Abstract
In the present study, the following was investigated: (a) The effect of ulvan on in vivo and in vitro biocontrol of Debaryomyces hansenii and Stenotrophomonas rhizophila against Fusarium proliferaum and (b) the effect of ulvan on in vivo and in vitro growth of D. hansenii and S. rhizophila and muskmelon quality parameters. The results showed that the biocontrol activity of D. hansenii and S. rhizophila could be enhanced by ulvan (5 g/L). The combination of ulvan and S. rhizophila resulted in a more effective control of fruit rot in comparison to fungicide benomyl. On in vitro growth of F. proliferatum, individual treatments of D. hansenii and S. rhizophila inhibited spore germination and mycelial growth with no statistical difference with the combined treatments. Ulvan does not have a direct effect on the in vivo and in vitro growth of D. hansenii and S. rhizophila. Furthermore, the combined treatments improve the natural disease incidence and quality parameters like weight, firmness, total soluble solids (TSS), and pH. These results suggest that the use of ulvan may be an effective method to improve the biological activity of D. hansenii and S. rhizophila.
1. Introduction
Muskmelon fruit (Cucumis melo L.) is commercialized worldwide because of its flavor and nutritional content [1]. During fruit ripening, it is easily perishable and susceptible to fungal pathogens during storage, transportation, and commercialization [2]. Fruit rot caused by Fusarium spp. is one of the most serious diseases of melon fruit, and it is generally controlled by applying synthetic fungicides [3]. However, indiscriminate use of synthetic fungicides causes environmental problems, puts humans at risk, and may proliferate fungicide resistance [4].
Biological control of postharvest disease is an effective and nonchemical alternative. It relies on the use of antagonist microorganisms which limit or stop the development of fungal pathogens [5]. In previous studies, the yeast Debaryomyces hansenii and the bacteria Stenotrophomonas rhizophila have showed good results and promissory characteristics as biological control agents. D. hansenii and S. rhizophila have significantly inhibited pathogens like Aspergillus spp., Fusarium spp., Colletotrichum spp., and Penicillium spp., among others [6,7,8,9,10]. D. hansenii has been considered as a potential biocontrol agent due to volatile organic compound (VOC) production, β-1, 3 glucanase and protease activity, inhibition of spore germination, and the competition for nutrients like saccharose, glucose, fructose and total carbohydrates [11]. S. rhizophila is another potential biocontrol agent because it produces lithic enzymes, siderophores, and secondary metabolites, which act as antifungal compounds [12,13].
Microbial antagonists, when applied individually, usually have a much lower level of effectiveness compared to that of synthetic fungicides [5]. Nonetheless, their activity can be enhanced by manipulation of the environment, using mixtures of beneficial organisms and physiological and genetic enhancement or biocontrol with other methods, such as low doses of fungicides and other chemicals [14]. Improvement of the biocontrol agents effect may result in direct inhibition of the pathogen, elicitation of systemic acquired resistance in the host tissue, and stimulation of the microbial antagonists [15]. Biological control agents (BCAs) combined with chemicals like sodium bicarbonate, harpin, quitosan, and ulvan have been demonstrated to provide enhanced characteristics in controlling fruit decay [16,17,18,19].
Ulvan is a polysaccharide isolated from green algae of the genus Ulva and it has been used as an alternative treatment for chemical fungicides [20]. These sulfated heteropolisacharide can reduce the disease severity of many pathogens of plants at concentrations of 5 g L−1 or less [19,20,21,22,23,24,25]. In apple, ulvan reduces the mycelial growth of Colletotrichum gloeosporioides, decreases disease severity to 66%, and increases peroxidase and glucanase activity in the host [22]. In Arabidopsis thaliana, ulvan increased nicotinamide adenine dinucleotide hydrogen (NADPH) oxidase activity and hydrogen peroxide levels [23]. In Medicago trucantula, ulvan is an efficient elicitor of resistance, which confers protection against Colletotrichum trifolii [24]. In tomato, oligoulvan reduced the severity caused by Fusarium oxysporum f. sp. lycopersici, stimulating phenyl alanine ammonia lyase, increasing the phenolic compounds, and inducing salicylic acid synthesis [25].
To our knowledge, no study has been conducted to determine the ability of ulvan to improve the biocontrol activity of the yeast D. hansenii and the bacteria S. rhizophila. In particular, the objectives of this study were to evaluate (a) the effect of ulvan on in vivo and in vitro control of D. hansenii and S. rhizophila against F. proliferaum and (b) the effect of ulvan on in vitro and in vivo growth of D. hansenii and S. rhizophila and muskmelon quality parameters.
2. Materials and Methods
2.1. Microorganisms and Fruit Materials
2.1.1. Fruit
Muskmelon (Cucumis melo L. var. reticulatus) fruit were collected from a commercial orchard located in El Pescadero, Baja California Sur, México. Fruit of uniform size at commercial maturity stage were selected and transported immediately to the laboratory. Fruit without physical damage or symptoms of fruit rot were disinfected with 2% (v/v) sodium hypochlorite for 2 min, washed with sterile distilled water, air dried at room temperature, and placed in plastic containers prior to use.
2.1.2. Pathogen Inoculum
The pathogen Fusarium proliferatum was previously isolated from infected melon fruits and maintained in potato dextrose agar (PDA, at dose of 39 g L−1) plates at 4 °C for storage [26]. To reactivate the culture and verify their pathogenicity, the pathogen was inoculated into wounded melon fruits and re-isolated onto PDA after infection was established. Spore suspension was obtained from 10 days old cultures PDA at 25 °C, and spore concentration was determined using a hemocytometer and adjusted to 104 spores/mL with sterile distilled water prior to use.
2.1.3. Antagonist Microorganisms
The antagonist microorganisms were obtained from the Centro de Investigaciones Biológicas del Noroeste (CIBNOR), La Paz Baja California Sur, México, and were originally isolated from the Ojo de Liebre hyperhyaline lagoon (27°35′ and 27°52′ north latitude and 113° 58′ and 114°0′ west latitude). D. hansenii and S. rhizophila were maintained in PDA and tripticase soy agar (TSA, at dose of 40 g L−1) plates respectively at 4 °C for storage. Liquid cultures of D. hansenii and S. rhizophila were grown in 250 mL Erlenmeyer flasks containing 50 mL of potato dextrose broth (PDB, at dose of 39 g L−1) and tripticase soy broth (TSB, at dose of 40 g L−1), respectively; both microorganisms had been inoculated with a loop of each culture and were incubated on a rotary shaker at 180 rpm and 27 °C. D. hansenii concentration was adjusted to 1 × 106 CFU mL−1 with a hemocytometer and the cells suspension of S. rhizophila was adjusted to 1 × 108 CFU mL−1 using a UV/V spectrophotometer (HACH, Dusseldorf, Germany) at 660 nm and absorbance of 1.
2.1.4. Chemical Treatments
Ulvan (OligoTech®, Elicityl Ltd., Crolles, France) solution was prepared at 5 g L−1 using sterile deionized water. The synthetic fungicide used in this study was benomyl at 1000 ppm and synthetic bactericide bactrol at 500 ppm.
2.2. Effect of Ulvan, D. hansenii, and S. rhizophila against F. proliferatum In Vivo
In this experiment, six equidistant 3 mm wounds in diameter were performed in each fruit and were inoculated with 20 µL of the following: (1) 1 × 106 CFU mL−1 D. hansenii; (2) 1 × 108 CFU mL−1 S. rhizophila; (3) 5 g L−1 ulvan; (4) 1 × 106 CFU mL−1 D. hansenii + 5 g L−1 ulvan; (5) 1 × 108 CFU mL−1 S. rhizophila + 5 g L−1 ulvan; (6) sterile distilled water (control); and (7) 1000 ppm benomyl. They were left to dry for 2 h and then a suspension (20 µL) of 1 × 104 spores mL−1 F. proliferatum was inoculated into each wound. Fruits were placed in plastic containers at 27 °C and 90% of relative humidity (RH) for 7 days. Disease control and lesion diameter (mm) were measured. Disease control (DC) was calculated by the formula: 100 − ([100 × Fi]/Tf), where Fi = number of infected fruits in each treatment and Tf = total of infected fruits in control treatment. Each treatment consisted of three fruits and was replicated ten times.
2.3. Effect of Ulvan, D. hansenii, and S. rhizophila against F. proliferatum In Vitro
2.3.1. Effect on Mycelial Growth
The effects of ulvan, D. hansenii, and S. rhizophila on the mycelial growth of F. proliferatum were assessed as described by Zhou et al. [27]. A 10 mm diameter hole was made in a 90 mm diameter plate containing 20 mL of nutrient broth (NB, at dose of 31 g/L). As treatments, 100 µL of: (1) 1 × 106 CFU mL−1 D. hansenii; (2) 1 × 108 CFU mL−1 S. rhizophila; (3) 5 g L−1 ulvan; (4) 1 × 106 CFU mL−1 D. hansenii + 5 g L−1 ulvan; (5) 1 × 108 CFU mL−1 S. rhizophila + 5 g L−1 ulvan; (6) sterile distilled water (control); and (7) 1000 ppm benomyl were deposited into the holes. After 2 h, 100 µL 1 × 104 spores mL−1 suspension of F. proliferatum was deposited into each hole. The plates were incubated at 27 °C for 7 days. The mycelial radial growth was measured with the ImageJ® program, which measured the relative area of the calibration parameter and the lesion site in pixels and converted the measurement of lesion site in mm2 based on the known value of the calibration. Inhibition percentage (I%) was calculated by the following equation: I% = (Gc − Gt)/Gc × 100, in which Gc means the radial growth of the pathogen in the control treatments and Gt means the radial growth of the pathogen with the treatments. Each treatment was replicated ten times.
2.3.2. Effect on Spore Germination
The effect of ulvan, D. hansenii, and S. rhizophila on spore germination was assayed according to the method of Mattiuz et al. [28]. We deposited 50 µL 1 × 104 spores mL−1 suspension of F. proliferatum into an Eppendorf® tube. Then, 50 µL of: (1) 1 × 106 CFU mL−1 D. hansenii; (2) 1 × 108 CFU mL−1 S. rhizophila; (3) 5 g L−1 ulvan; (4) 1 × 106 CFU mL−1 D. hansenii + 5 g L−1 ulvan; (5) 1 × 108 CFU mL−1 S. rhizophila + 5 g L−1 ulvan; (6) sterile distilled water (control); and (7) 1000 ppm benomyl were deposited into the Eppendorf® tubes. After incubation at 27 °C on a rotary shaker set at 180 rpm, an aliquot of 20 µL was taken every 12 h to observe the spore germination rate with an optical microscope (CARL ZEISS, Primo Star, Oberkochen, Germany). The experiment finished when control treatment reached a 100% germinated spores. A spore was considered as being germinating if its germ tube was longer than the spore itself. The germination inhibition was obtained by counting the number of germinated spores (NGS) among the first 100 spores observed. Each treatment was replicated ten times and inhibition ratio was calculated as being (100−NGS) × 100/100, and was expressed as a percentage (%).
2.4. Effect of Ulvan on Populations of D. hansenii and S. rhizophila in Vivo
The fruit were disinfected and wounded as described before. Then, the wounds were treated with 20 µL of a cell suspension of: (1) 1 × 106 CFU mL−1 D. hansenii; (2) 1 × 108 CFU mL−1 S. rhizophila; (3) 1 × 106 CFU mL−1 D. hansenii + 5 g L−1 ulvan; and (4) 1 × 108 CFU mL−1 S. rhizophila + 5 g L−1 ulvan, (5) 1 × 106 CFU mL−1 D. hansenii + 1000 ppm benomyl, and (6) 1 × 108 CFU mL−1 S. rhizophila + 500 ppm bactrol. Sterile distilled water was used as control. Fruits were placed in plastic containers at 27 °C and 90% RH. D. hansenii and S. rhizophila were recovered from the wounds 1 h after inoculation (time 0) and after 1, 2, 3, and 4 days. Wounded tissue was removed with an ethanol-flamed, 5 mm cork borer and ground in sterile mortar with 5 mL of sterile 0.05 M phosphate buffer (pH 7.0). The number of CFU of the yeast and bacteria was determined using the dilution plating technique. The results were expressed as Log10 CFU/wound. Each treatment consisted of three fruits and was replicated ten times.
2.5. Effect of Ulvan on the Growth of D. hansenii and S. rhizophila In Vitro
The experiment was conducted in petri plates (90 mm) of PDA for D. hansenii and TSA for S. rhizophila (20 mL per plate). One hundred milliliters of: 1 × 106 CFU mL−1 D. hansenii; or 1 × 108 CFU mL−1 S. rhizophila was spread with an ethanol-flamed glass rod. After 2 h, 4 equidistant holes (10 mm diameter) were made and 100 mL of: (1) 5 g L−1 ulvan for both microorganisms, (2) 1000 ppm benomyl for D. hansenii, or (3) 500 ppm bactrol for S. rhizophila were deposited in 4 holes. Sterile distilled water was used as control. The plates were incubated at 27 °C for 2 days. The number of CFU was determined by colony density and inhibition percentage (I%) was calculated by the following equation: I% = (Ga−Gu)/Gc × 100, in which Ga means the CFU of the antagonist in the control treatments and Gu means the CFU of the antagonist with chemicals. Each treatment was replicated ten times.
2.6. Effect of D. hansenii or S. rhizophila in Combination with Ulvan on Natural Disease Incidence
Intact fruit were saturated in treatment solutions as follows: (1) 1 × 106 CFU mL−1 D. hansenii; (2) 1 × 108 CFU mL−1 S. rhizophila; (3) 1 × 106 cells mL−1 D. hansenii + 5 g L−1 ulvan; (4) 1 × 108 CFU mL−1 S. rhizophila + 5 g L−1 ulvan; (5) sterile distilled water; and (6) 1000 ppm benomyl. Each fruit was kept soaked for 2 min, air dried at room temperature (25 °C) for 2 h, and packed in plastic containers at 27 °C and 90% relative humidity (RH) for 7 days. The numbers of decayed fruits were recorded, the incidence of decayed fruit was evaluated, and the quality parameters were determined. Each treatment was replicated ten times.
Determination of Quality Parameters
The weight loss (%), fruit firmness (N), total soluble solids (%), and pH were measured to evaluate the effect of ulvan, D. hansenii, and S. rhizophila on quality parameters. For weight loss, fruit from plastic containers were weighed before and after storage. Firmness values were determined by compression after application of a load of 9.8 N using the GY- texture Analyzer at two opposite sides of the equatorial region of the fruit. A homogeneous sample of fruit was prepared by crushing it in the mortar and pestle and a few drops of fruit juice were used for the total soluble solids (TSS) and pH. TSS was determined with a digital Abbe refractometer (PR—32, Atago Co., Tokyo, Japan) at room temperature. A few drops of the fruit juice were placed on the refractometer for measurement of total soluble solids percentage.
2.7. Statistical Analysis
To assess the advantage of in vivo combined postharvest treatments (BCAs + ulvan) with respect to the same treatments applied alone (BCAs or ulvan), the type of interaction (additive, synergistic, or antagonistic) was evaluated. The synergy factor (SF) was calculated according to the Abbott‘s formula [29]: SF = EO/EE; where EO and EE are, respectively, the observed and expected biocontrol percentage (C%) of the combination. EE was calculated as follows: (Ea + Eb) − (Ea × Eb/100), where Ea = C% of postharvest treatment (BCAs); Eb = C% of postharvest treatment b (ulvan). If SF = 1, the interaction between the combination treatments was identified as additive; if SF < 1, the interaction was antagonistic, and if SF > 1, the interaction was synergistic.
The data were processed by one-way analysis of variance (ANOVA). Statistical data analyses were performed using the software program STATISTICA 10.0, and the post hoc least significant difference Fisher test (p ≤ 0.05) was used for comparison of the means. When it was necessary, data were transformed into arcsine square root values to normalize distribution before analysis of variance.
3. Results
3.1. Effect of Ulvan, D. hansenii, and S. rhizophila on Muskmelon Fruit Rot by F. proliferatum
D. hansenii, S. rhizophila, and ulvan had a significant effect on disease control of fruit rot caused by F. proliferatum on muskmelon fruit stored at 27 °C for 7 days (Table 1). While ulvan treatment had a slight effect on disease control with 14.3%, in combination with S. rhizophila, it had the most effective disease control and was significant similar to benomyl treatment (64.3%). According to Abbott‘s formula [29], the treatments D. hansenii + ulvan and S. rhizophila + ulvan had synergistic effect in comparison with their individual treatments. On lesion diameter (Figure 1), single treatment of S. rhizophila was better than D. hansenii + ulvan treatment. S. rhizophila + ulvan were the most effective treatment on reducing lesion diameter, but not better than benomyl. These results suggest that ulvan enhances the biocontrol activity of D. hansenii and S. rhizophila against fruit rot caused by F. proliferatum in muskmelon fruit.

Table 1.
Effect of Debaryomyces hansenii, Stenotrophomonas rhizophila, and ulvan on disease control of muskmelon fruit rot by Fusarium proliferatum incubated at 27 °C for 7 days.
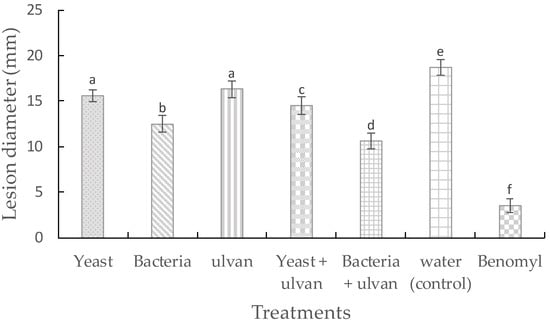
Figure 1.
Effects of the yeast Debaryomyces hansenii, the bacteria Stenotrophomonas rhizophila, and ulvan on lesion diameter (mm) of muskmelon fruit by Fusarium proliferatum during incubation at 27 °C for 7 days. Vertical bars are means ± standard deviation of ten replicates (three fruits each) Bars with different letters are significantly different (p ≤ 0.05) according to Fisher test.
3.2. Effect of D. hansenii, S. rhizophila, and Ulvan on Mycelial Growth and Spore Germination of F. proliferatum
On PDA media, ulvan had no inhibiting effect on the growth of F. proliferatum compared with the control (Figure 2a). Single treatments of D. hansenii and S. rhizophila significantly inhibited the growth of F. proliferatum in vitro. However, D. hansenii and S. rhizophila in combination with ulvan did not differ from single treatments with BCAs. The results on spore germination inhibition showed a similar pattern to mycelial growth inhibition against F. proliferatum (Figure 2b). These results suggest that ulvan does not have a direct effect on mycelial growth and spore germination inhibition of F. proliferatum.
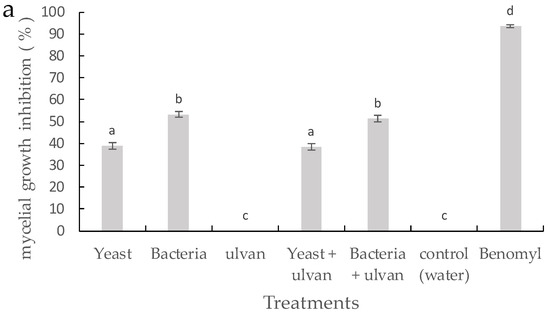
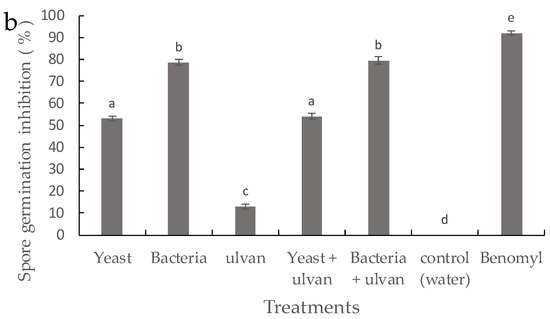
Figure 2.
Inhibitory effect of the yeast D. hansenii, the bacteria S. rhizophila, and ulvan on (a) mycelial growth and (b) spore germination rate of F. proliferatum on potato dextrose agar (PDA). Vertical bars are means ± standard deviation of ten replicates. Bars with different letters are significantly different (p ≤ 0.05) according to Fisher test.
3.3. Effect of Ulvan on the In Vivo Population Dynamics or in the In Vitro Growth of D. hansenii and S. rhizophila
Ulvan exhibited no significant negative effects both on the in vivo populations (Figure 3) and on the in vitro growth of D. hansenii and S. rhizophila (Figure 4). Both BCAs grew efficiently on muskmelon tissue during the period of time quantified. After 3 days of incubation, there were significant differences between S. rhizophila and S. rhizophila + ulvan treatments. It was found that for the rest of treatments during the period of incubation, there were no significant differences between single and addition of ulvan on treatments. Chemicals totally inhibited the in vivo growth of BCAs. These results suggest that ulvan does not have a direct effect on the in vivo and in vitro development of both BCAs.
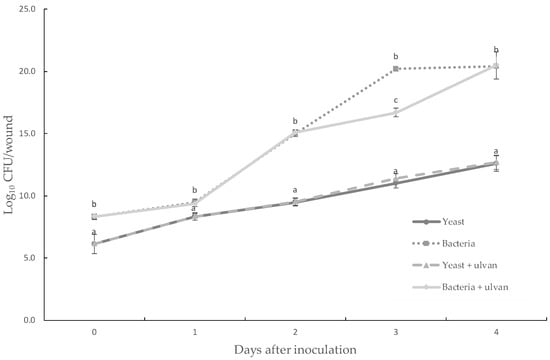
Figure 3.
Population dynamics of the yeast D. hansenii and the bacteria S. rhizophila with and without ulvan in wounds at 27 °C. Chemical treatments totally inhibited biological control agents (BCAs) since the first day after inoculation. Sterile distilled water was used as control. The data presented are the means ± standard deviation of ten replicates Different letters are significantly different (p ≤ 0.05) according to Fisher test.
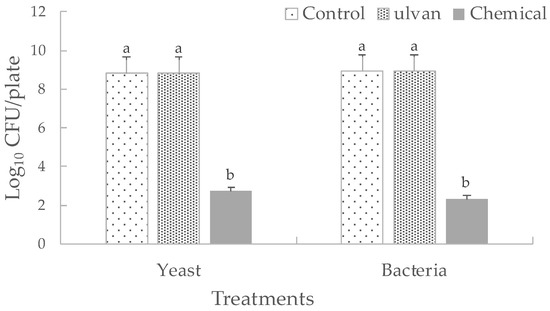
Figure 4.
Effect of ulvan on the yeast D. hansenii and the bacteria S. rhizophila on PDA plates for yeast and case soy broth (TSB) plates for bacteria incubated at 27 °C for 2 days. Chemical treatments consisted of benomyl for D. hansenii and bactrol for S. rhizophila. Sterile distilled water was used as control. Vertical bars are means ± standard deviation of ten replicates. Bars with different letters are significantly different (p ≤ 0.05) according to Fisher test.
3.4. Effect of D. hansenii, S. rhizophila, and Ulvan on Natural Disease Incidence and Quality Parameters
Fruit surfaces were inoculated to explore the preventive activity of D. hansenii and S. rhizophila alone or in combination with ulvan against fruit rot. Even though 70% of the fruits in the control developed decay symptoms after 7 days of storage, the disease incidence (DI) was significantly reduced with all treatments (Table 2). DI under treatment with S. rhizophila + ulvan (13.3%) was statistically similar to treatment with benomyl (10.0%). The combined treatments of BCAs with ulvan were more effective to reduce the disease incidence of muskmelon fruits than the single application. The quality parameters of three fruits from ten replicates were measured (Table 2). Treatment with benomyl and water (control) significantly increased weight loss and decreased the firmness of muskmelon fruit. On total soluble solids (TSS), no significant effects were observed. Single treatment of ulvan or the combination with D. hansenii or S. rhizophila decreased the pH level.

Table 2.
Effect of D. hansenii, S. rhizophila, and ulvan on natural disease incidence and quality parameters of muskmelon fruit after 7 days of incubation at 27 °C.
4. Discussion
The efficacy of biological control alternatives should be comparable to the level of control provided by conventional fungicides in order to obtain general acceptance [30]. Obtaining such high levels of control with biological control alternatives is difficult [15]. Thus, there is a tendency to promote an integrated system rather than a single one [31]. The results in the present study demonstrate that the combination of ulvan with D. hansenii or S. rhizophila can result in a significant enhanced control of fruit rot in muskmelon, compared with single treatments of D. hansenii, S. rhizophila, and ulvan (Figure 1) (Table 1). Previous studies have demonstrated that the biocontrol ability of different antagonist microorganisms to control postharvest diseases of fruits can be significantly improved by combining alternative, but compatible treatments [32]. Chitosan possess antifungal properties and the ability to elicit host defense responses; hence, it has been suggested as an effective additive to improve the biocontrol performance of the antagonistic yeasts Candida saitona and C. laurentii [33]. The combination of NaHCO3 and the bacterial antagonist Burkholderia spinosa is effective on the suppression of anthracnose, crown rote, and blossom end rot on banana [34].
To understand the direct effect of ulvan alone or in combination with D. hansenii and S. rhizophila on the growth of F. proliferatum, we investigated the effects of ulvan on mycelial growth and spore germination in vitro. In previous studies, ulvan promotes the conidial germination and appressoria formation of Colletotrichum gloeosporioides [35,36]. Nonetheless, our results showed that ulvan did not influence the mycelial growth of F. proliferatum. However, it slightly inhibited the spore germination of F. proliferatum, probably due to chemical PDA alteration (Figure 2). By contrast, single treatments of D. hansenii or S. rhizophila were found to be similar to the combined treatments with ulvan on spore germination inhibition. D. hansenii and S. rhizophila have the capacity to alleviate unfavorable conditions and grow efficiently [11,13]. Both BCAs probably reduce the slight spore germination by inhibition due to chemical alteration of the medium. It has been reported that ulvan did not exhibit any direct fungal activity against Alternaria brassicola, Colletotrichum lindemuthianum, and Uromyces appendiculatus [37,38,39]. These different results might be related to different sensitivities of fungal species to ulvan and dissimilar testing methods might explain these apparently contradictory results on fungal species.
We propose that the efficacy of ulvan in combination with D. hansenii and/or S. rhizophila in controlling fruit rot of muskmelon by F. proliferatum might be linked to the fruit-mediated mechanisms which increase defense response, like priming, PR proteins, and oxidative burst. Jaulneau et al. [40] elucidated that the ulvan-induced defense response on Medicago truncatula is mediated by the jasmonic acid signaling pathway. On wheat and rice, ulvan has a priming effect by increasing the initial oxidative burst and by enhancing the resistance against powdery mildew [41]. Cluzet et al. [24] concluded by a microarray that ulvan increases the expression of codificant genes to phytoalexins, PR proteins, and structural proteins. Although ulvan has a small effect on disease control, its effect is attributed to systemic acquired resistance (SAR) mechanisms and priming, which acts after systemic induced resistance (ISR) mechanisms [24,40,41]. Further investigation needs to be carried out in order to elucidate which mechanisms of resistance are induced in muskmelon fruit by ulvan.
During the last two decades, numerous BCAs have been isolated, identified, and applied to control postharvest decay of different fruits and vegetables [32]. It is crucial for BCAs to colonize fruit tissue more efficiently than the pathogen to compete for space and nutrients [42]. Moreover, our data showed that D. hansenii and S. rhizophila grew rapidly in muskmelon wounds (Figure 3). However, the direct antifungal activity of BCAs is an important mechanism of action during the colonization time and the efficacy of this mechanism relies on the rapid colonization level of BCAs, thereby inhibiting the early pathogenic process by fungi [42]. Previous studies have shown that D. hansenii significantly inhibited the mycelial growth of Monilinia fruticola (74.4%) and M. fructigena (44.1%), and S. rhizophila significantly inhibited the mycelial growth of C. gloeosporioides (93%) [43,8]. According to previous results, D. hansenii and S. rhizophila control the in vitro growth of F. proliferatum by mechanisms such as mycoparasitism by lytic enzymes and secondary metabolites excretion, e.g., surfactants and volatile organic compounds [44]. In addition, ulvan did not have any influence on the growth of the BCAs in vivo or in vitro (Figure 3 and Figure 4). To our knowledge, this is the first time that ulvan has been applied in combination with BCAs to control fungal diseases of fruit.
It is known that resistance in harvested fruit is associated with levels of senescence, as it drops considerably with the onset of tissue senescence [45]. In a previous study, the effect of combining Pichia membranefaciens and benzo-thiadiazole-7-carbothioic acid S-methyl ester (BTH) on the control of blue mold by Penicillium expansum in peach fruit showed that quality parameters were not impaired [31]. Ulvan in combination with both BCAs decreased natural disease incidence and significantly maintained the fruit firmness and weight (Table 2). An initial increase in the TSS content of fruit may be due to the hydrolysis of insoluble polysaccharide into simple sugars, but subsequently, TSS content decreased as the storage period increased, which is related to a higher respiratory process [46]. In our results, ulvan by the combined treatments with BCAs showed the highest TSS values by probably delaying the respiratory process. During muskmelon fruit maturation, the pH increases from approximately 5.3 to approximately 6.8 [45]. The increase in pH promotes the activity of the poligalacturonase enzyme that is related to the pathogenicity and virulence of F. proliferatum [47,48]. In our results, ulvan and the combined treatments with BCAs maintained the lowest pH values.
5. Conclusions
The results presented in our study showed that ulvan enhances the effect of D. hansenii and S. rhizophila in controlling fruit rot in muskmelon, but ulvan does not have direct effect on BCA growth. Also elucidated was the effect of ulvan on pathogenicity of F. proliferatum and development of fruit rot disease of muskmelon. The mode of action of both treatments may have complemented each other, whereby ulvan probably provides protection by muskmelon resistance induction, and BCAs inhibit fungal growth colonization by competition for space and direct antifungal activity. However, the mechanism by which ulvan enhanced the biocontrol efficacy of BCAs is complex and may be a result of several different interactions among ulvan, BCAs (D. hansenii or S. rhizophila) pathogen, and fruits. In this study, we found a new methodology to improve the performance of the antagonistic D. hansenii and S. rhizophila for controlling fruit rot in muskmelon during postharvest stage.
Author Contributions
Conceptualization, L.G.H.-M. and B.M.-A.; methodology, L.G.H.-M. and T.R.-G.; software, G.R.-E. and A.N.-G.; validation, L.G.H.-M., R.G.C.-C., and T.R.-G.; formal analysis, B.M.-A. and A.N.-G.; investigation, T.R.-G., L.G.H.-M., and B.M.-A.; resources, G.R.-E. and R.G.C.-C.; data curation, B.M.-A. and T.R.-G.; writing—original draft preparation, L.G.H.-M. and T.R.-G.; writing—review and editing, L.G.H.-M., A.N.-G., and G.R.-E.; visualization, T.R.-G.; supervision, R.G.C.-C. and A.N.-G.; project administration, L.G.H.-M.; funding acquisition, L.G.H.-M.
Funding
This research was funded by the grant project SEP-CONACYT 181972 and grant project Problemas nacionales 2015-01-352 CONACYT (Consejo Nacional de Ciencia y Tecnologia).
Acknowledgments
The CONACYT by grant awarded to T. Rivas Garcia and Michael Cordoba, a native English-speaking editor, for editing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sánchez-Estrada, A.; Tiznado-Hernández, M.E.; Ojeda-Contreras, A.J.; Valenzuela-Quintanar, A.I.; Troncoso-Rojas, R. Induction of enzymes and phenolic compounds related to the natural defense response of netted melon fruit by a bio-elicitor. J. Phytopathol. 2009, 157, 24–32. [Google Scholar] [CrossRef]
- Huang, K.; Zou, Y.; Luo, J.; Liu, Y. Combining UV-C treatment with biocontrol yeast to control postharvest decay of melon. Environ. Sci. Pollut. Res. 2015, 22, 14307–14313. [Google Scholar] [CrossRef] [PubMed]
- Jamalizadeh, M.; Etebarian, H.R.; Aminian, H.; Alizadeh, A. A review of mechanisms of action of biological control organisms against post-harvest fruit spoilage. EPPO Bull. 2011, 41, 65–71. [Google Scholar] [CrossRef]
- Mari, M.; Di Francesco, A.; Bertolini, P. Control of fruit postharvest diseases: old issues and innovative approaches. Stewart Postharvest Rev. 2014, 10, 1–4. [Google Scholar]
- Droby, S.; Wisniewski, M.; Macarisin, D.; Wilson, C. Twenty years of postharvest biocontrol research: Is it time for a new paradigm? Postharvest Biol. Technol. 2009, 52, 137–145. [Google Scholar] [CrossRef]
- Medina-Córdova, N.; López-Aguilar, R.; Ascencio, F.; Castellanos, T.; Campa-Córdova, A.I.; Angulo, C. Biocontrol activity of the marine yeast Debaryomyces hansenii against phytopathogenic fungi and its ability to inhibit mycotoxins production in maize grain (Zea mays L.). Biol. Control 2016, 97, 70–79. [Google Scholar] [CrossRef]
- Hernández-Montiel, L.G.; Ochoa, J.L.; Troyo-Diéguez, E.; Larralde-Corona, C.P. Biocontrol of postharvest blue mold (Penicillium italicum Wehmer) on Mexican lime by marine and citrus Debaryomyces hansenii isolates. Postharvest Biol. Technol. 2010, 56, 181–187. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Zulueta-Rodriguez, R.; Angulo, C.; Rueda-Puente, E.O.; Quiñonez-Aguilar, E.E.; Galicia, R. Marine yeasts and bacteria as biological control agents against anthracnose on mango. J. Phytopathol. 2017, 165, 833–840. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Gutierrez-Perez, E.D.; Murillo-Amador, B.; Vero, S.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G. Mechanisms employed by Debaryomyces hansenii in biological control of anthracnose disease on papaya fruit. Postharvest Biol. Technol. 2018, 139, 31–37. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Rivas-Garcia, T.R.; Romero-Bastidas, M.; Chiquito-Contreras, C.J.; Ruiz-Espinoza, F.H.; Chiquito-Contreras, R.G. Potencial antagónico de bacterias y levaduras marinas para el control de hongos fitopatógenos. REMEXCA 2018, 20, 4311–4321. [Google Scholar] [CrossRef]
- Medina-Córdova, N.; Rosales-Mendoza, S.; Hernández-Montiel, L.G.; Angulo, C. The potential use of Debaryomyces hansenii for the biological control of pathogenic fungi in food. Biol. Control 2018, 121, 216–222. [Google Scholar] [CrossRef]
- Berg, G.; Egamberdieva, D.; Lugtenberg, B.; Hagemann, M. Symbiotic plant–microbe interactions: Stress protection, plant growth promotion, and biocontrol by Stenotrophomonas. In Symbioses and Stress; Springer: Dordrecht, The Netherlands, 2010; pp. 445–460. [Google Scholar]
- Schmidt, C.S.; Alavi, M.; Cardinale, M.; Müller, H.; Berg, G. Stenotrophomonas rhizophila DSM14405T promotes plant growth probably by altering fungal communities in the rhizosphere. Biol. Fertil. Soils 2012, 48, 947–960. [Google Scholar]
- Nunes, C.A. Biological control of postharvest diseases of fruit. Eur. J. Plant Pathol. 2012, 133, 181–196. [Google Scholar] [CrossRef]
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Hao, W.; Luo, J.; Chen, S.; Hu, M.; Zhong, G. Combination of hot water, Bacillus amyloliquefaciens HF-01 and sodium bicarbonate treatments to control postharvest decay of mandarin fruit. Postharvest Biol. Technol. 2014, 88, 96–102. [Google Scholar] [CrossRef]
- Tang, J.; Liu, Y.; Li, H.; Wang, L.; Huang, K.; Chen, Z. Combining an antagonistic yeast with harpin treatment to control postharvest decay of kiwifruit. Biol. Control 2015, 89, 61–67. [Google Scholar] [CrossRef]
- Yu, T.; Yu, C.; Chen, F.; Sheng, K.; Zhou, T.; Zunun, M.; Abudu, O.; Yang, S.; Zheng, X. Integrated control of blue mold in pear fruit by combined application of chitosan, a biocontrol yeast and calcium chloride. Postharvest Biol. Technol. 2012, 69, 49–53. [Google Scholar] [CrossRef]
- Abouraïcha, E.; El Alaoui-Talibi, Z.; El Boutachfaiti, R.; Petit, E.; Courtois, B.; Courtois, J.; El Modafar, C. Induction of natural defense and protection against Penicillium expansum and Botrytis cinerea in apple fruit in response to bioelicitors isolated from green algae. Sci. Hort. 2015, 181, 121–128. [Google Scholar] [CrossRef]
- Stadnik, M.J.; Freitas, M.B.D. Algal polysaccharides as source of plant resistance inducers. Trop. Plant Pathol. 2014, 39, 111–118. [Google Scholar] [CrossRef]
- Siah, A.; Magnin-Robert, M.; Randoux, B.; Choma, C.; Rivière, C.; Halama, P.; Reignault, P. Natural agents inducing plant resistance against pests and diseases. In Natural Antimicrobial Agents; Merillon, J.M., Riviere, C., Eds.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-67045-4. [Google Scholar]
- Araujo, L.; Stadnik, M.J. Cultivar-specific and ulvan-induced resistance of apple plants to Glomerella leaf spot are associated with enhanced activity of peroxidases. Acta Sci. Agron. 2013, 35, 287–293. [Google Scholar] [CrossRef]
- de Freitas, M.B.; Stadnik, M.J. Ulvan-induced resistance in Arabidopsis thaliana against Alternaria brassicicola requires reactive oxygen species derived from NADPH oxidase. Physiol. Mol. Plant Pathol. 2015, 90, 49–56. [Google Scholar] [CrossRef]
- Cluzet, S.; Torregrosa, C.; Jacquet, C.; Lafitte, C.; Fournier, J.; Mercier, L.; Salamagne, S.; Briand, X.; Esquerre-Tugaye, M.T.; Dumas, B. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant Cell Environ. 2004, 27, 917–928. [Google Scholar] [CrossRef]
- El Modafar, C.; Elgadda, M.; El Boutachfaiti, R.; Abouraicha, E.; Zehhar, N.; Petit, E.; El Alaoui-Talibi, Z.; Courtois, J. Induction of natural defense accompanied by salicylic acid-dependent systemic acquired resistance in tomato seedlings in response to bioelicitors isolated from green algae. Sci. Hort. 2012, 138, 55–63. [Google Scholar] [CrossRef]
- Rivas-Garcia, T.; Hernandez-Montiel, L.G.; Murillo-Amador, B.; Nieto-Garibay, A.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G. Identification and characterization of Fusarium spp. from muskmelon in northwest Mexico. Biotecnia 2018, 20, 71–75. [Google Scholar]
- Zhou, Y.; Zhang, L.; Zeng, K. Efficacy of Pichia membranaefaciens combined with chitosan against Colletotrichum gloeosporioides in citrus fruits and possible modes of action. Biol. Control 2016, 96, 39–47. [Google Scholar] [CrossRef]
- Mattiuz, B.H.; Ducamp-Collin, M.N.; Mattiuz, C.F.M.; Vigneault, C.; Marques, K.M.; Sagoua, W.; Montet, D. Effect of propolis on postharvest control of anthracnose and quality parameters of ‘Kent’mango. Sci. Hort. 2015, 184, 160–168. [Google Scholar] [CrossRef]
- Levy, Y.; Benderly, M.; Cohen, Y.; Gisi, U.; Bassand, D. The joint action of fungicides in mixtures: Comparison of two methods for synergy calculation. EPPO Bulletin 1986, 16, 651–657. [Google Scholar] [CrossRef]
- Blackburn, D.; Shapiro-Ilan, D.I.; Adams, B.J. Biological control and nutrition: Food for thought. Biol. Control 2016, 97, 131–138. [Google Scholar] [CrossRef]
- Cao, S.; Yang, Z.; Hu, Z.; Zheng, Y. The effects of the combination of Pichia membranefaciens and BTH on controlling of blue mould decay caused by Penicillium expansum in peach fruit. Food Chem. 2011, 124, 991–996. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Meng, X.H.; Qin, G.Z.; Tian, S.P. Influences of preharvest spraying Cryptococcus laurentii combined with postharvest chitosan coating on postharvest diseases and quality of table grapes in storage. LWT-Food Sci. Technol. 2010, 43, 596–601. [Google Scholar] [CrossRef]
- De Costa, D.M.; Gunawardhana, H.M.D.M. Effects of sodium bicarbonate on pathogenicity of Colletotrichum musae and potential for controlling postharvest diseases of banana. Postharvest Biol. Technol. 2012, 68, 54–63. [Google Scholar] [CrossRef]
- Araújo, L.; Gonçalves, A.E.; Stadnik, M.J. Ulvan effect on conidial germination and appressoria formation of Colletotrichum gloeosporioides. Phytoparasitica 2014, 42, 631–640. [Google Scholar] [CrossRef]
- Gonçalves, A.E.; Stadnik, M.J. Interferência de ulvana no desenvolvimento e melanização de apressórios de Colletotrichum gloeosporioides. Trop. Plant Pathol. 2012, 37, 431–437. [Google Scholar] [CrossRef]
- de Freitas, M.B.; Ferreira, L.G.; Hawerroth, C.; Duarte, M.E.R.; Noseda, M.D.; Stadnik, M.J. Ulvan induce resistance against plant pathogenic fungi independently of their sulfation degree. Carbohydr. Polym. 2015, 133, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Paulert, R.; Talamini, V.; Cassolato, J.E.F.; Duarte, M.E.R.; Noseda, M.D.; Smania, A.; Stadnik, M.J. Effects of sulfated polysaccharide and alcoholic extracts from green seaweed Ulva fasciata on anthracnose severity and growth of common bean (Phaseolus vulgaris L.). J. Plant Dis. Protect. 2009, 116, 263–270. [Google Scholar] [CrossRef]
- Borsato, L.C.; Di Piero, R.M.; Stadnik, M.J. Mecanismos de defesa eliciados por ulvana contra Uromyces appendiculatus em três cultivares de feijoeiro. Trop. Plant Pathol. 2010, 35, 318–322. [Google Scholar] [CrossRef]
- Jaulneau, V.; Lafitte, C.; Jacquet, C.; Fournier, S.; Salamagne, S.; Briand, X.; Esquerre-Tugaye, M.T.; Dumas, B. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J. Biomed. Biotechnol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Paulert, R.; Ebbinghaus, D.; Urlass, C.; Moerschbacher, B.M. Priming of the oxidative burst in rice and wheat cell cultures by ulvan, a polysaccharide from green macroalgae, and enhanced resistance against powdery mildew in wheat and barley plants. Plant Pathol. 2010, 59, 634–642. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, M.; Żarowska, B.; Restuccia, C.; Cirvilleri, G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017, 61, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Garcia, T.; Hernandez-Montiel, L.G.; Murillo-Amador, B.; Nieto-Garibay, A.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G. Enhanced biocontrol of fruit rot on muskmelon by marine Debaryomyces hansenii and Stenotrophomonas rhizophila and their potential modes of action. Postharvest Biol. Technol. in press.
- Pech, J.C.; Bouzayen, M.; Latché, A. Climacteric fruit ripening: Ethylene-dependent and independent regulation of ripening pathways in melon fruit. Plant Sci. 2008, 175, 114–120. [Google Scholar] [CrossRef]
- Gupta, N.; Jain, S.K. Storage behavior of mango as affected by postharvest application of plant extracts and storage conditions. J. Food Sci. Technol. 2014, 51, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bruton, B.D.; Biles, C.L. Fusarium solani endo-polygalacturonase from decayed muskmelon fruit: Purification and characterization. Physiol. Mol. Plant Pathol. 1999, 54, 171–186. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 2nd ed.; Blackwell Pub.: Ames, IA, USA, 2006; p. 387. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).