Abstract
As microplastics (MPs) are an emerging pollution to farmland ecosystems, the research into the ecological and environmental effects of MPs need to be clarified urgently. Available nitrogen is the determining factor for productivity in most terrestrial ecosystems, especially for the farmland ecosystems with a high productivity. To explore the effects of MPs on soil available nitrogen in farmland soil, an incubation experiment was conducted by adding polyethylene MPs with different concentrations to farmland soil, which was collected from farmland in Fuqing, Fujian Province. The contents of three different nitrogen forms (nitrate, ammonium, and dissolved organic nitrogen) and soil dissolved organic carbon were measured, and the soil mineralization rate was calculated. Bacteria was quantified and bacterial community diversity indexes were measured. The results showed that the MPs addition (T1 and T2) had no significant effect on soil ammonium, nitrate and soil nitrogen mineralization rate compared to the control (p > 0.05). However, a significant increase was observed in soil dissolved organic carbon and dissolved organic nitrogen content (p < 0.05). It can be seen that the influence of PE MPs on the soil dissolved organic nitrogen is greater than that of inorganic nitrogen. The results of this study showed no major detrimental effects of MPs on the abundance of some bacterial families, whereas a significant change in soil bacterial evenness index was observed in T2 treatment compared to the treatment without MPs addition. In the background of current MPs pollution, the research results can provide a scientific basis for reducing nitrogen loss in soil and protecting farmland soil safety.
1. Introduction
Microplastics (MPs) represent an emerging pollutant to the global ecological environment [1]. Its high surface curvature could contribute to a high loading capacity for persistent organic pollutants and heavy metal [2,3]. The ecological effects of MPs have attracted considerable attention in recent years [4,5,6,7] and the deleterious effects on the ocean has received overwhelming attention compared to the terrestrial ecosystem [8,9]. Nevertheless, most plastic debris were released into terrestrial ecosystems, as has been reported. The amount of MPs on terrestrial environments was 4–23 folds larger than in the maritime ecosystem [10]. Indeed, Nizzetto et al. [11] suggested that farmland soils alone might accumulate more MPs than ocean. MPs presence is ubiquitous in terrestrial ecosystems as has several potential impacts for the ecosystem structure and function as well as for human health. For example, physiochemical alternations triggered by MPs could change soil microbe diversity, potentially influencing N fixing association in soil ecosystem [1]. Additionally, MPs exposure in humans could result in inflammatory and gene damage [12]. Therefore, it is urgently needed to investigate the deleterious ecological impacts of such small plastic particles in agricultural soil.
Nitrogen (N) is considered to be the primary growth-limited factor in various terrestrial ecosystems [13], especially for the agricultural ecosystem with a high productivity. The fact is that N in the soil mainly exists in the form of organic N, which is difficult to be absorbed by plants. Thus, the soil N mineralization rate plays a key role in N availability because plants can utilize the organic N when it is mineralized into inorganic N [14]. MPs arriving in agricultural systems can accumulated in the soil, resulting in changes in soil physical and biological properties, such as soil porosity, air circulation and carbon (C) [7,15]. These alternations in the soil structure and function driven by MPs could affect soil microbial activity and biodiversity [16,17], with potential impacts on soil N availability and mineralization [18]. At present, little research about the effects of MPs on soil available N has been conducted, but these studies yielded some inconsistent results. For example, Liu et al. [19] observed a significant change in soil soluble organic N (DON), soluble organic carbon (DOC) and nitrate N (NO3−-N) after the addition of 28% (w/w) polypropylene (PP) MPs (<180 μm) to a losses soil compared to the control. Chen et al. [20] suggested that the small pieces of a biological plastic termed polyactic acid (PLA) could decrease the concentration of ammonium, while increasing the contents of nitrate in soil. Boots et al. [21] indicated that the addition of polyethylene (PE) MPs affected the microbial activity related to N cycling and reducing N availability. The study by Green et al. [22] indicated that the present of PE microplastics can inhibit the activities of key enzymes in the soil N cycle, and then affect the soil N cycle. It has also been reported that PP MPs can directly inhibit N availability by chelating ammonium N in the soil through their surface functional groups [23]. The reason for these differences in results may be attributed to MPs type, morphology and their dose. To the best of our knowledge, the potential changes in soil biophysical properties triggered by MPs contamination have not been studied.
Plastics films are widely used in agriculture practice to maintain and conserve soil temperature and moisture around the world [24]. The global market for agricultural films was estimated at 4.6 million tons in 2013, and was expected to grow at a rate of 6.0% per year over the period 2013–2020 [25]. China is the largest consumer and producer of agricultural films. The usage of plastic films increased dramatically in China from 0.32 to 1.25 million tonnes from 1991 to 2011 [26]. PE is the most dominating raw material for agricultural films segment and accounted for about 60% of total market volume in 2013, potentially a major resource of MPs in farmland soil [25,27]. About 1270–2130 tons of MPs particles per million habitants per year were reported to be generated in European urban areas, while an annually MPs addition from sludge in European agricultural sites ups to 63,000–43,000 tons [5]. Around 7100–42,900 MPs particles per kg was estimated to be accumulated in agricultural soil in China [28]. The majority of MPs particle size is between 50 μm and 1 mm, accounting for 95% of the total MPs arriving in the agricultural soil [28].
To investigate the effects of MPs on soil available N in farmland soil, an incubation experiment was conducted by adding PE MPs (125–150μm) with different concentrations to farmland soil collected from a farmland in Fuqing, Fujian Province. This study analyzed the contents of three different N forms (nitrate, ammonium, and dissolved organic N (DON)) and soil dissolved organic carbon (DOC), and calculated the soil mineralization rate. The microbial diversity was also measured to illustrate how MPs alter the soil microbial community and functions [29]. The present study aims at revealing the influence of MPs on N availability in farmland soil and provides a scientific basis for risk management and control of farmland soil from MPs pollution.
2. Materials and Methods
2.1. Soil Sampling
The soil used in this experiment were sampled in Jiangjing Modern Agriculture Garden (25.5° N and 119.4° E) in Fujian Province, China, at an elevation of 4.6 m above sea level. This area climate is characterized by a subtropical monsoon climate with a mean annual temperature of 21.1 °C and an annual rainfall of 1450 mm. This soil type is sandy loam. The soil samples were collected in November 2021 after the pepper being harvested. Five sample soil cores (upper 0–15 cm) were randomly collected in a 20 m × 20 m plot in the above-mentioned agricultural garden. The collected soil was stored in a deepfreeze and transported to laboratory at the same day. After removing stones and plant residues, soil samples sieved on 2 mm to remove fine roots and divided into two subsamples: One part was used for the analysis of soil physical and chemical properties, and the other was stored in a refrigerator for the following incubation experiments. The soil properties are shown in Table 1.

Table 1.
Soil basic properties.
2.2. Experimental Design
Three treatments were established in this study: (1) CK: control (no MPs added); (2) T1: 1 g of MPs added (1% w/w); and (3) T2, 5 g MPs added (5% w/w). The MPs used in this experiment is made of Polyethylene (Cospheric, CA, USA). The MPs concentrations added into soil was based on the previous studies where MPs were observed to significantly accumulate up to 7% in the topsoil [30,31]. The soil and PE MPs were evenly mixed and placed in sterilized glass pots. The density of MPs is 0.96 g/cm3 and the particle size is 125 µm–150 µm. MPs were sterilized 3–4 times by using 95% absolute alcohol to remove other organic matter on the surface of MPs particles before adding to soil. The soils were slightly compacted by free gravity falling. The soil moisture was kept at 60% of field capacity throughout the experiment. The pots were covered by cling-film with some tiny holes to keep gas exchange, and then were incubated in a dark environment at 25 °C and a relative humidity of 75%. Samples from each pot were removed on 1, 2, 4, 6, 10, 15 and 30 days. Each treatment had five replicates. The soil samples were separated to three groups. One was kept in a refrigerator awaiting analysis for soil NH4+, NO3− and soil organic N. Another group was used to test soil basic properties. The third group was stored at −20 °C for the analysis of soil microbe.
2.3. Soil Physicochemical Properties
Soil pH was determined by using a pH meter (PHS-3C pH meter, Rex, Shanghai, China) in a 1:2.5 soil to water ratio. Total organic carbon (TOC) and total N (TN) were measure by using an element analyzer (Vario Isotope Cube, Germany). Mineral N was extracted with 2 M KCl at a soil-to-solution ratio of 1:5. The concentration of and were measured by a discrete chemistry analyzer (SmartChem 200, AMS Alliance, Rome, Italy). Soil DON and DOC were extracted with 0.5 mol L−1K2SO4 and quantified using a TOC analyzer (Shimadzu, Kyoto, Japan). Net N mineralization rate was measured as the difference between the final and initial inorganic N contents divided by the number of incubation days.
2.4. Soil Microbial Diversity
Soil samples were extracted to quantify the bacterial community diversity using high-throughput sequencing technology. The 0.5 g soil was extracted by a soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA). The hypervariable region V4-V5 of bacterial 16S rRNA genes were amplified with primer 515F (GTGCCAGCMGCCGCGG) and 907R (CCGTCAATTCMTTTRAGTTT). The conditions and procedures of Polymerase Chain Reaction (PCR) amplification are referred to this paper from Fei et al. [32], and the PCR quantification was performed, followed by the illustration from Wang et al. [33]. Purified amplicons were sequenced with an Illumina MiSeq platform (Illumina, San Diego, CA, USA) according to the standard protocols provided by Majorbio BioPharm Technology Co. Ltd. (Shanghai, China). The raw bacterial sequence in this study were deposited in National Center for Biotechnology Information (NCBI) Sequence Read Archive database (SUB12195744).
Alpha community diversity analysis (i.e., Chao1/ASV richness indexes, evenness indices and Shannon diversity estimator) was analyzed by applying the operational taxonomic unit (OTU) table in Quantitative Insights Into Microbial Ecology toolkit (QIIME). The indexes of diversity (Chao1, ASV, Shannon and Evenness indices) were analyzed.
2.5. Statistical Analysis
Values shown in figures and tables were presented with mean value (±standard deviation, SD). One-way ANOVA was used to determine the differences in soil DIN, DON, DOC and mineralization rates among treatments with a least significant difference (LSD) multiple comparisons test. Pearson correlation analysis was employed to test degrees of correlation among soil N mineralization rate and NH4+, NO3−, DON, DOC. All statistical analyses and figures were performed using Origin 8.5 (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. The Effects of MPs on Soil Inorganic N and Mineralization Rate
There was no significant difference in content among CK, T1 and T2 during the incubation period (p > 0.05) (Figure 1A). Similar to NH4+, NO3− contents except day 1, were observed to have no significant change after MPs addition (Figure 1B). The NO3− content was higher in T1 (13.58 ± 1.27 mgN·kg−1) and significantly lower in T2 than CK at the first day after MPs addition. Then nitrate contents declined quickly to 9.18 ± 0.09 mg N·kg−1 between days one and two among three treatments, following by a fluctuation from day two until the end of incubation. MPs additions had no significant effects on the soil net N mineralization rate in comparison to CK (p > 0.05).
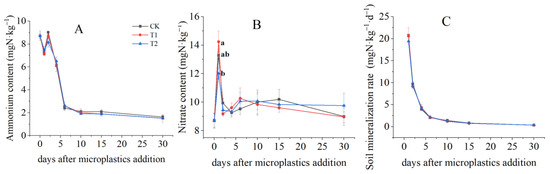
Figure 1.
Temporal variations of soil NH4+ (A), NO3− (B) contents and net mineralization rate (C). Letters designate significant differences among treatments (p < 0.05).
3.2. The Effects of MPs on Soil DON and DOC
MPs addition significantly affected DON contents (p < 0.05) (Figure 2A). MPs enhanced the release of DON throughout the incubation, as compared to the CK treatment. A significant increase (p < 0.05) of DON content was observed in T2 than T1 during the first six days after MPs addition. At day 6, DON content in T2 and T1 increased to 30.5 mg N·kg−1 and 33.7 mg N·kg−1, respectively, relative to CK. Between days 6 and 15, DON contents in T1 and T2 showed a significant decline tendency. The DON content decreased by 3.3% in T1 and 32.9% in T2. While increases of DON were observed in all the treatments from day 15. On day 30, the DON content was the highest in T1 (38.7 mg N·kg−1), followed by T2 (33.0 mg N·kg−1).
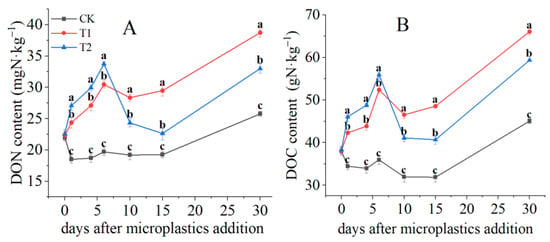
Figure 2.
Temporal variations of soil DON (A) and DOC contents (B). Letters designate significant differences among treatments (p < 0.05).
Soil DOC content showed a similar tendency with DON (Figure 2B). Compared to CK, the DOC content increased significantly in T2 and T1. Dramatic decline was observed at T2 and T1 from day 6 to day 15. The DOC content decreased to 40.7 g N·kg−1 and 48.6 g N·kg−1 in T2 and T1, respectively. Then, DOC content increased from day 15 to day 30 in all treatments. On day 30, the highest amount of DOC content was in T1 (66.13 g N·kg−1), followed by T2 (59.4 g N·kg−1).
3.3. Bacterial Community Diversity
As shown in Table 2, ASV, chao1 and Shannon indices did not show a significant difference (p > 0.05) in these three different treatments. While a significant difference was observed between the soil absent of MPs and 5% PE (T2) in Evenness index (p < 0.05). Nonetheless, there is no significant difference in the evenness index values between CK and T1 treatments.

Table 2.
Effects of MPs on the diversity of bacteria community.
4. Discussion
4.1. The Effects of MPs on Soil Inorganic N and Mineralization Rate
Available N, including inorganic N (nitrate and ammonium) and dissolved organic N, is regarded as an important limiting factor for productivity in most terrestrial ecosystems. The results showed that no or little changes in net mineralization rate and inorganic N content in farmland soil were observed after two levels (1% and 5%) PE MPs addition in comparison to CK throughout the incubation period. This result was consistent with the observation of Blocker et al. [34], where soil inorganic N and mineralization rates after 1% low density polyethylene (LDPE) MPs addition were not different from control. A similar result was found by Ren et al. [35], who added 1% MPs to soil and no or little changes was shown in the soil inorganic N. In contrast to our results, an experiment from Liu et al. [19] indicated an increase of nitrate content at a very high MPs addition concentration (28%), but they also found no difference in ammonium content between different treatments. Chen et al. [20] also found that 2% polyactic acid addition had no significant effects on soil nutrients, including soil nitrate and ammonium contents.
The responses of soil inorganic N to MPs additions were observed in different studies, which may result from the experimental conditions, MP types and addition levels. Previous studies have shown that MPs can alter soil physical and chemical properties, such as increasing soil porosity and changing the structure of soil aggregates [36,37,38], leading to alternation in soil microbial activity [39]. MPs addition levels were strongly correlated with microbial activity, affecting the release of soil N [40]. In comparison to the study by Liu et al. [19], we conducted incubation in a lower temperature (25 °C) and lower humidity (75%) without light, accounting for some of the differences. MPs breaking down to smaller plastics particles might accelerate UV-radiation under a light exposure condition [41,42]. Higher temperatures and higher humidity are beneficial for microbial activity [43]. In addition, the incubation term is an important reason to explain the differences in soil inorganic N. A short incubation term is so short that MPs could not decompose and display the effects on soil inorganic N.
4.2. The Effects of MPs on Soil DON and DOC
Soil soluble organic carbon and N are vital parts of soil organic matter and play an important role in soil nutrient biogeochemical cycling. Meanwhile, DON and DOC are sensitive indicators for the changes in soil quality [19,44]. Our results showed that soil DON and DOC content were significantly enhanced after MPs addition (p < 0.05). The tendencies along incubation time between DON and DOC were similar. Significant increases observed in soil DON and DOC at the first six days after MPs addition might due to the disturbance during soil and MPs mixing [19]. A decline tendency of DON and DOC from days 6 to 10 is also shown by the results of this study. Two reasons could be contributed to this phenomenon. On one hand, the consumption of resources and nutrients, such as soil organic matters, led to the decrease of microbial activity and quantity. On the other hand, the utilization of DON and DOC by microorganisms resulted in the decrease of soil DON and DOC contents. Plastic particles have a very high content of carbon [45], and this material will be decomposed slowly, contributing to a very high C: N ratio in soil. PE MPs could be utilized as C source by microorganisms and potentially enhanced microbial activities [46]. In turn, the increase in microbial activities further promoted soil organic matter decomposition, likely leading to the increases in soil DON and DOC contents from days 10 to the end of this incubation. However, it has to be emphasized that microbes are not only producers for soil DOC, but also a potential source of soil DOC [47] since the dead body of microorganisms was an important C source. Therefore, soil DON and DOC increased from days 15 to 30.
Currently, the impact of MPs on soil DON and DOC are inconsistent among different studies. For example, Liu et al. [19] indicated that the concentration of polypropylene MPs at 28% can promote the activity of hydrolase and increase the DOC content, a low concentration of 7% had no significant effect on soil DOC. In contrast to the studies conducted by Blocker et al. [34], the results suggested both PE (5%, w/w) and PLA (1%, w/w) additions did not have significant effects on DOC content. The reason for these differences in results may be attributed to the MPs type, morphology and their dose. Therefore, in order to better investigate the effects of MPs addition, further research about different MP types under various conditions is encouraged in the future.
4.3. The Effects of MPs on the Bacterial Community Diversity
These results reveal that the abundance of some bacterial families remains largely unaffected by PE addition throughout the incubation. Our results were consistent with the previous study conducted by Judy et al. [48], where no significant difference was observed in the bacterial community diversity under the 1% concentration addition with high-density polyethylene (HDPE), polyethylene terephthalate (PET), or polyvinyl chloride (PVC) MPs in comparison to the control. Notwithstanding, our study indicated a significant change in soil bacterial evenness index after MPs addition at a high concentration. This phenomenon may due to the number of dominant species in soil microorganisms at a high level of MPs addition, then an increase in the number of other species. Zhang et al. [31] suggested that MPs might promote the bacterial groups during the period of their own biodegradation, resulting in an alternation in bacterial community structure. However, the interactions between MPs and microorganisms were complicated, largely depending on soil properties, MP types and their dose [31,49].
5. Conclusions
This study investigated the effects of MPs on soil available N by adding PE MPs to farmland soil. Responses of soil ammonium, nitrate and N mineralization rate to MPs addition were negligible. However, significant increases were observed in soil DOC and DON contents with the existence of MPs. The abundance of bacterial families was not affected by MPs, whereas a significant change in soil bacterial evenness was observed in a high level of MPs addition. According to the findings, we concluded that the occurrence of MPs poses a profound effect on soil DON rather than soil inorganic N. While MPs had no significant influence on bacterial richness, the number of each bacterial species tends to be homogeneous. The effects of MPs on soil N availability depended on experimental conditions, incubation term, MPs types and dose. With regard to a sustainable release of MPs into farmland ecosystem, it is urgently needed to address the alternations in soil biogeochemical processes affected by MPs in relation to their features and clarify the microbial mechanism.
Author Contributions
W.L. performed the data analyses and wrote the manuscript; Z.C. contributed to the conception and design of the study; H.R. performed the experiment and data collection; D.X. contributed significantly to the analysis with constructive discussion and critical revision of this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 32001217), Natural Science Foundation of Fujian Province, China (Grant No. 2020J05242), and Fujian Universities and Colleges Engineering Research Center of Modern Facility Agriculture (Grant No. C1603904).
Data Availability Statement
The data that support the findings of this study are openly available. Currently, the raw bacterial sequence in our study were deposited in NCBI Sequence Read Archive (SRA) database (SUB12195744) and we will release immediately all these data if this manuscript is accepted.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Hoylaerts, M.F.; Hoet, P.H.M.; Vermylen, J.; Nemery, B. Size effect of intratracheally instilled particles on pulmonary inflammation and vascular thrombosis. Toxicol. Appl. Pharmacol. 2003, 186, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Wick, P.; Malek, A.; Manser, P.; Meili, D.; Maeder-Althaus, X.; Diener, L.; von Mandach, U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010, 118, 432–436. [Google Scholar] [CrossRef]
- Law, K.L.; Thompson, R.C. Microplastics in the seas. Science 2014, 345, 144–145. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.L.; Lwanga, E.H.; Eldridge, S.M.; Johnston, P.; Hu, H.W.; Geissen, V.; Chen, D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018, 627, 1377–1388. [Google Scholar] [CrossRef]
- Pinto da Costa, J.; Paço, A.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. Microplastics in soils: Assessment, analytics and risks. Environ. Chem. 2018, 16, 18–30. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastics in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef]
- Chae, Y.; An, Y.J. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 2018, 240, 387–395. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Devriese, L.; Galgani, F.; Robbens, J.; Janssen, C.R. Microplastics in sediments: A review of techniques, occurrence and effects. Mar. Environ. Res. 2015, 111, 5–17. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef] [PubMed]
- Forte, M.; Iachetta, G.; Tussellino, M.; Carotenuto, R.; Prisco, M.; De Falco, M.; Laforgia, V.; Valiante, S. Polystyrene nanoparticles internalization in human gastric adenocarcinoma cells. Toxicol. Vitr. 2016, 31, 126–136. [Google Scholar] [CrossRef] [PubMed]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Du, E.; Terrer, C.; Pellegrini, A.F.A.; Ahlstrom, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.H.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Gorlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Machado, A.A.S.; Valyi, K.; Rillig, M.C. Potential environmental impacts of an “underground revolution”: A response to Bender et al. Trends Ecol. Evol. 2016, 32, 8–10. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Halley, J.M.; Rillig, M.C. Extinction risk of soil biota. Nat. Commun. 2015, 6, 10. [Google Scholar] [CrossRef]
- Rousk, J.; Michelsen, A.; Rousk, J. Microbial control of soil organic matter mineralization responses to labile carbon in subarctic climate change treatment. Glob. Chang. Biol. 2016, 22, 4150–4161. [Google Scholar] [CrossRef]
- Liu, H.F.; Yang, X.M.; Liu, G.B.; Liang, C.T.; Xue, S.; Chen, H.; Ritsema, C.J.; Gerssen, V. Response of soil dissolved organic matter to microplasrics addition in Chinese loess soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Chen, H.P.; Wang, Y.H.; Sun, X.; Peng, Y.K.; Xiao, L. Mixing effect of polylactic acid microplastics and straw residue on soil property and ecological function. Chemosphere 2020, 243, 125271. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of microplastics in soil ecosystems: Above and below ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Boots, B.; Sigwart, J.; Jiang, S.; Rocha, C. Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling. Environ. Pollut. 2016, 208, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, S.; Sintim, H.Y.; Debruyn, J.M. Structural and functional responses of soil microbial communities to biodegradable plastic film mulching in two agroecosystems. BioRxiv 2019, 5, 650317. [Google Scholar]
- Wu, Y.; Huang, F.; Jia, Z.; Ren, X.; Cai, T. Response of soil water, temperature, and maize (Zea may L.) production to different plastic film mulching patterns in semi-arid areas of northwest China. Soil Tillage Res. 2017, 166, 113–121. [Google Scholar] [CrossRef]
- Market Analysis Report: Agricultural Films Market Size, Share & Trends Analysis Report by Raw Material (LDPE, LLDPE, Reclaims), by Application (Green House, Mulching, Silage), by Region, and Segment Forecasts, 2016–2024. Available online: https://www.grandviewreserch.com/industry-analysis/agricultural-films-market (accessed on 4 December 2022).
- Yearbook, C.S. China Statistical Yearbook from 1982 to 2012; China Statistics Press: Beijing, China, 2012. [Google Scholar]
- Liu, E.K.; He, W.Q.; Yan, C.R. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef]
- Zhang, G.S.; Liu, Y.F. The distribution of microplastics in soil aggregate fractions in southwestern China. Sci. Total Environ. 2018, 642, 12–20. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.Q.; Yan, C.R.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Fuller, S.; Gautam, A.A. Procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci. Technol. 2016, 11, 5774–5780. [Google Scholar] [CrossRef]
- Zhang, M.J.; Zhao, Y.R.; Qin, X.; Jia, W.Q.; Chai, L.W.; Huang, M.K.; Huang, Y. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 2019, 688, 470–478. [Google Scholar] [CrossRef]
- Fei, Y.F.; Huang, S.Y.; Zhang, H.B.; Tong, Y.Z.; Wen, D.S.; Xia, X.Y.; Wang, H.; Luo, Y.M.; Barceló, D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2019, 707, 135634. [Google Scholar] [CrossRef]
- Wang, J.; Luo, Y.M.; Teng, Y.; Ma, W.T.; Christie, P.; Li, Z.G. Soil contamination by phthalate esters in Chinese intensive vegetable production systems with different modes of use of plastic film. Environ. Pollut. 2013, 180, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Blocker, L.; Watson, C.; Wichern, F. Living in the plastic age-Different short-term microbial response to microplastics addition to arable soils with contrasting soil organic matter content and farm management legacy. Environ. Pollut. 2019, 267, 115468. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.W.; Tang, J.C.; Liu, X.M.; Liu, Q.L. Effects of microplastics on greenhouse gas emissions and the microbial community in fertilized soil. Environ. Pollut. 2020, 256, 113347. [Google Scholar] [CrossRef] [PubMed]
- Ladd, J.N.; Foster, R.C.; Skjemstad, J.O. Soil structure: Carbon and nitrogen metabolism. Geoderma 1993, 56, 401–434. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salanki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef]
- Zhang, F.; Li, M.; Qi, J.H.; Li, F.M.; Sun, G.J. Plastic film mulching increases soil respiration in ridge-furrow maize management. Arid. Land Res. Manag. 2015, 29, 432–453. [Google Scholar] [CrossRef]
- Naveed, M.; Herath, L.; Moldrup, P.; Arthur, E.; Nicolaisen, M.; Norgaard, T.; Ferre, T.P.A.; de Jonge, L.W. Spatial variability of microbial richness and diversity and relationships with soil organic carbon, texture and structure across an agricultural field. Appl. Soil Ecol. 2016, 103, 44–55. [Google Scholar] [CrossRef]
- Simon, J.; Klotz, M.G. Diversity and evolution of bioenergetic systems involved in microbial nitrogen compound transformations. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 114–135. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef]
- Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Occurrence and ecological impacts of microplastics in soil systems: A review. Bull. Environ. Contam. Toxicol. 2019, 102, 741–749. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Li, X.M.; Chen, Q.L.; He, C.; Shi, Q.; Chen, S.C.; Reid, B.J.; Zhu, Y.G.; Sun, G.X. Organic carbon amendments affect the chemodiversity of soil dissolved organic matter and its associations with soil microbial communities. Environ. Sci. Technol. 2019, 53, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Microplastic disguising as soil carbon storage. Environ. Sci. Technol. 2018, 52, 6079–6080. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A.; de Souza Machado, A.A.; Yang, G.W. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Marshall, M.R.; Zhao, J.; Gui, H.; Yang, Y.D.; Zeng, Z.H.; Jones, D.L.; Zhang, H.D. Microplastics as an emerging threat to plant and soil health in agroecosystems. Sci. Total Environ. 2021, 787, 147444. [Google Scholar] [CrossRef]
- Judy, J.D.; Williams, M.; Gregg, A.; Oliver, D.; Kumar, A.; Kookana, R.; Kirby, J.K. Microplastics in municipal mixed-waste organic outputs induce minimal short to long-term toxicity in key terrestrial biota. Environ. Pollut. 2019, 252, 522–531. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.H.; Li, Y.; Powell, T.; Wang, X.; Wang, G.Y.; Zhang, P.P. Microplastics as contaminants in the soil environment: A mini-review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).