Abstract
The amyloid cascade hypothesis has predominately been used to describe the pathogenesis of Alzheimer’s disease (AD) for decades, as Aβ oligomers are thought to be the prime cause of AD. Meanwhile, the neurotrophic factor hypothesis has also been proposed for decades. Accumulating evidence states that the amyloidogenic process and neurotrophic dysfunction are mutually influenced and may coincidently cause the onset and progress of AD. Meanwhile, there are intracellular regulators participating both in the amyloidogenic process and neurotrophic pathways, which might be the common original causes of amyloidogenesis and neurotrophic dysfunction. In this review, the current understanding regarding the role of neurotrophic dysfunction and the amyloidogenic process in AD pathology is briefly summarized. The mutual influence of these two pathogenesis pathways and their potential common causal pathway are further discussed. Therapeutic strategies targeting the common pathways to simultaneously prevent amyloidogenesis and neurotrophic dysfunction might be anticipated for the disease-modifying treatment of AD.
1. Introduction
Alzheimer’s disease (AD) is among the most common neurodegenerative disorders of the central nervous system in the elderly. The major neuropathological hallmarks of AD are amyloid plaques comprised of amyloid-β (Aβ) peptides, neurofibrillary tangles (NFTs) primarily composed of hyperphosphorylated tau proteins, selective basal forebrain cholinergic neuron (BFCN) degeneration and brain atrophy. Among the hypotheses for AD pathogenesis, the amyloid cascade hypothesis has dominated in the last few decades [1], and postulates that the abnormal accumulation of amyloid plaques causes neurodegeneration in various areas of the brain. Thus, Aβ oligomers are proposed to be the prime cause of AD, and toxic Aβ oligomer accumulation trigger the other pathologies, such as tau pathology, and inflammatory and synapse damage.
Another classic explanation for the pathogenesis of AD is the neurotrophic factor hypothesis [2,3], represented by the nerve growth factor (NGF) hypothesis [4,5]. Both laboratory and clinical research suggest that the neurotrophic status, supported by NGF and other neurotrophic factors (NTFs), plays a critical role in AD progression [6,7]. In the adult brain, enriched NGF expression appears to be restricted to few areas, of which the basal forebrain cholinergic system (BFCS) is representative. The BFCN plays a critical role in cognitive behavior and attention behavior. In addition to the classic characteristic changes observed in other neuronal systems and the presence of neuronal plaques and tangles, cholinergic deficits in the BFCN seem to be a principal element responsible for the memory loss typical of AD [8]. Significant cholinergic dysfunction and cholinergic neuronal degeneration are associated with cognitive deficits in AD [9,10].
The exact causes of AD are not fully understood and there is still no disease-modifying treatment that cures the disease or alters the disease process. Most of the theories, including the amyloid cascade hypothesis, tau hypothesis and inflammation hypothesis, explain AD pathogenesis from the perspective of overloaded toxicity or stress, whereas amyloidogenesis pathology seems more specific to AD and is better characterized. Complementary to this, the neurotrophic factor hypothesis tries to clarify AD pathogenesis in consideration of the shortage of neurotrophic outcomes. New theories, such as the traffic jam hypothesis, also suggest that upregulated amyloidogenesis and downregulated neurotrophic factor signaling mutually drive AD pathogenesis [11].
As both amyloidogenesis and NTFs are in charge of AD pathogenesis, balancing their neurotoxic and neurotrophic effects may be a considerable strategy for preventing AD. The neurotoxic roles of Aβ oligomers and the neurotrophic roles of NTFs on AD pathogenesis have been extensively reviewed [12,13,14,15,16]. In fact, the signaling pathway of NTFs can regulate amyloidogenesis [17,18], and proteins in the amyloidogenic process pathway affect the NTF signaling pathways [19,20]. Here, the current understanding of the role of NTFs and amyloidogenesis, as well as their interactions, in AD pathology are briefly summarized. The factors linked with both neurotrophic dysfunction and the amyloidogenic process in the pathology of AD are discussed. It is possible that there are various instances of co-operation between each well-characterized pathogenesis of AD that are interesting. It would be meaningful for these to be illustrated in the future to better understand AD pathology and treatment.
2. Amyloidogenesis and AD
The best-characterized histopathology hallmark in the AD brain is the extracellular plaques comprised of the Aβ peptides, which are proteolytic fragments of the amyloid precursor protein (APP) cleavage by β-secretases and γ-secretases. Generally, APP can be processed via α secretase (A disintegrin and metalloproteinases 10/17 (ADAM 10/17)) as a physiological pathway to begin the generation of α products (soluble APPα (sAPPα), c-terminal fragment CTFα), or via β secretase (β-site amyloid precursor protein cleaving enzyme-1 (BACE1) is the major one) as amyloidogenic pathways to begin the generation of β products (soluble APP β (sAPPβ) and C-terminal fragment CTFβ/C99) [21,22]. The cleavage of APP by BACE1 is the rate-limiting step in the generation of Aβ peptides, creating the C99 fragment that becomes a substrate for subsequent γ-secretase (consisting of presenilin, nicastrin, APH-1 and PEN2) to generate mature Aβ peptides. APP cleavage by BACE1 and γ-secretase both occur at the cell membrane and endocytic compartments, respectively. Combining APP cleavage by BACE1 with its processing within compartments of the endocytic pathway results in the overproduction of Aβ products and is proposed to be a pathological mechanism for AD [23].
The earliest Aβ aggregate appears in the parietal lobe, medial temporal lobe and frontal lobe, and then, it gradually exists in the entire neocortex, diencephalon, corpus striatum and more areas [24]. Accumulated clinical and laboratory research supports the assumption that amyloid plaques and soluble amyloid aggregates are the upstream cause of AD, and subsequently trigger the other pathologies, forming the amyloid cascade hypothesis. The general concept that Aβ is associated with cognitive impairment is confirmed by an 11-year follow-up study [25] and a meta-analysis study [26]. Progress in the Aβ-related pathobiology of AD has been extensively documented recently [14]. The amyloid cascade hypothesis is also facing challenges. The development of nearly all drugs targeting amyloid pathology has failed.
3. NTFs and AD
NTFs are a kind of growth factor secreted by neurons, glial cells and target tissues that promote the growth, differentiation and survival of nerve cells during the development and maintenance of the nervous system [27]. The hypothesis of NTFs in AD supports the idea that AD is caused by a deficiency in neurotrophic pathways, making NTF targeting a potential therapeutic tool for AD. The anticipated roles of NTFs in AD treatment have been discussed recently by various labs [28,29,30,31]. Most NTFs exert their effects via signaling through specific transmembrane receptor tyrosine kinases (RTKs) and are typically classified into the following groups: (1) Neurotrophins, the most-studied family of NTFs. The neurotrophins include NGF, brain-derived neurotrophic factor (BDNF) and neurotrophin-3/4/5 (NT-3/4/5). They can be associated with the corresponding high-affinity tropomyosin-related kinase receptors (Trks) to induce their tyrosine kinase activity and downstream signal transduction. NGF specifically binds to the TrkA receptor, BDNF and NT-4/5 specifically binds to the TrkB receptor, and NT-3 specifically binds to the TrkC receptor. Additionally, they can bind to the low-affinity p75 neurotrophic factor receptor (p75NTR) and induce apoptosis. (2) Glial cell-line-derived neurotrophic factor family ligands (GFLs), consisting of glial cell-line-derived neurotrophic factor (GDNF), neurturin (NRTN), artemin (ARTN) and persephin (PSPN). GFLs signal via a multicomponent receptor system consisting of a high-affinity ligand-binding glycosyl-phosphatidylinositol (GPI)-linked co-receptor (GFRα1-4) and the receptor tyrosine kinase (RET). GDNF, NRTN, ARTN and PSPN interact mainly with GFRα1, GFRα2, GFRα3 and GFRα4, respectively, to recruit and activate RET. (3) Neuropoietic cytokines, which are a big group of small secreted proteins, signal through a gp130 receptor complex, consisting of ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), etc. (4) Evolutionarily conserved cerebral dopamine neurotrophic factor (CDNF) and mesencephalic astrocyte-derived neurotrophic factor family (MANF). Besides the “classical” neurotrophic factors, there are growth factors with neurotrophic effects and newly classified NTFs, such as vascular endothelial growth factor (VEGF) and neurotrophic factor-α1 (NF-α1, also known as carboxypeptidase E or CPE), that have also been linked to AD [32,33]. The NTFs and growth factors which have altered expressions or mutations in AD patients are shown in Table 1.

Table 1.
Neurotrophic factors and growth factors altered in AD.
NGF was the first identified NTF and is one of the most researched NTFs associated with AD. Original research exploring the roles of NTFs on AD was initiated in NGF and has accordingly transitioned to clinical trials [68,69]. Dysfunction of the NGF pathway is tightly linked with profound and early BFCN degeneration [8,70,71,72,73]. Studies have proved that the expression of TrkA, but not p75NTR, is downregulated in the BFCNs and the cortex of AD patients [74,75], while the NGF-immunoreactive protein (which is now thought to be proNGF, the NGF precursor protein) level is elevated in the cortex and hippocampus and degraded in the basal forebrain [37,76,77]. This indicates that the retrograde transport of NGF mediated by TrkA is deficient, and hence, inadequate of neurotrophic signaling to maintain neuron survival [78,79]. It is now verified that proNGF is the major species in the adult human brain, whereas mature NGF is completely absent [80,81,82]. Indeed, proNGF is more sensitive to the balance of TrkA and p75NTR levels. proNGF acts as an NTF by activating TrkA-dependent signaling pathways in BFCNs that express normal levels of TrkA and p75NTR [70,80,83]. The decrease in TrkA levels might disturb the balance between TrkA and p75NTR for proNGF binding and result in increased proNGF/p75NTR interaction to induce apoptosis. Thus, the lack of survival signaling and the exacerbation of apoptosis signaling eventually promote neurodegeneration. BDNF is a key molecule to maintain hippocampal synaptic plasticity and memory storage which are damaged in AD. The deficits of BDNF have been linked with Aβ accumulation, tau phosphorylation, neuroinflammation and neuronal apoptosis, and the roles of the BDNF pathway in AD have been well-reviewed recently [84,85]. GDNF is critical for the development, survival and maintenance of midbrain dopaminergic neurons. The exogenous expression of GDNF in the astrocytes of aged rats was able to improve their cognitive deficits, and GDNF administration had a protective role against AD-like changes in animal models [86]. A recent study suggested that NT-3 promotes the neuronal differentiation of bone marrow-derived mesenchymal stem cells (proposed to be an effective therapy for neurodegenerative diseases, including AD) and improved cognitive function in an AD rat model [87].
4. Mutual Influence of Amyloidogenic Process and Neurotrophic Pathways
There is an interaction between the amyloidogenic process and neurotrophic pathways. Increased Aβ levels in AD trigger tau pathology; this leads to microtubule deficits and related disorders, such as defective microtubule assembly and axonal transport deficits, which are likely to affect the neurotrophic signaling of NTFs, followed by neuronal death, and eventually, disease [88,89]. Aβ can bind to p75NTR, resulting in impaired p75NTR polyubiquitination, TRAF6/p62/p75NTR interaction and NF-κB activation and inducing neuronal cell death [90,91]. Furthermore, Aβ abrogates the NGF-induced tyrosine phosphorylation and polyubiquitination of TrkA in PC12 cells and human hippocampal tissues, and hence, deactivates the downstream Ras/MAPK and PI3K/Akt signaling pathways [92]. Aβ decreases BDNF expression through variable pathways [93,94], and Aβ inhibits the expression level of the full-length TrkB receptor and the ability of BDNF to modulate neurotransmitter (GABA and glutamate) release and long-term potentiation in vitro [95,96]. On the other hand, the NGF and BDNF signaling pathways modulate the amyloidogenic route and Aβ production in the cultured hippocampal neurons of rats [17,97]. NGF deprivation in animal models results in Aβ accumulation/deposition, while NGF treatment ameliorates Aβ pathologic changes [98,99,100,101]. NGF administration upregulates the expression of ADAM10 and two enzymes (disintegrin and metalloprotease-17) with α-secretase activity and downregulates BACE1 expression, driving APP cleavage towards the non-amyloidogenic pathway [102,103,104]. NGF also regulates microglial homeostatic activities and prevents Aβ accumulation pathologies via its anti-inflammatory activity in the microglia [105].
Both in vitro and in vivo studies indicate that APP and the NGF/TrkA signaling pathway are interconnected. Furthermore, the APP protein exhibits direct binding with TrkA. The APP phosphorylation pattern is considered as a potential therapeutic target for AD as it determines APP binding to cytosolic interactors and intracellular trafficking, which will finally affect APP processing [20,106,107,108,109]. NGF stimulation promotes TrkA/APP interaction by increasing APP phosphorylation at Y682 and reducing APP phosphorylation at T668, to facilitate the transport of APP to the Golgi apparatus [110], to disturb APP interactions with BACE1 [111], to regulate TrkA activation and subcellular distribution [20,112]. Finally, it inhibits APP processing to Aβ and enhances NGF neurotrophic action. The regulation of APP phosphorylation by NGF and subsequent processes are potentially disrupted in AD patient brains, where the phosphorylation level of APP T668 is increased and the APP/TrkA interaction is reduced [111]. In fact, APP/TrkA interactions are present in brain tissues from normal rat, mouse, and human, but not in brain tissues from AD groups [20,111,112]. The aging pathway decreases TrkA expression levels, results in a TrkA-to-p75NTR receptor switch for NGF signaling and leads to Aβ peptide generation, potentially explaining why aging is a risk factor for AD [113,114,115]. NGF signaling through p75NTR increases both ceramide levels (it can regulate both the α- and β-cleavage of APP) and the steady-state levels of BACE1 and CTFβ, which is NGF dose-dependent and specific for p75NTR, not for TrkA [115]. Another study found that p75NTR upregulated BACE1 transcription and enhanced BACE1 activity on APP by activating c-Jun N-terminal kinase (JNK) in SHSY5Y cells [116].
5. Is There Common Pathway to Control Both the Amyloidogenic Process and the Neurotrophic Pathways?
Although it is clear that amyloidogenesis and neurotrophic dysfunctions are mutually influenced, it is hard to figure out which comes earlier, and whether they work independently, coordinately, simultaneously or successively in AD development. Soluble toxic Aβ oligomers, not the amyloid fibrils, are thought to be the principal pathogenic Aβ species in AD and can be identified in human cerebrospinal fluid (CSF) decades prior to AD onset [1,117]. BDNF mRNA levels and NGF metabolism are also dysregulated early in the pre-clinical stage of AD [7,118]. In addition to the most-studied rare risk genes directly participating in amyloidogenesis (such as APP, PSEN1 and PSEN2) for AD, genetic studies have identified dozens of risk genes, which can mostly be classified as participants in the endosomal trafficking pathways, the innate immune response pathways and the cholesterol metabolism pathways [119].
Both genetics and pathology started suggesting endosomal abnormalities and dysfunction as an early etiology in AD pathogenesis decades ago [120,121]. Furthermore, the recently developed traffic jam hypothesis proposes that the endosomal trafficking pathway is the universal cause in the multiple pathologies of AD [11,119,122]. According to the hypothesis, age-dependent endocytic trafficking dysfunction can alter Aβ production and clearance, neurotrophic signaling, etc. These alterations coordinately function in AD progression. In fact, among the products of these identified risk genes, there are regulators participating both in the control of amyloidogenic process and in the neurotrophic pathways, suggesting that amyloidogenesis and neurotrophic dysfunctions share common regulators, and thus, the common causal pathogenesis of AD. The regulators involved in controlling both amyloidogenesis and neurotrophic signaling are listed in Table 2. ApoE4, SORLA, sortilin, GGA3 and BIN1 can directly interact with targets (BACE1, APP, Aβ or TrkA et al.), whereas Arf6, CD2AP, retromers (including VPS35, VPS26 and VPS29) and Rab proteins act as the subsequent effectors in intracellular vesicle transport.

Table 2.
Regulators involved in the amyloidogenic process and the neurotrophic pathways in AD.
Apolipoprotein E (ApoE) is a kind of 34-kDa glycoprotein acting as cholesterol transporter to mediate the binding of lipoproteins or lipid complexes to specific cell-surface receptors. Of the three major ApoE isoforms (ApoE2, apoE3 and apoE4), ApoE4 has been identified as a major risk factor for AD, and the underlying mechanisms are widely investigated. ApoE4 controls Aβ aggregation, clearance, degradation, etc. via interactions with Aβ at multiple stages [123], while it suppresses BDNF mRNA expression by upregulating the nuclear translocation of histone deacetylases (HDACs) [93]. Thus, the high levels of ApoE4 in many AD patients may strengthen both Aβ neurotoxicity and the lack of BNDF neurotrophic signaling.
The sorting-related receptor with type-A repeats (SorLA, also named SorL1 or LR11) and sortilin are both vacuolar protein-sorting 10 protein (VPS10p) domain receptors involved in pleiotropic functions in intracellular cargo trafficking and signaling. SorLA has been linked tightly with APP cleavage by promoting APP trafficking away from the endosome, attenuating APP oligomerization, or inhibiting APP/BACE1 interactions [128,129,130,131,132,133]. SorLA directs the lysosomal targeting of Aβ peptides by binding with Aβ. SorLA also controls the sorting of GDNF and its receptors (GFRα1/RET) to regulate the subsequent neurotrophic activity [135], mediates the trafficking of TrkB to enhance the response of neurons to BDNF [136], and controls the activation of the EGFR/ERK/Fos signaling pathway to regulate neurite outgrowth and regeneration [137]. Sortilin facilitates BACE1 retrograde trafficking to the Golgi body to increase the cleavage of APP to Aβ [140,141], targets APP for lysosomal degradation and promotes APP cleavage by α-secretase for non-amyloidogenic processes [142]. Conversely, sortilin binds extracellular ApoE/Aβ complexes to facilitate their delivery to lysosomes for degradation [143,144]. Sortilin participates in the toxic effects of Aβ oligomers, as it acts as a receptor for oligomerized Aβ to mediate its endocytosis and induce apoptosis [145]. Sortilin also acts as receptor for ligands such as mature neurotrophins and proneurotrophins, known as neurotensin receptor-3 (NTR3) [149]. Sortilin interacts with Trk receptors to facilitate anterograde transport and neurotrophic signaling [146]. It can also serve as a co-receptor governing p75NTR binding with proNGF to induce cell death and neurodegeneration, acutely and chronically [147,148].
The Golgi-associated, gamma adaptin ear-containing, Arf-binding (GGA) proteins belong to a family of proteins that function as clathrin adaptors during intracellular vesicle trafficking. GGA1 and GGA3 control BACE1 degradation, recycling and axon transport, thus regulating BACE1 location and activity [151,152,153]. GGA3 also regulates the NGF pathway by enhancing TrkA post-endocytic recycling [154] or by rapidly recruiting p75NTR to the plasma membrane as a consequence of TrkA activation by NGF [155]. Arf6 is a partner of GGA proteins in regulating the macropinocytosis of APP in lysosomes [156], as well as TrkA and p75NTR trafficking [154,155]. The endocytic cargo-adaptor protein bridging integrator 1 (BIN1) and the scaffolding protein CD2AP are involved in the scission of BACE1 containing recycling carriers from early endosomes [160]. BIN1 also controls presynaptic neurotransmitter release [161], while CD2AP drives TrkA location to endosomes and the TrkA-induced AKT signaling pathway [163]. VPS35 is a critical component of the retromer cargo-recognition complex, which is involved in BACE1 endosome-to-Golgi retrieval transporting to inhibit Aβ production [165]. The Rab family small GTPase proteins, which function as master regulators of vesicular transport and membrane trafficking, have also been implicated in AD pathogenesis (recently reviewed by Zhang X. et al.) [167].
6. Targeting the Common Pathway for Preventing Both Amyloidogenesis and Neurotrophic Dysfunction
Research on disease-modifying treatments for AD has largely focused on preventing, eliminating or reducing amyloid plaque accumulation [1,12]. The neurotrophic factor hypothesis for AD pathogenesis supports the idea that targeting NTF signaling pathways is a potential therapeutic tool for AD. The anticipated roles of NTFs (typically NGF and BDNF) in AD treatment have been discussed recently by various labs [28,29,30]. Original research exploring the roles of NTFs on AD was initiated in NGF and has accordingly transitioned to clinical trials [68,69]. Although early clinical trials showed that NGF specifically affected AD patients, NGF cannot pass through the blood–brain barrier (BBB), and direct injection of NGF may cause adverse effects, such as pain and weight loss [2,168,169]. Tuszynski et al. initiated a clinical trial of NGF gene therapy in patients with early-stage AD and confirmed that NGF improves the function of degenerated neurons in the brain tissue of AD patients without obvious side effects [68,69]. Studies using encapsulated cell biodelivery (ECB) of NGF to the cholinergic basal forebrain of AD patients showed an increased NGF concentration at the target area with fewer off-target adverse effects [170,171].
In addition to the strategies directly targeting amyloid plaque accumulation or NFT signaling, the effectors simultaneously regulating both the neurotrophic pathway and amyloidogenic process may be promising targets for AD treatment. Recent research on the intracellular cargo transport deficiencies in AD suggests that improving the efficacy of intracellular trafficking may ameliorate AD pathology [11,119,122]. Recovering the normal expression level via gene therapy or specific drugs could be a choice to treat AD. Researchers have already verified that targeting specific retromers can ameliorate AD pathologies in mouse brain, including amyloid pathology, etc. [172,173,174,175]. Small molecules, such as pharmacological chaperones designed to modulate the stability of retromer complexes, including the VPS35/VPS29 interaction, have been tested for preventing APP cleavage by BACE1 [176,177]. Interestingly, Vps35 gene delivery into the central nervous system in mice significantly improves synaptic pathology and neuroinflammation, attenuating AD-induced alterations in spatial learning and working memory, significantly reducing Aβ levels and deposition and tau phosphorylation [173]. As the VPS26b/VPS29/VPS35 complex also controls the p75NTR/sortilin interaction, which may affect proNGF signaling, targeting VPS35 levels or the VPS35/VPS29 interaction might be effective in preventing amyloidogenesis and neurotrophic dysfunction simultaneously. On the other hand, the regulators may exhibit activity controlled by phosphorylation, epigenetic modification or GDP/GTP binding, etc. For instance, the phosphorylation status of GGA proteins controls their binding capacity with the ubiquitin tag or sorting signal of target cargo and, thus, decides the sorting fate of cargo. Targeting the activity of the regulators might compensate for the dysfunction results from abnormal expression levels. Therapeutic disease intervention targeting Rab GTPases through prenylation (the modulation of membrane association), GDP/GTP binding or exchange, and the inhibition of protein interactions, etc. has already been suggested [178].
7. Conclusions
Both the amyloidogenic process and neurotrophic dysfunction account for AD progression. No matter which comes earlier or whether they have a causal link, they possibly share common intracellular regulator deficits in AD. Recovering the expression levels using gene therapy or specific drugs, or modifying the activity of the regulators to compensate for the dysfunction resulting from abnormal expression levels or mutation, might be effective strategies for the treatment of AD (Figure 1).
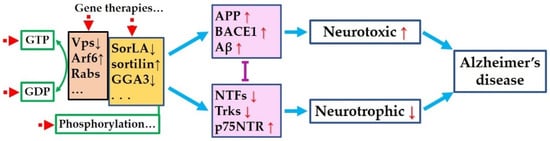
Figure 1.
Hypothetical common pathogenesis and targets for Alzheimer’s disease. Amyloidogenic process and neurotrophic pathways are mutually inhibited. Original deficit in any of the two pathways may dysregulate the other one and mutually aggravate each other, or coordinately cause or deteriorate AD. Abnormal expression or activities of the regulators (brown and yellow boxes) result in the dysregulated expression or activities of the key proteins (pink boxes), leads to overloaded neurotoxicity and degraded neurotrophic effects, and finally, causes Alzheimer’s disease. Targeting these regulators (red dotted arrows)—including recover the expression levels or correcting the mutations via gene therapies or specific drugs, or modifying the activity to compensate for the dysfunction resulting from abnormal expression levels or mutation—might be effective strategies to treat AD.
Author Contributions
Conceptualization, Q.W. and X.L.; writing—original draft preparation, F.J. and D.J.; writing—review and editing, Y.L., J.M., Q.W. and X.L.; funding acquisition, Y.L., Q.W. and X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC), grant number 31701247; the Students’ Innovation and Entrepreneurship Training Program of Shandong Province, grant number S202010443058; the Students’ Innovation and Entrepreneurship Training Program of Jining Medical University, grant number CX2020025; and the Natural Science Foundation of Shandong Province, grant number ZR2021QC039.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fa, M.; Amato, A.; Palmeri, A.; D’Adamio, L.; Grassi, C.; Devanand, D.P.; Honig, L.S.; et al. Role of Amyloid-beta and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimers Dis. JAD 2018, 64, S611–S631. [Google Scholar] [CrossRef] [PubMed]
- Appel, S.H. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann. Neurol. 1981, 10, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Dawbarn, D. The neurotrophins and their role in Alzheimer’s disease. Curr. Neuropharmacol. 2011, 9, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Iulita, M.F.; Cuello, A.C. Nerve growth factor metabolic dysfunction in Alzheimer’s disease and Down syndrome. Trends Pharmacol. Sci. 2014, 35, 338–348. [Google Scholar] [CrossRef]
- Cattaneo, A.; Calissano, P. Nerve growth factor and Alzheimer’s disease: New facts for an old hypothesis. Mol. Neurobiol. 2012, 46, 588–604. [Google Scholar] [CrossRef] [PubMed]
- Capsoni, S.; Ugolini, G.; Comparini, A.; Ruberti, F.; Berardi, N.; Cattaneo, A. Alzheimer-like neurodegeneration in aged antinerve growth factor transgenic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 6826–6831. [Google Scholar] [CrossRef]
- Pentz, R.; Iulita, M.F.; Ducatenzeiler, A.; Bennett, D.A.; Cuello, A.C. The human brain NGF metabolic pathway is impaired in the pre-clinical and clinical continuum of Alzheimers disease. Mol. Psychiatry 2020, 26, 6023–6037. [Google Scholar] [CrossRef]
- Hefti, F.; Weiner, W.J. Nerve growth factor and Alzheimer’s disease. Ann. Neurol. 1986, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Dean, R.L., III; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Aghourian, M.; Legault-Denis, C.; Soucy, J.P.; Rosa-Neto, P.; Gauthier, S.; Kostikov, A.; Gravel, P.; Bedard, M.A. Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [F-18]-FEOBV. Mol. Psychiatry 2017, 22, 1531–1538. [Google Scholar] [CrossRef]
- Kimura, N.; Yanagisawa, K. Traffic jam hypothesis: Relationship between endocytic dysfunction and Alzheimer’s disease. Neurochem. Int. 2018, 119, 35–41. [Google Scholar] [CrossRef]
- Kent, S.A.; Spires-Jones, T.L.; Durrant, C.S. The physiological roles of tau and Abeta: Implications for Alzheimer’s disease pathology and therapeutics. Acta Neuropathol. 2020, 140, 417–447. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef]
- Long, J.M.; Holtzman, D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Mufson, E.J.; Counts, S.E.; Ginsberg, S.D.; Mahady, L.; Perez, S.E.; Massa, S.M.; Longo, F.M.; Ikonomovic, M.D. Nerve Growth Factor Pathobiology during the Progression of Alzheimer’s Disease. Front. Neurosci. 2019, 13, 533. [Google Scholar] [CrossRef]
- Matrone, C.; Ciotti, M.T.; Mercanti, D.; Marolda, R.; Calissano, P. NGF and BDNF signaling control amyloidogenic route and Abeta production in hippocampal neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 13139–13144. [Google Scholar] [CrossRef]
- Zou, L.; Wang, Z.; Shen, L.; Bao, G.B.; Wang, T.; Kang, J.H.; Pei, G. Receptor tyrosine kinases positively regulate BACE activity and Amyloid-beta production through enhancing BACE internalization. Cell Res. 2007, 17, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Chen, Y.; Liu, Y.; Zhao, Y.; Liao, F.F.; Xu, H. APP regulates NGF receptor trafficking and NGF-mediated neuronal differentiation and survival. PLoS ONE 2013, 8, e80571. [Google Scholar] [CrossRef] [PubMed]
- Matrone, C.; Barbagallo, A.P.; La Rosa, L.R.; Florenzano, F.; Ciotti, M.T.; Mercanti, D.; Chao, M.V.; Calissano, P.; D’Adamio, L. APP is phosphorylated by TrkA and regulates NGF/TrkA signaling. J. Neurosci. 2011, 31, 11756–11761. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.G.; Stricker, R.; Lendeckel, U.; Bertram, I.; Dobrowolny, H.; Steiner, J.; Bogerts, B.; Reiser, G. Reduced neuronal co-localisation of nardilysin and the putative alpha-secretases ADAM10 and ADAM17 in Alzheimer’s disease and Down syndrome brains. Age 2009, 31, 11–25. [Google Scholar] [CrossRef]
- Vassar, R.; Kuhn, P.H.; Haass, C.; Kennedy, M.E.; Rajendran, L.; Wong, P.C.; Lichtenthaler, S.F. Function, therapeutic potential and cell biology of BACE proteases: Current status and future prospects. J. Neurochem. 2014, 130, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, S.H.; Callahan, J.W.; Mahuran, D.J. The role of the endosomal/lysosomal system in amyloid-beta production and the pathophysiology of Alzheimer’s disease: Reexamining the spatial paradox from a lysosomal perspective. J. Alzheimers Dis. 2004, 6, 53–65. [Google Scholar] [CrossRef]
- Sideris, D.I.; Danial, J.S.H.; Emin, D.; Ruggeri, F.S.; Xia, Z.; Zhang, Y.P.; Lobanova, E.; Dakin, H.; De, S.; Miller, A.; et al. Soluble amyloid beta-containing aggregates are present throughout the brain at early stages of Alzheimer’s disease. Brain Commun. 2021, 3, fcab147. [Google Scholar] [CrossRef]
- Donohue, M.C.; Sperling, R.A.; Petersen, R.; Sun, C.K.; Weiner, M.W.; Aisen, P.S.; Alzheimer’s Disease Neuroimaging Initiative. Association between Elevated Brain Amyloid and Subsequent Cognitive Decline among Cognitively Normal Persons. JAMA 2017, 317, 2305–2316. [Google Scholar] [CrossRef]
- Baker, J.E.; Lim, Y.Y.; Pietrzak, R.H.; Hassenstab, J.; Snyder, P.J.; Masters, C.L.; Maruff, P. Cognitive impairment and decline in cognitively normal older adults with high amyloid-beta: A meta-analysis. Alzheimers Dement. Diagn. Assess. Dis. Monit. 2017, 6, 108–121. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef]
- Lubke, J.H.; Idoon, F.; Mohasel-Roodi, M.; Alipour, F.; Hami, J.; Ehteshampour, A.; Mostafaee, H.; Sadeghi, A. Neurotrophic factors in Alzheimer’s disease: Pathogenesis and therapy. Acta Neurobiol. Exp. 2021, 81, 314–327. [Google Scholar]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 34. [Google Scholar] [CrossRef]
- Nasrolahi, A.; Javaherforooshzadeh, F.; Jafarzadeh-Gharehziaaddin, M.; Mahmoudi, J.; Asl, K.D.; Shabani, Z. Therapeutic potential of neurotrophic factors in Alzheimer’s Disease. Mol. Biol. Rep. 2022, 49, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wu, H.T.; Qin, X.Y.; Cao, C.; Liu, Y.; Cao, Z.Z.; Cheng, Y. Postmortem Brain, Cerebrospinal Fluid, and Blood Neurotrophic Factor Levels in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Mol. Neurosci. 2018, 65, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Karlsson, I.K.; Pedersen, N.L.; Hagg, S. Circulating insulin-like growth factors and Alzheimer disease: A mendelian randomization study. Neurology 2018, 90, e291–e297. [Google Scholar] [CrossRef]
- Cheng, Y.; Cawley, N.X.; Yanik, T.; Murthy, S.R.; Liu, C.; Kasikci, F.; Abebe, D.; Loh, Y.P. A human carboxypeptidase E/NF-alpha1 gene mutation in an Alzheimer’s disease patient leads to dementia and depression in mice. Transl. Psychiatry 2016, 6, e973. [Google Scholar] [CrossRef] [PubMed]
- Cuello, A.C.; Pentz, R.; Hall, H. The Brain NGF Metabolic Pathway in Health and in Alzheimer’s Pathology. Front. Neurosci. 2019, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.Y.; Cao, C.; Cawley, N.X.; Liu, T.T.; Yuan, J.; Loh, Y.P.; Cheng, Y. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease: A meta-analysis study (N = 7277). Mol. Psychiatry 2017, 22, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.Y.; Villemagne, V.L.; Laws, S.M.; Ames, D.; Pietrzak, R.H.; Ellis, K.A.; Harrington, K.D.; Bourgeat, P.; Salvado, O.; Darby, D.; et al. BDNF Val66Met, Abeta amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Narisawa-Saito, M.; Wakabayashi, K.; Tsuji, S.; Takahashi, H.; Nawa, H. Regional specificity of alterations in NGF, BDNF and NT-3 levels in Alzheimer’s disease. Neuroreport 1996, 7, 2925–2928. [Google Scholar] [CrossRef]
- Hock, C.; Heese, K.; Hulette, C.; Rosenberg, C.; Otten, U. Region-specific neurotrophin imbalances in Alzheimer disease: Decreased levels of brain-derived neurotrophic factor and increased levels of nerve growth factor in hippocampus and cortical areas. Arch. Neurol. 2000, 57, 846–851. [Google Scholar] [CrossRef]
- Airavaara, M.; Pletnikova, O.; Doyle, M.E.; Zhang, Y.E.; Troncoso, J.C.; Liu, Q.R. Identification of Novel GDNF Isoforms and cis-Antisense GDNFOS Gene and Their Regulation in Human Middle Temporal Gyrus of Alzheimer Disease. J. Biol. Chem. 2011, 286, 45093–45102. [Google Scholar] [CrossRef]
- Sharif, M.; Noroozian, M.; Hashemian, F. Do serum GDNF levels correlate with severity of Alzheimer’s disease? Neurol. Sci. 2021, 42, 2865–2872. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Miranda, A.S.; Barbosa, I.G.; Talib, L.L.; Diniz, B.S.; Gattaz, W.F.; Teixeira, A.L. Decreased Neurotrophic Support is Associated with Cognitive Decline in Non-Demented Subjects. J. Alzheimers Dis. 2015, 46, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Straten, G.; Eschweiler, G.W.; Maetzler, W.; Laske, C.; Leyhe, T. Glial cell-line derived neurotrophic factor (GDNF) concentrations in cerebrospinal fluid and serum of patients with early Alzheimer’s disease and normal controls. J. Alzheimers Dis. 2009, 18, 331–337. [Google Scholar] [CrossRef]
- Marksteiner, J.; Kemmler, G.; Weiss, E.M.; Knaus, G.; Ullrich, C.; Mechtcheriakov, S.; Oberbauer, H.; Auffinger, S.; Hinterholzl, J.; Hinterhuber, H.; et al. Five out of 16 plasma signaling proteins are enhanced in plasma of patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2011, 32, 539–540. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Brown, R.W.; Malone, H.M.; Burgess, K.C.; Gill, W.D.; Keasey, M.P.; Hagg, T. Ciliary neurotrophic factor is a key sex-specific regulator of depressive-like behavior in mice. Psychoneuroendocrinology 2019, 100, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Soilu-Hanninen, M.; Broberg, E.; Roytta, M.; Mattila, P.; Rinne, J.; Hukkanen, V. Expression of LIF and LIF receptor beta in Alzheimer’s and Parkinson’s diseases. Acta Neurol. Scand. 2010, 121, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, X.; Gao, K.; Lu, D.; Zhang, X.; Ma, C.; Ye, F.; Zhang, L. Cardiotrophin-1 (CTF1) ameliorates glucose-uptake defects and improves memory and learning deficits in a transgenic mouse model of Alzheimer’s disease. Pharm. Biochem. Behav. 2013, 107, 48–57. [Google Scholar] [CrossRef]
- Joshi, H.; Shah, J.; Abu-Hijleh, F.A.; Patel, V.; Rathbone, M.; Gabriele, S.; Gabriele, J.; Baranowski, D.; Molloy, D.; Frey, B.N.; et al. Decreased Expression of Cerebral Dopamine Neurotrophic Factor in Platelets of Probable Alzheimer Patients. Alzheimer Dis. Assoc. Disord. 2021, 36, 269–271. [Google Scholar] [CrossRef]
- Liu, X.C.; Qi, X.H.; Fang, H.; Zhou, K.Q.; Wang, Q.S.; Chen, G.H. Increased MANF Expression in the Inferior Temporal Gyrus in Patients with Alzheimer Disease. Front. Aging Neurosci. 2021, 13, 639318. [Google Scholar] [CrossRef]
- Kimura, K.; Wakamatsu, A.; Suzuki, Y.; Ota, T.; Nishikawa, T.; Yamashita, R.; Yamamoto, J.; Sekine, M.; Tsuritani, K.; Wakaguri, H.; et al. Diversification of transcriptional modulation: Large-scale identification and characterization of putative alternative promoters of human genes. Genome Res. 2006, 16, 55–65. [Google Scholar] [CrossRef]
- Mahoney, E.R.; Dumitrescu, L.; Moore, A.M.; Cambronero, F.E.; De Jager, P.L.; Koran, M.E.I.; Petyuk, V.A.; Robinson, R.A.S.; Goyal, S.; Schneider, J.A.; et al. Brain expression of the vascular endothelial growth factor gene family in cognitive aging and alzheimer’s disease. Mol. Psychiatry 2021, 26, 888–896. [Google Scholar] [CrossRef]
- Thomas, T.; Miners, S.; Love, S. Post-mortem assessment of hypoperfusion of cerebral cortex in Alzheimer’s disease and vascular dementia. Brain 2015, 138, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Provias, J.; Jeynes, B. Reduction in vascular endothelial growth factor expression in the superior temporal, hippocampal, and brainstem regions in Alzheimer’s disease. Curr. Neurovascular Res. 2014, 11, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Inai, T.; Mancuso, M.; Hashizume, H.; Baffert, F.; Haskell, A.; Baluk, P.; Hu-Lowe, D.D.; Shalinsky, D.R.; Thurston, G.; Yancopoulos, G.D.; et al. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am. J. Pathol. 2004, 165, 35–52. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chatterjee, M.; Twaalfhoven, H.; Del Campo Milan, M.; Teunissen, C.E.; Scheltens, P.; Fontijn, R.D.; van Der Flier, W.M.; de Vries, H.E. Vascular Endothelial Growth Factor remains unchanged in cerebrospinal fluid of patients with Alzheimer’s disease and vascular dementia. Alzheimers Res. Ther. 2018, 10, 58. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Alexopoulos, P.; Perneczky, R. Heart-type fatty acid binding protein and vascular endothelial growth factor: Cerebrospinal fluid biomarker candidates for Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 553–560. [Google Scholar] [CrossRef]
- Zhang, J.B.; Li, M.F.; Zhang, H.X.; Li, Z.G.; Sun, H.R.; Zhang, J.S.; Wang, P.F. Association of serum vascular endothelial growth factor levels and cerebral microbleeds in patients with Alzheimer’s disease. Eur. J. Neurol. 2016, 23, 1337–1342. [Google Scholar] [CrossRef]
- Mateo, I.; Llorca, J.; Infante, J.; Rodriguez-Rodriguez, E.; Fernandez-Viadero, C.; Pena, N.; Berciano, J.; Combarros, O. Low serum VEGF levels are associated with Alzheimer’s disease. Acta Neurol. Scand. 2007, 116, 56–58. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zeng, F.F.; Chen, Z.W.; Wang, C.Y.; Zhao, B.; Li, K.S. Vascular endothelial growth factor gene promoter polymorphisms and Alzheimer’s disease risk: A meta-analysis. CNS Neurosci. Ther. 2013, 19, 469–476. [Google Scholar] [CrossRef]
- Bjorkqvist, M.; Ohlsson, M.; Minthon, L.; Hansson, O. Evaluation of a previously suggested plasma biomarker panel to identify Alzheimer’s disease. PLoS ONE 2012, 7, e29868. [Google Scholar] [CrossRef]
- Sagare, A.P.; Bell, R.D.; Zhao, Z.; Ma, Q.; Winkler, E.A.; Ramanathan, A.; Zlokovic, B.V. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat. Commun. 2013, 4, 2932. [Google Scholar] [CrossRef]
- Sagare, A.P.; Sweeney, M.D.; Makshanoff, J.; Zlokovic, B.V. Shedding of soluble platelet-derived growth factor receptor-beta from human brain pericytes. Neurosci. Lett. 2015, 607, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Stopa, E.G.; Gonzalez, A.M.; Chorsky, R.; Corona, R.J.; Alvarez, J.; Bird, E.D.; Baird, A. Basic fibroblast growth factor in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 1990, 171, 690–696. [Google Scholar] [CrossRef]
- Mocali, A.; Cedrola, S.; Della Malva, N.; Bontempelli, M.; Mitidieri, V.A.; Bavazzano, A.; Comolli, R.; Paoletti, F.; La Porta, C.A. Increased plasma levels of soluble CD40, together with the decrease of TGF beta 1, as possible differential markers of Alzheimer disease. Exp. Gerontol. 2004, 39, 1555–1561. [Google Scholar] [CrossRef]
- Tesseur, I.; Zou, K.; Esposito, L.; Bard, F.; Berber, E.; Can, J.V.; Lin, A.H.; Crews, L.; Tremblay, P.; Mathews, P.; et al. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. J. Clin. Investig. 2006, 116, 3060–3069. [Google Scholar] [CrossRef]
- Decourt, B.; Lahiri, D.K.; Sabbagh, M.N. Targeting Tumor Necrosis Factor Alpha for Alzheimer’s Disease. Curr. Alzheimer Res. 2017, 14, 412–425. [Google Scholar] [CrossRef]
- Ostrowski, P.P.; Barszczyk, A.; Forstenpointner, J.; Zheng, W.; Feng, Z.P. Meta-Analysis of Serum Insulin-Like Growth Factor 1 in Alzheimer’s Disease. PLoS ONE 2016, 11, e0155733. [Google Scholar] [CrossRef] [PubMed]
- Fenton, H.; Finch, P.W.; Rubin, J.S.; Rosenberg, J.M.; Taylor, W.G.; Kuo-Leblanc, V.; Rodriguez-Wolf, M.; Baird, A.; Schipper, H.M.; Stopa, E.G. Hepatocyte growth factor (HGF/SF) in Alzheimer’s disease. Brain Res. 1998, 779, 262–270. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Thal, L.; Pay, M.; Salmon, D.P.; U, H.S.; Bakay, R.; Patel, P.; Blesch, A.; Vahlsing, H.L.; Ho, G.; et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat. Med. 2005, 11, 551–555. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Yang, J.H.; Barba, D.; U, H.S.; Bakay, R.A.; Pay, M.M.; Masliah, E.; Conner, J.M.; Kobalka, P.; Roy, S.; et al. Nerve Growth Factor Gene Therapy: Activation of Neuronal Responses in Alzheimer Disease. JAMA Neurol. 2015, 72, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Fahnestock, M.; Shekari, A. ProNGF and Neurodegeneration in Alzheimer’s Disease. Front. Neurosci. 2019, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.W.; Spreng, R.N. Alzheimer’s Disease Neuroimaging Initiative. Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat. Commun. 2016, 7, 13249. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, G.K.; Esiri, M.M.; Bowen, D.M.; Smith, C.C. Alzheimer’s disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J. Neurol. Sci. 1982, 57, 407–417. [Google Scholar] [CrossRef]
- Sobreviela, T.; Clary, D.O.; Reichardt, L.F.; Brandabur, M.M.; Kordower, J.H.; Mufson, E.J. TrkA-immunoreactive profiles in the central nervous system: Colocalization with neurons containing p75 nerve growth factor receptor, choline acetyltransferase, and serotonin. J. Comp. Neurol. 1994, 350, 587–611. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, S.D.; Che, S.; Wuu, J.; Counts, S.E.; Mufson, E.J. Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer’s disease. J. Neurochem. 2006, 97, 475–487. [Google Scholar] [CrossRef]
- Counts, S.E.; Nadeem, M.; Wuu, J.; Ginsberg, S.D.; Saragovi, H.U.; Mufson, E.J. Reduction of cortical TrkA but not p75(NTR) protein in early-stage Alzheimer’s disease. Ann. Neurol. 2004, 56, 520–531. [Google Scholar] [CrossRef]
- Crutcher, K.A.; Scott, S.A.; Liang, S.; Everson, W.V.; Weingartner, J. Detection of NGF-like activity in human brain tissue: Increased levels in Alzheimer’s disease. J. Neurosci. 1993, 13, 2540–2550. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Mufson, E.J.; Weingartner, J.A.; Skau, K.A.; Crutcher, K.A. Nerve growth factor in Alzheimer’s disease: Increased levels throughout the brain coupled with declines in nucleus basalis. J. Neurosci. 1995, 15, 6213–6221. [Google Scholar] [CrossRef]
- Mufson, E.J.; Conner, J.M.; Kordower, J.H. Nerve growth factor in Alzheimer’s disease: Defective retrograde transport to nucleus basalis. Neuroreport 1995, 6, 1063–1066. [Google Scholar] [CrossRef]
- Shekari, A.; Fahnestock, M. Retrograde axonal transport of BDNF and proNGF diminishes with age in basal forebrain cholinergic neurons. Neurobiol. Aging 2019, 84, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef]
- Fahnestock, M.; Yu, G.; Coughlin, M.D. ProNGF: A neurotrophic or an apoptotic molecule? Prog. Brain Res. 2004, 146, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Fahnestock, M.; Michalski, B.; Xu, B.; Coughlin, M.D. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol. Cell. Neurosci. 2001, 18, 210–220. [Google Scholar] [CrossRef]
- Masoudi, R.; Ioannou, M.S.; Coughlin, M.D.; Pagadala, P.; Neet, K.E.; Clewes, O.; Allen, S.J.; Dawbarn, D.; Fahnestock, M. Biological activity of nerve growth factor precursor is dependent upon relative levels of its receptors. J. Biol. Chem. 2009, 284, 18424–18433. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Pertusa, M.; Garcia-Matas, S.; Mammeri, H.; Adell, A.; Rodrigo, T.; Mallet, J.; Cristofol, R.; Sarkis, C.; Sanfeliu, C. Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol. Aging 2008, 29, 1366–1379. [Google Scholar] [CrossRef]
- Yan, Z.; Shi, X.; Wang, H.; Si, C.; Liu, Q.; Du, Y. Neurotrophin-3 Promotes the Neuronal Differentiation of BMSCs and Improves Cognitive Function in a Rat Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 629356. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Grundke-Iqbal, I.; Zaidi, T.; Merz, P.A.; Wen, G.Y.; Shaikh, S.S.; Wisniewski, H.M.; Alafuzoff, I.; Winblad, B. Defective brain microtubule assembly in Alzheimer’s disease. Lancet 1986, 2, 421–426. [Google Scholar] [CrossRef]
- Adalbert, R.; Milde, S.; Durrant, C.; Ando, K.; Stygelbout, V.; Yilmaz, Z.; Gould, S.; Brion, J.P.; Coleman, M.P. Interaction between a MAPT variant causing frontotemporal dementia and mutant APP affects axonal transport. Neurobiol. Aging 2018, 68, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Zhai, S.; Pilch, P.F.; Doyle, S.M.; Eisenhauer, P.B.; Fine, R.E.; Gilchrest, B.A. Binding of beta-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer’s disease. J. Clin. Investig. 1997, 100, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Zheng, C.; McGregor, W.C.; White, B.D.; Diaz-Meco, M.T.; Moscat, J.; Babu, J.R. TRAF6 and p62 inhibit amyloid beta-induced neuronal death through p75 neurotrophin receptor. Neurochem. Int. 2012, 61, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Geetha, T.; Gearing, M.; Babu, J.R. Amyloid beta-abrogated TrkA ubiquitination in PC12 cells analogous to Alzheimer’s disease. J. Neurochem. 2015, 133, 919–925. [Google Scholar] [CrossRef]
- Sen, A.; Nelson, T.J.; Alkon, D.L. ApoE4 and Abeta Oligomers Reduce BDNF Expression via HDAC Nuclear Translocation. J. Neurosci. 2015, 35, 7538–7551. [Google Scholar] [CrossRef]
- Rosa, E.; Fahnestock, M. CREB expression mediates amyloid beta-induced basal BDNF downregulation. Neurobiol. Aging 2015, 36, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Kemppainen, S.; Rantamaki, T.; Jeronimo-Santos, A.; Lavasseur, G.; Autio, H.; Karpova, N.; Karkkainen, E.; Staven, S.; Vicente Miranda, H.; Outeiro, T.F.; et al. Impaired TrkB receptor signaling contributes to memory impairment in APP/PS1 mice. Neurobiol. Aging 2012, 33, 1122.e23–1122.e39. [Google Scholar] [CrossRef]
- Jeronimo-Santos, A.; Vaz, S.H.; Parreira, S.; Rapaz-Lerias, S.; Caetano, A.P.; Buee-Scherrer, V.; Castren, E.; Valente, C.A.; Blum, D.; Sebastiao, A.M.; et al. Dysregulation of TrkB Receptors and BDNF Function by Amyloid-beta Peptide is Mediated by Calpain. Cereb. Cortex 2015, 25, 3107–3121. [Google Scholar] [CrossRef]
- Matrone, C.; Marolda, R.; Ciafre, S.; Ciotti, M.T.; Mercanti, D.; Calissano, P. Tyrosine kinase nerve growth factor receptor switches from prosurvival to proapoptotic activity via Abeta-mediated phosphorylation. Proc. Natl. Acad. Sci. USA 2009, 106, 11358–11363. [Google Scholar] [CrossRef]
- Cattaneo, A.; Capsoni, S.; Paoletti, F. Towards non invasive nerve growth factor therapies for Alzheimer’s disease. J. Alzheimers Dis. JAD 2008, 15, 255–283. [Google Scholar] [CrossRef]
- Capsoni, S.; Marinelli, S.; Ceci, M.; Vignone, D.; Amato, G.; Malerba, F.; Paoletti, F.; Meli, G.; Viegi, A.; Pavone, F.; et al. Intranasal “painless” human Nerve Growth Factor [corrected] slows amyloid neurodegeneration and prevents memory deficits in App X PS1 mice. PLoS ONE 2012, 7, e37555. [Google Scholar] [CrossRef]
- Tian, L.; Guo, R.; Yue, X.; Lv, Q.; Ye, X.; Wang, Z.; Chen, Z.; Wu, B.; Xu, G.; Liu, X. Intranasal administration of nerve growth factor ameliorate beta-amyloid deposition after traumatic brain injury in rats. Brain Res. 2012, 1440, 47–55. [Google Scholar] [CrossRef]
- Xu, C.J.; Wang, J.L.; Jin, W.L. The Emerging Therapeutic Role of NGF in Alzheimer’s Disease. Neurochem. Res. 2016, 41, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Fragkouli, A.; Tzinia, A.K.; Charalampopoulos, I.; Gravanis, A.; Tsilibary, E.C. Matrix metalloproteinase-9 participates in NGF-induced alpha-secretase cleavage of amyloid-beta protein precursor in PC12 cells. J. Alzheimers Dis. JAD 2011, 24, 705–719. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Y.L.; Ni, X.Q.; Li, N.; Zhang, B.H.; Fang, X.B. Enhancement of the nonamyloidogenic pathway by exogenous NGF in an Alzheimer transgenic mouse model. Neuropeptides 2014, 48, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xiao, Z.; Huang, J. C6 Glioma-Secreted NGF and FGF2 Regulate Neuronal APP Processing Through Up-Regulation of ADAM10 and Down-Regulation of BACE1, Respectively. J. Mol. Neurosci. 2016, 59, 334–342. [Google Scholar] [CrossRef]
- Rizzi, C.; Tiberi, A.; Giustizieri, M.; Marrone, M.C.; Gobbo, F.; Carucci, N.M.; Meli, G.; Arisi, I.; D’Onofrio, M.; Marinelli, S.; et al. NGF steers microglia toward a neuroprotective phenotype. Glia 2018, 66, 1395–1416. [Google Scholar] [CrossRef]
- Chang, K.A.; Kim, H.S.; Ha, T.Y.; Ha, J.W.; Shin, K.Y.; Jeong, Y.H.; Lee, J.P.; Park, C.H.; Kim, S.; Baik, T.K.; et al. Phosphorylation of amyloid precursor protein (APP) at Thr668 regulates the nuclear translocation of the APP intracellular domain and induces neurodegeneration. Mol. Cell. Biol. 2006, 26, 4327–4338. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakaya, T. Regulation of amyloid beta-protein precursor by phosphorylation and protein interactions. J. Biol. Chem. 2008, 283, 29633–29637. [Google Scholar] [CrossRef]
- Lee, M.S.; Kao, S.C.; Lemere, C.A.; Xia, W.M.; Tseng, H.C.; Zhou, Y.; Neve, R.; Ahlijanian, M.K.; Tsai, L.H. APP processing is regulated by cytoplasmic phosphorylation. J. Cell Biol. 2003, 163, 83–95. [Google Scholar] [CrossRef]
- Tamayev, R.; Zhou, D.W.; D’Adamio, L. The interactome of the amyloid beta precursor protein family members is shaped by phosphorylation of their intracellular domains. Mol. Neurodegener. 2009, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Canu, N.; Amadoro, G.; Triaca, V.; Latina, V.; Sposato, V.; Corsetti, V.; Severini, C.; Ciotti, M.T.; Calissano, P. The Intersection of NGF/TrkA Signaling and Amyloid Precursor Protein Processing in Alzheimer’s Disease Neuropathology. Int. J. Mol. Sci. 2017, 18, 1319. [Google Scholar] [CrossRef]
- Triaca, V.; Sposato, V.; Bolasco, G.; Ciotti, M.T.; Pelicci, P.; Bruni, A.C.; Cupidi, C.; Maletta, R.; Feligioni, M.; Nistico, R.; et al. NGF controls APP cleavage by downregulating APP phosphorylation at Thr668: Relevance for Alzheimer’s disease. Aging Cell 2016, 15, 661–672. [Google Scholar] [CrossRef]
- Canu, N.; Pagano, I.; La Rosa, L.R.; Pellegrino, M.; Ciotti, M.T.; Mercanti, D.; Moretti, F.; Sposato, V.; Triaca, V.; Petrella, C.; et al. Association of TrkA and APP Is Promoted by NGF and Reduced by Cell Death-Promoting Agents. Front. Mol. Neurosci. 2017, 10, 15. [Google Scholar] [CrossRef]
- Costantini, C.; Scrable, H.; Puglielli, L. An aging pathway controls the TrkA to p75NTR receptor switch and amyloid beta-peptide generation. EMBO J. 2006, 25, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Rossner, S.; Ueberham, U.; Schliebs, R.; Perez-Polo, J.R.; Bigl, V. The regulation of amyloid precursor protein metabolism by cholinergic mechanisms and neurotrophin receptor signaling. Prog. Neurobiol. 1998, 56, 541–569. [Google Scholar] [CrossRef]
- Costantini, C.; Weindruch, R.; Della Valle, G.; Puglielli, L. A TrkA-to-p75NTR molecular switch activates amyloid beta-peptide generation during aging. Biochem. J. 2005, 391, 59–67. [Google Scholar] [CrossRef]
- Saadipour, K.; Tiberi, A.; Lombardo, S.; Grajales, E.; Montroull, L.; Manucat-Tan, N.B.; LaFrancois, J.; Cammer, M.; Mathews, P.M.; Scharfman, H.E.; et al. Regulation of BACE1 expression after injury is linked to the p75 neurotrophin receptor. Mol. Cell. Neurosci. 2019, 99, 103395. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, H.; Tokuda, T.; Kasai, T.; Ishigami, N.; Hidaka, H.; Kondo, M.; Allsop, D.; Nakagawa, M. High-molecular-weight beta-amyloid oligomers are elevated in cerebrospinal fluid of Alzheimer patients. FASEB J. 2010, 24, 2716–2726. [Google Scholar] [CrossRef]
- Iulita, M.F.; Millon, M.B.B.; Pentz, R.; Aguilar, L.F.; Do Carmo, S.; Allard, S.; Michalski, B.; Wilson, E.N.; Ducatenzeiler, A.; Bruno, M.A.; et al. Differential deregulation of NGF and BDNF neurotrophins in a transgenic rat model of Alzheimer’s disease. Neurobiol. Dis. 2017, 108, 307–323. [Google Scholar] [CrossRef]
- Small, S.A.; Petsko, G.A. Endosomal recycling reconciles the Alzheimer’s disease paradox. Sci. Transl. Med. 2020, 12, eabb1717. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, A.M.; Peterhoff, C.M.; Troncoso, J.C.; Gomez-Isla, T.; Hyman, B.T.; Nixon, R.A. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: Differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 2000, 157, 277–286. [Google Scholar] [CrossRef]
- Offe, K.; Dodson, S.E.; Shoemaker, J.T.; Fritz, J.J.; Gearing, M.; Levey, A.I.; Lah, J.J. The lipoprotein receptor LR11 regulates amyloid beta production and amyloid precursor protein traffic in endosomal compartments. J. Neurosci. 2006, 26, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Small, S.A.; Simoes-Spassov, S.; Mayeux, R.; Petsko, G.A. Endosomal Traffic Jams Represent a Pathogenic Hub and Therapeutic Target in Alzheimer’s Disease. Trends Neurosci. 2017, 40, 592–602. [Google Scholar] [CrossRef]
- Chai, A.B.; Lam, H.H.J.; Kockx, M.; Gelissen, I.C. Apolipoprotein E isoform-dependent effects on the processing of Alzheimer’s amyloid-beta. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158980. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Scherzer, C.R.; Offe, K.; Gearing, M.; Rees, H.D.; Fang, G.; Heilman, C.J.; Schaller, C.; Bujo, H.; Levey, A.I.; Lah, J.J. Loss of apolipoprotein E receptor LR11 in Alzheimer disease. Arch. Neurol. 2004, 61, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Rudolph, I.M.; Willnow, T.E. Risk factor SORL1: From genetic association to functional validation in Alzheimer’s disease. Acta Neuropathol. 2016, 132, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.M.; Reiche, J.; Schmidt, V.; Gotthardt, M.; Spoelgen, R.; Behlke, J.; von Arnim, C.A.; Breiderhoff, T.; Jansen, P.; Wu, X.; et al. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2005, 102, 13461–13466. [Google Scholar] [CrossRef]
- Fjorback, A.W.; Andersen, O.M. SorLA is a molecular link for retromer-dependent sorting of the Amyloid precursor protein. Commun. Integr. Biol. 2012, 5, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.S.; Gustafsen, C.; Madsen, P.; Nyengaard, J.R.; Hermey, G.; Bakke, O.; Mari, M.; Schu, P.; Pohlmann, R.; Dennes, A.; et al. Sorting by the cytoplasmic domain of the amyloid precursor protein binding receptor SorLA. Mol. Cell. Biol. 2007, 27, 6842–6851. [Google Scholar] [CrossRef]
- Huang, T.Y.; Zhao, Y.; Li, X.; Wang, X.; Tseng, I.C.; Thompson, R.; Tu, S.; Willnow, T.E.; Zhang, Y.W.; Xu, H. SNX27 and SORLA Interact to Reduce Amyloidogenic Subcellular Distribution and Processing of Amyloid Precursor Protein. J. Neurosci. 2016, 36, 7996–8011. [Google Scholar] [CrossRef]
- Schmidt, V.; Baum, K.; Lao, A.; Rateitschak, K.; Schmitz, Y.; Teichmann, A.; Wiesner, B.; Petersen, C.M.; Nykjaer, A.; Wolf, J.; et al. Quantitative modelling of amyloidogenic processing and its influence by SORLA in Alzheimer’s disease. EMBO J. 2012, 31, 187–200. [Google Scholar] [CrossRef]
- Spoelgen, R.; von Arnim, C.A.; Thomas, A.V.; Peltan, I.D.; Koker, M.; Deng, A.; Irizarry, M.C.; Andersen, O.M.; Willnow, T.E.; Hyman, B.T. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and beta-secretase beta-site APP-cleaving enzyme. J. Neurosci. 2006, 26, 418–428. [Google Scholar] [CrossRef]
- Dumanis, S.B.; Burgert, T.; Caglayan, S.; Fuchtbauer, A.; Fuchtbauer, E.M.; Schmidt, V.; Willnow, T.E. Distinct Functions for Anterograde and Retrograde Sorting of SORLA in Amyloidogenic Processes in the Brain. J. Neurosci. 2015, 35, 12703–12713. [Google Scholar] [CrossRef]
- Glerup, S.; Lume, M.; Olsen, D.; Nyengaard, J.R.; Vaegter, C.B.; Gustafsen, C.; Christensen, E.I.; Kjolby, M.; Hay-Schmidt, A.; Bender, D.; et al. SorLA controls neurotrophic activity by sorting of GDNF and its receptors GFRalpha1 and RET. Cell Rep. 2013, 3, 186–199. [Google Scholar] [CrossRef]
- Rohe, M.; Hartl, D.; Fjorback, A.N.; Klose, J.; Willnow, T.E. SORLA-mediated trafficking of TrkB enhances the response of neurons to BDNF. PLoS ONE 2013, 8, e72164. [Google Scholar] [CrossRef]
- Stupack, J.; Xiong, X.P.; Jiang, L.L.; Zhang, T.M.; Zhou, L.S.; Campos, A.; Ranscht, B.; Mobley, W.; Pasquale, E.B.; Xu, H.X.; et al. Soluble SORLA Enhances Neurite Outgrowth and Regeneration through Activation of the EGF Receptor/ERK Signaling Axis. J. Neurosci. 2020, 40, 5908–5921. [Google Scholar] [CrossRef]
- Saadipour, K.; Yang, M.; Lim, Y.; Georgiou, K.; Sun, Y.; Keating, D.; Liu, J.; Wang, Y.R.; Gai, W.P.; Zhong, J.H.; et al. Amyloid beta(1)(-)(4)(2) (Abeta(4)(2)) up-regulates the expression of sortilin via the p75(NTR)/RhoA signaling pathway. J. Neurochem. 2013, 127, 152–162. [Google Scholar] [CrossRef]
- Andersson, C.H.; Hansson, O.; Minthon, L.; Andreasen, N.; Blennow, K.; Zetterberg, H.; Skoog, I.; Wallin, A.; Nilsson, S.; Kettunen, P. A Genetic Variant of the Sortilin 1 Gene is Associated with Reduced Risk of Alzheimer’s Disease. J. Alzheimers Dis. JAD 2016, 53, 1353–1363. [Google Scholar] [CrossRef]
- Finan, G.M.; Okada, H.; Kim, T.W. BACE1 retrograde trafficking is uniquely regulated by the cytoplasmic domain of sortilin. J. Biol. Chem. 2011, 286, 12602–12616. [Google Scholar] [CrossRef]
- Tan, J.; Evin, G. Beta-site APP-cleaving enzyme 1 trafficking and Alzheimer’s disease pathogenesis. J. Neurochem. 2012, 120, 869–880. [Google Scholar] [CrossRef]
- Gustafsen, C.; Glerup, S.; Pallesen, L.T.; Olsen, D.; Andersen, O.M.; Nykjaer, A.; Madsen, P.; Petersen, C.M. Sortilin and SorLA display distinct roles in processing and trafficking of amyloid precursor protein. J. Neurosci. 2013, 33, 64–71. [Google Scholar] [CrossRef]
- Carlo, A.S. Sortilin, a novel APOE receptor implicated in Alzheimer disease. Prion 2013, 7, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Carlo, A.S.; Gustafsen, C.; Mastrobuoni, G.; Nielsen, M.S.; Burgert, T.; Hartl, D.; Rohe, M.; Nykjaer, A.; Herz, J.; Heeren, J.; et al. The pro-neurotrophin receptor sortilin is a major neuronal apolipoprotein E receptor for catabolism of amyloid-beta peptide in the brain. J. Neurosci. 2013, 33, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Takamura, A.; Sato, Y.; Watabe, D.; Okamoto, Y.; Nakata, T.; Kawarabayashi, T.; Oddo, S.; Laferla, F.M.; Shoji, M.; Matsubara, E. Sortilin is required for toxic action of Abeta oligomers (AbetaOs): Extracellular AbetaOs trigger apoptosis, and intraneuronal AbetaOs impair degradation pathways. Life Sci. 2012, 91, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Vaegter, C.B.; Jansen, P.; Fjorback, A.W.; Glerup, S.; Skeldal, S.; Kjolby, M.; Richner, M.; Erdmann, B.; Nyengaard, J.R.; Tessarollo, L.; et al. Sortilin associates with Trk receptors to enhance anterograde transport and neurotrophin signaling. Nat. Neurosci. 2011, 14, 54–61. [Google Scholar] [CrossRef]
- Capsoni, S.; Amato, G.; Vignone, D.; Criscuolo, C.; Nykjaer, A.; Cattaneo, A. Dissecting the role of sortilin receptor signaling in neurodegeneration induced by NGF deprivation. Biochem. Biophys. Res. Commun. 2013, 431, 579–585. [Google Scholar] [CrossRef]
- Skeldal, S.; Matusica, D.; Nykjaer, A.; Coulson, E.J. Proteolytic processing of the p75 neurotrophin receptor: A prerequisite for signalling? Bioessays 2011, 33, 614–625. [Google Scholar] [CrossRef]
- Nykjaer, A.; Lee, R.; Teng, K.K.; Jansen, P.; Madsen, P.; Nielsen, M.S.; Jacobsen, C.; Kliemannel, M.; Schwarz, E.; Willnow, T.E.; et al. Sortilin is essential for proNGF-induced neuronal cell death. Nature 2004, 427, 843–848. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Ieraci, A.; Teng, H.; Dall, H.; Meng, C.X.; Herrera, D.G.; Nykjaer, A.; Hempstead, B.L.; Lee, F.S. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J. Neurosci. 2005, 25, 6156–6166. [Google Scholar] [CrossRef] [PubMed]
- Tesco, G.; Koh, Y.H.; Kang, E.L.; Cameron, A.N.; Das, S.; Sena-Esteves, M.; Hiltunen, M.; Yang, S.H.; Zhong, Z.Y.; Shen, Y.; et al. Depletion of GGA3 stabilizes BACE and enhances beta-secretase activity. Neuron 2007, 54, 721–737. [Google Scholar] [CrossRef] [PubMed]
- Lomoio, S.; Willen, R.; Kim, W.; Ho, K.Z.; Robinson, E.K.; Prokopenko, D.; Kennedy, M.E.; Tanzi, R.E.; Tesco, G. Gga3 deletion and a GGA3 rare variant associated with late onset Alzheimer’s disease trigger BACE1 accumulation in axonal swellings. Sci. Transl. Med. 2020, 12, eaba1871. [Google Scholar] [CrossRef]
- Kim, W.; Ma, L.; Lomoio, S.; Willen, R.; Lombardo, S.; Dong, J.; Haydon, P.G.; Tesco, G. BACE1 elevation engendered by GGA3 deletion increases beta-amyloid pathology in association with APP elevation and decreased CHL1 processing in 5XFAD mice. Mol. Neurodegener. 2018, 13, 6. [Google Scholar] [CrossRef]
- Li, X.; Lavigne, P.; Lavoie, C. GGA3 mediates TrkA endocytic recycling to promote sustained Akt phosphorylation and cell survival. Mol. Biol. Cell 2015, 26, 4412–4426. [Google Scholar] [CrossRef] [PubMed]
- Hickman, F.E.; Stanley, E.M.; Carter, B.D. Neurotrophin Responsiveness of Sympathetic Neurons Is Regulated by Rapid Mobilization of the p75 Receptor to the Cell Surface through TrkA Activation of Arf6. J. Neurosci. 2018, 38, 5606–5619. [Google Scholar] [CrossRef]
- Tang, W.; Tam, J.H.; Seah, C.; Chiu, J.; Tyrer, A.; Cregan, S.P.; Meakin, S.O.; Pasternak, S.H. Arf6 controls beta-amyloid production by regulating macropinocytosis of the Amyloid Precursor Protein to lysosomes. Mol. Brain 2015, 8, 41. [Google Scholar] [CrossRef]
- Perdigao, C.; Barata, M.A.; Burrinha, T.; Almeida, C.G. Alzheimer’s disease BIN1 coding variants increase intracellular Abeta levels by interfering with BACE1 recycling. J. Biol. Chem. 2021, 297, 101056. [Google Scholar] [CrossRef]
- Tan, M.S.; Yu, J.T.; Jiang, T.; Zhu, X.C.; Guan, H.S.; Tan, L. Genetic variation in BIN1 gene and Alzheimer’s disease risk in Han Chinese individuals. Neurobiol. Aging 2014, 35, 1781.e1–1781.e8. [Google Scholar] [CrossRef]
- Vardarajan, B.N.; Ghani, M.; Kahn, A.; Sheikh, S.; Sato, C.; Barral, S.; Lee, J.H.; Cheng, R.; Reitz, C.; Lantigua, R.; et al. Rare coding mutations identified by sequencing of Alzheimer disease genome-wide association studies loci. Ann. Neurol. 2015, 78, 487–498. [Google Scholar] [CrossRef]
- Ubelmann, F.; Burrinha, T.; Salavessa, L.; Gomes, R.; Ferreira, C.; Moreno, N.; Almeida, C.G. Bin1 and CD2AP polarise the endocytic generation of beta-amyloid. EMBO Rep. 2017, 18, 102–122. [Google Scholar] [CrossRef]
- De Rossi, P.; Nomura, T.; Andrew, R.J.; Masse, N.Y.; Sampathkumar, V.; Musial, T.F.; Sudwarts, A.; Recupero, A.J.; Le Metayer, T.; Hansen, M.T.; et al. Neuronal BIN1 Regulates Presynaptic Neurotransmitter Release and Memory Consolidation. Cell Rep. 2020, 30, 3520–3535.e3527. [Google Scholar] [CrossRef]
- Chen, H.; Wu, G.; Jiang, Y.; Feng, R.; Liao, M.; Zhang, L.; Ma, G.; Chen, Z.; Zhao, B.; Li, K.; et al. Analyzing 54,936 Samples Supports the Association between CD2AP rs9349407 Polymorphism and Alzheimer’s Disease Susceptibility. Mol. Neurobiol. 2015, 52, 1–7. [Google Scholar] [CrossRef]
- Harrison, B.J.; Venkat, G.; Lamb, J.L.; Hutson, T.H.; Drury, C.; Rau, K.K.; Bunge, M.B.; Mendell, L.M.; Gage, F.H.; Johnson, R.D.; et al. The Adaptor Protein CD2AP is a Coordinator of Neurotrophin Signaling-Mediated Axon Arbor Plasticity. J. Neurosci. 2016, 36, 4259–4275. [Google Scholar] [CrossRef]
- Small, S.A.; Kent, K.; Pierce, A.; Leung, C.; Kang, M.S.; Okada, H.; Honig, L.; Vonsattel, J.P.; Kim, T.W. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann. Neurol. 2005, 58, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Tang, F.L.; Hong, Y.; Luo, S.W.; Wang, C.L.; He, W.; Shen, C.; Jung, J.U.; Xiong, F.; Lee, D.H.; et al. VPS35 haploinsufficiency increases Alzheimer’s disease neuropathology. J. Cell Biol. 2011, 195, 765–779. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, Y.; Lee, H.J.; Kim, J.S.; Song, B.S.; Huh, J.W.; Lee, S.R.; Kim, S.U.; Kim, S.H.; Hong, Y.; et al. Implication of mouse Vps26b-Vps29-Vps35 retromer complex in sortilin trafficking. Biochem. Biophys. Res. Commun. 2010, 403, 167–171. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.Y.; Yancey, J.; Luo, H.; Zhang, Y.W. Role of Rab GTPases in Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 828–838. [Google Scholar] [CrossRef]
- Faustino, C.; Rijo, P.; Reis, C.P. Nanotechnological strategies for nerve growth factor delivery: Therapeutic implications in Alzheimer’s disease. Pharmacol. Res. 2017, 120, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Amadoro, G.; Latina, V.; Balzamino, B.O.; Squitti, R.; Varano, M.; Calissano, P.; Micera, A. Nerve Growth Factor-Based Therapy in Alzheimer’s Disease and Age-Related Macular Degeneration. Front. Neurosci. 2021, 15, 735928. [Google Scholar] [CrossRef]
- Fjord-Larsen, L.; Kusk, P.; Tornoe, J.; Juliusson, B.; Torp, M.; Bjarkam, C.R.; Nielsen, M.S.; Handberg, A.; Sorensen, J.C.; Wahlberg, L.U. Long-term delivery of nerve growth factor by encapsulated cell biodelivery in the Gottingen minipig basal forebrain. Mol. Ther. 2010, 18, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Eriksdotter-Jonhagen, M.; Linderoth, B.; Lind, G.; Aladellie, L.; Almkvist, O.; Andreasen, N.; Blennow, K.; Bogdanovic, N.; Jelic, V.; Kadir, A.; et al. Encapsulated cell biodelivery of nerve growth factor to the Basal forebrain in patients with Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 2012, 33, 18–28. [Google Scholar] [CrossRef]
- Qureshi, Y.H.; Berman, D.E.; Marsh, S.E.; Klein, R.L.; Patel, V.M.; Simoes, S.; Kannan, S.; Petsko, G.A.; Stevens, B.; Small, S.A. The neuronal retromer can regulate both neuronal and microglial phenotypes of Alzheimer’s disease. Cell Rep. 2022, 38, 110262. [Google Scholar] [CrossRef]
- Li, J.G.; Chiu, J.; Pratico, D. Full recovery of the Alzheimer’s disease phenotype by gain of function of vacuolar protein sorting 35. Mol. Psychiatry 2020, 25, 2630–2640. [Google Scholar] [CrossRef]
- Young, J.E.; Fong, L.K.; Frankowski, H.; Petsko, G.A.; Small, S.A.; Goldstein, L.S.B. Stabilizing the Retromer Complex in a Human Stem Cell Model of Alzheimer’s Disease Reduces TAU Phosphorylation Independently of Amyloid Precursor Protein. Stem. Cell Rep. 2018, 10, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Temkin, P.; Morishita, W.; Goswami, D.; Arendt, K.; Chen, L.; Malenka, R. The Retromer Supports AMPA Receptor Trafficking during LTP. Neuron 2017, 94, 74–82.e75. [Google Scholar] [CrossRef]
- Mecozzi, V.J.; Berman, D.E.; Simoes, S.; Vetanovetz, C.; Awal, M.R.; Patel, V.M.; Schneider, R.T.; Petsko, G.A.; Ringe, D.; Small, S.A. Pharmacological chaperones stabilize retromer to limit APP processing. Nat. Chem. Biol. 2014, 10, 443–449. [Google Scholar] [CrossRef]
- Berman, D.E.; Ringe, D.; Petsko, G.A.; Small, S.A. The Use of Pharmacological Retromer Chaperones in Alzheimer’s Disease and other Endosomal-related Disorders. Neurotherapeutics 2015, 12, 12–18. [Google Scholar] [CrossRef]
- Agola, J.O.; Jim, P.A.; Ward, H.H.; BasuRay, S.; Wandinger-Ness, A. Rab GTPases as regulators of endocytosis, targets of disease and therapeutic opportunities. Clin. Genet. 2011, 80, 305–318. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).