Abstract
Thermoplastic starch (TPS) blended with biodegradable polyesters such as polyhydroxybutyrate (PHB), polylactic acid (PLA), polybutylene succinate (PBS), and polycaprolactone (PCL) represents a promising route toward sustainable alternatives to petroleum-based plastics. TPS offers advantages related to abundance, low cost, and biodegradability, while polyesters provide improved mechanical strength, thermal stability, and barrier performance. However, the intrinsic incompatibility between hydrophilic TPS and hydrophobic polyesters typically leads to immiscible systems with poor interfacial adhesion and limited performance. This review critically examines recent advances in the development of TPS/polyester blends, with emphasis on compatibilization strategies based on chemical modification, natural and synthetic compatibilizers, bio-based additives, and reinforcing agents. Particular attention is given to the role of organic acids, essential oils, phenolic compounds, nanofillers, and natural reinforcements in controlling morphology, crystallinity, interfacial interactions, and thermal–mechanical behavior. In addition, the contribution of bioactive additives to antimicrobial and antioxidant functionality is discussed as an emerging multifunctional feature of some TPS/polyester systems. Finally, current limitations related to long-term stability, scalability, and life cycle assessment are highlighted, identifying key challenges and future research directions for the development of advanced biodegradable materials with tailored properties.
1. Introduction
The high dependence on conventional plastics has led to a massive growth in their production [1], as these materials offer a wide range of properties that are difficult to replicate in alternatives [2]. Approximately 45% of all plastics are used exclusively for food packaging [3]. However, their improper disposal practices have led to significant environmental contamination, due to their non-biodegradable [4] and recalcitrant nature [5]. Between the 1950s and 2015, about 6.3B tons of plastic were produced, of which only 21% have been managed by recycling or incineration [6]. Production continued to rise, reaching 359M tons in 2018 [7]. In addition, COVID-19 intensified plastic waste generation due to the massive use of disposable items [2].
This challenge is particularly critical in food packaging, where materials require resistance and flexibility, typically provided by petroleum-derived polymers [8]. These materials also pose risks to human health, due to the release of chemical and microplastics [9], which have been associated with cardiovascular diseases [10], reproductive disorders [11], and cancer [12]. These problems have led to the search for more sustainable and biodegradable alternatives [13], which can biodegrade more rapidly in natural environments [14], though early bioplastics often exhibited high production costs and inferior mechanical [15].
Starch is a low-cost [4], abundant [16], and fully biodegradable polysaccharide [1], composed of amylose and amylopectin [17]. Amylose consists of a linear chain with α-(1 → 4) bonds, while amylopectin has a branched structure, incorporating the same type of bonds as amylose in the main chain and α-(1 → 6) bonds in the branching zones [15]. Its semi-crystalline structure [18] varies among botanical sources such as potato [19], corn [20,21], sago, peach [5], cassava [14,22], yam [23,24,25], wheat [26,27], sweet potato [28], mango seeds [29], banana, rice [30], jackfruit seed [31], etc. However, the abundance of -OH groups renders starch highly hydrophilic [15], leading to moisture sensitivity [5] and limited mechanical performance [17,18].
Starch can be transformed into thermoplastic starch (TPS) when processed with plasticizers, heat, and shear [5], and modified through processes such as esterification [32], hydrolysis [33], crosslinking [34], and other chemical reactions [16]. To further enhance TPS properties, it is commonly blended with biopolyesters such as PHB, PLA, etc. [35]. These polyesters have become widely used due to their biodegradability [36], stiffness, barrier properties [37], thermal stability, and biocompatibility [38,39,40]. However, their production cost is relatively high [41], so it is an attractive option to blend them with other lower-cost polymers, such as TPS, to obtain more economical and environmentally friendly materials. Among these biopolyesters, polymers like PLA, PCL, and other PHAs show great potential in various applications, such as the packaging sector [42], medicine [43], etc., broadening the spectrum of sustainable alternatives.
In recent years, there has been an increasing interest in the use of naturally occurring agents, such as essential oils and organic acids, as they serve not only as compatibilizers in polymeric blends but also impart antimicrobial, antioxidant, or hydrophobic properties [44,45,46,47]. Such characteristics enable the development of more functional and value-added materials for applications in the food sector.
It should be noted that the TPS/polyester blends discussed in this review are reported in different physical forms depending on the processing method and target application. However, thin films are the most frequently investigated form, particularly in studies addressing morphology, barrier performance, and functional properties.
Considering this, the aim of this review is to critically analyze recent advances in TPS/biopolyester blends, with a particular emphasis on compatibilization strategies involving natural and synthetic agents, including chemical modifiers, reactive compatibilizers, bio-based additives, and reinforcements. This review discusses how these approaches influence interfacial interactions, morphology, and thermal–mechanical performance, as well as the emergence of bioactive functionalities, providing a comprehensive framework for the design of sustainable polymeric materials.
2. Thermoplastic Starch and Biodegradable Polyesters: Molecular Features, Sources, and Processing Approaches
TPS is a material produced from native starch (NS) plasticization [5]. By itself, NS cannot be called a thermoplastic [48], since the intermolecular forces among the -OH groups are very strong. However, the addition of a plasticizer, under conditions of heat and constant stress, can yield moldable behavior akin to those of a thermoplastic polymer [20]. A plasticizer is a compound added to a polymer to provide flexibility under conditions higher than the initial ones [49]. Various substances have been used for this purpose, such as urea, acetamides, sugars [5], glycerol [21], sorbitol, formamide [50], citric acid, polyethylene glycol [51], propylene glycol, xylitol, mannitol [52], glycerol monostearate [27,53], ethanolamine [54], maleic anhydride [14], water, etc. Vegetable and essential oils have also been employed, which can reduce the thermal degradation of NS and improve the processing conditions by lubrication [5]. The NS plasticization process is illustrated in Figure 1.
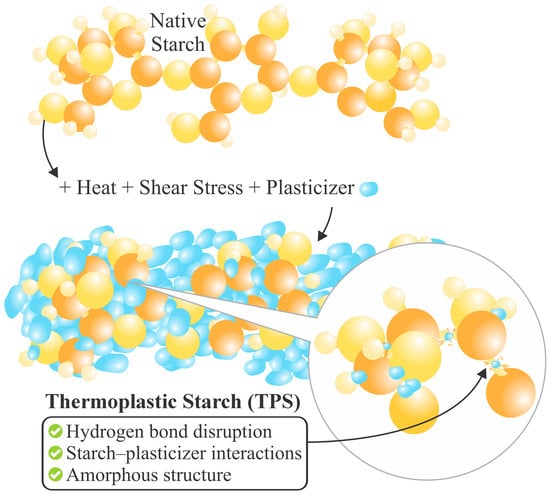
Figure 1.
Schematic representation of native starch plasticization under thermo-mechanical treatment and plasticizer addition, leading to TPS formation.
Under specified thermo-mechanical conditions, adding plasticizing agents results in the plasticizer molecules interspersing among the starch molecules, thereby disrupting a large part of the -OH bonds (see Figure 1) and reducing the intermolecular forces among them [49]. So, the interrelation between them would no longer be NS–NS, but NS–plasticizer [5], which transforms the NS semi-crystalline structure into an amorphous one, which is characteristic of TPS, as shown in Figure 2 [17]. This transition lowers the glass transition temperature (Tg) [52], melting point (Tm), and brittleness, while enhancing flexibility [48]. The plasticization process can be internal (when modifications are made to the chemical structure) or external (when the plasticizing agents do not produce new bonds) [52]. Therefore, it is not only the starch source alone that influences the ultimate material properties [55]; rather, the processing conditions, environment, and type of plasticizer also influence the mitigation of retrogradation [5]. Among the most used methods for TPS production are compression molding [56], casting, and extrusion [13,48]. Extrusion is particularly efficient due to the operational parameters and equipment design, which promote crystalline disruption and facilitate the production of a more homogeneous and structurally coherent material [48].
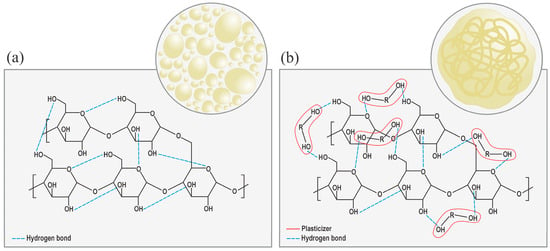
Figure 2.
(a) Native starch structure with intermolecular hydrogen bonds. (b) Plasticizer molecules disrupting starch–starch bonds and promoting starch–plasticizer interactions, yielding an amorphous TPS structure.
Poly(3-hydroxybutyrate) (PHB) is a biodegradable polyester [57]. It has been the most extensively studied compound of its kind [58] and belongs to the polyhydroxyalkanoate (PHA) family. It is a succession of 3-hydroxybutyrate monomers, consisting of methyl, carboxyl, and ester groups [59,60], as illustrated in Figure 3. This composition results in similar properties to those of some fossil-based plastics [60]. The production costs of this material depend on several factors, including cultivation methods, origin, and the processes used [58]. PHB can be extracted from various sources, such as shells, feathers, and even organic waste containing fish, oil, fruits, vegetables, dairy, rice, etc. [38]. There are even microorganisms (such as Ralstoniaeutropha) that can synthesize this type of material [61], as it is usually agglomerated in their cell membranes, reaching in some cases up to a little more than 80% by weight of the cell; therefore, the structure of this material can have certain variations, depending on the different classes of bacteria [58,62]. Its production is a response mechanism of certain microorganisms to stress, usually produced by nutrient restriction during cultivation [63]. There are other methods to obtain PHB, which involve the use of surfactants for chemical digestion, solvents (usually organic) for extraction, and fermentation. However, these tend to be more contaminating than the microorganism synthesis method [39,40].
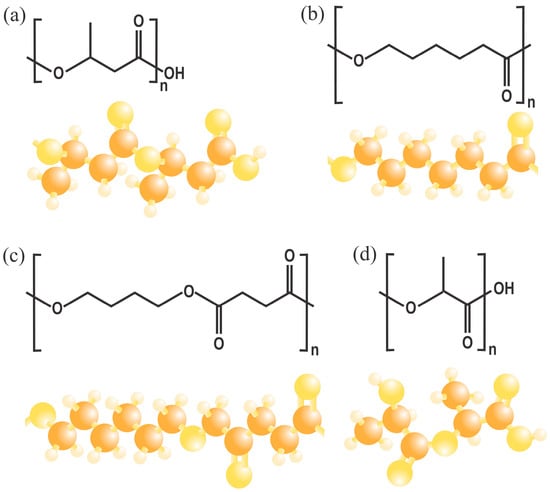
Figure 3.
Molecular structures of representative biodegradable polyesters: (a) poly(3-hydroxybutyrate) (PHB), (b) polycaprolactone (PCL), (c) poly(butylene succinate) (PBS), and (d) polylactic acid (PLA).
PLA is a biodegradable aliphatic polyester obtained from lactic acid by polycondensation from raw materials such as corn starch [42]. Its molecular structure is illustrated in Figure 3, which imparts a modulable crystallinity, making it possible to adjust its Tg and Tm for applications in packaging [64] and 3D printing [65], among other things. PLA can achieve transparency and stiffness comparable to that of some conventional polystyrenes, and it also possesses UV barrier properties [66]. However, it is a relatively brittle material with slow crystallization kinetics, which means that additives must be used to improve its mechanical properties, industrial productivity [67], and, in some cases, its biodegradability, since it must be subjected to industrial composting conditions to be completely degraded [68].
Another biodegradable polymer is PBS, which is synthesized by polycondensation of the monomers succinic acid and 1,4-butanediol, both obtained by fermentation [69]. In addition, it has a Tm close to 115 °C and a Tg of −22 °C [70], which gives it similar properties to polypropylene (PP) [71]. It also exhibits good processability by both extrusion and blow molding, which favors its use in agricultural applications [72]. However, in recent years, efforts have focused on developing polymeric blends to accelerate their degradation process and reduce high production costs [73]. PCL is a material produced by the ring opening of ε-caprolactone; its chemical structure is shown in Figure 3. It is a polymer with high flexibility and shelf life before degradation due to its low Tg and Tm [74], which makes it attractive for pharmaceutical applications.
2.1. Application Constraints and Processing Challenges
Polymers such as TPS can be produced at low cost, feature barrier properties, and are 100% biodegradable and compostable due to their natural occurrence [13,15,17,48,75]. PHB, being a biodegradable polymer, also poses inherent challenges that limit its applicability. Its thermal resistance is relatively low (See Table 1), constraining its use in applications that require stability at high temperatures. It is markedly more brittle than both biodegradable and synthetic polymers [37]; moreover, it is crystalline (up to 70%), which contributes to its brittle nature and susceptibility to fracture under high mechanical loads. Conversely, although PLA is a highly used biopolymer, its performance has limitations, with a Tg around 60 °C (Table 1) [76], which makes it necessary to exclude applications requiring high temperatures. Additionally, this material has a slow crystallization mechanism, which hinders rapid molding and, in some cases, prevents achieving the stiffness needed for high-performance packaging [77]. Being a highly stiff material, the lack of plastic deformation makes it prone to fracturing on impact [78].

Table 1.
Thermal, mechanical, and physical properties of common biopolyesters.
PBS, in contrast, is more ductile than PLA and offers good melt processability due to its relatively low Tm (115 °C). However, this also limits its application near that temperature threshold [69]. Additionally, PBS has high production costs and has the slowest biodegradation rate within this group, due to limited permeability to oxygen and water vapor [89]. On the other hand, PCL stands out for its flexibility and compatibility with textile materials but is limited in industrial applications by its low Tm and Tg, as shown in Table 1. These properties result in softening, even at elevated ambient temperatures, making it unsuitable for thermally demanding packaging [90]. Furthermore, its elastic modulus and tensile strength are significantly lower than those of other polyesters discussed. Although PCL is inherently biodegradable, its relatively slow decomposition rate restricts its suitability for sustainable biodegradability, and its relatively slow decomposition rate restricts its suitability for sustainable single-use applications [91].
Overall, each of these polyesters presents specific limitations. Recent research has demonstrated that blending them with other components, such as clay, TPS, plasticizers, and lubricants can modify their intrinsic properties, enabling the development of composites that combine the strengths of each constituent. This strategy broadens their potential application across multiple fields [56].
2.2. Functional Advantages and Potential Applications
Similarly, biodegradable polyesters have qualities that make them functional alternatives to synthetic plastics. PHB is a high-molar mass polymer [92], and has a Tm very close to PLA [93,94], ensuring that the elements manufactured with this material keep their original shape, even up to 132 °C [95]. It has antifungal properties [37], and is considered non-toxic [96], insoluble in water, piezoelectric, and more hydrophobic than starch [58]. Moreover, PHB can be chemically modified during processing through interaction with its functional groups or via reactions such as chlorination, hydroxylation, and methylation [97].
From an environmental standpoint, PHB is associated with a low ecological impact [38] and high biocompatibility, as it can degrade completely in a variety of active environments without leaving residues [37,94]. Its degradation can occur through enzymatic [98], aerobic (approximately 6 weeks), or anaerobic mechanisms, with the latter being the most efficient [60]. Remarkably, PHB’s performance is so comparable to conventional plastics that orange juice packaged in PHB containers has demonstrated similar stability to that achieved in high-density polyethylene (HDPE) bottles, indicating that product acidity is not an issue. Furthermore, its barrier properties regarding water vapor, flavor, odor, and CO2 have been found to be on par with those of PET and PVC [59].
PLA combines stiffness and transparency comparable to PET, and provides similar water vapor barrier performance. These features allow it to be used in applications such as thermoformed trays that preserve the freshness of high-moisture fruits, particularly when blended with antimicrobial agents, without compromising product visibility [99]. PBS, on the other hand, offers significantly higher elongation at break compared to other polymers (see Table 1), making it a strong candidate for film production while maintaining mechanical integrity up to approximately 115 °C [81]. In contrast, PCL is characterized by its excellent elongation at break and fast crystallization kinetics, which favor its use in the production of flexible components [100].
Given the inherent properties of TPS, it can be blended with hydrophobic polymers such as PP, PE [26], or the aforementioned biodegradable polyesters to reduce its hydrophilicity and combine the advantageous features of both phases. In recent years, numerous blends have been developed between TPS and polymers such as PP [17,21,101,102], PLA [103,104], PCL [13], PE [75,105], PHBV [106], PVA [107], PBSA [108], and PBS [109], among others.
Nonetheless, since TPS molecules are polar and most hydrophobic polymers are nonpolar, their blends may be heterogeneous when incompatibility arises between components. As illustrated in Figure 4, this incompatibility can be overcome by the use of compatibilizers, which improve phase dispersion and mechanical performance [5]. Such blends not only promote the development of biodegradable alternatives but also contribute to the reduction in fossil-based plastic consumption and greenhouse gas emissions [7].
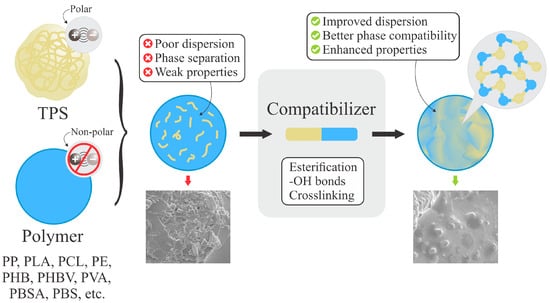
Figure 4.
Compatibilization in TPS/polymer blends. Adapted from [110] under the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/, accessed on 5 January 2026).
3. Morphological Limitations and Performance Constraints in Non-Compatibilized TPS and Biodegradable Polyester Blends
To improve the biodegradability and crystallinity properties of polyesters, blends with polymeric compounds have been developed recently, such as TPS, which helps to reduce brittleness and improve processability due to their amorphous nature [111]. To produce this type of film, the most used method has been compression molding, which involves placing the previously prepared formulation (usually by internal mixing or extrusion in previous stages) inside a hot mold to be compressed and form films or other solid forms [81]. Table 2 summarizes studies investigating the behavior of TPS blends with biodegradable polyesters and their deviations from the properties of the pure polymer matrices.

Table 2.
Processing methods and property responses of non-compatibilized TPS/biodegradable polyester blends.
Regarding non-compatible TPS/PHB blends, Lai et al. [92] used different types of starch plasticized with glycerol for their blending with PHB, achieving an increase in the mechanical properties compared to the main matrix (TPS); therefore, an appropriate degree of crystallization is crucial if a good performance in the blend is to be achieved. Additionally, the higher the glycerol content, the higher the water absorption. At higher PHB content, the blends exhibited more hydrophobic behavior, due to the molecular nature of PHB.
Thiré et al. [112] found that, in this type of blend, an increase in TPS concentration leads to a decrease in tensile strength, Young’s modulus, and elongation at break, due to the heterogeneous dispersion of starch in the PHB matrix. At the same time, thermal properties did not worsen with the addition of TPS. However, SEM showed phase immiscibility, which can be attributed to the incompatibility in the molecular groups of these two compounds. For TPS/PLA blends, a biphasic morphology has been previously reported with starch particles dispersed in the continuous PLA matrix, typically spherical and with sizes between 1 and 8 μm. This is a result of the low interfacial adhesion between the phases due to their differing polarities, which greatly limits their mechanical properties [113].
In other studies, TPS granules in non-compatibilized blends can reach up to 30 μm, which may act as a stress concentration point and favor premature failure due to crack generation. Given that the tensile strength decreases as TPS increases, the overall mechanical behavior dominates, despite the occasional minor improvement in elongation when compared to pure PLA (10–20%) [114]. Due to the stiffness and weak interfacial adhesion of both phases, non-compatibilized blends exhibited an elongation at break of less than 2% even with somewhat uniform dispersions, demonstrating their intrinsic brittleness. Furthermore, as the system’s TPS content still dominates the degradation rate, this absence of compatibilization has no discernible effect on the system’s degradability [115].
Ternary blends can be obtained when employing additional compounds in TPS/PLA blends. Vanovčanová et al. [116] made PLA/TPS/PHB blends, varying only PHB and PLA contents at a processing temperature profile of 160–190–170 °C, and, considering that PHB can degrade under such conditions, it was demonstrated that the PLA and TPS blend achieved improved performance in terms of processability, since the presence of PLA promotes a redistribution of the plasticizer from TPS toward the polyester-rich domains.
This redistribution reduces the effective exposure of PHB to thermally induced chain scission and enhances the melt stability of the blend. Additionally, it was found that the modifications in the mechanical properties resulted from thermal absorption that restores the molecular mobility lost during sub-Tg aging and promotes the relaxation of internal stresses. As mobility is recovered, the amorphous regions undergo structural reorganization, modifying the balance between stiffness and deformability [120].
According to reports, the type of starch employed in TPS/PBS blends may enhance the features of the polymeric blends even in the absence of compatibilization. Non-compatibilized blends made of waxy (WTPS, 0% amylose) and native (NTPS, 26% amylose) starch were assessed by Li et al. (2013) [117], who found that WTPS was easier to plasticize and allowed for better dispersion in PBS, which resulted in improved mechanical properties and higher processability compared to blends containing NTPS. As the PBS content increased, the blends showed higher strength and elongation, but lower stiffness. WTPS also reduced water absorption and improved thermal stability at high TPS contents, due to its more homogeneous morphology, demonstrating that the type of starch has a decisive influence on the properties of these types of blends [117].
Alternatively, non-compatible TPS/PCL blends can form limitedly miscible systems, since PCL usually acts as a continuous phase and TPS as a dispersed phase, even with a high TPS content, due to the higher fluidity of PCL in the blending processes [118]. In some cases, it has been possible to establish a certain level of compatibility promoted by -OH bonds [119], yet the blends remain significantly brittle since the interfacial adhesion is not strong. This means that PCL, although it reduces part of the moisture absorption, fails to prevent the retrogradation process after storage, which is more influenced by the environmental humidity than by the PCL content [118]. Finally, the crystallinity in this type of blend also depends on the starch source used, since the branched structure of amylopectin interferes with the crystalline order of PCL [119].
In general, non-compatibilized TPS/biodegradable polyester blends exhibit biphasic morphologies with poor interfacial adhesion, which adversely affects mechanical integrity and structural stability. Although each polymer contributes valuable attributes, their intrinsic immiscibility remains a major limitation, reinforcing the need for compatibilization strategies to achieve balanced and long-term performance.
4. Compatibilization and Reinforcement of TPS/Biopolyester Blends for Enhanced Properties
TPS and biopolyesters are high-potential materials for biodegradable plastics production [5]. For instance, polymers such as PHB degrade completely in both land and marine environments at temperatures from 21 °C, according to the TUV test [121,122]. Although polyesters provide enhanced stiffness and thermal resistance compared to TPS [58], most PHAs still exhibit brittleness and limited impact strength [123]. For this reason, they have been blended with other compounds such as PLA [116], PVA [124], or TPS [92]. However, these systems are often incompatible due to the differences in polarity and molecular structure, since TPS is hydrophilic [75], resulting in phase immiscibility and premature mechanical failure. Consequently, recent research has increasingly focused on compatibilization strategies to improve interfacial adhesion and stabilize TPS/biopolyester blends [122,125].
Compatibilizers are substances that can be strategically placed on contact surfaces to reduce interfacial tension and achieve improved integration between two or more components. The principle of compatibilization is based on interactions (chemical or physical) occurring among polymer functional groups. The compatibilizer agent has segments that bond or interact with one of the blend phases, while the other segments link to the second one [126], as shown in Figure 5. As a result, the interfacial energy decreases and the TPS polar group is more effectively dispersed within the PHB nonpolar phase, promoting finer phase distribution and enhanced interfacial adhesion [17]. Therefore, a compatible blend is characterized by a uniform distribution of the minority phase within the main matrix, along with a strong adhesion between the two phases. Hence, any applied force can be effectively distributed across all phase components, thereby achieving effective structural integration [102].
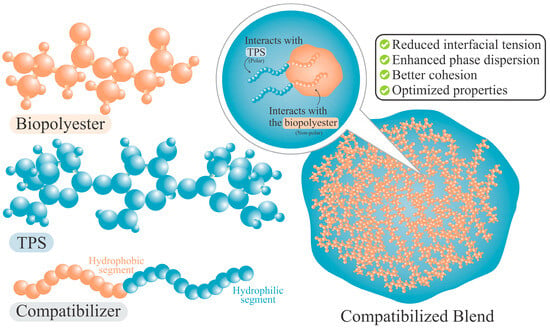
Figure 5.
Schematic of compatibilizer action in TPS/biopolyester blends.
As for polyesters, different types of compatibilizers have been employed, which include plasticizers, such as lubricants, nucleation agents [58], or even components to reduce the Tg [61]. Owing to the internal structure of polyesters, in some cases, nanofillers can be added to enhance the mechanical properties by penetrating the pore spaces of the matrix and thus improving the interfacial adhesion by providing a more exfoliated surface [75]. Furthermore, the addition of antioxidant components to the blends can stabilize them during processing and increase the viscosity of the matrix for better blending [81].
4.1. Compatibilization Using Conventional Agents and Functional Additives
By improving starch dispersion in PHB and water resistance, Liao and Wu [127] developed PHB/starch and PHB crosslinked with acrylic acid blends, resulting in notable improvements over the primary matrix. This was explained by the reaction between the carbonyl groups of the PHB graft and the -OH groups of the starch, which produced ester groups. Additionally, the compatibilized blend exhibited improved tensile strength at breakpoint with a starch content of up to 50 wt.%. A decrease in Tm accompanied by an increase in melting enthalpy (ΔHm) was observed as the starch content increased. This behavior is linked to the lower melt viscosity of starch compared to PHB, which reduces the temperature required for melting, while simultaneously promoting more efficient packing of crystalline domains, thereby increasing ΔHm. As a result, the morphology of the blends was more uniform compared to the non-compatibilized blend.
Similarly, another grafting-related investigation was conducted by Don et al. [124], where PHB was blended with a starch–polyvinyl acetate (PVAc) graft, achieving a grafting efficiency of 12.5% and complete miscibility in all formulations. As a result, an increase in the thermal properties of the material and a reduction in the molecular weight of PHB in processing were evidenced, resulting in a more tenacious PHB and improved interfacial adhesion. However, the compatibilizer used had a low biodegradability, which might negatively affect the blend in terms of degradation.
Florez et al. [128] performed TPS with plasma crosslinked PHB and sulfur hexafluoride (SF6) blends by compression molding to evaluate the material compatibilization, which resulted in a homogeneous distribution of PHB within the TPS matrix. The higher PHB content (even when modified) resulted in some agglomerations on the blend surface, which can become stress concentrators and adversely affect not only the interfacial adhesion but also other mechanical properties, thermal stability, crystallinity, and mechanical properties increased significantly compared to the TPS matrix. One exception was the elongation at break, which exhibited a decay. Therefore, this combination of materials offers promising results for applications that do not require high material elasticity.
Regarding TPS/PLA blends, one of the most prominent strategies involves incorporating grafted copolymers, as shown in Table 3. Trinh et al. [129] synthesized a starch copolymer grafted onto PLA by ring-opening polymerization, upon the incorporation of a 2.5–5 wt% in TPS/PLA blends (60/40), a remarkable morphological improvement was achieved, evidenced by a reduction in the size of PLA spherulites, greater homogeneity of phases, and significant enhancements in mechanical and barrier properties, with no need for covalent bonds between phases, thus facilitating the recyclability of the material.

Table 3.
Effects of different compatibilizers on the properties of TPS/biopolyester blends.
On the other hand, the use of citric acid (CA) as a compatibilizer and plasticizer in TPS/PLA blends has also been explored; this one can promote the partial esterification of starch and enhance its interaction with the PLA matrix. By adding a 5% CA ratio, it is possible to achieve a balance between thermal, mechanical, and barrier properties. This resulted in a higher level of structural fragmentation, which accelerated PLA’s biodegradability and strengthened its potential for sustainable applications because of its non-toxicity and widespread use in food applications [130].
In addition to organic compatibilization strategies, an alternative approach involves the use of organic fillers, like zeolite, combined with orienting procedures, as proposed by Rosa et al. [1]. These fillers offer a property profile that is difficult to achieve with polymeric modifiers alone [2] and can significantly enhance interphase compatibility. By reducing the size of the dispersed TPS phases and improving their dispersion within the PLA matrix, zeolite incorporation leads to positive results of up to 90% in oxygen barrier performance and 100% in tensile strength relative to the uncompatibilized blend. This demonstrates how an inorganic filler, such as zeolite, can effectively improve the properties without compromising the biodegradability of the system.
Reactive chemical modification is one of the most widely used compatibilization techniques for TPS/PBS blends. By adding PBS functionalized with terminal isocyanate groups, miscibility with TPS is significantly improved, with tensile strength up to 10 times that achieved with just 10% compatibilizer. This, in turn, reduces water absorption, increases hydrophobicity, and promotes a more uniform dispersion of PBS in the TPS matrix [132]. Similarly to the TPS/PLA samples, Beluci et al. [133] developed biodegradable TPS/PBS/PBAT films by extrusion, employing CA as a compatibilizer to enhance compatibility, mechanical strength, and opacity without affecting the color. In addition, better interaction of the components and a more ductile and rigid blend were exhibited, demonstrating that CA is useful for this purpose in different types of polyester.
Maleic anhydride and tartaric acid have also proven to be good compatibilizers for TPS/PBS blends in the presence of organotin catalysts, as they promote the formation and grafting of copolymers onto TPS, improving interfacial strength and adhesion, as previously confirmed by Fourier transform infrared spectroscopy (FTIR) [134]. On the other hand, reactive blends have also shown high efficacy in this function. Thajai et al. [135] incorporated chlorhexidine gluconate (CHG) into TPS for blending with PBS and epoxy resin. The reaction between the amino groups of CHG and the epoxies of the resin enhanced interfacial adhesion, resulting in improved mechanical strength, thermal stability, and hydrophobicity, which demonstrates the effectiveness of this blend for developing advanced bioplastics. Collazo-Bigliardi et al. [131] employed PCL with glycidyl methacrylate and maleic anhydride as compatibilizers in TPS blends. While preserving good mechanical integrity, the addition of 5% PCLG enhanced interfacial adhesion and dramatically decreased oxygen and vapor permeability. Thus, PCL can decrease matrix stiffness and increase elongation. Comparable qualities to LDPE were provided by PLA/TPS/PCL formulations, demonstrating the efficacy of PCL as a structural and flexibilizing agent [136].
Polyester inherently presents functional groups capable of interacting with similar segments in other polymeric components, which can facilitate interfacial adhesion in certain blend systems. In contrast, native starch exhibits limited compatibility with hydrophobic matrices. However, though esterification with chemical agents, its structure can be modified by introducing ester groups along the chain, thereby enhancing interfacial adhesion and better miscibility within polyester-based blends [109].
Its capacity to create ester linkages has led to extensive research into CA as a crosslinking agent in various starch matrices. Li et al. [137] examined the impact on wheat starch granules following a heat and moisture treatment after CA esterification. They achieved levels of 71% in both type A and type B granules, demonstrating a significant improvement in the synthesis of thermally resistant starch. The treatment was successful in altering starch crystallinity and increasing the degree of substitution, indicating efficient ester formation. Moreover, SEM showed collapsed granules due to structural modification, suggesting that CA esterification, especially when combined with this type of treatment, not only alters physicochemical properties but also offers thermal stability and digestibility in biodegradable film systems.
Similarly, CA-modified starch nanoparticles have been created that exhibit improved hydrophobicity and thermal stability through the formation of ester groups, as verified by FTIR. This illustrates how well CA works at the nano scale to modify materials to be compatible with hydrophobic ones [138], like polyester. Yang et al. [139] combined CA and oxidized palm oil oligomers to create composite materials based on fiber and starch. CA demonstrated high compatibility by improving interfacial compatibility and mechanical strength by esterification and crosslinking, significantly enhancing modulus and tensile strength with only 0.75% oxidized oil.
CA is also useful as a stabilizing agent in composite systems; its addition to tiger nut starch has been previously tested, obtaining esterification with a high content of resistant starch, decreased swelling, and solubility. This shows a reduction in crystallinity and an increase in humectability, improving its performance in Pickering solutions [140]. In fibrous starch and paper pulp composites, CA has also been shown to act as a crosslinker, by significantly improving the water resistance and mechanical strength of processed sheets [141], thereby reinforcing its potential to alter the processability and compatibility in relevant hybrid systems [142] for PHB–starch blends.
Beyond modifying individual granules, He et al. explored how CA, glycerol, and stearic acid (SA) functioned as plasticizers in TPS blended with PBAT, a polymer similar to PHB in its hydrophobic nature. It was observed that a 30% glycerol composition provided superior dispersion and yield of TPS compared to native starch [143]. SA is a compound that has also been attractive as a compatibilizer due to its ability to impart hydrophobicity and introduce long aliphatic chains into the starch structure through esterification [144,145]. An investigation of starch stearate sintering in aqueous urea/NaOH solutions reported substitution degree values higher than 0.065, lower crystallinity, higher thermal stability, and better emulsifying and moisture barrier properties than native starch [146]. Similarly, stearates were developed using a dry method under atmospheric conditions, in the absence of catalysts or solvents, with regenerated starch as the precursor, achieving a maximum degree of substitution of 0.3. Hence, the increase in this parameter led to improved emulsification capacity, freeze–thaw stability, and reduced moisture adsorption [147]. This suggests that esterification with SA is a scalable and efficient method for blending starch with PHB, where hydrophobic compatibility is essential for achieving phase dispersion and mechanical cohesion.
EA dual-modified cassava starch esters were synthesized by Lozano et al. [148]. In 2024, it was tested as a matrix in composites with microfibrillated cellulose. The results showed that the esterification method increases hydrophobicity and decreases the crystallinity of the phase. High substitution degree values, however, enhance barrier qualities but may also make some blending systems less compatible and more brittle [149]. Although CA and SA are different in structure and effect, both contribute positively to the mechanical and environmental performance of starch films. Thus, their suitability should be chosen based on the target application and process composition, as the benefits provided by each are complementary. CA improves crosslinking and structural rigidity, while SA can improve water resistance and hydrophobicity. This duality implies a potential strategy for the multifunctional compatibilization of starch with hydrophobic polymers [32], as summarized in Table 4.

Table 4.
Esterifying properties of citric acid vs. stearic acid.
In addition, it should be noted that, although there is a variety of compounds widely used as natural and synthetic additives, their safety for food contact depends on their chemical composition, concentration, and potential migration [150]. Some studies indicate that certain compounds can migrate into food; therefore, their limits must be validated according to the current regulations applicable to the area in which the material is developed. Thus, specific migration tests are essential to confirm their safe use in food packaging [151].
4.2. Phenolic and Oily Compounds as Natural Compatibilizers
If a polymer blend with improved properties is desired, the use of additive compatibilizing agents is essential. However, many of these may be detrimental to human health in applications involving direct contact with these plastics in consumer products, such as packaging. Therefore, promoting natural alternatives that fulfill the same compatibilization functions is necessary [152]. Previous studies have shown that many natural species of botanical origin can accomplish these purposes while extending the shelf life of the products inside [153]. In addition, these compounds have biodegradable properties, are easy to degrade, and are biocompatible, since they come from organic sources, which gives them a high potential as additives in polymeric blends [154].
Some bioactive compounds present in natural sources, such as polyphenols and even essential and conventional oils, have previously been employed as chemical additives [153]. Essential oils are scented products derived from plant sources, usually separated by steam, distillation, or other advanced techniques, and their lipidic nature allows them to be easily separated from the liquid phases without the need to use methods that modify their chemical structure [155]. These can be extracted from various plant spices, such as coriander [156], lavender [157], lemon [158], rosemary [159], mint [160], turmeric [161], eucalyptus [162], cinnamon [163], orange [164], moringa [165], garlic [166], clove [167], thyme [168], verbena [169], sage [170], chamomile [171], citronella [172], tangerine [173], oregano [174], and others.
Phenol-rich oils have been proven to be effective against a variety of pathogens, including resistant bacteria. Their mechanism of action involves altering cell membranes and inhibiting resistance systems, including cell–cell communication and biofilm formation. However, some aspects, such as light, temperature, or atmospheric oxygen, must be considered, as they may be affected due to the high tendency for oxidation reactions [175].
In the last few years, several oils used for addition to polyesters and TPS have been found to modify their properties depending on their extraction source (Table 5). Garcia-Garcia et al. [176] studied the effect of maleinized linseed oil (MLO) and epoxidized fatty acid ester (EFAE) at low concentrations in improving the toughness of PHB in industrial formulations. Tensile strength and elongation at break were enhanced by this addition, with EFAE exhibiting a somewhat higher strength. Additionally, the PHB matrix’s compatibility with these plasticizers reduced porosity, producing a homogeneous material with improved structural continuity. Furthermore, compared to the pure matrix, both components increased the degradation temperature and decreased the Tm of PHB, thereby increasing its processing window.

Table 5.
Effect of oily and phenolic compounds in biopolyesters blends.
Consequently, another study was also conducted using epoxidized linseed oil (MLO) and soybean oil (ESBO) to enhance the properties of PHB. MLO was found to be more effective than ESBO, particularly at a concentration of 10%, where improvements were evidenced in terms of increased elongation at break and energy absorbed per impact. However, the incorporation of both oils brought an improvement in the thermal stability of PHB through an increase in its degradation temperature, contributing to the optimization of formulations for industrial applications [79,177].
Other types of oil, such as essential oils, have also been used in blends with polyesters, like PHB, where even better properties, including antioxidant and antimicrobial effects, have been found. In research, three different types of essential oils (cinnamon, melaleuca, and citronella) were incorporated into PHB. The citronella–cinnamon and melaleuca–cinnamon oil combinations increased the flexibility of PHB, known to be a highly rigid material, and also inhibited the proliferation of various microorganisms. However, the melaleuca–citronella oil combination had no antimicrobial activity, and the PHB samples using it demonstrated minimal UV radiation transmission. Nevertheless, the addition of essential oils improved the crystallinity and thermal stability of PHB, while decreasing its melting temperature compared to the pure matrix. This particular type of composition has potential applications in the bioactive packaging industry [46].
Cinnamon oil variants, such as cinnamaldehyde, have been utilized as compatibilizing agents in PLA/PHB films, in conjunction with mono-caprylyl glycerate (MCG) and glycerol monolaurate (GML) as plasticizers. As a result, an improvement in the mechanical and active properties of the films was achieved, also exhibiting microbial activity, as demonstrated in an analysis performed with the preservation of a piece of salmon. Additionally, increases in tensile strength and antioxidant properties were observed as a result of the addition of cinnamaldehyde [64]. Albuquerque et al. [179] conducted a study to evaluate the antimicrobial effect of clove oil in PHB and bacterial cellulose blends in acetic acid. This time, such an addition enhanced the hydrophobic properties of the film and facilitated the blending of the components through a compatibilization effect, thereby improving the interactions between the phases. The microbial growth was reduced by up to 65%, while the mechanical and thermal properties showed an improvement, as evidenced by an increase in tensile strength and a higher degradation temperature. Thus, essential oils have multiple applications, and their performance can vary depending on the affinity with the components of the blend.
Another essential oil that is highly investigated is eugenol, which is not only antimicrobial and antioxidant, but also modifies the structure of PHB by processes such as extrusion [184]. A study analyzing the effect of adding eugenol to PHB in degradative processes revealed an antimicrobial oil-dependent behavior present in the matrix, which increased the crystallinity but caused a slight decrease in the mechanical properties. It should be noted that, at the time of degradation, the rate of polymer hydrolysis can vary depending on the degree of molecular disorder [180]. Garrido et al. developed biocomposites by blending TPS and PHB using organically modified montmorillonite (OMMT) as nanofiller and eugenol as a compatibilizing agent. An enhanced exfoliated surface and a sustained degradation temperature were achieved. However, there was a reduction in the Tm and elastic modulus. The crystallinity exhibited an increase, and both antifungal and antioxidant activity were evidenced by the effect of eugenol [35].
Finally, an eco-friendly method was suggested by Volpe et al. [183], who reported that used sunflower oil can function as an efficient plasticizer by partially substituting it for glycerin in starch grading. To reduce expenses and advance the circular economy, the addition of this fried oil enhanced TPS’s thermal stability and uniformity when combined with PLA. Although the study focused on PLA, the findings highlight the potential of conventional oils in the compatibilization of starch/polyesters blends, underlining that the ratio of oil to glycerol and the type of oil have a significant influence on the final performance and material properties.
Each essential oil modifies the polymer properties differently, depending on its nature and affinity. Similarly, the mechanism of action varies according to the components that comprise it. Since they all belong to the lipid group, the oxidation process in oils can be summarized in three phases: initiation, propagation, and termination [185].
Triplet oxygen (O2) is the ground state form of atmospheric oxygen, which is distinguished by the presence of two unpaired electrons with parallel spins. With a spin quantum number of 1, this is the most stable form of oxygen under normal circumstances. Additionally, unsaturated lipids contain bonds (C=C), which are relatively reactive. However, under standard conditions, the electrons in these bonds are paired in a state known as a singlet, i.e., the electrons are arranged in pairs with opposite spins, which would give a spin quantum number of 0 [186]. According to quantum chemistry, for a reaction to occur without the need for high activation energy, the total spin of the reactants must match the total spin of the products. Given the difference between the spin numbers of triplet oxygen and unsaturated lipids, for a direct reaction to occur, one or more of the unpaired electrons must reverse their spin [187]. These types of reactions are known as “spin forbidden” and are very unlikely to occur, as they require the spins to be reoriented, and this needs additional energy or specific conditions for oxygen to react with lipids [188].
Such a change in spin can be accomplished by additional energy supply via light, heat, catalysts, or singlet oxygen (1O2), a more reactive form of oxygen in which two electrons are paired in an excited state. During this state, oxygen can react more easily with lipids, as their spins are compatible, and here is where the initiation process takes place [188]. Subsequently, in the propagation stage, lipids are transformed into free radicals, which react again with the oxygen present in the atmosphere and then form new peroxide radicals. These remove hydrogen atoms from other lipid molecules, forming hydroperoxides, as illustrated in Figure 6a. This process is repetitive, and this mechanism causes the oxidation process to result in autocatalysis. The hydroperoxides formed are unstable compounds that usually decompose, producing a variety of volatile and non-volatile compounds, including radicals. The compounds produced usually influence oxidative enzymatic deterioration. During the termination phase, radicals combine and give rise to non-radical products, and any reaction that prevents propagation or removes free radicals from the system is fundamental to the mechanism of this phase [189].
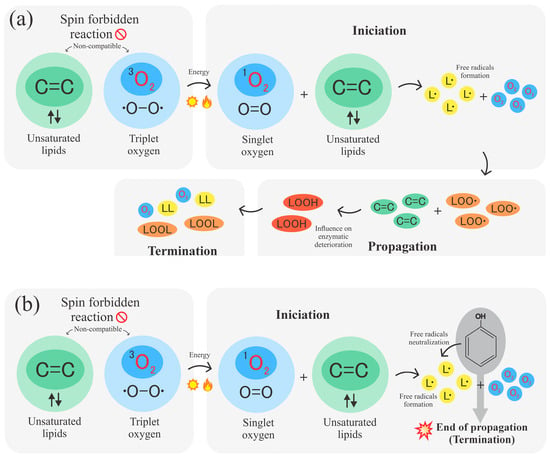
Figure 6.
(a) Lipid oxidation mechanism through initiation, propagation, and termination. (b) Antioxidant activity of phenolic compounds via free radical neutralization.
Oils, particularly the ones containing phenolic compounds, can act as antioxidants due to the presence of hydroxyl (-OH) groups in their structure, which can donate hydrogen atoms to free radicals, thus converting peroxide radicals into less reactive forms, stopping the propagation of the oxidation reaction, as shown in Figure 6b. In addition, these oils can bind to other radicals or react with each other to form non-radical species, contributing to the termination phase of lipid oxidation, where radicals combine and neutralize, effectively halting the oxidation chain [185]. So, theoretically, antioxidants reduce the development of the propagation phase in the oxidation mechanism, thereby shortening the path from initiation to termination.
Oils containing phenolic compounds are usually derived from specific plants. Among the most common phenolic compounds are eugenol [190], cardanol [191,192], cardol [193], thymol [194], and carvacrol [195]. Although widely recognized for their antioxidant and antimicrobial activity, these phenolic structures are also functional groups able to interact with polymer matrices, which is relevant when evaluating their potential roles in blend performance. Other essential oils, such as citrus oils, have a different chemical composition (terpenes), present different chemical compositions, but can still provide antioxidant effects [196].
These phenolic compounds may constitute up to 85% of the total oil composition [185], and their chemical reactivity contributes not only to bioactivity but also to possible interactions with polymer chains. Their natural origin [184] and antibacterial activity [197], in E. coli, S. aureus, and Eurotim amstelodami, among others, make them appealing multifunctional additives [184]. They occur in plants such as thyme, oregano, cashew [198], etc. Particularly, carvacrol, one of the main components of oregano oil [45], and thymol [199] have shown high oxidative and antimicrobial performance [200] due to their phenolic nature [201]. Cardanol and cardol, obtained from cashew nutshell liquid (CNSL) [202], present similar multifunctional characteristics.
Cashew contributes an important source of bioactive compounds [203] and is widespread across tropical regions. Although its chemical composition varies with species and environment [204,205,206,207,208], its shell liquid consistently contains anacardic acid, cardol, and cardanol as major constituents [209,210]. Extraction techniques such as cold pressing and solvent extraction [211] influence the proportion and functionality of these molecules.
Previous studies incorporating oils into polymeric matrices have shown that the antioxidant capacity of the phenolic components strongly dictates the overall activity of the oil [185]. Therefore, understanding their molecular interactions becomes essential when formulating polymer blends [212]. Recent studies revealed that cashew oil and its phenolic derivative cardanol can plasticize starch and serve as alternative modifiers for TPS, even with low or no traditional plasticizer loading [213,214]. This method is especially appealing for enhancing the functionality and processability of starch-based systems, including later blending with hydrophobic polyesters to create biodegradable composites. Its ability to undergo epoxidation or esterification [215,216] further expands its role from a simple plasticizer to a potential reactive modifier capable of promoting phase adhesion.
As a result, TPS modified with CNSL derivatives becomes more hydrophobic and more resistant to moisture [214]. The aromatic ring of cardanol may also provide some thermal and oxidative stability relative to common aliphatic plasticizers [215]. The hydrophobic features of the alkyl side chain [213,214] also better match the hydrophobic nature of polyesters by improving interfacial adhesion and retaining its molecular weight during melt extrusion by scavenging free radicals.
Regarding other oils, Fayyazbakhsh et al. [217] investigated how eucalyptol, limonene, and thymol affect PHB degradation. The results showed that samples containing 3% of these terpenoids slowed down the degradation process, achieving a maximum of 76.9% in 200 days, compared to the degradation of pure PHB, which reached 100% in 198 days. Due to its antibacterial qualities, eucalyptol also provided the greatest mechanical resistance and delayed biodegradation, followed by limonene and thymol. Although these terpenoids primarily act as bioactive modifiers, their influence on crystallinity and Tg indicates that they may also contribute indirectly to phase stability in polyester-rich systems.
In some blending processes, better performance has been evidenced in oils that are encapsulated prior to processing, as they are sensitive and can be affected during processes such as extrusion [218]. Additionally, this pretreatment enables the gradual release of the essential oil, which is crucial for maintaining prolonged antioxidant activity over time. It can also be useful to prevent the oil from acting as a plasticizer and weakening the structure of the main matrix [219]. Nanoencapsulation can enhance antimicrobial efficacy. A study performed encapsulation in oils of oregano (Origanum vulgare) and thyme (Thymus capitatus), with a composition of 73% carvacrol and 44% thymol in a PCL matrix using a nanoprecipitation method, resulting in high encapsulation efficiency and stability. Compared to pure oils, both thyme oil-loaded and oregano oil-loaded nano-capsules achieved higher antimicrobial activity than the oils in their pure state [220].
These components also exhibit a compatibilizing and antimicrobial effect in blends containing nanofillers. Campos-Requena et al. [221] carried out an investigation in which films of TPS nanocomposites with OMMT were prepared using a blend of carvacrol and thymol to create an antimicrobial packaging suitable for fruit preservation by the extrusion method, which exhibited a more exfoliated morphology compared to the main matrix. The mechanical properties increased along with the thermal properties, with a controlled release of the additive components, which decreased by 43%. In addition, it was shown that a combination of carvacrol and thymol could achieve higher antimicrobial activity compared to pure carvacrol, without affecting fruit quality parameters.
Therefore, polymeric blends with these types of compounds as compatibilizers have a high potential, particularly for the development of bioactive packaging for fresh foods [222], since they act as antimicrobial and antioxidant agents, extending their shelf life [223]. However, prior component studies are needed to verify their compatibility. Previous studies have reported that it is essential to have starches with large amorphous regions that facilitate the gelatinization of the starch, which can be achieved with a high amylose composition [56]. Also, further testing, especially on sensory factors and commercial acceptability, can further reinforce this premise [221].
4.3. Compatibilization and Property Enhancement Using Nanofillers and Natural Reinforcements
TPS/biopolyester blends have been widely studied in order to combine sustainability, processability, and functionality. However, the limited compatibility between the phases and the fragility of the resulting systems have motivated the incorporation of natural reinforcements or nanofillers to improve their performance. This general mechanism is illustrated in Figure 7, where the addition of nanofillers or reinforcements promotes barrier and morphological control, as well as enhanced mechanical performance through load transfer.
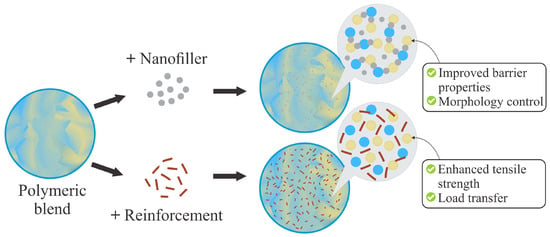
Figure 7.
Schematic representation of TPS/biopolyester blends modified with nanofillers/reinforcements.
Materials produced from PHB and TPS with the addition of jute fiber as reinforcement (up to 30 wt%) showed improved thermal stability and reduced water sensitivity [224]. However, the amount of glycerol should be controlled, since a high composition can reduce mechanical properties and increase water sensitivity due to its hydrophilic nature. On the other hand, the addition of fiber increases the material’s capacity to withstand stress, maintaining its strength even after hours of exposure in humid environments.
Nanofillers and essential oils have also been employed as compatibilizers in PHB and TPS blends. Garrido et al. performed blends with OMMT clay and eugenol, resulting in an exfoliated, rough surface and enhanced interfacial adhesion in the blends compared to the PHB matrix, attributed to the migration of the clay layers, which also improved the elastic modulus and the material’s stiffness up to 50 °C. However, the addition of clay produces a slight decrease in the degree of crystallinity, limiting the mobility in the bionanocomposite segments. Thus, this is a good alternative as long as the levels of both clay and TPS are moderated, therefore positioning PHB as a potential sustainable alternative in terms of synthetic polymers involving packaging applications [111,225].
Other studies have also explored the viability of improving the properties of TPS/PLA blends by using fibers to combine the advantages of both polymers. Serra-Parareda et al. [226] employed bleached Kraft fibers, and a 30% ratio increased Young’s modulus over 100% compared to the non-reinforced blend, achieving values of 5.54 GPa. Other types of fibers, like coconut fibers, also improve the interfacial compatibility and stiffness in the system, as evidenced by the formation of hydrogen bonds between the phases and the increase in PLA crystallinity [227]. In addition, the use of nanofillers such as OMMT enhances the properties of TPS/PLA blends, promoting exfoliated structures that improve the tensile strength, impact, and biodegradability of the material [228].
Regarding TPS/PBS, Boonprasith et al. [229] demonstrated that the addition of nanoclays significantly increased the tensile strength and improved the thermal properties, especially when a high proportion of TPS (75%) was used due to the intercalation and exfoliation of the clay in the main matrix. In contrast, the reinforcement mechanisms associated with natural fibers differ from those of inorganic nanoclays, as their efficiency depends primarily on the interfacial compatibility. Thus, various surface treatments such as alkali, silanization, acetylation, etc. have been used, significantly helping to improve stress transfer, leading to an increase in the elastic modulus and tensile strength [230]. On the other hand, for TPS/PCL blends reinforced with sisal-derived cellulose nanocrystals (Whiskers), surface changes in the nanomaterials promoted uniform dispersion and chemical interaction that resulted in an enhancement in crystallinity [231].
These results support the approach of blending biopolyesters with nanofillers and reinforcements to create bio-based materials with characteristics suited to different uses, offering a sustainable substitute for conventional plastics.
5. Future Perspectives
TPS blends with biodegradable polyesters such as PBH, PLA, PBS, and PCL have demonstrated promising thermal and mechanical performance. However, their long-term stability and industrial scalability remain unresolved challenges. Although many studies highlight property improvements through natural oils and compatibilizers, the reproducibility of these effects under real processing conditions is still inconsistent across authors. One of the key directions is the optimization of loading levels in TPS/polyester systems and techniques for incorporating oils into polymeric matrices, with a particular focus on the development of different advanced lubrication methods such as nanoencapsulation, which improves materials’ properties and ensures these are maintained over time, but the mechanisms governing their release kinetics and long-term interactions within these matrices require deeper investigation.
Although substantial progress has been made in developing bioplastics, most formulations remain confined to laboratory-scale demonstrations [232]. Therefore, it is essential to improve the methods used to add active compounds to achieve greater efficiency in the mechanical and thermal properties of the materials. However, due to the versatility and lower cost of fossil-based plastics, bioplastics are produced in limited quantities. Further research aimed towards improving these aspects, without a significant increase in their production cost, is therefore required to reduce the competitiveness gap between conventional plastics and renewable polymers [74], given that bioplastics have greater potential to offer environmental sustainability and a lower carbon footprint, and to contribute to the reduction in plastic waste accumulation [233].
Selective compatibilization approaches could improve phase dispersion and interfacial adhesion [234], yet most published work evaluates these improvements only qualitatively or under narrow experimental conditions. Future studies should systematically correlate morphology with mechanical, thermal, and functional performance to build predictive structure–property relationships.
Furthermore, the incorporation of advanced fabrication techniques, such as 3D printing, represents an emerging trend with great potential for the customized and scalable production of composites with complex geometries and properties tailored to specific applications [235], but their impact on TPS degradation kinetics and polyester crystallization has been insufficiently explored. In the field of nanotechnology, the development of nanocomposites based on TPS/polyester blends with fillers or nano-reinforcements is gaining relevance, especially with the incorporation of nanoparticles with antimicrobial properties, such as zinc oxide and copper oxide [236,237].
Life Cycle Assessment (LCA) Considerations for TPS/Polyester Blends
Although TPS blends with biodegradable polyesters such as PHB, PLA, PBS, and PCL have been thoroughly studied from a structural, thermal, and mechanical perspective, there is a significant gap in their environmental assessment using LCA [238]. This type of assessment is key to determining whether functional improvements achieved through compatibilization and the use of natural additives actually lead to sustainable benefits when considering aspects such as energy consumption [239], greenhouse gas emissions, and potential recyclability or compostability [240]. These interlinked factors can be better understood through an LCA framework of TPS/biopolyester blends, as illustrated in Figure 8.
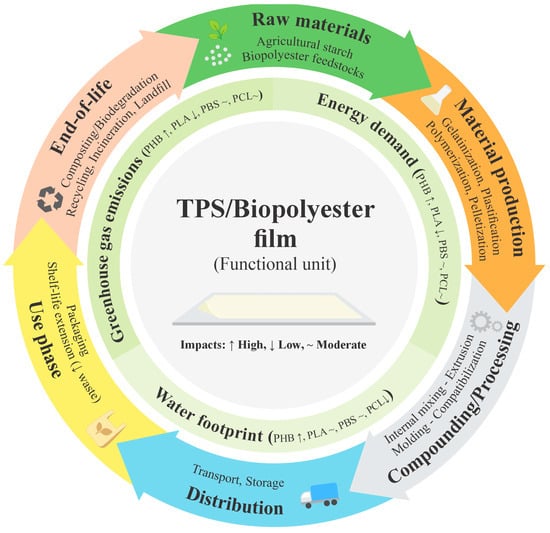
Figure 8.
Life cycle assessment (LCA) framework for TPS/biopolyester blends.
Some preliminary studies have indicated that blends with PLA could reduce the environmental impact of pure PLA [241]. However, in the case of TPS/PHB, the benefits are more uncertain due to the high energy demand of PHB production [242]. The use of synthetic compatibilizers or essential oils should also be carefully evaluated, as their incorporation may alter the environmentally expected balances [243].
A clear direction for future research is to integrate LCA approaches from the blend design phase, especially when scaling up to industrial applications, which would help prioritize formulations not only for performance but also for their overall environmental viability. This will allow early identification of the main impacts throughout the entire life cycle of the material, facilitating the selection of processes and additives that optimize both the functional properties and the overall sustainability of the product.
6. Conclusions
This review provides a comprehensive overview of recent advances in TPS/biopolyesters blends, with a particular emphasis on compatibilization strategies and the incorporation of bioactive functionalities. The literature consistently shows that the intrinsic incompatibility between hydrophilic TPS and hydrophobic polyesters remains a key challenge, directly affecting morphology, interfacial adhesion, and overall performance. Chemical modification of starch, reactive compatibilization, and the use of bio-based additives such as organic acids, oils, and phenolic compounds have proven effective in improving phase dispersion and tailoring thermal and mechanical behavior.
In addition, the integration of bioactive agents introduces multifunctionality to TPS/polyester systems, enabling antimicrobial and antioxidant responses while simultaneously influencing structural and physical properties. However, the reported effects strongly depend on additive type, concentration, and processing conditions, highlighting the need for systematic studies that link formulation, structure, and performance.
Despite significant progress, several challenges remain, including long-term stability, scalability of progressing routes, and the lack of standardized methodologies for evaluating biodegradation and bioactivity. Future research should focus on optimizing compatibilization efficiency using sustainable approaches, improving structure–property relationships, and assessing environmental performance through life cycle analysis. Addressing these aspects will be essential to advance TPS/polyester blends toward high-performance, sustainable polymeric materials suitable for a wide range of applications.
Author Contributions
E.M.-B.: Investigation, Formal analysis, Visualization, Writing—original draft preparation. M.J.A.-T.: Conceptualization, Investigation, Supervision, Review and Editing. A.F.J.: Conceptualization, Investigation, Supervision, Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an internal Call from Universidad Tecnológica de Bolívar (Cartagena, Colombia), ID INV05202504.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Universidad Tecnológica de Bolivar for funding this research. A.F.J. extends thanks to ANID for the support received through the FONDECYT REGULAR project 1231376.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Rosa, D.S.; Guedes, C.G.F.; Carvalho, C.L. Processing and Thermal, Mechanical and Morphological Characterization of Post-Consumer Polyolefins/Thermoplastic Starch Blends. J. Mater. Sci. 2007, 42, 551–557. [Google Scholar] [CrossRef]
- Horton, A.A. Plastic Pollution: When Do We Know Enough? J. Hazard. Mater. 2022, 422, 126885. [Google Scholar] [CrossRef]
- Wang, P.; Xiong, Z.Y.; Fei, P.; Cai, J.; Walayat, N.; Xiong, H. An Approach for Compatibilization of the Starch with Poly(Lactic Acid) and Ethylene-Vinyl Acetate-Glycidyl-Methacrylate. Int. J. Biol. Macromol. 2020, 161, 44–58. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; Liu, Q. Thermoplastic Starch Processing and Characteristics-A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1353–1370. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic Starches: Properties, Challenges, and Prospects. Starch-Starke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef]
- Tavanaie, M.A.; Ghahari, A.H. A Study on Melt Recycling of Bio-Based Polypropylene/Thermoplastic Starch Compound. J. Appl. Polym. Sci. 2021, 138, 51282. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Barrow, C.J.; Adhikari, B. The Future of Bioplastics in Food Packaging: An Industrial Perspective. Food Packag. Shelf Life 2024, 43, 101279. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Raps, H.; Cropper, M.; Bald, C.; Brunner, M.; Canonizado, E.M.; Charles, D.; Chiles, T.C.; Donohue, M.J.; Enck, J.; et al. The Minderoo-Monaco Commission on Plastics and Human Health. Ann. Glob. Health 2023, 89, 23. [Google Scholar] [CrossRef] [PubMed]
- Geppner, L.; Hellner, J.; Henjakovic, M. Effects of Micro- and Nanoplastics on Blood Cells in Vitro and Cardiovascular Parameters in Vivo, Considering Their Presence in the Human Bloodstream and Potential Impact on Blood Pressure. Environ. Res. 2025, 273, 121254. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Su, G. Plastic Packaging-Associated Chemicals and Their Hazards—An Overview of Reviews. Chemosphere 2023, 331, 138795. [Google Scholar] [CrossRef]
- Sheriff, S.S.; Yusuf, A.A.; Akiyode, O.O.; Hallie, E.F.; Odoma, S.; Yambasu, R.A.; Thompson-Williams, K.; Asumana, C.; Gono, S.Z.; Kamara, M.A. A Comprehensive Review on Exposure to Toxins and Health Risks from Plastic Waste: Challenges, Mitigation Measures, and Policy Interventions. Waste Manag. Bull. 2025, 3, 100204. [Google Scholar] [CrossRef]
- Carmona, V.B.; Corrêa, A.C.; Marconcini, J.M.; Mattoso, L.H.C. Properties of a Biodegradable Ternary Blend of Thermoplastic Starch (TPS), Poly(ε-Caprolactone) (PCL) and Poly(Lactic Acid) (PLA). J. Polym. Environ. 2015, 23, 83–89. [Google Scholar] [CrossRef]
- Obasi, H.C.; Igwe, I.O. Effects of Native Cassava Starch and Compatibilizer on Biodegradable and Tensile Properties of Polypropylene. Am. J. Eng. Res. 2014, 3, 96–104. [Google Scholar]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Escobar Macías, D. Thermoplastic Starch (TPS)/Polylactic Acid (PLA) Blending Methodologies: A Review. J. Polym. Environ. 2021, 30, 75–91. [Google Scholar] [CrossRef]
- Martinez Villadiego, K.; Arias Tapia, M.J.; Useche, J.; Ledesma, Y.; Leyton, A. Thermal and Morphological Characterization of Native and Plasticized Starches of Sweet Potato (Ipomoea batatas) and Diamante Yam (Dioscorea rotundata). J. Polym. Environ. 2020, 29, 871–880. [Google Scholar] [CrossRef]
- Bercini Martins, A.; Campomares Santana, R.M. Effect of Carboxylic Acids as Compatibilizer Agent on Mechanical Properties of Thermoplastic Starch and Polypropylene Blends. Carbohydr. Polym. 2016, 135, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Liu, Q.; Ding, T.; Han, Y.; Zhang, L.; Chen, D.; Tian, W. Ageing of Soft Thermoplastic Starch with High Glycerol Content. J. Appl. Polym. Sci. 2007, 103, 574–586. [Google Scholar] [CrossRef]
- Woźniak-Braszak, A.; Knitter, M.; Markiewicz, E.; Ingram, W.F.; Spontak, R.J. Effect of Composition on the Molecular Dynamics of Biodegradable Isotactic Polypropylene/Thermoplastic Starch Blends. ACS Sustain. Chem. Eng. 2019, 7, 16050–16059. [Google Scholar] [CrossRef]
- Jiugao, Y.; Ning, W.; Xiaofei, M. The Effects of Citric Acid on the Properties of Thermoplastic Starch Plasticized by Glycerol. Starch-Starke 2005, 57, 494–504. [Google Scholar] [CrossRef]
- Bercini Martins, A.; Capomares Santana, R.M. Structure-Properties Correlation in PP/Thermoplastic Starch Blends Containing Sustainable Compatibilizer Agent. Mater. Res. Express 2019, 6, 095336. [Google Scholar] [CrossRef]
- Obasi, H.C.; Igwe, I.O.; Ogbobe, O.; Madufor, I.C.; Egeolu, F.C. Analysis of the Mechanical and Degradation Performances of Selected Starch/Polypropylene Blends. J. Basic. Appl. Res. Int. 2015, 7, 42–52. [Google Scholar]
- Narváez-Gómez, G.; Figueroa-Flórez, J.; Salcedo-Mendoza, J.; Pérez-Cervera, C.; Andrade-Pizarro, R. Development and Characterization of Dual-Modified Yam (Dioscorea rotundata) Starch-Based Films. Heliyon 2021, 7, e06644. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Isolation, Composition, Structure, Properties, Modifications, and Uses of Yam Starch. Compr. Rev. Food Sci. Food Saf. 2015, 14, 357–386. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Chen, M.; Sun, L.; Wang, Y.; Yin, J.; Liu, J.; Sun, X.-Q.; Hang, Y.-Y. Genome-Wide Identification and Evolutionary Analysis of NBS-LRR Genes From Dioscorea Rotundata. Front. Genet. 2020, 11, 484. [Google Scholar] [CrossRef]
- Taguet, A.; Huneault, M.A.; Favis, B.D. Interface/Morphology Relationships in Polymer Blends with Thermoplastic Starch. Polymer 2009, 50, 5733–5743. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Yi, X.S.; Feng, Y. Effects of Glycerin and Glycerol Monostearate on Performance of Thermoplastic Starch. J. Mater. Sci. 2001, 36, 1809–1815. [Google Scholar] [CrossRef]
- Vithu, P.; Dash, S.K.; Rayaguru, K. Post-Harvest Processing and Utilization of Sweet Potato: A Review. Food Rev. Int. 2019, 35, 726–762. [Google Scholar] [CrossRef]
- Tesfaye, T.; Johakimu, J.K.; Chavan, R.B.; Sithole, B.; Ramjugernath, D. Valorisation of Mango Seed via Extraction of Starch: Preliminary Techno-Economic Analysis. Clean. Technol. Environ. Policy 2017, 20, 81–94. [Google Scholar] [CrossRef]
- Robyt, J.F. Starch: Structure, Properties, Chemistry, and Enzymology. In Glycoscience; Fraser-Reid, B., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin, Heidelberg, 2008; pp. 1437–1472. ISBN 978-3-540-30429-6. [Google Scholar]
- Nguyen, T.K.; That, N.T.T.; Nguyen, N.T.; Nguyen, H.T. Development of Starch-Based Bioplastic from Jackfruit Seed. Adv. Polym. Technol. 2022, 2022, 6547461. [Google Scholar] [CrossRef]
- Ruhul Amin, M.; Anannya, F.R.; Mahmud, M.A.; Raian, S. Esterification of Starch in Search of a Biodegradable Thermoplastic Material. J. Polym. Res. 2020, 27, 3. [Google Scholar] [CrossRef]
- He, H.-J.; Li, G.; Obadi, M.; Ou, X. An Overview on the Dry Heat Treatment (DHT) for Starch Modification: Current Progress and Prospective Applications. Curr. Res. Food Sci. 2025, 10, 101007. [Google Scholar] [CrossRef]
- Gerezgiher, A.G.; Szabó, T. Crosslinking of Starch Using Citric Acid. J. Phys. Conf. Ser. 2022, 2315, 012036. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez -Rivera, M.A.; Sanfuentes, E.A.; Peña-Farfal, C. Antioxidant and Antifungal Effects of Eugenol Incorporated in Bionanocomposites of Poly(3-Hydroxybutyrate)-Thermoplastic Starch. LWT 2018, 98, 260–267. [Google Scholar] [CrossRef]
- Prambauer, M.; Wendeler, C.; Weitzenböck, J.; Burgstaller, C. Biodegradable Geotextiles—An Overview of Existing and Potential Materials. Geotext. Geomembr. 2019, 47, 48–59. [Google Scholar] [CrossRef]
- Olkhov, A.A.; Kucherenko, E.L.; Zernova, Y.N.; Markin, V.S.; Kosenko, R.Y.; Filatova, A.G.; Vetcher, A.A.; Iordanskii, A.L. Production Methods and Biomedical Applications of Materials Based on Poly-3-Hydroxybutyrate and Its Compositions. Inorg. Mater. Appl. Res. 2024, 15, 1064–1076. [Google Scholar] [CrossRef]
- Gupta, S.; Nadda, A.K.; Gupta, A.; Singh, J.; Mulla, S.I.; Sharma, S. Transforming Wastes into High Value-Added Products: An Introduction. In Biopolymers: Recent Updates, Challenges and Opportunities; Nadda, A.K., Sharma, S., Bhat, R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–18. ISBN 978-3-030-98392-5. [Google Scholar]
- Vinayak, A.; Sharma, S.; Singh, G.B. Biopolymers from Industrial Waste. In Biopolymers: Recent Updates, Challenges and Opportunities; Nadda, A.K., Sharma, S., Bhat, R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 129–149. ISBN 978-3-030-98392-5. [Google Scholar]
- Kumawat, T.K.; Kumawat, V.; Sharma, S.; Sharma, V.; Pandit, A.; Kandwani, N.; Biyani, M. Sustainable Green Methods for the Extraction of Biopolymers; Springer International Publishing: Cham, Switzerland, 2022; pp. 73–110. [Google Scholar] [CrossRef]
- Umesh, M.; Sankar, S.A.; Thazeem, B. Fruit Waste as Sustainable Resources for Polyhydroxyalkanoate (PHA) Production. In Bioplastics for Sustainable Development; Kuddus, M., Roohi, Eds.; Springer: Singapore, 2021; pp. 205–229. ISBN 978-981-16-1823-9. [Google Scholar]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Comprehensive Review on Polylactic Acid (PLA)—Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef] [PubMed]
- Rahimkhoei, V.; Padervand, M.; Hedayat, M.; Seidi, F.; Dawi, E.A.; Akbari, A. Biomedical Applications of Electrospun Polycaprolactone-Based Carbohydrate Polymers: A Review. Int. J. Biol. Macromol. 2023, 253, 126642. [Google Scholar] [CrossRef]
- Ju, J.; Deng, Y.; Li, C.J.; Li, M. Antibacterial Activity of Essential Oil in Food System. In Essential Oils; Springer International Publishing: Cham, Switzerland, 2022; pp. 23–41. [Google Scholar]
- do Nascimento, L.D.; da Costa, K.S.; Cascaes, M.M.; de Aguiar Andrade, E.H. Encapsulation of Essential Oils by Spray-Drying: Antimicrobial Activity, and Applications in Food Preservation. In Essential Oils; Springer International Publishing: Cham, Switzerland, 2022; pp. 101–121. [Google Scholar]
- Rech, C.R.; Brabes, K.C.S.; Silva, B.E.B.; Martines, M.A.U.; Silveira, T.F.S.; Alberton, J.; Amadeu, C.A.A.; Caon, T.; Arruda, E.J.; Martelli, S.M. Antimicrobial and Physical–Mechanical Properties of Polyhydroxybutyrate Edible Films Containing Essential Oil Mixtures. J. Polym. Environ. 2021, 29, 1202–1211. [Google Scholar] [CrossRef]
- Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Ramos-Gómez, M.; Rodríguez-Muñoz, M.E.; Reynoso-Camacho, R.; Rocha-Uribe, A.; Roque-Rosales, M.R. Antioxidant Effect of Oregano (Lippia berlandieri v. Shauer) Essential Oil and Mother Liquors. Food Chem. 2007, 102, 330–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Rempel, C.; McLaren, D. Thermoplastic Starch. In Innovations in Food Packaging: Second Edition; Academic Press: Cambridge, MA, USA, 2014; pp. 391–412. ISBN 9780123946010. [Google Scholar]
- Somwanshi, S.B.; Dolas, R.T.; Wagh, V.D.; Kotade, K.B. Pharmaceutically Used Plasticizers: A Review. Eur. J. Biomed. Pharm. Sci. 2016, 3, 277–285. [Google Scholar]
- Kaseem, M.; Hamad, K.; Deri, F. Rheological and Mechanical Properties of Polypropylene/Thermoplastic Starch Blend. Polym. Bull. 2011, 68, 1079–1091. [Google Scholar] [CrossRef]
- Kaseem, M.; Hamad, K.; Deri, F. Thermoplastic Starch Blends: A Review of Recent Works. Polym. Sci. Ser. A 2012, 54, 165–176. [Google Scholar] [CrossRef]
- Byun, Y.; Zhang, Y.; Geng, X. Plasticization and Polymer Morphology. In Innovations in Food Packaging: Second Edition; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; pp. 87–108. ISBN 9780123946010. [Google Scholar]
- Kaur, L.; Singh, J.; Singh, N. Effect of Glycerol Monostearate on the Physico-Chemical, Thermal, Rheological and Noodle Making Properties of Corn and Potato Starches. Food Hydrocoll. 2005, 5, 839–849. [Google Scholar] [CrossRef]
- Schmitt, H.; Guidez, A.; Prashantha, K.; Soulestin, J.; Lacrampe, M.F.; Krawczak, P. Studies on the Effect of Storage Time and Plasticizers on the Structural Variations in Thermoplastic Starch. Carbohydr. Polym. 2015, 115, 364–372. [Google Scholar] [CrossRef]
- Stepto, R.F.T. Understanding the Processing of Thermoplastic Starch. Macromol. Symp. 2006, 245–246, 571–577. [Google Scholar] [CrossRef]
- Surendren, A.; Mohanty, A.K.; Liu, Q.; Misra, M. A Review of Biodegradable Thermoplastic Starches, Their Blends and Composites: Recent Developments and Opportunities for Single-Use Plastic Packaging Alternatives. Green Chem. 2022, 24, 8606–8636. [Google Scholar] [CrossRef]
- Kumar, D.P.; Nair, A.S.; Balakrishnan, P.; Gopi, S. Biopolymers from Renewable Sources. In Handbook of Biopolymers; Springer Nature: Singapore, 2023; pp. 27–56. [Google Scholar]
- Khosravi-Darani, K.; Bucci, D.Z.; Massoud, R. Microbial-Derived Biodegradable Polymers as Food Packaging Tool. In Biodegradable Polymer-Based Food Packaging; Springer Nature: Singapore, 2022; pp. 81–114. [Google Scholar]
- Stoica, M. Biodegradable Nanomaterials for Drink Packaging. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 609–632. [Google Scholar]
- Khosravi-Darani, K.; Yazdian, F. Polyhydroxyalkanoates (PHAs) in Food Packaging. In Biodegradable Polymer-Based Food Packaging; Springer Nature: Singapore, 2022; pp. 115–122. [Google Scholar]
- Sindhu, R.; Binod, P.; Pandey, A. Microbial Poly-3-Hydroxybutyrate and Related Copolymers. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 575–605. [Google Scholar]
- Gupta, S.; Ghosal, A.; Goswami, A.; Kumar Nadda, A.; Sharma, S.; Gupta, S.; Goswami, A.; Sharma, S.; Ghosal, A.; Nadda, A.K. The Scope of Biopolymers in Food Industry; Springer International Publishing: Cham, Switzerland, 2022; pp. 173–198. [Google Scholar] [CrossRef]
- Anjana; Raturi, G.; Shree, S.; Sharma, A.; Panesar, P.S.; Goswami, S. Recent Approaches for Enhanced Production of Microbial Polyhydroxybutyrate: Preparation of Biocomposites and Applications. Int. J. Biol. Macromol. 2021, 182, 1650–1669. [Google Scholar] [CrossRef]
- Ma, Y.; Li, L.; Wang, Y. Development of PLA-PHB-based Biodegradable Active Packaging and Its Application to Salmon. Packag. Technol. Sci. 2018, 31, 739–746. [Google Scholar] [CrossRef]
- Crupano, W.; Adrover-Monserrat, B.; Llumà, J.; Jerez-Mesa, R.; Travieso-Rodriguez, J.A. Investigating Mechanical Properties of 3D Printed Polylactic Acid/Poly-3-Hydroxybutyrate Composites. Compressive and Fatigue Performance. Heliyon 2024, 10, e38066. [Google Scholar] [CrossRef]
- Pirsa, S.; Sani, I.K.; Mirtalebi, S.S. Nano-Biocomposite Based Color Sensors: Investigation of Structure, Function, and Applications in Intelligent Food Packaging. Food Packag. Shelf Life 2022, 31, 100789. [Google Scholar] [CrossRef]
- Clarkson, C.M.; El Awad Azrak, S.M.; Schueneman, G.T.; Snyder, J.F.; Youngblood, J.P. Crystallization Kinetics and Morphology of Small Concentrations of Cellulose Nanofibrils (CNFs) and Cellulose Nanocrystals (CNCs) Melt-Compounded into Poly(Lactic Acid) (PLA) with Plasticizer. Polymer 2020, 187, 122101. [Google Scholar] [CrossRef]
- Moreno-Bohorquez, E.; Arias-Tapia, M.J.; Martínez-Villadiego, K.; Rhenals-Julio, J.D.; Jaramillo, A.F. Effect of Starch Variety and Environmental Conditions on the Aerobic Biodegradation of Citric Acid-Compatibilized Thermoplastic Starch/Polylactic Acid Blends. Polymers 2025, 17, 1295. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef]
- Choudhury, M.R.; Debnath, K. Green Composites: Introductory Overview; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–20. [Google Scholar]
- Klapiszewski, Ł.; Grząbka-Zasadzińska, A.; Borysiak, S.; Jesionowski, T. Preparation and Characterization of Polypropylene Composites Reinforced by Functional ZnO/Lignin Hybrid Materials. Polym. Test. 2019, 79, 106058. [Google Scholar] [CrossRef]
- Barletta, M.; Genovesi, A.; Desole, M.P.; Gisario, A. Melt Processing of Biodegradable Poly(Butylene Succinate) (PBS)—A Critical Review. Clean. Technol. Environ. Policy 2025, 27, 683–725. [Google Scholar] [CrossRef]
- Safari, M.; Pérez-Camargo, R.A.; Ballester-Bayarri, L.; Liu, G.; Mugica, A.; Zubitur, M.; Wang, D.; Müller, A.J. Biodegradable Binary Blends of Poly (Butylene Succinate) or Poly (ε-Caprolactone) with Poly (Butylene Succinate-Ran-ε-Caprolactone)Copolymers: Crystallization Behavior. Polymer 2022, 256, 125206. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent Advances in Biodegradable Polymers for Sustainable Applications. Npj Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Hammache, Y.; Serier, A.; Chaoui, S. The Effect of Thermoplastic Starch on the Properties of Polypropylene/High Density Polyethylene Blend Reinforced by Nano-Clay. Mater. Res. Express 2020, 7, 025308. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Kim, K.-W.; Yang, J.-Y.; Kim, B.-J. Enhanced Crystallization of Sustainable Polylactic Acid Composites Incorporating Recycled Industrial Cement. Polymers 2024, 16, 1666. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, J.; Liang, X.; Huang, Z.; Li, J.; Peng, S. Crystallization Behaviors Regulations and Mechanical Performances Enhancement Approaches of Polylactic Acid (PLA) Biodegradable Materials Modified by Organic Nucleating Agents. Int. J. Biol. Macromol. 2023, 233, 123581. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable Polylactic Acid and Its Composites: Characteristics, Processing, and Sustainable Applications in Sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, D.; Quiles-Carrillo, L.; Balart, R.; Torres-Giner, S.; Arrieta, M.P. Innovative Solutions and Challenges to Increase the Use of Poly(3-Hydroxybutyrate) in Food Packaging and Disposables. Eur. Polym. J. 2022, 178, 111505. [Google Scholar] [CrossRef]
- Amrutha, S.R.; Rejimon, P.K.; Suja, N.R.; Mart, A. Thermal Properties of Biopolymers. In Handbook of Biopolymers; Springer Nature: Singapore, 2023; pp. 1–28. [Google Scholar]
- Ribba, L.; Lorenzo, M.C.; Tupa, M.; Melaj, M.; Eisenberg, P.; Goyanes, S. Processing and Properties of Starch-Based Thermoplastic Matrix for Green Composites; Springer Nature: Singapore, 2021; pp. 63–133. [Google Scholar]
- Sakurai, T.; Nagakura, H.; Gondo, S.; Nojima, S. Crystallization of Poly(ɛ-Caprolactone) Blocks Confined in Crystallized Lamellar Morphology of Poly(ɛ-Caprolactone)-Block-Polyethylene Copolymers: Effects of Polyethylene Crystallinity and Confinement Size. Polym. J. 2013, 45, 436–443. [Google Scholar] [CrossRef]
- Briassoulis, D.; Tserotas, P.; Athanasoulia, I.-G. Alternative Optimization Routes for Improving the Performance of Poly(3-Hydroxybutyrate) (PHB) Based Plastics. J. Clean. Prod. 2021, 318, 128555. [Google Scholar] [CrossRef]
- Kato, S.; Ueda, T.; Aoshima, T.; Kosaka, N.; Nitta, S. BioPBSTM (Polybutylene Succinate); Springer: Cham, Switzerland, 2023; pp. 269–304. [Google Scholar]
- Labet, M.; Thielemans, W. Synthesis of Polycaprolactone: A Review. Chem. Soc. Rev. 2009, 38, 3484. [Google Scholar] [CrossRef]
- Greene, J.P. Microstructures of Polymers. In Automotive Plastics and Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–37. [Google Scholar]
- Yi, X.; Tong, J.; Zhang, X.; Zhu, J.; Liu, X.; Xian, G.; Li, Y.; Ding, F.; Rudd, C.; Liu, X.; et al. Biopolymers and Biocomposites. In Revolutionizing Aircraft Materials and Processes; Springer International Publishing: Cham, Switzerland, 2020; pp. 231–275. [Google Scholar]
- Freeland, B.; McCarthy, E.; Balakrishnan, R.; Fahy, S.; Boland, A.; Rochfort, K.D.; Dabros, M.; Marti, R.; Kelleher, S.M.; Gaughran, J. A Review of Polylactic Acid as a Replacement Material for Single-Use Laboratory Components. Materials 2022, 15, 2989. [Google Scholar] [CrossRef]
- Nath, D.; Misra, M.; Al-Daoud, F.; Mohanty, A.K. Studies on Poly(Butylene Succinate) and Poly(Butylene Succinate-Co-Adipate)-Based Biodegradable Plastics for Sustainable Flexible Packaging and Agricultural Applications: A Comprehensive Review. RSC Sustain. 2025, 3, 1267–1302. [Google Scholar] [CrossRef]
- Bhadran, A.; Shah, T.; Babanyinah, G.K.; Polara, H.; Taslimy, S.; Biewer, M.C.; Stefan, M.C. Recent Advances in Polycaprolactones for Anticancer Drug Delivery. Pharmaceutics 2023, 15, 1977. [Google Scholar] [CrossRef]
- Folino, A.; Karageorgiou, A.; Calabrò, P.S.; Komilis, D. Biodegradation of Wasted Bioplastics in Natural and Industrial Environments: A Review. Sustainability 2020, 12, 6030. [Google Scholar] [CrossRef]
- Lai, S.-M.; Don, T.-M.; Huang, Y.-C. Preparation and Properties of Biodegradable Thermoplastic Starch/Poly(Hydroxy Butyrate) Blends. J. Appl. Polym. Sci. 2006, 100, 2371–2379. [Google Scholar] [CrossRef]
- George, N.; Venugopal, B. Gas Barrier Properties of Biopolymers. In Handbook of Biopolymers; Springer Nature: Singapore, 2023; pp. 297–321. [Google Scholar]
- Paul, V.; Tripathi, A.D.; Maurya, K.K.; Pankaj; Dinesh; Rai, C. Introduction: Scope and Importance of Biodegradable Polymers. In Biodegradable Polymer-Based Food Packaging; Springer Nature: Singapore, 2022; pp. 1–11. [Google Scholar]
- McKeen, L.W. Renewable Resource, Sustainable and Biodegradable Polymers. In The Effect of UV Light and Weather on Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 425–438. [Google Scholar]
- Cecchi, T. Assessment of the Safety of BioBased Products. In Biobased Products from Food Sector Waste; Springer International Publishing: Cham, Switzerland, 2021; pp. 343–363. [Google Scholar]
- Cherian, R.M.; Kargarzadeh, H.; Rosli, N.A.; Jose, C.; Thomas, S. Tuning the Hydrophilic/Hydrophobic Behavior of Biopolymers. In Handbook of Biopolymers; Springer Nature: Singapore, 2023; pp. 367–401. [Google Scholar]
- Nagaraju, D.H.; Budagumpi, S.; Yhobu, Z. Biopolymer-Based Composites. In Handbook of Biopolymers; Springer Nature: Singapore, 2023; pp. 491–522. [Google Scholar]
- Darie-Niță, R.; Râpă, M.; Sivertsvik, M.; Rosnes, J.; Popa, E.; Dumitriu, R.; Marincaș, O.; Matei, E.; Predescu, C.; Vasile, C. PLA-Based Materials Containing Bio-Plasticizers and Chitosan Modified with Rosehip Seed Oil for Ecological Packaging. Polymers 2021, 13, 1610. [Google Scholar] [CrossRef]
- Meng, Z.; He, J.; Cai, Z.; Wang, F.; Zhang, J.; Wang, L.; Ling, R.; Li, D. Design and Additive Manufacturing of Flexible Polycaprolactone Scaffolds with Highly-Tunable Mechanical Properties for Soft Tissue Engineering. Mater. Des. 2020, 189, 108508. [Google Scholar] [CrossRef]
- Oliveira, C.F.d.P.; Fidalgo, N.N.; Valera, T.S.; Demarquette, N.R. Blends Polypropylene/Starch Thermoplastic; Associação Brasileira de Polímeros: São Carlos, Brazil, 2014. [Google Scholar]
- Pérez, M.A.; Rivas, B.L.Q.; Rodríguez-Llamazares, S. Polypropylene/Starch Blends: Study of Thermal and Morphological Properties. J. Chil. Chem. Soc. 2013, 58, 1643–1646. [Google Scholar] [CrossRef]
- Noivoil, N.; Yoksan, R. Oligo(Lactic Acid)-Grafted Starch: A Compatibilizer for Poly(Lactic Acid)/Thermoplastic Starch Blend. Int. J. Biol. Macromol. 2020, 160, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Wahab, M.K.A.; Uylan, D.N.; Ismail, H. Physical and Degradation Properties of Polylactic Acid and Thermoplastic Starch Blends—Effect of Citric Acid Treatment on Starch Structures. Bioresources 2017, 12, 3076–3087. [Google Scholar] [CrossRef]
- Al-Mulla, A.; Alfadhel, K.; Qambar, G.; Shaban, H. Rheological Study of Recycled Polypropylene–Starch Blends. Polym. Bull. 2013, 70, 2599–2618. [Google Scholar] [CrossRef]
- Muniyasamy, S.; Ofosu, O.; John, M.J.; Anandjiwala, R.D. Mineralization of Poly(Lactic Acid) (PLA), Poly(3-Hydroxybutyrate-Co-Valerate) (PHBV) and PLA/PHBV Blend in Compost and Soil Environments. J. Renew. Mater. 2016, 4, 133–145. [Google Scholar] [CrossRef]
- Maiti, S.; Ray, D.; Mitra, D. Role of Crosslinker on the Biodegradation Behavior of Starch/Polyvinylalcohol Blend Films. J. Polym. Environ. 2012, 20, 749–759. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, Y.; Shi, Q.; Guo, S. Study of the Preparation and Properties of TPS/PBSA/PLA Biodegradable Composites. J. Compos. Sci. 2021, 5, 48. [Google Scholar] [CrossRef]
- Liu, S.; Tang, S.; Lu, Y.; Su, T.; Wang, Z. Preparation of Esterified Starches with Different Amylose Content and Their Blending with Polybutylene Succinate. Int. J. Mol. Sci. 2024, 25, 6301. [Google Scholar] [CrossRef]
- Martínez-Villadiego, K.; Arias-Tapia, M.J.; Jaramillo, A.F. Improving Thermal Stability of Starches Native Cross-Linked with Citric Acid as a Compatibilizer for Thermoplastic Starch/Polylactic Acid Blends. Polym. Bull. 2024, 81, 13253–13274. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez-Rivera, M.; Fernández-Blázquez, J.P.; Monclús, M.; Peña-Farfal, C. Mechanical and morphological properties of poly(3-hydroxybutyrate)-thermoplastic starch/clay/eugenol bionanocomposites. J. Chil. Chem. Soc. 2020, 65, 4992–4997. [Google Scholar] [CrossRef]
- Thiré, R.M.S.M.; Ribeiro, T.A.A.; Andrade, C.T. Effect of Starch Addition on Compression-molded Poly(3-hydroxybutyrate)/Starch Blends. J. Appl. Polym. Sci. 2006, 100, 4338–4347. [Google Scholar] [CrossRef]
- Katanyoota, P.; Jariyasakoolroj, P.; Sane, A. Property Enhancement of Polylactic Acid/Thermoplastic Starch Blend Using Zeolite 5A Incorporation and Biaxial Stretching Process. Agric. Nat. Resour. 2023, 57, 191–200. [Google Scholar] [CrossRef]
- Huneault, M.A.; Li, H. Morphology and Properties of Compatibilized Polylactide/Thermoplastic Starch Blends. Polymer 2007, 48, 270–280. [Google Scholar] [CrossRef]
- Akrami, M.; Ghasemi, I.; Azizi, H.; Karrabi, M.; Seyedabadi, M. A New Approach in Compatibilization of the Poly(Lactic Acid)/Thermoplastic Starch (PLA/TPS) Blends. Carbohydr. Polym. 2016, 144, 254–262. [Google Scholar] [CrossRef]
- Vanovčanová, Z.; Alexy, P.; Feranc, J.; Plavec, R.; Bočkaj, J.; Kaliňáková, L.; Tomanová, K.; Perďochová, D.; Šariský, D.; Gálisová, I. Effect of PHB on the Properties of Biodegradable PLA Blends. Chem. Pap. 2016, 70, 1408–1415. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Lin, X.; Zhou, Y. Comparative Study on the Blends of PBS/Thermoplastic Starch Prepared from Waxy and Normal Corn Starches. Starch-Starke 2013, 65, 831–839. [Google Scholar] [CrossRef]
- Mina Hernandez, J.H. Effect of the Incorporation of Polycaprolactone (PCL) on the Retrogradation of Binary Blends with Cassava Thermoplastic Starch (TPS). Polymers 2020, 13, 38. [Google Scholar] [CrossRef]
- Estrada-Monje, A.; Alonso-Romero, S.; Zitzumbo-Guzmán, R.; Estrada-Moreno, I.A.; Zaragoza-Contreras, E.A. Thermoplastic Starch-Based Blends with Improved Thermal and Thermomechanical Properties. Polymers 2021, 13, 4263. [Google Scholar] [CrossRef]
- Guigo, N.; Sbirrazzuoli, N. Thermal Analysis of Biobased Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 399–429. [Google Scholar]
- Narancic, T.; Verstichel, S.; Reddy Chaganti, S.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Babu Padamati, R.; O’Connor, K.E. Biodegradable Plastic Blends Create New Possibilities for End-of-Life Management of Plastics but They Are Not a Panacea for Plastic Pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- Barron, A.; Sparks, T.D. Commercial Marine-Degradable Polymers for Flexible Packaging. iScience 2020, 23, 101353. [Google Scholar] [CrossRef]
- Goyal, C.; Rai, S.; Tripathi, A.D.; Rai, D.C. Microbial Biopolymers and Enzymes Involved in the Biosynthesis of PHAs. In Biodegradable Polymer-Based Food Packaging; Springer Nature: Singapore, 2022; pp. 155–178. [Google Scholar]
- Don, T.-M.; Chung, C.-Y.; Lai, S.-M.; Chiu, H.-J. Preparation and Properties of Blends from Poly(3-Hydroxybutyrate) with Poly(Vinyl Acetate)-Modified Starch. Polym. Eng. Sci. 2010, 50, 709–718. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Arai, S.; Arai, N. Design Strategy for Blends of Biodegradable Polyester and Thermoplastic Starch Based on a Molecular Dynamics Study of the Phase-Separated Interface. Carbohydr. Polym. 2024, 333, 122005. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.A.; Reghunadhan, A.; Maria, H.J.; Thomas, S. Role of Functional Polymers in the Compatibilization of Polymer Blends. In Reactive and Functional Polymers Volume Two; Springer International Publishing: Cham, Switzerland, 2020; pp. 5–21. [Google Scholar]
- Liao, H.-T.; Wu, C.-S. Performance of an Acrylic-Acid-Grafted Poly(3-Hydroxybutyric Acid)/Starch Bio-Blend: Characterization and Physical Properties. Des. Monomers Polym. 2007, 10, 1–18. [Google Scholar] [CrossRef]
- Florez, J.P.; Fazeli, M.; Simão, R.A. Preparation and Characterization of Thermoplastic Starch Composite Reinforced by Plasma-Treated Poly (Hydroxybutyrate) PHB. Int. J. Biol. Macromol. 2019, 123, 609–621. [Google Scholar] [CrossRef]
- Trinh, B.M.; Tadele, D.T.; Mekonnen, T.H. Robust and High Barrier Thermoplastic Starch—PLA Blend Films Using Starch-Graft-Poly(Lactic Acid) as a Compatibilizer. Mater. Adv. 2022, 3, 6208–6221. [Google Scholar] [CrossRef]
- Jozinović, A.; Kovač, M.; Ocelić Bulatović, V.; Kučić Grgić, D.; Miloloža, M.; Šubarić, D.; Ačkar, Đ. Biopolymeric Blends of Thermoplastic Starch and Polylactide as Sustainable Packaging Materials. Polymers 2024, 16, 1268. [Google Scholar] [CrossRef] [PubMed]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Using Grafted Poly(ε-Caprolactone) for the Compatibilization of Thermoplastic Starch-Polylactic Acid Blends. React. Funct. Polym. 2019, 142, 25–35. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Jiao, L.; Li, Y.-D.; Srinivasan, M.; Li, T.; Wang, Y.-Z. Bio-Based Blends of Starch and Poly(Butylene Succinate) with Improved Miscibility, Mechanical Properties, and Reduced Water Absorption. Carbohydr. Polym. 2011, 83, 762–768. [Google Scholar] [CrossRef]
- Beluci, N.d.C.L.; Santos, J.d.; de Carvalho, F.A.; Yamashita, F. Reactive Biodegradable Extruded Blends of Thermoplastic Starch and Polyesters. Carbohydr. Polym. Technol. Appl. 2023, 5, 100274. [Google Scholar] [CrossRef]
- Duc, V.M.; Giang, N.C. Morphology and Properties of Polybutylene Succinic and Cassava Starch Blend: Effect of Esterification Catalysts on Graft Copolymer Formation. Vietnam. J. Chem. 2021, 59, 90–97. [Google Scholar] [CrossRef]
- Thajai, N.; Rachtanapun, P.; Thanakkasaranee, S.; Punyodom, W.; Worajittiphon, P.; Phimolsiripol, Y.; Leksawasdi, N.; Ross, S.; Jantrawut, P.; Jantanasakulwong, K. Reactive Blending of Modified Thermoplastic Starch Chlorhexidine Gluconate and Poly(Butylene Succinate) Blending with Epoxy Compatibilizer. Polymers 2023, 15, 3487. [Google Scholar] [CrossRef]
- Menossi, M.; Salcedo, F.; Rivilli, N.; Nicolini, A.T.; Alvarez, V.A.; Ludueña, L.N. Biodegradable Mulch Films Based on Starch/Poly (Lactic Acid)/Poly (ε-Caprolactone) Ternary Blends. J. Polym. Environ. 2023, 31, 2114–2137. [Google Scholar] [CrossRef]
- Li, M.-N.; Xie, Y.; Chen, H.-Q.; Zhang, B. Effects of Heat-Moisture Treatment after Citric Acid Esterification on Structural Properties and Digestibility of Wheat Starch, A- and B-Type Starch Granules. Food Chem. 2019, 272, 523–529. [Google Scholar] [CrossRef]
- Miskeen, S.; Hong, J.S.; Choi, H.-D.; Kim, J.-Y. Fabrication of Citric Acid-Modified Starch Nanoparticles to Improve Their Thermal Stability and Hydrophobicity. Carbohydr. Polym. 2021, 253, 117242. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ching, Y.C.; Chuah, C.H.; Nguyen, D.H.; Liou, N.-S. Synthesis and Characterization of Starch/Fiber-Based Bioplastic Composites Modified by Citric Acid-Epoxidized Palm Oil Oligomer with Reactive Blending. Ind. Crops Prod. 2021, 170, 113797. [Google Scholar] [CrossRef]
- Lv, X.; Guo, C.; Ma, Y.; Liu, B. Effect of Citric Acid Esterification on the Structure and Physicochemical Properties of Tigernut Starch. Int. J. Biol. Macromol. 2022, 222, 2833–2842. [Google Scholar] [CrossRef]
- Itkor, P.; Singh, A.K.; Lee, M.; Boonsiriwit, A.; Lee, Y.S. Effects of Starch–Citric Acid Cross-Linking on the Fibrous Composites Using Waste Paper Pulp Material for Eco-Friendly Packaging. Biomass Convers. Biorefin 2024, 14, 14693–14705. [Google Scholar] [CrossRef]
- Gong, J.; Xu, W.; Zhang, C.; Zhu, Q.; Qin, X.; Zhang, H.; Liu, G. Effects of Esterification and Enzymatic Modification on the Properties of Wheat Starch and Dough. Food Hydrocoll. 2025, 158, 110509. [Google Scholar] [CrossRef]
- He, X.; Zhang, F.; Li, C.; Ding, W.; Jin, Y.; Tang, L.; Huang, R. Effect of Starch Plasticization on Morphological, Mechanical, Crystalline, Thermal, and Optical Behavior of Poly(Butylene Adipate-Co-Terephthalate)/Thermoplastic Starch Composite Films. Polymers 2024, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- Ojogbo, E.; Blanchard, R.; Mekonnen, T. Hydrophobic and Melt Processable Starch-Laurate Esters: Synthesis, Structure–Property Correlations. J. Polym. Sci. A Polym. Chem. 2018, 56, 2611–2622. [Google Scholar] [CrossRef]
- Boetje, L.; Lan, X.; Silvianti, F.; van Dijken, J.; Polhuis, M.; Loos, K. A More Efficient Synthesis and Properties of Saturated and Unsaturated Starch Esters. Carbohydr. Polym. 2022, 292, 119649. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, F.; Zhang, K.; Hu, J.; Xu, C.; Lin, Y.; Zhou, M.; Zhu, P. Synthesis of Long-Chain Fatty Acid Starch Esters in Aqueous Medium and Its Characterization. Eur. Polym. J. 2019, 119, 136–147. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Cheng, F.; Lin, Y.; Hu, J.; Zhu, P. Characterization and Properties of Long-Chain Fatty Acid Starch Esters Prepared with Regenerated Starch by Dry Method. Starch-Starke 2019, 71, 1900143. [Google Scholar] [CrossRef]
- Lozano, K.P.C.; Mendes, J.F.; Ferreira, L.F.; Martins, M.A.; Mendoza, J.S.; Mendes, R.F. Hydrophobic Bio-Composites of Stearic Acid Starch Esters and Micro Fibrillated Cellulose Processed by Extrusion. Ind. Crops Prod. 2024, 221, 119313. [Google Scholar] [CrossRef]
- Lin, Z.; Cheng, H.; He, K.; McClements, D.J.; Jin, Z.; Xu, Z.; Meng, M.; Peng, X.; Chen, L. Recent Progress in the Hydrophobic Modification of Starch-Based Films. Food Hydrocoll. 2024, 151, 109860. [Google Scholar] [CrossRef]
- Pack, E.C.; Lee, K.Y.; Jung, J.S.; Jang, D.Y.; Kim, H.S.; Koo, Y.J.; Lee, H.G.; Kim, Y.S.; Lim, K.M.; Lee, S.H.; et al. Determination of the Migration of Plastic Additives and Non-Intentionally Added Substances into Food Simulants and the Assessment of Health Risks from Convenience Food Packaging. Food Packag. Shelf Life 2021, 30, 100736. [Google Scholar] [CrossRef]
- Gupta, R.K.; Pipliya, S.; Karunanithi, S.; Eswaran, U.G.M.; Kumar, S.; Mandliya, S.; Srivastav, P.P.; Suthar, T.; Shaikh, A.M.; Harsányi, E.; et al. Migration of Chemical Compounds from Packaging Materials into Packaged Foods: Interaction, Mechanism, Assessment, and Regulations. Foods 2024, 13, 3125. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Pereira Santos, J.C.; Camilloto, G.P.; Miranda, A.L.; Druzian, J.I.; Guimarães, A.G. Development of Active Films Poly (Butylene Adipate Co-Terephthalate)—PBAT Incorporated with Oregano Essential Oil and Application in Fish Fillet Preservation. Ind. Crops Prod. 2017, 108, 388–397. [Google Scholar] [CrossRef]
- Zhang, W.; Azizi-Lalabadi, M.; Jafarzadeh, S.; Jafari, S.M. Starch-Gelatin Blend Films: A Promising Approach for High-Performance Degradable Food Packaging. Carbohydr. Polym. 2023, 320, 121266. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural Antioxidants-Based Edible Active Food Packaging: An Overview of Current Advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, A. Review on Essential Oil Extraction from Aromatic and Medicinal Plants: Techniques, Performance and Economic Analysis. Sustain. Chem. Pharm. 2022, 30, 100829. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-Based Active Food Packaging Materials: Sustainable Alternative to Conventional Petrochemical-Based Packaging Materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, A.; Zheng, Y.; Zhu, X.; Hu, Y.; Qu, X. Scented Solutions: Harnessing Lavender Essential Oil Liposomes for Enhanced Plywood Performance. Sustain. Chem. Pharm. 2024, 42, 101826. [Google Scholar] [CrossRef]
- Gonçalves, S.; Castro, J.; Almeida, A.; Monteiro, M.; Rodrigues, T.; Fernandes, R.; Matos, R.S. A Systematic Review of the Therapeutic Properties of Lemon Essential Oil. Adv. Integr. Med. 2024, 12, 100433. [Google Scholar] [CrossRef]
- Ziani, I.; Bouakline, H.; Bouknana, S.; Bentouhami, N.E.; Sher, F.; Ansar, S.; Fauconnier, M.-L.; Bnouham, M.; El Bachiri, A. Sustainable Management of Rosemary Wastewater and Essential Oil in Agri-Environmental Bioprocessing. Food Biosci. 2024, 62, 105263. [Google Scholar] [CrossRef]
- Jabbar, M.; Baboo, I.; Majeed, H.; Farooq, Z.; Palangi, V. Characterization and antibacterial application of peppermint essential oil nanoemulsions in broiler. Poult. Sci. 2024, 103, 104432. [Google Scholar] [CrossRef]
- Chauhan, R.; Vishvamitera, S.; Dhiman, D.; Sharma, S.K.; Kumar, R.; Kumar, D.; Singh, S. Growth, Essential Oil Yield and Biological Activities of Curcuma Caesia in Response to Sowing Time and Planting Geometry in the Non-Traditional Area of Western Himalayas. Sci. Hortic. 2024, 338, 113740. [Google Scholar] [CrossRef]
- Khaiper, M.; Poonia, P.K.; Redhu, I.; Verma, P.; Sheokand, R.N.; Nasir, M.; Tiwari, A.; Kumar, V. Chemical Composition, Antifungal and Antioxidant Properties of Seasonal Variation in Eucalyptus Tereticornis Leaves of Essential Oil. Ind. Crops Prod. 2024, 222, 119669. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, X.; Mao, S.; Fan, X.; Xu, Y.; Lu, C. Sustained-Release Composite Films Incorporated with Cinnamon Essential Oil: Characterization, Release Kinetics and Application for Cherry Tomato Preservation. J. Food Eng. 2025, 388, 112356. [Google Scholar] [CrossRef]
- Ricci, A.; Rodolfi, M.; Cirlini, M.; Ganino, T.; Bernini, V.; Lazzi, C. Fermentation of Orange Pomace: Impact on Essential Oil Composition and Antimicrobial Activity. Food Biosci. 2024, 61, 104677. [Google Scholar] [CrossRef]
- Monteiro, J.; Scotti-Campos, P.; Pais, I.; Figueiredo, A.C.; Viegas, D.; Reboredo, F. Elemental Composition, Total Fatty Acids, Soluble Sugar Content and Essential Oils of Flowers and Leaves of Moringa Oleifera Cultivated in Southern Portugal. Heliyon 2022, 8, e12647. [Google Scholar] [CrossRef]
- Martín-Cabrejas, I.; Goicoechea-Oses, E. Effect of Garlic Essential Oil on Sunflower Oil Oxidative Stability during Accelerated Storage Studied by FTIR Spectroscopy. Food Biosci. 2024, 62, 105012. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Barbosa-Nuñez, J.A.; Castillo-Herrera, G.A.; Estarrón-Espinosa, M.; Herrera-Rodríguez, S.E.; Espinosa-Andrews, H.; Álvarez, Á.H.; Martínez-Velázquez, M. Clove Essential Oil and Its Major Component, Eugenol: A Comparative Study of Their in Vitro Antioxidant and Anticancer Properties. Nat. Prod. Res. 2024, 39, 5395–5402. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Li, Y.; Song, Q.; Hu, Y.; Wang, Q.; Lu, S. Characterization of Thyme Essential Oil Microcapsules and Potato Starch/Pectin Composite Films and Their Impact on the Quality of Chilled Mutton. Food Chem. 2025, 464, 141692. [Google Scholar] [CrossRef]
- Cherif, M.; Rodrigues, N.; Veloso, A.C.A.; Zaghdoudi, K.; Pereira, J.A.; Peres, A.M. Kinetic-Thermodynamic Study of the Oxidative Stability of Arbequina Olive Oils Flavored with Lemon Verbena Essential Oil. LWT 2021, 140, 110711. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, R.; Gautam, R.D.; Chauhan, R.; Kumar, D.; Kumar, A.; Singh, S.; Singh, S. Multi-Environment Evaluation of Clary Sage (Salvia sclarea L.) Selections for Yield and Essential Oil Traits under Western Himalayan Conditions. J. Appl. Res. Med. Aromat. Plants 2024, 43, 100579. [Google Scholar] [CrossRef]
- Pant, Y.; Srivastava, S.; Lal, R.K.; Mishra, A.; Bawitlung, L.; Bhatt, D.; Gupta, P.; Yadav, S.; Bawankule, D.U.; Tyagi, V.; et al. Chemical Composition of γ-Irradiated German Chamomile (Matricaria recutita L.) Flower Essential Oils, Acetylcholinesterase Inhibitory Activity and Effects on Growth Inhibition of Staphylococcus Aureus and Candida Albicans. Biocatal. Agric. Biotechnol. 2024, 61, 103391. [Google Scholar] [CrossRef]
- de Souza Alves, M.; de Medeiros, E.A.D.P.; Pereira, C.d.S.B.; Cardoso, C.M.; Pontes, E.G.; dos Santos, A.M.; de Souza, M.A.A. Some Aspects Concerning Citronella Grass Essential Oil and the Agroecological Approach to Protecting Stored Cowpea Beans. J. Nat. Pestic. Res. 2024, 9, 100084. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, P.; You, N.; Xu, Y.; Zhang, Y.; Luan, P.; Lin, B.; Wang, Z.; Zhang, L. Preparation and Characterization of Corn Starch-Based Antimicrobial Indicator Films Containing Purple Corncob Anthocyanin and Tangerine Peel Essential Oil for Monitoring Pork Freshness. Int. J. Biol. Macromol. 2023, 251, 126320. [Google Scholar] [CrossRef]
- Avila-Sosa, R.; Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Palou, E.; López-Malo, A. Combinational Approaches for Antimicrobial Packaging: Chitosan and Oregano Oil. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; pp. 581–588. [Google Scholar] [CrossRef]
- Manaa, A.O.; Baghdadi, H.H.; El-Nikhely, N.A.; Heikal, L.A.; El-Hosseiny, L.S. Oregano Oil-Nanoemulsions: Formulation and Evaluation of Antibacterial and Anticancer Potentials. J. Drug Deliv. Sci. Technol. 2022, 78, 103978. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Fenollar, O.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of Mechanical Ductile Properties of Poly(3-Hydroxybutyrate) by Using Vegetable Oil Derivatives. Macromol. Mater. Eng. 2016, 302, 1600330. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Montanes, N.; Lopez-Martinez, J.; Balart, R. Plasticization Effects of Epoxidized Vegetable Oils on Mechanical Properties of Poly(3-hydroxybutyrate). Polym. Int. 2016, 65, 1157–1164. [Google Scholar] [CrossRef]
- Hosoda, N.; Tsujimoto, T.; Uyama, H. Plant Oil-Based Green Composite Using Porous Poly(3-Hydroxybutyrate). Polym. J. 2014, 46, 301–306. [Google Scholar] [CrossRef]
- Albuquerque, R.M.; Meira, H.M.; Silva, I.D.; Silva, C.J.G.; Almeida, F.C.G.; Amorim, J.D.; Vinhas, G.M.; Costa, A.F.S.; Sarubbo, L.A. Production of a Bacterial Cellulose/Poly(3-Hydroxybutyrate) Blend Activated with Clove Essential Oil for Food Packaging. Polym. Polym. Compos. 2021, 29, 259–270. [Google Scholar] [CrossRef]
- Rech, C.R.; da Silva Brabes, K.C.; Bagnara e Silva, B.E.; Bittencourt, P.R.S.; Koschevic, M.T.; da Silveira, T.F.S.; Martines, M.A.U.; Caon, T.; Martelli, S.M. Biodegradation of Eugenol-Loaded Polyhydroxybutyrate Films in Different Soil Types. Case Stud. Chem. Environ. Eng. 2020, 2, 100014. [Google Scholar] [CrossRef]
- Wang, H.-H.; Zhou, S.-J.; Xiong, S.-J.; Liu, Q.; Tian, H.; Yu, S.; Yuan, T.-Q. High-Performance Thermoplastic Starch/Poly(Butylene Adipate-Co-Terephthalate) Blends through Synergistic Plasticization of Epoxidized Soybean Oil and Glycerol. Int. J. Biol. Macromol. 2023, 242, 124716. [Google Scholar] [CrossRef]
- Gong, K.; Lu, Y.; Portela, A.; Farshbaf Taghinezhad, S.; Lawlor, D.; Connolly, S.; Hu, M.; Chen, Y.; Collins, M.N. A Comparative Study on the Compatibilization of Thermoplastic Starch/Polybutylene Succinate Blends by Chain Extender and Epoxidized Linseed Oil. Macromol. 2025, 5, 24. [Google Scholar] [CrossRef]
- Volpe, V.; De Feo, G.; De Marco, I.; Pantani, R. Use of Sunflower Seed Fried Oil as an Ecofriendly Plasticizer for Starch and Application of This Thermoplastic Starch as a Filler for PLA. Ind. Crops Prod. 2018, 122, 545–552. [Google Scholar] [CrossRef]
- Kerosenewala, J.; Vaidya, P.; Ozarkar, V.; Shirapure, Y.; More, A.P. Eugenol: Extraction, Properties and Its Applications on Incorporation with Polymers and Resins—A Review. Polym. Bull. 2023, 80, 7047–7099. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential Oils as Natural Additives to Prevent Oxidation Reactions in Meat and Meat Products: A Review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Richardson, T.; Korycka-Dahl, M. Lipid Oxidation. In Developments in Dairy Chemistry—2; Springer: Dordrecht, The Netherlands, 1983; pp. 241–363. [Google Scholar]
- Min, D.B.; Lee, H.-O. Chemistry of Lipid Oxidation. In Flavor Chemistry; Springer: Boston, MA, USA, 1999; pp. 175–187. [Google Scholar]
- Yanishlieva-Maslarova, N.V. Inhibiting Oxidation. In Antioxidants in Food; Woodhead Publishing Limited: Cambridge, UK, 2001; pp. 22–70. [Google Scholar] [CrossRef]
- Morales, M.T.; Przybylski, R. Olive Oil Oxidation. In Handbook of Olive Oil; Springer: Boston, MA, USA, 2000; pp. 459–490. [Google Scholar]
- Zeb, A. Chemistry of Phenolic Antioxidants. In Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer International Publishing: Cham, Switzerland, 2021; pp. 25–87. [Google Scholar]
- Caillol, S. The Future of Cardanol as Small Giant for Biobased Aromatic Polymers and Additives. Eur. Polym. J. 2023, 193, 112096. [Google Scholar] [CrossRef]
- Caillol, S. Cardanol: A Promising Building Block for Biobased Polymers and Additives. Curr. Opin. Green. Sustain. Chem. 2018, 14, 26–32. [Google Scholar] [CrossRef]
- De Lima, S.G.; Feitosa, C.M.; Cito, A.M.G.L.; Moita Neto, J.M.; Lopes, J.A.D.; Leite, A.S.; Brito, M.C.; Dantas, S.M.M.; Melo Cavalcante, A.A.C. Effects of Immature Cashew Nut-Shell Liquid (Anacardium occidentale) against Oxidative Damage in Saccharomyces Cerevisiae and Inhibition of Acetylcholinesterase Activity. Genet. Mol. Res. 2008, 7, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept of Antioxidants in Foods. In Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–23. [Google Scholar]
- Zeb, A. Phenolic Antioxidants in Herbs and Spices. In Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Springer International Publishing: Cham, Switzerland, 2021; pp. 225–238. [Google Scholar]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–359. [Google Scholar]
- El Omari, N.; Charfi, S.; Elmenyiy, N.; El Hachlafi, N.; Balahbib, A.; Chamkhi, I.; Bouyahya, A. Essential Oils for Combating Antimicrobial Resistance: Mechanism Insights and Clinical Uses. In Antimicrobial Resistance; Springer Nature: Singapore, 2022; pp. 323–355. [Google Scholar]
- Maran, S.; Yeo, W.W.Y.; Lim, S.-H.E.; Lai, K.-S. Plant Secondary Metabolites for Tackling Antimicrobial Resistance: A Pharmacological Perspective. In Antimicrobial Resistance; Springer Nature: Singapore, 2022; pp. 153–173. [Google Scholar]
- Soetjipto, H.; Aminu, N.R. Positive and Negative Impacts of the Use of Essential Oils in Food. In Essential Oils; Springer International Publishing: Cham, Switzerland, 2022; pp. 191–217. [Google Scholar]
- Mohamed, A.; El Galiou, O.; Zantar, S.; Arakrak, A.; Laglaoui, A.; Zerrouk, M.H. Edible Films and Coatings: Major Challenges and Potential Applications in Food Packaging. A Review. In Food Packaging: The Smarter Way; Springer Nature: Singapore, 2022; pp. 187–224. [Google Scholar]
- Gandova, V.; Lazarov, A.; Fidan, H.; Dimov, M.; Stankov, S.; Denev, P.; Ercisli, S.; Stoyanova, A.; Gulen, H.; Assouguem, A.; et al. Physicochemical and Biological Properties of Carvacrol. Open Chem. 2023, 21, 20220319. [Google Scholar] [CrossRef]
- Rojtman, E.; Denis, M.; Sirvent, C.; Lapinte, V.; Caillol, S.; Briou, B. Polyols from Cashew Nut Shell Liquid (CNSL): Corner-Stone Building Blocks for Cutting-Edge Bio-Based Additives and Polymers. Polym. Chem. 2024, 15, 4375–4415. [Google Scholar] [CrossRef]
- Chen, Y.; Li, N.; Guo, X.; Huang, H.; Garcia-Oliveira, P.; Sun, J.; Zhang, J.; Prieto, M.A.; Guo, Z.; Liu, C. The Nutritional and Bio-active Constituents, Functional Activities, and Industrial Applications of Cashew (Anacardium occidentale): A Review. Food Front. 2023, 4, 1606–1621. [Google Scholar] [CrossRef]
- Nair, K.P.P. Cashew Nut (Anacardium occidentale L.). In The Agronomy and Economy of Important Tree Crops of the Developing World; Elsevier: Amsterdam, The Netherlands, 2010; pp. 21–66. [Google Scholar]
- Yuliana, M.; Nguyen-Thi, B.T.; Faika, S.; Huynh, L.H.; Soetaredjo, F.E.; Ju, Y.-H. Separation and Purification of Cardol, Cardanol and Anacardic Acid from Cashew (Anacardium occidentale L.) Nut-Shell Liquid Using a Simple Two-Step Column Chromatography. J. Taiwan. Inst. Chem. Eng. 2014, 45, 2187–2193. [Google Scholar] [CrossRef]
- Wu, H.; Li, Q.; Yu, H.; Gu, M.; Wang, Y.; Xu, C.; Liao, Z. Comprehensive Utilization of Hainan Cashew Nut Shell: Process Optimization of Cashew Nut Shell Liquid Extraction and Cardanol Refinement by Catalytic Transfer Hydrogenation. Ind. Crops Prod. 2023, 203, 117168. [Google Scholar] [CrossRef]
- Cruz Reina, L.J.; López, G.-D.; Durán-Aranguren, D.D.; Quiroga, I.; Carazzone, C.; Sierra, R. Compressed Fluids and Soxhlet Extraction for the Valorization of Compounds from Colombian Cashew (Anacardium occidentale) Nut Shells Aimed at a Cosmetic Application. J. Supercrit. Fluids 2023, 192, 105808. [Google Scholar] [CrossRef]
- Salehi, B.; Gültekin-Özgüven, M.; Kırkın, C.; Özçelik, B.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Silva, T.G.d.; Coutinho, H.D.M.; Amina, B.; et al. Anacardium Plants: Chemical, Nutritional Composition and Biotechnological Applications. Biomolecules 2019, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Veeramanoharan, A.; Kim, S.-C. A Comprehensive Review on Sustainable Surfactants from CNSL: Chemistry, Key Applications and Research Perspectives. RSC Adv. 2024, 14, 25429–25471. [Google Scholar] [CrossRef]
- Krist, S. Cashew Oil. In Vegetable Fats and Oils; Springer International Publishing: Cham, Switzerland, 2020; pp. 191–196. [Google Scholar]
- Kyei, S.K.; Eke, W.I.; Nagre, R.D.; Mensah, I.; Akaranta, O. A Comprehensive Review on Waste Valorization of Cashew Nutshell Liquid: Sustainable Development and Industrial Applications. Clean. Waste Syst. 2023, 6, 100116. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased Materials for Active Food Packaging: A Review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Adjoumane, M.M.A.; Edja, A.F.; Doe-Mensah, J.; Boa, D.; Driscoll, M. Effect of Cardanol-Based Plasticizers on Thermoplastic Cassava (Manihot esculenta) Starch Properties. Ind. Crops Prod. 2023, 203, 117145. [Google Scholar] [CrossRef]
- Caicho-Caranqui, J.; Taipe, L.A.; Mena, K.A.; Ponce, S.; Mora, J.R.; Negrete-Bolagay, D.; Zamora-Mendoza, L.; Guerrero, V.H.; Ponton Bravo, P.I.; Pasquel, D.; et al. Towards Sustainable Bioplasticizers from Biomass to Polymers Applications: A Review. Sustain. Mater. Technol. 2025, 43, e01194. [Google Scholar] [CrossRef]
- Vallin, A.; Ferretti, F.; Campaner, P.; Monticelli, O.; Pellis, A. Environmentally Friendly Synthesis of Cardanol-Based Polyesters and Their Application as Poly(Lactic Acid) Additives. ACS Sustain. Chem. Eng. 2023, 11, 9654–9661. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, Z.; Xiong, Z.; Zhu, J. Preparation and Characterization of Thermoplastic Starches and Their Blends with Poly(Lactic Acid). Int. J. Biol. Macromol. 2015, 77, 273–279. [Google Scholar] [CrossRef]
- Fayyazbakhsh, A.; Koutný, M.; Kalendová, A.; Šašinková, D.; Julinová, M.; Kadlečková, M. Selected Simple Natural Antimicrobial Terpenoids as Additives to Control Biodegradation of Polyhydroxy Butyrate. Int. J. Mol. Sci. 2022, 23, 14079. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ghosh, T.; Zhang, W.; Rhim, J.-W. Recent Progress in PBAT-Based Films and Food Packaging Applications: A Mini-Review. Food Chem. 2024, 437, 137822. [Google Scholar] [CrossRef]
- Das, S.; Chaudhari, A.K.; Dwivedy, A.K.; Upadhyay, N.; Singh, V.K.; Singh, A.; Dubey, N.K. Nanoencapsulation Technology: Boon to Food Packaging Industries; Springer International Publishing: Cham, Switzerland, 2020; pp. 17–40. [Google Scholar]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and Antimicrobial Applications of Biopolymers: A Review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef]
- Campos-Requena, V.H.; Rivas, B.L.; Pérez, M.A.; Figueroa, C.R.; Figueroa, N.E.; Sanfuentes, E.A. Thermoplastic Starch/Clay Nanocomposites Loaded with Essential Oil Constituents as Packaging for Strawberries—In Vivo Antimicrobial Synergy over Botrytis Cinerea. Postharvest Biol. Technol. 2017, 129, 29–36. [Google Scholar] [CrossRef]
- Stoleru, E.; Irimia, A.; Butnaru, E. Bio-Based Bioplastics in Active Food Packaging. In Bioplastics for Sustainable Development; Springer: Singapore, 2021; pp. 347–379. [Google Scholar]
- Kusuma, H.S.; Sabita, A.; Putri, N.A.; Azliza, N.; Illiyanasafa, N.; Darmokoesoemo, H.; Amenaghawon, A.N.; Kurniawan, T.A. Waste to Wealth: Polyhydroxyalkanoates (PHA) Production from Food Waste for a Sustainable Packaging Paradigm. Food Chem. Mol. Sci. 2024, 9, 100225. [Google Scholar] [CrossRef]
- Belhassen, R.; Méndez, J.A.; Boufi, S.; López, J.P.; Puig, J.; Pèlach, A.; Mutjé, P. Preparation and Properties of Biocomposites Based on Jute Fibers and Blend of Plasticized Starch and Poly(Β-hydroxybutyrate). J. Appl. Polym. Sci. 2009, 114, 313–321. [Google Scholar] [CrossRef]
- Garrido-Miranda, K.A.; Rivas, B.L.; Pérez, M.A. Poly(3-hydroxybutyrate)–Thermoplastic Starch–Organoclay Bionanocomposites: Surface Properties. J. Appl. Polym. Sci. 2017, 134, 45217. [Google Scholar] [CrossRef]
- Serra-Parareda, F.; Delgado-Aguilar, M.; Espinach, F.X.; Mutjé, P.; Boufi, S.; Tarrés, Q. Sustainable Plastic Composites by Polylactic Acid-Starch Blends and Bleached Kraft Hardwood Fibers. Compos. B Eng. 2022, 238, 109901. [Google Scholar] [CrossRef]
- Chotiprayon, P.; Chaisawad, B.; Yoksan, R. Thermoplastic Cassava Starch/Poly(Lactic Acid) Blend Reinforced with Coir Fibres. Int. J. Biol. Macromol. 2020, 156, 960–968. [Google Scholar] [CrossRef]
- Ayana, B.; Suin, S.; Khatua, B.B. Highly Exfoliated Eco-Friendly Thermoplastic Starch (TPS)/Poly (Lactic Acid)(PLA)/Clay Nanocomposites Using Unmodified Nanoclay. Carbohydr. Polym. 2014, 110, 430–439. [Google Scholar] [CrossRef]
- Boonprasith, P.; Wootthikanokkhan, J.; Nimitsiriwat, N. Mechanical, Thermal, and Barrier Properties of Nanocomposites Based on Poly(Butylene Succinate)/Thermoplastic Starch Blends Containing Different Types of Clay. J. Appl. Polym. Sci. 2013, 130, 1114–1123. [Google Scholar] [CrossRef]
- Mochane, M.J.; Magagula, S.I.; Sefadi, J.S.; Mokhena, T.C. A Review on Green Composites Based on Natural Fiber-Reinforced Polybutylene Succinate (PBS). Polymers 2021, 13, 1200. [Google Scholar] [CrossRef]
- Campos, A.; Teodoro, K.B.R.; Teixeira, E.M.; Corrêa, A.C.; Marconcini, J.M.; Wood, D.F.; Williams, T.G.; Mattoso, L.H.C. Properties of Thermoplastic Starch and TPS/Polycaprolactone Blend Reinforced with Sisal Whiskers Using Extrusion Processing. Polym. Eng. Sci. 2013, 53, 800–808. [Google Scholar] [CrossRef]
- Nian, L.; Wang, M.; Sun, X.; Zeng, Y.; Xie, Y.; Cheng, S.; Cao, C. Biodegradable Active Packaging: Components, Preparation, and Applications in the Preservation of Postharvest Perishable Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2024, 64, 2304–2339. [Google Scholar] [CrossRef] [PubMed]
- Abang, S.; Wong, F.; Sarbatly, R.; Sariau, J.; Baini, R.; Besar, N.A. Bioplastic Classifications and Innovations in Antibacterial, Antifungal, and Antioxidant Applications. J. Bioresour. Bioprod. 2023, 8, 361–387. [Google Scholar] [CrossRef]
- Abhiram, Y.; Das, A.; Sharma, K.K. Green Composites for Structural and Non-Structural Applications: A Review. Mater. Today Proc. 2021, 44, 2658–2664. [Google Scholar] [CrossRef]
- Hlaváčiková, S.; Omaníková, L.; Horváth, V.; Alexy, P.; Jančovičová, V.; Baco, A.; Mikolajová, M.; Fogašová, M.; Tomanová, K.; Feranc, J.; et al. The Possibility of Using the Regranulate of a Biodegradable Polymer Blend Based on Polylactic Acid and Polyhydroxybutyrate in FDM 3D Printing Technology. Results Mater. 2024, 21, 100511. [Google Scholar] [CrossRef]
- Chong, W.J.; Shen, S.; Li, Y.; Trinchi, A.; Pejak Simunec, D.; Kyratzis, I.L.; Sola, A.; Wen, C. Biodegradable PLA-ZnO Nanocomposite Biomaterials with Antibacterial Properties, Tissue Engineering Viability, and Enhanced Biocompatibility. Smart Mater. Manuf. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Harnkarnsujarit, N.; Chongcharoenyanon, B.; Kwon, S.; Ko, S. Enhanced Properties of PBAT/TPS Biopolymer Blend with CuO Nanoparticles for Promising Active Packaging. Food Packag. Shelf Life 2023, 37, 101072. [Google Scholar] [CrossRef]
- Forfora, N.; Azuaje, I.; Kanipe, T.; Gonzalez, J.A.; Lendewig, M.; Urdaneta, I.; Venditti, R.; Gonzalez, R.; Argyropoulos, D. Are Starch-Based Materials More Eco-Friendly than Fossil-Based? A Critical Assessment. Clean. Environ. Syst. 2024, 13, 100177. [Google Scholar] [CrossRef]
- Broeren, M.L.M.; Kuling, L.; Worrell, E.; Shen, L. Environmental Impact Assessment of Six Starch Plastics Focusing on Wastewater-Derived Starch and Additives. Resour. Conserv. Recycl. 2017, 127, 246–255. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Environmental Impact of Bioplastic Use: A Review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Äkräs, L.; Madani, Z.; Silvenius, F.; Fazeli, M.; Lipponen, S.; Vapaavuori, J.; Seppälä, J. Development and Characterization of Polylactic Acid/Starch Biocomposites—From Melt Blending to Preliminary Life Cycle Assessment. Int. J. Biol. Macromol. 2024, 279, 135173. [Google Scholar] [CrossRef]
- Naranjo Vaseco, J.M. Design and Analysis of the Polyhydroxybutyrate (PHB) Production from Agroindustrial Wastes in Colombia; Universidad Nacional de Colombia: Manizales, Colombia, 2014. [Google Scholar]
- Ferraz, C.A.; Pastorinho, M.R.; Palmeira-de-Oliveira, A.; Sousa, A.C.A. Ecotoxicity of Plant Extracts and Essential Oils: A Review. Environ. Pollut. 2022, 292, 118319. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.