Abstract
β-cypermethrin (BCP) is a broad-spectrum insecticide known for its rapid efficacy. However, it is highly toxic to non-target organisms such as bees and fish, and its effectiveness is limited by a short duration of action. Improving the release profile of BCP is essential for reducing its environmental toxicity while preserving its effectiveness. In this study, hollow mesoporous silica nanoparticles (HMSNs) were synthesized using a self-templating method, and BCP-loaded HMSNs were prepared through physical adsorption. The structural and physicochemical properties of the nanoparticles were characterized using scanning electron microscopy (SEM), transmission electron microscopy (TEM), nitrogen adsorption–desorption analysis, Fourier transform infrared (FT-IR) spectroscopy, dynamic light scattering (DLS), and thermogravimetric analysis (TGA). The BCP release profile was assessed using the dialysis bag method. The results showed that the synthesized nanoparticles exhibited uniform morphology, thin shells, and large internal cavities. The HMSNs had a pore size of 3.09 nm, a specific surface area of 1318 m2·g−1, a pore volume of 1.52 cm3·g−1, and an average particle size of 183 nm. TEM, FT-IR, and TGA analyses confirmed the successful incorporation of BCP into the HMSNs, achieving a drug loading efficiency of 32.53%. The BCP-loaded nanoparticles exhibited sustained-release properties, with an initial burst followed by gradual release, extending efficacy for 30 days. Safety evaluations revealed minimal toxicity to maize seedlings, confirming the biocompatibility of the nanoparticles. These findings indicate that BCP-loaded HMSNs can enhance the efficacy of BCP while reducing its environmental toxicity, providing a biocompatible and environmentally friendly solution for pest control.
1. Introduction
Pesticides play a pivotal role in modern agriculture, safeguarding crops from pests and diseases. However, their widespread use has led to significant challenges, including high toxicity to non-target organisms, rapid degradation, and short efficacy periods, which contribute to environmental and ecological risks [1,2]. These issues underscore the need for innovative solutions to improve pesticide performance while minimizing their environmental impact [3]. Nanotechnology has emerged as a promising approach, revolutionizing pesticide delivery systems by enabling precise control over release mechanisms and minimizing adverse effects [4,5,6].
Recent advancements in nanotechnology have unlocked the potential of nanomaterials across diverse fields, including medicine, catalysis, and controlled drug delivery [7,8,9,10]. In agriculture, nanomaterials have gained traction for their ability to improve pesticide efficiency and reduce ecological harm. By leveraging mechanisms such as electrostatic interactions and covalent bonding, nanomaterials facilitate controlled and sustained pesticide release. For instance, cationic nanomaterials can gradually release anionic pesticide molecules as their charges weaken [11], while covalent bonds enable precise release upon cleavage [12]. These advanced systems improve pesticide stability and extend efficacy.
Among nanomaterials, mesoporous silica stands out as a highly versatile carrier due to its tunable pore size (2–50 nm), large surface area, excellent stability, and biocompatibility [13]. First synthesized in the 1990s [14,15], materials such as Mobil Composition of Matter No. 41 (MCM-41) and Santa Barbara Amorphous-15 (SBA-15) have since been developed for applications in agriculture, catalysis, and controlled release [16,17]. Their ease of functionalization further enhances their potential for pesticide delivery. Mesoporous silica nanoparticles (MSNs) provide additional advantages, offering tailored delivery through mesoporous channels. For example, Cao et al. [18] developed double-shelled hollow mesoporous silica (HMSNs) for loading the fungicide pyraclostrobin, achieving initial burst release followed by sustained release to effectively combat fungal infections. Cao et al. [19,20] further designed pH-sensitive (Aba@HMS@P) and temperature-sensitive (THI@HMS@P) MSNs to protect active ingredients from UV degradation and improve adhesion to crop leaves. Later, Liang et al. [21] created stimuli-responsive MSNs functionalized with starch, which provided photodegradation protection and enzyme-triggered release, effectively extending pest control durations. Recent studies have demonstrated the application of HMSNs in agriculture, such as Cao et al. [18] who used HMSNs to deliver pyraclostrobin for sustained release and improved antifungal activity. Similarly, Wang et al. [22] explored HMSNs for spirotetramat delivery, enhancing uptake and translocation in plants. These systems have demonstrated the ability to stabilize active ingredients, enhance release durations, and minimize environmental impact [23,24,25].
Mesoporous silica also plays a crucial role in agriculture, particularly for β-cypermethrin (BCP), a widely used pyrethroid insecticide. Despite its broad-spectrum activity and rapid action, BCP’s short efficacy period and high toxicity to non-target organisms pose significant challenges. However, BCP is selected for its unique combination of effectiveness against a wide range of pests, rapid action, and cost-effectiveness. Studies have shown that BCP undergoes significant environmental loss, primarily through mechanisms such as volatilization and photodegradation, which reduce its efficacy and increase environmental pollution [26,27,28]. This loss also contributes to decreased field efficiency, necessitating frequent reapplication and increasing both producer costs and environmental risks. While alternatives such as chlorpyrifos, deltamethrin, and malathion offer different benefits, they also come with their own set of challenges. Chlorpyrifos, for instance, has been linked to long-term environmental persistence and toxicity to aquatic life, while deltamethrin, though effective, can cause significant harm to beneficial insects such as pollinators [29,30]. Malathion, on the other hand, has a relatively higher toxicity to mammals, making it less ideal for sustainable pest management [31]. In contrast, BCP’s rapid degradation in the environment may reduce the long-term ecological impact, though its short efficacy period and toxicity to non-target organisms remain concerns [29]. Efforts to address these issues include Wang et al. [32], who developed biodegradable composite microcapsules (PBS/PLA 70:30) achieving 85.06% azoxystrobin and 90.18% difenoconazole encapsulation efficiency with 25-day sustained release, and Zou et al. [33], who demonstrated that HMSNs-based microcapsules could enhance BCP release precision (51% loading capacity, 82% cumulative release over 96 h) while reducing environmental toxicity (15-fold higher LC50 in zebrafish compared to conventional formulations).
Building on previous advancements, this study introduces several innovations to optimize the controlled release of BCP, providing a unique perspective on balancing environmental sustainability with agricultural efficiency. Unlike earlier studies that focused on specific carrier materials or single environmental triggers, we employed HMSNs synthesized via a self-templating method, using solid silica as a template and a sodium carbonate-mediated etching process. This approach ensures a uniform pore structure and high surface area, both essential for controlled release. BCP was loaded via physical adsorption, a straightforward method that avoids complex chemical modifications and preserves the carrier’s stability. Through comprehensive characterization using scanning electron microscopy (SEM), transmission electron microscopy (TEM), nitrogen adsorption–desorption analysis, Fourier transform infrared (FT-IR) spectroscopy, and thermogravimetric analysis (TGA), we demonstrate the ability of HMSNs to achieve controlled and prolonged release, reducing environmental impact while improving pest management efficiency. In this study, we developed and characterized HMSNs for encapsulation and sustained release of BCP, aiming to improve its agronomic efficiency and reduce environmental impacts. This approach may offer a sustainable alternative for pest control, minimizing the need for frequent reapplications and improving the safety of pesticide use in agriculture.
2. Materials and Methods
2.1. Materials and Instruments
The materials used in this study were as follows: β-cypermethrin (BCP, 95%, Anhui Fengle Agrochemical Co., Ltd., Hefei City, China), tetraethyl orthosilicate (TEOS), anhydrous ethanol, 25–28% ammonia solution, anhydrous sodium carbonate, N,N-dimethylformamide (DMF, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China), 36–38% hydrochloric acid (Yongfei Ltd., Huzhou City, China), dialysis bags (molecular weight cut-off 8000–14,000, Beijing Suolaibao Technology Co., Ltd., Beijing, China), dichloromethane, cetyltrimethylammonium bromide (CTAB), cyclohexane (Tianjin Fuchen Chemical Reagent Co., Ltd., Tianjin, China), and 4.5% BCP (Hebei Weiyuan Biochemical Co., Ltd., Shijiazhuang, China). Deionized water was used throughout the experiment.
The instruments employed in this study included a PS-100AL power-adjustable ultrasonic cleaner (Beijing Jinfu Renhao Technology Development Co., Ltd., Beijing, China), a constant-temperature water bath (Shanghai Yuhua Instruments Co., Ltd., Shanghai, China), an overhead stirrer (Shanghai Huxiang Industrial Co., Ltd., Shanghai, China), an H/T12MM desktop high-speed centrifuge (Hunan Hexi Instrument Equipment Co., Ltd., Changsha, China), a UV-1300 UV/visible spectrophotometer (Shanghai Meixi Instrument Co., Ltd., Shanghai, China), a D2PHASER X-ray diffractometer (Bruker, Billerica, MA, USA), an LSM 510 laser-scanning microscope (Carl Zeiss AG, Oberkochen, Germany), a JEM 2100F transmission electron microscope (Japan Electronics Co., Ltd., Akishima, Tokyo, Japan), an FT-IR spectrometer (Perkin Elmer Co., Ltd., Waltham, MA, USA), a Conta Autosorb-iQ specific surface area analyzer (Conta Instruments, Inc., Chatsworth, CA, USA), a TGA/DSC3+ simultaneous thermal analyzer (Mettler-Toledo Instruments Ltd., Greifensee, Switzerland), and a DynaPro NanoStar (Wyatt Technology, Santa Barbara, CA, USA).
2.2. Preparation of HMSNs
To prepare the HMSNs, 60 mL of anhydrous ethanol, 120 mL of deionized water, and 0.35 g of CTAB were mixed with an appropriate amount of cyclohexane and stirred thoroughly. Then, 2 mL of TEOS and 2 mL of ammonia solution were added to the mixture, which was stirred and allowed to react at room temperature. After the reaction was complete, the mixture was centrifuged and washed three times with anhydrous ethanol. The resulting precipitate was dried to obtain solid silica.
The solid silica was subsequently converted into HMSNs through selective etching, as described by Fang et al. [34]. Initially, an appropriate amount of solid silica was ultrasonically dispersed in 240 mL of deionized water. Then, 2.12 g of anhydrous sodium carbonate was added, and the mixture was heated and stirred at 65 °C for 5 or 7 h. The suspension was centrifuged, washed with deionized water, and redispersed in 240 mL of anhydrous ethanol containing hydrochloric acid. This mixture was stirred at 60 °C for 2 h to remove the template. The final HMSN product was obtained by washing and drying the precipitate (Figure 1).

Figure 1.
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images of hollow mesoporous silica nanoparticles (HMSNs) and β-cypermethrin-loaded hollow mesoporous silica nanoparticles (BCP/HMSNs) synthesized under different conditions. TEM image of HMSNs synthesized using water as the etching agent with anhydrous sodium carbonate at 65 °C for 5 h (A). TEM images of HMSNs synthesized by selective etching with anhydrous sodium carbonate for 7 h (B1,B2). SEM (C1) and TEM (C2) images of HMSNs synthesized by selective etching with anhydrous sodium carbonate for 5 h. SEM (D1) and TEM (D2) images of BCP/HMSNs synthesized by selective etching with anhydrous sodium carbonate for 5 h.
2.3. Preparation of β-Cypermethrin-Loaded HMSNs (BCP/HMSNs)
The BCP into HMSNs was achieved through physical adsorption. In this process, 50 mg of HMSNs were dispersed in dichloromethane using ultrasonic treatment. An appropriate amount of BCP was then added to the dispersion, which was stirred at 25 °C for 12 h. The mixture was then centrifuged to remove the supernatant, and the resulting precipitate was dried to obtain the BCP-loaded HMSNs (BCP/HMSNs).
2.4. Characterization of Nanoparticles
2.4.1. Morphology
The morphology and structural features of HMSNs and BCP/HMSNs were analyzed using SEM and TEM. SEM was performed at an accelerating voltage of 30 keV to observe the surface morphology of the nanoparticles. Samples were mounted on a sample holder using conductive adhesive, coated with a thin layer of gold for 30 s using an ion sputtering instrument, and subsequently imaged. TEM was performed at an accelerating voltage of 200 keV to analyze the internal structure and size distribution of the nanoparticles. For TEM analysis, samples were dispersed in absolute ethanol at a concentration of 2 mg·mL−1, and a 3–5 μL aliquot was deposited on a copper grid, dried in a desiccator to prevent contamination, and imaged under the microscope.
2.4.2. Specific Surface Area Determination
The nanoparticles were degassed at 200 °C for 4 h, and nitrogen was used as the analytical gas to measure the saturation pressure. The adsorption–desorption isotherm curve was obtained by measuring the adsorption volumes under various pressures, and the specific surface area and pore size were calculated. The Brunauer–Emmett–Teller (BET) equation was used to determine the specific surface area, while the Barrett–Joyner–Halenda (BJH) method was applied to calculate the pore size and pore size distribution.
2.4.3. HMSNs Size Measurement
The particle size of HMSNs and BCP/HMSNs was determined via dynamic light scattering (DLS) with a gallium-arsenide diode laser of 658 nm emission. Aqueous solutions of 100 mg L−1 nanoparticles were prepared for analysis.
2.4.4. FT-IR Spectroscopy
FT-IR spectral analysis was performed using the potassium bromide pellet method. Appropriate amounts of BCP, HMSNs, and BCP/HMSNs were sampled, and spectra were recorded over a wavelength range of 4000–400 cm−1.
2.4.5. TGA
Thermal stability was evaluated using a TGA/DSC3+ synchronous thermal analyzer. BCP, HMSNs, and BCP/HMSNs were analyzed within the temperature range of 20–800 °C at a heating rate of 10 °C·min−1 in a nitrogen atmosphere.
2.4.6. X-Ray Diffraction
X-ray diffraction (XRD) analysis was conducted to determine the crystalline phases of the original BCP, HMSNs, and BCP/HMSNs. The samples were evenly spread in the sample holder, and the diffraction patterns were recorded over a scanning range of 5–40°.
2.4.7. Release Performance of BCP/HMSNs
The release performance of BCP and BCP/HMSNs was assessed using the dialysis bag method. The samples were dissolved in a release medium (DMF:water = 6:4) and stirred at 100 rpm in the dark at 25 °C. At predetermined intervals, 5 mL of the release medium was removed and replaced with the same volume of fresh medium. The concentration of BCP in the medium was measured at 277 nm using a UV/visible spectrophotometer. The cumulative release percentage (Ep) was calculated using the following formula:
where Ep represents the cumulative release amount (%), Ve is the volume of the release medium removed (L), Ci is the mass concentration of BCP in the medium at the i-th sampling (mg·L−1), V0 is the total volume of the release medium (L), Cn is the mass concentration of BCP in the medium at the n-th sampling (mg·L−1), and mp is the total mass of the sample (mg).
2.4.8. HMSNs Safety Assessment
Maize seeds (Zea mays, variety Zhengdan 958) were obtained from the Qingyuan District Agricultural Trade Market in Baoding City, Hebei Province. The seeds were sown in pots filled with river sand that had been autoclaved at 121 °C and 1.2 MPa pressure for 30 min to ensure the elimination of any pathogens or contaminants. The seeds were germinated in a controlled environment with a temperature of 25 °C and maintained at a relative humidity of 60–70% for optimal germination. After germination, the plants were grown in a greenhouse under controlled conditions, with a constant temperature of 22–26 °C and a relative humidity of 70%. The plants were watered regularly with standard Hoagland nutrient solution to maintain soil moisture at a level suitable for maize growth. Additional nutrients were supplied using this solution once a week.
Maize plants with two leaves were sprayed with HMSNs and BCP/HMSNs at concentrations of 1, 2, 4, and 8 mg·L−1. Controls included maize plants treated with a 4.5% BCP emulsion at equivalent concentrations and untreated plants. Seven days after spraying, the plants were collected, washed, air-dried, and weed to determine the fresh weight of aboveground parts and roots. Subsequently, the plants were dried at 37 °C for 7 d to measure their dry weight. Each treatment was conducted in triplicate.
2.4.9. Biological Activity of BCP Nanoparticles
Indoor Biological Activity
Second-instar maize borer larvae (Ostrinia furnacalis) were used as model pests. These larvae were collected from local maize fields in the region and cultured under controlled conditions with a temperature of 25 °C and relative humidity of 60–70%. The larvae were reared on maize leaves until they reached the second-instar stage. Maize leaves were sprayed with test formulations of BCP/HMSNs at concentrations of 0.18, 0.135, and 0.09 mg·L−1. A 4.5% cypermethrin emulsion (0.18 mg·L−1) and a blank control were used as references. After 0, 3, 7, 10, 14, 20, and 30 d, treated leaves were collected, and 10 larvae were placed on each leaf. The larvae were allowed to feed for 24 h, and their mortality was recorded. Each treatment was repeated three times.
Field Biological Activity
The field experiment was conducted in an experimental plot size of 20 m2, with protective rows surrounding each plot to minimize cross-contamination. Each treatment was arranged in a randomized complete block design with three replications. Applications were carried out at a temperature of 22–25 °C and relative humidity of 60–70% to ensure uniform pesticide distribution. The time of application was between 8:00 AM and 10:00 AM to avoid temperature extremes and ensure stable conditions for pesticide dispersal. The treatment dosages are presented in Table 1. Initial pest populations were surveyed prior to pesticide application, and the number of live pests was recorded on days 1, 3, 10, and 20 post-application. Sampling was conducted at five randomly selected points within each plot, with five plants examined per point. Pest reduction rate (PRR) and control efficacy (CE) were calculated using the following formulas:

Table 1.
Drugs used in the drug test. BCP: β-cypermethrin; BCP/HMSNs: β-cypermethrin-loaded hollow mesoporous silica nanoparticles; N/A: not applicable.
PRR (%):
where Nbefore: Number of live pests before the application; Nafter: Number of live pests after the application.
CE (%):
where Nafter, treatment: Number of live pests after application in the treatment area; Nbefore, treatment: Number of live pests before application in the treatment area; Nafter, blank: Number of live pests after application in the blank (control) area; Nbefore, blank: Number of live pests before application in the blank (control) area.
2.5. Statistical Analysis
Statistical analyses were performed using SPSS 24.0 software. Before ANOVA, the data were tested for normality (Shapiro-Wilk test) and homoscedasticity (Levene test). One-way analysis of variance (ANOVA) was conducted to evaluate the effects of different treatments, including HMSNs, BCP/HMSNs, and conventional formulations, on maize seedling biomass (fresh and dry weights of roots and shoots), as well as the effects of different concentrations of BCP/HMSNs and 4.5% BCP emulsifiable concentrate on maize borer larvae mortality rates across various observation times. Tukey’s multiple comparison test was applied to identify significant differences among treatments at a significance level of p < 0.05. All statistical graphs, including bar plots for maize seedling biomass and mortality rates of maize borer larvae, were generated using GraphPad Prism software (version 10.1.0).
3. Results and Discussions
3.1. Characterization of Nanoparticle Morphologies
The morphologies of HMSNs and BCP/HMSNs were analyzed using SEM and TEM, as shown in Figure 1. The images clearly demonstrate the influence of different synthesis and processing conditions on the structural features of the nanoparticles. Figure 1A shows HMSNs synthesized by adding anhydrous sodium carbonate to water as the etching agent, without undergoing template removal via hot ethanol. The image reveals partially broken shells, indicating that the synthesis process resulted in structural instability. The lack of a template removal step likely left residual materials within the mesoporous structure, further contributing to the incomplete formation of hollow morphologies [35,36].
Figure 1(B1,B2) display TEM images of HMSNs synthesized with anhydrous sodium carbonate as the etching agent, followed by selective etching for 7 h and template removal via a 3-h reaction with hydrochloric acid in hot ethanol. These HMSNs exhibited thick shells, small internal cavities, and significant particle aggregation. The structural features suggest that the extended etching time led to excessive material dissolution, resulting in poorly defined shapes and severe adhesion between particles [37,38]. The lack of well-formed hollow structures and the presence of structural irregularities highlight the limitations of prolonged etching under these conditions [39].
In contrast, Figure 1(C1,C2) present SEM and TEM images of HMSNs synthesized by selective etching with anhydrous sodium carbonate for 5 h, followed by template removal via a 2-h reaction with hydrochloric acid in hot ethanol. The SEM and TEM images revealed that the HMSNs presented spherical morphology and homogeneous size distribution, indicating a successful and reproducible synthesis. These particles exhibit a more defined spherical morphology with thin shells, large internal cavities, and minimal aggregation. The reduced etching time allowed for better control over the dissolution process, resulting in intact and well-dispersed nanoparticles [37,40]. The improved structural uniformity and hollow cavity size are ideal for applications requiring high payload capacity and controlled release, as the thin shells facilitate diffusion while maintaining mechanical stability [40,41].
Figure 1(D1,D2) show SEM and TEM images of BCP-loaded HMSNs (BCP/HMSNs), highlighting the successful incorporation of BCP into the mesoporous silica nanoparticles. The TEM image (Figure 1(D2)) reveals distinct shadowed regions within the hollow cavities, indicating the presence of encapsulated BCP. The preserved spherical morphology and uniform dispersion of the BCP/HMSNs further confirm that the loading process did not compromise the structural integrity of the carrier.
These results demonstrate that synthesis conditions, including etching time and the template removal process, play a critical role in determining the structural properties and functional performance of HMSNs. Prolonged etching (7 h) led to structural irregularities, aggregation, and reduced cavity sizes, which are undesirable for applications requiring uniformity and stability [13]. By contrast, HMSNs synthesized with a 5-h etching time and appropriate template removal exhibited well-defined hollow morphologies with minimal adhesion, making them suitable for pesticide delivery [42].
3.2. Nanoparticle Mesoporous Parameter Analysis
The nitrogen adsorption–desorption curves and pore-size distributions of HMSNs synthesized under different etching conditions are shown in Figure 2. The nitrogen adsorption–desorption curves (Figure 2A) exhibit the characteristic shape of a type IV isotherm with a hysteresis loop, a hallmark of mesoporous materials [18]. This confirms the successful formation of mesoporous silica with well-defined pore structures. The pore-size distributions (Figure 2B) reveal significant variations depending on the etching time and temperature. For HMSNs synthesized under suboptimal conditions, such as longer etching times or higher temperatures, the pore diameters were predominantly concentrated in the range of 1–2 nm, which are too small for effective pesticide molecule encapsulation [43]. In contrast, HMSNs synthesized with a 5-h etching time exhibited larger and more uniform pore sizes concentrated in the range of 3–4 nm, indicating that these synthesis conditions are optimal for achieving a desirable mesoporous structure [44].
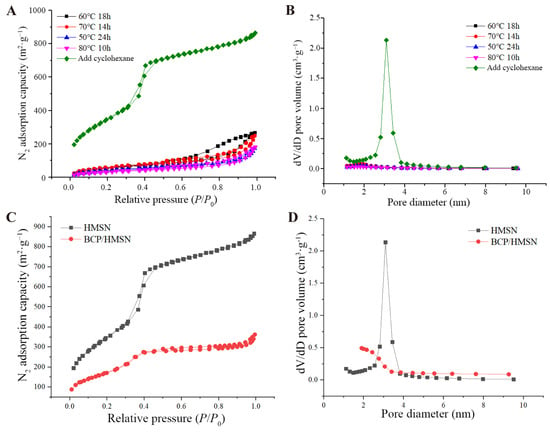
Figure 2.
Nitrogen adsorption-desorption isotherms and pore size distribution curves of hollow mesoporous silica nanoparticles (HMSNs) and β-cypermethrin-loaded hollow mesoporous silica nanoparticles (BCP/HMSNs) synthesized under various conditions. Nitrogen adsorption-desorption isotherms (A) and pore size distribution curves (B) of HMSNs synthesized under different etching times, temperatures, and with the addition of cyclohexane. Nitrogen adsorption-desorption isotherms (C) and pore size distribution curves (D) of HMSNs and BCP/HMSNs.
The addition of cyclohexane during the synthesis process played a critical role in enhancing the mesoporous properties of HMSNs [45]. As summarized in Table 2, HMSNs synthesized with cyclohexane and a 5-h etching time achieved a significantly higher specific surface area (1318 m2·g−1) and pore volume (1.52 cm3·g−1). These values are substantially higher than those of HMSNs synthesized under other conditions, which typically exhibited specific surface areas of 100–200 m2·g−1 and pore volumes below 0.5 cm3·g−1, with pore sizes smaller than 2 nm. Such limited mesoporous properties hinder pesticide molecules from entering the pores, leading to reduced loading efficiency. In contrast, the larger pore sizes and higher pore volumes observed in the optimized HMSNs are particularly advantageous for the adsorption and encapsulation of hydrophobic pesticide molecules such as BCP, as these structural features facilitate their diffusion into the mesoporous channels and increase the overall loading capacity.

Table 2.
Mesoporous structure characterization of β-cypermethrin-loaded hollow mesoporous silica nanoparticles (BCP/HMSNs). SBET: specific surface area (calculated by Brunauer-Emmett-Teller method); VBJH: pore volume (calculated by Barrett-Joyner-Halenda method); DBJH: pore diameter (calculated by Barrett-Joyner-Halenda method); N/A: not applicable.
The impact of BCP loading on the mesoporous properties of HMSNs is shown in Figure 2C,D and summarized in Table 2. The specific surface area and pore volume of HMSNs decreased significantly after BCP encapsulation, with the specific surface area decreasing from 1318 m2·g−1 to 920 m2·g−1 and the pore volume decreasing from 1.52 cm3·g−1 to 1.01 cm3·g−1. This reduction indicates that BCP molecules successfully entered the mesoporous structure, occupying internal cavities and reducing the available pore space. These results align with TEM observations in Figure 1D2, where shadowed regions within the hollow cavities confirmed the presence of encapsulated BCP. The encapsulation of BCP not only validates the effectiveness of the mesoporous structure for pesticide loading but also demonstrates the structural integrity of HMSNs during the loading process.
The mesoporous properties of HMSNs synthesized with a 5-h etching time provide significant advantages for pesticide delivery. The larger pore sizes of 3–4 nm facilitate the efficient diffusion and encapsulation of pesticide molecules, while the high pore volume ensures sufficient capacity for loading hydrophobic molecules like BCP [44]. These characteristics enable the nanoparticles to act as an efficient and sustained-release delivery system, reducing the environmental risks associated with conventional pesticide formulations. Moreover, the high specific surface area enhances the overall interaction between the pesticide molecules and the mesoporous carrier, further improving encapsulation efficiency and release performance.
3.3. Nanoparticle Size Analysis
The particle-size distributions of HMSNs and BCP/HMSNs are shown in Figure 3A. Both nanoparticles exhibit a size distribution predominantly concentrated in the range of 100–400 nm, with HMSNs and BCP/HMSNs showing average particle sizes of 183 nm and 189 nm, respectively. The slight increase in the particle size of BCP/HMSNs compared to HMSNs confirms that BCP molecules were successfully encapsulated within the pores of the HMSNs, rather than being merely adsorbed onto their external surfaces. This result aligns with the mesoporous properties observed earlier (Section 3.2), where reductions in specific surface area and pore volume were also consistent with internal loading of BCP molecules.
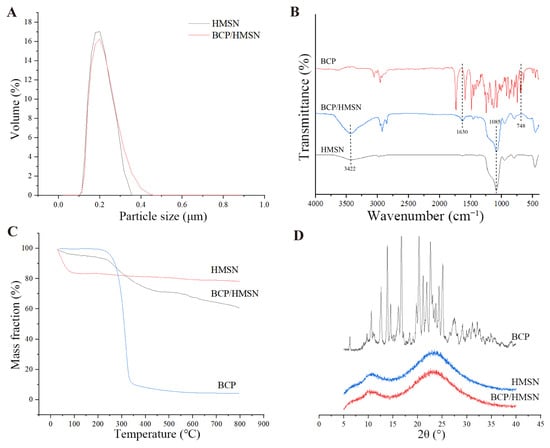
Figure 3.
Characterization of hollow mesoporous silica nanoparticles (HMSNs) and β-cypermethrin-loaded hollow mesoporous silica nanoparticles (BCP/HMSNs). Particle size distribution of HMSN and BCP/HMSNs (A). Fourier-transform infrared spectroscopy (FT-IR) spectra of β-cypermethrin (BCP), HMSNs, and BCP/HMSNs (B). Thermogravimetric analysis (TGA) curves of β-cypermethrin, HMSNs, and BCP/HMSNs (C). X-ray diffraction (XRD) patterns of β-cypermethrin, HMSNs, and BCP/HMSNs (D).
It is important to note that the particle sizes measured using DLS reflect the hydrated diameters of the nanoparticles, which are slightly larger than the dry particle sizes typically observed in TEM images [46]. The hydration layer surrounding each nanoparticle contributes to the measured size and plays a critical role in stabilizing the nanoparticles in aqueous environments. This stability is particularly advantageous for agricultural applications, as it ensures uniform dispersion of the particles in water-based pesticide formulations, minimizing aggregation and improving application efficiency on crop surfaces [47].
The particle size of both HMSNs and BCP/HMSNs falls within the optimal range for nanoparticle-based pesticide delivery systems. Nanoparticles with sizes in the 100–400 nm range are small enough to penetrate plant surfaces, including stomata and leaf cuticles, yet large enough to avoid translocation to non-target plant tissues or environmental dispersion [48]. Additionally, this size range enhances the adhesion of nanoparticles to hydrophobic leaf surfaces, reducing spray drift and improving the precision of pesticide application. The slight increase in particle size observed after BCP loading does not negatively affect the performance of the nanoparticles and instead confirms the structural integrity of HMSNs as carriers, which maintain their size uniformity and stability even after encapsulation.
3.4. FT-IR Spectroscopy Results
The FT-IR spectra of BCP, HMSNs, and BCP/HMSNs are shown in Figure 3B. The FT-IR spectrum of HMSNs exhibits several characteristic peaks that confirm the successful formation of the mesoporous silica framework. A strong and broad absorption band at 1085 cm−1 corresponds to the antisymmetric stretching vibration of Si–O–Si bonds, while a peak at 804 cm−1 is attributed to the symmetric stretching and bending vibrations of Si–O. Additionally, a broad absorption band at 3422 cm−1 is associated with the antisymmetric stretching vibrations of O–H groups from structural water adsorbed on the nanoparticle surface. Notably, no absorption peaks were observed in the 2800–3000 cm−1 range, which corresponds to the C–H stretching vibrations of organic compounds. This absence confirms that residual organic substances, such as CTAB and cyclohexane, were successfully removed during the synthesis process, ensuring the purity and functionality of the mesoporous silica nanoparticles.
The FT-IR spectrum of BCP/HMSNs closely resembles that of HMSNs, with the characteristic peaks at 1085 cm−1 (Si–O–Si stretching) and 3422 cm−1 (O–H stretching) remaining unchanged. This indicates that the encapsulation of BCP within HMSNs occurs through physical adsorption rather than chemical bonding. The stability of the silica framework and the absence of new peaks related to chemical interactions between BCP and HMSNs confirm that the encapsulation process does not compromise the chemical integrity of either the carrier or the active ingredient. These findings highlight the suitability of HMSNs as stable carriers for pesticide delivery, as they do not alter the chemical properties of the encapsulated BCP.
Additional peaks observed in the spectrum of BCP/HMSNs provide direct evidence of BCP loading. A peak at 748 cm−1 corresponds to the stretching vibration of the C–Cl bond, a characteristic feature of BCP, while the peak at 1630 cm−1 is attributed to the stretching vibration of the carbonyl group (C=O). The presence of these peaks confirms the successful incorporation of BCP within the HMSNs, further validating the encapsulation process. The alignment of these peaks with those in the FT-IR spectrum of pure BCP demonstrates that the physical adsorption mechanism preserves the chemical integrity of the active ingredient, ensuring its efficacy during application.
The FT-IR results confirm the suitability of HMSNs as a carrier for hydrophobic pesticides like BCP. The mesoporous silica nanoparticles exhibit excellent stability, with no chemical interactions between the carrier and the active ingredient. The successful removal of organic residues ensures that the structural and functional properties of HMSNs are not compromised, while the observed characteristic peaks of BCP indicate efficient encapsulation within the mesoporous framework.
3.5. TGA Results
The thermogravimetric (TG) curves of BCP, HMSNs, and BCP/HMSNs are presented in Figure 3C, demonstrating the thermal stability and encapsulation efficiency of the nanoparticles. The samples were heated from 20 °C to 800 °C to evaluate their thermal decomposition profiles.
The TG curve of pure BCP showed a sharp mass loss beginning at approximately 180 °C, with nearly complete degradation observed by 800 °C. This rapid decline in mass is attributed to the thermal decomposition of the organic structure of BCP under high-temperature conditions, consistent with previously reported thermal behaviors of similar pesticide molecules [49,50]. This thermal instability highlights the susceptibility of BCP to degradation, underscoring the necessity of a protective carrier system to enhance its thermal stability during storage and application.
In contrast, HMSNs exhibited excellent thermal stability across the tested temperature range, with minimal mass loss. A small, sharp decrease in mass (15.56%) was observed below 100 °C, which is attributed to the evaporation of surface-adsorbed water and the decomposition of residual molecules from the synthesis process [51]. Beyond this point, the mass remained nearly constant, confirming the robustness of the silica framework even under elevated temperatures. This stability supports the use of HMSNs as a reliable carrier for pesticide delivery, especially in conditions where thermal resistance is critical.
The TG curve of BCP/HMSNs showed a total mass loss of 38.09%, significantly higher than that of unloaded HMSNs. This additional mass loss is attributed to the decomposition of encapsulated BCP molecules. By subtracting the baseline mass loss of HMSNs, the loading efficiency of BCP within the mesoporous silica nanoparticles was calculated to be approximately 32.53%, consistent with the results obtained from other characterization techniques, such as nitrogen adsorption–desorption analysis (Section 3.2) and TEM imaging (Section 3.1). The agreement among these methods provides robust evidence of successful BCP encapsulation within the mesoporous structure.
The encapsulation of BCP within HMSNs also demonstrated improved thermal stability compared to pure BCP. The delayed onset of thermal degradation for BCP/HMSNs suggests that the mesoporous silica framework provides a protective effect, shielding the encapsulated pesticide molecules from direct exposure to thermal stress.
3.6. XRD Results
The XRD patterns of BCP, HMSNs, and BCP/HMSNs are shown in Figure 3D. The XRD pattern of pure BCP exhibited sharp and distinct diffraction peaks at 12.58°, 13.85°, 16.70°, 20.28°, 22.60°, and 25.10°, which are characteristic of its crystalline structure. These well-defined peaks indicate that BCP exists in a highly ordered crystalline form, consistent with its molecular arrangement in the raw material.
In contrast, the XRD pattern of HMSNs displayed no sharp diffraction peaks, showing only a broad and diffuse pattern, which is a hallmark of amorphous materials [52,53]. This confirms that HMSNs possess an amorphous silica structure, as expected for mesoporous silica nanoparticles [44]. The absence of crystalline peaks for BCP in the BCP/HMSNs sample suggests that the BCP molecules were successfully encapsulated within the mesoporous structure of HMSNs. This encapsulation likely prevents the detection of crystalline diffraction peaks due to the shielding effect of the silica matrix or the disruption of the ordered crystalline structure [54].
When combined with other results, such as FT-IR, which confirms the presence of intact BCP molecules, and TEM, which shows the successful encapsulation of BCP into the internal cavities of HMSNs, the XRD data suggest that the crystalline BCP may have experienced structural rearrangement or dispersion due to spatial confinement within the mesoporous silica. Furthermore, the TGA results indicate effective encapsulation without significant chemical alteration, further supporting the conclusion that BCP is securely loaded into the silica matrix. While the absence of diffraction peaks might indicate a loss of crystalline order, additional techniques such as differential scanning calorimetry (DSC) are required to confirm whether BCP has been fully transformed into an amorphous state [55].
These findings highlight the capacity of HMSNs to encapsulate and protect hydrophobic pesticide molecules like BCP, potentially altering their structural properties in the process. This ability to suppress crystalline diffraction peaks enhances the functional versatility of HMSNs as carriers for controlled-release pesticide formulations.
3.7. In Vitro Release Behavior of BCP and BCP/HMSNs
The in vitro release behavior of free BCP and BCP/HMSNs in a DMF–water solution was analyzed to assess the controlled-release capabilities of the mesoporous silica nanoparticles, and the results are shown in Figure 4. The calibration curve for BCP in the release medium (Figure 4A) exhibited excellent linearity (R2 = 0.9995), confirming the reliability and accuracy of the quantitative measurements used to monitor the release profiles. Figure 4B shows the cumulative release profiles of pure BCP and BCP/HMSNs in the release medium. Due to the poor water solubility of BCP, a solvent-based medium was chosen to ensure effective dissolution and release measurement [56]. DMF–water solution was selected over lower-boiling-point solvents such as methanol due to its compatibility and stability during prolonged release experiments [57].
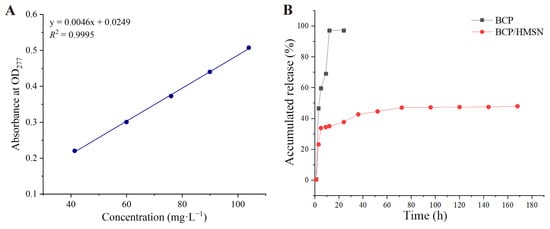
Figure 4.
Standard curve and cumulative release profile of β-cypermethrin (BCP). Standard curve of BCP in dimethylformamide (DMF)-water solution (A). Cumulative release rate of BCP in DMF-water solution (B).
The release profiles reveal significant differences in the release behavior of pure BCP and BCP/HMSNs. Pure BCP exhibited a rapid release, with over 95% of the pesticide released within 12 h, approaching near-complete dissolution. This rapid release is characteristic of unencapsulated BCP molecules, which are fully exposed to the medium and dissolve freely without any diffusion barriers [58].
In contrast, the release of BCP from BCP/HMSNs was much slower, with a cumulative release rate of approximately 50% over 170 h. The release profile of BCP/HMSNs can be divided into two distinct phases. The initial burst release observed in the first 5 h (Figure 4B) is attributed to the desorption of a small fraction of BCP molecules loosely adsorbed on the external surface of HMSNs. These surface-bound molecules are not deeply encapsulated within the mesoporous framework, making them readily available for diffusion into the release medium. This phenomenon is commonly observed in nanoparticle-based systems where a portion of the active ingredient remains physically attached to the outer surface during the loading process [59].
The majority of BCP molecules, however, are encapsulated within the mesopores of HMSNs, as confirmed by the XRD results, which showed no detectable crystalline diffraction peaks for BCP in BCP/HMSNs. This absence of crystalline peaks indicates that most BCP molecules are either fully confined within the mesoporous structure or have experienced a loss of crystallinity due to the spatial constraints of the silica framework. This deep encapsulation explains the sustained-release behavior observed after the initial burst phase, where BCP molecules gradually diffuse from the mesopores into the medium over an extended period.
This dual distribution of BCP—minor surface adsorption and predominant internal encapsulation—aligns with both the rapid initial release phase and the slower sustained-release phase observed in the cumulative release profile. It also highlights the ability of HMSNs to regulate pesticide release by controlling molecular diffusion through their mesoporous structure, ensuring a balance between immediate efficacy and prolonged action.
3.8. Safety Evaluation of Nanoparticles
The safety evaluation of HMSNs and BCP/HMSNs on the roots and shoots of maize seedlings is shown in Figure 5. In contrast to the blank control group, treatment with the 4.5% BCP emulsifiable concentrate did not result in significant differences in the growth of maize seedlings, indicating that the conventional pesticide formulation had minimal observable effects on maize growth under the tested conditions. This suggests that the conventional pesticide, despite its insecticidal effectiveness, does not significantly affect the overall growth of maize seedlings.
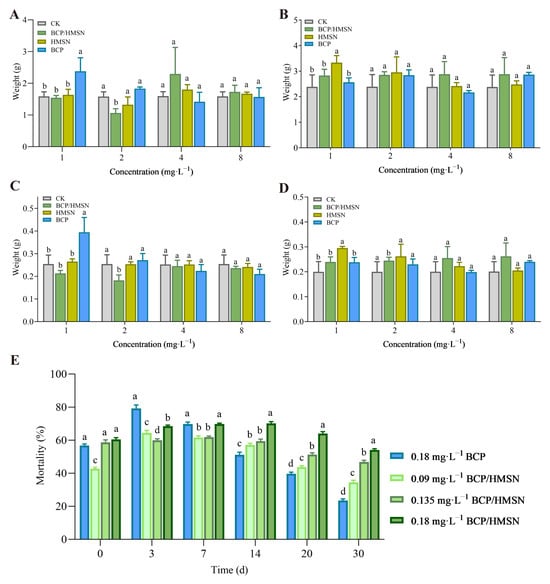
Figure 5.
Safety evaluation of hollow mesoporous silica nanoparticles (HMSNs) and β-cypermethrin-loaded hollow mesoporous silica nanoparticles (BCP/HMSNs) on the roots and shoots of maize seedlings, and insecticidal performance. Fresh weight of roots (A) and shoots (B) of maize seedlings after treatment. Dry weight of roots (C) and shoots (D) of maize seedlings after treatment. Mortality of maize borer larvae with different concentrations of 4.5% BCP/HMSNs and 4.5% β-cypermethrin (BCP) emulsion at different times (E). Statistical significance (p < 0.05) among the data (mean ± SD, n = 3) from different treatments was determined by one-way ANOVA with Tukey’s multiple comparisons test. Significant differences are denoted by different letters (e.g., a, b, c, d) above the bars.
In contrast, the application of HMSNs and BCP/HMSNs had varying impacts on maize growth, with a noticeable positive influence on shoot biomass, particularly with 1 mg·L−1 BCP and 4 mg·L−1 BCP/HMSN. As shown in Figure 5A, both 1 mg·L−1 BCP and 4 mg·L−1 BCP/HMSN significantly increased the fresh weight of maize roots compared to the control group. However, treatment with 2 mg·L−1 BCP/HMSN resulted in a significant decrease in root fresh weight compared to the control. No significant differences were observed in root fresh weight among the other treatments. Regarding the fresh weight of maize shoots, 1 mg·L−1 BCP was found to significantly increase shoot fresh weight, as shown in Figure 5B. No significant differences in shoot fresh weight were observed between other concentrations of treatments. For root dry weight, 1 mg·L−1 BCP treatment significantly increased the dry weight of maize roots, whereas the application of 1 mg·L−1 and 2 mg·L−1 BCP/HMSN resulted in a significant decrease in root dry weight (Figure 5C). No significant differences in root dry weight were observed at other treatment concentrations. For the dry weight of maize shoots, 1 mg·L−1 HMSN significantly increased shoot dry weight (Figure 5D), while no significant differences were observed in shoot dry weight among the other treatments.
The observed enhancement in shoot growth, particularly in response to 1 mg/L BCP, may be attributed to the potential of nanoparticles to influence plant microenvironmental factors such as chlorophyll content and protein synthesis [60]. Studies have shown that certain nanoparticles can act as growth stimulants by improving nutrient absorption and metabolic activity in plants [61,62]. The mesoporous structure of HMSNs may facilitate a controlled release of essential micronutrients, promoting enhanced shoot development [61]. Moreover, the encapsulation of BCP within HMSNs appears to mitigate the phytotoxic effects commonly associated with conventional pesticide applications, thus reducing stress on the maize seedlings and fostering growth.
This positive effect on the aboveground parts of maize seedlings is particularly significant for agricultural applications, as it highlights the dual benefits of HMSNs and BCP/HMSNs. In addition to their controlled-release properties for pesticides, these nanoparticles may also act as growth promoters, addressing the common trade-off between pest control and plant health. By minimizing the phytotoxic impact of BCP while promoting shoot growth, HMSNs and BCP/HMSNs offer a promising approach for improving crop productivity and sustainability in pesticide applications.
3.9. Bioassay of BCP-Loaded Particles
The bioassay results demonstrated that BCP/HMSNs exhibit prolonged insecticidal efficacy compared to the conventional 4.5% BCP emulsifiable concentrate. The results in Figure 5E and Table 3 reveal distinct differences in the mortality rates of maize borer larvae over time, highlighting the controlled-release properties of BCP/HMSNs.

Table 3.
Pest mortality in the field with different concentrations of β-cypermethrin-loaded hollow mesoporous silica nanoparticles (BCP/HMSNs) or 4.5% β-cypermethrin (BCP) emulsion over time. PRR: pest reduction rate; CE: control efficacy.
The 4.5% BCP emulsifiable concentrate displayed rapid insecticidal action, with the highest mortality rate of 79.2% observed at 3 days post-application (Figure 5E). However, the efficacy of the emulsifiable concentrate declined sharply after this peak, with a pest mortality rate of only 23.5% at 30 d (Figure 5E). This pattern reflects the quick release and depletion of the active ingredient, a characteristic limitation of conventional pesticide formulations [63,64].
In contrast, BCP/HMSNs exhibited a more sustained insecticidal effect. At concentrations of 0.09 mg·L−1, 0.135 mg·L−1, and 0.18 mg·L−1, the mortality rates of maize borer larvae at 30 d were 34.4%, 46.8%, and 54.1%, respectively (Figure 5E). The mortality rates of 0.18 mg·L−1 BCP/HMSNs remained significantly higher than those of other treatments at both 20 and 30 d. The gradual decline in efficacy over time suggests that the nanoparticles effectively regulate the diffusion of BCP molecules, providing consistent pesticide concentrations in the environment. Additionally, the sustained-release behavior ensures that BCP/HMSNs maintain pest control efficacy over an extended period, reducing the need for frequent reapplication. Specifically, the insecticidal efficacy of 0.18 mg·L−1 BCP/HMSNs at days 0, 3, 7, 14, 20, and 30 were 60.4%, 68.5%, 69.7%, 70.2%, 64.1%, and 54.1%, respectively, demonstrating the sustained action of the nanoparticles over time.
Field bioassays further validated these findings. As shown in Table 3, there were no significant differences in PRR and CE between treatments with BCP/HMSNs and 4.5% BCP emulsifiable concentrate on the first day. However, by day 20, the differences became statistically significant. Notably, the treatments with 5 g·667−1·m−2 and 10 g·667−1·m−2 of BCP/HMSNs achieved 100% PRR and CE, corroborating the sustained-release properties observed in Figure 5E.
The superior insecticidal performance of BCP/HMSNs is consistent with their controlled-release properties observed in in vitro release experiments (Section 3.7). The initial burst release phase ensures immediate pest control, while the sustained-release phase prolongs efficacy, addressing the challenges of short activity durations associated with conventional pesticides. Furthermore, the encapsulation of BCP within HMSNs provides protection against environmental factors such as UV radiation and volatilization, enhancing the stability of the pesticide in field conditions [65].
The results highlight the dual advantages of BCP/HMSNs in pest control: immediate and effective action combined with prolonged efficacy. This dual-phase release mechanism significantly reduces the frequency of pesticide applications, minimizing labor costs and environmental contamination. Moreover, the enhanced efficacy of BCP/HMSNs at lower concentrations (e.g., 0.135 mg·L−1 and 0.18 mg·L−1) suggests the potential for dose reduction, contributing to more sustainable and environmentally friendly agricultural practices.
In summary, the bioassay results confirm the superior insecticidal performance and prolonged efficacy of BCP/HMSNs compared to the conventional emulsifiable concentrate. These findings underscore the potential of mesoporous silica nanoparticles as a versatile platform for controlled-release pesticide formulations, offering significant benefits in improving agricultural productivity while reducing environmental impact.
4. Conclusions
This study demonstrates that HMSNs are effective carriers for the controlled release of BCP, offering significant improvements in both pest control and environmental sustainability. The self-templating method used to prepare HMSNs ensures greater structural control, leading to efficient release and enhanced drug-loading capacity (32.53%). The sustained release over 170 h, with approximately 50% of BCP released, provides prolonged efficacy, maintaining ~50% pest mortality after 30 days—significantly outperforming conventional emulsifiable concentrates. This extended release reduces the need for reapplications, thus lowering operational costs and minimizing contamination risks. The technology also shows low phytotoxicity in maize seedlings, confirming its agricultural viability as both a pesticide carrier and plant growth stimulant. This work advances the field by introducing a self-templating method that offers greater control over the release process. Future research should focus on improving drug-loading efficiency, developing responsive release mechanisms for diverse agricultural conditions, and addressing scalability and environmental impact. Further studies on field applications and adaptations to other active ingredients will help position HMSNs as a sustainable, cost-effective solution for precise pesticide delivery in agriculture.
Author Contributions
Conceptualization: X.L., Z.K., M.L., T.G. and D.Z. Methodology: M.L., T.G., D.Z. and X.L. Software: M.L. Validation: M.L. and X.L. Formal analysis: M.L. and Z.Z. Investigation: T.G. Resources: X.L. and Z.K. Data curation: M.L. Writing—Original draft preparation: M.L. Writing—Review and editing: L.X., Z.Z., X.L., Z.K., T.G. and D.Z. Visualization: Z.Z. Supervision: X.L. and Z.K. Funding acquisition: X.L. and Z.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Hebei Agriculture Research System (HBCT2024180403).
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yang, S.; Liu, J.; Bi, X.; Ning, Y.; Qiao, S.; Yu, Q.; Zhang, J. Risks Related to Heavy Metal Pollution in Urban Construction Dust Fall of Fast-Developing Chinese Cities. Ecotoxicol. Environ. Saf. 2020, 197, 110628. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Zhao, X.; Zhu, W.; Sun, W.; Qiu, Y.; Zhang, J. Species Differences in the Green-up Date of Typical Vegetation in Inner Mongolia and Climate-Driven Mechanism Based on Process-Based Phenology Models. Sci. Total Environ. 2022, 834, 155260. [Google Scholar] [CrossRef]
- Su, J.; Cheng, C.; Guo, Y.; Xu, H.; Ke, Q. OMS-2-Based Catalysts with Controllable Hierarchical Morphologies for Highly Efficient Catalytic Oxidation of Formaldehyde. J. Hazard. Mater. 2019, 380, 120890. [Google Scholar] [CrossRef]
- Belagalla, N.; Kumar, A.; Badekhan, A.; Chaturvedi, K.; Kumar, A.; Panigrahi, C.K.; Sachan, K.; Singh, B.V. Enhancing Agricultural Efficiency and Biodiversity Conservation through Nano Pesticides; a Focus on Food Research. J. Adv. Biol. Biotechnol. 2024, 27, 319–335. [Google Scholar] [CrossRef]
- Opdensteinen, P.; Charudattan, R.; Hong, J.C.; Rosskopf, E.N.; Steinmetz, N.F. Biochemical and Nanotechnological Approaches to Combat Phytoparasitic Nematodes. Plant Biotechnol. J. 2024, 22, 2444–2460. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Anil, A.; Sakthivel, N. Chapter 9—Plant Growth-Promoting Bacteria as a Potential Source for Nanoparticles. In Nanoparticles Synthesis by Soil Microbes; Kumar, A., Ghosh, S., Singh, J., Thongmee, S., Eds.; Plant and Soil Microbiome; Academic Press: Cambridge, MA, USA, 2025; pp. 191–213. ISBN 978-0-443-21692-3. [Google Scholar]
- Sari Yilmaz, M. Synthesis of Novel Amine Modified Hollow Mesoporous Silica@mg-al Layered Double Hydroxide Composite and Its Application in CO2 Adsorption. Microporous Mesoporous Mater. 2017, 245, 109–117. [Google Scholar] [CrossRef]
- Mahmoodzade, E.; Meshkani, F.; Rezaei, M.; Rastegarpanah, A. Preparation and Improvement of Nickel Catalyst Supported Ordered Mesoporous Spherical Silica for Thermocatalytic Decomposition of Methane. J. Energy Inst. 2020, 93, 2488–2496. [Google Scholar] [CrossRef]
- Lin, Z.; Gong, C.; Tang, L.; Cao, B.; Kong, F.; Wang, Z.; Bi, Y. Study on Preparation and in Vitro Anti-Tumor Activity of Chitosan-Modified Mesoporous Silica Hybrids by GPTMS Cross-Linking Agent. React. Funct. Polym. 2021, 169, 105072. [Google Scholar] [CrossRef]
- Zhou, J.; Zhai, Y.; Xu, J.; Zhou, T.; Cen, L. Microfluidic Preparation of PLGA Composite Microspheres with Mesoporous Silica Nanoparticles for Finely Manipulated Drug Release. Int. J. Pharm. 2021, 593, 120173. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Z.; Zhang, L.; Li, Y.; Yang, J.; Shen, J.; Wang, J.; Niu, Y.; Xiao, Z.; Chen, L.; et al. Preparation of Hollow Mesoporous Silica Nanorods for Encapsulating and Slowly Releasing Eugenol. Chin. Chem. Lett. 2020, 31, 3135–3138. [Google Scholar] [CrossRef]
- Zhang, T.; Lu, Z.; Wang, J.; Shen, J.; Hao, Q.; Li, Y.; Yang, J.; Niu, Y.; Xiao, Z.; Chen, L.; et al. Preparation of Mesoporous Silica Nanoparticle with Tunable Pore Diameters for Encapsulating and Slowly Releasing Eugenol. Chin. Chem. Lett. 2021, 32, 1755–1758. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, G.; Guo, Z.; Wang, M.; Qi, C.; Chen, G.; Huang, X.; Yan, S.; Xu, D. Stimuli-Responsive Pesticide Carriers Based on Porous Nanomaterials: A Review. Chem. Eng. J. 2023, 455, 140167. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef]
- Huang, C.; Wei, G.; Cui, L.; Zhou, Z.; Du, X. Gaseous Thermal Conductivity Studies on Mesoporous Silica Particles Based on a Bimodal-Pore Distribution Model. Int. J. Therm. Sci. 2021, 160, 106668. [Google Scholar] [CrossRef]
- Van Der Voort, P.; Benjelloun, M.; Vansant, E.F. Rationalization of the Synthesis of SBA-16: Controlling the Micro- and Mesoporosity. J. Phys. Chem. B 2002, 106, 9027–9032. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, H.; Zhou, Z.; Xu, C.; Shan, Y.; Lin, Y.; Huang, Q. Fluorophore-Free Luminescent Double-Shelled Hollow Mesoporous Silica Nanoparticles as Pesticide Delivery Vehicles. Nanoscale 2018, 10, 20354–20365. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; He, S.; Xiao, Y.; Qin, X.; Zhang, Y.; Li, D.; Ma, H.; You, H.; Li, J. Fabrication of a Hollow Mesoporous Silica Hybrid to Improve the Targeting of a Pesticide. Chem. Eng. J. 2019, 364, 361–369. [Google Scholar] [CrossRef]
- Gao, Y.; Xiao, Y.; Mao, K.; Qin, X.; Zhang, Y.; Li, D.; Zhang, Y.; Li, J.; Wan, H.; He, S. Thermoresponsive Polymer-Encapsulated Hollow Mesoporous Silica Nanoparticles and Their Application in Insecticide Delivery. Chem. Eng. J. 2020, 383, 123169. [Google Scholar] [CrossRef]
- Liang, Y.; Gao, Y.; Wang, W.; Dong, H.; Tang, R.; Yang, J.; Niu, J.; Zhou, Z.; Jiang, N.; Cao, Y. Fabrication of Smart Stimuli-Responsive Mesoporous Organosilica Nano-Vehicles for Targeted Pesticide Delivery. J. Hazard. Mater. 2020, 389, 122075. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, W.; Meng, Z.; Fan, T.; Yang, C.; Wang, J.; Chen, X. Development of Spirotetramat Nanoparticles Based on Mesoporous Silica: Improving the Uptake and Translocation of Spirotetramat in Plants. Environ. Sci. Pollut. Res. 2023, 30, 12618–12627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ke, J.; Gou, K.; Guo, Y.; Xu, X.; Li, S.; Li, H. Amino Functionalized Mesoporous Silica with Twisted Rod-like Shapes: Synthetic Design, in Vitro and in Vivo Evaluation for Ibuprofen Delivery. Microporous Mesoporous Mater. 2020, 294, 109896. [Google Scholar] [CrossRef]
- Fujimoto, K.; Watanabe, K.; Ishikawa, S.; Ishii, H.; Suga, K.; Nagao, D. Pore Expanding Effect of Hydrophobic Agent on 100 Nm-Sized Mesoporous Silica Particles Estimated Based on Hansen Solubility Parameters. Colloids Surf. Physicochem. Eng. Asp. 2021, 609, 125647. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, C.; Zhang, S.; Zheng, L.; Li, F.; Cao, C.; Cao, L.; Huang, Q. Fungicide-Loaded Mesoporous Silica Nanoparticles Promote Rice Seedling Growth by Regulating Amino Acid Metabolic Pathways. J. Hazard. Mater. 2022, 425, 127892. [Google Scholar] [CrossRef]
- Gupta, S.; Gajbhiye, V.T.; Sharma, R.K.; Gupta, R.K. Dissipation of Cypermethrin, Chlorpyriphos, and Profenofos in Tomato Fruits and Soil Following Application of Pre-Mix Formulations. Environ. Monit. Assess. 2011, 174, 337–345. [Google Scholar] [CrossRef]
- Katagi, T. Photodegradation of Pesticides on Plant and Soil Surfaces. In Reviews of Environmental Contamination and Toxicology: Continuation of Residue Reviews; Ware, G.W., Ed.; Springer: New York, NY, USA, 2004; pp. 1–78. ISBN 978-1-4419-9098-3. [Google Scholar]
- Ghosh, S.; Crist, K. Modeling Volatilization Emissions of Soil-Applied Pesticides under Agricultural Field Conditions. Heliyon 2022, 8, e11810. [Google Scholar] [CrossRef]
- Badr, A.M. Organophosphate Toxicity: Updates of Malathion Potential Toxic Effects in Mammals and Potential Treatments. Environ. Sci. Pollut. Res. 2020, 27, 26036–26057. [Google Scholar] [CrossRef]
- Basak, M.; Choudhury, R.A.; Goswami, P.; Dey, B.K.; Laskar, M.A. A Review on Non-Target Toxicity of Deltamethrin and Piperonyl Butoxide: Synergist. J. Pharm. Res. Int. 2021, 33, 85–89. [Google Scholar] [CrossRef]
- dos Santos, A.A.; Naime, A.A.; de Oliveira, J.; Colle, D.; dos Santos, D.B.; Hort, M.A.; Moreira, E.L.G.; Suñol, C.; de Bem, A.F.; Farina, M. Long-Term and Low-Dose Malathion Exposure Causes Cognitive Impairment in Adult Mice: Evidence of Hippocampal Mitochondrial Dysfunction, Astrogliosis and Apoptotic Events. Arch. Toxicol. 2016, 90, 647–660. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Wang, Y.; Zhang, Y.; Li, X. Compound Pesticide Controlled Release System Based on the Mixture of Poly(Butylene Succinate) and PLA. J. Microencapsul. 2018, 35, 494–503. [Google Scholar] [CrossRef]
- Zou, W.; Zhao, Y.; Deng, Y.; Zhang, H.; Mao, Z.; Xiong, Y.; He, J.; Zhao, Q. Preparation of Layered Beta-Cypermethrin-Carrying Microcapsules from Pickering Emulsion of Hollow Mesoporous Silica Nanoparticles. Mater. Today Commun. 2022, 31, 103695. [Google Scholar] [CrossRef]
- Fang, X.; Chen, C.; Liu, Z.; Liu, P.; Zheng, N. A Cationic Surfactant Assisted Selective Etching Strategy to Hollow Mesoporous Silica Spheres. Nanoscale 2011, 3, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.P.; Mou, C.Y. Structural and Morphological Control of Cationic Surfactant-Templated Mesoporous Silica. Acc. Chem. Res. 2002, 35, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Guo, L.; He, Q.; Chen, F.; Zhou, J.; Feng, J.; Shi, J. Hollow/Rattle-Type Mesoporous Nanostructures by a Structural Difference-Based Selective Etching Strategy. ACS Nano 2010, 4, 529–539. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.-H.; Na, J.; Lee, C.-H.; Sun, Z.; Wang, S.-B.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.-Z.; et al. Nanoarchitectured Structure and Surface Biofunctionality of Mesoporous Silica Nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Shi, Y.; Zhang, Y. Structure-Dependent Adsorptive or Photocatalytic Performances of Solid and Hollow Dendritic Mesoporous Silica & Titania Nanospheres. Microporous Mesoporous Mater. 2020, 305, 110326. [Google Scholar] [CrossRef]
- Li, W.; Yue, Q.; Deng, Y.; Zhao, D. Ordered Mesoporous Materials Based on Interfacial Assembly and Engineering. Adv. Mater. 2013, 25, 5129–5152. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, X.; Cheng, M.; Wang, Y.; Li, C.; Hu, S. Facile Preparation of Redox-Responsive Hollow Mesoporous Silica Spheres for the Encapsulation and Controlled Release of Corrosion Inhibitors. Prog. Org. Coat. 2019, 136, 105302. [Google Scholar] [CrossRef]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of Mesoporous Silica Nanoparticles for Oral Drug Delivery—Current Status and Perspective of MSNs Drug Carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef]
- Zhu, M.; Ou, X.; Tang, J.; Shi, T.; Ma, X.; Wang, Y.; Wu, X.; Li, Q.X.; Hua, R. Uptake, Distribution and Translocation of Imidacloprid-Loaded Fluorescence Double Hollow Shell Mesoporous Silica Nanoparticles and Metabolism of Imidacloprid in Pakchoi. Sci. Total Environ. 2021, 787, 147578. [Google Scholar] [CrossRef]
- Kaziem, A.E.; Yang, L.; Lin, Y.; Xu, H.; Zhang, Z. β-Glucan-Functionalized Mesoporous Silica Nanoparticles for Smart Control of Fungicide Release and Translocation in Plants. ACS Omega 2022, 7, 14807–14819. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Zhang, Y.; Hong, T.; Cui, J.; Zhao, Y.; Wang, Z. Degradable ZIF-8/Silica Carriers with Accropode-like Structure for Enhanced Foliar Affinity and Responsive Pesticide Delivery. Chem. Eng. J. 2024, 489, 151301. [Google Scholar] [CrossRef]
- Yadav, C.K.; Bhattarai, A.; Chaudhary, Y. Transmission Electron Microscopy and Dynamic Light Scattering-Fundamental Perspective. Cognition 2024, 6, 9–16. [Google Scholar] [CrossRef]
- Rodriguez-Loya, J.; Lerma, M.; Gardea-Torresdey, J.L. Dynamic Light Scattering and Its Application to Control Nanoparticle Aggregation in Colloidal Systems: A Review. Micromachines 2024, 15, 24. [Google Scholar] [CrossRef]
- Kong, X.-P.; Zhang, B.-H.; Wang, J. Multiple Roles of Mesoporous Silica in Safe Pesticide Application by Nanotechnology: A Review. J. Agric. Food Chem. 2021, 69, 6735–6754. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Arena, M.; Auteri, D.; Barmaz, S.; Brancato, A.; Brocca, D.; Bura, L.; Carrasco Cabrera, L.; Chiusolo, A.; Civitella, C.; et al. Peer Review of the Pesticide Risk Assessment of the Active Substance Alpha-Cypermethrin. EFSA J. 2018, 16, e05403. [Google Scholar] [CrossRef]
- Chen, K.; Mackie, J.C.; Kennedy, E.M.; Dlugogorski, B.Z. Determination of Toxic Products Released in Combustion of Pesticides. Prog. Energy Combust. Sci. 2012, 38, 400–418. [Google Scholar] [CrossRef]
- Alam, Z.; Ghamami, S.; Baghshahi, S. Synthesis and Characterization of Hollow Mesoporous Silica Nanocomposites Containing Phosphorescent Pigment and Doxycycline. Nano Sel. 2023, 4, 192–201. [Google Scholar] [CrossRef]
- Jarmolińska, S.; Feliczak-Guzik, A.; Nowak, I. Synthesis, Characterization and Use of Mesoporous Silicas of the Following Types SBA-1, SBA-2, HMM-1 and HMM-2. Materials 2020, 13, 4385. [Google Scholar] [CrossRef]
- Zhuravlev, L.T. The Surface Chemistry of Amorphous Silica. Zhuravlev Model. Colloids Surf. Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Wibowo, D.; Hui, Y.; Middelberg, A.P.J.; Zhao, C.-X. Interfacial Engineering for Silica Nanocapsules. Adv. Colloid Interface Sci. 2016, 236, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, E.A. A Comparative Study of the Method of Williamson Hall and the Pattern of Cadmium Oxide Nanoparticles for X-Rays. Turk. J. Comput. Math. Educ. TURCOMAT 2021, 12, 881–889. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Bellisai, G.; Bernasconi, G.; Binaglia, M.; Brancato, A.; Cabrera, L.C.; Castellan, I.; Castoldi, A.F.; Chiusolo, A.; Crivellente, F.; et al. Review of the Existing Maximum Residue Levels for Cypermethrins According to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2023, 21, e07800. [Google Scholar] [CrossRef] [PubMed]
- Bou-Chacra, N.; Melo, K.J.C.; Morales, I.A.C.; Stippler, E.S.; Kesisoglou, F.; Yazdanian, M.; Löbenberg, R. Evolution of Choice of Solubility and Dissolution Media after Two Decades of Biopharmaceutical Classification System. AAPS J. 2017, 19, 989–1001. [Google Scholar] [CrossRef]
- Wang, S. Ordered Mesoporous Materials for Drug Delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. Control of Encapsulation Efficiency and Initial Burst in Polymeric Microparticle Systems. Arch. Pharm. Res. 2004, 27, 1–12. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Plant Response to Silver Nanoparticles: A Critical Review. Crit. Rev. Biotechnol. 2022, 42, 973–990. [Google Scholar] [CrossRef]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in Mesoporous Silica Nanoparticles for Targeted Stimuli-Responsive Drug Delivery. Expert Opin. Drug Deliv. 2015, 12, 319–337. [Google Scholar] [CrossRef]
- Yang, J.; Cao, W.; Rui, Y. Interactions between Nanoparticles and Plants: Phytotoxicity and Defense Mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-Guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Ugbede Itodo, H. Controlled Release of Herbicides Using Nano-Formulation: A Review. J. Chem. Rev. 2019, 1, 130–138. [Google Scholar] [CrossRef]
- Subroto, E.; Andoyo, R.; Indiarto, R. Solid Lipid Nanoparticles: Review of the Current Research on Encapsulation and Delivery Systems for Active and Antioxidant Compounds. Antioxidants 2023, 12, 633. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).