Abstract
Chinese cabbage (Brassica rapa L.) is one of the most important and highly nutritious vegetables in China belonging to the Brassicaceae family. Flowering or bolting is one of the most critical developmental stages in flowering plants. For the spring-sown Chinese cabbage, late-bolting is desirable over early-bolting according to consumer preferences. We determined the inheritance pattern of the late-bolting trait using F1 and F2 generated from a cross between ‘SY2004’ (late-bolting) and ‘CX14-1’ (early-bolting). The genetic analysis revealed that the late-bolting to early-bolting trait was controlled by an incomplete dominant gene that we named BrLb-1. Furthermore, we performed bulked segregant analysis (BSA) via whole genome re-sequencing and the results showed that this gene was harbored on the chromosome A07 at the intersections of 20,070,000 to 25,290,000 bp and 20,330,000 to 25,220,000, an interval distance of 4.89 Mb. In this candidate interval, totals of 2321 and 1526 SNPs with non-synonymous mutations, and 229 and 131 InDels with frameshift mutations, were found between the parents and the bulked pools, respectively. Furthermore, we identified three putative candidate genes for the late-bolting trait, including BraA07g029500, BraA07g029530 and BraA07g030360, which code for the AGAMOUS-like MADS-box protein AGL12, a pentatricopeptide repeat-containing protein and NAC transcription factor 29, respectively; however, further functional analysis is required. These genetic variants could be utilized for the further development of molecular markers for marker-assisted breeding in Chinese cabbage.
1. Introduction
Chinese cabbage (Brassica rapa L.) is one of the most important and widely consumed vegetables in China that belongs to the Brassicaceae family [1]. It is nutritionally rich and considered the most important source of mineral nutrients by the Chinese people [2]. This vegetable has shown significant morphological diversity, including a heading type (heading Chinese cabbage, B. rapa L. subsp. pekensis) with leafy heads and a non-heading type (pakchoi, B. rapa subsp. chinensis) with smooth and flat leaves [3,4,5]. The heading type Chinese cabbage is the most popular in China, Korea and Japan [6,7]. In addition, it is also used to prepare Korean ‘Kimchi’, a fermented traditional side dish [7,8]. Moreover, the non-heading type also includes flowering Chinese cabbage (B. rapa ssp. Chinensis var. parachinensis) with elongated, tender and thick stalks with fast bolting [6,9]. These morphotypes are also partially regulated by the genes associated with flowering time [10].
The most critical developmental switch in the life cycle of an angiosperm is the transition from the vegetative to reproductive stage, specifically flowering [11,12,13]. This transition is regulated by the several environmental and endogenous signals [12,14]. The regulation of flowering time has been well studied in Arabidopsis, a close relative of the Brassica species, with over 180 genes identified through functional analysis [12,15]. It has at least six major pathways, namely, photoperiod, vernalization, ambient temperature, age, autonomous, and gibberellin [9,12,15]. It has been reported that flowering time is regulated by the transcriptional activation of FLOWERING LOCUS T (FT), which is repressed by FLOWERING LOCUS C (FLC) via interaction with the genes associated with the vernalization pathway, and activated by the zinc finger transcription factor (TF) gene CONSTANS CONSTANS (CO) through interactions with the genes related to the circadian clock and the photoperiod pathway [16,17]. Schiessl et al. identified 35 flowering-related genes in the Brassica species, five of which were orthologous to known Arabidopsis genes in the flowering regulating pathway [16]. There were two FT genes (A02 and A07) and three FLC genes (A02, A03 and A10) homologous with Arabidopsis have been identified in B. rapa [16,18]. Kim et al. [18] reported that BrFLC1 and BrFLC2 were highly expressed compared to BrFLC3 in vernalized Chinese cabbage. Moreover, BrVIN3.1 and BrFLC1 were reported as the key factors for flowering time variation in B. rapa [19]. FRIGIDA (FRI) isa code for a plant-specific protein that triggers the expression of FLC and inhibits flowering [20]. On the other hand, vernalization induces the expression of VERNALIZATION INSENSITIVE 3 (VIN3), VERNALIZATION 1 (VRN1) and VERNALIZATION 2 (VRN2), which suppress the FLC [21,22,23,24]. In B. rapa, BrVIN3.1 and BrFLC1 have been reported as the important factors for flowering time variation [19]. Another study reported that a natural mutation at the 5’ splice site in intron 6 of BrFLC1 on A10 was linked to flowering time variation in B. rapa [25]. Recently, six genes involved in the ‘plant hormone and signal transduction’ pathway were reported as candidates for late bolting in Chinese cabbage, including BraA02g009130, indole-3-acetic acid-induced protein ARG7; BraA09g058230, auxin-responsive protein SAUR41; BraA07g032960, serine/threonine-protein kinase BSK11; BraA10g019860, auxin-induced protein 15A; BraA08g012630, unknown and BraA01g009450, abscisic acid receptor PYR1 [26].
Previous studies have detected QTLs for flowering time across most chromosomes in both B. rapa and the closely related vegetable B. oleracea. For example, in B. rapa, three flowering QTLs have been detected by [27] on A02 (FLQTL-1, FLQTL-2) and A08 (FLQTL-3). Among those, only FLQTL-1 was reported as a major QTL and BrFLC2 was mapped at the upper part of A02 [27]. Ajisaka et al. [28] identified a QTL ‘BN007-1’ via BSA which was linked to late-bolting in Chinese cabbage cv ‘Osaka Shirona Bansei’. Osborn et al. [10] reported that two QTLs ‘VFRl’ on LG 02 and ‘VFR2’ on LG 08 were associated with the late-bolting trait in B. rapa; the same is true [29] of the flowering QTL ‘COR6.6’ on LG 2 in B. rapa. In B. oleracea, a total of 11 QTLs for flowering time have been reported on C01, C02, C03, C05 and C09 [30]. Moreover, two flowering-time QTLs on C07 and C08 in cauliflower × Brussels sprouts have been reported [31]. Okazaki et al. [32] identified a total of six QTLs on five different chromosomes (two on C02 and one on each of C03, C06, C09 and C09) and a major QTL was detected on C02, where BoFLC2 was located. Recently, a major QTL, ‘Ef2.1’, linked to early flowering in ‘broccoli × cabbage’ was detected by Su et al. [19], while BolGRF6 was found as the candidate gene in broccoli.
Prolonged cold temperature with a long day causes a developmental shift from a vegetative stage to a reproductive stage, resulting in early bolting without reaching the proper harvesting stage, which is a major problem in Chinese cabbage cultivation [12]. Consequently, late bolting is a more desirable trait than early flowering in spring-sown Chinese cabbage.
A limited report described the late-bolting trait gene in Chinese cabbage. In the present study, we crossed a late-bolting (‘SY2004’) heading Chinese cabbage (B. rapa var. pekensis) with an early-bolting (‘CX14-1’) flowering Chinese cabbage (B. rapa ssp. chinensis var. parachinensis) for F1 and F2 generation, and then performed bulked segregant analysis (BSA) in F2 via whole-genome re-sequencing using the Illumina Hiseq 2000 platform to determine the inheritance of the late-bolting trait and to map the BrLb-1 in Chinese cabbage.
2. Materials and Methods
2.1. Plant Materials
In this study, we used a double haploid (DH) late-bolting heading Chinese cabbage line (Brassica rapa var. pekinensis) ‘SY2004’ as P1, and an early-bolting flowering Chinese cabbage DH line (Brassica rapa var. parachinensis) ‘CX14-1’ as P2 which is vernalization-independent (Figure 1). The seeds of these lines were obtained from the Institute of Horticulture, Henan Academy of Agricultural Sciences, Zhengzhou, China, and developed F1 plants by crossing them. Furthermore, the F2 population was obtained via the self-pollination of F1 plants. All the plants were grown in the experimental field at Yuanyang (longitude 113°97′ E and 35°5′ N latitude), Henan province, China.

Figure 1.
Phenotype of ‘CX14-1’ (P1, early-bolting), ‘SY2004’ (P2, late-bolting) and their F1 (mid-bolting).
2.2. Phenotypic Analysis
The DH line ‘CX14-1’ initiated floral bud formation much earlier and exhibited a more rapid flower-stalk growth compared to ‘SY2004’ (Figure 1). The stem heights of the P1 (SY2004), P2 (CX14-1), F1 and F2 plants were measured to analyze the inheritance pattern of the early-bolting trait in Chinese cabbage (Table 1). A chi-square test was performed on the F2 population (425 plants).

Table 1.
Phenotype of the early-to late-bolting trait in a four-generation family, and the chi-square test ratio of segregation in the F2 population.
2.3. Bulk Segreant Analysis (BSA)
Genomic DNA was isolated from fresh young leaves through the cetyltrimethyl ammonium bromide (CTAB) method [33]. The concentration, integrity and purity of the DNA samples were determined with a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and agarose gel electrophoresis. We prepared two DNA pools including early-bolting bulk (EB-bulk) and late-bolting bulk (LB-bulk) for BSA analysis by mixing the same quantity of DNA from 30 plants of each early- and late-bolting Chinese cabbage F2 population. Moreover, the parental lines (P1 and P2) were also sequenced along with the pooled samples. A total of four paired-end libraries with 200 to 500 bp insert sizes were constructed for whole-genome re-sequencing with the Illumina Hiseq2000 platform by the Biomarker Technologies Corporation (Beijing, China) following the manufacturer’s instructions (Illumina, Hayward, CA, USA). The adaptor sequences were removed and low-quality bases were filtered out. Thereafter, high-quality clean reads were aligned to the Brassica rapa (Chiifu-401) v.3.0 version reference genome (Brassica database (BRAD), http://brassicadb.org/brad/ (accessed on 21 January 2021)) using the BWA software [34]. The alignment files were converted to SAM/BAM files with SAM tools. The GATK (genome analysis toolkit) [35] was implemented to detect single nucleotide polymorphisms (SNPs) and insertions/deletions (InDels). The elimination of PCR duplication was carried out using Picard (http://sourceforge.net/projects/picard/ (accessed on 21 January 2021)). The accuracy of the obtained variants was determined by local re-alignment, recalibration and variant calling (SNP and InDel) with GATK. SNPs were strictly filtered (SNP cluster filtering: if there were 2 SNPs within 5 bp; SNP within 5 bp near InDel; and InDel filtering: if the distance between two InDel wasless than 10 bp) [36]. The variants (SNPs and InDels) were compared between the samples using a Venn diagram. The SnpEff software was used to annotate the variants (SNP, Small InDel) [37].
The Euclidean distance (ED) algorithm was used to detect significant differences between the EB-bulk and the LB-bulk and to evaluate the regions associated with this trait [38]. The median and 3SD of all locus fitting values were taken as the correlation threshold of the analysis [38]. The SNP-index was used to find significant differences in genotype frequencies between the S- and L-pools, using the ΔSNP-index or ΔInDel index statistics [38,39]. The putative coding genes within the candidate interval were annotated via the BLAST [40] software using multiple databases: NR [41], Swiss-Prot [42], GO [43], KEGG [44], and COG [45].
2.4. Gene Expression Analysis
Total RNA was isolated from 100 mg finely powdered leaves of the Chinese cabbage parental population (P1 and P2) with a commercial kit ‘RNeasy mini’ (Qiagen, Valencia, CA, USA) according to the manufacturer’s guidelines. The quantity, integrity and purity of the RNA samples were determined with a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) followed by agarose gel electrophoresis. Afterwards, 1 μg of RNA was converted into cDNA using SuperScript III following the manufacturer’s protocol (Invitrogen, Gaithersburg, MD, USA). The expression of candidate genes was determined by quantitative real-time PCR (RT-qPCR) ‘LightCycler®480II’ (Roche, Mannheim, Germany). A total of 45 ng cDNA was used as templates for the RT-qPCR assay with gene-specific primers using ‘2× SyGreen Mix (qPCRBIO Lo-ROX)’ (PCR Biosystems, London, UK) and the thermocycling condition was adjusted to95 °C for 5 min, 45 cycles of 95 °C for 10 s, and 60 °C for 10 s, 72 °C (15 s). Ct values were analyzed with the LightCycler®480II software (Roche, Germany). The efficiency of each gene-specific primer was determined using pooled cDNA samples via standard curve analysis. Finally, the expression of genes was normalized using a comparative 2−ΔΔCt method [46] with BrGAPDH [47] as a reference gene.
3. Results
3.1. Phenotypic Evaluation and Inheritance of the Flowering Trait
All the plants from ‘CX14-1’ (P1), F1 (P1 × P2) and ‘SY2004’ (P2) exhibited early-bolting (vernalization-independent), mid-bolting (intermediate) and late-bolting (vernalization-dependent), respectively (Figure 1, Table 1 and Table S8). Among the 425 F2 population, 90, 209 and 126 plants showed early-, mid- and late-bolting, respectively (Table 1). The genetic analysis revealed that the segregation ratio of late-bolting individuals in the F2 population supported the Mendelian 1:2:1 inheritance. The results suggest that the early-bolting trait is controlled by an incomplete dominant gene, which we named Brassica rapa late bolting 1, or BrLb-1.
3.2. Bulked Segregant Analysis
A total of 64.24 Gb reads were generated by whole-genome re-sequencing. After filtering, we obtained a total of 63.58 Gb clean reads with 40.61x coverage, of which 98.57% were mapped to the Chinese cabbage reference genome Brassica rapa (Chiifu-401) v3.0 version reference genome (Brassica database (BRAD), http://brassicadb.org/brad/ (accessed on 28 January 2021)), while the genome coverage was 94.12% and the average coverage depth was 27.25x. A total of 1580,458 SNPs were obtained between the two parents (‘SY2004’ and ‘CX14-1’), of which 113,067 (7.15%) SNPs were non-synonymous, while 167,793 SNPs were obtained between the two bulks (EB bulk and LB bulk). Of these, 8722 (5.2%) SNPs caused non-synonymous mutations (Table S1). Moreover, a total of 557,544 and 62,777 small InDels were obtained between the two parents and two bulks, respectively (Table S2). With the SNP-index correlation algorithm, a candidate region related to traits was obtained, with a total length of 5.22 Mb. Furthermore, the SNP and InDel variations of the samples and BSA correlation analysis results were plotted using ‘Circos’ (http://circos.ca/ (accessed on 28 January 2021)) and are shown in Figure 2.
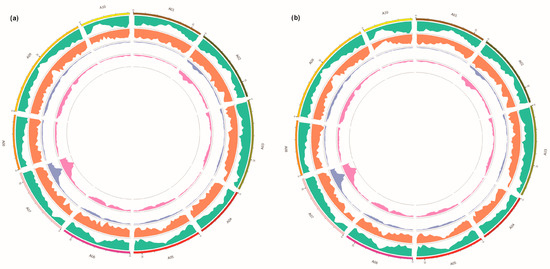
Figure 2.
Visualization the distribution of SNPs (a) and InDels (b) between samples on the chromosomes. From the outside to the inside: the first circle, chromosome coordinates; the second circle, gene distribution; the third circle, InDel density distribution; the fourth circle, ED value distribution; the fifth circle, ΔInDel-index value distribution.
The BrLb-1 was mapped to the region from 18,970,000 to 28,420,000 bp (9.45 Mb) on chromosome A07 based on SNPs and the ED algorithm (Figure 3). A total of 1839 genes, including 1144 genes with non-synonymous mutant SNP sites, were obtained within 9.45 Mb based on the median + 3SD of the fitting values of all the sites as the correlation threshold (0.43) (Table S3). Furthermore, the SNP index analysis revealed that BrEb-1 was mapped to between 20,070,000 and 25,290,000 bp (5.22 Mb) on A07 (Figure 4). Within this region, a total of 964 genes, including 600 non-synonymous mutant genes, were obtained (Table S4). Consequently, we identified an intersection from 20,070,000 to 25,290,000 bp on A07. On the other hand, we mapped BrEb-1 between 18,970,000 and 28,740,000 bp (9.48 Mb) on A07 based on the InDels and ED algorithms (Figure 5). We recognized an intersection from 20,330,000 to 25,220,000 (4.89 Mb) on that chromosome according to the InDel index (Figure 6). The result of the intersection of SNP and InDel revealed that there was a candidate region linked to the BrLb-1 within 4.89 Mb on chromosome A07.
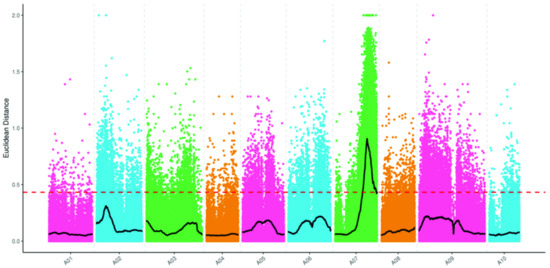
Figure 3.
Mapping BrEb-1 by the Euclidean distance (ED) algorithm, using the SNP approach. The abscissa represents the chromosome name; the colored dots represent the ED value of each SNP locus; the black line indicates the fitted ED value; the red dashed line represents the significant correlation threshold (threshold = 0.43). The higher the ED value, the greater the effect.
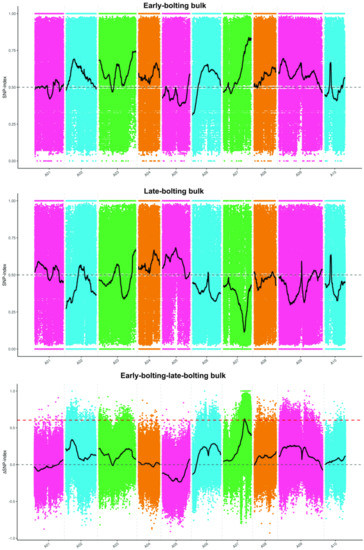
Figure 4.
Mapping BrEb-1 by the SNP index method. The abscissa represents the chromosome name; the colored dots represent the calculated SNP index (or ΔSNP index); the black line indicates the fitted SNP index. The top panel represents the distribution of SNP index values for early-bolting (recessive) bulk; the middle panel indicates the distribution of SNP index values for late-bolting (dominant) bulk; the bottom panel represents the distribution of ΔSNP index values; the red dashed line represents the significant correlation threshold.
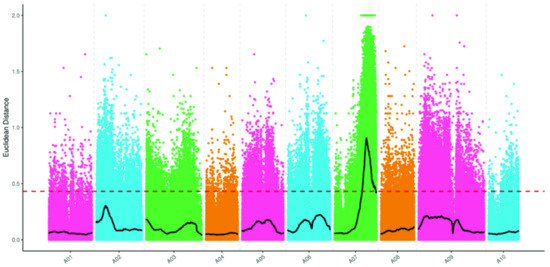
Figure 5.
Mapping BrEb-1 by the Euclidean distance (ED) algorithm, using the InDel approach. The abscissa represents the chromosome name; the colored dots represent the ED value of each InDel locus; the black line indicates the fitted ED value; the red dashed line represents the significant correlation threshold (threshold = 0.43). The higher the ED value, the greater the effect.
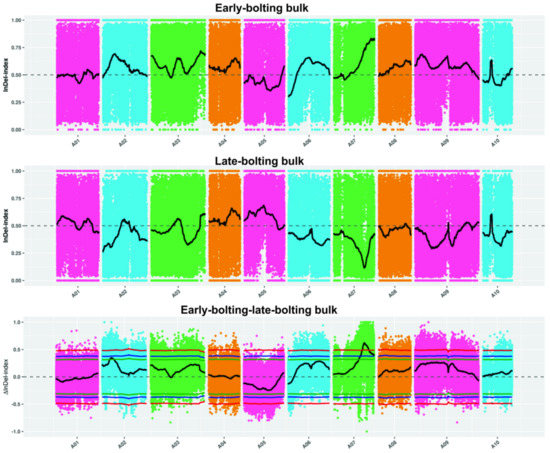
Figure 6.
Mapping BrEb-1 by the InDel index method. The abscissa represents the chromosome name; the colored dots represent the calculated InDel index (or ΔInDel index); the black line indicates the fitted SNP index. The top panel represents the distribution of InDel index values for early-bolting (recessive) bulk; the middle panel indicates the distribution of InDel index values for late-bolting bulk (dominant); the bottom panel represents the distribution of ΔInDel index values; the threshold line (red) was 0.99; the blue line represents a threshold line with a confidence of 0.95; the green line represents a threshold line with a confidence of 0.90.
A total of 887 genes were annotated in the candidate region, among which there were 557 non-synonymous mutant genes annotated between parents and 147 frameshift mutant genes (Table S5). Moreover, within the 4.89 Mb region on chromosome A07, 2321 and 1526 SNPs with non-synonymous mutations were obtained between the parents and the mixed pools, respectively (Table S5). These SNPs are likely to be directly related to BrLb-1. Likewise, 229 and 131 InDels with frameshift mutations were found between the parents and the bulk pools, respectively, which were likely to be directly related to the BrLb-1 (Table S5). Additionally, we checked the expression of these candidate genes via RT-qPCR using gene-specific primers (Table S7). The results showed that the transcript levels of these genes were up-regulated in late-bolting compared to early-bolting genotypes (Figure 7).
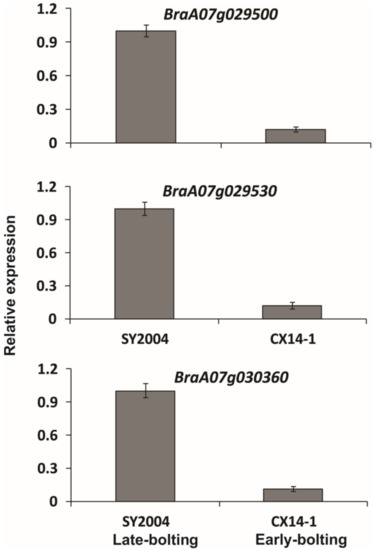
Figure 7.
Relative expression of three genes in the leaves of early- and late-bolting Chinese cabbage double haploid lines. Error bars represent ±SE of the means of the four techniques with three biological replicates.
4. Discussion
Flowering-related traits such as early- and late-bolting in Chinese cabbage are important for the seed industry as well as consumer preferences. Little is known about the molecular mechanism and inheritance patterns for this trait in Chinese cabbage. In the present study, we highlighted the late-bolting to early-bolting trait and elucidated the inheritance pattern as well as putative candidate genes linked to the trait. We found that the late-bolting trait is controlled by an incomplete dominant gene for early-bolting. Incomplete dominance inheritance patterns have been seen for traits in diverse species including B. napus [48,49], rice [50] and tomato [51].
Bulked segregant analysis (BSA) was first used to map disease resistance genes in lettuce [52]. For BSA, bulked DNA samples are prepared from individuals within a segregating population which contrasts for a particular trait, such as bulks of resistant and susceptible individuals. Comparing the genotype of these bulks across the genome can identify markers linked to the trait under investigation [51,52,53,54]. The next-generation sequencing technology is the most powerful sequencing platform that allows for rapid re-sequencing of the complete genome of different organisms, including plants. By using the BSA method, we first mapped the late-bolting trait (BrLb-1) of Chinese cabbage via whole-genome BSA to chromosome A07 from 20,330,000 to 25,220,000 (4.89 Mb). In this region, we identified a total of 3847 SNPs causing the non-synonymous mutations and which were likely to be directly related to BrLb-1 (Table S5). Similarly, a total of 360 InDels resulted in the frameshift mutations and were likely to be directly related to the BrLb-1 (Table S5). To further narrow down the candidate region for BrLb-1, we would need to utilize the variants (SNPs and InDels) generated via our re-sequencing results. A similar BSA method was reported by [55], where a yellow-green leaf (ygl-1) of cabbage was mapped to C01 and further fine mapped using InDel markers. Yu et al. [56] mapped the clubroot resistance gene (Rcr1) against P. brassicae pathotype 3 to A03 of B. rapa (pak choy) using BSA. RNA-seq and SNP markers were used to narrow down the candidate genes.
A total of 557 and 147 non-synonymous mutant genes and frameshift mutant genes, respectively, were identified in the candidate region (chromosome A07) via a BLAST search of multiple databases including NR, Swiss-Prot, GO, KEGG, etc. (Table S5).
Schiessl et al. [16] reported a total of 35 flowering time orthologues in Brassica species after comparison with known pathways associated with Arabidopsis flowering time. The AGAMOUS-like MADS-box protein AGL12 is involved in late-flowering phenotypes and root development in Arabidopsis [57]. The Arabidopsis gene LONG VEGETATIVE PHASE 1 (LOV), which codes for a NAC transcription factor, regulates flowering time and cold response [58], and other Arabidopsis NAC genes have also been reported to regulate flowering time [59]. Pentatricopeptide repeat-containing proteins (PPR) play important developmental roles in plants [60,61,62,63]. During our searches of gene annotations in the BrEb-1 candidate region, we identified three promising candidate genes. BraA07g029500, BraA07g029530 and BraA07g030360 code for AGL-12, PPR and NAC transcription factor 29, respectively. Moreover, the variant (SNP/InDel) analysis of these candidate genes revealed that there were four non-synonymous SNPs in BraA07g030360 (22213851: G/A, T/I; 22214367: T/A, D/V) and BraA07g029530 (21784475: T/C, S/P; 21786168: C/T, P/L), while four SNPs were found in an intron of BraA07g029500 (Table S6). In addition, all these candidate genes showed small InDels (Table S6). The transcript levels of the three candidate genes were all up-regulated in late-bolting compared to early-bolting genotypes (Figure 7).
The results suggest that these candidate genes might be candidates for the late-bolting trait in Chinese cabbage. In the future, the genetic variants (SNPs and InDels) presented in this study could be a valuable resource for further fine mapping of BrLb-1. The results of this study could be useful for developing molecular marks and marker-assisted breeding for the late-bolting to early-bolting trait in Chinese cabbage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12051048/s1, Table S1: SNPs are non-synonymous in the two parents and two bulks (EB bulk and LB bulk); Table S2: Small InDels in the two parents and two bulks; Table S3: Genetic information between 18.97 MB and 28.42 MB; Table S4: Genetic information between 20.07 MB and 25.29 MB; Table S5: Genetic information within the candidate region of both parents; Table S6: Variant (SNP/InDel) analysis of these candidate genes; Table S7: Primers used for gene expression analysis through RT-qPCR. Table S8: The stem height of the SY2004, CX14-1, F1 and F2 population.
Author Contributions
Conceptualization, X.Z. and Y.Y.; investigation, Z.W. and Y.Z.; formal analysis, S.Y., S.A.S. and J.L.; validation, H.S. and Z.Y.; writing—original draft, X.W. and M.A.R.; writing—review and editing, X.Z. and Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (31801874), Programs for Science and Technology Development of Henan Province (212102110124), the Fund for Independent Innovation from Henan Academy of Agricultural Sciences (2022ZC21), the China Agriculture Research System (CARS-23-G-16) and the Sci-Tech Innovation Team of Henan Academy Agricultural Sciences (2022TD06).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The clean data from fine mapping have been deposited into the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra/ (accessed on 21 May 2021)) with accession number PRJNA817372.
Acknowledgments
The authors extend their appreciation for the support from the Institute of Horticulture, Henan Academy of Agricultural Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, J.; Yuan, Y.-X.; Zhang, X.-W.; Zhao, J.; Song, X.; Li, Y.; Li, X.; Sun, R.; Koornneef, M.; Aarts, M.G.M.; et al. Mapping QTLs for mineral accumulation and shoot dry biomass under different Zn nutritional conditions in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Soil 2008, 310, 25–40. [Google Scholar] [CrossRef][Green Version]
- Ma, G.; Li, Y.; Jin, Y.; Du, S.; Kok, F.J.; Yang, X. Assessment of intake inadequacy and food sources of zinc of people in China. Public Health Nutr. 2007, 10, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Deng, B.; Lou, P.; Wu, J.; Sun, R.; Xu, Z.; Vromans, J.; Koornneef, M.; Bonnema, G. Genetic relationships within Brassica rapa as inferred from AFLP fingerprints. Theor. Appl. Genet. 2005, 110, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Hanelt, P. Mansfeld’s Encyclopedia of Agricultural and Horticultural Crops; Springer: Berlin/Heidelberg, Germany, 2001; p. 539. [Google Scholar]
- Sun, X.X.; Luo, S.; Luo, L.; Wang, X.; Chen, X.; Lu, Y.; Shen, S.; Zhao, J.; Bonnema, G. Genetic analysis of Chinese cabbage reveals correlation between rosette leaf and leafy head variation. Front. Plant Sci. 2018, 9, 1455. [Google Scholar] [CrossRef]
- Cheng, F.; Wu, J.; Wang, X. Genome triplication drove the diversification of Brassica plants. Hortic. Res. 2014, 1, 14024. [Google Scholar] [CrossRef]
- Bong, Y.-S.; Shin, W.-J.; Gautam, M.K.; Jeong, Y.-J.; Lee, A.-R.; Jang, C.-S.; Lim, Y.-P.; Chung, G.-S.; Lee, K.-S. Determining the geographical origin of Chinese cabbages using multielement composition and strontium isotope ratio analyses. Food Chem. 2012, 135, 2666–2674. [Google Scholar] [CrossRef]
- Lee, M.-K.; Chun, J.-H.; Byeon, D.H.; Chung, S.-O.; Park, S.U.; Park, S.; Arasu, M.V.; Al-Dhabi, N.A.; Lim, Y.-P.; Kim, S.-J. Variation of glucosinolates in 62 varieties of Chinese cabbage (Brassica rapa L. ssp. pekinensis) and their antioxidant activity. LWT Food Sci. Technol. 2014, 58, 93–101. [Google Scholar] [CrossRef]
- Huang, X.; Lei, Y.; Guan, H.; Hao, Y.; Liu, H.; Sun, G.; Chen, R.; Song, S. Transcriptomic analysis of the regulation of stalk development in flowering Chinese cabbage (Brassica campestris) by RNA sequencing. Sci. Rep. 2017, 7, 15517. [Google Scholar] [CrossRef]
- Osborn, T.C.; Kole, C.; Parkin, I.A.P.; Sharpe, A.G.; Kuiper, M.; Lydiate, D.J.; Trick, M. Comparison of flowering time genes in Brassica rapa, B. napus and Arabidopsis thaliana. Genetics 1997, 146, 1123–1129. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, D.; He, Y. FRIGIDA establishes a local chromosomal environment for FLOWERING LOCUS C mRNA production. Nat. Plants 2018, 4, 836–846. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, L.; Liu, B.; Hu, Y.; Cheng, F.; Liang, J.; Aarts, M.G.M.; Wang, X.; Wu, J. A transposon insertion in FLOWERING LOCUS T is associated with delayed flowering in Brassica rapa. Plant Sci. 2015, 241, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Duan, W.; Huang, Z.; Liu, G.; Wu, P.; Liu, T.; Li, Y.; Hou, X. Comprehensive analysis of the flowering genes in Chinese cabbage and examination of evolutionary pattern of CO-like genes in plant kingdom. Sci. Rep. 2015, 5, 14631. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Abe, M. Regulation of reproductive development by non-coding RNA in Arabidopsis: To flower or not to flower. J. Plant Res. 2012, 125, 693–704. [Google Scholar] [CrossRef]
- Fornara, F.; de Montaigu, A.; Coupland, G. SnapShot: Control of flowering in Arabidopsis. Cell 2010, 141, 550–550.e2. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, S.V.; Huettel, B.; Kuehn, D.; Reinhardt, R.; Snowdon, R.J. Flowering Time Gene Variation in Brassica Species Shows Evolutionary Principles. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Blümel, M.; Dally, N.; Jung, C. Flowering time regulation in crops-what did we learn from Arabidopsis? Curr. Opin. Biotechnol. 2015, 32, 121–129. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, B.S.; Kwon, S.J.; Kim, J.; Lim, M.H.; Park, Y.D.; Kim, D.Y.; Suh, S.C.; Jin, Y.M.; Ahn, J.H.; et al. Delayed flowering time in Arabidopsis and Brassica rapa by the overexpression of FLOWERING LOCUS C (FLC) homologs isolated from Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Cell Rep. 2007, 26, 327–336. [Google Scholar] [CrossRef]
- Su, T.; Wang, W.; Li, P.; Zhang, B.; Li, P.; Xin, X.; Sun, H.; Yu, Y.; Zhang, D.; Zhao, X.; et al. A Genomic Variation Map Provides Insights into the Genetic Basis of Spring Chinese Cabbage (Brassica rapa ssp. pekinensis) Selection. Mol. Plant 2018, 11, 1360–1376. [Google Scholar] [CrossRef]
- Johanson, U.; West, J.; Lister, C.; Michaels, S.; Amasino, R.; Dean, C. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 2000, 290, 344–347. [Google Scholar] [CrossRef]
- Sung, S.; Amasino, R.M. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 2004, 427, 159–164. [Google Scholar] [CrossRef]
- Gendall, A.R.; Levy, Y.Y.; Wilson, A.; Dean, C. The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 2001, 107, 525–535. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kaya, H.; Goto, K.; Iwabuchi, M.; Araki, T. A Pair of Related Genes with Antagonistic Roles in Mediating Flowering Signals. Science 1999, 286, 1960–1962. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, R.J.; Hong, L.; Michaels, S.; Amasino, R.M. FRIGIDA-ESSENTIAL 1 interacts genetically with FRIGIDA and FRIGIDA-LIKE 1 to promote the winter-annual habit of Arabidopsis thaliana. Development 2005, 132, 5471–5478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-X.; Wu, J.; Sun, R.-F.; Zhang, X.-W.; Xu, D.-H.; Bonnema, G.; Wang, X.-W. A naturally occurring splicing site mutation in the Brassica rapa FLC1 gene is associated with variation in flowering time. J. Exp. Bot. 2009, 60, 1299–1308. [Google Scholar] [CrossRef]
- Wei, X.; Rahim, M.A.; Zhao, Y.; Yang, S.; Wang, Z.; Su, H.; Li, L.; Niu, L.; Harun-Ur-Rashid, M.; Yuan, Y.; et al. Comparative Transcriptome Analysis of Early- and Late-Bolting Traits in Chinese Cabbage (Brassica rapa). Front. Genet. 2021, 12, 590830. [Google Scholar] [CrossRef]
- Zhao, J.; Kulkarni, V.; Liu, N.; Pino, D.; Carpio, D.; Bucher, J.; Bonnema, G. BrFLC2 (FLOWERING LOCUS C) as a candidate gene for a vernalization response QTL in Brassica rapa. J. Exp. Bot. 2010, 61, 1817–1825. [Google Scholar] [CrossRef]
- Ajisaka, H.; Kuginuki, Y.; Yui, S.; Enomoto, S.; Hirai, M. Identification and mapping of a quantitative trait locus controlling extreme late bolting in Chinese cabbage (Brassica rapa L. ssp. pekinensis syn. campestris L.) using bulked segregant analysis. Euphytica 2001, 118, 75–81. [Google Scholar] [CrossRef]
- Teutonico, R.A.; Osborn, T.C. Mapping loci controlling vernalization requirement in Brassica rapa. Theor. Appl. Genet. 1995, 91, 1279–1283. [Google Scholar] [CrossRef]
- Rae, A.M.; Howell, E.C.; Kearsey, M.J. More QTL for flowering time revealed by substitution lines in Brassica oleracea. Heredity 1999, 83, 586–596. [Google Scholar] [CrossRef]
- Sebastian, R.L.; Kearsey, M.J.; King, G.J. Identification of quantitative trait loci controlling developmental characteristics of Brassica oleracea L. Theor. Appl. Genet. 2002, 104, 601–609. [Google Scholar] [CrossRef]
- Okazaki, K.; Sakamoto, K.; Kikuchi, R.; Saito, A.; Togashi, E.; Kuginuki, Y.; Matsumoto, S.; Hirai, M. Mapping and characterization of FLC homologs and QTL analysis of flowering time in Brassica oleracea. Theor. Appl. Genet. 2007, 114, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Report. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Reumers, J.; De Rijk, P.; Zhao, H.; Liekens, A.; Smeets, D.; Cleary, J.; Van Loo, P.; Van Den Bossche, M.; Catthoor, K.; Sabbe, B.; et al. Optimized filtering reduces the error rate in detecting genomic variants by short-read sequencing. Nat. Biotechnol. 2012, 30, 61–68. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly Austin 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Hill, J.T.; Demarest, B.L.; Bisgrove, B.W.; Gorsi, B.; Su, Y.C.; Yost, H.J. MMAPPR: Mutation Mapping Analysis Pipeline for Pooled RNA-seq. Genome Res. 2013, 23, 687–697. [Google Scholar] [CrossRef]
- Fekih, R.; Takagi, H.; Tamiru, M.; Abe, A.; Natsume, S.; Yaegashi, H.; Sharma, S.; Sharma, S.; Kanzaki, H.; Matsumura, H.; et al. MutMap+: Genetic Mapping and Mutant Identification without Crossing in Rice. PLoS ONE 2013, 8, e68529. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs; Oxford University Press: Oxford, UK, 1997; Volume 25. [Google Scholar]
- Yangyang, D.; Jianqi, L.; Songfeng, W.; Zhu, Y.; Yaowen, C.; Fuchu, H. Integrated nr Database in Protein Annotation System and Its Localization. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-JSJC200605025.htm (accessed on 4 June 2020).
- Bairoch, A.; Apweiler, R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000, 28, 45–48. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kord, H.; Shakib, A.M.; Daneshvar, M.H.; Azadi, P.; Bayat, V.; Mashayekhi, M.; Zarea, M.; Seifi, A.; Ahmad-Raji, M. RNAi-mediated down-regulation of SHATTERPROOF gene in transgenic oilseed rape. 3 Biotech 2015, 5, 271–277. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Wang, M.L. Inheritance and agronomic performance of an apetalous flower mutant in Brassica napus L. Euphytica 2004, 137, 381–386. [Google Scholar]
- Ni, X.; Huang, J.; Ali, B.; Zhou, W.; Zhao, J. Genetic analysis and fine mapping of the LOBED-LEAF 1 (BnLL1) gene in rapeseed (Brassica napus L.). Euphytica 2015, 204, 29–38. [Google Scholar] [CrossRef]
- Shao, Y.; Pan, C.; Chen, Z.; Zuo, S.; Zhang, Y.; Pan, X. Fine mapping of an incomplete recessive gene for leaf rolling in rice (Oryza sativa L.). Chin. Sci. Bull. 2005, 50, 2466–2472. [Google Scholar] [CrossRef]
- Moreau, P.; Thoquet, P.; Olivier, J.; Laterrot, H.; Grimsley, N. Genetic mapping of Ph-2, a single locus controlling partial resistance to Phytophthora infestans in tomato. Mol. Plant Microbe Interact. 1998, 11, 259–269. [Google Scholar] [CrossRef]
- Michelmore, R.W.; Paran, I.; Kesseli, R.V. Identification of markers linked to disease-resistance genes by bulked segregant analysis: A rapid method to detect markers in specific genomic regions by using segregating populations. Proc. Natl. Acad. Sci. USA 1991, 88, 9828–9832. [Google Scholar] [CrossRef]
- Quarrie, S.A.; Lazić-Jančić, V.; Kovačević, D.; Steed, A.; Pekić, S. Bulk segregant analysis with molecular markers and its use for improving drought resistance in maize. J. Exp. Bot. 1999, 50, 1299–1306. [Google Scholar] [CrossRef]
- Lv, J.; Fu, Q.; Lai, Y.; Zhou, M.; Wang, H. Inheritance and gene mapping of spotted to non-spotted trait gene CmSp-1 in melon (Cucumis melo L. var. chinensis Pangalo). Mol. Breed. 2018, 38, 1–9. [Google Scholar] [CrossRef]
- Liu, X.; Yang, C.; Han, F.; Fang, Z.; Yang, L.; Zhuang, M.; Lv, H.; Liu, Y.; Li, Z.; Zhang, Y. Genetics and fine mapping of a yellow-green leaf gene (ygl-1) in cabbage (Brassica oleracea var. capitata L.). Mol. Breed. 2016, 36, 82. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, X.; Huang, Z.; Chu, M.; Song, T.; Falk, K.C.; Deora, A.; Chen, Q.; Zhang, Y.; McGregor, L.; et al. Identification of Genome-Wide Variants and Discovery of Variants Associated with Brassica rapa Clubroot Resistance Gene Rcr1 through Bulked Segregant RNA Sequencing. PLoS ONE 2016, 11, e0153218. [Google Scholar] [CrossRef] [PubMed]
- Tapia-López, R.; García-Ponce, B.; Dubrovsky, J.G.; Garay-Arroyo, A.; Pérez-Ruíz, R.V.; Kim, S.H.; Acevedo, F.; Pelaz, S.; Alvarez-Buylla, E.R. An AGAMOUS-related MADS-box gene, XAL1 (AGL12), regulates root meristem cell proliferation and flowering transition in Arabidopsis. Plant Physiol. 2008, 146, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Y.; Kim, Y.; Kim, S.Y.; Lee, J.S.; Ahn, J.H. Control of Flowering Time and Cold Response by a NAC-Domain Protein in Arabidopsis. PLoS ONE 2007, 2, e642. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.-Q.; Ma, Z.-Y.; Huang, H.-W.; Mo, H.; Zhao, T.-T.; Li, L.; Cai, T.; Chen, S.; Ma, L.; He, X.-J. Two novel NAC transcription factors regulate gene expression and flowering time by associating with the histone demethylase JMJ14. Nucleic Acids Res. 2015, 43, 1469–1484. [Google Scholar] [CrossRef]
- Saha, D.; Prasad, A.M.; Srinivasan, R. Pentatricopeptide repeat proteins and their emerging roles in plants. Plant Physiol. Biochem. 2007, 45, 521–534. [Google Scholar] [CrossRef]
- Bentolila, S.; Alfonso, A.A.; Hanson, M.R. A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proc. Natl. Acad. Sci. USA 2002, 99, 10887–10892. [Google Scholar] [CrossRef]
- Barkan, A.; Small, I. Pentatricopeptide Repeat Proteins in Plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, F.; Wang, X.; Wang, T.; Su, R.; Hong, D.; Yang, G. A pentatricopeptide repeat protein restores nap cytoplasmic male sterility in Brassica napus. J. Exp. Bot. 2017, 68, 4115–4123. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).