ward, C. Fully integrating pathophysiological insights in COPD: An updated working disease model to broaden therapeutic vision. Eur. Respir. Rev. 2021, 30. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.M.; Patel, I.S.; Wilks, M.; Donaldson, G.C.; Wedzicha, J.A. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Murphy, T.F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 2008, 359, 2355–2365. [Google Scholar] [CrossRef]
- Desai, H.; Eschberger, K.; Wrona, C.; Grove, L.; Agrawal, A.; Grant, B.; Yin, J.; Parameswaran, G.I.; Murphy, T.; Sethi, S. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014, 11, 303–309. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, Y.; Li, X.; Chen, S.; Zhao, Z.; He, F.; Zou, W.; Luo, Q.; Li, W.; Pan, Y.; et al. Lung function and incidence of chronic obstructive pulmonary disease after improved cooking fuels and kitchen ventilation: A 9-year prospective cohort study. PLoS Med. 2014, 11, e1001621. [Google Scholar] [CrossRef]
- Siddharthan, T.; Pollard, S.L.; Jackson, P.; Robertson, N.M.; Wosu, A.C.; Rahman, N.; Padalkar, R.; Sekitoleko, I.; Namazzi, E.; Alupo, P.; et al. Effectiveness of low-dose theophylline for the management of biomass-associated COPD (LODOT-BCOPD): Study protocol for a randomized controlled trial. Trials 2021, 22, 213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).