Abstract
The effects of humic acid on plant yield, essential oil content, the composition of essential oil and the antimicrobial activity of Origanum vulgare L. subsp. hirtum (Link.) (cv. Tinmaz) cultivated in 2017 and 2018 under Eskisehir ecological conditions were evaluated. Three humic acid (HA) doses in response to 50.0 L ha−1 (HA 50), 30.0 L ha−1 (HA 30) and 0.0 L ha−1 (HA 0, as control) were applied to soil at the vegetative stage and beginning of the blooming stage of the plant throughout each harvest in both years. Essential oil composition was determined using GC-FID/GC-MS. The antibacterial and antifungal activity were determined by the well-diffusion method. Fresh herb yield, dry herb yield and dry leaf yield were highest at HA 50 both years, although essential oil content increased in 2017 at both HA 50 and HA 30, but was greatest at HA 50 in the second year. The essential oil content differences between the control dose and 50.0 L HA ha−1 were 0.46% and 0.42% in 2017 and 2018, respectively. The antimicrobial activity of the essential oil samples against two bacteria species (Enterococcus faecalis and Staphylococcus aureus) and two yeast species (Candida albicans and Candida parapisilosis) was generally higher than that of the control drugs, and the activity increased with increasing HA doses. Analysis of the essential oil components showed that the carvacrol and γ-Terpinene ratios generally increased as the HA doses increased to 50.0 L HA ha−1. Soil HA applications could be recommended for higher quality, plant yield and antimicrobial activity of Origanum vulgare L. subsp. hirtum.
1. Introduction
Some medicinal and aromatic plants possess antimicrobial activity due to the presence of essential oils (EOs) [1,2,3,4,5]. EOs contain components that are effective against different organisms such as bacteria and fungi [6,7,8,9,10] and are therefore especially important in controlling plant diseases in the field and postharvest [11,12]. Pesticides are commonly used to effectively control plant diseases, despite their harmful effects for human health and the environment. Toxic residues are formed as a result of using synthetic fungicides or bactericides. In addition, new resistant strains of microorganisms may develop due to the intensive usage of pesticides. Therefore, the use of natural substances such as EOs, which are safer for consumers and the environment, are preferred [13,14,15]. Similarly, in medicine, increased resistance to antibiotics have led researchers to investigate the antimicrobial activities of medicinal and aromatic herbs [16,17,18].
Origanum vulgare L. subsp. hirtum (Link.) Letswaart is a perennial plant which is widely used in agriculture, the pharmaceutical, cosmetic and food industry. Many studies have reported that the EOs of oregano and its primary component carvacrol were efficient in antimicrobial activity [19,20,21]. It has been stated that there are some factors, such as environmental, genetic and agronomic which may affect the chemical variability of oregano EOs’ composition. Particularly agronomic factors are known to have a large influence on the quality and yield of the plant [22,23,24].
Many agronomic methods are used to increase plant yield and quality and to improve the soil properties. Among these agronomic methods is the application of humic substances to the soil. HAs are the organic substances that increase the chemical and biological properties of the soil and root environment [25,26,27,28,29]. This substance added to the soil has both direct and indirect effects on the growth of plants. While the direct effects are promoting the development of root systems and the uptake of mineral elements applied to the soil by converting them into absorbable forms, the indirect effect is the improvement of the physical and chemical texture of the soil. As a result, HA increases the rooting, flowering, fruiting and the yield of the plant [30,31,32,33].
The application of humic substances to medicinal and aromatic plants has gained prominence recently. The fresh and dry herb weight and essential oil rates of Origanum syriacum L. increased with increasing humic acid doses [34], and increasing HAD increased the EOs of Thymus vulgaris L. [35]. Although the literature lacks data on the effects of HS on yield traits and essential oil content of Origanum vulgare L. plants cultivated especially at field conditions, a pot trial was conducted with a single dose of HS and revealed significant differences and high values of fresh weight and essential oil content [24]. In addition, no research has been conducted on the effects of HS on the antibacterial and antifungal activity of Origanum vulgare L. In a recent pot trial, basil plants were treated with three different HS doses, which functioned as an effective biostimulant and improved the production and bioactive features of basil EOs. In addition, the effects of HS on the antibacterial activity of basil EOs were found to be significantly high compared to the control doses of HS and the essential oil composition changed with the maximum dose which induced a concentration increase in eugenol and methyl-eugenol [36]. On the other hand, different ecological conditions of essential oil components may depend on day length, climatic factors, plant development period, photoperiod and vegetation period [37].
It is of great interest to investigate the effects of HA on plant yield, the composition of EOs and the antimicrobial activity of Origanum vulgare L. subsp. hirtum (Link.) (cv. Tinmaz) cultivated in the harvest seasons of 2017 and 2018 under Eskisehir ecological conditions. The objectives of this research were to study the effects of three HA doses applied to soil (i) on the fresh herb yield, dry herb yield, dry leaf yield, essential oil content and (ii) on the antifungal and antibacterial activity and composition of EOs of Origanum vulgare L. subsp. hirtum (Link.) (cv. Tinmaz) which have not been studied previously.
2. Materials and Methods
2.1. Site Conditions
Field experiments were carried out in two growing seasons (2017 and 2018) at the Faculty of Agriculture (Eskisehir Osmangazi University, Turkey. N 39°48′, E 30°31′, altitude 789 m).
In both years, the soil samples (0–30 cm) were taken from each plot at the begin of the vegetation period. They were dried, sieved through a 2 mm stainless sieve and analyzed for pH (1:2.5 soil:water), electrical conductivity (EC, 1:2.5 soil:water) [38], lime (Scheibler calcimeter), organic matter [39], available K (1N ammonium acetate, pH 7), available P (sodium bicarbonate method) [40] and texture (hydrometer method). Fe, Cu, Mn and Zn concentrations were analyzed [41] with an atomic absorption spectrometer (Analytik Jena novAA 350, Jena, Germany).
The soil of the research area was alkaline, loam texture soil. It was moderately calcareous with low content of organic matter and salt concentration. P2O5 content of the soil was insufficient. The contents of K2O and Fe were moderately sufficient. An insufficient content of Zn and sufficient contents of Mn and Cu were obtained (Table 1).

Table 1.
Soil characteristics of the research area in 2017 and 2018.
Meteorological data were obtained by the weather station at Eskisehir (Figure 1 and Figure 2). Total rainfall amount (221.6 and 229.2 mm) and average air temperature (16.48–17.64 °C) were similar during the growing season in 2017 and 2018. The total rainfall amount was higher in May to July 2018 compared to the same months of 2017 and long term (Figure 1). As seen in Figure 2, the mean temperature during the growing season of 2018 was slightly higher compared to the long term and 2017. The humidity rate, which was between 66.54 and 67.70% for both years, was rather moderate.
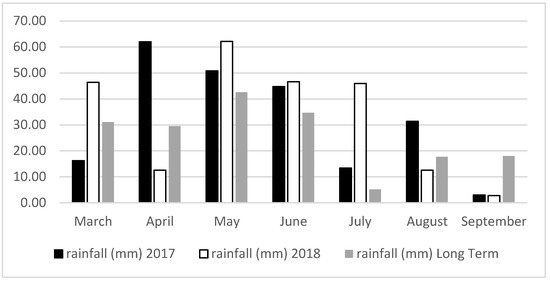
Figure 1.
The average rainfall (mm) amount during the experimental period at Eskisehir in 2017, 2018 and long term.
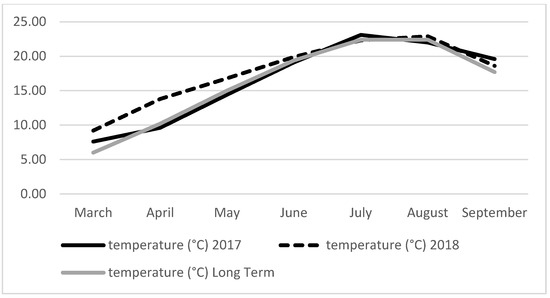
Figure 2.
The average air temperature (°C) during the experimental period at Eskisehir in 2017, 2018 and long term.
2.2. Experiment Setup
The seeds of Origanum vulgare subsp. hirtum (cv. Tınmaz), obtained from Atatürk Horticultural Central Research Institute, Yalova, were used as experimental material. Voucher specimens were deposited at the Herbarium of Department of Field Crops, Agricultural Faculty, Eskisehir Osmangazi University, Eskişehir. Seeds were sown into multi pots with a mixture of sand, mulch and manure (1:1:1) in April 2016. Seedlings were transplanted in June 2016 to the research field at an inter-row spacing of 40 cm and a 20 cm intra-row spacing. The experiment was arranged in a randomized complete block design with three replications. The plants were 2 and 3 years old in the first (2017) and second year (2018) of the research, respectively.
The weeds were controlled by hand weeding. The plants were irrigated once a week during the summer season of both years. No pesticides and chemical fertilizers were used. Harvest (1. Harvest and 2. Harvest) was carried out manually two times at the blooming stage [22,23] in both years. Harvest in the first year was carried out in June and September, whereas in the second year, the harvest was carried out in July and October. The plants matured late in the second year compared to the first year. This is because the total rainfall amount (154.8 mm) was higher in May to July 2018 compared to the same months of 2017 (109.0 mm) (Figure 1). The vegetative growth lasted longer and the blooming time was delayed in the second year compared to the first year. Therefore, 1. H and 2. H. were carried out one month later in 2018. The harvest cut height was 15 cm from the soil surface No frost damage was observed in both years.
Three HA doses, 50.0 L ha−1 (HA 50), 30.0 L ha−1 (HA 30) and 0.0 L ha−1 (HA 0, as control), were applied to soil from a liquid HA source which included 15% of total organic matter, 12% fulvic acid + HA (H + F) and 5% potassium oxide (water soluble). HA was applied to the soil after dissolving in deionized water at the appropriate doses given above, two times during the growing season each year. The first application was carried out at the vegetative stage and the second at the beginning of the blooming stage of the plant throughout each harvest in both years.
2.3. Experimental Procedure
2.3.1. Yield Traits
Fresh herb yield (kg ha−1), dry herb yield (kg ha−1) and dry leaf yield (kg ha−1) were investigated in 2017 and 2018. The inner two rows were harvested for each plot. Fresh herb yield was weighed just after the harvest. In order to find the dry herb yield, 500 g of fresh herbs per plot was dried at room temperature for a week and then dried at 35 °C for 24 h. Dry herb yield was weighed after the drying process. The leaves and flowers were separated from the stems and weighed for the dry leaf yield.
2.3.2. Essential Oil Content
Dried leaves were stored in paper sacks until distillation and were distilled using a Clevenger apparatus. Essential oil content (%) of the plants was determined by a volumetric method (mL 100 g−1) [42]. The mean content of essential oil for each application was obtained from 3 parallels. Essential oils were stored at 4 °C until the gas chromatography GC-FID/GC-MS analysis.
2.3.3. Antimicrobial Activity
The antibacterial and antifungal activities of the Origanum vulgare samples were determined by the well-diffusion method. Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Candida albicans ATCC 14053 and Candida parapisilosis ATCC 22019 were used to investigate the antibacterial and antifungal activities of the studied samples. The bacterial (S.aureus ATCC 29213, E. faecalis ATCC 29212) and fungal (C. albicans ATCC 14053, C. parapisilosis ATCC 22019) subcultures, were obtained from the Faculty of Medicine, Eskisehir Osmangazi University, Turkey.
Staphylococcus aureus and Enterococcus faecalis liquid cultures were prepared in brain heart infusion broth for their antibacterial activity tests, and Candida albicans and Candida parapisilosis were prepared in Sabouraud Dextrose Broth for their antifungal activity tests. Approximately 1 mL of the 24 h broth cultures containing 106 cfu mL−1 was placed in sterile Petri dishes. Moltent Mueller Hinton Agar (15 mL) kept at 45 °C was then poured into the Petri dishes and allowed to solidify. Six-millimeter diameter holes were then punched carefully using a sterile cork borer and completely filled with the test solutions. The plates were incubated for 24 h at 37 °C. Then, the inhibition zone that appeared around the holes in each plate was measured [43,44,45].
2.3.4. Composition of Essential Oil
GC-MS conditions: The composition of essential oil (%) was analyzed using a capillary GC and GC-MS (Agilent Technologies, Inc., Santa Clara, CA, USA) system. HP-Innowax FSC column (Hewlett-Packard-HP, Palo Alto, CA, USA) (60 m × 0.25 mm i.d., with 0.25 μm film thickness). Helium was used as a carrier gas (0.8 mL/min). The GC oven temperature was kept at 60 °C for 10 min and programmed to 220 °C at a rate of 4 °C/min, and kept constant at 220 °C for 10 min and then programmed to 240 °C at a rate of 1 °C/min. The split flow was adjusted at 40 mL min−1 with 40:1 split ratio. The injector temperature was set at 250 °C. Mass spectra were taken at 70 eV with the mass range of m/z 35–450.
GC-FID conditions: Agilent 6890N GC system fitted with an FID detector was used and set at a temperature of 300 °C. To obtain the same elution order with GC-MS, simultaneous auto-injection was carried out on a duplicate of the same column applying the same operational conditions. Relative percentage amounts of the separated compounds were calculated from FID chromatograms by using Agilent ChemStation Plus® software with peak integration process.
Identification of essential oil components was performed by comparing their mass spectra with those in the Baser Library of Essential Oil Constituents, Wiley GC-MS Library, Adams Library, MassFinder Library and confirmed by comparing their retention indices. In order to calculate the relative retention indices (RRI), a homologous series of n-alkanes were used as a reference. The relative percentages of the separated compounds were calculated from FID chromatograms.
2.4. Statistical Analysis
The experiment was arranged as two factors (HA doses and harvest times) in a randomized complete block design with three replications. Data of the yield and yield components were analyzed by analysis of variance (ANOVA) using SPSS for Windows (versions 20.0). The differences among the means were compared using the LSD values (p < 0.05 and p < 0.01).
3. Results
3.1. Yield Traits and Essential Oil Content
Fresh herb yield, dry herb yield, dry leaf yield and essential oil content were significantly affected by different humic acid doses (HAD) and harvest times (HT) in both years. The interaction of HAD x HT was statistically significant for fresh leaf yield in both years (Table 2).

Table 2.
Analysis of variance of HAD and HT on fresh herb yield (kg ha−1), dry herb yield (kg ha−1) and dry leaf yield (kg ha−1) of O. vulgare subsp. hirtum L. in 2017 and 2018.
As demonstrated in Table 3, mean values within the same column and mean values within the same row with different letters were significantly different at p < 0.05. The mean values of fresh herb yield, dry herb yield, dry leaf yield and essential oil content at the 1. Harvest were higher compared to the mean values at the 2. Harvest in both years.

Table 3.
Effects of HAD and HT on fresh herb yield (kg ha−1), dry herb yield (kg ha−1) and dry leaf yield (kg ha−1) of O. vulgare subsp. hirtum L. in 2017 and 2018.
Fresh herb yield, dry herb yield and dry leaf yield increased with increasing doses of HA 50 at both harvests in both years. All mean values of HA 50 were higher than those of the control doses HA 0 and HA 30. Essential oil content was highest at HA 50 an HA 30 in 2017, and, similarly, it was highest at HA 50 in the second year (Table 3). The differences in the essential oil content between the control dose and HA 50 in 2017 and 2018 were 0.46% and 0.42%, respectively.
3.2. Antimicrobial Activity and Chemical Composition of O. vulgare subsp. hirtum Essential Oil
The antimicrobial activity of the essential oils obtained from plant samples after 1. H and 2. H in both trial years (2017 and 2018) was evaluated and it was determined that the essential oil samples showed antimicrobial activity against two bacteria species (Enterococcus faecalis and, Staphylococcus aureus and two yeast species (Candida albicans and Candida parapisilosis). The antimicrobial effects against S. aureus and C. parapisilosis obtained from HA 30 and HA 50 were particularly evident. The antimicrobial activity of the essential oil against E. faecalis, S. aureus, C. albicans and C. parapisilosis in 2017 showed an increase at HA 30 and HA 50 for both harvests compared to the control dose. In 2018, the antimicrobial activity of the essential oil was particularly effective against S. aureus and C. parapisilosis at HA 30 and HA 50 for both harvests compared to the control dose (Table 4). C. parapisilosis yeast species exhibited the lowest antimicrobial inhibition zone with 1.0–1.3 mm, which was obtained from the control dose. The essential oils obtained from HA 30 and HA 50 worked against E. faecalis and C. albicans in the first year and the activity was highest at HA 50. However, the activity between HA0, HA 50 and HA 30 was similar in the second year. Generally, the antimicrobial activity of the essential oil against bacteria strains was higher compared to the yeast species. Among the yeast strains, the essential oil had a higher antifungal effect against C. albicans compared to C. parapisilosis (Table 4).

Table 4.
Antimicrobial activity of O. vulgare subsp. hirtum L. according to HT and HAD in 2017 and 2018.
The results of the antimicrobial activity of the control drugs are given in Table 5. Vancomycin, Levofloxacin and Cefepime were employed as standard antibacterial agents, and Fluconazole as an antifungal agent. Significant differences existed between the antimicrobial effects of the control compounds. The essential oil samples were found to have a similar and higher antimicrobial activity at some samples than Levoflaxacin and Cefepime control compounds. The antimicrobial inhibition zone of the essential oil of HA 50 against S. aureus was higher (2.8–3.5 mm) than Levoflaxacin in both years, particularly for the 2. H (Table 4). In both years, it was likely more effective (3.7–4.2 mm) than Cefepime against E. faecalis for both harvests. Vancomycin exhibited high antimicrobial effects against S. aureus, and the effect of the essential oil against E. faecalis, particularly for 2. H (4.0–4.2 mm) in both years (Table 4). Fluconazole showed lower activity compared to the essential oil against C. albicans (2.2–3.5 mm). The activity of the essential oil, even at HA 0, was higher than that of Fluconazole. Similarly, the antimicrobial inhibition zone of the essential oil of HA 30 and HA 50 against C. parapisilosis was higher (1.3–2.5 mm) (Table 4) than Fluconazole in both years (Table 5).

Table 5.
Antimicrobial activity of control drugs.
The antimicrobial activity of the essential oils belonging to 1. H and 2. H in both trial years (2017 and 2018) was shown in Figure 3. It was determined that the essential oil samples showed antimicrobial activity against Enterococcus faecalis, Staphylococcus aureus, Candida albicans and Candida parapisilosis.
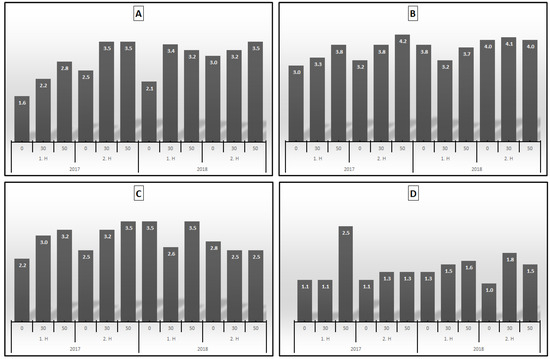
Figure 3.
Antimicrobial inhibition zone (mm) of (A): Staphylococcus aureus; (B): Enterococcus faecalis; (C): Candida albicans; (D): Candida parapisilosis according to HT and HAD in 2017 and 2018.
Analysis of the essential oil components showed that the ratios of “carvacrol and γ-Terpinene” generally increased as the HAD increased, and this increase was more pronounced compared to HA 0 (56.4% and 7.8%), HA 30 (61.4% and 9.8%) and HA 50 (69.2% and 8.2%) obtained from 2. H in 2017, respectively (Table 6). In 2018, the ratio of “carvacrol and γ-Terpinene” also increased with increasing doses of HA at both harvest times. The increase was more pronounced compared to HA 0 (72.7% and 3.3%), at the dose of HA 30 (77.4% and 6.5%) and HA 50 (75.3% and 7.5%) obtained from 1. H, respectively, and at the dose of HA 0 (63.9% and 4.6%) and HA 30 (72.6% and 5.4%) obtained from 2. H, respectively (Table 7). The ratios of carvacrol and γ-Terpinene obtained from HA 50 of the 2. H in 2018 was lower and so was the content of the essential oil. In both years, it was observed that both contents of EOs and carvacrol obtained from the 1. H were higher than those from the 2. H (Table 3 and Table 7).

Table 6.
Essential oil components of Origanum vulgare subsp. hirtum according to the different HT and HAD in 2017.

Table 7.
Essential oil components of Origanum vulgare subsp. hirtum according to the different HT and HAD in 2018.
4. Discussion
4.1. Yield Traits and Essential Oil Content
Humic substances are natural products that have beneficial effects on soil and plant growth. It was stated that they interact directly with physiological processes and have positive effects on the development of plants. The mean values of fresh herb yield, dry herb yield and dry leaf yield of the first harvest (1. H) were higher than those of the second harvest (2. H) in both years due to the climatic differences between the harvest times. The 1. H. occurred during mid-summer, whereas the 2. H. was during autumn in both years. Due to the mean temperatures for the months in mid-summer being high (22.4 °C), the yield of fresh herb was high (Table 3). The mean temperature of autumn was 19.6 °C. The higher temperatures during mid-summer positively influenced the dry herb yield and dry leaf yield. The higher the fresh herb yield, the higher the dry yields. Similarly, Ref. [46] found a higher fresh herb yield, drug herb yield, drug leaf yield and essential oil content of Origanum onites in June compared to the yield values obtained in September. Likewise, the yield and essential oil content of Salvia offıcinalis leaves changed monthly and was highest in July [47]. In this study, the fresh and dry herb yield values of the second year were higher than the first year. This is due to the plants’ growing season. In the first year, a 2-year-old plant was used, whereas in the second year, it was a 3-year-old plant. The growth of the 2-year-old plant was weaker as it was only planted one year prior, so the biomass and root production were lower compared to the 3-year-old plant. Likewise, the total rainfall amount (154.8 mm) was higher in May to July in 2018 compared to the same months in 2017 (109.0 mm) (Figure 1). Although the vegetative growth lasted longer and the blooming time was delayed in the second year compared to the first year, it produced more biomass and this resulted in higher fresh and dry herb yields.
Ref. [24] reported that the essential oil rate was higher in thyme plants treated with HA compared to the control plants. Ref. [48] applied mycorrhiza and HA to the basil plants and stated that these substances increased plant growth, essential oil yield and changed the chemical composition. Although the literature lacks data on the effects of HAD on the yield traits and essential oil content of cultivated Origanum vulgare L. plants, particularly in field conditions, research was conducted with one single dose of potassium humate which showed significant differences and high values of fresh weight and essential oil content for two growing seasons and two harvests of Origanum vulgare L. plants cultivated in pots [24]. Similarly, Ref. [34] applied four levels of potassium humate doses to Origanum syriacum L. under saline conditions and found significant increases in fresh herb weight per plant, dry herb weight per plant and essential oils for both years and harvests compared to the control dose. Fresh and dry herb yield and essential oil content varied by a factor of 2–3 between the lowest and highest values. In addition, Ref. [35] examined the effects of various HA levels (control, 50, 75 and 100 g m−2) on the chemical composition of Thymus vulgaris L. and determined that the essential oil content increased with the increase in HA levels compared to the control dose. Ref. [36] conducted a pot trial with basil plants, applying HS from the residues of green artichoke compost at three different rates (10, 50, 100 mg L−1). Similar to the results of our research, yield values and essential oil content increased with increasing HAD. They found the HS to be an effective biostimulant to improve the production and bioactive features of basil essential oil. Fresh weight biomass increased with increasing doses of humic substances compared to the control dose. HS behaved as a biostimulant in the rhizosphere of the plants.
Recently, numerous studies involving different medicinal and aromatic plants have been conducted. A study was conducted on the Nepeta species regarding the growth and essential oil responses to potassium humate and harvest time and showed the highest essential oil content and yield. The highest contents of p-cymene, citronellol and geraniol were obtained from the application of potassium humate [49]. Likewise, a study on the effect of humic substances on the growth and essential oil quality of the cultivated Aloysia tripylla showed that the flowering period was shortened and the percentage of terpenes of the EO increased, leading to an optimization of the quality of the EOs [50]. The application of three biostimulators at two concentrations significantly increased the growth attributes of marjoram plants, including yield components, fresh and dry weights of the herb and essential oil yield compared to the control plants. High levels of HA resulted in high contents of cis-sabinene hydrate. As a result, HA was recommended for improving plant growth, oil yield and primary components of Majorana hortensis [51]. A pot trial, which was conducted with Nigella sativa, stated that the application of HA increased the seed yield of the plant, the essential oil content and main essential oil components (pcymene, gama terpinene and thymoquinone) [52].
4.2. Antimicrobial Activity and Chemical Composition of O. vulgare subsp. hirtum Essential Oil
In this study, the application of HA substances to soil resulted in an increase in the essential oil content and some main components of oregano and positively affected antimicrobial activity. Both years demonstrated the synergistic effects of the essential oil compounds on the activity against all bacterial and yeast strains. The antimicrobial effect of EOs may be because of the impairment of various enzyme systems, involved in component synthesis and energy production [53]. It is clear that the effects of location, genotype, climate, soil characteristics, fertilization, irrigation and harvest time on the antimicrobial activity were different, as reported by many researchers [4,24,37,48,54]. In a study examining the effect of different concentrations of oregano essential oil on biofilms of Staphylococcus aureus and Escherichia coli bacteria species, it was stated that these EOs reduce the biofilm level [55]. Likewise, the antibacterial activity of different concentrations of thyme essential oil, examined with disc and well-diffusion tests against Staphylococcus aureus and other species, was rather high [56]. Similarly, Ref. [57] reported that different O. vulgare extracts showed antimicrobial activity against many microorganism species, including S.aureus, E.faecalis bacteria species and C. albicans yeast species. Ref. [1] investigated the chemical composition and antibacterial properties of EOs obtained from the above-ground parts of four different Origanum species. It was determined that the EOs have strong antibacterial activity and suggested the possibility of using EOs or some of their ingredients as natural food preservatives. Ref. [4] tested the antimicrobial activities of the EOs obtained from the O. vulgare plants, which were harvested twice in October and June with and without irrigation and fertilization, and they tested the EOs against different bacteria species, detecting some antibacterial activity.
EOs contain substances known as therapeutic and antimicrobial agents such as thymol and carvacrol, both of which are used for their antifungal and antibacterial properties and as flavoring agents [58,59]. It is thought that the effective antimicrobial activity obtained in this study was due to the high carvacrol rates. Some researchers indicate that these compounds are particularly responsible for antimicrobial activity [60,61,62] and most studies have stated that the antimicrobial effect of the thyme plant is due to its high thymol and carvacrol contents [5,63,64,65]. The antimicrobial effects of thyme essential oil and the control compound “ampicillin” were tested against bacteria and yeast species and as a result the essential oil was found to be high compared to the control compound [20].
Similarly, the antibacterial activities of water, ethanolic and methanolic extracts of EOs obtained from O. vulgare plants against Gram-positive and Gram-negative microorganisms were investigated and the results were compared with those of control compounds such as Vancomycin, Erythromycin, Cloxacillin, Ciprofloxacin and Streptomycin. It was determined that all extracts exhibit pronounced antibacterial effects and there are significant differences between the effects of extracts and control drugs [66].
In this study, as HAD increased, a higher ratio of EOs was obtained in general and this may have caused an increase in carvacrol synthesis. Carvacrol is a monoterpenic alcohol which is isolated from the oils of marjoram, Origanum, summer savory and thyme, and utilized as a disinfectant. Carvacrol is reportedly used as a flavoring agent in a number of foods and beverages [42]. It is also recognized as a broad-spectrum antimicrobial effective component against bacteria, yeast and fungi, and it is suggested that the use of natural antimicrobial agents can be an effective alternative or complementary to the control of microorganisms [59,60,65,67]. Similar to our findings, Ref. [35] reported that HA positively affected the quantity and quality of chemical compositions, particularly thymol, biological activities and essential oil content in T. vulgaris and they strongly recommended its use to promote plant growth. In a study on the antifungal activity of the EOs of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. against clinical isolates of oral Candida spp., the high content of phenolic monoterpenes compounds was found to be effective [68]. Many research studies on Origanum vulgare showed high antimicrobial activity against Streptococcus pneumonia, Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Rhodotorula spp. and Candida albicans. It was stated that carvacrol-rich Origanum EOs showed potent inhibitory effects on the growth of common food-borne pathogenic bacteria [68,69,70,71,72,73,74].
In this study, it was observed that the carvacrol ratio obtained from the 1. H was higher than that obtained from the 2. H each year. This difference is due to the fact that the two harvest times had different climatic properties and therefore the content of essential oil obtained from the 1. H was higher than the 2. H, as stated previously (Table 2). Considering that the 1. H in 2018 occurred in July, whereas the 2. H was in October, the essential oil content (2.59%) and the ratio of carvacrol (50.5%) were decreased (Table 3 and Table 7). Different ecological conditions of essential oil components may depend on day length, climatic factors, plant development period, photoperiod and vegetation period [15]. In a study, the increase in temperature was accompanied by an increase in carvacrol [75]. In addition, sunlight may play an important role in the biosynthesis of monoterpenes; generally, the greater the intensity and duration of sunlight exposure, the greater the effects on the accumulation of monoterpenes [76], because many enzymes of the secondary metabolite pathways are UV-B-dependent [77].
5. Conclusions
The application of HA to soil significantly influenced the plant yield and essential oil content of Origanum vulgare subsp. hirtum. Fresh herb yield, dry herb yield and dry leaf yield increased with increasing doses of HA 50 for both harvests in both years. All mean values of HA 50 were higher than those of the control dose. Essential oil content was highest at HA 50 and HA 30 in 2017, and, similarly, it was highest at HA 50 in the second year. The essential oil content differences between the control dose and HA 50 in 2017 and 2018 were 0.46% and 0.42%, respectively. The antimicrobial activity and main component, carvacrol, of the essential oil increased with increasing doses of HA. Increased HAD resulted in a higher EO content of oregano and this may have caused higher carvacrol synthesis. Carvacrol percentages obtained from the 1. H and HA applications in the first year were not different but the values for 2. H were higher (61.4–69.2%) compared to the control dose (56.4%). In the second year, a higher carvacrol percentage was obtained from the 1. H and HA applications (75.3–77.4%) compared to the control dose (72.7%). The synergistic effects of the essential oil compounds on the activity against all bacterial and yeast strains were evident in both years. Oregano EOs showed antimicrobial activity against two bacteria species (Enterococcus faecalis and Staphylococcus aureus) and two yeast species (Candida albicans and Candida parapisilosis). The antimicrobial effects against S. aureus and C. parapisilosis obtained from HA 30 and HA 50 were particularly evident.
HA 50 could be recommended for higher essential oil quality, plant yield and antimicrobial activity. The antimicrobial inhibition zone of bacteria and yeast species increased with the increase in HAD. In conclusion, essential oil from Origanum vulgare subsp. hirtum can be used to combat the increasing resistance of antibiotics in the clinic and can be an alternative in the food preservation. In addition, the quality and antimicrobial activity of the essential oil could be increased via HA applications. Eventually, it will be necessary to test EOs using a variety of bacterial and fungal subcultures and different serial dilution methods. It is anticipated that the results presented in this study will contribute to other research on the antimicrobial potential of other different aromatic and medicinal plants.
Author Contributions
Conceptualization, Z.A.; methodology, Z.A., A.G. and M.K.; resources, Z.A. and A.G.; data curation, Z.A., A.G. and M.K.; writing—original draft preparation, Z.A. and A.G.; writing—review and editing, Z.A.; formal analysis, Z.A., A.G. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All new research data were presented in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baydar, H.; Sagdiç, O.; Özkan, G.Ü.; Karadoğan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microb. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, L.; Najafgholi, H.M.; Biouki, R.Y.; Moghaddam, M. Chemical composition and antimicrobial activity of Origanum vulgare subsp. viride essential oils cultivated in two different regions of Iran. J. Essent. Oil Bear. Plants 2018, 21, 1062–1075. [Google Scholar] [CrossRef]
- Fournomiti, M.; Kimbaris, A.; Mantzourani, I.; Plessas, S.; Sinapidou, E.; Panopoulou, M.; Bezirtzoglou, E.; Alexopoulos, A. Antimicrobial Activity of Essential Oils Extracted from Cultivated Oregano (Origanum vulgare), Sage (Salvia officinalis), Thyme (Thymus vulgaris) and Rosemary (Rosmarinus officinalis) against Clinical Isolates of Escherichia coli, Klebsiella oxytoca, Klebsiella pneumonia and Listeria monocytogenes. SF J. Agric. Crop Manag. 2020, 1, 1–9. [Google Scholar]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents-Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Grohs, B.M.; Kunz, B. Use of spices for the stabilization of fresh portioned pork. Food Control 2000, 11, 433–436. [Google Scholar] [CrossRef]
- Lambert, R.J.W.; Skandamis, P.N.; Coote, P.; Nychas, G.J.E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. In-vitro activity of Melaleuca alternifolia (Tea tree) oil against dermatophytes and other filamentous fungi. J. Antimicrob. 2002, 50, 195–199. [Google Scholar] [CrossRef]
- Lanciotti, R.; Gianotti, A.; Patrignani, N.; Belleti, N.; Guerzoni, M.E.; Gardini, F. Use of natural aroma compounds to improve shelf-life of minimally processed fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. Potential application of spice and herb extracts as natural preservatives in cheese. J. Med. Food 2011, 14, 284–290. [Google Scholar] [CrossRef]
- Selvam, S.P.; Dharini, S.; Puffy, S. Antifungal activity and chemical composition of thyme, peppermint and citronella oils in vapour phase against avocado and peach postharvest pathogens. J. Food Saf. 2013, 33, 86–93. [Google Scholar]
- Plavšic, D.V.; Škrinjar, M.M.; Psodorov, Ð.B.; Pezo, L.L.; Milovanovic, I.L.J.; Psodorov, D.Ð.; Kojic, P.S.; Kocic, T.D. Chemical structure and antifungal activity of mint essential oil components. J. Serb. Chem. Soc. 2020, 85, 1149–1161. [Google Scholar] [CrossRef] [Green Version]
- Bautista-Baños, S.; Hernandez-Lauzardo, A.N.; Velázquez-Del Valle, M.G.; Hernandez-Lopez, M.; Ait-Barka, E.; Bosquez-Molina, E.; Wilson, C.L. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Marques, J.L.; Volcão, L.M.; Funck, G.D.; Kroning, I.S.; Silva, W.P.; Fiorentinia, A.M.; Ribeiro, G.A. Antimicrobial Activity of Essential Oils of Origanum vulgare L. and Origanum majorana L. Against Staphylococcus aureus isolated from Poultry Meat. Ind. Crops Prod. 2015, 77, 444–450. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Giovanelli, E.; Deans, S.G.; Eaglesham, E. Antibacterial and antioxidant properties of Mediterranean aromatic plants. Ind. Crops Prod. 1993, 2, 47–50. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Deans, S.G. Antimicrobial effects of hydrophilic extracts of Pelargonium species (Geraniaceae). Lett. Appl. Microbiol. 1996, 23, 205–207. [Google Scholar] [CrossRef]
- Naz, R.; Ayub, H.; Nawaz, S.; Islam, Z.U.; Yasmin, T.; Bano, A.; Wakeel, A.; Zia, S.; Roberts, T.H. Antimicrobial activity, toxicity and anti-inflammatory potential of methanolic extracts of four ethnomedicinal plant species from Punjab, Pakistan. BMC Complement Altern Med. 2017, 17, 302. [Google Scholar] [CrossRef]
- Langeveld, W.T.; Veldhuizen, E.J.; Burt, S.A. Synergy between essential oil components and antibiotics: A review. Crit. Rev. Microbiol. 2014, 40, 76–94. [Google Scholar] [CrossRef]
- Ultee, E.; Smid, J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Özkalp, B.; Sevgi, F.; Özcan, M.; Özcan, M.M. The antibacterial activity of essential oil of oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 8, 272–274. [Google Scholar]
- Béjaoui, A.; Boulila, A.; Boussaid, M. Chemical composition and biological activities of essential oils and solvent extracts of Origanum vulgare subsp. glandulosum Desf. from Tunisia. J. Med. Plants Res. 2013, 7, 2429–2435. [Google Scholar]
- Shiyab, S.; Shatnawi, M.; Shibli, R.; Al-Zweiri, M.; Akash, M.; Aburijai, T. Influence of developmental stage on yield and composition of Origanum syriacum L. oil by multivariate analysis. J. Med. Plants Res. 2012, 6, 2985–2994. [Google Scholar]
- Baranauskienė, R.; Venskutonis, P.R.; Dambrauskienė, E.; Viškelis, P. Harvesting time influences the yield and oil composition of Origanum vulgare L. ssp. vulgare and ssp. Hirtum. Ind. Crops Prod. 2013, 49, 43–51. [Google Scholar] [CrossRef]
- Al Ahl, H.A.H.S.; Hasnaa, S.A. Hendawy, Effect of potassium humate and nitrogen fertilizer on herb and essential oil of oregano under different irrigation intervals. J. Appl. Sci. 2009, 2, 319–323. [Google Scholar]
- Gümüş, İ.; Şeker, C. Influence of humic acid applications on soil physicochemical properties. Solid Earth 2015, 7, 2481–2500. [Google Scholar]
- Fahramand, M.; Moradi, H.; Noori, M.; Sobhkhizi, A.; Adibian, M.; Abdollahi, S.; Rigi, K. Influence of humic acid on increase yield of plants and soil properties. Int. J. Farming Allied Sci. 2014, 3, 339–341. [Google Scholar]
- Kütük, C.; Çaycı, G.; Baran, A.; Başkan, O. Effect of humic acid on some soil properties. In Proceedings of the International Symposium on Desertification, Konya, Turkey, 13–17 June 2000; Volume 25, pp. 324–328. [Google Scholar]
- Bleam, W.F. Chapter 7—Natural Organic Matter. In Soil and Environmental Chemistry; Academic Press: Cambridge, MA, USA, 2016; pp. 333–384. [Google Scholar]
- Pukalchik, M.; Kydralieva, K.; Yakimenko, O.; Fedoseeva, E.; Terekhova, V. Outlining the potential role of humic products in modifying biological properties of the soil—A review. Front. Environ. Sci. 2019, 7, 80. [Google Scholar] [CrossRef]
- Liu, C.; Cooper, R.J.; Bowman, D.C. Humic acid application affects photosynthesis, root development, and nutrient content of creeping bentgrass. HortScience 1998, 33, 1023–1025. [Google Scholar] [CrossRef] [Green Version]
- Khattab, M.M.; Shaban, A.E.; El-Shrief, A.H.; Mohamed, A.E.D. Effect of humic acid and amino acids on pomegranate trees under deficit irrigation. I: Growth, flowering and fruiting. J. Hortic. Sci. Ornam. Plants 2012, 4, 253–259. [Google Scholar]
- Sangeetha, M.; Singaram, P.; Devi, R.D. Effect of lignite humic acid and fertilizers on the yield of onion and nutrient availability. In Proceedings of 18th World Congress of Soil Science, Philadelphia, PA, USA, 9–15 July 2006. [Google Scholar]
- Khaled, H.; Fawy, H.A. Effect of different levels of humic acids on the nutrient content, plant growth, and soil properties under conditions of salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef]
- Hanfy, M.R.; ElShafay, R.M.M.A.; Ali, M.A.M.; Abdallah, S.A.S. Effect of humic acid and acetyl salicylic acid on improving productivity of oregano (Origanum syriacum L.) plant irrigated with saline water. Menoufia J. Plant Prod. 2019, 4, 305–317. [Google Scholar] [CrossRef]
- Noroozisharaf, A.; Kaviani, M. Effect of soil application of humic acid on nutrients uptake, essential oil and chemical compositions of garden thyme (Thymus vulgaris L.) under greenhouse conditions. Physiol. Mol. Biol. Plants 2018, 24, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Verrillo, M.; Cozzolino, V.; Spaccini, R.; Piccolo, A. Humic substances from green compost increase bioactivity and antibacterial properties of essential oils in Basil leaves. Chem. Biol. Technol. Agric. 2021, 8, 28. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M. Characterization of several aromatic plants grown in northern Italy. Flavour Fragr. J. 1993, 8, 115–122. [Google Scholar] [CrossRef]
- Rowell, D.R. Soil Science: Methods and Applications; Longman: Harlow, UK, 1996. [Google Scholar]
- Walkley, A.; Black, L.A. An examination of the Degtjareff method for determining soil organic metter and a proposed madification of the chramic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Olsen, S.R.; Dean, L.A. Phosphorus. In Methods of Soil Analysis, 1st ed.; Black, C.A., Ed.; Part 2. Chemical and Microbiological Properties; Agronomy Series No. 9 (Part 2); American Society of Agronomy, Inc.: Madison, WI, USA, 1965; pp. 1035–1049. [Google Scholar]
- Lindsay, W.L.; Norwell, W.A. Development of a DTPA soil test for Zn, Fe, Mn and Cd. J. Soil Sci. Soc. Am. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Wichtel, M. Die Pharmakognostich-Chemische Analyse; Band 12; Akademische Verlagsgesselschaft: Frankfurt am Main, Germany, 1971; p. 12. [Google Scholar]
- Approved Standard M2-A7 NCCLS; Performance Standarts for Antimicrobial Disc Susceptility Tests. NCCLS National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2000.
- İlkimen, H.; Gülbandılar, A. Investigation of Antimicrobial Effects of Lavender, Sage Tea, Thyme and Chamomile. Türk Mikrobiyol. Cem. Derg. 2018, 48, 241–246. [Google Scholar] [CrossRef]
- Collins, C.M.; Lyne, P.M. Microbiologicial Methots; Buttermorths & Co., Ltd.: London, UK, 1987; p. 450. [Google Scholar]
- Sonmez, C. Effect Of Different Harvest Times On Some Yield and Essentıal Oil Characteristics in Origanum onites L. Turk. J. Field Crops 2019, 24, 106–110. [Google Scholar] [CrossRef]
- Putievsky, E.; Ravid, U.; Duda, I.N. The influence of season and harvest frequency on essential oil and herbal yields from a pure clone of sage grown under cultivated conditions. J. Nat. Prod. 1986, 49, 326–329. [Google Scholar] [CrossRef]
- Morelli, F.; Ferarrese, L.; Munhoz, C.L.; Alberton, O. Antimicrobial activity of essential oil and growth of Ocimum basilicum (L.) inoculated with mycorrhiza and humic substances applied to soil. Genet. Mol. Res. 2017, 16, 16039710. [Google Scholar] [CrossRef]
- Mohamed, H.F.; Mahmoud, A.A.; Alatawi, A.; Hegazy, M.H.; Astatkie, T.; Ahl, S.A.; Hussein, A.H. Growth and essential oil responses of Nepeta species to potassium humate and harvest time. Acta Physiol. Plant. 2018, 40, 204. [Google Scholar] [CrossRef]
- Sardashti, A.R.; Assadi-Khanoki, A. Humic Substances Effect and Climatic Tensions on the Growth and Essential Oil Quality of the Cultivated Aloysia triphylla (Iran). Jordan J. Agric. Sci. 2021, 17, 355–376. [Google Scholar] [CrossRef]
- El-Khateeb, M.A.; El-Attar, A.B.; Nour, R.M. Application of plant biostimulants to improve the biological responses and essential oil production of marjoram (Majorana hortensis, Moench) plants. Middle East J. Agric. Res. 2017, 6, 928–941. [Google Scholar]
- Ariafar, S.; Forouzandeh, M. Evaluation of humic acid application on biochemical composition and yield of black cumin under limited irrigation condition. Bull. Soc. R. Sci. Liège 2017, 86, 13–24. [Google Scholar] [CrossRef]
- Conner, D.E.; Beuchat, L.R. Recovery of heat-stressed yeasts in media containing plant oleoresins. J. Appl. Bacteriol. 1985, 59, 49–55. [Google Scholar] [CrossRef]
- Juarez, C.R.; Craker, L.E.; Mendoza, R.D.L.N.R.; Aguilar-Castıllo, J.A. Humic substances and moisture content in the production of biomass and bioactive constituents of Thymus vulgaris L. Rev. Fitotec. Mex. 2011, 34, 183–188. [Google Scholar]
- Oral, N.B.; Vatansever, L.; Aydın, B.D.; Sezer, Ç.; Güven, A.; Gülmez, M.; Başer, K.H.C.; Kürkçüoğlu, M. Effect of oregano essential oil on biofilms formed by Staphylococci and Escherichia coli. Kafkas Univ. Vet. Fak Derg. 2010, 16 (Suppl. A), S23–S29. [Google Scholar]
- Cattelan, M.G.; de Castilhos, M.B.M.; Sales, P.J.P.; Hoffmann, F.L. Antibacterial activity of oregano essential oil against foodborne pathogens. Nutr. Food Sci. 2013, 43, 169–174. [Google Scholar] [CrossRef]
- Licina, B.Z.; Stefanovic, O.D.; Vasic, S.M.; Radojevic, I.D.; Dekic, M.S.; Comic, L.R. Biological activities of the extracts from wild growing Origanum vulgare L. Food Control 2013, 33, 498–504. [Google Scholar] [CrossRef]
- Marchese, A.; Arciola, C.R.; Coppo, E.; Barbieri, R.; Barreca, D.; Chebaibi, S.; Daglia, M. The natural plant compound carvacrol as an antimicrobial and anti-biofilm agent: Mechanisms, synergies and bio-inspired anti-infective materials. Biofouling 2018, 34, 630–656. [Google Scholar] [CrossRef]
- Kilic, T. Analysis of Essential Oil Composition of Thymbra spicata var. spicata: Antifungal, Antibacterial and Antimycobacterial Activities. Z. Nat. C 2006, 61, 324–328. [Google Scholar]
- Béjaoui, A.; Chaabane, H.; Jemli, M.; Boulila, A.; Boussaid, M. Essential oil composition and antibacterial activity of Origanum vulgare subsp. glandulosum Desf. at different phenological stages. J. Med. Food 2013, 16, 1115–1120. [Google Scholar] [PubMed]
- Adam, K.; Sivropoulou, A.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia and Salvia fruticosa essential oils against human pathogenic fungi. J. Agric. Food Chem. 1998, 46, 1739–1745. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Dambolena, J.S.; Zygadlo, J.A.; Rubinstein, H. Antifumonisin activity of natural phenolic compounds: A structure-property-activity relationship study. Int. J. Food Microbiol. 2011, 145, 140–146. [Google Scholar] [CrossRef]
- Ahmad, R.G.; Babak, B. Effects of phenological stages on herbage yield and quality/quantity of oil in garden thyme (Thymus vulgaris L.). J. Med. Plants Res. 2011, 5, 6085–6089. [Google Scholar]
- Nostro, A.; Papalia, T. Antimicrobial activity of carvacrol: Current progress and future prospectives. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Jaber, N.N. Antimicrobial efficacy of oregano extracts. Basrah J. Vet. Res. 2012, 11, 23–31. [Google Scholar] [CrossRef]
- Vardar-Unlu, G.; Yagmuroglu, A.; Unlu, M. Evaluation of in vitro activity of carvacrol against Candida albicans strains. Nat. Prod. Res. 2010, 24, 1189–1193. [Google Scholar] [CrossRef]
- Baj, T.; Biernasiuk, A.; Wróbel, R.; Malm, A. Chemical composition and in vitro activity of Origanum vulgare L., Satureja hortensis L., Thymus serpyllum L. and Thymus vulgaris L. essential oils towards oral isolates of Candida albicans and Candida glabrata. Open Chem. 2020, 18, 108–118. [Google Scholar] [CrossRef]
- Ebani, V.V.; Nardoni, S.; Bertelloni, F.; Pollera, C.; Pistelli, L.; Mancianti, F. In vitro antimicrobial activity of selected essential oils against bacteria and yeasts isolated from the genital tract of mares. Nat. Prod. Res. 2022, 36, 2648–2653. [Google Scholar] [CrossRef]
- Mohsen, L.; Jaber, H.; Kamel, W.M. Antibacterial Activity of the Essential Oil Isolated from Origanum vulgare L. (Lamiaceae) Against Multi-Drug Resistant Bacteria. IJDDT 2022, 12, 81–84. [Google Scholar]
- Hao, Y.; Kang, J.; Yang, R.; Li, H.; Cui, H.; Bai, H.; Shi, L. Multidimensional exploration of essential oils generated via eight oregano cultivars: Compositions, chemodiversities, and antibacterial capacities. Food Chem. 2022, 374, 131629. [Google Scholar] [CrossRef]
- Kryvtsova, M.; Hrytsyna, M.; Salamon, I. Chemical Compositıon and Antimicrobial Properties of Essential Oil from Origanum vulgare L. in Different Habitats. Biotechnol. Acta 2020, 13, 64–72. [Google Scholar] [CrossRef]
- Tsitlakidou, P.; Papachristoforou, A.; Tasopoulos, N.; Matzara, A.; Hatzikamari, M.; Karamanoli, K.; Mourtzinos, I. Sensory analysis, volatile profiles and antimicrobial properties of Origanum vulgare L. essential oils. Flavour Fragr. J. 2022, 37, 43–51. [Google Scholar] [CrossRef]
- Bešta-Gajević, R.; Karalija, E.; Jerković-Mujkić, A.; Karadža, D.; Smajlović-Skenderagić, L.; Dahija, S. Antimicrobial and antioxidant activity of the extracts from Origanum vulgare L. growing wild in Bosnia and Herzegovina. Genet. Appl. 2018, 2, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Novak, J.; Lukas, B.; Franz, C. Temperature influences thymol and carvacrol differentially in Origanum spp.(Lamiaceae). J. Essent. Oil Res. 2010, 22, 412–415. [Google Scholar] [CrossRef]
- Sharafzadeh, S. Growth and secondary metabolites of basil, mint and thyme as affected by light. Int. J. Pharma Bio Sci. 2012, 3, 43–46. [Google Scholar]
- Kun, D.N.; Chappell, J.; Boudet, A.; Hahlbrock, K. Induction of phenylalanine ammonia-lyase and 4-coumarate:CoA ligase mRNAs in cultured plant cells by UV light or fungal elicitor. Proc. Natl. Acad. Sci. USA 1984, 81, 1102–1106. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).