Abstract
Sleep Disordered Breathing (SDB) and Alzheimer’s Disease (AD) are strongly associated clinically, but it is unknown if they are mechanistically associated. Here, we review data covering both the cellular and molecular responses in SDB and AD with an emphasis on the overlapping neuroimmune responses in both diseases. We extensively discuss the use of animal models of both diseases and their relative utilities in modeling human disease. Data presented here from mice exposed to intermittent hypoxia indicate that microglia become more activated following exposure to hypoxia. This also supports the idea that intermittent hypoxia can activate the neuroimmune system in a manner like that seen in AD. Finally, we highlight similarities in the cellular and neuroimmune responses between SDB and AD and propose that these similarities may lead to a pathological synergy between SDB and AD.
1. Introduction
Although neurodegenerative diseases like Alzheimer’s Disease (AD), are strongly associated with sleep disordered breathing (SDB), the cellular and physiologic mechanisms underlying this relationship remain poorly understood. Thus, the goals of this review are to: (1) briefly summarize current knowledge about SDB and AD to call attention to gaps in knowledge, (2) present experimental evidence supporting the hypothesis that the neuroimmune system is a common point of convergence in both SDB and AD pathologies, and (3) highlight the paucity of understanding of basic cellular mechanisms underlying the intersection between SDB and pathology in AD, with a focus on the neuroimmune response.
2. Neurodegeneration Is Common in Both SDB and AD
2.1. Sleep Disordered Breathing
Sleep disordered breathing (SDB) occurs when individuals repetitively stop breathing during sleep, often hundreds of times each night. There are three major types of SDB: (1) obstructive sleep apnea (OSA) where the upper airway either partially or fully collapses during inspiration, (2) central sleep apnea where respiratory rhythm generating neurons in the brainstem fail to send sufficient signals to upper airway pharyngeal dilator muscles and chest wall respiratory pump muscles, and (3) mixed or complex sleep apnea, which involves a combination of both obstructive and central apneas. OSA is by far the most common form of SDB, the prevalence of which is increased in individuals with larger neck circumferences, often due to adipose tissue deposition around the neck that creates anatomical impediments on the airway [1,2,3,4,5]. Sleep apnea is estimated to affect ~17–26% of the adult population in the US, with up to 80% of people with the disorder remaining undiagnosed [6,7,8]. The prevalence in the United States of mild, moderate, and severe OSA is 10%, 3.8% and 6.5% respectively [9], with the remainder of individuals effected by sleep apnea having central or mixed apneas. It is thought that some obstructive events may begin as a central event first [10,11]. Cessations in breathing due either to central or obstructive apneas can cause intermittent hypoxia (IH). IH results when breathing resumes between episodes of recurrent apneas or hypopneas, reductions in breathing, causing intermittent re-oxygenation of blood and tissues [12,13]. IH is thought to be causal of several morbidities associated with SDB including cardiovascular remodeling, metabolic disorder, sympathetic nervous system hyperactivation, and cognitive impairments [9].
2.2. Neurodegeneration in SDB
Cognitive impairments in individuals with SDB present as attention deficits, memory loss, and decreased executive functioning [14]. These cognitive impairments correlate with neuroanatomical changes and gray matter loss, based on magnetic resonance imaging morphometry [15]. The central nervous system (CNS) regions most affected by SDB are associated with memory, attention, and higher cognitive function and include the hippocampus; frontal, parietal, and temporal cortices; and brainstem regions including the nucleus tractus solitaries [16]. There is also gray matter loss in cerebellar, motor area, and limbic regions associated with upper airway motor regulation and cognitive processing [17]. Accordingly, exposure of rodents to IH recapitulates some but not all aspects of neuronal loss observed in human SDB. A seminal paper by Gozal et al. demonstrated cortical and hippocampal neuronal apoptosis in rats exposed to IH; this neuronal loss correlated with spatial learning deficits [18]. As the first study to report neurocognitive deficits in an animal model of IH, it sparked intense research efforts into the neuropathological consequences and molecular mechanisms underlying IH-induced CNS injury leading to similar observations in mice and other rat SDB paradigms [19,20,21,22,23,24,25]. While rodent IH models do not display neuronal apoptosis in all CNS regions observed in human SDB patients, this could be due to the short durations of experimental IH exposures (usually only weeks), and/or the absence of other morbidities of SDB such as sleep fragmentation and intermittent hypercapnia that typify human SDB. Sleep fragmentation refers to the overall amount and distribution of wakefulness during a total sleep period.
SDB is associated with neurodegenerative diseases; over 50% of patients with AD experience SDB [26]. Despite the prevalence, the impact of SDB on the progression of neurodegenerative and genetic disorders is less well understood. A study in a small population of AD patients with OSA found that continuous positive airway pressure improved cognitive scores in the fields of memory and attention [27], suggesting that some of the cognitive deficits exhibited in these patients could be slowed or even reversed by treating the OSA aspect. Although these studies need to be repeated in larger patient cohorts, evidence from animal IH studies support this general notion. For example, IH exposure increases cerebral and hippocampal amyloid beta (Aβ) burden [28,29,30] and hypoxia induces tau hyperphosphorylation [30,31,32], two pathologic hallmarks of AD that will be discussed further below.
2.3. Experimental Models of SDB
Rodent models mimicking key SDB aspects, most commonly IH, have been developed to better understand mechanisms of SDB [33,34,35,36]. Importantly, IH alone can recapitulate most of the OSA-associated morbidities observed in humans [37], thus disentangling the complexities of OSA in animal models. The hypoxia exposure paradigms used in rodents most often range between 5% and 11% O2, while the cycle frequency, cycle length, and daily duration depend on whether rats or mice are used. Because mice are efficient at physiologically adapting to hypoxia, inducing pathologic IH requires lower O2 levels, more episodes per hour, and longer total exposure times than rats [18,38,39,40]. Sleep fragmentation, a common IH co-morbidity [41,42], can also be modeled in rodents using mechanical devices that interrupt sleep without causing sleep deprivation or reduction in total sleep [43,44,45,46,47,48]. Sleep fragmentation alone, like IH, can also recapitulate some negative aspects of SDB including hypertension and metabolic disturbances [49,50]. Recent studies have begun to tease apart the individual physiologic effects of IH and sleep fragmentation [51].
2.4. Alzheimer’s Disease
AD is the most prevalent form of dementia among the elderly. As many as 5 million Americans, about 1 in 10 people over the age of 65, have AD [52]. In the United States, AD is ranked as the 6th leading cause of death [53]. Most observational and experimental evidence to date indicates that AD is initiated by the accumulation of extracellular plaques composed of Aβ leading to progressive accumulation and aggregation of hyperphosphorylated tau into neurofibrillary tangles (NFTs) [54]. These plaques and NFTs then contribute to neuronal loss, brain atrophy, and ventricular enlargement [55,56]. Clinically, AD is characterized by progressive loss of memory, emotional and cognitive changes, and diminished reaction to stimuli [57]; typically, onset of noticeable symptoms occurs after 60 years of age. Although family history, genetics, environment, lifestyle and SDB are all likely contributing factors to disease development and progression, there is no definitive predictive factor for AD [30,57].
Of the various hypotheses on the etiology of AD pathogenesis, the foremost three are the: (1) amyloid, (2) tau, and (3) infectious origin hypotheses. The amyloid hypothesis states that the deposition of extracellular plaques, which are primarily composed of Aβ peptides, is the causative agent underlying AD pathology [58]. Although the amyloid hypothesis is the most widely accepted, it does not explain all aspects of AD pathogenesis [59,60]. The second major hypothesis is the tau hypothesis. This hypothesis posits that tau hyperphosphorylation and accumulation into intraneuronal NFTs is the causative agent of AD [61]. The tau hypothesis can explain questions and gaps left by the amyloid hypothesis, but it fails explain the full progression of AD pathology on its own. The final prominent hypothesis for AD pathogenesis is the infectious origin model. This hypothesis proposes that the initiating step in AD development is a pathogenic insult, either viral or bacterial, that triggers the formation of amyloid plaques and NFTs resulting in the onset of AD [62]. The infectious origin hypothesis is relatively new and much of the data supporting it remains circumstantial.
2.5. Experimental Models of AD
Several animal models of AD have been developed and have significantly contributed to our understanding of AD pathogenesis, foremost among which is the mouse. Due to the genetic manipulability of mice, several models with altered tau, such as the P301S line PS19, Aβ models including the 5XFAD and APPPS1, or mice expressing both altered tau and Aβ such as the 3xTg mice [63,64,65,66] have been developed to elucidate individual aspects of AD pathology including the formation of NFT or Aβ plaques. More complex models combine both Aβ and NFT aspects; for example, using an Aβ mouse model as a base, then seeding the brain with NFTs to explore their combined effects on AD pathology [67]. Many of these models also exhibit behavioral defects alongside cellular responses, particularly microgliosis and microglial activation, which closely mimic those observed in humans with AD. In addition to mouse models, several non-human primate and other rodent models exist. However, the use of these models has been much more limited [68].
3. Neuroinflammation in AD and SDB
3.1. Microglia and the Inflammasome in AD and SDB
One of the primary functions of microglia is to identify and resolve oxidative stress and inflammation in the CNS. Novel imaging techniques (including non-invasive two-photon in vivo microscopy) have shown the normal state of microglia to be anything but “quiescent” or “resting,” as they are highly dynamic cells; constantly surveying the brain parenchyma with their processes to interact with neurons, synapses, axons, other glial cells, and blood vessels [69,70,71,72,73]. Microglia are involved in synaptic plasticity, neurogenesis, learning and memory and functional brain connectivity via their synaptic pruning activities [74,75]. Since neuroinflammation occurs in both AD and SDB, microglia have become a recent focus of research, separately, in the AD and SDB fields.
While it has been known for more than a century that microglia are both activated and proliferative in AD, their contribution to AD pathology remains incompletely understood [76]. The identification of several single nucleotide polymorphisms in microglial genes associated with increased risk for developing AD [77,78,79] prompted extensive follow-up work that has firmly implicated microglia as key players in AD etiology [76].
Microglial function in AD is complex, having both detrimental [80,81] and neuroprotective activities [82,83,84,85]. In early AD, microglia in Aβ models work to clear amyloid plaques but also release pro-inflammatory cytokines and produce apoptosis-associated speck-like protein containing a CARD (ASC specks). ASC specks in microglia are hallmarks of inflammasome activation and seed new Aβ plaque formation [80,81,83,84,85]. In 5XFAD mice, inhibiting this inflammasome activity early on can decrease pathology [86]. Depletion of microglia from 5XFAD mice by pharmacological inhibition of the colony stimulating factor 1 receptor with PLX3397 improved hippocampal-dependent contextual memory and lessened neuronal loss, but it did not alter plaque load [87,88]. However, these findings are complicated by the fact that most of the remaining (non-depleted) microglia were heavily associated with Aβ plaques [88,89]. Therefore, the implications of these studies on determining the role of microglia in AD remain unclear. In tau mouse AD models, microglia enhance neurodegeneration [90]. However, in more complex AD models in which both Aβ and NFTs are present in a single animal, microglia appear to surround Aβ plaques and limit their neurotoxic impact resulting in reduced formation of NFTs [67]. Taken together, these data suggest a complicated role for microglia in the response to AD pathology.
3.2. Astrocytes in AD and SDB
Astrocytes are a population of cells in the CNS that normally regulate the integrity of the blood brain barrier and sustain normal tissue and cellular function by maintaining neuronal cell function and regulating tissue homeostasis. Although less studied, astrocytes also play a role in AD. They proliferate, lose their exclusive spatial autonomy and are associated with both tau NFTs and Aβ plaques [91,92,93,94,95,96,97]. Reactive astrocytes also produce Aβ and inflammatory cytokines that, together with those produced by microglia, contribute to enhanced neuroinflammation in the AD brain [93]. Reactive astrocytes surround amyloid plaques [98] and the magnitude of astrogliosis may correlate with plaque number [99,100,101,102]. However, it has also been reported that astrocytes associate with tau and NFTs, and reactive astrocyte response more closely correlates with NFT burden than plaque burden [91,97]. Some astrocytic processes become part of the insoluble Aβ plaque that they surround, and their other hypertrophic processes make abnormal contacts with nearby blood vessels and neurons [99]. However, astrocytes that are further away from the amyloid plaques undergo atrophy [101,103,104]. Because astrocytes become differentially activated or atrophied based on their proximity to the Aβ plaques [104,105,106,107,108], potentially prior to plaque formation, astrocytes may also contribute to AD pathology.
While considerably less is known about astrocytes in the context of SDB, a recent study of individuals with OSA showed that they had elevated levels of S100B in serum, a marker of astrocyte activation [109]. Patients with OSA also exhibit increased levels of midbrain tissue glutamate as detected by 2D magnetic resonance spectroscopy, implicating excitotoxicity and astrocyte activation [110]. Exposure of mice to IH also causes astrogliosis [18,111], but to date, there have been no studies investigating the contributions of astrocytes to IH or AD-induced neuropathology. In the one recent study currently available, in an AD mouse model (APP-PS1) IH exposure did not affect Aβ levels or plaque load, but it did increase astrogliosis (based on glial-fibrillary acidic protein; GFAP) staining [111].
3.3. Molecular Mechanisms of Neuroinflammation in AD and SDB
Chronic inflammasome activation is common in early onset AD patients [80] with NOD-, LRR-, and pyrin containing 3 (NLRP3) inflammasomes contributing to Aβ plaque and tau pathology in the context of Alzheimer’s Disease [80,81,112,113]. In primary microglial cultures, exposure to soluble Aβ oligomers and protofibrils results in inflammasome activation [114]. APP/PS1/NLRP3 and APP/PS1/Caspase-1 deficient mice were protected from spatial memory deficits despite having no significant difference in total brain IL-1β [80]. Although loss of NLRP3 inflammasome activation is associated with reduced tau hyperphosphorylation and improved spatial memory in mice [112], intrahippocampal injections of ASC-specks themselves resulted in the spreading Aβ pathology in double mutant APP-PSEN1 mice [81], underscoring the complexity of microglia in AD. β-hydroxybutyrate (BHB) is a byproduct of ketogenesis that is known to inhibit the NLRP3 inflammasome [115]. BHB levels are significantly lower in AD patients [86]. Supplementing 5XFAD mice with BHB resulted in significantly less plaques and less complex microglia when compared to untreated 5XFADs [86]. Along with fewer ASC specks and lowered levels of Caspase-1 in the cortex, this suggests that BHB decreases microgliosis and is an inhibitor of the inflammasome [86].
Although microglial activation of the inflammasome can cause inflammation in the brain, to date no studies have examined the impact of the inflammasome in SDB. However, one recent study started to tease apart the relationship between IH and the inflammasome. This group utilized bone-marrow derived macrophages from lean and obese mice and subsequently exposed them to IH in vitro [116]. The IH exposure increased the inflammatory profile of both sets of bone-marrow derived macrophages [116]. Pretreatment with a toll-like receptor 4 (TLR4) inhibitor also prevented IH-induced inflammation [116]. In bone-marrow derived macrophages from NLRP3-/- mice, there was no increase in the inflammatory profile due to IH exposure [116]. This study starts to uncover the role that TLR4/NLRP3 signaling roles play in exposure to IH. Our group has also examined the effects of IH on microglial inflammatory and TLR4 gene expression levels in microglia in mice in vivo, following exposure to IH for up to 14 days [117]. We found that microglial inflammatory gene expression was differentially increased in immunomagnetically isolated microglia in a region-dependent manner, and that TLR4 gene expression was similarly increased [117]. Surface TLR4 protein levels of microglia, as detected by flow cytometry, trended towards being increased at seven days of IH exposure and were significantly increased by 14 days, an effect that persisted until at least four weeks of IH exposure (Figure 1). Similarly, microglial surface CD45 protein levels were significantly increased within seven days of IH exposure, persisting through to 28 days (Figure 2). We also found that protein expression of neuronal caspase 3 was increased within seven days of IH exposure and persisted for the 28-day experimental duration (Figure 3), indicating that microglial activation and neuronal apoptosis are temporally associated. These data support the idea that microglia become activated in response to IH in vivo, and that microglial TLR4 signaling and inflammasome activation may be enhanced.
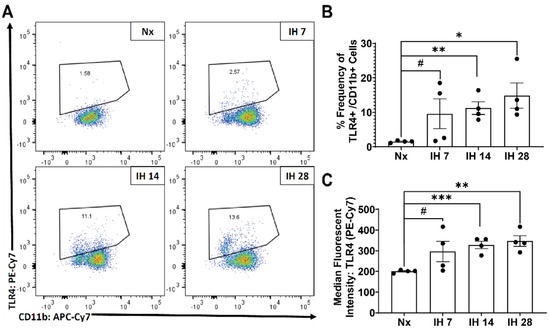
Figure 1.
Microglial surface expression of active TLR4/MD2 is significantly increased by IH exposure in vivo. Mice were exposed to normoxia (room air; Nx), or IH for 7, 14, or 28 days. 14 h following the last hypoxic exposure, hippocampal tissue was dissociated, and single cell suspensions were stained with anti-TLR4/MD2 and anti-CD11b antibodies for analysis by flow cytometry. (A) Representative dot plots of TLR4/MD2 vs. CD11b immunofluorescence. (B) Frequency of TLR4/MD2+ cells as a percentage of total CD11b+ cells. (C) Average mean fluorescent intensity of TLR4/MD2 in CD11b+ cells. Statistical significance was determined by student’s t-tests. * p < 0.05; ** p < 0.01; *** p < 0.001; # 0.05 < p< 0.12.
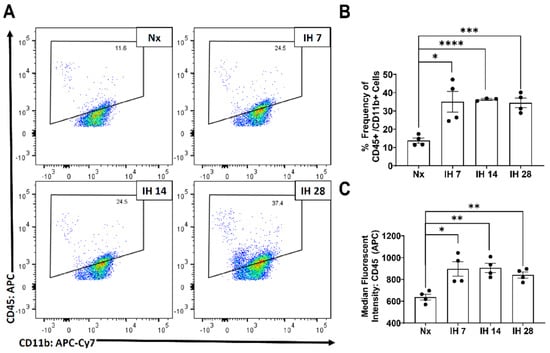
Figure 2.
Microglial CD45 expression increases following IH exposure in vivo. Mice were exposed to normoxia (room air; Nx), or IH for 7, 14, or 28 days. 14 h following the last hypoxic exposure, hippocampal tissue was dissociated, and single cell suspensions were stained with anti-CD45 and anti-CD11b antibodies for analysis by flow cytometry. (A) Representative dot plots of CD45 vs. CD11b immunofluorescence. (B) Frequency of CD45+/CD11b+ cells. (C) Average mean fluorescent intensity CD45 in CD11b+ cells. Statistical significance was determined by student’s t-tests. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
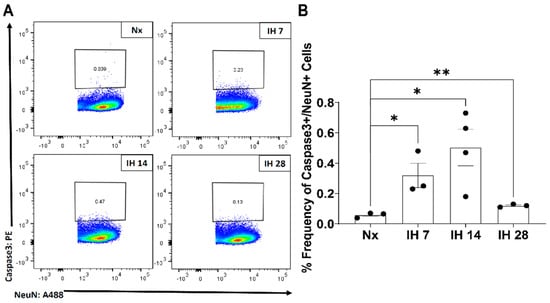
Figure 3.
IH increases neuronal caspase-3 levels in the mouse hippocampus. Mice were exposed to normoxia (room air; Nx), or IH for 7, 14, or 28 days. 14 h following the last hypoxic exposure, hippocampal tissue was dissociated, and single cell suspensions were stained with anti-caspase 3 and anti-NeuN antibodies for analysis by flow cytometry. (A) Representative dot plots of caspase 3+/NeuN+ cells. (B) Frequency of caspase 3+/NeuN+ cells. Statistical significance was determined by student’s t-tests. * p < 0.05; ** p < 0.01.
4. Common Mechanisms of SDB and AD
4.1. Bidirectional Effects of SDB and AD on One Another
In the CNS, many of the cellular processes, including the activation of microglia, are shared between SDB and AD. Only recently has the association between these processes in SDB and AD been investigated. Evidence suggests that individuals with SDB are at a higher risk for also having some form of neurodegenerative disease [12,118,119,120,121,122]. When examining institutionalized patients with dementia, it was determined that greater than 70% of those patients also had SDB, with over half of them having moderate or severe SDB [123]. This is important because this rate is nearly three times that expected in the general population, suggesting that there may be an interaction between SDB and dementia. Another study that examined post-mortem human brains (from cognitively normal individuals) found that while SDB severity was a strong predictor of Aβ plaque load in the hippocampus, it was not correlated with hippocampal NFT burden, nor Aβ plaque or NFT burden in the brainstem, where respiratory neural control resides [30]. Thus, SDB may contribute to large Aβ plaque loads, at least in pre-clinical stages of AD progression. Intriguingly, although SDB patients were also recently found to exhibit reduced CSF soluble Aβ [124,125,126,127] and increased tau proteins in CSF [125] and plasma [128,129], as well as increased Aβ plaques by PET scan [127,130,131,132,133], not all studies are consistent [127]. For example, decreased Aβ 1-42 and increased tau was reported in CSF from individuals with OSA, which also correlated with increased memory impairment [125]. However, unlike with OSA, the frequency of central sleep apnea in AD is less well understood, and the association with AD is thought to be less common than with OSA [134]. Due to the distinct cellular and pathological similarities between SDB and AD, we propose that these seemingly unrelated diseases may interact synergistically to enhance neurodegeneration and disease pathology. We also suggest that SDB-induced neurodegeneration precedes AD symptom onset, effectively setting the stage for exacerbated AD progression. In the sections below, we will describe the evidence including sleep disturbances, sexual dimorphism, common risk factors, and inflammation–particularly involving the neuroimmune system–that support the idea that SDB and AD may have common etiologies.
4.2. Sleep Disturbances
Unsurprisingly, SDB increases sleep disturbances and worsens the quality of sleep that is experienced, leading to daytime sleepiness, which is associated with deficits in working memory and vigilance [119]. Interestingly, sleep disturbances including insomnia, circadian rhythm alterations, and nocturnal agitation are also among the earliest behavioral changes associated with AD development, and they typically worsen during the development of the disease [121,122,134,135,136,137,138,139]. People reporting short sleep durations (<6 h) have increased incidence of hypertension, obesity, type II diabetes, cardiovascular disease, and stroke [140,141,142,143]. Sleep studies have shown that AD patients with cognitive deficits are more likely to have significantly increased sleep disturbances than AD patients with mild or no cognitive impairments, demonstrating that as AD-associated dementia severity increases, so too does the number of sleep disturbances [8]. Experimental evidence from both human studies and animal models have further highlighted the importance of sleep in AD progression, as the clearance of Aβ occurs mostly during sleep and as little as one night of disrupted sleep can lead to an increase in detectable Aβ levels in the brain [144,145]. Additionally, CSF tau, an important AD biomarker, increases following sleep deprivation in humans and in a mouse model of chronic sleep deprivation tau spreading was increased following sleep deprivation [146]. In the future, additional studies taking advantage of new technologies and approaches such as sleep monitoring by smart watches will allow us to further refine our understanding of the link between sleep disturbances and the development of AD.
Given that sleep disturbances are common in AD, and that SDB is associated with exacerbated AD related pathological changes, we suggest that SDB enhances and/or accelerates AD pathology. Patients with severe SDB exhibit hippocampal atrophy, as is observed in AD [30,147,148,149], and rats with reduced REM sleep showed reduced neuronal arborization in the hippocampus [150] and increased neuronal apoptosis in the locus coeruleus, laterodorsal tegmentum, pedunculopontine tegmentum, and medial preoptic area [140,151]. Rodents exposed to chronic sleep deprivation have decreased volume in brainstem respiratory nuclei (NTS and parabrachial nucleus) [152], in the medial prefrontal cortex [153], in the dorsal and CA2/CA3 hippocampal regions [154], and in the CA1 and dentate gyrus hippocampal regions [155], indicating a detrimental effect on key brain regions involved in cognition and autonomic nervous system function. 5XFAD mice exhibit increased sleep fragmentation [156].
4.3. Sex Hormones and Gender Differences
Interestingly, both SDB and AD have prominent sexual dimorphism. For SDB in general, men are two to three times more likely to be diagnosed than women, with diagnoses increasing in both sexes with age [6,7,157,158,159]. In the United States, 17–24% of men but only 9% of women aged 50–70 years old experience sleep apnea [6,7]. Men have both increasing incidence and severity of SDB as they age [7,157]. Regardless of age, increased SDB severity in men can be predicted by increased obesity, body mass index, waist-hip ratio, and neck circumference [7,157,160,161]. Many male patients who have SDB and are obese have high body mass index or waist-hip ratio, or larger neck circumference can decrease the severity of SDB by weight loss [162]. Women, unlike males, exhibit no positive correlation between obesity (body mass index) and OSA; OSA severity is not correlated to waist-hip ratio or neck circumference, nor is SDB affected by weight loss [157,162]. Women tend to be older than men at their initial diagnosis of OSA, and present with less severe disease [162].
Women with poly-cystic ovarian syndrome (PCOS), typically pre-menopausal with higher testosterone levels, tend to develop OSA at higher rates than women without PCOS, which suggests that sex hormones may play a role in OSA development [163]. Additionally, women with PCOS and men have similar patterns of adipose tissue deposition and size of airway structures [164,165]. Although the incidence of OSA does not change in premenopausal women, it increases during and after menopause [7,158,162,166,167]. However, despite this increased incidence of OSA in post-menopausal women, OSA severity does not worsen, nor does it become associated with obesity, like it is in men [7,158,162,166,167], suggesting sexually dimorphic mechanisms in SDB etiology. The increase in OSA incidence in post-menopausal women may be due to the reductions in circulating estrogen levels (and consequent loss of its neuroprotective effects) and would be consistent with the increased incidence of SDB in younger women with PCOS in which there are higher levels of testosterone, and relatively lower levels of estrogen.
Both OSA and AD are sexually dimorphic, with OSA affecting more men than premenopausal women, and AD being more prevalent in women than men [168]. The primary risk factor for developing AD is age [169]. In men, the risk of developing AD begins to rise in midlife and increases steadily thereafter [170]. Like SDB development and progression, women tend to be somewhat “protected” from AD until menopause, at which point OSA prevalence in women quickly approaches and surpasses that in men [170]. Interestingly, mirroring what is observed with OSA, younger women with PCOS are at an increased risk for developing AD [171]. Further, male AD patients have slower cognitive decline than women [172]. Accordingly, we hypothesize that sex hormones and/or sex differences play a pivotal role in the progression of these distinct diseases.
4.4. Common Risk Factors and Comorbidities for SDB and AD
Another overlapping aspect of both SDB and AD are disease-associated risk factors and comorbidities (Table 1). Clinical studies have shown that men with OSA are at an increased risk for developing comorbidities compared to women [158]. However, in both sexes, SDB leads to an increased risk for health disorders including cardiovascular and neurodegenerative disorders [158]. Additionally, both SDB and AD are often accompanied by hypertension [139,173,174]. Regarding known sex differences, men with OSA are more often diagnosed with hypertension, tend to have more severe hypertension, and are likely to have more apneas per hour compared to women with OSA [175,176]. Conversely, women with OSA tend to have milder hypertension that is unrelated to the number of apneas per hour [176]. This is similar to findings in rodent models of SDB where male rats exposed to IH have increased mean arterial pressures, whereas female rats do not [177]; ovariectomized females behaved similarly to males [178]. We hypothesize that since hypertension also exacerbates inflammation and oxidative stress via increased vascular dysfunction, patients with OSA and comorbid hypertension may be at increased risk of subsequently developing AD or other neurodegenerative diseases.

Table 1.
Factors commonly observed in Alzheimer’s Disease and Sleep Disordered Breathing. “X” denotes strong experimental evidence exists; “?” denotes a potential connection.
SDB and AD are often accompanied by increased inflammation and oxidative stress [117,139,169,173,174,179,180,181,182]. Studies in children with OSA have identified increases in circulating inflammatory markers [183] as well as the AD markers Aβ and presenilin 1 levels [184]. However, these studies were done in pediatric populations, not in aged adults who would be more likely to develop AD. Both women and men with OSA (without other comorbidities) display increased inflammatory biomarkers in blood or exhaled breath [185,186,187,188,189,190,191], as well as increased markers of oxidative stress, such as eNOS, HIF-1α, and VEGF [179]. Although many studies have measured indices of circulating systemic inflammation in individuals with OSA, few have examined CNS inflammation in humans with OSA.
Rodents exposed to IH exhibit increases in both peripheral and central inflammatory mediators as well as enhanced markers of oxidative stress [19,117,118,139,192,193,194]. IH exposure also significantly increases neuronal loss in the hippocampus and prefrontal cortex [18,19,118,194,195] indicating that repetitive hypoxic events during SDB can cause oxidative stress and inflammation in CNS regions involved in cognition. Coincidentally, oxidative stress and inflammation associated with AD are also detected in CNS regions including the hippocampus [139,196], entorhinal cortex [169,197], rostral ventrolateral medulla and solitary tract nucleus [198,199], the same regions in which OSA induces grey matter loss in the human brain (see Neurodegeneration in SDB section above on pg. 2). Together these data illustrate that similar areas of the brain are affected in both SDB and AD, underscoring the possibility that these diseases may act synergistically to worsen the pathology of the other.
4.5. Studying SDB in AD Animal Models
There are very few animal studies that have investigated the intersection of AD and OSA. One available study exposed 3xTg AD mice to IH and found increased intraneuronal Aβ amyloid production [29]. Another group looked at the effect of 4-weeks of IH on astrocytes and Aβ levels in APP-PS1 mice. They found that the Aβ levels remained unchanged, but there was a significant increase in GFAP positive astrocytes, indicative of a pro-inflammatory response [111]. This robust astrogliosis was found in several CNS regions: near blood vessels, and in the neocortex, corpus callosum, CA1 and CA2, dentate gyrus, thalamus, and striatum, an effect that was not observed as severely in the age matched WT controls [111]. Although these studies have begun to fill in the gaps related to the AD and SDB relationship, our ongoing studies aim to identify the role of microglia in the context of long term (months) IH exposure by assessing neuroimmune cell changes, microglia marker differences, and astrocyte and microglia morphologic alterations that regulate the CNS immune response.
5. Summary and Closing Thoughts
Here we have summarized the evidence supporting our hypothesis that there are several commonalities between AD and SDB pathology, including their co-morbid prevalence, the similar regions of brain impacted by neurodegeneration, the involvement of sleep disruption, the neuroinflammation that is driven by both inflammatory activation of microglia and aberrant astrocyte function, as well as common hormonal and sex-dependent influences. Consequently, we propose that microglial activation, via an inflammasome-dependent mechanism, bridges both disorders, possibly leading to reciprocal and synergistic exacerbation of both diseases. Although several recent studies have begun to interrogate the relationship between SDB and AD in human subjects, animal studies are needed to further probe mechanistic interactions. Additionally, data from rodent studies support the need for future experiments that explore not only the functional contributions of microglia and astrocyte activities to this interaction, but also the underlying cellular signaling pathways. Based on the AD literature, we posit that inflammasome activation will be a key point of convergence in uniting both pathologies; however, the evidence is sparse at best in the SDB literature to support this notion. Further studies are necessary in SDB models to understand the cell types that contribute to neuroinflammation and neurodegeneration, the cellular signaling pathways recruited to do so, and how those pathways intersect and link SDB and AD.
6. Experimental Methods
6.1. Intermittent Hypoxia (IH) Exposure
Adult male C57/BL6 mice were obtained from Harlan Laboratories (Madison, WI, USA) and housed in AAALAC-accredited facilities with 12 h:12 h light-dark conditions. All animal exposures were performed using a commercially designed system (BioSpherix, Redfield, NY, USA). Animals were housed in standard polycarbonate cages with access to food and water ad libitum and maintained in a specialized chamber (12 × 20 × 30 inches). Oxygen and carbon dioxide concentrations were continuously monitored by an O2 and CO2 analyzer and were changed by a computerized system controlling the gas outlets (Oxycycler model G2 and Watview software). During the sleep cycle (lights on), O2 concentrations were modified to generate a cyclical pattern of 6% and 21% O2 every 90 s (IH) [200,201]. During their wake period (lights off), O2 concentration was maintained at 21%. Airflow was sufficient to prevent CO2 accumulation, maintaining a concentration below 0.3%. Normoxic control animals were housed inside a chamber with circulating 21% O2 to mimic the IH exposure.
6.2. Tissue Isolation and Fixation for Flow Cytometry
CNS tissues were dissociated as previously described [202]. Briefly, mice were euthanized with an overdose of isoflurane and perfused intra-aortically with cold 0.1 M phosphate buffered saline (PBS). The frontal cortex and hippocampus were dissected out, placed into cold Hank’s Buffered Salt Solution (HBSS; Cellgro, Herndon, VA, USA) on ice. Tissue was mechanically dissociated and pushed through a pre-moistened 100 µM cell strainer with a syringe plunger and washed with cold HBSS supplemented with 0.01 mg/mL DNase (Worthington Biochemicals, Lakewood, NJ, USA). Dissociated tissues were resuspending in 26% Percoll (GE Healthcare, Waukesha, WI, USA) in 0.1 M PBS and centrifuged at 850× g for 15 min to remove myelin. Samples were fixed in a modified zinc-based fixative (mZBF) (0.5% zinc chloride, 0.5% zinc trifluroacetate, 0.05% calcium acetate in 0.1 M Tris-HCl, pH 6.4–6.7) [203] and glycerol (1:1) and stored at −20 °C overnight or until ready to be stained for flow cytometry [203,204].
6.3. Staining for Flow Cytometry
Fixed samples were washed 3x in ice-cold PBS. Cell surface protein stains CD11b-APC-Cy7 (1:150; eBiosciences, San Diego, CA, USA) and the active TLR4/MD2 complex (TLR4-PE-Cy7) (1:25; Biolegend, San Diego, CA, USA) were performed on ice in 50 µL of 1X PBS supplemented with 0.1% BSA for 25 min. Cells were washed 3x and resuspended in a permeabilization buffer (1X PBS + 0.2% saponin + 0.1% BSA). Intracellular staining was performed in 50 µL permeabilization buffer on ice for 45 min for NeuN-Alexa488 (1:500; Millipore, Billerica, MA, USA) and caspase-3-PE (1:20; BD Biosciences, San Jose, CA, USA). Samples were washed and resuspended in the permeabilization buffer containing DAPI (1 µg/mL; Invitrogen, Carlsbad, CA, USA) to identify cells with intact nuclei. Cells were analyzed on a BD LSR II and FACSDiva software (BD Biosciences). FCS files were analyzed via FlowJO software v10.8.0. Cells were gated to live cell singlets as previously described [205].
6.4. Statistical ANALYSIS
All statistical analyses were performed using GraphPad PRISM version 9.1.0. The Grubb’s Test was used to identify outliers. Statistical significance (set at p < 0.05) was determined using Student’s t-tests.
Author Contributions
Conceptualization, T.K.U. and J.J.W.; methodology, A.C.E.; investigation, S.M.C.S.; formal analysis, A.O.K. and S.M.C.S.; figure preparation, A.O.K.; writing-original draft preparation A.C.E., K.M.M., T.K.U., J.J.W.; writing-review and editing A.C.E., K.M.M., A.O.K., T.K.U., J.J.W.; funding acquisition, T.K.U. and J.J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded NIH R01AG070973 (T.K.U.), R21AG068652 (T.K.U.), R01HL142752 (J.J.W.), R01HL142752 NIA supplement (J.J.W., T.K.U.), P30-AG534255 UWADRCDP (T.K.U.).
Institutional Review Board Statement
All animal experimental procedures were performed according to the NIH guidelines set forth in the Guide for the Care and Use of Laboratory Animals and were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee (Protocol #V005173). All efforts were made to minimize animal distress and reduce the numbers used, while permitting the formation of statistically reliable conclusions.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hoffstein, V.; Mateika, S. Differences in Abdominal and Neck Circumferences in Patients with and without Obstructive Sleep Apnoea. Eur. Respir. J. 1992, 5, 377–381. [Google Scholar] [PubMed]
- Davies, R.J.O.; Ali, N.J.; Stradling, J.R. Neck Circumference and Other Clinical Features in the Diagnosis of the Obstructive Sleep Apnoea Syndrome. Thorax 1992, 47, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Kurian, S.; Malik, V.; Mohan, A.; Banga, A.; Pandey, R.M.; Handa, K.K.; Mukhopadhyay, S. A Stepped Approach for Prediction of Obstructive Sleep Apnea in Overtly Asymptomatic Obese Subjects: A Hospital Based Study. Sleep Med. 2004, 5, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Skatrud, J.; Peppard, P.E. Risk Factors for Obstructive Sleep Apnea in Adults. JAMA 2004, 291, 2013–2016. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Taheri, S. Excess Weight and Sleep-Disordered Breathing. J. Appl. Physiol. 2005, 99, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Snyder, B.; Cunningham, R.L. Sex Differences in Sleep Apnea and Comorbid Neurodegenerative Diseases. Steroids 2018, 133, 28–33. [Google Scholar] [CrossRef]
- Chang, H.-P.; Chen, Y.-F.; Du, J.-K. Obstructive Sleep Apnea Treatment in Adults. Kaohsiung J. Med. Sci. 2020, 36, 7–12. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Smith, C.A. Pathophysiology of Human Ventilatory Control. Eur. Respir. J. 2014, 44, 495–512. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Xie, A.; Patz, D.S.; Wang, D. Physiology in Medicine: Obstructive Sleep Apnea Pathogenesis and Treatment—Considerations beyond Airway Anatomy. J. Appl. Physiol. 2014, 116, 3–12. [Google Scholar] [CrossRef]
- Dempsey, J.A.; Veasey, S.C.; Morgan, B.J.; O’donnell, C.P. Pathophysiology of Sleep Apnea. Physiol. Rev. 2010, 90, 47–112. [Google Scholar] [CrossRef]
- Zhang, S.X.L.; Wang, Y.; Gozal, D. Pathological Consequences of Intermittent Hypoxia in the Central Nervous System. Compr. Physiol. 2012, 2, 1767–1777. [Google Scholar] [CrossRef]
- Jackson, M.L.; Howard, M.E.; Barnes, M. Cognition and Daytime Functioning in Sleep-Related Breathing Disorders. Prog. Brain Res. 2011, 190, 53–68. [Google Scholar] [CrossRef]
- Zimmerman, M.E.; Aloia, M.S. A Review of Neuroimaging in Obstructive Sleep Apnea. J. Clin. Sleep Med. 2006, 2, 461–471. [Google Scholar] [CrossRef]
- Celle, S.; Peyron, R.; Faillenot, I.; Pichot, V.; Alabdullah, M.; Gaspoz, J.-M.; Laurent, B.; Barthélémy, J.-C.; Roche, F. Undiagnosed Sleep-Related Breathing Disorders Are Associated with Focal Brainstem Atrophy in the Elderly. Hum. Brain Mapp. 2009, 30, 2090–2097. [Google Scholar] [CrossRef]
- Macey, P.M.; Henderson, L.A.; Macey, K.E.; Alger, J.R.; Frysinger, R.C.; Woo, M.A.; Harper, R.K.; Yan-Go, F.L.; Harper, R.M. Brain Morphology Associated with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2002, 166, 1382–1387. [Google Scholar] [CrossRef]
- Gozal, D.; Daniel, J.M.; Dohanich, G.P. Behavioral and Anatomical Correlates of Chronic Episodic Hypoxia during Sleep in the Rat. J. Neurosci. 2001, 21, 2442–2450. [Google Scholar] [CrossRef]
- Xu, W.; Chi, L.; Row, B.W.; Xu, R.; Ke, Y.; Xu, B.; Luo, C.; Kheirandish, L.; Gozal, D.; Liu, R. Increased Oxidative Stress Is Associated with Chronic Intermittent Hypoxia-Mediated Brain Cortical Neuronal Cell Apoptosis in a Mouse Model of Sleep Apnea. Neuroscience 2004, 126, 313–323. [Google Scholar] [CrossRef]
- Machaalani, R.; Waters, K.A. Postnatal Nicotine and/or Intermittent Hypercapnic Hypoxia Effects on Apoptotic Markers in the Developing Piglet Brainstem Medulla. Neuroscience 2006, 142, 107–117. [Google Scholar] [CrossRef]
- Nanduri, J.; Nanduri, R.P. Cellular Mechanisms Associated with Intermittent Hypoxia. Essays Biochem. 2007, 43, 91–104. [Google Scholar] [CrossRef]
- Douglas, R.M.; Ryu, J.; Kanaan, A.; del Carmen Rivero, M.; Dugan, L.L.; Haddad, G.G.; Ali, S.S. Neuronal Death during Combined Intermittent Hypoxia/Hypercapnia Is Due to Mitochondrial Dysfunction. Am. J. Physiol. Cell Physiol. 2010, 298, 1594–1602. [Google Scholar] [CrossRef]
- Daulatzai, M.A. Quintessential Risk Factors: Their Role in Promoting Cognitive Dysfunction and Alzheimer’s Disease. Neurochem. Res. 2012, 37, 2627–2658. [Google Scholar] [CrossRef]
- Chou, Y.-T.; Zhan, G.; Zhu, Y.; Fenik, P.; Panossian, L.; Li, Y.; Zhang, J.; Veasey, S. C/EBP Homologous Binding Protein (CHOP) Underlies Neural Injury in Sleep Apnea Model. Sleep 2013, 36, 481–492. [Google Scholar] [CrossRef]
- Yuan, X.; Guo, X.; Deng, Y.; Zhu, D.; Shang, J.; Liu, H. Chronic Intermittent Hypoxia-Induced Neuronal Apoptosis in the Hippocampus Is Attenuated by Telmisartan through Suppression of INOS/NO and Inhibition of Lipid Peroxidation and Inflammatory Responses. Brain Res. 2015, 1596, 48–57. [Google Scholar] [CrossRef]
- Bliwise, D.L. Sleep Disorders in Alzheimer’s Disease and Other Dementias. Clin. Cornerstone 2004, 6, S16–S28. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Palmer, B.W.; Cooke, J.R.; Corey-Bloom, J.; Fiorentino, L.; Natarajan, L.; Liu, L.; Ayalon, L.; He, F.; Loredo, J.S. Cognitive Effects of Treating Obstructive Sleep Apnea in Alzheimer’s Disease: A Randomized Controlled Study. J. Am. Geriatr. Soc. 2008, 56, 2076–2081. [Google Scholar] [CrossRef]
- Ng, K.M.; Lau, C.F.; Fung, M.L. Melatonin Reduces Hippocampal β-Amyloid Generation in Rats Exposed to Chronic Intermittent Hypoxia. Brain Res. 2010, 1354, 163–171. [Google Scholar] [CrossRef]
- Shiota, S.; Takekawa, H.; Matsumoto, S.-E.; Takeda, K.; Nurwidya, F.; Yoshioka, Y.; Takahashi, F.; Hattori, N.; Tabira, T.; Mochizuki, H.; et al. Chronic Intermittent Hypoxia/Reoxygenation Facilitate Amyloid-Generation in Mice. J. Alzheimer’s Dis. 2013, 37, 325–333. [Google Scholar] [CrossRef]
- Owen, J.E.; Benediktsdottir, B.; Cook, E.; Olafsson, I.; Gislason, T.; Robinson, S.R. Alzheimer’s Disease Neuropathology in the Hippocampus and Brainstem of People with Obstructive Sleep Apnea. Sleep 2021, 44, zsaa195. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, L.F.; Meng, F.T.; Du, X.; Zhou, J.N. Acute Hypoxia Promote the Phosphorylation of Tau via ERK Pathway. Neurosci. Lett. 2010, 474, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-E.; Yang, X.; Li, L.; Sui, X.; Tian, Q.; Wei, W.; Wang, J.; Liu, G. Hypoxia-Induced Tau Phosphorylation and Memory Deficit in Rats. Neurodegener. Dis. 2014, 14, 107–116. [Google Scholar] [CrossRef]
- Fletcher, E.C.; Lesske, J.; Behm, R.; Miller, C.C., 3rd; Stauss, H.; Unger, T. Carotid Chemoreceptors, Systemic Blood Pressure, and Chronic Episodic Hypoxia Mimicking Sleep Apnea. J. Appl. Physiol. 1992, 72, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.C.; Lesske, J.; Qian, W.; Miller, C.C., 3rd; Unger, T. Repetitive, Episodic Hypoxia Causes Diurnal Elevation of Blood Pressure in Rats. Hypertension 1992, 19, 555–561. [Google Scholar] [CrossRef]
- Lim, D.C.; Brady, D.C.; Po, P.; Chuang, L.P.; Marcondes, L.; Kim, E.Y.; Keenan, B.T.; Guo, X.; Maislin, G.; Galante, R.J.; et al. Simulating Obstructive Sleep Apnea Patients’ Oxygenation Characteristics into a Mouse Model of Cyclical Intermittent Hypoxia. J. Appl. Physiol. 2015, 118, 544–557. [Google Scholar] [CrossRef]
- Chopra, S.; Polotsky, V.Y.; Jun, J.C. Sleep Apnea Research in Animals. Past, Present, and Future. Am. J. Respir. Cell Mol. Biol. 2016, 54, 299–305. [Google Scholar] [CrossRef]
- Dewan, N.A.; Nieto, F.J.; Somers, V.K. Intermittent Hypoxemia and OSA: Implications for Comorbidities. Chest 2015, 147, 266. [Google Scholar] [CrossRef] [PubMed]
- Jochmans-Lemoine, A.; Villalpando, G.; Gonzales, M.; Valverde, I.; Soria, R.; Joseph, V. Divergent Physiological Responses in Laboratory Rats and Mice Raised at High Altitude. J. Exp. Biol. 2015, 218, 1035–1043. [Google Scholar] [CrossRef]
- Jochmans-Lemoine, A.; Shahare, M.; Soliz, J.; Joseph, V. HIF1α and Physiological Responses to Hypoxia Are Correlated in Mice but Not in Rats. J. Exp. Biol. 2016, 219, 3952–3961. [Google Scholar] [CrossRef]
- Arias-Reyes, C.; Soliz, J.; Joseph, V. Mice and Rats Display Different Ventilatory, Hematological, and Metabolic Features of Acclimatization to Hypoxia. Front. Physiol. 2021, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Kimoff, R.J. Sleep Fragmentation in Obstructive Sleep Apnea. Sleep 1996, 19 (Suppl. 9), S61–S66. [Google Scholar] [CrossRef]
- Montgomery-Downs, H.E.; Crabtree, V.M.; Gozal, D. Cognition, Sleep and Respiration in at-Risk Children Treated for Obstructive Sleep Apnoea. Eur. Respir. J. 2005, 25, 336–342. [Google Scholar] [CrossRef]
- Ramesh, V.; Nair, D.; Zhang, S.X.L.; Hakim, F.; Kaushal, N.; Kayali, F.; Wang, Y.; Li, R.C.; Carreras, A.; Gozal, D. Disrupted Sleep without Sleep Curtailment Induces Sleepiness and Cognitive Dysfunction via the Tumor Necrosis Factor-α Pathway. J. Neuroinflamm. 2012, 9, 91. [Google Scholar] [CrossRef]
- McKenna, J.T.; Tartar, J.L.; Ward, C.P.; Thakkar, M.M.; Cordeira, J.W.; McCarley, R.W.; Strecker, R.E. Sleep Fragmentation Elevates Behavioral, Electrographic and Neurochemical Measures of Sleepiness. Neuroscience 2007, 146, 1462–1473. [Google Scholar] [CrossRef]
- Sinton, C.M.; Kovakkattu, D.; Friese, R.S. Validation of a Novel Method to Interrupt Sleep in the Mouse. J. Neurosci. Methods 2009, 184, 71–78. [Google Scholar] [CrossRef]
- Tartar, J.L.; Ward, C.P.; McKenna, J.T.; Thakkar, M.; Arrigoni, E.; McCarley, R.W.; Brown, R.E.; Strecker, R.E. Hippocampal Synaptic Plasticity and Spatial Learning Are Impaired in a Rat Model of Sleep Fragmentation. Eur. J. Neurosci. 2006, 23, 2739–2748. [Google Scholar] [CrossRef]
- Ward, C.P.; McCoy, J.G.; McKenna, J.T.; Connolly, N.P.; McCarley, R.W.; Strecker, R.E. Spatial Learning and Memory Deficits Following Exposure to 24 h of Sleep Fragmentation or Intermittent Hypoxia in a Rat Model of Obstructive Sleep Apnea. Brain Res. 2009, 1294, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Kaushal, N.; Gozal, D. Sleep Fragmentation Differentially Modifies EEG Delta Power during Slow Wave Sleep in Socially Isolated and Paired Mice. Sleep Sci. 2009, 2, 64–75. [Google Scholar]
- McAlpine, C.S.; Swirski, F.K. Circadian Influence on Metabolism and Inflammation in Atherosclerosis. Circ. Res. 2016, 119, 131–141. [Google Scholar] [CrossRef]
- Maki, K.A.; Burke, L.A.; Calik, M.W.; Watanabe-Chailland, M.; Sweeney, D.; Romick-Rosendale, L.E.; Green, S.J.; Fink, A.M. Sleep Fragmentation Increases Blood Pressure and Is Associated with Alterations in the Gut Microbiome and Fecal Metabolome in Rats. Physiol. Genom. 2020, 52, 280–292. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Almendros, I.; Díaz-García, E.; Toledano, V.; Casitas, R.; Galera, R.; López-Collazo, E.; Farre, R.; Gozal, D.; García-Rio, F. Differential Effect of Intermittent Hypoxia and Sleep Fragmentation on PD-1/PD-L1 Upregulation. Sleep 2020, 43, zsz285. [Google Scholar] [CrossRef]
- 2020 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef]
- Kockanek, K.D.; Xu, J.; Arias, E. Mortality in the United States; NCHS Data Brief 2020; NCHS: Hyattsville, MD, USA, No. 355; 2020; pp. 1–8. [Google Scholar]
- Long, J.M.; Holtzman, D.M. Leading Edge Review Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell 2019, 179, 312–339. [Google Scholar] [CrossRef] [PubMed]
- Bobinski, M.; Wegiel, J.; Tarnawski, M.; Bobinski, M.; Reisberg, B.; de Leon, M.J.; Miller, D.C.; Wisniewski, H.M. Relationships between Regional Neuronal Loss and Neurofibrillary Changes in the Hippocampal Formation and Duration and Severity of Alzheimer Disease. J. Neuropathol. Exp. Neurol. 1997, 56, 414–420. [Google Scholar] [CrossRef]
- Silbert, L.C.; Quinn, J.F.; Moore, M.M.; Corbridge, E.; Ball, M.J.; Murdoch, G.; Sexton, G.; Kaye, J.A. Changes in Premorbid Brain Volume Predict Alzheimer’s Disease Pathology. Neurology 2003, 61, 487–492. [Google Scholar] [CrossRef]
- Alzheimer’s Disease Fact Sheet|National Institute on Aging. Available online: https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet (accessed on 29 September 2021).
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Farías, G.; Moraels, I.; Navarrete, L. The Revitalized Tau Hypothesis on Alzheimer Disease. Arch. Med. Res. 2010, 41, 226–231. [Google Scholar] [CrossRef]
- Ashraf, G.M.; Tarasov, V.V.; Makhmutova, A.; Chubarev, V.N.; Avila-Rodriguez, M.; Bachurin, S.O.; Aliev, G. The Possibility of an Infectious Etiology of Alzheimer Disease. Mol. Neurobiol. 2019, 56, 4479–4491. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; van Eldik, L.; et al. Intraneuronal β-Amyloid Aggregates, Neurodegeneration, and Neuron Loss in Transgenic Mice with Five Familial Alzheimer’s Disease Mutations: Potential Factors in Amyloid Plaque Formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Yoshiyama, Y.; Higuchi, M.; Zhang, B.; Huang, S.M.; Iwata, N.; Saido, T.C.C.; Maeda, J.; Suhara, T.; Trojanowski, J.Q.; Lee, V.M.Y. Synapse Loss and Microglial Activation Precede Tangles in a P301S Tauopathy Mouse Model. Neuron 2007, 53, 337–351. [Google Scholar] [CrossRef]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles: Intracellular Aβ and Synaptic Dysfunction. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef]
- Radde, R.; Bolmont, T.; Kaeser, S.A.; Coomaraswamy, J.; Lindau, D.; Stoltze, L.; Calhoun, M.E.; Jäggi, F.; Wolburg, H.; Gengler, S.; et al. Aβ42-Driven Cerebral Amyloidosis in Transgenic Mice Reveals Early and Robust Pathology. EMBO Rep. 2006, 7, 940. [Google Scholar] [CrossRef] [PubMed]
- Leyns, C.E.G.; Gratuze, M.; Narasimhan, S.; Jain, N.; Koscal, L.J.; Jiang, H.; Manis, M.; Colonna, M.; Lee, V.M.Y.; Ulrich, J.D.; et al. TREM2 Function Impedes Tau Seeding in Neuritic Plaques. Nat. Neurosci. 2019, 22, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Wisniewski, T. Alzheimer’s Disease: Experimental Models and Reality. Acta Neuropathol. 2016, 133, 155–175. [Google Scholar] [CrossRef]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Neuroscience: Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP Mediates Rapid Microglial Response to Local Brain Injury in Vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting Microglia Directly Monitor the Functional State of Synapses In Vivo and Determine the Fate of Ischemic Terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef]
- Tremblay, M.-È.; Lowery, R.L.; Majewska, A.K. Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol. 2010, 8, e1000527. [Google Scholar] [CrossRef]
- Schain, A.J.; Hill, R.A.; Grutzendler, J. Label-Free in Vivo Imaging of Myelinated Axons in Health and Disease with Spectral Confocal Reflectance Microscopy. Nat. Med. 2014, 20, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Stevens, B. Microglia Emerge as Central Players in Brain Disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Savage, J.C.; Hui, C.W.; Bisht, K.; Tremblay, M.-È. Microglia across the Lifespan: From Origin to Function in Brain Development, Plasticity and Cognition. J. Physiol. 2017, 595, 1929–1945. [Google Scholar] [CrossRef] [PubMed]
- Ulland, T.K.; Colonna, M. TREM2—A Key Player in Microglial Biology and Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 667–675. [Google Scholar] [CrossRef]
- Emahazion, T.; Feuk, L.; Jobs, M.; Sawyer, S.L.; Fredman, D.; St Clair, D.; Prince, J.A.; Brookes, A.J. SNP Association Studies in Alzheimer’s Disease Highlight Problems for Complex Disease Analysis. Trends Genet. 2001, 17, 407–413. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.K.; Younkin, S.; et al. TREM2 Variants in Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 117–127. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 Associated with the Risk of Alzheimer’s Disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 Is Activated in Alzheimer’s Disease and Contributes to Pathology in APP/PS1 Mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Venegas, C.; Kumar, S.; Franklin, B.S.; Dierkes, T.; Brinkschulte, R.; Tejera, D.; Vieira-Saecker, A.; Schwartz, S.; Santarelli, F.; Kummer, M.P.; et al. Microglia-Derived ASC Specks Cross-Seed Amyloid-β in Alzheimer’s Disease. Nature 2017, 552, 355–361. [Google Scholar] [CrossRef]
- Landel, V.; Baranger, K.; Virard, I.; Loriod, B.; Khrestchatisky, M.; Rivera, S.; Benech, P.; Féron, F. Temporal Gene Profiling of the 5XFAD Transgenic Mouse Model Highlights the Importance of Microglial Activation in Alzheimer’s Disease. Mol. Neurodegener. 2014, 9, 33. [Google Scholar] [CrossRef]
- Condello, C.; Yuan, P.; Schain, A.; Grutzendler, J. Microglia Constitute a Barrier That Prevents Neurotoxic Protofibrillar Aβ42 Hotspots around Plaques. Nat. Commun. 2015, 6, 6175. [Google Scholar] [CrossRef]
- Wang, Y.; Ulland, T.K.; Ulrich, J.D.; Song, W.; Tzaferis, J.A.; Hole, J.T.; Yuan, P.; Mahan, T.E.; Shi, Y.; Gilfillan, S.; et al. TREM2-Mediated Early Microglial Response Limits Diffusion and Toxicity of Amyloid Plaques. J. Exp. Med. 2016, 213, 667–675. [Google Scholar] [CrossRef]
- Yuan, P.; Condello, C.; Keene, C.D.; Wang, Y.; Bird, T.D.; Paul, S.M.; Luo, W.; Colonna, M.; Baddeley, D.; Grutzendler, J. TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron 2016, 90, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. β-Hydroxybutyrate Inhibits Inflammasome Activation to Attenuate Alzheimer’s Disease Pathology. J. Neuroinflamm. 2020, 17, 280. [Google Scholar] [CrossRef]
- Elmore, M.R.P.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-Stimulating Factor 1 Receptor Signaling Is Necessary for Microglia Viability, Unmasking a Microglia Progenitor Cell in the Adult Brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef]
- Spangenberg, E.E.; Lee, R.J.; Najafi, A.R.; Rice, R.A.; Elmore, M.R.P.; Blurton-Jones, M.; West, B.L.; Green, K.N. Eliminating Microglia in Alzheimer’s Mice Prevents Neuronal Loss without Modulating Amyloid-b Pathology. Brain 2016, 139, 1265–1281. [Google Scholar] [CrossRef]
- Casali, B.T.; MacPherson, K.P.; Reed-Geaghan, E.G.; Landreth, G.E. Microglia Depletion Rapidly and Reversibly Alters Amyloid Pathology by Modification of Plaque Compaction and Morphologies. Neurobiol. Dis. 2020, 142, 104956. [Google Scholar] [CrossRef]
- Leyns, C.E.G.; Ulrich, J.D.; Finn, M.B.; Stewart, F.R.; Koscal, L.J.; Serrano, J.R.; Robinson, G.O.; Anderson, E.; Colonna, M.; Holtzman, D.M. TREM2 Deficiency Attenuates Neuroinflammation and Protects against Neurodegeneration in a Mouse Model of Tauopathy. Proc. Natl. Acad. Sci. USA 2017, 114, 11524–11529. [Google Scholar] [CrossRef] [PubMed]
- Ingelsson, M.; Fukumoto, H.; Newell, K.L.; Growdon, J.H.; Hedley–Whyte, E.T.; Frosch, M.P.; Albert, M.S.; Hyman, B.T.; Irizarry, M.C. Early Aβ Accumulation and Progressive Synaptic Loss, Gliosis, and Tangle Formation in AD Brain. Neurology 2004, 62, 925–931. [Google Scholar] [CrossRef]
- Wilhelmsson, U.; Bushong, E.A.; Price, D.L.; Smarr, B.L.; Phung, V.; Terada, M.; Ellisman, M.H.; Pekny, M. Redefining the Concept of Reactive Astrocytes as Cells That Remain within Their Unique Domains upon Reaction to Injury. Proc. Natl. Acad. Sci. USA 2006, 103, 17513–17518. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Molecular Dissection of Reactive Astrogliosis and Glial Scar Formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2009, 119, 7–35. [Google Scholar] [CrossRef]
- Anderson, M.A.; Ao, Y.; Sofroniew, M.v. Heterogeneity of Reactive Astrocytes. Neurosci. Lett. 2014, 565, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Jakobs, T.C. Structural Remodeling of Astrocytes in the Injured CNS. Neuroscientist 2011, 18, 567–588. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Mielke, M.L.; Gómez-Isla, T.; Betensky, R.A.; Growdon, J.H.; Frosch, M.P.; Hyman, B.T. Reactive Glia Not Only Associates with Plaques but Also Parallels Tangles in Alzheimer’s Disease. Am. J. Pathol. 2011, 179, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Nagele, R.G.; D’Andrea, M.R.; Lee, H.; Venkataraman, V.; Wang, H.Y. Astrocytes Accumulate Aβ42 and Give Rise to Astrocytic Amyloid Plaques in Alzheimer Disease Brains. Brain Res. 2003, 971, 197–209. [Google Scholar] [CrossRef]
- Wisniewski, H.M.; Wegiel, J. Spatial Relationships between Astrocytes and Classical Plaque Components. Neurobiol. Aging 1991, 12, 593–600. [Google Scholar] [CrossRef]
- Overmyer, M.; Helisalmi, S.; Soininen, H.; Laakso, M.; Riekkinen Sr., P.; Alafuzoff, I. Reactive Microglia in Aging and Dementia: An Immunohistochemical Study of Postmortem Human Brain Tissue. Acta Neuropathol. 1999, 97, 383–392. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Olabarria, M.; Chvatal, A.; Verkhratsky, A. Astroglia in Dementia and Alzheimer’s Disease. Cell Death Differ. 2008, 16, 378–385. [Google Scholar] [CrossRef]
- Simpson, J.E.; Ince, P.G.; Lace, G.; Forster, G.; Shaw, P.J.; Matthews, F.; Savva, G.; Brayne, C.; Wharton, S.B. Astrocyte Phenotype in Relation to Alzheimer-Type Pathology in the Ageing Brain. Neurobiol. Aging 2010, 31, 578–590. [Google Scholar] [CrossRef]
- Senitz, D.; Reichenbach, A.; Smith Jr, T. Surface Complexity of Human Neocortical Astrocytic Cells: Changes with Development, Aging, and Dementia. J. Hirnforsch 1995, 36, 531–537. [Google Scholar]
- Olabarria, M.; Noristani, H.N.; Verkhratsky, A.; Rodríguez, J.J. Concomitant Astroglial Atrophy and Astrogliosis in a Triple Transgenic Animal Model of Alzheimer’s Disease. Glia 2010, 58, 831–838. [Google Scholar] [CrossRef]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Dewachter, I.; Walter, J.; Klockgether, T.; van Leuven, F. Focal Glial Activation Coincides with Increased BACE1 Activation and Precedes Amyloid Plaque Deposition in APP [V717I] Transgenic Mice. J. Neuroinflamm. 2005, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-Y.; Vadhwana, B.; Verkhratsky, A.; Rodríguez, J.J. Early Astrocytic Atrophy in the Entorhinal Cortex of a Triple Transgenic Animal Model of Alzheimer’s Disease. ASN Neuro 2011, 3, 271–279. [Google Scholar] [CrossRef]
- Carter, S.F.; Schöll, M.; Almkvist, O.; Wall, A.; Engler, H.; Långström, B.; Nordberg, A. Evidence for Astrocytosis in Prodromal Alzheimer Disease Provided by 11C-Deuterium-L-Deprenyl: A Multitracer PET Paradigm Combining 11C-Pittsburgh Compound B and 18F-FDG. J. Nucl. Med. 2012, 53, 37–46. [Google Scholar] [CrossRef]
- Rodriguez-Vieitez, E.; Ni, R.; Gulyás, B.; Tóth, M.; Häggkvist, J.; Halldin, C.; Voytenko, L.; Marutle, A.; Nordberg, A. Astrocytosis Precedes Amyloid Plaque Deposition in Alzheimer APPswe Transgenic Mouse Brain: A Correlative Positron Emission Tomography and in Vitro Imaging Study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1119–1132. [Google Scholar] [CrossRef]
- Rezaei, F.; Abbasi, H.; Sadeghi, M.; Imani, M.M. The Effect of Obstructive Sleep Apnea Syndrome on Serum S100B and NSE Levels: A Systematic Review and Meta-Analysis of Observational Studies. BMC Pulm. Med. 2020, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Macey, P.M.; Sarma, M.K.; Prasad, J.P.; Ogren, J.A.; Aysola, R.; Harper, R.M.; Thomas, M.A. Obstructive Sleep Apnea Is Associated with Altered Midbrain Chemical Concentrations. Neuroscience 2017, 363, 76–86. [Google Scholar] [CrossRef]
- Macheda, T.; Roberts, K.; Lyons, D.N.; Higgins, E.; Ritter, K.J.; Lin, A.-L.; Alilain, W.J.; Bachstetter, A.D. Chronic Intermittent Hypoxia Induces Robust Astrogliosis in an Alzheimer’s Disease-Relevant Mouse Model HHS Public Access. Neuroscience 2019, 398, 55–63. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 Inflammasome Activation Drives Tau Pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Hanslik, K.L.; Ulland, T.K. The Role of Microglia and the Nlrp3 Inflammasome in Alzheimer’s Disease. Front. Neurol. 2020, 11, 570711. [Google Scholar] [CrossRef]
- Lučiūnaitė, A.; Mcmanus, R.M.; Jankunec, M.; Rácz, I.; Dansokho, C.; Dalgėdienė, I.; Schwartz, S.; Brosseron, F.; Michael, I.; Heneka, T.; et al. Soluble Aβ Oligomers and Protofibrils Induce NLRP3 Inflammasome Activation in Microglia. J. Neurochem. 2020, 155, 650–661. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.D.; et al. The Ketone Metabolite β-Hydroxybutyrate Blocks NLRP3 Inflammasome–Mediated Inflammatory Disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Fitzpatrick, S.F.; King, A.D.; O’Donnell, C.; Roche, H.M.; Ryan, S. Mechanisms of Intermittent Hypoxia-Mediated Macrophage Activation—Potential Therapeutic Targets for Obstructive Sleep Apnoea. J. Sleep Res. 2021, 30, e13202. [Google Scholar] [CrossRef]
- Smith, S.M.C.; Friedle, S.A.; Watters, J.J. Chronic Intermittent Hypoxia Exerts CNS Region-Specific Effects on Rat Microglial Inflammatory and TLR4 Gene Expression. PLoS ONE 2013, 8, e81584. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.; Dayyat, E.A.; Zhang, S.X.; Wang, Y.; Gozal, D. Intermittent Hypoxia-Induced Cognitive Deficits Are Mediated by NADPH Oxidase Activity in a Murine Model of Sleep Apnea. PLoS ONE 2011, 6, e19847. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, K.; Baril, A.A.; Gagnon, J.F.; Fortin, M.; Décary, A.; Lafond, C.; Desautels, A.; Montplaisir, J.; Gosselin, N. Cognitive Impairment in Obstructive Sleep Apnea. Pathol. Biol. 2014, 62, 233–240. [Google Scholar] [CrossRef]
- Osorio, R.S.; Gumb, T.; Pirraglia, E.; Varga, A.W.; Lu, S.; Lim, J.; Wohlleber, M.E.; Ducca, E.L.; Koushyk, V.; Glodzik, L.; et al. Sleep-Disordered Breathing Advances Cognitive Decline in the Elderly. Neurology 2015, 84, 1964–1971. [Google Scholar] [CrossRef] [PubMed]
- Yeh, N.C.; Tien, K.J.; Yang, C.M.; Wang, J.J.; Weng, S.F. Increased Risk of Parkinson’s Disease in Patients with Obstructive Sleep Apnea: A Population-Based, Propensity Score-Matched, Longitudinal Follow-up Study. Medicine 2016, 95, e2293. [Google Scholar] [CrossRef]
- Chou, P.-S.; Lai, C.-L.; Chou, Y.-H.; Chang, W.-P. Sleep Apnea and the Subsequent Risk of Parkinson’s Disease: A 3-Year Nationwide Population-Based Study. Neuropsychiatr. Dis. Treat. 2017, 13, 959–965. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Klauber, M.R.; Butters, N.; Parker, L.; Kripke, D.F. Dementia in Institutionalized Elderly: Relation to Sleep Apnea. J. Am. Geriatr. Soc. 1991, 39, 258–263. [Google Scholar] [CrossRef]
- Ju, Y.-E.S.; Finn, M.B.; Sutphen, C.L.; Herries, E.M.; Jerome, G.M.; Ladenson, J.H.; Crimmins, D.L.; Fagan, A.M.; Holtzman, D.M. Obstructive Sleep Apnea Decreases Central Nervous System–Derived Proteins in the Cerebrospinal Fluid. Ann. Neurol. 2016, 80, 154–159. [Google Scholar] [CrossRef]
- Liguori, C.; Mercuri, N.B.; Izzi, F.; Romigi, A.; Cordella, A.; Sancesario, G.; Placidi, F. Obstructive Sleep Apnea Is Associated With Early but Possibly Modifiable Alzheimer’s Disease Biomarkers Changes. Sleep 2017, 40, zsx011. [Google Scholar] [CrossRef]
- Liguori, C.; Mercuri, N.B.; Nuccetelli, M.; Izzi, F.; Cordella, A.; Bernardini, S.; Placidi, F. Obstructive Sleep Apnea May Induce Orexinergic System and Cerebral β-Amyloid Metabolism Dysregulation: Is It a Further Proof for Alzheimer’s Disease Risk? Sleep Med. 2019, 56, 171–176. [Google Scholar] [CrossRef]
- Mullins, A.E.; Kam, K.; Parekh, A.; Bubu, O.M.; Osorio, R.S.; Varga, A.W. Obstructive Sleep Apnea and Its Treatment in Aging: Effects on Alzheimer’s Disease Biomarkers, Cognition, Brain Structure and Neurophysiology. Neurobiol. Dis. 2020, 145, 105054. [Google Scholar] [CrossRef]
- Bu, X.-L.; Liu, Y.-H.; Wang, Q.-H.; Jiao, S.-S.; Zeng, F.; Yao, X.-Q.; Gao, D.; Chen, J.-C.; Wang, Y.-J. Serum Amyloid-Beta Levels Are Increased in Patients with Obstructive Sleep Apnea Syndrome. Sci. Rep. 2015, 5, 13917. [Google Scholar] [CrossRef]
- Motamedi, V.; Kanefsky, R.; Matsangas, P.; Mithani, S.; Jeromin, A.; Brock, M.S.; Mysliwiec, V.; Gill, J. Elevated Tau and Interleukin-6 Concentrations in Adults with Obstructive Sleep Apnea. Sleep Med. 2018, 43, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.P.; Yager, C.; Brandt, J.; Smith, G.S.; Zhou, Y.; Mathur, A.; Kumar, A.; Brašić, J.R.; Wong, D.F.; Wu, M.N. Objectively Measured Sleep and β-Amyloid Burden in Older Adults: A Pilot Study. SAGE Open Med. 2014, 2, 211454652. [Google Scholar] [CrossRef]
- Yun, C.-H.; Lee, H.-Y.; Lee, S.K.; Kim, H.; Seo, H.S.; Bang, S.A.; Kim, S.E.; Greve, D.N.; Au, R.; Shin, C.; et al. Amyloid Burden in Obstructive Sleep Apnea. J. Alzheimer’s Dis. 2017, 59, 21–29. [Google Scholar] [CrossRef]
- Elias, A.; Cummins, T.; Tyrrell, R.; Lamb, F.; Dore, V.; Williams, R.; Rosenfeld, J.V.; Hopwood, M.; Villemagne, V.L.; Rowe, C.C. Risk of Alzheimer’s Disease in Obstructive Sleep Apnea Syndrome: Amyloid-β and Tau Imaging. J. Alzheimer’s Dis. 2018, 66, 733–741. [Google Scholar] [CrossRef] [PubMed]
- André, C.; Rehel, S.; Kuhn, E.; Landeau, B.; Moulinet, I.; Touron, E.; Ourry, V.; le Du, G.; Mézenge, F.; Tomadesso, C.; et al. Association of Sleep-Disordered Breathing with Alzheimer Disease Biomarkers in Community-Dwelling Older Adults: A Secondary Analysis of a Randomized Clinical Trial. JAMA Neurol. 2020, 77, 716–724. [Google Scholar] [CrossRef]
- Boeve, B.F. Update on the Diagnosis and Management of Sleep Disturbances in Dementia. Sleep Med. Clin. 2008, 3, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Buratti, L.; Viticchi, G.; Falsetti, L.; Cagnetti, C.; Luzzi, S.; Bartolini, M.; Provinciali, L.; Silvestrini, M. Vascular Impairment in Alzheimer’s Disease: The Role of Obstructive Sleep Apnea. J. Alzheimer’s Dis. 2014, 38, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Stark, C.D.; Stark, R.J. Sleep Apnoea and the Neurologist. Pract. Neurol. 2017, 17, 21–27. [Google Scholar] [CrossRef]
- Johnson, S.M.; Randhawa, K.S.; Epstein, J.J.; Gustafson, E.; Hocker, A.D.; Huxtable, A.G.; Baker, T.L.; Watters, J.J. Gestational Intermittent Hypoxia Increases Susceptibility to Neuroinflammation and Alters Respiratory Motor Control in Neonatal Rats. Respir. Physiol. Neurobiol. 2018, 256, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.E.S.; Videnovic, A.; Vaughn, B.V. Comorbid Sleep Disturbances in Neurologic Disorders. CONTINUUM Lifelong Learn. Neurol. 2017, 23, 1117–1131. [Google Scholar] [CrossRef]
- Snyder, B.; Shell, B.; Cunningham, J.T.; Cunningham, R.L. Chronic Intermittent Hypoxia Induces Oxidative Stress and Inflammation in Brain Regions Associated with Early-Stage Neurodegeneration. Physiol. Rep. 2017, 5, e13258. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Giles, W.H.; Croft, J.B.; Bliwise, D.L. Habitual Sleep Patterns and Risk for Stroke and Coronary Heart Disease: A 10-Year Follow-up from NHANES I. Neurology 1997, 48, 904–911. [Google Scholar] [CrossRef]
- Ayas, N.T.; White, D.P.; Al-Delaimy, W.K.; Manson, J.E.; Stampfer, M.J.; Speizer, F.E.; Patel, S.; Hu, F.B. A Prospective Study of Self-Reported Sleep Duration and Incident Diabetes in Women. Diabetes Care 2003, 26, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Leproult, R.; van Cauter, E. Effects of Poor and Short Sleep on Glucose Metabolism and Obesity Risk. Nat. Rev. Endocrinol. 2009, 5, 253–261. [Google Scholar] [CrossRef]
- Chen, J.-C.; Brunner, R.L.; Ren, H.; Wassertheil-Smoller, S.; Larson, J.C.; Levine, D.W.; Allison, M.; Naughton, M.J.; Stefanick, M.L. Sleep Duration and Risk of Ischemic Stroke in Postmenopausal Women. Stroke 2008, 39, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Kojori, E.; Wang, G.; Wiers, C.; Demiral, S.; Guo, M.; Kim, S.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef]
- Wang, C.; Holtzman, D. Bidirectional relationship between sleep and Alzheimer’s disease: Role of amyloid, tau, and other factors. Neuropsychopharmacology 2019, 45, 104–120. [Google Scholar] [CrossRef]
- Holth, J.; Fritschi, S.; Wang, C.; Pedersen, N.; Cirrito, J.; Mahan, T.; Finn, M.; Manis, M.; Geerling, J.; Fuller, P.; et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019, 363, 880–884. [Google Scholar] [CrossRef]
- Macey, P.M.; Kumar, R.; Woo, M.A.; Valladares, E.M.; Yan-Go, F.L.; Harper, R.M. Brain Structural Changes in Obstructive Sleep Apnea. Sleep 2008, 31, 967. [Google Scholar] [PubMed]
- Canessa, N.; Castronovo, V.; Cappa, S.F.; Aloia, M.S.; Marelli, S.; Falini, A.; Alemanno, F.; Ferini-Strambi, L. Obstructive Sleep Apnea: Brain Structural Changes and Neurocognitive Function before and after Treatment. Am. J. Respir. Crit. Care Med. 2012, 183, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.E.; BenediktsdÓttir, B.; Gislason, T.; Robinson, S.R. Neuropathological Investigation of Cell Layer Thickness and Myelination in the Hippocampus of People with Obstructive Sleep Apnea. Sleep 2019, 42, zsy199. [Google Scholar] [CrossRef]
- Giri, S.; Ranjan, A.; Kumar, A.; Amar, M.; Mallick, B.N. Rapid Eye Movement Sleep Deprivation Impairs Neuronal Plasticity and Reduces Hippocampal Neuronal Arborization in Male Albino Rats: Noradrenaline Is Involved in the Process. J. Neurosci. Res. 2021, 99, 1815–1834. [Google Scholar] [CrossRef]
- Biswas, S.; Mishra, P.; Mallick, B.N. Increased Apoptosis in Rat Brain after Rapid Eye Movement Sleep Loss. Neuroscience 2006, 142, 315–331. [Google Scholar] [CrossRef]
- Kamali, A.-M.; Noorafshan, A.; Karimi, F.; Karbalay-Doust, S.; Nami, M. The Impact of Chronic Sleep Restriction on Neuronal Number and Volumetric Correlates of the Dorsal Respiratory Nuclei in a Rat Model. Sleep 2017, 40, zsx072. [Google Scholar] [CrossRef]
- Noorafshan, A.; Karimi, F.; Karbalay-Doust, S.; Kamali, A.M. Using Curcumin to Prevent Structural and Behavioral Changes of Medial Prefrontal Cortex Induced by Sleep Deprivation in Rats. EXCLI J. 2017, 16, 520. [Google Scholar] [CrossRef]
- Novati, A.; Hulshof, H.J.; Koolhaas, J.M.; Lucassen, P.J.; Meerlo, P. Chronic Sleep Restriction Causes a Decrease in Hippocampal Volume in Adolescent Rats, Which Is Not Explained by Changes in Glucocorticoid Levels or Neurogenesis. Neuroscience 2011, 190, 145–155. [Google Scholar] [CrossRef]
- Noorafshan, A.; Karimi, F.; Kamali, A.M.; Karbalay-Doust, S.; Nami, M. Restorative Effects of Curcumin on Sleep-Deprivation Induced Memory Impairments and Structural Changes of the Hippocampus in a Rat Model. Life Sci. 2017, 189, 63–70. [Google Scholar] [CrossRef]
- Sethi, M.; Joshi, S.S.; Webb, R.L.; Beckett, T.L.; Donohue, K.D.; Murphy, M.P.; O’Hara, B.F.; Duncan, M.J. Increased Fragmentation of Sleep–Wake Cycles in the 5XFAD Mouse Model of Alzheimer’s Disease. Neuroscience 2015, 290, 80–89. [Google Scholar] [CrossRef]
- Punjabi, N.M. The Epidemiology of Adult Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Pien, G.W.; Weaver, T.E. Gender Differences in the Clinical Manifestation of Obstructive Sleep Apnea. Sleep Med. 2009, 10, 1075–1084. [Google Scholar] [CrossRef]
- Semelka, M.; Wilson, J.; Floyd, R. Diagnosis and Treatment of Obstructive Sleep Apnea in Adults. Am. Fam. Physician 2016, 94, 355–361. [Google Scholar] [PubMed]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Exosome and Macrophage Crosstalk in Sleep-Disordered Breathing-Induced Metabolic Dysfunction. Int. J. Mol. Sci. 2018, 19, 3383. [Google Scholar] [CrossRef]
- Melamed, K.H.; Goldhaber, S.Z. Obstructive Sleep Apnea. Circulation 2015, 132, e114–e116. [Google Scholar] [CrossRef][Green Version]
- Basoglu, O.K.; Tasbakan, M.S. Gender Differences in Clinical and Polysomnographic Features of Obstructive Sleep Apnea: A Clinical Study of 2827 Patients. Sleep Breath. 2017, 22, 241–249. [Google Scholar] [CrossRef]
- Helvaci, N.; Karabulut, E.; Demir, A.U.; Yildiz, B.O. Polycystic Ovary Syndrome and the Risk of Obstructive Sleep Apnea: A Meta-Analysis and Review of the Literature. Endocr. Connect. 2017, 6, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Randeva, H.S.; Tan, B.K.; Weickert, M.O.; Lois, K.; Nestler, J.E.; Sattar, N.; Lehnert, H. Cardiometabolic Aspects of the Polycystic Ovary Syndrome. Endocr. Rev. 2012, 33, 812–841. [Google Scholar] [CrossRef]
- Sathish, V.; Martin, Y.N.; Prakash, Y.S. Sex Steroid Signaling: Implications for Lung Diseases. Pharmacol. Ther. 2015, 150, 94–108. [Google Scholar] [CrossRef]
- Young, T.; Finn, L.; Austin, D.; Peterson, A. Menopausal Status and Sleep-Disordered Breathing in the Wisconsin Sleep Cohort Study. Am. J. Respir. Crit. Care Med. 2003, 167, 1181–1185. [Google Scholar] [CrossRef]
- Young, T.; Rabago, D.; Zgierska, A.; Austin, D.; Finn, L. Objective and Subjective Sleep Quality in Premenopausal, Perimenopausal, and Postmenopausal Women in the Wisconsin Sleep Cohort Study. Sleep 2003, 26, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Beam, C.R.; Kaneshiro, C.; Jang, J.Y.; Reynolds, C.A.; Pedersen, N.L.; Gatz, M. Differences Between Women and Men in Incidence Rates of Dementia and Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 64, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer Disease. Cold Spring Harbor Perspect. Med. 2012, 2, a006239. [Google Scholar] [CrossRef]
- Yaffe, K.; Laffan, A.M.; Harrison, S.L.; Redline, S.; Spira, A.P.; Ensrud, K.E.; Ancoli-Israel, S.; Stone, K.L. Sleep-Disordered Breathing, Hypoxia, and Risk of Mild Cognitive Impairment and Dementia in Older Women. JAMA 2011, 306, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Rasgon, N.L.; Kenna, H.A. Insulin Resistance in Depressive Disorders and Alzheimer’s Disease: Revisiting the Missing Link Hypothesis. Neurobiol. Aging 2005, 26, 103–107. [Google Scholar] [CrossRef]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical Epidemiology of Alzheimer’s Disease: Assessing Sex and Gender Differences. Clin. Epidemiol. 2014, 2014, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L.; Prabhakar, N.R. Neural Regulation of Hypoxia-Inducible Factors and Redox State Drives the Pathogenesis of Hypertension in a Rodent Model of Sleep Apnea. J. Appl. Physiol. 2015, 119, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- May, A.M.; Mehra, R. Obstructive Sleep Apnea: Role of Intermittent Hypoxia and Inflammation. Semin. Respir. Crit. Care Med. 2014, 35, 531–544. [Google Scholar] [CrossRef]
- Hedner, J.; Bengtsson-Boström, K.; Peker, Y.; Grote, L.; Råstam, L.; Lindblad, U. Hypertension Prevalence in Obstructive Sleep Apnoea and Sex: A Population-Based Case–Control Study. Eur. Respir. J. 2006, 27, 564–570. [Google Scholar] [CrossRef]
- Yu, Q.; Yin, G.; Zhang, P.; Song, Z.; Chen, Y.; Zhang, D.; Hu, W. Distinct Associations between Hypertension and Obstructive Sleep Apnea in Male and Female Patients. PLoS ONE 2014, 9, e113076. [Google Scholar] [CrossRef]
- Knight, W.D.; Little, J.T.; Carreno, F.R.; Toney, G.M.; Mifflin, S.W.; Cunningham, J.T. Chronic Intermittent Hypoxia Increases Blood Pressure and Expression of FosB/ΔFosB in Central Autonomic Regions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R131–R139. [Google Scholar] [CrossRef]
- Hinojosa-Laborde, C.; Mifflin, S.W. Sex Differences in Blood Pressure Response to Intermittent Hypoxia in Rats. Hypertension 2005, 46, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, E.; Bakker, J.P.; Clarke, D.N.; Csizmadia, E.; Kocher, O.; Veves, A.; Tecilazich, F.; O’Donnell, C.P.; Ferran, C.; Malhotra, A. Molecular Biomarkers of Vascular Dysfunction in Obstructive Sleep Apnea. PLoS ONE 2013, 8, e70559. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, M.E.; Landreth, G.E. Inflammation, Apoptosis, and Alzheimer’s Disease. Neuroscientist 2002, 8, 276–283. [Google Scholar] [CrossRef]
- Harrison, D.G.; Guzik, T.J.; Lob, H.E.; Madhur, M.S.; Marvar, P.J.; Thabet, S.R.; Vinh, A.; Weyand, C.M. Inflammation, Immunity, and Hypertension. Hypertension 2011, 57, 132–140. [Google Scholar] [CrossRef]
- Baumgart, M.; Snyder, H.M.; Carrillo, M.C.; Fazio, S.; Kim, H.; Johns, H. Summary of the Evidence on Modifiable Risk Factors for Cognitive Decline and Dementia: A Population-Based Perspective. Alzheimer’s Dement. 2015, 11, 718–726. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Sans Capdevila, O.; Kheirandish, E.; Gozal, D. Elevated Serum Aminotransferase Levels in Children at Risk for Obstructive Sleep Apnea. Chest 2008, 133, 92–99. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Philby, M.F.; Alonso-Álvarez, M.L.; Terán-Santos, J.; Gozal, D. Biomarkers of Alzheimer Disease in Children with Obstructive Sleep Apnea: Effect of Adenotonsillectomy. Sleep 2016, 39, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Carpagnano, G.E.; Kharitonov, S.A.; Resta, O.; Foschino-Barbaro, M.P.; Gramiccioni, E.; Barnes, P.J. Increased 8-Isoprostane and Interleukin-6 in Breath Condensate of Obstructive Sleep Apnea Patients. Chest 2002, 122, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Bouloukaki, I.; Mermigkis, C.; Tzanakis, N.; Kallergis, E.; Moniaki, V.; Mauroudi, E.; Schiza, S.E. Evaluation of Inflammatory Markers in a Large Sample of Obstructive Sleep Apnea Patients without Comorbidities. Mediat. Inflamm. 2017, 2017, 4573756. [Google Scholar] [CrossRef] [PubMed]
- De la Peña Bravo, M.; Serpero, L.D.; Barceló, A.; Barbé, F.; Agustí, A.; Gozal, D. Inflammatory Proteins in Patients with Obstructive Sleep Apnea with and without Daytime Sleepiness. Sleep Breath. 2007, 11, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Minoguchi, K.; Tazaki, T.; Yokoe, T.; Minoguchi, H.; Watanabe, Y.; Yamamoto, M.; Adachi, M. Elevated Production of Tumor Necrosis Factor-α by Monocytes in Patients With Obstructive Sleep Apnea Syndrome. Chest 2004, 126, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, T.U.; Kokturk, O.; Bukan, N.; Bilgihan, A. The Relationship between Serum Cytokine Levels with Obesity and Obstructive Sleep Apnea Syndrome. Cytokine 2004, 28, 87–91. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of Plasma Cytokines in Disorders of Excessive Daytime Sleepiness: Role of Sleep Disturbance and Obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Hopper, K.; Lotsikas, A.; Lin, H.-M.; Kales, A.; Chrousos, G.P. Sleep Apnea and Daytime Sleepiness and Fatigue: Relation to Visceral Obesity, Insulin Resistance, and Hypercytokinemia. J. Clin. Endocrinol. Metab. 2000, 85, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Briançon-Marjollet, A.; Monneret, D.; Henri, M.; Hazane-Puch, F.; Pepin, J.-L.; Faure, P.; Godin-Ribuot, D. Endothelin Regulates Intermittent Hypoxia-Induced Lipolytic Remodelling of Adipose Tissue and Phosphorylation of Hormone-Sensitive Lipase. J. Physiol. 2016, 594, 1727–1740. [Google Scholar] [CrossRef]
- Rosenzweig, I.; Williams, S.C.R.; Morrella, M.J. The Impact of Sleep and Hypoxia on the Brain: Potential Mechanisms for the Effects of Obstructive Sleep Apnea. Curr. Opin. Pulm. Med. 2014, 20, 565–571. [Google Scholar] [CrossRef]
- Sapin, E.; Peyron, C.; Roche, F.; Gay, N.; Carcenac, C.; Savasta, M.; Levy, P.; Dematteis, M. Chronic Intermittent Hypoxia Induces Chronic Low-Grade Neuroinflammation in the Dorsal Hippocampus of Mice. Sleep 2015, 38, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Row, B.W.; Kheirandish, L.; Liu, R.; Guo, S.Z.; Qiang, F.; Brittian, K.R. Increased Susceptibility to Intermittent Hypoxia in Aging Rats: Changes in Proteasomal Activity, Neuronal Apoptosis and Spatial Function. J. Neurochem. 2003, 86, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Mayeux, R. Alzheimer Disease: Epidemiology, Diagnostic Criteria, Risk Factors and Biomarkers. Biochem. Pharmacol. 2014, 88, 640–651. [Google Scholar] [CrossRef]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; del Tredici, K. Staging of Alzheimer Disease-Associated Neurofibrillary Pathology Using Paraffin Sections and Immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.T.; Knight, W.D.; Mifflin, S.W.; Nestler, E.J. An Essential Role for ΔFosB in the Median Preoptic Nucleus in the Sustained Hypertensive Effects of Chronic Intermittent Hypoxia. Hypertension 2012, 60, 179–187. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Y.-C.; Wang, Y.-J.; Tseng, G.-F. Ascorbic Acid and α-Tocopherol Supplement Starting Prenatally Enhances the Resistance of Nucleus Tractus Solitarius Neurons to Hypobaric Hypoxic Challenge. Brain Struct. Funct. 2011, 216, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish, L.; Row, B.W.; Li, R.C.; Brittian, K.R.; Gozal, D. Apolipoprotein E-Deficient Mice Exhibit Increased Vulnerability to Intermittent Hypoxia-Induced Spatial Learning Deficits. Sleep 2005, 28, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Tagaito, Y.; Polotsky, V.Y.; Campen, M.J.; Wilson, J.A.; Balbir, A.; Smith, P.L.; Schwartz, A.R.; O’Donnell, C.P. A Model of Sleep-Disordered Breathing in the C57BL/6J Mouse. J. Appl. Physiol. 2001, 91, 2758–2766. [Google Scholar] [CrossRef]
- Smith, S.M.C.; Kimyon, R.S.; Watters, J.J. Cell-Type-Specific Jumonji Histone Demethylase Gene Expression in the Healthy Rat CNS: Detection by a Novel Flow Cytometry Method. ASN Neuro 2014, 6, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Lykidis, D.; van Noorden, S.; Armstrong, A.; Spencer-Dene, B.; Li, J.; Zhuang, Z.; Stamp, G.W.H. Novel Zinc-Based Fixative for High Quality DNA, RNA and Protein Analysis. Nucleic Acids Res. 2007, 35, e85. [Google Scholar] [CrossRef] [PubMed]
- Jensen, U.B.; Owens, D.M.; Pedersen, S.; Christensen, R. Zinc Fixation Preserves Flow Cytometry Scatter and Fluorescence Parameters and Allows Simultaneous Analysis of DNA Content and Synthesis, and Intracellular and Surface Epitopes. Cytom. Part A 2010, 77, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, E.A.; Wang, T.; Vanderplow, A.M.; Cherukuri, S.; Cahill, M.E.; Watters, J.J. Neonatal Intermittent Hypoxia Induces Lasting Sex-Specific Augmentation of Rat Microglial Cytokine Expression. Front. Immunol. 2019, 10, 1479. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).