Sensitive and Stable NCF/GO/Au@Ag SERS Substrate for Trace Detection of Polycyclic Aromatic Hydrocarbons
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of NCF/GO/Au@Ag Substrate
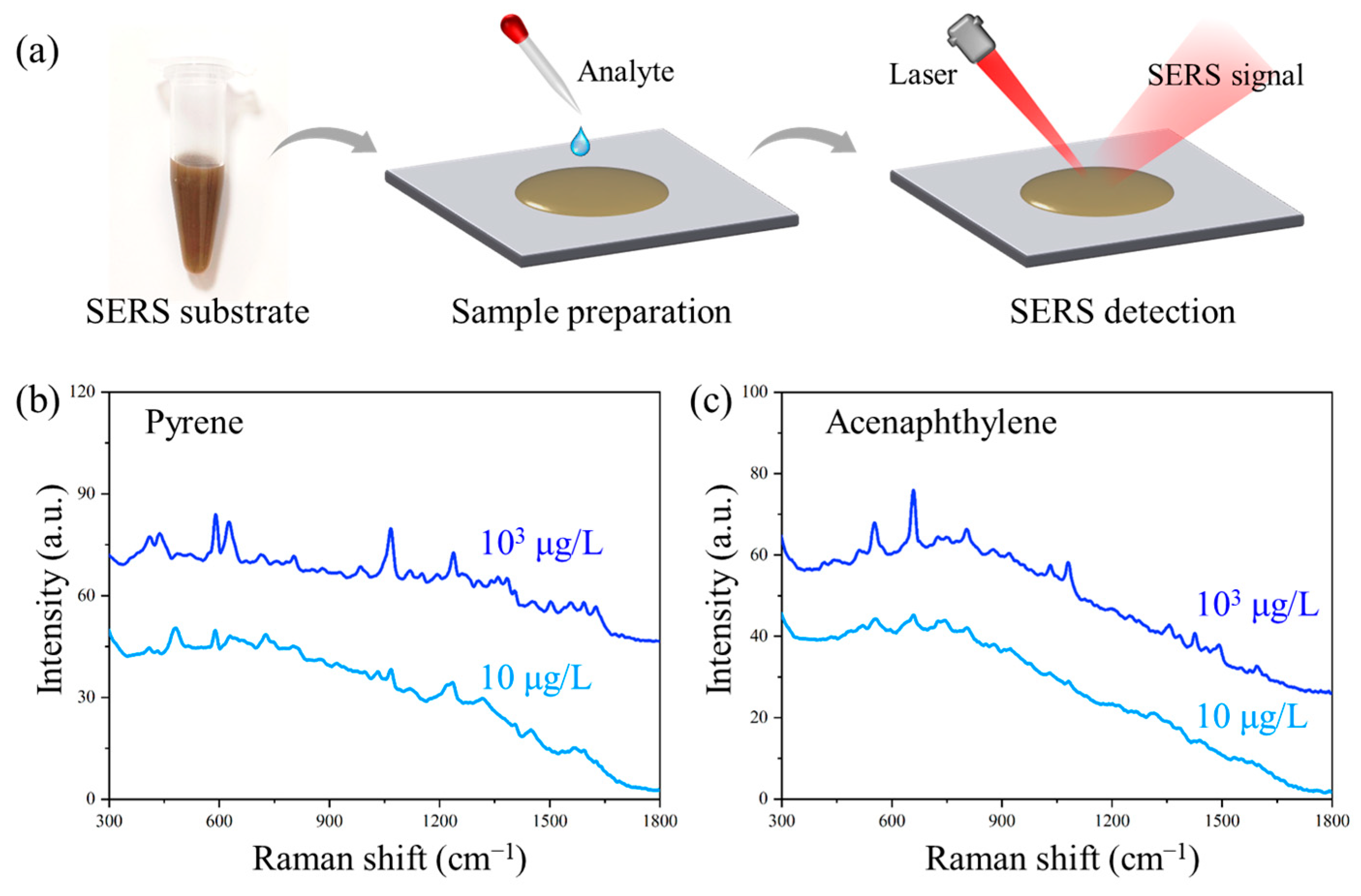
2.3. Sample Preparation and SERS Detection
2.4. Apparatus and Measurements
2.5. FEM Simulation and Data Analysis
3. Results and Discussion
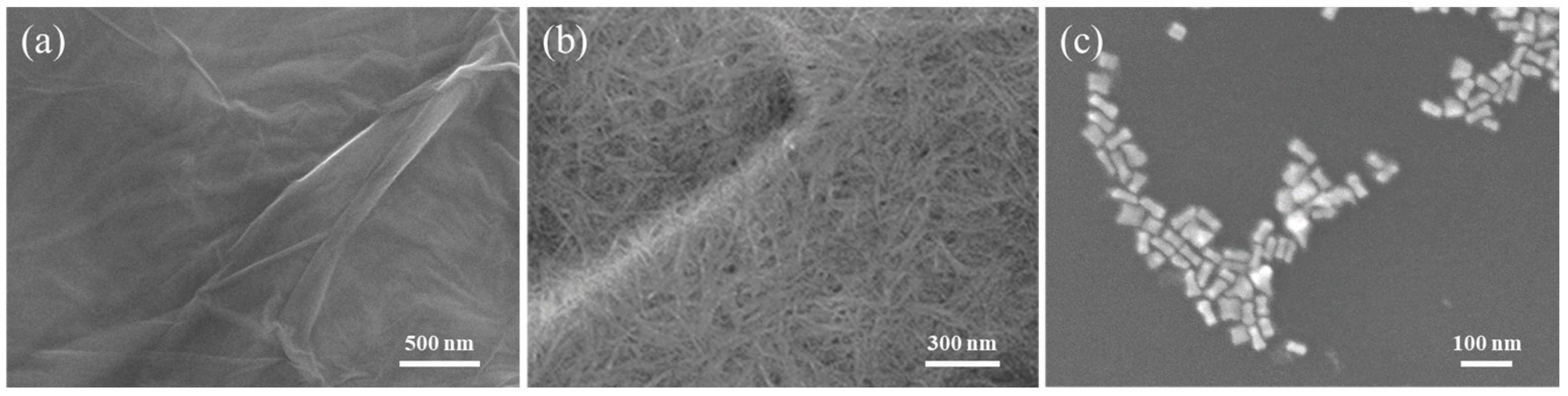
3.1. Characterization of NCF/GO/Au@Ag Substrate
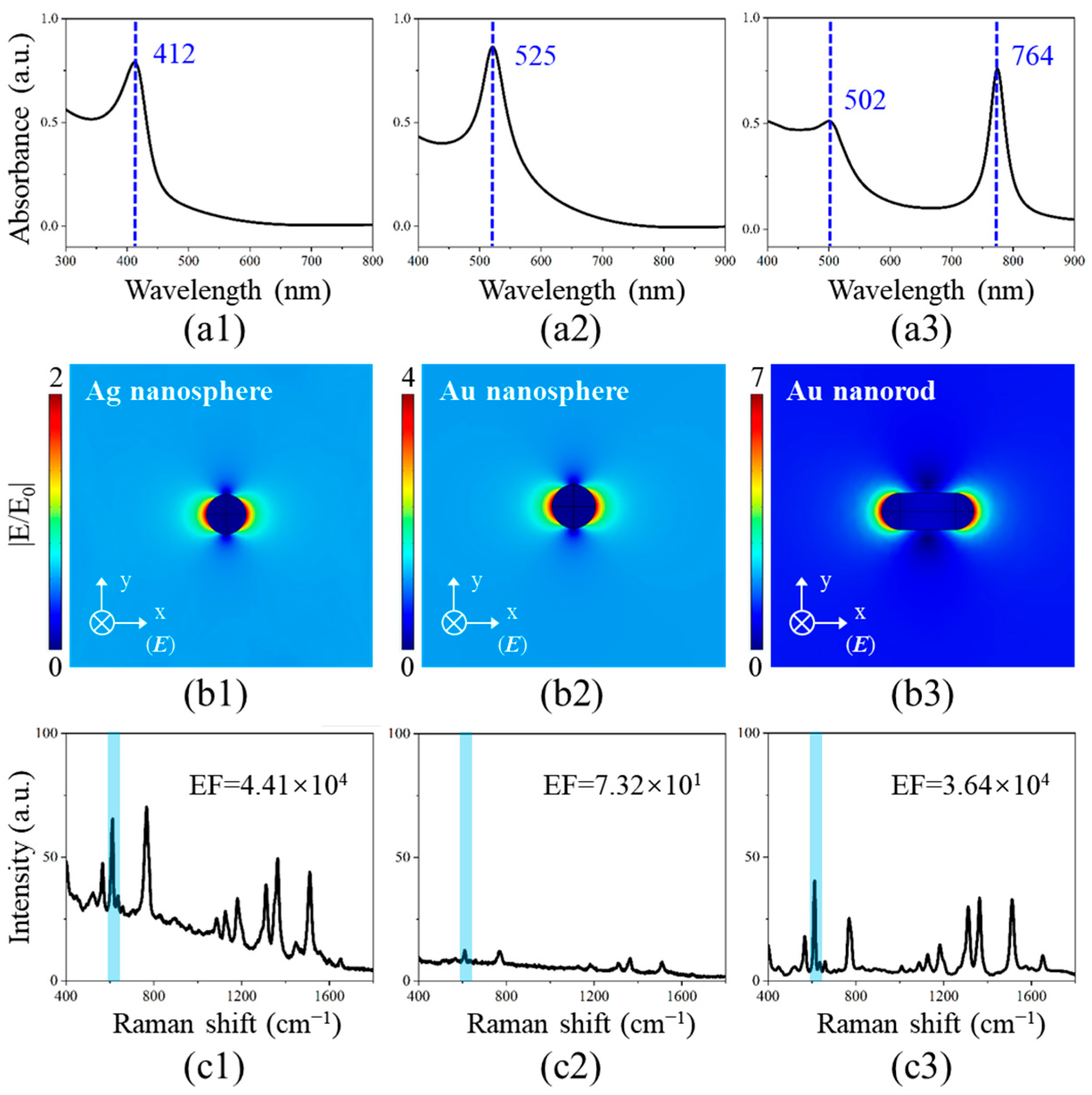
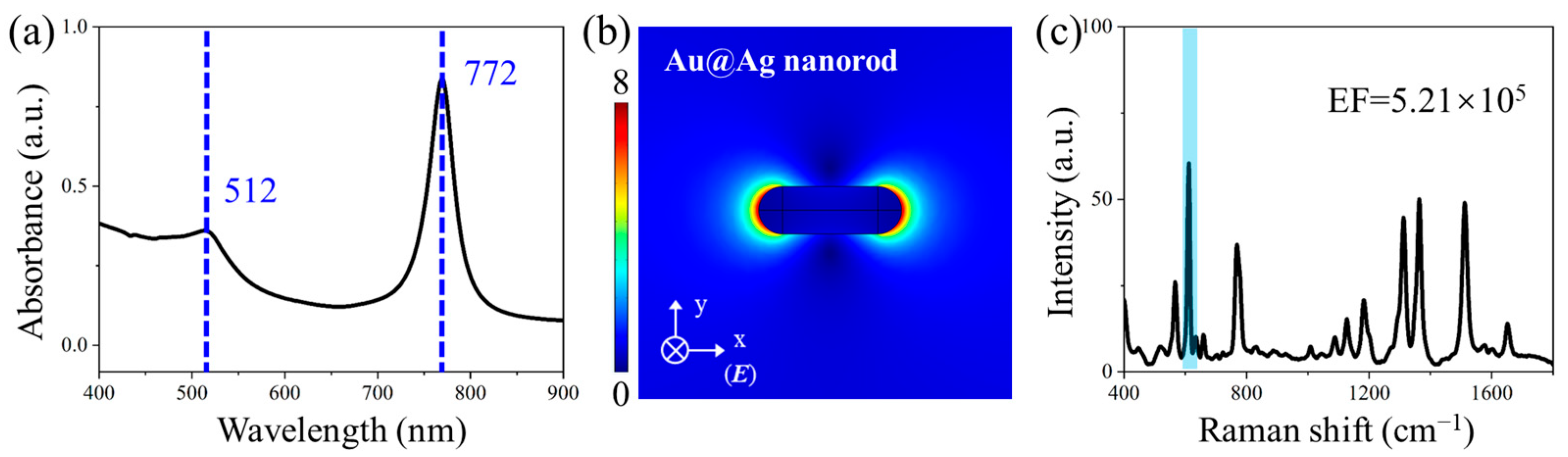
3.2. LSPR Characteristics of Noble Metal Nanostructures
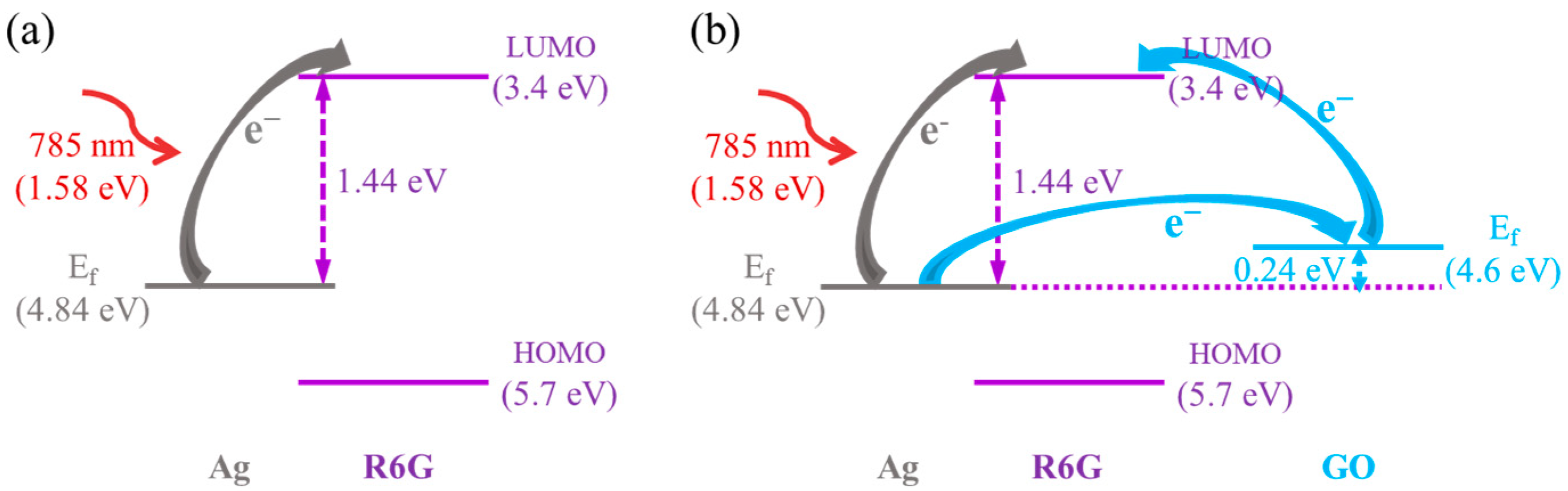
3.3. Synergistic Enhancement Mechanisms for GO
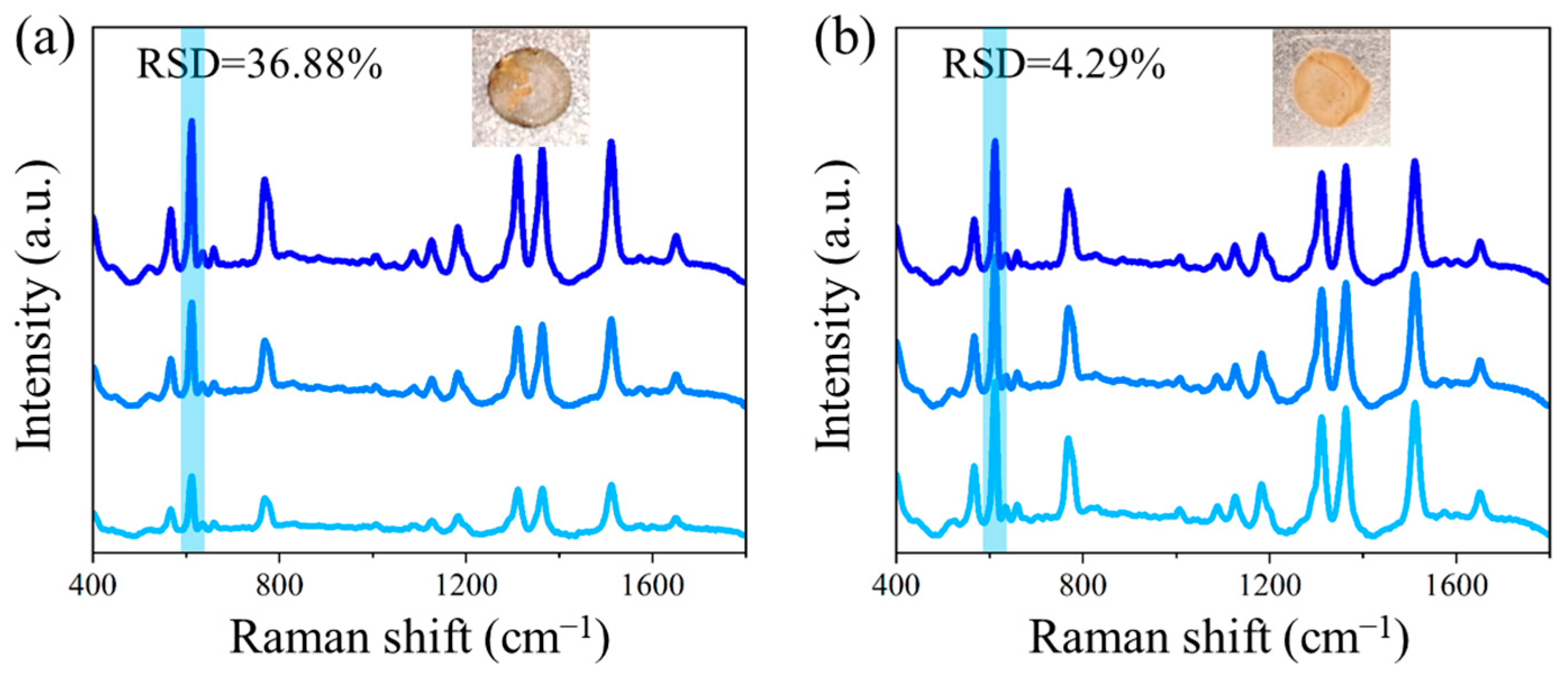
3.4. The Signal Stabilization Effect of NCF
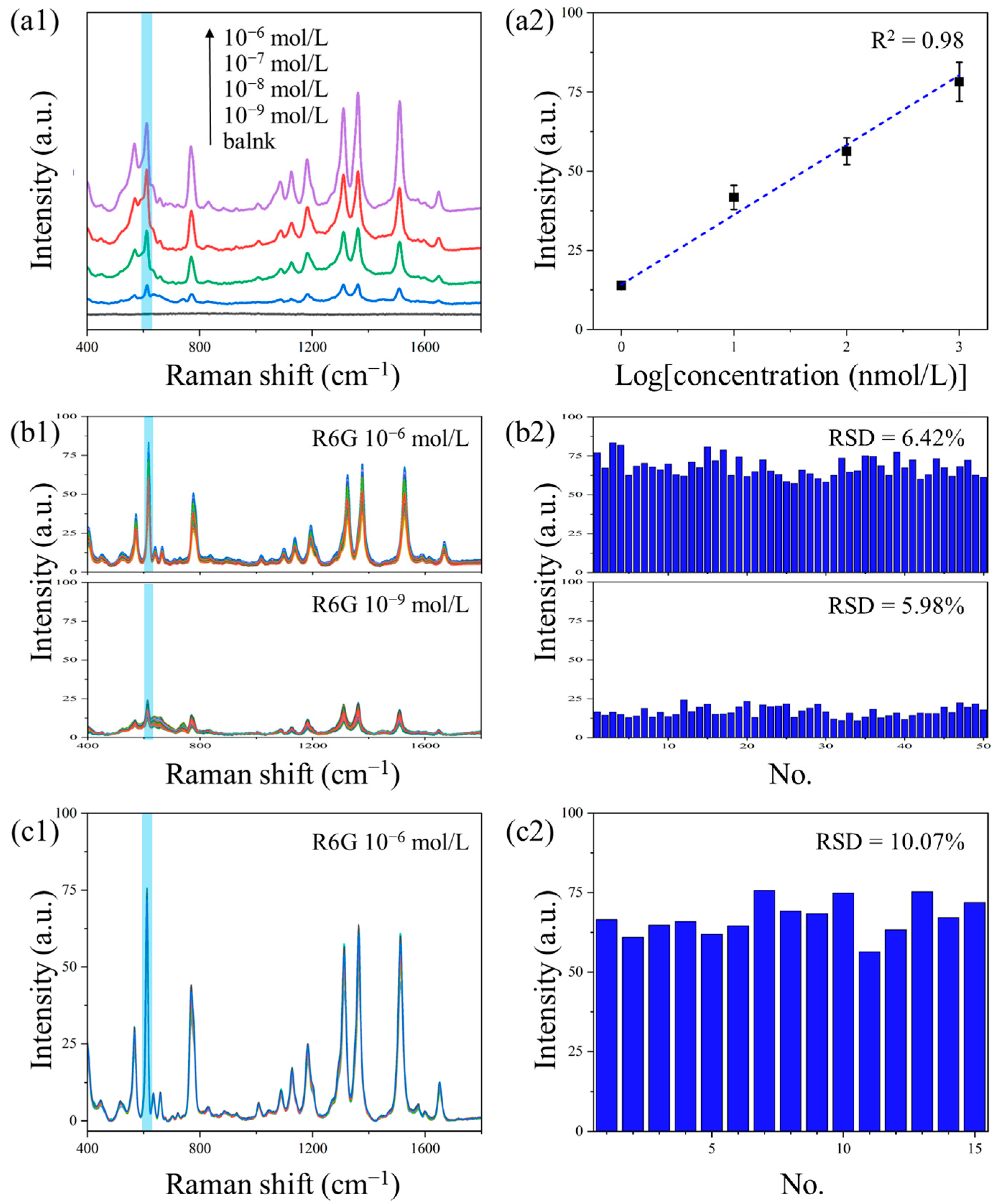
3.5. The Performance of NCF/GO/Au@Ag Substrate
3.6. Trace Detection of PAHs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vijayanand, M.; Ramakrishnan, A.; Subramanian, R.; Issac, P.K.; Nasr, M.; Khoo, K.S.; Rajagopal, R.; Greff, B.; Azelee, N.I.W.; Jeon, B.; et al. Polyaromatic hydrocarbons (PAHs) in the water environment: A review on toxicity, microbial biodegradation, systematic biological advancements, and environmental fate. Environ. Res. 2023, 227, 115716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nie, M.; Diwu, Z.; Chang, F.; Nie, H.; Zhang, B.; Bai, X.; Yin, Q. Toxicity evaluation of the metabolites derived from the degradation of phenanthrene by one of a soil ubiquitous PAHs-degrading strain Rhodococcus qingshengii FF. J. Hazard. Mater. 2021, 415, 125657. [Google Scholar] [CrossRef]
- Qin, D.; Zhao, M.; Wang, J.; Lian, Z. Selective extraction and detection of norfloxacin from marine sediment and seawater samples using molecularly imprinted silica sorbents coupled with HPLC. Mar. Pollut. Bull. 2020, 150, 110677. [Google Scholar] [CrossRef]
- Temerdashev, Z.; Prasad, S.; Musorina, T.; Chervonnaya, T.; Arutyunyan, Z. Simultaneous dispersive liquid–liquid microextraction and determination of different polycyclic aromatic hydrocarbons in surface water. Molecules 2022, 27, 8586. [Google Scholar] [CrossRef]
- Ma, T.; Shen, X.; Yang, C.; Qian, H.L.; Pang, Y.H.; Yan, X.P. Covalent immobilization of covalent organic framework on stainless steel wire for solid-phase microextraction GC-MS/MS determination of sixteen polycyclic aromatic hydrocarbons in grilled meat samples. Talanta 2019, 201, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Liamtsau, V.; Fan, C.; Liu, G.; McGoron, A.J.; Cai, Y. Speciation of thioarsenicals through application of coffee ring effect on gold nanofilm and surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2020, 1106, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Cheng, L.; Ding, S.; Wang, G.; Choo, J.; Chen, L. SERS-based test strips: Principles, designs and applications. Biosens. Bioelectron. 2021, 189, 113360. [Google Scholar] [CrossRef]
- Fraser, J.P.; Postnikov, P.; Miliutina, E.; Kolska, Z.; Valiev, R.; Svorcík, V.; Lyutakov, O.; Ganin, A.Y.; Guselnikova, O. Application of a 2D molybdenum telluride in SERS detection of biorelevant molecules. ACS Appl. Mater. Interfaces 2020, 12, 47774–47783. [Google Scholar] [CrossRef]
- He, J.; Song, G.; Wang, X.; Zhou, L.; Li, J. Multifunctional magnetic Fe3O4/GO/Ag composite microspheres for SERS detection and catalytic degradation of methylene blue and ciprofloxacin. J. Alloys Compd. 2022, 893, 162226. [Google Scholar] [CrossRef]
- Sarma, D.; Medhi, A.; Mohanta, D.; Nath, P. Electrochemically deposited bimetallic SERS substrate for trace sensing of antibiotics. Microchim. Acta 2024, 191, 14. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, R.; Li, X.; Mao, X.; Xu, S.; Zhou, N.; Li, S.; Mao, H.; Huang, C. A SERS sensor based on 3D nanocone forests capable of intelligent classification of aquatic product dyes. J. Mater. Chem. C 2023, 11, 14237–14247. [Google Scholar] [CrossRef]
- He, Q.; Han, Y.; Huang, Y.; Gao, J.; Gao, Y.; Han, L.; Zhang, Y. Reusable dual-enhancement SERS sensor based on graphene and hybrid nanostructures for ultrasensitive lead (II) detection. Sens. Actuators B-Chem. 2021, 341, 130031. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, X.; Lai, K.; Huang, Y.; Rasco, B.A.; Fan, Y. Gold nanorods as surface-enhanced Raman spectroscopy substrates for rapid and sensitive analysis of allura red and sunset yellow in beverages. J. Agric. Food Chem. 2018, 66, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, H.; Zhang, J.; Zhou, J.; Jiang, T. Recyclable SERS monitoring of food quality based on the shrubby morphology of titania oxide-triggered electromagnetic “hotspots”. Appl. Surf. Sci. 2022, 604, 154456. [Google Scholar] [CrossRef]
- Raveendran, J.; Docoslis, A. Detection and quantification of toxicants in food and water using Ag-Au core-shell fractal SERS nanostructures and multivariate analysis. Talanta 2021, 231, 122383. [Google Scholar] [CrossRef]
- Atta, S.; Li, J.Q.; Vo-Dinh, T. Multiplex SERS detection of polycyclic aromatic hydrocarbon (PAH) pollutants in water samples using gold nanostars and machine learning analysis. Analyst 2023, 148, 5105–5116. [Google Scholar] [CrossRef]
- Shan, J.; Zhang, Y.; Wang, J.; Ren, T.; Wang, X. Microextraction based on microplastic followed by SERS for on-site detection of hydrophobic organic contaminants, an indicator of seawater pollution. J. Hazard. Mater. 2020, 400, 123202. [Google Scholar] [CrossRef]
- Yu, Z.; Grasso, M.F.; Sorensen, H.H.; Zhng, P. Ratiometric SERS detection of polycyclic aromatic hydrocarbons assisted by β-cyclodextrin-modified gold nanoparticles. Microchim. Acta 2019, 186, 391. [Google Scholar] [CrossRef]
- Qiu, M.; Tang, L.; Wang, J.; Xu, Q.; Zheng, S.; Weng, S. SERS with flexible β-CD@AuNP/PTFE Substrates for in situ detection and identification of PAH residues on fruit and vegetable surfaces combined with lightweight network. Foods 2023, 12, 3096. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.; Wang, J.; Zou, Y.; Liu, T.; Zhang, Y.; Liu, G.; Tian, Z. Trace detection of polycyclic aromatic hydrocarbons in environmental waters by SERS. Spectrochim. Acta A 2020, 234, 118250. [Google Scholar] [CrossRef]
- Sánchez-Purrà, M.; Carré-Camps, M.; Puig, H.D.; Bosch, I.; Gehrke, L.; Hamad-Schifferli, K. Surface-Enhanced Raman Spectroscopy-Based Sandwich Immunoassays for Multiplexed Detection of Zika and Dengue Viral Biomarkers. ACS Infect. Dis. 2017, 3, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Salcedo, W.J.; Rahman, M.M.; Brolo, A.G. Surface-enhanced Raman scattering from bowtie nanoaperture arrays. Surf. Sci. 2018, 676, 39–45. [Google Scholar] [CrossRef]
- Han, C.; Yao, Y.; Wang, W.; Qu, L.; Bradley, L.; Sun, S.; Zhao, Y. Rapid and Sensitive Detection of Sodium Saccharin in Soft Drinks by Silver Nanorod Array SERS Substrates. Sens. Actuators B-Chem. 2017, 251, 272–279. [Google Scholar] [CrossRef]
- Mackenzie, M.; Chi, H.; Varma, M.; Pal, P.; Kar, A.; Paterson, L. Femtosecond laser fabrication of silver nanostructures on glass for surface enhanced Raman spectroscopy. Sci. Rep. 2019, 9, 17058. [Google Scholar] [CrossRef]
- Wu, T.; Lin, Y.W. Surface-enhanced Raman scattering active gold nanoparticle/nanohole arrays fabricated through electron beam lithography. Appl. Surf. Sci. 2018, 435, 1143–1149. [Google Scholar] [CrossRef]
- Yang, J.; Song, G.; Zhou, L.; Wang, X.; You, L.; Li, J. Applied surface science highly sensitively detecting tetramethylthiuram disulfide based on synergistic contribution of metal and semiconductor in stable Ag/TiO2 core-shell SERS substrates. Appl. Surf. Sci. 2021, 539, 147744. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Hu, X.; Han, F.; Zhu, C. Silver-nanoparticles/graphene hybrids for effective enrichment and sensitive SERS detection of polycyclic aromatic hydrocarbons. Spectrochim. Acta A 2020, 228, 117783. [Google Scholar] [CrossRef]
- Yan, S.; An, R.; Zou, Y.; Yang, N.; Zhang, Y. Fabrication of polymer colloidal/Au composite nanofilms for stable and reusable SERS-active substrates with highly-dense hotspots. Sens. Actuators B-Chem. 2020, 302, 127107. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Zhang, C.; Weng, G.; Zhao, J.; Li, J.; Zhao, J. Synthesis of colloidal gold nanobones with tunable negative curvatures at end surface and their application in SERS. J. Nanopart. Res. 2017, 19, 364. [Google Scholar] [CrossRef]
- Liu, B.; Han, G.; Zhang, Z.; Liu, R.; Jiang, C.; Wang, S.; Han, M. Shell thickness-dependent Raman enhancement for rapid identification and detection of pesticide residues at fruit peels. Anal. Chem. 2012, 84, 255–261. [Google Scholar] [CrossRef]
- Deepak, F.L. Metal Nanoparticles and Clusters: Advances in Synthesis, Properties and Applications; Springer: Cham, Switzerland, 2018; pp. 89–122. [Google Scholar]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; EI-Sayed, M.E. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. Indian J. Chem. A 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Gans, R. Über die Form ultramikroskopischer Goldteilchen. Ann. Phys. 1912, 342, 881–900. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Yu, X.; Sun, Q.; Huang, M.; Liu, T.; Chen, J. Sensitive and Stable NCF/GO/Au@Ag SERS Substrate for Trace Detection of Polycyclic Aromatic Hydrocarbons. Polymers 2025, 17, 1716. https://doi.org/10.3390/polym17121716
Kong L, Yu X, Sun Q, Huang M, Liu T, Chen J. Sensitive and Stable NCF/GO/Au@Ag SERS Substrate for Trace Detection of Polycyclic Aromatic Hydrocarbons. Polymers. 2025; 17(12):1716. https://doi.org/10.3390/polym17121716
Chicago/Turabian StyleKong, Lili, Xinna Yu, Qifang Sun, Meizhen Huang, Tianyuan Liu, and Jie Chen. 2025. "Sensitive and Stable NCF/GO/Au@Ag SERS Substrate for Trace Detection of Polycyclic Aromatic Hydrocarbons" Polymers 17, no. 12: 1716. https://doi.org/10.3390/polym17121716
APA StyleKong, L., Yu, X., Sun, Q., Huang, M., Liu, T., & Chen, J. (2025). Sensitive and Stable NCF/GO/Au@Ag SERS Substrate for Trace Detection of Polycyclic Aromatic Hydrocarbons. Polymers, 17(12), 1716. https://doi.org/10.3390/polym17121716






