Abstract
The objective of this study was to explore the effects of plant growth-promoting rhizobacteria (PGPR), strain Bacillus licheniformis, with softwood biochar amendment on potato growth and water use efficiency (WUE) under a deficit irrigation (DI) regime. A pot experiment was conducted in a greenhouse. The results showed that PGPR improved leaf gas exchange rates, including photosynthesis rate, stomatal conductance and transpiration rate at early seedling stage, while tended to depress these parameters gradually until final harvest. The effects of biochar on plant leaf physiology, plant growth and WUE were not evident. Plants were more affected by DI than PGPR inoculation and biochar amendment. DI significantly decreased leaf gas exchange rates after exposure to water treatment for around three weeks, and the negative effect was eliminated at final harvest. At final harvest, DI significantly decreased leaf area, specific leaf area, dry mass of leaf and stem, total dry mass, dry mass increment and plant water use. The synergistical effect of PGPR strain Bacillus licheniformis and DI on plant growth and WUE were not observed in our study. WUE was solely improved by DI, indicating that, compared to PGPR inoculation, DI was a more effective measure to enhance plant WUE.
1. Introduction
Water shortage is a huge challenge for agriculture as it threatens water and food security. The global population is predicted to increase to around 10 billion by 2050, and half will live in water-scarce regions [1,2], and, at the same time, demand for food and agricultural water will double, whereas the availability of fresh water is predicted to decline by 50% [3,4]. Thus, it is urgent to promote the development of water-saving agriculture aimed at improving plant water use efficiency (WUE) with acceptable crop yield [5,6]. Du et al. summarized and proposed deficit irrigation (DI) as a promising strategy to resolve the contradiction between water shortage and crop yield [7]. DI strategy allows plants to experience water deficit by reducing irrigation during crop growth period, or by withdrawing irrigation at certain stages when plants are insensitive to water deficit, with acceptable yield loss and optimized WUE [7,8,9]. DI strategy has been successfully adopted in various crops and vegetables, including potatoes [10,11,12].
DI limits plant growth by reducing leaf gas exchange rates, disturbing plant water relations, and inducing reactive oxygen species [13,14]. Meanwhile, plants have developed mechanisms to prevent water loss, distribute water to vital organs, maintain cellular water content and adapt to periods of drought [15]. Plants grown under water deficit had weaker ability in nutrient uptake, as a result of reduced transpiration and impaired active transport and membrane permeability. The degree and duration of DI could affect plant response; for example, moderate DI could induce chemical signals (mainly root-to-shoot ascorbic acid, ABA) pathway to optimize stomatal aperture, which was mainly modulated by turgor pressure under severe DI [16]. As stomatal conductance (gs) and transpiration rate (Tr) are more sensitive to DI, compared to photosynthetic rate (An), the plant could maintain higher intrinsic water use efficiency (WUEi, An/gs) and instantaneous water use efficiency (WUET, An/Tr) under a moderate DI regime [16,17]. The response of plant to DI is also related to the time and frequency of irrigation [18], and increasing irrigation frequency and reducing irrigation amount could lead to improved potato yield and WUE [19].
Apart from DI, increasing evidence indicates that implementing plant growth-promoting rhizobacteria (PGPR) is also an efficient method to improve plant WUE. PGPR can colonize plant roots to promote plant growth through direct mechanisms, such as phytohormone synthesis, nitrogen fixation and phosphorus dissolving, and through indirect mechanisms referring to the resistance of biotic and abiotic stresses. PGPR contains different kinds of bacteria communities, among which the Pseudomonas and Bacillus spp. have been identified as the predominant [20]. The gram-positive spore forming Bacillus is one of the most promising PGPR, gaining increasing attention due to its inherent stability and extended shelf life [21]. Bacillus spp. can improve rhizosphere essential nutrient (such as P and N) availability by converting the complex form of nutrient to a simple one [20], thus reducing the application of traditional fertilizers and related pollution, thereby promoting sustainable development of agriculture [22]. Inoculating drought-tolerant Bacillus spp. can increase the populations of bacteria on plant roots and stimulate root exudation to promote plant growth [23]. Additionally, recent research reported that Bacillus licheniformis (FMCH001) could improve WUE of maize up to 46% in both well-watered (90% field capacity) and drought-stressed (65% field capacity) plants in greenhouses [14]. However, in field practice, due to the strong competition of indigenous soil microorganisms, PGPR colonization of plant roots is difficult and affects its growth-promoting role. Biochar provides a potential habitat for PGPR colonization [24].
Biochar is the product of thermal degradation of organic materials in a vacuum environment, and it has been described as a soil amendment to improve the physical and chemical properties of soil and strengthen the ability of soil to hold nutrients and water [25,26,27]. As a kind of porous media, biochar has the ability to change soil biological community composition and abundance [28], and may accelerate nutrient cycles and further affect plant growth [29].
Up to now, the individual or two-factor effect of DI, PGPR or biochar amendment on plant growth, physiology and WUE has been intensively studied [9,14,27,30], whereas little is known about the combined effects of PGPR with biochar amendment on plant growth and WUE under the DI regime. Therefore, the objective of this study was to investigate whether PGPR with biochar amendment could alleviate the negative effect of DI on plant growth, and synergistically improve plant WUE.
2. Materials and Methods
2.1. Experimental Setup
The pot experiment was conducted in a greenhouse located in the Northwest A&F University, Yangling, Shaanxi, China (34°20″ N, 108°04″ E and altitude of 521 m). Temperature and relative humidity during the experiment are shown in Figure 1. The columned pots used in the experiment were 30 cm in height, 15 cm in inner diameter, with a volume of 5.30 L. In order to maintain ventilation, 5 mm-apertures were punched with a distance of 3 cm in the bottom of the pot. The soil in the study was taken from a local field (0–20 cm layer), and was classified as silty clay loam soil with gravimetric field capacity (θf) and pH of 26% and 8.0, respectively. The contents of organic matter, rapidly available nitrogen and rapidly available K were 6.77 g kg−1, 127.72 mg kg−1 and 205 mg kg−1 respectively. The soil was sieved through 0.5 cm mesh and air-dried before filling the pots. The fertilizers, 0.50 g N, 0.30 g K and 0.24 g P, were supplied to each pot in the forms of urea (N 46.67%) and KH2PO4, and thoroughly mixed with the soil when filling the pots.
Figure 1.
(a) The maximum and minimum daily temperature (T, °C) and (b) the daily maximum and minimum relative humidity (RH, %) in the greenhouse during the treatment period.
Standard softwood biochar came from the UK Biochar Research Centre, University of Edinburgh, School of GeoSciences, UK, with pH, EC, CEC, total C, total N and total K of 7.91, 90 μS cm−1, 3.15 cmol kg−1, 8.56 g kg−1, <0.01 g kg−1 and 0.03 g kg−1, respectively. The biochar was ground into powder and sieved through 0.45 mm mesh to ensure thorough mixing with the soil. For half of the pots, 6 kg air-dried soil was used; for the other half, 2% biochar (i.e., 120 g) mixed with 5.88 kg soil was used, both with volume-weight of 1.25 g cm−3. The field capacity of soil with biochar amendment was also 26%, with organic matter, rapidly available nitrogen, rapidly available K and pH of 27.96 g kg−1, 138.66 mg kg−1, 234 mg kg−1 and 8.1, respectively.
Potato tubers (Jinshu 16) were germinated in a plastic box covered with a towel at room temperature from 15 May to 2 June, 2020. Tubers were transplanted on 3rd June into pots with/without biochar amendment. Half of the tubers were inoculated with plant growth-promoting rhizobacteria (PGPR) strain Bacillus licheniformis (supplied by the Shaanxi Agricultural Science and Technology Co., Ltd. SAEN, Xi’an, China) by immersing the tuber in bacteria diluent which contained an average Bacillus licheniformis count of 1.0 × 106 CFU per ml for 10 s. The other half were immersed in distilled water for 10 s and transplanted into the pots, thus resulting in 4 treatments. Each treatment was replicated 12 times. After transplanting, the pots were irrigated to field capacity. Thereafter, soil water was kept at field capacity for four weeks until plants grew to around 10 leaves. At this point, 4 plants of each treated plant were harvested to investigate the effect of biochar amendment and PGPR inoculation on plant physiology and growth. After that, 4 plants of each treatment were irrigated to field capacity, and the other 4 of each treatment were subjected to DI by withholding irrigation from the pots for two days. Thereafter, the irrigation amount was cut to 70% of that of the well-watered ones. The water treatment lasted for 1 month, and all plants were harvested on 1st August. Pots were weighed around 5:30 pm to evaluate soil water status. The water content (g g−1) during the treatment is shown in Figure 2.
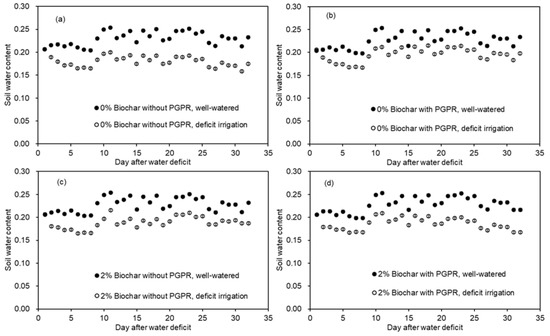
Figure 2.
Changes of soil water content (%, g g−1) in pots of potatoes exposed to two irrigation regimes with or without PGPR inoculation under two biochar amendments. (a) 0% biochar without PGPR; (b) 0% biochar with PGPR; (c) 2% biochar without PGPR; (d) 2% biochar with PGPR. The water amount for deficit irrigation was 70% of that of the well-watered ones.
2.2. Measurement
2.2.1. Leaf Gas Exchange and Stomatal Density
On the first harvest day (1 July) and 6, 19 and 30 days after initiation of irrigation treatment (DAIT), leaf gas exchange rates, including net photosynthetic rate (An, μmol m−2s−1), stomatal conductance (gs, mol m−2s−1) and transpiration rate (Tr, mmol m−2 s−1) were measured on the upper canopy of fully expanded leaves between 9:00 and 11:00 with a portable photosynthetic system (LiCor-6800, LI-Cor, Lincoln, NE, USA). The chamber leaf temperature, photon flux density and CO2 concentration of the system were set as 25 °C, 1200 μmol m−2 s−1 and 400 ppm, respectively. Intrinsic water use efficiency (WUEi, μmol mol−1) and instantaneous water use efficiency (WUET, mmol mol−1) were determined as An/gs and An/Tr, respectively.
Stomatal density (SD, mm−2) in the leaf was measured with silica gel and clear nail varnish to make an impression of the epidermis and removed to the slide with scotch tape. The slide was placed on an electron microscope (BA210, Motic, Xiamen, China), and pictures were taken using image editing software (Leica Microsystems, version 2.5.0, CMS GmbH, Zürich, Switzerland). SD was expressed as the number of stomata per mm2.
2.2.2. Chlorophyll, Flavonoids and Nitrogen Balance Index
Chlorophyll (Chl), flavonoids (flav) and nitrogen balance index (NBI) were measured with a polyphenol chlorophyll meter (Dualex Scientific, Force A, Orsay, France) on the first harvest day, 6 DAIT, 18 DAIT and 27 DAIT.
2.2.3. Plant Water Relations
Leaf water potential (Ψl) was determined before noon with a pressure chamber (Soil Moisture Equipment, SEC, Santa Barbara, CA, USA) on the first and the final harvest day. Relative water content (RWC) was solely measured on the first harvest day following the method described in [17].
2.2.4. Leaf Area and Specific Leaf Area
Plant leaf area (LA, cm2) was measured with a leaf area meter (LICOR 3100, LI-Cor, NE, USA). Specific leaf area (SLA, cm2 g−1) was determined as the ratio of LA to leaf dry mass.
2.2.5. Plant Dry Mass, Dry Mass Increment, Water Use and Water Use Efficiency
Plant dry mass (DM, g) was determined after samples were dried to a constant weight in an oven at 75 °C for 2 days. Dry mass increment (ΔDM, g) was the difference of dry mass between the first and the final harvest. Water use (WU, L) was calculated based on the irrigation amount and the change of soil water content in the pot between the first and the final harvest. Plant water use efficiency (WUE, kg m−3) was the ratio of ΔDM to WU during the irrigation treatment period.
2.2.6. Leaf Nitrogen Concentration and Nitrogen Use Efficiency
Leaf samples were totally dried, then thoroughly ground into powder and analyzed for total N concentration using the Kjeldahl method (Kjeltec 2300, FOSS Tecator. Höganäs, Sweden). Nitrogen use efficiency (NUE) was determined as the ratio of DM to N uptake.
2.3. Data Analysis and Statistics
The data collected at first harvest were subject to two-way Analysis of Variance (ANOVA) to analyze the effect of biochar and PGPR on plant physiology and growth. The data collected after the first harvest were subject to three-way ANOVA to obtain insight into the effect of biochar and PGPR on plant physiology and growth under the reduced irrigation regime. Data were compared using the Duncan Test in SPSS 21 software package (Version 21.0, IBM SPSS, Armonk, NY, USA). Graphs were plotted in Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA).
3. Results
3.1. Effect of Biochar and PGPR on Plant Physiology and Growth at First Harvest
3.1.1. Leaf Gas Exchange and Stomatal Density
At first harvest, biochar amendment had no effect on leaf gas exchange, including photosynthesis rate (An), stomatal conductance (gs) and transpiration rate (Tr), while PGPR inoculation significantly improved these parameters (Figure 3). Leaf intrinsic water use efficiency (WUEi) and instantaneous water use efficiency (WUET) were not affected by biochar amendment or PGPR inoculation at the first harvest (Figure 4).
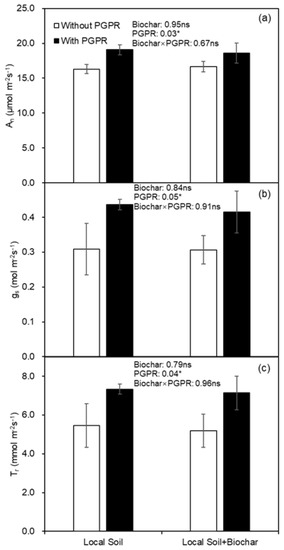
Figure 3.
(a) Photosynthesis rate (An), (b) stomatal conductance (gs) and (c) transpiration rate (Tr) of potato leaf as affected by biochar amendment and PGPR inoculation at first harvest. * indicates significance effect at p < 0.05 level, and ns indicates the effect was not statistically significant at p < 0.05 level.
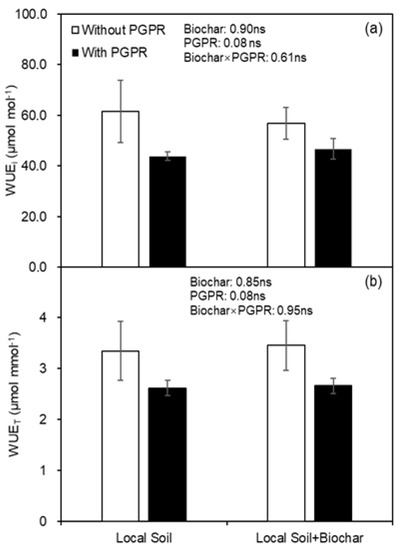
Figure 4.
(a) Intrinsic water use efficiency (WUEi) and (b) instantaneous water use efficiency (WUET) of potato leaf as affected by biochar amendment and PGPR inoculation at first harvest. ns indicates the effect was not statistically significant at p < 0.05 level.
In relation to 0% biochar amendment, 2% biochar amendment significantly improved stomatal density (SD) by 12.76% (Table 1).

Table 1.
Stomata density (SD), chlorophyll (Chl), flavonoids (flav), nitrogen balance index (NBI), relative water content (RWC), leaf water potential (Ψl), leaf area (LA), specific leaf area (SLA), dry mass of leaf (DMleaf) and stem (DMstem) and total dry mass (DM) as affected by biochar amendment and PGPR inoculation at first harvest.
3.1.2. Chlorophyll, Flavonoids and Nitrogen Balance Index
Biochar amendment and PGPR inoculation had no effect on leaf chlorophyll content (Chl). Flavonoids content (Flav) was significantly improved by biochar amendment and PGPR inoculation. Nitrogen balance index (NBI) was lower in plants grown with 2% biochar amendment compared to that with 0% biochar amendment (Table 1).
3.1.3. Plant Water Relations
Relative water content (RWC) and leaf water potential (Ψl) were not affected by biochar amendment or PGPR inoculation (Table 1).
3.1.4. Leaf Area, Specific Leaf Area and Dry Mass
Leaf area (LA) was lower in plants grown with 2% biochar amendment compared to those grown with 0% biochar amendment. Specific leaf area (SLA), dry mass of leaf (DMleaf) and stem (DMstem) and total dry mass (DM) were not affected by biochar amendment or PGPR inoculation (Table 1).
3.2. Effect of Biochar and PGPR on Plant Physiology and Growth under Reduced Irrigation Regime
3.2.1. Leaf Gas Exchange
Six days after the initiation of irrigation treatment (6 DAIT), 2% biochar significantly increased leaf An in relation to 0% biochar amendment (Figure 5a), while having no effect on An on 19 DAIT (Figure 5b) or 30 DAIT (Figure 5c). Biochar amendment had no effect on gs (Figure 5d–f) or Tr (Figure 5g–i). PGPR inoculation had no significant effect on An, gs and Tr on 6 DAIT or 19 DAIT, while significantly decreasing these parameters on 30 DAIT (Figure 5a–i). Deficit irrigation (DI) significantly decreased An, gs and Tr on 6 DAIT and 19 DAIT, while the negative effect was eliminated on 30 DAIT (Figure 5a–i).
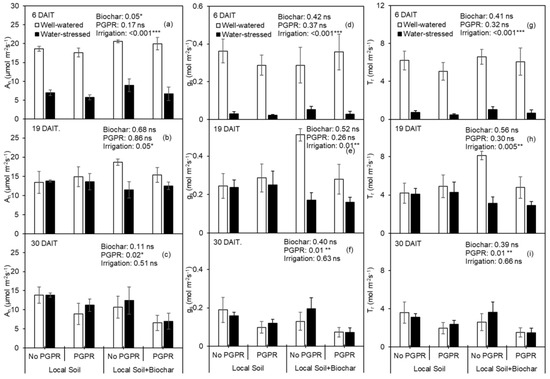
Figure 5.
(a–c) Photosynthesis rate (An), (d–f) stomatal conductance (gs) and (g–i) transpiration rate (Tr) of potato leaf as affected by biochar amendment and PGPR inoculation at 6 days after initiation of irrigation treatment (DAIT), 19 DAIT and 30 DAIT, respectively. *, ** and *** indicate significance effect at p < 0.05, p < 0.01 and p < 0.001 level, respectively, and ns indicates the effect was not statistically significant at p < 0.05 level.
Biochar amendment had no effect on WUEi or WUET on 6 DAIT, 19 DAIT and 30DAIT (Figure 6a–f). The improved WUEi by PGPR inoculation was observed on 30 DAIT (Figure 6c). Reduced irrigation regime significantly improved WUEi and WUET on 6 DAIT, while had no effect on WUEi or WUET on 19 DAIT or 30 DAIT (Figure 6).
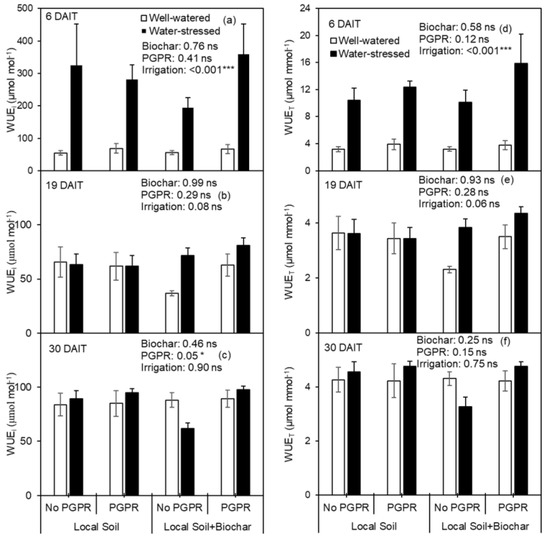
Figure 6.
(a–c) Intrinsic water use efficiency (WUEi) and (d–f) instantaneous water use efficiency (WUET) of potato leaf as affected by biochar amendment and PGPR inoculation after DI exposed. * and *** indicate significance effect at p < 0.05 and p < 0.001 level, respectively, and ns indicates the effect was not statistically significant at p < 0.05 level.
3.2.2. Chl, Flav and NBI
Compared to 0% biochar amendment, 2% biochar amendment increased Flav content. Reduced irrigation regime significantly improved Chl and Flav content. PGPR inoculation significantly improved Flav content, thereby decreasing NBI (Table 2).

Table 2.
Change of chlorophyll (Chl), flavonoids (Flav), nitrogen balance index (NBI), leaf water potential (Ψl), leaf area (LA), leaf dry mass (DMleaf), stem dry mass (DMstem), specific leaf area (SLA), total dry mass (DM), dry mass increment (ΔDM), water use (WU), water use efficiency (WUE), leaf N concentration ([N]leaf) and nitrogen use efficiency (NUE) as affected by biochar, PGPR and irrigation at final harvest.
3.2.3. Ψl, LA and SLA
Ψl was significantly affected by Biochar × PGPR × Irrigation interaction. Ψl was highest in plants grown with 2% biochar amendment and PGPR inoculation under DI condition (Table 2).
LA and SLA were significantly decreased by DI (Table 2).
3.2.4. DM, WU and WUE
DMleaf, DMstem, DM, DM increment (ΔDM) and water use (WU) were significantly decreased by DI, and WUE was significantly enhanced by DI (Table 2). PGPR inoculation improved DMleaf and DM, while having no effect on ΔDM, WU or WUE. Biochar amendment had no effect on tissue DM, ΔDM, WU or WUE (Table 2).
3.2.5. [N]leaf and NUE
[N]leaf was solely affected by Biochar × PGPR interaction. PGPR inoculation significantly improved [N]leaf under conditions of 0% biochar amendment, and the result was reversed under conditions of 2% biochar amendment. Compared to local soil, 2% biochar amendment lowered plant NUE. Biochar × PGPR interaction affected NUE, which was decreased by PGPR inoculation when plants were grown under local soil.
4. Discussion
Numerous studies have shown that plant growth-promoting rhizobacteria (PGPR) perform an environmentally-friendly solution for sustainable agricultural development, and PGPR inoculation has become an integral part of agroecosystem management [31,32]. The strain Bacillus licheniformis has been reported as having multi-functional traits, including auxin production and exopolysaccharide secretion, and Bacillus licheniformis could be used to alleviate drought stress in arid regions without the application of agrochemicals and chemical fertilizers [33]. In this study, the effects of PGPR inoculation and biochar amendment on potato leaf physiology, growth and WUE under a deficit irrigation (DI) regime were investigated. The PGPR strain Bacillus licheniformis improved leaf gas exchange rates, including photosynthesis rate (An), stomatal conductance (gs) and transpiration rate (Tr) at early seedling stage (Figure 3), while these parameters tended to gradually depress until the final harvest (Figure 5). The reversed results from a different growth stage were unexpected, and the results were different from Akhtar et al., who found no significant effect of Bacillus licheniformis on An or gs of maize, inoculated by coating seeds with LB media and plating serial dilutions [14]. The inconsistent effects of PGPR could be related to crop species, inoculation methods and periods. It was well accepted that chlorophyll content (Chl) and N content in the leaf ([N]leaf) could reflect photosynthetic capacity [34]. However, in this present study, the depression of An induced by PGPR at the final harvest was not in accordance with the change of Chl or [N]leaf, as Chl and [N]leaf were not affected by PGPR (Table 2). There should be other mechanisms involved in the regulation of gas exchange. Nitrogen balance index (NBI), which is the ratio of Chl to flavonoids (Flav), was based on crop canopy fluorescence index under photoexcitation, and was considered to be a good indicator for evaluating plant nitrogen status [35]. We noticed that at the final harvest the change of An was in accordance with the change of NBI, indicating that PGPR could influence An by the modulation of NBI. However, the reason for the negative modulation of NBI by PGPR still remains unclear.
Biochar as a kind of soil amendment has shown great potential to improve soil fertility and protect plants from various soil borne pathogens, hereby enhancing plant growth [25,36]. The positive effect of biochar on plant growth has been reported in vegetables and crops, including tomatoes [37], wheat [38] and potatoes [39]. However, in our study, even though in the early seedling stage 2% softwood biochar amendment enhanced stomata density (Table 1), it had no significant effect on gs, An or Tr (Figure 3), and the effects of biochar on plant leaf physiology, plant growth and WUE were not evident (Table 2; Figure 5 and Figure 6). The insignificant effect of softwood biochar on plant growth was also reported in [27]. This was mainly related to the property of softwood biochar [40], which possesses lower nutrient contents, including N, P, K and lower pH, as described in the Materials and Method part. Thus, soil physicochemical properties were not improved by softwood biochar amendment [27]. Literature has documented that plant response to biochar amendment varies with biochar amount, type, plant species and even time [27,39,41,42,43]. Soil structure could be improved in the following two ways: one is soil aggregation, due to the binding agents from oxidation of biochar over time [44,45], and the other is the restructuring of soil and biochar particles, resulting in wider pore size distribution [46]. In our study, two months might be too short a time to improve soil structure, further making little sense to plant growth.
Plant growth and WUE were more affected by DI than PGPR inoculation and biochar amendment. Though the negative effects of DI on leaf gas exchange rates faded away at the final harvest (Figure 5), DI led to significant decrease in leaf area (LA) and plant DM (Table 2). This was in agreement with Jefferies and MacKerron, who presented the point that the first morphological response of potato plants to soil water deficit was reduction in LA [47]. Similar results were also reported by Sun et al. [48] and Liu et al. [17]. Plants exposed to DI could stimulate the synthesis of abscisic acid (ABA) in the roots, which could be transported through the transpiration stream to the shoots to suppress leaf expansion and stomata opening [13]. As gs and Tr are more susceptible to DI than An [17], plants could possess higher intrinsic water use efficiency (WUEi, An/gs) and instantaneous water use efficiency (WUET, An/Tr) under moderate DI (Figure 6a,d). For potato plants at the vegetative growth stage, decreased LA and Tr will not only reduce the plant’s whole transpiration, but also decelerate the crop photosynthetic rate as a result of less light interception [49], hereby further depressing plant WU and DM (Table 2). In our study, compared to well-watered plants, DI plants consumed 35.7% less water with 25.8% reduction in ΔDM, thus resulting in an improved WUE (Table 2).
The synergistical effect of the PGPR strain Bacillus licheniformis and DI on plant growth and WUE were not seen in our study. The result was contrary with [50], who reported that the inoculation of PGPR improved the growth and physiology of maize under DI. We noticed that in soil with 0% biochar amendment, leaf area was increased by PGPR inoculation under well-watered conditions, but not under DI (Table 2). The ineffectiveness of these bacteria on plant growth and WUE under the DI regime could be associated with the colonization of PGPR. Even though PGPR has the potential to promote plant growth, by inducing the production of phytohormones, chelating compounds, siderophores, N2 fixation, phosphate solubilization and other mechanisms [51], strong competition with indigenous soil microorganisms could lead to uncompetitive colonization in roots, especially under a DI regime. The dominant bacterial community in the rhizosphere should also be focused on in future study.
5. Conclusions
Our study shows that plant leaf physiology, plant growth and WUE were mainly affected by DI strategy rather than PGPR Bacillus licheniformis inoculation or softwood biochar amendment. Though the negative effect of DI on An, gs and Tr was gradually eliminated with progress in the growth stages, DI significantly decreased leaf area, total dry mass and dry mass increment at the final harvest. The synergistical effect of PGPR and DI on plant growth and WUE were not observed in our study. WUE was solely affected by DI. In relation to well-watered plants, DI plants consumed 35.7% less water with 25.8% reduction in dry mass increment, hereby improving plant WUE.
Author Contributions
Conceptualization, J.L. and F.L.; methodology, F.L.; software, J.L.; formal analysis, J.L. and J.Z.; investigation, M.Z., H.W., Z.C., N.Y. and J.D.; data curation, J.L. and Z.W.; writing—original draft preparation, J.L.; writing—review and editing, T.H. and F.L.; supervision, J.L.; project administration, J.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 52009112, China Postdoctoral Science Foundation, grant number 2020M683576 and Innovation Training Program for College students, grant number S202010712370.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Hongchao Li, the legal representative of the Shaanxi Agricultural Science and Technology Co., Ltd. SAEN, Xi’an, China for supplying PGPR materials in the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koncagül, E.; Tran, M.; Connor, R.; Uhlenbrook, S. World Water Development Report 2020—Water and Climate Change; SC-2018/WS/5; UNESCO WWAP: Paris, France, 2018. [Google Scholar]
- Kölbel, J.; Strong, C.; Noe, C.; Reig, P. Mapping Public Water Management by Harmonizing and Sharing Corporate Water Risk Information. Technical Note. World Research Institute (WRI). 2018. Available online: www.wri.org/publication/mapping-public-water (accessed on 21 October 2020).
- Gleick, P. The World Water 2000–2001: The Biennial Report on Freshwater Resources; Island Press: Washington, DC, USA, 2000. [Google Scholar]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Hao, X.; Du, T.; Tong, L.; Su, X.; Lu, H.; Li, X.; Huo, Z.; Li, S.; Ding, R. Improving agricultural water productivity to ensure food security in China under changing environment: From research to practice. Agric. Water Manag. 2017, 179, 5–17. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Kang, S.; Zhang, J.; Davies, W. Deficit irrigation and sustainable water-resource strategies in agriculture for China’s food security. J. Exp. Bot. 2015, 66, 2253–2269. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, L.; Liang, Y.; Hu, X.; Cai, H.; Gu, B. Effect of limited irrigation on yield and water use efficiency of winter wheat in the Loess Plateau of China. Agric. Water Manag. 2002, 55, 203–216. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J. Controlled alternate partial root-zone irrigation: Its physiological consequences and impact on water use efficiency. J. Exp. Bot. 2004, 55, 2437–2446. [Google Scholar] [CrossRef]
- Ahmadi, S.; Andersen, M.; Plauborg, F.; Poulsen, R.; Jensen, C.; Sepaskhah, A.; Hansen, S. Effects of irrigation strategies and soils on field-grown potatoes: Gas exchange and xylem [ABA]. Agric. Water Manag. 2010, 97, 1486–1494. [Google Scholar] [CrossRef]
- Carli, C.; Yuldashev, F.; Khalikov, D.; Condori, B.; Mares, V.; Monneveux, P. Effect of different irrigation regimes on yield, water use efficiency and quality of potato (Solanum tuberosum L.) in the lowlands of Tashkent, Uzbekistan: A field and modeling perspective. Field Crops Res. 2014, 163, 90–99. [Google Scholar] [CrossRef]
- Elhani, S.; Haddadi, M.; Csákvári, E.; Zantar, S.; Hamim, A.; Villányi, V.; Douaik, A.; Bánfalvi, Z. Effect of partial root-zone drying and deficit irrigation on yield, irrigation water-use efficiency and some potato (Solanum tuberosum L.) quality traits under glasshouse conditions. Agric. Water Manag. 2019, 224, 105748. [Google Scholar] [CrossRef]
- Liu, F.; Jensen, C.; Shahanzari, A.; Andersen, M.; Jacobsen, S. ABA regulated stomatal control and photosynthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 2005, 168, 831–836. [Google Scholar] [CrossRef]
- Akhtar, S.; Amby, D.; Hegelund, J.; Fimognari, L.; Großkinsky, D.; Westergaard, J.; Müller, R.; Moelbak, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Andersen, M.; Jacobsen, S.; Jensen, C. Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environ. Exp. Bot. 2005, 54, 33–40. [Google Scholar]
- Liu, J.; Hu, T.; Fang, L.; Peng, X.; Liu, F. CO2 elevation modulates the response of leaf gas exchange to progressive soil drying in tomato plants. Agric. Forest Meteorol. 2019, 268, 181–188. [Google Scholar] [CrossRef]
- Kassem, M. Effect of drip irrigation frequency on soil moisture distribution and water use efficiency for spring potato planted under drip irrigation in a sandy soil. Irrig. Drain. 2008, 142, 144–151. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, F.; Liu, H.; Yuan, B. Potato evapotranspiration and yield under different drip irrigation regimes. Irrig. Sci. 2004, 23, 133–143. [Google Scholar] [CrossRef]
- Kang, S.; Radhakrishnan, R.; Lee, K.; You, Y.; Ko, J.; Kim, J.; Lee, I. Mechanism of plant growth promotion elicited by Bacillus sp. LKE15 in oriental melon. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2015, 65, 637–647. [Google Scholar]
- Leser, T.; Knarreborg, A.; Worm, J. Germination and outgrowth of Bacillus subtilis and Bacillus licheniformis spores in the gastrointestinal tract of pigs. J. Appl. Microbiol. 2008, 104, 1025–1033. [Google Scholar] [CrossRef]
- Gouda, S.; Kerry, R.; Das, G.; Paramithiotis, S.; Shin, H.; Patra, J. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 2016, 131–140. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth, osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14. [Google Scholar]
- Lehmann, J.; Rillig, M.; Thies, J.; Masiello, V.; Hockaday, W.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 1–12. [Google Scholar]
- Gou, M.; Qu, Z.; Yang, X.; Zhang, D. Study on the effect of biochar on saving water, preserving fertility and tomato yield. Trans. Chin. Soc. Agric. Mach. 2013, 45, 137–142. [Google Scholar]
- Liu, X.; Wei, Z.; Ma, Y.; Liu, J.; Liu, F. Effects of biochar amendment and reduced irrigation on growth, physiology, water-use efficiency and nutrients uptake of tobacco (Nicotiana tabacum L.) on two different soil types. Sci. Total Environ. 2021, 770, 144769. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Sparovek, G.; Longo, R.; Melo, W.; Crowley, D. Bacterial diversity of terra preta and pristine forest soil from the Western Amazon. Soil Biol. Biochem. 2007, 39, 648–690. [Google Scholar] [CrossRef]
- Compant, S.; Clément, S.; Sessitsch, A. Plant growth-promoting bacteria in the rhizo- and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Zoppellari, F.; Malusá, E.; Chitarra, W.; Lovisolo, C.; Spanna, F.; Bardi, L. Improvement of drought tolerance in maize (Zea mays L.) by selected rhizospheric microorganisms. Ital. J. Agrometeorol. 2014, 1, 5–18. [Google Scholar]
- Kumar, S.; Pandey, P.; Maheshwari, D. Reduction in dose of chemical fertilizers and growth enhancement of sesame (Sesamum indicum L.) with application of rhizospheric competent Pseudomonas aeruginosa LES4. Eur. J. Soil Biol. 2009, 45, 334–340. [Google Scholar] [CrossRef]
- Verma, J.; Yadav, J.; Tiwari, K.; Kumar, A. Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol. Eng. 2013, 51, 282–286. [Google Scholar] [CrossRef]
- Lim, J.; Kim, S. Introduction of drought stress resistance by multi-functional PGPR bacillus licheniformis K11 in pepper. Plant Pathol. J. 2013, 29, 201–208. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Andersen, M.; Jensen, C. Comparative effects of partial root-zone drying and deficit irrigation on nitrogen uptake in potatoes (Solanum tuberosum L.). Irrig. Sci. 2009, 27, 443–448. [Google Scholar] [CrossRef]
- Cartelate, A.; Gerovic, Z.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.; Barbottin, A.; Jeuffroy, M.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Upadhyay, K.; Dhami, N.; Sharma, P.; Neupane, J.; Shrestha, J. Growth and yield responses of potato (Solanum tuberosum L.) to biochar. Agraarteadus 2020, 31, 244–253. [Google Scholar]
- Vaccari, F.; Maienza, A.; Miglietta, F.; Baronti, S.; Lonardo, S.; Giagnoni, L.; Lagomarsino, A.; Pozzi, A.; Pusceddu, E.; Ranieri, R.; et al. Biochar simulates plant growth but not fruit yield of processing tomato in a fertile soil. Agric. Ecosyst. Environ. 2015, 207, 163–170. [Google Scholar] [CrossRef]
- Akhtar, S.; Andersen, M.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Kassaye, K.; Boulange, J.; Kurebito, S.; Tokunari, T.; Saito, H.; Watanabe, H. The role of biochar in improving soil properties, water retention and potato yield in a Fluvisol under temperate monsoon climate. Soil Use Manag. 2022, 38, 1069–1083. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.; Thomas, B.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Upadhyay, K.; George, D.; Swift, R.; Galea, V. The influence of biochar on growth of lettuce and potato. J. Integr. Agric. 2014, 13, 541–546. [Google Scholar] [CrossRef]
- Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Kätterer, T.; Roobroeck, D.; Andrén, O.; Kimutai, G.; Karltun, E.; Kirchmann, H.; Nyberg, G.; Vanlauwe, B.; Nowina, K. Biochar addition persistently increased soil fertility and yields in maize-soybean rotations over 10 years in sub-humid regions of Kenya. Field Crop. Res. 2019, 235, 18–26. [Google Scholar] [CrossRef]
- Burrell, L.; Zehetner, F.; Rampazzo, N.; Wimmer, B.; Soja, G. Long term effects of biochar on soil physical properties. Geoderma 2016, 282, 96–102. [Google Scholar] [CrossRef]
- Dong, X.; Guan, T.; Li, G.; Lin, Q.; Zhao, X. Long-term effects of biochar amount on the content and composition of organic matter in soil aggregates under field conditions. J. Soil Sediment. 2016, 16, 1481–1497. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.; Gonnermann, H. Biochar particle size, shape, and porosity act together to influence soil water properties. PLoS ONE 2017, 12, e0179079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferies, R.; Mackerron, D. Radiation interception and growth of irrigated and drought-stressed potato (Solanum tuberosum). Field Crop. Res. 1989, 22, 101–112. [Google Scholar] [CrossRef]
- Sun, Y.; Yan, F.; Cui, X.; Liu, F. Plasticity in stomatal size and density of potato leaves under different irrigation and phosphorus regimes. J. Plant Physiol. 2014, 171, 1248–1255. [Google Scholar] [CrossRef]
- Liu, F.; Shahnazri, A.; Andersen, M.; Jacobsen, S. Physiological responses of potato (Solanum tuberosum L.) to partial root-zone drying: ABA signalling, leaf gas exchange, and water use efficiency. J. Exp. Bot. 2006, 57, 3727–3735. [Google Scholar] [CrossRef] [Green Version]
- Ullah, N.; Ditta, A.; Khalid, A.; Mehmood, S.; Rizwan, M.; Ashraf, M.; Mubeen, F.; Imtiaz, M.; Iqbal, M. Integrated effect of algal biochar and plant growth promoting rhizobacteria on physiology and growth of maize under deficit irrigations. J. Soil Sci. Plant Nut. 2020, 20, 346–356. [Google Scholar] [CrossRef]
- Saharan, B.; Nehra, V. Plant growth promoting rhizobacteria: A critical review. Life Sci. Med. Res. 2011, 21, 1–30. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).