Abstract
This study aimed at the development and validation of an accurate, more affordable, and precise digital imaging resazurin-based fungicide sensitivity colorimetric assay (COL-assay) for fungal plant pathogens from the genera Mycosphaerella and Pyricularia. This proposed digital imaging assay was based on colorimetric estimates of resazurin reduction, which was used as a metabolic indicator of fungal respiration activity on microplate cultures. As fungal model systems, we used the yellow and black Sigatoka pathogens [Mycosphaerella musicola (Mm) and M. fijiensis (Mf), respectively] and the wheat blast pathogen, Pyricularia oryzae Triticum lineage (PoTl), which were previously characterized for QoI, DMI, and SDHI fungicide sensitivity. We then compared the classical spectrophotometry detection assay (SPEC-assay) with the proposed COL-assay based on the analyses of digital images of the microplates’ cultures captured with mobile phone cameras on a handmade trans-illuminator built for poorly equipped labs. Qualitatively, in terms of accuracy, there was full correspondence between the SPEC-assay and the COL-assay according to the fungal EC50 or the relative growth classes on QoI, SDHI, and DMI fungicides for both Mycosphaerella and Pyricularia pathogens. We also observed a strong to very strong correlation coefficient between the COL-assay and the SPEC-assay fungicide sensitivity values for the QoI azoxystrobin, the SDHI fluxapyroxad, and the DMI tebuconazole. Our conclusion was that the COL-assay had a similar accuracy as the SPEC-assay (i.e., resulted in similar fungicide-sensitivity categories for both resistant or sensitive fungal isolates) and high precision. By openly sharing here the COL-assay’s full methodology, and the blueprints of the handmade trans-illuminator, we foresee its adoption by poorly equipped labs throughout the country as an affordable venue for monitoring the fungicide resistance status of populations of important fungal plant pathogens such as M. fijiensis, M. musicola, and P. oryzae Triticum and Oryza lineages.
1. Introduction
Fungicide sensitivity assessments of fungal pathogen populations are essential for monitoring the emergence of resistance to high-risk site-specific fungicides and reducing its spread in the agroecosystem [1,2,3]. This is especially important to prolong the efficacy of recently launched fungicide active molecules for the management of important crop diseases in agricultural fields [3]. Classical methods for assessing the fungicides baseline sensitivity of fungal populations are based on determining the EC50 (half maximal effective fungicide concentration) derived either from measurements of mycelial growth or spore germination in artificial culture media (for necrotroph or hemibiotroph fungi, which are able to grow in artificial medium) amended with different doses of fungicides [4,5,6,7]. For biotrophs these assays are conducted in planta [8,9], which is outside of the scope of our study. Most of these methods are laborious, and time and resources-consuming. Alternatively, the assessment of fungicide sensitivity can be performed measuring the respiratory activity of fungal plant pathogens based on the reduction of the metabolic indicator resazurin (RZ) or alamar blue (“ready to use” resazurin dye solution) in microplates [10,11,12].
The metabolic indicator RZ is a non-toxic dye that acts as an intermediate electron acceptor in the mitochondrial transport chain without interfering with normal electron transferring [13]. The RZ dye is extensively used for sensitivity assays of fungi, bacteria, mammalian cells, and other organisms to several chemical compounds as an indicator of metabolic activity [14]. The reduced form (pink color) of RZ predominates under high respiratory activity while the oxidized form (blue color) is associated with low cellular respiration [15,16]. Therefore, in these assays, analyses of fungal plant pathogens’ respiratory activity can be performed under optimum growth conditions on microplates by determining both the reduced (resazurin, RD) and the fluorescent oxidized (resorufin, OX) forms of RZ under spectrophotometry (i.e., the spectrum of absorbance λRD at 600 ηm and at λOX at 570 ηm) [10] or spectrofluorometry (i.e., the spectrum of absorbance λRD excitation at 530–560 ηm and at λRD emission at 590 ηm) [17].
For assessing the fungicide sensitivity status of fungal populations of important fungal plant pathogens from the genera Mycosphaerella and Pyricularia, we aimed to develop a digital imaging assay, based on colorimetric estimates of resazurin reduction (RD) as a direct indicator of fungal respiration activity derived from growth on liquid cultures in microplates, like the method described by Borra and collaborators [18]. As fungal model systems, we used the yellow and black Sigatoka pathogens [Mycosphaerella musicola (Mm) and M. fijiensis (Mf), respectively [19]] and the wheat or the rice blast pathogens Pyricularia oryzae Triticum lineage (PoTl) and P. oryzae Oryza lineage (PoOl) [20], which were previously characterized for QoI, DMI, or SDHI fungicides sensitivity [6,7,19].
The method from Borra and collaborators [18] used digital images obtained with CCD cameras to evaluate the reduction of resazurin or alamarBlue in microplates. These images were decomposed into three sets of grays from the original red (R), green (G), and blue (B) spectra. The intensities of gray tones were obtained with the ImageLab software (Bio-Rad® Laboratories, Hercules, CA, USA) or with the Adobe Photoshop® software (Adobe Inc., San Jose, CA, USA) where the reduced RZ was inferred from images in the G spectrum while the oxidized RZ from images in the R spectrum. As an opensource choice, the ReadPlate3.0 plugin [21] of the software ImageJ ([22,23], available at http://fiji.sc/ (accessed on 1 April 2022)) enables the automatic determination of the color spectra variation from all 96 wells of a microplate at once, with the possibility of reading in four different filters: green, red, blue, and gray. In fact, ReadPlate3.0/ImageJ has been widely used in many distinct colorimetric assays [21,24,25].
The success of a digital imaging assay depends on prior optimization of the fungal culturing conditions, which includes medium composition, medium pH, amount and type of initial inoculum, and incubation time, as well as the optimization for the RZ dye reaction to reflect the fungal respiratory activity [5,6,7,11,12,26,27,28,29].
Regarding the culture media, its suitableness for measurements of respiratory activity with the RZ dye is fungal dependent. For instance, potato dextrose (PD) medium was considered suitable in fungicide resistance assays of Monilinia fructicola to the DMI fenbuconazole based on measurements [11]. However, while PD was unsuitable for RZ-based QoI azoxystrobin sensitivity assays for Alternaria alternata, the CM medium was considered more appropriate for that assay [12]. This was associated with the PD medium’s high carbohydrates content, which triggered a high secretion of acids by the fungus A. alternata metabolism during growth. Because this high acidity condition accelerated RZ reduction, without a buffer adjusting and keeping the medium’s pH around neutrality, PD might be unsuitable for RZ-based fungicide sensitivity assays for A. alternata [12,26].
As for the incubation period, the recommendation is to assess the respiratory activity at its maximum level, which occurs during the log (exponential) phase of the fungal growth [26]. The few RZ-based fungicide sensitivity assays conducted with plant pathogens so far are based on 24-h incubation assessments [11,12]. However, particularly for Pyricularia, the assessment of fungicide sensitivity based on the evaluation of the fungus respiration activity in this short 24-h incubation period could result in very different phenotypes from those obtained previously [5,6,7,28], making the former relevant data incomparable with any data recently generated. This is because previous assays for QoI, DMI, and SDHI sensitivity for Pyricularia were based on fungal mycelial growth from five days incubation on liquid medium in microtiter plates. The same applies to QoI and DMI fungicide sensitivity assays for Mycosphaerella, which takes from seven to 10 days incubation [27,29].
In our study, after the experimental conditions have been optimized, including medium composition and pH, fungal inoculum concentration, incubation time, and conditions, we tested the hypothesis that a digital imaging assay based on colorimetric estimates of resazurin reduction (RD) as an indicator of fungal respiration activity (COL-assay) is as accurate and precise for fungicide sensitivity testing as the spectrophotometric assay (SPEC-assay), at similar conditions, for the plant pathogenic fungal species from the genus Myscosphaerella and Pyricularia. We then compared the classical spectrophotometry detection assay (SPEC-assay) with the proposed colorimetric assay (COL-assay) based on analyses of digital images captured with mobile phone cameras on a handmade trans-illuminator built for poorly equipped labs. For validation of the COL-assay, in comparison with the SPEC-assay, we determined the accuracy of the phenotypes of a group of resistant and sensitive isolates from Myscosphaerella and Pyricularia, previously characterized for sensitivity to the site-specific fungicides QoIs, DMIs, and SDHIs.
2. Materials and Methods
2.1. Fungal Strains
To carry out this study, we selected 13 isolates from the black or yellow Sigatoka fungal pathogens Mycosphaerella fijiensis or M. musicola with previous information about their sensitivity status to QoI [19], DMI, and SDHI fungicides derived from pilot studies in our lab. We also selected 14 isolates from the wheat or rice blast pathogens Pyricularia oryzae Triticum (PoTl) or Oryza (PoOl) lineages, previously classified according to their resistance category to QoI [5], DMI [7], and SDHI [6,30] fungicides. These Mycosphaerella and Pyricularia isolates were selected from our fungal collections spanning 2007, 2017, 2018, and 2019 sampling years from either banana plantations or cereal (wheat or rice) fields from Central-southern Brazil (Table 1).

Table 1.
Mycosphaerella and Pyricularia isolates selected for this study, their fungicide resistance categories, and doses of QoI, DMI, and SDHI fungicides tested.
2.2. Fungal Inoculum, Microplate Cultures Preparation, and Incubation Conditions for Fungicide Sensitivity Testing
Sensitivity tests to QoI, DMI, and SDHI fungicides were conducted with flat-bottomed 96-well microtiter plates (Kasvi, Parganas North, West Bengal, India) using the mycelial fragments protocol [29,31]. (Appendix A) Isolates of M. fijiensis and M. musicola were reactivated on PDA medium (20.7 g L−1 potato dextrose, 15 g L−1 agar) supplemented with chloramphenicol and streptomycin (50 µg mL−1 of each). Isolates of Pyricularia oryzae Triticum and Oryza lineages were reactivated in oatmeal agar medium (30 g L−1 oatmeal, 25 g L−1 agar) supplemented with 100 µg mL−1 chloramphenicol. After 10 days of growth at 25 °C and 12 h photoperiod, for both Mycosphaerella and Pyricularia, fungal mycelium fragments were transferred to 1.5 mL microtubes containing 0.5 mL of 0.1 mm diameter glass beads. A total of 1000 μL of distilled water was added to the mixture, which was bead-beatered in a Fast-Prep apparatus for 20 s at speed 4 m s−1. This beating cycle resulted in a suspension of small mycelial fragments, which were diluted in 10 mL of distilled water. The final concentration of the mycelial fragment’s suspension was adjusted to 104 mL−1 based on Neubauer chamber counts. The fungicides tested at distinct doses were mixed with PD medium prepared with 0.025M phosphate buffer, and final pH adjusted to 5.0. The total volume dispensed in each microplate well was 150 µL as follows: 100 µL of PD medium with and without fungicides at distinct doses, 50 µL of fungal propagules suspension. The experimental design was completely randomized with eight reps and each experiment was repeated once. The microplate with fungal liquid cultures were wrapped in plastic film and incubated at 25 °C in the dark and shaken at 150 rpm for five days for Pyricularia or 10 days for Mycosphaerella, when the fungal growth reached its maximum.
2.3. Spectrophotometric Assay (SPEC-Assay) for Fungicide Sensitivity Testing Based on Measuring the Reduction of Resazurin, a Metabolic Indicator for Fungal Respiration Activity
After completing the incubation period for maximum fungal growth, 50 µL of resazurin at 160 µM was added to each microplate well to obtain a final concentration of 40 µM (to a final volume of 200 µL) and initial absorbance readings at 569 ηm (Abs569 ηm at T0) were taken using a microplate reader device (Multiskan™ FC Microplate Photometer, Thermo Fisher Scientific, Waltham, MA, USA) for spectrophotometric estimates of RZ reduction (SPEC-assay). Subsequently, the microplates were kept at 25 °C under complete dark for a 4 h-reaction-period for Pyricularia or a 24 h-reaction-period for Mycosphaerella, when final absorbance readings were then taken at the same wavelengths (Abs569 ηm at T4 or T24).
2.4. Resazurin-Based Colorimetric Assays (COL-Assay) for Fungicide Sensitivity Testing Using a Handmade Trans-Illuminator with Attached Mobile Phone Digital Cameras
A handmade trans-illuminator was built according to Delfino [21] (Figure 1 and Figure 2) using 9-mm-thick medium-density wood fiber (MDF), painted in matte black (80 cm high × 30 cm wide × 25 cm deep), with a front door with the same height and width. A 7.5 cm diameter hole at the top of the trans-illuminator was made to attach a mobile phone digital camera. The entire interior of the trans-illuminator was covered with photographic diffuser film (Zcod®, São José, Brazil). A 20 cm × 20 cm × 2.5 cm LED lamp (Vanyluz™, São Paulo, Brazil) was installed inside, at its base. The lamp is activated by a side switch, and the power supply is 110 V. A small mark was made in the center of the LED lamp base (Figure 1B) for correctly positioning the microplate (Figure 1C). Once the light is activated (Figure 1D), the front door is closed, and the mobile phone digital camera is positioned in the top hole (Figure 1E). We applied the camera function that allows the screen to be centered in the microplate image captured, using “guides” (Figure 1E, arrow). The camera settings and the zoom were standardized, so that the images were captured under the same conditions and size (Figure 1F). A technical drawing of the handmade trans-illuminator for microplates is presented in Figure 2.
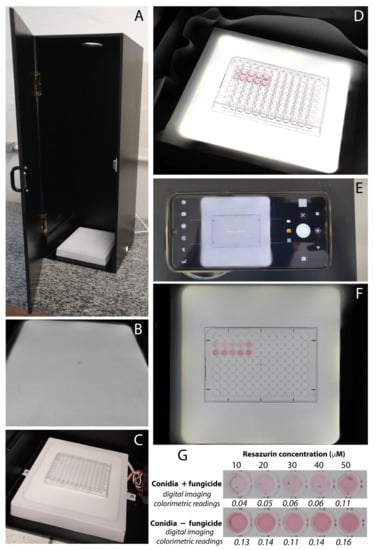
Figure 1.
(A) Handmade transilluminator for the colorimetric resazurin-based digital-imaging assay (COL-assay) developed by our group for this study. (B) Photography diffuser film covering square led lamp (marked at the center). (C) Microplate positioned above the LED lamp. (D) LED lamp turned on. (E) Positioning of the mobile phone digital camera over the top hole of the transilluminator. (F) Positioning of the experimental microplate at the center of the LEd lamp. (G) Digital images data obtained using ReadPlate3.0 plugin from Imagej software and sensitivity analysis on a preliminary test using suspension of mycelia fragments as fungal inoculum from the PoOl isolate 363 growing in PD medium supplemented or not with the fungicide azoxystrobin at 10 µg mL−1. Values shown at the bottom of each individual well are the digital-imaging-estimated colorimetric values and the pink color intensity indicates the differences in fungal respiration.
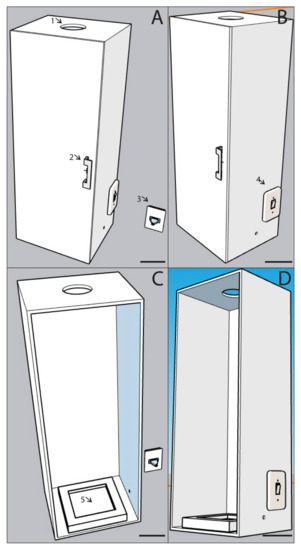
Figure 2.
Technical drawing of the transilluminator for the colorimetric resazurin-based digital-imaging assay (COL-assay) developed for capturing fungal respiration activity from mycelial growth on microplates. (A) Closed door view. 1: camera top hole. 2: door handle. 3: power plug. (B) Side view. 4: light switch. (C) Inside view. 5: square led lamp. (D) Side with door opened. Scale bars = 10 cm. The transilluminator was made with medium density wood fiber (MDF) 90 mm thick, painted in matte black color, 80 cm high, 30 cm wide, 25 cm deep.
After completing the incubation period defined for the fungal respiration resazurin-based fungicide sensitivity testing for either Mycosphaerella or Pyricularia, colorimetric estimation of RZ reduction from each individual fungal culture microplate well was determined using the ReadPlate3.0 application from the ImageJ software, with background correction (Acorr, Lachine, QC, Canadan), as described by Delfino [21].
2.5. Resazurin Oxidation-Reduction Gradient Testing for Substantiating the Assays
This experiment was conducted using an oxidation-reduction gradient resulting from the mixture of completely oxidized (OR, blue color) to completely reduced (RD, pink color) states of RZ, with the purpose of substantiating both spectrophotometric (SPEC-assay) and the digital imaging assay based on colorimetric estimates (COL-assay) of resazurin reduction. The reduced RZ solution was obtained by autoclaving the oxidized RZ for 15 min and allowing it to cool to room temperature in the dark [18]. The experiments were conducted in 96-well microplates. In each individual microplate well a total of 100 µL of PD medium prepared with 0.025M phosphate buffer, final pH 5.0 and 50 µL of M. musicola or P. oryzae Triticum lineage inoculum suspension (104 mycelial fragments.mL−1 or water as the negative control) were added. After a 5 day-incubation period for Pyricularia or a 10 day-incubation period for Mycosphaerella at 25 °C in the dark and under shaking at 150 rpm, 50 µL of RZ 160 µM were added to obtain a final concentration of 40 µM RZ in the following proportions of the reduced/oxidized forms (Figure 3).
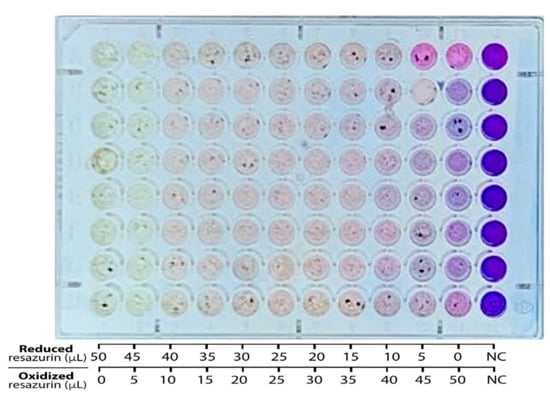
Figure 3.
Oxidation-reduction gradient obtained with the mixture of the reduced and oxidized forms of the resazurin solution at the final concentration of 40 µM *.* Mixtures of reduced and oxidized forms of RZ and respective volumes added in the microplate wells containing PD medium prepared with 0.025 M phosphate buffer, final pH 5.0 and 50 µL of M. musicola or P. oryzae Triticum lineage inoculum suspension (104 mycelial fragments·mL−1 or water as the negative control). RD = reduced resazurin (autoclaved for 15 min). OR = oxidized resazurin (no autoclaving). NC = negative control (no mycelium fragments added).
Initial absorbance readings at 569 ηm (Abs569 ηm at T0) were taken for the SPEC-assay estimates of RZ reduction, as described earlier in item 2.3. The microplate was also digitally photographed using a mobile phone camera attached to the transilluminator designed for colorimetric (COL-assay) estimation of RZ reduction at T0 using ReadPlate3.0 from ImageJ as described earlier in item 2.4. Subsequently, the microplates were kept at 25 °C under complete dark for a 4 h-reaction-period for Pyricularia or a 24 h-reaction-period for Mycosphaerella, when final absorbance readings were taken at the same wavelengths (Abs569 ηm at T4 or T24) and the microplates were then digitally photographed also at T4 or T24 for colorimetric estimation of RZ reduction.
2.6. Accuracy and Precision of the Resazurin-Based Colorimetric Assay (COL-Assay) Based on a Handmade Trans-Illuminator for Fungicide Sensitivity Testing
The objective of this phase of the study was to compare the classical spectrophotometry detection assay (SPEC-assay) with the proposed colorimetric assay (COL-assay) based on analyses of digital images captured with mobile phone cameras on a handmade trans-illuminator built. We aimed to determine the accuracy and precision of the COL-assay for fungicide sensitivity testing with both Mycosphaerella and Pyricularia fungal pathogens. For digital images capture we used an iPhone 11 with iOS16 operating system, both from Apple Inc. (Cupertino, CA, USA), with a 12MP wide camera with ƒ/1.8 aperture.
The fungicide sensitivity tests were conducted with flat-bottomed 96-well microtiter plates (Kasvi, Parganas North, West Bengal, India) using the mycelial fragments protocol, microplate cultures preparation, and incubation conditions for fungicide sensitivity testing as described earlier in this study in item 2.2. Each microplate well was filled with 50 μL of inoculum suspension and 100 μL of PD broth [20.7 g L−1 of potato dextrose (Kasvi), 1 L of distilled water)] amended with different concentrations of the QoI, DMI, or SDHI fungicides as described in Table 1.
We applied both the SPEC- and COL-assays’ protocols for fungicide sensitivity testing based on measuring the RZ reduction, as described earlier in items 2.3 and 2.4. For Mycosphaerella, The RZ reduction was estimated as follows:
where, RRMycosphaerella = relative reduction of resazurin; T = Absorbance at 569 ηm for the SPEC-assay or colorimetric reading for the COL-assay; T0 = reading at time zero (immediately after adding resazurin to the fungal 10-day-old liquid culture in buffered PD medium); T24 = reading at time 24 (24 h after adding resazurin).
RRMycosphaerella = T0 − T24
For Pyricularia, The RZ reduction was estimated as follows:
where, RRPyricularia= relative reduction of resazurin; T0 = reading at time zero (immediately after adding resazurin to the fungal 5-day-old liquid culture in buffered PD medium); T4 = reading at time 4 (4 h after adding resazurin).
RRPyricularia = T0 − T4
Based on the RZ reduction (RR) estimates we were able to determine the effective fungicide concentration for inhibiting 50% of the fungal respiration activity (EC50, in µg mL−1) using the macro ED50 plus v1.0 [32] for Excel (Microsoft™, Redmond, WA, USA). This macro uses a logarithmic function for estimating the EC50 by a dose-response curve between log (doses) and RR values. We also determined the relative growth (RG) of fungal cultures at discriminatory doses of fungicides as described earlier (Table 1). We calculated the EC50 and RG values based on RR determined by both SPEC- and COL-assays.
2.7. Statistical Analysis
Analysis of variance (ANOVA) by the F test and means comparison were performed using the R software with the statistical libraries agricolae and laercio [33].
As a measure of accuracy of the resazurin-based colorimetric assay (COL-assay), we applied the Scott–Knott test (at α ≤ 0.05 probability) to compare means with the resazurin-based spectrophotometric assay (SPEC-assay). The boxplot figures depicting the correspondence between fungicide resistance categories (based on EC50 values or relative growth estimates) as a measure of accuracy of the COL-assay in comparison with the SPEC-assay were built using the R software library tidyverse 1.3.1 [33], which included the packages ggplot2 3.3.5, purrr 0.3.4, tibble 3.1.6, dplyr 1.0.7, tidyr 1.1.4, stringr 1.4.0, readr 2.1.0, and forcats 0.5.1, and the functions ggplot, geom_boxplot, stat_summary, geom_jitter, ggtitle, theme, and geom_text.
As a measure of precision of the COL-assay, we built and compared the simple linear and the polynomial (quadratic) regression models to predict the correspondence between the relative growth or EC50 estimates from the resazurin-based spectrophotometric assay at 569 ηm (SPEC-assay) on the basis of the COL-assay measurements. The data were randomly split into a training set (77.5% of the data, for building the predictive models) and a test set (22.5%, for evaluating the models), setting a random seed for reproducibility. Next, the R function predict [specifying the model, the data set, the option interval = “confidence”] was used for predicting outcome values and the corresponding 95% confidence interval reflecting the uncertainty around the mean predictions for each regression model. The comparisons among regression models’ performance were achieved by analyzing the RMSE and the R2 metrics [34]. The RMSE represents the model prediction error, that is the average difference between the observed outcome values and the predicted outcome values. The R2 represents the squared correlation between the observed and predicted outcome values. The best model chosen between linear and quadratic was the one with the lowest RMSE and the highest R2. For depicting a scatter plot containing a regression line and the confidence interval band for the model chosen, we used the R package ggplot2 3.3.5.
The whole set of colors palette chosen to build all figures with accessibility are color-blind safe and print friendly, using the resources from Color Brewer 2.0 available at the URL https://colorbrewer2.org/#type=sequential&scheme=BuGn&n=3 (accessed on 1 April 2022).
3. Results
3.1. Resazurin Oxidation-Reduction Gradient Testing for Substantiating the Assays
The oxidation-reduction experiment reflected a perfect color gradient spanning the completely oxidized (OR, blue) to the completely reduced (RD, pink) states of RZ, used for calibrating both the spectrophotometric (SPEC-assay) and the digital imaging assay based on colorimetric estimates (COL-assay) of resazurin reduction (Figure 3). This observation was corroborated by the detection of a negatively significant correlation between the proportion of oxidized to reduced RZ and the measurements of absorbance at 569 nm (A) or the colorimetric readings (B) with p ≤ 0.01 and Adj.R2 = 0.94 (Figure 4).
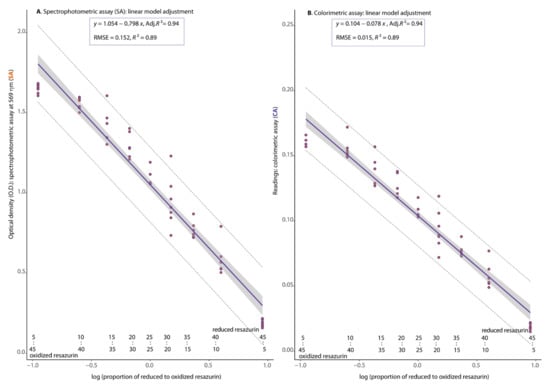
Figure 4.
Simple linear regression models predicting the correspondence between the optical density (O.D.) estimates from the resazurin-based spectrophotometric assay at 569 nm (SA = SPEC-assay) or the readings from the resazurin-based colorimetric assay (CA = COL-assay) on the basis of the proportion of reduced to oxidized resazurin (RZ) for Mycosphaerella musicola.
3.2. Accuracy and Precision of the Resazurin-Based Colorimetric Assay (COL-Assay) on a Handmade Trans-Illuminator for Fungicide Sensitivity Testing
From the qualitative point of view of the phenotyping, by which we inferred accuracy, the group of fungal isolates associated with the Sigatoka disease complex M. fijiensis (JA2.24, JA3.9a, and SA6) and M. musicola (ISC92, ISR6, and ISR21) were similarly characterized as resistant or sensitive to QoI or DMI fungicides using either the SPEC-assay (SA) or the COL-assay (CA) (Figure 5A,C). For the SDHI fungicide, in particular (Figure 5B), although the between assays EC50 values for fluxapyroxad were significantly different by the Scott–Knott test (at α ≤ 0.05) for three of the isolates tested, the resistance category was not mistakenly inferred. However, quantitatively, either by the SPEC- or the COL-assay, significant phenotypic differences were detected between resistant and sensitive isolates of M. fijiensis and M. musicola to all three fungicide groups (Figure 5). This joint observation demonstrated the accuracy of the COL-assay fungicide sensitivity testing.
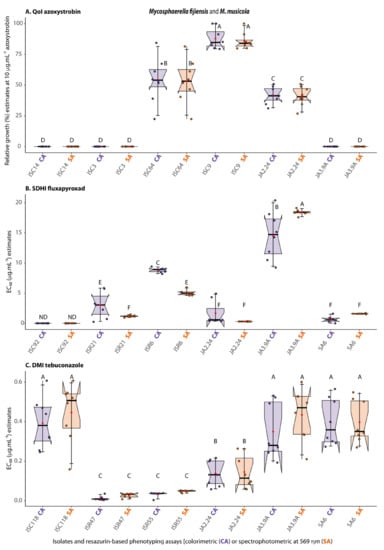
Figure 5.
Correspondence between fungicide resistance categories (based on EC50 values or relative growth estimates) as a measure of accuracy of the resazurin-based colorimetric assay (CA = COL-assay, purple boxplots) in comparison with the classical spectrophotometry detection assay (SA = SPEC-assay, orange boxplots) for testing sensitivity of Mycosphaerella fijiensis or M. musicola to QoI (A), SDHI (B), or DMI (C) fungicides a,b. a The COL-assay was based on analyses of digital images of experimental microplates captured with mobile phone cameras on a handmade trans-illuminator designed for colorimetric estimation of RZ reduction from T0 to T24 using the ReadPlate3.0 in ImageJ developed by Delfino [21] as described in item 2.6 from this study. b The figure depicts boxplots with the medians represented by black lines across the notches, the average as red circles along the whisker lines, and the jittered data points in purple or orange to avoid data overplotting. The F statistics for treatment effect was significant at least at p ≤ 0.05. Boxplots with the same letter on top (from A to F) indicate that the means for the particular isolate or resazurin-based phenotyping assay are not significantly different by the Scott–Knott test at α ≤ 0.05.
From the quantitative point of view, by which we measured the precision of the assay, in general, for the three fungicides tested, the EC50 or the values of relative growth in discriminatory doses of fungicides for the COL-assay were significantly and positively correlated with the values from the SPEC-assay (with Adj.R2 varying from 0.76 for the SDHI, 0.81 for the DMI, to 0.99 for the QoI fungicides) (Figure 6A–C), indicating the fair to high precision of the assay.
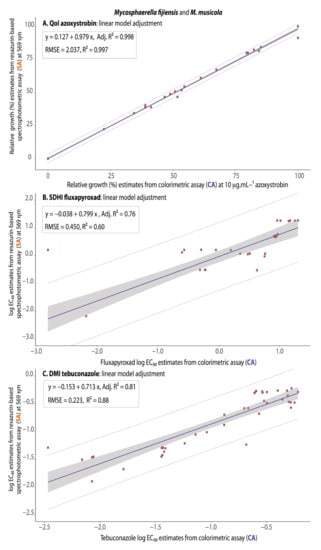
Figure 6.
Simple linear regression models predicting the correspondence between the log EC50 or fungus relative growth estimates from the spectrophotometric assay at 569 nm (SA = SPEC-assay) on the basis of log EC50 or relative growth estimates from the colorimetric assay (CA = COL-assay) for QoI (A), SDHI (B), or DMI (C) fungicide sensitivity testing for Mycosphaerella fijiensis and M. musicola a,b. a The rectangle within each figure contains the corresponding linear equations, the adjusted R2 (Adj.R2) as a measure of precision of the colorimetric assay, and the model fit parameters (RMSE and R2). In all three cases the linear regression had the best fit as a predicting model, with the lowest RMSE and the highest R2. b The regression lines were presented in purple, the confidence interval band of the prediction model in gray and the distribution of the values by orange circles contained within the limits of dotted lines.
A similar outcome was observed for the fungicide sensitivity testing conducted with isolates of the wheat or rice blast pathogens (PoTl or PoOl), previously characterized according to their resistance category to QoI [5], SDHI [6,30], or DMI [7,28] fungicides. Qualitatively, in terms of accuracy, there was full correspondence between the SPEC-assay and the COL-assay according to the fungal relative growth classes on QoI, SDHI, and DMI fungicides (Figure 7A–C) inferred for the group of PoTl (12.1.015, 12.1.037, 12.1.045i, 12.1.130, 12.1.183, 12.1.299, 12.1.312, 18MGH19, 18MGH25, or 18SPK6) and PoOl isolates (421, 656, or 704). In addition, either by the COL-assay or by the SPEC-assay, significant phenotypic differences were detected between resistant and sensitive isolates of PoTl or PoOl to all the three fungicide groups.
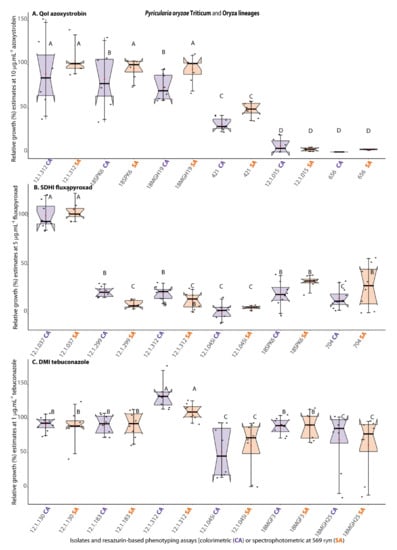
Figure 7.
Correspondence between fungicide resistance categories (based on relative growth estimates) as a measure of accuracy of the resazurin-based colorimetric assay (COL-assay, purple boxplots) in comparison with the classical spectrophotometry detection assay (SPEC-assay, orange boxplots) for testing sensitivity of Pyricularia oryzae Triticum (PoTl) or P. oryzae Oryza lineage (PoOl) to QoI, (A), SDHI (B), and DMI (C) fungicides a,b. a The COL-assay was based on analyses of digital images of experimental microplates captured with mobile phone cameras on a handmade trans-illuminator designed for colorimetric estimation of RZ reduction from T0 to T4 using the ReadPlate3.0 in ImageJ developed by Delfino [21], as described in item 2.6 from this study. b The figure depicts boxplots with the medians represented by black lines across the notches, the average as red circles along the whisker lines, and the jittered data points in purple or orange to avoid data overplotting. The F statistics for treatment effect was significant at least at p ≤ 0.05. Boxplots with the same letter on top (from A to D) indicate that the means for the particular isolate or resazurin-based phenotyping assay are not significantly different by the Scott–Knott test at α ≤ 0.05.
Qualitatively, considering the measure of precision of the assay, the values of relative growth in discriminatory doses of fungicides for the COL-assay were significantly and positively correlated with the values from the SPEC-assay for all three fungicides, fitting to either linear or quadratic models (with Adj.R2 varying from 0.60 for the DMI, 0.83 for the QoI, to 0.84 for the SDHI) (Figure 8), also indicating the fair to high precision of the assay.
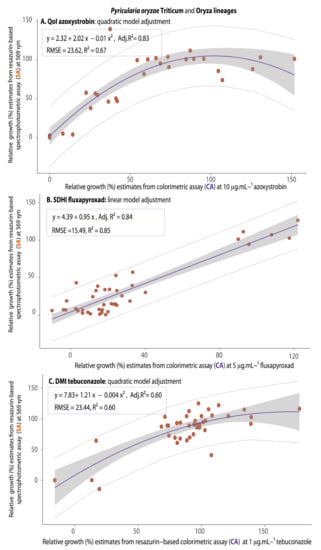
Figure 8.
Regression models predicting the correspondence between the fungus relative growth estimates from the SPEC-assay at 569 nm (SA) on the basis of relative growth estimates from the COL-assay (CA) for QoI (A), SDHI (B), and DMI (C) fungicide sensitivity testing for Pyricularia oryzae Triticum lineage and P.oryzae Oryza lineage a,b. a The rectangle within each figure contains the corresponding linear equations, the adjusted R2 (Adj.R2) as a measure of precision of the colorimetric assay, and the model fit parameters (RMSE and R2). For the QoI (A) and DMI fungicide (C) the quadratic regression had the best fit as a predicting model while for SDHI (B) the linear regression had the best fit, with the lowest RMSE and the highest R2. b The regression lines were presented in purple, the confidence interval band of the prediction model in gray and the distribution of the values by orange circles contained within the limits of dotted lines.
4. Discussion
The main objective of our study was the development of an accurate, affordable, and precise resazurin-based digital imaging colorimetric assay for the assessment of fungicide sensitivity status of populations of fungal plant pathogens.
Preliminary steps for the optimization of the fungal culture conditions were taken to aid the development of our RZ-based digital imaging colorimetric assay. For instance, the testing of three culture media (PD [5,19], SN [35], and V8 medium [36]), at three different pHs (5.0, 6.0, or 7.0), for RZ-based sensitivity assays to determine QoI, DMI, and SDHI fungicides for Mycosphaerella and Pyricularia, indicated the suitability of PD medium for both fungal pathogens with an initial pH 5.0 (data not shown). Differing from other assays reported [11,12], in our RZ-based assay we have chosen to use buffered PD medium prepared with 0.025M phosphate buffer, to avoid significant changes in its pH during fungal growth and when supplementing the media with distinct fungicide concentrations.
Considering the amount of fungal inoculum for our RZ-based fungicide resistance colorimetric assays, we checked the effect of variable concentrations of fungal propagules (for both Mycosphaerella and Pyricularia). There was a positive correlation between respiratory activity measured as RZ reduction and fungal inoculum concentration (from 0 to 105 propagules.mL−1), validating the sensitivity of the assay (data not shown). For the subsequent RZ-based assays conducted in this study, the concentration of 104 propagules.mL−1 was chosen as an initial inoculum, which matched previous non-RZ-based fungicide sensitivity assays with these pathogens [5,6,7,19,27,28,29].
As for the incubation period, in our proposed RZ-based fungicide sensitivity assays we kept the same incubation period from five to 10 days, as reported in previous studies with Pyricularia and Mycosphaerella. We then amended the liquid medium of the fungal growing colonies with the RZ dye and subsequently measured the respiration activity at four to 24 h, for Pyricularia or Mycosphaerella, respectively, after incubation at 25 °C under completely dark conditions [5,6,7,19,27,28,29].
After the experimental conditions had been optimized, we tested the hypothesis that the colorimetric assay (COL-assay) was as accurate and precise for fungicide sensitivity testing as the spectrophotometric assay (SPEC-assay), under similar conditions. The fungicide sensitivity was assessed based on a direct measure of fungal respiratory activity in distinct fungicide doses, by quantifying the reduction of the metabolic indicator resazurin (RZ), as extensively reported for other systems [10,11,12]. The equivalence between the estimates of optical density values from the SPEC-assay and the readings from the COL-assay with the corresponding levels of RZ reduction (Figure 4) was the first indication that the COL-assay developed here can be useful for fungicide resistance phenotyping.
As model systems, we phenotyped the black and yellow Sigatoka fungal pathogens M. fijiensis and M. musicola and the wheat or the rice fungal pathogens P. oryzae Triticum and Oryza lineages. For the COL-assay we built a handmade trans-illuminator for capturing digital images of the experimental 96-well microplates with an attached mobile phone camera (Figure 1 and Figure 2).
The COL-assay required the use of the opensource software Image J “ReadPlate” plugin, which allows the user to define a grid of circular regions superimposed upon the microplate image. A grid is subsequently created by defining the number of rows and columns of the microplate, delimiting the pixel coordinates of each well (e.g., well A1 to H12) and the diameter of each analysis circle [37].
Similar techniques based on image analysis using ImageJ have been used to evaluate a variety of analytical targets, from residues of organophosphates, as well as forensically and clinically relevant compounds [38,39,40,41].
Using previously optimized experimental conditions, which included medium composition, pH, concentration of fungal inoculum, incubation conditions, and period, we then tested the hypothesis that the COL-assay was as accurate and precise as the SPEC-assay for fungicide sensitivity testing at similar conditions.
In general, for Mycosphaerella, the COL-assay performed as accurately as the SPEC-assay in cataloging fungal population according to fungicide resistance categories (based on EC50 values or relative growth in discriminatory doses of the active ingredients) to the three major fungicide classes tested: QoI—strobilurin (azoxystrobin), SDHI (fluxapyroxad), and the DMI—triazole (tebuconazole). Therefore, all the Mycosphaerella isolates were classified in the exact same resistance or sensitivity category in both methods (Figure 5). The COL-assay also performed as precisely as the SPEC-assay in determining levels of fungicide resistance as indicated by the correspondence between EC50 values or fungal relative fungal growth estimates from the two assays for all the three fungicides classes (Figure 6). In all three cases, the trend line plotted from the distribution of the data pairs from the two assays indicated a positive linear correlation. From a merely statistical point of view (by which correlation coefficients could be classified as weak/moderate/strong/very strong), we observed a very strong correlation coefficient between the COL-assay and the SPEC-assay values for the QoI azoxystrobin (R2 =0.997) and strong for fluxapyroxad (R2 = 0.60) and tebuconazole (R2 = 0.88) [42] (Figure 6).
Similar to the Mycosphaerella pathogens, correspondence between the COL- and the SPEC-assays was also detected for the resistance categories of the wheat or rice blast Pyricularia pathogens PoTl and PoOl to the QoI azoxystrobin, SDHI fluxapyroxad, and DMI tebuconazole, indicating both accuracy and precision (Figure 7 and Figure 8).
Taking all this information together, our study explored the applicability of a digital image colorimetric assay (COL-assay), associated with the measurement of resazurin activity during fungal growth, using a hand-made apparatus for the digital documentation of reactions in fungicide resistance detection experiments for both Mycosphaerella and Pyricularia pathogens. Since the the COL-assay has been shown to be accurate and precise, it is an affordable choice for poorly equipped laboratories with no access to a spectrophotometric microplate reader, and for end users such as extension plant pathologists with little experience in digital image processing. For instance, the COL assay specific costs require the framing of the handmade transilluminator (which includes carpentry: US$ 50; painting: US$ 20; lamp, cable and power plug acquisition: US$ 25; electrician and other services: US$ 10; totaling: US$ 105) and the purchase of a cell phone device (US$ 583), totaling US$ 688. In comparison, the SPEC assay requires the availability of a microplate spectrophotometer containing the specific 569 and 620 ηm filters for the readings. Our model (Multiskan™ FC Microplate Photometer, from Thermo Fisher Scientific, Waltham, MA, USA) costs US$ 8070. Therefore, the COL-assay could result in saving up to US$ 7382 in equipment costs.
By openly sharing its full methodology and the blueprints of the trans-illuminator we foresee its adoption throughout the country as a phenotyping platform for monitoring the fungicide resistance status of populations of several other important necrotrophic or hemibiotrophic fungal plant pathogens, besides M. fijiensis, M. musicola, and P. oryzae. In Brazil, these relevant pathogens include, as examples, Alternaria alternata on tangerines [43], Botrytis cinerea on strawberries [44], Colletotrichum accutatum on apples [45], Colletotrichum truncatum and Corynespora cassiicola on soybeans [46,47,48], Lasiodiplodia theobromae on papaya [49], Monilinia fruticola on stone fruits [50], Phyllosticta citricarpa on citrus [51], and Ramulariopsis gossypii and R. pseudoglycines on cotton [52]. This fungicide resistance phenotyping platform will certainly require successful optimization and validation of the COL-assay for such a broad range of pathosystems.
Such a countrywise fungicide resistance detection platform would deliver free access to data on the status of fungicide resistance and guide smart decisions, in real time (i.e., during the ongoing cropping seasons) on reducing or limiting the spraying of high-risk fungicides for which resistance has been detected.
5. Conclusions
The fungicide sensitivity assay developed here based on colorimetric analyses of digital images of fungal growth on microtitter plates allowed the accurate and precise assessment of fungicide resistance phenotype from several samples of two fungal pathogen species.
The handmade trans-illuminator developed for the colorimetric assays has low costs, thus offering an affordable alternative of large-scale fungicide resistance phenotyping in poorly equipped labs in Brazil.
Author Contributions
Conceptualization, P.C.C., S.I.M., T.C.S. and S.N.C.V.; methodology, T.C.S., P.C.C., S.N.C.V., F.G.A.J., T.Y.K.O. and A.G.S.; software, F.G.A.J. and P.C.C.; validation, T.C.S.; formal analysis, T.C.S. and P.C.C.; investigation, T.C.S., F.G.A.J., S.N.C.V., S.I.M., F.S.C.J., T.Y.K.O. and A.G.S.; resources, A.A.P.C., P.C.C. and M.C.G.G.; data curation, T.C.S., S.I.M. and P.C.C.; writing, T.C.S., P.C.C. and S.I.M.; writing—review and editing, T.C.S., S.I.M., S.N.C.V., M.C.G.G., A.A.P.C., R.P.L.J., W.C.J.J. and P.C.C.; visualization, M.C.G.G., R.P.L.J., W.C.J.J. and P.C.C.; supervision, P.C.C. and S.I.M.; project administration, P.C.C.; funding acquisition, A.A.P.C. and P.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP (São Paulo Research Foundation, Brazil), Grant/Award Numbers: 2017/50456-1, 2018/21197-0, 2019/12509-1, 2020/07611-9, 2021/03402-9, and 2022/12661-0; in partnership with Fundação Araucária (F.A., Paraná, Brazil) to A.A.P.C. (FPS2020011000057); CNPq (Brazilian National Council for Scientific and Technological Development), Grant/Award Numbers: Pq-1D 313825/2018-1 and Pq-1C 311895/2022-0; CAPES (Coordination for the Improvement of Higher Level Personnel), Grant/Award Numbers: CAPES/Pro-equipment Program 775202/2012, CAPES/AUXPE Program 88881.593505/2020-01—UNESP Ilha Solteira Campus, CAPES PrInt Program/UNESP, CAPES studentship Program 001); Newton Fund /BBSRC (Biotechnology and Biological Sciences Research Council, United Kingdom), Grant/Award Number: BB/S018867/2 awarded by BBSRC under the BBSRC-FAPESP Antimicrobial Resistance and Insecticide Pest Resistance in Livestock and Agriculture Program. T.Y.K.O. and F.G.A.J. were supported by a M.Sc. research scholarship from CAPES. S.N.C.V., A.G.S. and T.C.S. were supported by Ph.D scholarships from CAPES or UNIVESP.
Informed Consent Statement
The Brazilian Ministry of Environment/National System for the Management of Genetic Heritage and Associated Traditional Knowledge—SisGen, issued the Certificates # A64D0EA and A100786 authorizing the scientific activities associated with the collection of botanical and fungal material from the banana and wheat agroecosystems in the Cerrado´s and Atlantic Forest´s biomes and access the phenotypic diversity of Mycosphaerella and Pyricularia species.
Data Availability Statement
Upon publication, the phenotypic data presented in this study will be publicly available at Mendeley Data repository at https://doi.org/10.17632/rcjy95gbvg.1 (accessed on 16 December 2022).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A. Reagents and Media for Resazurin-Based Fungicide Sensitivity Assays
Phosphate buffer at 0.025 M: 2.18 g of K2HPO4, 0.68 g of KH2PO4, to a final volume of 1 L distilled water. Use this buffer to replace water for media preparation.
Potato dextrose (PD) medium: 27g PD Kasvi®, 0.05g of Chloramphenicol, 0.05 g of Streptomycin, 0.078g of salicylhydroxamic acid, to a final volume of 1L of 0.025M phosphate buffer.
SN medium: 1 g of KH2PO4, 1 g of KNO3, 0.5 g of MgSO4 · 7H2O, 0.5g of KCl, 0.2 g of glucose, 0.2 g of sucrose, 0.05 g of chloramphenicol, 0.05 g of streptomycin, 0.078 g of salicylhydroxamic acid, to a final volume of 1L of 0.025M phosphate buffer.
V8 medium: 100 mL of V8 juice, 1 g of CaCO3, 0.05 g of chloramphenicol, 0.05 g of streptomycin, 0.078 g of salicylhydroxamic acid, complete with 0.025 M phosphate buffer to a final volume of 1 L.
NaOH solution at 1 M for pH adjustment of the media: 0.399 g of NaOH in 10 mL of distilled water.
HCl solution at 4M for pH adjustment of the media: Dilute 84 mL of concentrated HCl in water to a final volume of 250 mL.
Note about medium preparation: Use as little NaOH and HCl as possible adding drop by drop in the medium to reach the desired pH. After preparing the medium, adjust the medium´s pH before autoclaving. After autoclaving, add the chloramphenicol and salicylhydroxamic acid (SHAM), and adjust the final pH again. These steps are essential otherwise the medium will have a very dark color after autoclaving, which can interfere with the experimental results.
Resazurin solution: Prepare Resazurin (Sigma-Aldrich®) (RZ) stock solution at 800 µM (= 0.02% w/v): 0.01 g in 50 mL of potassium phosphate buffer. Perform filtration of the stock solution using 0.22 µM filter and syringe.
Prepare RZ working solution: dilute to 160 µM. For example, to prepare 50 mL of 160 µM RZ, add 10 mL of 800 µM RZ stock solution + 40 mL of potassium phosphate buffer.
Potassium phosphate buffer at 0.1 M, pH 7.0: For a final volume of 50 mL mix 0.1 M K2HPO4 (0.435 g/25 mL water) + 0.1 M KH2PO4 (0.135 g/25 mL water).
Note about preparing working resazurin solutions: RZ solutions should not be exposed to direct light. Preparation and storage can be performed using 50 mL Falcon tubes, wrapped in aluminum foil. Store RZ solutions in the refrigerator at 10 °C (~20 months) or at −20 °C (indefinite time). Heat RZ solution for 10 min in a water bath at 37 °C before use (if stored at −20 °C, thaw it first).
The recommended final RZ concentration is 40 µM. Therefore mix 50 µL RZ solution at 160 µM with 100 µL medium and 50 µL of inoculum suspension, to a final volume of 200 µL.
References
- Kleinkauf, N.; Verweij, P.E.; Arendrup, M.C.; Donnelly, P.J.; Cuenca-Estrella, M.; Fraaije, B.A.; Melchers, W.J.G.; Adriaenssens, N.; Kema, G.H.J.; Ullmann, A.; et al. Risk Assessment on the Impact of Environmental Usage of Triazoles on the Development and Spread of Resistance to Medical Triazoles in Aspergillus Species; Technical report/European Centre for Disease Prevention and Control; ECDC Europäisches Zentrum für die Prävention und die Kontrolle von Krankheiten: Stockholm, Sweden, 2013; ISBN 978-92-9193-444-7. [Google Scholar]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The Evolution of Fungicide Resistance. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 90, pp. 29–92. ISBN 978-0-12-802275-7. [Google Scholar]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2022, 71, 150–169. [Google Scholar] [CrossRef]
- Casado, P.S.; De Carvalho, G.; Ceresini, P.C.; Castroagudín, V.L.; Sabbag, O.J.; Vicentini, S.N.C.; Maciel, J.L.N. Método eficiente, baseado em leitores de microplaca, para detecção de resistência a fungicidas triazóis (IDM) e estrobirulinas (IQe) em populações do patógeno da brusone do trigo. Summa Phytopathol. 2018, 44, 236–244. [Google Scholar] [CrossRef]
- Castroagudín, V.L.; Ceresini, P.C.; de Oliveira, S.C.; Reges, J.T.A.; Maciel, J.L.N.; Bonato, A.L.V.; Dorigan, A.F.; McDonald, B.A. Resistance to QoI Fungicides Is Widespread in Brazilian Populations of the Wheat Blast Pathogen Magnaporthe Oryzae. Phytopathol. 2015, 105, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, S.N.C.; Casado, P.S.; de Carvalho, G.; Moreira, S.I.; Dorigan, A.F.; Silva, T.C.; Silva, A.G.; Custódio, A.A.P.; Gomes, A.C.S.; Maciel, J.L.N.; et al. Monitoring of Brazilian wheat blast field populations reveals resistance to QoI, DMI, and SDHI fungicides. Plant Pathol. 2022, 71, 304–321. [Google Scholar] [CrossRef]
- Poloni, N.M.; Carvalho, G.; Vicentini, S.; Dorigan, A.; Maciel, J.L.; McDonald, B.A.; Moreira, S.; Hawkins, N.; Fraaije, B.A.; Kelly, D.E.; et al. Widespread distribution of resistance to triazole fungicides in Brazilian populations of the wheat blast pathogen. Plant Pathol. 2021, 70, 436–448. [Google Scholar] [CrossRef]
- De Mello, F.E.; Mathioni, S.M.; Fantin, L.H.; Rosa, D.D.; Antunes, R.F.D.; Filho, N.R.C.; Duvaresch, D.L.; Canteri, M.G. Sensitivity assessment and SDHC-I86F mutation frequency of Phakopsora pachyrhizi populations to benzovindiflupyr and fluxapyroxad fungicides from 2015 to 2019 in Brazil. Pest Manag. Sci. 2021, 77, 4331–4339. [Google Scholar] [CrossRef]
- Müller, M.A.; Stammler, G.; De Mio, L.L.M. Multiple resistance to DMI, QoI and SDHI fungicides in field isolates of Phakopsora pachyrhizi. Crop. Prot. 2021, 145, 105618. [Google Scholar] [CrossRef]
- Barua, P.; You, M.P.; Bayliss, K.; Lanoiselet, V.; Barbetti, M.J. A rapid and miniaturized system using Alamar blue to assess fungal spore viability: Implications for biosecurity. Eur. J. Plant Pathol. 2017, 148, 139–150. [Google Scholar] [CrossRef]
- Cox, K.D.; Quello, K.; Deford, R.J.; Beckerman, J.L. A Rapid Method to Quantify Fungicide Sensitivity in the Brown Rot Pathogen Monilinia fructicola. Plant Dis. 2009, 93, 328–331. [Google Scholar] [CrossRef]
- Vega, B.; Liberti, D.; Harmon, P.F.; Dewdney, M.M. A Rapid Resazurin-Based Microtiter Assay to Evaluate QoI Sensitivity for Alternaria alternata Isolates and Their Molecular Characterization. Plant Dis. 2012, 96, 1262–1270. [Google Scholar] [CrossRef]
- Page, B.; Page, M.; Noel, C. A new fluorometric assay for cytotoxicity measurements in-vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]
- Mania, D.; Hilpert, K.; Ruden, S.; Fischer, R.; Takeshita, N. Screening for Antifungal Peptides and Their Modes of Action in Aspergillus nidulans. Appl. Environ. Microbiol. 2010, 76, 7102–7108. [Google Scholar] [CrossRef]
- Prakash, G.; Boopathy, M.; Selvam, R.; Kumar, S.J.; Subramanian, K. The effect of anthracene-based chalcone derivatives in the resazurin dye reduction assay mechanisms for the investigation of Gram-positive and Gram-negative bacterial and fungal infection. New J. Chem. 2018, 42, 1037–1045. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Balbaied, T.; Moore, E. Resazurin-Based Assay for Quantifying Living Cells during Alkaline Phosphatase (ALP) Release. Appl. Sci. 2020, 10, 3840. [Google Scholar] [CrossRef]
- Borra, R.C.; Lotufo, M.A.; Gagioti, S.M.; de Mesquita Barros, F.; Andrade, P.M. A simple method to measure cell viability in proliferation and cytotoxicity assays. Braz. Oral Res. 2009, 23, 255–262. [Google Scholar] [CrossRef]
- Oliveira, T.Y.K.; Silva, T.C.; Moreira, S.I.; Christiano, F.S.; Gasparoto, M.C.G.; Fraaije, B.A.; Ceresini, P.C. Evidence of Resistance to QoI Fungicides in Contemporary Populations of Mycosphaerella fijiensis, M. musicola and M. thailandica from Banana Plantations in Southeastern Brazil. Agronomy 2022, 12, 2952. [Google Scholar] [CrossRef]
- Ceresini, P.C.; Castroagudín, V.L.; Rodrigues, F.; Rios, J.A.; Aucique-Pérez, C.E.; Moreira, S.I.; Croll, D.; Alves, E.; de Carvalho, G.; Maciel, J.L.N.; et al. Wheat blast: From its origins in South America to its emergence as a global threat. Mol. Plant Pathol. 2019, 20, 155–172. [Google Scholar] [CrossRef]
- Delfino, J.M. ReadPlate, version 3.0; ImageJ Plugin; School of Pharmacy & Biochemistry, University of Buenos Aires: Buenos Aires, Argentina, 2020. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Colzani, H.; Rodrigues, Q.E.A.G.; Fogaça, C.; Gelinski, J.M.L.N.; Pereira-Filho, E.R.; Borges, E.M. Determinação de fosfato em refrigerantes utilizando um scanner de mesa e análise automatizada de dados: Um exemplo didático para ensino de química. Química Nova 2017, 40, 833–839. [Google Scholar] [CrossRef]
- Da Silva, E.K.; dos Santos, V.B.; Resque, I.S.; Neves, C.A.; Moreira, S.G.C.; Franco, M.D.O.K.; Suarez, E.W.T. A fluorescence digital image-based method using a 3D-printed platform and a UV-LED chamber made of polyacid lactic for quinine quantification in beverages. Microchem. J. 2020, 157, 104986. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.I.S.; Bibiano, L.B.J.; Da Silva, G.F.; Hanada, R.E.; Mizubuti, E.S.G. Baseline sensitivity of Brazilian Mycosphaerella fijiensis isolates to protectant and systemic fungicides. Trop. Plant Pathol. 2014, 39, 172–177. [Google Scholar] [CrossRef]
- Dorigan, A.F.; De Carvalho, G.; Poloni, N.M.; Negrisoli, M.M.; Maciel, J.L.N.; Ceresini, P.C. Resistance to triazole fungicides in Pyricularia species associated with invasive plants from wheat fields in Brazil. Acta Sci. Agron. 2019, 41, 39332. [Google Scholar] [CrossRef]
- Brito, F.S.D.; Santos, J.R.P.; Azevedo, V.C.R.; Peixouto, Y.S.; de Oliveira, S.A.; Ferreira, C.F.; Haddad, F.; Amorim, E.P.; Fraaije, B.; Miller, R.N.G. Genetic Diversity and Azole Fungicide Sensitivity in Pseudocercospora musae Field Populations in Brazil. Front. Microbiol. 2020, 11, 99. [Google Scholar] [CrossRef]
- Vicentini, S.N.C.; Moreira, S.I.; da Silva, A.G.; de Oliveira, T.Y.K.; Silva, T.C.; Junior, F.G.A.; Krug, L.D.; de Paiva Custódio, A.A.; Júnior, R.P.L.; Teodoro, P.E.; et al. Efflux Pumps and Multidrug-Resistance in Pyricularia oryzae Triticum Lineage. Agronomy 2022, 12, 2068. [Google Scholar] [CrossRef]
- Peláez Montoya, J.E.; Vásquez David, L.E.; Diaz Brito, T.J.; Castañeda Sánchez, D.A.; Rodríguez Beltrán, E.; Arango Isaza, R.E. Use of a Micro Title Plate Dilution Assay to Measure Activity of Antifungal Compounds against Mycosphaerella Fijiensis. Rev. Fac. Nac. Agron. Medellín 2006, 59, 3425–3433. [Google Scholar]
- Vargas, M.H. ED50plus v1.0. Available online: https://archive.org/details/ed50v10_zip (accessed on 2 March 2022).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Bruce, P.C.; Bruce, A.; Gedeck, P. Practical Statistics for Data Scientists: 50+ Essential Concepts Using R and Python, 2nd ed.; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2020; ISBN 978-1-4920-7289-8. [Google Scholar]
- Leslie, J.F.; Summerell, B.A.; Bullock, S. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 978-0-470-27646-4. [Google Scholar]
- Hanada, R.E.; Gasparotto, L.; Pereira, J.C.R. Esporulação de Mycosphaerella fijiensis em diferentes meios de cultura. Fitopatol. Bras. 2002, 27, 170–173. [Google Scholar] [CrossRef]
- Parker, R.W.; Wilson, D.J.; Mace, C.R. Open software platform for automated analysis of paper-based microfluidic devices. Sci. Rep. 2020, 10, 11284. [Google Scholar] [CrossRef]
- Shah, M.M.; Ren, W.; Irudayaraj, J.; Sajini, A.A.; Ali, M.I.; Ahmad, B. Colorimetric Detection of Organophosphate Pesticides Based on Acetylcholinesterase and Cysteamine Capped Gold Nanoparticles as Nanozyme. Sensors 2021, 21, 8050. [Google Scholar] [CrossRef]
- Hwang, J.; Kwon, D.; Lee, S.; Jeon, S. Detection of Salmonella bacteria in milk using gold-coated magnetic nanoparticle clusters and lateral flow filters. RSC Adv. 2016, 6, 48445–48448. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, L.Q.; Peng, C.; Chen, Z.; Xu, C. A new development of measurement of 19-Nortestosterone by combining immunochromatographic strip assay and ImageJ software. Food Agric. Immunol. 2009, 20, 1–10. [Google Scholar] [CrossRef]
- Amaral, C.; Antunes, W.; Moe, E.; Duarte, A.G.; Lima, L.M.P.; Santos, C.; Gomes, I.L.; Afonso, G.S.; Vieira, R.; Teles, H.S.S.; et al. A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples. Sci. Rep. 2021, 11, 16430. [Google Scholar] [CrossRef]
- Callegari-Jacques, S.M. Bioestatística: Princípios e Aplicações; Artmed: Porto Alegre, RS, Brazil, 2007; ISBN 978-85-363-0092-4. [Google Scholar]
- Chitolina, G.M.; Silva-Junior, G.J.; Feichtenberger, E.; Pereira, R.G.; Amorim, L. Distribution of Alternaria alternata isolates with resistance to quinone outside inhibitor (QoI) fungicides in Brazilian orchards of tangerines and their hybrids. Crop. Prot. 2021, 141, 105493. [Google Scholar] [CrossRef]
- Maia, J.N.; Beger, G.; Pereira, W.V.; De Mio, L.L.M.; da Silva Silveira Duarte, H. Gray mold in strawberries in the Paraná state of Brazil is caused by Botrytis cinerea and its isolates exhibit multiple-fungicide resistance. Crop. Prot. 2021, 140, 105415. [Google Scholar] [CrossRef]
- Moreira, R.R.; Hamada, N.A.; Peres, N.A.; De Mio, L.L.M. Sensitivity of the Colletotrichum acutatum Species Complex from Apple Trees in Brazil to Dithiocarbamates, Methyl Benzimidazole Carbamates, and Quinone Outside Inhibitor Fungicides. Plant Dis. 2019, 103, 2569–2576. [Google Scholar] [CrossRef]
- Dias, M.D.; Dias-Neto, J.J.; Santos, M.D.; Formento, A.N.; Bizerra, L.V.; Fonseca, M.E.N.; Boiteux, L.S.; Café-Filho, A.C. Current Status of Soybean Anthracnose Associated with Colletotrichum truncatum in Brazil and Argentina. Plants 2019, 8, 459. [Google Scholar] [CrossRef]
- Boufleur, T.R.; Ciampi-Guillardi, M.; Tikami, Í.; Rogério, F.; Thon, M.R.; Sukno, S.A.; Júnior, N.S.M.; Baroncelli, R. Soybean anthracnose caused by Colletotrichum species: Current status and future prospects. Mol. Plant Pathol. 2021, 22, 393–409. [Google Scholar] [CrossRef]
- De Mello, F.E.; Lopes-Caitar, V.S.; Xavier-Valencio, S.A.; da Silva, H.P.; Franzenburg, S.; Mehl, A.; Verreet, J.; Balbi-Peña, M.I.; Marcelino-Guimaraes, F.C.; Godoy, C.V. Resistance of Corynespora cassiicola from soybean to QoI and MBC fungicides in Brazil. Plant Pathol. 2022, 71, 373–385. [Google Scholar] [CrossRef]
- Li, Y.; Tsuji, S.S.; Hu, M.; Câmara, M.P.S.; Michereff, S.J.; Schnabel, G.; Chen, F. Characterization of difenoconazole resistance in Lasiodiplodia theobromae from papaya in Brazil. Pest Manag. Sci. 2020, 76, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Dutra, P.S.S.; Lichtemberg, P.D.S.F.; Martinez, M.B.; Michailides, T.J.; De Mio, L.L.M. Cross-Resistance Among Demethylation Inhibitor Fungicides with Brazilian Monilinia fructicola Isolates as a Foundation to Discuss Brown Rot Control in Stone Fruit. Plant Dis. 2020, 104, 2843–2850. [Google Scholar] [CrossRef] [PubMed]
- Possiede, Y.; Gabardo, J.; Kava-Cordeiro, V.; Galli-Terasawa, L.; Azevedo, J.; Glienke, C. Fungicide resistance and genetic variability in plant pathogenic strains of Guignardia citricarpa. Braz. J. Microbiol. 2009, 40, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Mathioni, S.M.; de Mello, F.E.; Antunes, R.F.D.; Duvaresch, D.L.; Milanesi, D.F.; Brommonschenkel, S.H.; Pinho, D.B.; Rosa, D.D. Species Determination and CYTB-G143A Monitoring of Ramulariopsis spp. Isolated from Cotton in Brazil. Plant Health Prog. 2022, 23, 4–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).