Abstract
Increasing energy demands and fossil fuel consumption causing global warming has motivated research to find alternative energy sources such as biofuels. Giant reed (Arundo donax L.), a lignocellulosic, perennial, rhizomatous grass has been proposed as an important bioenergy crop for advanced biofuel in the Mediterranean area. Anaerobic digestion for advanced biomethane seems to be a promising approach. However, the presence of lignin in lignocellulosic biomass represents the main obstacle to its production (due to its recalcitrance). Thus, to use effectively lignocellulosic biomass in anaerobic digestion, one or more pretreatment steps are needed to aid microorganisms access to the plant cell wall. To this end, the present study investigated the effect of fungal pretreatment of giant reeds obtained from two different harvesting time (autumn and winter) on biomethane production by anaerobic digestion using two white rot fungi (Pleurotus ostreatus and Irpex lactus, respectively). The highest biomass lignin degradation after 30 days incubation with P. ostreatus in both autumn (27.1%) and winter (31.5%) harvest time. P. ostreatus pretreatment showed promising results for anaerobic digestion of giant reed achieving a cumulative yield of 130.9 NmL g−1 VS for the winter harvest, whereas I. lacteus showed a decrease in methane yield as compared with the untreated biomass (77.4 NmL g−1 VS and 73.3 NmL g−1 VS for winter and autumn harvest, respectively). I. lacteus pretreatment resulted in a loss of both holocellulose and lignin, indicating that this strain was less selective than P. ostreatus. Further studies are necessary to identify white rot fungi more suitable to lignocellulosic biomass and optimize biological pretreatment conditions to reduce its duration.
1. Introduction
The growing world population and global demand for food and energy are rapidly increasing, resulting in a consequent depletion of fossil fuels and environmental concerns such as global warming, greenhouse gas emission, and land use changes [1]. These factors have greatly contributed to investigations into production and use of non-conventional fuels (such as biodiesel, bioethanol and biogas) originating from bio-renewable sources to reduce use of fossil fuels and mitigate their adverse environmental effects on air, soil and water quality.
Among various technologies to produce biofuels, anaerobic digestion (AD) is considered as the most cost-effective and environmentally friendly [2,3].
AD is a biological process in which organic substrate is decomposed by microorganisms under oxygen free conditions to produce biogas (50–75% CH4 and 25–50% CO2). Moreover, in addition to the biogas, AD produces the digestate, a nutrient rich fertilizer that can replace common mineral fertilizers.
Advanced AD using a larger portfolio of non-food feedstock, such as agricultural residues, dedicated crops, lignocellulosic biomass, sewage sludge, animal manure, urban and industrial organic waste and microalgae, allows one to produce clean energy utilizing much more organic materials than conventional feedstocks (i.e., sugar and starch-based crops), [4].
Lignocellulosic feedstock is one of the most abundant organic resources derived from agricultural residuals, forestry, urban wastes, and dedicated energy crops. It represents a renewable resource which is widely available and rich in complex carbohydrates. These characteristics make it a promising candidate for second generation bioenergy production in order to reduce dependency on limited fossil fuels sources, greenhouse gas emissions, and environmental pollutions [5].
An ideal energy crop for biogas production should have high biomass yields, show adaptability to varying environments even under low requirement of energy, water, and nutrients and do not directly compete with food or feed crops for the exploitation of limited agricultural land resources [6].
Giant reed (Arundo donax L.) is a lignocellulosic, perennial, rhizomatous grass widespread in the Mediterranean area which is considered a promising energy crop for southern Europe [7,8,9]. As a perennial crop, giant reed can positively affect soil quality, since it contributes to reduce the risk of soil erosion and to increase the soil organic matter content [10]. Giant reed shows many advantages when compared to other energy crops, such as (i) the adaptability to different types of environments, soils, and growing conditions; (ii) the high biomass production; and (iii) the low input required for its cultivation (use of irrigation, fertilizers, pesticides) [7]. It is considered a drought-tolerant species that can achieve high biomass yields also under high salinity conditions [11]. It can be grown in marginal or sub-marginal lands reducing competition with food crops for soil use [12,13] and has recently been proposed as energy crops for producing biogas [7,8,14,15,16].
Lignocellulosic biomass consists primarily of cellulose, hemicellulose, and lignin, and the linkage of these components create a highly resistant and recalcitrant structure to anaerobic degradation [17]. Thus, pretreatment is a necessary step to overcome lignocellulosic recalcitrance in order to improve methane production from lignocellulosic substrates [18].
Lignocellulosic biomass pretreatments include different methods (e.g., physical, chemical, thermal, thermo-chemical, biological, etc.). In general, all methods have as major effect the change of structure to increase the accessible area by enzymes and microbes. Compared with physical and chemical pretreatment, biological methods are more environment friendly and low-cost, consume less energy and require simple equipment, do not produce inhibitors, and do not require chemicals input [19].
The commonly microorganisms involved in biological pretreatment are filamentous fungi, mainly white-rot fungi (WRF) due to their ability to grow on the biomass at mild conditions of temperature and selectively degrade lignin from the holocellulose (cellulose and hemicellulose) surface without added chemicals [20,21].
WRF are able to degrade lignin through the action of lignin-degrading enzymes synthetized during an oxidative process, such as lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase. WRF have also a hydrolytic mechanism that cause substantial cellulose loss due to the high cellulolytic and hemicellulolytic enzyme activity [22].
The ability of several WRF to degrade polymers of lignocellulose biomass has been widely studied and Pleurotus ostreatus, Phanerochaete chrysosposrium, Trametes versicolor, Irpex lacteus, Ganoderma lucidum, Bjerkandera adusta are some of the most efficient wood-decaying fungi which selectively degrade lignin, leaving high cellulose residue [23,24]. However, there is a lack of research on fungal pretreatment of giant reed biomass to date.
The objectives of this study were to: (1) evaluate the effects of two different harvesting time (autumn and winter) on giant reed biomass production; (2) compare fungal pretreatment of giant reed using two WRF (Pleurotus ostreatus and Irpex lactus); and (3) evaluate the effect of fungal pretreatment of giant reed biomass on biomethane production by anaerobic digestion.
2. Materials and Methods
2.1. Agronomic Data
The field trial was carried out at the Experimental farm of the University of Catania, Italy (10 m a.s.l., 37°25′ N lat., 15°03′ E long.) in a typical xerofluvent soil. The Giant reed (Arundo donax L.) field was established in 1997, using plantlets obtained from nodal cuttings at a plant density of 2.5 plants m−2. Further details are reported in Cosentino et al. (2014) [8]. From 2002 the field was managed without any fertilisation, irrigation or other agronomic input.
From 2011 and up to present, plots were split to differentiate harvest times, namely autumn and winter on three replicated plots of 134 m2 (8 × 17 m) each treatment were differentiated. Thus, the autum growing season refers to the growth of crops that were harveted in the previous autumn (12 months growth), while the winter growing season to the crops that were harvested the previous winter (12 months growth). The biomass used in this study was obtained from the 2020/2021 growing season, which represents the plantation’s 23rd year. The date of harvests were 12 September and 11 February for the autumn and winter harvest times, respectively. During the autumn and winter growing seasons, meteorological conditions and potential evapotranspiration (ET0) were continuously measured using a weather station connected to a data logger (Delta-T, WS-GP1 Compact) and a Class A evaporation pan (mm d−1).
At harvest, edge plants were removed in each plot and the aboveground biomass from a sampling area of 6 m2 (3 × 2 m) was weighted. A sample of plants was collected in order to subdivide the plants in stems and leaves.
Fresh sub-samples were randomly collected, weighted, and dried to a constant weight at 65 °C to determine the dry biomass yield which was referred to as the unit land area (DMY, Mg ha−1).
2.2. Characterization of Feedstock
After being oven-dried at 65 °C, samples of giant reed biomass (GRB) was milled and stored for chemical analysis and pretreatment.
The total solids (TS), volatile solids (VS), and chemical composition of feedstock were determined before pretreatment. TS correspond to the residue after drying the sample at 105 °C to constant weight. It is expressed in percent of the sample initial weight. Dry residue is, then, burnt 5 h at 550 °C. VS are the combusted organic matter (expressed in % TS), whereas residue after ignition is the mineral matter (ash). TS and VS measurements were determined in duplicates [25].
The total fiber composition was determined as neutral detergent soluble (NDS), neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) according to Van Soest method [26] through a Fiber Analyzers (Fibertec Velp Scientifica, model FIWE). The hemicellulose and cellulose contents were calculated as the difference between the NDF and ADF, and the ADF and ADL, respectively. To determine the ash, ADL residue was ignited in muffle furnace at 550 °C for 5 h and lignin content was calculated as the difference between ADL and ash. The analyses were performed in triplicate.
2.3. Inoculum Preparation
Two fungal strains were compared: Pleurotus ostreatus (MUT00002977) and Irpex lactus (MUT00005918), purchased from Mycotheca Universitatis Taurinensis (MUT) of the Department of Life Sciences and Systems Biology, University of Turin (Turin, Italy).
Fungi were activated on Malt Extract Agar plates and incubated at 26 °C for 7 days. Sterile GRB colonized with P. ostreatus and I. lactus was used as inoculum for the fungal pretreatment experiments.
To prepare the inoculum, 30 g (dry basis) of GRB were placed in 0.5 L reactors, in which deionized water was added to obtain a moisture content of 70%. Reactors were autoclaved at 121 °C for 20 min followed by cooling down to room temperature. Subsequently, four agar discs of seven-day-old mycelia (approximately 1 cm in diameter) were aseptically added to sterilized GRB and incubated at 26 °C until full colonization. At the end of colonization, which occurred four weeks after the start of incubation, fungal-colonized GRB was thoroughly mixed and used as inoculum for the successive fungal pretreatment of GRB.
2.4. Fungal Pretreatments
Sterile GRB and inoculum (fungal-colonized GRB) were mixed and added to 0.5 L reactors. Fungal pretreatments were performed at 30% (dry weight basis) inoculum ratio. Deionized water was added to reach 70% moisture content. Reactors were covered with cotton plugs and incubated at 26 °C for 30 days. Sterile GRB was considered as a negative control.
Samples were collected at day 10, 20, and 30, dried at 65 °C in a ventilated oven until constant weight, and subjected to composition analysis for cellulose, hemicellulose, and lignin content. The dry matter loss and degradation of cellulose, hemicellulose, and lignin during the pretreatment were expressed as percentage of the initial dry weight and fiber fractions before fungal pretreatment.
2.5. Biochemical Methane Potential (BMP) Tests
The BMP test was performed by an automatic methanogenic potential detection system (AMPTS II, Automatic Methane Potential Test System, Bioprocess Control AB, Lund, Sweden). The AMPTS II is a standardized laboratory set-up specially designed for automatic BMP determination of any biodegradable material. It consists of 15 parallel reactors and the same number of gas flow meters (flow cells) attached to a detection unit for online, automatic data acquisition.
The experiment was conducted in reactors of 500 mL each, in which substrates and inoculum were mixed at a ratio of 1:3 in terms of grams of VS at mesophilic conditions (38 ± 1 °C) with continuously mixing. All tests were performed in triplicate. The inoculum was originally obtained from an anaerobic digester located in Sicily and maintained in a reactor in the laboratory. To remove large and undigested particles the inoculum was filtered through a 2 mm porosity sieve and then it was stabilized in an incubator at 38 °C for 5 days.
TS and VS were determined both for the organic substrate and the inoculum as reported above.
Each reactor was connected to a 80 mL trap bottle of 3 M sodium hydroxide solution used for absorbing CO2 from the raw gas. The remaining gas after scrubbing passed to ultra-low gas flow meters which were connected to the data analytical and acquisition system. The BMP test was run for 30 days.
Additionally, blank samples, only containing inoculum, were incubated. The resulting methane production of the substrate was determined by subtracting methane production of the blank (inoculum) from the substrate sample (substrate + inoculum). The final value of cumulative methane production at the end of the test was defined as the experimental BMP of the substrate.
2.6. Biomethane Potential Per Hectare
The biomethane yields per hectare of giant reed (m3 CH4 ha−1) was calculated as the product of biomethane potential and dry biomass yield expressed in volatile solid (gVS ha−1).
2.7. Statistical Analysis
Data were analyzed according to the randomized block design. Before conducting the ANOVA, the Bartlett’s test was run to verify the assumption of homogeneity of variances. Biomass dry matter yield, biomass composition, and yield content of the hemicellulose, cellulose, ADL, ash and NDS, were analyzed by one-way ANOVA with harvest time as fixed effect. The biomethane yield was analyzed by a two-way ANOVA with fungal pretreatment and harvest time as fixed effect. Degradation of giant reed dry matter, hemicellulose, cellulose and acid detergent lignin after 10, 20, and 30-day fungal pretreatment, the daily and cumulated biomethane of untreated and fungal pretreated giant reed after 30-day incubation were analyzed by the repeated measure ANOVA. Time represented the within-factor, while the fungal pretreatment and harvest time the between-factor effect (SPSS, PASW Statistics 18). When data failed Mauchly’s test for sphericity, the univariate results were adjusted by using the Greenhouse-Geisser Epsilon and the Huynh-Feldt Epsilon correction factors. When univariate results satisfied sphericity tests for within-subject effects, the F-values and associated p-values for between-subject effects were tested. Differences between means were evaluated for significance using the Tukey test at 95% confidence level.
3. Results
3.1. Meteorological Conditions
During the autumn growing season annual average temperature was 23.9 °C for the maximum, 12.8 °C for the minimum and 18.1 °C for the mean temperature (Figure 1). The winter season was cooler, 23.4 °C, 12.3 °C, and 17.6 °C for the maximum, the minimum, and the mean air temperature, respectively.
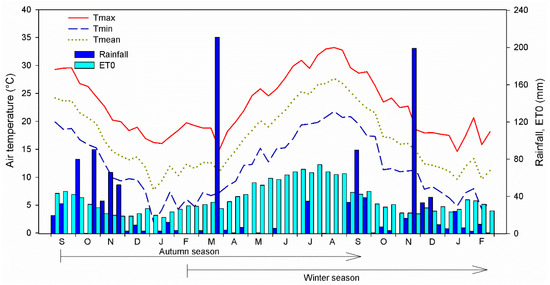
Figure 1.
Meteorological trend (air temperature and rainfall) and reference evapotranspiration (ET0) at the autumn and winter growing season of giant reed (Arundo donax) grown at the experimental farm of the University of Catania, Catania, Italy (10 m a.s.l., 37°25′ N lat., 15°03′ E long.).
Rainfall was more abundant in the winter than in the autumn season (776.4 and 674.6 mm, respectively). The reference evapotranspiration (ET0) was higher in the autumn than in the winter season (1505.2 and 1465.8 mm, respectively), with an average of 4.12 and 4.02 mm day−1, respectively.
3.2. Biomass Composition and Yield Components
The analysis of variance (ANOVA) of the harvest time main effect showed significant differences for ADL and ash biomass components, while hemicellulose, cellulsose, and NDS did not differ (Table 1). Total aboveground biomass yield (DMY) and yield components (i.e., dry biomass composition yield) were significanly affected by harvest time (except for the ADL yield).

Table 1.
One-way ANOVA for main effect (harvest) on biomass dry matter yield (DMY), hemicellulose content and yield (H, % and Y) cellulose content and yield (C, % and Y), lignin content and yield (ADL, % and Y), neutral detergent soluble content and yield (NDS, % and Y), ash content and yield (ASH, % and Y). Degree of freedom (df) and adjusted mean square significance: p ≤ 0.001 (***), p ≤ 0.01 (**), p ≤ 0.05 (*), Not significant (ns).
The ADL content was 10.4% w/w in winter and 9.6% w/w in autumn harvest, while ash content was higher in autumn than winter (1.2 and 0.7% w/w, respectively). Although not significant, hemicellulose and NDS content were higher in autumn (29.1 and 24.2% w/w, respectively) than winter (29.0 and 23.9% w/w, respectively), while the cellulose content was 36.9 and 35.9% w/w in winter and autumn, respectively (Figure 2A).
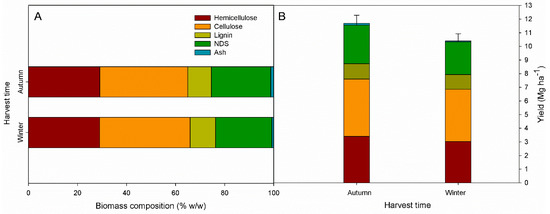
Figure 2.
(A) Biomass composition (% w/w) and (B) aboveground dry matter yield and yield components (Mg ha−1) of giant reed (Arundo donax) at the autumn and winter harvest regimes.
The aboveground dry matter yield (DMY) was higher in autumn than winter, 11.6 Mg ha−1 and 10.4 Mg ha−1, respectively (Fugure 2B). The autumn harvest produced higher yield components: cellulose represented the largest part of giant reed yield, reaching 4.2 Mg ha−1 in autumn and 3.8 Mg ha−1 in winter harvest, followed by hemicellulose (3.4 and 3.0 Mg ha−1 for autumn and winter, respectively), NDS (2.8 and 2.4 Mg ha−1 for autumn and winter, respectively), ADL (1.1 and 1.0 Mg ha−1 for autumn and winter, respectively), and ash (0.14 and 0.08 Mg ha−1 for autumn and winter, respectively).
3.3. Pretreatment Effects on Lignocellulosic Biomass
Losses of cellulose, hemicellulose, and lignin, with a consequent reduction of organic matter, can be used to evaluate the degradation pattern of different white-rot fungi. The ANOVA showed that biomass chemical composition was significantly modified by fungi growth (Table 2).

Table 2.
Repeated measure ANOVA on degradation of giant reed dry matter (DM), hemicellulose (H), cellulose (C) and acid detergent lignin (ADL) during 10, 20, and 30-day fungal pretreatment (I. lacteus and P. ostreatus) in winter and autumn harvest. Degree of freedom (df) and adjusted mean square significance: p ≤ 0.001 (***), p ≤ 0.01 (**), p ≤ 0.05 (*), Not significant (ns).
The effect of pretreatment was significant on cellulose and lignin content, while harvest time was significant on dry matter, hemicellulose, cellulose and lignin. Significant interactions “pretreatment × harvest time” were observed for dry matter, hemicellulose, and cellulose.
The degradation of dry matter, cellulose, hemicellulose, and lignin in GRB increased with time for both P. ostreatus and I. lacteus treatment (Figure 3).
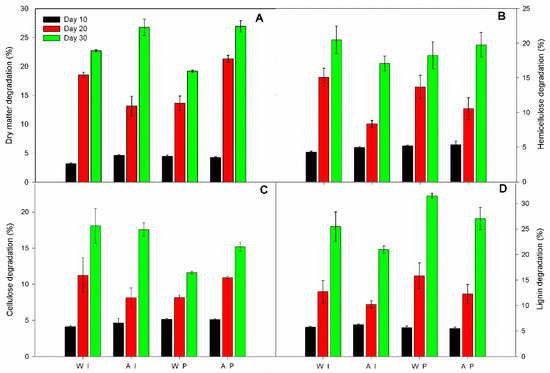
Figure 3.
Degradation (%) of giant reed components: (A) dry matter, (B) hemicellulose, (C) cellulose, and (D) lignin during 10, 20 and 30-day fungal pretreatment in winter and autumn I. lacteus pretreatment (WI and AI, respectively), and winter and autumn P. ostreatus pretreatment (WP and AP, respectively). Significant interaction (LSDint p ≤ 0.05) for: (i) dry matter (2.76), hemicellulose (2.85), cellulose (1.33), and ADL (3.03).
A high percentage of degradation of dry matter was observed for the biomass of autumn harvest for both P. ostreatus (26.9%) and I. lacteus (26.7%) treatment after 30 days of incubation (Figure 3A). For hemicellulose and cellulose, maximum degradation rates were observed for I. lacteus in the winter harvest with a loss of 20.5% and 18.1%, respectively (Figure 3B,C). The highest value of lignin loss was obtained by P. ostreatus in both autumn (27.1%) and winter (31.5%) harvest time (Figure 3D).
Hemicellulose and lignin were degraded more than cellulose during fungal pretreatment, mainly with P. ostreatus. This is confirmed by selectivity value, defined as lignin degradation over cellulose loss. It is important to evaluate the selective lignin-degrading capability of white rot fungi. The highest selectivity value of 2.7 with lignin degradation of 31.5% was reached from P. ostreatus, indicating this strain a selectively degrading hemicellulose and lignin over cellulose. The low degradation of cellulose during the pretreatment has a positive impact on the anaerobic digestion process since cellulose is considered the main substrate for anaerobic microorganisms to produce biogas.
3.4. Methane Production
The ANOVA showed that daily and cumulative biomethane production were significantly influenced by the incubation time, the pretreatment time, and the harvest time. Significant interactions “pretreatment × harvest time” were also observed (Table 3).

Table 3.
Repeated measure ANOVA on daily and cumulated biomethane (DCH4 and ∑CH4, respectively) of untreated and fungal pretreated giant reed in winter and autumn harvest after 30-day incubation. Degree of freedom (df) and adjusted mean square significance: p ≤ 0.001 (***), p ≤ 0.01 (**), p ≤ 0.05 (*), Not significant (ns).
Daily production (NmL g−1 VS d−1) and cumulative methane production (NmL g−1 VS) during anaerobic digestion of untreated and fungal pretreated giant reed are displayed in Figure 4. The daily production curves for the pretreated samples showed the same trend for both harvesting time for each fungal strain used (Figure 4A). The daily biomethane peaks (15.6 and 12.6 NmL g−1 VS d−1) were highest in the biomass pretreated by P. ostreatus after 17 days of digestion for winter and autumn harvest, respectively. Winter giant reed pretreated by I. lacteus showed the maximum peak (6.2 NmL g−1 VS d−1) after 18 days incubation, while the autumn one reached the peak of 6.1 NmL g−1 VS d−1 after 23 days.
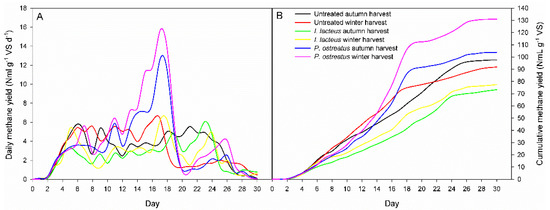
Figure 4.
(A) Daily methane yield (NmL g−1 VS d−1) and (B) cumulative methane yield (NmL g−1 VS) for GRB under harvest regimes (autumn and winter) and fungal pretreatments (I. lacteus and P. ostreatus). Significant interaction (LSDint p ≤ 0.05) for: (i) DCH4 (1.34) and ∑CH4 (7.22).
Cumulative biomethane production was observed for 30 days until biomethane yield reached a plateau at the end of exponential phase (Figure 4B). The initial lag phase lasted around three days until the complete adaptation of the bacterial flora to the lignocellulosic substrate. The methane yield obtained for the untreated GRB of autumn and winter harvest was 97.6 NmL g−1 VS and 91.8 NmL g−1 VS, respectively. P. ostreatus pretreated GRB achieved the highest BMP values, 130.9 NmL g−1 VS and 103.8 NmL g−1 VS for the winter and the autumn harvest, respectively, showing an improvement of the anaerobic digestion after fungal pretreatment. On the contrary, the pretreatment using I. lacteus was uneffective and produced lower cumulative methane yield than the untreated giant reed, 77.4 NmL g−1 VS and 73.3 NmL g−1 VS for winter and autumn harvest, respectively. I. lacteus pretreatment resulted in a loss of both holocellulose and lignin, indicating that this strain was less selective than P. ostreatus.
3.5. Methane Yields Per Hectare
The ANOVA showed that pretreatment, harvest time and interaction were significant on biomethane yield (BMY) (Table 4).

Table 4.
Two-way ANOVA on biomethane yield (BMY) of untreated and fungal pretreated giant reed in winter and autumn harvests. Degree of freedom (df) and adjusted mean square significance: p ≤ 0.001 (***), p ≤ 0.01 (**), Not significant (ns).
The BMY in the untreated GRB was greater for the autumn than the winter harvest (1078.4 m3 CH4 ha−1 and 905.8 m3 CH4 ha−1, respectively) as consequence of the higher biochemical methane potential and higher dry biomass yield of autumn harvest time (Figure 5).
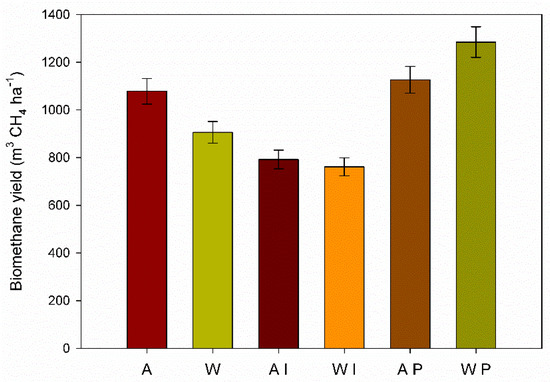
Figure 5.
Biomethane yield (m3 CH4 ha−1) of untreated GRB (autumn, A; winter, W), autumn and winter pretreated with I. lacteus (AI and WI, respectively), autumn and winter pretreated with P. ostreatus (AP and WP, respectively). Significant interaction (LSDint p ≤ 0.05) for BMY (18.73).
P. ostreatus pretreatment of the winter GRB showed the highest biomethane yield per hectare (1284.5 m3 CH4 ha−1), followed by the autumn P. ostreatus pretreated GRB (1126.5 m3 CH4 ha−1). Despite the lowest dry biomass yield of winter, the biomethane production per hectare depended mostly on the higher BMP showed by winter GRB pretreated by P. ostreatus. On the other hand, I. lacteus pretreated GRB yielded the lowest biomethane (791.9 m3 CH4 ha−1 and 761.4 m3 CH4 ha−1 for the autumn and the winter harvest, respectively).
4. Discussion
Giant reed showed a higher dry biomass yield in autumn than winter harvest (11.7 Mg DM ha−1 and 10.4 Mg DM ha−1, respectively). This production was in line with data reported by previous long-term trials on giant reed [11,14,27,28]. The higher yield in autumn harvest was linked to the higher leaves yield. Indeed, leaves decreased during the winter period due to senescence and losses (data not shown).
The composition analysis of the raw material showed differences in the lignocellulosic matrix by changing harvest time, with a greater content of hemicellulose, NDS and ash in autumn and a greater amount of lignin and cellulose in winter harvest. Biomass yield and composition agree with values reported for this species by Scordia et al., 2020 and Zanetti et al., 2019 [29,30]. The feedstock collected in winter is characterized by a loss of hemicellulose associated with lignin deposition and mineral translocation (i.e., both ash and soluble compounds) to rhizomes during the cold season (which then remobilizes them to the aboveground part during the subsequent growing season) [31].
Comparing the composition of giant reed to other feedstocks used for anaerobic digestion, the cellulose content is similar to that of maize stover and rice straw but higher than maize silage and wheat straw. The hemicellulose content is similar to the rice and wheat straw and higher than maize. The lignin content is similar to the maize stover and lower than the rice and wheat straw [32,33,34,35]. High concentrations of cellulose and hemicellulose confirm the wide interest in biochemical conversion and anaerobic digestion of giant reed for advanced biomethane production.
This study proved that giant reed methanogenic potential could be increased by the use of a pretreatment that reduce the lignin content with a minimum loss of cellulose and hemicellulose. Several pretreatments carried out on giant reed biomass were performed using physical methods, chemicals (acids, bases or solvents), or severe conditions (high temperature and/or pressure). Di Girolamo et al. (2013) reported the effects of hydrothermal pretreatments performed at varying times, temperatures, and catalysts on giant reed, determining that hydrothermal pretreatments without acid catalyst contributed to increase methane yield of giant reed (average, +12%) whereas pretreatments with H2SO4 incurred a methanogenic inhibition [36]. A recent work studied the effects of a milling pretreatment on giant reed stems, reporting a methane yield of 89.5 Nm3 t−1 VS for untreated giant reed stems (whereas the processed material reached a methane production of 212.8 Nm3 t−1 VS) [37].
Fungal pretreatment with P. ostreatus and I. lacteus employed here changed the composition of GRB. In particular, the effect of time was significant on dry matter, cellulose, hemicellulose, and lignin losses, showing the maximum percentage of degradation after 30 days of pretreatment. Previous studies reported similar results for P. ostreatus pretreatment applied to corn stover and rice straw [38,39] and the effect of other white rot fungi used on lignocellulosic biomass such as rice straw, wheat straw, and corn stover [33,40,41]. However, very few studies described the effect of fungal pretreatment on giant reed biomass [42,43].
P. ostreatus is the most studied white rot fungus for its ability to produce hydrolytic ligninolytic enzymes in different lignocellulosic biomass. Mustafa et al. [33] found that P. ostreatus treatment of rice straw at 75% moisture content and 20-day incubation time led to a lignin degradation of 33.4%. Taniguchi et al. [44] comparing four different strains of white-rot fungi (Trametes versicolor, Phanerochaete chrysosporium, P. ostreatus and Ceriporiopsis subvermispora) to pretreat rice straw, reported P. ostreatus as the most efficient to selectively degrade the lignin but not the holocellulose component, with 41% degradation of lignin and residual amounts of cellulose and hemicellulose of 83% and 52% as compared with the untreated rice straw, respectively.
Previous studies reported promising results in terms of selectivity also for I. lacteus. For example, Yu et al. [45] investigated the effect of fungal pretreatment with I. lacteus on sodium hydroxide pretreatment of corn stalks under mild reaction condition, and reported a lignin degradation of 11.8% after 10 days of pretreatment, showing a selective lignin-degrading ability for I. lacteus. However, as reported by Wan and Li (2012), fungal degradation rate varies with different feedstocks and fungal selectivity depends on the species and on the pretreatment time [22].
In the present experiments, I. lacteus showed a lower lignin degradation (21% and 25.5% for autumn and winter biomass, respectively) and a greater cellulose loss (17.6% and 18.1% for autumn and winter biomass, respectively) than P. ostreatus, which reported a lignin loss of 27.1% and 31.5% for autumn and winter biomass, respectively, and a cellulose degradation of 15.7% and 11.6% for autumn and winter biomass, respectively. I. lacteus pretreatment resulted in a major consumption of holocellulose. Holocellulose losses during pretreatment led to a reduced biomethane yield through anaerobic digestion. The low methane yields obtained using the I. lacteus strain were due to the degradation of the all components during the pretreatment. The P. ostreatus pretreatment showed promising results for anaerobic digestion of giant reed, reaching a cumulative yield of 130.9 NmL g−1 VS for the winter harvest, whereas I. lacteus showed a decrease in methane yield as compared with the untreated control.
Regarding the daily production trend, the main differences observed were due to the fibrous composition of the biomass after pretreatment. The pretreatment step allowed one to modify the lignocellulosic composition removing lignin, ultimately increasing the polysaccharides accessible surface area. Thus, after the conversion of readily-available soluble fraction (i.e., NDS content), pretreated samples were more susceptible to an enzymatic attack, resulting in a better digestibility of cellulose. This was observed on P. ostreatus that reached the highest biomethane yield thanks to the selective lignin consumption that led to a higher cellulose proportion in the digested biomass. On the contrary, I. lacteus showed a reduction of methane production due to the elevate content of cellulose degraded during pretreatment.
It is worth mentioning that the untreated autumn biomass produced higher biomethane due to a higher proportion of leaves which contain a higher number of soluble substances and a lower content of lignin compared to the untreated winter harvest time. The winter P. ostreatus pretreated GRB, on the contrary, showed an increased biomethane yield compared to the autumn GRB due to the greater content of lignin degraded during pretreatment that led to a rise of soluble and cellulose fraction suitable for anaerobic digestion.
In accordance with other studies, P. ostreatus confirmed its ability to improve anaerobic digestion showing an outstanding degrading lignin rate [33,39,40,44,46]. Regarding the performance of I. lacteus, the results suggested that this strain did not improve the methane production but rather caused a decrease of cumulative yield compared to the control. Similarly, other works reported I. lacteus as non-selective due to an extended consumption of polysaccharides over lignin [46].
The biomethane yield obtained here is quite low if compared with that produced by the common feedstock such as maize and sorghum. However, from an environmental point of view, the present plantation has not received any agronomic input (e.g., irrigation, fertilization, weed or pest control) for nearly 20 years. Hence, cultivating perennial grasses, such as giant reed, for anaerobic digestion can contribute to more long-term environmental benefits than the most productive annual feedstock.
5. Conclusions
This study highlighted the potential of giant reed to produce satisfactory dry biomass yield and biomethane production even after 23-year of cultivation in the semiarid Mediterranean environment and in the absence of agronomic input.
The white-rot fungus P. ostreatus showed high values of lignin degradation in both autumn and winter harvest time and enhanced the methane yield of recalcitrant giant reed biomass during anaerobic digestion. In contrast, pretreatment using I. lacteus produced lower cumulative methane yield than the untreated giant reed due to the high percentage of holocellulose lost during the pretreatment.
The application of a biological pretreatment, using white-rot fungi, allows one to improve the methane yield degrading lignin from lignocellulosic biomass through a safe and environmentally friendly pretreatment without the requirements of high energy, chemicals, or expensive instruments necessary in other widely used pretreatment methods such as physical and thermochemical processes.
However, further studies are necessary to ascertain the best harvest time window to accumulate the highest biomass dry matter yield and components while preserving the plantation in the long-term. In addition, more work should still be carried out on the appropriateness of white-rot to identify those more suitable to a wide spectrum of lignocellulosic feedstock and optimize biological pretreatment conditions to reduce its duration.
Author Contributions
Conceptualization, A.P. and G.T.; methodology, A.P. and G.T.; investigation, A.P., S.A.C. and G.T.; data curation, A.P. and S.A.C.; writing—original draft preparation, A.P. and G.T.; writing—review and editing, A.P., G.T. and D.S.; supervision, S.L.C. and G.T.; funding acquisition, G.T., D.S. and S.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This paper is part of a project that has received funding from the University of Catania by the PIA.CE.RI. 2020-2022 Linea 2-CROP2FUEL project (5A722192164).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture Trends and Challenges. Rome. 2017. Available online: https://www.fao.org/publications/fofa (accessed on 15 February 2022).
- Lim, J.W.; Ge, T.; Tong, Y.W. Monitoring of Microbial Communities in Anaerobic Digestion Sludge for Biogas Optimisation. Waste Manag. 2018, 71, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of Agricultural Biomass for Anaerobic Digestion: Current State and Challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Bacenetti, J.; Fusi, A.; Negri, M.; Guidetti, R.; Fiala, M. Environmental Assessment of Two Different Crop Systems in Terms of Biomethane Potential Production. Sci. Total Environ. 2014, 466–467, 1066–1077. [Google Scholar] [CrossRef]
- Scordia, D.; Cosentino, S.L. Perennial Energy Grasses: Resilient Crops in a Changing European Agriculture. Agriculture 2019, 9, 169. [Google Scholar] [CrossRef] [Green Version]
- Maucieri, C.; Camarotto, C.; Florio, G.; Albergo, R.; Ambrico, A.; Trupo, M.; Borin, M. Bioethanol and Biomethane Potential Production of Thirteen Pluri-Annual Herbaceous Species. Ind. Crops Prod. 2019, 129, 694–701. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The Development and Current Status of Perennial Rhizomatous Grasses as Energy Crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Scordia, D.; Sanzone, E.; Testa, G.; Copani, V. Response of Giant Reed (Arundo donax L.) to Nitrogen Fertilization and Soil Water Availability in Semi-Arid Mediterranean Environment. Eur. J. Agron. 2014, 60, 22–32. [Google Scholar] [CrossRef]
- Angelini, L.G.; Ceccarini, L.; Bonari, E. Biomass Yield and Energy Balance of Giant Reed (Arundo donax L.) Cropped in Central Italy as Related to Different Management Practices. Eur. J. Agron. 2005, 22, 375–389. [Google Scholar] [CrossRef]
- Cosentino, S.; Copani, V.; Scalici, G.; Scordia, D.; Testa, G. Soil Erosion Mitigation by Perennial Species Under Mediterranean Environment. Bio. Energy Res. 2015, 8, 1538–1547. [Google Scholar] [CrossRef]
- Angelini, L.G.; Ceccarini, L.; o Di Nasso, N.N.; Bonari, E. Comparison of Arundo donax L. and Miscanthus x Giganteus in a Long-Term Field Experiment in Central Italy: Analysis of Productive Characteristics and Energy Balance. Biomass Bioenergy 2009, 33, 635–643. [Google Scholar] [CrossRef]
- Fernando, A.L.; Costa, J.; Barbosa, B.; Monti, A.; Rettenmaier, N. Environmental Impact Assessment of Perennial Crops Cultivation on Marginal Soils in the Mediterranean Region: 24th European Biomass Conference and Exhibition (EUBCE). Biomass Bioenergy 2018, 111, 174–186. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; D’Agosta, G.M.; Sanzone, E.; Mantineo, M. First Results on Evaluation of Arundo donax L. Clones Collected in Southern Italy. Ind. Crops Prod. 2006, 2, 212–222. [Google Scholar] [CrossRef]
- Alexopoulou, E.; Zanetti, F.; Scordia, D.; Zegada-Lizarazu, W.; Christou, M.; Testa, G.; Cosentino, S.L.; Monti, A. Long-Term Yields of Switchgrass, Giant Reed, and Miscanthus in the Mediterranean Basin. BioEnergy Res. 2015, 8, 1492–1499. [Google Scholar] [CrossRef]
- Dragoni, F.; Ragaglini, G.; Corneli, E.; Nassi o Di Nasso, N.; Tozzini, C.; Cattani, S.; Bonari, E. Giant Reed (Arundo donax L.) for Biogas Production: Land Use Saving and Nitrogen Utilisation Efficiency Compared with Arable Crops. Ital. J. Agron. 2015, 10, 192. [Google Scholar] [CrossRef] [Green Version]
- Corno, L.; Pilu, R.; Adani, F. Arundo donax L.: A Non-Food Crop for Bioenergy and Bio-Compound Production. Biotechnol. Adv. 2014, 32, 1535–1549. [Google Scholar] [CrossRef]
- Monlau, F.; Barakat, A.; Trably, E.; Dumas, C.; Steyer, J.-P.; Carrère, H. Lignocellulosic Materials into Biohydrogen and Biomethane: Impact of Structural Features and Pretreatment. Crit. Rev. Environ. Sci. Technol. 2013, 43, 260–322. [Google Scholar] [CrossRef]
- Taherzadeh, M.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Fang, Z.; Guo, F. Impact and Prospective of Fungal Pre-Treatment of Lignocellulosic Biomass for Enzymatic Hydrolysis. Biofuels Bioprod. Biorefin. 2012, 6, 335–350. [Google Scholar] [CrossRef]
- Wagner, A.; Lackner, N.; Mutschlechner, M.; Prem, E.; Markt, R.; Illmer, P. Biological Pretreatment Strategies for Second-Generation Lignocellulosic Resources to Enhance Biogas Production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef] [Green Version]
- Vasco-Correa, J.; Shah, A. Techno-Economic Bottlenecks of the Fungal Pretreatment of Lignocellulosic Biomass. Fermentation 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Wan, C.; Li, Y. Fungal Pretreatment of Lignocellulosic Biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Noonari, A.A.; Mahar, R.B.; Sahito, A.R.; Brohi, K.M. Effects of Isolated Fungal Pretreatment on Bio-Methane Production through the Co-Digestion of Rice Straw and Buffalo Dung. Energy 2020, 206, 118107. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sluiter, A. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples: Laboratory Analytical Procedure (LAP). Tech. Rep. 2008, 9, 1–6. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Pritoni, G.; Monti, A. Long-Term Effects of Nitrogen Fertilization on Giant Reed (Arundo donax L.) Biomass Productivity. In Proceedings of the European Biomass Conference and Exhibition, Berlin, Germany, 6–10 June 2011; pp. 152–157. [Google Scholar]
- Piccitto, A.; Corinzia, S.A.; Scordia, D.; Calcagno, S.; Ciaramella, B.R.; Patanè, C.; Cosentino, S.L.; Testa, G. Biomethane Potential of an Old Plantation of Giant Reed Genotypes with Two Irrigation Levels. In Proceedings of the European Biomass Conference and Exhibition, Online Meeting, 6 July 2020; pp. 234–237. [Google Scholar]
- Scordia, D.; Calcagno, S.; Piccitto, A.; Corinzia, A.S.; Testa, G.; Ciaramella, B.R.; Cosentino, S.L. Advanced Biomethane Production by Arundo donax under Changing Harvest Time and Nitrogen Fertilization. In Proceedings of the European Biomass Conference and Exhibition, Online Meeting, 6 July 2020; pp. 222–227. [Google Scholar]
- Zanetti, F.; Scordia, D.; Calcagno, S.; Acciai, M.; Grasso, A.; Cosentino, S.L.; Monti, A. Trade-off between Harvest Date and Lignocellulosic Crop Choice for Advanced Biofuel Production in the Mediterranean Area. Ind. Crops Prod. 2019, 138, 111439. [Google Scholar] [CrossRef]
- Bell, G.P. Ecology and Management of Arundo donax, and Approaches to Riparian Habitat Restoration in Southern California. In Plant Invasions: Studies from North America and Europe; Brock, J.H., Wade, M., Pysek, P., Green, D., Eds.; Blackhuys Publishers: Leiden, The Netherlands, 1997; pp. 103–113. [Google Scholar]
- Nowicka, A.; Zieliński, M.; Dębowski, M.; Dudek, M. Progress in the Production of Biogas from Maize Silage after Acid-Heat Pretreatment. Energies 2021, 14, 8018. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of Fungal and Milling Pretreatments for Enhancing Rice Straw Biogas Production during Solid-State Anaerobic Digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- McKendry, P. Energy Production from Biomass (Part 1): Overview of Biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Grigatti, M.; Barbanti, L.; Angelidaki, I. Effects of Hydrothermal Pre-Treatments on Giant Reed (Arundo donax) Methane Yield. Bioresour. Technol. 2013, 147, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Dell’Omo, P.P.; Spena, V.A. Mechanical Pretreatment of Lignocellulosic Biomass to Improve Biogas Production: Comparison of Results for Giant Reed and Wheat Straw. Energy 2020, 203, 117798. [Google Scholar] [CrossRef]
- Ding, C.; Wang, X.; Li, M. Evaluation of Six White-Rot Fungal Pretreatments on Corn Stover for the Production of Cellulolytic and Ligninolytic Enzymes, Reducing Sugars, and Ethanol. Appl. Microbiol. Biotechnol. 2019, 103, 5641–5652. [Google Scholar] [CrossRef] [PubMed]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V.; Goel, R. Fungal Pretreatment and Associated Kinetics of Rice Straw Hydrolysis to Accelerate Methane Yield from Anaerobic Digestion. Bioresour. Technol. 2019, 286, 121368. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.M.; Poulsen, T.G.; Sheng, K. Fungal Pretreatment of Rice Straw with Pleurotus Ostreatus and Trichoderma Reesei to Enhance Methane Production under Solid-State Anaerobic Digestion. Appl. Energy 2016, 180, 661–671. [Google Scholar] [CrossRef]
- van Kuijk, S.J.A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; Cone, J.W. The Effect of Particle Size and Amount of Inoculum on Fungal Treatment of Wheat Straw and Wood Chips. J. Anim. Sci. Biotechnol. 2016, 7, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Xu, F.; Ge, X.; Li, Y. Comparison between Ensilage and Fungal Pretreatment for Storage of Giant Reed and Subsequent Methane Production. Bioresour. Technol. 2016, 209, 246–253. [Google Scholar] [CrossRef] [Green Version]
- Majeed, S.; Hafeez, F.Y.; Li, X.; Salama, E.-S.; Ji, J.; Malik, K. Evaluation of Fungal and Sonication Pretreatments to Improve Saccharification Yield of Arundo donax. Int. J. Agric. Biol. 2020, 24, 9. [Google Scholar]
- Taniguchi, M.; Suzuki, H.; Watanabe, D.; Sakai, K.; Hoshino, K.; Tanaka, T. Evaluation of Pretreatment with Pleurotus Ostreatus for Enzymatic Hydrolysis of Rice Straw. J. Biosci. Bioeng. 2005, 100, 637–643. [Google Scholar] [CrossRef]
- Yu, H.; Du, W.; Zhang, J.; Ma, F.; Zhang, X.; Zhong, W. Fungal Treatment of Cornstalks Enhances the Delignification and Xylan Loss during Mild Alkaline Pretreatment and Enzymatic Digestibility of Glucan. Bioresour. Technol. 2010, 101, 6728–6734. [Google Scholar] [CrossRef]
- Basinas, P.; Rusín, J.; Chamrádová, K.; Malachová, K.; Rybková, Z.; Novotný, Č. Fungal Pretreatment Parameters for Improving Methane Generation from Anaerobic Digestion of Corn Silage. Bioresour. Technol. 2022, 345, 126526. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).