Abstract
Non-small-cell lung cancer (NSCLC) is one of the most common malignancies and the leading causes of cancer-related death worldwide. Despite many therapeutic advances in the past decade, NSCLC remains an incurable disease for the majority of patients. Molecular targeted therapies and immunotherapies have significantly improved the prognosis of NSCLC. However, the vast majority of advanced NSCLC develop resistance to current therapies and eventually progress. In this review, we discuss current and potential therapies for NSCLC, focusing on targeted therapies and immunotherapies. We highlight the future role of metabolic therapies and combination therapies in NSCLC.
1. Non-Small Cell Lung Cancer
In recent decades, lung cancer has decreased in incidence and mortality, but it remains the second most commonly detected cancer in the world [1]. Lung cancer can be divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) according to different pathological types, and NSCLC accounts for 80–85% of all lung cancers [2] (Figure 1).
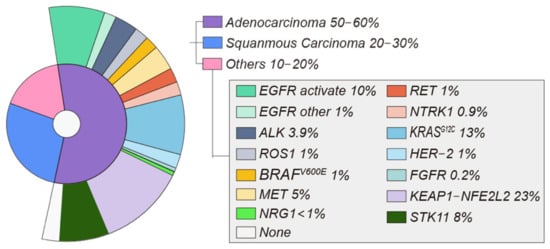
Figure 1.
In total, 50–60% of NSCLC cases are adenocarcinomas, whereas 20–30% are squamous cell carcinomas. Adenocarcinoma molecular alterations related to targetable oncogenic drivers. Incidences of oncogenic driver alterations extracted from study results [6,9,10].
The treatment of lung cancer involves surgery, radio-chemotherapy, immunotherapy, and targeted approaches with antiangiogenic monoclonal antibodies and tyrosine kinase inhibitors (TKIs) if tumors harbor a specific mutation [3]. NSCLC is often diagnosed at an advanced stage and has a poor prognosis [4]. Approximately 60% of patients with advanced NSCLC subtypes have specific molecular changes that may be suitable for targeted therapy [5].
Nowadays, next-generation sequencing technologies have enabled a comprehensive genetic characterization of NSCLC [6,7]. Therefore, clinicians have benefited from understanding cancer genetics.
At present, the actionable mutations with US Food and Drug Administration (FDA)-approved therapies include epidermal growth factor receptor (EGFR)-activating mutations, anaplastic lymphoma kinase (ALK) rearrangements, ROS1 gene fusions, BRAFV600E mutations, and neurotrophic tropomyosin-related kinase (NTRK) gene fusions. Other emerging targeted therapies for NSCLC target gene aberrations associated with ERBB2 (HER2), mesenchymal–epithelial transition (MET), rearranged during transfection (RET), KRASG12C, NeuReGulin 1(NRG1), and the fibroblast growth factor receptor (FGFR) [8]. Other therapeutic targets include metabolic targets (KEAP1-NFE2L2, STK11) and immune targets (PD-1, PD-L1, CTLA-4).
2. Molecular Targets
2.1. EGFR
2.1.1. EGFR p.L858R and EGFR Exon 19 Deletion
The EGFR gene is a tyrosine kinase receptor with a mutation rate of approximately 10% in patients with NSCLC (Figure 1) [11]. EGFR p.L858R and a 15 bp deletion in exon 19 are the two most frequent mutations in NSCLC, accounting for about 85–90% over all EGFR mutations [12].
The identification of EGFR-activating mutations in NSCLC drastically changed the disease’s clinical landscape. EGFR TKIs are now established first-line treatments for NSCLC with EGFR mutations. Compared to platinum-based chemotherapies, the primary EGFR TKIs showed advantages in progression-free survival (PFS) and overall survival (OS) (Table 1) [13,14]. Following the development of next-generation EGFR-targeted therapies, patients with EGFR-mutant tumors already have an OS of over three years [15].

Table 1.
Part of the key randomized trials in EGFR-positive NSCLC.
However, the clinical efficacy of these initial inhibitors was ultimately limited by the development of drug resistance, in particular the T790M gatekeeper residue mutation [18]. In order to solve the problem caused by drug resistance, more irreversible mutant-selective inhibitors including afatinib [19], osimertinib [20], dacomitinib, and rociletinib [21] were developed as second or third EGFR inhibitors. Osimertinib, a third-generation TKI, was originally approved for the treatment of T790M-positive resistance [22]. In patients with first-generation EGFR TKI resistance caused by T790M, osimertinib demonstrated a superior objective response rate (ORR) and PFS than standard chemotherapy [22]. Therefore, it was recently approved for the first-line therapeutic drug of EGFR-mutant lung cancer [23].
Despite ORRs as high as 70–80% in the early stages of treatment, resistance inevitably occurs. Unfortunately, acquired resistance to osimertinib has been linked to mutations in EGFR, loss of T790M, and the bypass of pathways via KRAS, MET, and PIK3CA [24,25]. Targeted therapeutic strategies are being evaluated in numerous trials following EGFR TKI resistance. In the current environment, cytotoxic chemotherapy is the most common treatment option. Notably, immune checkpoint inhibitor therapy is lacking in efficacy in EGFR-mutant NSCLC [26]. There are a large number of third-generation EGFR TKIs which have been developed, such as nazartinib (NCT02335944), lazertinib (NCT04248829, NCT04077463, NCT04487080) and aumolertinib (NCT04923906), in ongoing clinical trials [27].
2.1.2. EGFR Exon 20 Insertion Mutations
Exon 20 of the EGFR kinase domain contains around 10% of the EGFR activating mutations (EGFR ins20) [28] (Figure 1). Early generations of EGFR TKIs did not show the same sensitivity to EGFR ins20 mutations as classical activating EGFR mutations.
With significant advancements in sequencing technology, the success gained in overcoming these systemic hurdles has substantially increased the speed of recent breakthroughs in EGFR ins20 targeted medicines. An example of this is the recent approval of amivantamab by FDA [29] (Figure 2).
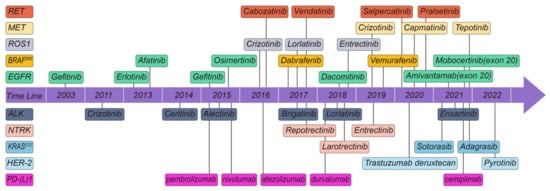
Figure 2.
Timeline of targeted drugs which FDA approved for oncogene-driven NSCLC.
A recent retrospective study of 6290 NSCLC patients found that patients with EGFR ins20 tumors had longer OS and time to treatment cessation with platinum chemotherapy compared with patients without targeted mutations [30].
2.1.3. Other EGFR Mutations
A number of unusual EGFR mutations have also been identified, including G719X, S768I, and L861Q. A multicenter, phase II study, in which 37 patients were enrolled, demonstrated favorable activity with manageable toxicity of osimertinib used for those with harboring uncommon mutations. These patients were given osimertinib as their first-line therapeutic drug in 61% of cases. L861Q, S768I, and other mutations were the next most common, followed by G719X. The ORR was 50% (95% CI, 33% to 67%). The median PFS was 8.2 months (95% CI, 5.9 to 10.5 months) [31].
A recent extensive study discovered a novel atypical EGFR mutation named very rare EGFR mutations. In this study, researchers found that treatment responses in very rare EGFR mutations were highly heterogeneous. It is worth noting that there was no significant tendency for co-occurring TP53 mutations to adversely affect the outcome in EGFR-TKI-treated patients in the exon 20 insertion mutations group or the in very rare EGFR mutations group [32].
2.2. ALK
The first case of NSCLC with ALK rearrangement was reported in 2007 [33]. ALK is a tyrosine kinase that belongs to the insulin receptor superfamily of transmembrane receptors [34]. ALK- rearrangement NSCLC accounts for approximately 3–8% of NSCLC [35] (Figure 1).
Similar to EGFR-targeted therapy, TKI-targeting ALK is the first-line therapeutic drug for ALK-rearranged advanced NSCLC. Since the first ALK-TKI crizotinib was developed, patient prognosis has improved considerably [36]. Currently, five TKIs are approved by the FDA. Since the FDA approval of crizotinib [37] in 2011, ceritinib [38] was approved in 2014, alectinib [39] in 2015, brigatinib [40] in 2017, and lorlatinib [41] in 2018 (Figure 2).
Of these, crizotinib is the most widely investigated. Several clinical trials including at least two-phase III trials showed that crizotinib was superior to chemotherapy in patients with advanced NSCLC that were positive for ALK rearrangements [42,43] (Table 2).

Table 2.
Part of the key randomized trials in ALK-positive NSCLC.
Subsequently, PFS benefit was reported in phase III trials for several next-generation ALK TKIs in treatment-naive patients. Ceritinib and chemotherapy were compared [44]. Subsequently, other ALK TKIs, alectinib [45], brigatinib [46], ensartinib [48], and lorlatinib [47] were compared with crizotinib. Following the experience with osimertinib in NSCLC patients with EGFR mutations, the second-generation ALK TKIs have replaced crizotinib as the first-line treatment for advanced-stage ALK-rearranged NSCLC.
In ASCEND-4 [44], of 376 untreated patients, 189 received ceritinib and 187 received chemotherapy. Ceritinib significantly enhanced PFS compared with platinum-based chemotherapy (median PFS, 16.6 months vs. 8.1 months, respectively; HR, 0.55 [95% CI, 0.42–0.73]; p < 0.00001).
Researchers randomly assigned patients with metastatic lung cancer who expressed the ALK gene to receive either alectinib or crizotinib as a first therapeutic drug in a phase III randomized study (n = 303). Compared with crizotinib, alectinib markedly improved the rate of investigator-assessed PFS (12-month event-free survival rate, 68.4% vs. 48.7%, respectively; HR, 0.47 [95% CI, 0.34–0.65]; p < 0.001) [45].
Based on these data, in the main therapy of ALK-positive NSCLC, alectinib exhibited greater effectiveness and decreased toxicity.
The randomization of 275 patients in another phase III trial found that 137 received brigatinib and 138 received crizotinib. The median follow-up in the brigatinib group was 11.0 months and 9.3 months in the crizotinib group in the first interim analysis (99 incidents). Compared with crizotinib, brigatinib had a higher rate of PFS (estimated 12-month PFS of 67% vs. 43%; HR of 0.49 [95% CI, 0.33–0.74]; p < 0.001) [46].
Based on these data, brigatinib outperforms crizotinib in terms of effectiveness, tolerability, and quality of life, making it a viable first-line therapeutic drug for ALK-positive NSCLC.
Despite the fact that alectinib and brigatinib have a median duration of chronic control of more than two years, drug resistance persists.
Based on previous data, initial phase III results with lorlatinib, an ALK-TKI of the third generation, showed promising results in this setting. In this trial, patients were randomly assigned to receive first-line lorlatinib (n = 149) or crizotinib (n = 147). The ORR was 76% vs. 58%, and 12-month PFS was 78% vs. 39%; HR, 0.28 [95% CI, 0.19–0.41]; p < 0.001 [47]. These results may redefine the new potential standard of care in the first-line setting.
In an open-label, multicenter phase III study, ensartinib, an oral tyrosine kinase inhibitor of ALK, showed systemic and central nervous system improvement for patients with ALK-positive NSCLC. In this trial, 290 patients were randomized to accept ensartinib or crizotinib. In the intent-to-treat population, ensartinib had a considerably longer median PFS than crizotinib (25.8 vs. 12.7 months; HR, 0.51 [95% Cl, 0.35–0.72]; p < 0.001) [48]. Ensartinib did not achieve its median PFS in the modified intention-to-treat population, whereas crizotinib group was 12.7 months (95% CI, 8.9–16.6 months; HR, 0.45 [95% CI, 0.30–0.66]; p < 0.001). Ensartinib did not improve PFS for patients without brain metastases compared to crizotinib, which achieved 16.6 months (at 12 months: 4.2% with ensartinib vs. 23.9% with crizotinib; cause-specific HR, 0.32 [95% CI, 0.16–0.63]; p = 0.001) [48].
Based on these data, a new treatment option available for patients with NSCLC who were ALK-positive was ensartinib.
Approximately 50–60% of patients with ALK kinase-domain mutations are resistant to second-generation ALK TKIs [49]. It may be important to note that ALK fusion variants may play a role in the development of ALK inhibitor resistance on-target [50]. Several recurrent acquired mutations in ALK confer resistance by decreasing drug binding, including I1171T/N/S, V1180L, G1269A and G1202R [51]. Studies have shown that ALKG1202R mutations are more common in tumors harboring EML4-ALK variant 3 (exon 6a/b of EML4 fused with exon 20 of ALK) than variant 1 (exon 13 of EML4 fused with exon 20 of ALK) [52].
There is no evidence that other TKIs other than lorlatinib can overcome resistance associated with G1202R [53]. Consistent with its broad ALK mutation coverage and CNS penetration, loratinib showed significant overall and intracranial activity ALK tyrosine kinase inhibitor in both ALK-positive NSCLC primary patients and in crizotinib second-generation therapy [41]. Moreover, loratinib has a robust and durable response and a high objective intracranial response in previously treated Chinese patients with ALK-positive NSCLC [54]. However, approximately 25–30% of patients who are treated with alectinib develop G1202R resistance, while 10–15% have I1171X resistance [49].
The latest ALK inhibitor developed by researchers is XMU-MP-5, which is designed to overcome crizotinib resistance mutations, such as L1196M and G1202R. It has achieved promising results in in vitro experiments [55].
Neuroendocrine transformation (NET) is another mechanism of ALK TKI resistance in NSCLC. In a study of 15 NET patients, immunohistochemistry showed p53/Rb inactivation in 72.7% of pre-NET lesions. Therefore, for patients with NET progression, p53/Rb risk prediction should be performed in addition to genomic assessment [56].
Up to now, in first-line therapy, there are already many ALK TKI alternatives, but the best upfront treatment option remains controversial [57,58].
A patient with metastatic ALK-rearranged NSCLC acquired crizotinib resistance due to a mutation in the ALK kinase domain, as shown in an investigation. This acquired mutation conferred lorlatinib resistance, but it also restored sensitivity to crizotinib [59].
There are no firm findings on the treatment sequence after first-line therapy, and further research is needed.
2.3. ROS1
Similar to ALK, ROS1 possesses tyrosine kinase activity and is a member of the insulin receptor family [60]. NSCLC was the second solid tumor found to harbor ROS1 rearrangements. Rearrangement of this gene is found in 1–2% of NSCLC, but more frequently in young women with adenocarcinoma who never smoked [61] (Figure 1).
The ROS1 gene was first implicated in chromosomal rearrangements in NSCLC in 2007 [62]. Since then, oncogenic ROS1 rearrangements have become a well-established therapeutic target in NSCLC.
The FDA-approved crizotinib as the first TKI to treat ROS1-rearranged NSCLC (Figure 2). This approval was based on efficacy and safety results from the expansion cohort of the phase I crizotinib study, which revealed an ORR of 72% and a median PFS of 19.2 months in advanced ROS1-rearranged NSCLC [61].
East Asian patients with ROS1-positive advanced NSCLC who had undergone three or fewer lines of previous systemic therapy were included in a phase II, open-label, controlled trial. A total of 127 individuals were included in the evaluations of effectiveness and safety, with 49.6% still taking medication at the end of the data collection period. With 17 full replies and 74 partial responses, the ORR was 71.7% (95% CI, 63.0–79.3%) [63]. Researchers reported similar ORR of 70% (95% CI, 51–85%) and median PFS of 20.0 months (95% CI, 10.1-not reached) for 34 European patients with advanced ROS1-positive NSCLC in a recent single-arm, multicenter, phase II trial [64].
In spite of the high response rates of crizotinib, the majority of patients eventually succumb to disease progression in part as a result of inadequate drug CNS penetration and/or the development of ROS1 resistant mutations [65].
The second-generation TKI ceritinib is indicated for the treatment of NSCLC that is resistant to crizotinib and those that have not been treated with TKIs. There was a 67% ORR (95% CI, 48–81%) with a 21-month DOR (95% CI, 17–25 months) and a 19.3-month median PFS (95% CI, 1–37 months) in a phase II trial of 30 crizotinib-naive patients with ROS1-positive NSCLC [66]. Despite the fact that ceritinib may be a promising treatment option for ROS1-positive NSCLC based on this study, several crizotinib-resistant ROS1 mutations such as G2032R, D2033N, L1951R, and S1986Y/F cannot be overcome by ceritinib [67,68].
ROS1 and ALK TKI lorlatinib displays strong penetration into the CNS and is effective against a wide range of ROS1 resistance mutations in preclinical studies.
Currently, during the first-in-man phase I multicenter study, 12 patients with ROS1-positive NSCLC received at least one dose of lorlatinib. Finally, 6 (50%) of the 12 patients obtained an objective response (95% CI, 21–79). Meanwhile, lorlatinib showed both systemic and intracranial activity in patients with advanced ROS1-positive NSCLC [69]. In one of the latest studies, when compared to the crizotinib group, the ORR of lorlatinib was higher (76% [95% CI, 68–83%] vs. 58% [95% CI, 49–66%]) [47]. Among those with measurable brain metastases, the intracranial response was 82% (95% CI, 57–96%) of loratinib group and 23% (95% CI, 5–54%) of the crizotinib group [49]. In particular, it was found that 71% of patients treated with lorlatinib had an intracranial complete response [49]. However, since lipid levels are often altered with lorlatinib, adverse events were more common than with crizotinib [47].
Entrectinib is a multikinase inhibitor with activity against ROS1, ALK, and TRK [70]. A substantial concentration of it reaches the central nervous system because of its ability to cross the blood-brain barrier [71]. An integrated review of three phase I-II trials showed that entrectinib is active and provides long-term disease control in patients with ROS1 fusion-positive NSCLC. It is well tolerated and has a controllable safety profile, making it suitable for long-term therapy in these patients [72].
2.4. BRAF
The RAS-RAF-MEK-ERK signaling pathway is one of the most frequently mutated in human tumors [73]. BRAF is one of the most frequently mutated kinases in human cancer, especially melanoma, with activating mutations in BRAF seen in 40–50% of malignancies. Smokers are more likely to have somatically activating BRAFV600E mutations, which have been reported in 1–2% of NSCLC [35] (Figure 1).
BRAF mutations do not coincide with other oncogenic alterations in a significant number of lung adenocarcinomas.
As the use of next-generation sequencing in clinical practice becomes more frequent, oncologists often find BRAFnon-V600 mutations in their patients’ malignancies, but they are unsure of feasible therapeutic alternatives for optimum therapy. Dabrafenib or vemurafenib, which are single-agent BRAF inhibitors, have had rather short-term responses [74,75]. In patients with metastatic BRAFV600E mutant NSCLC, 59 patients were enrolled in a phase II study investigating combination pathway blocking with dabrafenib (a BRAF inhibitor) and trametinib (a MEK inhibitor). This study was successful, with a response rate of 64% and a median PFS of 11 months [76].
These data backed up a molecularly focused approach for patients with BRAF-mutant lung cancer. The FDA has approved dabrafenib and trametinib for the treatment of metastatic NSCLC exhibited the BRAFV600E mutation [77] (Figure 2).
Another trial launched by the French National Cancer Institute (INCA) assessed the effectiveness and safety of vemurafenib in tumors with diverse BRAF mutations [78]. The trial recruited 118 patients (101 with BRAFV600E mutations and 17 with BRAFnonV600E mutations), with a median follow-up of 23.9 months. There was no objective response in the BRAFnonV600E cohort, hence it was halted. 43/96 individuals in the BRAFV600E cohort exhibited objective responses. The median response duration was 6.4 months, while the median PFS was 5.2 months (95% CI, 3.8–6.8 months), and the median OS was 10 months (95% CI, 6.8–15.7 months). The safety profile of vemurafenib was consistent with earlier studies [78]. These data indicated that BRAFV600E mutations should be a routine biomarker screened of NSCLC, and vemurafenib may be the novel BRAF-mutation targeted drug.
2.5. MET
In epithelial cells, the MET receptor plays a role in tyrosine kinase signaling [79].
MET exon 14 skipping mutations are seen in 3–4% of patients with adenocarcinomas and 1–2% of individuals with another NSCLC histology [80] (Figure 1).
MET TKIs are classified as type I, II and III. Inhibitors of type Ia (such as crizotinib) block ATP binding to prevent receptor phosphorylation/activation. Capmatinib, tepo-tinib, savolitinib, and AMG 337 are type Ib inhibitors that are more effective against MET than type Ia inhibitors. Unlike ATP-binding sites, type III inhibitors (such as tivantinib) bind to allosteric sites [81].
Capmatinib was tested in 364 patients with stage IIIB/IV NSCLC in the GEOMETRY mono-1 trial. Patients were divided into groups depending on their past treatment regimens and MET status. In patients with a MET exon 14 skipping mutation, the study found that overall, 41% (95% CI, 29–53%) of 69 patients who had previously had one or two lines of medication responded, compared to 68% (95% CI, 48–84%) of 28 patients who had never received treatment. Furthermore, the median duration of response (DOR) was 9.7 months [82].
Based on these data, the FDA has approved capmatinib for treating patients with metastatic NSCLC with an exon 14 skipping mutation (Figure 2).
Tepotinib monotherapy was investigated prospectively in individuals with advanced NSCLC with MET exon 14 skipping mutations in the VISION open-label Phase II trial. At least 9 months of follow-up were conducted on 152 patients who received tepotinib. According to an independent analysis, based on the combined biopsy group, 46% of patients responded (95% CI, 36–57%) and the median DOR was 11.1 months (95% CI, 7.2 to NE).
The response rate for 66 patients in the liquid biopsy group was 48% (95% CI, 36–61%) and for 60 patients in the tissue biopsy group was 50% (95% CI, 37–63%). Both approaches had favorable outcomes in 27 cases. Regardless of previous therapy for advanced or metastatic cancer, the investigator-assessed response rate of 56% (95% CI, 45–66%) was identical [83].
Based on these data, tepotinib was granted breakthrough therapy designation by the FDA in September 2019 for the treatment of metastatic NSCLC patients with MET exon 14 skipping mutations who progressed following platinum-based cancer therapy (Figure 2).
Crizotinib was used in the METROS single-arm research, which included 26 patients (each) in two cohorts having ROS1 or MET rearrangements and one or more previous chemotherapy regimens. FISH was used to centrally evaluate ROS1 rearrangement and MET amplification. The researchers used direct sequencing to confirm the MET mutation at the end of the trial. The major trial endpoint was RR, which was 27% (of those with MET), with a PFS of 4.4 months and 13 patients reporting SAEs (50%) [69].
Treatments for patients with MET exon 14 skipping mutations are being revolutionized by MET TKIs. However, resistance to MET TKIs limits the magnitude and DOR. Several mechanisms of resistance to MET TKI have been established, such as targeted resistance driven by mutations in kinase structural domains that affect drug-receptor binding or its ATP affinity, amplification of MET exon 14 mutant alleles, and activation-mediated off-target resistance bypass signaling [84,85,86,87]. Therefore, to effectively target NSCLC, it is crucial to identify the mechanisms of resistance to MET TKIs.
2.6. RET
In 1–2% of NSCLC patients, RET gene rearrangements can enhance tumor development [88] (Figure 1). Patients with nondriver mutation NSCLCs, such as those with ROS, are younger and more likely to be light smokers or non-smokers.
Multiple TKIs that target RET have shown minimal therapeutic benefit in patients with RET rearrangements in their malignancies, includes cabozatinib [89] (tumor response rate of 28% and median PFS of 5.5 months) and vendatinib [90] (response rate of 18% and median PFS of 4.5 months).
For patients with advanced RET fusion and previous platinum-based therapy, a phase II study showed that selpercatinib had a long-term effectiveness. The ORR was 64% (95% CI, 54–73%) in the first 105 patients with RET fusion-positive NSCLC who had previously received at least platinum-based chemotherapy. The median response time was 17.5 months (95% CI, 12.0 could not be evaluated), and 63% of the responses were still active after 12.1 months of follow-up. The number of patients who had an objective response was 85% (95% CI, 70–94%) among 39 previously untreated patients, and 90% of the responses continued for 6 months. The percentage with an objective intracranial response was 91% (95% CI, 59–100%) among 11 patients with demonstrable central nervous system metastases at enrolment. At the same time, the toxic effects of selpercatinib were low-grade [91].
Based on these data, selpercatinib had durable efficacy for patients with RET fusion-positive NSCLC.
Investigators presented results from ARROW, a continuing noncomparative phase I or II study with pralsetinib, at the ASCO 2020 (and 2019) conference. ARROW is a phase 1/2 multi-cohort, open-label trial conducted at 71 locations (community and academic cancer centers) in 13 countries. From 17 March 2017 through 22 May 2020, pralsetinetib was given to 92 patients with RET fusion-positive NSCLC, and 29 patients who had never received treatment were given the drug by 11 July 2019. OS was documented in 53 (61%; [95% CI, 50–71]) of 87 patients who had previously received platinum-based chemotherapy, with 5 patients achieving a full response, and 19 (70%; 50–86) of 27 treatment-naive patients achieving a complete response [92].
Based on these data, FDA approval to pralsetinib for adult patients with metastatic RET fusion-positive NSCLC (Figure 2).
2.7. NTRK1
The NTRK1 gene encodes tropomyosin-related kinase A [93]. NTRK gene abnormalities have been found in fewer than 1% of NSCLC [94] (Figure 1).
The NTRK mutation treatment drug larotrectinib was developed using a clinical trial that enrolled patients with any kind of NTRK gene rearrangements which showed a tumor response rate of 75% and 12-month PFS rate of 55%. As a result, larotrectinib was approved for treatment of NTRK-altered cancers regardless of their primary site by FDA (Figure 2). FDA approved the case series in which four patients with lung cancer were included as part of the FDA approval process [95].
Entrectinib was administered to patients with NTRK-positive tumors of various histology in a second pooled analysis, and Doebele and colleagues reported that 31 (57%; [95% CI, 43.2–70.8%]) of 54 patients treated with entrectinib achieved an objective response, with a median PFS of 11.2 months (95% CI, 8.0–14.9 months) and a median DOR of 10.4 months (95% CI, 7.1 to not estimable) [96].
The FDA approved entrectinib in August 2019 for the therapeutic drugs of metastatic ROS1-positive NSCLC and NTRK-positive tumors in both adults and children (Figure 2). Since larotrectinib was approved by the FDA in November 2018 (Figure 2), two TKIs have been licensed for NTRK gene fusion-positive solid tumors.
Most patients take first-generation TRK inhibitors well, with toxicity profiles typified by infrequent off-tumor, on-target side effects (attributable to TRK inhibition in non-malignant tissues). Despite the fact that many patients have long-term disease control, advanced-stage NTRK fusion-positive malignancies eventually develop resistance to TRK inhibition. Resistance might be caused by the acquisition of NTRK kinase domain mutations.
Following that, second-generation TRK inhibitors such as LOXO-195 and TPX-0005, which are now in clinical studies, can overcome some resistance mutations [97,98].
2.8. KRAS
Because of the lack of a deep binding pocket, NSCLC with oncogenic KRAS mutations is notoriously hard to treat with small molecule inhibitors [100]. Sotorisib, a first-in-class KRASG12C small molecule inhibitor, has been approved for irreversible inhibition of KRASG12C via an interaction with the P2 pocket by the FDA. Sotorasib showed a 37% ORR and a median PFS of 6.7 months in the Code Brea K 100 phase II single-arm study [101]. KRYSTAL-1 phase I and II data, which have demonstrated a 45% ORR, have led the FDA to designate adagrasib as a breakthrough therapeutic drug [102] (Figure 2).
These data showed that combinatorial techniques can help increase response persistence, and several studies are currently underway to investigate the impact of checkpoint inhibitors and other MAPK pathway inhibitors.
2.9. HER2
Trastuzumab emtansine(T-DM1), a HER2-targeted antibody-drug combination, has demonstrated tumor responses in these patients (n = 18; response rate of 44% and median PFS of 5 months) [104].
Meanwhile, T-DM1 was studied in a phase II study in patients with advanced HER2-overexpressing NSCLC who had previously been treated. In this study, 59 patients received T-DM1 (29 IHC 2+, 20 IHC 3+). In the IHC 2+ cohort, no treatment responses were detected. In the IHC 3+ cohort, four partial responses were observed (ORR, 20% [95% CI, 5.7–43.7%]). The clinical benefit rates in the IHC 2+ and 3+ groups were 7% and 30%, respectively. Respondents took 2.9, 7.3, 8.3, and 10.8 months to complete their responses. Between groups, median PFS and OS were similar [105].
Seventy-eight individuals were enrolled in a multicenter study to assess effectiveness and safety. In this study, the 6-month PFS rate was 49.5% (95% CI, 39.2–60.8%). Pyrotinib had 19.2% of ORR (95% CI, 11.2–30.0%), median OS of 10.5 months (95% CI, 8.7–12.3 months), and median PFS of 5.6 months (95% CI, 2.8–8.4 months). Meanwhile, there was a median DOR of 9.9 months (95% CI, 6.2–13.6 months). With HER2 mutations in exon 20 and without exon 20, ORRs were 17.7% and 25.0%, respectively. Afatinib exposure and brain metastases at baseline had no effect on ORR, PFS, or OS. As the illness progressed, the loss of HER2 mutations and the development of amplification in EGFR and HER2 were discovered [106].
Based on these data, in NSCLC with exon 20 or non-exon 20 HER2 mutations, pyrotinib showed promising effectiveness and tolerable tolerability.
Trastuzumab (T-DXd) is a novel antibody–drug conjugate that targets HER2. In an ongoing multicenter study called DESTINY-Lung01, the effectiveness of T-DXd in treating patients with non-squamous NSCLC overexpressing HER2 or containing a HER2-activating mutation is being tested [107]. The ORR was 61.9% (95% CI, 45.6–76.4%) at data cutoff. The following results confirm its long-lasting anti-cancer activity: the median DOR was 9.3 months (95% CI, 5.7–14.7 months), median PFS was 8.2 months (95% CI, 6.0–11.9 months) and median OS was 17.8 months (95% CI, 13.8–22.1 months) [108]. These ideal outcomes demonstrated durable responses in NSCLC with HER2-mutation.
Recently, FDA granted accelerated approval to fam-trastuzumab deruxtecan-nxki for HER2-mutant NSCLC (Figure 2). Efficacy for accelerated approval was based on DESTINY-Lung02 (NCT04644237). The available, substantial results showed that the confirmed ORR was 58% (95% CI, 43–71 months) and the median DOR was 8.7 months (95% CI, 7.1 months to NE).
3. Molecular Targets with Potential Therapeutics
3.1. NRG1
Studies have shown that NRG1 fusions are another rare but potentially actionable class of alterations in NSCLC. However, NRG1 fusions are rare overall (<1% in NSCLC) [109] (Figure 1).
Given the rarity of NRG1 fusions, clinical experience with NRG1-directed therapies remains limited. Although anti-ERBB3 mAb therapy (GSK2849330) achieved a durable response in abnormal responders to NRG1 rearranged IMA in an in vitro assay, a trial indicated that no response to anti-ERBB2 treatment (afatinib) was achieved in four NRG1 rearranged IMA patients (including the index patient after GCK2849330) [110]. Tarloxotinib is a prodrug that takes advantage of tumor hypoxia to produce high levels of the potent covalent pan-HER tyrosine kinase inhibitor tarloxotinib effector (tarloxotinib-E) in the tumor microenvironment. A recent trial tested the clinical response to tarloxotinib [111].
3.2. FGFR
The fibroblast growth factor receptors (FGFRs) are involved in cell growth, development, and cancer growth. NSCLC has an amplification of FGFR1 in about 0.2% of cases, which raises the possibility that the novel anti-cancer drug could target FGFR [112] (Figure 1). However, acquired resistance to this type of treatment remains a significant therapeutic issue.
To establish whether FGFR is a potential target, researchers examined at the response of NSCLC cell lines to the new selective FGFR inhibitor CPL304110 and the probable mechanism of acquired resistance. In this study, despite considerable genetic variations between CPL304110-sensitive and resistant cell lines, as well as elevated p38 expression/phosphorylation, they also observed increased MAPK gene expression. They discovered that inhibiting p38 restored CPL304110 sensitivity in these cells. Furthermore, the p38 MAPK-overexpressing parental cells showed reduced sensitivity to inhibition of FGFR, suggesting that p38 MAPK is responsible for resistance to a new FGFR inhibitor [112].
Based on these data, the p38 MAPK pathway may point to a new avenue for NSCLC targeted treatment.
4. Metabolic Targets
4.1. KEAP1-NFE2L2
Activation of the KEAP1-NFE2L2 pathway increases tumor growth and aggressiveness [113,114]. KEAP1-NFE2L2 mutations are estimated to be found in 23% of NSCLC based on the Cancer Genome Atlas Network (TCGA) [9] (Figure 1).
In recent years, KEAP1-NFE2L2 mutations have been found to have multiple prognostic implications. KEAP1-NFE2L2 was shown to be a predictive biomarker of radiation resistance [115]. In addition, KEAP1-NFE2L2 deletion has been identified as a mechanism of acquired resistance to targeted therapy in EGFR-mutant tumors [116].
As the prognostic impact mutations of KEAP1-NFE2L2 continue to evolve, many experiments have sprung up to develop therapies targeting this pathway. The compound sapanisertib (TAK-228), which inhibits both mTORC1 and mTORC2, was the first therapy for cancer patients who carried KEAP1-NFE2L2 mutations [117]. A study of cancer models performed in squamous cell lung showed that KEAP1-NFE2L2 mutant lung cancer cells were sensitive to inhibition by TAK-228 but not by everolimus or rapamycin [118].
KEAP1-NFE2L2 mutant tumors are dependent on glutaminolysis and inhibition of glutaminase leads to reduced growth and radiation sensitization of KEAP1-NFE2L2 mutant tumors [119]. In the BeGIN trial (NCT03872427), the glutaminase inhibitor (CB-839) is being investigated in patients with advanced solid tumors with mutations in KEAP1-NFE2L2, STK11/LKB1, or NF1. In addition, CB-839 is also being tested in a phase II randomized trial (NCT04265534) to compare it with chemotherapy and pembrolizumab as standard-of-care in patients with advanced KEAP1-NFE2L2 mutant non-squamous NSCLC.
4.2. STK11
STK11 is a key upstream activator of AMP-activated protein kinases that can affect the regulation of glucose and fat Metabolism, cell growth and homeostasis [120]. STK11 mutations occur in 8–39% of NSCLC [121] (Figure 1).
Because both STK11 and KEAP1 mutations are highly prevalent in NSCLCs with KRAS mutations, STK11 and KEAP1 mutations have mainly been evaluated in KRAS-mutated patients. Mutations in STK11 and KEAP1 genes, as well as the presence of other mutations, are associated with poor outcomes in patients treated with ICI for NSCLC. Skoulidis and colleagues found that as compared to KRAS wild-type, concurrent STK11 mutations result in significantly reduced ORR, PFS, and OS [122].
Similar to KEAP1, STK11 may affect the prognosis of NSCLC. Recently, a retrospective analysis was performed on 2276 patients with NSCLC who received different types of therapy, including PD-1/PD-L1 in beta-blockers, chemotherapy, and tyrosine kinase inhibitors. Regardless of whether KRAS mutations were present or absent, STK11 and KEAP1 mutations were associated with poor prognoses in all treatment categories. In patients with both STK11 and KEAP1 mutations, the prognosis was worse than in those with only one mutation, suggesting an additive effect [123].
However, STK11 mutations in NSCLC have not been studied clinically, so a comprehensive evaluation of these patients is not be possible.
5. Immune Targets
5.1. PD-1 and PD-L1
In 1992, researchers identified a novel programmed death-1 (PD-1) growth factor in mouse T cell tumors. PD-1, which belongs to the regulatory B7-CD28 receptor superfamily, is considered to be a novel kind of cell protein that is widely expressed on the surface of T cells and is closely related to apoptotic growth factors [124]. The main ligand of PD-1 is programmed death ligand 1 (PD-L1), and the two exert immunomodulatory effects through binding. Studies have demonstrated that PD-L1 expression is elevated in a range of malignancies, including NSCLC, and that this is linked to shorter patient terrible prognosis and survival [125]. In recent years, immune checkpoint inhibitors (ICIs) have exerted antitumor effects through the mechanism of immune checkpoints, especially targeting the PD-1 and PD-L1 pathways. However, the selection of the beneficiary population remains a challenge.
PD-1 is targeted by the monoclonal antibody nivolumab, which is composed of all-human IgG4 antibodies [126]. In several open-label trials (CheckMate 057, CheckMate 026, CheckMate 017) [127,128,129], the results of the trials showed that nivolumab has a longer OS and better safety profile than chemotherapy. A pooled analysis of CheckMate 017 and CheckMate 057 studies showed that five-year pooled OS rates of nivolumab compared to docetaxel were 13.4% vs. 2.6% and 5-year PFS rates of nivolumab compared to docetaxel were 8.0% vs. 0%. Furthermore, nivolumab had a 28% lower death rate than docetaxel, and its adverse reactions were also lower than docetaxel’s.
Pembrolizumab, a PD-1 receptor agonist, inhibits the interactions between PD-L1 and PD-L2. KEYNOTE-001 showed an ideal result in that the median OS was 22.3 months in treatment-naive patients and 10.5 months in previously treated patients [130]. KEYNOTE-024 then identified pembrolizumab as an effective first-line treatment for patients with advanced NSCLC with PD-L1 TPS ≥ 50% [131]. Immediately after KEYNOTE-024, KEYNOTE-042 further expanded the population considered to benefit from pembrolizumab treatment [132]. KEYNOTE-042 suggested that pembrolizumab could be extended as first-line therapy to patients with locally advanced or metastatic NSCLC.
Inhibiting PD-1 and PD-L1 receptor interaction by targeting PD-L1 protein, atezolizumab is an IgG1 monoclonal antibody. In an open-label tail called POPLAR which enrolled 287 patients with advanced NSCLC who had progressed after platinum-based chemotherapy. Atezolizumab group had a better medium OS of 12.6 months than docetaxel group whose medium OS was 9.7 months [133]. Subsequently, a multicenter trial called OAK further demonstrated the efficacy and safety of atezolizumab. The median OS of atezolizumab was 13.8 months versus 9.6 months compared to docetaxel [134]. Patients treated with atezolizumab had fewer grade 3 or 4 adverse events compared to patients treated with docetaxel. In 2020, a trial called IMpower110 showed atezolizumab as a first-line treatment with NSCLC with PD-L1 expression, regardless of histologic type [135].
Avelumab is a monoclonal antibody that targets PD-L1 in humans. According to its use in first-line treatment of patients with advanced NSCLC, avelumab showed safety and tolerability when expressing antitumor activity [136]. In this trial, the median PFS was 4.0 months (95% CI, 2.7–5.4 months) and the 6-month PFS rate was 38.5% (95% CI, 30.7–46.3%). The median OS was 14.1 months (95% CI, 11.3–16.9 months) and the 12-month OS rate was 56.6% (95% CI, 48.2–64.1%). In patients with platinum-treated PD-L1+ NSCLC, avelumab did not significantly prolong OS as compared with docetaxel in the primary analysis [137], a 2-year follow-up data of JAVELIN LUNG 200 showed that OS rates were doubled with avelumab in subgroups with higher PD-L1 expression (greater than or equal to 50% and greater than or equal to 80%) [138].
Another PD-L1 inhibitor, durvalumab, targets CD80 and PD-1 by blocking its binding to PD-L1. In a study called PACIFIC of durvalumab after chemoradiotherapy in stage III NSCLC [139]. The median PFS from randomization was 16.8 months (95% CI, 13.0–18.1 months) with durvalumab versus 5.6 months (95% CI, 4.6–7.8) with the placebo (HR, 0.52 [95% CI, 0.42–0.65]; p < 0.001). The study reconfirms that durvalumab therapy resulted in significantly longer OS than the placebo [140]. The latest OS and PFS data from PACIFIC further validate the benefit of durvalumab in NSCLC [141,142,143,144].
By inhibiting PD-1, cemiplimab stimulates an anticancer response. In EMPOWER-Lung 1, a multicenter trial which examined cemiplimab of advanced NSCLC with PD-L1 of at least 50%, cemiplimab significantly improved OS and PFS compared with chemotherapy [145]. The median OS of cemiplimab group (n = 283) was not reached (95% CI, 17.9 months to not evaluable), while it was 14.2 months in those who received chemotherapy (n = 280). Moreover, cemiplimab had a higher median PFS than chemotherapy (8.2 moths vs. 5.7 months). A new treatment option is available for advanced NSCLC with PD-L1 of at least 50%.
Tislelizumab is a novel PD-1-targeted antibody designed to have reduced binding to Fc-gamma receptors, in an effort to overcome a potential mechanism of resistance to PD-1 inhibitor antibody therapy [146].
Similar to the targeted drugs above, immunotherapy drugs are also inevitably limited by drug resistance. Host, tumor cells, and the immune microenvironment interact to cause tumor immunotherapy resistance [147]. Currently, the most important and effective method of delaying or reversing immune resistance is through a combination therapy strategy [148].
5.2. CTLA-4
Another important immune checkpoint is CTLA-4. CTLA-4 is a ligand or receptor for tumor cell-immune cell interactions and acts as an immunomodulator [149]. Treatment against CTLA-4 plays an important role in melanoma [150]. Similar to PD-1/PD-L1, ICI anti-CTLA4 has a significant role in NSCLC [151].
Ipilimumab, an inhibitor of CTLA-4, may promote T-cell activation and subsequent anti-tumor immunity. In an early study involving patients with advanced NSCLC, median OS with nivolumab plus ipilimumab was 17.1 months (95% CI, 15.0–20.1 months) compared with chemotherapy that was 14.9 months (95% CI, 12.7–16.7 months) [152].
In CheckMate 227, Patients with advanced NSCLC who received first-line nivolumab plus ipilimumab for a minimum of four years have continued to show durable long-term efficacy even after stopping immunotherapy for at least two years. The four-year OS rate with nivolumab plus ipilimumab was 29%, while chemotherapy was 18% [153].
Tremelimumab is a fully human anti-CTLA-4, IgG2 mAb. In a phase Ib trial, tremelimumab was initially studied in combination with the anti-PD-L1 drug durvalumab in the treatment of advanced NSCLC [154].
As a result of these findings, the Phase III MYSTIC Trial was conducted, which tested durvalumab alone or in combination with tremelimumab as an upfront strategy for patients with advanced NSCLC. The median OS was 16.3 months (95% CI, 12.2–20.8 months) in the durvalumab group versus 12.9 months (95% CI, 10.5–15.0 months) in the chemotherapy group (HR, 0.76 [97.54% CI, 0.56–1.02]) [155].
6. Combined Immune-Therapies with Targets
6.1. STING
Although immunotherapy in combination with chemotherapy is the current standard of care for NSCLC, clinical response varies. The stimulator of interferon genes (STING) may be activated by cytosolic DNA fragments [156].
By activating STING-mediated antitumor, DNA damage and anti-PD-L1 synergistic immune responses can be induced by treatment with DNA damage response inhibitors in the syngeneic model of SCLC [157]. Based on this study, Carminia and colleagues explored the profile of immune-related genes related to STING pathway activation in NSCLC. The considerable result is that STING pathway activation in NSCLC is characteristic of predictive immunotherapy response and is enhanced by cisplatin treatment, suggesting that possible predictive biomarkers and mechanisms can improve response to chemoimmunotherapy combinations [158].
In a study which explores the overall prognosis of NSCLC, the investigators found that high STING expression was associated with improved OS in patients with local adenocarcinoma and may be a potential biomarker for NSCLC [159].
6.2. LAG3
Lymphocyte-activation gene 3 (LAG3) is the most promising immune checkpoint next to PD-1 and CTLA-4. Fibrin-like protein 1 (FGL1), a liver-secreted protein, is a major LAG-3 functional ligand independent of MHC-II [160].
In NSCLC, the high expression of FGL1 and LAG3 was associated with a poorer five-year OS, respectively [161]. In metastatic NSCLC characterized by high plasma FGL1 levels, the poor outcome after anti-PD-1 treatment suggests that FGL1 may play a role in tumor immune resistance. Higher FGL1 expression may induce resistance to gefitinib [162].
A novel anti-LAG3 drug, ieramilimab (LAG525), is currently being investigated in two trials as a monotherapy or in combination with PDR001, an experimental anti-PD-1 drug. Patients with solid tumors, including NSCLC, were tested for safety and antitumor activity of LAG525 as a single agent or combined with PDR001. Twelve patients responded to the combination with a durable response and an acceptable safety profile [163].
An ongoing phase II study combining pembrolizumab and IMP321 to treat patients with untreated, unresectable, or metastatic NSCLC is investigating this combination [161].
6.3. TIGIT
TIGIT (T-cell immunoreceptor with Ig and ITIM domains) gene encodes immunoglobulin proteins of the poliovirus receptor family of inhibitory immune receptors [164].
Overexpression of TIGIT in NSCLC may be associated with increased levels of other immunosuppressive receptors (such as PD-1 and LAG-3) and decreased levels of activator receptors [165].
CITYSCAPE is a phase II, randomized, double-blind, placebo-controlled trial. 135 eligible patients were randomly assigned to receive tiragolumab (a TIGIT inhibitor) plus atezolizumab (n = 67) or a placebo plus atezolizumab (n = 68). At a median follow-up of 5.9 months, 11 patients had an objective response in the tiragolumab plus atezolizumab group versus the placebo plus atezolizumab group. The median PFS was 5.4 months in the tiragolumab plus atezolizumab group, and 3.6 months in the placebo plus atezolizumab group [166]. Based on these data, tiragolumab plus atezolizumab is a promising immunotherapy combination for the treatment of previously untreated locally advanced unresectable or metastatic NSCLC.
Studies are ongoing to evaluate the efficacy and safety of pembrolizumab (MK-3475) PLUS chemotherapy in combination with anti-TIGIT vibostolimab (MK-7684), MK-5890, or MK-4830 in newly treated participants with advanced SCC or non-SCC (NCT 04165798).
7. Molecular Testing
With so many authorized targeted drugs, molecular profiling must be quick and accurate. Despite this, genetic testing is still not widely used in clinical practice.
Single biomarker assays have traditionally been performed using immunohistochemistry, fluorescence in situ hybridization, polymerase chain reaction, and Sanger sequencing methods [167]. Since it is relatively easy to implement, has a quick turnaround time, and is relatively inexpensive, it is suitable for a wide range of applications. However, as the number of molecular biomarkers grows, multiplexed assays, particularly NGS, are becoming more popular for evaluating several biomarkers in a single workflow. Tissue yield and attrition are important factors in NSCLC, as diagnostic specimens are frequently acquired from tiny biopsies [168]. As a result, the use of liquid biopsies to analyze circulating tumor DNA has exploded. However, emerging assays must be compared to existing molecular tests to demonstrate their clinical validity, practicability, and cost-effectiveness. In recent years, several studies have revealed that NGS is cost-effective in treating LUAD when biomarkers other than ALK, EGFR, and ROS1 are analyzed [169]. Even in Asian cultures, where EGFR mutations are the most common driver mutation, this has been confirmed [170]. However, before implementing this in various settings, health-care resource use, prescription prices, and long-term patient outcomes must be considered. NGS has already been found to have superior sensitivity when compared to single-gene focused assays [171,172,173]. Current molecular tests for oncogenic drivers are shown in Figure 3.
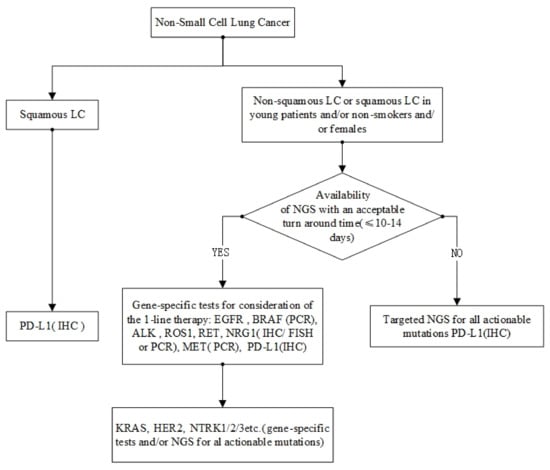
Figure 3.
Current molecular tests for oncogenic drivers.
It is possible that many new biomarkers have no recognized gold standard methods of detection. Furthermore, there may be discrepancies between tests, such as for RET rearrangements, RNA-based vs. DNA-based NGS for MET exon 14 alterations [174] and NGS vs. fluorescence in situ hybridization [175]. Tests based on NGS panels, such as those in tissue and plasma, have been approved as companion testing for several targeted medicines.
8. Discussion
Despite the current good progress in the treatment of NSCLC at the time of writing, FDA-approved therapeutics are EGFR, ALK, ROS1, BRAFV600E, MET, RET, NTRK, KRASG12C, HER2 and PD-1 molecular abnormalities [176] (Figure 2). NSCLC continues to have the highest incidence and mortality rate of any disease [1].
When compared to chemotherapy, biomarker-targeted therapies have demonstrated great potential in NSCLC, drug resistance is a problem that never goes away [177,178]. As a result, a deeper understanding of the molecular basis of drug resistance is critical in order to delay or even prevent resistance.
Despite these challenges, the future aspect of the NSCLC treatment landscape does look bright, with multiple promising new therapies targeting newly labeled proteins and less prone to acquiring resistance, many of which are even in clinical studies. Innovative therapies targeting other immune checkpoint receptors, such as Toll-like receptors 9 (DV281 and CpG ODN) [179,180], various neoadjuvant therapies, combined immune-therapies have shown significant results in preclinical studies and are currently under clinical investigation.
With a better understanding of the molecular heterogeneity of NSCLC and an increased clinical use of NGS, the development of novel targeted drugs will continue to accelerate. Recent deep multiset sequencing of oncogenic drivers, such as proteome sequencing, may reveal further molecular signatures related to response and resistance to therapies and novel targets [181]. These features will allow the development of therapies with higher selectivity and specificity for targets of interest.
In summary, the identification of actionable targets in NSCLC has changed the way many patients are managed and treated. A growing number of active phase II/III studies are showing promise in combating medication resistance and discovering new viable drugs. The current research into the genetic and epigenomic basis of cancer ecosystems will lead to the development of new, highly specific targeted drugs. In a word, biomarker-target therapies are potential and powerful weapon to treat even overcome NSCLC.
Author Contributions
Conceptualization, H.G., J.Z. and C.Q.; methodology, H.Y. and T.L.; software, H.G.; validation, S.T. (Shengjie Tang); formal analysis, J.Z., C.Q. and S.T. (Shoujun Tang); resources, H.G. and H.Z.; data curation, H.H., S.T. (Shengjie Tang) and S.T. (Shoujun Tang); writing—original draft preparation, H.G., J.Z. and C.Q.; writing—review and editing, H.G., J.Z. and C.Q.; visualization, H.H.; supervision, H.Z.; project administration, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Chengdu University of Traditional Chinese Medicine: YYZX2020093.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Meng, G.X.; Liu, X.W.; Ma, T.; Sun, G.; He, H. Deep-LC: A Novel Deep Learning Method of Identifying Non-Small Cell Lung Cancer-Related Genes. Front. Oncol. 2022, 12, 949546. [Google Scholar] [CrossRef]
- Soo, R.; Stone, E.; Cummings, K.; Jett, J.; Field, J.; Groen, H.; Mulshine, J.; Yatabe, Y.; Bubendorf, L.; Dacic, S.; et al. Scientific Advances in Thoracic Oncology 2016. J. Thorac. Oncol. 2017, 12, 1183–1209. [Google Scholar] [CrossRef]
- Solito, S.; Marigo, I.; Pinton, L.; Damuzzo, V.; Mandruzzato, S.; Bronte, V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann. N. Y. Acad. Sci. 2014, 1319, 47–65. [Google Scholar] [CrossRef]
- Deb, D.; Moore, A.C.; Roy, U.B. The 2021 Global Lung Cancer Therapy Landscape. J. Thorac. Oncol. 2022, 17, 931–936. [Google Scholar] [CrossRef]
- Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014, 511, 543–550. [CrossRef]
- George, J.; Lim, J.; Jang, S.; Cun, Y.; OzRETić, L.; Kong, G.; Leenders, F.; Lu, X.; Fernández-Cuesta, L.; Bosco, G.; et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Li, T.; Kung, H.-J.; Mack, P.C.; Gandara, D.R. Genotyping and genomic profiling of non-small-cell lung cancer: Implications for current and future therapies. J. Clin. Oncol. 2013, 31, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Johnson, A.; Ross, J.S.; Miller, V.A.; Ali, S.M.; Schrock, A.B.; Gadgeel, S.M. Detection of Known and Novel FGFR Fusions in Non-Small Cell Lung Cancer by Comprehensive Genomic Profiling. J. Thorac. Oncol. 2019, 14, 54–62. [Google Scholar] [CrossRef]
- Hsu, W.H.; Yang, J.C.H.; Mok, T.S.; Loong, H.H. Overview of current systemic management of EGFR-mutant NSCLC. Ann. Oncol. 2018, 29, i3–i9. [Google Scholar] [CrossRef] [PubMed]
- Rotow, J.; Bivona, T.G. Understanding and targeting resistance mechanisms in NSCLC. Nat. Rev. Cancer 2017, 17, 637–658. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Shimokawa, M.; Seto, T.; Morita, S.; Yatabe, Y.; Okamoto, I.; Tsurutani, J.; Satouchi, M.; Hirashima, T.; Atagi, S.; et al. Final overall survival results of WJTOG3405, a randomized phase III trial comparing gefitinib versus cisplatin with docetaxel as the first-line treatment for patients with stage IIIB/IV or postoperative recurrent EGFR mutation-positive non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1978–1984. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann. Oncol. 2015, 26, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, -Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Tan, E.H.; O’Byrne, K.; Zhang, L.; Hirsh, V.; Boyer, M.; Yang, J.C.H.; Mok, T.; Lee, K.H.; Lu, S.; et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase IIb LUX-Lung 7 trial. Ann. Oncol. 2017, 28, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef]
- Jia, Y.; Yun, C.-H.; Park, E.; Ercan, D.; Manuia, M.; Juarez, J.; Xu, C.; Rhee, K.; Chen, T.; Zhang, H.; et al. Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors. Nature 2016, 534, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.A.; Pao, W. Targeted therapies: Afatinib—New therapy option for EGFR-mutant lung cancer. Nat. Rev. Clin. Oncol. 2013, 10, 551–552. [Google Scholar] [CrossRef]
- Liang, W.; Zhong, R.; He, J. Osimertinib in EGFR-Mutated Lung Cancer. N. Engl. J. Med. 2021, 384, 675. [Google Scholar] [CrossRef]
- Sequist, L.V.; Soria, J.-C.; Goldman, J.W.; Wakelee, H.A.; Gadgeel, S.M.; Varga, A.; Papadimitrakopoulou, V.; Solomon, B.J.; Oxnard, G.R.; Dziadziuszko, R.; et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 1700–1709. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.M.E.; et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Yang, J.C.H.; Lee, C.K.; Kurata, T.; Kim, D.-W.; John, T.; Nogami, N.; Ohe, Y.; Mann, H.; Rukazenkov, Y.; et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, N.; Ou, Q.; Xiang, Y.; Jiang, T.; Wu, X.; Bao, H.; Tong, X.; Wang, X.; Shao, Y.W.; et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 3097–3107. [Google Scholar] [CrossRef]
- StarRETt, J.H.; Guernet, A.A.; Cuomo, M.E.; Poels, K.E.; van Alderwerelt van Rosenburgh, I.K.; Nagelberg, A.; Farnsworth, D.; Price, K.S.; Khan, H.; Ashtekar, K.D.; et al. Drug Sensitivity and Allele Specificity of First-Line Osimertinib Resistance Mutations. Cancer Res. 2020, 80, 2017–2030. [Google Scholar] [CrossRef]
- Lee, C.K.; Man, J.; Lord, S.; Cooper, W.; Links, M.; Gebski, V.; Herbst, R.S.; Gralla, R.J.; Mok, T.; Yang, J.C.-H. Clinical and Molecular Characteristics Associated with Survival Among Patients Treated with Checkpoint Inhibitors for Advanced Non-Small Cell Lung Carcinoma: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 210–216. [Google Scholar] [CrossRef]
- Andrews Wright, N.M.; Goss, G.D. Third-generation epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8, S247–S264. [Google Scholar] [CrossRef] [PubMed]
- Meador, C.B.; Sequist, L.V.; Piotrowska, Z. Targeting Exon 20 Insertions in Non-Small Cell Lung Cancer: Recent Advances and Clinical Updates. Cancer Discov. 2021, 11, 2145–2157. [Google Scholar] [CrossRef] [PubMed]
- Romero, D. Amivantamab is effective in NSCLC harbouring EGFR exon 20 insertions. Nat. Rev. Clin. Oncol. 2021, 18, 604. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, N.J.; Schoenfeld, A.J.; Flynn, J.; Falcon, C.J.; Rizvi, H.; Rudin, C.M.; Kris, M.G.; Arcila, M.E.; Heller, G.; Yu, H.A.; et al. Response to Standard Therapies and Comprehensive Genomic Analysis for Patients with Lung Adenocarcinoma with Exon 20 Insertions. Clin. Cancer Res. 2021, 27, 2920–2927. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Lim, S.H.; An, H.J.; Kim, K.H.; Park, K.U.; Kang, E.J.; Choi, Y.H.; Ahn, M.S.; Lee, M.H.; Sun, J.-M.; et al. Osimertinib for Patients with Non-Small-Cell Lung Cancer Harboring Uncommon EGFR Mutations: A Multicenter, Open-Label, Phase II Trial (KCSG-LU15-09). J. Clin. Oncol. 2020, 38, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Janning, M.; Süptitz, J.; Albers-Leischner, C.; Delpy, P.; Tufman, A.; Velthaus-Rusik, J.L.; Reck, M.; Jung, A.; Kauffmann-Guerrero, D.; Bonzheim, I.; et al. Treatment outcome of atypical EGFR mutations in the German National Network Genomic Medicine Lung Cancer (nNGM). Ann. Oncol. 2022, 33, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Golding, B.; Luu, A.; Jones, R.; Viloria-Petit, A.M. The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol. Cancer 2018, 17, 52. [Google Scholar] [CrossRef]
- Kris, M.G.; Johnson, B.E.; Berry, L.D.; Kwiatkowski, D.J.; Iafrate, A.J.; Wistuba, I.I.; Varella-Garcia, M.; Franklin, W.A.; Aronson, S.L.; Su, P.-F.; et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014, 311, 1998–2006. [Google Scholar] [CrossRef] [PubMed]
- Katayama, R.; Lovly, C.M.; Shaw, A.T. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: A paradigm for precision cancer medicine. Clin. Cancer Res. 2015, 21, 2227–2235. [Google Scholar] [CrossRef]
- Shaw, A.T.; Yasothan, U.; Kirkpatrick, P. Crizotinib. Nat. Rev. Drug Discov. 2011, 10, 897–898. [Google Scholar] [CrossRef]
- Ceritinib gains FDA approval for lung cancer. Cancer Discov. 2014, 4, 753–754. [CrossRef][Green Version]
- Larkins, E.; Blumenthal, G.M.; Chen, H.; He, K.; Agarwal, R.; Gieser, G.; Stephens, O.; ZahALKa, E.; Ringgold, K.; Helms, W.; et al. FDA Approval: Alectinib for the Treatment of Metastatic, ALK-Positive Non-Small Cell Lung Cancer Following Crizotinib. Clin. Cancer Res. 2016, 22, 5171–5176. [Google Scholar] [CrossRef]
- American Association for Cancer Research. Brigatinib Approved, but Treatment Role Uncertain. Cancer Discov. 2017, 7, OF3. [Google Scholar] [CrossRef][Green Version]
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.-C.; Gadgeel, S.M.; et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef]
- Solomon, B.J.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; Tang, Y.; et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Shaw, A.T.; Bauer, T.M.; de Marinis, F.; Felip, E.; Goto, Y.; Liu, G.; Mazieres, J.; Kim, D.-W.; Mok, T.; Polli, A.; et al. First-Line Lorlatinib or Crizotinib in Advanced -Positive Lung Cancer. N. Engl. J. Med. 2020, 383, 2018–2029. [Google Scholar] [CrossRef]
- Horn, L.; Wang, Z.; Wu, G.; Poddubskaya, E.; Mok, T.; Reck, M.; Wakelee, H.; Chiappori, A.A.; Lee, D.H.; Breder, V.; et al. Ensartinib vs Crizotinib for Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1617–1625. [Google Scholar] [CrossRef]
- Gainor, J.F.; Dardaei, L.; Yoda, S.; Friboulet, L.; Leshchiner, I.; Katayama, R.; Dagogo-Jack, I.; Gadgeel, S.; Schultz, K.; Singh, M.; et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov. 2016, 6, 1118–1133. [Google Scholar] [CrossRef]
- Song, Z.; Lian, S.; Mak, S.; Chow, M.Z.-Y.; Xu, C.; Wang, W.; Keung, H.Y.; Lu, C.; Kebede, F.T.; Gao, Y.; et al. Deep RNA Sequencing Revealed Fusion Junctional Heterogeneity May Predict Crizotinib Treatment Efficacy in ALK-Rearranged NSCLC. J. Thorac. Oncol. 2022, 17, 264–276. [Google Scholar] [CrossRef]
- Noé, J.; Lovejoy, A.; Ou, S.-H.I.; Yaung, S.J.; Bordogna, W.; Klass, D.M.; Cummings, C.A.; Shaw, A.T. ALK Mutation Status Before and After Alectinib Treatment in Locally Advanced or Metastatic ALK-Positive NSCLC: Pooled Analysis of Two Prospective Trials. J. Thorac. Oncol. 2020, 15, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Zhu, V.W.; Yoda, S.; Yeap, B.Y.; Schrock, A.B.; Dagogo-Jack, I.; Jessop, N.A.; Jiang, G.Y.; Le, L.P.; Gowen, K.; et al. Impact of EML4-ALK Variant on Resistance Mechanisms and Clinical Outcomes in ALK-Positive Lung Cancer. J. Clin. Oncol. 2018, 36, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Solomon, B.J.; Besse, B.; Bauer, T.M.; Lin, C.-C.; Soo, R.A.; Riely, G.J.; Ou, S.-H.I.; Clancy, J.S.; Li, S.; et al. Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2019, 37, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhou, Q.; Liu, X.; Du, Y.; Fan, Y.; Cheng, Y.; Fang, J.; Lu, Y.; Huang, C.; Zhou, J.; et al. Lorlatinib for Previously Treated ALK-Positive Advanced NSCLC: Primary Efficacy and Safety From a Phase 2 Study in People’s Republic of China. J. Thorac. Oncol. 2022, 17, 816–826. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, Z.; Zhu, S.-J.; Huang, X.; Zhuang, Z.; Li, Y.; Deng, Z.; Gao, L.; Hong, X.; Zhang, T.; et al. A new ALK inhibitor overcomes resistance to first- and second-generation inhibitors in NSCLC. EMBO Mol. Med. 2022, 14, e14296. [Google Scholar] [CrossRef]
- Chu, X.; Xu, Y.; Li, Y.; Zhou, Y.; Chu, L.; Yang, X.; Ni, J.; Li, Y.; Guo, T.; Zheng, Z.; et al. Neuroendocrine transformation from EGFR/ALK-wild type or TKI-naïve non-small cell lung cancer: An under-recognized phenomenon. Lung Cancer 2022, 169, 22–30. [Google Scholar] [CrossRef]
- Camidge, D.R. Lorlatinib Should Not be Considered as the Preferred First-Line Option in Patients with Advanced ALK Rearranged NSCLC. J. Thorac. Oncol. 2021, 16, 528–531. [Google Scholar] [CrossRef]
- Nagasaka, M.; Ou, S.-H.I. Lorlatinib Should Be Considered as the Preferred First-Line Option in Patients with Advanced ALK-Rearranged NSCLC. J. Thorac. Oncol. 2021, 16, 532–536. [Google Scholar] [CrossRef]
- Shaw, A.T.; Friboulet, L.; Leshchiner, I.; Gainor, J.F.; Bergqvist, S.; Brooun, A.; Burke, B.J.; Deng, Y.-L.; Liu, W.; Dardaei, L.; et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N. Engl. J. Med. 2016, 374, 54–61. [Google Scholar] [CrossRef]
- Savic, S.; Rothschild, S.; Bubendorf, L. Lonely Driver ROS1. J. Thorac. Oncol. 2017, 12, 776–777. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.H.; Bang, Y.J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Rikova, K.; Guo, A.; Zeng, Q.; Possemato, A.; Yu, J.; Haack, H.; Nardone, J.; Lee, K.; Reeves, C.; Li, Y.; et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007, 131, 1190–1203. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Yang, J.C.-H.; Kim, D.-W.; Lu, S.; Zhou, J.; Seto, T.; Yang, J.-J.; Yamamoto, N.; Ahn, M.-J.; Takahashi, T.; et al. Phase II Study of Crizotinib in East Asian Patients with ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 1405–1411. [Google Scholar] [CrossRef]
- Michels, S.; Massutí, B.; Schildhaus, H.-U.; Franklin, J.; Sebastian, M.; Felip, E.; Grohé, C.; Rodriguez-Abreu, D.; Abdulla, D.S.Y.; Bischoff, H.; et al. Safety and Efficacy of Crizotinib in Patients with Advanced or Metastatic ROS1-Rearranged Lung Cancer (EUCROSS): A European Phase II Clinical Trial. J. Thorac. Oncol. 2019, 14, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Tseng, D.; Yoda, S.; Dagogo-Jack, I.; Friboulet, L.; Lin, J.J.; Hubbeling, H.G.; Dardaei, L.; Farago, A.F.; Schultz, K.R.; et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in -Positive Non-Small-Cell Lung Cancer. JCO Precis. Oncol. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Lim, S.M.; Kim, H.R.; Lee, J.-S.; Lee, K.H.; Lee, Y.-G.; Min, Y.J.; Cho, E.K.; Lee, S.S.; Kim, B.-S.; Choi, M.Y.; et al. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients with Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J. Clin. Oncol. 2017, 35, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Engelman, J.A.; Shaw, A.T. Acquired resistance to crizotinib from a mutation in CD74-ROS1. N. Engl. J. Med. 2013, 369, 1173. [Google Scholar] [CrossRef]
- Facchinetti, F.; Loriot, Y.; Kuo, M.-S.; Mahjoubi, L.; Lacroix, L.; Planchard, D.; Besse, B.; Farace, F.; Auger, N.; Remon, J.; et al. Crizotinib-Resistant ROS1 Mutations Reveal a Predictive Kinase Inhibitor Sensitivity Model for ROS1- and ALK-Rearranged Lung Cancers. Clin. Cancer Res. 2016, 22, 5983–5991. [Google Scholar] [CrossRef]
- Shaw, A.T.; Felip, E.; Bauer, T.M.; Besse, B.; Navarro, A.; Postel-Vinay, S.; Gainor, J.F.; Johnson, M.; Dietrich, J.; James, L.P.; et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: An international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017, 18, 1590–1599. [Google Scholar] [CrossRef]
- Menichincheri, M.; Ardini, E.; Magnaghi, P.; Avanzi, N.; Banfi, P.; Bossi, R.; Buffa, L.; Canevari, G.; Ceriani, L.; Colombo, M.; et al. Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), c-ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) inhibitor. J. Med. Chem. 2016, 59, 3392–3408. [Google Scholar] [CrossRef]
- Ardini, E.; Menichincheri, M.; Banfi, P.; Bosotti, R.; De Ponti, C.; Pulci, R.; Ballinari, D.; Ciomei, M.; Texido, G.; Degrassi, A.; et al. Entrectinib, a Pan-TRK, ROS1, and ALK Inhibitor with Activity in Multiple Molecularly Defined Cancer Indications. Mol. Cancer Ther. 2016, 15, 628–639. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; de Braud, F.; Rolfo, C.; Ahn, M.-J.; Wolf, J.; et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 261–270. [Google Scholar] [CrossRef]
- Roskoski, R. Allosteric MEK1/2 inhibitors including cobimetanib and trametinib in the treatment of cutaneous melanomas. Pharmacol. Res. 2017, 117, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Hyman, D.; Puzanov, I.; Subbiah, V.; Faris, J.; Chau, I.; Blay, J.; Wolf, J.; Raje, N.; Diamond, E.; Hollebecque, A.; et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015, 373, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Kim, T.; Mazieres, J.; Quoix, E.; Riely, G.; Barlesi, F.; Souquet, P.; Smit, E.; Groen, H.; Kelly, R.; et al. Dabrafenib in patients with BRAF(V600E)-positive advanced non-small-cell lung cancer: A single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 642–650. [Google Scholar] [CrossRef]
- Planchard, D.; Smit, E.; Groen, H.; Mazieres, J.; Besse, B.; Helland, Å.; Giannone, V.; D’Amelio, A.; Zhang, P.; Mookerjee, B.; et al. Dabrafenib plus trametinib in patients with previously untreated BRAF-mutant Metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1307–1316. [Google Scholar] [CrossRef]
- Odogwu, L.; Mathieu, L.; Blumenthal, G.; Larkins, E.; Goldberg, K.B.; Griffin, N.; Bijwaard, K.; Lee, E.Y.; Philip, R.; Jiang, X.; et al. FDA Approval Summary: Dabrafenib and Trametinib for the Treatment of Metastatic Non-Small Cell Lung Cancers Harboring Mutations. Oncologist 2018, 23, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Cropet, C.; Montané, L.; Barlesi, F.; Souquet, P.J.; Quantin, X.; Dubos-Arvis, C.; Otto, J.; Favier, L.; Avrillon, V.; et al. Vemurafenib in non-small-cell lung cancer patients with BRAF and BRAF mutations. Ann. Oncol. 2020, 31, 289–294. [Google Scholar] [CrossRef]
- Eder, J.P.; Vande Woude, G.F.; Boerner, S.A.; LoRusso, P.M. Novel therapeutic inhibitors of the c-MET signaling pathway in cancer. Clin. Cancer Res. 2009, 15, 2207–2214. [Google Scholar] [CrossRef]
- Schrock, A.B.; Frampton, G.M.; Suh, J.; Chalmers, Z.R.; Rosenzweig, M.; Erlich, R.L.; Halmos, B.; Goldman, J.; Forde, P.; Leuenberger, K.; et al. Characterization of 298 Patients with Lung Cancer Harboring MET Exon 14 Skipping Alterations. J. Thorac. Oncol. 2016, 11, 1493–1502. [Google Scholar] [CrossRef]
- Vansteenkiste, J.F.; Van De Kerkhove, C.; Wauters, E.; Van Mol, P. Capmatinib for the treatment of non-small cell lung cancer. Expert Rev. Anticancer Ther. 2019, 19, 659–671. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in Exon 14-Mutated or -Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non-Small-Cell Lung Cancer with Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Heist, R.S.; Sequist, L.V.; Borger, D.; Gainor, J.F.; Arellano, R.S.; Le, L.P.; Dias-Santagata, D.; Clark, J.W.; Engelman, J.A.; Shaw, A.T.; et al. Acquired Resistance to Crizotinib in NSCLC with MET Exon 14 Skipping. J. Thorac. Oncol. 2016, 11, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Kobayashi, Y.; Suda, K.; Koga, T.; Nishino, M.; Ohara, S.; Chiba, M.; Shimoji, M.; Tomizawa, K.; Takemoto, T.; et al. Sensitivity and Resistance of MET Exon 14 Mutations in Lung Cancer to Eight MET Tyrosine Kinase Inhibitors In Vitro. J. Thorac. Oncol. 2019, 14, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Suzawa, K.; Offin, M.; Lu, D.; Kurzatkowski, C.; Vojnic, M.; Smith, R.S.; Sabari, J.K.; Tai, H.; Mattar, M.; Khodos, I.; et al. Activation of KRAS Mediates Resistance to Targeted Therapy in MET Exon 14-mutant Non-small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 1248–1260. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Offin, M.; Brannon, A.R.; Chang, J.; Chow, A.; Delasos, L.; Girshman, J.; Wilkins, O.; McCarthy, C.G.; Makhnin, A.; et al. Exon 14-altered Lung Cancers and MET Inhibitor Resistance. Clin. Cancer Res. 2021, 27, 799–806. [Google Scholar] [CrossRef]
- Gautschi, O.; Milia, J.; Filleron, T.; Wolf, J.; Carbone, D.P.; Owen, D.; Camidge, R.; Narayanan, V.; Doebele, R.C.; Besse, B.; et al. Targeting RET in Patients with RET-Rearranged Lung Cancers: Results From the Global, Multicenter RET Registry. J. Clin. Oncol. 2017, 35, 1403–1410. [Google Scholar] [CrossRef]
- Drilon, A.; Rekhtman, N.; Arcila, M.; Wang, L.; Ni, A.; Albano, M.; Van Voorthuysen, M.; Somwar, R.; Smith, R.S.; Montecalvo, J.; et al. Cabozantinib in patients with advanced RET-rearranged non-small-cell lung cancer: An open-label, single-centre, phase 2, single-arm trial. Lancet Oncol. 2016, 17, 1653–1660. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.K.; Ahn, M.J.; Kim, D.W.; Sun, J.M.; Keam, B.; Kim, T.M.; Heo, D.S.; Ahn, J.S.; Choi, Y.L.; et al. Vandetanib in pretreated patients with advanced non-small cell lung cancer-harboring RET rearrangement: A phase II clinical trial. Ann. Oncol. 2017, 28, 292–297. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in Fusion-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; Cassier, P.A.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Indo, Y.; Tsuruta, M.; Hayashida, Y.; Karim, M.A.; Ohta, K.; Kawano, T.; Mitsubuchi, H.; Tonoki, H.; Awaya, Y.; Matsuda, I. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat. Genet. 1996, 13, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Hofman, P. Next-Generation Sequencing with Liquid Biopsies from Treatment-Naïve Non-Small Cell Lung Carcinoma Patients. Cancers 2021, 13, 2049. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; DeMETri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or Metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A.; Nagasubramanian, R.; Blake, J.F.; Ku, N.; Tuch, B.B.; Ebata, K.; Smith, S.; Lauriault, V.; Kolakowski, G.R.; Brandhuber, B.J.; et al. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov. 2017, 7, 963–972. [Google Scholar] [CrossRef]
- Drilon, A.; Ou, S.-H.I.; Cho, B.C.; Kim, D.-W.; Lee, J.; Lin, J.J.; Zhu, V.W.; Ahn, M.-J.; Camidge, D.R.; Nguyen, J.; et al. Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov. 2018, 8, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Xue, J.Y.; Lito, P. Targeting KRAS(G12C): From Inhibitory Mechanism to Modulation of Antitumor Effects in Patients. Cell 2020, 183, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Salgia, R.; Pharaon, R.; Mambetsariev, I.; Nam, A.; Sattler, M. The improbable targeted therapy: KRAS as an emerging target in non-small cell lung cancer (NSCLC). Cell Rep. Med. 2021, 2, 100186. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Riely, G.J.; Ou, S.H.I.; Rybkin, I.; Spira, A.; Papadopoulos, K.; Sabari, J.K.; Johnson, M.; Heist, R.S.; Bazhenova, L.; Barve, M.; et al. 99O_PR KRYSTAL-1: Activity and preliminary pharmacodynamic (PD) analysis of adagrasib (MRTX849) in patients (Pts) with advanced non–small cell lung cancer (NSCLC) harboring KRASG12C mutation. J. Thorac. Oncol. 2021, 16, S751–S752. [Google Scholar] [CrossRef]
- Stephens, P.; Hunter, C.; Bignell, G.; Edkins, S.; Davies, H.; Teague, J.; Stevens, C.; O’Meara, S.; Smith, R.; Parker, A.; et al. Lung cancer: Intragenic ERBB2 kinase mutations in tumours. Nature 2004, 431, 525–526. [Google Scholar] [CrossRef]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-Trastuzumab Emtansine for Patients with HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J. Clin. Oncol. 2018, 36, 2532–2537. [Google Scholar] [CrossRef]
- Peters, S.; Stahel, R.; Bubendorf, L.; Bonomi, P.; Villegas, A.; Kowalski, D.M.; Baik, C.S.; Isla, D.; Carpeno, J.D.C.; Garrido, P.; et al. Trastuzumab Emtansine (T-DM1) in Patients with Previously Treated HER2-Overexpressing Metastatic Non-Small Cell Lung Cancer: Efficacy, Safety, and Biomarkers. Clin. Cancer Res. 2019, 25, 64–72. [Google Scholar] [CrossRef]
- Song, Z.; Li, Y.; Chen, S.; Ying, S.; Xu, S.; Huang, J.; Wu, D.; Lv, D.; Bei, T.; Liu, S.; et al. Efficacy and safety of pyrotinib in advanced lung adenocarcinoma with HER2 mutations: A multicenter, single-arm, phase II trial. BMC Med. 2022, 20, 42. [Google Scholar] [CrossRef]
- Smit, E.F.; Nakagawa, K.; Nagasaka, M.; Felip, E.; Goto, Y.; Li, B.T.; Pacheco, J.M.; Murakami, H.; Barlesi, F.; Saltos, A.N.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients with HER2-mutated Metastatic non-small cell lung cancer (NSCLC): Interim results of DESTINY-Lung01. J. Clin. Oncol. 2020, 38, 9504. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in -Mutant Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, L.; Plenker, D.; Osada, H.; Sun, R.; Menon, R.; Leenders, F.; Ortiz-Cuaran, S.; Peifer, M.; Bos, M.; Daßler, J.; et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014, 4, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Somwar, R.; Mangatt, B.P.; Edgren, H.; Desmeules, P.; Ruusulehto, A.; Smith, R.S.; Delasos, L.; Vojnic, M.; Plodkowski, A.J.; et al. Response to ERBB3-Directed Targeted Therapy in -Rearranged Cancers. Cancer Discov. 2018, 8, 686–695. [Google Scholar] [CrossRef]
- Estrada-Bernal, A.; Le, A.T.; Doak, A.E.; Tirunagaru, V.G.; Silva, S.; Bull, M.R.; Smaill, J.B.; Patterson, A.V.; Kim, C.; Liu, S.V.; et al. Tarloxotinib Is a Hypoxia-Activated Pan-HER Kinase Inhibitor Active Against a Broad Range of HER-Family Oncogenes. Clin. Cancer Res. 2021, 27, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Zarczynska, I.; Gorska-Arcisz, M.; Cortez, A.J.; Kujawa, K.A.; Wilk, A.M.; Skladanowski, A.C.; Stanczak, A.; Skupinska, M.; Wieczorek, M.; Lisowska, K.M.; et al. p38 Mediates Resistance to FGFR Inhibition in Non-Small Cell Lung Cancer. Cells 2021, 10, 3363. [Google Scholar] [CrossRef] [PubMed]
- DeNicola, G.M.; KarRETh, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Moriguchi, T.; Saigusa, D.; Baird, L.; Yu, L.; Rokutan, H.; Igarashi, K.; Ebina, M.; Shibata, T.; Yamamoto, M. NRF2 Intensifies Host Defense Systems to Prevent Lung Carcinogenesis, but After Tumor Initiation Accelerates Malignant Cell Growth. Cancer Res. 2016, 76, 3088–3096. [Google Scholar] [CrossRef] [PubMed]
- Binkley, M.S.; Jeon, Y.-J.; Nesselbush, M.; Moding, E.J.; Nabet, B.Y.; Almanza, D.; Kunder, C.; Stehr, H.; Yoo, C.H.; Rhee, S.; et al. Mutations Predict Lung Cancer Radiation Resistance That Can Be Targeted by Glutaminase Inhibition. Cancer Discov. 2020, 10, 1826–1841. [Google Scholar] [CrossRef]
- Yu, H.A.; Suzawa, K.; Jordan, E.; Zehir, A.; Ni, A.; Kim, R.; Kris, M.G.; Hellmann, M.D.; Li, B.T.; Somwar, R.; et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin. Cancer Res. 2018, 24, 3108–3118. [Google Scholar] [CrossRef]
- Shibata, T.; Saito, S.; Kokubu, A.; Suzuki, T.; Yamamoto, M.; Hirohashi, S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010, 70, 9095–9105. [Google Scholar] [CrossRef]
- Paik, P.K.; Ahn, L.S.H.; Ginsberg, M.S.; Plodkowski, A.J.; Kim, R.; Doyle, L.A.; Rudin, C.M. Targeting NFE2L2/KEAP1 mutations in advanced NSCLC with the TORC1/2 inhibitor TAK-228. J. Clin. Oncol. 2019, 37, 9085. [Google Scholar] [CrossRef]
- Romero, R.; Sayin, V.I.; Davidson, S.M.; Bauer, M.R.; Singh, S.X.; LeBoeuf, S.E.; Karakousi, T.R.; Ellis, D.C.; Bhutkar, A.; Sánchez-Rivera, F.J.; et al. KEAP1 loss promotes KRAS-driven lung cancer and results in dependence on glutaminolysis. Nat. Med. 2017, 23, 1362–1368. [Google Scholar] [CrossRef]
- Krishnamurthy, N.; Goodman, A.M.; Barkauskas, D.A.; Kurzrock, R. STK11 alterations in the pan-cancer setting: Prognostic and therapeutic implications. Eur. J. Cancer 2021, 148, 215–229. [Google Scholar] [CrossRef]
- Roosan, M.R.; Mambetsariev, I.; Pharaon, R.; Fricke, J.; Husain, H.; Reckamp, K.L.; Koczywas, M.; Massarelli, E.; Bild, A.H.; Salgia, R. Usefulness of Circulating Tumor DNA in Identifying Somatic Mutations and Tracking Tumor Evolution in Patients with Non-small Cell Lung Cancer. Chest 2021, 160, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Goldberg, M.E.; Greenawalt, D.M.; Hellmann, M.D.; Awad, M.M.; Gainor, J.F.; Schrock, A.B.; Hartmaier, R.J.; Trabucco, S.E.; Gay, L.; et al. Mutations and PD-1 Inhibitor Resistance in -Mutant Lung Adenocarcinoma. Cancer Discov. 2018, 8, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Papillon-Cavanagh, S.; Doshi, P.; Dobrin, R.; Szustakowski, J.; Walsh, A.M. and mutations as prognostic biomarkers in an observational real-world lung adenocarcinoma cohort. ESMO Open 2020, 5, e000706. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Mu, C.Y.; Huang, J.A.; Chen, Y.; Chen, C.; Zhang, X.G. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med. Oncol. 2011, 28, 682–688. [Google Scholar] [CrossRef]
- Khanna, P.; Blais, N.; Gaudreau, P.-O.; Corrales-Rodriguez, L. Immunotherapy Comes of Age in Lung Cancer. Clin. Lung Cancer 2017, 18, 13–22. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients with Advanced Non-Small-Cell Lung Cancer Treated with Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.K.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or Metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Verschraegen, C.F.; Jerusalem, G.; McClay, E.F.; Iannotti, N.; Redfern, C.H.; Bennouna, J.; Chen, F.L.; Kelly, K.; Mehnert, J.; Morris, J.C.; et al. Efficacy and safety of first-line avelumab in patients with advanced non-small cell lung cancer: Results from a phase Ib cohort of the JAVELIN Solid Tumor study. J. Immunother. Cancer 2020, 8, e001064. [Google Scholar] [CrossRef]
- Barlesi, F.; Vansteenkiste, J.; Spigel, D.; Ishii, H.; Garassino, M.; de Marinis, F.; Özgüroğlu, M.; Szczesna, A.; Polychronis, A.; Uslu, R.; et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): An open-label, randomised, phase 3 study. Lancet Oncol. 2018, 19, 1468–1479. [Google Scholar] [CrossRef]
- Park, K.; Özgüroğlu, M.; Vansteenkiste, J.; Spigel, D.; Yang, J.C.H.; Ishii, H.; Garassino, M.; de Marinis, F.; Szczesna, A.; Polychronis, A.; et al. Avelumab Versus Docetaxel in Patients with Platinum-Treated Advanced NSCLC: 2-Year Follow-Up From the JAVELIN Lung 200 Phase 3 Trial. J. Thorac. Oncol. 2021, 16, 1369–1378. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Gray, J.E.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; Cho, B.C.; et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J. Thorac. Oncol. 2020, 15, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Spira, A.; Raben, D.; Planchard, D.; Cho, B.C.; Özgüroğlu, M.; Daniel, D.; Villegas, A.; Vicente, D.; Hui, R.; et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. 2020, 31, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year Survival with Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update from the PACIFIC Trial. J. Thorac. Oncol. 2021, 16, 860–867. [Google Scholar] [CrossRef]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Wu, Y.-L. Tislelizumab: An investigational anti-PD-1 antibody for the treatment of advanced non-small cell lung cancer (NSCLC). Expert Opin. Investig. Drugs 2020, 29, 1355–1364. [Google Scholar] [CrossRef]
- Ciciola, P.; Cascetta, P.; Bianco, C.; Formisano, L.; Bianco, R. Combining Immune Checkpoint Inhibitors with Anti-Angiogenic Agents. J. Clin. Med. 2020, 9, 675. [Google Scholar] [CrossRef]
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11, 320. [Google Scholar] [CrossRef]
- WALKer, L.S.K.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef]
- Chen, D.; Menon, H.; Verma, V.; Guo, C.; Ramapriyan, R.; Barsoumian, H.; Younes, A.; Hu, Y.; Wasley, M.; Cortez, M.A.; et al. Response and outcomes after anti-CTLA4 versus anti-PD1 combined with stereotactic body radiation therapy for Metastatic non-small cell lung cancer: RETrospective analysis of two single-institution prospective trials. J. Immunother. Cancer 2020, 8, e000492. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Ramalingam, S.S.; Ciuleanu, T.-E.; Lee, J.-S.; Urban, L.; Caro, R.B.; Park, K.; Sakai, H.; Ohe, Y.; Nishio, M.; et al. First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J. Thorac. Oncol. 2022, 17, 289–308. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.; Goldberg, S.B.; Balmanoukian, A.; Chaft, J.E.; Sanborn, R.E.; Gupta, A.; Narwal, R.; Steele, K.; Gu, Y.; Karakunnel, J.J.; et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: A multicentre, phase 1b study. Lancet Oncol. 2016, 17, 299–308. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.-J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab with or without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Barber, G.N. STING: Infection, inflammation and cancer. Nat. Rev. Immunol 2015, 15, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Rodriguez, B.L.; Chen, L.; Corte, C.M.D.; Morikawa, N.; Fujimoto, J.; Cristea, S.; Nguyen, T.; Diao, L.; Li, L.; et al. Targeting DNA Damage Response Promotes Antitumor Immunity through STING-Mediated T-cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019, 9, 646–661. [Google Scholar] [CrossRef]
- Della Corte, C.M.; Sen, T.; Gay, C.M.; Ramkumar, K.; Diao, L.; Cardnell, R.J.; Rodriguez, B.L.; Stewart, C.A.; Papadimitrakopoulou, V.A.; Gibson, L.; et al. STING Pathway Expression Identifies NSCLC with an Immune-Responsive Phenotype. J. Thorac. Oncol. 2020, 15, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Raaby Gammelgaard, K.; Sandfeld-Paulsen, B.; Godsk, S.H.; Demuth, C.; Meldgaard, P.; Sorensen, B.S.; Jakobsen, M.R. cGAS-STING pathway expression as a prognostic tool in NSCLC. Transl. Lung Cancer Res. 2021, 10, 340–354. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347.e12. [Google Scholar] [CrossRef]
- Puhr, H.C.; Ilhan-Mutlu, A. New emerging targets in cancer immunotherapy: The role of LAG3. ESMO Open 2019, 4, e000482. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, X.; Hou, L.; Zhao, J.; Zhou, F.; Chu, X.; Wu, Y.; Zhou, C.; Su, C. Epidermal growth factor receptor tyrosine kinase inhibitor remodels tumor microenvironment by upregulating LAG-3 in advanced non-small-cell lung cancer. Lung Cancer 2021, 153, 143–149. [Google Scholar] [CrossRef]
- Hong, D.S.; Schoffski, P.; Calvo, A.; Sarantopoulos, J.; Ochoa De Olza, M.; Carvajal, R.D.; Prawira, A.; Kyi, C.; Esaki, T.; Akerley, W.L. Phase I/II study of LAG525±spartalizumab (PDR001) in patients (pts) with advanced malignancies. J Clin. Oncol. 2018, 36, 3012. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Nemunaitis, M.; Senzer, N.; Snitz, P.; Bedell, C.; Kumar, P.; Pappen, B.; Maples, P.; Shawler, D.; Fakhrai, H. Phase II trial of Belagenpumatucel-L, a TGF-β2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther. 2009, 16, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020, 200, 108–119. [Google Scholar] [CrossRef]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; HiRET, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef]
- Kalemkerian, G.P.; Narula, N.; Kennedy, E.B.; Biermann, W.A.; Donington, J.; Leighl, N.B.; Lew, M.; Pantelas, J.; Ramalingam, S.S.; Reck, M.; et al. Molecular Testing Guideline for the Selection of Patients with Lung Cancer for Treatment with Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 911–919. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef]
- Layfield, L.J.; Hammer, R.D.; White, S.K.; Furtado, L.V.; Schmidt, R.L. Molecular Testing Strategies for Pulmonary Adenocarcinoma: An Optimal Approach with Cost Analysis. Arch. Pathol. Lab. Med. 2019, 143, 628–633. [Google Scholar] [CrossRef]
- Tan, A.C.; Lai, G.G.Y.; Tan, G.S.; Poon, S.Y.; Doble, B.; Lim, T.H.; Aung, Z.W.; Takano, A.; Tan, W.L.; Ang, M.-K.; et al. Utility of incorporating next-generation sequencing (NGS) in an Asian non-small cell lung cancer (NSCLC) population: Incremental yield of actionable alterations and cost-effectiveness analysis. Lung Cancer 2020, 139, 207–215. [Google Scholar] [CrossRef]
- Drilon, A.; Wang, L.; Arcila, M.E.; Balasubramanian, S.; Greenbowe, J.R.; Ross, J.S.; Stephens, P.; Lipson, D.; Miller, V.A.; Kris, M.G.; et al. Broad, Hybrid Capture-Based Next-Generation Sequencing Identifies Actionable Genomic Alterations in Lung Adenocarcinomas Otherwise Negative for Such Alterations by Other Genomic Testing Approaches. Clin. Cancer Res. 2015, 21, 3631–3639. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-Y.; Kim, S.H.; Lee, Y.-S.; Lee, S.-Y.; Hwang, J.-A.; Kim, J.Y.; Yoon, S.J.; Lee, G.K. Comparison of targeted next-generation sequencing with conventional sequencing for predicting the responsiveness to epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) therapy in never-smokers with lung adenocarcinoma. Lung Cancer 2014, 85, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Tuononen, K.; Mäki-Nevala, S.; Sarhadi, V.K.; Wirtanen, A.; Rönty, M.; Salmenkivi, K.; Andrews, J.M.; Telaranta-Keerie, A.I.; Hannula, S.; Lagström, S.; et al. Comparison of targeted next-generation sequencing (NGS) and real-time PCR in the detection of EGFR, KRAS, and BRAF mutations on formalin-fixed, paraffin-embedded tumor material of non-small cell lung carcinoma-superiority of NGS. Genes Chromosom. Cancer 2013, 52, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.D.; Lomboy, A.; Lawrence, C.A.; Yourshaw, M.; Bocsi, G.T.; Camidge, D.R.; Aisner, D.L. DNA-Based versus RNA-Based Detection of MET Exon 14 Skipping Events in Lung Cancer. J. Thorac. Oncol. 2019, 14, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Seet, A.O.L.; Lai, G.G.Y.; Lim, T.H.; Lim, A.S.T.; Tan, G.S.; Takano, A.; Tai, D.W.M.; Tan, T.J.Y.; Lam, J.Y.C.; et al. Molecular Characterization and Clinical Outcomes in RET-Rearranged NSCLC. J. Thorac. Oncol. 2020, 15, 1928–1934. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2022 update. Pharmacol. Res. 2022, 175, 106037. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-generation EGFR and ALK inhibitors: Mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Brahmer, J.; Antonia, S.; Mok, T.; Peters, S. Managing Resistance to Immune Checkpoint Inhibitors in Lung Cancer: Treatment and Novel Strategies. J. Clin. Oncol. 2022, 40, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Spira, A.I.; Johnson, M.; Bazhenova, L.; Leach, J.; Cummings, A.L.; Candia, A.; Coffman, R.L.; Janatpour, M.J.; Janssen, R.; et al. A Phase Ib Open-Label, Multicenter Study of Inhaled DV281, a TLR9 Agonist, in Combination with Nivolumab in Patients with Advanced or Metastatic Non-small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 4566–4573. [Google Scholar] [CrossRef]
- Otsuka, T.; Nishida, S.; Shibahara, T.; Temizoz, B.; Hamaguchi, M.; Shiroyama, T.; Kimura, K.; Miyake, K.; Hirata, H.; Mizuno, Y.; et al. CpG ODN (K3)-toll-like receptor 9 agonist-induces Th1-type immune response and enhances cytotoxic activity in advanced lung cancer patients: A phase I study. BMC Cancer 2022, 22, 744. [Google Scholar] [CrossRef] [PubMed]
- Gillette, M.A.; Satpathy, S.; Cao, S.; Dhanasekaran, S.M.; Vasaikar, S.V.; Krug, K.; Petralia, F.; Li, Y.; Liang, W.-W.; Reva, B.; et al. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell 2020, 182, 200–225.e35. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).