Abstract
Pyrethrins are the most widely used insecticide class in olive groves with organic management. Although there are data sets about insect pests of stored products and human parasites developing resistance to pyrethrins, there is no information on the long-term effect on olive agroecosystems. A field method based on the experimental induction of sublethal effects by means of insecticide application, and the monitoring of the response of insects through post-treatment sampling, has recently been developed. This method has allowed for the detection of populations behaviorally resistant to organophosphates in integrated pest management (IPM) and conventional crops. With the application of a similar methodology, this study aimed to verify the possible reaction of natural enemies in organic crops, using pyrethrins as an inducing insecticide. The study was carried out in 2019 in two olive groves in southern Spain (Jaén, Andalusia), one of them being IPM and the other being an organic production system. The results did not allow for verification of the behavioral resistance in populations of natural enemies of both IPM and organic management against pyrethrins, while against dimethoate, behavioral resistance was verified in IPM management. The possible causes involved in obtaining these results are discussed.
1. Introduction
After being applied to crops, insecticides are subjected to environmental factors (light, heat, water, and wind), which cause gradual degradation and dispersion of their residues, which implies that affected insects are exposed to sublethal doses over time [1,2,3,4]. A sublethal dose/concentration is defined as inducing no apparent mortality in the experimental population [4,5]. In general, insecticide dose/concentrations under the median lethal (LD50/LC50) are considered to induce sublethal effects [5]. The induced effects produce alterations that affect physiological, biological, and behavioral processes, among which agitation, hyperreflexia, irritability, and repellency have been reported [3,4,5,6,7,8,9]. These effects allow individuals affected by doses lower than the LD50 to avoid new contact with insecticide residues and to escape their toxic action.
The repellent effect of nonselective insecticides is a characteristic reported for organochlorines [10,11], pyrethroids [11,12,13,14,15], organophosphates [11,16], and carbamates [11]. Although the precise behavioral responses of insects in the field are elusive and difficult to measure [17], experiments carried out in olive groves by monitoring populations of natural enemies (Aeolothrips intermedius (Bagnall, 1934) (Thysanoptera: Aeolothripidae); Chrysoperla agilis (Henry et al., 2003) (Neuroptera: Chrysopidae); Harraphidia laufferi (Navás, 1915) (Raphidioptera, Raphidiidae); Anthocoris nemoralis (Fabricius, 1794) (Hemiptera, Anthocoridae); Orius laevigatus (Fieber, 1860) (Hemiptera, Anthocoridae)) after insecticide applications have observed repellency in crops submitted to frequent dimethoate application [18,19], which has been used in conventional pest management and IPM in recent decades [20,21]. The applied method is based on the monitoring of insect populations by means of the deployment of adherent traps, installed immediately after insecticide application. In the results, negative deviations have been observed in the capture rates of beneficial insects in traps of organic crops after experimental insecticide application. On the contrary, positive deviation is found in agrosystem populations regularly submitted to synthetic insecticide applications, which is indicative of the repellency reaction exhibited by behaviorally resistant lineages. This repellent effect, manifested by insect populations after repeated synthetic insecticide applications, has been interpreted as the first barrier of a detoxification mechanism [9,12,22,23,24,25]. As frequently reported [5,9,26], in these agrosystems, the environmental pressure represented by the regular use of insecticides has led to a gradual and progressive purification of behaviorally resistant lineages.
Despite the decisive effect of sublethal doses [3], their effect has been underestimated [27,28] and the need for more precise evaluations of their impact has been highlighted [4,29]. Their importance acquires greater relevance in the characterization of organic crops, compared to those based on the use of synthetic insecticides. The importance of organic cultivation in recent years [30,31] is reflected in the 5% average annual increase in the cultivated area, currently representing 10% of the total olive grove cultivation area in Spain [32]. Compared to traditional agriculture, the higher quality of products of organic origin leads to greater respect for the environment, as well as the progressive elimination of synthetic chemical products. Regarding pest control, biodiversity stimulation in organic crops will have an impact on the improvement of the entomophagous activity of natural enemies, which has been verified in the case of Prays oleae (Bernard, 1788) (Lepidoptera: Praydidae) [33].
In order to promote conversion to organic agricultural management regimes among farmers, the regional government grants economic subsidies. In addition to these benefits, higher prices for organic olive oil have been set in the market, which have increased by 15–30% [34]. In recent years, the granting of economic subsidies by the regional government has triggered a 40% increase in the area of organic olive groves in relation to the year 2001 [35]. Among the requirements demanded by the Regional Administration to grant the certification of organic olive groves, crops with this type of management regime must undergo periodic inspections, which allow the determination of pesticide residues in olive oil. However, alternative and/or complementary procedures are required. In this sense, the absence of behavioral resistance in the beneficial insects in relation to synthetic insecticides is a distinctive characteristic of organic pest management with respect to conventional management and IPM [33]. This has been the determining element for the development of a reliable strategy for its characterization from a behavioral point of view. This method is based on the experimental application of an organophosphate insecticide in a reduced sector of problem olive groves, where changes in capture rates are subsequently monitored, during the following two weeks, for which adherent chromotropic traps are used. Although its relative abundance is lower in olive groves usually treated with synthetic insecticides, the capture rates in traps are higher after treatment application. This increase in capture rates is attributed to the repellency reaction generated by insecticide application, which occurs when insect populations in agrosystems are frequently exposed to synthetic insecticides. This fact constitutes an essential difference with respect to organic olive groves, in which the relative abundance is higher and the post-treatment capture rates of beneficial insects are lower, as a consequence of the absence of behaviorally resistant lineages.
Among organic alternatives to conventional synthetic insecticides, natural pyrethrins, obtained from Dalmatian pyrethrum (Tanacetum cinerariifolium), have important advantages such as their low toxicity for mammals [36,37,38] and short environmental persistence [39] due to the action of UV rays, so their effect on agroecosystems is of very short duration [40,41]. The lower persistence and toxicity to humans make pyrethrins more ecologically acceptable than the widely used conventional agricultural synthetic insecticides [42]. Among the sublethal effects of pyrethrins, the reduction in longevity and fecundity in certain parasitoid hymenopterans [43] and their repellent action on various Diptera species, such as Aedes aegypti (Linnaeus, 1762) (Diptera: Culicidae) or Aedes albopictus Skuse, 1895 (Diptera: Culicidae) [44,45,46], have been frequently reported. Most data on the resistance to pyrethrins come from programs for protection against dipteran stings [44,45,46,47,48,49,50,51,52], as well as conservation programs for stored agricultural products [53,54,55,56,57]. In the latter, cross-resistance against organophosphates and pyrethrins has been detected [58], which suggests the hypothesis that a similar effect may be occurring in olive growing given the frequent behavioral resistance to dimethoate [59,60]. With this study, the application of the monitoring induction technique is proposed, which has been very useful in detecting lineages resistant to synthetic insecticides, to detect possible behavioral resistance of insects to pyrethrins, as it is the most commonly applied insecticide in organic olive growing.
2. Materials and Methods
2.1. Description of the Study Area
This study was carried out in two olive groves in the municipality of Jaén (Andalusia, southern Spain) in the spring of 2019, during the oviposition period of the phyllophagous generation of P. oleae in olive flowers, a time of great activity of the auxiliary entomofauna [61,62]. The selected olive groves (Figure 1) correspond to two different types of pest management: IPM and organic management (ORG).
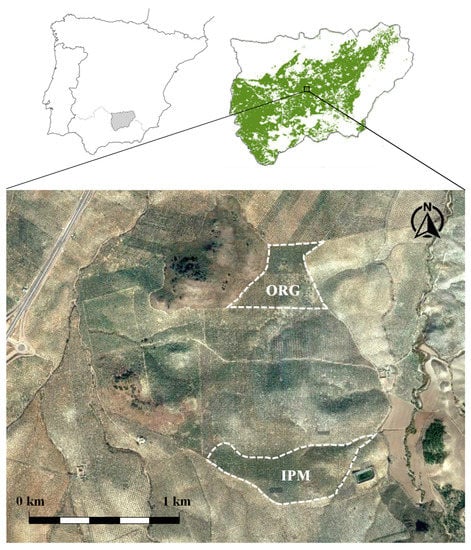
Figure 1.
Location of the selected olive groves in the municipality of Jaén: Integrated pest management (IPM) and organic (ORG). Source: Own elaboration using the Google Earth Pro geographic information system.
The integrated pest management (IPM) olive grove has an area of 20 ha (37°52′12.55″ N, 3°34′03.33″ W). Since 1995, the management of this olive grove has been carried out in accordance with the principles of integrated pest management. The 28-year-old olive trees belong to the Picual variety and are arranged in a 10 × 10 m configuration. The soil is fertilized with organic and mineral nutrients twice a year. In addition, foliar fertilization is annually carried out using crystalline urea (nitrogen content of 46%), potassium sulfate, and natural amino acids (arginine, glycine, threonine, and proline). Pest control is only carried out when the population levels of pest species exceed the action threshold of damage established in integrated production regulations. When this occurs, the insecticides used are Dimethoate 40% © (IRAC Group 1B) for the control of the olive fly Bactrocera oleae (Rossi, 1790) (Diptera: Tephritidae), the olive bark beetle Phloeotribus scarabaeoides (Bernard, 1788) (Coleoptera: Curculionidae), and the olive moth P. oleae, and chlorpyrifos 48% © (IRAC Group 1B) for the control of the branch borer Euzophera pinguis (Haworth, 1811) (Lepidoptera: Pyralidae) (Table 1).

Table 1.
Products applied in the control of the main pests and the annual application regime in both olive groves (IPM and organic).
The organic (ORG) olive grove has an area of 17 ha (37°52′21.11″ N, 3°34′29.46″ W). This olive grove has had organic certification since 2007. The olive trees, of the Picual variety, are 28 years old and are arranged in a 10 × 10 m configuration. The soil is biannually fertilized with natural organic nutrients, and foliar treatments are annually carried out with biostimulants authorized for use in organic farming (CE Regulation No. 889/2008). The insecticides used for pest control are of natural origin, authorized in Annex II of the EC Regulation No. 889/2008 (Table 1). For the control of B. oleae, the annual patching technique is used, and 0.024% spinosad (IRAC Group 5) is applied in combination with a protein hydrolysate. Additionally, and in order to manage resistance to spinosad [63,64,65,66,67], in two out of every five years, this treatment is complemented with the application of 4% pyrethrins (IRAC Group 11A). For the control of P. oleae, homogeneous spray application of a Bacillus thuringiensis var. aizawaii (IRAC Group 3A) formulation is performed, with a frequency of three out of every four years. Furthermore, in years with the occurrence of strong attack, which occurs at an average frequency of one out of every four years [61], this application is replaced by a homogeneous spray of 4% natural pyrethrins. The control of E. pinguis is based on a massive trapping technique using delta traps baited with synthetic pheromone [68]. Finally, to control P. scarabaeoides, bait trunks are used during the period of attack on pruning logs. These trunks are destroyed before the emergence of the offspring.
It is noteworthy that the two olive groves are cultivated in rainfed systems and that, in both, the growth of a spontaneous vegetation cover is encouraged, controlled with mechanical mowing at specific times. For this reason, a well-preserved cover has developed in both olive groves, where the majority of species are Senecio vulgaris Linnaeus, Diplotaxis virgata Candolle, Bromus madritensis Linnaeus, Lolium rigidum Gaudin, Hordeum leporinum Linnaeus, Sinapis alba Linnaeus, Anacyclus clavatus Desfontaines, and Crepis sancta Linnaeus. The olive groves of both crops are pruned every two years.
2.2. Experimental Design
Both olive groves (organic and IPM) were divided into three blocks, establishing in each of them three plots of 40 × 40 m (16 olive trees), and the minimum distance between plots of the same block was 200 m (Figure 2). Within each block, two of the three plots were randomly selected to apply the two insecticides under study. Thus, in each olive grove (organic and MIP), a plot of each block was sprayed with 40% Dimethoate © (400 g/L) (BASF) at a concentration of 0.1% (v/v); another plot of each block was sprayed with nonsystemic bioinsecticide Abanto © (4% pyrethrins), a natural compound allowed in organic agriculture (CE No. 889/2008); and finally, the third plot of each block was established as a control, for which distilled water was applied. These experimental treatments were carried out on 14 May under calm atmospheric conditions and with a wind speed of less than 5 km/h. Treatments were carried out using a MATABI Evolution 16 © hydraulic sprayer. The area treated with insecticides was equivalent to 4.8% and 5.6% of the total area of IPM and organic olive groves, respectively, being that the latter has maintained the organic cultivation certification. It should be noted that, during this work, in the rest of the two olive groves considered, no type of insecticide application was carried out since this could interfere with the results.
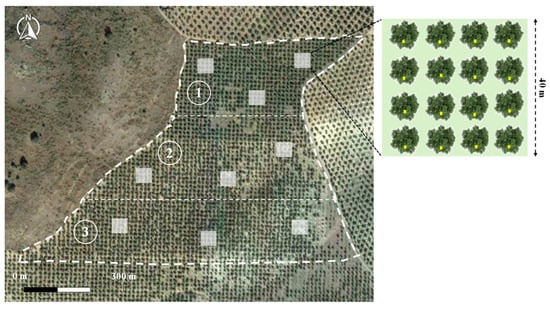
Figure 2.
Distribution of the plots into the three organic olive grove blocks. Source: Own elaboration using the Google Earth Pro geographic information system.
In all plots of both olive groves, the main natural enemy species of olive tree pests were monitored. To do this, the passive sampling technique was carried out using adherent yellow chromotropic traps (20 × 40 cm). This sampling methodology has provided excellent results and stands out for being easily replicable [33,62,69,70,71]. Traps were immediately placed after experimental insecticide applications, at the rate of one per olive tree (16 traps per plot) and at a height of 1.5 m in the N sector of each tree. Since their placement, the traps were weekly renewed, establishing two sampling intervals: 14–21 May and 21–28 May. After their renewal, the traps were temporarily stored in a cold room (4 °C). In each sampling interval and from each block of both olive groves, 6 traps of each plot were randomly selected to be observed. This subsample of traps from each block was used in order to avoid the lack of randomization of the observed samples and prevent pseudoreplication effects. Therefore, in each sampling interval the number of traps observed of each treatment (dimethoate, pyrethrins, and control) in each olive grove was 18. The traps were examined by means of a binocular magnifying glass for the taxonomic determination and quantification of beneficial species captured. For this, those species associated with at least one olive pest species [72,73,74,75,76] were taken into account.
To obtain an estimate of the relative abundance of the different species in both types of olive grove management systems, the capture values recorded in the control plots were considered, as they were free of any alteration caused by insecticide applications.
2.3. Statistical Analysis
For the statistical analysis of the data, the Statgraphics Centurion XVII statistical package (2016) has been used. The normality of the distributions was verified by the Shapiro–Wilk normality test. Since the data set does not fit to a normal distribution, the Mann–Whitney U test was applied to determine significant differences between the captured values obtained from the different natural enemy species in control plots of the two management regimes. To determine significant differences among the captured values obtained from the species of beneficial insects in plots of the different treatments (dimethoate, pyrethrins, and control), the Kruskal–Wallis test was used. Once statistically significant differences were determined using the Kruskal–Wallis test, the Mann–Whitney U test was used to compare the catch values of the natural enemies species between the pair of treatments.
3. Results
3.1. Analysis of the Relative Abundance of Beneficial Insects (Control Plots)
Among the captured species, 12 species of natural enemies were selected (Table 2), which are relatively common in the entomofauna associated with olive grove pests in southern Spain [33,72,77,78]. Individuals of these 12 species of natural enemies were captured in both olive groves. Due to its greater abundance, the A. intermedius predator stands out, which is a cosmopolitan species common in a wide range of crops [33,79,80]. Most of the captured species (nine species) are natural enemies of the olive moth P. oleae, which, as indicated, during sampling, is in the oviposition period corresponding to the anthophagous generation. Among parasitoids are the following hymenopterans predominate: Tetrastichus cesirae (Russo, 1938), Elasmus flabellatus (Fonscolombe, 1832) (Eulophidae), Ageniaspis fuscicollis (Dalman, 1820) (Encyrtidae), and Diadegma semiclausum (Hellen, 1949) (Ichneumonidae). Despite the predatory importance of chrysopids in the control of P. oleae [81,82,83,84], the species Ch. agilis (Henry et al., 2003) has presented exceptionally low values, most likely because its predatory activity takes place mainly during the oviposition period corresponding to the carpophagous generation, which occurs during the months of June and July [85].

Table 2.
Identified natural enemy species; olive grove pests that prey/parasitize and bibliographical references.
The relative abundance of beneficial species in the olive groves of both types of management is represented in Figure 3 (control plots). All species considered presented relative abundance values much higher in the organic olive grove compared to the IPM olive grove. Figure 4 represents the results of the comparison test for the capture values of beneficial species with the highest relative abundance. In all of them, the existence of significant differences in favor of the organic olive grove stands out (p < 0.05).
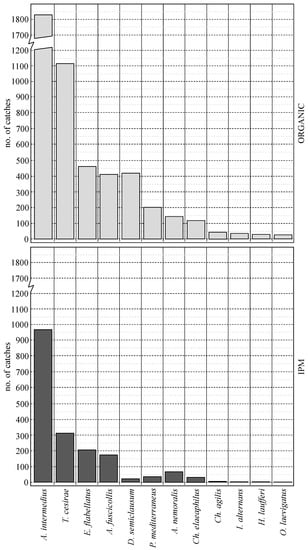
Figure 3.
Abundance values obtained by the species in the control plots of the organic (light color) and IPM (dark color) olive groves.
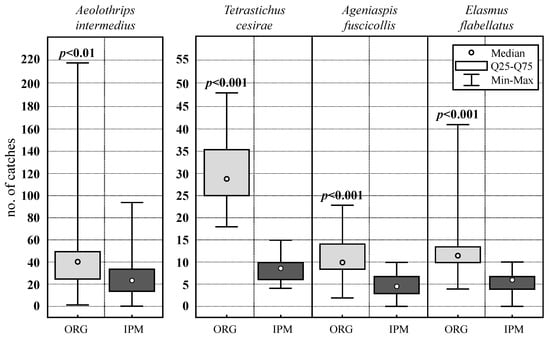
Figure 4.
Statistic capture values (median, quartile 25, quartile 75, maximum, and minimum) of the major beneficial species in the control plots of organic (light color) and IPM (dark color) olive groves. The level of statistical significance obtained from the Mann–Whitney U test is also indicated (p-value).
3.2. Behavioral Resistance Assessment (Plots Treated with Dimethoate/Pyrethrins)
Figure 5 shows the statistic capture values of the main species in the control plots and the plots treated with both types of insecticides. The results of the Kruskal–Wallis test allowed rejecting the null hypothesis of equality of medians of capturing data in the three experimental treatments of both olive groves: A. intermedius (ORG: Chi-square = 16.21; df = 2; p < 0.001; IPM: Chi-square = 14.22; df = 2; p < 0.001), T. cesirae (ORG: Chi-square = 66.16; df = 2; p < 0.001; df = 2; IPM: Chi-square = 72.79; df = 2; p < 0.001), A. fuscicollis (ORG: Chi-square = 42.14; df = 2; p < 0.001; IPM: Chi-square = 57.50; df = 2; p < 0.001), E. flabellatus (ORG: Chi-square = 44.13; df = 2; p < 0.001; IPM: Chi-square = 71.49; df = 2; p < 0.001).
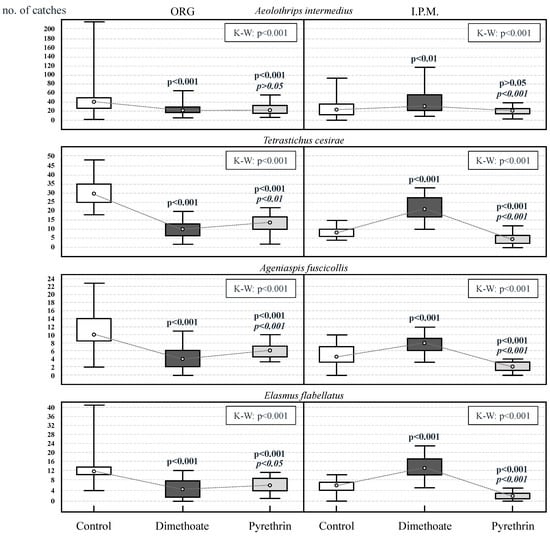
Figure 5.
Statistic capture values (median, quartile 25, quartile 75, maximum and minimum) of the main beneficial species in the control plots (white color), treated with organophosphate Dimethoate 40% © (dark color) and pyrethrins (light color), of the two olive groves (organic/IPM). The p-value (Kruskal–Wallis test) is indicated in the upper right corner. The p-values indicated above the boxplots correspond to the comparison with respect to the control plot (bold letters) and between the two insecticides (italic letters).
In the IPM olive grove, a greater number of captures were registered, for all the species, in those plots treated with dimethoate, in which the values were significantly higher than those registered in the control plots (p < 0.05). On the contrary, those plots treated with pyrethrins presented the minimum capture values, being generally significantly lower than those observed in the control plots (p < 0.05).
In the organic olive grove, the beneficial insect species presented higher post-treatment capture rates in the control plots, where the values were significantly higher than those observed for the plots treated with either of the two insecticides under study.
4. Discussion
In the first part of this study, which corresponds to the data from the control plots, which during the study remained free of any insecticide action. The capture data of the plots reflect the long-term effect that the use of each of the two compared insecticides is having on the relative abundance of the selected species. The greater abundance of beneficial species in the organic olive grove, compared to the IPM olive grove, agrees with the results of studies carried out in olive groves in Spain [33,86] and Portugal [87]. Given that the main difference between the types of management (IPM and organic) mainly affects the insecticide used and the application frequency, the results are consistent with those reported by Santos et al. [87], since the lethality produced by dimethoate on beneficial fauna is the cause of an important alteration in the agroecosystem balance in IPM olive groves [88,89]. This effect, together with the absence of dimethoate selectivity, has been indicated even for concentrations lower than the LD50 [90], causing significant alterations in the reproductive process of surviving individuals [91], which significantly reduces their success rate. In this sense, Pascual et al. [92] and Nikolova et al. [80] point out that the reduction in the abundance of natural enemies is an obvious consequence of the regular application of synthetic pesticides in agroecosystems. This dependence on synthetic insecticide applications for pest control negatively affects the predatory efficacy of natural enemies, such as the lacewing larvae [33]. In contrast, the higher population of natural enemies in organic olive groves results in higher predatory activity [33].
As presented in our results on IPM management, which is regularly treated with dimethoate, the experimental application of this insecticide triggers an increase in the number of captures in all the selected natural enemy species in relation to the control plots. As indicated before, among the sublethal effects of insecticides, several alterations in behavior are mentioned, such as agitation, hyperreflexia, irritability, and repellency [3,4,5,6,7,8,9]. Affected individuals have a tendency to move towards the insecticide-free surfaces, which explains a higher rate of capture in the sticky traps of the treated plots, so these results are in line with what has been previously observed [18,19,33]. Therefore, in the IPM olive grove, the increase in post-treatment capture values reflects the existence of a repellent effect, which leads insects to areas free of the pesticide. This is a characteristic effect of populations submitted to selection pressure, which favors lineages better adapted to avoid the insecticidal action. This implies an increase in the capture rate in plots usually treated with this insecticide. Several authors have pointed out the acquisition of behavioral resistance, as a behavior modification, developed by populations frequently exposed to sublethal doses [3,5,6,17,26]. It is important to emphasize that the increase in the number of individuals in the traps in the treated areas can be misleading when interpreting it as a result of an increase in abundance in the olive groves after the application of dimethoate. Obviously, it would be an erroneous statement, since it would be due to the reaction induced in the behavior of the individuals affected by sublethal doses, and their reaction of fleeing towards surfaces free of the insecticide, as a survival mechanism, which is very different from a reaction due to its chromatic attraction. Repeated exposure to sublethal doses is therefore a necessary condition for the gradual acquisition of behavioral resistance and explains the absence of increased capture rates in organically managed plots experimentally treated with dimethoate since insects lack this capacity. Regarding the real insecticide dose absorbed by insects, it is impossible to determine the proportion of them that receive sublethal doses [93]. In certain species of insects, and particularly in species of medical importance, such as malaria vectors, the existence of “behavioral resistance” has been indicated as a result of repeated exposure to sublethal insecticide concentrations. However, authors such as Chareonviriyaphap et al. [17] have proposed the term “behavioral avoidance”, which, unlike resistance, is the result of an innate, natural, and involuntary response or capacity. They proposed that, unlike behavioral resistance, behavioral avoidance can play an important role in reducing selection pressure, thus slowing the emergence and spread of physiological resistance. However, in this study, it could be concluded that the effect is related to acquired behavioral resistance since in the organic olive grove, where dimethoate had not been used prior to this experiment, insects lack a repellent reaction, which represents the main difference with respect to the insect populations in the IPM olive grove.
In both types of management (IPM and organic), plots experimentally treated with pyrethrins showed a decrease in the post-treatment capture rate compared to the control plots. On the one hand, in the organic olive grove, the absence of repellency of beneficial insects against pyrethrins is consistent with the scarcity of reports on resistance to this insecticide in the field [94]. On the other hand, in the IPM olive grove, experimental pyrethrin application corresponds to a reduction in the capture rate, an opposite effect to the dimethoate application, which makes it possible to rule out the possibility of cross-resistance of insects against both insecticides. A plausible explanation for the lack of repellency with respect to pyrethrins is probably attributed to their large knockdown effect [38,39,95], which involves rapid movement paralysis and eventual death [38]. In addition to this effect, the rapid waste degradation and its short persistence, of a few days or only a few hours [39,44,96,97], imply a specific action in relation to that of conventional insecticides; therefore, under these conditions, the population of affected insects must be much lower, which leads to conclude that the selective pressure generated is greatly mitigated and practically imperceptible. This may explain why most of the literature on acquired resistance to pyrethrins corresponds to studies on their application in the control of pests of stored agricultural products [53,54,55,56,57], where storage conditions considerably limit the action of environmental factors that degrade pyrethrins.
5. Conclusions
The application of the induction-monitoring technique for the detection of behaviorally resistant lineages in olive groves under IPM and organic pest management, through the experimental application of pyrethrins and dimethoate as inducing insecticides, allowed for the detection of the existence of lineages in the IPM olive grove resistant to dimethoate, although not to pyrethrin. In the organic olive grove, where the product most widely used is pyrethrin, the relative abundance of beneficial insects was notably higher. Thus, no repellency reaction was detected in relation to both insecticides under study.
Author Contributions
Conceptualization, J.A.G.-G. and R.G.-R.; methodology, R.G.-R.; validation, J.A.G.-G. and M.S.-P.; formal analysis, J.A.G.-G.; investigation, J.A.G.-G.; resources, J.A.G.-G.; data curation, J.A.G.-G. and R.G.-R.; writing—original draft preparation, J.A.G.-G., R.G.-R. and M.S.-P.; writing—review and editing, J.A.G.-G. and R.G.-R.; visualization, R.G.-R.; supervision, R.G.-R.; project administration, R.G.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stark, J.D.; Jepson, P.C.; Mayer, D.F. Limitations to use of topical toxicity data for predictions of pesticide side effects in the field. J. Econ. Entomol. 1995, 88, 1081–1088. [Google Scholar] [CrossRef]
- Lund, A.E.; Hollingworth, R.M.; Shankland, D.L. Chlordimeform: Plant protection by a sublethal, noncholinergic action on the central nervous system. Pestic. Biochem. Physiol. 1979, 11, 117–128. [Google Scholar] [CrossRef]
- Haynes, K.F. Sublethal effects of neurotoxic insecticides on insect behavior. Annu. Rev. Entomol. 1988, 33, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- De França, S.M.; Breda, M.O.; Barbosa, D.R.; Araujo, A.M.; Guedes, C.A. The sublethal effects of insecticides in insects. In Biological Control of Pest and Vector Insects; IntechOpen: London, UK, 2017; pp. 23–39. [Google Scholar]
- Lee, C.Y. Sublethal effects of insecticides on longevity, fecundity and behaviour of insect pests: A review. J. Biosci. 2000, 11, 107–112. [Google Scholar]
- Kongmee, M.; Prabaripai, A.; Akratanakul, P.; Bangs, M.J.; Chareonviriyaphap, T. Behavioral responses of Aedes aegypti (Diptera: Culicidae) exposed to deltamethrin and possible implications for disease control. J. Med. Entomol. 2004, 41, 1055–1063. [Google Scholar] [CrossRef]
- Correa, Y.D.C.G.; Faroni, L.R.; Haddi, K.; Oliveira, E.E.; Pereira, E.J.G. Locomotory and physiological responses induced by clove and cinnamon essential oils in the maize weevil Sitophilus zeamais. Pestic. Biochem. Physiol. 2015, 125, 31–37. [Google Scholar] [CrossRef]
- Haddi, K.; Oliveira, E.E.; Faroni, L.R.; Guedes, D.C.; Miranda, N.N. Sublethal exposure to clove and cinnamon essential oils induces hormetic-like responses and disturbs behavioral and respiratory responses in Sitophilus zeamais (Coleoptera: Curculionidae). J. Econ. Entomol. 2015, 108, 2815–2822. [Google Scholar] [CrossRef]
- Kennedy, J.S. The excitant and repellent effects on mosquitos of sub-lethal contacts with DDT. Bull. Entomol. Res. 1947, 37, 593–607. [Google Scholar] [CrossRef]
- Rolff, J.; Reynolds, S. (Eds.) Insect Infection and Immunity: Evolution, Ecology, and Mechanisms (No. 25); Oxford University Press on Demand: Oxford, UK, 2009. [Google Scholar]
- Van Dame, R.; Meled, M.; Colin, M.E.; Belzunces, L.P. Alteration of the homing-flight in the honey bee Apis mellifera L. Exposed to sublethal dose of deltamethrin. Environ. Toxicol. Chem. Int. J. 1995, 14, 855–860. [Google Scholar] [CrossRef]
- Pike, K.S.; Mayer, D.F.; Glazer, M.; Kious, C. Effects of permethrin on mortality and foraging behavior of honey bees in sweet corn. Environ. Entomol. 1982, 11, 951–953. [Google Scholar] [CrossRef]
- Quisenberry, S.S.; Lockwood, J.A.; Byford, R.L.; Wilson, H.K.; Sparks, T.C. Pyrethroid Resistance in the Horn Fly., Haematobia irritans (L.) (Diptera: Muscidae). J. Econ. Entomol. 1984, 77, 1095–1098. [Google Scholar] [CrossRef]
- Haynes, K.F.; Li, W.G.; Baker, T.C. Control of pink bollworm moth (Lepidoptera: Gelechiidae) with insecticides and pheromones (attracticide): Lethal and sublethal effects. J. Econ. Entomol. 1986, 79, 1466–1471. [Google Scholar] [CrossRef]
- Moore, R.F. Behavioral and biological effects of NRDC-161 as factors in control of the boll weevil. J. Econ. Entomol. 1980, 73, 265–267. [Google Scholar] [CrossRef]
- Chareonviriyaphap, T.; Bangs, M.J.; Suwonkerd, W.; Kongmee, M.; Corbel, V.; Ngoen-Klan, R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites Vectors 2013, 6, 280. [Google Scholar] [CrossRef] [Green Version]
- González-Ruiz, R.; Varela, J.L.M. Chemical control of Prays oleae (Lep., Yponomeutidae) and its influence on natural enemies of olive grove pests. Phytoma España 2000, 115, 24–30. (In Spanish) [Google Scholar]
- Gómez-Guzmán, J.A.; García-Marín, F.J.; Sáinz-Pérez, M.; González-Ruiz, R. Behavioural Resistance in Insects: Its Potential Use as Bio Indicator of Organic Agriculture. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 95, p. 042038. [Google Scholar]
- Alvarado, M.; Civantos, M.; Duran, J.M. Plagas. In El Cultivo del Olivo; Junta de Andalousia and Editiones Mundi-Prensa: Madrid, Spain, 1997; pp. 401–459. [Google Scholar]
- Civantos, M. Defensa Fitosanitaria en Sistemas de Producción Integrada. In Cultivo del Olivar en Zonas de Especial Protección Ambiental. Informaciones Técnicas 65/99; Consejería de Agricultura y Pesca; Junta de Andalucía: Seville, Spain, 1999. [Google Scholar]
- Gerold, J.L.; Laarman, J.J. Behavioural responses to contact with DDT in Anopheles atroparvus. Nature 1967, 215, 518–520. [Google Scholar] [CrossRef]
- Gould, F. Role of behavior in the evolution of insect adaptation to insecticides and resistant host plants. Bull. ESA 1984, 30, 34–41. [Google Scholar] [CrossRef]
- Lockwood, J.A.; Sparks, T.C.; Story, R.N. Evolution of insect resistance to insecticides: A reevaluation of the roles of physiology and behavior. Bull. ESA 1984, 30, 41–51. [Google Scholar] [CrossRef]
- Pluthero, F.G.; Singh, R.S. Insect behavioural responses to toxins: Practical and evolutionary considerations. Can. Entomol. 1984, 116, 57–68. [Google Scholar] [CrossRef]
- Singh, J.P.; Marwaha, K.K. Effect of sublethal concentrations of some insecticides on growth and development of maize stalk borer, Chilo partellus (Swinhoe) larvae. Shashpa 2000, 7, 181–186. [Google Scholar]
- Kevan, P.G. Pollinators as bioindicators of the state of the environment: Species, activity and diversity. Agric. Ecosyst. Environ. 1999, 74, 373–393. [Google Scholar] [CrossRef]
- Thompson, H.M. Behavioural effects of pesticides in bees—Their potential for use in risk assessment. Ecotoxicology 2003, 12, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Guedes, N.M.P.; Guedes, R.N.C.; Ferreira, G.H.; Silva, L.B. Flight take-off and walking behavior of insecticide-susceptible and–resistant strains of Sitophilus zeamais exposed to deltamethrin. Bull. Entomol. Res. 2009, 99, 393–400. [Google Scholar] [CrossRef]
- Prodescon, S.A. Characterization of the Spanish Organic Production Sector in Terms of Value and Market, Referring to the Year 2015; Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente: Madrid, Spain, 2016. [Google Scholar]
- Moral, A.M.; Uclés, D.F.; Jurado, E.B.; Viruel, M.J.M. The commitment to organic farming in the olive grove. A market for the future. Boletín Inst. Estud. Giennenses 2017, 216, 353–376. [Google Scholar]
- ESYRCE. Encuesta Sobre Superficie y Rendimientos de los Cultivos. 2020. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/esyrce/ (accessed on 11 July 2021).
- Gómez-Guzmán, J.A.; Sainz-Pérez, M.; González-Ruiz, R. Induction of sublethal effects for the characterization of Olive groves under different pest management systems. Rev. Bras. Frutic. 2021, 43. [Google Scholar] [CrossRef]
- Poolred. Available online: http://www.poolred.com/ (accessed on 7 September 2021).
- Henry, C.S.; Brooks, S.J.; Thierry, D.; Duelli, P.; Johnson, J.B.; McEwen, P.K.; New, T.R.; Whittington, A.E. The common green lacewing (Chrysoperla carnea s. lat.) and the sibling species problem. In Lacewings in the Crop Environment; Cambridge University Press: Cambridge, UK, 2001; pp. 29–42. [Google Scholar]
- Regnault-Roger, C.; Philogène, B.J.R.; Vincent, C. (Eds.) Produits phytosanitaires insecticides d’origine végétale: Promesses d’hier et d’aujourd’hui. In Biopesticides D’origine Végétale; Tec&Doc Editions: London, UK, 2002; pp. 1–18. [Google Scholar]
- Zapata, N.; Medina, P.; Viñuela, E.; Budia, F. Toxicidad de malation, pimetrocina, piretrinas naturales+ PBO y triflumuron en adultos del parasitoide Psyttalia concolor (Szepligeti)/(Hym.: Braconidae) según el modo de aplicación. Bol. San. Veg. Plagas 2005, 31, 111–118. [Google Scholar]
- Lafargue, G.L.; Medina, J.M.A.; Acosta, A.L.; Llanes, Y.M. Piretrinas y Piretroides. Anu. Cienc. UNAH 2018, 16, 4–13. [Google Scholar]
- Henry, C.W.; Shamsi, S.A.; Warner, I.M. Separation of natural pyrethrum extracts using micellar electrokinetic chromatography. J. Chromatogr. A 1999, 863, 89–103. [Google Scholar] [CrossRef]
- Soderlund, D.M.; Bloomquist, J.R. Neurotoxic actions of pyrethroid insecticides. Annu. Rev. Entomol. 1989, 34, 77–96. [Google Scholar] [CrossRef]
- Crosby, D.G. Environmental Fate of Pyrethrins; Oxford University Press: New York, NY, USA, 1995; pp. 194–213. [Google Scholar]
- Lybrand, D.B.; Xu, H.; Last, R.L.; Pichersky, E. How plants synthesize Pyrethrins: Safe and biodegradable insecticides. Trends Plant Sci. 2020, 25, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.V.; Holle, S.G.; Hutchison, W.D.; Koch, R.L. Lethal and sublethal effects of conventional and organic insecticides on the parasitoid Trissolcus japonicus, a biological control agent for Halyomorpha halys. Front. Insect Sci. 2021, 1, 5. [Google Scholar] [CrossRef]
- Todd, G.D.; Wohlers, D.; Citra, M.J. Toxicological Profile for Pyrethrins and Pyrethroids; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2003. [Google Scholar]
- Liu, F.; Wang, Q.; Xu, P.; Andreazza, F.; Valbon, W.R.; Bandason, E.; Chen, M.; Yan, R.; Feng, B.; Smith, L.B.; et al. A dual-target molecular mechanism of pyrethrum repellency against mosquitoes. Nat. Commun. 2021, 12, 2553. [Google Scholar] [CrossRef]
- Yan, R.; Zhou, Q.; Xu, Z.; Wu, Y.; Zhu, G.; Wang, M.; Guo, Y.; Dong, K.; Chen, M. Pyrethrins elicit olfactory response and spatial repellency in Aedes albopictus. Pest Manag. Sci. 2021, 77, 3706–3712. [Google Scholar] [CrossRef]
- Fine, B.C. Pattern of pyrethrin-resistance in houseflles. Nature 1961, 191, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.G. Effects of synergists on bendiocarb and pyrethrins resistance in the German cockroach (Dictyoptera: Blattellidae). J. Econ. Entomol. 1987, 80, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, R.; Henn, T. Botanical insecticides and insecticidal soaps. In Handbook of Integrated Pest Management for Turf and Ornamentals; CRC Press: Boca Raton, FL, USA, 1994; pp. 541–555. ISBN 9780138752798. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9780138752798-58/botanical-insecticides-insecticidal-soaps-richard-weinzierl-tess-henn (accessed on 26 January 2022).
- Cochran, D.G. Resistance to pyrethrins in the German cockroach: Inheritance and gene-frequency estimates in field-collected populations (Dictyoptera: Blattellidae). J. Econ. Entomol. 1994, 87, 280–284. [Google Scholar] [CrossRef]
- Kaufman, P.E.; Scott, J.G.; Rutz, D.A. Monitoring insecticide resistance in house flies (Diptera: Muscidae) from New York dairies. Pest Manag. Sci. 2001, 57, 514–521. [Google Scholar] [CrossRef]
- Speare, R.; Koehler, J.M. A case of pubic lice resistant to pyrethrins. Aust. Fam. Physician 2001, 30, 572–574. [Google Scholar]
- Fine, B.C. The present status of resistance to pyrethroid insecticides. Pyrethrum Post 1963, 7, 18–21. [Google Scholar]
- Lloyd, C.J.; Parkin, E.A. Further studies on a pyrethrum-resistant strain of the granary weevil, Sitophilus granarius (L.). J. Sci. Food Agric. 1963, 14, 655–663. [Google Scholar] [CrossRef]
- Lloyd, C.J. The toxicity of pyrethrins and five synthetic pyrethroids, to Tribolium castaneum (Herbst), and susceptible and pyrethrin-resistant Sitophilus granarius (L.). J. Stored Prod. Res. 1973, 9, 77–92. [Google Scholar] [CrossRef]
- Zettle, J.L.; McDonald, L.L.; Redlinger, L.M.; Jones, R.D. Plodia interpunctella and Cadra cautella resistance in strains to malathion and synergized pyrethrins. J. Econ. Entomol. 1973, 66, 1049–1050. [Google Scholar] [CrossRef]
- Zettler, L.J. Pesticide resistance in Tribolium castaneum and T. confusum (Coleoptera: Tenebrionidae) from flour mills in the United States. J. Econ. Entomol. 1991, 84, 763–767. [Google Scholar] [CrossRef]
- Lloyd, C.J.; Ruczkowski, G.E. The cross-resistance to pyrethrins and eight synthetic pyrethroids, of an organophosphorus-resistant strain of the rust-red flour beetle Tribolium castaneum (herbst). Pestic. Sci. 1980, 11, 331–340. [Google Scholar] [CrossRef]
- Stasinakis, P.; Katsares, V.; Mavragani-Tsipidou, P. Organophosphate Resistance and Allelic Frequencies of Esterases in the Olive Fruit Fly Bactrocera oleae (Diptera: Tephritidae). J. Agric. Urban Entomol. 2001, 18, 157–168. [Google Scholar]
- Skouras, P.G.; Margaritopoulos, J.T.; Seraphides, N.A.; Ioannides, I.M.; Kakani, E.G.; Mathiopoulos, K.D.; Tsitsipis, J.A. Organophosphate resistance in olive fruit fly, Bactrocera oleae, populations in Greece and Cyprus. Pest Manag. Sci. 2007, 63, 42–48. [Google Scholar] [CrossRef]
- Ramos, P.; Ramos, J.M. Veinte años de observaciones sobre la depredación oófaga en Prays oleae Bern. Granada (España), 1970–1989. Boletín Sanid. Veg. Plagas 1990, 16, 119–127. [Google Scholar]
- Gullan, P.J.; Cranston, P.S. The Insect: An Outline of Entomology; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Kranthi, K.R.; Ali, S.S.; Banerjee, S.K. Baseline toxicity of spinosad on the cotton bollworm, Helicoverpa armigera (Hub.), in India. Resist. Pest Manag. 2000, 11, 9–12. [Google Scholar]
- Moulton, J.K.; Pepper, D.A.; Dennehy, T.J. Beet armyworm (Spodoptera exigua) resistance to spinosad. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 842–848. [Google Scholar] [CrossRef]
- Mau, R.F.; Gusukuma-Minuto, L. Diamondback moth resistance to spinosad (Success and Tracer, Dow Agro-Sciences) in Hawaii: Confirmation, review of causal factors and establishment of a mitigation plan. In Proceedings of the 5th International Seminar on Technology of Cole Crops Production, Celaya, Guanajuato, Mexico, 17–18 May 2001; pp. 75–80. [Google Scholar]
- Gunning, R.V.; Balfe, M.E. Spinosad resistance in Australian Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). In Proceedings of the 10th IUPAC International Congress on the Chemistry of Crop Protection, Basel, Switzerland, 4–9 August 2002; p. 290. [Google Scholar]
- García, V.B.Q. La Resistencia a Spinosad en Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Ph.D. Thesis, Universidad Politécnica de Cartagena, Cartagena, Spain, 2007. [Google Scholar]
- Bollero, A.L.; Moya, J.H.; Macías, V.V.; Mohedano, D.P.; Moya, J.C.H. Introducción al Olivar Ecológico en Andalucía; Instituto de Investigación y Formación Agraria y Pesquera, Junta de Andalucía: Seville, Spain, 2017. [Google Scholar]
- Delvare, G.; Aberlenc, H.P.; Adis, J.; Springate, N.D.; Stork, N.E.; Didham, R.K.; Basset, Y. A review of methods for sampling arthropods in tree canopies. Canopy Arthropods 1997, 27, 52. [Google Scholar]
- Young, M. Insects in flight. In Insect Sampling in Forest Ecosystems; Blackwell Publishing: Hoboken, NJ, USA, 2005; pp. 116–145. [Google Scholar]
- Dimitrova, A.; Livieratos, I.; Gkisakis, V. Trapping methodologies for functional canopy arthropod diversity in olive agroecosystem. In Proceedings of the 2nd Mediterranean Forum Research and Innovation as Tools for Sustainable Agriculture, Food & Nutrition Security, Bari, Italy, 18–20 September 2018. [Google Scholar]
- Arambourg, Y. Traité D’entomologie Oléicole; Conseil Oléicole International: Madrid, Spain, 1986. [Google Scholar]
- Hodkinson, I.D.; Hughes, M.K. La Fitofagia en los Insectos; Oikos-Tau: Barcelona, Spain, 1993. [Google Scholar]
- Andrés-Cantero, F. Enfermedades y Plagas del Olivo, 4th ed.; Riquelme y Vargas Ediciones, SL Jaén: Jaen, Spain, 2001; p. 646. [Google Scholar]
- Guerrero García, A. Nueva Olivicultura; Mundi-Prensa: Madrid, Spain, 2003; Volume 5, p. 225. [Google Scholar]
- Burrack, H.J.; Fornell, A.M.; Connell, J.H.; O’Connell, N.V.; Phillips, P.A.; Vossen, P.M.; Zalom, F.G. Intraspecific larval competition in the olive fruit fly (Diptera: Tephritidae). Environ. Entomol. 2009, 38, 1400–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, M. Contribución al estudio de la entomocenosis de Prays oleae Bern. (Lep. Hyponomeutidae) en Granada (España). Acta Oecol. 1981, 2, 27–35. [Google Scholar]
- Varela, J.L.M.; González-Ruiz, R. Bases Metodológicas para la Evaluación del Impacto Ocasionado por las Aplicaciones Insecticidas Sobre los Enemigos Naturales de las Plagas del Olivo (II); Phytoma España: València, Spain, 1999; pp. 32–42. [Google Scholar]
- De Liñán, C. Entomología Agroforestal, Insectos y Ácaros que Dañan Montes, Cultivos y Jardines; Ediciones Aerotécnicas: Madrid, Spain, 1998. [Google Scholar]
- Nikolova, I.; Georgieva, N.; Tahsin, N. Toxicity of neem and pyrethrum products applied alone and in combination with different organic products to some predators and their population density. Rom. Agric. Res. 2015, 32, 291–301. [Google Scholar]
- Canard, M. Chrysopides (Neuroptera) Récoltés Dans les Oliveraies en Grèce; Bio. Gall. Hell: Toulouse, France, 1979. [Google Scholar]
- Alrouechdi, K. Les Chrysopides (Neuroptera) Récoltés dans une Oliveraie du sud-est de la France; Acta Oecologica/Oecologia Aplicata: Paris, France, 1980. [Google Scholar]
- Campos, M.; Ramos, P. Some relationships between the number of Prays oleae eggs laid on olive fruits and their predation by Chrysoperla carnea. In Integrated Pesticide Control Olive-Groves, Proceedings of the CEC/FAO/IOBC International Joint Meeting, Pisa, Italy, 3–6 April 1984; Balkema for CEC: Rotterdam, The Netherlands, 1985; pp. 237–241. [Google Scholar]
- Bozsik, A.; González-Ruiz Ruíz, R.; Lara, B.H. Distribution of the Chysoperla carnea Complex in Southern Spain (Neuroptera: Chrysopidae). In Protecția Mediului: Analele Universității din Oradea, Fascicula; Oradea, Romania, 2009; Volume 14, pp. 60–65. Available online: https://protmed.uoradea.ro/facultate/anale/protectia_mediului/2009/agr/11.Bozsikv%20Andras%202.pdf (accessed on 1 January 2022).
- González-Ruiz, R.G.; Al-Asaad, S.; Bozsik, A. Influencia de las masas forestales en la diversidad y abundancia de los crisópidos (Neur.: “Chrysopidae”) del olivar. Cuad. Soc. Española Cienc. For. 2008, 26, 33–38. [Google Scholar]
- González-Ruiz, R.; Gómez-Guzmán, J.A. Agricultural management greatly affects the beneficial entomofauna of the olive groves. Am. J. Biomed. Sci. Res. 2019, 1, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.A.; Pereira, J.A.; Torres, L.M.; Nogueira, A.J. Evaluation of the effects, on canopy arthropods, of two agricultural management systems to control pests in olive groves from north-east of Portugal. Chemosphere 2007, 67, 131–139. [Google Scholar] [CrossRef]
- Petacchi, R.; Minnocci, A. Impact of different Bactrocera oleae (Gmel) control strategies on olive-grove entomofauna. In Proceedings of the II International Symposium on Olive Growing, Jerusalem, Israel, 1 January 1994; ISHS Acta Horticulturae 356. Volume 356, pp. 399–402. [Google Scholar] [CrossRef]
- Rodríguez, E.; Peña, A.; Raya, A.J.S.; Campos, M. Evaluation of the effect on arthropod populations by using deltamethrin to control Phloeotribus scarabaeoides Bern. (Coleoptera: Scolytidae) in olive orchards. Chemosphere 2003, 52, 127–134. [Google Scholar] [CrossRef]
- Araya, M.; Araya, J.; Guerrero, M. Efectos de algunos insecticidas en dosis subletales sobre adultos de Aphidius ervi Haliday (Hymenoptera: Aphidiidae). Boletín Sanid. Vegetal. Plagas 2004, 30, 247–254. [Google Scholar]
- Umoru, P.A.; Powell, W. Sub-lethal effects of the insecticides pirimicarb and dimethoate on the aphid parasitoid Diaeretiella rapae (Hymenoptera: Braconidae) when attacking and developing in insecticide-resistant hosts. Biocontrol Sci. Technol. 2002, 12, 605–614. [Google Scholar] [CrossRef]
- Pascual, S.; Cobos, G.; Seris, E.; Sánchez-Ramos, I.; González-Núñez, M. Spinosad bait sprays against the olive fruit fly (Bactrocera oleae (Rossi)): Effect on the canopy non-target arthropod fauna. Int. J. Pest Manag. 2014, 60, 258–268. [Google Scholar] [CrossRef]
- Cochran, D.G. Insecticide Resistance. Understanding and Controlling the German Cockroach; Oxford University Press: New York, NY, USA, 1995; pp. 171–192. [Google Scholar]
- Busvine, J.R. Resistance to pyrethrins. Bull. World Health Organ. 1960, 22, 592. [Google Scholar] [PubMed]
- Morales, J.; Budia, F.; Viñuela, E. Efectos secundarios de cinco insecticidas sobre los diferentes estadios de desarrollo del parasitoide Hyposoter didymator (Thunberg) (Hymenoptera: Ichneumonidae). Bol. San. Veg. Plagas 2004, 30, 773–782. [Google Scholar]
- Schalk, J.M.; Shepard, B.M.; Stoner, K.A. Response of caterpillar pests and the parasite Diadegma insulare to collard cultivars and a pyrethrin insecticide. HortScience 1993, 28, 308–310. [Google Scholar] [CrossRef] [Green Version]
- Viñuela, E.; Handel, U.; Vogt, H. Evaluación en campo de los efectos secundarios de dos plaguicidas de origen botánico, una piretrina natural y un extracto de neem, sobre Chrysoperla carnea Steph. (Neuroptera: Chrysopidae). Bol. San. Veg. Plagas 1996, 22, 97–106. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).