Abstract
To model the rates of interfacial polycondensations, the rates of reaction of benzoyl chloride and methyl chloroformate with various aliphatic monoamines in acetonitrile were determined at 25 °C. Buffering with picric acid slowed these extremely fast reactions so the rate constants could be determined from the rate of disappearance of picrate ion. The rates of the amine reactions correlated linearly with their Swain-Scott nucleophilicities.
1. Introduction
Interfacial polycondensation is an important polymerization technique, recently used for membrane formation [1] and encapsulation [2]. It involves the extremely rapid reaction of diacid chlorides or bis-chloroformates with diamines. Although this process has been known for over fifty years [3,4,5], measurement of the rate constants has not yet been reported. This is not surprising since amines are among the most nucleophilic molecules while acid chlorides are among the most electrophilic. Besides the usual problems in measuring rate constants for ultrafast reactions, reactions often become heterogeneous and the components lack UV absorption for stopped flow kinetics.
We worked with monoamines to simplify the kinetics. A requirement was that the amines be reversibly masked to decrease their instantaneous concentrations. This can conveniently be done in water solution using buffer solutions of varying pH. A first step in this area was noticing that methyl chloroformate hydrolyzed in water at room temperature with a half-life of about one hour [6,7]. This made it possible to examine the rates of reaction with amines under buffered conditions in water. To avoid competitive reaction with buffer components, a series of sterically hindered buffers was synthesized [8]. The rate constants obtained by this technique were very high, up to 104 L/mol∙s.
Subsequently, Castro and Moodie [9] used a pH-stat to follow the reactions, finding EtNH2 k2 = 390 L∙mol−1∙s−1. Ahnfeldt and Harting [10] gave a rate constant of 1.1 × 102 L∙mol−1∙s−1 for ethyl chloroformate—butylamine. These values are consistent with our previous ones. However, other acid chlorides cannot be measured by these techniques because they hydrolyze in water too rapidly.
We utilized a novel technique to determine the rate constants in organic solvents: amine picrates were reacted with acid chlorides in acetonitrile solution. The reactions were followed by UV, which monitored the disappearance of picrate ion. In this way the concentration of free amine is buffered to very low concentrations which allow rate measurements. Picrate ion was selected because its 2,6-dinitro groups sterically hinder its capacity for competitive nucleophilic behavior. Building on our exploratory studies on the base strengths of amines in nonprotolytic solvents [11], extensive elegant research on acid-base reactions in acetonitrile solution by the groups of Kolthoff [12,13,14,15] and Coetzee [16] in the 1960s led to the evaluation of the dissociation constants of picric acid and of amine salts in acetonitrile. These data were essential for interpreting our results.
2. Experimental Section
Chemicals were used as received from Aldrich. Picrates were prepared by mixing equimolar quantities of the amine and picric acid in ether. In most cases the salt precipitated and was filtered; in the remaining cases the solution was evaporated and the salt was dried in a vacuum desiccator over P2O5. Serial dilutions of the amine picrates were used to crate a calibration curve which was used to calculate the molar absorbtivity with Beer’s Law. In a glass cuvette various volumes and concentrations of the acid chloride were added to 1.5 mL of the amine picrate solution. The solution was mixed for 2 s. The absorbance dropped immediately because of dilution and then a smooth reaction decay curve occurred. An Ocean Optics Chem 2000 UV-VIS spectrometer recorded absorbance values at 375 nm every second until the reaction was finished. The absorbance values were then converted to concentrations using Beer’s Law and plotted according to the rate equation.
3. Results and Discussion
The following rate equation was derived
2∙ln([P−]0 + [P−]t) − ln[P−]t = 0.5Ct + ln 4 [P-]o
where P− = picrate anion and
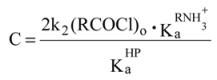
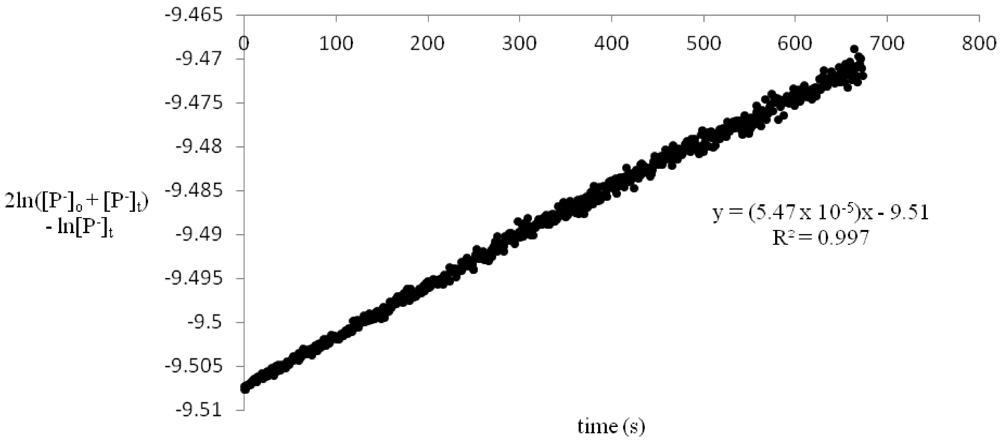
Figure 1.
2ln([P−]o + [P−]t) − ln[P−]t vs. time for reaction of 3.12 × 10−5 M benzylamine picrate with 1.18 × 10−2 M benzoyl chloride.
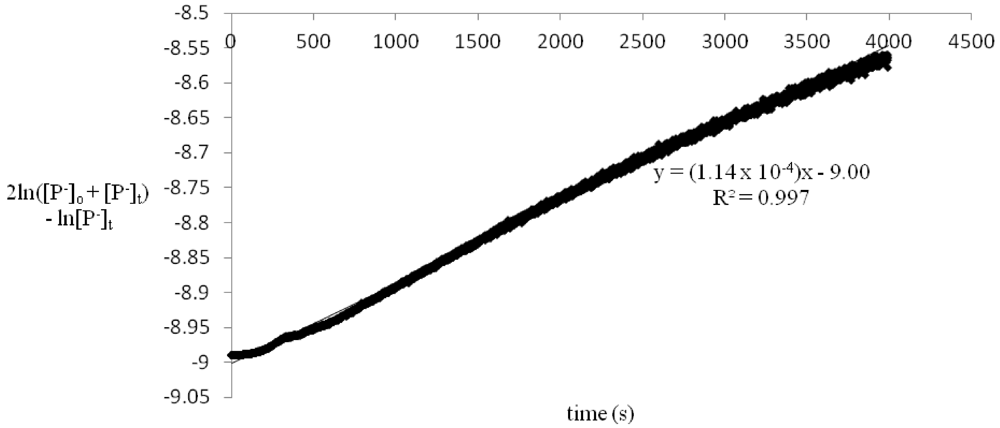
Figure 2.
2ln([P−]o + [P−]t) − ln[P−]t vs. time for reaction of 2.40 × 10−5 M benzylamine picrate with 1.18 × 10−2 M methyl chloroformate.
The kinetic data resulting from the averages of several runs at varying concentrations are summarized in Table 1 with the amines listed in order of increasing nucleophilicity. Their rates of reaction with benzoyl chloride and methyl chloroformate are given. The reactions were followed for >3 half-lives. Amines of lower basicity gave measurable rates of reaction. Amines of higher basicity are too highly protonated by picric acid to permit them to react; piperidine and pyrrolidine, the strongest bases, overcome this barrier because of their extremely high nucleophilicity. Aliphatic amines, strongly basic but less nucleophilic, react slowly. Aniline, on the other hand, is less strongly protonated, but its low nucleophilicity gave measurable rates.

Table 1.
Reaction rates of amine picrates with acid chlorides.
| Amine in amine picrate | Methyl chloroformate | Benzoyl chloride |
|---|---|---|
| k2, L∙mol−1∙s−1 | k2, L∙mol−1∙s−1 | |
| Aniline | 5.6 ± 0.6 × 10−3 | 1.0 ± 0.1 × 100 |
| Benzylamine | 1.2 ± 0.1 × 103 | 5.0 ± 2 × 103 |
| Ethyl 1-piperazinecarboxylate | 3.1 ± 0.1 × 103 | 4.9 ± 3 × 104 |
| Morpholine | 6.6 ± 2.9 × 102 | 3.7 ± 0.6 × 104 |
| Piperidine | 6.7 ± 0.6 × 105 | 1.7 ± 1 × 106 |
| Pyrrolidine | 8.6 × 106 | 1.1 ± 0.8 × 107 |
Tetrabutylammonium picrate and N-ethyldiisopropylammonium picrate reacted at negligibly slow rates, showing that the sterically hindered, weakly basic picrate anion does not compete as a nucleophile.
Methyl chloroformate was always less reactive than benzoyl chloride. This is reasonable since electron donation from OMe to the carbonyl group in the ester, deactivating it, exceeds that of phenyl to the carbonyl group in the acid chloride.
The rate constants for reactions of amines with methyl chloroformate in acetonitrile were substantially higher than those found earlier for the same reactions in water. This is reasonable since in water the amines are hydrated, sterically and electronically diminishing their reactivity.
The rate constants for both benzoyl chloride and methyl chloroformate were shown to follow the Swain-Scott equation log(k/ko) = sn, where s is the substrate constant and n is the nucleophile constant [17]. We showed that aliphatic amines follow this linear free energy relationship and were able to assign nucleophilicity values n to a variety of aliphatic amines [18]. Figure 3 plots n vs. logk2 for the present results with benzoyl chloride and Figure 4 with methyl chloroformate. The correlations are good over 7 powers of 10.
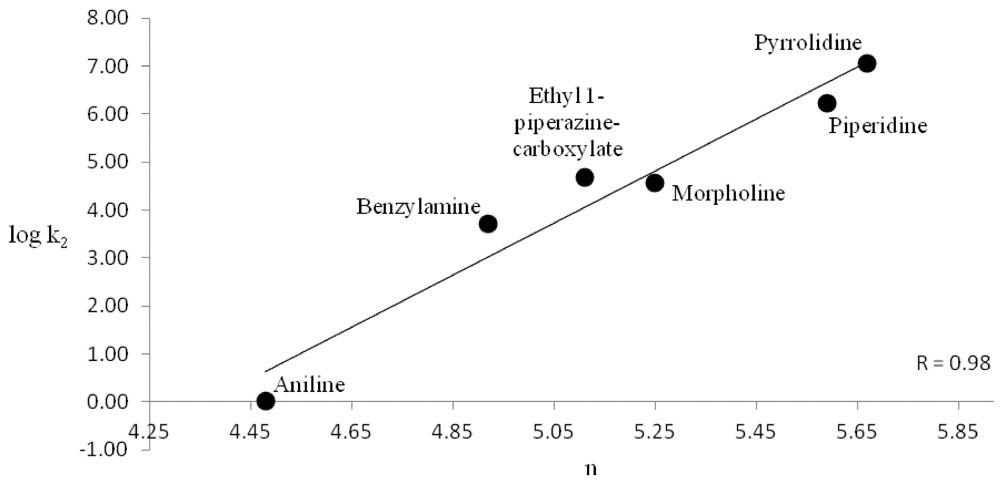
Figure 3.
Swain-Scott nucleophilicity n vs. log k2 for benzoyl chloride–amine reactions.
The case of aniline allows a direct comparison with a literature value. This amine reacts slowly enough to measure by conventional methods. Bose and Hinshelwood [19] showed that benzoylation of m-chloroaniline in polar benzonitrile proceeded with rate constant 0.16 L∙mol−1∙s−1. Multiplying this value by 10 to correct it to aniline gives 1.6 L∙mol−1∙s−1, which is in reasonable agreement with our value of 1.0 L∙mol−1∙s−1.
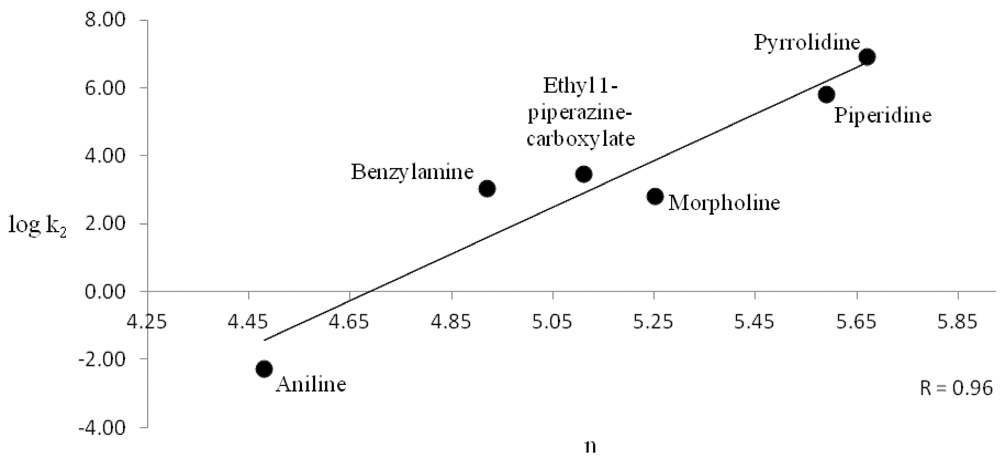
Figure 4.
Swain-Scott nucleophilicity n vs. log k2 for methyl chloroformate—amine reactions.
4. Conclusions
The primary amines ethylamine and butylamine reacted too slowly to measure under these conditions. The reason is that while they are about as basic as the secondary amines used here, they are substantially less reactive; picric acid protonates them so highly that the free amine is present in too low concentrations to react. A solution to this problem should be the use of a sterically hindered phenol weaker than picric acid. Conversely, more reactive aliphatic acid chlorides will require a stronger acid than picric.
In interfacial polymerization, the extremely high rates found for the free amines are much faster than the rate of mixing; they even approach the diffusion control limit at which molecules react on contact. Thus the rate of decrease of diamine in the water layer is a measure of the polymerization rate as well.
The kinetics technique used here is very simple and flexible. It enables the measurement of hitherto inaccessible aminolysis rates for a variety of readily hydrolyzable carbonyl, sulfonyl, and phosphoryl chlorides.
Acknowledgment
We are indebted to Solvay Advanced Polymers for support of this work.
References
- Feng, C.; Khulbe, K.C.; Matsuura, T. Applications of nanofibers and nanofiber membranes via electrospinning interfacial polymerization. J. Appl. Polym. Sci. 2010, 115, 756–776. [Google Scholar] [CrossRef]
- Shan, G.-R.; Cao, Z.-H. Polymeric nanocapsules by interfacial miniemulsion polymerization. In Miniemulsion Polymerization Technology; Mittal, V., Ed.; Wiley: New York, NY, USA, 2010; pp. 97–138. [Google Scholar]
- Morgan, P.W. Development of low temperature polycondensation processes and aromatic polyamides. J. Polym. Sci. Polym. Symp. 1982, 72, 27–37. [Google Scholar] [CrossRef]
- Wittbecker, E.L.; Morgan, P.W. Interfacial polymerization I. J. Polym. Sci. A 1996, 34, 521–529. [Google Scholar] [CrossRef]
- Morgan, P.W.; Kwolek, S.L. Interfacial polymerization II. Fundamentals of polymer formation and liquid interfaces. J. Polym. Sci. A 1996, 34, 531–559. [Google Scholar] [CrossRef]
- Boehme, H.; Schuerhoff, W. Ueber die hydrolyse organischer halogenverbindingen in gemischen von wasser und dioxan. Chem. Ber. 1951, 84, 28–47. [Google Scholar] [CrossRef]
- Hall, H.K., Jr. Mechanisms of hydrolysis of carbonyl chlorides. J. Am. Chem. Soc. 1955, 77, 5993–5996. [Google Scholar] [CrossRef]
- Hall, H.K., Jr. Sterically hindered phenolic buffers. Application to determination of rates of amidation of ethyl chloroformate. J. Am. Chem. Soc. 1957, 79, 5439–5441. [Google Scholar] [CrossRef]
- Castro, E.A.; Moodie, R.B. Kinetics of hydrolysis and aminolysis of methyl chloroformate in aqueous solution. J. Chem. Soc. Perkin Trans. II 1974. [Google Scholar] [CrossRef]
- Ahnfelt, N.; Hartrey, P. GC analysis of primary and secondary amines after derivatization with chloroformates in buffered aqueous solution. Acta Pharm. Sueca 1980, 17, 307–318. [Google Scholar]
- Hall, H.K., Jr. Potentiometric determination of the base strengths of amines in nonprotolytic solvents. J. Phys. Chem. 1956, 60, 63–70. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Bruckenstein, S.; Chantooni, M.K., Jr. Acid-base equilibria in acetonitrile; pKa of various acids. J. Am. Chem. Soc. 1961, 83, 3927–3935. [Google Scholar]
- Kolthoff, I.M.; Chantooni, M.K., Jr. Molecular acid-base dissociations in acetonitrile. J. Am. Chem. Soc. 1965, 87, 1004–1012. [Google Scholar] [CrossRef]
- Kolthoff, I.M.; Chantooni, M.K., Jr. Calibration of the glan electrode in acetonitrile; dissociation constant of picric acid. J. Am. Chem. Soc. 1965, 87, 4428–4436. [Google Scholar]
- Kolthoff, I.M.; Chantooni, M.K., Jr. Effect of water on the dissociation constant of picric acid in acetonitrile. The pKa of picric acid in acetonitrile is 11.0. J. Am. Chem. Soc. 1969, 91, 6907–6910. [Google Scholar] [CrossRef]
- Coetzee, J.F.; Padmanabhan, G.R. Properties of bases in acetonitrile as solvent. IV. proton acceptor power and homoconjugation of mono- and diamines. J. Am. Chem. Soc. 1965, 87, 5005–5010. [Google Scholar] [CrossRef]
- Swain, C.G.; Scott, C.B. Quantitative correlation of relative rates. Comparison of hydroxide ion with other nucleophilic reagents toward alkyl halides, esters, epoxides and acyl halides. J. Am. Chem. Soc. 1953, 75, 141–147. [Google Scholar] [CrossRef]
- Hall, H.K., Jr. Correlation of the nucleophilic reactivity of aliphatic amines. J. Am. Chem. Soc. 1964, 29, 3539–3544. [Google Scholar]
- Bose, A.N.; Hinshelwood, C.N. Benzoylation of aniline in different solvents. J. Chem. Soc. 1958, 4085–4092. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).