Abstract
Salinization is an important soil environmental problem, which severely restricts the sustainable production of cucumbers. Therefore, how to improve the salt tolerance of cucumbers is a global problem. Grafting improves the resistance of crops, and calcium ion (Ca2+) weakens the permeability of the plasma membrane. In this paper, grafting cucumber with NaCl-free treatment was the control treatment (CK). Under salt stress, grafting combined different concentrations of CaCl2 and non-grafted (NG) were considered as treatments. The synergistic effect of grafting and Ca2+ to relieve salt stress on cucumber seedlings was investigated. The results revealed that grafting (G), Ca2+, and their interaction significantly influenced plant growth, osmotic adjustment substances, enzyme activities, and iron distribution. Under salt stress, grafting increased the absorption of potassium ion (K+) and Ca2+ in cucumber stems and leaves, but compared with NG, it significantly reduced the accumulation of Na+ in those parts by 61.58–89.40%. Moreover, supplication suitable Ca2+ content had a similar effect. Supplemental Ca2+ promoted the shoot and root biomass. The 10 mM L−1 Ca2+ had the highest biomass, compared with CK and NG, an increase of 49.95% and 20.47%, respectively; the lowest sodium ion (Na+). The highest Ca2+ accumulation in cucumber stem and leaves was found in 10 mM L−1 Ca2+ treatment. Supplemental Ca2+ increased free proline (Pro) and decreased malondialdehyde (MDA) content during the entire salt stress period. At 11 days, compared with 0 mM L−1 Ca2+ treatment, pro content was increased by 4.70–25.31, and MDA content was decreased by 1.08–4.90 times, respectively. Superoxide dismutase (SOD) activity, relative growth rate of plant height (PH), and stem volume (SV), and K+/Na+ and K+/Ca2+ in cucumber leaves had significantly negative correlations with a salt damage score. The combination of grafting and supplemental 5–20 mM L−1 Ca2+ relieved salt damage to cucumber seedlings. The best synergistic effect was obtained with grafting and 10 mM L−1 Ca2+ treatment.
1. Introduction
Soil salinization has been a globally alarming ecological problem. According to statistics, about 20% of cultivated agriculture lands and half of all irrigated areas are affected by salt damage worldwide []. The area of saline-alkali soil constantly expands, and soil salinization is aggravated, thereby seriously threatening food safety worldwide [,,]. In China, 26.68 million hm2 lands are exposed to salinization threats, which accounts for 25% of arable areas []. How to use these salinized soils, expand planting area, and increase crop yield has become an essential issue for sustainable production and meeting people’s food demands.
Salinity stress is destructive abiotic stress with deleterious effects on agricultural production and ecological environment balance. Salinity causes osmotic stress and ionic toxicity, which threaten plant growth [,,]. Under salt stress, salt ions destroy the integrity of the plasma membrane of plants, intensify the peroxidation of the plasma membrane, increase cell membrane permeability, and hinder the normal occurrence and transmission of calcium ion (Ca2+) signaling [,]. Soil salinity induces ion or osmotic stress, which causes water deficit, stomatal closure, and reduced photosynthesis and biomass accumulation [,]. Salt stress causes the ion imbalance of plants, which restricts crop growth and reduces crop yield [,]. Sodium ion (Na+) is the primary ion of salt damages for plant growth, which inhibits potassium ion (K+) and Ca2+ influx into the cell from the soil [,,]. Most plants accumulate excessive salt ions in leaves and other organs with an active metabolism, thereby destroying the osmotic adjustment of the cell and reducing plant growth biomass accumulation [].
Grafting is a primary agronomic measure in vegetable production. Grafting promotes the tolerance of crops to abiotic stress (low potassium, low temperature, salt stress, and ion toxicity) and reduces the yield loss [,]. Compared with non-grafting seedlings, grafting increases water content and the photosynthesis of leaves and improves plant biomass and yield []. Under salt stress, grafting promotes the transmission of nutrients to stems and leaves and increases nutrient absorption and utilization of plants []. Grafting decreases Na+ adsorption by stems and leaves, increases K+ adsorption by leaves, and maintains Na+/K+ balance in leaves. Grafting increases the production of antioxidant and osmotic adjustment substances and improves plant resistance to salt stress [,].
As the second messenger, calcium plays a critical role in plant survival under various abiotic stresses by increasing plant stress resistance [,]. Calcium needed for the healthy growth of plants becomes inadequate under salt stress, because the absorption and utilization of Ca2+ in the plant is obstructed by salt ions []. Supplemental calcium can increase the cell-protective enzyme activities, decrease the content of reactive oxygen species, and alleviate the reactive oxygen species-induced damages on a plant cell caused by peroxidation of the plasma membrane and membrane lipid [,]. Elevated Ca2+ concentration in the nutrient solution can lighten the adverse effects of NaCl by inhibiting Na+ uptake, by maintaining K+/Na+ selectivity and enriching Ca2+ content in roots []. Moreover, calcium can maintain membrane integrity and improve photosynthesis efficiency and PSII activity []. Therefore, supplemental an appropriate calcium concentration alleviates nutrition stress caused by inadequate Ca2+ and maintains the stability of ion metabolism in the cell.
In relation to salinity tolerance, the response of grafted trees to saline conditions was determined. Although grafting has been extensively applied in cucumber cultivation [,], there are few studies about combined grafting and supplemental calcium practices on the salt tolerance of cucumber, and there is still a lack of sufficient scientific proof as to whether the addition of Ca2+ in combination with grafting on the pumpkin rootstock can provide an effective way to increase cucumber salt tolerance [].
Cucumber (Cucumis sativus L.), a major vegetable worldwide, is sensitive to salt stress because of its shallow roots. Cucumber’s sustainable production is severely affected by the rising secondary salinization of soil, due to continuous cropping and excessive fertilization. In this study, we combined grafting and supplemental calcium practices under salt stress. We aimed to do the following: (1) explore the effect of Ca2+ and grafting on osmotic adjustment substances and relevant enzyme activities; (2) reveal the effect of grafting and Ca2+ on ion distribution and salt damages for cucumber seedlings; and (3) discover the interaction effect of grafting and Ca2+ on the alleviation of salt damages. Our results provide science-based recommendations for sustainable production of cucumber under salt stress.
2. Materials and Methods
2.1. Site Description and Experiment Design
The study was carried out in a solar greenhouse of Ningxia University in Yinchuan, China (32.5° N, 119.31° E). Cucumber seeds were selected from a variety widely used locally (C. sativus cv. ‘Deer No. 99’) and purchased from Tianjin Derui Seeding Co., Ltd., Tianjin, China. Pumpkin seeds (Cucurbita ficifolia Bouché) were provided by Kunming Jingfeng Seedlings Co., Ltd., Kunming, China. The cucumber seeds were immersed in water for 4 h at room temperature, incubated in the dark at 28 °C for 1 day and sowed in the compound substrate under a 12 h light/12 h dark cycle. Pumpkin seeds were immersed for 8 h at room temperature, incubated in the dark at 28 °C for 2 days, and sown in the compound substrate under a 12 h light/12 h dark cycle, and sown in the compound substrate when the cucumber seedling cotyledon was flattened. Grafting was conducted when pumpkin seedling cotyledon flattened. The grafted seedlings sown in the compound substrate and were irrigated by freshwater during the first 14 days, cultivated in 50% Yamazaki (NH4+-N: 0.50 mM L−1, NO3−-N: 6.50 mM L−1, P: 0.50 mM L−1, K: 3.00 mM L−1, Ca: 1.75 mM L−1, Mg: 1.00 mM L−1, S: 1.00 mM L−1) solution for 7 days, and finally grown in 50% Yamazaki solution until the three-leaf stage. Cucumber seedlings were subjected to calcium and salt stresses when they had three leaves; salt stresses were treated with 100 mM L−1 NaCl. Grafted cucumber seedlings without NaCl and CaCl2 were used as CK. Under salt stress, grafting combined different concentrations of CaCl2 (0, 5, 10, 20, and 30 mM L−1) and non-grafted (NG) were considered as treatments, a total of eight treatments. Each treatment had three repetitions, and each repetition had 50 plants. The nutrient solution was changed every 2 days, and NaCl and CaCl2 were replaced simultaneously.
2.2. Osmotic Adjustment Substances, Enzyme Activities, and Salt Damage Score
Five representative plants were collected from each repetition of each treatment at 0, 1, 3, 7, and 11 days. The functional leaves of the labeled plants were collected and packed with aluminum foil paper and stored in liquid nitrogen immediately. The leaf samples were used to detect free proline (Pro), malondialdehyde (MDA), soluble sugar (SS) content, superoxide dismutase (SOD) and peroxidase (POD) activities, relative electrical conductivity, injury degree, and salt damage score. MDA content in leaves was analyzed by using the thiobarbituric acid method; pro content was determined by sulfosalicylic acid method; SOD activity was assayed by the method of nitro blue tetrazolium; POD activity was detected using guaiacol test by following the change of absorbance at 470 nm []. Salt damage scores were measured from 8–12 days under salt stress and were estimated according to the standards discussed by Zhong et al. []. The relative electrical conductivity and the injury degree of the plasma membrane were assayed using an EC meter and calculated using Equations (1) and (2) [], as follows:
where L indicates the relative electrical conductivity; C1 and C2 represent the initial electrical conductivity and final electrical conductivity of leaves, respectively; D indicates the injure degree of leaves; and L and Lck represent the relative electrical conductivity of leaves in treatments and CK, respectively.
2.3. Cucumber Seedling Growth and Na+, Ca2+, and K+ Distributions
Plant height (PH) and stem diameter (SD) of cucumber seedlings were measured at 0, 3, 7, and 11 days under salt stress. Relative growth rates of plant height (RGR-PH) and stem volume (RGR-SV) were calculated using Equations (3) and (4) [], as follows:
where h1 and h2 are the value of PH at t1 and t2, respectively. d1 and d2 are the value of SD at t1 and t2, respectively.
Five plants were sampled randomly from each replicate at 11 days under salt stress. Pumpkin root, pumpkin stem, cucumber stem and cucumber leaves were separated for the grafting cucumber seedling. Non-grafting seedlings were cut into cucumber root, cucumber stem and cucumber leaves. Plant samples were washed with deionized water and dried with paper. Plant samples were deactivated for 15 min at 105 °C and dried at 75 °C for 48 h to constant weight. The dry plant samples were used to assay Na+, K+, and Ca2+, which were estimated by flame photometry (JENWAY, PFP7), as described by []. The dry shoot biomass of grafted cucumber seedlings was the sum of cucumber stem, cucumber leaves, and pumpkin stem. The dry root weight of non-grafting cucumber seedling was the sum of cucumber stem and leaves.
2.4. Principal Component Analysis
Principal component analysis (PCA) is a widely used statistical method that avoids magnitude of different indicator units and improves interpretability while minimizing information loss; the parameters were selected based on Pearson’s correlation analysis using SPSS 20.0. The high eigenvectors contributed by the indices were selected for PCA [].
2.5. Statistical Analysis
Statistical analysis was implemented using Excel 2010 and SPSS 20.0. One-way ANOVA was used to analyze the mean value. Significance analysis was performed by Tukey analysis at the p < 0.05 level. Two-way ANOVA was used to investigate the influence of grafting, Ca2+, and their interaction on plant growth, osmotic adjustment substances, enzyme activities, and ion distribution. PCA was conducted to analyze the differences of treatments, main contributing factors, and relations among various indices.
3. Results
3.1. Cucumber Seedling Growth
Shoot biomass was significantly influenced by grafting, Ca2+, and their interaction (all p < 0.01; Table 1), and root biomass was affected by grafting (p < 0.01, Table 1). Under salt stress, shoot and root biomass of grafting cucumber seedling ascended and reduced with increasing supplemental Ca2+ concentration under salt stress. Shoot and root biomass were higher under the 10 mM L−1 Ca2+ treatment than in other treatments. Compared with CK and NG, shoot biomass increased by 60.58% and 59.69%, and root biomass increased by 15.37% and 12.16% in 10 mM L−1 Ca2+ treatment, respectively. The RGR-PH was higher in CK and 10 mM L−1 Ca2+, and RGR-SV was lower in NG compared with other treatments. The treatments (Ca2+ ≤ 10 mM L−1) had higher RGR-PH and RGR-SV, whereas other treatments (Ca2+ ≥ 15 mM L−1) had the opposite trend (Figure 1).

Table 1.
ANOVA of the effects of grafting (G), Ca2+, and interaction of G and Ca2+ on osmotic adjustment substance and enzyme activity-related parameters, ion distribution-related parameters, and plant growth-related characteristics. The values shown are F-values. * p < 0.05; ** p < 0.01; ns, not significant.

Figure 1.
Cucumber seedling shoot biomass, root biomass, relative growth rate of plant height (PH), and stem volume (SV) under grafting and Ca2+ treatments under salt stress. Bars represent standard errors. Different letters indicate a significant difference in the same parameter between treatments at p < 0.05.
3.2. Enzyme Activities and Osmotic Adjustment Substances
SOD and POD activities were significantly influenced by grafting, Ca2+, and their interaction (all p < 0.01, Table 1). Under salt stress, the CK showed the lowest POD and SOD activities. Moreover, the SOD activity was higher in NG than in the grafting treatment during the early period (1–3 days), at the 7 day; compared with NG, SOD activity significantly increased by 109.17% in 10 mM L−1 Ca2+ treatment. There was no significant difference among other grafting treatments and NG. In the later period (11 days), the opposite trend was observed in the early period. The POD activity of NG was higher than those of grafting treatments during the entire period under salt stress. SOD and POD activities increased and decreased with the increase of Ca2+ concentration under salt stress. Compared with CK and 0 mM L−1 Ca2+, 10 mM L−1 Ca2+ treatments increased SOD activity by 56.45% and 109.17% in the 10 mM L−1 Ca2+ treatment at 1 and 7 days, respectively, under salt stress. Moreover, compared with CK, SOD activity increased by 77.68% and 90.31% in 15 mM L−1 Ca2+ and 20 mM L−1 Ca2+ treatments at 11 days under salt stress, respectively. The POD activity was higher in 5 and 20 mM L−1 Ca2+ treatment at 3 and 7 days, and the effect lasted until 11 days under salt stress (Figure 2).
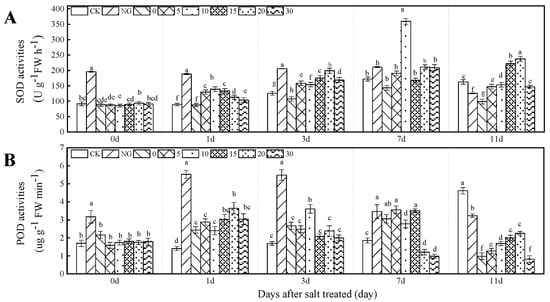
Figure 2.
SOD and POD activities of cucumber seeding leaves as affected by grafting and Ca2+ treatments under salt stress. Bars represent standard errors. Different letters indicate a significant difference in the same parameter between treatments at p < 0.05.
Compared with grafting treatments, NG had lower Pro and SS content and medium MDA content. The 30 mM L−1 Ca2+ treatment had the highest Pro content at 1, 3, and 7 days, they were 14.69, 2.52, and 1.22 times of CK, respectively, and 20 mM L−1 Ca2+ treatment had the highest Pro content at 11 days under salt stress. The MDA content was reduced in 10 mM L−1 ≤ Ca2+ ≤ 20 mM L−1 treatments compared with CK and 0 mM L−1 Ca2+ treatment at 1, 7, and 11 days under salt stress. The content of SS was proportional to the time of salt stress, compared with NG, SS content increased by 121.47%, 133.67% and 17.54% in 0, 15, 20 mM L−1 Ca2+ treatments at 11 days, respectively.
3.3. Injury Degree and Salt Damage Score
The relative electrical conductivity, injury degree, and salt damage score were significantly influenced by grafting, Ca2+, and their interaction (all p < 0.01, Table 1). The relative electrical conductivity of leaves was higher in NG treatment than those in grafting treatments in the first 3 days under salt stress. Compared with 0 mM L−1 Ca2+ treatment, relative electrical conductivity increased by 51.03%, 40.00% in NG treatment, at 1, 3 days, respectively. NG had a higher injury degree than grafting treatments at the later period under salt stress, when Ca2+ concentration was less than 20 mM L−1. Under salt stress, the relative electrical conductivity was lower in 10 and 20 mM L−1 Ca2+ treatments than those in CK and 0 mM L−1 Ca2+ at 1, 7, and 11 days. The lowest relative electrical conductivity was found in 10 mM L−1 Ca2+ at 1 and 3 days, Compared with 0 mM L−1 Ca2+ treatments, relative electrical conductivity decreased by 43.14% and 7.2% in the 10 mM L−1 Ca2+ treatment at 1 and 3 days under salt stress, respectively. The lowest relative electrical conductivity was detected in 5 mM L−1 Ca2+ treatment at 7 and 11 days. Compared with 0 mM L−1 Ca2+ treatment, relative electrical conductivity decreased by 2.77%, 22.17% in 5 mM L−1 Ca2+ treatment, at 7, 11 days. The 30 mM L−1 Ca2+ had the highest relative electrical conductivity in the late stage of salt stress (Figure 3A). The injury degree increased with increasing salt stress time and had a positive relationship with Ca2+ concentration. Thus, 5 and 10 mM L−1 Ca2+ treatments reduced injury degree under salt stress (Figure 3B).
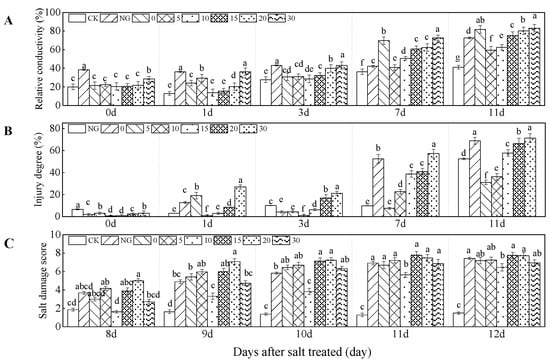
Figure 3.
Relative conductivity, injury degree, and salt damage degree of cucumber seedlings as affected by grafting and Ca2+ treatments under salt stress. Bars represent standard errors. Different letters indicate a significant difference in the same parameter between treatments at p < 0.05.
In the first 8 days under salt stress, grafting with non-supplemental Ca2+ resulted in a lower salt damage score than NG. Salt damage score increased with time under salt stress. Salt damage score decreased and increased with increasing Ca2+ concentration. The lowest salt damage score was obtained from the treatment of 10 mM L−1 Ca2+, which was lower by 33.55% and 37.89% compared with scores obtained from 0 mM L−1 Ca2+ and NG treatments, respectively. The lowest salt damage degree score was found in 10 mM L−1 Ca2+ treatment (Figure 3C).
3.4. Na+, Ca2+, and K+ Content Distribution in Plants
The Na+, Ca2+, and K+ concentrations in plants were significantly influenced by grafting, Ca2+, and their interaction (all p < 0.01, Table 1). Compared with the NG, grafting promoted the adsorption of Na+, Ca2+, and K+ in plants and decreased the adsorption of Na+ by 61.58–89.40% in cucumber stem and leaves. Moreover, grafting improved the adsorption of Ca2+ and K+ in cucumber stems and leaves.
Na+ content of pumpkin root and stem had a positive relationship with Ca2+ concentration. The 10 mM L−1Ca2+ treatment had the highest Na+ content in pumpkin root and stem. The Na+ contents of pumpkin root and stem accounted for 57.01%, 80.80%, 62.88%, and 80.71% of the total Na+ content in the 10, 20, and 30 mM L−1 Ca2+ treatments. Na+ content in cucumber stem increased and decreased with increasing Ca2+ concentration. The lowest Na+ content was discovered in 30 mM L−1 Ca2+ treatment, followed by 10, 20, and 15 mM L−1 Ca2+ treatments. Na+ contents decreased by 75.56%, 73.89%, 46.77%, and 21.35% compared with 0 mM L−1 Ca2+ treatment. Generally, 10 and 15 mM L−1 Ca2+ treatments had a lower Na+ content in cucumber leaves than in other treatments (Figure 4A).
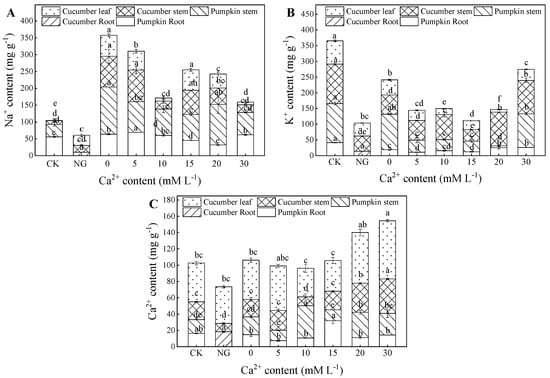
Figure 4.
Content distributions of Na+, Ca2+, and K+ in the plant, as affected by grafting and Ca2+ treatments under salt stress. Bars represent standard errors. Different letters indicate a significant difference in the same parameter between treatments at p < 0.05.
The lowest K+ content in pumpkin root and stem was found in 20 mM L−1 Ca2+ treatment, followed by 10 and 15 mM L−1 Ca2+. The treatment’s K+ content in pumpkin root and stem accounted for 21.22%, 33.93%, and 41.35% of the total K+ content in plants, successively. The 0 mM L−1 Ca2+ treatment had the highest K+ content in pumpkin root and stem. The highest K+ content was detected in cucumber stem and leaves in 20 mM L−1 Ca2+. In contrast, the lowest K+ content was detected in cucumber stem and leaves in 5 and 15 mM L−1 Ca2+ treatments (Figure 4B).
The lowest Ca2+ content in pumpkin root and stem was detected in the treatment with 0 mM L−1 Ca2+, followed by 15 mM L−1. Under these same treatments, the Ca2+ contents in pumpkin root and stem accounted for 47.70%, 57.23% and 65.54% of the total Ca2+ content, respectively. The highest Ca2+ content in cucumber stem and leaves was obtained under the treatment with 10 mM L−1 Ca2+, followed by 15, 20, and 30 mM L−1 Ca2+ treatments. Compared with 0 mM L−1 Ca2+ treatment, the Ca2+ content increased by 64.21%, 23.57%, 15.16%, and 11.48% in 5, 10, 15, 20, and 30 mM L−1 Ca2+ treatments, respectively (Figure 4C).
3.5. K+/Na+ and Ca2+/Na+ in Various Parts of Cucumber Seedlings
K+/Na+ and Ca2+/Na+ in cucumber leaves were significantly influenced by grafting, Ca2+, and their interaction (all p < 0.01, Table 2). Compared with non-salt treatment, salt stress decreased the K+/Na+ and Ca2+/Na+ of cucumber leaves significantly. Moreover, compared with NG, grafting with non-supplemental Ca2+ increased K+/Na+ and Ca2+/Na+ under salt stress. K+/Na+ of pumpkin stem and root decreased and increased with the increasing duration of salt stress, whereas Ca2+/Na+ was converse. K+/Na+ and Ca2+/Na+ in cucumber leaves and stem increased and decreased with increasing Ca2+ concentration. Compared with 0 mM L−1 Ca2+ treatments, K+/Na+ in pumpkin stem and root was lower in the ≤20 mM L−1 Ca2+ treatments, and Ca2+/Na+ was lower in the 5 mM L−1 Ca2+ treatments. Cucumber stem and leaves had higher K+/Na+ in 10 mM L−1 Ca2+ treatment and had higher Ca2+/Na+ in 10 and 30 mM L−1 Ca2+ compared with 0 mM L−1 Ca2+ treatment (Table 2).

Table 2.
Ratios of K+/Na+ and Ca2+/Na+ in different parts of grafted plants and no-graft plants under salt stress. The same letter in the same parameter and column denotes no significant difference (p < 0.05) by the least significant difference (LSD) test. Values are means ± standard error (SE) (n = 3).
3.6. PCA
The salt damage score was significantly negatively correlated with SOD, RGR-SV, and Ca2+/Na+ of cucumber leaves. The injury degree had a strongly negative correlation with SOD and RGR-PH. POD had a significantly negative relationship with the Ca2+ concentration of cucumber leaves. Pro was significantly positively correlated with SS and Ca2+ concentration of cucumber leaves. The shoot biomass had a strongly positive relationship with K+/Na+ and Ca2+/Na+ of cucumber leaves (Figure 5A).
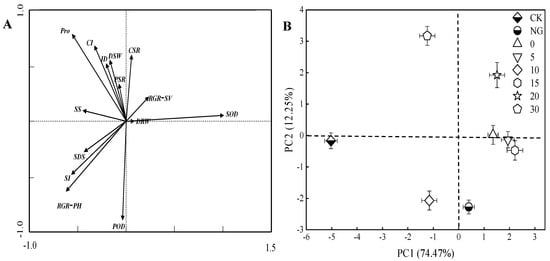
Figure 5.
Principal component analysis of osmotic adjustment substance and enzyme activity-related parameters, ion distribution-related parameters, and plant growth characteristics. Arrow direction and length indicate correlation and strength, respectively. Bars represent standard errors. ID, injury degree; POD, peroxidase; SOD, superoxide dismutase; Pro, proline; SS, soluble sugar; SI, sodium ion content of cucumber leaf; CI, calcium ion content of cucumber leaf; SDS, salt damage score; DSW, dry shoot weight; DRW, dry root weight; PSR, potassium and sodium ratio of leaf; CSR, calcium and sodium ratio of leaf; RGH-PH, relative growth rate of plant height relative growth rate; RGH-SV, relative growth rate of stem volume.
PCA was calculated based on the parameters, including plant growth, enzyme activities of oxidization system, osmotic adjustment substances, Na+, Ca2+, and K+ content, and salt damage score. PC1 and PC2 explained 74.47% and 12.25% of the variance. A total of 86.72% variation was found in the two PCs. The SOD, root biomass, SS, salt damage score had a higher weight in PC1. Ca2+, Ca2+/Na+, and K+/Na+ of cucumber leaves, pro, injury degree, shoot biomass, and RGR-PH had a higher weight in PC2. CK and 10 mM L−1 Ca2+ treatment located in the IV quadrant, which was separated from other treatments. NG, 0 mM L−1, 5 mM L−1, and 15 mM L−1 Ca2+ treatments were located in the I quadrant. The 30 mM L−1 Ca2+ treatment was located in the III quadrant, which was separated from the other treatments (Figure 5B).
4. Discussion
4.1. Effects of Grafting and Ca2+ on Oxidization System
Grafting is widely used to promote tolerance to abiotic stress and improves crop productivity. Grafting alleviates salt stress, increases antioxidant activity to maintain osmotic balance, and relieves oxidative damage to leaf cells [,]. This paper showed that grafting with 10 mM L−1 Ca2+ treatment increased SOD activity compared with NG in the late period under salt stress (Figure 2A). The SS content of the 15 mM L−1 Ca2+ treatment increased by 17.20–99.56% compared with NG during the salt stress period (Figure 3). Similarly, Huang et al. [] discovered a relatively higher sugar content of tomato fruits under grafting compared with NG. POD and SOD activities increased and decreased with increasing Ca2+ concentration under salt stress (Figure 2), probably because Ca2+ could strengthen the capacity to scavenge active oxygen and adaptation to abiotic stress by increasing SOD and POD activities [,]. However, excessive Ca2+ concentration stimulates the plants’ production of a large amount of active oxygen, which could destroy the activity sites of enzymes and weaken enzyme activity [,]. Similar to SOD and POD activities, MDA increased, decreased, and finally increased with time (Figure 6B), thereby reflecting that excessive Ca2+ concentration could stimulate the growth of MDA in cells and intensify salt damage []. SS and pro could adjust osmotic balance of membrane and strengthen resistance to salt stress for plants []. Pro and SS contents increased with the duration of salt stress. Pro and SS contents were higher in the 10–15 mM L−1 Ca2+ treatment than in other treatments (Figure 6A,C).
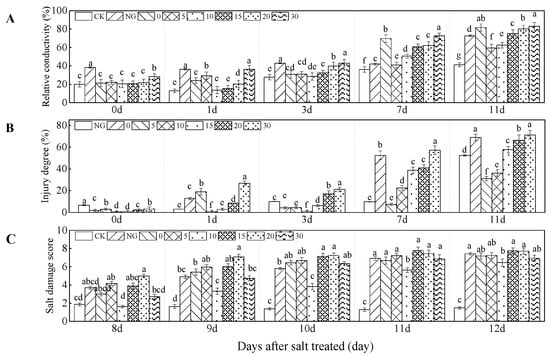
Figure 6.
Free proline, malondialdehyde, and soluble sugar contents of cucumber seeding leave as affected by grafting and Ca2+ treatments under salt stress. Bars represent standard errors. Different letters indicate a significant difference in the same parameter between treatments at p < 0.05.
4.2. Effects of Grafting and Ca2+ on Salt Damage Score
Salt stress-induced damage influences the growth of plants by applying an osmotic and ion pressure []. The relative electrically conductive, injury degree, and salt damage score increased with Ca2+ concentrations. Moreover, those metrics were lower in grafting with Ca2+ treatments than in the CK under salt stress. The lowest injury degree and salt damage score were found in 5 and 10 mM L−1 Ca2+, but these scores were highest in 20 and 30 mM L−1 Ca2+ (Figure 3). A possible reason for this phenomenon is that excessive Na+ replaced Ca2+ on the plasma membrane, thereby causing the loss of Ca2+ and K+ in cells and resulting in salt stress-induced damages [,]. Another possible reason is that H2O2 and O2− accumulated in plants gradually under salt stress []. The appropriate Ca2+ concentration helped eliminate H2O2 and O2− in the plant cell, but excessive Ca2+ reduced the POD and SOD activities and increased H2O2 and O2− accumulation. Therefore, excessive Ca2+ concentration intensifies the destruction of cell membrane structure by the electrolyte outflow and by increasing the salt damage score [,].
4.3. Effects of Grafting and Ca2+ on Ion Absorption and Distribution
The paper showed that compared with NG, grafting improved the adsorption of Na+, Ca2+, and K+ in plants, decreased the adsorption of Na+ in cucumber stem and leaves, and promoted the adsorptions of K+ and Ca2+ in cucumber stems and leaves. Compared with NG, grafting reduced the adsorption of Na+ by 61.58–89.40% in cucumber stem and leaves, but increased the adsorption of K+ and Ca2+ by 25.21–207.21% and 0.71–102.36% in plants, respectively (Figure 4). However, grafting had a significant influence on the accumulation of Ca2+ in cucumber leaves and affected the distribution of Ca2+ in other parts of plants (Table 1). A previous study showed that abundant Na+ accumulated in the root system and stem of NG seedlings first; this was then transported to the leaves, finally resulting in ion toxicity []. Grafting could inhibit the accumulation of Na+ and Cl− in the scion, which indicated that grafting could shield NaCl ions in plant leaves and relieve damage caused by salt stress [,]. In this paper, compared with non-supplemental Ca2+, supplemental Ca2+ reduced the adsorption of Na+ in plants. The highest Ca2+ and the lowest Na+ contents were detected in cucumber stems and leaves in the 10 mM L−1 Ca2+ treatment, and Ca2+ accumulation accounted for 52.30% of the total amount in plants. Moreover, supplemental Ca2+ increased the adsorption of K+ and Ca2+ in cucumber stems and leaves and relieved salt damage (Figure 4). These results agree with the findings of Lei et al. [], who also found similar responses of cucumber plants to salt stress, supplementary Ca2+ in both the grafted plants increased the shoot K+, Ca2+ content and this phenomenon appeared to be related with the decreased shoot Na+ content. Grafting and supple-mental Ca2+ changed the distribution of salt ions to maintain the healthy metabolism of the cell; this could be the reason why this phenomenon occurs []. Moreover, supplemental Ca2+ could relieve antagonism among Na+, K+, and Ca2+ and reduce the competition between Na+, K+, and Ca2+, thereby increasing the adsorption of K+ and Ca2+ in plants [], and the pumpkin rootstock-grafted plants might have higher ability to restrict K+ efflux [].
4.4. Effects of Grafting and Ca2+ on Plant Growth
In this paper, shoot and root biomass were higher in the 10, 15, and 20 mM L−1 Ca2+ treatments than in NG (Figure 1), probably because rootstock increased the absorption of water and nutrition for plants by the developed root. Moreover, grafting strengthened the production of endogenous hormones and activity of the scion of plants []. Supplemental Ca2+ increased shoot and root biomass, and the appropriate Ca2+ concentration increased relative growth rates of PH and SV, possibly because grafting strengthened salt resistance, increased the absorption of nutrients, and promoted plant growth, thereby resulting in increased plant biomass []. Ca2+ could increase plant biomass by promoting root and stem growth [].
5. Conclusions
Grafting, supplemental Ca2+, and their interaction significantly influenced enzyme activities of oxidative system and osmotic adjustment substances. Grafting and supplemental Ca2+ also greatly influenced plant growth and ion distribution. Under salt stress, grafting relieved the salt damages score in the early stage (8 days) and reduced injury degree at the late phase (7 and 11 days). Grafting promoted the adsorption of Na+, Ca2+, and K+ in plants. It decreased accumulation of Na+ in cucumber stem and leaves. It improved the accumulation of K+ and Ca2+ in cucumber stems and leaves. Supplemental Ca2+ increased shoot and root biomass, and the appropriate Ca2+ concentration increased the relative growth rate of PH and SV. Moreover, supplemental Ca2+ increased the accumulation of Na+ in pumpkin stem and root and Ca2+ and K+ in cucumber stems and leaves. A comprehensive analysis showed that the combination of grafting and 5–20 mM L−1 Ca2+ relieved salt damage to cucumber seedlings, and grafting and 10 mM L−1 Ca2+ exerted the best synergistic effect. This study provides important suggestions to the production of salinization areas. In addition, affected by genotype, the grafting, supplemental Ca2+ and their interactions may have certain differences among different cucumber varieties.
Author Contributions
X.W. and Z.L. made the same contribution to the paper and should be regarded as co-first authors. X.Z., J.L., L.T., and Z.L. conceived and designed the experiments. X.Z., X.W., Z.L., G.Y., and Y.G. performed the research and analyzed the data. X.Z. and X.W. wrote the paper. All authors interpreted the data and contributed to drafting the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the National Natural Science Foundation of China (31760593), the Horticulture Science Program of Western First-class Discipline Project in Ningxia (030900002104), the Science and Technique Innovation Leader Program of Ningxia (KJT2017001), Helan Mountain Scholars in Ningxia University (2020) and Graduate Innovation Project of Ningxia University (GIP2020040).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
CI, calcium ion content of cucumber leaf; CSR, calcium and sodium ratio of leaf; DRW, dry root weight; DSW, dry shoot weight; G, grafting; ID, injury degree; MDA, malondialdehyde; NG, non-grafted; PCA, principal component analysis; PH, plant height; POD, peroxidase; Pro, proline; PSR, potassium and sodium ratio of leaf; PSII, pigment system; RGR-PH, relative growth rate plant height; RGR-SV, relative growth rate stem volume; SD, stem diameter; SDS, salt damage score; SI, sodium ion content of cucumber leaf; SOD, superoxide dismutase; SS, soluble sugar; SV, stem volume.
References
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Maximova, E.; Fuggi, A.; Carillo, P. Durum Wheat Roots Adapt to Salinity Remodeling the Cellular Content of Nitrogen Metabolites and Sucrose. Front. Plant Sci. 2016, 7, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Butcher, K.; Wick, A.F.; Desutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Feng, L.; Xu, W.; Sun, N.; Mandal, S.; Wang, H.; Geng, Z. Efficient improvement of soil salinization through phytoremediation induced by chemical remediation in extreme arid land northwest China. Int. J. Phytoremediat. 2019, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.F.; Guo, S.R.; Jiao, Y.S.; Zhang, R.H. The effects of exogenous nitric oxide on growth, active oxygen metabolism and photosynthetic characteristics in cucumber seedling under NaCl stress. Front. Agric. China 2007, 1, 308–314. [Google Scholar] [CrossRef]
- Bot, P.J.; Abbasi, G.H.; Akhtar, J.; Anwar-Ul-Haq, M.; Malik, W. Exogenous potassium differentially mitigates salt stress in tolerant and sensitive maize hybrids. Pak. J. Bot. 2015, 46, 135–146. [Google Scholar]
- Petronia, C.; Chiara, C.; Veronica, D.M.; Carmen, A.; Stefania, D.P.; Youssef, R. Morpho-anatomical, physiological and biochemical adaptive responses to saline water of Bougainvillea spectabilis Willd. Trained to different canopy shapes. Agric. Water Manag. 2018, 212, 12–22. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Salahshoor, F.; Kazemi, F. Effect of calcium on reducing salt stress in seed germination and early growth stage of Festuca ovina L. Plant Soil Environ. 2016, 62, 460–466. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, J.Z.; Chen, X.H. Effect of durative low temprature on morphological and physiological characteristics of cucumber seedling. North. Hortic. 2010, 16, 1–3. [Google Scholar]
- Porcel, R.; Aroca, R.; Ruiz-Lozano, J.M. Salinity stress alleviation using arbuscular mycorrhizal fungi. A review. Agron. Sustain. Dev. 2012, 32, 181–200. [Google Scholar] [CrossRef]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Deb, S.K.; Sharma, P.; Shukla, M.K.; Sammis, T.W.; Ashigh, J. Drip-irrigated Pecan Seedlings Response to Irrigation Water Salinity. Hortscience 2013, 48, 1548–1555. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Saied, A.S.; Keutgen, A.J.; Noga, G. The influence of NaCl salinity on growth, yield and fruit quality of strawberry cvs. ‘Elsanta’ and ‘Korona’. Sci. Hortic. 2005, 103, 289–303. [Google Scholar] [CrossRef]
- Youssef, R.; Kyriacou, M.C.; Giuseppe, C. Vegetable Grafting: A Toolbox for Securing Yield Stability under Multiple Stress Conditions. Front. Plant Sci. 2017, 8, 2255. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; He, S.; Hua, B.; Zhen, A.; Liu, Z. Improving cucumber tolerance to major nutrients induced salinity by grafting onto Cucurbita ficifolia. Environ. Exp. Bot. 2010, 69, 32–38. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Leonardi, C.; Bie, Z. Role of grafting in vegetable crops grown under saline conditions. Sci. Hortic. 2010, 127, 147–155. [Google Scholar] [CrossRef]
- Savvas, D.; Öztekin, G.B.; Tepecik, M.; Ropokis, A.; Tüzel, Y.; Ntatsi, G.; Schwarz, D. Impact of grafting and rootstock on nutrient-to-water uptake ratios during the first month after planting of hydroponically grown tomato. J. Hortic. Sci. Biotechnol. 2017, 92, 294–302. [Google Scholar] [CrossRef]
- Plaut, Z.; Edelstein, M.; Ben-Hur, M. Overcoming salinity barriers to crop production using traditional methods. Crit. Rev. Plant Sci. 2013, 32, 250–291. [Google Scholar] [CrossRef]
- Cha-Um, S.; Singh, H.P.; Samphumphuang, T.; Kirdmanee, C. Calcium-alleviated salt tolerance in indica rice (‘Oryza sativa’ L. spp. ‘indica’): Physiological and morphological changes. Aust. J. Crop Sci. 2012, 6, 176–182. [Google Scholar]
- Liu, G. Role of nitric oxide and calcium signaling in oxalate-induced resistance of cucumber leaves to Pseudoperonospora cubensis. Acta Bot. Boreali Occident. Sin. 2019, 32, 969–974. [Google Scholar]
- Roy, P.R.; Tahjib-Ul-Arif, M.; Polash, M.A.S.; Hossen, M.Z.; Hossain, M.A. Physiological mechanisms of exogenous calcium on alleviating salinity-induced stress in rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2019, 25, 611–624. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Tang, R.J.; Zhao, F.G.; Garcia, V.J.; Kleist, T.J.; Yang, L.; Zhang, H.X.; Luan, S. Tonoplast CBL–CIPK calcium signaling network regulates magnesium homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, 3134–3139. [Google Scholar] [CrossRef]
- Lei, B.; Huang, Y.; Xie, J.J.; Liu, Z.X.; Zhen, A.; Fan, M.L.; Bie, Z.L. Increased cucumber salt tolerance by grafting on pumpkin rootstock and after application of calcium. Biol. Plant. 2013, 58, 179–184. [Google Scholar] [CrossRef]
- Moez, H.; Chantal, E.; Mariama, N.; Laurent, L.; Khaled, M. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef]
- Chen, G.; Wang, R. Effects of salinity on growth and concentrations of sodium, potassium, and calcium in grafted cucumber seedlings. Acta Hortic. 2008, 771, 217–224. [Google Scholar] [CrossRef]
- Alizadeh, M.; Singh, S.K.; Patel, V.B.; Bhattacharya, R.C.; Yadav, B.P. In vitro responses of grape rootstocks to NaCl. Biol. Plant. 2010, 54, 381–385. [Google Scholar] [CrossRef]
- Feng, G.J. Plant Physiology Experiment Guide, 3rd ed.; Higher Education Press: Beijing, China, 2006; pp. 87–92. [Google Scholar]
- Zhong, X.; Lin, L.; Liang, H. The improvement of electric conductivity method-the measurement of hurt degree of plant tissue under stress. J. Biol. 2003, 20, 45–63. [Google Scholar]
- Kingsbury, R.W.; Epstein, E.; Pearcy, R.W. Physiological responses to salinity in selected lines of wheat. Plant Physiol. 1984, 74, 417–423. [Google Scholar] [CrossRef]
- Baligar, V.C.; Schaffert, R.E.; Santos, H.L.D.; Pitta, G.V.E.; Filho, A.F.D.C.B. Growth and Nutrient Uptake Parameters in Sorghum as Influenced by Aluminum. Agron. J. 1993, 85, 1068–1074. [Google Scholar] [CrossRef]
- Li, G.; Wang, W.; Chen, Z.; Hu, Z.; Leng, P. Effect of calcium on ion contents in different organs and absorption of K+ and Na+ in the root tips of Mesembryanthemum crystallinum L. under NaCl stress. Plant Soil Environ. 2018, 36, 282–290. [Google Scholar]
- Chaturvedi, K.; Sharma, N.; Yadav, S.K. Composite edible coatings from commercial pectin, corn flour and beetroot powder minimize post-harvest decay, reduces ripening and improves sensory liking of tomatoes. Int. J. Biol. Macromol. 2019, 133, 284–293. [Google Scholar] [CrossRef]
- Albacete, A.; Martinez-Andujar, C.; Ghanem, M.E.; Acosta, M.; Sanchez-Bravo, J.; Asins, M.J.; Cuartero, J.; Lutts, S.; Dodd, I.C.; Perez-Alfocea, F. Rootstock-mediated changes in xylem ionic and hormonal status are correlated with delayed leaf senescence, and increased leaf area and crop productivity in salinized tomato. Plant Cell Environ. 2010, 32, 928–938. [Google Scholar] [CrossRef]
- Huang, W.; Liao, S.; Lv, H.; Khaldun, A.B.M.; Wang, Y. Characterization of the growth and fruit quality of tomato grafted on a woody medicinal plant, Lycium chinense. Sci. Hortic. 2015, 197, 447–453. [Google Scholar] [CrossRef]
- Brookes, P.S. Calcium, ATP and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Tian, X.; He, M.; Wang, Z.; Zhang, J.; Song, Y.; He, Z.; Dong, Y. Application of nitric oxide and calcium nitrate enhances tolerance of wheat seedlings to salt stress. Plant Growth Regul. 2015, 77, 343–356. [Google Scholar] [CrossRef]
- Fan, H.F.; Guo, S.R.; Jiao, Y.S.; Zhang, R.H.; Li, J. Ameliorating effects of exogenous Ca2+ on foxtail millet seedlings under salt stress. Funct. Plant Biol. 2019, 46, 407–416. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.; Singer, J.W.; Fessehaie, A.; Krebs, S.L.; Arora, R. Seasonal changes in photosynthesis, antioxidant systems and ELIP expression in a thermonastic and non-thermonastic Rhododendron species: A comparison of photoprotective strategies in overwintering plants. Plant Sci. 2009, 177, 607–617. [Google Scholar] [CrossRef]
- Li, L.; Zhu, T.; Liu, J.; Zhao, C.; Li, L.; Chen, M. An orthogonal test of the effect of NO3−, PO43−, K+, and Ca2+ on the growth and ion absorption of Elaeagnus angustifolia L. seedlings under salt stress. Acta Physiol. Plant. 2019, 41, 1–11. [Google Scholar] [CrossRef]
- Chen, X.J.; Chen, G.; Chang, X.C.; Tursun, Z.; Jian-Ping, L.I.; Hao, X.Y.; Gao, S.Q.; Huang, Q.S. Physiological Response Mechanism of Corn Seedlings under Salt. Acta Agric. Zhejiangensis 2020, 32, 1141–1148. [Google Scholar] [CrossRef]
- Fan, M.; Bie, Z.; Krumbein, A.; Schwarz, D. Salinity stress in tomatoes can be alleviated by grafting and potassium depending on the rootstock and K-concentration employed. Sci. Hortic. 2011, 130, 615–623. [Google Scholar] [CrossRef]
- Grigore, M.N.; Boscaiu, M.; Llinares, J.; Vicente, O. Mitigation of Salt Stress-Induced Inhibition of Plantago crassifolia Reproductive Development by Supplemental Calcium or Magnesium. Not. Bot. Horti Agrobot. Cluj Napoca 2012, 40, 58–66. [Google Scholar] [CrossRef][Green Version]
- Saeidi-Sar, S.; Abbaspour, H.; Afshari, H.; Yaghoobi, S.R. Effects of ascorbic acid and gibberellin A3 on alleviation of salt stress in common bean (Phaseolus vulgaris L.) seedlings. Acta Physiol. Plant. 2013, 35, 667–677. [Google Scholar] [CrossRef]
- Liu, J.; Niu, Y.; Zhang, J.; Zhou, Y.; Ma, Z.; Huang, X. Ca2+ channels and Ca2+ signals involved in abiotic stress responses in plant cells: Recent advances. Plant Cell Tissue Organ Cult. 2018, 132, 413–424. [Google Scholar] [CrossRef]
- Qian, H.F.; Peng, X.F.; Han, X.; Ren, J.; Zhan, K.Y.; Zhu, M. The stress factor, exogenous ascorbic acid, affects plant growth and the antioxidant system in Arabidopsis thaliana. Russ. J. Plant Physiol. 2014, 61, 467–475. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Cheng, Y.; Zhou, T.; Duan, X.; Gong, M.; Zou, Z. Generation of reactive oxygen species and their functions and deleterious effects in plants. Acta Bot. Boreali Occident. Sin. 2014, 34, 1916–1926. [Google Scholar]
- Huang, Y.; Bie, Z.; Liu, P.; Niu, M.; Zhen, A.; Liu, Z.; Lei, B.; Gu, D.; Lu, C.; Wang, B. Reciprocal grafting between cucumber and pumpkin demonstrates the roles of the rootstock in the determination of cucumber salt tolerance and sodium accumulation. Sci. Hortic. 2013, 149, 47–54. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, Y.; Chun-Mei, H.; Liu, Z.; Wei, G. Effects of Salt Stress on Biomass Formation and Ion Partition in Hydroponicaly—Cultured Grafted Cucumber. Acta Bot. Boreali Occident. Sin. 2006, 26, 2500–2505. [Google Scholar]
- Zhu, S.; Guo, S. Effects of grafting on K+, Na+ contents and distribution of watermelon (Citrullus vulgaris Schrad.) seedlings under salt stress. Acta Hortic. Sin. 2009, 36, 814–820. [Google Scholar] [CrossRef]
- Anisur, R.; Kamrun, N.; Mirza, H.; Masayuki, F. Calcium Supplementation Improves Na+/K+ Ratio, Antioxidant Defense and Glyoxalase Systems in Salt-Stressed Rice Seedlings. Front. Plant Sci. 2016, 7, 609. [Google Scholar] [CrossRef]
- Cuin, T.A.; Betts, S.A.; Chalmandrier, R.; Shabala, S. A root’s ability to retain K+ correlates with salt tolerance in wheat. J. Exp. Bot. 2008, 59, 2697–2706. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Jawad, R.; Kumar, P.; Rea, E.; Cardarelli, M. The effectiveness of grafting to improve NaCl and CaCl2 tolerance in cucumber. Sci. Hortic. 2013, 164, 380–391. [Google Scholar] [CrossRef]
- Gong, B.; Li, X.; Vandenlangenberg, K.M.; Wen, D.; Sun, S.; Wei, M.; Li, Y.; Yang, F.; Shi, Q.; Wang, X. Overexpression of S-adenosyl-l-methionine synthetase increased tomato tolerance to alkali stress through polyamine metabolism. Plant Biotechnol. J. 2014, 12, 694–708. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.P.; Ye, J.; Gao, W.; Qiao, Y.J.; Dai, C.J.; Zhao, Y.X.; Shi, S.J. Effects of exogenous Ca2+ on stomatal traits, photosynthesis, and biomass of maize seedings under salt stress. Chin. J. Appl. Ecol. 2019, 30, 923–930. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).