Abstract
The aim of this study was to evaluate physiological responses and elemental composition of three salt tolerant alfalfa (Medicago sativa L.) cultivars, ‘Halo’, ‘Bridgeview’, ‘Rugged’, and two intolerant cultivars ‘Rangelander’ and ‘Vernal’ under five salinity levels (0 dSm−1, 4 dSm−1, 8 dSm−1, 12 dSm−1 and 16 dSm−1) in a sand based hydroponic system in the greenhouse. The germination percentage among the cultivars was highest for ‘Halo’ under salt stress. ‘Rugged’ and ‘Halo’ had higher seed vigor than the other cultivars in 16 dSm−1 EC. Among the alfalfa cultivars, ‘Rugged’ had the highest chlorophyll content at 0–12 dSm−1 EC. There was variation for root (p = 0.01) and shoot (p = 0.03) biomass among the alfalfa cultivars. Salt stress reduced (p < 0.001) plant height and shoot biomass, with 4.2% and 7.9% reduction for each 1 dS m−1 increase, respectively. Shoot biomass showed a positive correlation with plant height (p < 0.001, r = 0.80), chlorophyll content (p < 0.001, r = 0.56), root biomass (p < 0.001, r = 0.51), but was not correlated with seed vigor. This study demonstrated that seed vigor in the germination stage can not be used to predict salt tolerance of alfalfa at mature growth stages, however plant height and leaf chlorophyll content can serve as physiological markers for high shoot biomass selection at mature growth stages under salt stress.
1. Introduction
Soil salinity is a major restriction in crop production, limiting plant growth and contributing to land degradation globally [1,2]. Salinization threatens the agricultural productivity in the Great Plains of North America, affecting about 6 million ha of agricultural land in the Canadian Prairies alone [3,4,5]. To reduce further salinization, it is essential to grow deep-rooted perennial crops such as alfalfa (Medicago sativa L.) to provide permanent cover in saline areas where annual crop production is limited. Alfalfa is moderately tolerant to salinity [6,7], and its deep root system can keep the water table low, thus preventing salt-laden groundwater from “recharging” the topsoil with salt ions. Beside this, growing alfalfa in salt affected land supports livestock industry, providing a steady stream of protein-rich feed supplies [8,9]. Unfortunately, alfalfa becomes increasingly susceptible to salt stress above 8 ds/m of soil salinity [5], with the germination and seedling stages of alfalfa being most sensitive to salt stress [10]. Therefore, genetic improvement of alfalfa to salt tolerance is an important research topic for expanding its adaptation to the salt-affected areas.
Stepphun et al. [5] reported that alfalfa breeders relied on the germination rate of alfalfa in saline substrate as a selection indicator for salt tolerance, as the germination responses of plants to soil salinity determine their early survival rate in saline environments. Because of this selection method, the majority of the current salt-tolerant alfalfa cultivars showed improved tolerance at the germination stage [2]. Although it is critical to tolerate salt stress at early growth stages, plant selection at mature growth stages could improve it’s long-term adaptation and forage productivity. As no correlation has been found between germination and post germination stages’ salinity tolerance [11,12], there is a need for evaluation of different salt-tolerant alfalfa cultivars from germination to mature growth stages and regrowth phase to identify specific traits for plant selection, and further understand salt tolerance mechanisms among alfalfa populations.
Although the reduction of growth rate and shoot biomass is common, high genetic diversity exists among alfalfa populations under salt stress [5]. The responses to salt stress may vary between salt tolerant and sensitive alfalfa cultivars due to their genetic make-up. Salt tolerant alfalfa cultivars maintain growth through the exclusion of ions from leaves during the early phase of salt stress at mature growth stages [13,14,15,16]. However, exposure to high salt stress induced an increase in sodium and chlorine, which in turn decreased calcium and potassium levels in alfalfa [17,18,19]. Increased concentrations of sodium and chloride ions in the cytoplasm can disrupt cellular processes, exerting damage to the photosynthetic apparatus [1,19]. This means the maintenance of the regular photosynthetic rate is an important trait for salt tolerant alfalfa cultivars. Alfalfa populations selected for improved salt tolerance showed greater leaf production under salt treatment compared to their unselected initial population [20]. Ashrafi et al. [19] reported that salt tolerant alfalfa cultivars were characterized by low sodium and magnesium contents and high potassium, nitrogen, phosphorus, calcium, zinc, and copper contents.
In this study, we hypothesized that there must be specific genotypic variation among alfalfa cultivars in response to salinity at different growth stages. The response of five alfalfa cultivars was studied at germination and post germination stages by evaluating seed vigor, phenotypic and physiological traits at different gradients of salt concentrations, as well as elemental composition through inductively coupled plasma-mass spectroscopy (ICP-MS).
2. Materials and Methods
2.1. Plant Materials
The plant materials included alfalfa cultivars with contrasting tolerances to salinity, namely, salt tolerant: ‘Halo’, ‘Bridgeview’, ‘Rugged’, and salt intolerant: ‘Rangelander’ and ‘Vernal’. ‘Halo’ is a saline tolerant, synthetic cultivar of 192 clones, sequentially selected for germination, seedling growth and mature plant regrowth under repeated irrigation with 100mM NaCl solution in the greenhouse and registered in the United States (www.naaic.org, accessed on 5 October 2020) [5]. Furthermore, ‘Halo’ is highly resistant to anthracnose, bacterial wilt, Fusarium wilt, Verticillium wilt, Aphanomyces root rot, Phytophthora root rot, pea aphid, root knot nematode (Meloidogyne hapla), spotted alfalfa aphid, and stem nematode (www.naaic.org, accessed on 5 October 2020). ‘Bridgeview’ is a salt tolerant alfalfa cultivar developed by the Agriculture and Agri-Food Canada (AAFC) Lethbridge Research Centre. The cultivar ‘Bridgeview’ has a high level of salinity tolerance as well as winter hardiness. ‘Bridgeview’ is a 226-clone synthetic developed from polycross of seven alfalfa cultivars, namely ‘Apica’, ‘AC Blue J’, ‘Barrier’, ‘Beaver’, ‘Heinrichs’, ‘Rangelander’, and ‘Roamer’ [21]. ‘Rugged’ is a synthetic cultivar from 200 parental clones selected for salinity tolerance during germination and tolerance to continuous grazing [5]. Furthermore, ‘Rugged’ has high resistance to bacterial wilt, Verticillium wilt, Fusarium wilt, Phytophthora root rot, anthracnose (race 1), Aphanomyces root rot (race 1), pea aphid, and moderate resistance to stem nematode and Aphanomyces root rot (race 2) (www.naaic.org, accessed on 5 October 2020). ‘Vernal’ is a synthetic cultivar developed at the University of Wisconsin, selected for adaptation to the northern states and Canada with good winter hardiness and bacterial wilt resistance [22]. ‘Vernal’ is a salt susceptible alfalfa cultivar [10,23]. ‘Rangelander’ is a creeping rooted alfalfa that persists under long-term grazing, which was developed by AAFC research center, Swift Current, SK. ‘Rangelander’ is a 15-clone synthetic cultivar developed through mass selection for good persistence from ‘Rambler’, ‘Roamer’, ‘Drylander’, and strains of Medicago falcata growing in competition with crested wheatgrass over 8 years [24]. ‘Rangelander’ is a salt sensitive alfalfa cultivar [5,10,25].
2.2. Experimental Design
2.2.1. Germination Study
The experiments were conducted over a gradient of five salinity levels; 0 dS m−1, 4 dS m−1, 8 dS m−1, 12 dS m−1, 16 dS m−1. EC was maintained through NaCl additions. The germination experimental design was 5 (cultivar) × 5 (salinity) factorial arrangement in a randomized complete block design (RCBD) with four replications under day/night (12/12 h) temperatures of 20/10 °C. Twenty-five alfalfa seeds were imbibed on top of two layers of filter paper (Whatman 597) in 9 cm diameter sterilized plastic Petri dishes moistened with 5 mL distilled water or 5 mL of respective saline concentrations. The germination test was carried out in the germination cabinet (CMP 6010, Conviron, China). The germination experiment was repeated twice. The experimental conditions of germination are described by Bhattarai et al. [15]. From the germination experiment germination percentage, germination rate, the length of seedling, and seed vigor were recorded and calculated using the following formulas (1) and (2) [26,27].
2.2.2. Greenhouse Study
The post germination experimental design was a split-plot arrangement in a randomized complete block design (RCBD), with the salinity treatment being a main plot and cultivar being a sub-plot factor. The post germination test was carried out in the College of Agriculture and Bioresources greenhouse at the University of Saskatchewan (45 Innovation Blvd., Saskatoon, SK, Canada). In the greenhouse, natural light was supplemented with high pressure sodium halogen lamps for a total of 490–550 μM s−1 m−2 PAR with a 16 h photoperiod. Temperature of 21/16 °C (day/night) was maintained during the study. Each treatment combination was replicated four times. For each replication, three pots were used as one experimental unit, with each pot seeded with five seeds of each alfalfa cultivar. Pots were later thinned to two plants per pot after 5 weeks. The entire experiment consisted of 600 plants. The post germination experiments were repeated twice. The experimental conditions of greenhouse are described in details by Bhattarai et al. [15]. Plant height of all individual plants were measured 5 times at 14 d interval beginning from the first day of salt treatment. After reaching the targeted salt concentrations at 4 weeks, plant injury was scored with a 1–5 scale based on chlorotic spots and necrosis on the leaf and stem surfaces: 1—(no injury), 2—(<25% injury), 3—(26–50% injury), 4—(51–75% injury) and 5—(>75% injury).
Readings of chlorophyll content were obtained using the Chlorophyll Meter; SPAD-502 (Konica Minolta Sensing, Osaka, Japan). Readings were taken in all individual plants at 7 d intervals five times after reaching the targeted salt concentrations at 4 weeks. Three fully expanded leaflets were randomly chosen from each plant to take the chlorophyll content readings and values were averaged.
Whole plants were harvested after 12 weeks of growth in the greenhouse. Shoot biomass was harvested manually at 3 cm stubble height and weighed for fresh shoot biomass. Similarly, fresh root biomass was determined after washing roots with tap water and shade drying. After measuring fresh biomass, shoot and root samples were dried at 60 °C for 48 h in a forced air oven and weighed for dry weight determinations.
Stress tolerance indices of alfalfa cultivars were determined based on shoot dry weight using formula (3) [28].
where Yc and Ys are shoot dry weight of an individual plant under no salt stress control and salinity stress, respectively. is the shoot dry weight means of all genotypes under control condition.
At the end of the experiments, surviving plants were counted and expressed as number of surviving plants × 100/number of total plants.
Crude protein (CP) content was determined using whole plant shoot samples. Dried shoot samples were ground in a Willey Mill (Thomas-Wiley, Philadelphia, PA, USA) to pass through a 1 mm mesh screen (Cyclone Mill, UDY Mfg, Fort Collins, CO, USA). The ground samples were stored in plastic bags prior to CP quantification. Nitrogen content was determined using LECO CN628 Element Analyzer (LECO, St. Joseph, MI, USA). Crude protein was calculated as CP = nitrogen concentration (%) × 6.25.
Leaf and root tissues were sampled from the five alfalfa cultivars grown under salt stress for 8 weeks. Three randomly chosen pots were sampled for each cultivar from each salt concentration. Leaf, and root tissues were harvested separately, totaling 225 samples. The samples were ground using the procedure described above for CP determination and stored in plastic bags prior to element quantification. Quantification of elements (sodium, chlorine, phosphorus, potassium, calcium, magnesium, sulphur, iron, copper, zinc, manganese) in leaf and root tissues of alfalfa was done by ICP-MS (Agilent 7500ce, Palo Alto, CA, USA) using two technical replicates.
2.3. Statistical Analyses
For statistical analysis, analysis of variance (ANOVA) was performed on the data using Proc Mixed in SAS software version 9.4 (http://www.sas.com/, accessed on 5 February 2020). Tukey’s multiple comparison test was applied to compare means at the significance level of p < 0.05. Pearson correlation coefficient was also calculated among germination and post germination traits under salt stress.
3. Results
3.1. Germination Percentage and Germination Rate
The ANOVA showed that seed germination percentage was significantly (p < 0.001) affected by the interaction between salinity and cultivar. The germination percentage was the highest for ‘Vernal’ at 0 dS m−1 (90%), but ‘Halo’ had the highest germination percentage under salt stresses, ranging from 73.0–86.5% (Figure 1A). The germination percentage of ‘Rugged’ was lower than ‘Halo’, ‘Bridgeview’, and Vernal from 0–12 dS m−1, but its germination percentage was not negatively affected by salinity (Figure 1A). At 16 dS m−1 salinity, ‘Halo’ had the highest germination rate followed by ‘Rugged’, ‘Bridgeview’, and ‘Vernal’. The salt intolerant cultivar ‘Rangelander’ had the lowest germination rate in all treatments.
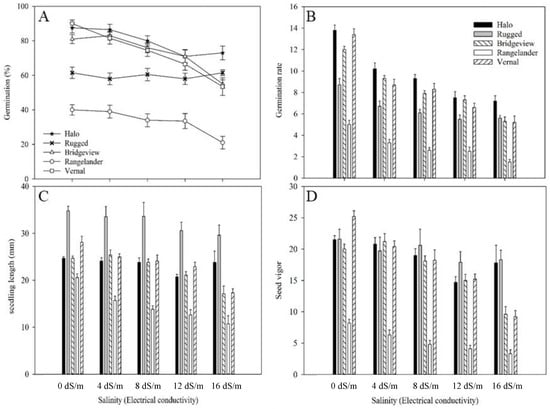
Figure 1.
(A) Cumulated seed germination (%), (B) germination rate, (C) seedling length (mm) and (D) seed vigor of five alfalfa cultivars under five gradients of salinity: 0 dS m−1, 4 dS m−1, 8 dS m−1, 12 dS m−1, 16 dS m−1 (error bar represents standard errors of means).
Seed germination rate (p < 0.001) was significantly affected by the interaction between salinity and cultivar (Figure 1B). Germination rate decreased with increasing salinity (p < 0.001) for all five alfalfa cultivars. The germination rate of ‘Halo’ was highest among all cultivars at all salinity levels, ranging from 7.18–13.77 (Figure 1B). The germination rate of ‘Rangelander’ was the lowest among all cultivars at all salinity levels (Figure 1B). ‘Bridgeview’ had higher germination rate than ‘Rugged’ in all treatments except at 16 dS m−1 salinity.
3.2. Seedling Length and Seed Vigor
Seedling length was significantly (p = 0.043) affected by the interaction between salinity and cultivar. Salinity had no effect on the seedling length of ‘Rugged’, whereas a significant effect was observed for the remaining cultivars. The seedling length of ‘Rugged’ was the longest among the cultivars at different salinities, ranging from 29.6–34.8 mm, while it was the shortest for ‘Rangelander’, ranging from 10.7–20.5 mm (Figure 1C). ‘Halo’, ‘Bridgeview’, and ‘Vernal’ had similar seedling length at 0–12 dS m−1, but it was longer for ‘Halo’ than the other two cultivars at 16 dS m−1 salinity.
Seed vigor was significantly affected by salinity (p < 0.001), cultivar (p < 0.001) and their interaction (p < 0.001). At 0 dS m−1, ‘Vernal’ showed the highest seed vigor (25.17) which was similar to ‘Bridgeview’ (20.00), ‘Halo’ (21.55), and ‘Rugged’ (21.55) (Figure 1D). At 16 dS m−1, seed vigor was the highest for ‘Rugged’ (18.3) and ‘Halo’ (17.8), intermediate for ‘Bridgeview’ (9.6) and ‘Vernal’ (9.2), and the lowest for ‘Rangelander’ (3.2) (Figure 1D).
3.3. Plant Height
Although alfalfa continued to grow taller over the course of the experiment, plant height was significantly different among the salt treatments after 10 weeks of growth. At 10 and 12 weeks, plant height decreased with the increase in salinity. Average plant height at 16 dS m−1 was about half the plant height of controls (Figure 2). There was significant variation observed between alfalfa cultivars (p < 0.001) for plant heights after 12 weeks of growth. Among cultivars, plant height at 12th week was highest for ‘Vernal’ at 0 dS m−1 (54.3 cm), 8 dS m−1 (33.6 cm), 12 dS m−1 (29.3 cm), 16 dS m−1 (27.7 cm), while ‘Rugged’ was the tallest at 4 dS m−1 (41.8 cm). However, the growth rate of ‘Vernal’ was lowest among all cultivars (data not shown).
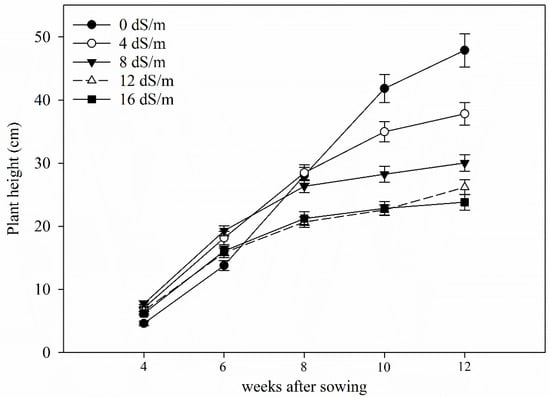
Figure 2.
Average plant height (cm) of alfalfa plants at different stages of growth under five gradients of salinity (Electrical conductivities of 0 dS m−1, 4 dS m−1, 8 dS m−1, 12 dS m−1, 16 dS m−1) (salt stress was applied on 4 weeks old plant; error bar represents standard errors of means).
3.4. Chlorophyll Content
There was significant variation observed among the alfalfa cultivars (p < 0.001) for chlorophyll content after 12 weeks of growth. Plants in salinity levels 0 dS m−1, 4 dS m−1, and 8 dS m−1 had similar chlorophyll content. However, there were significant reductions in chlorophyll at 12 dS m−1, and 16 dS m−1 (Figure 3). The relative chlorophyll content after 12 weeks of sowing was the lowest for ‘Rangelander’ at all salinity levels and highest for ‘Rugged’ at salinity levels from 0–12 dS m−1 (data not shown).
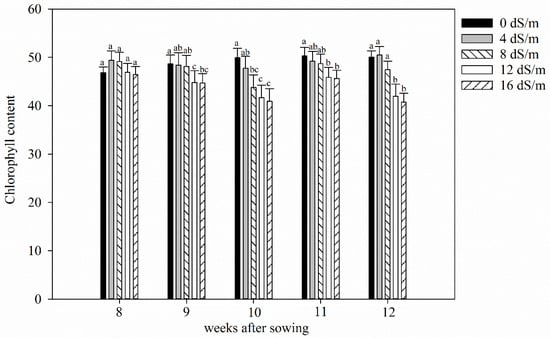
Figure 3.
Average chlorophyll content (SPAD value) of alfalfa plants at different stages of growth under five gradient of salt stresses (Electrical conductivities of 0 dS m−1, 4 dS m−1, 8 dS m−1, 12 dS m−1, 16 dS m−1) (salt stress was applied on 4 weeks old plant; error bar represents standard errors of means; means followed by same letter are not significantly different at p < 0.05).
3.5. Plant Injury Score and Plant Survival
Plant injury score increased with increasing salinity (p < 0.001). It also varied among the alfalfa cultivars (p = 0.007), but there was no salinity × cultivar interaction effect on plant injury score, indicating a similar trend for all cultivars (Table 1). ‘Rugged’ showed the highest level of injury at 4 dS m−1 (2.3), 12 dS m−1 (3.3), and 16 dS m−1 (3.6). ‘Rangelander’ showed the highest level of injury at 8 dS m−1 (2.9) (Table 1), while ‘Halo’ showed the least injuries at 4 dS m−1 (1.7), 8 dS m−1 (2.1) and 12 dS m−1 (2.7). Finally, ‘Bridgeview’ showed the least injuries at 16 dS m−1 (2.7) (Table 1).

Table 1.
Mean value (2-yr) of plant injury, survival, crude protein, dry biomass yield of alfalfa cultivars under five gradients of salinity 0 dS m−1, 4 dS m−1, 8 dS m−1, 12 dS m−1, 16 dS m−1.
Plant survival was significantly affected by salinity (p < 0.001), but no significant variation was observed among the cultivars. All alfalfa plants survived at the control and 4 dS m−1, whereas 94% of alfalfa plants survived at 8 dS m−1. The survival rate decreased at 12 dS m−1 (73%) and 16 dS m−1 (57%) after 12 weeks (Table 1). At 16 dS m−1 salinity, 34% of alfalfa plants survived (data not shown). As a result of this poor survival, biomass yield was not reported for 16 dS m−1.
3.6. Crude Protein
Crude protein was significantly affected by salinity (p < 0.001), but no significant variation was observed among the cultivars. Crude protein content of all alfalfa cultivars increased with increase in salinity except in ‘Rangelander’ which showed the highest CP at 8 dS m−1 and ‘Vernal’ which showed the least CP at 4 dS m−1 (Table 1). At high salt stress of 12 dS m−1, ‘Halo’ (21.7%) had numericallyhighest CP followed by ‘Vernal’ (20.0%), ‘Bridgeview’ (19.6%) and ‘Rugged’ (18.7%) with the least CP ‘Rangelander’ (17.2%) ranked last (Table 1).
3.7. Root and Shoot Biomass and Salt Tolerant Index
There were significant effects of salinity (p < 0.001) and cultivar (p < 0.05) on root biomass (Table 1). Root biomass was the highest at 4 dS m−1 (1.84 g plant−1) which was non-significantly different from the control treatment (1.81 g plant−1). Root biomass was reduced with increase in salinity after 4 dS m−1 to 0.72 g plant−1 at 12 dS m−1 (Table 1). Root biomass at 0 dS m−1, 4 dS m−1, 8 dS m−1 and 12 dS m−1 was highest for ‘Halo’ (2.5 g plant−1), ‘Vernal’ (2.4 g plant−1), ‘Vernal’ (1.2 g plant−1) and ‘Halo’ (0.9 g plant−1), respectively (Table 1). At high salt stress of 12 dS m−1, ‘Halo’ (0.9 g plant−1) had the highest root biomass followed by ‘Vernal’ (0.8 g plant−1), ‘Bridgeview’ (0.7 g plant−1), and ‘Rangelander’ (0.7 g plant−1). This value was with least for ‘Rugged’ (0.5 g plant−1).
There were significant effects of salinity on shoot biomass (p < 0.001). Additionally, there was significant variation among the alfalfa cultivars for shoot biomass (p = 0.03). Shoot biomass was 3.32 g plant−1 in the control treatment, which was reduced to 1.12 g plant−1 at 12 dS m−1 (Table 1). Shoot biomass at 0 dS m−1, 4 dS m−1, 8 dS m−1, and 12 dS m−1 was the highest for ‘Halo’ (4.4 g plant−1), ‘Vernal’ (2.2 g plant−1), ‘Rugged’ (1.5 g plant−1), and ‘Halo’ (1.4 g plant−1), respectively (Table 1). In the high salt stress of 12 dS m−1, ‘Halo’ (1.4 g plant−1) had the highest shoot biomass followed by ‘Vernal’ (1.2 g plant−1) and ‘Rangelander’ (1.2 g plant−1) while it was lowest in ‘Rugged’ (0.9 g plant−1) and ‘Bridgeview’ (0.9 g plant−1).
The salt-tolerance index based on shoot biomass showed that ‘Halo’ was the most tolerant among the five cultivars at the salinity levels of 4 dS m−1 and 12 dS m−1 whereas ‘Rugged’ showed greater tolerance at 8 dS m−1 followed by ‘Halo’ (Table 2).

Table 2.
Salt tolerance index of alfalfa cultivars based on shoot biomass yield.
3.8. Correlation among the Measured Variables
Plant height had a significant positive correlation with leaf chlorophyll content (p < 0.001, r = 0.59), shoot biomass (p < 0.001, r = 0.80), root biomass (p < 0.001, r = 0.51), germination rate (p < 0.001, r = 0.28), seed vigor (p < 0.05, r = 0.21). Shoot biomass showed a significant positive correlation with leaf chlorophyll content (p < 0.001, r = 0.56), root biomass (p < 0.001, r = 0.51), but no correlation was observed with germination rate or seed vigor. Root biomass showed a significant positive correlation with chlorophyll content (p < 0.05, r = 0.22) and germination rate (p < 0.05, r = 0.22). Plant injury score showed a significant negative correlation with plant height (p < 0.001, r = −0.29), leaf chlorophyll content (p < 0.001, r = −0.38), shoot biomass (p < 0.01, r = −0.29), root biomass (p < 0.01, r = −0.29), and germination rate (p < 0.001, r = −0.34), and seed vigor (p < 0.05, r = −0.16) (Table 3).

Table 3.
Pearson correlation coefficient among the traits measured at germination and post germination stages of alfalfa under salt stress (upper triangular matrix represent positive and negative correlation coefficient and lower triangular matrix represents significant level).
3.9. Elemental Composition of Alfalfa
3.9.1. Leaf
The elemental composition in leaf tissue of alfalfa cultivars at each level of salinity as revealed by ICP-MS is shown in Table 4. At 0 dS m−1, there was significant variation among alfalfa cultivars for sodium (p < 0.001) and sulphur (p = 0.01). At 4 dS m−1 there was significant variation among alfalfa cultivars for sulphur (p = 0.03), potassium (p = 0.001), and iron (p = 0.01). At 8 dS m−1, there was significant variation among alfalfa cultivars for sodium (p = 0.003), sulphur (p = 0.002), potassium (p < 0.001), chloride (p = 0.02), phosphorus (p < 0.001), magnesium (p = 0.02), copper (p = 0.001), and manganese (p = 0.01). At 12 dS m−1, there was significant variation among alfalfa cultivars for sodium (p = 0.01), sulphur (p = 0.01), potassium (p = 0.03), magnesium (p = 0.06), copper (p = 0.01), manganese (p < 0.001), and zinc (p < 0.001). At 16 dS m−1, there was significant variation among alfalfa cultivars for sodium (p < 0.001), sulphur (p = 0.01), potassium (p < 0.001), chloride (p = 0.003), phosphorus (p < 0.001), calcium (p < 0.001), copper (p = 0.01), and manganese (p < 0.001). In leaf tissue at 12 dS m−1, sodium concentration was the highest for ‘Rugged’, followed by ‘Rangelander’, ‘Bridgeview’, ‘Halo’ and ‘Vernal’. Likewise, at 12 dS m−1 salinity, chlorine concentration was the highest for ‘Rangelander’, followed in order by ‘Bridgeview’, ‘Vernal’, ‘Halo’, and ‘Rugged’.

Table 4.
Analysis of variance and mean value of elemental concentrations (mg L−1) in leaf tissue of five alfalfa cultivars (H, ‘Halo’; Ru, ‘Rugged’; B, ‘Bridgeview’; Ra, ‘Rangelander’; V, ‘Vernal’) under five gradients of salt stresses (Electrical conductivities of 0 dS m−1, 4 dS m−1, 8 dS m−1, 12 dS m−1 and 16 dS m−1) as revealed by inductively coupled plasma-mass spectroscopy.
3.9.2. Root
The elemental composition in root tissue of alfalfa cultivars at each level of salinity as revealed by ICP-MS is shown in Table 5. At 0 dS m−1, there was significant variation among alfalfa cultivars for sodium (p = 0.005), potassium (p = 0.001), magnesium (p < 0.001), sulphur (p = 0.009), and manganese (p = 0.01). At 4 dS m−1, there was significant variation among alfalfa cultivars for all measured elements except iron (p = 0.15). At 8 dS m−1, there was significant variation among alfalfa cultivars for all measured elements except potassium (p = 0.72) and calcium (p = 0.06). At 12 dS m−1, there was significant variation among alfalfa cultivars for potassium (p = 0.02), sulphur (p = 0.003), iron (p < 0.001), copper (p < 0.001), zinc (p < 0.001), and manganese (p < 0.001). At 16 dS m−1, there was significant variation among alfalfa cultivars for phosphorus (p = 0.009), calcium (p < 0.001), sulphur (p = 0.004), copper (p = 0.007), zinc (p = 0.02), and manganese (p < 0.001). At 12 dS m−1 salinity, the sodium and chlorine concentrations were the highest for ‘Rangelander’, followed by ‘Vernal’, ‘Bridgeview’, ‘Halo’, and ‘Rugged’ in root tissues.

Table 5.
Analysis of variance and mean value of elemental concentrations (mg L−1) in root tissue of five alfalfa cultivars (H, ‘Halo’; Ru, ‘Rugged’; B, ‘Bridgeview’; Ra, ‘Rangelander’; V, ‘Vernal‘) under five gradients of salt stresses (Electrical conductivities of 0 dS m−1, 4 dS m−1, 8 dS m−1, 12 dS m−1 and 16 dS m−1) as revealed by inductively coupled plasma-mass spectroscopy.
4. Discussion
All crops are routinely affected by a broad range of biotic and abiotic stresses in natural growth conditions. This results in difficulty in dissecting a single component of plant stress in the field. In this study, we used a sand based hydroponic experiment in the greenhouse to understand the physiological responses of five alfalfa cultivars with varying tolerances to salt stress at germination and post-germination stages of growth. The findings of this study can provide useful physiological indicators for screening alfalfa germplasms for salt tolerant in future alfalfa genetic improvement.
As expected, the seed vigor and seedling length of salt tolerant alfalfa cultivars ‘Halo’ and ‘Rugged’ were greater as compared to other cultivars in 16 dS m−1 because ‘Rugged’ was selected for tolerance to salinity during germination and ‘Halo’ for tolerance to salinity at germination and mature growth stages [5]. Similarly, another salt tolerant cultivar ‘Bridgeview’ was also selected for high seed vigor and forage yield as compared to ‘Rangelander’ [21]. In our study, ‘Bridgeview’ showed higher seed vigor than ‘Rangelander’, which was lower than ‘Halo’ and ‘Rugged’. The salt intolerant alfalfa cultivar ‘Vernal’ had the highest average final plant height under salinity 8–16 dS m−1. This might be because the reduction in plant height may represent a strategy adopted by salt tolerant alfalfa cultivars to survive under salt stress by reallocating assimilates to support mechanisms that promote plant survival. Salt tolerant alfalfa cultivar ‘Halo’ had the highest biomass and the lowest plant injury score in this study, which is in agreement with previous studies by Steppuhn et al. [5] and Bertrand et al. [29]. The decrease in chlorophyll content under salinity has been considered a typical symptom of oxidative stress because of either slow synthesis or fast breakdown [30]. This study suggests that in the early stages of salt stress alfalfa does not undergo significant chlorophyll reduction. Therefore, to effectively screen alfalfa germplasm using plant injury scores and chlorophyll content as markers it may be necessary to expose plants to extended periods of salt stress. Interestingly, we also found that crude protein concentration increased under higher salinity levels, which suggests that the selection for salt tolerant alfalfa germplasm will also select for improved forage quality.
It is important to emphasize that the definition of salt resistance varies between growth stages; during the germination stage resistance is based on survival, whereas in later developmental stages resistance is usually based on relative growth reduction [31]. This study suggests selecting for higher seed vigor with lower plant injury and higher biomass yield can be used for selecting salt tolerant alfalfa genotypes for population development. This study suggests truncation selection, aka independent culling, as a breeding strategy for developing salt tolerant alfalfa cultivars at different growth stages. This is because seedling length and seed vigor showed no correlation with shoot biomass. This result is similar to previous reports on the correlation between germination and post-germination stages [11,12]. To successfully develop salt tolerant alfalfa cultivars through truncation selection, germplasms should be selected for high seed vigor followed by low plant injury score and high biomass in later growth stages. During truncation selection, population size is severely reduced due to the high selection intensity for each trait. This selection approach might reduce genetic variation significantly, thus, starting with diverse germplasm is desirable. A number of studies have reported that salt tolerance in alfalfa populations can be improved if initial genetic variability is high for traits associated with salt tolerance [5,10].
There are different strategies used by plant species to cope with salt toxicity. Some plants can accumulate salt ions in vacuoles, while some exclude salts through the roots [1,2]. Increasing potassium uptake is also a strategy for coping with sodium ion toxicity [32]. Alfalfa exposed to salt stress for 8 weeks showed accumulations of sodium in leaves at higher salinity levels (8–12 dS m−1), which contradicts Wand and Han [33] who found a higher accumulation of sodium in roots than in leaves sampled after 15 days of salt stress (120 mmol L−1 NaCl). This difference was likely because of varying responses of cultivars to salinity in addition to the length of salt exposure. For two salt-tolerant cultivars, Bhattarai et al. [15] found the pattern of chlorine accumulation for ‘Halo’ was root > stem~leaf at 8 dSm−1, and root~leaf > stem at 12 dSm−1, potentially preventing an elemental overload injury in leaf tissues. In contrast, for ‘Vernal’ chlorine accumulation was leaf > stem~root at 8 dSm−1. Rahman et al. [23] found alfalfa under 50 mM and 100 mM NaCl showed an ion-exclusion salt tolerance mechanism. Potassium and sodium, being monovalent cations, are generally considered as competitive elements for uptake and transport [34]. Our study, however, found an increasing concentration of sodium with increases in salinity while potassium concentrations did not show any trend relative to the salinity gradients. In leaf tissue of alfalfa, calcium concentration decreased with an increase in salinity levels which is similar to the result reported by Younesi et al. [35], suggesting a sodium induced calcium deficiency.
There are contradictory explanations for decreased, increased, or unchanged phosphorus uptake in response to salinization in different plant species [36], indicating a complex interaction between salinity and phosphorus uptake. This study found phosphorus concentration in leaf tissue of all alfalfa increased with the increase in salinity from 0 dS m−1 to 12 dS m−1. Phosphorus is involved in several key functions in plants such as photosynthesis, transformation of sugars and starches, and nutrient movement. In contrast to Ashrafi et al. [19] who observed increased magnesium content in leaf and root tissues under salt stress, our study showed decreased content in leaf tissue and increased content in root tissue as compared to controls. Magnesium is essential for many cellular enzymes functioning and is also the central atom of the chlorophyll molecule [37]. Therefore, decrease in magnesium in leaf tissue in our study may suggest a decrease in photosynthesis under salt stress. Sulphur containing compounds play important role in plant defense against stresses [38]. We found sulphur content increased with increases in salt stress in leaf tissue. It is difficult to explain the influences of salt stress on micro-element concentrations because of relatively smaller differences between control and stressed plants [39]. Manganese is an essential micronutrient involved in photosystem II (PS II), providing electrons for photosynthesis. We found the accumulation of both manganese and zinc in alfalfa increased with increasing salinity up to 12 dS m−1. In particular, salt-tolerant alfalfa cultivars in our study accumulated more manganese than intolerant alfalfa at 12 dS m−1. Manganese and zinc were assumed to scavenge the free radical superoxide (O2−) and hydrogen peroxide (H2O2), thereby providing defense against oxidative stress [40,41]. However, a high salinity level of 16 dS m−1 had a detrimental effect on manganese and zinc accumulation eventually affecting photosynthesis and plant growth. Alfalfa showed higher copper concentration in root tissues than leaf tissues and the highest concentration at 12 dS m−1. Super-oxide dismutase which contains copper and zinc as metal components [42] detoxifies reactive oxygen species (ROS). Decrease in micro-elements under salt stress might have detrimental effects on this ROS scavenging ability. The finding of this ionome study is crucial in understanding alfalfa’s response to salt stress.
5. Conclusions
Salinity reduced shoot and root growth of all five alfalfa cultivars, but the magnitude of growth reduction varied among the cultivars. The variability in response to salinity among alfalfa cultivars indicated a potential for further plant selection for future breeding. ‘Halo’ is a promising cultivar based on high salt tolerance index, high biomass, and low plant injury score relative to other cultivars under greenhouse conditions. The approach in this paper might facilitate the selection of salt tolerant alfalfa genotypes based on physiological traits for the development of salt tolerant cultivars, which could improve alfalfa productivity in saline regions. This study found that indirect selection for improved germination and seedling vigor may not be an effective method for improving forage biomass yield. Rather, a sequential selection based on high seed vigor at germination followed by low plant injury and high biomass in later growth stages could be an effective strategy for improving salinity tolerance. As alfalfa is a perennial forage legume, further investigation is needed to assess the response of alfalfa to salinity during re-growth. Additionally, this study was conducted in a sand-based hydroponics system in the greenhouse, therefore further study is needed to validate these results in field conditions.
Author Contributions
B.B. conceived the project; S.B., B.B. designed experiments; B.B. prepared the study materials; S.B. performed experiments; S.B., B.B. analyzed data; S.B. wrote a first version of the manuscript and S.L., B.B. substantially contributed to the last version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The research was funded by NSERC Discovery grant (RGPIN-2016-06082). We would like to thank greenhouse staff at the College of Agriculture and Bioresources, University of Saskatchewan. We would also like to thank Barry Goetz and Rocky Kundu for their technical help in ICP-MS analysis and Siyang Shen for his help during greenhouse study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Bhattarai, S.; Biswas, D.; Fu, Y.-B.; Biligetu, B. Morphological, Physiological, and Genetic Responses to Salt Stress in Alfalfa: A Review. Agronomy 2020, 10, 577. [Google Scholar] [CrossRef]
- Steppuhn, H. What is soil salinity? In Proceedings of the Soil Salinity Assessment Workshop, Alberta Agriculture, Lethbridge, AB, Canada, 30 March 1996; pp. 1–5. [Google Scholar]
- Wiebe, B.H.; Eilers, R.G.; Eilers, W.D.; Brierley, J.A. Application of a risk indicator for assessing trends in dryland salinization risk on the Canadian Prairies. Can. J. Soil Sci. 2007, 87, 213–224. [Google Scholar] [CrossRef]
- Steppuhn, H.; Acharya, S.N.; Iwaasa, A.D.; Gruber, M.; Miller, D.R. Inherent responses to root-zone salinity in nine alfalfa populations. Can. J. Plant Sci. 2012, 92, 235–248. [Google Scholar] [CrossRef]
- Maas, E.V.; Hoffman, G.J. Crop salt tolerance-current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar] [CrossRef]
- Diaz, F.J.; Grattan, S.R.; Reyes, J.A.; de la Roza-Delgado, B.; Benes, S.E.; Jimenez, C.; Dorta, M.; Tejedor, M. Using saline soil and marginal quality water to produce alfalfa in arid climates. Agric. Water Manag. 2018, 199, 11–21. [Google Scholar] [CrossRef]
- Goplen, B.P.; Baenziger, H.; Bailey, L.D.; Gross, A.T.H.; Hanna, M.R.; Michaud, R.; Richards, K.W.; Waddington, J. Agriculture Canada: Growing and Managing Alfalfa in Canada; Agriculture Canada: Burnaby, BC, USA, 1982; p. 1705. [Google Scholar]
- Yuegao, H.; Cash, D. Global status and development trends of alfalfa. In Alfalfa Management. Guide for Ningxia; Cash, D., Ed.; United Nations Food and Agriculture Organization: Beijing, China, 2009; pp. 1–14. [Google Scholar]
- Peel, M.D.; Waldron, B.L.; Jensen, K.B.; Chatterton, N.J.; Horton, H.; Dudley, L.M. Screening for salinity tolerance in alfalfa. Crop Sci. 2004, 44, 2049–2053. [Google Scholar] [CrossRef]
- Al-Niemi, T.S.; Campbell, W.F.; Rumbaugh, M.D. Response of alfalfa cultivars to salinity during germination and post-germination growth. Crop Sci. 1992, 32, 976–980. [Google Scholar] [CrossRef]
- Johnson, D.W.; Smith, S.E.; Dobrenz, A.K. Selection for increased forage yield in alfalfa at different NaCl levels. Euphytica 1992, 60, 27–35. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Teuber, L.R.; Phillips, D.A. Lucerne (Medicago sativa L.) selected for vigor in a nonsaline environment maintained growth under salt stress. Crop Pasture Sci. 1989, 40, 1253–1259. [Google Scholar] [CrossRef]
- McKimmie, T.; Dobrenz, A.K. Ionic concentrations and water relations of alfalfa seedlings differing in salt tolerance. Agron. J. 1991, 83, 363–367. [Google Scholar] [CrossRef]
- Bhattarai, S.; Liu, N.; Karunakaran, C.; Tanino, K.K.; Fu, Y.-B.; Coulman, B.; Warkentin, T.; Biligetu, B. Tissue specific changes in elements and organic compounds of alfalfa (Medicago sativa L.) cultivars differing in salt tolerance under salt stress. J. Plant Physiol. 2021, 264, 153485. [Google Scholar] [CrossRef]
- Khorshidi, M.B.; Yarnia, M.; Hassanpanah, D. Salinity effect on nutrients accumulation in alfalfa shoots in hydroponic condition. J. Food Agric. Environ. 2009, 7, 787–790. [Google Scholar]
- Li, R.; Shi, F.; Fukuda, K.; Yang, Y. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2010, 56, 725–733. [Google Scholar] [CrossRef]
- Cornacchione, M.V.; Suarez, D.L. Emergence, forage production, and ion relations of alfalfa in response to saline waters. Crop Sci. 2015, 55, 444–457. [Google Scholar] [CrossRef]
- Ashrafi, E.; Razmjoo, J.M.; Zahedi, M. Effect of salt stress on growth and ion accumulation of alfalfa (Medicago sativa L.) cultivars. J. Plant Nutr. 2018, 41, 818–831. [Google Scholar] [CrossRef]
- Anower, R.M.; Mott, I.W.; Peel, M.D.; Wu, Y. Characterization of physiological responses of two alfalfa half-sib families with improved salt tolerance. Plant Physiol. Biochem. 2013, 71, 103–111. [Google Scholar] [CrossRef]
- Acharya, S.N.; Steppuhn, H. Bridgeview alfalfa. Can. J. Plant Sci. 2012, 92, 203–206. [Google Scholar] [CrossRef]
- Graber, L.F. Registration of vernal alfalfa. Agronomy J. 1956, 48, 587. [Google Scholar] [CrossRef][Green Version]
- Rahman, M.A.; Alam, I.; Kim, Y.G.; Ahn, N.Y.; Heo, S.H.; Lee, D.G.; Liu, G.; Lee, B.H. Screening for salt responsive proteins in two contrasting alfalfa cultivars using a comparative proteome approach. Plant Physiol. Biochem. 2015, 89, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Heinrichs, D.H.; Lawrence, T.; McElgunn, J.D. Rangelander alfalfa. Research Note. Can. J. Plant Sci. 1979, 59, 491–492. [Google Scholar] [CrossRef]
- Gruber, M.; Xia, J.; Yu, M.; Steppuhn, H.; Wall, K.; Messer, D.; Sharpe, A.; Acharya, S.; Wishart, D.; Johnson, D.; et al. Transcript analysis in two alfalfa salt tolerance selected breeding populations relative to a non-tolerant population. Genome 2017, 60, 104–127. [Google Scholar] [CrossRef] [PubMed]
- Maguire, J.D. Speed of germination in selection and evaluation for seedling vigour. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiplication. Crop Sci. 1973, 3, 630–633. [Google Scholar] [CrossRef]
- Fernandez, G.C.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetable and Other Food Crops in Temperature and Water Stress, Taiwan, 13–18 August 1992; Kuo, C.G., Ed.; AVRDC Publication: Tainan, Taiwan, 1992; pp. 257–270. [Google Scholar]
- Bertrand, A.; Dhont, C.; Bipfubusa, M.; Chalifour, F.P.; Drouin, P.; Beauchamp, C.J. Improving salt stress responses of the symbiosis in alfalfa using salt-tolerant cultivar and rhizobial strain. Appl. Soil Ecol. 2015, 87, 108–117. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Lauchli, A.; Grattan, S.R. Plant growth and development under salinity stress. In Advances in Molecular Breeding toward Drought and Salt Tolerant Crops; Jenks, M.A., Hasegawa, P.M., Jain, S.M., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 1–32. [Google Scholar]
- Serrano, R.; Mulet, J.M.; Rios, G.; Marquez, J.A.; Leube, M.P.; Mendizabal, I.; Pascual-Ahuir, A.; Proft, M.; Ros, R.; Montesinos, C. A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot. 1999, 50, 1023–1036. [Google Scholar] [CrossRef]
- Wang, X.S.; Han, J.G. Effects of NaCl and silicon on ion distribution in the roots, shoots and leaves of two alfalfa cultivars with different salt tolerance. Soil Sci. Plant Nutr. 2007, 53, 278–285. [Google Scholar] [CrossRef]
- Chen, Z.; Cuin, T.A.; Zhou, M.; Twomey, A.; Naidu, B.P.; Shabala, S. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef]
- Younesi, O.; Chaichi, M.R.; Postini, K. Salt tolerance in alfalfa following inoculation with Pseudomonas. Middle East J. Sci. Res. 2013, 16, 101–107. [Google Scholar]
- Grattan, S.R.; Grieve, C.M. Mineral element acquisition and growth response of plants grown in saline environments. Agric. Ecosyst. Environ. 1992, 38, 275–300. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Nazar, R.; Iqbal, N.; Masood, A.; Syeed, S.; Khan, N.A. Understanding the significance of sulphur in improving salinity tolerance in plants. Environ. Exp. Bot. 2011, 70, 80–87. [Google Scholar] [CrossRef]
- Tozlu, I.; Moore, G.A.; Guy, C.L. Effect of increasing NaCl concentration on stem elongation, dry mass production, and macro- and micro-nutrient accumulation in Poncirus trifoliata. Aust. J. Plant Physiol. 2000, 27, 35–42. [Google Scholar]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Ducic, T.; Polle, A. Transport and detoxification of manganese and copper in plants. Braz. J. Plant Physiol. 2005, 17, 103–112. [Google Scholar] [CrossRef]
- Culotta, V.C.; Yang, M.; O’Halloran, T.V. Activation of superoxide dismutases: Putting the metal to the pedal. Biochim. Biophys. Acta. 2006, 1763, 747–758. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).