Changes in Soil Microbiome Composition and Tomato Plant’s Physiological Response to Water Deficit and Excess
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Physiological Analyses
2.2.1. Leaf Gas Exchange
2.2.2. Chlorophyll Fluorescence
2.2.3. Leaf Color Determination
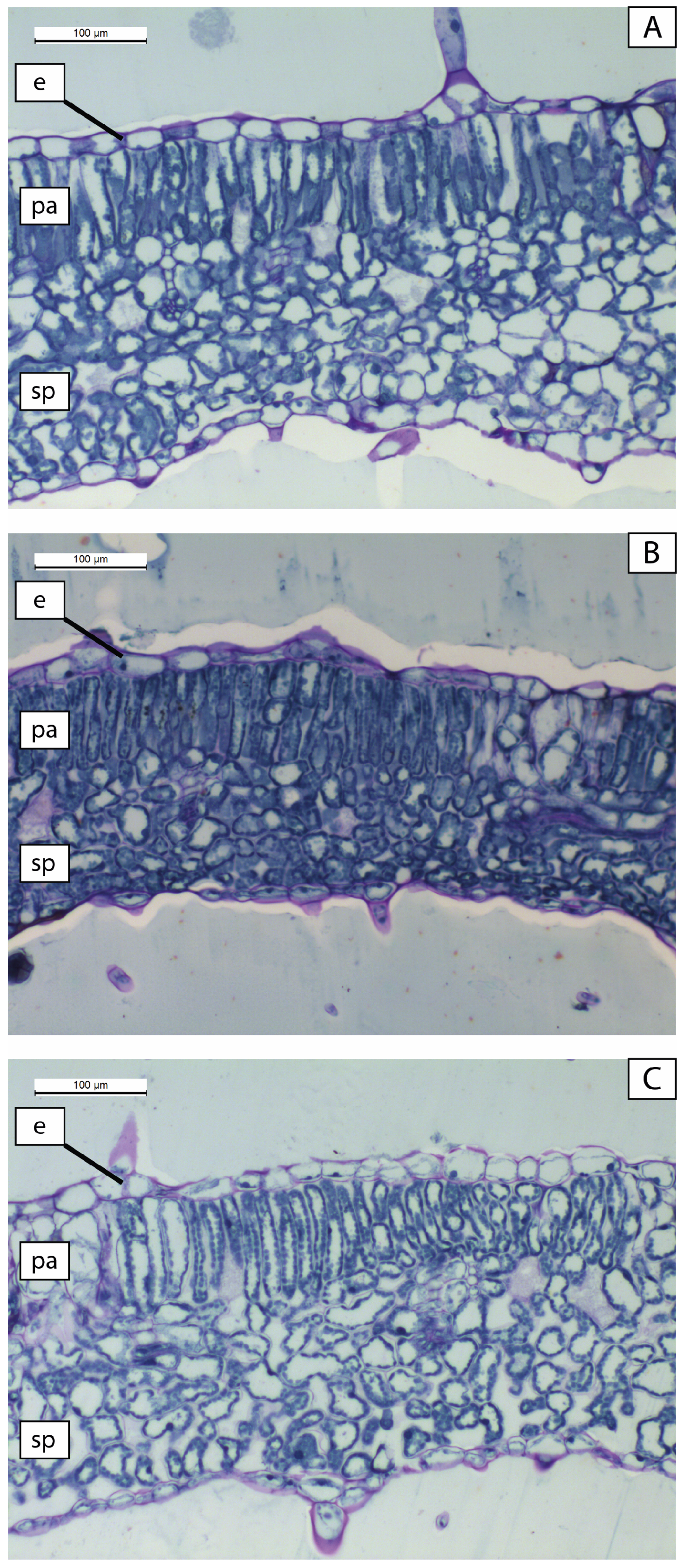
2.3. Leaf Anatomical Structure
2.4. Leaf Biochemical Characterization
2.4.1. Chlorophyll and Carotenoids Content
2.4.2. Total Polyphenol Content and DPPH Assay
2.4.3. Proline Extraction and Quantification
2.4.4. Lipid Peroxidation (TBARS Assay)
2.5. Fruit Yield and Size
2.6. Microbiological Analyses
2.6.1. Community-Level Physiological Profiling (CLPP)
2.6.2. DNA Extraction
2.6.3. Metataxonomic Profiling of the Bacterial Community of Soil
2.7. Soil Respiration
2.8. Statistical Analysis
3. Results and Discussions
3.1. Physiological Measurements
3.2. Leaf Anatomical Structure
3.3. Leaf Biochemical Characterization
3.4. Fruit Yield and Size
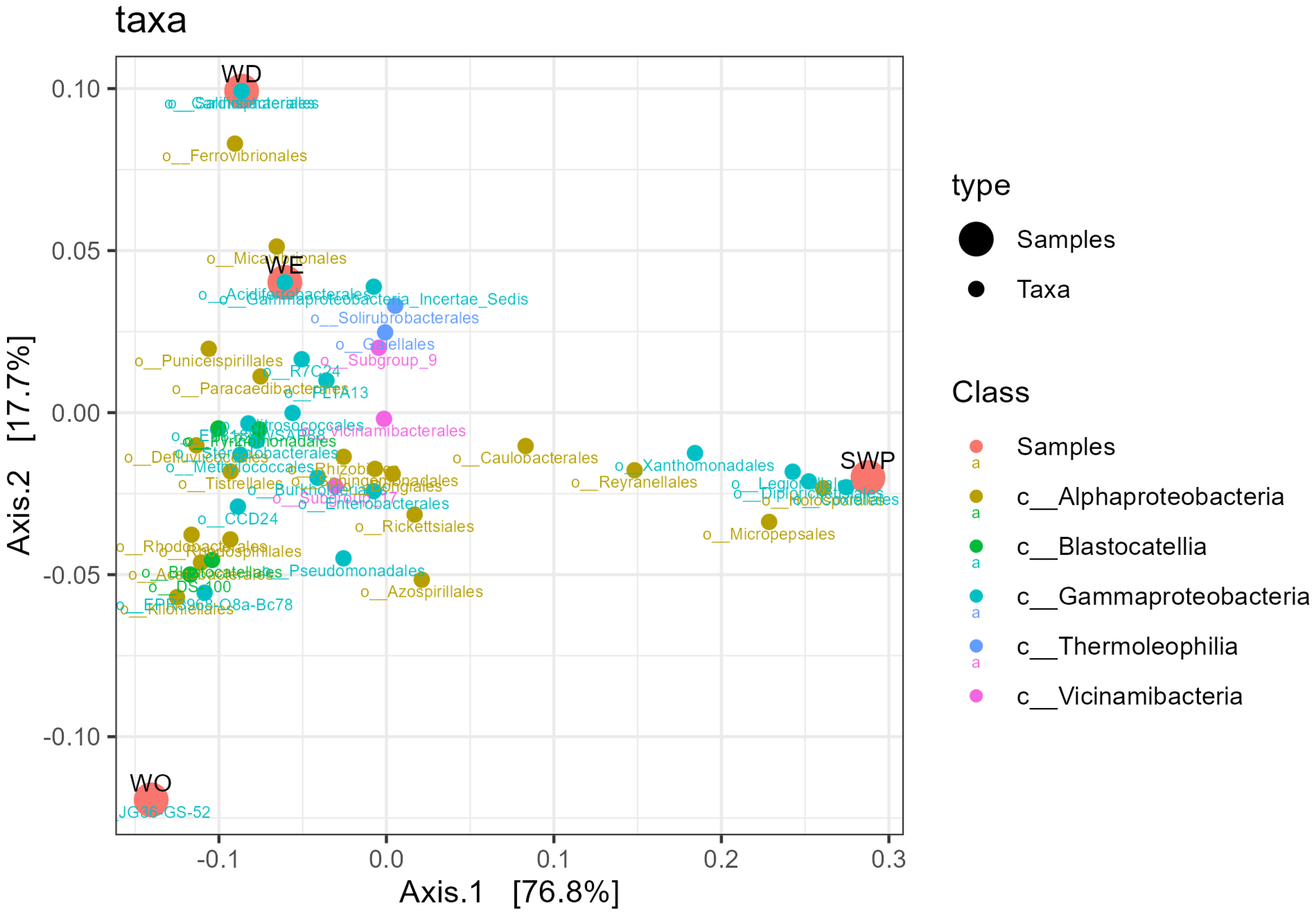
3.5. Soil Rhizosphere: Composition Diversity
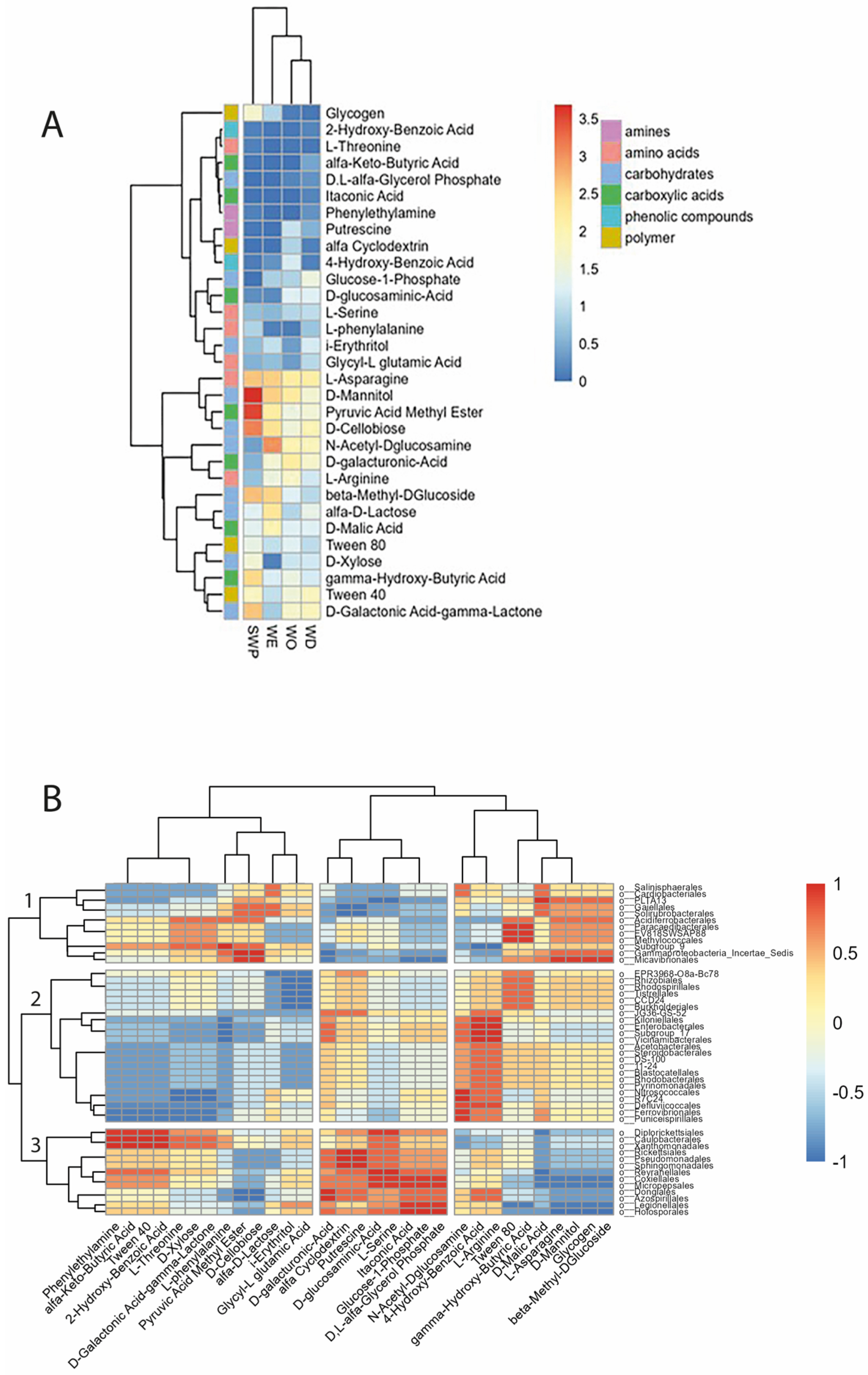
3.6. Soil Microbial Functionality
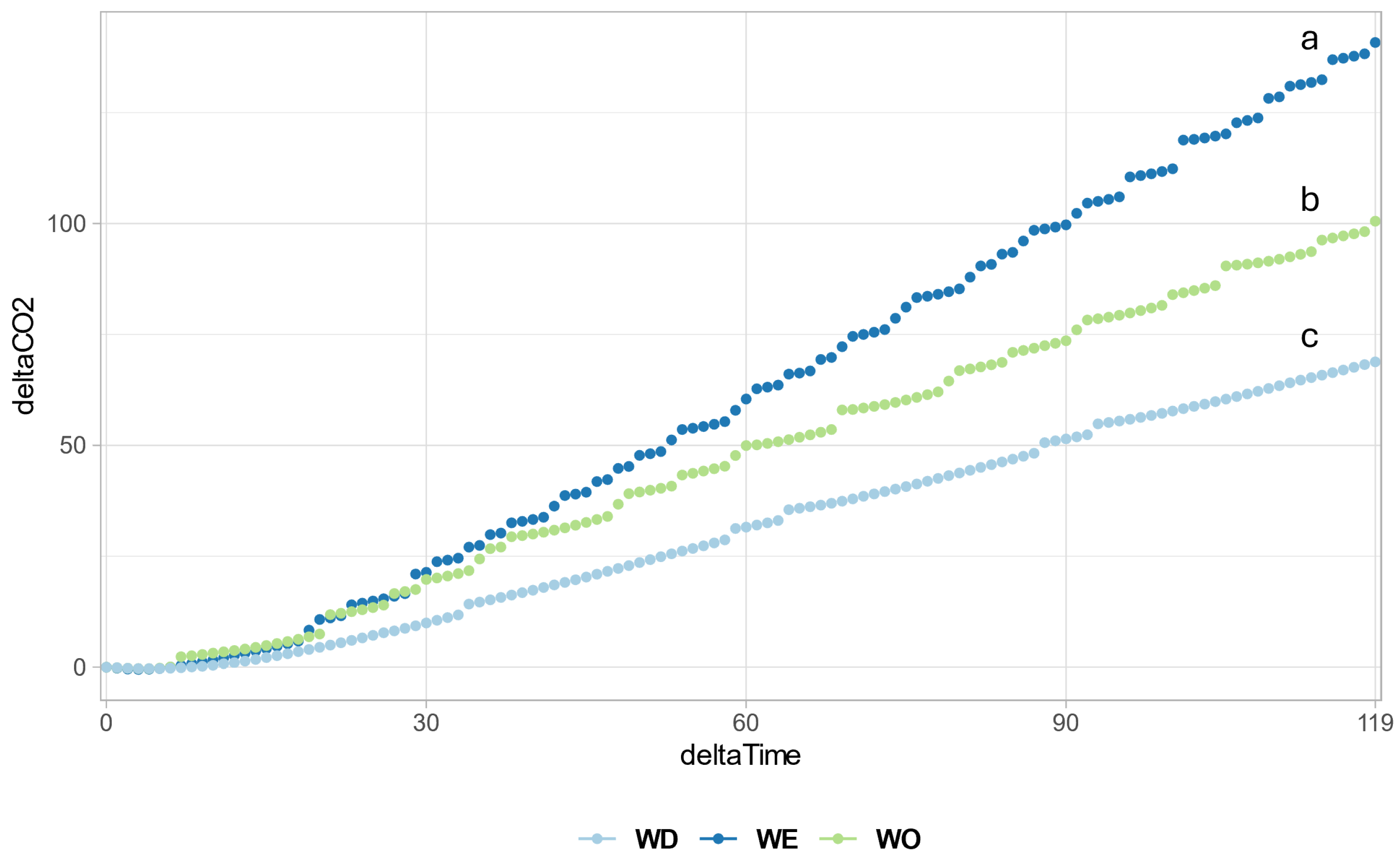
3.7. Soil Respiration
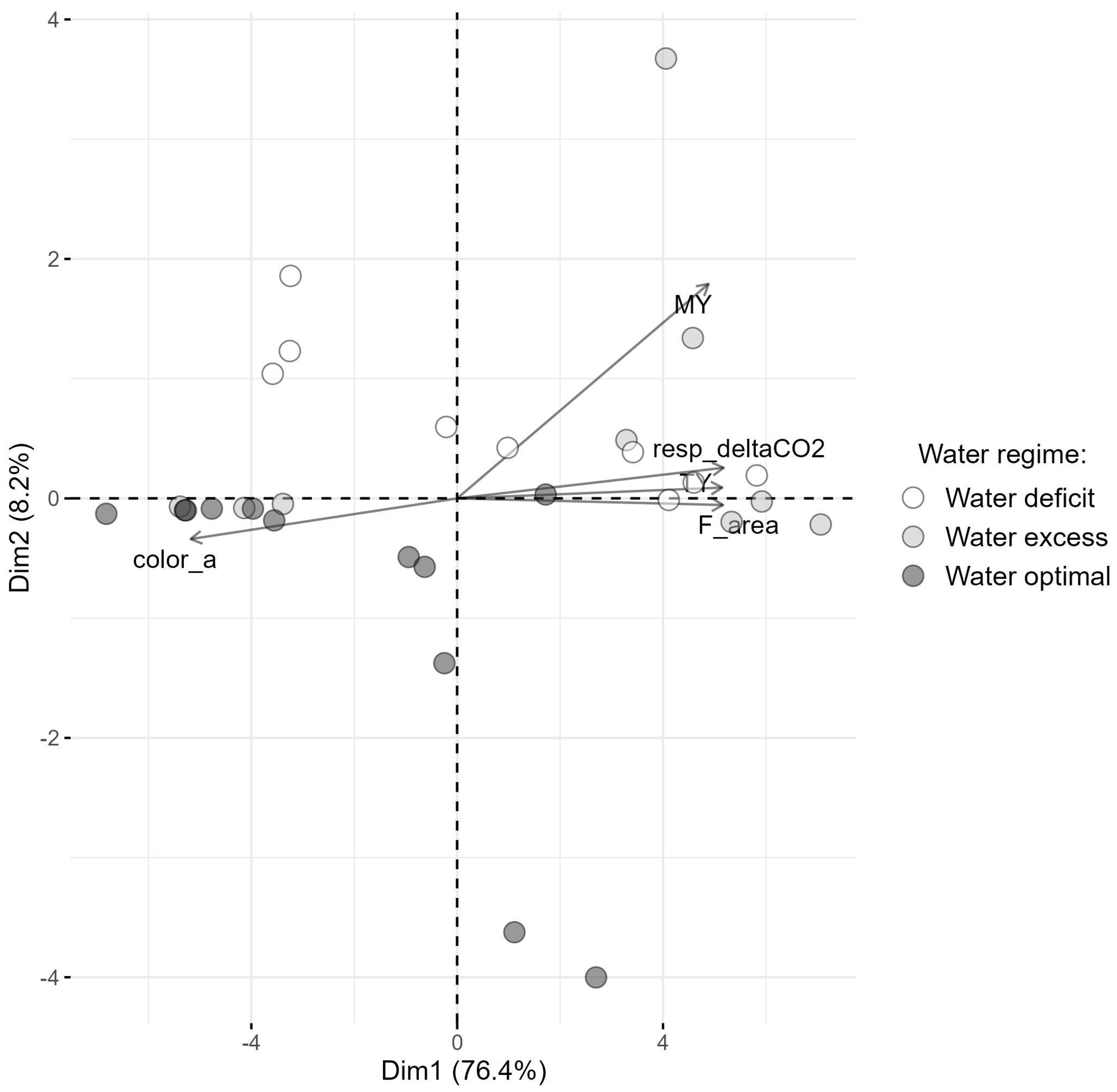
3.8. Correlation Between Rhizosphere Composition and Plant Physiology and Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yuan, X.; Li, S.; Chen, J.; Yu, H.; Yang, T.; Wang, C.; Huang, S.; Chen, H.; Ao, X. Impacts of Global Climate Change on Agricultural Production: A Comprehensive Review. Agronomy 2024, 14, 1360. [Google Scholar] [CrossRef]
- Kour, D.; Yadav, A.N. Bacterial Mitigation of Drought Stress in Plants: Current Perspectives and Future Challenges. Curr. Microbiol. 2022, 79, 248. [Google Scholar] [CrossRef]
- Livi-Bacci, M. The Crowded Rims of the Mediterranean Sea. Rend. Lincei 2024, 35, 343–350. [Google Scholar] [CrossRef]
- Vatter, J.; Wagnitz, P.; Schmiester, J.; Hernandez, E. Drought Risk: The Global Thirst for Water in the Era of Climate Crisis; Hartwig, L., Ed.; WWF Germany: Berlin, Germany, 2019. [Google Scholar]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A Review on Drought Stress in Plants: Implications, Mitigation and the Role of Plant Growth Promoting Rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Starke, R.; Bastida, F.; Abadía, J.; García, C.; Nicolás, E.; Jehmlich, N. Ecological and Functional Adaptations to Water Management in a Semiarid Agroecosystem: A Soil Metaproteomics Approach. Sci. Rep. 2017, 7, 10221. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental Stress Destabilizes Microbial Networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- FAO. Agricultural Production Statistics Agricultural Production Statistics 2000–2020. In FAOSTAT Analytical Briefs, No. 79.; FAO: Rome, Italy, 2022. [Google Scholar]
- Giuliani, M.M.; Nardella, E.; Gagliardi, A.; Gatta, G. Deficit Irrigation and Partial Root-Zone Drying Techniques in Processing Tomato Cultivated under Mediterranean Climate Conditions. Sustainability 2017, 9, 2197. [Google Scholar] [CrossRef]
- Du, Y.D.; Niu, W.Q.; Gu, X.B.; Zhang, Q.; Cui, B.J. Water- and Nitrogen-Saving Potentials in Tomato Production: A Meta-Analysis. Agric. Water Manag. 2018, 210, 296–303. [Google Scholar] [CrossRef]
- Cammarano, D.; Jamshidi, S.; Hoogenboom, G.; Ruane, A.C.; Niyogi, D.; Ronga, D. Processing Tomato Production Is Expected to Decrease by 2050 Due to the Projected Increase in Temperature. Nat. Food 2022, 3, 437–444. [Google Scholar] [CrossRef]
- Nemeskéri, E.; Neményi, A.; Bocs, A.; Pék, Z.; Helyes, L. Physiological Factors and Their Relationship with the Productivity of Processing Tomato under Different Water Supplies. Water 2019, 11, 586. [Google Scholar] [CrossRef]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of Deficit Irrigation on Biomass, Yield, Water Productivity and Fruit Quality of Processing Tomato under Semi-Arid Mediterranean Climate Conditions. Sci. Hortic. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Nangare, D.D.; Singh, Y.; Kumar, P.S.; Minhas, P.S. Growth, Fruit Yield and Quality of Tomato (Lycopersicon esculentum Mill.) as Affected by Deficit Irrigation Regulated on Phenological Basis. Agric. Water Manag. 2016, 171, 73–79. [Google Scholar] [CrossRef]
- Takács, S.; Pék, Z.; Csányi, D.; Daood, H.G.; Szuvandzsiev, P.; Palotás, G.; Helyes, L. Influence of Water Stress Levels on the Yield and Lycopene Content of Tomato. Water 2020, 12, 2165. [Google Scholar] [CrossRef]
- Tramblay, Y.; Somot, S. Future Evolution of Extreme Precipitation in the Mediterranean. Clim. Change 2018, 151, 289–302. [Google Scholar] [CrossRef]
- Karlen, D.L.; Sojka, R.E.; Robbins, M.L. Influence of Excess Soil-water and n Rates on Leaf Diffusive Resistance and Storage Quality of Tomato Fruit. Commun. Soil. Sci. Plant Anal. 1983, 14, 699–708. [Google Scholar] [CrossRef]
- He, D.; Gao, R.; Dong, H.; Liu, X.; Ren, L.; Wu, Q.; Yao, Q.; Zhu, H. Structure, Variation and Assembly Processes of Bacterial Communities in Different Root-Associated Niches of Tomato under Periodic Drought and Nitrogen Addition. Pedosphere 2024, 34, 892–904. [Google Scholar] [CrossRef]
- Gaete, A.; Pulgar, R.; Hodar, C.; Maldonado, J.; Pavez, L.; Zamorano, D.; Pastenes, C.; González, M.; Franck, N.; Mandakovic, D. Tomato Cultivars With Variable Tolerances to Water Deficit Differentially Modulate the Composition and Interaction Patterns of Their Rhizosphere Microbial Communities. Front. Plant Sci. 2021, 12, 688533. [Google Scholar] [CrossRef]
- Sillo, F.; Marino, G.; Franchi, E.; Haworth, M.; Zampieri, E.; Pietrini, I.; Fusini, D.; Mennone, C.; Centritto, M.; Balestrini, R. Impact of Irrigation Water Deficit on Two Tomato Genotypes Grown under Open Field Conditions: From the Root-Associated Microbiota to the Stress Responses. Ital. J. Agron. 2022, 17, 2130. [Google Scholar]
- Vurro, F.; Manfredi, R.; Bettelli, M.; Bocci, G.; Cologni, A.L.; Cornali, S.; Reggiani, R.; Marchetti, E.; Coppedè, N.; Caselli, S.; et al. In Vivo Sensing to Monitor Tomato Plants in Field Conditions and Optimize Crop Water Management. Precis. Agric. 2023, 24, 2479–2499. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer Netherlands: Dordrecht, The Netherlands, 2004. [Google Scholar]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999; Volume 198. [Google Scholar]
- Rodolfi, M.; Barbanti, L.; Giordano, C.; Rinaldi, M.; Fabbri, A.; Pretti, L.; Casolari, R.; Beghé, D.; Petruccelli, R.; Ganino, T. The Effect of Different Organic Foliar Fertilization on Physiological and Chemical Characters in Hop (Humulus lupulus L., Cv Cascade) Leaves and Cones. Appl. Sci. 2021, 11, 6778. [Google Scholar] [CrossRef]
- Dere, Ş.; Güneş, T.; Sivaci, R. Spectrophotometric Determination of Chlorophyll-A, B and Total Caretenoid Contents of Some Algae Species Using Different Solvents. Turk. J. Bot. 1998, 22, 13–18. [Google Scholar]
- Schiavon, M.; Dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Selenium Fertilization Alters the Chemical Composition and Antioxidant Constituents of Tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Potestio, S.; Giannelli, G.; Degola, F.; Vamerali, T.; Fragni, R.; Cocconi, E.; Sandei, L.; Visioli, G. Salt Stress Mitigation and Improvement in Fruit Nutritional Characteristics of Tomato Plants: New Opportunities from the Exploitation of a Halotorelant Agrobacterium Strain. Plant Stress. 2024, 13, 100558. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Marchioni, I.; Pistelli, L.; Ferri, B.; Copetta, A.; Ruffoni, B.; Pistelli, L.; Najar, B. Phytonutritional Content and Aroma Profile Changes During Postharvest Storage of Edible Flowers. Front. Plant Sci. 2020, 11, 590968. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.; Wang, D.; Wang, M.; Liao, C.; Yang, X.; Liu, F. Decoupled Linkage between Soil Carbon and Nitrogen Mineralization among Soil Depths in a Subtropical Mixed Forest. Soil. Biol. Biochem. 2017, 109, 135–144. [Google Scholar] [CrossRef]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a Prokaryotic Universal Primer for Simultaneous Analysis of Bacteria and Archaea Using Next-Generation Sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar]
- Robeson, M.S.; O’Rourke, D.R.; Kaehler, B.D.; Ziemski, M.; Dillon, M.R.; Foster, J.T.; Bokulich, N.A. RESCRIPt: Reproducible Sequence Taxonomy Reference Database Management. PLoS Comput. Biol. 2021, 17, e1009581. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhao, C.; Zhao, Z.; Yu, B.; Zhou, T. Soil Respiration and the Contribution of Root Respiration of Cotton (Gossypium hirsutum L.) in Arid Region. Acta Ecol. Sin. 2015, 35, 17–21. [Google Scholar] [CrossRef]
- Kassambara, A. _rstatix: Pipe-Friendly Framework for Basic Statistical Tests_. R Package Version 0.7.2. 2023. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 25 January 2025).
- Lenth, R. _emmeans: Estimated Marginal Means, aka Least-Squares Means_. R Package Version 1.11.0. 2023. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 25 January 2025).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks CA, USA, 2019. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Josse, J.; Husson, F. MissMDA: A Package for Handling Missing Values in Multivariate Data Analysis. J. Stat. Softw. 2016, 70, 1–31. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. GGally: Extension to “Ggplot2”. R Package Version 2.2.1. Available online: https://ggobi.github.io/ggally/authors.html (accessed on 29 January 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 29 January 2025).
- Chaves, M.M.; Pereira, J.S.; Maroco, J.; Rodrigues, M.L.; Ricardo, C.P.P.; Osório, M.L.; Carvalho, I.; Faria, T.; Pinheiro, C. How Plants Cope with Water Stress in the Field. Photosynthesis and Growth. Ann. Bot. 2002, 89, 907. [Google Scholar] [CrossRef]
- Patanè, C.; Corinzia, S.A.; Testa, G.; Scordia, D.; Cosentino, S.L. Physiological and Agronomic Responses of Processing Tomatoes to Deficit Irrigation at Critical Stages in a Semi-Arid Environment. Agronomy 2020, 10, 800. [Google Scholar] [CrossRef]
- Fiebig, A.; Dodd, I.C. Inhibition of Tomato Shoot Growth by Over-Irrigation Is Linked to Nitrogen Deficiency and Ethylene. Physiol. Plant 2016, 156, 70–83. [Google Scholar] [CrossRef]
- Ashraf, M.; Arfan, M. Gas Exchange Characteristics and Water Relations in Two Cultivars of Hibiscus Esculentus under Waterlogging. Biol. Plant 2005, 49, 459–462. [Google Scholar] [CrossRef]
- Panda, D.; Rao, D.N.; Sharma, S.G.; Strasser, R.J.; Sarkar, R.K. Submergence Effects on Rice Genotypes during Seedling Stage: Probing of Submergence Driven Changes of Photosystem 2 by Chlorophyll a Fluorescence Induction O-J-I-P Transients. Photosynthetica 2006, 44, 69–75. [Google Scholar] [CrossRef]
- Mihaljević, I.; Viljevac Vuletić, M.; Tomaš, V.; Horvat, D.; Zdunić, Z.; Vuković, D. Psii Photochemistry Responses to Drought Stress in Autochthonous and Modern Sweet Cherry Cultivars. Photosynthetica 2021, 59, 517–528. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A. Polyphasic Chlorophyll a Fluorescence Transient in Plants and Cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently Asked Questions about Chlorophyll Fluorescence, the Sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of γ-Tocopherol Methyl Transferase Gene in Transgenic Brassica Juncea Plants Alleviates Abiotic Stress: Physiological and Chlorophyll a Fluorescence Measurements. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Antunović Dunić, J.; Mlinarić, S.; Pavlović, I.; Lepeduš, H.; Salopek-Sondi, B. Comparative Analysis of Primary Photosynthetic Reactions Assessed by OJIP Kinetics in Three Brassica Crops after Drought and Recovery. Appl. Sci. 2023, 13, 3078. [Google Scholar] [CrossRef]
- Gomes, M.T.G.; da Luz, A.C.; dos Santos, M.R.; do Carmo Pimentel Batitucci, M.; Silva, D.M.; Falqueto, A.R. Drought Tolerance of Passion Fruit Plants Assessed by the OJIP Chlorophyll a Fluorescence Transient. Sci. Hortic. 2012, 142, 49–56. [Google Scholar] [CrossRef]
- Jedmowski, C.; Brüggemann, W. Imaging of Fast Chlorophyll Fluorescence Induction Curve (OJIP) Parameters, Applied in a Screening Study with Wild Barley (Hordeum Spontaneum) Genotypes under Heat Stress. J. Photochem. Photobiol. B 2015, 151, 153–160. [Google Scholar] [CrossRef]
- Sousaraei, N.; Mashayekhi, K.; Mousavizadeh, S.J.; Akbarpour, V.; Medina, J.; Aliniaeifard, S. Screening of Tomato Landraces for Drought Tolerance Based on Growth and Chlorophyll Fluorescence Analyses. Hortic. Environ. Biotechnol. 2021, 62, 521–535. [Google Scholar] [CrossRef]
- Meng, L.L.; Song, J.F.; Wen, J.; Zhang, J.; Wei, J.H. Effects of Drought Stress on Fluorescence Characteristics of Photosystem II in Leaves of Plectranthus Scutellarioides. Photosynthetica 2016, 54, 414–421. [Google Scholar] [CrossRef]
- Botyanszka, L.; Zivcak, M.; Chovancek, E.; Sytar, O.; Barek, V.; Hauptvogel, P.; Halabuk, A.; Brestic, M. Chlorophyll Fluorescence Kinetics May Be Useful to Identify Early Drought and Irrigation Effects on Photosynthetic Apparatus in Field-Grown Wheat. Agronomy 2020, 10, 1275. [Google Scholar] [CrossRef]
- Kołton, A.; Kȩska, K.; Czernicka, M. Selection of Tomato and Cucumber Accessions for Waterlogging Sensitivity Throughmorpho-Physiological Assessment at an Early Vegetative Stage. Agronomy 2020, 10, 1490. [Google Scholar] [CrossRef]
- Ihl, M.; Shene, C.; Scheuermann, E.; Bifani, V. Correlation for Pigment Content through Colour Determination Using Tristimulus Values in a Green Leafy Vegetable, Swiss Chard. J. Sci. Food Agric. 1994, 66, 527–531. [Google Scholar] [CrossRef]
- Madeira, A.C.; Ferreira, A.; De Varennes, A.; Vieira, M.I. SPAD Meter versus Tristimulus Colorimeter to Estimate Chlorophyll Content and Leaf Color in Sweet Pepper. Commun. Soil. Sci. Plant Anal. 2003, 34, 2461–2470. [Google Scholar] [CrossRef]
- Sam, O.; Jeréz, E.; Dell’Amico, J.; Ruiz-Sanchez, M.C. Water Stress Induced Changes in Anatomy of Tomato Leaf Epidermes. Biol. Plant 2000, 43, 275–277. [Google Scholar] [CrossRef]
- Scippa, G.S.; Di Michele, M.; Onelli, E.; Patrignani, G.; Chiatante, D.; Bray, E.A. The Histone-like Protein H1-S and the Response of Tomato Leaves to Water Deficit. J. Exp. Bot. 2004, 55, 99–109. [Google Scholar] [CrossRef]
- Hasanagić, D.; Koleška, I.; Kojić, D.; Vlaisavljević, S.; Janjić, N.; Kukavica, B. Long Term Drought Effects on Tomato Leaves: Anatomical, Gas Exchange and Antioxidant Modifications. Acta Physiol. Plant 2020, 42, 1–14. [Google Scholar] [CrossRef]
- Massimi, M. Tomato (Lycopersicon esculentum Mill.) Anatomical, Physiological, Biochemical and Production Responses to Drought Stress-A Mini-Review Essay. Int. J. Hortic. Sci. 2021, 27, 40–45. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.d.M.; Blasco, B.; Leyva, R.; Romero, L.; Ruiz, J.M. Antioxidant Response Resides in the Shoot in Reciprocal Grafts of Drought-Tolerant and Drought-Sensitive Cultivars in Tomato under Water Stress. Plant Sci. 2012, 188, 89–96. [Google Scholar] [CrossRef]
- Aghaie, P.; Hosseini Tafreshi, S.A.; Ebrahimi, M.A.; Haerinasab, M. Tolerance Evaluation and Clustering of Fourteen Tomato Cultivars Grown under Mild and Severe Drought Conditions. Sci. Hortic. 2018, 232, 1–12. [Google Scholar] [CrossRef]
- Obadi, A.; Alharbi, A.; Alomran, A.; Alghamdi, A.G.; Louki, I.; Alkhasha, A. Effect of Biochar Application on Morpho-Physiological Traits, Yield, and Water Use Efficiency of Tomato Crop under Water Quality and Drought Stress. Plants 2023, 12, 2355. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive Oxygen Species Homeostasis and Signalling during Drought and Salinity Stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar]
- Oukarroum, A.; Schansker, G.; Strasser, R.J. Drought Stress Effects on Photosystem i Content and Photosystem II Thermotolerance Analyzed Using Chl a Fluorescence Kinetics in Barley Varieties Differing in Their Drought Tolerance. Physiol. Plant 2009, 137, 188–199. [Google Scholar] [CrossRef]
- Vicente, O.; Boscaiu, M. Flavonoids: Antioxidant Compounds for Plant Defence... and for a Healthy Human Diet. Not. Bot. Horti Agrobot. Cluj. Napoca 2018, 46, 992. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants: An Overview. Plant Metab. Regul. Under Environ. Stress 2018, 153–167. [Google Scholar] [CrossRef]
- Moles, T.M.; Mariotti, L.; De Pedro, L.F.; Guglielminetti, L.; Picciarelli, P.; Scartazza, A. Drought Induced Changes of Leaf-to-Root Relationships in Two Tomato Genotypes. Plant Physiol. Biochem. 2018, 128, 24–31. [Google Scholar] [CrossRef]
- Rigano, M.M.; Arena, C.; Di Matteo, A.; Sellitto, S.; Frusciante, L.; Barone, A. Eco-Physiological Response to Water Stress of Drought-Tolerant and Drought-Sensitive Tomato Genotypes. Plant Biosyst. 2016, 150, 682–691. [Google Scholar] [CrossRef]
- Tahi, H.; Wahbi, S.; El Modafar, C.; Aganchich, A.; Serraj, R. Changes in Antioxidant Activities and Phenol Content in Tomato Plants Subjected to Partial Root Drying and Regulated Deficit Irrigation. Plant Biosyst. 2008, 142, 550–562. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Rubio-Wilhelmi, M.; Cervilla, L.M.; Blasco, B.; Rios, J.J.; Rosales, M.A.; Romero, L.; Ruiz, J.M. Genotypic Differences in Some Physiological Parameters Symptomatic for Oxidative Stress under Moderate Drought in Tomato Plants. Plant Sci. 2010, 178, 30–40. [Google Scholar] [CrossRef]
- Taylor, C.B. Proline and Water Deficit: Ups, Downs, Ins, and Outs. Plant Cell 1996, 8, 1221. [Google Scholar] [CrossRef][Green Version]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal 2013, 19, 998–1011. [Google Scholar]
- Alvarez, M.E.; Savouré, A.; Szabados, L. Proline Metabolism as Regulatory Hub. Trends Plant Sci. 2022, 27, 39–55. [Google Scholar]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [PubMed]
- Jureková, Z.; Németh-Molnár, K.; Paganová, V. Physiological Responses of Six Tomato (Lycopersicon esculentum Mill.) Cultivars to Water Stress. J. Hortic. For. 2011, 3, 294–300. [Google Scholar]
- Patanè, C.; Scordia, D.; Testa, G.; Cosentino, S.L. Physiological Screening for Drought Tolerance in Mediterranean Long-Storage Tomato. Plant Sci. 2016, 249, 25–34. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Gomes, F.P.; Oliva, M.A.; Mielke, M.S.; Almeida, A.A.F.; Aquino, L.A. Osmotic Adjustment, Proline Accumulation and Cell Membrane Stability in Leaves of Cocos Nucifera Submitted to Drought Stress. Sci. Hortic. 2010, 126, 379–384. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging Causes Early Modification in the Physiological Performance, Carotenoids, Chlorophylls, Proline, and Soluble Sugars of Cucumber Plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef]
- Tian, L.; Bi, W.; Liu, X.; Sun, L.; Li, J. Effects of Waterlogging Stress on the Physiological Response and Grain-Filling Characteristics of Spring Maize (Zea mays L.) under Field Conditions. Acta Physiol. Plant 2019, 41, 63. [Google Scholar] [CrossRef]
- Yin, J.; Niu, L.; Li, Y.; Song, X.; Ottosen, C.O.; Wu, Z.; Jiang, F.; Zhou, R. The Effects of Waterlogging Stress on Plant Morphology, Leaf Physiology and Fruit Yield in Six Tomato Genotypes at Anthesis Stage. Veg. Res. 2023, 3, 31. [Google Scholar] [CrossRef]
- Liu, C.; Lan, C.; Li, C.; Li, C.; Huang, J. Exogenous Spermidine and Calcium Alleviate Waterlogging Stress in Cherry Tomato at the Seedling Stage. Sci. Hortic. 2023, 307, 111504. [Google Scholar] [CrossRef]
- Fujita, M.; Hasanuzzaman, M. Approaches to Enhancing Antioxidant Defense in Plants. Antioxidants 2022, 11, 925. [Google Scholar] [CrossRef]
- Kuscu, H.; Turhan, A.; Ozmen, N.; Aydinol, P.; Demir, A.O. Optimizing Levels of Water and Nitrogen Applied through Drip Irrigation for Yield, Quality, and Water Productivity of Processing Tomato (Lycopersicon esculentum Mill.). Hortic. Environ. Biotechnol. 2014, 55, 103–114. [Google Scholar] [CrossRef]
- Patanè, C.; Siah, S.; Pellegrino, A.; Cosentino, S.L.; Siracusa, L. Fruit Yield, Polyphenols, and Carotenoids in Long Shelf-Life Tomatoes in Response to Drought Stress and Rewatering. Agronomy 2021, 11, 1943. [Google Scholar] [CrossRef]
- Cerasola, V.A.; Perlotti, L.; Pennisi, G.; Orsini, F.; Gianquinto, G. Potential Use of Superabsorbent Polymer on Drought-Stressed Processing Tomato (Solanum Lycopersicum L.) in a Mediterranean Climate. Horticulturae 2022, 8, 718. [Google Scholar] [CrossRef]
- Millones-Chanamé, C.E.; de Oliveira, A.M.S.; de Castro, E.M.; Maluf, W.R. Inheritance of Blossom End Rot Resistance Induced by Drought Stress and of Associated Stomatal Densities in Tomatoes. Euphytica 2019, 215, 120. [Google Scholar]
- Molefe, R.R.; Amoo, A.E.; Babalola, O.O. Communication between Plant Roots and the Soil Microbiome; Involvement in Plant Growth and Development. Symbiosis 2023, 90, 231–239. [Google Scholar] [CrossRef]
- Mhete, M.; Eze, P.N.; Rahube, T.O.; Akinyemi, F.O. Soil Properties Influence Bacterial Abundance and Diversity under Different Land-Use Regimes in Semi-Arid Environments. Sci. Afr. 2020, 7, e00246. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere Bacteriome Structure and Functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Kalam, S.; Basu, A.; Ahmad, I.; Sayyed, R.Z.; El-Enshasy, H.A.; Dailin, D.J.; Suriani, N.L. Recent Understanding of Soil Acidobacteria and Their Ecological Significance: A Critical Review. Front. Microbiol. 2020, 11, 580024. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Tang, F.; Wang, J.; Dong, J.; Xing, J.; Shi, F. Drought-Induced Recruitment of Specific Root-Associated Bacteria Enhances Adaptation of Alfalfa to Drought Stress. Front. Microbiol. 2023, 14, 1114400. [Google Scholar] [CrossRef]
- Hazarika, S.N.; Thakur, D. Actinobacteria. Benef. Microbes Agro-Ecol. Bact. Fungi 2020, 21, 443–476. [Google Scholar] [CrossRef]
- Simmons, T.; Styer, A.B.; Pierroz, G.; Gonçalves, A.P.; Pasricha, R.; Hazra, A.B.; Bubner, P.; Coleman-Derr, D. Drought Drives Spatial Variation in the Millet Root Microbiome. Front. Plant Sci. 2020, 11, 518243. [Google Scholar] [CrossRef]
- Mukhtar, S.; Mehnaz, S.; Malik, K.A. Microbial Diversity in the Rhizosphere of Plants Growing under Extreme Environments and Its Impact on Crop Improvement. Environ. Sustain. 2019, 2, 329–338. [Google Scholar] [CrossRef]
- Huber, K.J.; Vieira, S.; Sikorski, J.; Wüst, P.K.; Fösel, B.U.; Gröngröft, A.; Overmann, J. Differential Response of Acidobacteria to Water Content, Soil Type, and Land Use During an Extended Drought in African Savannah Soils. Front. Microbiol. 2022, 13, 750456. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 Efflux from Soil and Review of Partitioning Methods. Soil. Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Chen, L.F.; He, Z.B.; Wu, X.R.; Du, J.; Zhu, X.; Lin, P.F.; Tian, Q.Y.; Kong, J.Q. Linkages between Soil Respiration and Microbial Communities Following Afforestation of Alpine Grasslands in the Northeastern Tibetan Plateau. Appl. Soil. Ecol. 2021, 161, 103882. [Google Scholar] [CrossRef]





| Organic matter | 19.6 g·kg−1 |
| pH | 8.2 |
| Total N | 1.14 g·kg−1 |
| Assimilable P (P2O5) | 26 mg·kg−1 |
| Exchangeable K | 0.3 meq kg−1 |
| Irrigation Level | Upper Epidermis Cell Area (µm2) | Palisade Tissue (%) | Spongy Tissue (%) |
|---|---|---|---|
| WD | 634.68 ± 52.35 a | 32.12 ± 0.75 b | 67.88 ± 0.75 b |
| WO | 405.04 ± 48.59 b | 27.72 ± 0.86 c | 72.29 ± 0.86 a |
| WE | 619.73 ± 78.97 a | 37.98 ± 1.31 a | 62.02 ± 1.31 c |
| Irrigation | Chl a | Chl b | Chl tot | Car |
|---|---|---|---|---|
| WD | 7.27 ± 0.37 b | 2.78 ± 0.16 b | 10.05 ± 0.53 b | 2.02 ± 0.06 b |
| WO | 7.87 ± 0.19 b | 2.87 ± 0.09 b | 10.74 ± 0.28 b | 2.19 ± 0.07 ab |
| WE | 9.58 ± 0.27 a | 3.46 ± 0.09 a | 13.03 ± 0.32 a | 2.37 ± 0.08 a |
| Irrigation | TPC | DPPH | Proline | MDA |
|---|---|---|---|---|
| WD | 2.98 ± 0.21 a | 26.39 ± 1.52 b | 0.85 ± 0.03 a | 3.84 ± 0.09 a |
| WO | 2.56 ± 0.24 ab | 40.53 ± 3.96 a | 0.47 ± 0.07 b | 3.15 ± 0.30 a |
| WE | 1.88 ± 0.07 b | 41.50 ± 0.10 a | 0.81 ± 0.06 a | 3.42 ± 0.22 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galaverni, M.; Hadj Saadoun, J.; Ganino, T.; Levante, A.; Rodolfi, M.; Marchioni, I.; Bettera, L.; Beghè, D.; Lazzi, C. Changes in Soil Microbiome Composition and Tomato Plant’s Physiological Response to Water Deficit and Excess. Agronomy 2025, 15, 915. https://doi.org/10.3390/agronomy15040915
Galaverni M, Hadj Saadoun J, Ganino T, Levante A, Rodolfi M, Marchioni I, Bettera L, Beghè D, Lazzi C. Changes in Soil Microbiome Composition and Tomato Plant’s Physiological Response to Water Deficit and Excess. Agronomy. 2025; 15(4):915. https://doi.org/10.3390/agronomy15040915
Chicago/Turabian StyleGalaverni, Martina, Jasmine Hadj Saadoun, Tommaso Ganino, Alessia Levante, Margherita Rodolfi, Ilaria Marchioni, Luca Bettera, Deborah Beghè, and Camilla Lazzi. 2025. "Changes in Soil Microbiome Composition and Tomato Plant’s Physiological Response to Water Deficit and Excess" Agronomy 15, no. 4: 915. https://doi.org/10.3390/agronomy15040915
APA StyleGalaverni, M., Hadj Saadoun, J., Ganino, T., Levante, A., Rodolfi, M., Marchioni, I., Bettera, L., Beghè, D., & Lazzi, C. (2025). Changes in Soil Microbiome Composition and Tomato Plant’s Physiological Response to Water Deficit and Excess. Agronomy, 15(4), 915. https://doi.org/10.3390/agronomy15040915










