Abstract
Under-exploited crops such as Lablab purpureus are regarded a pathway towards alleviating the food and nutritional security in Sub-Saharan Africa. This study aimed at evaluating the morpho-agronomic diversity present in 277 lablab accessions based on 38 morpho-agronomic traits. The experiment was laid out in an Augmented design across two main cropping seasons in Tanzania. Qualitative data was analysed using pivot tables. The Generalized Linear Model (PROC GLM), Agglomerative Hierarchical Clustering (AHC) and Principal Component Analysis (PCA) were used to analyse variation of 14 quantitative traits. The findings revealed the presence of wide variability of the qualitative traits in the studied accessions. Significant differences were observed among accessions, between seasons, the interaction of blocks and season, and the season and accession effects in most of the traits. Most of the traits had high significant differences in relation to contrast among accessions, among checks and between accessions and checks. The first five principal components cumulatively accounted for 61.89% of the total variability among the accessions studied. Furthermore, cluster analysis grouped the accessions into four major clusters. This results suggest the 14 morpho-agronomic traits can successfully discriminate and show presence of wide diversity vital for selection and hybridization program of lablab species.
1. Introduction
Food insecurity, malnutrition, climate change and unpredictability, and the continent’s booming population growth rate are all major concerns in Sub-Saharan Africa. Implementing nutrient-dense, affordable, climate-resilient, and sustainable food systems can help us meet a number of objectives, including ending hunger, ensuring food security, promoting nutrition, and advancing sustainable agriculture []. Agricultural diversification has been deemed to be a fundamental component in achieving the Sustainable Development Goal of ending world hunger. It includes both food production and dietary diversity. Whilst production diversification centers on the supply of diversified and nutritious food systems as well as mitigating the impacts of climate change, diet diversification addresses hunger and malnutrition in a manner that is accessible, economical, and sustainable []. Leveraging underutilized and underexploited crops like lablab (Lablab purpureus (L.) Sweet), which is highly nutrient-dense, can thrive in marginal areas, and is resilient to biotic and abiotic stresses, is vital to diversify diets, amplify sustainable farming, and mitigate climate change and its effects.
Lablab (Lablab purpureus (L.) Sweet) is a versatile crop with numerous advantages, including the ability of its major plant parts, including leaves, fresh seeds, and dry seeds, to be consumed. It is nutrient packed, with vital elements such as protein 18–25% [,], lysine 6–7% [], fiber 3.35–8% [,], Thiamine 0.5–1.130 mg/100 gm [], Iron 155 mg/100 g [], phosphorus (P) 27–57 mg/100 g [], Potassium (K) 132–297 mg/100 g [], Calcium (Ca) 130 mg/100 gm []. Regardless of the fact that lablab is nutritious; its consumption is constrained by its anti-nutritional compounds. This include tannins 0.33–3.84 mg/100 g, phytates 723 mg/100 gm and trypsin inhibitors 13 TIU/mg [,,]. Lablab is also regarded a valuable animal feed in the form of fresh leaves, herbage and silage that boost livestock production [,,]. Other advantages of lablab include soil conservation crop [], intercrop [], pharmaceutical importance [,], green manure [,] and climate resilient crop [,]. However, limited farmers’ preferred lablab varieties exist. Production of lablab is constrained by pests and diseases, lack of improved varieties, poor marketability, poor soil fertility, competition from related legume crops, and fluctuating climatic conditions [,]. Moreover, the limited information on the genetic diversity of the germplasm found in Africa impede lablab advancement []. Being a neglected crop, lablab improvement lag as compared to the counterpart leguminous crops. Research on genetic diversity has not been done with current research initiatives focusing on its use for soil conservation, intercrop and animal feed [,]. Effective breeding program depend on proper characterization and evaluation of existing germplasm in order to identify elite genotypes and novel germplasm with important traits for biotic and abiotic stresses. Studies on farmer’s collections (landraces) and germplasm from gene banks serve as avenues for improving lablab crop.
Several morphological studies on lablab have been conducted outside of Africa including [] in USA, [,,,] in India and [] in Nepal with contrasting results pertaining genetic diversity. Despite limited genetic variability, improved varieties have been developed particularly in India, owing to the existence of well-established breeding programs.
However, few genetic diversity studies [] in Kenya and [] in Tanzania have been explored in Africa with distinct variation of the studied accessions. Previous research only investigated a smaller number of accessions, making it difficult to draw firm conclusions about the entire existing germplasm. Breeders and research scientists need robust morphological studies on lablab accessions conserved by farmers as well as breeding resources available in research institutions to provide them the knowledge required to be prioritized in breeding programs.
In this context, this study aims to investigate genetic variation and describe morpho-agronomic traits of lablab germplasm in Tanzania in order to improve its production and development. Knowledge on genetic variation and characteristics in lablab germplasm is critical for germplasm resource conservation, selection of superior cultivars, development of new varieties, and determining a pre-breeding program.
2. Materials and Methods
2.1. Site Description
Morphological and agronomic studies of lablab accessions were conducted at the Nelson Mandela African Institution of Science and Technology (NM-AIST) experimental field in Northern Tanzania. NM-AIST lies at Latitude 03°02′17.0″ S and Longitude 037°35′24.9″ E at an elevation of 1106 m.a.s.l. The average maximum temperature ranges from 22 °C to 28 °C while the average minimum temperature ranges from 12 °C to 15 °C respectively []. The experiment was carried out in Phaeozem soils [] with pH 6.5 (deionized water: soil 1: 2.5), total carbon 17.1 g/kg, Nitrogen 1.4 g/kg, Phosphorus 7.9 mg/kg, Potassium 3.4 Cmol/kg, magnesium 4.9 Cmol/kg and Calcium 24 Cmol/kg per 0–30 cm soil depth []. Rainfall is generally consistent, ranging from 800–1400 mm/year and bimodal []. The research was set up over two growing seasons: short rains (November 2019–March 2020) and long rains (March 2020–October 2020).
2.2. Experimental Design and Layout
The experimental plot was initially mechanically ploughed followed by harrowing until a fine tilt was achieved. The experiment was then set up in an augmented randomized complete block design as generated by the statistical tool available at Indian Agricultural Statistical Research Institute (IASRI) website []. Three hundred and twenty (320) accessions of lablab collected world-wide and maintained at NMAIST were used in the study (Appendix A). Three checks, two Indian released varieties (HA-3 (C1), HA-4 (C2)) and Kenyan released variety ELDO-KT (C3) were used. The three checks were replicated twice in each block and the total number of experiment units were 390. Based on the design, 390 accessions including checks were distributed randomly in ten blocks of 39 rows each. Each row representing one accession (treatment) of 10 seed (one seed per hole) with a spacing of 45 cm between the holes and 70 cm between the lines. Total experimental field was 40 m × 30 m. Each block size was 30 m in length and 5 m in width and 1 m between each block. Normal agronomic practices (pest and disease control, weeding and irrigation) were done when necessary.
2.3. Data Collection
From the 320 accessions (317 test accessions and 3 checks), data was collected on 271 test accessions. The remaining 46 test accessions had poor germination, did not grow to vegetative stages and some were attacked by pests and diseases. Thus data was collected on total of 277 accessions. A total of 38 morphological traits (24 qualitative and 14 quantitative) were recorded as per [] lablab descriptor sheet. The 24 qualitative traits were visually assayed and include hypocotyl colour, cotyledon colour, stem pigmentation, leaf vein colour, leaf anthocyanin, leaf colour, leaf hairiness, leaf shape, growth habit, branch orientation, flower characteristics (bud, standard petal, wing petal and keel) colour, raceme position, pod characteristics (curvature, pubescence, fragrance, constriction, colour, attachment and colour at physiological maturity) and seed characteristics (fresh seed colour, dry seed shape and dry seed colour) were expressed as percentages. Data on quantitative traits were based on 5 randomly selected plants. Number of primary branches, number of secondary branches, number of buds per node, number of buds per raceme, number of buds per node, number of racemes per plant, fresh pods per plant, number of locules and number of seeds per pod were counted for the 5 tagged plants. Days to 50% flowering was recorded for days from sowing to when 50% of the plants in each accession had produced flowers. Raceme length, fresh pod length and fresh pod width was measured using a metre rod and expressed in centimetres (cm). Seed yield per plant and hundred seed weight were measured using a weighing balance and expressed in grams (g) (Appendix B).
2.4. Statistical Analysis
Descriptive statistics were obtained for qualitative parameters using the Pivot table in MS Excel. Generalized linear model procedure (GLM PROC) of the SAS software (SAS Institute Inc. 14.1. SAS/IML® 14.1 User’s Guide. Cary, North Carolina State, USA: SAS Institute Inc) was used to analyse the accession, block, season and accession, and block vs. accession. The Agglomerative Hierarchical Clustering (AHC) and Principal Component Analysis (PCA), in XLSTAT -Base version 21.1.57988.0 were used to analyse the accession vs season and accession vs season interactions, and the clustering and variations among studied accessions. Phenotypic, genotypic and environmental variance components and their coefficients of variation, broad sense heritability (H2) and genetic advance as percent of mean (GAM) were estimated by using R software version 4.1.2 []. Phenotypic Coefficient of Variation (PCV) and Genotypic Coefficient of Variation (GCV) estimates were classified as low (0–10%), moderate (11–20%), and high (>20%) as per []. Additionally, heritability estimates were categorized as low 0–30%, medium 30–60% and high >60% as per Mat Sulaiman, et al. [], heritability estimates are While the genetic advance as a percentage of mean (GAM) was classified as low 0–10%, medium 10–20%, and high >20% [].
3. Results
3.1. Morphological Characterization of Qualitative Characters
3.1.1. Variation of Characters at Vegetative Stage
Characters like hypocotyl color, cotyledon color, stem pigmentation, leaf vein color, leaf anthocyanin color, leaf color, form, growth habit, and branch orientation were assessed during the vegetative stage. 100 percent of the accessions had green hypocotyl color.
In terms of cotyledon color, 79.4% were green and 20.6% were white. For the various accessions, the stem pigmentation ranged from almost solid (2.5%), extensive (0.7%), localized to nodes (2.2%), to no pigmentation (94.6%). In regards to leaf vein color, green (98.6%) prevailed over purple (1.4%). Only 3.2% of the accessions exhibited some coloration, whereas the majority of them (96.8%) lacked leaf anthocyanin. Purple (0.7%), light green (1.1%), dark green (1.8%), and green (96.4%) were the different leaf colors.
The accessions varied significantly in terms of leaf hairiness, with glabrous (5.1%), moderate (12.3%), high (16.6%), and low (66.1%) pubescence. Regarding leaf shape round shape (1.4%) was outnumbered by ovate leaf shape (98.6%). Indeterminate growth habit made up the majority of the accessions (62.1%), followed by determinate (22%) and semi-determinate (15.9%). Most accessions had first lateral branches spreading in the ground (56.7%), short erect lateral branches (39%) and branches perpendicular to main stem (4.3%). (Figure 1).
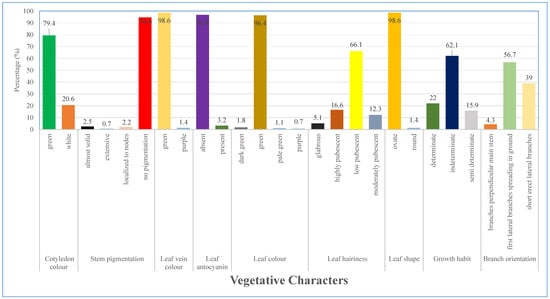
Figure 1.
Variations in selected vegetative characters of lablab accessions.
3.1.2. Variation of Characters at Flowering Stage
Qualitative characters such as flower bud color, standard petal color, wing petal color, keel petal color, and raceme position were included in the list of flowering features. White (39.7%), cream (39%) and purple (21.3%) were the most common flower bud, standard petal, wing, and keel color variations; raceme position on the plant showed that 71.1% were intermediate, 23.8% completely emerged from the canopy, and 5.1% appeared within the canopy (Figure 2).
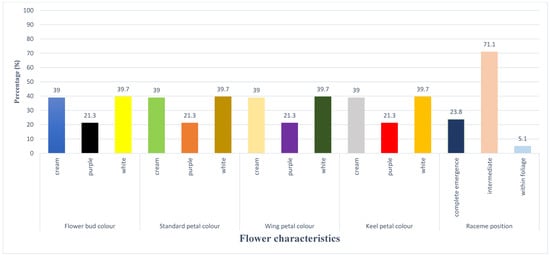
Figure 2.
Variations in selected flowering characters of lablab accessions Variation of characters at podding stage.
3.1.3. Variation of Pod Characteristics
The majority of the accessions studied had straight pods (92.4%), compared to slightly curved (4.3%) and curved (3.2%). Most accessions’ pods had glabrous pubescence (80.9%), with pubescent accessions 11.6% and moderate pubescence were 7.6%. in relation to fragrance, 10.5% of pods had a strong fragrance, 59.6% were moderate, 16.6% were low, and 13.4% were without any fragrance. Majority of the accessions (9.39%) had constricted pods, while 6.1% had slight constriction. The most prevalent pod color (95.3%) was green, followed by green with purple suture (3.2%) and white (1.4%). The pods with intermediate attachment were (96.8%), while 1.8% had pendant attachment and 1.4% were erect. At the time of pod maturity, 98.6% of the accessions had tan pods, while only 1.4% were brown coloured (Figure 3).
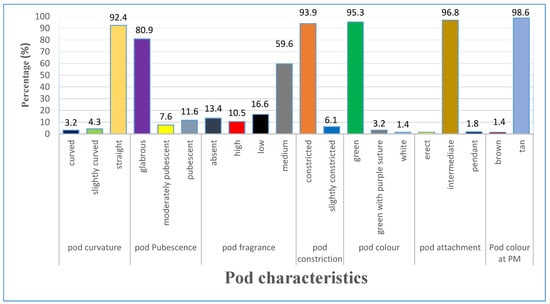
Figure 3.
Variation in selected pod characteristics of lablab accessions.
3.1.4. Variation of Seed Characters
Green colour (99.6%) was dominant over purple coloration (0.4%) in relation to freshly harvested seeds. Oval seed shape made up the majority of the dry seed (92.8%), with 7.2% being round seeded. Black (26.7%), white (20.2%), cream (19.5%), and brown (0.4%) were the most prevalent dry seed colors (Figure 4). Selected qualitative characters are shown visually as in Figure 5.
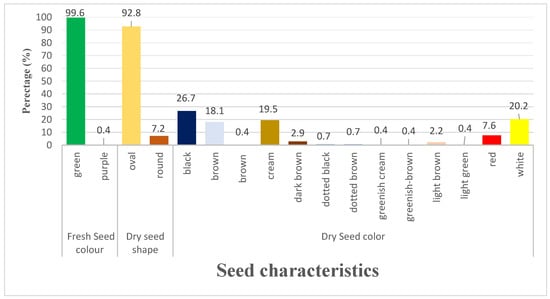
Figure 4.
Variation in seed characters of lablab accessions.


Figure 5.
Pictorial presentation of the different morphological characters of lablab.
3.2. Diversity in Quantitative Characters of Lablab Accessions
3.2.1. Descriptive Analysis, Genetic Variability, and Heritability of Quantitative Traits of Lablab Accessions
Descriptive statistics reveal presence of a wide variability in the 14 quantitative traits of the lablab accession studied. The highest mean value was recorded in yield per plant (112.88 g) while least mean value was in primary branches (1.7 cm). The least number of primary branches was in D302 (0.6) while D67 had the highest 3.02. The days to 50% flowering ranged from 44 days for D264 to 180 days in D113. The number of secondary branches ranged from 6.94 in D117 to 42.37 in D26. In relation to individual trait, buds per node ranged from 1.57 cm (D107) to 3.27 cm in D299. The number of buds per raceme ranged from 6.03 (D54) to 20.21 (D393). The number of racemes per plant ranged from 4 in D148 to 23 in D229. Accession D145 had the shortest pod length (4.12 cm) as compared to D119 which had the longest pods (11.65 cm). Fresh pod width value was the least in D102 (0.69 cm) as compared to the 2.8 cm in D119. Accession D354 had the least number of locules (3) while D64 had the highest number (6). In regards to raceme length, D77 had the shortest raceme length (6.37 cm) as compared to 25.11 cm in D281. The number of seeds per pod ranged from 3 in D354 to 6 in D64. Few number of pods were recorded in accession D107 (14.67) while maximum pod number was in D134 (334.34). The yield per plant was the least in accession D25 (5.56 gm) and the highest in D134 (413.16) (Table 1). The least hundred seed weight value was in D393 (18.05 g) as compared to 47.52 g in D92.

Table 1.
Descriptive analysis, genetic variability and heritability values of quantitative characters of lablab accessions across two cropping seasons in Tanzania.
The phenotypic coefficient of variation (PCV), genotypic coefficient of variation (GCV), broad sense of heritability (Hbs), and genetic advance as percent mean (GAM) were used to calculate genetic variability in studies (Table 2). PCV estimates were, on average, higher than GCV values for all 14 quantitative features that were assessed. Low GCV values were observed for the number of buds per raceme (4.46%), the number of locules per pod (5.79%), the number of seeds per pod (5.79%), the width of the fresh pod (7.54%), and the length of the raceme (7.85%). While the number of racemes (22.05%), secondary branches (29.62%), days to 50% flowering (29.64%), number of pods (46.35%), and yield per plant recorded higher estimates, the number of primary branches (14.2%), number of buds per node (14.88%), and fresh pod length (16.81%) recorded medium estimates.

Table 2.
Mean squares from ANOVA for 14 quantitative traits of lablab across two cropping seasons in Northern Tanzania.
Low PCV values were reported in the number of locules per pod (5.79%) and number of seeds per pod (5.79%). Fresh pod width (13.5%), number of buds per raceme (14.3%), hundred seed weight (15.14%), number of buds per node (18.73%), raceme length (18.86%), and fresh pod length (19.56%) all revealed medium values. Whereas, the remaining traits, number of primary branches (23.41%), number of racemes (26.34%), days to 50% flowering (29.83%), number of secondary branches (35.01%), number of pods (52.32%) and yield per plant (61.41%) had high PCV values.
Low heritability estimates were observed for the number of buds per raceme (9.72%) and raceme length (17.32%), medium (primary branches 36.79 and fresh pod width 31.25%) and maximum 100% in locules per pod and seed per pod. In genetic advance as a percentage of mean (GAM), high estimates was recorded for yield per plant 105.31%, number of pods 84.7% and days to 50% flowering 60.75%, while least GAM was found in buds per raceme 2.87%, raceme length 6.74% and fresh pod width 8.7% (Table 1). Traits such as days to 50% flowering, number of secondary branches, number of racemes, number of pods and yield per plant had high estimates in all the variability components including PCV, GCV, broad sense of heritability and GAM.
3.2.2. Analysis of Variance of the Quantitative Traits across the Two Cropping Seasons
The analysis of variance of the quantitative traits revealed significant differences (p < 0.001) in all the characters except fresh pod width (Table 2). All traits, with the exception of the number of secondary branches, showed substantial seasonal effects. All traits showed high significant differences between the accessions, with the exception of the fresh pod width. Additionally, only the number of racemes, buds per node, hundred seed weight, principal branches, and number of secondary branches were significantly impacted by the interactions between block and season. In the interaction effects of season and accessions, only the days to 50% flowering, hundred seed weight, number of racemes, locules per pod, seeds per pod, and yield per plant exhibited significant variations. All of the tested quantitative traits showed a high coefficient of genetic determination (R2). Fresh pod width showed a high coefficient of variation (86.69) while the number of seeds and locules per pod had the lowest value (0). Significant differences in all traits were observed among checks, with the exception of the number of buds per raceme, fresh pod width, number of locules per pod, and number of seeds per pod. Additionally, the selected traits significantly varied between checks and accessions, with the exception of fresh pod width (Table 3).

Table 3.
Analysis of the differences and interactions among accessions, among control and between accessions and checks across the two cropping seasons.
3.2.3. Principal Component Analysis of the Quantitative Traits of Lablab Accessions
The findings of the quantitative qualities were analyzed using PCA, and 5 components were found to have eigen values greater than 1. Cumulative variability of 61.89% was contributed by the five major quantitative character components. Only 3 of these 5 major factors effectively made a significant contribution to the total variation. It is evident that the first main component, with an eigen value of 2.62 and a contribution of 18.70%, was the dominant one based on the factor pattern and summary description in (Table 4). Three features, namely locules per pod, seeds per pod, and fresh pod length, made up the majority of the first primary component (PC1). The second main component (PC2) with Eigen value of 2.03 and 33.17% cumulative variability was governed by yield per plant, number of pods, hundred seed weight and days to 50% flowering. The third component (PC3) was governed by number of primary branches and fresh pod width with eigen value 1.56 and a cumulative variability of 44.35%. Number of racemes, days to 50% flowering, and number of secondary branches were the dominant traits in PC4 with an eigen value of 1.40 and a cumulative variability of 54.33%. The fifth component (PC5) was mainly comprised of raceme length, number of primary branches, and number of secondary branches with eigen value of 1.06 and a cumulative percentage of 61.89%. PC1 and PC2 combined accounted for 33.17% of the PCA scatter plot (Figure 6). The distinct variation among the quantitative traits of the germplasm study is shown in the scatter plot. The number of locules per pod, the number of seeds per pod, and the length of the fresh pods were all positively correlated.

Table 4.
Eigen values, proportion variability and quantitative traits that accounted for the first five principal components of lablab accessions.
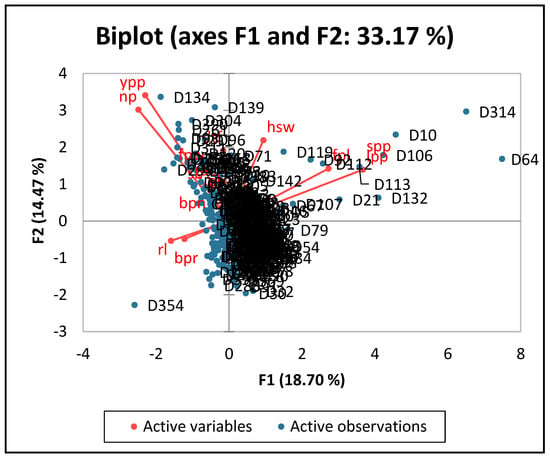
Figure 6.
Scatter plot diagram of principal component analysis of quantitative traits of lablab.
Similarly, the number of pods per plant, the yield per plant, and the weight of a hundred seeds were positively correlated.
3.2.4. Cluster Analysis of Selected Quantitative Traits of Lablab Accessions
Cluster analysis aids in determining the existing variation among genotypes and group/cluster them based on similarity of their descriptions. These clusters reduces the number of individual accessions and arrange them based on similarity index thus forming a dendrogram. Euclidean distance and the Wards technique were used to perform the cluster analysis of the 14 quantitative traits of the lablab accessions, and a dendrogram was generated (Figure 7). According to the study, the 277 accessions can be clustered into 4 main groups with weak association between them and the place of origin. Cluster III (C3) had the highest number of accessions (144) followed by Cluster I (C1) (105) and Cluster II (C2) (20) and finally cluster IV (C4) (8 accessions) (Figure 7). Maximum cluster distance (296.22) was evident between cluster II and cluster IV, followed by cluster I and cluster II (263.34), then cluster II and cluster III (216.07), cluster III and IV (81.35), cluster I and Cluster III (60.65) and least inter-cluster distance was between cluster I and IV (46.4). Cluster I was dominated by buds per node, buds per raceme, days to 50% flowering, fresh pod length, hundred seed weight, primary branches and raceme length. Cluster II comprised of number of pods and yield per plant. Cluster III was majorly fresh pod width, number of racemes and secondary branches. Cluster IV was made up of locules per pod and seeds per pod.
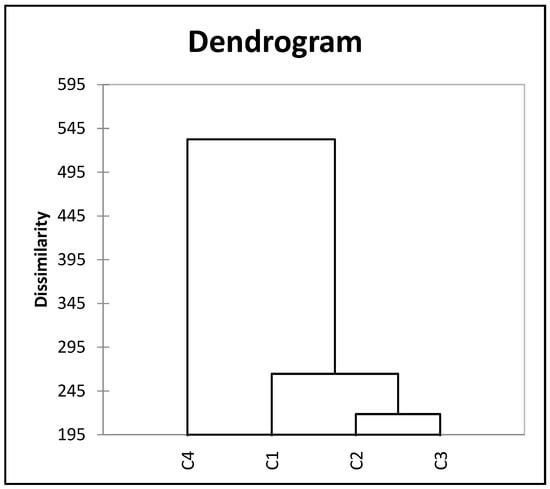
Figure 7.
Cluster dendogram of 274 lablab accessions based on mean values of 14 quantitative traits over two cropping seasons.
4. Discussion
Wide inter- and intra-variability within a specific germplasm indicate a wide reservoir of breeding material for improving a given crop species. Germplasm diversity studies helps to characterize evaluating and distinguishing different characters in a germplasm. Effective and efficient characterization program lays down a strong foundation for development of new varieties []. Morphological characterization is the preliminary stage that generates key information by assessing both the qualitative and quantitative traits.
4.1. Qualitative Traits
This study revealed wide variability in the qualitative characters of the lablab accessions. Qualitative characters are considered the initial selection parameter used by breeders and farmers to evaluate a given germplasm collection. These characters are easy to ascertain and score, mainly genetically controlled and provide unique description of the given germplasm []. Wide polymorphism in the study accessions were observed in leaf hairiness, growth habit, branch orientation, flower characteristics, raceme position, pod fragrance and dry seed colour parameters only. Each of the other characters was dominated by a single attribute with frequencies of more than 80%. The use of the mono-morphic characters in characterization of lablab germplasm may provide a simple and affordable way to distinguish accessions. Similar results were reported by Vaijayanthi, et al. [].
Polymorphism was evident in the leaf hairiness with majority of the accessions exhibiting low pubescence. Pubescence act as plant protective mechanism against insect pests that could feed on them []. In contrast to Vaijayanthi, et al. [], who reported 30% of accessions had strong pubescence, the results of this investigation showed a lower percentage (16%). This could possibly be deduced by the study using fewer accessions. Limited information exist on pubescence of lablab leaves and their significance [].
Growth habit is a principal factor in lablab breeding []. Majority of the accessions studied revealed indeterminate growth habit. The high number of indeterminate and semi-determinate accessions were expected since the collections were obtained from farmers and local gene banks of which little varietal improvement have been done. Similarly, research centres such as International Livestock Research Institutes (ILRI) where some accessions were sourced from have been predominantly prioritizing lablab as a livestock feed and cover crop []. With this emphasis, the previous research could have been tailored towards indeterminate accessions due to dense fresh biomass content. Indeterminate habit is associated with prolonged reproductive cycle and staggered pod maturity []. In Tanzania, however, farmer participatory breeding study on lablab revealed that farmers preferred determinate accessions []. Farmers reported that determinate varieties are preferable due to its shorter maturation cycles and potential to intercrop with maize or sorghum for more efficient utilization of the available land []. Successful breeding of determinate lablab varieties in India such as HA-4 and HA-3 has been documented It has been reported that determinate lablab varieties like HA-4 and HA-3 have been successfully developed in India [,]. However, scanty research has been undertaken in Africa to enhance the development of determinate varieties [].
Flower attribute is controlled by a single gene and is considered stable in its expression in a given species []. In this study, white, cream, and purple were the three color variation with white being the dominant. Flower colour act as a visible morphological marker to identify different accessions within a specific germplasm collection. A strong association has been reported between flower colour and seed coat colour. According to Ewansiha, et al. [], there exists a direct association between purple flowers and black seed coats while white colour were correlated with white, green and cream seeds. According to Letting, Venkataramana and Ndakidemi [], farmers preferred white seeded varieties for their own consumption while black seeds were mainly for market purposes. In Tanzania, breeding of accessions with white flower relating to cream or white seeds would be easily adopted for human consumption.
Pod characterization described variation in shape, pubescence, fragrance, constriction and its attachment in the studied accessions. Most of the examined accessions possessed fragrance levels between moderate and strong, which was a characteristic most commonly used for vegetable purposes in Asian countries []. High fragrance in lablab is related to presence of 2 fatty acids namely: Trans 2-Dodecenoic acid and Trans 2-Tetradecenoic acids are perceived to be controlled by fewer genes []. Most of the accessions in this study exhibited constricted pods, a critical trait in legume crops. Compared to varieties with flat seeds, those with restricted pods shatter less frequently []. Pod pubescence plays an important role in plant defense against insect damage and injury []. Trichomes, or hairs, are a physical defense mechanism for plants against insect attack. Thus, pubescence on the pod surface can help a species defend itself from pest attack []. The majority of the tested accessions had glabrous pods.
In most of the accessions, seed characteristics such as seed colour and shape varied distinctively. Seed coat is a monogenic trait which can be used as a marker to identify and characterize a given accession []. In Tanzania, seed colour influences consumer acceptability of lablab with majority of farmers preferring white coloured seeds for consumption and black seeds for commercial purposes []. Black seeded varieties are associated with presence of anti-nutritional factors which makes them less palatable []. Seed production programmes rely on seed colour to identify admixtures in a given seed lot. In this study, majority of the accessions were black coloured which can be attributed to farmers’ collections which were mainly grown for commercial purposes to the ready market in Kenya. Similar findings in relation to seed characteristic have been reported by Singh and Abhilash [], Maass and Usongo [] and Islam, Rahman and Hossain [].
Qualitative characters act as diagnostic markers for germplasm characterization to identify and classify accessions thus reducing duplication of samples as well as during labelling. They are usually easy to score, exhibit stable expression, selectively neutral and governed by single or oligogenic genes []. Qualitative traits are integral during Distinctiveness Uniformity and Stability (DUS) tests before variety release, an aspect required for plant protection rights.
4.2. Quantitative Characters
Crop improvement programs require heritable variation of the major morpho-agronomic characters of a given species. Selection of appropriate accessions for a breeding program rely on the extent of variation especially in the yield and yield related traits []. Designing an effective breeding program depends on existing variation in the germplasm, extent of genetic variability and the genetic gain within the tested germplasm [].
The mean performance in the quantitative traits revealed existence of a wide range of variation. Quantitative traits such as days to 50% flowering, number of secondary branches, number of buds per raceme, number of racemes, fresh pod length, raceme length, number of pods yield per plant and hundred seed weight showed a significant difference between maximum and minimum values illustrating presence of a wide variability among the accessions. The accessions tested originated from various countries, have undergone various selection pressures, and have grown in diverse environmental conditions, all of which contributed to variations in the genetic constitution translating to genetic diversity.
Phenotypic variability of individuals in a given population is influenced by genotypic and environmental factors. In the current study, all the traits had lower GCV than PCV values. However, there were narrow differences indicating low environmental influence and stable expression of these traits, a vital aspect during selection of traits of interest. These results corroborate previous findings on lablab indicating close estimates for PCV and GCV [,]. Higher PCV and GCV values were found in days to 50% flowering, number of secondary branches, number of racemes, number of pods and yield per plant suggesting a wide range of selection of accessions for this traits. Lower PCV and GCV values for number of buds per raceme, fresh pod width, number of locules per pod, raceme length and number of seeds per pod indicate small improvement of this traits can be attained.
The inheritance of a trait from one generation to the next is integral when making a selection. Heritability estimates is crucial when selecting lablab accessions. High heritability values were observed in this study for 10 out of 14 traits. It is simple to select characteristics with high heritability estimates when improving crops. High estimates in PCV, GCV, broad sense of heritability, and GAM were found for the number of secondary branches, number of racemes, days to 50% flowering, number of pods, and yield per plant. This implies that these morpho-agronomic features are controlled by additive gene action and are suitable for continuous selection during lablab improvement programs. Hadavani, et al. [] revealed high PCV, GCV and GAM for number of pods per plant, seed yield per plant, and fresh pod length. To facilitate lablab improvement, effective breeding programs should consider yield and yield contributing traits.
Pooled analysis of variance revealed high significant mean squares attributed to study accession in all the 14 quantitative traits an indicator of a wide genetic variability within germplasm examined. Farmer preferred traits such as pests and disease resistance, high yielding, early maturity and market preference are critical in lablab breeding []. Yield and yield related attributes are key traits when breeding especially with a focus on adoption by farmers. High yielding varieties are of high economic value as compared to low yielding. Current existing varieties in Africa and specifically Tanzania are landraces with low yielding potential []. In this study, D134, D390, D93 and D304 were potential accessions which can put forward for future breeding considerations. Identification of high yielding accessions paves way for selection and breeding of varieties easily adopted by farmers.
Early maturity varieties are closely associated with short flowering durations. Early maturing types are preferred by breeders and growers in ecological zones with short rainfall []. For areas with prolonged rainfall regimes, late maturing varieties are desirable. Early maturing cultivars are becoming more and more preferred because of climate change and variability, optimal use of land resources for the following cropping season, reduced insect infestation. On the other hand, late maturing types generally produce higher yields and, owing to their leaf deposits, enrich the soil []. Early maturing accessions, including D264, HA-4, HA-3, D28, and D163, were found in this study and can be used in future breeding programs. In this study, early maturing accessions such as D264, HA-4, HA-3, D28 and D163 were identified and can be forwarded for further breeding programmes. Conversely, late maturing varieties such as D113, D244, D162, D35 and D308 can be candidate accessions for utilisation as cover crops and soil conservation strategies.
PCA variables with eigen values of large magnitude depict a strong influence of a given trait. During selection, traits that contribute significant variation in the PCA defines the variability of the germplasm collection []. In this study, yield per plant, number of pods per plant, number of seeds per pod, number of locules per pod, fresh pod length and hundred seed weight showed high factor values. Lablab breeding strategies should focus on the qualities indicated above because they account for a large portion of phenotypic variance. Comparably, PCA identifies appropriate parental germplasm for the development of segregants for a specific quantitative trait locus essential for variety development. The current research was carried out across two cropping seasons; therefore, multi-location trials done over a number of years are necessary to gather more comprehensive data on the performance of the accessions. The PCA analysis results are in agreement with Singh, et al. [], [,].
A two-dimensional scatter plot generated using the first two principal components reveals the accessions’ dispersion in the four quadrats. For future hybridization breeding programs, accessions located further away from the origin should be considered because they have a more diversified genetic make-up. This includes D134 and D64 with six seeds per pod, D134 and D390 with a high yield per plant and the most pods, D92 and D10 with the most seeds per pound, D281 and D13 with the longest racemes, and D229 and D53 with the most racemes. This PCA information reveals traits that contributed most variability in a given germplasm collection which can be utilized for selecting parental material for breeding []. These results were in accordance with findings by Singh, Rajan, Kumar and Soni [] and Venkatesha, et al. [].
Cluster analysis aids in ascertaining genotypes contrasting for different traits which can provide information for lablab improvement. Cluster analysis was unable to discriminate accessions based on their geographic origin an indication that no relationship exist between geographic origin and the genetic diversity of lablab species []. Existing variations could be attributed to genotype, environment and the interaction of genotype and environment. Accessions from cluster I and cluster IV which showed maximum inter-cluster distance are divergent from one other and can be potential parental lines for hybridization and heterosis. The accessions from clusters with minimum inter-cluster distances reveal a close association thus undesirable for recombination during lablab improvement. The cluster analysis findings corroborate previous reports on lablab [,,]. Lablab improvement depends on comprehensive characterization and evaluation of agro-morphological data which generates vital information on genetic diversity and relationships of the germplasm present in Tanzania.
5. Conclusions
Genetic diversity is important when developing varieties with farmer preferred traits. This study acts as a baseline towards breeding and research on genetic diversity of lablab. From this study, a wide variability among studied accessions was observed and could be used to identify accessions with novel traits. Genetic variability is imperative during lablab crop improvement programs. The findings from this study reveal presence of genetic heterogeneity in the morpho-agronomic characters of lablab accessions in Tanzania. Selection of accessions for breeding towards farmer and consumer preffered traits rely on the wide variation in a given germplasm pool. It is critical to use accessions from diverse clusters in selection and hybridization operations. The current information generated from this study forms a baseline for future breeding of lablab. More molecular diversity research is required to supplement these findings and offer a solid foundation for lablab breeding.
Author Contributions
Conceptualization and designing the experiments, P.B.V.; experimentation, data collection, data analysis, and manuscript writing; F.K.L.; supervision, reviewing and editing the manuscript P.A.N.; made the final internal review and revised the final draft of the manuscript, P.B.V. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Regional Universities Forum for Capacity Building in Agriculture (RUFORUM) through the Doctoral Regional Research Grant Number RU/2020/GTA/DRG/024. The research also received partial funding support from Centre for Research, Agricultural Advancement, Teaching Excellence, and Sustainability in Food and Nutrition Security (CREATES-FNS) through the Nelson Mandela African Institution of Science and Technology and the World Bank. Additionally, the research received funding through a capacity building competitive grant training the next generation of scientists provided by the Carnegie Cooperation for New York through the Regional Universities Forum for Capacity Building in Agriculture (RUFORUM).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was funded by the Regional Universities Forum for Capacity Building in Agriculture RUFORUM) through the Graduate Training Assistantship. Additionally, the research received funding through a capacity building competitive grant training the next generation of scientists provided by the Carnegie Cooperation for New York through the Regional Universities Forum for Capacity Building in Agriculture (RUFORUM). The authors also acknowledge the Centre for Research, Agricultural Advancement, Teaching Excellence and Sustainability in Food and Nutrition Security (CREATESFNS), the Nelson Mandela African Institution of Science and Technology and the World Bank).
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Lablab descriptor sheet.
Table A1.
Lablab descriptor sheet.
| 1. Vegetative | |
| 1.1 Emerging cotyledon | 1 = White 2 = Green 3 = Purple |
| 1.2 Hypocotyl colour | 1 = Green 2 = Purple |
| 1.3 Stem pigmentation | 0 = No pigment 3 = Localized to nodes 5 = Extensive 7 = solid |
| 1.4 Leaf vein colour | 1 = Green 2 = Purple |
| 1.5 Leaf colour | 1 = Pale green 3 = Green 5 = Dark green 7 = Purple 9 = Dark Purple |
| 1.6 Leaf hairiness | 0 = Glabrous 3 = Low pubescent 5 = Moderately pubescent 7 = Highly pubescent |
| 1.7 Leaf shape | 1 = Round 3 = Ovate |
| 1.8 Growth habit | 1 = Determinate 2 = Semi determinate 3 = Indeterminate |
| 1.9 Primary branches | Average from 5 randomly chosen plants |
| 1.10 Secondary branches | Average from 5 randomly chosen plants |
| 1.11 Branch orientation | 3 = Short and erect lateral branches 5 = Branches tending to be perpendicular to main stem 7 = First lateral branches long and spreading over ground |
| 2. Inflorescence | |
| 2.1 Days to 50% flowering | Days from sowing to 50 % of the plant produce flower |
| 2.2 Flower bud colour | 1 = white 2 = Cream 3 = Light Yellow 4 = Pink 5 = Purple |
| 2.3 Standard petal colour | 1 = white 2 = Cream 3 = Light Yellow 4 = Pink 5 = Purple |
| 2.4 Wing petal colour | 1 = white 2 = Cream 3 = Light Yellow 4 = Pink 5 = Purple |
| 2.5 Keel petal colour | 1 = white 2 = Cream 3 = Light Yellow 4 = Pink 5 = Purple |
| 2.6 Number of flower buds/raceme | Average of 5 randomly chosen plants |
| 2.7 Number of buds/node | Average of 5 randomly chosen plants |
| 2.8 Number of racemes/plant | Average of 5 randomly chosen plants |
| 2.9 Raceme length | Average of 5 randomly chosen plants (cm) |
| 2.10 Raceme position/emergence | 3 = Within foliage 5 = Intermediate 7 = complete emergence from leaf canopy |
| 3. Fruit | |
| 3.1 Fresh pod curvature | 0 = Straight 3 = Slightly curved 5 = Curved |
| 3.2 Fresh pod pubescence | 0 = Glabrous 3 = Moderately pubescent 5 = Pubescent |
| 3.3 Fresh pod fragrance | 0 = Absent 1 = Low 2 = Medium 3 = high |
| 3.4 Fresh pod length | Average of 5 randomly chosen pods (cm ) |
| 3.5 Fresh pod width | Average of 5 randomly chosen pods (cm ) |
| 3.6 Fresh pod constriction | 0 = No constriction 3 = Slightly constricted 5 = constricted |
| 3.7 Fresh pod colour | 1 = White 2 = Cream 3 = Green 4 = Green with purple suture 5 = Purple 6= Dark Purple 7 = Red |
| 3.8 Fresh pod attachment | 1 = Erect, 2 = Intermediate, 3 = Pendant |
| 3.9 Number of fresh pods/plant | Average number of pods from 10 randomly chosen plants |
| 3.10 Number of locules/ fresh pod | Average of 5 randomly chosen pods |
| 3.11 Number of seeds/fresh pod | Average of 5 randomly chosen pods |
| 3.12 Pod color at physiological maturity | 3 = Tan 5 = Brown 7 = others (specify) |
| 4. Seed | |
| 4.1 Dry seed colour | 1 = White 2 = Green 3 = Cream 3 = Purple 5 = Brown 6 = Black |
| 4.2 Dry seed hilum colour | 1 = White 2 = Tan 3 = Others (specify) |
| 4.3 Dry seed shape | 1 = Round 2 = Oval 3 = Flat 4 = Others (specify) |
| 4.4 Dry 100 seed weight | Average weight of 100 seeds chosen at random (g) |
| 4.5 Dry seed yield/plant | Average of 5 plants chose (g) |
Appendix B

Table A2.
Seed accessions and their places of origin.
Table A2.
Seed accessions and their places of origin.
| S/N | Accession | Place of Origin | S/N | Accession | Place of Origin |
|---|---|---|---|---|---|
| 1 | D139 | Australia | 42 | D350 | China |
| 2 | D56 | Australia | 43 | D68 | China |
| 3 | D61 | Australia | 44 | D165 | Columbia |
| 4 | D62 | Australia | 45 | D112 | Denmark |
| 5 | D73 | Australia | 46 | D353 | ECHO Collection |
| 6 | D74 | Australia | 47 | D354 | ECHO Collection |
| 7 | D75 | Australia | 48 | D355 | ECHO Collection |
| 8 | D76 | Australia | 49 | D356 | ECHO Collection |
| 9 | D77 | Australia | 50 | D358 | ECHO Collection |
| 10 | D78 | Australia | 51 | D359 | ECHO Collection |
| 11 | D79 | Australia | 52 | D360 | ECHO Collection |
| 12 | D80 | Australia | 53 | D361 | ECHO Collection |
| 13 | D81 | Australia | 54 | D362 | ECHO Collection |
| 14 | D83 | Australia | 55 | D363 | ECHO Collection |
| 15 | D84 | Australia | 56 | D365 | ECHO Collection |
| 16 | D85 | Australia | 57 | D367 | ECHO Collection |
| 17 | D128 | Bangladesh | 58 | D368 | ECHO Collection |
| 18 | D28 | Bangladesh | 59 | D369 | ECHO Collection |
| 19 | D30 | Bangladesh | 60 | D370 | ECHO Collection |
| 20 | D31 | Bangladesh | 61 | D371 | ECHO Collection |
| 21 | D32 | Bangladesh | 62 | D372 | ECHO Collection |
| 22 | D34 | Bangladesh | 63 | D373 | ECHO Collection |
| 23 | D35 | Bangladesh | 64 | D374 | ECHO Collection |
| 24 | D37 | Bangladesh | 65 | D375 | ECHO Collection |
| 25 | D38 | Bangladesh | 66 | D376 | ECHO Collection |
| 26 | D39 | Bangladesh | 67 | D377 | ECHO Collection |
| 27 | D40 | Bangladesh | 68 | D378 | ECHO Collection |
| 28 | D41 | Bangladesh | 69 | D379 | ECHO Collection |
| 29 | D43 | Bangladesh | 70 | D381 | ECHO Collection |
| 30 | D45 | Bangladesh | 71 | D382 | ECHO Collection |
| 31 | D46 | Bangladesh | 72 | D383 | ECHO Collection |
| 32 | D47 | Bangladesh | 73 | D384 | ECHO Collection |
| 33 | D48 | Bangladesh | 74 | D385 | ECHO Collection |
| 34 | D49 | Bangladesh | 75 | D387 | ECHO Collection |
| 35 | D50 | Bangladesh | 76 | D390 | ECHO Collection |
| 36 | D51 | Bangladesh | 77 | D142 | Ethiopia |
| 37 | D55 | Cambodia | 78 | D143 | Ethiopia |
| 38 | D64 | Cambodia | 79 | D144 | Ethiopia |
| 39 | D65 | Cambodia | 80 | D145 | Ethiopia |
| 40 | D127 | China | 81 | D146 | Ethiopia |
| 41 | D347 | China | 82 | D147 | Ethiopia |
| 83 | D148 | Ethiopia | 126 | D131 | India |
| 84 | D150 | Ethiopia | 127 | D132 | India |
| 85 | D152 | Ethiopia | 128 | D133 | India |
| 86 | D164 | Ethiopia | 129 | D134 | India |
| 87 | D58 | Ethiopia | 130 | D188 | India |
| 88 | D290 | Farmers collection(Tanzania) | 131 | D193 | India |
| 89 | D291 | Farmers collection(Tanzania) | 132 | D194 | India |
| 90 | D292 | Farmers collection(Tanzania) | 133 | D197 | India |
| 91 | D293 | Farmers collection(Tanzania) | 134 | D199 | India |
| 92 | D294 | Farmers collection(Tanzania) | 135 | D201 | India |
| 93 | D295 | Farmers collection(Tanzania) | 136 | D206 | India |
| 94 | D296 | Farmers collection(Tanzania) | 137 | D212 | India |
| 95 | D297 | Farmers collection(Tanzania) | 138 | D215 | India |
| 96 | D298 | Farmers collection(Tanzania) | 139 | D220 | India |
| 97 | D299 | Farmers collection(Tanzania) | 140 | D229 | India |
| 98 | D300 | Farmers collection(Tanzania) | 141 | D240 | India |
| 99 | D302 | Farmers collection(Tanzania) | 142 | D241 | India |
| 100 | D303 | Farmers collection(Tanzania) | 143 | D242 | India |
| 101 | D304 | Farmers collection(Tanzania) | 144 | D243 | India |
| 102 | D305 | Farmers collection(Tanzania) | 145 | D244 | India |
| 103 | D306 | Farmers collection(Tanzania) | 146 | D245 | India |
| 104 | D308 | Farmers collection(Tanzania) | 147 | D246 | India |
| 105 | D310 | Farmers collection(Tanzania) | 148 | D247 | India |
| 106 | D311 | Farmers collection(Tanzania) | 149 | D248 | India |
| 107 | D312 | Farmers collection(Tanzania) | 150 | D249 | India |
| 108 | D313 | Farmers collection(Tanzania) | 151 | D250 | India |
| 109 | D314 | Farmers collection(Tanzania) | 152 | D251 | India |
| 110 | D315 | Farmers collection(Tanzania) | 153 | D252 | India |
| 111 | D391 | Farmers collection(Tanzania) | 154 | D253 | India |
| 112 | D392 | Farmers collection(Tanzania) | 155 | D254 | India |
| 113 | D393 | Farmers collection(Tanzania) | 156 | D255 | India |
| 114 | D397 | Farmers collection(Tanzania) | 157 | D256 | India |
| 115 | D166 | Germany | 158 | D257 | India |
| 116 | D167 | Germany | 159 | D258 | India |
| 117 | HA3-1 | India | 160 | D259 | India |
| 118 | HA3-2 | India | 161 | D260 | India |
| 119 | HA-4-1 | India | 162 | D261 | India |
| 120 | HA-4-2 | India | 163 | D262 | India |
| 121 | D111 | India | 164 | D263 | India |
| 122 | D119 | India | 165 | D264 | India |
| 123 | D120 | India | 166 | D265 | India |
| 124 | D122 | India | 167 | D266 | India |
| 125 | D126 | India | 168 | D267 | India |
| 169 | D268 | India | 212 | D26 | Lao People’s Democratic Republic |
| 170 | D269 | India | 213 | D69 | Malaysia |
| 171 | D270 | India | 214 | D70 | Malaysia |
| 172 | D271 | India | 215 | D21 | Philippines |
| 173 | D135 | India | 216 | D22 | Philippines |
| 174 | D181 | India | 217 | D24 | Philippines |
| 175 | D272 | India | 218 | D116 | South Africa |
| 176 | D273 | India | 219 | D117 | South Africa |
| 177 | D274 | India | 220 | D104 | Tanzania |
| 178 | D275 | India | 221 | D105 | Tanzania |
| 179 | D276 | India | 222 | D106 | Tanzania |
| 180 | D277 | India | 223 | D107 | Tanzania |
| 181 | D278 | India | 224 | D108 | Tanzania |
| 182 | D279 | India | 225 | D109 | Tanzania |
| 183 | D280 | India | 226 | D156 | Tanzania |
| 184 | D281 | India | 227 | D158 | Tanzania |
| 185 | D282 | India | 228 | D159 | Tanzania |
| 186 | D283 | India | 229 | D160 | Tanzania |
| 187 | D284 | India | 230 | D161 | Tanzania |
| 188 | D285 | India | 231 | D162 | Tanzania |
| 189 | D286 | India | 232 | D163 | Tanzania |
| 190 | D287 | India | 233 | D169 | Tanzania |
| 191 | D288 | India | 234 | D170 | Tanzania |
| 192 | D289 | India | 235 | D172 | Tanzania |
| 193 | D52 | India | 236 | D173 | Tanzania |
| 194 | D53 | India | 237 | D71 | Tanzania |
| 195 | D54 | India | 238 | D72 | Tanzania |
| 196 | D130 | Indonesia | 239 | D95 | Tanzania |
| 197 | D25 | Indonesia | 240 | D1 | Thailand |
| 198 | D174 | Japan | 241 | D10 | Thailand |
| 199 | D176 | Japan | 242 | D11 | Thailand |
| 200 | D183 | Japan | 243 | D13 | Thailand |
| 201 | D185 | Japan | 244 | D14 | Thailand |
| 202 | ELDO-KT-1 | Kenya | 245 | D15 | Thailand |
| 203 | ELDO-KT-2 | Kenya | 246 | D16 | Thailand |
| 204 | D129 | Kenya | 247 | D17 | Thailand |
| 205 | D138 | Kenya | 248 | D19 | Thailand |
| 206 | D168 | Kenya | 249 | D2 | Thailand |
| 207 | D346 | Kenya | 250 | D20 | Thailand |
| 208 | D348 | Kenya | 251 | D3 | Thailand |
| 209 | D349 | Kenya | 252 | D4 | Thailand |
| 210 | D351 | Kenya | 253 | D5 | Thailand |
| 211 | D352 | Kenya | 254 | D6 | Thailand |
| 255 | D7 | Thailand | |||
| 256 | D8 | Thailand | |||
| 257 | D9 | Thailand | |||
| 258 | D319 | TPRI Collection | |||
| 259 | D343 | TPRI Collection | |||
| 260 | D121 | Uganda | |||
| 261 | D113 | Ukraine | |||
| 262 | D102 | Unknown | |||
| 263 | D86 | Unknown | |||
| 264 | D88 | Unknown | |||
| 265 | D89 | Unknown | |||
| 266 | D90 | Unknown | |||
| 267 | D91 | Unknown | |||
| 268 | D92 | Unknown | |||
| 269 | D93 | Unknown | |||
| 270 | D94 | Unknown | |||
| 271 | D96 | Unknown | |||
| 272 | D98 | Unknown | |||
| 273 | D66 | Uzbekistan | |||
| 274 | D67 | Vietnam | |||
| 275 | D124 | Zambia | |||
| 276 | D125 | Zambia | |||
| 277 | D123 | Zimbabwe |
References
- FAO; UNICEF; WFP; WHO. Transforming Food Systems for Affordable Healthy Diets; FAO: Rome, Italy, 2021; p. 240.
- Li, X.; Yadav, R.; Siddique, K.H.M. Neglected and Underutilized Crop Species: The Key to Improving Dietary Diversity and Fighting Hunger and Malnutrition in Asia and the Pacific. Front. Nutr. 2020, 7, 593711. [Google Scholar] [CrossRef] [PubMed]
- Kilonzi, S.M. Physicochemical and Functional Characterisation of Three Lablab Bean (Lablab purpureus L.(Sweet)) Varieties Grown in Kenya. Ph.D. Dissertation, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya, 2020. Available online: http://localhost/xmlui/handle/123456789/5409 (accessed on 4 September 2022).
- Purwanti, E.; Prihanta, W.; Fauzi, A. Nutritional Content Characteristics of Dolichos lablab L. Accessions in Effort to Investigate Functional Food Source. In Proceedings of the 6th International Conference on Community Development (ICCD 2019), Bandar Seri Begawan, Indonesia, October 2019; Available online: https://www.atlantis-press.com/proceedings/iccd-19/125919047 (accessed on 4 September 2022).
- Letting, F.K.; Venkataramana, P.B.; Ndakidemi, P.A. Breeding potential of lablab [Lablab purpureus (L.) Sweet]: A Review on Characterization and Bruchid Studies towards Improved Production and Utilization in Africa. Genet. Resour. Crop Evol. 2021, 68, 3081–3101. [Google Scholar] [CrossRef]
- Kimno, S.; Kinyua, M.; Pkania, K.; Emmy, C. Evaluation of Proximate and Mineral Composition of Mutant Dolichos Lablab (Lablab purpureus L. Sweet) Accessions in Kenya. J. Exp. Agric. Int. 2021, 43, 72–80. [Google Scholar] [CrossRef]
- Sulaiman, A.; Lawal, A. A proximate, mineral composition and anti-nutritional factors of the aerial parts of Lablab purpureus (L.) sweet. Bayero J. Pure Appl. Sci. 2018, 11, 37–40. [Google Scholar] [CrossRef]
- Naeem, M.; Shabbir, A.; Ansari, A.A.; Aftab, T.; Khan, M.M.A.; Uddin, M. Hyacinth bean (Lablab purpureus L.)–An underutilised crop with future potential. Sci. Hortic. 2020, 272, 109551. [Google Scholar] [CrossRef]
- Kilonzi, S.M.; Makokha, A.O.; Kenji, G.M. Physical characteristics, proximate composition and anti-nutritional factors in grains of lablab bean (Lablab purpureus) genotypes from Kenya. J. Appl. Biosci. 2017, 114, 11289–11298. [Google Scholar] [CrossRef]
- Morrison, M.M. Nutrient Composition of Cooked Lablab Bean Varieties for Improving Nutrition and Food Security in Tanzania. Ph.D. Dissertation, Sokoine University of Agriculture, Morogoro, Tanzania, 2019. Available online: https://www.suaire.sua.ac.tz/handle/123456789/3045 (accessed on 4 September 2022).
- Tulu, A.; Yadav, K.R.; Geleti, D. Supplementary value of two Lablab purpureus cultivars and concentrate mixture to natural grass hay basal diet based on feed intake, digestibility, growth performance and net return of Horro sheep. Int. J. Livest. Prod. 2018, 9, 140–150. [Google Scholar] [CrossRef]
- Ishiaku, Y.; Hassan, M.; Tanko, R.; Abdu, S.; Musa, A. Feed Quality of Silage made fromForage Sorghum (Sorghum almum Parodi) and Lablab (Lablab purpureus L. Sweet) in Shika, Nigeria. Niger. J. Anim. Sci. Technol. 2020, 3, 91–95. [Google Scholar]
- Wangila, A.J.; Gachuiri, C.K.; Muthomi, J.W.; Ojiem, J.O. Biomass yield and quality of fodder from selected varieties of lablab. Online J. Anim. Feed. Res. 2021, 11, 28–35. [Google Scholar] [CrossRef]
- Bekele, B.; Ademe, D.; Gemi, Y.; Habtemariam, T. Evaluation of Intercropping Legume Covers with Maize on Soil Moisture Improvement in Misrak Azerinet Berbere woreda, SNNPR, Ethiopia. Water Conserv. Sci. Eng. 2021, 6, 145–151. [Google Scholar] [CrossRef]
- Nord, A.; Miller, N.R.; Mariki, W.; Drinkwater, L.; Snapp, S. Investigating the diverse potential of a multi-purpose legume, Lablab purpureus (L.) Sweet, for smallholder production in East Africa. PLoS ONE 2020, 15, e0227739. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.K.; Rai, N.; Pandey-Rai, S. Unlocking Pharmacological and Therapeutic Potential of Hyacinth Bean (Lablab purpureus L.). In Role of OMICS Based Biology, Biotic and Abiotic Elicitors; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Shahed-Al-Mahmud, M.; Chen, X.; Chen, T.-H.; Liao, K.-S.; Lo, J.M.; Wu, Y.-M.; Ho, M.-C.; Wu, C.-Y.; Wong, C.-H. A carbohydrate-binding protein from the edible Lablab beans effectively blocks the infections of influenza viruses and SARS-CoV-2. Cell Rep. 2020, 32, 108016. [Google Scholar] [CrossRef] [PubMed]
- Jibat, G.; Gerkabo, H. Effects of green manure legumes and their termination time on yield of maize and soil chemical properties. Arch. Agron. Soil Sci. 2021, 67, 1–13. [Google Scholar] [CrossRef]
- Okumu, O.O.; Muthomi, J.; Ojiem, J.; Narla, R.; Nderitu, J. Effect of Lablab Green Manure on Population of Soil Microorganisms and Establishment of Common Bean (Phaseolus vulgaris L.). Am. J. Agric. Sci. 2018, 5, 44–54. [Google Scholar]
- Missanga, J.S.; Venkataramana, P.B.; Ndakidemi, P.A. Recent developments in Lablab purpureus genomics: A focus on drought stress tolerance and use of genomic resources to develop stress-resilient varieties. Legume Sci. 2021, 3, e99. [Google Scholar] [CrossRef]
- Grotelüschen, K. Lablab purpureus (L.) Sweet: A Promising Multipurpose Legume for Enhanced Drought Resistance and Improved Household Nutritional Status in Smallholder Farming Systems of Eastern Kenya. Ph.D. Dissertation, Georg-August University, Göttingen, Germany, 2014. [Google Scholar]
- Forsythe, C. Exploring the Viability of Re-Introducing Lablab purpureus (L.) Sweet as a Multifunctional Legume in Northern Tanzania. Ph.D. Dissertation, Swedish University of Agricultural Sciences, Alnarp, Sweden, 2019. [Google Scholar]
- Letting, F.K.; Venkataramana, P.B.; Ndakidemi, P.A. Farmers’ Participatory Plant Selection of Lablab [Lablab purpureus (L.) Sweet] in Tanzania. Front. Plant Sci. 2022, 13, 1429. [Google Scholar] [CrossRef]
- Miller, N.; Mariki, W.; Nord, A.; Snapp, S. Cultivar selection and management strategies for Lablab purpureus (L.) Sweet in Africa. In Handbook of Climate Change Resilience; Filho, W.L., Ed.; Springer Nature Switzerland AG: Gewerbestrasse, Switzerland, 2018; Volume 2, pp. 1–14. [Google Scholar]
- Morris, J.B. Morphological and Reproductive Characterization in Hyacinth Bean, Lablab purpureus (L.) Sweet Germplasm with Clinically Proven Nutraceutical and Pharmaceutical Traits for Use as a Medicinal Food. J. Diet. Suppl. 2009, 6, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Rahman, M.M.; Hossain, T. Physico-morphological variation in hyacinth bean [Lablab purpureus (L.) Sweet]. Bangladesh J. Agric. Res. 2010, 35, 431–438. [Google Scholar] [CrossRef][Green Version]
- Shrikrishna, P.; Ramesh, S. Visually assayable morphological descriptors-based establishment of distinctiveness [D], uniformity [U] and stability [S] of dolichos bean (Lablab purpureus L. Sweet var. Lignosus) genotypes. Plant Genet. Resour. 2020, 18, 1–4. [Google Scholar] [CrossRef]
- Kumar, A. Quantitative analysis of some germplasms of lablab bean in Uttar Pradesh. Int. J. Environ. Agric. Biotechnol. 2017, 2, 238643. [Google Scholar] [CrossRef]
- Vishnu, V.S.; Radhamany, P.M. Assessment of variability in Lablab purpureus (L.) Sweet germplasm based on quantitative morphological and biochemical traits. Genet. Resour. Crop Evol. 2022, 69, 1535–1546. [Google Scholar] [CrossRef]
- Ram, K.C.; Joshi, B.; Dahal, S. Diversity Analysis and Physico-Morphlogical Characteritics of Indigenous Germplasm of Lablab Bean. J. Nepal Agric. Res. Counc. 2016, 2, 15. [Google Scholar] [CrossRef][Green Version]
- Ngure, D.; Kinyua, M.; Kiplagat, O. Morphological and microsatellite characterization of improved Lablab purpureus genotypes. J. Plant Breed. Crop Sci. 2021, 13, 23–34. [Google Scholar]
- Chawe, K. Morphological Screening and Farmers’ Acceptability of Selected Lablab Bean (Lablab purpureus) Accessions in Moshi District, Tanzania. Ph.D. Dissertation, NM-AIST, Arusha, Tanzania, 2019. Available online: https://dspace.nm-aist.ac.tz/bitstream/handle/20.500.12479/245/MSc_LiSE_Kissa_Chawe_2019.pdf?sequence=1&isAllowed=y (accessed on 4 September 2022).
- Massawe, P.I.; Mtei, K.M.; Munishi, L.K.; Ndakidemi, P.A. Improving soil fertility and crops yield through maize-legumes (common bean and Dolichos lablab) intercropping systems. J. Agric. Sci. 2016, 8, 148–163. [Google Scholar] [CrossRef]
- Meya, A.; Ndakidemi, P.; Mtei, K.; Swennen, R.; Merckx, R. Optimizing Soil Fertility Management Strategies to Enhance Banana Production in Volcanic Soils of the Northern Highlands, Tanzania. Agronomy 2020, 10, 289. [Google Scholar] [CrossRef]
- International FertilizerI Development Centre IFDC. Tanzania Fertilizer Assessment; International FertilizerI Development Centre IFDC: Dar es Salaam, Tanzania, 2012. [Google Scholar]
- IASRI. Augmented Block Designs. Available online: http://www.iasri.res.in/design/AugmentedDesigns/ (accessed on 6 June 2019).
- Byregowda, M.; Girish, G.; Ramesh, S.; Mahadevu, P.; Keerthi, C. Descriptors of Dolichos bean (Lablab purpureus L.). J. Food Legumes 2015, 28, 203–214. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Sarif, H.; Rafii, M.; Ramli, A.; Yusuff, O.; Musa, H.; Rahim, H.; Mohd, Z.; Chukwu, S.; Samuel, Z. Genetic diversity and variability among pigmented rice germplasm using molecular marker and morphological traits. Biotechnol. Biotechnol. Equip. 2020, 34, 747–762. [Google Scholar] [CrossRef]
- Mat Sulaiman, N.N.; Rafii, M.; Duangjit, J.; Izan, S.; Phumichai, C.; Yusuff, O.; Datta, D.; Musa, I. Genetic Variability of Eggplant Germplasm Evaluated under Open Field and Glasshouse Cropping Conditions. Agronomy 2020, 10, 436. [Google Scholar] [CrossRef]
- Govindaraj, M.; Vetriventhan, M.; M, S. Importance of Genetic Diversity Assessment in Crop Plants and Its Recent Advances: An Overview of Its Analytical Perspectives. Genet. Res. Int. 2015, 2, 431487. [Google Scholar] [CrossRef]
- Fu, Y.-B. Understanding crop genetic diversity under modern plant breeding. Theor. Appl. Genet. 2015, 128, 2131–2142. [Google Scholar] [CrossRef]
- Vaijayanthi, P.V.; Ramesh, S.; Gowda, M.; Mohan Rao, A.; Gowda, J.; Ramappa, H.K.; Keerthi, C.M.; Rajendra Prasad, B.S. Development and Validation of a Core Set of Dolichos Bean Germplasm. Int. J. Veg. Sci. 2014, 21, 150527094502000. [Google Scholar] [CrossRef]
- Jennings, J.; Foster, J. Legume Structure and Morphology. In Forages: The Science of Grassland Agriculture, 7th ed.; Kenneth, M.C., Moore, J., Nelson, C.J., Redfearn, D.D., Eds.; John Wiley and Sons Ltd: Hoboken, NJ, USA, 2020; Volume 2, pp. 51–64. [Google Scholar]
- Vaijayanthi, P.; Ramesh, S.; Gowda, M.B.; Rao, A.M.; Keerthi, C.; Reena, G. Genetic variability for morpho-metric traits in Dolichos bean (Lablab purpureus L. Sweet) germplasm. J. Food Legumes 2015, 28, 5–10. [Google Scholar] [CrossRef][Green Version]
- Jagadeesh, B.; CS, B.M.; Girish, G. Screening of Dolichos germplasm for pod borers and bruchids. Environ. Ecol. 2008, 26, 2288–2290. [Google Scholar]
- Keerthi, C.; Ramesh, S.; Byregowda, M.; Rao, A.M.; Prasad, B.R.; Vaijayanthi, P. Genetics of growth habit and photoperiodic response to flowering time in dolichos bean (Lablab purpureus (L.) Sweet). J. Genet. 2014, 93, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Whitbread, A.; Ayisi, K.; Mabapa, P.; Odhiambo, J.; Maluleke, N.; Pengelly, B.C. Evaluating Lablab purpureus (L.) Sweet germplasm to identify short-season accessions suitable for crop and livestock farming systems in southern Africa. Afr. J. Range Forage Sci. 2011, 28, 21–28. [Google Scholar] [CrossRef]
- Krylova, E.; Khlestkina, E.; Burlyaeva, M.; Vishnyakova, M. Determinate growth habit of grain legumes: Role in domestication and selection, genetic control. Ecol. Genet. 2020, 18, 43–58. [Google Scholar] [CrossRef]
- Keerthi, C.; Ramesh, S.; Byregowda, M.; Rao, A.M.; Rajendra Prasad, B.S.; Vaijayanthi, P.V. Further evidence for the genetic basis of qualitative traits and their linkage relationships in dolichos bean (Lablab purpureus L.). J. Genet. 2016, 95, 89–98. [Google Scholar] [CrossRef]
- Kamau, E.; Kinyua, M.; Waturu, C.; Karanja, D. Genotypic variability, heritability and path analysis of yield components of determinate lablab (Lablab purpureus (L.) Sweet) inbred lines in Kenya. Afr. J. Plant Sci. 2021, 15, 266–276. [Google Scholar] [CrossRef]
- Ewansiha, S.; Chiezey, U.F.; Tarawali, S.; Iwuafor, E. Morpho-phenological variation in Lablab purpureus. Trop. Grassl. 2007, 41, 277–284. [Google Scholar]
- Uday, K.H.R.; Gowda, B.M.; Ramesh, S.; Vasundhara, M. Characterization and Identification of Dolichos Bean (Lablab purpureus L. sweet) Recombinant Inbred Lines (RIL) with High Pod Yield and High Pod Fragrance. Int. J. Pure Appl. Biosci. 2017, 5, 428–436. [Google Scholar] [CrossRef]
- Patil, A.S.; Popovsky, S.; Levy, Y.; Chu, Y.; Clevenger, J.; Ozias-Akins, P.; Hovav, R. Genetic insight and mapping of the pod constriction trait in Virginia-type peanut. BMC Genet 2018, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-X.; Hartman, G.L. Characterization of Insect Resistance Loci in the USDA Soybean Germplasm Collection Using Genome-Wide Association Studies. Front. Plant Sci. 2017, 8, 670. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Abhilash, P. Varietal dataset of nutritionally important Lablab purpureus (L.) sweet from Eastern Uttar Pradesh, India. Data Brief 2019, 24, 103935. [Google Scholar] [CrossRef] [PubMed]
- Maass, B.L.; Usongo, M.F. Changes in seed characteristics during the domestication of the lablab bean (Lablab purpureus (L.) Sweet: Papilionoideae). Aust. J. Agric. Res. 2007, 58, 9–19. [Google Scholar] [CrossRef]
- Serpico, D. Beyond quantitative and qualitative traits: Three telling cases in the life sciences. Biol. Philos. 2020, 35, 34. [Google Scholar] [CrossRef]
- Savitha, B.N.; Ravikumar, R.L.; Shinde, D.G. Characterization and genetic diversity analysis in field bean [Lablab purpureus (L.) Sweet] collections of Karnataka. J. Food Legumes 2012, 25, 18–24. [Google Scholar]
- Kumar, U.; Prasad, K.; Tiwari, R.; Ghosh, S.; Sinha, B.; Yadav, L. Estimation of Genetic Variability and Genetic Divergence in Dolichos Bean [Lablab purpureus (L.) Sweet.] Genotypes. Legume Res. Int. J. 2021, 44, 916–920. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, A.; Dhillon, N.; Jamwal, M. Genetic variability of morphological and yield traits in Dolichos bean (Lablab purpureus L.). Afr. J. Agric. Res. 2013, 8, 1022–1027. [Google Scholar] [CrossRef]
- Hadavani, J.K.; Mehta, D.R.; Lata, J.R.; Ghetiya, K.P. Genetic Variability Parameters in Indian Bean (Lablab purpureus L.). Int. J. Pure Appl. Biosci. 2018, 6, 164–168. [Google Scholar] [CrossRef]
- Nkhata, W.; Shimelis, H.; Melis, R.; Chirwa, R.; Mzengeza, T.; Mathew, I.; Shayanowako, A. Population structure and genetic diversity analyses of common bean germplasm collections of East and Southern Africa using morphological traits and high-density SNP markers. PLoS ONE 2020, 15, e0243238. [Google Scholar] [CrossRef] [PubMed]
- Gadissa, F.; Abebe, M.; Bekele, T. Agro-morphological traits-based genetic diversity assessment in Ethiopian barley (Hordeum vulgare L.) landrace collections from Bale highlands, Southeast Ethiopia. Agric. Food Secur. 2021, 10, 58. [Google Scholar] [CrossRef]
- Singh, S.; Rajan, S.; Kumar, D.; Soni, V.K. Genetic Diversity Assessment in Dolichos Bean (Lablab purpureus L.) Based on Principal Component Analysis and Single Linkage Cluster Analysis. Legume Res. Int. J. 2021, 1, LR-4561. [Google Scholar] [CrossRef]
- Shibli, M.; Rasul, M.G.; Islam, A.; Saikat, M.; Haque, M.M. Genetic diversity of country bean (Lablab purpureus) genotypes collected from the coastal regions of Bangladesh. J. Hortic. Postharvest Res. 2021, 4, 93–104. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Felenji, H. Evaluating Diversity among Potato Cultivars Using Agro-Morphological and Yield Components in Fall Cultivation of Jiroft Area. Am. Eurasian J. Agric. Environ. Sci. 2011, 11, 655–662. [Google Scholar]
- Chinnegowda, V.S.; Ganapathy, K.N.; Gowda, M.B.; Gowda, P.H.R.; Mahadevu, P.; Govindappa, G.; Chandrashekar, A. Variability and Genetic Structure among Lablab Bean Collections of India and their Relationship with Exotic Accessions. Vegetos Int. J. Plant Res. 2013, 26, 121. [Google Scholar] [CrossRef]
- Magalingam, V.; Yassin, M.; Selvan, R. Genetic Variability and Character Association in Dolichos Bean. SAARC J. Agric. 2014, 11, 161–171. [Google Scholar] [CrossRef]
- Raj, N.; Anitha, P.; Pradeepkumar, T.; Jose, D.; Beena, V.; Laly, C. Genetic Divergence Analysis in Dolichos Bean (Lablab purpureus var. typicus). Res. J. Agric. Sci. 2021, 12, 195–199. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).