Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Chemicals and Reagents
2.3. Measurement of Chlorophyll Fluorescence Parameters
2.4. Measurement of Growth Parameters
2.5. Analysis of Chlorophyll (Chl) Content
2.6. Analysis of Proline Content
2.7. Analysis of Ascorbic Acid Content
2.8. Analysis of Total Phenol and Total Flavonoid Content
2.9. Measurement of Antioxidant Activities
2.10. Statistical Analysis
3. Results and Discussion
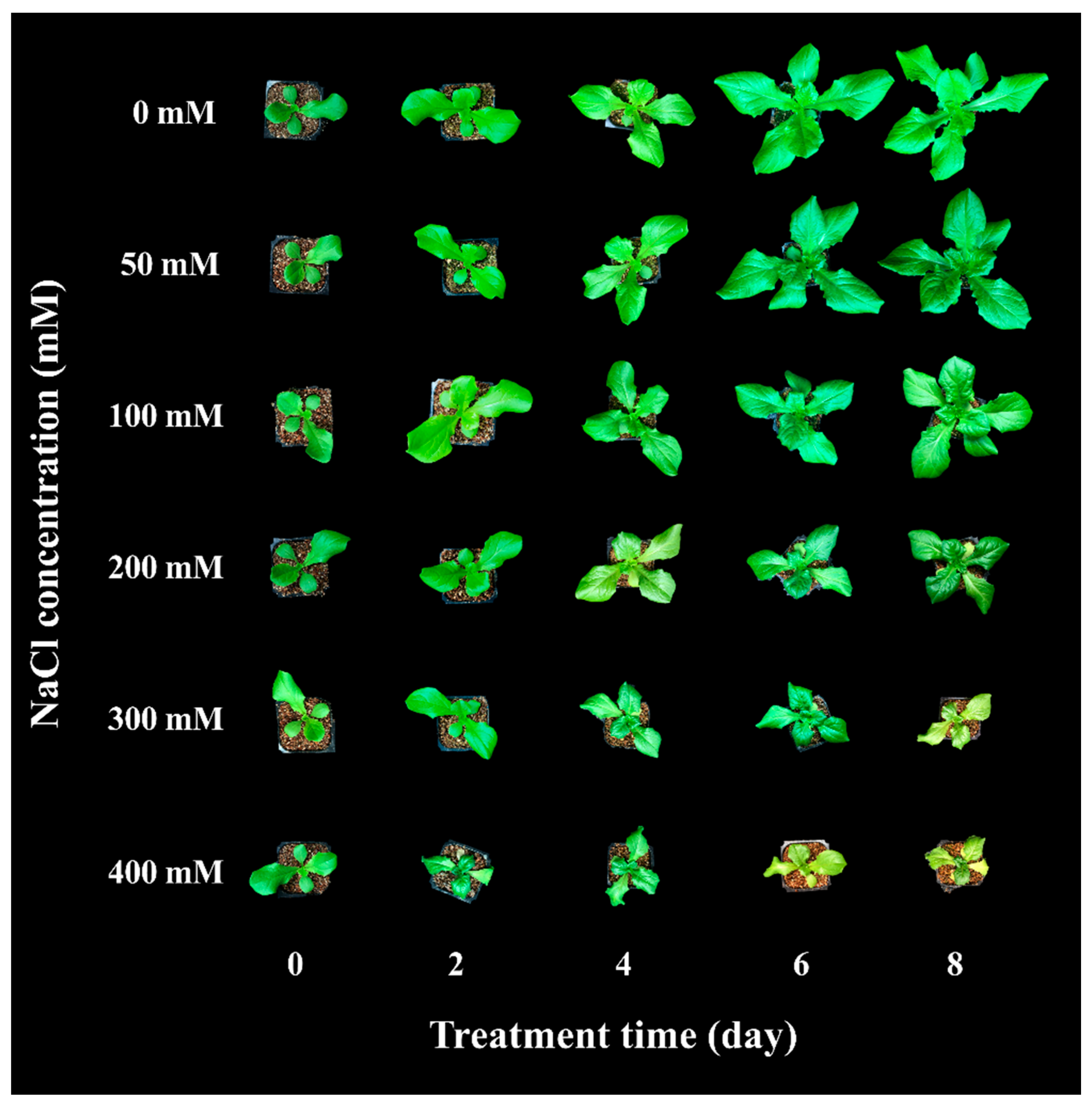
3.1. Effect of NaCl Concentration in Plant Growth Parameters
3.2. Effect of NaCl Concentration in Chlorophyll Fluorescence (CF) Parameters
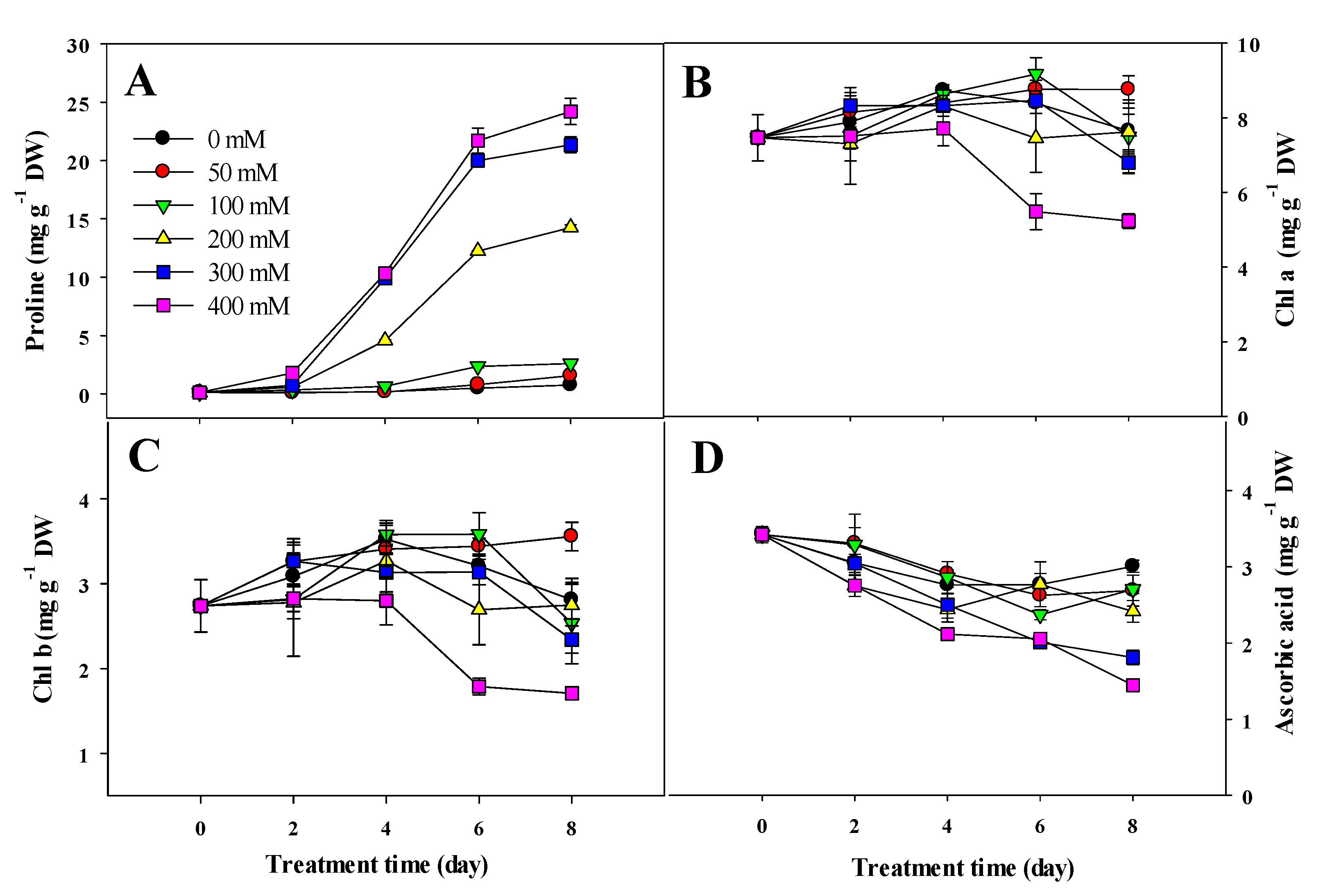
3.3. Variation in Proline and Chlorophyll Content
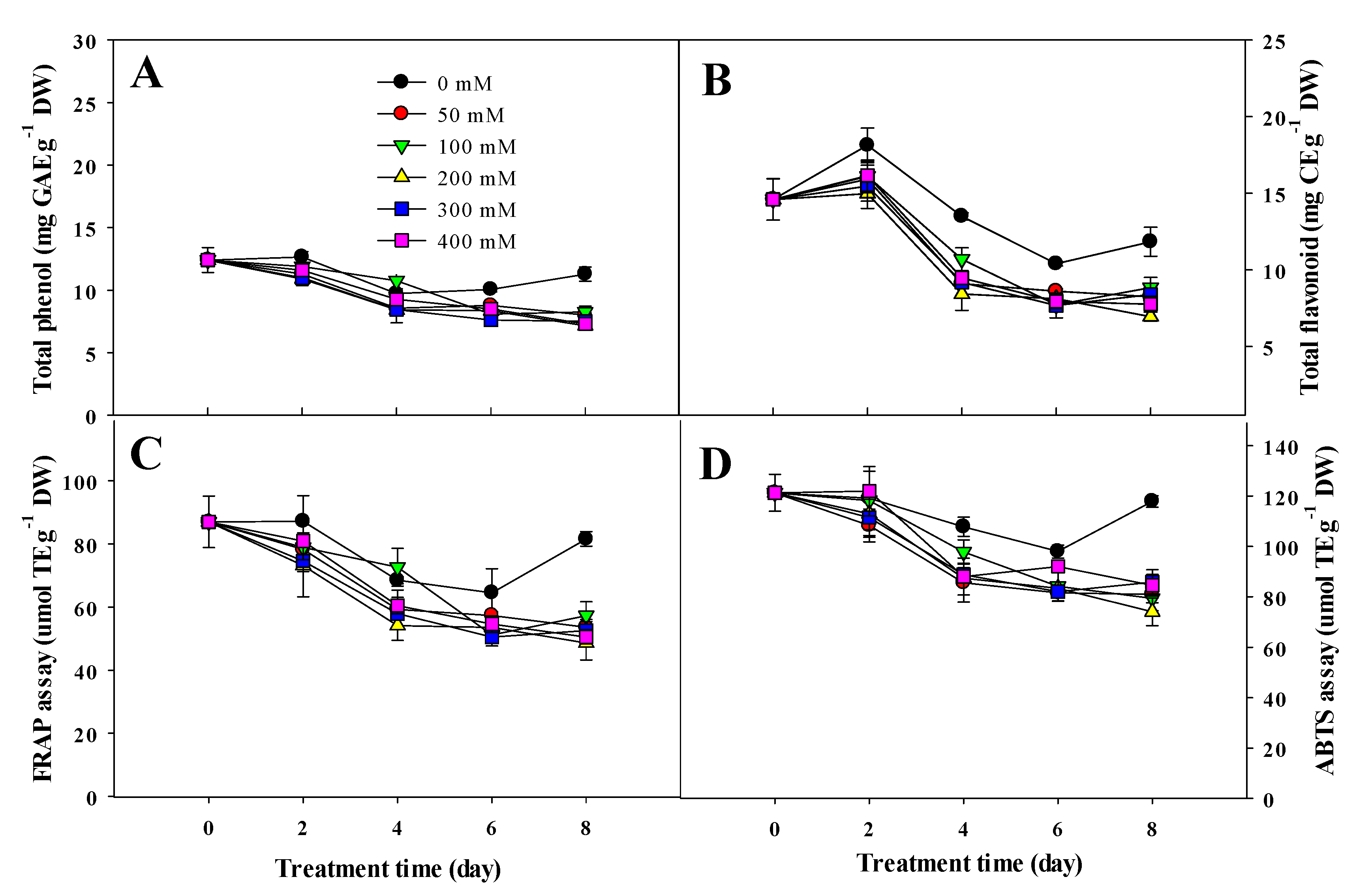
3.4. Variation in Ascorbic Acid, Total Phenol, Total Flavonoid Content, and Antioxidant Activity
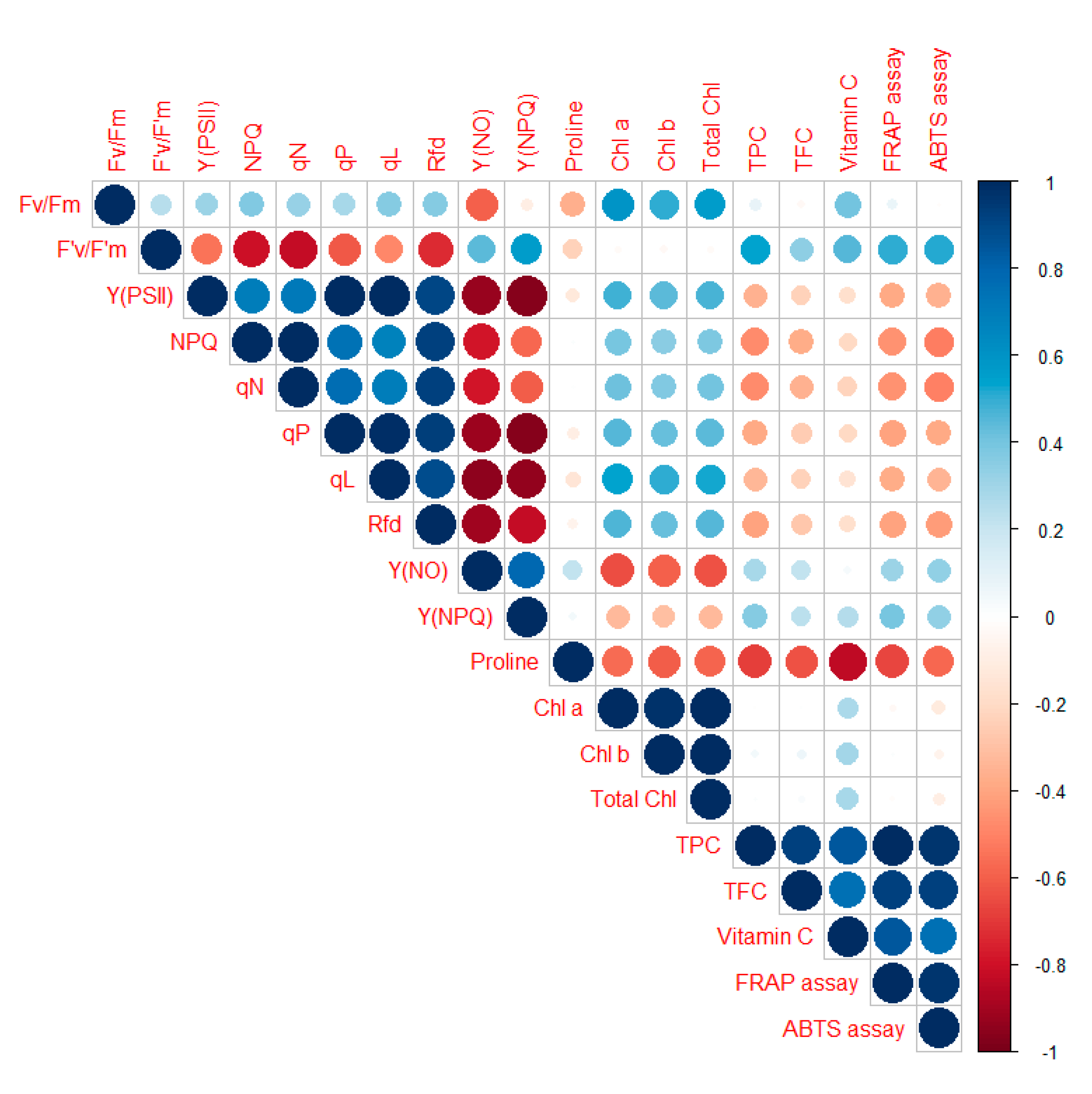
3.5. Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO (Food and Agriculture Organization). Agricultural Statistical Database for 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 26 August 2020).
- Camejo, D.; Frutos, A.; Mestre, T.C.; Pinero, M.D.C.; Rivero, R.M.; Martinez, V. Artificial light impacts the physical and nutritional quality of lettuce. Hortic. Environ. Biotechnol. 2020, 61, 69–82. [Google Scholar] [CrossRef]
- Baslam, M.; Pascual, I.; Sanchez-Diaz, M.; Erro, J.; Garcia-Mina, J.M.; Goicoechea, N. Improvement of nutritional quality of greenhouse-grown lettuce by Arbuscular Mycorrhizal fungi is conditioned by the source of phosphorus nutrition. J. Agric. Food Chem. 2011, 59, 11129–11140. [Google Scholar] [CrossRef] [PubMed]
- Soerjomataram, I.; Oomen, D.; Lemmens, V.; Oenema, A.; Benetou, V.; Trichopoulou, A.; Coebergh, J.W.; Barendregt, J.; de Vries, E. Increased consumption of fruit and vegetables and future cancer incidence in selected European countries. Eur. J. Cancer 2010, 46, 2480–2563. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Melnyk, J.P.; Tsao, R.; Marcone, M.F. How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Res. Int. 2011, 44, 14–22. [Google Scholar] [CrossRef]
- Al-Maskri, A.; Al-Kharusi, L.; Al-Miqbali, H.; Khan, M.M. Effect of salinity stress on growth of lettuce (Lactuca sativa) under closed-recycle nutrient film technique. Int. J. Agric. Biol. 2010, 12, 377–380. [Google Scholar]
- Fu, W.; Li, P.; Wu, Y. Effects of different light intensities on chlorophyll fluorescence characteristics and yield in lettuce. Sci. Hortic. 2012, 135, 45–51. [Google Scholar] [CrossRef]
- Sofo, A.; Lundegardh, B.; Martensson, A.; Manfra, M.; Pepe, G.; Sommella, E.; De Nisco, M.; Tenore, G.C.; Campiglia, P.; Scopa, A. Different agronomic and fertilization systems affect polyphenolic profile, antioxidant capacity and mineral composition of lettuce. Sci. Hortic. 2016, 204, 106–115. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Ortuno, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef]
- Aroca, R.; Ruiz-Lozano, J.M.; Zamarreno, A.M.; Paz, J.A.; Garcia-Mina, J.M.; Pozo, M.J.; Lopez-Raez, J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013, 170, 47–55. [Google Scholar] [CrossRef]
- Evelin, H.; Giri, B.; Kapoor, R. Ultrastructural evidence for AMF mediated salt stress mitigation in Trigonella foenum-graecum. Mycorrhiza 2013, 23, 71–86. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- De Oliveira, A.B.; Alencar, N.L.M.; Gomes-Filho, E. Comparison between the water and salt stress effects on plant growth and development. In Responses of Organisms to Water Stress; Akinci, S., Ed.; IntechOpen: London, UK, 2013; pp. 67–94. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global plant-responding mechanisms to salt stress: Physiological and molecular levels and implications in biotechnology. Critic. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef]
- Negrao, S.; Schmockel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Nemeskeri, E.; Helyes, L. Physiological responses of selected vegetable crop species to water stress. Agronomy 2019, 9, 447. [Google Scholar] [CrossRef]
- Bauriegel, E.; Brabandt, H.; Garber, U.; Herppich, W.B. Chlorophyll fluorescence imaging to facilitate breeding of Bremia lactucae-resistant lettuce cultivars. Comput. Electron. Agric. 2014, 105, 74–82. [Google Scholar] [CrossRef]
- Chisari, M.; Todaro, A.; Barbagallo, R.N.; Spagna, G. Salinity effects on enzymatic browning and antioxidant capacity of fresh-cut baby Romaine lettuce (Lactuca sativa L. cv. Duende). Food Chem. 2010, 119, 1502–1506. [Google Scholar] [CrossRef]
- Simko, I.; Jimenez-Berni, J.A.; Furbank, R.T. Detection of decay in fresh-cut lettuce using hyperspectral imaging and chlorophyll fluorescence imaging. Postharvest Biol. Technol. 2015, 106, 44–52. [Google Scholar] [CrossRef]
- Adhikari, N.D.; Simko, I.; Mou, B. Phenomic and physiological analysis of salinity effects on lettuce. Sensors 2019, 19, 4814. [Google Scholar] [CrossRef]
- Garrido, Y.; Tudela, J.A.; Marin, A.; Mestre, T.; Martinez, V.; Gil, M.I. Physiological, phytochemical and structural changes of multi-leaf lettuce caused by salt stress. J. Sci. Food Agric. 2014, 94, 1592–1599. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water affects the nutritional and visual properties of Romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Cho, M.C.; Lee, J.G. Evaluation of chlorophyll fluorescence parameters and proline content in tomato seedlings grown under different salt stress conditions. Hortic. Environ. Biotechnol. 2020, 61, 433–443. [Google Scholar] [CrossRef]
- Warren, C.R. Rapid measurement of chlorophylls with a microplate reader. J. Plant Nutr. 2008, 31, 1321–1332. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Spinola, V.; Mendes, B.; Camara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Lee, J.G. Ripening-dependent changes in antioxidants, color attributes, and antioxidant activity of seven tomato (Solanum lycopersicum L.) cultivars. J. Anal. Method Chem. 2016, 2016, 5498618. [Google Scholar] [CrossRef]
- Jo, J.S.; Bhandari, S.R.; Kang, G.H.; Lee, J.G. Comparative analysis of individual glucosinolates, phytochemicals, and antioxidant activities in broccoli breeding lines. Hortic. Environ. Biotechnol. 2016, 57, 392–403. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Qin, L.; Guo, S.; Ai, W.; Tang, Y.; Cheng, Q.; Chen, G. Effect of salt stress on growth and physiology in amaranth and lettuce: Implications for bio-regenerative life support system. Adv. Space Res. 2013, 51, 476–482. [Google Scholar] [CrossRef]
- Goussi, R.; Manaa, A.; Derbali, W.; Cantamessa, S.; Abdelly, C.; Barbato, R. Comparative analysis of salt stress, duration and intensity, on the chloroplast ultrastructure and photosynthetic apparatus in Thellungiella salsuginea. J. Photochem. Photobiol. B 2018, 183, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mou, B. Evaluation of lettuce genotypes for salinity tolerance. HortScience 2015, 50, 1441–1446. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Giannakoula, A.; Ilias, I.; Zamanidis, P. Alleviation of drought and salinity stresses on growth, physiology, bioachemistry and quality of two Cucumis sativus L. cultivars by Si application. Braz. J. Bot. 2016, 39, 531–539. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Ilias, I.F.; Giannakoula, A.; Theocharidou, I. Effect of water stress and NaCl triggered changes on yield, physiology, biochemistry of broad bean (Vicia faba L.) plants and on quality of harvested pods. Biologia 2014, 69, 1010–1017. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Gorbe, E.; Calatayud, A. Applications of chlorophyll fluorescence imaging technique in horticultural research—A review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Kim, J.H.; Bhandari, S.R.; Chae, S.Y.; Cho, M.C.; Lee, J.G. Application of maximum quantum yield, a parameter of chlorophyll fluorescence, for early detection of bacterial wilt in tomato seedlings. Hortic. Environ. Biotechnol. 2019, 60, 821–829. [Google Scholar] [CrossRef]
- Woo, N.S.; Badger, M.R.; Pogson, B.J. A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods 2008, 4, 27. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Kim, Y.H.; Lee, J.G. Detection of temperature stress using chlorophyll fluorescence parameters and stress-related chlorophyll and proline content in paprika (Capsicum annuum L.) seedlings. Hortic. Sci. Technol. 2018, 36, 619–629. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Z.; Yang, J.; Ni, X.; Zhu, B. Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environ. Exp. Bot. 2009, 66, 270–278. [Google Scholar] [CrossRef]
- Stepien, P.; Johnson, G.N. Contrasting responses of photosynthesis to salt stress in the glycophyte Arabisopsis and the halophyte Thellungiella: Role of plastid terminal oxidase as an alternative election sink. Plant Physiol. 2009, 149, 1154–1165. [Google Scholar] [CrossRef]
- Acosta-Motos, J.R.; Diaz-Vivancos, P.; Alvarez, S.; Fernandez-Garcia, N.; Sanchez-Blanco, M.J.; Hernandez, J.A. NaCl-induced physiological and bioachemical adaptative mechanisms in the ornamental Myrtus communis L. plants. J. Plant Physiol. 2015, 183, 41–51. [Google Scholar] [CrossRef]
- Song, X.; Zhou, G.; Ma, B.L.; Wu, W.; Ahmad, I.; Zhu, G.; Yan, W.; Jiao, X. Nitrogen application improved photosynthetic productivity, chlorophyll fluorescence, yield and yield components of two oat genotypes under saline conditions. Agronomy 2019, 9, 115. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Langsdorf, G.; Lenk, S.; Buschmann, C. Chlorophyll fluorescence imaging of photosynthetic activity with the flash-lamp fluorescence imaging system. Photosynthetica 2005, 43, 355–369. [Google Scholar] [CrossRef]
- Trovato, M.; Mattioli, R.; Costantino, P. Multiple roles of proline in plant stress tolerance and development. Rend. Lincei. Sci. Fis. Nat. 2008, 19, 325–346. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef]
- Chen, Z.; Cuin, T.A.; Zhou, M.; Twomey, A.; Naidu, B.P.; Shabala, S. Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J. Exp. Bot. 2007, 58, 4245–4255. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Bartha, C.; Fodorpataki, L.; Martinez-Ballesta, M.C.; Popescu, O.; Carvajal, M. Sodium accumulation contributes to salt stress tolerance in lettuce cultivars. J. App. Bot. Food Qual. 2015, 88, 42–48. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Taffouo, V.D.; Nouck, A.H.; Dibong, S.D.; Amougou, A. Effects of salinity stress on seedling growth, numeral nutrients, and total chlorophyll of some tomato (Lycopersicum esculentum L.) cultivars. Afr. J. Biotechnol. 2010, 9, 5366–5372. [Google Scholar]
- Silveira, J.A.G.; Carvalho, F.E.L. Proteomics, photosynthesis and salt resistance in crops: An integrative view. J. Proteom. 2016, 143, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Neocleous, D.; Koukounaras, A.; Siomos, A.S.; Vasilakakis, M. Assessing the salinity effects on mineral composition and nutritional quality of green and red “baby” lettuce. J. Food Qual. 2014, 37, 1–8. [Google Scholar] [CrossRef]
- Hand, M.J.; Taffouo, V.D.; Nouck, A.E.; Nyemene, K.P.J.; Tonfack, L.B.; Meguekam, T.L.; Youmbi, E. Effects of salt stress on plant growth, nutrient partitioning, chlorophyll content, leaf relative water content, accumulation of osmolytes and antioxidant compounds in pepper (Capsicum annuum L.) cultivars. Not. Bot. Horti Agrobo. 2017, 45, 481–490. [Google Scholar] [CrossRef]
- Zribi, L.; Fatma, G.; Fatma, R.; Salwa, R.; Hassan, N.; Neejib, R.M. Application of chlorophyll fluorescence for the diagnosis of salt stress in tomato “Solanum lycopersicum (variety Rio Grande)”. Sci. Hortic. 2009, 120, 367–372. [Google Scholar] [CrossRef]

| Parameter | Formula | Description |
|---|---|---|
| Fv/Fm | (Fm − F0)/Fm | Maximum quantum yield of PSII photochemistry measured in the dark-adapted state |
| Fv′/Fm′ | (F′m − F′0)/F′m | Exciton transfer efficiency from antenna pigments to the reaction center of photosystem II (PSII) in the light-adapted state |
| Y(PSII) | (F′m − Fs)/F′m | Effective quantum yield of photochemical energy conversion in PSII |
| NPQ | (Fm − F′m)/F′m | Non photochemical quenching of maximum fluorescence |
| qP | (F′m − Fs)/(F′m − F′0) | Photochemical quenching of PSII |
| qN | (Fm − F′m)/(Fm − F′0) | Coefficient of non-photochemical quenching of variable fluorescence |
| qL | qP x F0/Fs | Coefficient of photochemical quenching of variable fluorescence based on the lake model of PSII |
| Y(NO) | 1/[NPQ + 1 + qL(Fm/F0 − 1)] | Quantum yield of non-regulated energy dissipation in PSII |
| Y(NPQ) | 1 − ϕPSII − ϕNO | Quantum yield of regulated energy dissipation in PSII |
| Rfd | (Fm − Fs)/Fs | Ratio of fluorescence decline |
| NaCl Concentration | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Epicotyl Length (cm) | True Leaf Number | Leaf Length (cm) | Leaf Width (cm) | Leaf Length/Leaf Width |
|---|---|---|---|---|---|---|---|
| 0 mM | 3.65 ± 0.34 f | 0.22 ± 0.01 e | 0.48 ± 0.08 e | 6.60 ± 0.55 e | 11.12 ± 0.60 f | 5.30 ± 0.35 f | 2.10 ± 0.09 c |
| 50 mM | 3.09 ± 0.35 e | 0.20 ± 0.02 d | 0.38 ± 0.04 d | 6.60 ± 0.55 e | 10.20 ± 0.33 e | 4.78 ± 0.50 e | 2.15 ± 0.19 c |
| 100 mM | 2.54 ± 0.20 d | 0.20 ± 0.02 d | 0.30 ± 0.00 c | 6.00 ± 0.00 d | 8.58 ± 0.38 d | 4.38 ± 0.11 d | 1.96 ± 0.08 b |
| 200 mM | 1.59 ± 0.19 c | 0.16 ± 0.02 c | 0.30 ± 0.00 c | 5.00 ± 0.00 c | 6.80 ± 0.37 c | 3.72 ± 0.18 c | 1.83 ± 0.06 ab |
| 300 mM | 0.91 ± 0.12 b | 0.12 ± 0.02 b | 0.20 ± 0.00 b | 3.80 ± 0.45 b | 5.48 ± 0.38 b | 3.14 ± 0.13 b | 1.74 ± 0.07 a |
| 400 mM | 0.48 ± 0.08 a | 0.08 ± 0.01 a | 0.10 ± 0.00 a | 2.60 ± 0.55 a | 4.86 ± 0.19 a | 2.62 ± 0.15 a | 1.86 ± 0.06 ab |
| CF Parameters | NaCl Concentration (N) | Treatment Time (T) | N × T | |||
|---|---|---|---|---|---|---|
| F-Value | Significance | F-Value | Significance | F-Value | Significance | |
| Fv/Fm | 16.46 | *** | 7.98 | *** | 8.67 | *** |
| F′v/F′m | 9.07 | *** | 39.72 | *** | 1.66 | ** |
| Y(PSII) | 21.53 | *** | 89.95 | *** | 12.39 | *** |
| NPQ | 18.75 | *** | 37.60 | *** | 4.06 | *** |
| qN | 14.62 | *** | 30.95 | *** | 2.92 | *** |
| qP | 22.40 | *** | 103.74 | *** | 13.07 | *** |
| qL | 20.88 | *** | 95.11 | *** | 13.59 | *** |
| Rfd | 28.08 | *** | 70.05 | *** | 8.50 | *** |
| Y(NO) | 28.56 | *** | 55.76 | *** | 15.13 | *** |
| Y(NPQ) | 10.24 | *** | 64.49 | *** | 7.56 | *** |
| Parameters | NaCl Concentration (N) | Treatment Time (T) | N × T | |||
|---|---|---|---|---|---|---|
| F-Value | Significance | F-Value | Significance | F-Value | Significance | |
| Total phenol | 18.64 | *** | 168.52 | *** | 4.07 | *** |
| Total flavonoid | 22.91 | *** | 294.53 | *** | 2.46 | ** |
| Ascorbic acid | 47.92 | *** | 170.84 | *** | 9.91 | *** |
| Chlorophyll a | 17.07 | *** | 11.23 | *** | 4.66 | *** |
| Chlorophyll b | 18.14 | *** | 15.34 | *** | 4.36 | *** |
| Total chlorophyll | 18.34 | *** | 13.19 | *** | 4.75 | *** |
| FRAP assay | 12.93 | *** | 114.26 | *** | 2.79 | *** |
| ABTS assay | 16.76 | *** | 131.66 | *** | 3.74 | *** |
| Proline | 3779 | *** | 4250 | *** | 711 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities. Agronomy 2020, 10, 1627. https://doi.org/10.3390/agronomy10111627
Shin YK, Bhandari SR, Jo JS, Song JW, Cho MC, Yang EY, Lee JG. Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities. Agronomy. 2020; 10(11):1627. https://doi.org/10.3390/agronomy10111627
Chicago/Turabian StyleShin, Yu Kyeong, Shiva Ram Bhandari, Jung Su Jo, Jae Woo Song, Myeong Cheoul Cho, Eun Young Yang, and Jun Gu Lee. 2020. "Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities" Agronomy 10, no. 11: 1627. https://doi.org/10.3390/agronomy10111627
APA StyleShin, Y. K., Bhandari, S. R., Jo, J. S., Song, J. W., Cho, M. C., Yang, E. Y., & Lee, J. G. (2020). Response to Salt Stress in Lettuce: Changes in Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities. Agronomy, 10(11), 1627. https://doi.org/10.3390/agronomy10111627







