Defense Enzymes in Mycorrhizal Tomato Plants Exposed to Combined Drought and Heat Stresses
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Biological Materials
2.2. Arbuscular Mycorrhizal Fungal Inoculum
2.3. Growth Conditions and Experimental Design
2.4. Assessment of Tomato Plant Biomass
2.5. Root Colonization by AMF
2.6. Determination of H2O2 Content
2.7. Determination of Malondialdehyde (MDA) Content
2.8. Defense Enzymatic Activity Determination
2.8.1. Glutathione S-Transferase
2.8.2. Catalase
2.8.3. Peroxidase
2.8.4. Polyphenol Oxidase
3. Results
3.1. Plant Growth and Mycorrhizal Colonization
3.2. Accumulation of Hydrogen Peroxide and Lipid Peroxidation
3.3. Defense Enzyme Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AM | arbuscular mycorrhizal |
| AMF | arbuscular mycorrhizal fungi |
| APX | ascorbate peroxidase |
| CAT | catalase |
| drought + heat stress | D + H |
| drought + heat shock | D + HS |
| FW | Fresh weight |
| GHS | reduced glutathione |
| GR | glutathione reductase |
| GST | glutathione-S-transferase |
| MDA | malondialdehyde |
| non-stress | NoS |
| POD | peroxidase |
| PPO | polyphenol oxidase |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
References
- Dubey, P.K.; Singh, G.S.; Abhilash, P.C. Agriculture in a changing climate. J. Clean. Prod. 2016, 113, 1046–1047. [Google Scholar] [CrossRef]
- Dubey, R.K.; Tripathi, V.; Dubey, P.K.; Singh, H.B.; Abhilash, P.C. Exploring rhizospheric interactions for agricultural sustainability: The need of integrative research on multi-trophic interactions. J. Clean. Prod. 2016, 115, 362–365. [Google Scholar] [CrossRef]
- Dubey, P.K.; Singh, A. Adaptive agricultural practices for Rice-Wheat cropping system in Indo- Gangetic plains of India. Agroecosyst. Spec. Group CEM IUCN Newsl. 2017, 1, 13–17. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). IPCC, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; 1535p. [Google Scholar]
- Ploeg, D.V.; Heuvelink, E. Influence of sub-optimal temperature on tomato growth and yield: A review. J. Hortic. Sci. Biotechnol. 2005, 80, 652–659. [Google Scholar] [CrossRef]
- Ideal Temperatures for Growing Tomatoes. Allot. Gard. Available online: https://www.allotment-garden.org/vegetable/how-to-grow-your-own-tomatoes/ideal-temperatures-for-growing-tomatoes/ (accessed on 13 August 2020).
- Hameed, A.; Wu, Q.-S.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Lone, H.A.; Ahmad, P. Role of AM Fungi in Alleviating Drought Stress in Plants. In Use of Microbes for the Alleviation of Soil Stresses: Volume 2: Alleviation of Soil Stress by PGPR and Mycorrhizal Fungi; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; pp. 55–75. ISBN 978-1-4939-0721-2. [Google Scholar]
- Osório, M.L.; Osório, J.; Vieira, A.C.; Gonçalves, S.; Romano, A. Influence of enhanced temperature on photosynthesis, photooxidative damage, and antioxidant strategies in Ceratonia siliqua L. seedlings subjected to water deficit and rewatering. Photosynthetica 2011, 49, 3–12. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Zou, Y.-N.; Wu, Q.-S. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 2017, 7, 42335. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E.; Zou, Z. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef]
- Abdel Latef, A.A.H.; Mostofa, M.G.; Rahman, M.M.; Abdel-Farid, I.B.; Tran, L.-S.P. Extracts from Yeast and Carrot Roots Enhance Maize Performance under Seawater-Induced Salt Stress by Altering Physio-Biochemical Characteristics of Stressed Plants. J. Plant Growth Regul. 2019, 38, 966–979. [Google Scholar] [CrossRef]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive Oxygen Species (ROS): Beneficial Companions of Plants’ Developmental Processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. Cell Mol. Biol. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Neuser, J.; Metzen, C.C.; Dreyer, B.H.; Feulner, C.; van Dongen, J.T.; Schmidt, R.R.; Schippers, J.H.M. HBI1 Mediates the Trade-off between Growth and Immunity through Its Impact on Apoplastic ROS Homeostasis. Cell Rep. 2019, 28, 1670.e3–1678.e3. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. PPB 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Al-Huqail, A.A.; Wirth, S.; Egamberdieva, D. The Interaction between Arbuscular Mycorrhizal Fungi and Endophytic Bacteria Enhances Plant Growth of Acacia gerrardii under Salt Stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef]
- Wu, H.-H.; Zou, Y.-N.; Rahman, M.M.; Ni, Q.-D.; Wu, Q.-S. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Horn, S.; Hempel, S.; Verbruggen, E.; Rillig, M.C.; Caruso, T. Linking the community structure of arbuscular mycorrhizal fungi and plants: A story of interdependence? ISME J. 2017, 11, 1400–1411. [Google Scholar] [CrossRef]
- Mello, A.; Balestrini, R. Recent Insights on Biological and Ecological Aspects of Ectomycorrhizal Fungi and Their Interactions. Front. Microbiol. 2018, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Guo, W.; Bi, N.; Guo, J.; Wang, L.; Zhao, J.; Zhang, J. Arbuscular mycorrhizal fungi affect the growth, nutrient uptake and water status of maize (Zea mays L.) grown in two types of coal mine spoils under drought stress. Appl. Soil Ecol. 2015, 88, 41–49. [Google Scholar] [CrossRef]
- Bowles, T.M.; Jackson, L.E.; Cavagnaro, T.R. Mycorrhizal fungi enhance plant nutrient acquisition and modulate nitrogen loss with variable water regimes. Glob. Chang. Biol. 2018, 24, e171–e182. [Google Scholar] [CrossRef] [PubMed]
- Pedranzani, H.; Rodríguez-Rivera, M.; Gutiérrez, M.; Porcel, R.; Hause, B.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 2016, 26, 141–152. [Google Scholar] [CrossRef]
- Chang, W.; Sui, X.; Fan, X.-X.; Jia, T.-T.; Song, F.-Q. Arbuscular Mycorrhizal Symbiosis Modulates Antioxidant Response and Ion Distribution in Salt-Stressed Elaeagnus angustifolia Seedlings. Front. Microbiol. 2018, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Xu, L.; Li, T.; Wu, Z.; Feng, H.; Yu, M.; Zhang, X.; Chen, B. Arbuscular mycorrhiza enhances drought tolerance of tomato plants by regulating the 14-3-3 genes in the ABA signaling pathway. Appl. Soil Ecol. 2018, 125, 213–221. [Google Scholar] [CrossRef]
- Chitarra, W.; Pagliarani, C.; Maserti, B.; Lumini, E.; Siciliano, I.; Cascone, P.; Schubert, A.; Gambino, G.; Balestrini, R.; Guerrieri, E. Insights on the Impact of Arbuscular Mycorrhizal Symbiosis on Tomato Tolerance to Water Stress. Plant Physiol. 2016, 171, 1009–1023. [Google Scholar] [CrossRef] [PubMed]
- Jia-Dong, H.; Tao, D.; Hui-Hui, W.; Ying-Ning, Z.; Qiang-Sheng, W.; Kamil, K. Mycorrhizas induce diverse responses of root TIP aquaporin gene expression to drought stress in trifoliate orange. Sci. Hortic. 2019, 243, 64–69. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) Grasses via Altering Antioxidant Enzyme Activities and Photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.T.; Fu, J.J.; Sun, Y.F.; Xu, Y.M.; Miao, Y.J.; Xu, Y.F.; Hu, T.M. Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus nutans Griseb. S. Afr. J. Bot. 2016, 104, 21–29. [Google Scholar] [CrossRef]
- Azcón, R.; del Carmen Perálvarez, M.; Roldán, A.; Barea, J.-M. Arbuscular Mycorrhizal Fungi, Bacillus cereus, and Candida parapsilosis from a Multicontaminated Soil Alleviate Metal Toxicity in Plants. Microb. Ecol. 2010, 59, 668–677. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.; Gianinazzi-Pearson, V. Evaluation of VA infection levels in root systems. In Physiological and Genetical Aspects of Mycorrhizae; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular-arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Rathmell, W.G.; Sequeira, L. Soluble peroxidase in fluid from the intercellular spaces of tobacco leaves. Plant Physiol. 1974, 53, 317–318. [Google Scholar] [CrossRef]
- Fehrmann, H.; Diamond, A.E. Studies on Auxins in the Phytophthora Disease of the Potato Tuber I. Role of indole-acetic acid in pathogenesis. J. Phytopathol. 1967, 59, 83–100. [Google Scholar] [CrossRef]
- Tricker, P.J.; El Habti, A.; Schmidt, J.; Fleury, D. The physiological and genetic basis of combined drought and heat tolerance in wheat. J. Exp. Bot. 2018, 69, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Derr, D.; Tringe, S.G. Building the crops of tomorrow: Advantages of symbiont-based approaches to improving abiotic stress tolerance. Front. Microbiol. 2014, 5, 283. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; Heijden, M.G.A. Van der the role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef]
- Liu, S.; Guo, X.; Feng, G.; Maimaitiaili, B.; Fan, J.; He, X. Indigenous arbuscular mycorrhizal fungi can alleviate salt stress and promote growth of cotton and maize in saline fields. Plant Soil 2016, 398, 195–206. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of Plant Growth, Photosynthesis, Antioxidation and Osmosis by an Arbuscular Mycorrhizal Fungus in Watermelon Seedlings under Well-Watered and Drought Conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef]
- Ye, L.; Zhao, X.; Bao, E.; Cao, K.; Zou, Z. Effects of Arbuscular Mycorrhizal Fungi on Watermelon Growth, Elemental Uptake, Antioxidant, and Photosystem II Activities and Stress-Response Gene Expressions Under Salinity-Alkalinity Stresses. Front. Plant Sci. 2019, 10, 863. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Aroca, R.; Chaumont, F.; Ruiz-Lozano, J.M. Enhanced Drought Stress Tolerance by the Arbuscular Mycorrhizal Symbiosis in a Drought-Sensitive Maize Cultivar Is Related to a Broader and Differential Regulation of Host Plant Aquaporins than in a Drought-Tolerant Cultivar. Front. Plant Sci. 2017, 8, 1056. [Google Scholar] [CrossRef] [PubMed]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (In)Organic Adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef]
- Ruíz-Sánchez, M.; Armada, E.; Muñoz, Y.; García de Salamone, I.E.; Aroca, R.; Ruíz-Lozano, J.M.; Azcón, R. Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef]
- Hazzoumi, Z.; Moustakime, Y.; hassan Elharchli, E.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi (AMF) and water stress on growth, phenolic compounds, glandular hairs, and yield of essential oil in basil (Ocimum gratissimum L.). Chem. Biol. Technol. Agric. 2015, 2, 10. [Google Scholar] [CrossRef]
- Tuo, X.-Q.; He, L.; Zou, Y.-N. Alleviation of Drought Stress in White Clover after Inoculation with Arbuscular Mycorrhizal Fungi. Not. Bot. Horti Agrobot. Cluj Napoca 2017, 45, 220–224. [Google Scholar] [CrossRef]
- Mathur, S.; Tomar, R.S.; Jajoo, A. Arbuscular Mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019, 139, 227–238. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Mirshad, P.P.; Puthur, J.T. Arbuscular mycorrhizal association enhances drought tolerance potential of promising bioenergy grass (Saccharum arundinaceum retz.). Environ. Monit. Assess. 2016, 188, 425. [Google Scholar] [CrossRef]
- Ara, N.; Nakkanong, K.; Lv, W.; Yang, J.; Hu, Z.; Zhang, M. Antioxidant enzymatic activities and gene expression associated with heat tolerance in the stems and roots of two cucurbit species (“Cucurbita maxima” and “Cucurbita moschata”) and their interspecific inbred line “Maxchata”. Int. J. Mol. Sci. 2013, 14, 24008–24028. [Google Scholar] [CrossRef]
- Sheikh-Mohamadi, M.H.; Etemadi, N.; Nikbakht, A.; Arab, M.; Majidi, M.M.; Pessarakli, M. Antioxidante defence system and physiological responses of Iranian crested wheatgrass (Agropyron cristatum L.) to drought and salinity stress. Acta Physiol. Plant. 2017, 39, 245. [Google Scholar] [CrossRef]
- Tommasino, E.; López Colomba, E.; Carrizo, M.; Grunberg, K.; Quiroga, M.; Carloni, E.; Griffa, S.; Ribotta, A.; Luna, C. Individual and combined effects of drought and heat on antioxidant parameters and growth performance in Buffel grass (Cenchrus ciliaris L.) genotypes. S. Afr. J. Bot. 2018, 119, 104–111. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yi, X.; Hu, Z.; Hu, M.; Chen, S.; Dong, X.; Gong, L. RNAi-mediated knockdown of catalase causes cell cycle arrest in SL-1 cells and results in low survival rate of Spodoptera litura (Fabricius). PLoS ONE 2013, 8, e59527. [Google Scholar] [CrossRef]
- Tyagi, J.; Varma, A.; Pudake, R.N. Evaluation of comparative effects of arbuscular mycorrhiza (Rhizophagus intraradices) and endophyte (Piriformospora indica) association with finger millet (Eleusine coracana) under drought stress. Eur. J. Soil Biol. 2017, 81, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, J.; Chu, X.; Sun, Y.; Zhou, H.; Hu, T. Nitric oxide mediates abscisic acid induced light-tolerance in leaves of tall fescue under high-light stress. Sci Hortic. 2013, 162, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, Y.H.; Liu, B.W.; Liu, Q.; Wen, S.Y.; Ao, B.; Lin, Z.Q.; Zheng, Y.L.; Yang, W.Z.; Chu, X.T.; et al. Arbuscular mycorrhiza fungus improved growth, antioxidant defense, and endogenous hormones in tall fescue under low-light stress. S. Afr. J. Bot. 2019, 127, 43–50. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef]
- Guajardo, E.; Correa, J.A.; Contreras-Porcia, L. Role of abscisic acid (ABA) in activating antioxidant tolerance responses to desiccation stress in intertidal seaweed species. Planta 2016, 243, 767–781. [Google Scholar]
- Rani, B.; Madan, S.; POOJA, K.S.; Sharma, K.D.; Kumari, N.; Kumar, A. Mitigating the effect of drought stress on yield in wheat (Triticum aestivum) using arbuscular mycorrhiza fungi (Glomus mosseae). Indian J. Agric. Sci. 2018, 88, 95–100. [Google Scholar]
- Huang, Y.-M.; Srivastava, A.K.; Zou, Y.-N.; Ni, Q.-D.; Han, Y.; Wu, Q.-S. Mycorrhizal-induced calmodulin mediated changes in antioxidant enzymes and growth response of drought-stressed trifoliate orange. Front. Microbiol. 2014, 5, 682. [Google Scholar]
- Benhiba, L.; Fouad, M.O.; Essahibi, A.; Ghoulam, C.; Qaddoury, A. Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees 2015, 29, 1725–1733. [Google Scholar]
- Essahibi, A.; Benhiba, L.; Babram, M.A.; Ghoulam, C.; Qaddoury, A. Influence of arbuscular mycorrhizal fungi on the functional mechanisms associated with drought tolerance in carob (Ceratonia siliqua L.). Trees 2018, 32, 87–97. [Google Scholar]
- Al-Arjani, A.-B.F.; Hashem, A.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata Boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef]
- Ding, N.; Wang, A.; Zhang, X.; Wu, Y.; Wang, R.; Cui, H.; Huang, R.; Luo, Y. Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biol. 2017, 17, 225. [Google Scholar]
- Cetinkaya, H.; Tasci, E.; Dinler, B.S. Regulation of glutathione S-transferase enzyme activity with salt pre-treatment under heat stress in maize leaves. Res. Plant Biol. 2014, 4, 45–56. [Google Scholar]
- Kellős, T.; Tímár, I.; Szilágyi, V.; Szalai, G.; Galiba, G.; Kocsy, G. Stress hormones and abiotic stresses have different effects on antioxidants in maize lines with different sensitivity. Plant Biol. 2008, 10, 563–572. [Google Scholar]
- Halušková, L.; Valentovičová, K.; Huttová, J.; Mistrík, I.; Tamás, L. Effect of abiotic stresses on glutathione peroxidase and glutathione S-transferase activity in barley root tips. Plant Physiol. Biochem. 2009, 47, 1069–1074. [Google Scholar]
- Jiang, H.-W.; Liu, M.-J.; Chen, I.-C.; Huang, C.-H.; Chao, L.-Y.; Hsieh, H.-L. A Glutathione S-Transferase Regulated by Light and Hormones Participates in the Modulation of Arabidopsis Seedling Development. Plant Physiol. 2010, 154, 1646–1658. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Yuan, Z.; Hu, X.; Lu, M.; Wang, W.; Wang, Y. Identification of proteins regulated by ABA in response to combined drought and heat stress in maize roots. Acta Physiol. Plant 2013, 35, 501–513. [Google Scholar] [CrossRef]
- Cabral, C.; Ravnskov, S.; Tringovska, I.; Wollenweber, B. Arbuscular mycorrhizal fungi modify nutrient allocation and composition in wheat (Triticum aestivum L.) subjected to heat-stress. Plant Soil 2016, 408, 385–399. [Google Scholar] [CrossRef]
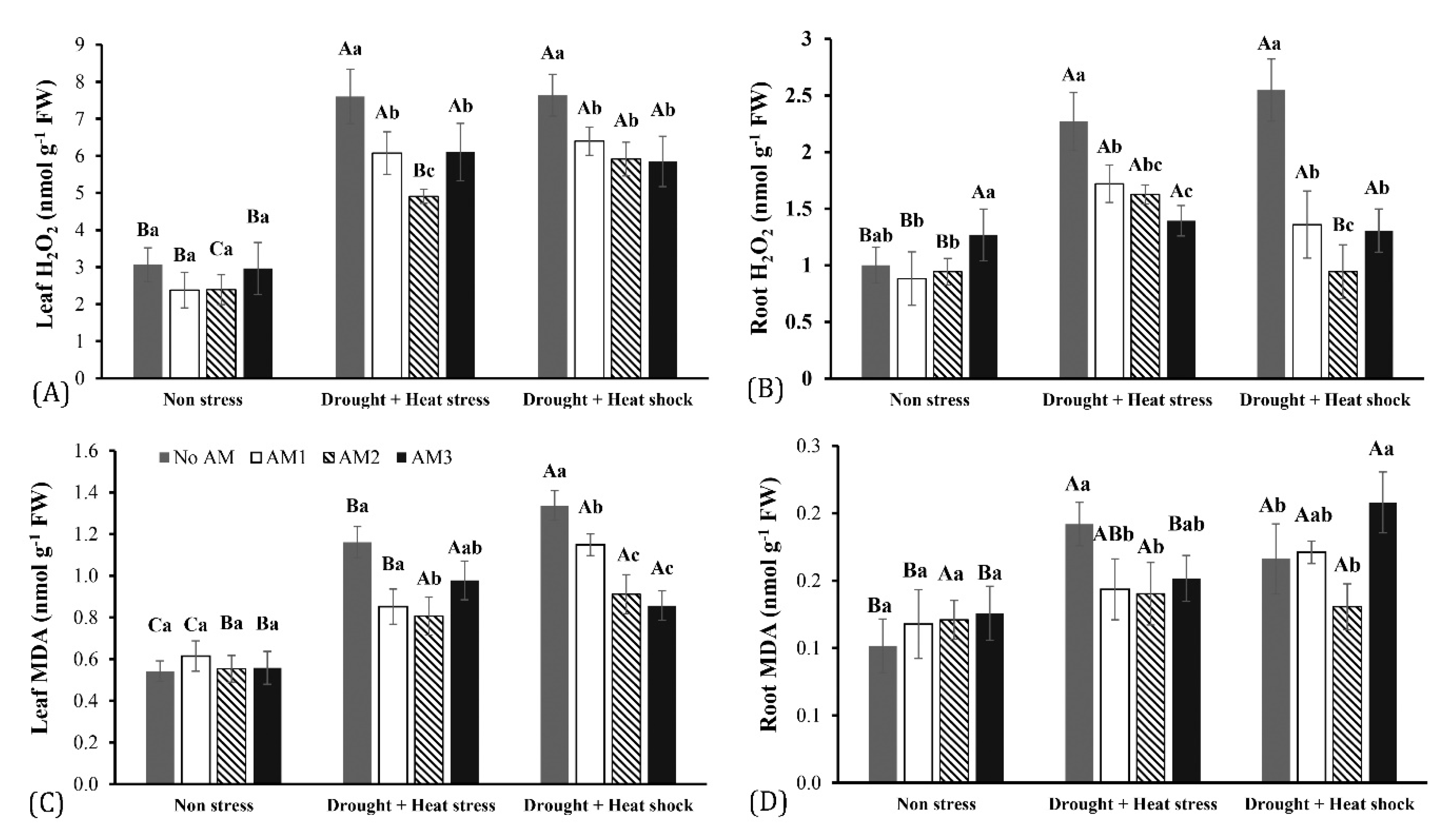

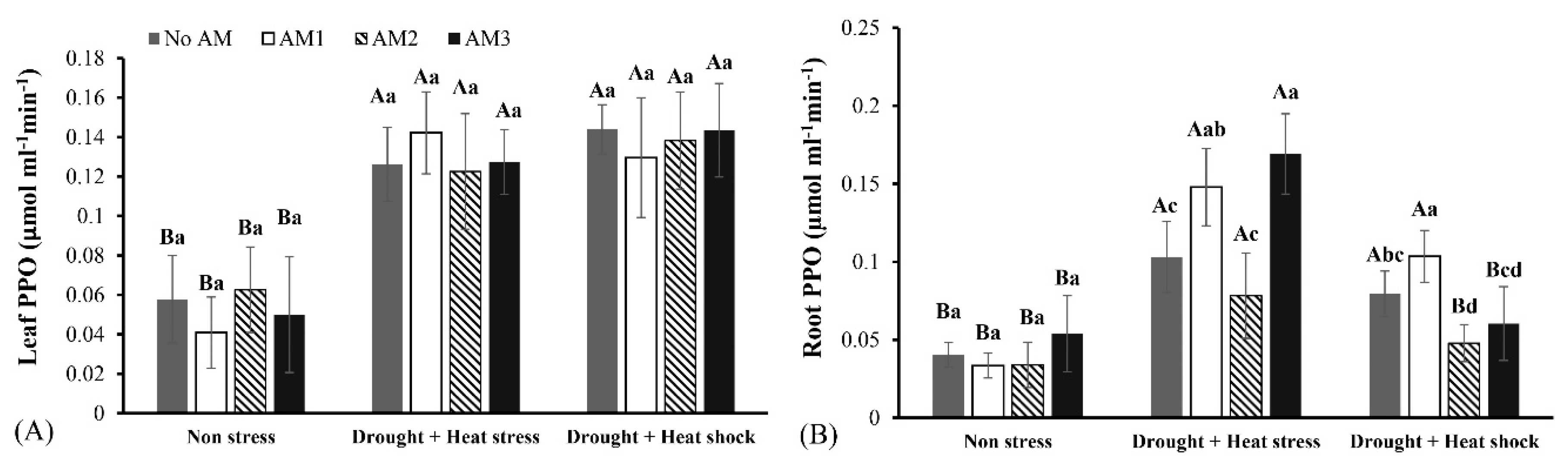

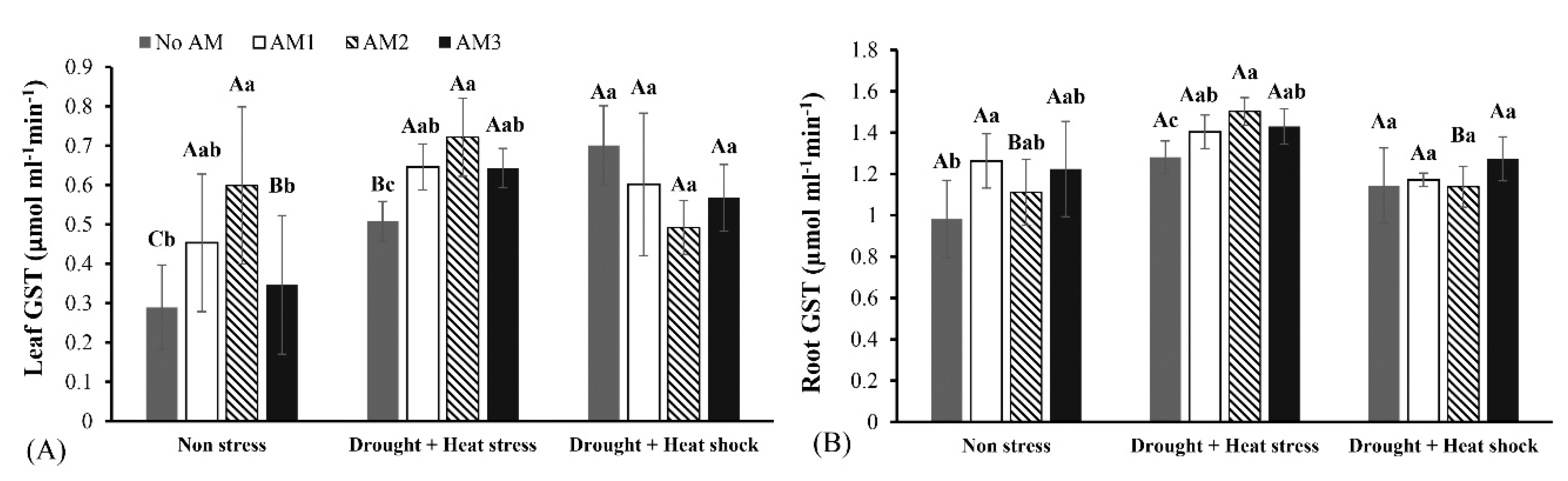
| Parameters | Variables | Mean Squares | F-Value | Df | p-Value |
|---|---|---|---|---|---|
| Fresh shoot biomass | AM inoculation (M) | 4.548 | 7.42 | 3 | ** 0.0006 |
| Stress application (S) | 131.045 | 213.85 | 2 | *** <0.0001 | |
| M*S | 3.819 | 6.23 | 6 | ** 0.0002 | |
| Leaf H2O2 accumulation | AM inoculation (M) | 4.991 | 18.09 | 3 | *** <0.0001 |
| Stress application (S) | 114.779 | 415.85 | 2 | *** <0.0001 | |
| M*S | 2.26 | 8.19 | 6 | *** <0.0001 | |
| Root H2O2 accumulation | AM inoculation (M) | 0.7 | 13.07 | 3 | *** <0.0001 |
| Stress application (S) | 3.894 | 72.67 | 2 | *** <0.0001 | |
| M*S | 0.551 | 10.28 | 6 | *** <0.0001 | |
| Leaf MDA | AM inoculation (M) | 0.152 | 19.89 | 3 | *** <0.0001 |
| Stress application (S) | 1.081 | 140.73 | 2 | *** <0.0001 | |
| M*S | 0.074 | 9.74 | 6 | *** <0.0001 | |
| Root MDA | AM inoculation (M) | 0.002 | 5.3413 | 3 | ** 0.0037 |
| Stress application (S) | 0.012 | 30.1518 | 2 | *** <0.0001 | |
| M*S | 0.002 | 5.7469 | 6 | *** <0.0001 | |
| Leaf CAT | AM inoculation (M) | 3752.957 | 12.44 | 3 | *** <0.0001 |
| Stress application (S) | 20,880.46 | 69.23 | 2 | *** <0.0001 | |
| M*S | 1688.4 | 5.6 | 6 | *** <0.0001 | |
| Root CAT | AM inoculation (M) | 1951.394 | 12.46 | 3 | *** <0.0001 |
| Stress application (S) | 16,928.51 | 108.05 | 2 | *** <0.0001 | |
| M*S | 1110.936 | 7.09 | 6 | *** <0.0001 | |
| Leaf POD | AM inoculation (M) | 346.791 | 15.76 | 3 | *** <0.0001 |
| Stress application (S) | 6526.13 | 296.5 | 2 | *** <0.0001 | |
| M*S | 202.406 | 9.2 | 6 | *** <0.0001 | |
| Root POD | AM inoculation (M) | 2609.255 | 23.94 | 3 | *** <0.0001 |
| Stress application (S) | 19,807.52 | 181.7 | 2 | *** <0.0001 | |
| M*S | 1465.06 | 13.44 | 6 | *** <0.0001 | |
| Leaf PPO | AM inoculation (M) | 0.003 | 6.96 | 3 | *** <0.0001 |
| Stress application (S) | 0.08 | 155.71 | 2 | *** <0.0001 | |
| M*S | 0.001 | 2.21 | 6 | * 0.0155 | |
| Root PPO | AM inoculation (M) | 0.002 | 9.76 | 3 | *** <0.0001 |
| Stress application (S) | 0.083 | 297.48 | 2 | *** <0.0001 | |
| M*S | 0.002 | 8.39 | 6 | *** <0.0001 | |
| Leaf GST | AM inoculation (M) | 0.054 | 3.59 | 3 | ** 0.0023 |
| Stress application (S) | 0.244 | 16.02 | 2 | *** <0.0001 | |
| M*S | 0.092 | 6.05 | 6 | *** <0.0001 | |
| Root GST | AM inoculation (M) | 0.043 | 2.62 | 3 | * 0.0185 |
| Stress application (S) | 0.52 | 31.37 | 2 | *** <0.0001 | |
| M*S | 0.045 | 2.77 | 6 | ** 0.0026 |
| Stress Condition | AM Inoculation | Fresh Plant Biomass (g plant−1) | AM Colonization (%) |
|---|---|---|---|
| No stress | No AM | 17.21 ± 0.33 Aa | 0 |
| R. irregularis | 17.86 ± 0.84 Aa | 58.87 ± 6.90 | |
| F. mosseae | 17.17 ± 1.41 Aa | 47.27 ± 7.35 | |
| F. coronatum | 17.23 ± 1.37 Aa | 48.01 ± 7.11 | |
| Drought + heat stress | No AM | 11.38 ± 0.43 Bb | 0 |
| R. irregularis | 12.28 ± 0.66 Ba | 63.82 ± 11.47 | |
| F. mosseae | 12.02 ± 0.89 Ba | 53.16 ± 6.44 | |
| F. coronatum | 11.79 ± 0.59 Bba | 48.36 ± 9.07 | |
| Drought + heat shock | No AM | 12.31 ± 0.34 Cb | 0 |
| R. irregularis | 12.75 ± 1.12 Bb | 52.09 ± 9.45 | |
| F. mosseae | 15.72 ± 0.46 Aa | 55.84 ± 8.14 | |
| F. coronatum | 12.18 ± 0.47 Bb | 51.03 ± 7.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddidi, I.; Duc, N.H.; Tonk, S.; Rápó, E.; Posta, K. Defense Enzymes in Mycorrhizal Tomato Plants Exposed to Combined Drought and Heat Stresses. Agronomy 2020, 10, 1657. https://doi.org/10.3390/agronomy10111657
Haddidi I, Duc NH, Tonk S, Rápó E, Posta K. Defense Enzymes in Mycorrhizal Tomato Plants Exposed to Combined Drought and Heat Stresses. Agronomy. 2020; 10(11):1657. https://doi.org/10.3390/agronomy10111657
Chicago/Turabian StyleHaddidi, Imane, Nguyen Hong Duc, Szende Tonk, Eszter Rápó, and Katalin Posta. 2020. "Defense Enzymes in Mycorrhizal Tomato Plants Exposed to Combined Drought and Heat Stresses" Agronomy 10, no. 11: 1657. https://doi.org/10.3390/agronomy10111657
APA StyleHaddidi, I., Duc, N. H., Tonk, S., Rápó, E., & Posta, K. (2020). Defense Enzymes in Mycorrhizal Tomato Plants Exposed to Combined Drought and Heat Stresses. Agronomy, 10(11), 1657. https://doi.org/10.3390/agronomy10111657





