An Evaluation of the Dough Rheology and Bread Quality of Replacement Wheat Flour with Different Quinoa Particle Sizes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Flours
2.3. The Process of Bread Making
2.4. Dough and Bread Characteristics Analysis
2.4.1. Dough Rheological Measurements
2.4.2. Bread Physical and Textural Parameters
2.5. Factorial Design and Statistics
3. Results
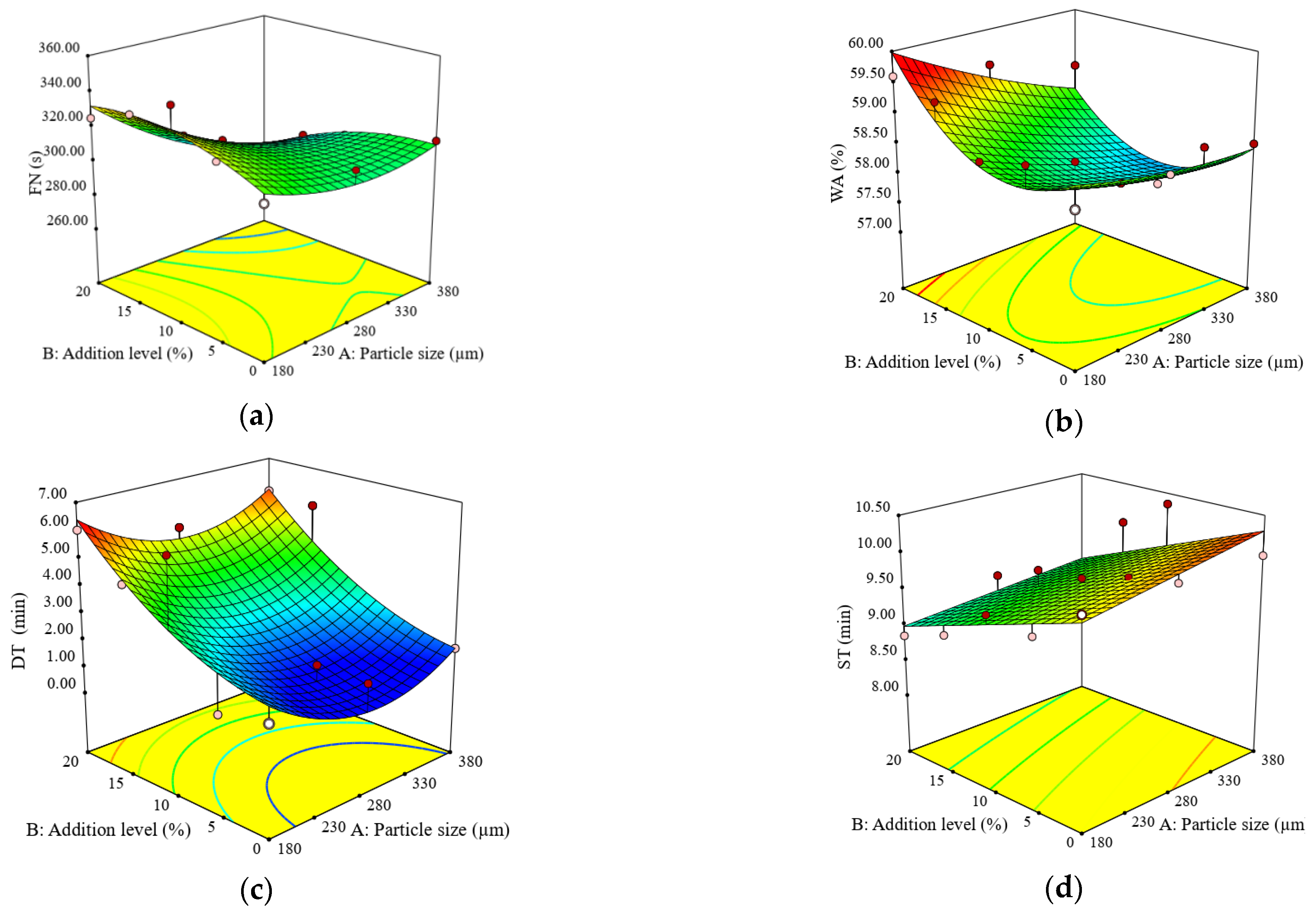
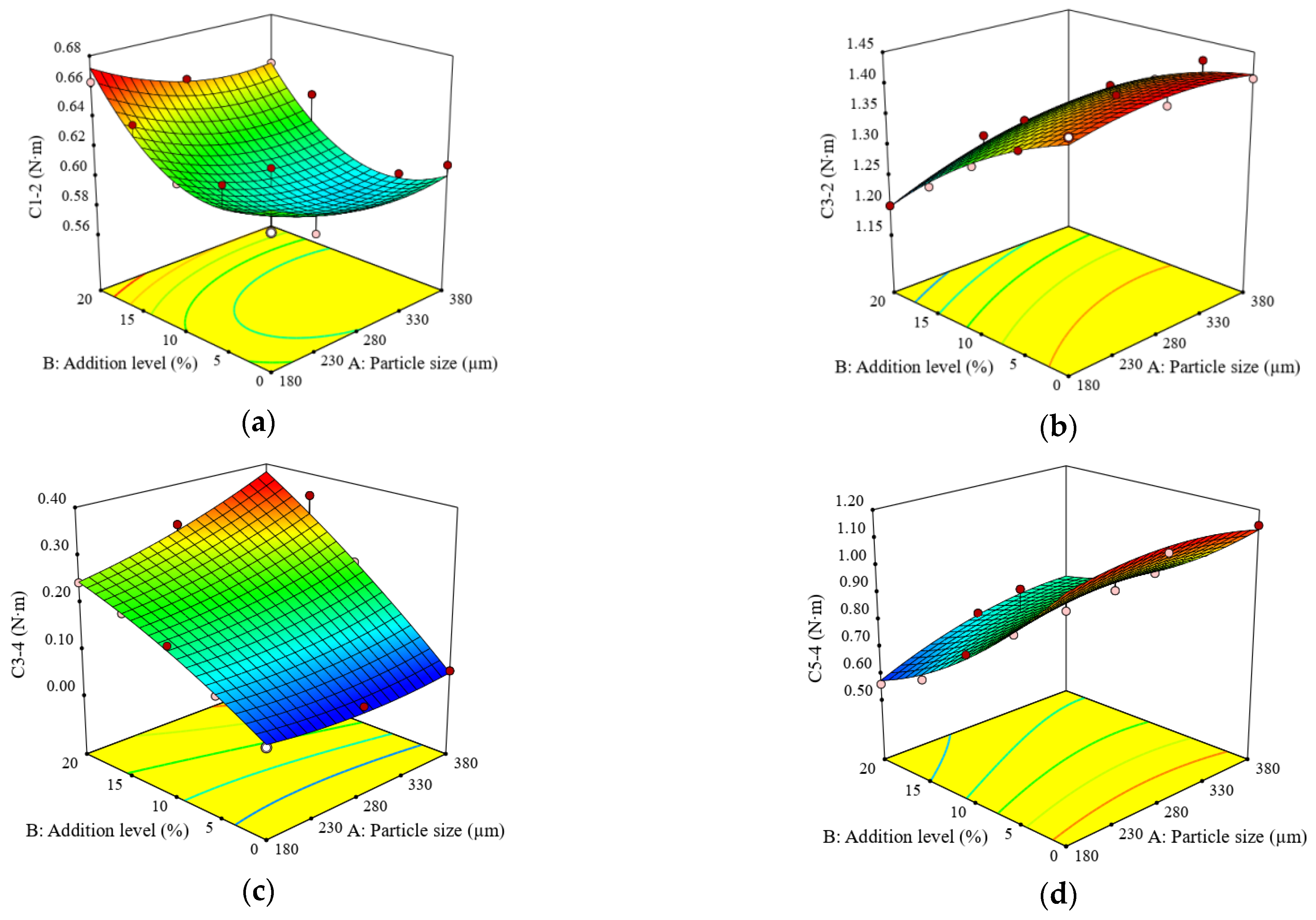
3.1. Impact of Particle Size and Addition Level of Quinoa Flour in Wheat Flour on FN Index, Dough Rheological Properties during Mixing and Heating-Cooling Cycle, and Dough Biaxial Extension
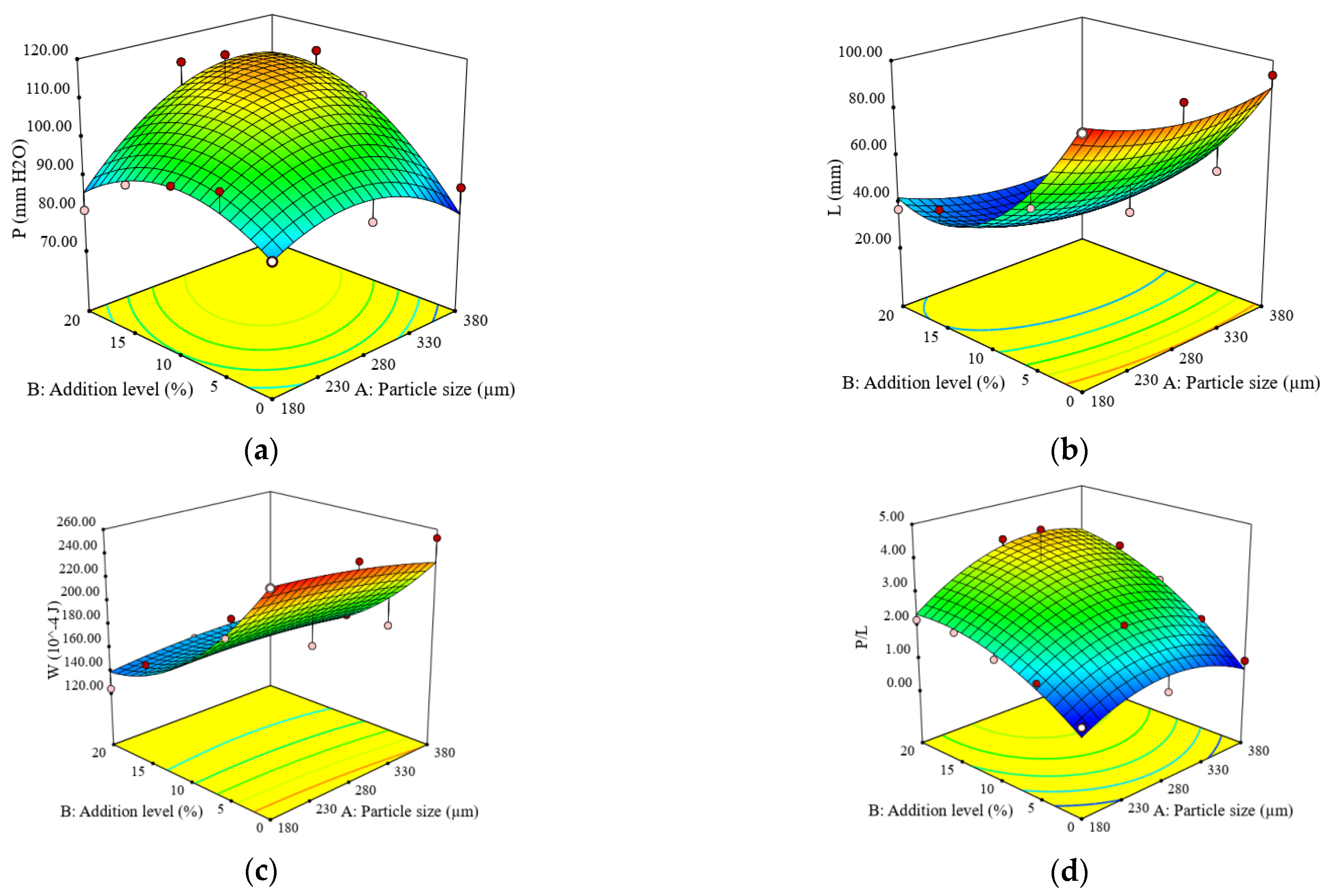
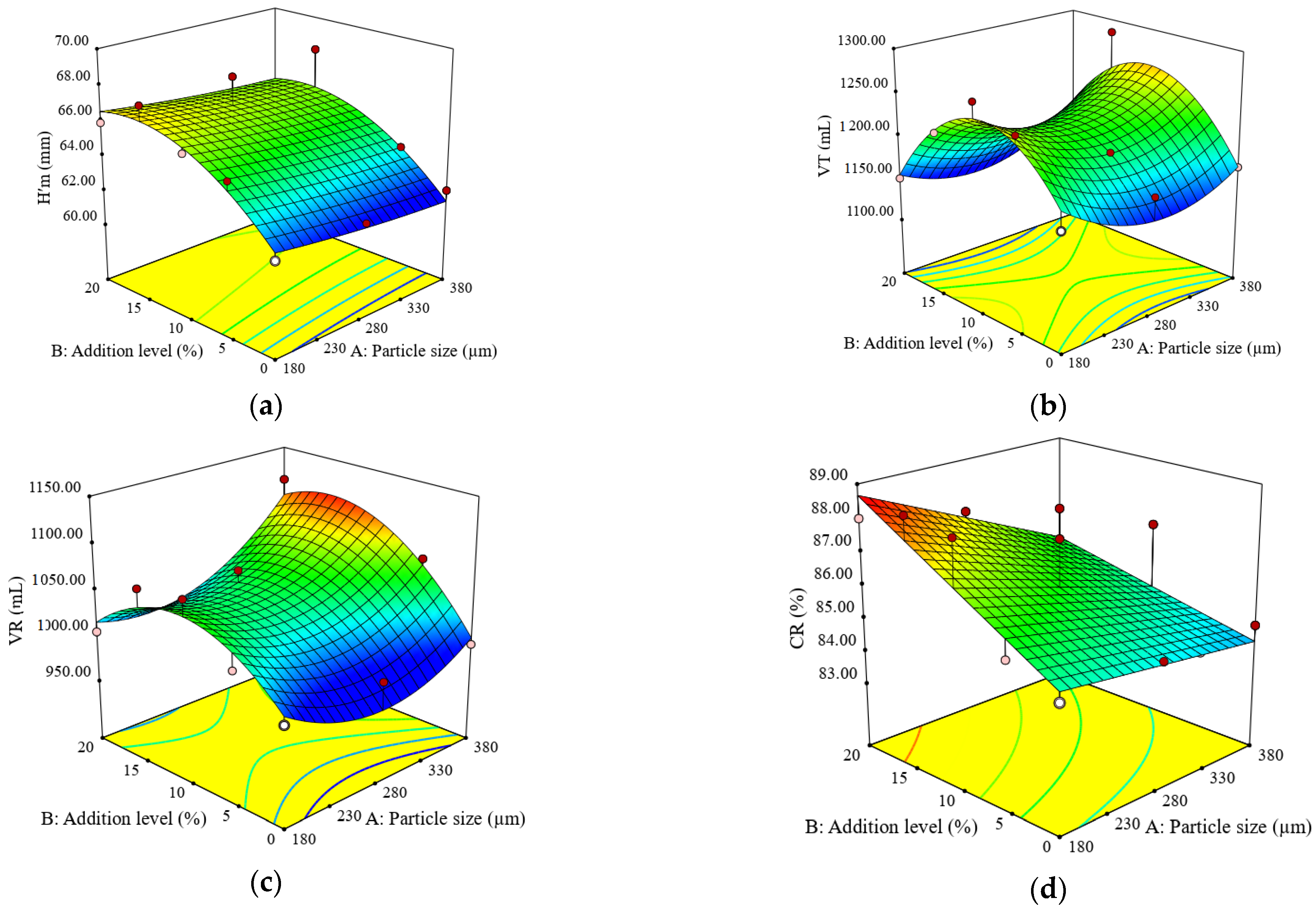
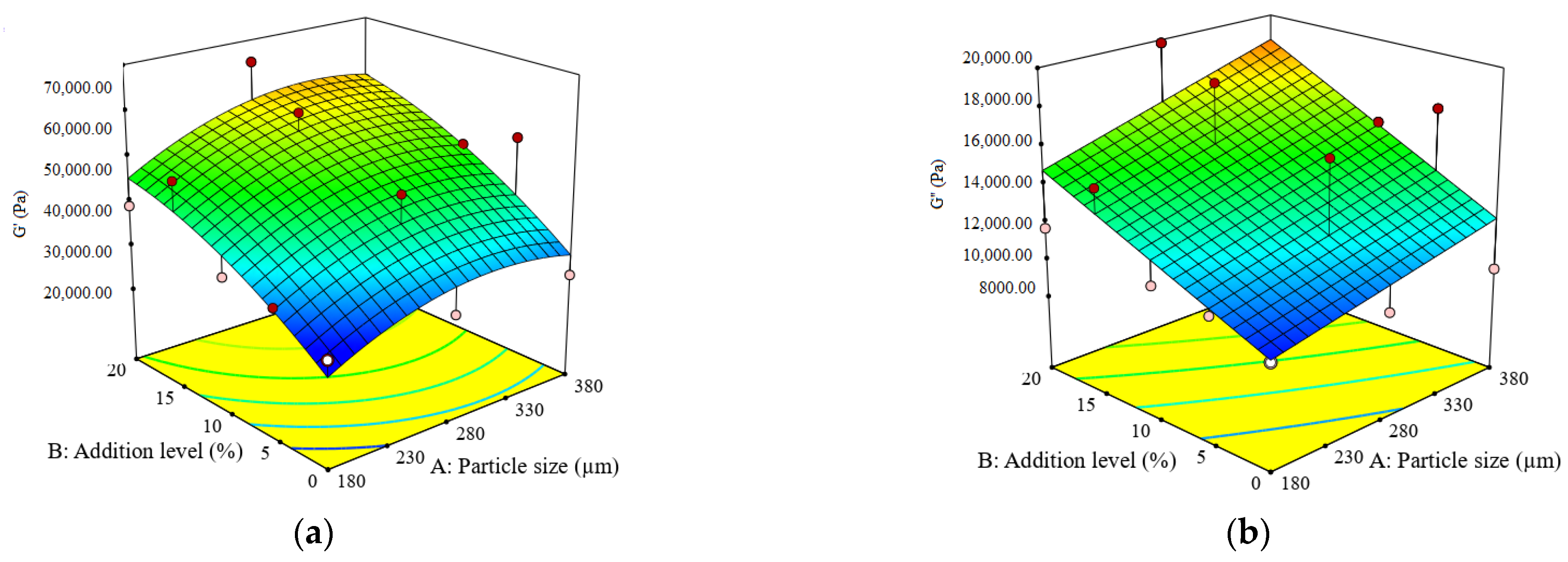
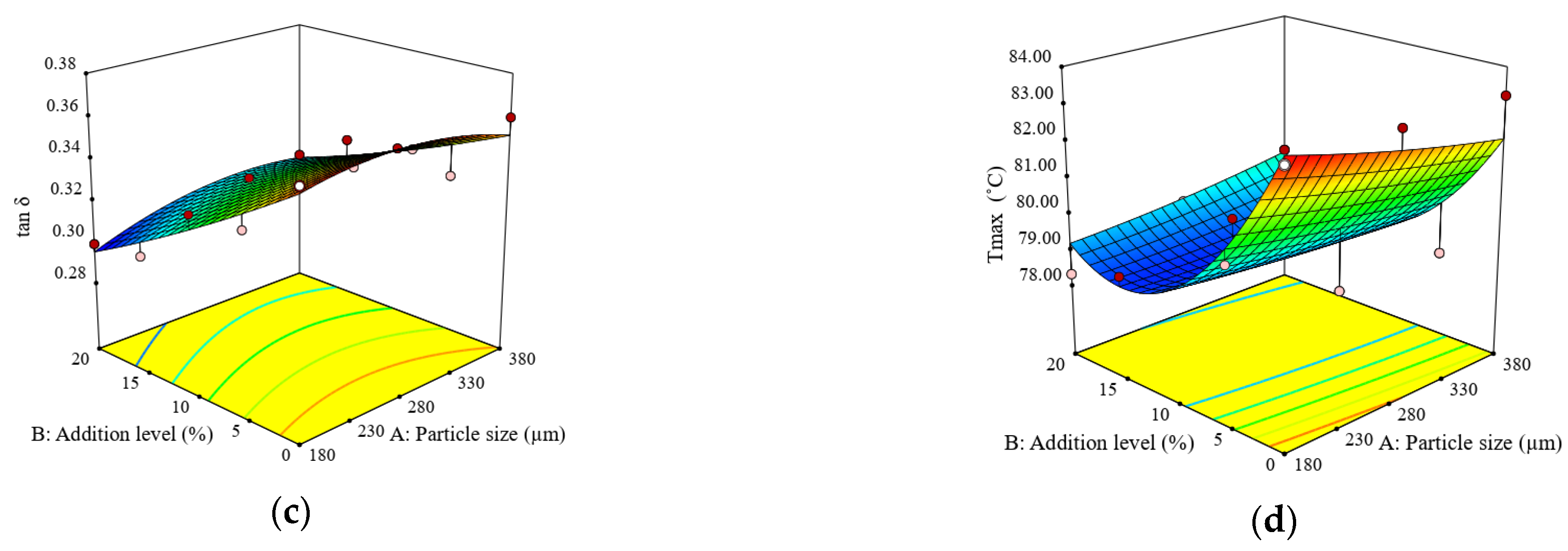
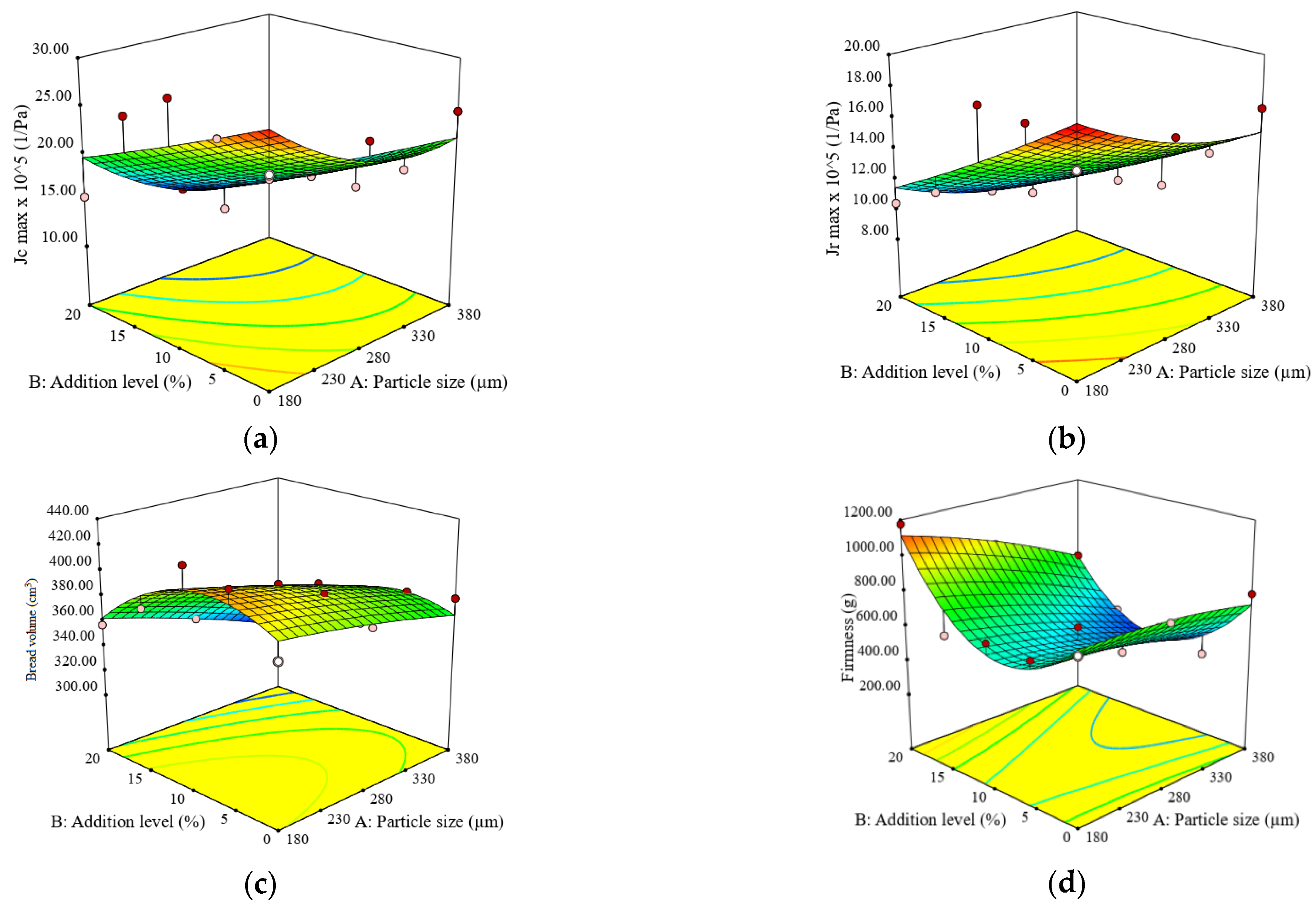
3.2. Impact of Particle Size and Addition Level of Quinoa Flour in Wheat Flour on Dough Fermentation, Dynamic Rheological Properties, and Bread Characteristics
3.3. Optimization of the Addition Level for the Particle Sizes of the Studied Quinoa Flour and Evaluation of the Baking Characteristics of the Wheat-Quinoa Composite Flour and the Quality of the Bread
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz-Penella, J.M.; Wronkowska, M.; Soral-Smietana, M.; Haros, M. Effect of whole amaranth flour on bread properties and nutritive value. LWT-Food Sci. Technol. 2013, 50, 679–685. [Google Scholar] [CrossRef]
- Iglesias-Puig, E.; Monedero, V.; Haros, M. Bread with whole quinoa flour and bifidobacterial phytases improve contribution to dietary mineral intake and their bioavailability without substantial loss of bread quality. LWT–Food Sci. Technol. 2015, 60, 71–77. [Google Scholar] [CrossRef]
- Ramesh, D.; Prakash, J. Nutritional and functional properties of amaranth grain flour fractions obtained by differential sieving. Prog. Chem. Biochem. Res. 2020, 3, 272–286. [Google Scholar]
- Zhang, D.; Moore, W.R. Wheat bran particle size effects on bread baking performance and quality. J. Sci. Food Agric. 1999, 79, 805–809. [Google Scholar] [CrossRef]
- Noort, M.W.J.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality–Evidence for fibre–protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Cai, L.; Choi, I.; Hyun, J.N.; Jeong, Y.K.; Baik, B.K. Influence of bran particle size on bread-baking quality of whole grain wheat flour and starch retrogradation. Cereal Chem. 2014, 91, 65–71. [Google Scholar] [CrossRef]
- Li, S.H.; Zhao, W.; Li, P.L.; Min, G.; Zhang, A.X.; Zhang, J.L.; Liu, J.K. Effects of different cultivars and particle sizes of non-degermed millet flour fractions on the physical and texture properties of Chinese steamed bread. Cereal Chem. 2020, 97, 661–669. [Google Scholar] [CrossRef]
- Coţovanu, I.; Mironeasa, C.; Mironeasa, S. Insights into the Potential of Buckwheat Flour Fractions in Wheat Bread Dough. Appl. Sci. 2022, 12, 2302. [Google Scholar] [CrossRef]
- Coţovanu, I.; Mironeasa, S. Features of Bread Made from Different Amaranth Flour Fractions Partially Substituting Wheat Flour. Appl. Sci. 2022, 12, 897. [Google Scholar] [CrossRef]
- Niu, M.; Hou, G.G.; Wang, L.; Chen, Z. Effects of superfine grinding on the quality characteristics of whole-wheat flour and its raw noodle product. J. Cereal Sci. 2014, 60, 382–388. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Liu, B.; Zhao, H.; Zhou, F.; Zhang, B. An external addition of soy protein isolate hydrolysate to sourdough as a new strategy to improve the quality of chinese steamed bread. J. Food Qual. 2016, 39, 3–12. [Google Scholar] [CrossRef]
- Ahmed, J.; Ptaszek, P.; Basu, S. Influence of fibers and particle size distribution on Food Rheology. In Advances in Food Rheology and Its Applications; Woodhead Publishing: Cambridge, UK, 2016. [Google Scholar]
- Penella, J.S.; Collar, C.; Haros, M. Effect of wheat bran and enzyme addition on dough functional performance and phytic acid levels in bread. J. Cereal Sci. 2008, 48, 715–721. [Google Scholar] [CrossRef]
- Coțovanu, I.; Batariuc, A.; Mironeasa, S. Characterization of Quinoa Seeds Milling Fractions and Their Effect on the Rheological Properties of Wheat Flour Dough. Appl. Sci. 2020, 10, 7225. [Google Scholar] [CrossRef]
- Coţovanu, I.; Ungureanu-Iuga, M.; Mironeasa, S. Investigation of Quinoa Seeds Fractions and Their Application in Wheat Bread Production. Plants 2021, 10, 2150. [Google Scholar] [CrossRef]
- Xiong, L.; Zhang, B.; Niu, M.; Zhao, S. Protein polymerization and water mobility in whole-wheat dough influenced by bran particle size distribution. LWT-Food Sci. Technol. 2017, 82, 396–403. [Google Scholar] [CrossRef]
- Ahmed, J.; Thomas, L.; Arfat, Y.A. Functional, rheological, microstructural and antioxidant properties of quinoa flour in dispersions as influenced by particle size. Food Res. Int. 2019, 116, 302–311. [Google Scholar] [CrossRef]
- Wang, X.; Lao, X.; Bao, Y.; Guan, X.; Li, C. Effect of whole quinoa flour substitution on the texture and in vitro starch digestibility of wheat bread. Food Hydrocoll. 2021, 119, 106840. [Google Scholar] [CrossRef]
- Koziol, M.J. Quinoa: A potential new oil crop. In New Crops; Janick, J., Simon, J.E., Eds.; Wiley: New York, NY, USA, 1993; pp. 328–336. [Google Scholar]
- Steffolani, M.E.; León, A.E.; Pérez, G.T. Study of the physicochemical and functional characterization of quinoa and kañiwa starches. Stärke 2013, 65, 976–983. [Google Scholar] [CrossRef]
- Demin, M.A.; Vucelic-Radovic, B.V.; Banjac, N.R. Buckwheat and Quinoa seeds as supplements in wheat bread production. Hem. Ind. 2013, 67, 115–121. [Google Scholar] [CrossRef]
- Poutanen, K.; Sozer, N.; Della Valle, G. How can technology help to deliver more of grain in cereal foods for a healthy diet? J. Cereal Sci. 2014, 59, 327–336. [Google Scholar] [CrossRef]
- SR 90:2007; Wheat Flour. Analysis Method. Romanian Standards Association: Bucharest, Romania, 2007.
- ICC. Standard Methods of the International Association for Cereal Chemistry. Methods 104/1, 110/1, 136, 105/2, 171, 121, 107/1, 173; International Association for Cereal Chemistry: Vienna, Austria, 2010. [Google Scholar]
- Coțovanu, I.; Mironeasa, S. Impact of different amaranth particle size addition level on wheat flour dough rheology and bread features. Foods 2021, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Coțovanu, I.; Stoenescu, G.; Mironeasa, S. Amaranth Influence on Wheat Flour Dough Rheology: Optimal Particle Size and Amount of Flour Replacement. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 366–373. [Google Scholar] [CrossRef]
- Rasper, V.F.; Walker, C.E. Quality evaluation of cereals and cereal products. In Handbook of Cereal Science and Technology; Kulp, K., Ponte, J.G., Eds.; Marcel Dekker: New York, NY, USA, 2000; Chapter 16. [Google Scholar]
- Iuga, M.; Mironeasa, C.; Mironeasa, S. Oscillatory rheology and creep-recovery behaviour of grape seed-wheat flour dough: Effect of grape seed particle size, variety and addition level. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2019, 76, 40–51. [Google Scholar] [CrossRef]
- Iuga, M.; Boestean, O.; Ghendov-Mosanu, A.; Mironeasa, S. Impact of dairy ingredients on wheat flour dough rheology and bread properties. Foods 2020, 9, 828. [Google Scholar] [CrossRef]
- Struyf, N.; Verspreet, J.; Courtin, C.M. The effect of amylolytic activity and substrate availability on sugar release in non-yeasted dough. J. Cereal Sci. 2016, 69, 111–118. [Google Scholar] [CrossRef]
- Wang, H.; Xu, K.; Liu, X.; Zhang, Y.; Xie, X.; Zhang, H. Understanding the structural, pasting and digestion properties of starch isolated from frozen wheat dough. Food Hydrocoll. 2021, 111, 106168. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, D.; Guo, L.; Yao, Y.; Yao, X.; Wang, Z.; Wu, K.; Cao, X.; Gao, X. Effects of Quinoa Flour on Wheat Dough Quality, Baking Quality, and in vitro Starch Digestibility of the Crispy Biscuits. Front. Nutr. 2022, 9, 846808. [Google Scholar] [CrossRef]
- Sundarram, A.; Murthy, T.P.K. α-amylase production and applications: A review. J. Appl. Environ. Microbiol. 2014, 2, 166–175. [Google Scholar] [CrossRef]
- Drakos, A.; Kyriakakis, G.; Evageliou, V.; Protonotariou, S.; Mandala, I.; Ritzoulis, C. Influence of jet milling and particle size on the composition, physicochemical and mechanical properties of barley and rye flours. Food Chem. 2017, 215, 326–332. [Google Scholar] [CrossRef]
- Rao, B.D.; Anis, M.; Kalpana, K.; Sunooj, K.V.; Patiul, J.V.; Ganesh, T. Influence of milling methods and particle size hydration properties of sorghum flour and quality of sorghum biscuits. LWT-Food Sci. Technol. 2016, 67, 8–13. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Srichuwong, S.; Reuhs, B.L.; Hamaker, B.R. Quinoa (Chenopodium quinoa W.) and amaranth (Amaranthus caudatus L.) provide dietary fibres high in pectic substances and xyloglucans. Food Chem. 2015, 167, 490–496. [Google Scholar] [CrossRef]
- Haros, C.M.; Sanz-Penella, J.M. Food uses of whole pseudocereals. In Pseudocereals: Chemistry and Technology; Haros, M., Schoenlechner, R., Eds.; Wiley: Oxford, UK, 2017; pp. 163–192. [Google Scholar]
- Mironeasa, S.; Iuga, M.; Zaharia, D.; Mironeasa, C. Optimization of grape peels particle size and flour substitution in white wheat flour dough. Sci. Study Res. Chem. Eng. Biotechnol. Food Ind. 2019, 20, 29–42. [Google Scholar]
- Carr, N.O.; Daniels, N.W.; Frazier, P.J. Lipid interactions in breadmaking. Crit. Rev. Food Sci. Nutr. 1992, 31, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Mironeasa, S.; Zaharia, D.; Codină, G.G.; Ropciuc, S.; Iuga, M. Effects of grape peels addition on mixing, pasting and fermentation characteristics of dough from 480 wheat flour type. Bull. UASVM Food Sci. Technol. 2018, 75, 27–35. [Google Scholar] [CrossRef]
- Martínez-Villaluenga, C.; Peñas, E.; Hernández-Ledesma, B. Pseudocereal grains: Nutritional value, health benefits and current applications for the development of gluten-free foods. Food Chem. Toxicol. 2020, 137, 111178. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, F. Formulation and Quality Attributes of Quinoa Food Products. Food Bioprocess Technol. 2016, 9, 49–68. [Google Scholar] [CrossRef]
- Liu, X.L.; Mu, T.H.; Sun, H.N.; Zhang, M.; Chen, J.W. Influence of potato flour on dough rheological properties and quality of steamed bread. J. Integr. Agric. 2016, 15, 2666–2676. [Google Scholar] [CrossRef]
- BeMiller, J.N. Starches: Molecular and granular structures and properties. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; BeMiller, J.N., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 159–189. [Google Scholar]
- Coțovanu, I.; Mironeasa, S. Buckwheat seeds: Impact of milling fractions and addition level on wheat bread dough rheology. Appl. Sci. 2021, 11, 1731. [Google Scholar] [CrossRef]
- Cappelli, A.; Cini, E.; Guerrini, L.; Masella, P.; Angeloni, G.; Parenti, A. Predictive models of the rheological properties and optimal water content in doughs: An application to ancient grain flours with different degrees of refining. J. Cereal Sci. 2018, 83, 229–235. [Google Scholar] [CrossRef]
- Hager, A.S.; Wolter, A.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.K.; Czerny, M. Investigation of product quality, sensory profile and ultrastructure of breads made from a range of commercial gluten-free flours compared to their wheat counterparts. Eur. Food Res. Technol. 2012, 235, 333–344. [Google Scholar] [CrossRef]
- Föste, M.; Nordlohne, S.D.; Elgeti, D.; Linden, M.H.; Heinz, V.; Jekle, M.; Becker, T. Impact of quinoa bran on gluten-free dough and bread characteristics. Eur. Food Res.Technol. 2014, 239, 767–775. [Google Scholar] [CrossRef]
- Solaesa, Á.G.; Villanueva, M.; Vela, A.J.; Ronda, F. Protein and lipid enrichment of quinoa (cv. Titicaca) by dry fractionation. Techno-functional, thermal and rheological properties of milling fractions. Food Hydrocoll. 2020, 105, 105770. [Google Scholar] [CrossRef]
- Sun, J.J.; Wei, X.P.; Qiu, Y.Y.; Luan, G.Z.; Shi, L.; Eizo, T. Effect of dry method gelatinization on the stress relaxation properties of oat flour dough. J. Chin. Cereals Oils Assoc. 2016, 31, 23–27. [Google Scholar]
- Kurek, M.A.; Sokolova, N. Optimization of bread quality with quinoa flour of different particle size and degree of wheat flour replacement. Food Sci. Technol. 2020, 40, 307–314. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Shehata, M.G.; Mehany, T.; Zeitoun, M.A. Nutritional, Physicochemical, and Sensorial Evaluation of Flat Bread Supplemented with Quinoa Flour. Int. J. Food Sci. 2019, 2019, 4686727. [Google Scholar] [CrossRef]
- Wolter, A.; Hager, A.S.; Zannini, E.; Arendt, E.K. In vitro starch digestibility and predicted glycaemic indexes of buckwheat, oat, quinoa, sorghum, teff and commercial gluten-free bread. J. Cereal Sci. 2013, 58, 431–436. [Google Scholar] [CrossRef]
| (a) | ||||||||
| Run | Falling Number | Mixolab | ||||||
| FN (s) | WA (%) | DT (min) | ST (min) | C1-2 (N m) | C3-2 (N m) | C3-4 (N m) | C5-4 (N m) | |
| 1 | 277.00 ± 8.41 i | 59.00 ± 1.04 abc | 5.72 ± 0.06 c | 8.40 ± 0.16 h | 0.65 ± 0.03 ab | 1.27 ± 0.05 gh | 0.36 ± 0.01 e | 0.73 ± 0.03 g |
| 2 | 292.00 ± 5.35 h | 57.50 ± 0.75 c | 5.57 ± 0.04 d | 9.93 ± 0.09 b | 0.63 ± 0.03 bc | 1.34 ± 0.03 def | 0.35 ± 0.00 a | 0.80± 0.02 f |
| 3 | 300.00 ± 6.56 fg | 57.30 ± 0.81 c | 2.13 ± 0.06 g | 10.35 ± 0.10 a | 0.56 ± 0.03 f | 1.37 ± 0.05 bcd | 0.23 ± 0.01 c | 0.87 ± 0.03 d |
| 4 | 306.00 ± 8.25 e | 58.20 ± 1.81 bc | 1.67 ± 0.01 h | 9.93 ± 0.12 b | 0.59 ± 0.02 de | 1.42 ± 0.03 ab | 0.12 ± 0.00 e | 1.00 ± 0.02 b |
| 5 | 312.00 ± 9.25 d | 58.50 ± 1.11 bc | 1.69 ± 0.07 h | 9.96 ± 0.39 b | 0.61 ± 0.01 cd | 1.41 ± 0.02 a | 0.05 ± 0.00 f | 1.15 ± 0.04 a |
| 6 | 299.50 ± 11.32 g | 59.40 ± 0.92 a | 5.18 ± 0.20 e | 9.32 ± 0.22 e | 0.65 ± 0.02 ab | 1.27 ± 0.02 gh | 0.31 ± 0.00 e | 0.71 ± 0.02 g |
| 7 | 304.00 ± 9.47 efg | 58.10 ± 0.82 bc | 1.55 ± 0.06 i | 9.58 ± 0.27 cd | 0.61 ± 0.02 cd | 1.32 ± 0.02 fg | 0.22 ± 0.00 cd | 0.86 ± 0.01 de |
| 8 | 304.00 ± 9.47 ef | 58.20 ± 1.17 bc | 1.47 ± 0.07 j | 9.65 ± 0.40 c | 0.61 ± 0.01 cd | 1.35 ± 0.02 cde | 0.18 ± 0.00 d | 0.83 ± 0.02 e |
| 9 | 299.00 ± 27.15 g | 58.10 ± 1.47 bc | 1.70 ± 0.05 h | 9.85 ± 0.17 b | 0.57 ± 0.02 ef | 1.40 ± 0.02 abc | 0.12 ± 0.00 e | 0.96 ± 0.02 b |
| 10 | 312.00 ± 8.75 d | 58.50 ± 0.92 bc | 1.69 ± 0.02 h | 9.96 ± 0.16 b | 0.61 ± 0.03 cd | 1.41 ± 0.06 a | 0.05 ± 0.01 f | 1.15 ± 0.03 a |
| 11 | 325.00 ± 13.41 c | 59.60 ± 1.16 a | 6.02 ± 0.07 b | 8.85 ± 0.15 g | 0.66 ± 0.01 a | 1.20 ± 0.02 i | 0.24 ± 0.00 c | 0.56 ± 0.01 i |
| 12 | 334.50 ± 5.13 b | 59.40 ± 1.28 a | 4.60 ± 0.03 f | 9.07 ± 0.32 f | 0.64 ± 0.01 ab | 1.26 ± 0.02 h | 0.21 ± 0.00 cd | 0.64 ± 0.01 h |
| 13 | 347.00 ± 9.25 a | 58.70 ± 1.10 ab | 6.13 ± 0.06 a | 9.55 ± 0.12 f | 0.62 ± 0.03 cd | 1.32 ± 0.05 ef | 0.18 ± 0.01 d | 0.80 ± 0.03 f |
| 14 | 325.50 ± 14.25 c | 58.90 ± 1.57 ab | 1.28 ± 0.14 k | 9.48 ± 0.15 d | 0.63 ± 0.02 abc | 1.36 ± 0.01 bcde | 0.12 ± 0.01 e | 0.93 ± 0.01 c |
| 15 | 312.00 ± 10.75 d | 58.50 ± 1.05 bc | 1.69 ± 0.06 h | 9.96 ± 0.38 b | 0.61 ± 0.01 cd | 1.41 ± 0.03 a | 0.05 ± 0.00 f | 1.15 ± 0.04 a |
| (b) | ||||||||
| Run | Alveograph | Rheofermentometer | ||||||
| P (mm H2O) | L (mm) | W (×10−4) (J) | P/L (adim.) | H‘m (mm) | TV (mL) | VR (mL) | CR (%) | |
| 1 | 113 ± 1.49 b | 35 ± 0.56 i | 142 ± 1.96 g | 3.21 ± 0.05 d | 65.10 ± 2.22 e | 1168 ± 43.32 g | 1113 ± 39.86 a | 86.70 ± 2.68 e |
| 2 | 104 ± 1.76 c | 33 ± 0.76 j | 158 ± 2.62 f | 3.39 ± 0.04 c | 68.10 ± 2.80 a | 1285 ± 18.35 a | 1101 ± 13.11 b | 83.80 ± 1.18 i |
| 3 | 103 ± 1.69 cd | 40 ± 0.55 gh | 166 ± 1.90 e | 2.65 ± 0.03 h | 62.80 ± 3.50 c | 1245 ± 36.04 b | 1090 ± 37.44 d | 87.00 ± 2.19 d |
| 4 | 89 ± 1.49 fg | 47 ± 0.74 d | 168 ± 5.69 e | 1.80 ± 0.03 j | 63.70 ± 3.03 g | 1220 ± 16.68 d | 1068 ± 18.71 d | 83.40 ± 1.26 j |
| 5 | 87 ± 2.66 g | 94 ± 3.67 a | 253 ± 9.87 a | 0.93 ± 0.03 l | 62.00 ± 1.18 i | 1168 ± 18.69 g | 991 ± 15.86 i | 84.80 ± 1.36 g |
| 6 | 113 ± 1.85 b | 29 ± 0.80 k | 145 ± 5.15 g | 3.92 ± 0.04 b | 65.40 ± 2.89 d | 1140 ± 15.46 i | 997 ± 17.76 hi | 87.40 ± 1.17 c |
| 7 | 118 ± 1.40 a | 26 ± 0.80 l | 174 ± 5.42 d | 4.53 ± 0.03 a | 67.80 ± 2.65 ab | 1190 ± 17.89 f | 1055 ± 18.61 e | 84.50 ± 1.37 h |
| 8 | 101 ± 2.44 cd | 42 ± 2.82 fg | 174 ± 0.12 d | 2.35 ± 0.03 g | 64.30 ± 1.12 f | 1185 ± 23.36 f | 1035 ± 18.75 f | 87.40 ± 1.70 c |
| 9 | 103 ± 2.11 cd | 43 ± 1.09 ef | 174 ± 6.59 d | 2.44 ± 0.04 f | 63.90 ± 1.28 fg | 1200 ± 20.45 e | 995 ± 39.60 hi | 84.60 ± 1.47 h |
| 10 | 87 ± 1.16 g | 94 ± 0.27 a | 253 ± 1.34 a | 0.93 ± 0.03 l | 62.00 ± 1.71 i | 1168 ± 28.60 g | 991 ± 24.08 i | 84.80 ± 2.31 g |
| 11 | 81 ± 1.38 h | 37 ± 1.32 hi | 124 ± 2.60 h | 2.18 ± 0.02 h | 65.90 ± 1.43 c | 1150 ± 26.58 h | 1005 ± 23.12 h | 88.00 ± 1.81 b |
| 12 | 92 ± 2.06 f | 44 ± 1.06 de | 158 ± 6.08 e | 2.22 ± 0.04 h | 67.60 ± 2.70 b | 1220 ± 29.48 d | 1070 ± 26.06 d | 88.50 ± 1.68 a |
| 13 | 96 ± 0.65 e | 53 ± 0.48 c | 180 ± 0.96 c | 1.87 ± 0.01 i | 65.80 ± 1.24 c | 1270 ± 26.85 b | 1077 ± 9.61 c | 88.30 ± 1.76 f |
| 14 | 99 ± 1.30 d | 59 ± 0.40 b | 204 ± 4.72 b | 1.55 ± 0.03 k | 65.20 ± 2.82 de | 1250 ± 11.97 c | 1024 ± 9.86 g | 85.40 ± 0.81 a |
| 15 | 87 ± 2.61 g | 94 ± 3.57 a | 253 ± 9.61 a | 0.93 ± 0.03 l | 62.00 ± 1.15 i | 1168 ± 21.02 g | 991 ± 15.86 i | 84.80 ± 1.70 g |
| (c) | ||||||||
| Run | Rheometer | Bread Characteristics | ||||||
| G′ (Pa) | G″ (Pa) | tan δ (adim.) | Tmax (°C) | Jcmax (×10−5) (Pa−1) | Jrmax (×10−5) (Pa−1) | BV (cm3) | BF (g) | |
| 1 | 55,380 ± 542 c | 16,525 ± 200 d | 0.3108 ± 0.01 j | 81.76 ± 1.55 a | 15.22 ± 0.44 g | 10.92 ± 0.31 h | 316.65 ± 7.71 l | 727.50 ± 9.89 d |
| 2 | 45,430 ± 422 h | 14,870 ±180 g | 0.3315 ± 0.01 g | 79.36 ± 1.27 b | 12.57 ± 0.34 hi | 8.85 ± 0.24 m | 358.63 ± 4.88 h | 459.00 ± 10.76 k |
| 3 | 47,360 ± 976 g | 15,565 ± 359 f | 0.3350 ± 0.01 f | 82.36 ± 3.21 ab | 13.05 ± 0.58 h | 9.53 ± 0.32 k | 335.11 ± 5.63 k | 428.00 ± 12.58 l |
| 4 | 52,790 ± 224 d | 17,125 ±101 c | 0.3210 ± 0.00 i | 79.63 ± 1.43 b | 16.69 ± 0.26 f | 12.77 ± 0.24 e | 372.80 ± 6.69 g | 350.00 ± 17.00 m |
| 5 | 26,370 ± 237 m | 9488 ± 85 m | 0.3698 ± 0.00 a | 83.24 ± 2.16 ab | 24.50 ± 0.42 d | 16.62 ± 0.37 c | 378.20 ± 14.75 f | 786.00 ± 7.07 c |
| 6 | 65,040 ± 143 a | 19,840 ± 77 a | 0.3080 ± 0.00 k | 81.48 ± 2.36 ab | 12.82 ± 0.48 hi | 9.13 ± 0.12 l | 339.10 ± 6.20 j | 932.00 ± 4.69 b |
| 7 | 57,130 ± 319 b | 18,470 ± 102 b | 0.3250 ± 0.00 h | 80.38 ± 1.61 b | 12.31 ± 0.14 i | 10.06 ± 0.18 j | 372.70 ± 6.39 g | 637.50 ± 4.18 g |
| 8 | 34,865 ± 316 j | 11,935 ± 104 i | 0.3498 ± 0.00 c | 83.24 ± 2.00 ab | 17.32 ± 0.25 f | 12.48 ± 0.40 f | 389.58 ± 14.37 d | 596.00 ± 6.29 i |
| 9 | 47,380 ± 191 f | 16,300 ± 80 e | 0.3440 ± 0.00 d | 79.36 ± 1.51 b | 20.52 ± 0.44 e | 14.68 ± 0.09 d | 392.67 ± 10.35 c | 537.00 ± 12.61 j |
| 10 | 26,370 ± 710 m | 9488 ± 227 m | 0.3400 ± 0.01 a | 81.95 ± 1.31 ab | 24.50 ± 0.13 d | 16.62 ± 0.41 c | 378.20 ± 4.16 f | 786.00 ± 30.71 c |
| 11 | 39,020 ± 541 i | 11,680 ± 204 j | 0.3600 ± 0.01 e | 82.46 ± 1.65 ab | 15.40 ± 0.63 g | 10.41 ± 0.35 i | 357.13 ± 7.48 i | 1176.00 ± 4.62 a |
| 12 | 48,890 ± 297 e | 14,780 ± 75 h | 0.3080 ± 0.00 b | 80.15 ± 1.76 b | 25.48 ± 0.50 c | 12.19 ± 0.20 g | 381.24 ± 6.28 e | 630.00 ± 11.91 h |
| 13 | 32,860 ± 621 k | 10,853 ± 224 k | 0.3350 ± 0.01 f | 82.79 ± 1.49 ab | 28.67 ± 0.38 a | 18.55 ± 0.36 a | 424.35 ± 4.70 a | 675.50 ± 13.24 e |
| 14 | 31,600 ± 297 l | 10,510 ± 133 l | 0.3300 ± 0.00 g | 80.48 ± 1.45 b | 26.24 ± 0.52 b | 18.38 ± 0.22 b | 417.58 ± 5.16 b | 671.00 ± 5.24 f |
| 15 | 26,370 ± 316 m | 9488 ± 104 m | 0.3698 ± 0.00 a | 83.24 ± 2.33 ab | 24.50 ± 0.38 d | 16.62 ± 0.46 c | 378.20 ± 14.37 f | 786.00 ± 6.29 c |
| Factors | Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Falling Number | Mixolab | Alveograph | ||||||||||
| FN (s) | WA (%) | DT (min) | ST (min) | C1-2 (N⋅m) | C3-2 (N⋅m) | C3-4 (N⋅m) | C5-4 (N⋅m) | P (mm H2O) | L (mm) | W (×10−4) (J) | P/L (adim.) | |
| Constant | 307.99 | 58.06 | 1.93 | 9.59 | 0.59 | 1.36 | 0.18 | 0.87 | 109.07 | 35.18 | 171.43 | 3.10 |
| A | −15.65 *** | −0.46 ** | −0.29 | 0.16 | −0.01 * | 0.02 ** | 0.03 ** | 0.04 ** | 4.20 | −3.80 | −3.20 | 0.31 |
| B | −4.53 | 0.32 * | 2.05 * | −0.48 ** | 0.02 ** | −0.08 ** | 0.12 *** | −0.22 *** | 7.07 * | −27.20 *** | −50.13 *** | 1.15 ** |
| A × B | −11.95 ** | −0.24 | −0.002 | −0.04 | −0.001 | 0.01* | 0.03 ** | 0.04 * | 7.70 * | −0.30 | 7.20 | 0.30 |
| A2 | 9.25 * | 0.10 | 1.33 | - | 0.011 | −0.01 | 0.01 | −0.04 | −9.20 * | 6.80 | −3.40 | −0.75 * |
| B2 | −8.38 | 0.80** | 0.77 | - | 0.03 * | −0.02 | −0.01 | 0.06 * | −9.33 * | 23.24 ** | 25.14 * | −0.53 |
| Model evaluation | ||||||||||||
| R2 | 0.89 | 0.79 | 0.71 | 0.57 | 0.78 | 0.98 | 0.98 | 0.97 | 0.79 | 0.93 | 0.88 | 0.86 |
| Adj.-R2 | 0.83 | 0.67 | 0.55 | 0.45 | 0.66 | 0.97 | 0.96 | 0.95 | 0.67 | 0.89 | 0.81 | 0.79 |
| p-value | 0.0004 | 0.0074 | 0.0269 | 0.0213 | 0.008 | ˂0.0001 | ˂0.0001 | ˂0.0001 | 0.0076 | ˂0.0001 | 0.0006 | 0.0011 |
| Factors | Parameters | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rheofermentometer | Rheometer | Bread Parameters | ||||||||||
| H‘m (mL) | VT (mL) | VR (mL) | CR (%) | G′ (Pa) | G″ (Pa) | tan δ (adim.) | Tmax (°C) | Jcmax (×10−5) (Pa−1) | Jrmax (×10−5) (Pa−1) | BV (cm3) | BF (g) | |
| Constant | 65.50 | 1216.65 | 1040.36 | 85.96 | 47885.10 | 13794.50 | 0.3338 | 78.78 | 17.40 | 12.47 | 388.68 | 521.46 |
| A | −0.48 | 2.80 | 19.60 * | −0.93 * | 4859.00 | 1626.15 | 0.0025 | −0.16 | −3.83 ** | −1.75 * | −19.71 ** | −118.85 ** |
| B | 2.10 ** | −4.47 | 28.20 ** | 1.25 * | 12022.67 ** | 2889.80 * | −0.0255 *** | −1.61 ** | −4.88 ** | −3.57 ** | −20.93 ** | 74.87 * |
| A × B | 0.04 | 13.10 | 20.30 | - | 807.00 | 316.50 | 0.0051 | 0.52 | −0.37 | 0.32 | −5.88 | −74.85 |
| A2 | 0.14 | 37.80 * | 38.40 ** | - | −5550.00 | - | −0.0074 | 0.10 | 2.74 | 0.89 | −2.46 | −28.75 |
| B2 | −1.65 | −80.10 ** | −51.52 ** | - | −3456.19 | - | 0.0021 | 2.29 ** | 0.18 | 0.24 | −28.45 * | 352.48 *** |
| Model evaluation | ||||||||||||
| R2 | 0.71 | 0.81 | 0.87 | 0.53 | 0.68 | 0.52 | 0.89 | 0.77 | 0.74 | 0.78 | 0.81 | 0.90 |
| Adj.-R2 | 0.54 | 0.71 | 0.79 | 0.40 | 0.50 | 0.39 | 0.83 | 0.65 | 0.60 | 0.65 | 0.71 | 0.85 |
| (p-value) | 0.0279 | 0.0043 | 0.0010 | 0.0355 | 0.0405 | 0.0374 | 0.0005 | 0.0098 | 0.0171 | 0.0088 | 0.0042 | 0.0003 |
| Parameters | Control Sample | O_QL | O_QM | O_QS |
|---|---|---|---|---|
| 100% WF | 9.13% | 10.57% | 10.25% | |
| FN (s) | 312.00 ± 5.25 b | 303.47 ± 7.23 c | 308.29 ± 6.25 bc | 331.71 ± 7.45 a |
| Water absorption (%) | 58.50 ± 0.02 a | 57.70 ± 0.38 b | 58.04 ± 0.52 ab | 58.55 ± 4.47 a |
| Development time (min) | 1.69 ± 0.15 d | 2.73 ± 0.25 b | 1.77 ± 0.14 c | 3.28 ± 0.24 a |
| Stability (min) | 9.96 ± 0.65 a | 9.82 ± 0.37 b | 9.62 ± 0.56 c | 9.48 ± 0.74 d |
| C1-2 (N∙m) | 0.61 ± 0.02 a | 0.59 ± 0.01 a | 0.59 ± 0.02 a | 0.61 ± 0.02 a |
| C3-2 (N∙m) | 1.41 ± 0.02 a | 1.37 ± 0.01 ab | 1.36 ± 0.05 ab | 1.33 ± 0.07 b |
| C3-4 (N∙m) | 0.05 ± 0.04 d | 0.21 ± 0.02 a | 0.17 ± 0.01 b | 0.15 ± 0.03 c |
| C5-4 (N∙m) | 1.15 ± 0.03 a | 0.90 ± 0.03 b | 0.89 ± 0.02 b | 0.81 ± 0.04 b |
| P (mm H2O) | 87.00 ± 5.75 d | 102.13 ± 6.41 b | 108.44 ± 3.25 a | 95.57 ± 6.52 c |
| L (mm) | 91.00 ± 8.58 a | 41.86 ± 4.02 c | 37.50 ± 2.78 d | 49.91 ± 3.45 b |
| W (×10−4) (J) | 253.00 ± 20.14 a | 170.41 ± 17.30 d | 175.59 ± 12.14 c | 179.58 ± 11.12 b |
| P/L (adim.) | 0.95 ± 0.05 d | 2.47 ± 0.15 b | 3.00 ± 0.25 a | 1.91 ± 0.08 c |
| H’m (mm) | 62.00 ± 4.25 b | 64.87 ± 3.52 a | 65.32 ± 3.47 a | 65.80 ± 3.47 a |
| VT (mL) | 1168.00 ± 89.56 c | 1255.01 ± 75.12 a | 1216.49 ± 75.65 b | 1252.54 ± 7.50 a |
| VR (mL) | 991.00 ± 85.25 d | 1091.70 ± 20.18 a | 1037.77 ± 74.85 c | 1057.10 ± 8.25 b |
| CR (%) | 84.80 ± 2.50 b | 84.94 ± 1.34 b | 85.86 ± 4.75 b | 95.57 ± 4.45 a |
| G′ (Pa) | 26,370.00 ± 180.00 a | 45,583.51 ± 427.52 a | 46,901.51 ± 285.69 a | 35,868.67 ± 269.17 a |
| G″ (Pa) | 9488.00 ± 74.58 d | 15,030.90 ± 120.58 a | 13,563.39 ± 350.45 b | 11,814.53 ± 200.47 c |
| tan δ (adim.) | 0.362 ± 0.02 a | 0.331 ± 0.01 c | 0.336 ± 0.02 b | 0.328 ± 0.01 c |
| Tmax (°C) | 83.24 ± 0.55 a | 78.88 ± 1.06 b | 78.92 ± 1.78 b | 79.37 ± 4.25 b |
| Jcmax (×10−5) (Pa−1) | 24.50 ± 2.35 a | 16.95 ± 1.25 b | 17.79 ± 1.32 b | 24.58 ± 1.25 a |
| Jrmax (×10−5) (Pa−1) | 16.62 ± 2.40 a | 12.01 ± 0.85 d | 12.75 ± 1.00 c | 15.64 ± 1.23 b |
| Bread volume (cm3) | 372.20 ± 15.25 c | 369.34 ± 17.78 c | 390.16 ± 16.78 b | 407.46 ± 17.85 a |
| Bread firmness (g) | 786.00 ± 68.52 a | 379.06 ± 20.36 d | 517.72 ± 35.78 c | 597.64 ± 45.14 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coţovanu, I.; Mironeasa, S. An Evaluation of the Dough Rheology and Bread Quality of Replacement Wheat Flour with Different Quinoa Particle Sizes. Agronomy 2022, 12, 2271. https://doi.org/10.3390/agronomy12102271
Coţovanu I, Mironeasa S. An Evaluation of the Dough Rheology and Bread Quality of Replacement Wheat Flour with Different Quinoa Particle Sizes. Agronomy. 2022; 12(10):2271. https://doi.org/10.3390/agronomy12102271
Chicago/Turabian StyleCoţovanu, Ionica, and Silvia Mironeasa. 2022. "An Evaluation of the Dough Rheology and Bread Quality of Replacement Wheat Flour with Different Quinoa Particle Sizes" Agronomy 12, no. 10: 2271. https://doi.org/10.3390/agronomy12102271
APA StyleCoţovanu, I., & Mironeasa, S. (2022). An Evaluation of the Dough Rheology and Bread Quality of Replacement Wheat Flour with Different Quinoa Particle Sizes. Agronomy, 12(10), 2271. https://doi.org/10.3390/agronomy12102271







